- 1Department of Central Laboratory, Liaocheng People’s Hospital, Liaocheng, China
- 2Nursing Department, Liaocheng People’s Hospital, Liaocheng, China
- 3Department of Traditional Chinese Medicine, Liaocheng People’s Hospital, Liaocheng, China
Hospital-acquired pressure ulcers (HAPUs) are still an important worldwide issue related to the safety and quality of patient care, which are among the top five adverse events reported. Patients who develop HAPUs have longer stays in the hospital than necessary, are at a greater risk of infections, and are more likely to die. Surgical patients are prone to developing PUs because they often remain immobile for extended periods of time, and their surgical procedures may limit the flow of blood oxygen and nutrition and lead to a decrease in muscle tone. Mesenchymal stem cells (MSCs) represent an attractive stem cell source for tissue regeneration in clinical applications, which have been demonstrated to improve wound healing through re-epithelialization, increased angiogenesis, and granulation tissue formation. Here, we present the case of an emergency surgical patient who developed an ulcer on the right heel during hospitalization. The human umbilical cord Wharton’s jelly-derived MSCs (WJ-MSCs) re-suspended in platelet-rich plasma (PRP) were injected into ulcer margins. Four days after the WJ-MSC application, the patient showed progressive healing of the PU. From days 4 to 33, granulation tissue formation and re-epithelialization were clearly observed. The ulcer was almost healed completely on day 47, and the pain in the patient’s wound area also decreased. Thus, intradermal transplantation of WJ-MSCs and PRP was safe and effective for treatment in patients with pressure ulcers. WJ-MSCs, together with PRP, may offer a promising treatment option for wound healing.
Introduction
Pressure ulcers (PUs), also known as pressure injuries, pressure sores, or bedsores, are defined as a type of injury that breaks down the skin or underlying soft tissues, usually over a bony prominence or related to a medical or other device (Gillespie et al., 2014; Chaboyer et al., 2016). The injury usually occurs as a result of intense and/or prolonged pressure or pressure in combination with shear (Gaspar et al., 2019). With the aging of the population worldwide, the number of patients with PUs is increasing every year (Mervis and Phillips, 2019). Hospital-acquired PUs (HAPUs) in the hospital context remain an important worldwide issue related to the safety and quality of patient care, ranking among the top five adverse events reported (Brandeis et al., 2001; Gillespie et al., 2014). The incidence and prevalence rates of PUs among hospitalized patients vary greatly, ranging from less than 3% to over 30% (Nixon et al., 2006; Schuurman et al., 2009). Once PUs form, conservative management is usually indicated for less severe ulcers (stages I and II); the current therapeutic approaches for severe ulcers (stages III and IV) are surgery and negative pressure (Cushing and Phillips, 2013). Given the current challenges in prevention and management strategies for PU patients, new perspectives, technologies, and more effective treatments must be further considered.
With the development of stem cell therapy and tissue engineering in recent years, mesenchymal stem cells (MSCs) have shown promising outcomes in treating chronic wounds such as pressure ulcers, radiation-related skin injuries, severe skin burns, and non-healing diabetic ulcers (Portas et al., 2016; Pelizzo et al., 2018; Wu et al., 2018; Han et al., 2019; Torres-Guzman et al., 2023). The main sources are umbilical cord-derived MSCs (UC-MSCs), bone marrow-derived MSCs (BM-MSCs), and adipose tissue-derived MSCs (AD-MSCs) (Wang et al., 2016). Among these, the umbilical cords are easier to obtain and culture MSCs due to noninvasive collection, cost-effectiveness, productivity, and ethical access (Arno et al., 2014). In particular, the umbilical cord Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) have been demonstrated to be young, immunomodulatory, and non-tumorigenic cells with lower immunogenicity, faster proliferation, and greater ex vivo expansion capabilities than other human MSC sources, such as BM-MSCs and AD-MSCs (Nekanti et al., 2010). Arno et al. proved that human WJ-MSCs can promote wound healing by paracrine signaling in an excisional full-thickness skin murine model (Arno et al., 2014). WJ-MSCs possess the capacity to be induced to differentiate into sweat gland-like cells, which would be potential in sweat gland restoration after skin injury and improvement in cutaneous regeneration (Xu et al., 2012). Meanwhile, WJ-MSCs decrease liver, kidney, and lung fibrosis, which play a more important role in promoting skin regeneration (Moodley et al., 2009; Tsai et al., 2009; Du et al., 2012). Thus, the clinical application of WJ-MSCs has been shown to accelerate wound healing, and they have great potential in treating PUs.
In recent years, platelet-rich plasma (PRP), also named autologous platelet gel, has raised a great deal of interest in promoting angiogenesis and increasing the healing rate of PUs (Volakakis et al., 2019; Uçar and Çelik, 2020). It is obtained from the peripheral blood of the patient with elevated concentrations of platelets and extensively adapted and applied to acute and chronic wounds (Xiao et al., 2021). Recent findings proposed that platelets, growth factors, cytokines, chemokines, and fibrin in PRP gel can aid in wound healing and skin regeneration by interacting with fibroblasts, promoting the production of collagen fiber, facilitating cell recruitment, and increasing keratinocyte migration (Martínez et al., 2015; Hu et al., 2024). The use of PRP, especially in combination with MSCs, may present therapeutic potential in the treatment of wound healing through preserving or enhancing the tissue regenerative properties of transplanted cells (Tang et al., 2015). Many studies have proved PRP has the optimal effect on the growth of BM-MSCs and AD-MSCs (Kocaoemer et al., 2007; Cervelli et al., 2012; Murphy et al., 2012). In addition, PRP could prolong the survival and retention of transplanted MSCs, which may be a powerful tool to attract cell populations in the wound area (Lamo-Espinosa et al., 2020). Therefore, MSCs, together with PRP, may be an advantageous approach to treating PUs.
