
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol., 21 February 2025
Sec. Biomaterials
Volume 13 - 2025 | https://doi.org/10.3389/fbioe.2025.1504883
 Anniek M. C. Gielen1,2*
Anniek M. C. Gielen1,2* Niels M. Leijten1
Niels M. Leijten1 Payal P. S. Balraadjsing1,3
Payal P. S. Balraadjsing1,3 Hedwig M. Braakhuis1
Hedwig M. Braakhuis1 Hannah Abee1,4
Hannah Abee1,4 Jacobus J. Arts4,5
Jacobus J. Arts4,5 Annemarie P. van Wezel2
Annemarie P. van Wezel2 Agnes G. Oomen1,2
Agnes G. Oomen1,2 Nick R. M. Beijer1
Nick R. M. Beijer1Orthopedic hip implant failure due to adverse events, such as infection, are still a major problem leading to high morbidity and mortality. Over the years, various innovative biomaterials have been investigated to improve safety and functionality of implants. Although novel biomaterials show initial promising results, many fail at the (later) stages of safety testing. We performed a literature review serving as a first step in a Safe-by-Design (SbD) approach. SbD is a strategy which includes safety considerations at early development stages and that streamlines the pre-clinical safety assessment of innovative medical implants. In a SbD approach, the standard safety assessment of medical implants (e.g., ISO10993) is complemented with insights on cell-biomaterial interactions allowing for a better in vivo response prediction. As a first step, these insights are based on existing information from literature. Therefore, in this review, correlations between implant biomaterial surface properties and key biological processes, relevant for the success and safety of titanium hip implants, are investigated. In particular, the influence of biomaterial roughness, wettability and pore size on key biological processes for a hip implant (osseointegration, bacterial adhesion and the immune response) are examined. Although it was found that no ideal combination of properties exist to satisfy the key biological processes simultaneously, the gathered insights provide directions for the development of safe and functional biomaterials. Altogether, an assessment of the different aspects of safety at early development stages within an SbD approach can improve biomaterial functionality and thus safety.
Orthopedic hip implants are widely used medical devices that can contribute immensely to the quality of life of patients. In the Netherlands, yearly, over 30 thousand hip replacements were performed (LROI DAR, 2020), for a population of 16,8 to 17,2 million between 2014 and 2019 respectively (Netherlands, 2024). The annual need for implants is expected to grow due to an aging population. Besides the positive contributions, there is a potential risk of adverse outcomes such as implant infection or implant loosening. Adverse outcomes after implantation are common causes of revision. Based on Dutch data between 2014 and 2019, for 1 out of 8 hip replacements, revisions were performed, with 22.8% due to loosening of the acetabulum component (surface in the pelvis), 19.1% attributed to infection and 18.9% to loosening of the femur component (LROI DAR, 2020). These adverse outcomes pose a health risk to the patient and increase the economic burden to the healthcare system (OECD, 2021). Adverse outcomes are influenced by patient characteristics, surgeon expertise as well as implant design. While retrospective studies on implant failure often focus on factors like gender, fracture type and femur size, they rarely consider implant material design (Roerink et al., 2024).
It is important that biological processes that influence adverse outcomes are considered during the safety assessment of orthopedic hip implants. Knowledge on key biological processes can feed into a Safe-by-Design (SbD) framework and be implemented into the design phase (Schmutz et al., 2020). For example, implant infection occurs when microorganisms adhere and colonize the implant surface and surrounding tissue, leading to tissue damage and loss of implant function (Rosman et al., 2021). Loosening of the femur component could be due to lack of osseointegration or due to a strong inflammatory response, compromising surrounding tissue (Nobles et al., 2021). Implementing key biological processes such as bacterial adhesion and osseointegration early into the design phase of novel implants allows innovators to incorporate safety at an early stage of development. SbD can help to streamline innovation and ultimately increase patient safety.
In this review, we propose a Safe-by-Design approach for medical implants, focusing on innovative antimicrobial orthopedic hip implants, with titanium as primary focus biomaterial including modifications by coating. We structure existing information that can be used as a first step in SbD. To that end, literature reviews (listed in Supplementary Table S1) focusing on one of three well-studied biomaterial surface properties (roughness, wettability and pore size),are assessed to determine their relationship to three key biological processes (osseointegration, bacterial adhesion and immune response). This approach will allow to identify directions for implant biomaterial development at early innovation stages, that can be further detailed by in vitro and ultimately in vivo testing related to these key biological processes.
Literature search strings have been developed tailored to various combinations of biomaterial properties and biological processes. PubMed and Embase served as the primary databases for this effort. Snowball citations were employed to identify additional relevant literature. Exclusion criteria were established to filter out studies involving mandibular implantation, skull implantation and induced animal models.
Safe and Sustainable by Design (SSbD) is currently actively promoted by the European Commission as part of the European Green Deal and the Chemicals Strategy for Sustainability to strive for a prosperous society without harm to humans or the environment caused by hazardous materials or chemicals (European et al., 2022a; European et al., 2022b). The SSbD concept refers to anticipating risks and uncertainties concerning human and environmental safety early on in the innovation and development process. It addresses the safety and sustainability of the material/product as well as the associated processes throughout the whole cycle of innovation. Safety and sustainability is integrated in product development in an iterative manner to include information on safety, functionality and other aspects such as costs or sustainability early on. It thereby prevents as much as possible, failure later on in the development process (van de Poel and Robaey, 2017; Tavernaro et al., 2021; van Gelder et al., 2021). This paper will primarily focus on safety considerations, within the framework of Safe-by-Design (SbD). While sustainability remains an important topic, our initial emphasis will be directed towards ensuring patient safety by minimizing adverse outcomes. SbD was first introduced in the nanotechnology field to address potential risks induced by novel nanomaterials (Dekkers et al., 2020), and has been introduced in other sectors (e.g., chemical industry) (van Dijk et al., 2022). This paper applies the SbD principles to medical implants.
To bring the SbD approach for medical implants into practice, safety and functionality need to be integrated early into the development phase. Key safety aspects for the application specific implant need to be identified. The novel SbD strategy (Figure 1) for medical implants starts with a clinical need or question as input and a successful implant as a result. The very first SbD step is gathering implant and application specific information through existing literature on cell-biomaterial interactions, biomaterial chemical safety and retrospective studies. Additional information will be gathered through application specific in vitro assays. SbD aims to put safety parallel to implant optimization. By simultaneously characterizing the implant biomaterial and to test them for application-specific functionalities together with their biological safety, early in the process. Through this approach, complications later in the development process, e.g., during later stage in vivo tests, and ultimately in patients, might be avoided.
