
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 26 June 2024
Sec. Biomechanics
Volume 12 - 2024 | https://doi.org/10.3389/fbioe.2024.1401899
This article is part of the Research Topic Computational and Experimental Approaches on Soft Tissues Biomechanics and Mechanobiology View all 18 articles
Background: The bone repair requires the bone scaffolds to meet various mechanical and biological requirements, which makes the design of bone scaffolds a challenging problem. Novel triply periodic minimal surface (TPMS)-based bone scaffolds were designed in this study to improve the mechanical and biological performances simultaneously.
Methods: The novel bone scaffolds were designed by adding optimization-guided multi-functional pores to the original scaffolds, and finite element (FE) method was used to evaluate the performances of the novel scaffolds. In addition, the novel scaffolds were fabricated by additive manufacturing (AM) and mechanical experiments were performed to evaluate the performances.
Results: The FE results demonstrated the improvement in performance: the elastic modulus reduced from 5.01 GPa (original scaffold) to 2.30 GPa (novel designed scaffold), resulting in lower stress shielding; the permeability increased from 8.58 × 10−9 m2 (original scaffold) to 5.14 × 10−8 m2 (novel designed scaffold), resulting in higher mass transport capacity.
Conclusion: In summary, the novel TPMS scaffolds with multi-functional pores simultaneously improve the mechanical and biological performances, making them ideal candidates for bone repair. Furthermore, the novel scaffolds expanded the design domain of TPMS-based bone scaffolds, providing a promising new method for the design of high-performance bone scaffolds.
The demand for high performance artificial bone implants is growing due to the rising prevalence of bone diseases and traumas (Kurtz et al., 2007; Campana et al., 2014; Wallace et al., 2017). Porous bone scaffolds are considered ideal for bone repair because their porosity and stiffness can be adjusted to mimic human bone. Additionally, a microscopic porous scaffold can provide a suitable physiological environment for bone ingrowth (Peng et al., 2022). Recently, studies have used triply periodic minimal surface (TPMS) as a porous structure to design bone scaffold (Davoodi et al., 2020; Jiang et al., 2020; Vijayavenkataraman et al., 2020). The advantage of TPMS lies in its ability to easily adjust pore sizes using control equations, allowing the mechanical properties of the scaffolds to closely resemble those of human bone (Maconachie et al., 2019). Moreover, TPMS can provide micropores larger than 300.0 μm to allow for bone growth. Barba et al. (2019) concluded that a 300.0–600.0 μm pore size is best for osseointegration since it facilitates vascularization and cell growth. Consequently, TPMS-based bone scaffolds are being extensively studied and designed to address clinical challenges. On the other hand, additive manufacturing (AM) provides an ideal solution for manufacturing highly complex geometries such as TPMS scaffolds (Askari et al., 2020). AM describes a range of processes used to fabricate components directly from a digital representation of the intended geometry by the layer wise combination of a common source material (Zhai et al., 2014). As a result, TPMS-based bone scaffolds manufactured using AM have become the ideal choice for bone implants.
However, there are two problems with TPMS-based scaffolds that urgently need to be solved—the stress shielding effect caused by the high elastic modulus and the insufficient mass transport capacity caused by the low permeability. A bone scaffold should have an elastic modulus similar to that of the human bone to avoid the stress shielding effect. This effect occurs when the load is predominantly borne by the bone scaffold, leading to loosening at the interface (Barba et al., 2019). Although the elastic modulus of a TPMS scaffold is lower than that of the traditional cubic structure, it is still higher than that of cancellous bone. Sevilla et al. (2007) reported that the elastic modulus of cancellous bone is 1.08 GPa. Wu et al. (2018) investigated the elastic modulus of cancellous bone under different loading directions. The results indicated that the modulus of cancellous bone is 3.47 GPa in the longitudinal direction and 2.57 ± 0.28 GPa in the transverse direction. While there are discrepancies in reported elastic moduli of cancellous bone across studies, it is generally agreed that a bone scaffold’s elastic modulus should not exceed 3.00 GPa to align with cancellous bone. However, the modulus of a Schwarz P TPMS scaffold with 70% porosity is 5.60 GPa (Khaleghi et al., 2021), which is greater than that of cancellous bone. Additionally, the permeability of cancellous bone is in the range of 3.66
In recent years, there have been efforts to enhance the performance of TPMS scaffolds through innovative design approaches. The most common design method is structural optimization, which aims to find the best design scheme based on specific goals and constraints. Moreover, the integration of structural optimization with additive manufacturing (AM) presents a realm of creative opportunities for bone scaffold design (Tan et al., 2017). This method has been applied in clinic to fabricate devices such as pelvic protheses (Iqbal et al., 2019), craniofacial prostheses (Sutradhar et al., 2016), and femoral stem protheses (Arabnejad et al., 2017; Sun et al., 2018). Additionally, some studies have designed hybrid TPMS scaffolds and functional graded TPMS scaffolds to improve the performances. For example, Liu et al. (2022) designed a hybrid TPMS structure to increase the permeability to 1.20
In order to address the problems of poor controllability and limited optimization objectives in the optimal design of TPMS bone scaffolds, we propose a novel design method: introducing a new geometric variable, that is, optimization-guided multi-functional pore. The novel TPMS scaffolds with optimization-guided multi-functional pores were designed to address the problems of high elastic modulus and low permeability in this study. The functions of these pores are: to reduce the elastic modulus, to improve the permeability, and to broaden the design domain. Therefore, we name it “multi-functional pores” to represent these functions. In addition, the position of multi-function pore is not random, but determined under the guidance of optimization theory. After determining the position of the multi-function pore, change its radius to evaluate the effect of different size pores on the performance. The introduction of this multi-functional pore solves many difficulties in the design of TPMS bone scaffolds. First, the multi-functional pore is designed under the optimization framework of reducing the elastic modulus of the bone scaffold, and the elastic modulus of the new designed bone scaffold must be reduced to avoid the stress shielding effect. In the previous design methods, the performance of the new structure cannot be predicted before the design. Therefore, the proposed design method has higher controllability and efficiency. Second, there are few design variables of TPMS structure at present. By introducing the new variable of multi-function pore, the design domain of TPMS structure can be broadened, and the design of high-performance TPMS bone scaffold can be realized. To investigate the performances of the novel designed bone scaffolds, all the scaffolds were fabricated from Ti6Al4V using selective laser melting (SLM). Experiments and FE simulations were used to evaluate the elastic modulus, permeability, and anisotropy of the structures.
In this section, the method to design the novel TPMS scaffolds with multi-functional pores is first detailed. Then, the experimental and simulation methods for evaluating the elastic modulus and permeability behaviors of the TPMS scaffolds are illustrated. In the end, the anisotropic elastic response of the new scaffold is evaluated using the numerical homogenization method.