Despite the increasing availability of WJ-MSCs or PRP for improving and accelerating wound healing, the therapeutic effects of the combination of WJ-MSCs and PRP on PUs are still rarely reported and need to be confirmed. With the aim of developing a more effective therapeutic regime for PUs in the future, we present a case of an emergency surgical patient with a PU on the right heel. The purpose of this study is to present a review of the existing literature on PUs, and the clinical outcome of combination therapy will be critically discussed to explore their therapeutic potential for the patient.
Case presentation
The study was conducted at Liaocheng People’s Hospital and approved by the Ethics Committee of Liaocheng People’s Hospital (2021094). This report was conducted in accordance with the CARE (CAse REport) guidelines.
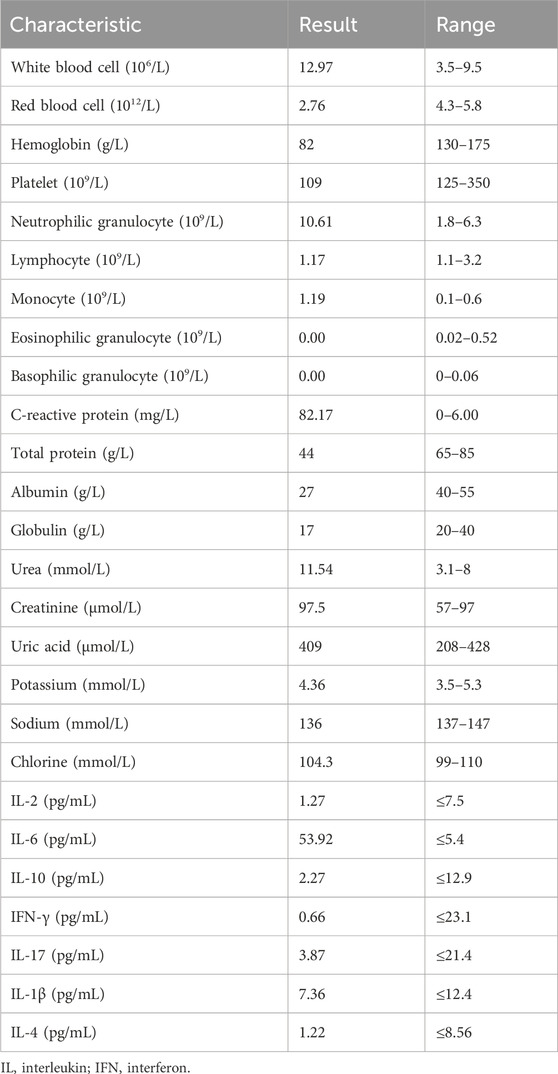
On 3 July 2023, a 49-year-old man was admitted to emergency surgery at Liaocheng People’s Hospital because of multiple trauma to the right lower limb. Three hours before his admission to our hospital, the patient was accidentally injured by a steel plate impact during the work process. Therefore, the patient was admitted to the hospital for an emergency surgery operation. Past medical history includes good health with no history of diabetes, hypertension, heart disease, or cerebrovascular disease. The patient denied a history of hepatitis, tuberculosis, and other infectious diseases, as well as any history of surgery, trauma, blood transfusion, or drug allergy. The physical examination showed a body temperature of 36.7°C, blood pressure of 105/60 mmHg, and a pulse of 107 beats per minute. The admission X-ray showed a comminuted fracture and soft tissue injury of the proximal right femur and right distal femoral fracture. Laboratory examinations: Table 1 shows the patient’s laboratory test results after admission to our hospital. The levels of C-reactive protein and interleukin-6 (IL-6) were significantly elevated.
The patient was diagnosed with a right lower limb traumatic fracture accompanied by arterial, venous, and nerve damage. Upon admission, the patient immediately underwent emergency surgery. The patient was identified to be at a high risk of PU development based on the Braden pressure injury scale, and he developed a PU despite preventive measures. On 11 August, on hospital day 14, he developed a PU (stage I) on the right heel. Once the PU developed, a series of intervention methods were added to the prevention protocols: rinsing the wound with physiologic saline, application of collagenase, silver ion dressing, hydrocolloid dressing, and sterile hydrogel dressing, whenever necessary, in order to remove the necrotic tissue. Low-air-loss mattresses, raising the lower limbs, and nutritional supplementation were used to optimize wound healing. Despite these preventive measures, the size and depth of the PU increased with evidence of deep tissue injury in the wound tract. On 9 November, on hospital day 130, the PU progressed to stage IV (2 × 3 cm). Thus, apart from topical wound care, nutritional support, and continuation of preventive care during hospitalization, we undertook the combined use of WJ-MSCs and PRP with intradermal injection in ulcer margins.
Cell culture and characterization
The clinical grade WJ-MSCs were generated under good manufacturing practice (GMP) conditions with standard operating procedures, as previously described by Zhang et al. (2020). The WJ-MSCs were supplied by the Central Laboratory of Liaocheng People’s Hospital, which has been certified by the National Institutes for Food and Drug Control of China (authorization numbers: SH201900594 and SH201900597). WJ-MSCs were obtained from Wharton’s jelly of the umbilical cords of infants delivered full-term by normal labor. In brief, under sterile conditions, the umbilical cord membrane was stripped, and the umbilical cord’s two arteries and one vein were removed to retain the Wharton’s jelly. The Wharton’s jelly was cut into 1-mm3 pieces and then cultured in Dulbecco’s modified Eagle media: Nutrient Mixture F-12 (Gibco, United States) supplemented with 10% fetal bovine serum (FBS) (Gibco, United States). Collected tissues were cultured at 37°C in a 5% CO2 incubator with saturated humidity. The culture medium was changed every 3 days. WJ-MSCs were digested and passaged with 0.25% trypsin (Sigma, United States) when they reached 80%–90% confluence.