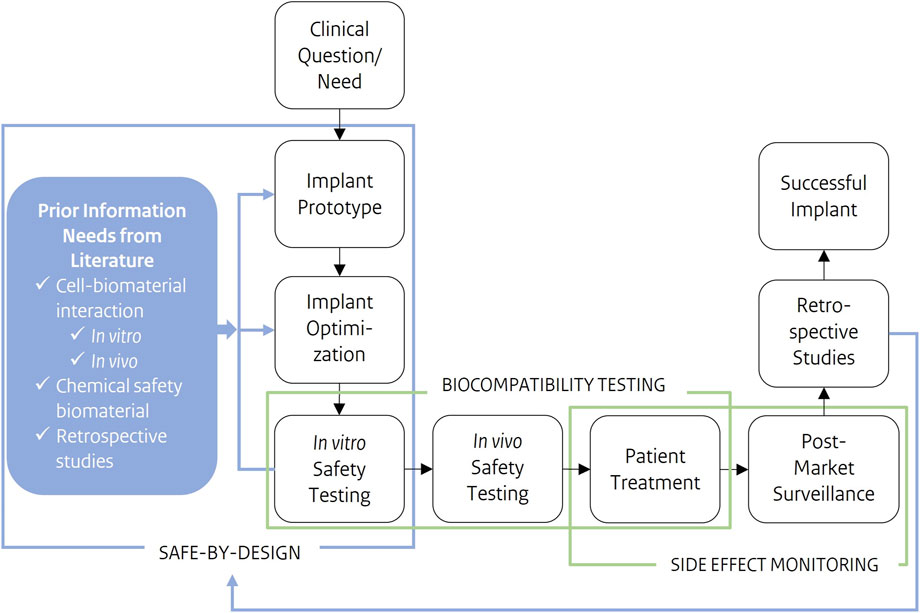
Figure 1. Schematic representation of a development process of a novel medical implant, from clinical questions/need as input to a successful implant as output. Safe-by-Design is applied early in the development process. Prior information needs, such as cell-biomaterial interactions or the chemical safety of components, are gathered preceding product development and optimization. These safety considerations are further integrated into implant development and optimization. Where safety testing results, feed design choices in implant prototype and optimization. Cell-biomaterial interactions are the prior information needs this study focusses on.
Before market entry, the biocompatibility of an implant, i.e., ‘the ability of a material to perform with an appropriate host response in a specific application’ (Williams, 2021), needs to be thoroughly investigated to minimize the risk of adverse effects. Requirements for the market entry of medical devices have been captured in the EU in the Medical Device Regulations (MDR), which aims to identify and monitor significant adverse events involving medical devices. The safety assessment and testing methods of (innovative) medical technologies are addressed in the standards of the International Organization for Standardization (ISO). The non-biological functionalities, (e.g., mechanical loading of a hip implant) and general biological safety are assessed. The general biological safety testing for medical implants is described in the ISO 10993 series. ISO 10993 addresses traditional toxicity endpoints, including separate standards for irritation, sensitization, cyto- and genotoxicity (ISO 10993, 2018). However, these standards mainly focus on the chemical safety of leachables of a biomaterial rather than direct biomaterial contact as occurs in the body, both for in vitro and in vivo testing. The exception is ISO10993-6, which is the standard for in vivo local effects after implantation, assessing tissue effects and interactions for the first time. Testing primarily leachables beforehand provides a standardized reflection of the chemical safety of the material and the manufacturing process. However, by solely relying on the leachables test in vitro, insights into the impact of implant material properties itself on the designated biological environment in vitro are missed and only will come across with in vivo testing of the whole product (Jurczak et al., 2024).
In addition to current practices, using existing knowledge on cell-biomaterial interactions as well as incorporating obtained in vitro cell-biomaterial interaction into safety assessment might provide insights predictive for the implant performance in vivo (Salthouse et al., 2022). This will lead to more targeted and probably fewer animal tests, as well as an increase in the overall success of the implant throughout the rest of the development process. ISO 10993 warrants characterization of the physical form and characteristics (e.g., geometry and surface roughness) of the material, however this is not yet related to any biological effect in vitro in the early stage testing. In the Safe-by-Design approach introduced here, existing in vitro/vivo information from literature and in vitro cell-biomaterial interaction assessment will be integrated into the early stages of development, allowing for directions that provide predictive insights into the implant’s in vivo performance.
The implant and the surrounding tissue form a system, where the interactions within the system are dependent on the implant biomaterial physical and chemical properties as well as the biological features of the surrounding tissue and host (Williams, 2019). The host response to an implant involves complex interactions between different cell types and properties of the biomaterial (Zhu et al., 2021; Rahmati et al., 2020). These cell-biomaterial interactions and subsequent signaling pathways are key in determining the tissue-specific compatibility of a biomaterial (Rahmati et al., 2020). For the (re)design of safe and functional implants, it is therefore essential to know the key biological processes involved in specific applications and the influence of biomaterial properties on these key processes on tissue and cellular level (Stich et al., 2022). It would be very valuable to take this knowledge into account in the safety standards, however, this state-of-the-art knowledge still needs to find its way to the existing and regulatory standards (Lackington et al., 2022).
Furthermore, different applications require different implant-tissue interactions, as these differ between, e.g., an orthopedic implant and a pacemaker. One needs to understand which key biological processes are relevant to consider, as well as their relationship with implant properties. Understanding this will give innovators directions towards safer and functional implants. Therefore, safety assessment should be based on the unique properties of the biomaterial and the intended use of the implant, where a SbD approach can help to guide innovators towards the most optimal biological outcome.
To be safe and functional, an innovative antimicrobial orthopedic hip implant needs proper bone integration (osseointegration), an appropriate local immune response and minimize bacterial colonization on the implant surface. These three processes, i.e., osseointegration, immune response and bacterial adhesion, are here referred as the key biological processes. The processes are all connected to the prevalence of adverse outcomes related to hip implants, such as aseptic loosening or biomaterial-associated infection, making them valuable processes to investigate (Nobles et al., 2021).
Osseointegration is the stable anchorage of an orthopedic implant due to direct bone-to-implant contact without the interposition of nonbone tissue and is essential for the clinical success of orthopedic hip implants (Albrektsson and Johansson, 2001; Kim et al., 2017; Morinaga et al., 2009). Implants with proper osseointegration allow osteoblasts to adhere, proliferate and create a favorable microenvironment by secreting specific matrix proteins (Saldaña et al., 2011). It is regarded as one of the most decisive factors for long-term success of an hip implant and determined by several factors, such as biomaterial roughness (Stoilov et al., 2022).
The immune response related to implantation of a hip implant plays, in addition to osseointegration, a pivotal role in determining the clinical outcome (Salthouse et al., 2022). Upon implantation, implant biomaterials are recognized as foreign, initiating a complex cascade of events called the foreign body response (FBR). While a diverse set of immune cells and factors are involved in the FBR (Christo et al., 2015) this review focusses on macrophages since they are important players in the initial phase of inflammation (Batool et al., 2021). Macrophages adapt to their local microenvironment, influenced by biomaterial properties, such as wettability and roughness, and can then polarize to different phenotypes (Chen et al., 2022). Tissue damage due to implantation leads to an initial inflammatory response with more pro-inflammatory phenotyped macrophages. On the contrary, a more anti-inflammatory macrophage phenotype promotes healing and regeneration of the tissue (Xie et al., 2020). Of importance is a timely switch from pro-towards anti-inflammatory to allow for the formation of new bone tissue (He et al., 2020). As such this first macrophage response is a determinant for the biological outcome (Salthouse et al., 2022). Implant biomaterial design should thus take into account the macrophage response, and optimize this by tweaking the biomaterial properties (Bu et al., 2022; Dong et al., 2022).
