
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioinform. , 01 June 2023
Sec. Network Bioinformatics
Volume 3 - 2023 | https://doi.org/10.3389/fbinf.2023.1189723
This article is part of the Research Topic Approaches to modelling complex biological systems View all 5 articles
Computational modeling has emerged as a critical tool in investigating the complex molecular processes involved in biological systems and diseases. In this study, we apply Boolean modeling to uncover the molecular mechanisms underlying Parkinson’s disease (PD), one of the most prevalent neurodegenerative disorders. Our approach is based on the PD-map, a comprehensive molecular interaction diagram that captures the key mechanisms involved in the initiation and progression of PD. Using Boolean modeling, we aim to gain a deeper understanding of the disease dynamics, identify potential drug targets, and simulate the response to treatments. Our analysis demonstrates the effectiveness of this approach in uncovering the intricacies of PD. Our results confirm existing knowledge about the disease and provide valuable insights into the underlying mechanisms, ultimately suggesting potential targets for therapeutic intervention. Moreover, our approach allows us to parametrize the models based on omics data for further disease stratification. Our study highlights the value of computational modeling in advancing our understanding of complex biological systems and diseases, emphasizing the importance of continued research in this field. Furthermore, our findings have potential implications for the development of novel therapies for PD, which is a pressing public health concern. Overall, this study represents a significant step forward in the application of computational modeling to the investigation of neurodegenerative diseases, and underscores the power of interdisciplinary approaches in tackling challenging biomedical problems.
The interpretation of omics data is crucial to develop meaningful hypotheses to understand complex disease mechanisms (García Del Valle et al., 2019). To interpret these data, pathway databases play a role in providing an overview of the processes involved in disease mechanisms. Community-driven initiatives, such as disease maps, have been established to encode disease mechanisms in a computable form (Mazein, 2018). The disease maps can be further visualized through the use of dedicated biocuration and visualization tools (Wiese, 2004; Funahashi, 2007; Kuperstein, 2013; Gawron, 2016; Balci, 2020). The information provided by pathway databases and disease maps can then be utilized to develop computational models, which are crucial in advancing our understanding of the dynamic properties of complex diseases (Silk, 2014). By integrating experimental data with computational models, researchers can identify key molecular players and pathways involved in disease initiation and progression, and predict the effects of therapeutic interventions. Therefore, the use of disease maps and computational modeling represents a promising approach in the field of biomedical research, with potential implications for the development of personalized medicine and precision therapies Dynamic modeling approaches such as Boolean or Multi-valued models, Petri nets, and Ordinary Differential Equations (ODEs) (Walter, 1998; Dubrova, 2006; Aalst, 2009) can be used to represent the complex dynamics of biological systems and diseases. However, parameterizing these models remains a significant challenge (Ilea, 2012). Logical models provide a valuable alternative for researchers, as they can be more easily constructed and parameterized specially with limited amount of data (Naldi, 2018).
By utilizing logical models, disease maps can be encoded in a computable form, and the dynamics of diseases can be analyzed in a more straightforward manner. These logical models can be further integrated with experimental data to create more comprehensive and accurate representations of disease dynamics. This approach has the potential to facilitate the discovery of novel drug targets and the development of personalized medicine.
Therefore, further research is needed to better understand the molecular mechanisms underlying diseases and advance personalized medicine. By leveraging community-driven initiatives such as disease maps and computational modeling approaches, researchers can gain a deeper understanding of complex diseases, and ultimately, develop more effective treatments.
Logical modeling in systems biology is a mathematical approach that uses Boolean algebra to represent the interactions between components in a biological system (Albert and Thakar, 2014). Logical modeling is a valuable tool for understanding the behavior of cellular networks and gene regulation networks, as well as for predicting the effects of perturbations such as drug treatments (Maldonado et al., 2017). There are several types of logical modeling used in systems biology, including Boolean models (BMs), probabilistic BMs, Petri nets, and rule-based models (Aalst, 2009).
Boolean models are the most commonly used type of logical modeling in systems biology (Albert and Thakar, 2014). Boolean models represent variables with binary values of one (ON) or zero (OFF) (Albert and Thakar, 2014). The behavior of the output biomolecules is described by Boolean functions (BFs) that define the interactions between the inputs. Updating schemes determine the order in which BFs are calculated (Hemedan et al., 2022). BFs have been primarily used to describe gene regulation, but they have also been applied to signaling networks using various logical formalisms, including Boolean, differential, and fuzzy equations (Terfve, 2012; Bloomingdale, 2018; Eduati, 2020).Boolean models provide a qualitative representation of the system and its interactions, without the need for detailed kinetic information (Aalst, 2009). However, in some cases, presenting data in a simple BM may not provide the best description of the biological system, and integration with other quantitative methods may be necessary (Maldonado et al., 2017).
In this study, we utilized Boolean modeling to represent the complex molecular mechanisms underlying Parkinson’s disease (PD). By using the Parkinson’s disease map Fujita et al. (2014) PD mechanisms in a computable form, we simulated the dynamics of the disease and proposed potential drug targets. By abstracting the disease mechanisms in a logical form, we can simulate disease dynamics and identify potential therapeutic targets, ultimately facilitating the development of personalized medicine. For example, LRRK2 mutations have been found to increase the aggregation of cytosolic proteins, leading to apoptosis and cell dysfunction, which could be targeted by therapeutic interventions (Gopalakrishna and Alexander, 2015).
The PD map is translated into BMs in an automated fashion using CaSQ tool (Aghamiri et al., 2020). The complexity of disease pathways is studied by simulating the effect of genomic perturbations from omics data. BMs are created at different scales of the complexities to ensure its ability and reliability to simulate disease mechanisms. These different levels of complexity refer to the different scales at which disease pathways can be represented and analyzed. First, the simple and known mechanisms are simulated to investigate the model ability to represent the already known behaviour. Further, the BMs are used to re-simulate complex molecular interventions data, comparing the results with the literature. Creating and validating BMs at different levels of complexity is a crucial step towards developing accurate simulations of biological processes. Gradually increasing the complexity of the model allows us to identify key factors that impact the accuracy of the simulation, such as specific biological components and interactions, and refine the model accordingly. Through this process, we can better understand the complexity of disease mechanisms and develop more reliable results that can ultimately contribute to the development of effective treatments. BMs were created in different modelling formats, facilitating the interoperability and the simulation across different tools. This includes SBML-qual, which is used for creating, storing and exchanging qualitative models (Chaouiya et al., 2013). Analysis of the models’ structural and dynamic properties was used to verify their accuracy. The dynamic verification and sensitivity analysis results showed that the BFs accurately represented the original interactions and the model was robust against single perturbations. The validation was performed by simulating models in different scales of complexity. We analyzed and validated a number of pathways based on the literature and experimental evidence. These included the TCA cycle, dopamine transcription, FOXO3, and the Wnt-PI3k/AKT signaling pathway. Our results demonstrated the utility of Boolean modeling in simulating the behavior of these pathways and predicting the effects of perturbations, providing valuable insights into the molecular mechanisms underlying PD.
The diagrams analyzed in this paper were obtained from the MINERVA Platform (Gawron, 2016) (see the Supplementary Material). This platform provides the capability to export specific diagrams from the map. The diagrams in CellDesigner SBML format were then automatically transformed into SBML-qual format using the CaSQ (CellDesigner as SBML-qual) tool. CaSQ uses specific rewriting rules to reduce diagrams from Process Description to Activity Flow notation, and to infer the logical functions and translate the interactions. Aghamiri et al. (2020).
Rule 1: In a reaction, where a receptor and a ligand form complex, the receptor reactant is removed.
Rule 2: In a reaction, where two proteins form a complex, the reactants are removed and modifiers are linked directly to the product.
Rule 3: Inactive forms of a biomolecule in a single reaction are removed (do not participate in other interactions).
Rule 4: In a single transport reaction a reactant is removed and the reactions are linked to the product (in case product and reactant are the same).
The translation process involves converting the Process Description notation into the Activity Flow notation (see Figure 1). Biomolecules in Process Description diagrams may exist in various states (such as phosphorylated or methylated), however, during the translation to the Activity Flow notation, each biomolecule is represented as a single node with its different states depicted as distinct logical states of the node, such as active or inactive.
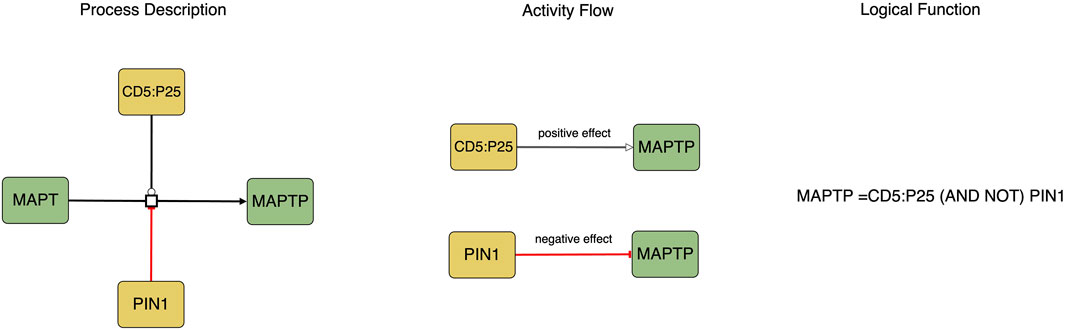
FIGURE 1. This figure represents the causal molecular interactions in both Process Description and Activity Flow. The logic equation depicted in the Process Description indicates that the activity of MAPTP as a product is determined by the presence of CD5:P25 and the absence of PIN1.
The structural and functional correctness of BM was evaluated by analyzing the interactions between the biomolecules. To this end the topological features of the BM were analyzed as a network. To do so, Simple Interaction Format (SIF) was created as a simple way to represent pairwise interactions among biomolecules in a network graph. To transform an SBML model into SIF format, the model is first converted into a graph representation with nodes representing biomolecules and edges representing interactions between biomolecules. Relevant information such as the source and target nodes of each edge is then extracted from the graph representation and mapped to the SIF format. The resulting SIF representation is a list of pairwise interactions between nodes, which can be useful for evaluating the structural correctness of biological models. Established tools Trinh and Kwon (2019), were employed to analyze the network, including the in/out degree, feedback loops, and centrality measures. Among the centrality measures, betweenness and stress centrality were utilized to quantify the significance of each node in the network.
Betweenness centrality measures the importance of a node as an intermediary between other nodes, by quantifying how frequently a node lies on the shortest path between pairs of other nodes in the network. Stress centrality measures the importance of a node in connecting other nodes by quantifying the number of shortest paths that pass through the node. (Ma’ayan 2011; Ashtiani et al., 2018).