WJ-MSCs isolated from the Wharton’s jelly of the umbilical cord were examined to confirm their MSC characteristics. Flow cytometry for MSC surface markers (CD90+, CD73+, CD105+, CD45–, and CD34–) was performed, as previously described by Zhang et al. (2020). Cell surface marker analysis by flow cytometry was performed by incubating WJ-MSCs (2 × 105) with a combination of antibodies: CD90-fluorescein isothiocyanate (FITC, 555595), CD73-phycoerythrin (PE, 550257), CD105-phycoerythrin (PE, 560839), CD45-(APC, 555485), and CD34-phycoerythrin-Cy (PE-Cy, 5555823). These cells were analyzed by fluorescence-activated cell sorting using a Becton-Dickinson instrument (BD, San Diego, CA). The rates of apoptosis and phenotype were analyzed using a FACSCanto Cytometer (BD Biosciences, San Jose, CA) and DIVA software (BD Biosciences, San Jose, CA).
A series of verification tests were conducted before cell therapy, including cell morphology (fibroblast-like adherent cells), cell viability assay (>90% live cells measured by trypan blue assay), purity by FACS (≥95% CD90+, CD73+, CD105+, CD34−, and CD45− cells), and endotoxin and mycoplasma and sterility testing (−).
Blood collection and platelet-rich plasma preparation
Approximately 40 mL of whole venous blood was collected from the forearm vein using 5-mL tubes containing 3.2% sodium citrate, and a 100-μL sample was separated for whole blood platelet count, and the remaining blood was used to produce PRP by a modified two-step centrifugation method, as previously described by Amable et al. (2013). The PRP preparation procedure consisted of two centrifugation steps. First, whole blood was centrifuged at 300 × g for 5 min at 18°C. Under sterile conditions, the upper fraction of each tube was separated, transferred into a new sterile tube, and centrifuged the second time at 700 × g for 17 min at 18°C. After the two centrifugation steps, the upper yellow solution (platelet-poor plasma) was removed, resulting in approximately 5 mL of PRP, which was used to re-suspend WJ-MSCs for subsequent use.
Ulcer assessment
According to treatment and maintenance procedures for pressure ulcers, the depth, surface area, and tissue type of the ulcer were used as criteria for wound healing. After the pressure wounds were evaluated by the investigators, the wounds with necrotic tissue were removed by an international wound therapist before cell treatment. The debrided ulcers were re-observed and visualized using a mobile phone with a digital camera before the maintenance. The digital picture was taken with standardized settings at a constant distance before the patient was treated. The length, width, and depth of the wound in centimeters were measured and recorded with a disposable wound ruler before maintenance.
Wound treatment
The WJ-MSCs were cultured and passaged until they were available in the amounts required to be administered to the patient. For cell treatment, cells were harvested, washed three times with PBS, and re-suspended in prepared autologous PRP to be administered within 24 h harvesting of the cells.
Any associated comorbidities of the patient were treated as indicated to optimize and improve the patient’s health and general condition. During treatment, routine laboratory tests were conducted to ensure the stability of the patient’s nutritional status. Before cell therapy, normal saline was used to perform radical debridement of the PU, combined with sharp debridement, if necessary, to remove necrotic tissue and residual foreign bodies from the wound as much as possible. The wound cultures were harvested to exclude local infections. Clinicians performed cell therapy, in which WJ-MSCs (5 × 106) re-suspended in PRP were injected into ulcer margins. Before injection, the wound was locally anesthetized, and the injection point was determined according to the extent of wound involvement. After single-dose administration of WJ-MSCs combined with PRP, the wound was covered with a transparent and nonadherent dressing. During the period of cell treatment, local creams or dressings with potential healing action were not allowed to be used.
Wound analysis
During the 7-week study period, the treatment’s effectiveness was observed and assessed weekly with wound area measurements from the digital images using the same digital camera on a mobile phone. On days 8, 13, 19, 26, 33, and 40 after cell treatment, wound measurements, including length, width, maximal diameter, and circumference, were taken, and wound closure was examined in a timely manner. Wound closure was considered complete when the entire surface area was covered with epithelial tissue. To underline the reliability of the analysis, this process was performed in a blinded manner by two independent researchers showing minimal interindividual variation, and the average of their measurements was used.
Results
Adverse events
No serious adverse events or complications derived from the procedures or treatments were noted within 2 hours after transplantation. In addition, no delayed hypersensitivity or secondary infections were detected after treatment.
Clinical effects
Healing progress after the use of WJ-MSCs and PRP in the patient was monitored and evaluated until the wound healed completely. The wound area was observed and assessed before starting therapy. Following that, the treatment with WJ-MSCs and PRP was initiated. A file was created for the patient, which included the healing progress and the date of transition to the granulation and epithelialization stages. The digital images displaying the healing process are shown in Figure 1. The initial wound surface area was 6 cm2, and the observation time after cell treatment lasted 47 days. Four days after the WJ-MSCs application, the patient showed progressive healing of the PU. From days 4 to 33, granulation tissue formation and re-epithelialization were clearly observed. On day 33, the wound surface area was 3.68 cm2. The ulcer was almost healed completely on day 47, and the pain in the patient’s wound area also decreased. The epithelialization period was found to be quicker than ulcer conventional treatment like negative pressure or advanced moist wound therapies (Roubelakis et al., 2014). Re-epithelialization is a term used to describe the resurfacing of a wound with new epithelium. It is one of the key steps in the process of skin wound repair, which starts 24 h after injury and continues until the healing process covers the entire surface of the wound (Chang et al., 2022). Furthermore, the epithelialization time of skin wounds largely depends on the specific condition of the wound, such as the location, size, depth, microbial contamination, patient health status, genetics, and epigenetics (Sorg et al., 2017). A comparative study of collagenase and hydrogel in maintaining debridement and wound closure in institutionalized adults with pressure ulcers showed all patients (n = 3) in the hydrogel group achieved complete epithelialization with a mean of 32.6 days, and 9 out of 10 patients in the collagenase group achieved complete epithelialization with a mean of 45 days (Milne et al., 2012).