Biomaterial-associated infections remain a major challenge in designing and developing orthopedic hip implants (Pandey, 2022). Bacterial adhesion is a complex process where different type of physical-chemical interactions are involved, these interactions are dependent on bacterial and biomaterial properties (Kreve and Reis, 2021; Filipović et al., 2020; Yang et al., 2022). The process begins with a reversible and unstable adhesion phase, followed by the second phase where bacterial firmly anchor to the surface and form a biofilm (Dong et al., 2022; Filipović et al., 2020; Yang et al., 2022). In biofilms, bacteria are embedded in a protective matrix, consisting of extracellular polysaccharides, matrix proteins and extracellular DNA, where they can survive even under harsh conditions (Li et al., 2023). Bacteria in biofilms are difficult to treat with conventional antibiotics due to limited penetration into the protective biofilm matrix, metabolically reduced phenotypes and the development of antibiotic resistance. Implant surface design with antimicrobial functionalities which minimize bacterial adhesion, such as non-adhesive or bactericidal surfaces, can aid in the prevention of biomaterial-associated infections. Antimicrobial implant technologies differ in biomaterial properties that have an influence on key biological processes, thus influence the safety as well as the functionality of the orthopedic hip implant (Yang et al., 2022).
These three key biological processes, osseointegration, immune response and bacterial adhesion, collectively contribute to the safety and functionality of the hip implant. Therefore, all processes should be considered simultaneously during implant design and optimization to minimize adverse outcomes. The biological response to an implant is predominantly influenced by its biomaterial surface properties (Chen et al., 2016). For instance, the physicochemical properties of the biomaterial surface directly affect the cellular behavior of the surrounding tissue (Ren et al., 2021). Important physicochemical surface properties of titanium hip implant specifically are roughness, wettability and pore size (Figure 2) (Ren et al., 2021). The relevance of each of these surface properties is addressed in more detail below.
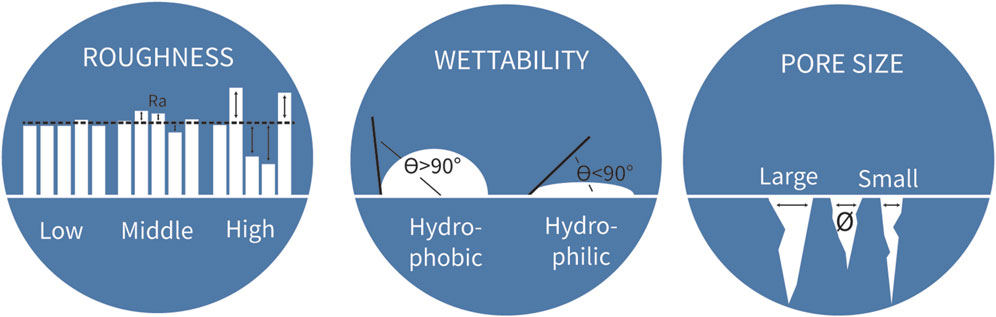
Figure 2. Three biomaterial properties to consider for implant biomaterial design. Measure for roughness is the average peaks and valleys expressed (Ra) in the unit µm. The angle of a water drop determines the wettability of the biomaterial. The unit for pore size is the average diameter of the pores.
Roughness is a measure of the texture of a surface, expressed in average peaks and valleys as the profile roughness parameter Ra, with µm as unit (Figure 2). This surface property has been researched extensively in relation to osseointegration and bacterial adhesion. In literature, roughness is distinguished at the micro and nanoscale. The microscale roughness dictates tissue level interactions and improves mechanical anchorage of the implant in the bone tissue. Nanoscale roughness activates biological responses at the cell and protein level (Nobles et al., 2021; Stich et al., 2022; Albrektsson and Wennerberg, 2019). Figure 3 summarizes the findings of eight literature reviews, where the upper part of the graph shows in vitro data and the lower part in vivo data.
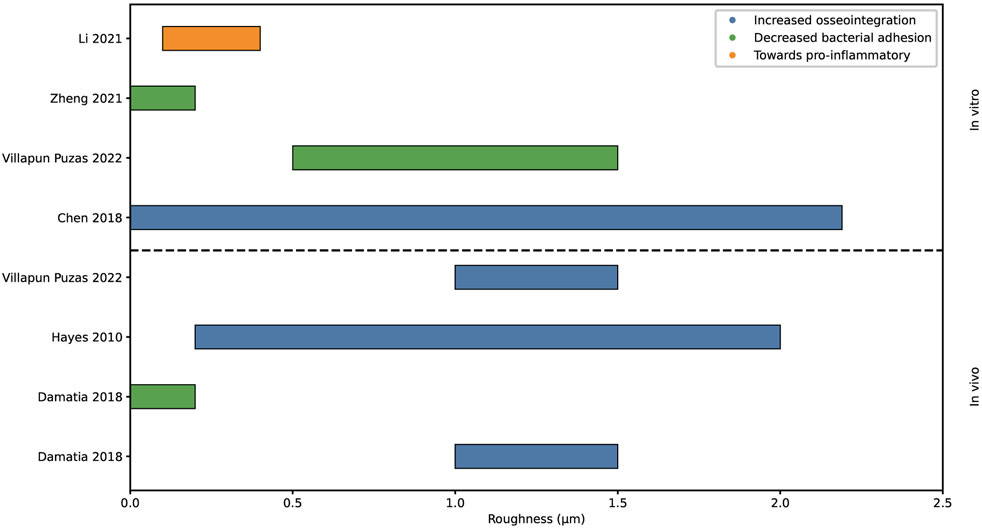
Figure 3. Graphical representation of the proposed surface roughness values for increased osseointegration, decreased bacterial adhesion and polarization macrophage. Analyzed with in vivo and in vitro methods.
Hayes et al. hypothesized about a so-called roughness window. Within this effective roughness window, which ranges from 200 to 2000 nm, cells reacted optimally resulting in increased osseointegration (Hayes and Richards, 2010). Chen et al. spoke of an optimum range of roughness as well, showing that a roughness over 2.19 µm inhibits osteoblastic adhesion. Therefore, a roughness below 2.19 µm is considered to stimulate osseointegration (Chen et al., 2018; Anselme et al., 2000).
On the other hand, increased roughness increases the colonization of bacteria (Yang et al., 2022). The grooves can provide shelter for bacteria against antibiotic treatment (Damiati et al., 2018). Villapun Puzas et al. also stated that bacterial attachment generally increased with an increasing roughness, additionally they specified that a roughness between 0.5 and 1.5 µm achieves a limited colonization of bacteria (Villapun et al., 2022). Whereas Zheng et al and Damiati et al. observed limited bacterial adhesion at a roughness below 0.2 µm, however Zheng et al states that a so called threshold roughness of 0.2 µm is currently debatable due to contradicting studies (Filipović et al., 2020; Damiati et al., 2018; Zheng et al., 2021).
Li et al. explored the findings for the influence of roughness onto the immune response and found in vitro data suggesting a macrophage phenotype polarization towards pro-inflammatory with increasing roughness from 100 to 400 nm (Li et al., 2021).
Altogether, several studies have proposed an optimal roughness window for osseointegration, demonstrating an consensus of optimal roughness values within literature as can be seen in Figure 3. However, there is no agreement between studies on the optimal roughness value for reduced bacterial adhesion. In vitro research has explored the macrophage response to roughness, yet the identification of an optimal roughness value for an optimal immune response remains elusive.