Betweenness centrality:
where V is the set of nodes in the network, σ(s, t) is the total number of shortest paths from node s to node t, and σ(s, t|v) is the number of those shortest paths that pass through node v.
Stress centrality:
where V is the set of nodes in the network, and gst(v) is the number of shortest paths from node s to node t that pass through node v. Molecular targets for perturbation were prioritised based on their centrality and sensitivity measures. First, we computed betweenness centrality and stress centrality scores for all nodes in the network and identified the top-ranked nodes with high centrality values. Next, we simulated perturbations (Knockouts and overexpressions) to the network and measured the similarity and identity based distances between the original and perturbed states to assess the sensitivity of each node to the perturbations. By combining the centrality and sensitivity scores, we prioritized the molecular targets for perturbation that were highly central and sensitive.
The updating schemes of Boolean functions (BFs) were evaluated and compared, including synchronous updating, asynchronous updating (Garg et al. (2008).
The synchronous updating scheme updates all biomolecules simultaneously according to their BFs (Garg et al. (2008); Wang (2012).
The asynchronous scheme updates the variables in a non-synchronous manner. The new value of each variable σ is denoted by an asterisk and is given by:
Here, the values of the inputs on the right-hand side of the equation can be either current or prior, depending on the individual timescales (Wang, 2012).
Probabilistic Boolean models (PBMs) was also be used to update BFs in a probabilistic manner by assigning probabilities to BFs and updating biomolecules based on these probabilities (Grieb, 2015; Schwab, 2020). A PBM can be described as follows:
where Xt is the state of the network at time t, xt is a vector of binary values representing the state of each node in the network at time t, pai is the set of parent nodes of node i in the network, and θi is a vector of probabilities representing the conditional probability distribution of node i given its parent nodes. The equation states that the probability of the network being in state xt at time t, given its previous states and the network structure, is the product of the conditional probabilities of each node in the network.
Synchronous updating scheme was simulated using BoolNet tool in which all the biomolecules in the network updated simultaneously, based on the states of their regulators (Müssel, 2010). Asynchronous updating scheme was simulated using pyMaBoSS Stoll et al. (2017), see Stochastic Boolean model simulation section.
The behavior of a BM under different update schemes was visualized through state transition graphs (see section 5 in the supplementary file), which represent all possible states of the system and the transitions between them (Garg et al., 2008). The state transition graph illustrates the range of outcomes for a given initial condition based on the update scheme used. Both update schemes demonstrated the ability to simulate expected system behavior.
Attractors are states in a state transition graph that have no outgoing edges and can be stable or complex. The basin of attraction is the set of states that lead to an attractor and can provide insight into potential biological scenarios (Klemm and Bornholdt, 2005; Hopfensitz, 2012). During the verification of models, several search algorithms were examined to compare their speed and reachability. One method used was an exhaustive search, which aimed to find all possible attractors by performing synchronous transitions between states. To increase efficiency, a SAT-based technique was employed, where the problem of attractor identification was framed as a Boolean satisfiability problem (SAT). This allowed for determining if a particular formula was satisfiable or not and to limit the search to loops of a specific length (Biere, 2008). The SAT-based technique involves representing the state of the network as Boolean variables, and representing the Boolean rules that govern the transitions between states as logical constraints. Finding solutions to the SAT problem that correspond to attractors in the network allows for the attractor search.
The SAT-based technique can be formulated mathematically as follows:
Let x1, x2, …, xn be the Boolean variables that represent the state of the network, where xi is either 0 or 1. Let f1, f2, …, fm be the Boolean functions that represent the transitions between states, where each function is a logical constraint that depends on the values of the Boolean variables. The goal of the SAT-based technique is to find a set of values for the Boolean variables that satisfy the logical constraints and correspond to an attractor in the network. This can be represented as a SAT problem as follows:
Find x1, x2, …, xn such that
The SAT problem can be efficiently solved using SAT solvers to determine the attractors in the network.
Another approach, the decomposition method, aimed to optimize speed and reduce complexity by breaking down the model into strongly connected components (SCCs). The method can be represented mathematically as:
where f(x) is the original problem, n is the number of subproblems, and gi(xi) is the solution to each subproblem. The subproblems are usually defined so that they can be solved independently and then combined to obtain a solution to the original problem. The decomposition method is an approximation of the Boolean system and may not work well in all cases, depending on the network simulated.
In addition, a heuristic search and an asynchronous search were conducted. The heuristic search involved selecting a subset of possible states as initial conditions and performing synchronous transitions until an attractor was reached. The asynchronous search used random asynchronous transitions to identify steady states and complex attractors from the chosen initial conditions. To evaluate cyclic attractors, we performed multiple simulation runs starting from different random initial states until all cyclic attractors were identified. We identified all the attractors present in the system during each simulation run and considered them as potential steady states or complex attractors that the system could reach under certain initial conditions. By performing multiple simulation runs and identifying all the attractors present in the system, we were able to gain a more comprehensive understanding of the system’s behavior and the potential biological scenarios it could represent.
A perturbation analysis was conducted to evaluate the effect on the topological robustness, dynamic resilience, and attractors reached by the models (Trinh and Kwon, 2019). Specially, we focused on node perturbations, which altering the state of a single biomolecule through knockout and overexpressions.
The evaluation was performed by performing sensitivity analysis on a selected set of models, examining each biomolecule. Sensitivity analysis is a technique that assesses how changes in a model or system’s inputs affect its output, in this case, the two attractors reached by the model (unperturbed and perturbed). To quantify the difference between the two attractors, similarity-based distance and identity-based distance were used. Similarity-based distance assessed the similarity between the two attractors by taking into account the common and unique states in both. The similarity-based distance between the unperturbed attractor (Aunpert) and the perturbed attractor (Apert) can be defined as:
Identity-based distance, on the other hand, measured the percentage of states present in both attractors.
The simulations of the specified biological models were conducted using pyMaBoSS, a python API for the MaBoSS software (Stoll et al., 2017). This framework provides a tool for probabilistic Boolean modeling and simulation of biological systems through discrete/continuous time Markov processes. It operates by utilizing a Monte Carlo algorithm that simulates the system’s evolution over time based on the initial conditions of the biomolecules and the interactions between them.
pyMaBoSS performs asynchronous updates through a random walk simulation technique, in which a single biomolecule is selected and updated at each step, followed by repeating this process to obtain a sample of the attractors. Asynchronous transitions are used in pyMaBOSS to determine steady states and complex attractors from the specified initial states.
In pyMaBoSS, a biological system is represented by a model of interconnected Boolean variables, with each variable representing the state of a biomolecule (e.g. present or absent, active or inactive). The interactions between the variables are defined by Boolean rules, which indicate the impact of one variable’s state on another. The Monte Carlo algorithm randomly samples the possible states of the system at each time step, based on the current state of the system, the probabilities of each state, and the interactions between the variables. By simulating the system over multiple time steps, pyMaBoSS estimates the probability of each state at each time point (Stoll et al., 2017).
We investigated the dynamic behavior of molecular mechanisms in Parkinson’s disease (PD) and their response to changes. To achieve this goal, a variety of BM formats - including SBML-qual - were created and analyzed using a range of tools. The validity of the BMs was evaluated by examining their structural and dynamic properties. The results of this assessment demonstrated that the models displayed consistent structural and dynamic properties, suggesting their use in study of the dynamics of PD.
To construct the Boolean models of PD mechanisms, we first selected a group of PD map diagrams for downstream modeling and verification. We carefully chose diagrams that were directly relevant to PD-related phenotypes, as outlined in Table 1 (see also Supplementary Figures S1–S6 i n the supplementary file). Once we identified the relevant pathways from the PD map, we exported them in CellDesigner SBML format, allowing for further analysis and modeling. The CellDesigner SBML diagrams provided us with a detailed representation of the biological pathways involved in PD, which we used to construct our Boolean models.
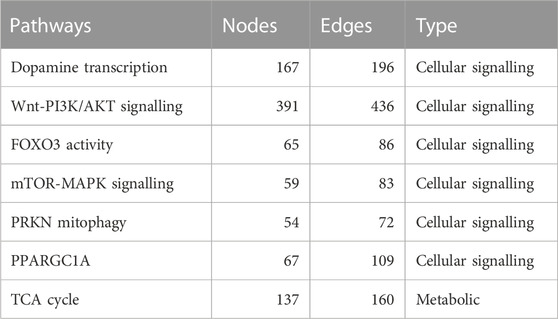
TABLE 1. This table presents a summary of the selected pathways with their nodes, and edges in several important cellular signaling and metabolic pathways. The pathways listed include dopamine transcription, Wnt-PI3K/AKT signaling, FOXO3 activity, mTOR-MAPK signaling, PRKN mitophagy, PPARGC1A, and the TCA cycle. The number of nodes and edges for each pathway is also provided.
To generate the SBML-qual models necessary for our modeling work, we used CaSQ. This software allowed us to convert the CellDesigner SBML diagrams into a SBML-qual model that facilitated the interoperability between modeling tools. With these Boolean models, we analysed the PD mechanisms to explore the underlying biology of the disease and gain insights into potential therapeutic targets.
To verify the accuracy and reliability of BMs, both structural and dynamic aspects of the model must be examined. First, we evaluated the interactions and relationships between the model’s components, to examine how they reflect the underlying biological system. For dynamic verification, we analysed the model’s behavior over time and compared it with the known biological behaviour represented in the literature.
We aimed to identify molecules that can serve as potential targets and intervention points in pathways. To achieve this, we conducted a structural and sensitivity analysis. We selected the molecules with the highest betweenness centralities of each respective model (top quantile). These molecules were proposed to play a role in maintaining the stability of the system as they are located at the intersection of multiple paths. The table below (Table 2) shows the identified molecules and their corresponding betweenness centralities.