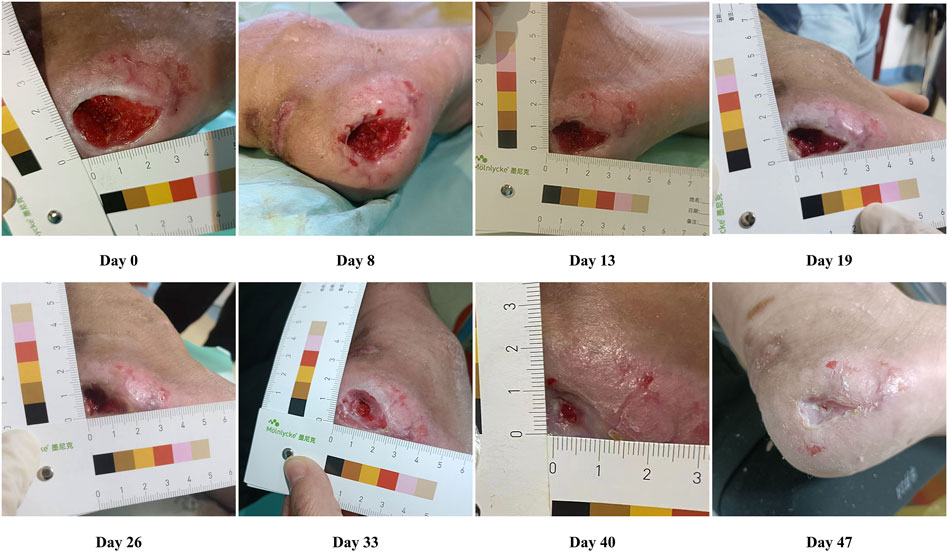
Figure 1. Representative photographs showing the healing progress of pressure ulcers on days 8, 13, 19, 26, 33, and 40 after cell treatment.
During 12 months of follow-up, the resolved ulcers have not recurred again, and no adverse event was reported. To summarize, an accelerated healing process with increased neovascularization in the ulcerative area was identified after cell therapy.
Discussion
Chronic wounds are one of the major health issues, predominantly affecting older individuals around the world. Chronic dermal ulcers, such as lower limb vein ulcers, diabetes foot ulcers, and PUs, can lead to loss of function and productivity, anxiety, and a decrease in the quality of life (Snyder et al., 2016). Debridement is a key component of wound care, which involves removing necrotic tissue, foreign objects, bacterial growth, and callus from chronic wounds to stimulate the granulation, epithelialization, and proliferation of the wound. The methods of debridement include surgery debridement, autolysis, enzymatic degradation, chemicals, biological surgery, and mechanical dehydration (Waycaster et al., 2018). These methods can be used alone or in combination to optimize the process of debridement. The choice of debridement procedures depends on multiple factors, including patient status, wound characteristics, the likelihood of infection, and available medical expertise, as well as resources (Waycaster and Milne, 2013).
PUs that occur in the hospital are commonly referred to as HAPUs, which are considered an important indicator of the quality of hospital patient care (Spector et al., 2016). Patients who develop HAPUs have longer stays in the hospital than necessary, and they are at a greater risk of infections and are more likely to die (Lyder et al., 2012; Braga et al., 2013). Surgical patients are prone to developing PUs because they often remain immobile for extended periods of time, and their surgical procedures may limit the flow of blood oxygen and nutrition and lead to a decrease in muscle tone. Li et al. (2020) reported the pooled hospital-acquired pressure injuries rate among 1,893,593 patients was 8.4%, and the most common stages were stage I (43.5%) and stage II (28.0%). Most body sites affected by ulcers in surgical patients are commonly found on the sacrum, coccyx, heel, and back of the head. HAPUs can be partially prevented by postoperative turning and early mobilization after the surgical procedure, minimizing shear force and pressure through appropriate positioning, and improving blood and oxygen flow (Feuchtinger et al., 2005; Spector et al., 2016). Prevention of PUs is one of the most important indicators of the quality of healthcare services. However, it is noteworthy that the frequency of HAPUs for surgical patients is still high.
In this study, we reported a surgical patient who developed an ulcer during hospitalization. This study evaluated the safety and efficacy of intradermal injection of WJ-MSCs re-suspended in PRP in improving and accelerating wound healing. The obvious side effects are not found in the treatment of cell transplantation, indicating it was well-tolerated and safe. Our results showed that the administration of WJ-MSCs resulted in improved granulation, facilitated re-epithelialization, and accelerated wound closure. Compared with traditional treatment, MSC treatment can shorten the healing time of ulcers. The healing time of PU varies and largely depends on the stage of the PU and the patient’s physiologic condition. Moreover, prolonged non-healing of ulcers during hospitalization can increase hospitalization costs. HAPUs can add approximately 44% to the cost of major surgical hospital stays, but the amount varies depending on the total cost during hospitalization (Spector et al., 2016). A study of 19,889 older adults in 51 nursing homes found that 75% of PUs (stage II) and 17% of PUs (stages III or IV) healed in 8 weeks, and it is worth noting that 23% of PUs (stage II) and 48% of PUs (stage IV) had not healed after 1 year (Brandeis et al., 1990). Although surgical treatment for pressure ulcer patients (stages III and IV) typically leads to faster healing, roughly 6 weeks of bed rest is still required, and the recurrence rates of ulcers are high (Feldman and McCauley, 2018).