TPMS is a minimal surface that can extend periodically in three directions, and its topological shape is determined by functional expressions. Common TPMS structures include Schwarz P, Gyroid, Diamond, I-WP, etc. (Blanquer et al., 2017). It is worth mentioning that the concept of minimal surface was first proposed by the scientist Schwarz in 1883 (Strömberg, 2021), so Schwarz P is also one of the most classical and widely used types of TPMS structures. Besides, this structure has been shown to have a more stable curvature to promote cell growth (Blanquer et al., 2017). Therefore, this paper chooses Schwarz P as the basis of structural design. The 3D Schwarz P structure was formed by adding the thickness of the minimal surface (Figure 1A). The Schwarz P structure can be characterized by the following mathematical function (Khaleghi et al., 2021):
where
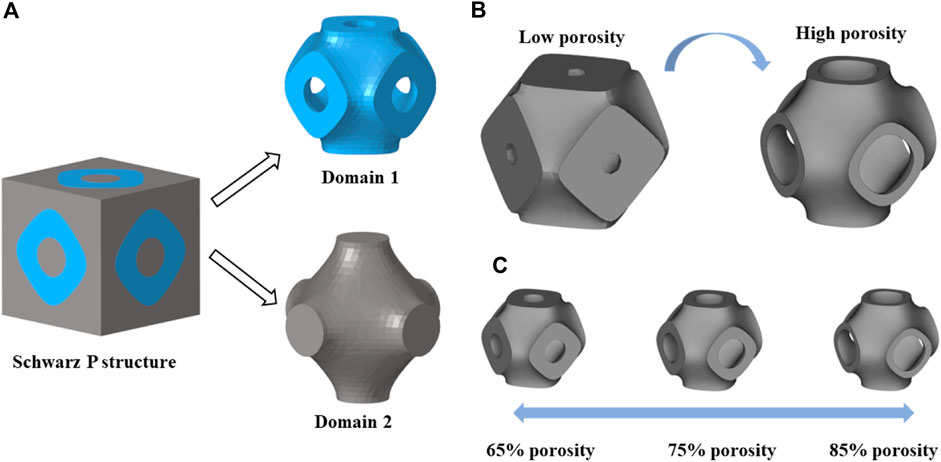
Figure 1. (A) Two domains divided by a Schwarz P structure. (B) Schwarz P structures with different porosities obtained by changing the constant
By adjusting the constants in Eq. 1, only structures with different porosities can be obtained, that is, porosity is the only variable when designing the scaffolds. However, the mass transport capacity of the scaffold depends on the pore size and the obstructed area (Ali et al., 2020). Therefore, the multi-functional pores were added to the scaffold to improve the permeability. These multi-functional pores were designed and guided by structural optimization method, which is detailed in this section.
Mechanical parameters, including the elastic modulus and anisotropy, are crucial factors for structural design (Feng et al., 2021), and they can be obtained through the constitutive relation. The constitutive relation of a TPMS structure is given by Eq. 2 (Lee et al., 2017):
where the stress and strain can be expressed in matrix form:
The stiffness matrix can be expressed as Eq. 4:
Moreover, Schwarz P is a cubic symmetric structure with three independent elastic constants, which means that
where
The energies of two load cases can be defined as shown in Eq. 7 (Ma et al., 2021):
where
The elastic modulus of the Schwarz P structure can be obtained as Eq. 9 (Feng et al., 2021):
Based on Eqs 8, 9, the elastic modulus of the Schwarz P structure can be expressed as Eq. 10:
where
To match the mechanical properties of cancellous bone, it is necessary to reduce the elastic modulus. Thus, the optimization objection is to find the minimum value of Eq. 10, which can be expressed as Eq. 11:
where
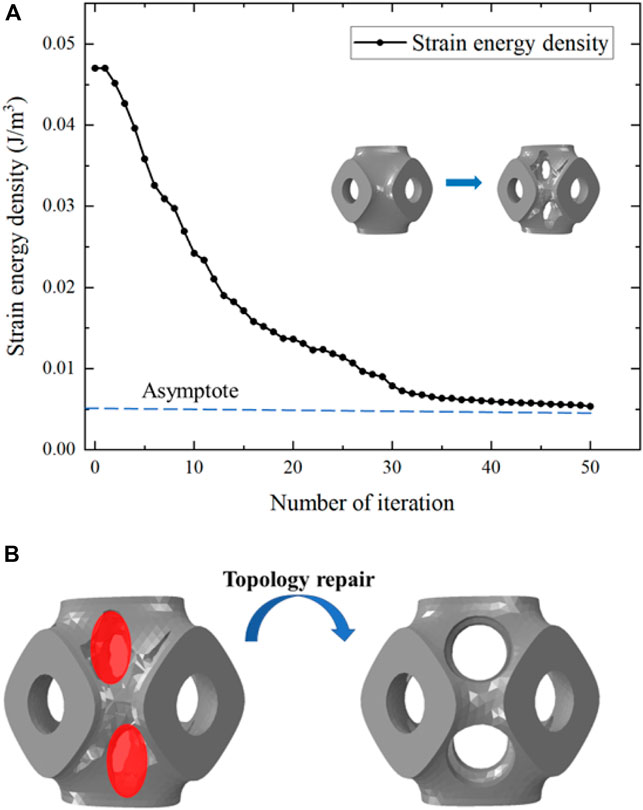
Figure 2. (A) Evolution of the strain energy density during the structural design. (B) Illustration of the geometric repair process.
The disadvantage of the SIMP method was that the boundary of the structure’s topology was not sufficiently clear and the structure was not able to be directly fabricated (Strömberg, 2021). Therefore, topology repair was necessary to ensure the scaffolds can be fabricated. This repair process involved adding multi-functional pores to the scaffolds where material had been removed, determined through ABAQUS calculations (Supplementary Figure 2.2B). To illustrate the structural design process, multi-functional pores with varying radii (0.1 mm, 0.2 mm, 0.3 mm, and 0.4 mm) were added (Figure 3). To maintain the cubic symmetry of the scaffold, eight multi-functional pores were added in each unit cell. P654 is taken as an example to illustrate the naming method for each scaffold: the letter “P” stands for the Schwarz P scaffold, the number “65” stands for the 65% porosity scaffold before the optimization, and “4” stands for the 0.4-mm radius of the multi-functional pores.
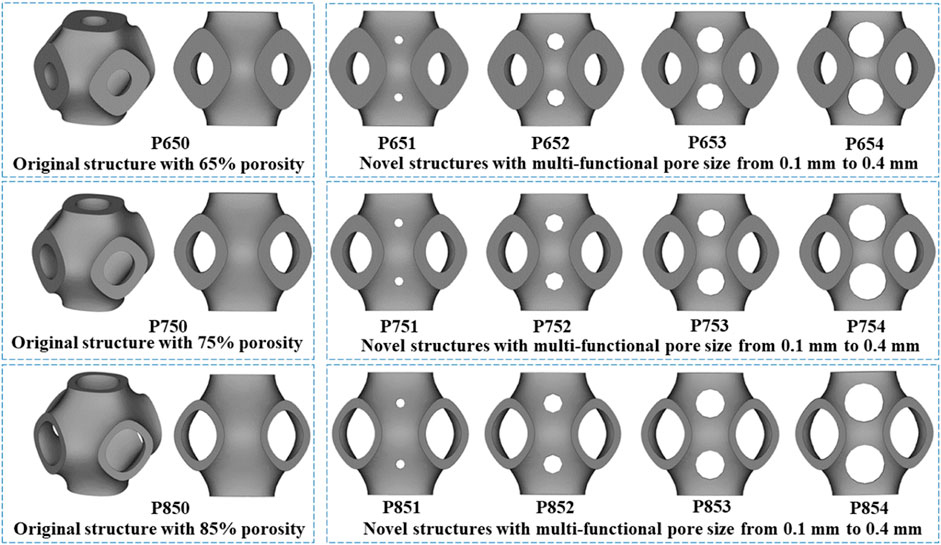
Figure 3. Three original scaffolds before optimization (named P650, P750, and P850) and the corresponding optimized novel scaffolds with multi-functional pore sizes from 0.1 mm to 0.4 mm (named P651–P654, P751–P754, and P851–P854).