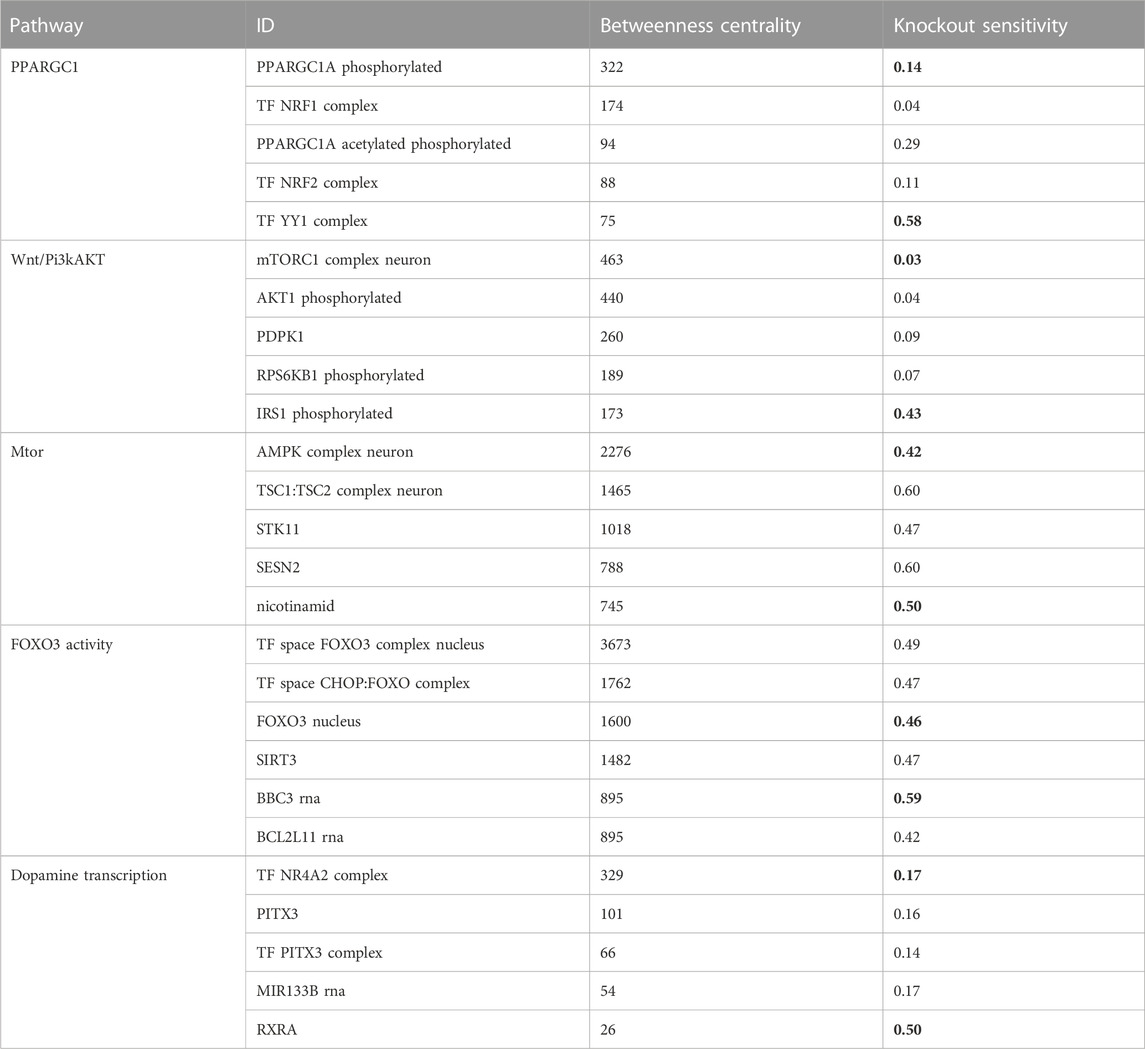
TABLE 2. The table displays the betweenness centrality values and knockout sensitivity for key pathways and relevant biomolecules. The bolded valued indicate that biomolecules with high betweenness have low knockout sensitivity, and vice versa, while some with low betweenness have high knockout sensitivity.
The second step of our analysis aimed to evaluate the sensitivity of the identified high central molecules to perturbations, specifically gene knockouts. We investigated whether the levels of the molecules are reliable measures to estimate the effect of perturbation and to identify potential targets for intervention.
We found that most of the high central molecules showed less sensitivity against knockouts, indicating their robustness in maintaining the system’s stability. This observation suggests that targeting these molecules may not have a significant impact on the overall system stability. However, this does not necessarily mean that they are not potential targets for intervention, as their perturbation may have downstream effects that affect other pathways or processes (see Figure 2).
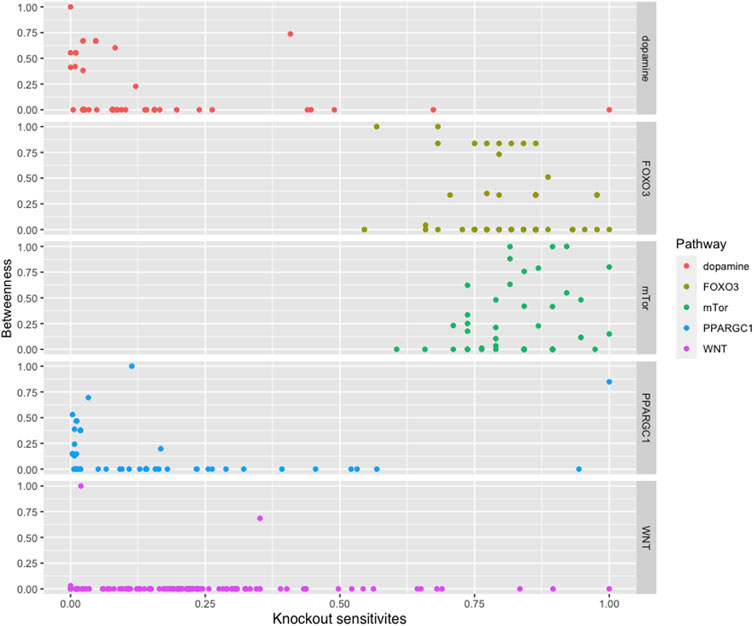
FIGURE 2. The figure displays a comparison between the betweenness centralities and sensitivity measures in selected pathways. While most pathways exhibit high betweenness centralities, their sensitivity measures are low. This suggests that betweenness centrality may not be a reliable indicator of the significance of biomolecules in these pathways.
To further investigate the reliability of the identified molecules as potential targets for intervention, we identified the common molecules that have significant centralities and/or sensitivities and also have reported biological importance (see Tables 3, 4). These perturbations were selected based on known biological scenarios to assess the model’s ability to reproduce the known behavior.
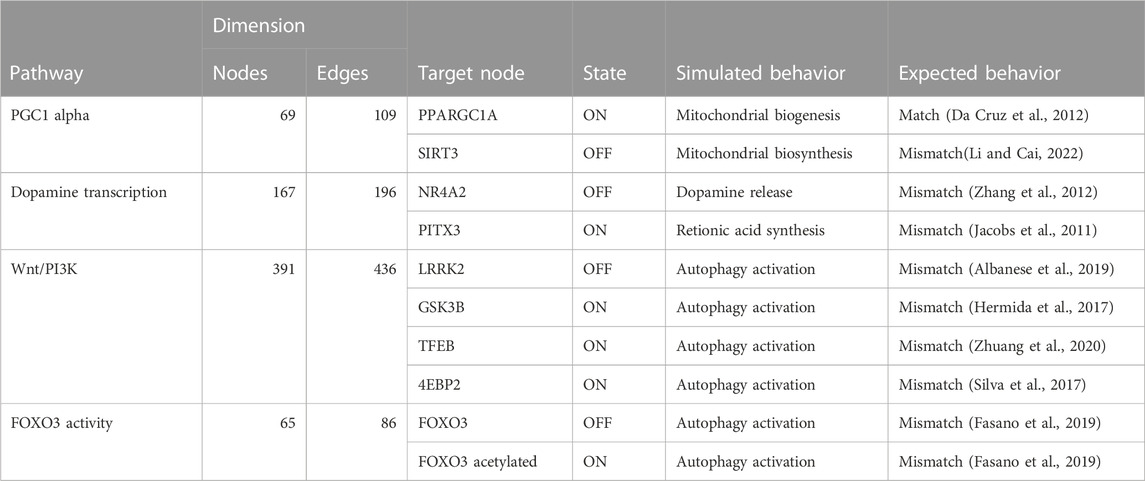
TABLE 3. The table compares the simulated behavior of decomposed Boolean models to expected behavior based on published literature. The table includes information on the pathways, the number of nodes and edges in each network, the target node, the state of the target node (ON or OFF), and the simulated and expected behavior for each pathway.
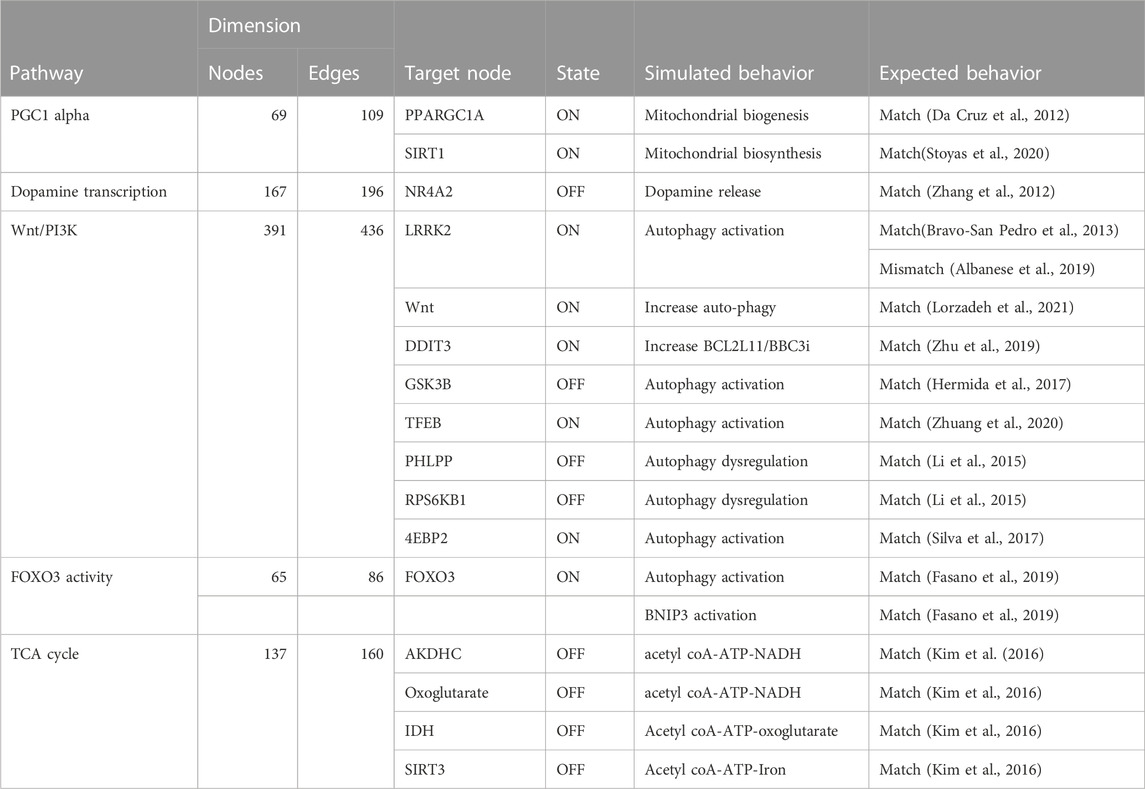
TABLE 4. The table compares the simulated behavior of several Boolean models to expected behavior based on published literature. The table includes information on the pathways, the number of nodes and edges in each network, the target node, the state of the target node (ON or OFF), and the simulated and expected behavior for each pathway.