MSCs represent an attractive stem cell source for tissue regeneration in clinical applications, which have been demonstrated to improve wound healing through re-epithelialization, increased angiogenesis, and granulation tissue formation. At the wound site, MSCs play an active role in modulating the inflammatory environment, enhancing angiogenesis, and promoting the migration of keratinocytes and recruitment of other host cells, which can contribute to the generation of granulation tissue, promote angiogenesis and epithelialization, and attenuate scar formation (Kamolz et al., 2014; Laverdet et al., 2014). The mechanisms of MSC-based treatment for wound healing have not been fully elucidated, but it is widely believed that the treatment effects mainly depend on their paracrine effects (Figure 2). MSCs can secrete a large number of soluble factors, such as growth factors, anti-inflammatory cytokines, antimicrobial peptides, chemokines, and exosomes, to enhance the growth, survival, and function of wound repair cells. To determine the therapeutic potential of MSCs for PUs, several clinical trials have shown improved and accelerated wound healing after the transplantation of MSCs (Yoshikawa et al., 2008; Sarasúa et al., 2011). PRP is widely used in wound healing, and it provides a release of multiple functional growth factors which are beneficial for skin wound healing and cell proliferation, including platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin growth factor 1 (IGF-1), and interleukins (Marx, 2001; Arnalich et al., 2016). Moreover, PRP can boost the potency of transplanted MSCs by serving as a cellular scaffold used in clinical applications. When administered as a co-adjuvant to MSCs, PRP stimulates cell proliferation, preserves differentiation potential, and does not affect any lineage differentiation in a controlled, nontumorigenic manner (Mahmoudian-Sani et al., 2018). PRP can serve as a regulatory factor for cell migration and wound healing, promoting the migration of MSCs and the wound-healing process. The possible mechanism involved in the increased growth of the MSCs is that PRP leads to an increase in the number of cells at the transplantation site by increasing cell proliferation and utilizing the MSCs further. The combined effect of MSCs and PRP has been evaluated in a preclinical trial by Lian et al. (2014), which showed that wound healing rates were significantly higher in the BM-MSCs plus PRP group than in the other groups. Their results found that the combined administration of BM-MSCs and PRP can accelerate wound healing by enhancing cell proliferation, increasing angiogenesis, affecting the infiltration response, and inducing TGF-β1 expression.
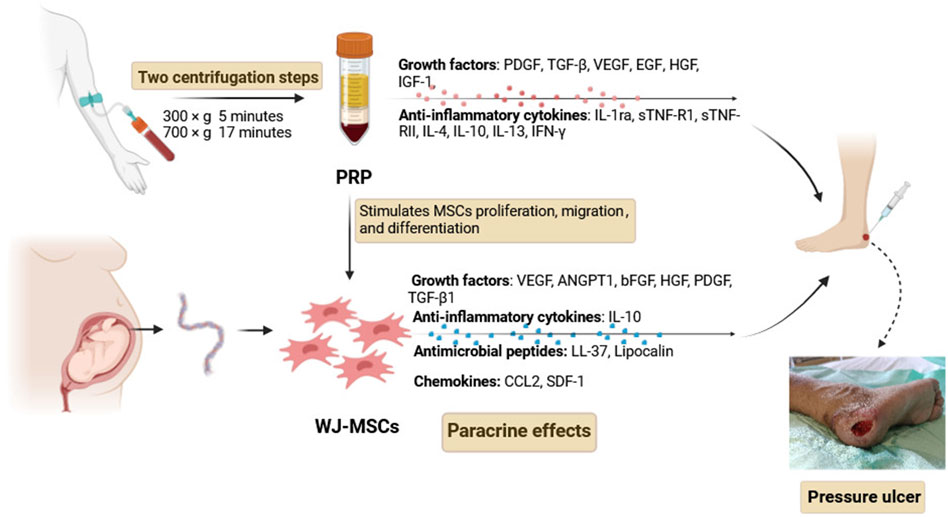
Figure 2. Mechanisms of WJ-MSCs and PRP treatment for wound healing. Abbreviations: PDGF, platelet-derived growth factor; TGF-β, transforming growth factor β; EGF, epidermal growth factor; HGF, hepatocyte growth factor; IGF-1, insulin growth factor-1; VEGF, vascular endothelial growth factor; ANGPT1, angiopoietin 1; bFGF, basic fibroblast growth factor; IL-1ra, interleukin-1 receptor antagonist; sTNF-R1, soluble tumor necrosis factor receptor 1; sTNF-RII, soluble tumor necrosis factor receptor II; IL-4, interleukin-4; IL-10, interleukin-10; IL-13, interleukin-13; IFN-γ, interferon-γ; CCL2, chemokine (C-C motif) ligand 2 (CCL2); SDF-1, stromal cell-derived factor-1.
The present study is still subject to several limitations. First, the number of patients recruited in this study is small, and only one patient was enrolled. A placebo group was not designed due to ethical reasons. Therefore, in future clinical trials, using standard wound care as the control group to validate the safety and efficiency of MSC-based therapy is reasonable. Second, ulcer measurements with a ruler and multiplying to calculate the total surface area of the ulcer are more likely to lead to an overestimation of the calculated surface. Efforts to minimize potential biases in wound measurements were made by two independent researchers. In addition, standardizing photographs is very difficult because the distance between the photographs recorded and the injury varied. Despite an extensive search across databases, there are many differences in clinical trials that lead to direct comparisons of the outcomes between different studies, including cell sources, doses, administration route, and wound types and staging. Due to the limited number of randomized controlled clinical trials conducted so far, there is an urgent need for high-quality clinical research in the future, especially multicenter, randomized, double-blind, and placebo-controlled clinical trials.