Wettability is defined as the ability of a material to maintain contact with a liquid. It is expressed as the contact angle of a drop of water; a hydrophilic surface exhibits an angle below 90°, whereas hydrophobic surfaces have a water contact angle greater than 90° (Figure 2) (Nobles et al., 2021; Damiati et al., 2018). The wettability of a surface is a major driving force for the adsorption of proteins (Barberi and Spriano, 2021; Mariani et al., 2019) which plays a critical role in mediating tissue integration outcomes for implants, influencing cell and bacteria adhesion and proliferation on the implant biomaterial surface immediately post-implantation (Barberi and Spriano, 2021). The spatial conformation of proteins is very different between hydrophobic and hydrophilic surfaces due to protein unfolding, resulting in different biochemical and physicochemical behavior (Barberi and Spriano, 2021; Mitra, 2020). All included reviews covered in vitro studies only (Figure 4).

Figure 4. Graphical representation of influence of wettability categories onto the biological categories osseointegration, bacterial adhesion and polarization of macrophages. The location of the author represents the combination of the categories placed on the y- and x-axis. For example, Villapun Puzas et al. described an increase in bacterial adhesion for hydrophilic surfaces. All studies represent in vitro methods.
All three studies on osseointegration align and provide evidence that hydrophilic surfaces promote osseointegration (Chen et al., 2018; Miron et al., 2023; Lee et al., 2019).
Villapun Puzas et al. have found however, that also bacteria have an increased proliferation on hydrophilic surfaces compared to hydrophobic surfaces (Villapun et al., 2022). Several studies have explored wettability as a strategy to reduce bacterial attachment. Zheng et al. presented that superhydrophobic and superhydrophilic surfaces serve as effective means against bacterial adhesion (Zheng et al., 2021), which are not displayed in Figure 4. They noted that findings on the influence of wettability on bacterial adhesion are inconsistent. Moreover, this relationship is influenced by the hydrophobicity of bacteria, which differs among species. Generally, hydrophobic bacterial tend to adhere to hydrophobic surfaces, while hydrophilic bacteria prefer hydrophilic surfaces (Filipović et al., 2020).
Wettability and the related protein adsorption are a big driving force for macrophage polarization. Several literature reviews describe that hydrophilic surfaces drive the macrophage polarization towards an anti-inflammatory phenotype in vitro (He et al., 2020; Li et al., 2021; Miron et al., 2023; Lee et al., 2019; Abaricia et al., 2021; Antmen et al., 2021; Abaricia et al., 2020). Moreover, Li et al. and Antmen et al. presented literature that found an increase in pro-inflammatory phenotype factors as a reaction to hydrophobic surfaces (Li et al., 2021; Antmen et al., 2021).
In conclusion, hydrophilic surfaces promote cellular and bacterial adhesion through the appropriate spatial conformation of proteins, and stimulate an anti-inflammatory environment for macrophages.
Porous materials or porous surface coatings help promote cellular attachment, vascularization and transport of nutrients and thus porosity plays an essential role during the early stages of osseointegration (Bandyopadhyay et al., 2023). Porosity increases the surface area for potential cell adhesion and helps with the interlocking between host tissue and implant. Porosity is defined as a measure of spaces in a material, where the pore size is the average diameter of the spaces (Figure 2). The percentage of porosity and the size of the pores have limitations. During implant design it should be considered that pores and porosity throughout the structure decrease structural stability. Therefore, porous structures should be tested on their mechanical strength to withstand in vivo stresses (Zhu et al., 2021; Bandyopadhyay et al., 2023; Falchete do Prado et al., 2018). The size of the pores of a biomaterial dictate the cellular interactions; small pore sizes limit cellular migration into the material, therefore cells tend to grow only on the outer surfaces. Larger pore sizes restrict the total surface area for cells to attach to but stimulate cellular migration into the biomaterial (Chen et al., 2018). Thus, this would suggest there is a pore size range where cellular migration, adhesion and vascularization is optimal.
Chen et al. and Nobles et al. stated that an appropriate pore size is between 100–600 μm and 100–700 µm respectively, for cell ingrowth and adhesion (Nobles et al., 2021; Chen et al., 2018). Chen et al. also noted favorable pore sizes of around 100–135 µm for osteoblast adhesion. However, it's important to clarify that the optimal pore size for bone regeneration may not necessarily align with that for osteoblast adhesion (Chen et al., 2018). Whereas Gu et al. reviewed several studies of porous structures in animal models (Gu et al., 2022). Their findings suggested pore sizes ranging from 500 to 600 µm and a porosity of 80%–90% is most optimal for osseointegration on titanium scaffolds tested in vivo. Karageorgiou et al. observed increased osteogenesis for scaffolds with pore sizes above 300 μm, due to the possibility of vascularization (Karageorgiou and Kaplan, 2005). They pointed out that the upper limit of scaffold pore sizes and porosity is set by mechanical restraints. Hussain et al. found macroporous scaffolds, with a pore size range from 250 to 500 μm, to be essential for bone regeneration due to the proper osteoblast attachment, angiogenesis and integration of host tissue (Hussain et al., 2024). While Hussain et al.’s findings are primarily oriented towards tissue engineering applications rather than orthopedic hip implants, there exists potential to learn from this field.
The porosity of biomaterials can be designed to influence the macrophage phenotype, Lee et al. found an increase in anti-inflammatory macrophages with pores of diameters between 100 and 200 nm (Lee et al., 2019). He et al. stated that pores and porosity influence oxygen supply after implantation by affecting vascularization. Limited oxygen supply results in inflammation affecting the bone remodeling. They found an anti-inflammatory phenotype with increasing pore size (He et al., 2020). However, as Nobles et al. raised, the porosity and pore size should be optimized for its intended application, dependent on important biological processes, since there is no “one-size-fits-all” (Nobles et al., 2021).
Figure 5 shows both in vitro and in vivo research investigating the impact of pore sizes onto the key biological processes. In conclusion, the impact on osseointegration has been extensively studied, revealing overlapping findings. However, the research into the effect on bacterial adhesion and macrophage polarization has been limited.
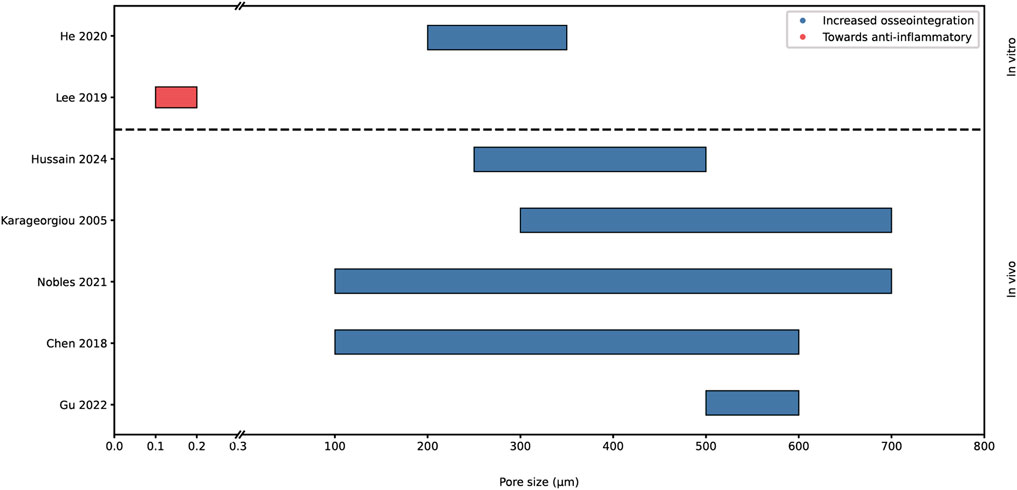
Figure 5. Graphical representation of proposed optimal pore sizes for increased osseointegration and observed polarization of macrophage. Analyzed with in vivo and in vitro data. The x-axis is broken up into 0-0.3 µm and 0.3-800 µm.