A group of Schwarz P structures with 65% porosity (P650–P654) were selected for manufacturing to carry out mechanical tests on them. Besides, in this process, it can also be proved that the designed new TPMS bone scaffold can be manufactured and has practical clinical significance. It is worth noting that all the TPMS bone scaffolds designed at 65% (P650-P654), 75% (P750-P754) and 85% (P850-P854) porosity meet the manufacturing requirements:
To evaluate the mechanical behavior of the novel scaffolds, both FE simulations and mechanical experiments were performed. Based on the obtained elastic moduli, the Zener anisotropy indexed were calculated. The elastic moduli and Zener anisotropy indexes were used to evaluate the performance of the novel scaffolds.
For the FE simulations, the boundary condition of
The boundary condition of
According to the derivation in Section 2.2,
where the sigma-bar of the stress
The scaffolds after topology repair were meshed using C3D8R elements with all element size of 0.01 mm. In order to ensure that the results based on this element size can be converged, the convergence analysis is shown in the Figure 4. The smaller the size of the element is, the greater number of meshes is, and the result tends to converge. Therefore, four different element sizes of 0.04 mm, 0.03 mm, 0.02 mm and 0.01 mm are taken to calculate the effective elastic modulus. According to the results obtained, the elastic modulus is 5.59 GPa when the element size is 0.02 mm and 5.58 GPa when the element size is 0.01 mm. Therefore, it can be considered that the result is convergent when the element size is 0.01 mm. At present, the material of bone scaffold is Ti6Al4V, which is widely used in clinic, that is, elastic modulus is 110.0 GPa and Poisson’s ratio is 0.3 (Montazerian et al., 2017). Then, the FE simulations were carried out in ABAQUS with an input elastic modulus of 110.0 GPa and Poisson’s ratio of 0.3. Eventually, the elastic moduli were calculated using Eq. 9.
The Zener anisotropy index, which is commonly used to evaluate the anisotropic properties of materials (Chen et al., 2019), is given by Eq. 15:
After the FE analyses with the boundary conditions in Eq. 12 and Eq. 13, the stiffness matrix C was obtained, and then the Zener anisotropy index was calculated using Eq. 15. Next, the 3D anisotropic elastic responses of the Schwarz P scaffolds were visualized in MATLAB.
Quasi-static uniaxial compression tests were performed using INSTRON 5985 (Instron Company, Massachusetts, United States) and the loading speed was 0.5 mm/min according to the mechanical test standard ISO13314 (Ma et al., 2020). The samples were placed at the center of the lower fixture. The lower plate of the fixture remained fixed, while the upper plate was loaded at 0.5 mm/min, and the force and displacement were recorded using the sensors of the equipment (Figure 5). The stress was calculated by dividing the measured force by the cross-sectional area of the sample, and the strain was calculated by dividing the displacement by the height of the sample in the loading direction. The elastic modulus was calculated from the slope of the linear part of the stress–strain curve, and the yield stress was calculated using the 0.2% offset method. One camera was mounted in front of the samples to record the whole deformation process.
Mass transport capacity (measured by permeability) is very important for bone scaffolds, because a high mass transport capacity is beneficial for the transmission in the scaffold, which is crucial for the growth of cells. Computational fluid dynamics (CFD) was used to simulate the process of transmission using COMSOL (v6.0, COMSOL Multiphysics, Stockholm, Sweden). The permeabilities of the novel scaffolds with multi-functional pores were calculated to evaluate the mass transport capacity.
All the structures were arrayed to 2
Darcy’s law is given by Eq. 16:
where
The pressure drop between the inlet and outlet can be obtained from CFD calculation, and the permeability (
where
In this section, the geometric characteristics of the Schwarz P scaffolds are discussed first, including the difference in size between the designed and the SLM fabricated multi-functional pores. Then, the mechanical properties of all Schwarz P scaffolds under loading cases
The SLM-fabricated structures are visualized in Figure 7A, and the sizes of the multi-functional pores are in the increasing order from the original structure to the novel optimized structure (P650–P654). To assess the accuracy of fabrication, the discrepancy between the fabricated specimens and the theoretical designs was examined, with particular focus on the P654 structure (Figure 7B) through a scanning electron microscope (SEM). The designed diameter of the multi-functional pore of P654 was 0.80 mm, and the theoretical observation size of the diameter was calculated to be 0.56 mm with the equation
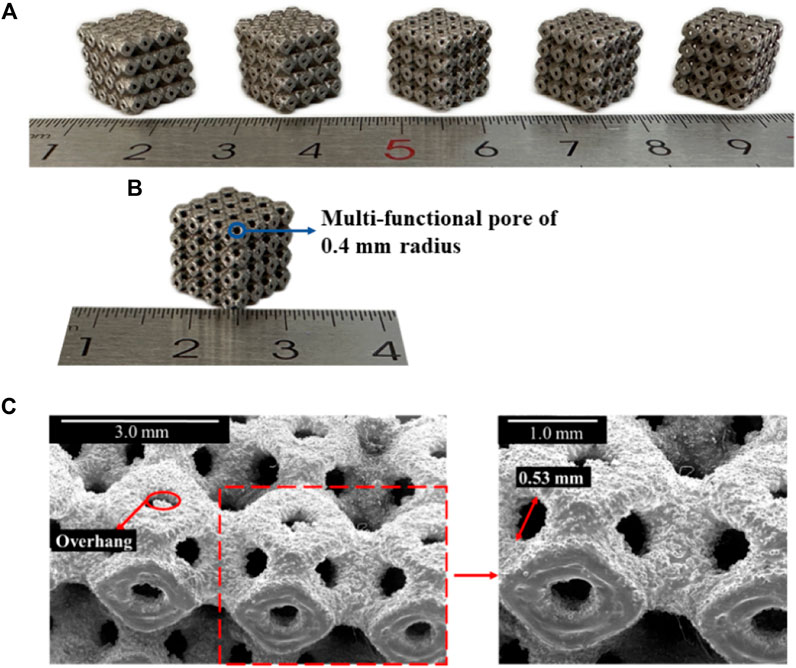
Figure 7. (A) SLM-fabricated Ti6Al4V specimens for P650–P654 scaffolds (P650 to P654 from left to right). (B) SLM-fabricated Ti6Al4V specimen for P654. (C) SEM image showing details of the fabricated sample of P654.
