- 1Centre for Biomaterials and Tissue Engineering (CBIT), Universitat Politècnica de València, València, Spain
- 2Hematology and Transplant Center, University Hospital “San Giovanni di Dio e Ruggi d’Aragona”, Salerno, Italy
- 3Department of Medicine and Surgery, University of Salerno, Baronissi, Italy
Editorial on the Research Topic
A new age for articular cartilage: from bench to beside of tissue engineering and regenerative medicine in cartilage tissue repair
1 Introduction
Articular cartilage is a key part of the synovial joints, which also include the synovial cavity and joint capsule. Various events can cause articular cartilage lesions, such as traumas, sports-related activities, occupational accidents, or autoimmune diseases. These can lead to chronic conditions known as osteoarthritis (OA) because cartilage has a limited blood supply and low metabolic activity, preventing it from healing on its own (Richmond et al., 2013). Detailly, OA is characterized by structural deterioration of hyaline cartilage with subsequent exposure of underlying bones (Man and Mologhianu, 2014). Current clinical management of articular cartilage injuries and OA represents a significant challenge in orthopedics. Available treatments are largely conservative (e.g., pharmacotherapy, arthrocentesis, and physiotherapy) or surgical, primarily focusing on relieving pain and inflammatory symptoms to decrease functional disability and complications (Żylińska et al., 2018). However, no existing medications can completely heal or regenerate cartilage lesions.
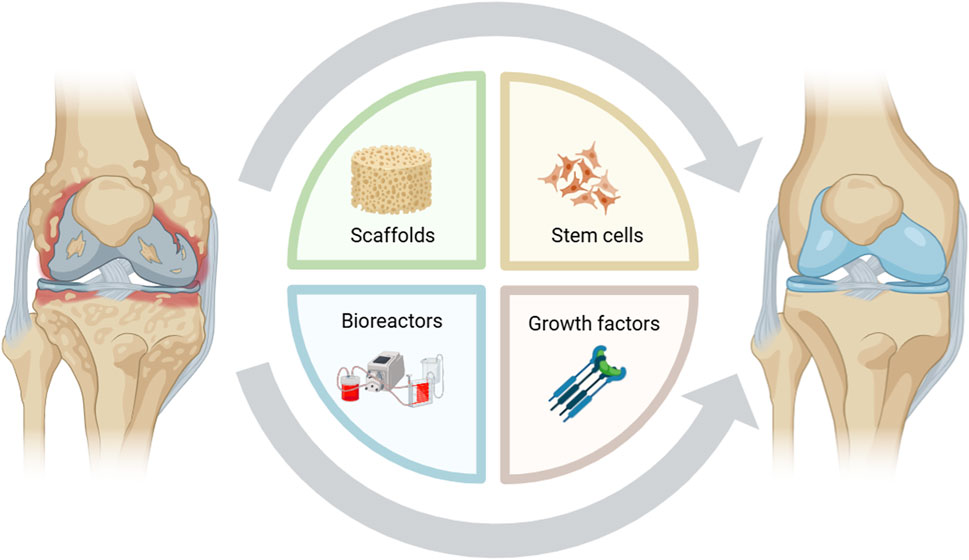
Tissue engineering is an innovative and promising therapeutic approach in which cells, scaffolds, and chemical or mechanical signals are organized to mimic biological tissues. Once optimized in vitro, synthetic tissues can be grafted to damaged sites to guide regeneration (Figure 1). Interactive and bioengineered supports, also termed 3-dimensional (3D) scaffolds, are implanted to stimulate new cartilage formation and promote stem cell differentiation toward a chondrogenic phenotype. 3D scaffolds, composed of biocompatible and biodegradable polymers, can host stem cells and growth factors to generate a bioengineered tissue mimicking physiological conditions; however, biomaterials must comply with strict requirements to be adopted in clinical settings, including biocompatibility, biodegradability, and low immunogenicity (Song et al., 2018). Moreover, these biomaterials should exhibit suitable mechanical properties to avoid structural degradation once implanted in vivo. Therefore, the proper choice of biomaterials and human stem cells, as well as chemical and mechanical stimuli, is critical to the success of tissue engineering (Lamparelli et al., 2022).
In this research topic of Frontiers in Bioengineering and Biotechnology, we gathered original works and reviews that provide state-of-the-art about cartilage tissue engineering, focusing on recent 3D in vitro models, including organoids under static or dynamic conditions or employing innovative scaffold-based or high-density cultures. Furthermore, we included bibliometric analysis for exploring research interest in OA joint distraction treatments and original articles on the biological significance of microRNAs in mitigating inflammation and fibrosis in 3D in vitro OA models.