The relationship between biomaterial properties and the key biological processes is crucial for determining the clinical success of an implant. A better understanding of the cell-biomaterial interactions can greatly contribute to the implant design phase. Therefore, this review has examined existing literature reviews to identify possible safe innovation ranges. Extensive research has been done on the effect of biomaterial properties on osseointegration, finding consistent ranges of optimal biomaterial properties. Contrarily, no consistent information could be found regarding bacterial adhesion and immune response, except for hydrophilic surfaces promoting anti-inflammatory phenotypes in macrophages. In the Safe-by-Design approach, the gathered information helps to identify potential hazards or innovation windows early on (Dekkers et al., 2020), as summarized in Table 1. The cells of Table 1 are color categorized on the level of evidence found supporting the identified interactions displayed in the table cells.
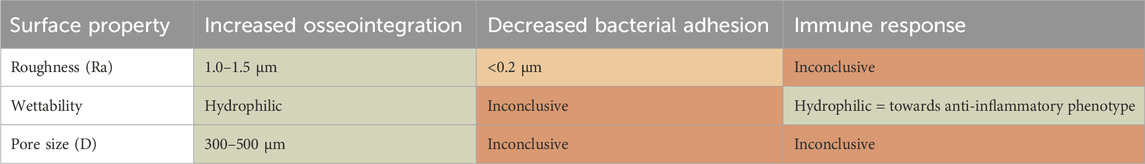
Table 1. Overview on relation between the biomaterial property design space as related to the three key biological processes. In context of Safe-by-Design the results are categorized in convincing (green), indication (orange) and inconclusive (red) information.
This literature review reveals that while individual biomaterial property optima may be present for distinct biological processes, there is insufficient data to conclude on an optimal range to satisfy all key biological processes. Yet, these insights provide directions for safer and functional biomaterial innovations and form the first step in a Safe-by-Design framework. As a next step, the impact of the biomaterial properties on key biological properties should be tested using various in vitro assays, to provide more detailed insight and reduce uncertainty (Salthouse et al., 2022). The SbD approach is a balancing act between different aspects. The approach helps to better understand potential negative impacts of design choices and provides innovators the option to discuss trade-offs between different material impacts.
For innovators to successfully apply a SbD strategy, information is key. The more knowledge available and gathered in an early stage of innovation, the more efficient the product development and safety assessment can be performed. Where retrospective studies, focused on existing implant material design (Puijk et al., 2023), can provide valuable insights by creating a feedback loop from existing data to inform and improve future implant innovations. As mentioned, there exists no optimal property value to maximize all biological processes. However, this knowledge can function as a guide during the design phase and safety assessment. As seen for orthopedic hip implants, if the biomaterial roughness deviates for the optimal osseointegration roughness range, aimed at limiting bacterial adhesion, safety assessments should prioritize evaluating the osseointegration capabilities of the biomaterial. Conversely, if the design is within the optimal osseointegration roughness range but also promoting bacterial adhesion, additional preventive (designing) strategies may be considered to fight bacterial adhesion. The SbD approach can be applied to any implant type or application, where different key biological processes are involved and the properties to be varied are dependent on the type of biomaterial used.
In this manuscript, we highlighted a selection of three material properties within the design space and three biological processes that are acutely influenced by these properties. However, long-term effects, such as biomaterial degradation, must also be considered, as they can significantly impact tissue reactions. For instance, while metal wear particles from metal-on-metal hip implants can cause adverse effects (Dapunt et al., 2014), bioactive degradation products may actively support tissue regeneration (Rahaman et al., 2014). This interplay between material properties and biological responses illustrates the complexity of biomaterials and medical implants. Although the current study focused on a Safe-by-Design (SbD) approach for the surface properties of titanium orthopedic implants, the challenges are likely to grow with the development of more innovative biomaterials and tissue-engineering constructs. These may involve combinations of materials with distinct properties, the integration of bioactive components, or even cell-loaded constructs (Todros et al., 2021).
In nanomaterial research, Tavernaro et al. (Tavernaro et al., 2021) stated “A Safe-by-Design strategy strives for negligible human safety risks through an acceptable balance between safety, product functionality and, as far as possible, costs”. The implementation of SbD in various industries often involves substituting harmful compounds with less harmful alternatives, sometimes at the expense of product functionality. In contrast, implants present a case where safety and functionality are inherently interconnected. Implant functionalities, such as an antimicrobial surface, contribute to implant safety by avoiding biomaterial-associated infections (see Figure 6). For this focus-application, the common causes of implant failure are implant loosening and infection (Khalifa and Bakr, 2021). These causes are linked to the selected key biological processes and are relevant to both safety and functionality. Paying attention to the influence of biomaterial properties on these key biological processes during the design and development process, within a SbD approach, will increase the safety of implants. Figure 6 illustrates the phenomena associated with functionality, safety, or both. Phenomena like osseointegration, immune acceptance and antimicrobial functionalities contribute to both functionality and safety.
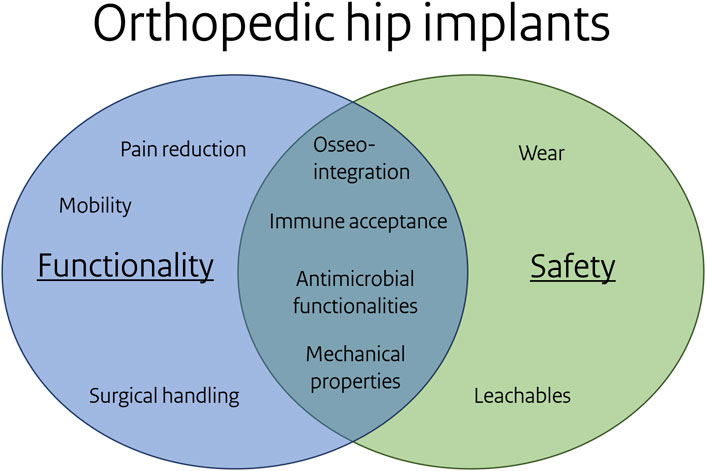
Figure 6. Overlap of functionality and safety for the orthopedic hip focus application. Note: some items under functionality and safety can be related, e.g. particular leachables reduce bacterial adhesion or affect osseointegration, mechanical properties may link to wear.