Conclusion
The treatment of WJ-MSCs together with autologous PRP improves and accelerates PU healing in comparison with traditional treatment. WJ-MSCs, together with PRP, may offer a promising treatment option for wound healing. A deeper understanding of the interaction mechanism between WJ-MSCs and PRP, including the migration and homing of WJ-MSCs to the tissue of injury, and understanding how autologous PRP regulates and supports the activation and differentiation of WJ-MSCs during the wound-healing process will help optimize treatment plans for patients with chronic wounds. In the future, further studies, especially large-scale and multicenter studies, are necessary to confirm the efficacy of using WJ-MSCs or PRP alone or a combination of both in the treatment of PUs and determine the optimal application plan for patients to improve survival and quality of life.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Liaocheng People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CZ: investigation, writing–original draft, and writing–review and editing. LJ: conceptualization, writing–original draft, and writing–review and editing. XQ: investigation, resources, and writing–original draft. WZ: data curation, project administration, and writing–original draft. SC: conceptualization, investigation, supervision, and writing–original draft. CY: data curation, investigation, validation, and writing–original draft. MM: conceptualization, formal analysis, investigation, supervision, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amable, P. R., Carias, R. B., Teixeira, M. V., da Cruz Pacheco, I., Corrêa do Amaral, R. J., Granjeiro, J. M., et al. (2013). Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 4 (3), 67. doi:10.1186/scrt218
Arnalich, F., Rodriguez, A. E., Luque-Rio, A., and Alio, J. L. (2016). Solid platelet rich plasma in corneal surgery. Ophthalmol. Ther. 5 (1), 31–45. doi:10.1007/s40123-016-0051-9
Arno, A. I., Amini-Nik, S., Blit, P. H., Al-Shehab, M., Belo, C., Herer, E., et al. (2014). Human Wharton's jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res. Ther. 5 (1), 28. doi:10.1186/scrt417
Braga, I. A., Pirett, C. C., Ribas, R. M., Gontijo Filho, P. P., and Diogo Filho, A. (2013). Bacterial colonization of pressure ulcers: assessment of risk for bloodstream infection and impact on patient outcomes. J. Hosp. Infect. 83 (4), 314–320. doi:10.1016/j.jhin.2012.11.008
Brandeis, G. H., Berlowitz, D. R., and Katz, P. (2001). Are pressure ulcers preventable? A survey of experts. Adv. skin wound care 14 (5), 244–248. doi:10.1097/00129334-200109000-00011
Brandeis, G. H., Morris, J. N., Nash, D. J., and Lipsitz, L. A. (1990). The epidemiology and natural history of pressure ulcers in elderly nursing home residents. Jama 264 (22), 2905–2909. doi:10.1001/jama.1990.03450220071025
Cervelli, V., Scioli, M. G., Gentile, P., Doldo, E., Bonanno, E., Spagnoli, L. G., et al. (2012). Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem cells Transl. Med. 1 (3), 206–220. doi:10.5966/sctm.2011-0052
Chaboyer, W., Bucknall, T., Webster, J., McInnes, E., Gillespie, B. M., Banks, M., et al. (2016). The effect of a patient centred care bundle intervention on pressure ulcer incidence (INTACT): a cluster randomised trial. Int. J. Nurs. Stud. 64, 63–71. doi:10.1016/j.ijnurstu.2016.09.015
Chang, C. W., Christian, M., Chang, D. H., Lai, F., Liu, T. J., Chen, Y. S., et al. (2022). Deep learning approach based on superpixel segmentation assisted labeling for automatic pressure ulcer diagnosis. PloS one 17 (2), e0264139. doi:10.1371/journal.pone.0264139
Cushing, C. A., and Phillips, L. G. (2013). Evidence-based medicine: pressure sores. Plastic Reconstr. Surg. 132 (6), 1720–1732. doi:10.1097/PRS.0b013e3182a808ba
Du, T., Cheng, J., Zhong, L., Zhao, X. F., Zhu, J., Zhu, Y. J., et al. (2012). The alleviation of acute and chronic kidney injury by human Wharton's jelly-derived mesenchymal stromal cells triggered by ischemia-reperfusion injury via an endocrine mechanism. Cytotherapy 14 (10), 1215–1227. doi:10.3109/14653249.2012.711471
Feldman, D., and McCauley, J. (2018). Mesenchymal stem cells and transforming growth factor-β3 (TGF-β3) to enhance the regenerative ability of an albumin scaffold in full thickness wound healing. J. Funct. biomaterials 9 (4), 65. doi:10.3390/jfb9040065
Feuchtinger, J., Halfens, R. J., and Dassen, T. (2005). Pressure ulcer risk factors in cardiac surgery: a review of the research literature. Heart and lung J. Crit. care 34 (6), 375–385. doi:10.1016/j.hrtlng.2005.04.004
Gaspar, S., Peralta, M., Marques, A., Budri, A., and Gaspar de Matos, M. (2019). Effectiveness on hospital-acquired pressure ulcers prevention: a systematic review. Int. wound J. 16 (5), 1087–1102. doi:10.1111/iwj.13147
Gillespie, B. M., Chaboyer, W. P., McInnes, E., Kent, B., Whitty, J. A., and Thalib, L. (2014). Repositioning for pressure ulcer prevention in adults. Cochrane database Syst. Rev. 2014 (4), Cd009958. doi:10.1002/14651858.CD009958.pub2