In the Wnt Pi3kAKT pathway, we identified molecules such as ROCK2, EIF4E, IGFR1, IGF1, and INS that can compensate for the absence of PDPK1, RPS6KB1 phosphorylated (Figure 3). The presence of such alternative molecules can maintain the overall function of the pathway when such a scenario arises. These compensatory paths may be exploited to develop targeted interventions for pathological conditions.
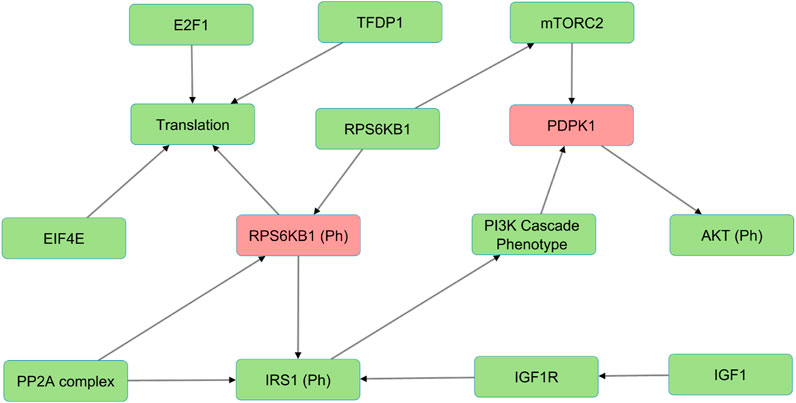
FIGURE 3. The figure illustrates an example of alternative molecules in the Wnt/PI3K-AKT model (shown in green) that compensate for PDPK1 and RPS6KB1 phosphorylated knockouts (shown in red) and reduce sensitivities. These compensatory molecules enable the pathway to continue functioning despite the loss of the phosphorylated proteins and reduce the overall sensitivity of the pathway to perturbations.
In this study, we aimed to identify stable states, known as attractors, within the analyzed pathways using Boolean modeling. To ensure the biological relevance of the identified attractors, a thorough literature search was conducted. The search aimed to identify experimental evidence for the molecular states and interactions observed in the identified attractors. The findings of this literature search were summarized in Tables 3, 4, which present the expected behavior of the molecules and their corresponding stable states. The relevance of each stable state was evaluated based on its correspondence to known biological states, its association with PD, and its relevance to the analyzed pathways.
Our results indicated that the attractors produced by decomposition-based approaches (in methods) were not biologically relevant for all pathways (see Figure 4; Table 3). This discrepancy was attributed to their aggressive decomposition which resulted in overly fragmented models (available in the Gitlab repository).
FIGURE 4. The figure illustrates the attractor pattern of decomposed WNT-Pi3k/AKT. The yellow and green colours represent the OFF and ON states of the molecules in the attractor.
However, the attractors produced by Heuristic and SAT solver algorithms were found to be viable for all pathways except for the Wnt/PI3K pathway (see Table 4). Therefore, we deemed Heuristic and SAT solver algorithms more suitable for further analysis.
We validated the model outputs by comparing them to the literature. We first selected targets for the pathways and perturbed them according to scenarios suggested by literature. We then simulated the changes in the corresponding outputs. This was achieved through: i) running a simulation with modeling tools, including probablistic Boolean modelling tools and the CellCollective platform Helikar (2012); Trinh and Kwon (2019); Stoll et al. (2017). ii) Comparing the computed attractors by modeling tools with the differential expressed molecules reported in corresponding published dataset (Zhang et al., 2012) and literature as shown in Table 4. The validation process has yielded both matching and mismatched scenarios between the model outputs and pertinent literature. Further exploration into the causes of the observed mismatches will contribute to an enhanced understanding of the limitations and strengths of the model. Further, the comparison to literature, as described, represents a crucial step in the ongoing refinement of our comprehension of the pathways and perturbations. The validation scenarios, results, and interpretation for the selected pathways are discussed in subsequent sections.
The TCA cycle, a metabolic pathway occurring in the mitochondria of cells, is disrupted in Parkinson’s disease (PD), potentially contributing to its development (Shen et al., 2020). This pathway is an ideal candidate for validating the BM approach due to the extensive literature and data available for comparison, as well as its well-studied and understood nature (Ahn et al., 2017). Furthermore, the TCA cycle involves the transformation of multiple metabolites, which can be monitored through techniques such as fluxomics, providing a means of evaluating the BM’s accuracy by comparing predicted and experimentally observed metabolite levels. Given its central role in various cellular processes, a BM of the TCA cycle could have broad and far-reaching implications.
The validity and precision of the TCA cycle BM are established through a comparison with literature and fluxomics data. To run the simulation, the knockouts of isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase, and the pyruvate dehydrogenase complex are selected as the initial state parameters based on prior research (Ahn et al., 2017). Using temporal-fluxomics data, the levels of alpha-ketoglutarate dehydrogenase activity and the nucleotide triphosphates GTP and GDP’s impact on ATP levels are modeled using CellCollective. The simulation results are then compared to experimental data as described below.
As shown in the fluxomics data, the validated results based on literature indicate that the simulation of increased activation of acetyl CoA, NADH, and PDKs leads to an increase in phosphorylation reaction, subsequently reducing the activity of the pyruvate dehydrogenase complex (PDC). This finding supports the hypothesis that PDC deficiency, a potential target for therapeutic intervention in age-related diseases, arises from the activation of the phosphorylation reaction involving these substances (Stacpoole, 2012). The deficiency of PDC, caused by KGDHC knockout, has a significant impact on the production of succinic semialdehyde (Sergi and Parayil Sankaran, 2021). This deficiency results in decreased levels of succinic acid and succinyl CoA, leading to a decline in ATP and GTP in mitochondria (Gibson et al., 2003; Shi et al., 2011). The knockout of isocitrate dehydrogenase leads to a decrease in ATP levels and a disruption in the oxidative decarboxylation catalysis of isocitrate into alpha ketoglutarate, resulting in mitochondrial dysfunction and dopaminergic neurotoxicity (Kim et al., 2016). In PD, key regulators such as oxoglutaric acid, glutamate hydrogenase 1 (GLUD), and ATP levels are disturbed in response to SIRT3 knockout, which directly impacts mitochondrial function (Shen et al., 2020). These findings are summarized in Table 4.
The cellular metabolites undergo fluctuations during the cell cycle, adapting to changes within the cell. The impact of alpha KGDH, GTP, and GDP on ATP levels are modeled in the simulation. The simulated ATP activity levels match the measured concentrations in synchronized HeLa cells every 3 h following release from growth arrest
One of the pathways validated in this study was the dopamine transcription pathway, known to be disrupted in Parkinson’s disease (PD) (Barneda-Zahonero et al., 2012).
NR4A2 were selected as the key elements in this validation, as they are proteins that play crucial roles in the development and maintenance of neurotransmitters and various cellular processes (Barneda-Zahonero et al., 2012; Li et al., 2020).
In the simulation, the effects of perturbations to NR4A2 and SIRT1 on dopamine release and mitochondrial biosynthesis were observed as activity levels using CellCollective. These results, confirmed through a comprehensive attractor analysis (available in the Gitlab repository), demonstrate the following behavior in line with literature (Table 4):
• The production of brain-derived neurotrophic factor (BDNF), crucial for the survival and growth of neurons, was impacted by the NR4A2 knockout. This result aligns with previous research showing that NR4A2 is involved in the stimulation of BDNF production in response to the neurotransmitter N-methyl-D-aspartate (NMDA) (Barneda-Zahonero et al., 2012).
• The NR4A2 knockout was also observed to affect other molecules such as GCH1, TH, DDC, SLC18A2, SLC6A3, and DRD2, which play significant roles in the development and maintenance of neurotransmitters through various targets, as previously reported in Jankovic et al. (2005); Kadkhodaei et al. (2013); Jacobs et al. (2009).
The Wnt-PI3K/AKT pathway was evaluated in this study as it has been reported to be impacted by mutations in Parkinson’s disease (PD) (Bravo-San Pedro et al., 2013; Rabanal-Ruiz et al., 2017; Madureira et al., 2020). The key elements of the pathway considered during the validation were LRRK2 G2019S mutation, Wnt, DDIT3, GSK3B, TFEB, PHLPP, RPS6KB1, and 4EBP2, which are involved in the development and progression of PD (Bravo-San Pedro et al., 2013; Poret and Guziolowski, 2018; Zhu et al., 2019; McCabe et al., 2020).
The simulation scenario involved perturbing these biomolecules and observing the effects on autophagy and neuron survival as activity levels using CellCollective. These results were confirmed through an exhaustive attractor search analysis. The results showed the following behaviors that are in line with the published literature (Table 4, 5):
• The simulation results showed that overexpression of the LRRK2 G2019S mutant increased autophagy (Bravo-San Pedro et al., 2013).
• This increase was found to be mediated by the inhibition of mTORC1/2, which are proteins that regulate autophagy (Poret and Guziolowski, 2018; McCabe et al., 2020). These findings suggest that the interconnectedness of amino acid sensing, mTORC1 signaling, and autophagy may offer a promising approach for treating PD (Rabanal-Ruiz et al., 2017).
• Activating both 4EBP2 and TFEB was found to increase autophagy activity more than activating them separately (Decressac et al., 2013; Moors et al., 2017; Franco-Juárez et al., 2022). Specifically, activating both of these proteins in combination resulted in a 10.7% and 13.6% increase in autophagy compared to activating them individually.
• In addition, the combination of inhibiting the proteins RPS6KB1 and PHLPP and activating TFEB significantly decreased neuronal death and the active state of autophagy (Li et al., 2015). This combination resulted in a 96.3% decrease in neuronal death.
• Both activating the Wnt protein and inhibiting the protein GSK3B were found to increase autophagy (Hermida et al., 2017; Yang et al., 2018; Palomer et al., 2019). The combination of these modulations could improve our understanding of therapeutic protocols for neurological diseases by promoting neurogenesis and autophagy (Siegle, 2018). These findings suggest that targeting these proteins could be a promising approach for developing treatments for neurological disorders.