This review is not systematic, but illustrates the use of the SbD framework and its potential outcomes. Search strings were used to identify material property–biological response relationships, with exclusions like mandibular implantation, skull implantation, and induced animal models. While more data is needed for firm conclusions, the findings offer a starting point for biomaterial innovation using Safe-by-Design. The methodology relies predominantly on literature reviews and articles that analyzed data and identified trends within a specific property range. Consensus could be found for optimal property ranges for osseointegration in literature reviews. However, the influence of the selected properties on macrophage polarization and bacterial adhesion lacked sufficient clarity to do so. Only a few reviews identified specific property ranges that reliably induced a particular biological outcome for these two processes, resulting in limited inclusion of information in this review. This highlights the uncertainty surrounding the relationship between biomaterial properties and macrophage polarization and bacterial adhesion, emphasizing the necessity for further research in this area. Additionally, variability exists among the studies included in the literature reviews discussing in this manuscript. This variability includes the type of tests used, in vitro or in vivo methods, and the choice of animal model or cell types. The studies focused on different biomaterials, primarily titanium, with an emphasis on design considerations for specific applications. Since not all properties can be modified on titanium alone, the scope includes coatings and similar solutions. As a result, mapping the influence of biomaterial properties on several biological processes for one particular application is challenging. Nevertheless, these overviews of existing data can give an indication on the influence of one biomaterial property on several important biological processes. The raised variability observed between studies also highlights the need for standardizing the gathering and reporting of findings. The use of different models, measurement tools and techniques can greatly impact the results. Further information on the included studies can be found in the Supplementary Table S1.
A remaining challenge for in vitro safety assessment, is the lack of optimized and validated methods for the complex evaluation of key biological processes (Przekora, 2019). The standardized in vitro and in vivo tests described in ISO 10993 are usually one of the last steps in the process before patient trials. The in vitro methods mostly concern leachables and therefore cannot address the complexity of the direct interaction between the biomaterial and the host (Williams, 2017). To better understand the local events at the implant biomaterial surfaces, more advanced and application-specific in vitro tests are necessary, e.g., a standardized in vitro test for osseointegration assessment (Antmen et al., 2022). These can be used in the SbD approach after consideration of the existing information related to biomaterial properties and key biological processes. Furthermore, understanding biomaterial properties and the associated host tissue responses will guide the choice of biocompatibility tests. In vitro testing does not replace in vivo testing in animals and in humans for medical device approval. However, this can function as a preliminary evaluation to identify and address safety risks and hazard alerts during the development process, minimizing the number and expense of in vivo testing (Bernard et al., 2018).
During implant design, numerous biomaterial properties can be fine-tuned to align with the key biological processes pivotal for the success of the implant within a specific application. A comprehensive understanding of this intricate relationship between biological processes and biomaterial properties is crucial to optimize both components synergistically. A Safe-by-Design approach supports innovators during the design phase, providing essential tools to develop implants that prioritize safety at the beginning of development. This study introduced the application of Safe-by-Design for orthopedic titanium hip implants, emphasizing the advantages from early-stage knowledge integration. One important aspect is the relationship between biomaterial properties (roughness, wettability and pore size) and key biological processes (osseointegration, immune response and bacterial adhesion), serving as a cornerstone in implant design that yield optimal outcomes. From literature, this study has demonstrated that for certain combinations of biomaterial properties and key biological processes, an optimal property value for an optimal biological response can be found. Such a range was hypothesized by various authors for roughness and pore size, influencing osseointegration. Hydrophilic surfaces seem to drive an anti-inflammatory phenotype, combined with increased osseointegration and unfortunately increased bacterial adhesion. Consistent information regarding the impact of pore size and surface roughness on bacterial adhesion and macrophage polarization could not be found. A property range to satisfy all key biological processes has not been identified. This complex interaction between biomaterial properties and biological processes emphasizes the need for a careful Safe-by-Design approach in the precise design of medical implants. In Safe-by-Design the prior information needs focus on application-specific key biological processes linked to common adverse outcomes, as well as those crucial for implant success and safety. Application-specific in vitro tests will be selected and applied aiming to optimize the implant biomaterial and select the most suitable candidate for future in vivo studies, saving time and resources. Collectively, these steps can serve to guide design and innovation.
AG: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. NL: Conceptualization, Writing–review and editing. PB: Writing–review and editing. HB: Writing–review and editing. HA: Writing–review and editing. JA: Funding acquisition, Writing–review and editing. AvW: Writing–review and editing. AO: Conceptualization, Writing–review and editing. NB: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This publication is part of the project DARTBAC (with project number NWA.1292.19.354) of the research program NWA-ORC which is (partly) financed by the Dutch Research Council (NWO).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1504883/full#supplementary-material
Abaricia, J. O., Shah, A. H., Chaubal, M., Hotchkiss, K. M., and Olivares-Navarrete, R. (2020). Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 243, 119920-. doi:10.1016/j.biomaterials.2020.119920
Abaricia, J. O., Farzad, N., Heath, T. J., Simmons, J., Morandini, L., and Olivares-Navarrete, R. (2021). Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 133, 58–73. doi:10.1016/j.actbio.2021.04.021
Albrektsson, T., and Johansson, C. (2001). Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 10, S96–S101. doi:10.1007/s005860100282
Albrektsson, T., and Wennerberg, A. (2019). On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 21 (S1), 4–7. doi:10.1111/cid.12742
Anselme, K., Bigerelle, M., Noel, B., Dufresne, E., Judas, D., Iost, A., et al. (2000). Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 49 (2), 155–166. doi:10.1002/(sici)1097-4636(200002)49:2<155::aid-jbm2>3.3.co;2-a
Antmen, E., Vrana, N. E., and Hasirci, V. (2021). The role of biomaterials and scaffolds in immune responses in regenerative medicine: macrophage phenotype modulation by biomaterial properties and scaffold architectures. Biomaterials Sci. 9 (24), 8090–8110. doi:10.1039/d1bm00840d
Antmen, E., Muller, C. B., Calligaro, C., Dupret-Bories, A., Barthes, J., Lavalle, P., et al. (2022). In vitro two-step granuloma formation model for testing innate immune response to implants and coatings. Biomater. Adv. 138, 212872-. doi:10.1016/j.bioadv.2022.212872
Bandyopadhyay, A., Mitra, I., Goodman, S. B., Kumar, M., and Bose, S. (2023). Improving biocompatibility for next generation of metallic implants. Prog. Mater. Sci. 133, 101053. doi:10.1016/j.pmatsci.2022.101053
Barberi, J., and Spriano, S. (2021). Titanium and protein adsorption: an overview of mechanisms and effects of surface features. Materials, 14(7):1590. doi:10.3390/ma14071590
Batool, F., Özçelik, H., Stutz, C., Gegout, P.-Y., Benkirane-Jessel, N., Petit, C., et al. (2021). Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 12, 20417314211041428. doi:10.1177/20417314211041428
Bernard, M., Jubeli, E., Pungente, M. D., and Yagoubi, N. (2018). Biocompatibility of polymer-based biomaterials and medical devices – regulations, in vitro screening and risk-management. Biomaterials Sci. 6 (8), 2025–2053. doi:10.1039/c8bm00518d
Bu, W., Wu, Y., Ghaemmaghami, A. M., Sun, H., and Mata, A. (2022). Rational design of hydrogels for immunomodulation. Regen. Biomater. 9, rbac009. doi:10.1093/rb/rbac009
Chen, Z., Klein, T., Murray, R. Z., Crawford, R., Chang, J., Wu, C., et al. (2016). Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 19 (6), 304–321. doi:10.1016/j.mattod.2015.11.004
Chen, S., Guo, Y., Liu, R., Wu, S., Fang, J., Huang, B., et al. (2018). Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surfaces B Biointerfaces 164, 58–69. doi:10.1016/j.colsurfb.2018.01.022
Chen, L., Yao, Z., Zhang, S., Tang, K., Yang, Q., Wang, Y., et al. (2022). Biomaterial-induced macrophage polarization for bone regeneration. Chin. Chem. Lett. 34, 107925. doi:10.1016/j.cclet.2022.107925
Christo, S. N., Diener, K. R., Bachhuka, A., Vasilev, K., and Hayball, J. D. (2015). Innate immunity and biomaterials at the nexus: friends or foes. BioMed Res. Int. 2015, 1–23. doi:10.1155/2015/342304
Damiati, L., Eales, M. G., Nobbs, A. H., Su, B., Tsimbouri, P. M., Salmeron-Sanchez, M., et al. (2018). Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 9, 2041731418790694. doi:10.1177/2041731418790694
Dapunt, U., Giese, T., Lasitschka, F., Reinders, J., Lehner, B., Kretzer, J. P., et al. (2014). On the inflammatory response in metal-on-metal implants. J. Transl. Med. 12 (1), 74. doi:10.1186/1479-5876-12-74
Dekkers, S., Wijnhoven, S. W. P., Braakhuis, H. M., Soeteman-Hernandez, L. G., Sips, AJAM, Tavernaro, I., et al. (2020). Safe-by-Design part I: proposal for nanospecific human health safety aspects needed along the innovation process. NanoImpact 18, 100227-. doi:10.1016/j.impact.2020.100227
Dong, J., Wang, W., Zhou, W., Zhang, S., Li, M., Li, N., et al. (2022). Immunomodulatory biomaterials for implant-associated infections: from conventional to advanced therapeutic strategies. Biomaterials Res. 26 (1), 72. doi:10.1186/s40824-022-00326-x
European, C., Joint Research, C., Caldeira, C., Farcal, L., Garmendia Aguirre, I., Mancini, L., et al. (2022a). Safe and sustainable by design chemicals and materials: framework for the definition of criteria and evaluation procedure for chemicals and materials. Luxembourg: Publications Office of the European Union.