Han, Y., Li, X., Zhang, Y., Han, Y., Chang, F., and Ding, J. (2019). Mesenchymal stem cells for regenerative medicine. Cells 8 (8), 886. doi:10.3390/cells8080886
Hu, Z., Xv, H., Feng, A., Wang, S., and Han, X. (2024). Efficacy and safety of platelet-rich plasma for pressure ulcers: a systematic review and meta-analysis of randomized controlled trials. Int. J. Low. Extrem. wounds, 15347346241227001. doi:10.1177/15347346241227001
Kamolz, L. P., Keck, M., and Kasper, C. (2014). Wharton's jelly mesenchymal stem cells promote wound healing and tissue regeneration. Stem Cell Res. Ther. 5 (3), 62. doi:10.1186/scrt451
Kocaoemer, A., Kern, S., Klüter, H., and Bieback, K. (2007). Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem cells (Dayt. Ohio) 25 (5), 1270–1278. doi:10.1634/stemcells.2006-0627
Lamo-Espinosa, J. M., Blanco, J. F., Sánchez, M., Moreno, V., Granero-Moltó, F., Sánchez-Guijo, F., et al. (2020). Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J. Transl. Med. 18 (1), 356. doi:10.1186/s12967-020-02530-6
Laverdet, B., Micallef, L., Lebreton, C., Mollard, J., Lataillade, J. J., Coulomb, B., et al. (2014). Use of mesenchymal stem cells for cutaneous repair and skin substitute elaboration. Pathologie-biologie 62 (2), 108–117. doi:10.1016/j.patbio.2014.01.002
Li, Z., Lin, F., Thalib, L., and Chaboyer, W. (2020). Global prevalence and incidence of pressure injuries in hospitalised adult patients: a systematic review and meta-analysis. Int. J. Nurs. Stud. 105, 103546. doi:10.1016/j.ijnurstu.2020.103546
Lian, Z., Yin, X., Li, H., Jia, L., He, X., Yan, Y., et al. (2014). Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann. dermatology 26 (1), 1–10. doi:10.5021/ad.2014.26.1.1
Lyder, C. H., Wang, Y., Metersky, M., Curry, M., Kliman, R., Verzier, N. R., et al. (2012). Hospital-acquired pressure ulcers: results from the national medicare patient safety monitoring system study. J. Am. Geriatrics Soc. 60 (9), 1603–1608. doi:10.1111/j.1532-5415.2012.04106.x
Mahmoudian-Sani, M. R., Rafeei, F., Amini, R., and Saidijam, M. (2018). The effect of mesenchymal stem cells combined with platelet-rich plasma on skin wound healing. J. Cosmet. dermatology 17 (5), 650–659. doi:10.1111/jocd.12512
Martínez, C. E., Smith, P. C., and Palma Alvarado, V. A. (2015). The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front. physiology 6, 290. doi:10.3389/fphys.2015.00290
Marx, R. E. (2001). Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 10 (4), 225–228. doi:10.1097/00008505-200110000-00002
Mervis, J. S., and Phillips, T. J. (2019). Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J. Am. Acad. Dermatology 81 (4), 881–890. doi:10.1016/j.jaad.2018.12.069
Milne, C. T., Ciccarelli, A., and Lassy, M. (2012). A comparison of collagenase to hydrogel dressings in maintenance debridement and wound closure. Wounds a Compend. Clin. Res. Pract. 24 (11), 317–322.
Moodley, Y., Atienza, D., Manuelpillai, U., Samuel, C. S., Tchongue, J., Ilancheran, S., et al. (2009). Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. pathology 175 (1), 303–313. doi:10.2353/ajpath.2009.080629
Murphy, M. B., Blashki, D., Buchanan, R. M., Yazdi, I. K., Ferrari, M., Simmons, P. J., et al. (2012). Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 33 (21), 5308–5316. doi:10.1016/j.biomaterials.2012.04.007
Nekanti, U., Rao, V. B., Bahirvani, A. G., Jan, M., Totey, S., and Ta, M. (2010). Long-term expansion and pluripotent marker array analysis of Wharton's jelly-derived mesenchymal stem cells. Stem cells Dev. 19 (1), 117–130. doi:10.1089/scd.2009.0177
Nixon, J., Nelson, E. A., Cranny, G., Iglesias, C. P., Hawkins, K., Cullum, N. A., et al. (2006). Pressure relieving support surfaces: a randomised evaluation. Health Technol. Assess. Winch. Engl. 10 (22), 1–163. doi:10.3310/hta10220
Pelizzo, G., Avanzini, M. A., Mantelli, M., Croce, S., Maltese, A., Vestri, E., et al. (2018). Granulation tissue-derived mesenchymal stromal cells: a potential application for burn wound healing in pediatric patients. J. stem cells Regen. Med. 14 (1), 53–58. doi:10.46582/jsrm.1401007
Portas, M., Mansilla, E., Drago, H., Dubner, D., Radl, A., Coppola, A., et al. (2016). Use of human cadaveric mesenchymal stem cells for cell therapy of a chronic radiation-induced skin lesion: a case report. Radiat. Prot. Dosim. 171 (1), 99–106. doi:10.1093/rpd/ncw206
Roubelakis, M. G., Trohatou, O., Roubelakis, A., Mili, E., Kalaitzopoulos, I., Papazoglou, G., et al. (2014). Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. Rep. 10 (3), 417–428. doi:10.1007/s12015-013-9494-8
Sarasúa, J. G., López, S. P., Viejo, M. A., Basterrechea, M. P., Rodríguez, A. F., Gutiérrez, A. F., et al. (2011). Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J. spinal cord Med. 34 (3), 301–307. doi:10.1179/2045772311y.0000000010
Schuurman, J. P., Schoonhoven, L., Defloor, T., van Engelshoven, I., van Ramshorst, B., and Buskens, E. (2009). Economic evaluation of pressure ulcer care: a cost minimization analysis of preventive strategies. Nurs. Econ. 27 (6), 390–415.