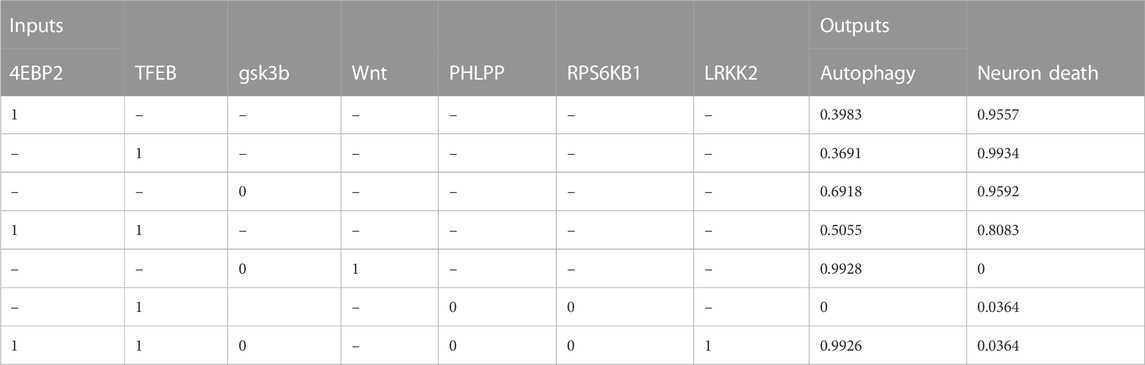
TABLE 5. The table shows the relationship between molecular target interventions and the probabilities of autophagy and neuron death. The data includes the levels of seven different inputs, which are known to play a role in these processes. The table presents the resulting probability of autophagy and neuron death for each combination of input levels.
The FOXO3 activity pathway was also evaluated in this study due to its dysregulation in Parkinson’s disease (PD) (Pino et al., 2014). The key element considered in this validation was the FOXO3 biomolecule, which plays important roles in autophagy, cell cycle progression, apoptosis, and stress resistance in PD (Pino et al., 2014; Fasano et al., 2019; Cheng, 2022).
The simulation scenario involved perturbing FOXO3 and observing the effects on autophagy and RNA-mediated biomolecules as activity levels using CellCollective. These results were confirmed through an exhaustive attractor search analysis. The results showed the following behaviors that are in line with published literature (Table 4):
• The simulation results showed that FOXO3 activation increased autophagy in mitochondria (Fasano et al., 2019).
• FOXO3 activation also activated different biomolecules involved in RNA-mediated mechanisms, including BECN1, GABARAPL1, MAP1LC3A, BNIP3, ATG12, and MUL1, which are known to be important regulators of autophagy (Hou et al., 2020).
In order to validate the relevance of biomolecules for studying PD mechanisms, four pathways were analyzed, including one metabolic and three signaling pathways. The simulated behavior of these pathways was compared to the expected behavior based on published literature (Table 4). While the behavior of the pathways was largely consistent with published literature, some discrepancies were observed in the Wnt/PI3K pathway, specifically in the simulated behavior of LRRK2. The results suggested that they are trustworthy indicators of biological processes, as they align with available data. Nonetheless, there was a mismatch in the Wnt-PI3K/AKT signaling pathway wre the model did not match published literature, leading to its revision.
Several factors could contribute to these discrepancies, such as differences in experimental conditions or protocols, the use of different LRRK2 models or cellular systems, the complexity of the autophagy pathway, and limited understanding of the exact mechanisms by which LRRK2 regulates the autophagy pathway. To address these discrepancies, corrective measures were taken, including modifying the Boolean function to better represent the interactions between LRRK2, ARFGAP1, and autophagy (Stafa et al., 2012). These results suggest that the simulated behavior of the pathways is largely consistent with published literature.
In this study, Boolean modeling was used to examine the complexity of PD by simulating the dynamic interactions between various biomolecules. These models were used to test hypotheses about the role of certain biomolecules in PD progression, by simulating the effects of these biomolecules and comparing the results to experimental or observational data. Additionally, the models were used to investigate the impact of multiple perturbations on the disease and to identify patterns that may not be evident from experimental or observational data alone.
The Boolean models were automatically constructed using the CaSQ tool (Aghamiri et al., 2020). One of the key benefits of using the CaSQ tool is its ability to apply specific rewriting rules to simplify the model and make it more manageable. Another advantage is its ability to translate diagrams into the SBML-qual format, which is a widely adopted standard in the systems biology community for representing qualitative dynamic systems. This format allows for the description of the model structure (biomolecules and interactions) and the mathematical equations describing their behavior over time, making it easy to share and compare models with other researchers.
The transformation of the diagrams into Simple Interaction Format (SIF) using the CaSQ tool was also beneficial, as it allowed for the use of various tools to analyze the diagrams. By using different tools to analyze the diagrams, researchers can gain a more comprehensive view of the disease, leading to an improved understanding of its underlying mechanisms.
The reduction of Process Description notation into Activity Flow notation and inference of logical functions from interactions, in combination with the use of SBML-qual format, resulted in the creation of models that are both accurate and computationally efficient. This makes the generated models suitable for studying large and complex systems, and allows for a deeper understanding of the system and more accurate predictions about its behavior. Therefore, the constructed Boolean models have the potential to be expanded to study larger and more complex systems.
It’s worth mentioning that using specific knowledge resources, such as PD map, has the advantage of providing a high quality of disease-specific knowledge. This is because the diagrams are focused on a specific disease and are created and reviewed by experts in the field. This expertise and specificity make the generated models more reliable.
The construction of models in compliance with systems biology standards enhances their compatibility with various tools and programs, leading to improved reproducibility and easier pipeline development. By adhering to these standards, researchers can ensure that their models are easily comprehended and integrated with other models and data sources in a consistent and dependable manner. Inadequate modeling details can lead to erroneous predictions. Thus, it is crucial to prioritize model quality during construction to minimize false positives during simulation. Verification is a critical aspect of the modeling process, as it confirms the reliability of the model and its ability to make accurate predictions and inform decision-making.
This study evaluated the BMs by demonstrating their ability to replicate experimentally validated studies from literature. The results, as shown in Table 4, indicate that the simulated behavior of the models aligns with expected behavior under both original and perturbed conditions. The simulation of known perturbations confirms the models’ ability to recreate known pathological conditions, enhances understanding of these conditions, and enables the identification of a reliable set of biomolecules and translation rules. The selection of PD map diagrams for downstream modeling and verification is crucial in comprehending the biological mechanisms underlying PD and identifying potential targets for therapy development. These diagrams were chosen based on their relevance to PD phenotypes such as mitochondrial dysfunction, dopamine dysregulation, alpha-synuclein aggregation, neuroinflammation, and oxidative stress (Antony et al., 2013; MacMahon Copas et al., 2021).
The TCA cycle model from the PD map was used to validate the Boolean modeling approach. The TCA cycle is a widely studied pathway with ample experimental data on enzyme activity and metabolite levels, making it possible to compare BM predictions with experimental data and assess the accuracy and reliability of the model. Additionally, dysregulation of the TCA cycle is linked to oxidative stress, inflammation, and cell death, which are all hallmarks of Parkinson’s disease (Stacpoole, 2012; Kim et al., 2016; Sergi and Parayil Sankaran, 2021).
The structural and functional roles of key molecules in the TCA cycle BM were analyzed. This involved examining the involved enzymes and cofactors, as well as the reactions they catalyze and the intermediates they produce. The regulatory mechanisms controlling the activity of these molecules were also considered to predict their impact on the TCA cycle (Kafkia et al., 2022). The effects of overexpression and knockout of the regulatory mechanisms controlling the activity of the TCA cycle enzymes were modeled in the TCA cycle BM. The results from the structural analysis were used to calculate the sensitivity of the TCA cycle to knockouts and overexpressions by simulating the impact of knockouts and overexpressions of specific biomolecules on the overall activity of the TCA cycle. Literature was reviewed to find experimental studies that perturbed TCA cycle molecules in model organisms, such as yeast or mice, to verify the results (Lee et al., 2011; Wongkittichote et al., 2019). Selected perturbations in the Boolean model resulted in the following findings supported by the literature (Table 4):
• Activation of acetyl CoA, NADH, and PDKs in silico increased the phosphorylation reaction, reducing the activity of the pyruvate dehydrogenase complex (PDC), which led to decreased levels of succinic semialdehyde and succinic acid (Stacpoole, 2012; Sergi and Parayil Sankaran, 2021).
• Simulated KGDHC knockout predicted a deficiency in succinic acid and succinyl CoA and a downstream decrease in ATP and GTP levels (Gibson et al., 2003; Shi et al., 2011).
• Simulated isocitrate dehydrogenase knockout resulted in decreased ATP production and inhibited the oxidative decarboxylation of isocitrate, with decreased levels of oxoglutarate observed in response to L-glutamate (Huergo and Dixon, 2015; Kim et al., 2016).
• Knockout of SIRT3 downregulated oxoglutaric acid, glutamate hydrogenase 1 (GLUD), and ATP levels, directly impacting mitochondrial function (Shen et al., 2020).
• The simulation of the effect of alpha KGDH and GTP, GDP on ATP levels was validated using a probabilistic BM based on temporal-fluxomics data, which describes the oscillations of metabolites in the TCA. The simulated activity levels matched the measured concentrations in synchronized HeLa cells at various time points post-release from growth arrest (Table 6).
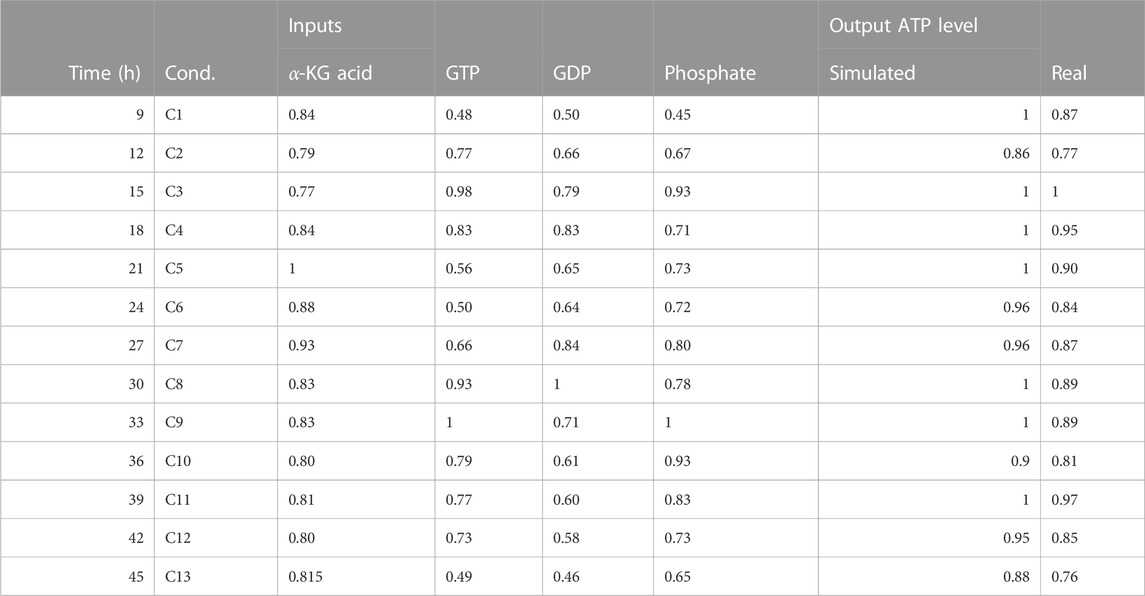
TABLE 6. This table presents data on the ATP activity in the TCA (tricarboxylic acid) cycle with fluxomics integration. The data includes the time in hours, the condition (labeled as “Cond”), and the levels of various inputs (alpha-ketoglutaric acid, GTP, GDP, and phosphate). The table also includes both simulated and real measurements of ATP levels.