The overall distribution of the von Mises stress under the tensile load condition
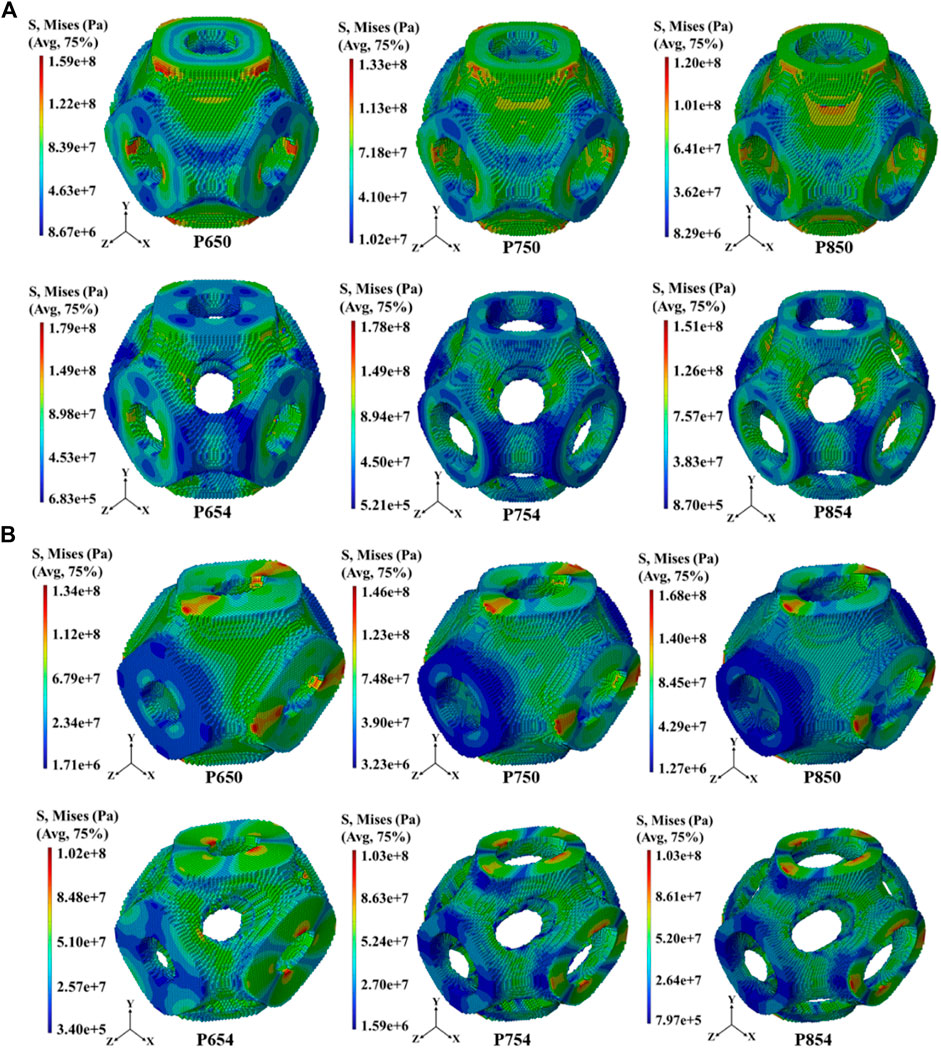
Figure 8. (A) Distribution of von Mises stress in the scaffolds before and after structural design at 65%, 75%, and 85% porosities under the uniaxial tensile load condition
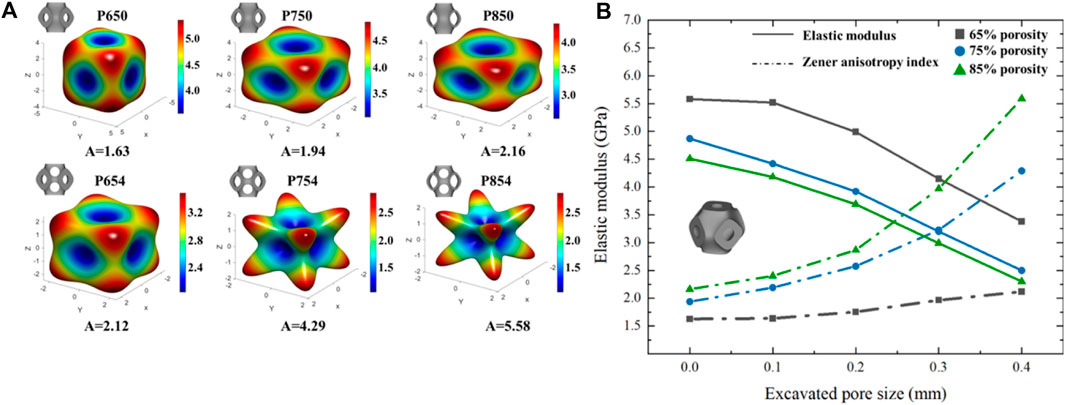
Figure 9. (A) Three-dimensional elastic modulus surface for different Schwarz P scaffolds. (B) Variation trend of structural elastic modulus and Zener anisotropy index with the increase of multi-functional pore size under different porosities.
The uniaxial compressive deformation behavior of the novel designed scaffold was basically consistent with that of the original scaffold. Both P650 and P654 scaffolds showed a shear band at the compressive strain of 0.15, which can be attributed to the slip. At the compressive strain of 0.40, the first fracture positions of the P650 and P654 structures were marked in the experimental results. Specifically, at this stage, the P654 structure exhibited a V-shaped shear band, while the P650 structure displayed a single diagonal shear band. Moreover, the fracture characteristics differed between the two structures. The fracture surface of the P650 specimen appeared smooth, whereas that of the P654 specimen was relatively rough, as depicted in Figure 10.
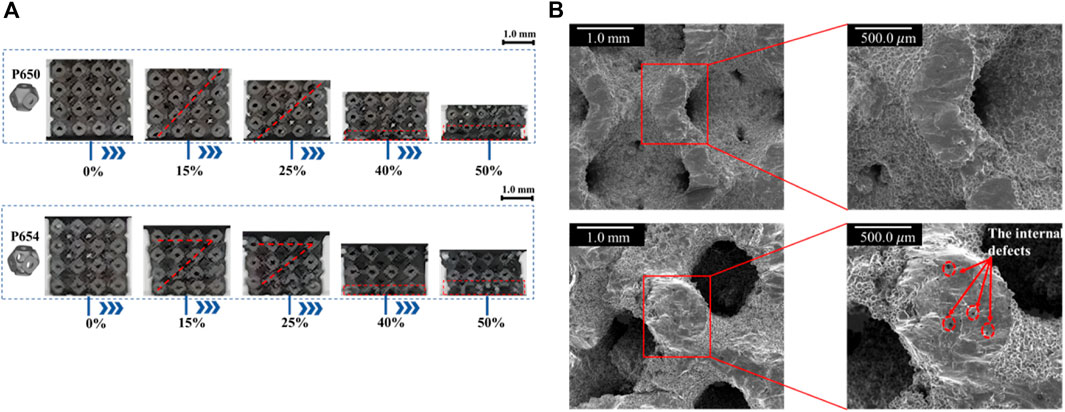
Figure 10. (A) Deformation behaviors of P650 and P654. (B) SEM views of the compressive fracture surfaces of P650 (upper) and P654 (lower).
In Figure 11, the deformation behaviors of the P650 and P654 structures can be observed to follow a pattern consisting of three main stages: the elastic stage, plateau stage, and densification stage. Both curves started in the linear elastic stage, dropped sharply after reaching the peak stress, began a long plateau stage, and finally entered the densification stage from the strain of 0.4. In addition, the strains experienced by both structures at the ultimate compressive strength were found to be approximately the same.
The elastic modulus of the P650 scaffold was 5.58 GPa and that of the P654 structure was 3.38 GPa, showing the same trend as the FE simulation results. In addition, the yield strength of the P650 structure was 292.0 MPa, and that of the P654 structure was 153.0 MPa. The error between the measured elastic modulus and the FE simulation result was 9.0% for the P650 structure and 8.3% for the P654 structure (Figure 12).