Snyder, R. J., Fife, C., and Moore, Z. (2016). Components and quality measures of DIME (devitalized tissue, infection/inflammation, moisture balance, and edge preparation) in wound care. Adv. skin wound care 29 (5), 205–215. doi:10.1097/01.ASW.0000482354.01988.b4
Sorg, H., Tilkorn, D. J., Hager, S., Hauser, J., and Mirastschijski, U. (2017). Skin wound healing: an update on the current knowledge and concepts. Eur. Surg. Res. Eur. Chir. Forschung Recherches Chir. Eur. 58 (1-2), 81–94. doi:10.1159/000454919
Spector, W. D., Limcangco, R., Owens, P. L., and Steiner, C. A. (2016). Marginal hospital cost of surgery-related hospital-acquired pressure ulcers. Med. care 54 (9), 845–851. doi:10.1097/mlr.0000000000000558
Tang, X. B., Dong, P. L., Wang, J., Zhou, H. Y., Zhang, H. X., and Wang, S. Z. (2015). Effect of autologous platelet-rich plasma on the chondrogenic differentiation of rabbit adipose-derived stem cells in vitro. Exp. Ther. Med. 10 (2), 477–483. doi:10.3892/etm.2015.2528
Torres-Guzman, R. A., Avila, F. R., Maita, K., Garcia, J. P., De Sario, G. D., Borna, S., et al. (2023). Mesenchymal stromal cell healing outcomes in clinical and pre-clinical models to treat pressure ulcers: a systematic review. J. Clin. Med. 12 (24), 7545. doi:10.3390/jcm12247545
Tsai, P. C., Fu, T. W., Chen, Y. M., Ko, T. L., Chen, T. H., Shih, Y. H., et al. (2009). The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transplant. 15 (5), 484–495. doi:10.1002/lt.21715
Uçar, Ö., and Çelik, S. (2020). Comparison of platelet-rich plasma gel in the care of the pressure ulcers with the dressing with serum physiology in terms of healing process and dressing costs. Int. wound J. 17 (3), 831–841. doi:10.1111/iwj.13344
Volakakis, E., Papadakis, M., Manios, A., Ioannou, C. V., Zoras, O., and de Bree, E. (2019). Platelet-rich plasma improves healing of pressure ulcers as objectively assessed by digital planimetry. Wounds a Compend. Clin. Res. Pract. 31 (10), 252–256.
Wang, S., Yang, H., Tang, Z., Long, G., and Huang, W. (2016). Wound dressing model of human umbilical cord mesenchymal stem cells-alginates complex promotes skin wound healing by paracrine signaling. Stem cells Int. 2016, 1–8. doi:10.1155/2016/3269267
Waycaster, C., Carter, M. J., Gilligan, A. M., Mearns, E. S., Fife, C. E., and Milne, C. T. (2018). Comparative cost and clinical effectiveness of clostridial collagenase ointment for chronic dermal ulcers. J. Comp. Eff. Res. 7 (2), 149–165. doi:10.2217/cer-2017-0066
Waycaster, C., and Milne, C. T. (2013). Clinical and economic benefit of enzymatic debridement of pressure ulcers compared to autolytic debridement with a hydrogel dressing. J. Med. Econ. 16 (7), 976–986. doi:10.3111/13696998.2013.807268
Wu, Q., Lei, X., Chen, L., Zheng, Y., Huang, H., Qian, C., et al. (2018). Autologous platelet-rich gel combined with in vitro amplification of bone marrow mesenchymal stem cell transplantation to treat the diabetic foot ulcer: a case report. Ann. Transl. Med. 6 (15), 307. doi:10.21037/atm.2018.07.12
Xiao, Wf, Yang, Y. T., Xie, W. Q., He, M., Liu, D., Cai, Z. J., et al. (2021). Effects of platelet-rich plasma and bone marrow mesenchymal stem cells on meniscal repair in the white-white zone of the meniscus. Orthop. Surg. 13 (8), 2423–2432. doi:10.1111/os.13089
Xu, Y., Huang, S., Ma, K., Fu, X., Han, W., and Sheng, Z. (2012). Promising new potential for mesenchymal stem cells derived from human umbilical cord Wharton's jelly: sweat gland cell-like differentiative capacity. J. tissue Eng. Regen. Med. 6 (8), 645–654. doi:10.1002/term.468
Yoshikawa, T., Mitsuno, H., Nonaka, I., Sen, Y., Kawanishi, K., Inada, Y., et al. (2008). Wound therapy by marrow mesenchymal cell transplantation. Plastic Reconstr. Surg. 121 (3), 860–877. doi:10.1097/01.prs.0000299922.96006.24
Keywords: hospital-acquired pressure ulcers, mesenchymal stem cells, platelet-rich plasma, treatment, transplantation
Citation: Zhou C, Jiao L, Qiao X, Zhang W, Chen S, Yang C and Meng M (2024) Combined treatment of umbilical cord Wharton’s jelly-derived mesenchymal stem cells and platelet-rich plasma for a surgical patient with hospital-acquired pressure ulcer: a case report and literature review. Front. Bioeng. Biotechnol. 12:1424941. doi: 10.3389/fbioe.2024.1424941
Received: 30 April 2024; Accepted: 10 June 2024;
Published: 09 July 2024.
Edited by:
Bruce Alan Bunnell, University of North Texas Health Science Center, United StatesReviewed by:
Stacey Schutte, University of Cincinnati, United StatesAlena Ribeiro Alves Peixoto Medrado, Federal University of Bahia (UFBA), Brazil
Copyright © 2024 Zhou, Jiao, Qiao, Zhang, Chen, Yang and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Meng, bWVuZ21pbjIwMTFAMTI2LmNvbQ==; Chunling Yang, MTMzNDYyNTUwNzlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Changhui Zhou1†
Changhui Zhou1† Min Meng
Min Meng