The TCA cycle BM used in this study was capable of reproducing molecular activity that affects ATP levels and mitochondrial function. These results indicate that the TCA BM is a trustworthy tool for depicting the general picture of energy metabolism and can shed light on the mechanisms behind the oscillations of cellular metabolites. Nevertheless, this evaluation has some limitations, such as the possibility of missing relevant data in the literature search or not accurately reflecting all complex interactions within cells. Additionally, the context of evaluating the TCA cycle may differ based on the organism or tissue being studied. Despite these limitations, the primary goal of validating the TCA cycle BM is to ensure its accuracy in reflecting the underlying biological processes.
The NR4A2 gene plays a critical role in the regulation of dopamine and the growth and preservation of neurons. This gene is expressed in various tissues, including the brain, and has been implicated in several vital biological processes, such as neuron survival, mitochondrial biogenesis, and apoptosis (Chen et al., 2020). When modeled using in silico methods, the knockout of the NR4A2 gene correctly predicts the decrease in BDNF production (Barneda-Zahonero et al., 2012) and the alteration of expression of other genes involved in neurotransmitter metabolism and transport, including dopamine (Jankovic et al., 2005; Jacobs et al., 2009; Kadkhodaei et al., 2013). Activation of the SIRT1 gene, which is also involved in neurotransmitter metabolism, leads to improved mitochondrial biogenesis, a process required for the formation of new mitochondria.
The Pi3K/AKT pathway and Wnt signaling are known to be critical for dopaminergic neurogenesis, as well as for crucial developmental processes and the aging process, which is a major risk factor PD (Marchetti et al., 2020; Long et al., 2021). Both pathways share common downstream targets, suggesting the possibility of crosstalk and potential synergistic therapeutic approaches. For instance, both pathways can regulate the activity of GSK3B, a signaling protein, and are involved in autophagy, protein translation, and neuronal survival (Castelo-Branco et al., 2004; Hermida et al., 2017). Numerous studies have validated the content and structure of these pathways. Of note, Pi3K/AKT pathway and Wnt signaling have been shown to play a significant role in PD pathogenesis. The Pi3K/AKT pathway has been implicated in modulating neuronal survival, while Wnt signaling has been implicated in both neuroprotection and neurodegeneration. Additionally, the interaction of the two pathways with alpha-synuclein, a protein that aggregates and forms Lewy bodies, a hallmark feature of PD, suggests a complex interplay between these pathways in PD pathogenesis. Therefore, a deeper understanding of the interaction between the Pi3K/AKT pathway and Wnt signaling in PD pathogenesis could provide novel insights into disease mechanisms and novel therapeutic strategies (Marchetti et al., 2020; Long et al., 2021).
The following points outline key findings from modeling studies on the role of various molecular pathways in Parkinson’s disease (PD):
• The LRRK2 gene, particularly its G2019S mutant form, has been associated with an increased risk of developing PD (Madureira et al., 2020). Overexpression of the LRRK2 G2019S mutant has been shown to enhance autophagy, a process involving the degradation and recycling of cellular biomolecules, through the inhibition of mTORC1/2 (Bravo-San Pedro et al., 2013; Poret and Guziolowski, 2018; Zhu et al., 2019; McCabe et al., 2020). Simulations of LRRK2 overexpression predicted the reactivation of mTORC1, which is consistent with proposed interactions between amino acid sensing, mTORC1 signaling, and autophagy (Rabanal-Ruiz et al., 2017).
• TFEB, a transcription factor that regulates autophagy, has been shown to have complex effects on this process, activating autophagy while also activating protein synthesis inducers such as EIF4E and RPS6KB1 (Decressac et al., 2013). Simulations of TFEB overexpression predicted a neuroprotective effect through increased autophagy activity, while joint activation of TFEB and protein synthesis inhibitor 4EBP2 led to a further increase in autophagy activity (Decressac et al., 2013; Zhuang et al., 2020; Franco-Juárez et al., 2022). The inhibition of RPS6KB1 and PHLPP and activation of TFEB significantly decreased neuronal death (Decressac et al., 2013).
• The Wnt signaling pathway and GSK3B have also been implicated in PD. Simulated overexpression of Wnt protein and inactivation of GSK3B were shown to increase autophagy (Momčilović et al., 2014; Moya et al., 2014; Awad et al., 2017). Simulations of GSK3B inhibition and overexpression indicated its role in regulating neurogenesis, consistent with reported findings (Castelo-Branco et al., 2004; Toledo et al., 2017). Modulating Wnt signaling and GSK3B together may hold potential as a neuroprotective treatment in early stages of PD progression (Castelo-Branco et al., 2004; Momčilović et al., 2014; Moya et al., 2014; Awad et al., 2017; Toledo et al., 2017).
In conclusion, the validation study of the Wnt/PI3K pathway has confirmed existing literature findings and has also generated novel hypotheses regarding the combined modulation of its components. However, it is important to note that the relationship between the discussed biomolecules and neurogenesis and autophagy is complex and may depend on specific cell types, activity of other signaling pathways, or the stage of disease progression. Further studies are needed to fully elucidate the mechanisms underlying the interactions between these signaling pathways and their downstream targets, and to determine their potential as therapeutic targets in PD. Nonetheless, the findings presented in this study provide a promising avenue for further exploration and potential development of novel treatments for PD.
The FOXO3 activity pathway has been identified as a critical mediator of mitochondrial homeostasis (Fasano et al., 2019). The BM of FOXO3 activation have revealed that this pathway specifically promotes autophagy in mitochondria, through the activation of various biomolecules, including BECN1, GABARAPL1, MAP1LC3A, BNIP3, ATG12, and MUL1 (Hou et al., 2020). These biomolecules have been established as key regulators of autophagy. Thus, the activation of these biomolecules in response to FOXO3 activation highlights a close interconnection between RNA-mediated pathways and FOXO3 activity in the regulation of autophagy. These findings have the potential to improve our understanding of the complex mechanisms involved in the regulation of autophagy and its role in maintaining cellular health. Further investigations are required to clarify the extent and specifics of this relationship, which could enhance the development of targeted therapeutic strategies for disorders related to mitochondrial dysfunction and autophagy.
Translational medicine aims to bridge the gap between basic research and clinical practice by applying scientific findings to medical practice. One way to achieve this goal is through the use of modeling and simulation techniques. These techniques use existing knowledge from bench experiments and disease-relevant omics datasets to develop new hypotheses about the disease and propose improved therapies and diagnostics. The Boolean modeling approach, which is based on systems biology and systems medicine, is particularly useful because it allows for iterative improvements in understanding through a continuous cycle of data-driven modeling and model-driven experimentation. The Boolean modeling approach has the potential to generate therapy-related hypotheses by designing perturbation experiments to compare model attractors to the disease signature. This allows the identification of the basins of attraction that could alleviate the pathological signature of the disease and suggest the best combinations of targets to achieve a healthy state.
This study offers valuable insights into the potential use of a standardized SBML-qual format for Parkinson’s disease pathway models. Through the use of the CaSQ translation tool, the authors were able to generate models that can inform future research efforts. Moreover, the study highlights the importance of accurate and relevant data input, appropriate assumptions and algorithms, and further integration with omics data for improving the accuracy and relevance of modeling and simulation approaches in understanding disease mechanisms and proposing targeted therapies. We also emphasize the need for disease-specific datasets to accurately parametrize models and better understand individual dysregulations in different disease subtypes. This personalized approach has the potential to lead to the development of more effective treatments tailored to the unique characteristics of each disease subtype.
While the study acknowledges certain limitations, such as the high-level representation of observable disease effects and variations in disease mechanism dynamics between patients, it highlights the potential for continued improvement in modeling and simulation techniques to advance translational medicine and improve disease diagnosis and therapy. By addressing these limitations and incorporating more precise and individualized data, modeling and simulation approaches can provide a powerful tool for advancing our understanding of disease progression and the development of targeted therapeutic approaches.
Boolean modeling presents a promising opportunity to understand the complexity of the disease and propose potential therapeutic strategies. By modeling the dynamic interactions between various biomolecules, researchers can gain insight into the underlying mechanisms of the disease, test hypotheses about the role of particular biomolecules, explore the impact of multiple perturbations, and identify patterns that may not be apparent from experimental or observational data alone. However, to leverage the full potential of Boolean models, researchers must address several challenges, such as improving the accuracy and predictive power of these models. To address these challenges, researchers can apply model refinement, data integration, and parameter optimization techniques. Additionally, model stratification is a promising future direction for the field, which has the potential to increase the accuracy of the models and allow for more precise treatments.
To enhance the accuracy of Boolean models, it is crucial to improve their interoperability, annotations, and reproducibility. Standardized annotations provide more information about the model, making it easier to understand and use. Improved interoperability can help integrate different models and datasets, leading to more accurate predictions. Reproducibility can validate the results of the model and increase confidence in its predictive power.
By addressing these challenges and enhancing these parameters, we can improve the accuracy and reliability of Boolean models, ultimately leading to better predictions and insights into the underlying mechanisms of complex diseases such as Parkinson’s disease. As such, Boolean modeling is a promising tool for future research in this field, and its continued development will enable the discovery of novel therapeutic strategies and advance our understanding of complex diseases.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
AH: investigation, writing–original draft. RS: supervision, writing–review and editing. MO: conceptualization, review and editing, supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 733100: SYSCID—A systems medicine approach to chronic inflammatory diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbinf.2023.1189723/full#supplementary-material
Aalst, W. (2009). Petri nets. In Encyclopedia of database systems In, L. LIU, and M. ÖZSU(Boston, MA: Springer US. 2103–2108.