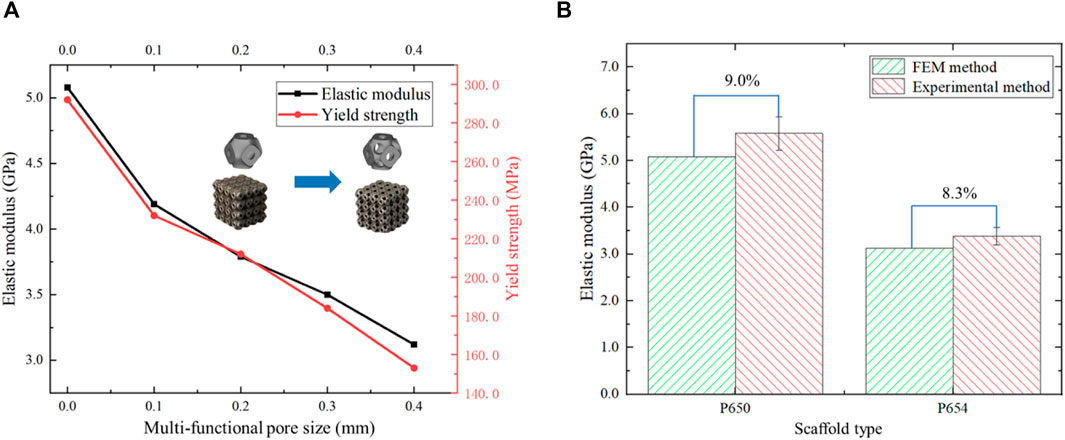
Figure 12. (A) Elastic moduli and yield strengths of scaffolds P650 to P654 calculated from experimental data. (B) Error in the elastic moduli of scaffolds P650 and P654 calculated by mechanical experiment and FE analysis.
The pressure field in the entire model was homogenized along the vertical direction, indicating that the same pressure drop could be obtained by selecting any cross section. Since the multi-functional pores facilitated the fluid flow, the chosen cross-section position was strategically placed to intersect the centers of these pores, as illustrated in Figure 13.
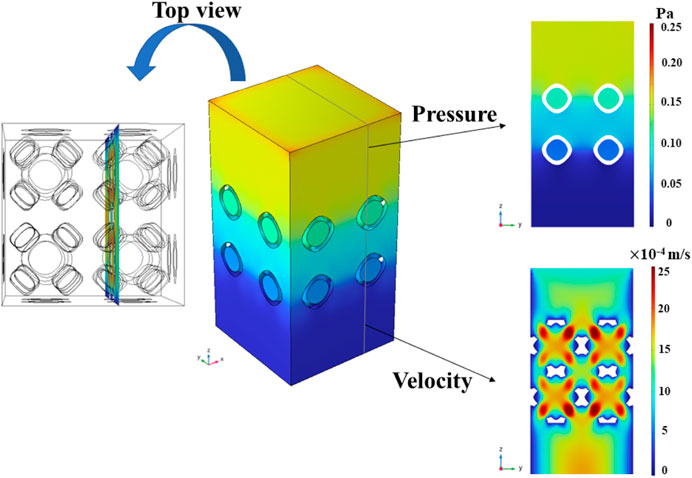
Figure 13. Pressure field and local flow velocity for the chosen cross section in the representative CFD model with symmetrical boundary conditions.
With an increase in the radius of the multi-functional pores, the fluid velocity experienced a significant rise, culminating in a maximum velocity 2.8 × 10−3 m/s in the P654 scaffold (Figure 14A). In addition, the pressure drop decreased with the increase of the radius of the multi-functional pore (Figure 14B). According to Darcy’s law shown in Eq. 17, the permeability of the scaffold was greatly improved by adding the multi-functional pores.
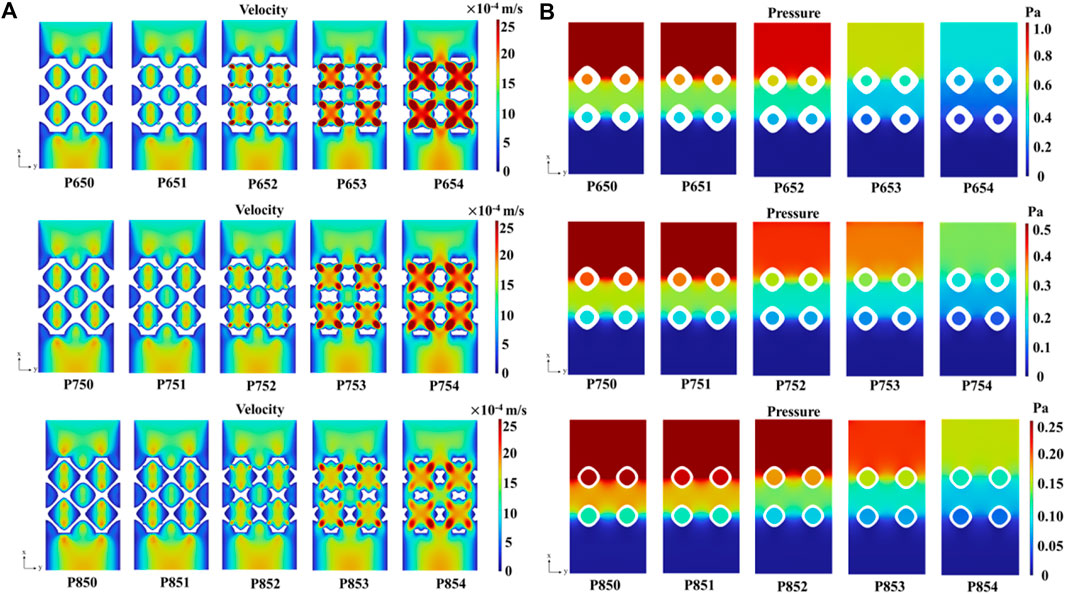
Figure 14. (A) Velocity field in the Schwarz P scaffolds with symmetrical boundary conditions. (B) Pressure distribution in the Schwarz P scaffolds with the symmetrical boundary conditions.
The permeabilities of the scaffolds increased with the increase of porosity (Figure 15). For example, the permeability of the P650 structure was 8.58
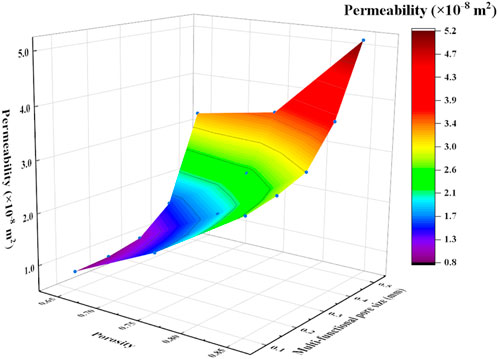
Figure 15. Effects of porosity and radius of multi-functional pores on the permeability of Schwarz P scaffolds.
Finally, the elastic moduli and permeabilities of the scaffolds were compared with those of cancellous bone (Figure 16). As a result of the design process, the coordinate points gradually shifted from the upper left corner (higher elastic modulus and lower permeability) of the graph to the lower right corner (lower elastic modulus and higher permeability). The elastic moduli and permeabilities of three structures, P754, P853, and P854, had been optimized to fall within the range observed for cancellous bone.
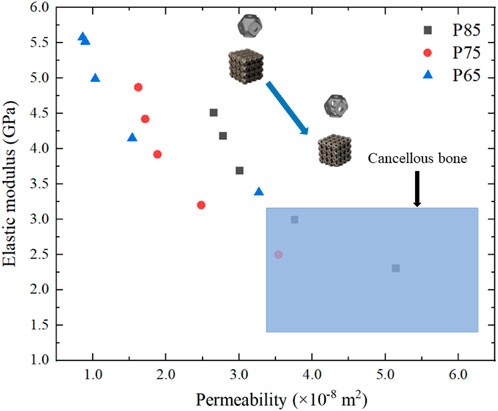
Figure 16. Optimization of the elastic modulus and permeability of Schwarz P scaffold and comparison with those of cancellous bone (the blue area is the range of the elastic modulus and permeability of cancellous bone).
Novel TPMS structures were designed and fabricated in this study. The morphological properties, mechanical properties, and mass transport capacities of the structures were investigated using FE simulations and mechanical experiments. In this section, the calculated results of the novel scaffolds are discussed and compared with those of cancellous bone, proving that the novel scaffolds in this study are ideal candidates for clinical applications.