European, C., Joint Research, C., Caldeira, C., Farcal, R., Moretti, C., Mancini, L., et al. (2022b). Safe and sustainable by design chemicals and materials – review of safety and sustainability dimensions, aspects, methods, indicators, and tools. Luxembourg: Publications Office of the European Union.
Falchete do Prado, R., Esteves, G. C., De, E. L., Santos, S., Griti Bueno, D. A., Alves Cairo, C. A., et al. (2018). In vitro and in vivo biological performance of porous Ti alloys prepared by powder metallurgy. PLoS ONE 13 (5), e0196169. doi:10.1371/journal.pone.0196169
Filipović, U., Dahmane, R. G., Ghannouchi, S., Zore, A., and Bohinc, K. (2020). Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 283, 102228. doi:10.1016/j.cis.2020.102228
Gu, Y., Sun, Y., Shujaat, S., Braem, A., Politis, C., and Jacobs, R. (2022). 3D-printed porous Ti6Al4V scaffolds for long bone repair in animal models: a systematic review. J. Orthop. Surg. Res. 17 (1), 68. doi:10.1186/s13018-022-02960-6
Hayes, J. S., and Richards, R. G. (2010). Surfaces to control tissue adhesion for osteosynthesis with metal implants:in vitroandin vivostudies to bring solutions to the patient. Expert Rev. Med. Devices 7 (1), 131–142. doi:10.1586/erd.09.55
He, J., Chen, G., Liu, M., Xu, Z., Chen, H., Yang, L., et al. (2020). Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater. Sci. Eng. C 108, 110411. doi:10.1016/j.msec.2019.110411
Hussain, Z., Mehmood, S., Liu, X., Liu, Y., Wang, G., and Pei, R. (2024). Decoding bone-inspired and cell-instructive cues of scaffolds for bone tissue engineering. Eng. Regen. 5 (1), 21–44. doi:10.1016/j.engreg.2023.10.003
ISO 10993, (2018). European committee for standardization. 5. Brussels: Biological evaluation of medical devices
Jurczak, K. M., van der Boon, T. A. B., Devia-Rodriguez, R., Schuurmann, R. C. L., Sjollema, J., van Huizen, L., et al. (2024). Recent regulatory developments in EU Medical Device Regulation and their impact on biomaterials translation. Bioeng. and Transl. Med., e10721. doi:10.1002/btm2.10721
Karageorgiou, V., and Kaplan, D. (2005). Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26 (27), 5474–5491. doi:10.1016/j.biomaterials.2005.02.002
Khalifa, A. A., and Bakr, H. M. (2021). Updates in biomaterials of bearing surfaces in total hip arthroplasty. Arthroplasty 3 (1), 32–38. doi:10.1186/s42836-021-00092-6
Kim, C. S., Kim, J. H., Kim, B., Park, Y. S., Kim, H. K., Tran, H. T., et al. (2017). A specific groove pattern can effectively induce osteoblast differentiation. Adv. Funct. Mater. 27 (44). doi:10.1002/adfm.201703569
Kreve, S., and Reis, A. C. D. (2021). Bacterial adhesion to biomaterials: what regulates this attachment? A review. Jpn. Dent. Sci. Rev. 57, 85–96. doi:10.1016/j.jdsr.2021.05.003
Lackington, W. A., Fleyshman, L., Schweizer, P., Elbs-Glatz, Y., Guimond, S., and Rottmar, M. (2022). The response of soft tissue cells to Ti implants is modulated by blood-implant interactions. Mater. Today Bio 15. doi:10.1016/j.mtbio.2022.100303
Lee, J., Byun, H., Madhurakkat Perikamana, S. K., Lee, S., and Shin, H. (2019). Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthc. Mater. 8 (4), 1801106. doi:10.1002/adhm.201801106
Li, J., Jiang, X., Li, H., Gelinsky, M., and Gu, Z. (2021). Tailoring materials for modulation of macrophage fate. Adv. Mater. 33 (12), 2004172. doi:10.1002/adma.202004172
Li, P., Yin, R., Cheng, J., and Lin, J. (2023). Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int. J. Mol. Sci. 24 (14), 11680. doi:10.3390/ijms241411680
Mariani, E., Lisignoli, G., Borzì, R. M., and Pulsatelli, L. (2019). Biomaterials: foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 20 (3), 636. doi:10.3390/ijms20030636
Miron, R. J., Bohner, M., Zhang, Y., and Bosshardt, D. D. (2023). “Osteoinduction and osteoimmunology: emerging concepts,” in Periodontology 2000.
Mitra, S. P. (2020). Protein adsorption on biomaterial surfaces: subsequent conformational and biological consequences-A review. J. Surf. Sci. Technol. 36 (2), 970–1893. doi:10.18311/jsst/2020/23282
Morinaga, K., Kido, H., Sato, A., Watazu, A., and Matsuura, M. (2009). Chronological changes in the ultrastructure of titanium-bone interfaces: analysis by light microscopy, transmission electron microscopy, and micro-computed tomography. Clin. Implant Dent. Relat. Res. 11 (1), 59–68. doi:10.1111/j.1708-8208.2008.00093.x
Nobles, K. P., Janorkar, A. V., and Williamson, R. S. (2021). Surface modifications to enhance osseointegration–Resulting material properties and biological responses. J. Biomed. Mater. Res. Part B Appl. Biomaterials 109 (11), 1909–1923. doi:10.1002/jbm.b.34835
OECD, (2021). Health at a glance 2021: OECD indicators OECDiLibrary2021. Available at: https://www.oecd-ilibrary.org/sites/8b492d7a-en/index.html?itemId=/content/component/8b492d7a-en (Accessed June 19, 2023).