Aghamiri, S. S., Singh, V., Naldi, A., Helikar, T., Soliman, S., and Niarakis, A. (2020). Automated inference of Boolean models from molecular interaction maps using CaSQ. Bioinformatics 36, 4473–4482. doi:10.1093/bioinformatics/btaa484
Ahn, E., Kumar, P., Mukha, D., Tzur, A., and Shlomi, T. (2017). Temporal fluxomics reveals oscillations in TCA cycle flux throughout the mammalian cell cycle. Mol. Syst. Biol. 13, 953. doi:10.15252/msb.20177763
Albanese, F., Novello, S., and Morari, M. (2019). Autophagy and LRRK2 in the aging brain. Front. Neurosci. 13, 1. doi:10.3389/fnins.2019.01352
Albert, R., and Thakar, J. (2014). Boolean modeling: A logic-based dynamic approach for understanding signaling and regulatory networks and for making useful predictions. Wiley Interdiscip. Rev. Syst. Biol. Med. 6, 353–369. doi:10.1002/wsbm.1273
Antony, P. M. A., Diederich, N. J., Krüger, R., and Balling, R. (2013). The hallmarks of Parkinson’s disease. FEBS J. 280, 5981–5993. doi:10.1111/febs.12335
Ashtiani, M., Salehzadeh-Yazdi, A., Razaghi-Moghadam, Z., Hennig, H., Wolkenhauer, O., Mirzaie, M., et al. (2018). A systematic survey of centrality measures for protein-protein interaction networks. BMC Syst. Biol. 12, 80. doi:10.1186/s12918-018-0598-2
Awad, O., Panicker, L. M., Deranieh, R. M., Srikanth, M. P., Brown, R. A., Voit, A., et al. (2017). Altered differentiation potential of gaucher’s disease iPSC neuronal progenitors due to wnt/β-catenin downregulation. Stem Cell Rep. 9, 1853–1867. doi:10.1016/j.stemcr.2017.10.029
Balci, H. (2020). Newt: A comprehensive web-based tool for viewing, constructing and analyzing biological maps. Bioinformatics.
Barneda-Zahonero, B., Servitja, J.-M., Badiola, N., Miñano-Molina, A. J., Fadó, R., Saura, C. A., et al. (2012). Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival. J. Biol. Chem. 287, 11351–11362. doi:10.1074/jbc.M111.272427
Biere, A. (2008). PicoSAT essentials. J. Satisfiability, Boolean Model. Comput. 4, 75–97. doi:10.3233/SAT190039
Bloomingdale, P. (2018). Boolean network modeling in systems pharmacology. J. Pharmacokinet. Pharmacodyn. 45, 159–180. doi:10.1007/s10928-017-9567-4
Bravo-San Pedro, J. M., Niso-Santano, M., Gómez-Sánchez, R., Pizarro-Estrella, E., Aiastui-Pujana, A., Gorostidi, A., et al. (2013). The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell. Mol. life Sci. CMLS 70, 121–136. doi:10.1007/s00018-012-1061-y
Castelo-Branco, G., Rawal, N., and Arenas, E. (2004). GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J. Cell Sci. 117, 5731–5737. doi:10.1242/jcs.01505
Chaouiya, C., Bérenguier, D., Keating, S. M., Naldi, A., van Iersel, M. P., Rodriguez, N., et al. (2013). SBML qualitative models: A model representation format and infrastructure to foster interactions between qualitative modelling formalisms and tools. BMC Syst. Biol. 7, 135. doi:10.1186/1752-0509-7-135
Chen, L., Fan, F., Wu, L., and Zhao, Y. (2020). The nuclear receptor 4A family members: Mediators in human disease and autophagy. Cell. Mol. Biol. Lett. 25, 48. doi:10.1186/s11658-020-00241-w
Cheng, Z. (2022). FoxO transcription factors in mitochondrial homeostasis. Biochem. J. 479, 525–536. doi:10.1042/BCJ20210777
Da Cruz, S., Parone, P. A., Lopes, V. S., Lillo, C., McAlonis-Downes, M., Lee, S. K., et al. (2012). Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab. 15, 778–786. doi:10.1016/j.cmet.2012.03.019
Decressac, M., Mattsson, B., Weikop, P., Lundblad, M., Jakobsson, J., and Björklund, A. (2013). TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc. Natl. Acad. Sci. 110, E1817–E1826. doi:10.1073/pnas.1305623110
Dubrova, E. (2006). Random multiple-valued networks: Theory and applications. 36th Int. Symposium Multiple-Valued Log.6, 27. doi:10.1109/ISMVL.2006.36
Eduati, F. (2020). Patient-specific logic models of signaling pathways from screenings on cancer biopsies to prioritize personalized combination therapies. Mol. Syst. Biol. 16, 8664. doi:10.15252/msb.20188664
Fasano, C., Disciglio, V., Bertora, S., Lepore Signorile, M., and Simone, C. (2019). FOXO3a from the nucleus to the mitochondria: A round trip in cellular stress response. Cells 8, E1110. doi:10.3390/cells8091110
Franco-Juárez, B., Coronel-Cruz, C., Hernández-Ochoa, B., Gómez-Manzo, S., Cárdenas-Rodríguez, N., Arreguin-Espinosa, R., et al. (2022). TFEB; beyond its role as an autophagy and lysosomes regulator. Cells 11, 3153. doi:10.3390/cells11193153
Fujita, K. A., Ostaszewski, M., Matsuoka, Y., Ghosh, S., Glaab, E., Trefois, C., et al. (2014). Integrating pathways of Parkinson’s disease in a molecular interaction map. Mol. Neurobiol. 49, 88–102. doi:10.1007/s12035-013-8489-4
Funahashi, A. (2007). “CellDesigner: A graphical biological network editor and workbench interfacing simulator,” in Introduction to systems biology. C. Sangdun (Totowa, NJ: Humana Press), 422–434.
Garg, A., Di Cara, A., Xenarios, I., Mendoza, L., and De Micheli, G. (2008). Synchronous versus asynchronous modeling of gene regulatory networks. Bioinforma. Oxf. Engl. 24, 1917–1925. doi:10.1093/bioinformatics/btn336
García Del Valle, E. P., Lagunes García, G., Prieto Santamaría, L., Zanin, M., Menasalvas Ruiz, E., and Rodríguez-González, A. (2019). Disease networks and their contribution to disease understanding: A review of their evolution, techniques and data sources. J. Biomed. Inform. 94, 103206. doi:10.1016/j.jbi.2019.103206
Gawron, P. (2016). MINERVA-a platform for visualization and curation of molecular interaction networks. NPJ Syst. Biol. Appl. 2, 16020. doi:10.1038/npjsba.2016.20
Gibson, G. E., Kingsbury, A. E., Xu, H., Lindsay, J. G., Daniel, S., Foster, O. J. F., et al. (2003). Deficits in a tricarboxylic acid cycle enzyme in brains from patients with Parkinson’s disease. Neurochem. Int. 43, 129–135. doi:10.1016/s0197-0186(02)00225-5
Gopalakrishna, A., and Alexander, S. A. (2015). Understanding Parkinson disease: A complex and multifaceted illness. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses 47, 320–326. doi:10.1097/JNN.0000000000000162
Grieb, M. (2015). Predicting variabilities in cardiac gene expression with a boolean network incorporating uncertainty. PLOS ONE 10, 0131832. doi:10.1371/journal.pone.0131832
Helikar, T. (2012). The Cell Collective: Toward an open and collaborative approach to systems biology. BMC Syst. Biol. 6, 96.
Hemedan, A. A., Niarakis, A., Schneider, R., and Ostaszewski, M. (2022). Boolean modelling as a logic-based dynamic approach in systems medicine. Comput. Struct. Biotechnol. J. 20, 3161–3172. doi:10.1016/j.csbj.2022.06.035
Hermida, M. A., Dinesh Kumar, J., and Leslie, N. R. (2017). GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 65, 5–15. doi:10.1016/j.jbior.2017.06.003
Hopfensitz, M. (2012). Multiscale binarization of gene expression data for reconstructing Boolean networks. IEEE/ACM Trans. Comput. Biol. Bioinform 9, 487–498. doi:10.1109/TCBB.2011.62
Hou, X., Watzlawik, J. O., Fiesel, F. C., and Springer, W. (2020). Autophagy in Parkinson’s disease. J. Mol. Biol. 432, 2651–2672. doi:10.1016/j.jmb.2020.01.037
Huergo, L. F., and Dixon, R. (2015). The emergence of 2-oxoglutarate as a master regulator metabolite. Microbiol. Mol. Biol. Rev. MMBR 79, 419–435. doi:10.1128/MMBR.00038-15
Ilea, M. (2012). Ordinary differential equations with applications in molecular biology. Rev. Med. Chir. Soc. Med. Nat. Iasi 116, 347–352.