The error between the fabricated and designed values was 5.4%, indicating that the fabricated scaffolds were of good quality. However, it is necessary to discuss the possible reasons for the error. First, overhang structures were observed (Supplementary Figure 3.1C), which were the defects in the printing process generated by powder attachment. The side attachment was due to the partial melting of the powder around the contour line by laser tracking (Yan et al., 2012) and the bottom attachment was due to the improper thickness of the adjacent layers (Tian et al., 2017). Manufacturing defects such as overhang structures and residual powder are inevitable, and the same phenomena have been observed in other studies (Fan et al., 2021; Qiu et al., 2023). Second, the accuracy of the STL model and the internal structure of the specimen can also affect the manufacturing precision, but these factors are beyond the scope of this study. Although we can eliminate errors as much as possible, due to the limitations of AM technology, errors cannot be completely avoided. The error analysis of AM technology is another complex research field. Therefore, it may be possible to avoid errors by printing multiple times in the future, but in this study, considering the acceptable error of the multi-functional pore (5.4%), it is believed that the fabricated scaffolds meet the requirements of clinical applications.
The FE analysis results revealed that the novel scaffolds designed in this study do not produce stress concentration near the multi-functional pores, which will benefit the long-term service of the scaffolds (Supplementary Figure 3.2). Large stress will inevitably be generated near the multi-functional pores, but such a stress concentration phenomenon is not obvious, and it will not cause the structure to be damaged at the multi-functional pores. It is important to highlight that, in this study, mixed boundary conditions were employed instead of periodic boundary conditions to determine the scaffolds’ elastic moduli. This choice not only enhances computational efficiency but has also been validated for its reliable calculation accuracy in previous research (Feng et al., 2021; Baghous et al., 2023; Peng et al., 2023).
The Zener anisotropy indexes (
The deformation behavior of the newly designed structure closely mirrored that of the original structure, as illustrated in Supplementary Figure 3.4. Within the strain range of 0–0.1, both structures exhibited stress-strain relationships that were predominantly linear. Notably, at a strain of 0.1, the stresses of P650 and P654 experienced significant drops to 27.4% and 51.3% of their peak stress levels, respectively. This phenomenon was related to the brittle failure of pillars in the Ti6Al4V lattice treated by SLM (Kadkhodapour et al., 2017), and this same phenomenon has been observed in previous studies. For example, Qiu et al. (2023) reported that the stress dropped to 39.4% of the peak stress, and Rezapourian et al. (Rezapourian et al., 2023) reported that the stress dropped to 8.9% of the peak stress. As the stress decreased, both the P650 and P654 structures exhibited a shear band along a diagonal direction of 45°, and P654 produced an additional horizontal shear band (Supplementary Figure 3.4A). The shear band along the diagonal was caused by the diagonal distribution of the maximum local curvature of the Schwarz P structure (Fan et al., 2021). In the plateau stage, the stresses were lower than the ultimate strength and increased in waves. Subsequently, the two structures entered the densification stage, and the stress–strain curve showed an upward trend and reached its initial peak. As the strain increased, the fracture zone of the structure continued to expand, resulting in a state of collapse in the lower layer and a state of yield in the upper layer. The P650 structure displayed smooth and flat fracture characteristics, indicating that the structure’s fracture type was primarily due to tensile deformation (Supplementary Figure 3.4B). Furthermore, some internal defects were observed on the fracture cross section of the P654 structure, which may have led to the formation of the horizontal shear band of P654. Similar internal defects have been documented in other studies as well, indicating a potential influence on the mechanical behavior and failure mode of the structure (Fan et al., 2021; Qiu et al., 2023).
The compressive strength and yield strength of the novel scaffolds were compared with those of cancellous bone, indicating that the novel scaffolds had sufficient strength. The compressive strength of cancellous bone is 5.8 MPa and the yield strength of cancellous bone is 4.1 MPa (Syahrom et al., 2011). The P654 structure had compressive strength of 165.0 MPa and yield strength of 153.0 MPa. Therefore, the novel scaffolds met the strength requirements. The error between the FE method and experimental method was 9.0% for the P650 structure and 8.3% for the P654 structure. The error between the two methods evaluated by Jia et al. (2020) fluctuated between 8.0% and 20.0%, so the error in this study was within the acceptable range.
With the increase of the radius of the multi-functional pore, the velocity of the fluid in the scaffold increased gradually (Supplementary Figure 3.8A). When the radius of the multi-functional pore reached 0.4 mm, the internal structure was completely connected, which significantly improved the permeability of the scaffold. In the past, the method to improve permeability was to increase porosity, but the possible porosity for a certain TPMS scaffold (Maskery et al., 2018) is limited to a certain range. For example, the permeability of P650 was 8.58
After adding the multi-functional pores to the scaffolds, the elastic moduli and permeabilities of P754, P853, and P854 fall within the corresponding ranges of cancellous bone (Supplementary Figure 3.10). In addition, with the progress of adding multi-functional pores, the change of permeability was much larger than the change of elastic modulus. From the P650 scaffold to the P654 scaffold, the elastic modulus decreased by 39.0% and the permeability increased by 281.0%. Previous studies have shown that the permeability of a TPMS scaffold is more sensitive to pore size than is the elastic modulus (Sutradhar et al., 2016). The results shown in Supplementary Figure 3.10 also prove that the design method proposed in this study can expand the design domain of TPMS structure. To be specific, since the TPMS structure is controlled by mathematical equation such as Eq. 1, it means that the variable parameters in Eq. 1 is the only variable, which leads to great limitations in the design domain of TPMS structures. To solve this problem, novel TPMS structures such as functional graded TPMS structures are designed in previous studies to broaden the design domain (Fan et al., 2021; Liu et al., 2022). The optimal design method proposed in this study introduced a new variable: multi-functional pore, it can broaden the design domain of TPMS structure, so more different TPMS bone scaffolds can be designed. As shown in Supplementary Figure 3.10, the bone scaffolds that possess elastic modulus and permeability meet the requirements of cancellous bone can be designed by this method, which are new scaffolds cannot be obtained in the previous design domain.
Although promising results were obtained in this study, there are still some limitations that should be resolved in the future. First, the novel design method proposed in this study is only suitable for symmetric structures such as TPMS structures. Because the TPMS structures are symmetrical, its stiffness matrix
To address the problems of high elastic modulus and low permeability in current bone scaffolds, novel TPMS bone scaffolds with optimization-guided multi-functional pores were designed in this study. The performances of the novel TPMS scaffolds were investigated using experimental characterization and numerical simulations. The main conclusions are as follows:
(1) The effective elastic modulus reduced from 5.58 GPa (original scaffold) to 3.38 GPa (novel designed scaffold), resulting in lower stress shielding.
(2) The multi-functional pores greatly improved the mass transport capacities of the TPMS scaffolds by providing new pores on the walls. The permeability increased from 8.58 × 10−9 m2 (original scaffold) to 5.14 × 10−8 m2 (novel designed scaffold)
(3) The deformation mode of the novel TPMS bone scaffolds at 65% porosity remained unchanged, which ensured that the new scaffolds can be used as stably as the previous scaffolds. The compressive strength and yield strength of the structure met the clinical requirements, i.e., the scaffolds need to have enough strengths to ensure that they will not break easily.