Pandey, L. M. (2022). Design of biocompatible and self-antibacterial titanium surfaces for biomedical applications. Curr. Opin. Biomed. Eng. 25, 100423. doi:10.1016/j.cobme.2022.100423
Przekora, A. (2019). The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 97, 1036–1051. doi:10.1016/j.msec.2019.01.061
Puijk, R., Rassir, R., Sierevelt, I. N., Spekenbrink-Spooren, A., Nelissen, RGHH, and Nolte, P. A. (2023). Association between surface modifications for biologic fixation and aseptic loosening of uncemented total knee arthroplasties. J. Arthroplasty 38 (12), 2605–2611.e1. doi:10.1016/j.arth.2023.05.094
Rahaman, M. N., Bal, B. S., and Huang, W. (2014). Review: emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater. Sci. Eng. C 41, 224–231. doi:10.1016/j.msec.2014.04.055
Rahmati, M., Silva, E. A., Reseland, J. A., Heyward, C., and Haugen, H. J. (2020). Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 49 (15), 5178–5224. doi:10.1039/d0cs00103a
Ren, B., Wan, Y., Liu, C., Wang, H., Yu, M., Zhang, X., et al. (2021). Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: an in vitro and in vivo study. Mater. Sci. Eng. C 118, 111505-. doi:10.1016/j.msec.2020.111505
Roerink, A. M. C., Nelissen, RGHH, Holder, C., Graves, S. E., Dunbar, M., Bohm, E., et al. (2024). Sex-based differences in risk of revision for infection after hip, knee, shoulder, and ankle arthroplasty in osteoarthritis patients: a multinational registry study of 4,800,000 implants. Acta Orthop. 95, 730–736. doi:10.2340/17453674.2024.42183
Rosman, C. W. K., van Dijl, J. M., and Sjollema, J. (2021). “Interactions between the foreign body reaction and Staphylococcus aureus biomaterial-associated infection,” in Winning strategies in the derby on biomaterial implant surfaces. doi:10.1080/1040841X.2021.2011132
Saldaña, L., Bensiamar, F., Boré, A., and Vilaboa, N. (2011). In search of representative models of human bone-forming cells for cytocompatibility studies. Elsevier, 4210–4221.
Salthouse, D., Novakovic, K., Hilkens, C. M. U., and Ferreira, A. M. (2022). Interplay between biomaterials and the immune system: challenges and opportunities in regenerative medicine. Acta Biomater. 155, 1–18. doi:10.1016/j.actbio.2022.11.003
Schmutz, M., Borges, O., Jesus, S., Borchard, G., Perale, G., Zinn, M., et al. (2020). A methodological safe-by-design approach for the development of nanomedicines. Front. Bioeng. Biotechnol. 8, 258. doi:10.3389/fbioe.2020.00258
Stich, T., Alagboso, F., Křenek, T., Kovářík, T., Alt, V., and Docheva, D. (2022). Implant-bone-interface: reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. and Transl. Med. 7 (1), e10239–e. doi:10.1002/btm2.10239
Stoilov, M., Stoilov, L., Enkling, N., Stark, H., Winter, J., Marder, M., et al. (2022). Effects of different titanium surface treatments on adhesion, proliferation and differentiation of bone cells: an in vitro study. J. Funct. Biomaterials, 13(3):143. doi:10.3390/jfb13030143
Tavernaro, I., Dekkers, S., Soeteman-Hernández, L. G., Herbeck-Engel, P., Noorlander, C., and Kraegeloh, A. (2021). Safe-by-Design part II: a strategy for balancing safety and functionality in the different stages of the innovation process. NanoImpact 24, 100354. doi:10.1016/j.impact.2021.100354
Todros, S., Todesco, M., and Bagno, A. (2021). Biomaterials and their biomedical applications: from replacement to regeneration. Processes 9 (11), 1949. doi:10.3390/pr9111949
van de Poel, I., and Robaey, Z. (2017). Safe-by-Design: from safety to responsibility. NanoEthics 11 (3), 297–306. doi:10.1007/s11569-017-0301-x
van Dijk, J., Flerlage, H., Beijer, S., Slootweg, J. C., and van Wezel, A. P. (2022). Safe and sustainable by design: a computer-based approach to redesign chemicals for reduced environmental hazards. Chemosphere 296, 134050. doi:10.1016/j.chemosphere.2022.134050
van Gelder, P., Klaassen, P., Taebi, B., Walhout, B., van Ommen, R., van de Poel, I., et al. (2021). Safe-by-Design in engineering: an overview and comparative analysis of engineering disciplines. Int. J. Environ. Res. Public Health 18, 6329, doi:10.3390/ijerph18126329
Villapun, P. V. M., Carter, L. N., Schröder, C., Colavita, P. E., Hoey, D. A., Webber, M. A., et al. (2022). Surface free energy dominates the biological interactions of postprocessed additively manufactured Ti-6Al-4V. Washington, DC: ACS biomaterials science and engineering.
Williams, D. F. (2017). Biocompatibility pathways: biomaterials-induced sterile inflammation, mechanotransduction, and principles of biocompatibility control. ACS Biomaterials Sci. Eng. 3 (1), 2–35. doi:10.1021/acsbiomaterials.6b00607
Williams, D. F. (2019). Biocompatibility in clinical practice: predictable and unpredictable outcomes. Prog. Biomed. Eng. 1 (1), 013001. doi:10.1088/2516-1091/ab22cc
Williams, D. F. (2021). Assessing the triad of biocompatibility, medical device functionality and biological safety. Med. Devices and Sensors 4 (1), e10150–e. doi:10.1002/mds3.10150
Xie, Y., Hu, C., Feng, Y., Li, D., Ai, T., Huang, Y., et al. (2020). Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 7 (3), 233–245. doi:10.1093/rb/rbaa006
Yang, K., Shi, J., Wang, L., Chen, Y., Liang, C., Yang, L., et al. (2022). Bacterial anti-adhesion surface design: surface patterning, roughness and wettability: a review. J. Mater. Sci. and Technol. 99, 82–100. doi:10.1016/j.jmst.2021.05.028
Zheng, S., Bawazir, M., Dhall, A., Kim, H.-E., He, L., Heo, J., et al. (2021). Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 9, 643722. doi:10.3389/fbioe.2021.643722
Keywords: osseointegration, immune response, bacterial adhesion, surface properties, Safe-by-Design
Citation: Gielen AMC, Leijten NM, Balraadjsing PPS, Braakhuis HM, Abee H, Arts JJ, van Wezel AP, Oomen AG and Beijer NRM (2025) Utilizing biomaterial surface properties to improve orthopedic hip implant safety and function in a Safe-by-Design approach. Front. Bioeng. Biotechnol. 13:1504883. doi: 10.3389/fbioe.2025.1504883
Received: 03 October 2024; Accepted: 31 January 2025;
Published: 21 February 2025.
Edited by:
Masoud Mozafari, University of Oulu, FinlandReviewed by:
Raj Hazra, North Dakota State University, United StatesCopyright © 2025 Gielen, Leijten, Balraadjsing, Braakhuis, Abee, Arts, van Wezel, Oomen and Beijer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anniek M. C. Gielen, QW5uaWVrLmdpZWxlbkByaXZtLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.