Jacobs, F. M. J., van Erp, S., van der Linden, A. J. A., von Oerthel, L., Burbach, J. P. H., and Smidt, M. P. (2009). Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Dev. Camb. Engl. 136, 531–540. doi:10.1242/dev.029769
Jacobs, F. M. J., Veenvliet, J. V., Almirza, W. H., Hoekstra, E. J., von Oerthel, L., van der Linden, A. J. A., et al. (2011). Retinoic acid-dependent and -independent gene-regulatory pathways of Pitx3 in meso-diencephalic dopaminergic neurons. Dev. Camb. Engl. 138, 5213–5222. doi:10.1242/dev.071704
Jankovic, J., Chen, S., and Le, W. D. (2005). The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog. Neurobiol. 77, 128–138. doi:10.1016/j.pneurobio.2005.09.001
Kadkhodaei, B., Alvarsson, A., Schintu, N., Ramsköld, D., Volakakis, N., Joodmardi, E., et al. (2013). Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc. Natl. Acad. Sci. U. S. A. 110, 2360–2365. doi:10.1073/pnas.1221077110
Kafkia, E., Andres-Pons, A., Ganter, K., Seiler, M., Smith, T. S., Andrejeva, A., et al. (2022). Operation of a TCA cycle subnetwork in the mammalian nucleus. Sci. Adv. 8, eabq5206. doi:10.1126/sciadv.abq5206
Kim, H., Kim, S. H., Cha, H., Kim, S. R., Lee, J. H., and Park, J.-W. (2016). IDH2 deficiency promotes mitochondrial dysfunction and dopaminergic neurotoxicity: Implications for Parkinson’s disease. Free Radic. Res. 50, 853–860. doi:10.1080/10715762.2016.1185519
Klemm, K., and Bornholdt, S. (2005). Stable and unstable attractors in Boolean networks. Phys. Rev. E 72, 055101. doi:10.1103/PhysRevE.72.055101
Kuperstein, I. (2013). NaviCell: A web-based environment for navigation, curation and maintenance of large molecular interaction maps. BMC Syst. Biol. 7, 100. doi:10.1186/1752-0509-7-100
Lee, Y. J., Jang, J. W., Kim, K. J., and Maeng, P. J. (2011). TCA cycle-independent acetate metabolism via the glyoxylate cycle in Saccharomyces cerevisiae. Yeast 28, 153–166. doi:10.1002/yea.1828
Li, H.-Y., and Cai, Z.-Y. (2022). SIRT3 regulates mitochondrial biogenesis in aging-related diseases. J. Biomed. Res. 37, 1–12. doi:10.7555/JBR.36.20220078
Li, X., Feng, Y., Wang, X.-X., Truong, D., and Wu, Y.-C. (2020). The critical role of SIRT1 in Parkinson’s disease: Mechanism and therapeutic considerations. Aging Dis. 11, 1608–1622. doi:10.14336/AD.2020.0216
Li, Y., Zhang, J., and Yang, C. (2015). UNC-51-like kinase 1 blocks S6k1 phosphorylation contributes to neurodegeneration in Parkinson’s disease model in vitro. Biochem. Biophysical Res. Commun. 459, 196–200. doi:10.1016/j.bbrc.2015.02.008
Long, H.-Z., Cheng, Y., Zhou, Z.-W., Luo, H.-Y., Wen, D.-D., and Gao, L.-C. (2021). PI3K/AKT signal pathway: A target of natural products in the prevention and treatment of alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 12, 1. doi:10.3389/fphar.2021.648636
Lorzadeh, S., Kohan, L., Ghavami, S., and Azarpira, N. (2021). Autophagy and the Wnt signaling pathway: A focus on wnt/β-catenin signaling. Biochimica Biophysica Acta. Mol. Cell Res. 1868, 118926. doi:10.1016/j.bbamcr.2020.118926
Ma’ayan, A. (2011). Introduction to network analysis in systems biology. Sci. Signal. 4, 5. doi:10.1126/scisignal.2001965
MacMahon Copas, A. N., McComish, S. F., Fletcher, J. M., and Caldwell, M. A. (2021). The pathogenesis of Parkinson’s disease: A complex interplay between astrocytes, microglia, and T lymphocytes? Front. Neurology 12, 1. doi:10.3389/fneur.2021.666737
Madureira, M., Connor-Robson, N., and Wade-Martins, R. (2020). LRRK2: Autophagy and lysosomal activity. Front. Neurosci. 14, 498. doi:10.3389/fnins.2020.00498
Maldonado, E. M., Leoncikas, V., Fisher, C. P., Moore, J. B., Plant, N. J., and Kierzek, A. M. (2017). Integration of genome scale metabolic networks and gene regulation of metabolic enzymes with physiologically based pharmacokinetics. CPT pharmacometrics Syst. Pharmacol. 6, 732–746. doi:10.1002/psp4.12230
Marchetti, B., Tirolo, C., L’Episcopo, F., Caniglia, S., Testa, N., Smith, J. A., et al. (2020). Parkinson’s disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell 19, e13101. doi:10.1111/acel.13101
Mazein, A. (2018). Systems medicine disease maps: Community-driven comprehensive representation of disease mechanisms. NPJ Syst. Biol. Appl. 4, 21. doi:10.1038/s41540-018-0059-y
McCabe, M. P., Cullen, E. R., Barrows, C. M., Shore, A. N., Tooke, K. I., Laprade, K. A., et al. (2020). Genetic inactivation of mTORC1 or mTORC2 in neurons reveals distinct functions in glutamatergic synaptic transmission. eLife 9, e51440. doi:10.7554/eLife.51440
Momčilović, O., Liu, Q., Swistowski, A., Russo-Tait, T., Zhao, Y., Rao, M. S., et al. (2014). Genome wide profiling of dopaminergic neurons derived from human embryonic and induced pluripotent stem cells. Stem Cells Dev. 23, 406–420. doi:10.1089/scd.2013.0412
Moors, T. E., Hoozemans, J. J. M., Ingrassia, A., Beccari, T., Parnetti, L., Chartier-Harlin, M.-C., et al. (2017). Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol. Neurodegener. 12, 11. doi:10.1186/s13024-017-0154-3
Moya, N., Cutts, J., Gaasterland, T., Willert, K., and Brafman, D. A. (2014). Endogenous WNT signaling regulates hPSC-derived neural progenitor cell heterogeneity and specifies their regional identity. Stem Cell Rep. 3, 1015–1028. doi:10.1016/j.stemcr.2014.10.004
Müssel, C. (2010). BoolNet–an R package for generation, reconstruction and analysis of Boolean networks. Bioinforma. Oxf. Engl. 26, 1378–1380. doi:10.1093/bioinformatics/btq124
Naldi, A. (2018). Logical modeling and analysis of cellular regulatory networks with GINsim 3.0. Front. Physiol. 9, 1.
Palomer, E., Buechler, J., and Salinas, P. C. (2019). Wnt signaling deregulation in the aging and alzheimer’s brain. Front. Cell. Neurosci. 13, 227. doi:10.3389/fncel.2019.00227
Pino, E., Amamoto, R., Zheng, L., Cacquevel, M., Sarria, J.-C., Knott, G. W., et al. (2014). FOXO3 determines the accumulation of α-synuclein and controls the fate of dopaminergic neurons in the substantia nigra. Hum. Mol. Genet. 23, 1435–1452. doi:10.1093/hmg/ddt530
Poret, A., and Guziolowski, C. (2018). Therapeutic target discovery using boolean network attractors: Improvements of kali. R. Soc. Open Sci. 5, 1. doi:10.1098/rsos.171852
Rabanal-Ruiz, Y., Otten, E. G., and Korolchuk, V. I. (2017). mTORC1 as the main gateway to autophagy. Essays Biochem. 61, 565–584. doi:10.1042/EBC20170027
Schwab, J. (2020). Concepts in boolean network modeling: What do they all mean? Comput. Struct. Biotechnol. J. 18, 571–582. doi:10.1016/j.csbj.2020.03.001
Sergi, C., and Parayil Sankaran, B. (2021). “Succinic semialdehyde dehydrogenase deficiency,” in StatPearls (treasure island (FL) (Florida: StatPearls Publishing).
Shen, Y., Wu, Q., Shi, J., and Zhou, S. (2020). Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 132, 110928. doi:10.1016/j.biopha.2020.110928
Shi, Q., Xu, H., Yu, H., Zhang, N., Ye, Y., Estevez, A. G., et al. (2011). Inactivation and reactivation of the mitochondrial α-ketoglutarate dehydrogenase complex. J. Biol. Chem. 286, 17640–17648. doi:10.1074/jbc.M110.203018
Siegle, L. (2018). A Boolean network of the crosstalk between IGF and Wnt signaling in aging satellite cells. PloS One 13, 0195126. doi:10.1371/journal.pone.0195126
Silk, D. (2014). Model selection in systems biology depends on experimental design. PLOS Comput. Biol. 10, 1003650. doi:10.1371/journal.pcbi.1003650
Silva, B. J. d. A., Barbosa, M. G. d. M., Andrade, P. R., Ferreira, H., Nery, J. A. d. C., Côrte-Real, S., et al. (2017). Autophagy is an innate mechanism associated with leprosy polarization. PLoS Pathog. 13, e1006103. doi:10.1371/journal.ppat.1006103
Stacpoole, P. W. (2012). The pyruvate dehydrogenase complex as a therapeutic target for age-related diseases. Aging Cell 11, 371–377. doi:10.1111/j.1474-9726.2012.00805.x
Stafa, K., Trancikova, A., Webber, P. J., Glauser, L., West, A. B., and Moore, D. J. (2012). GTPase activity and neuronal toxicity of Parkinson’s disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet. 8, e1002526. doi:10.1371/journal.pgen.1002526
Stoll, G., Caron, B., Viara, E., Dugourd, A., Zinovyev, A., Naldi, A., et al. (2017). MaBoSS 2.0: An environment for stochastic boolean modeling. Bioinformatics 33, 2226–2228. doi:10.1093/bioinformatics/btx123
Stoyas, C. A., Bushart, D. D., Switonski, P. M., Ward, J. M., Alaghatta, A., Tang, M.-B., et al. (2020). Nicotinamide pathway-dependent Sirt1 activation restores calcium homeostasis to achieve neuroprotection in spinocerebellar ataxia type 7. Neuron 105, 630–644.e9. doi:10.1016/j.neuron.2019.11.019
Terfve, C. (2012). CellNOptR: A flexible toolkit to train protein signaling networks to data using multiple logic formalisms. BMC Syst. Biol. 6, 133.
Toledo, E. M., Gyllborg, D., and Arenas, E. (2017). Translation of WNT developmental programs into stem cell replacement strategies for the treatment of Parkinson’s disease. Br. J. Pharmacol. 174, 4716–4724. doi:10.1111/bph.13871
Trinh, H.-C., and Kwon, Y.-K. (2019). RMut: R package for a boolean sensitivity analysis against various types of mutations. PLOS ONE 14, 0213736. doi:10.1371/journal.pone.0213736
Wang, R.-S. (2012). Boolean modeling in systems biology: An overview of methodology and applications. Phys. Biol. 9, 055001. doi:10.1088/1478-3975/9/5/055001
Wiese, R. (2004). “yFiles — visualization and automatic layout of graphs,” in Graph drawing software, mathematics and visualization J. M. In, and P. Mutzel (Berlin, Heidelberg: Springer), 173–191.
Wongkittichote, P., Cunningham, G., Summar, M. L., Pumbo, E., Forny, P., Baumgartner, M. R., et al. (2019). Tricarboxylic acid cycle enzyme activities in a mouse model of methylmalonic aciduria. Mol. Genet. Metabolism 128, 444–451. doi:10.1016/j.ymgme.2019.10.007
Yang, L., Wang, H., Liu, L., and Xie, A. (2018). The role of insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson’s disease dementia. Front. Neurosci. 12, 73. doi:10.3389/fnins.2018.00073
Zhang, L., Le, W., Xie, W., and Dani, J. A. (2012). Age-related changes in dopamine signaling in Nurr1 deficient mice as a model of Parkinson’s disease. Neurobiol. Aging 33, 1001. e7–16. doi:10.1016/j.neurobiolaging.2011.03.022
Zhu, Z., Yang, C., Iyaswamy, A., Krishnamoorthi, S., Sreenivasmurthy, S. G., Liu, J., et al. (2019). Balancing mTOR signaling and autophagy in the treatment of Parkinson’s disease. Int. J. Mol. Sci. 20, E728. doi:10.3390/ijms20030728
Keywords: Boolean modelling, molecular mechanisms, systems biology, drug target, Parkinsion’s disease
Citation: Hemedan AA, Schneider R and Ostaszewski M (2023) Applications of Boolean modeling to study the dynamics of a complex disease and therapeutics responses. Front. Bioinform. 3:1189723. doi: 10.3389/fbinf.2023.1189723
Received: 19 March 2023; Accepted: 18 May 2023;
Published: 01 June 2023.
Edited by:
Mehdi Pirooznia, Johnson & Johnson, United StatesReviewed by:
Gautier Stoll, INSERM U1138 Centre de Recherche des Cordeliers (CRC), FranceCopyright © 2023 Hemedan, Schneider and Ostaszewski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Abdelmonem Hemedan, YWhtZWQuaGVtZWRhbkB1bmkubHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.