(4) The novel scaffolds expanded the design domain of TPMS-based bone scaffolds, providing a promising new method for the design of high-performance bone scaffolds.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
JJ: Funding acquisition, Investigation, Writing–review and editing. YH: Methodology, Software, Writing–original draft. XP: Methodology, Writing–original draft. CW: Investigation, Writing–review and editing. HZ: Supervision, Writing–review and editing. YL: Conceptualization, Formal Analysis, Methodology, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China [grant numbers 12211530062 and 12072066], State Key Laboratory of Structural Analysis for Industrial Equipment [grant number GZ22105], Natural Science Foundation of Liaoning Province [grant numbers 2022-MS-443 and 2022-YGJC-21], Dalian Medical Science Research Program Project [grant number 2111005], Dalian University of Technology and Affiliated Central Hospital joint research fund [grant numbers 2022ZXYG45 and DUT23YG217] and the DUT-BSU Joint Institute fund [grant number ICR2303].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2024.1401899/full#supplementary-material
Ali, D., Ozalp, M., Blanquer, S., and Onel, S. (2020). Permeability and fluid flow-induced wall shear stress in bone scaffolds with TPMS and lattice architectures: a CFD analysis. Eur. J. Mechanics-B/Fluids 79, 376–385. doi:10.1016/j.euromechflu.2019.09.015
Al-Ketan, O., and Abu Al-Rub, R. K. (2019). Multifunctional mechanical metamaterials based on triply periodic minimal surface lattices. Adv. Eng. Mater. 21 (10), 1900524. doi:10.1002/adem.201900524
Arabnejad, S., Johnston, B., Tanzer, M., and Pasini, D. (2017). Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 35 (8), 1774–1783. doi:10.1002/jor.23445
Askari, M., Hutchins, D., Thomas, P., Astolfi, L., Watson, R. L., Abdi, M., et al. (2020). Additive manufacturing of metamaterials: a review. Addit. Manuf. 36, 101562. doi:10.1016/j.addma.2020.101562
Augat, P., Link, T., Lang, T., Lin, J. C., Majumdar, S., and Genant, H. K. (1998). Anisotropy of the elastic modulus of trabecular bone specimens from different anatomical locations. Med. Eng. Phys. 20 (2), 124–131. doi:10.1016/S1350-4533(98)00001-0
Baghous, N., Barsoum, I., and Al-Rub, R. (2023). Generalized yield surface for sheet-based triply periodic minimal surface lattices. Int. J. Mech. Sci. 252, 108370. doi:10.1016/j.ijmecsci.2023.108370
Barba, D., Alabort, E., and Reed, R. (2019). Synthetic bone: design by additive manufacturing. Acta Biomater. 97, 637–656. doi:10.1016/j.actbio.2019.07.049
Blanquer, S., Werner, M., Hannula, M., Sharifi, S., Lajoinie, G. P. R., Eglin, D., et al. (2017). Surface curvature in triply-periodic minimal surface architectures as a distinct design parameter in preparing advanced tissue engineering scaffolds. Biofabrication 9 (2), 025001. doi:10.1088/1758-5090/aa6553
Campana, V., Milano, G., Pagano, E., Barba, M., Cicione, C., Salonna, G., et al. (2014). Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J. Mater. Sci. Mater. Med. 25, 2445–2461. doi:10.1007/s10856-014-5240-2
Chen, Z., Xie, Y., Wu, X., Wang, Z., Li, Q., and Zhou, S. (2019). On hybrid cellular materials based on triply periodic minimal surfaces with extreme mechanical properties. Mater. Des. 183, 108109. doi:10.1016/j.matdes.2019.108109
Davoodi, E., Montazerian, H., Khademhosseini, A., and Toyserkani, E. (2020). Sacrificial 3D printing of shrinkable silicone elastomers for enhanced feature resolution in flexible tissue scaffolds. Acta Biomater. 117, 261–272. doi:10.1016/j.actbio.2020.10.001
Dendorfer, S., Maier, H., Taylor, D., and Hammer, J. (2008). Anisotropy of the fatigue behaviour of cancellous bone. J. Biomechanics 41 (3), 636–641. doi:10.1016/j.jbiomech.2007.09.037
Fan, X., Tang, Q., Feng, Q., Ma, S., Song, J., Jin, M., et al. (2021). Design, mechanical properties and energy absorption capability of graded-thickness triply periodic minimal surface structures fabricated by selective laser melting. Int. J. Mech. Sci. 204, 106586. doi:10.1016/j.ijmecsci.2021.106586
Feng, J., Liu, B., Lin, Z., and Fu, J. (2021). Isotropic porous structure design methods based on triply periodic minimal surfaces. Mater. Des. 210, 110050. doi:10.1016/j.matdes.2021.110050
Iqbal, T., Wang, L., Li, D., Dong, E., Fan, H., Fu, J., et al. (2019). A general multi-objective topology optimization methodology developed for customized design of pelvic prostheses. Med. Eng. Phys. 69, 8–16. doi:10.1016/j.medengphy.2019.06.008
Jia, H., Lei, H., Wang, P., Meng, J., Li, C., Zhou, H., et al. (2020). An experimental and numerical investigation of compressive response of designed Schwarz Primitive triply periodic minimal surface with non-uniform shell thickness. Extreme Mech. Lett. 37, 100671. doi:10.1016/j.eml.2020.100671
Jiang, H., Le, B., Bednarcyk, B., Scarpa, F., and Chen, Y. (2020). Bioinspired multilayered cellular composites with enhanced energy absorption and shape recovery. Addit. Manuf. 36, 101430. doi:10.1016/j.addma.2020.101430
Kadkhodapour, J., Montazerian, H., Darabi, A. C., Zargarian, A., and Schmauder, S. (2017). The relationships between deformation mechanisms and mechanical properties of additively manufactured porous biomaterials. J. Mech. Behav. Biomed. Mater. 70, 28–42. doi:10.1016/j.jmbbm.2016.09.018
Kang, J., Dong, E., Li, D., Dong, S., Zhang, C., and Wang, L. (2020). Anisotropy characteristics of microstructures for bone substitutes and porous implants with application of additive manufacturing in orthopaedic. Mater. Des. 191, 108608. doi:10.1016/j.matdes.2020.108608
Khaleghi, S., Dehnavi, F., Baghani, M., Safdari, M., Wang, K., and Baniassadi, M. (2021). On the directional elastic modulus of the TPMS structures and a novel hybridization method to control anisotropy. Mater. Des. 210, 110074. doi:10.1016/j.matdes.2021.110074
Kurtz, S., Ong, K., Lau, E., Mowat, F., and Halpern, M. (2007). Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 89 (4), 780–785. doi:10.2106/JBJS.F.00222
Lee, D., Khan, K., and Al-Rub, R. (2017). Stiffness and yield strength of architectured foams based on the Schwarz Primitive triply periodic minimal surface. Int. J. Plasticity 95, 1–20. doi:10.1016/j.ijplas.2017.03.005
Liu, Z., Gong, H., Gao, J., and Liu, L. (2022). Topological design, mechanical responses and mass transport characteristics of high strength-high permeability TPMS-based scaffolds. Int. J. Mech. Sci. 217, 107023. doi:10.1016/j.ijmecsci.2021.107023
Ma, Q., Zhang, L., Ding, J., Qu, S., Fu, J., Zhou, M., et al. (2021). Elastically-isotropic open-cell minimal surface shell lattices with superior stiffness via variable thickness design. Addit. Manuf. 47, 102293. doi:10.1016/j.addma.2021.102293