In their review, Zeng et al. have summarized advancements in new OA models using cartilage organoids, 3D structures that mimic cartilage architecture and physiological functions. These models are beneficial for disease modeling, drug screening, and regenerative medicine purposes. However, cartilage organoids still face many challenges before their application in clinical research and regenerative medicine because they poorly reproduce in vivo functions, distribute nutrients, growth factors, and oxygen diffusion at the core of 3D masses, and ensure proper interactions between different cell types (Zeng et al.).
In their original article, Peng et al. conducted a bibliometric analysis of joint distraction within OA treatment research, examining over 450 scholarly articles, mostly from the United States, as leaders in international collaboration, publication count, and citation frequency. However, most studies focused on nonsurgical interventions and joint arthroscopy procedures, with limited research on joint distraction for OA treatment (Peng et al.).
The work by Pfeifer et al. explores the expression of microRNA-140 in equine synovial-membrane-derived mesenchymal stem cells-derived extracellular vesicles under inflammatory conditions in 2D- and 3D-OA in vitro models, as this microRNA has anti-inflammatory and anti-fibrotic properties and is produced in response to inflammation to quickly restore homeostasis, especially under 3D conditions (Pfeifer et al.).
The review by Cui et al. deals with common osteochondral staining methods, criteria for high-quality histological images, and current histological scoring systems for osteochondral regeneration with tissue-engineered grafts in synovial joint pathological degeneration. They emphasize the importance of assessing the cartilage layer, new bone formation, and graft–host interface through histological staining, and suggest including the subchondral bone plate in the scoring system for osteochondral regeneration (Cui et al.).
Finally, Cheng et al. use a 3D printing technology to fabricate a triphasic scaffold of PLA/PCL-PLGA/Mg(OH)₂, composed of a cartilage layer, an osteochondral interface, and a bone layer, and filled with Velvet antler polypeptides, a bioactive peptide, for promoting in vitro osteogenesis and chondrogenesis of fibrocartilage stem cells. This biocompatible scaffold could represent a promising tool for bone and cartilage tissue engineering in osteochondral defects treatment, likely due to its hierarchical structure that creates distinct microenvironments for cartilage and bone tissues (Cheng et al.).
Author contributions
PG: Writing–original draft, Writing–review and editing. VG: Writing–original draft, Writing–review and editing. EL: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Lamparelli, E. P., Ciardulli, M. C., Scala, P., Scognamiglio, M., Charlier, B., Di Pietro, P., et al. (2022). Lipid nano-vesicles for thyroid hormone encapsulation: a comparison between different fabrication technologies, drug loading, and an in vitro delivery to human tendon stem/progenitor cells in 2D and 3D culture. Int. J. Pharm. 624, 122007. doi:10.1016/j.ijpharm.2022.122007
Man, G. S., and Mologhianu, G. (2014). Osteoarthritis pathogenesis - a complex process that involves the entire joint. J. Med. Life 7 (1), 37–41.
Richmond, S. A., Fukuchi, R. K., Ezzat, A., Schneider, K., Schneider, G., and Emery, C. A. (2013). Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J. Orthop. Sports Phys. Ther. 43 (8), 515–B19. doi:10.2519/jospt.2013.4796
Song, R., Murphy, M., Li, C., Ting, K., Soo, C., and Zheng, Z. (2018). Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel Ther. 12, 3117–3145. doi:10.2147/DDDT.S165440
Keywords: articular cartilage, tissue engineering, regenerative medicine, tissue repair, personalized medicine
Citation: Gentile P, Giudice V and Lamparelli EP (2024) Editorial: A new age for articular cartilage: from bench to beside of tissue engineering and regenerative medicine in cartilage tissue repair. Front. Bioeng. Biotechnol. 12:1519455. doi: 10.3389/fbioe.2024.1519455
Received: 29 October 2024; Accepted: 21 November 2024;
Published: 29 November 2024.
Edited and reviewed by:
Ranieri Cancedda, Independent Researcher, Genova, ItalyCopyright © 2024 Gentile, Giudice and Lamparelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erwin Pavel Lamparelli, ZWxhbXBhcmVsbGlAdW5pc2EuaXQ=
 Piergiorgio Gentile
Piergiorgio Gentile Valentina Giudice
Valentina Giudice Erwin Pavel Lamparelli
Erwin Pavel Lamparelli