Ma, S., Song, K., Lan, J., and Ma, L. (2020). Biological and mechanical property analysis for designed heterogeneous porous scaffolds based on the refined TPMS. J. Mech. Behav. Biomed. Mater. 107, 103727. doi:10.1016/j.jmbbm.2020.103727
Maconachie, T., Leary, M., Lozanovski, B., Zhang, X., Qian, M., Faruque, O., et al. (2019). SLM lattice structures: properties, performance, applications and challenges. Mater. Des. 183, 108137. doi:10.1016/j.matdes.2019.108137
Maskery, I., Aremu, A., Parry, L., Wildman, R., Tuck, C., and Ashcroft, I. (2018). Effective design and simulation of surface-based lattice structures featuring volume fraction and cell type grading. Mater. Des. 155, 220–232. doi:10.1016/j.matdes.2018.05.058
Montazerian, H., Davoodi, E., Asadi-Eydivand, M., Kadkhodapour, J., and Solati-Hashjin, M. (2017). Porous scaffold internal architecture design based on minimal surfaces: a compromise between permeability and elastic properties. Mater. Des. 126, 98–114. doi:10.1016/j.matdes.2017.04.009
Peng, X., Huang, Q., Zhang, G., Li, J., Zhang, X., Lu, Y., et al. (2022). Compensating the anisotropic mechanical properties of electron beam melting-based Gyroid scaffolds using structural design. Int. J. Mech. Sci. 226, 107442. doi:10.1016/j.ijmecsci.2022.107442
Peng, X., Huo, Y., Zhang, G., Cheng, L., Lu, Y., Li, J., et al. (2023). Controlled mechanical and mass-transport properties of porous scaffolds through hollow strut. Int. J. Mech. Sci. 248, 108202. doi:10.1016/j.ijmecsci.2023.108202
Qiu, N., Zhang, J., Li, C., Shen, Y., and Fang, J. (2023). Mechanical properties of three-dimensional functionally graded TPMS structures. Int. J. Mech. Sci. 1, 108118. doi:10.1016/j.ijmecsci.2023.108118
Qureshi, Z., Al-Omari, S., Elnajjar, E., Al-Ketan, O., and Al-Rub, R. A. (2021). Using triply periodic minimal surfaces (TPMS)-based metal foams structures as skeleton for metal-foam-PCM composites for thermal energy storage and energy management applications. Int. Commun. Heat Mass Transf. 124, 105265. doi:10.1016/j.icheatmasstransfer.2021.105265
Rabiatul, A., Fatihhi, S., Md, S., Zakaria, Z., Harun, M. N., Kadir, M. R. A., et al. (2021). Fluid–structure interaction (FSI) modeling of bone marrow through trabecular bone structure under compression. Biomechanics Model. Mechanobiol. 20, 957–968. doi:10.1007/s10237-021-01423-x
Rezapourian, M., Jasiuk, I., Saarna, M., and Hussainova, I. (2023). Selective laser melted Ti6Al4V split-P TPMS lattices for bone tissue engineering. Int. J. Mech. Sci. 251, 108353. doi:10.1016/j.ijmecsci.2023.108353
Santos, J., Pires, T., Gouveia, B., Castro, A. P., and Fernandes, P. R. (2020). On the permeability of TPMS scaffolds. J. Mech. Behav. Biomed. Mater. 110, 103932. doi:10.1016/j.jmbbm.2020.103932
Sevilla, P., Aparicio, C., Planell, J. A., and Gil, F. (2007). Comparison of the mechanical properties between tantalum and nickel–titanium foams implant materials for bone ingrowth applications. J. Alloys Compd. 439 (1-2), 67–73. doi:10.1016/j.jallcom.2006.08.069
Soro, N., Attar, H., Brodie, E., Veidt, M., Molotnikov, A., and Dargusch, M. S. (2019). Evaluation of the mechanical compatibility of additively manufactured porous Ti–25Ta alloy for load-bearing implant applications. J. Mech. Behav. Biomed. Mater. 97, 149–158. doi:10.1016/j.jmbbm.2019.05.019
Strömberg, N. (2021). Optimal grading of TPMS-based lattice structures with transversely isotropic elastic bulk properties. Eng. Optim. 53 (11), 1871–1883. doi:10.1080/0305215X.2020.1837790
Sun, C., Wang, L., Kang, J., and Jin, Z. (2018). Biomechanical optimization of elastic modulus distribution in porous femoral stem for artificial hip joints. J. Bionic Eng. 15, 693–702. doi:10.1007/s42235-018-0057-1
Sutradhar, A., Park, J., Carrau, D., Nguyen, T. H., Miller, M. J., and Paulino, G. H. (2016). Designing patient-specific 3D printed craniofacial implants using a novel topology optimization method. Med. Biol. Eng. Comput. 54, 1123–1135. doi:10.1007/s11517-015-1418-0
Syahrom, A., Abdul, K., Abdullah, J., and Öchsner, A. (2011). Mechanical and microarchitectural analyses of cancellous bone through experiment and computer simulation. Med. Biol. Eng. Comput. 49, 1393–1403. doi:10.1007/s11517-011-0833-0
Tan, X., Tan, Y., Chow, C., Tor, S., and Yeong, W. (2017). Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: a state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 76, 1328–1343. doi:10.1016/j.msec.2017.02.094
Tian, Y., Tomus, D., Rometsch, P., and Wu, X. (2017). Influences of processing parameters on surface roughness of Hastelloy X produced by selective laser melting. Addit. Manuf. 13, 103–112. doi:10.1016/j.addma.2016.10.010
Vijayavenkataraman, S., Kuan, L., and Lu, W. (2020). 3D-printed ceramic triply periodic minimal surface structures for design of functionally graded bone implants. Mater. Des. 191, 108602. doi:10.1016/j.matdes.2020.108602
Wallace, I., Worthington, S., Felson, D., Jurmain, R. D., Wren, K. T., Maijanen, H., et al. (2017). Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. 114 (35), 9332–9336. doi:10.1073/pnas.1703856114
Wauthle, R., Van Der Stok, J., Yavari, S., Van Humbeeck, J., Kruth, J. P., Zadpoor, A. A., et al. (2015). Additively manufactured porous tantalum implants. Acta Biomater. 14, 217–225. doi:10.1016/j.actbio.2014.12.003
Wu, D., Isaksson, P., Ferguson, S. J., and Persson, C. (2018). Young’s modulus of trabecular bone at the tissue level: a review. Acta Biomater. 78, 1–12. doi:10.1016/j.actbio.2018.08.001
Yan, C., Hao, L., Hussein, A., and Raymont, D. (2012). Evaluations of cellular lattice structures manufactured using selective laser melting. Int. J. Mach. Tools Manuf. 62, 32–38. doi:10.1016/j.ijmachtools.2012.06.002
Zhai, Y., Lados, D., and Lagoy, J. (2014). Additive manufacturing: making imagination the major limitation. Jom 66, 808–816. doi:10.1007/s11837-014-0886-2
Keywords: bone scaffold, multi-functional pore, additive manufacturing, mechanical behavior, mass transport capacity
Citation: Jiang J, Huo Y, Peng X, Wu C, Zhu H and Lyu Y (2024) Design of novel triply periodic minimal surface (TPMS) bone scaffold with multi-functional pores: lower stress shielding and higher mass transport capacity. Front. Bioeng. Biotechnol. 12:1401899. doi: 10.3389/fbioe.2024.1401899
Received: 16 March 2024; Accepted: 07 June 2024;
Published: 26 June 2024.
Edited by:
Lei Fan, Marquette University, United StatesCopyright © 2024 Jiang, Huo, Peng, Wu, Zhu and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongtao Lyu, eW9uZ3Rhb2x1QGRsdXQuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.