
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Bioeng. Biotechnol., 01 February 2024
Sec. Tissue Engineering and Regenerative Medicine
Volume 12 - 2024 | https://doi.org/10.3389/fbioe.2024.1345343
 Giorgia Codispoti
Giorgia Codispoti Melania Carniato
Melania Carniato Silvia Brogini*
Silvia Brogini* Alessia Romanelli
Alessia Romanelli Lucia Martini
Lucia Martini Gianluca Giavaresi
Gianluca Giavaresi Matilde Tschon
Matilde TschonBackground: Rotator cuff tears (RCTs), resulting from degeneration or trauma of the shoulder tendons, are one of the main causes of shoulder pain. In particular, massive RCTs represent 40% of all injuries, require surgical treatment, and are characterized by poor clinical outcomes and a high rate of failure. In recent years, the use of biological decellularized patches for augmentation procedures has received great interest owing to their excellent self-integration properties, improving healing and, thus, presenting an innovative therapeutic option. However, the findings from clinical studies have emerged with conflicting viewpoints regarding the benefits of this procedure, as an excessive tension load might compromise the integrity of the tendon-to-bone connection when the patch exhibits low elasticity or insufficient strength. This could prevent the healing process, leading to unpredictable results in clinical practice.
Methods: This systematic review was conducted following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines across three databases (PubMed, Scopus, and Web of Knowledge) to underline the results obtained in preclinical studies involving animal models of RCT surgeries that utilized the biological decellularized matrix augmentation technique in the last 5 years.
Results: Thirteen articles were included after the screening, and the SYRCLE tools were applied to assess the risk of bias in in vivo studies. Open-surgery techniques were conducted to create tendon defects or detachment in different animal models: rat (31%), rabbit (46%), dog (15%), and sheep (8%). Patches decellularized with non-standardized protocols were used in 77% of studies, while commercially available matrices were used in 15%. Of the studies, 31% used allogenic patches, 61% used xenogenic patches, and 8% utilized both xenogenic and autologous patches.
Conclusion: Overall, this review provides a comprehensive overview of the use of acellular patches and their effective therapeutic potential in rotator cuff (RC) repair at the preclinical level with the aim of expanding the strategies and matrices available for surgeons.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023468716.
Rotator cuff disorders include a wide spectrum of pathologies related to the different anatomical structures that make up the rotator cuff, and their incidence increases with age. In humans, the rotator cuff consists of the subscapular, teres minor, supraspinatus, and infraspinatus muscles and their tendons at the neck of the humeral head, which provide dynamic stabilization of the glenohumeral joint and, thus, shoulder motion. Rotator cuff injuries are usually degenerative conditions due to trauma or degeneration of the shoulder tendons and are the most common cause of pain and fatigue (Zhou et al., 2023). Rotator cuff tears (RCTs), which include partial or full-thickness injuries, can range in size from small to massive; full-thickness injuries are also classified by tear size into small (<1 cm in length), medium (from 1 to 3 cm), large (from 3 to 5 cm), and massive (greater than 5 cm) tears and by Patte or modified Patte classifications according to MRI measurements (tendon retraction in the frontal plane, supraspinatus atrophy, and supraspinatus muscle fat infiltration) (Lippe et al., 2012; Dang and Davies, 2018). The modified Patte classification can predict both the risk of re-tear after surgery and tendon irreparability (Guo et al., 2020). Despite recent advancements in surgical techniques, fixation biomaterials, and rehabilitation programs, massive RCTs represent 40% of all injuries and often have a poor clinical outcome and a high rate of failure compared to smaller RCTs (Di Benedetto et al., 2021; Yang et al., 2022). A successful outcome for small- and medium-size partial or full-thickness tears is obtained by non-surgical or conservative options, such as periscapular or deltoid musculature strengthening and functional rehabilitation to increase the range of joint motion and restore muscle strength and joint coordination (Kuhn et al., 2013). In addition to physiotherapy, glucocorticoid injections can be used to reduce inflammation and pain although they can cause spontaneous tendon rupture and slow healing (Dang and Davies, 2018; Zhou et al., 2023). Surgical treatment is considered based on factors such as the size, thickness, and muscle quality of the tear. It is the preferred option for lesions that cannot be treated with conservative options, particularly in young patients. Various surgical strategies have been proposed for the treatment of massive tears, including arthroscopic debridement followed by biceps tenotomy or tenodesis and subacromial decompression, complete or partial tear repair, tendon transfer, arthroscopic superior capsular reconstruction, and total arthroplasty. Advances in surgical techniques for the repair of RCTs have made it possible to achieve the goals of reducing pain, restoring function and motion, restoring the biomechanical properties of the rotator cuff, and promoting healing. Nevertheless, surgical treatment of massive RCTs has a high failure rate (up to and over 90% of cases (McElvany et al., 2015)) due to fat infiltration, tension on the repaired site, reduction of the acromion humeral distance, and patient characteristics, as reported in many studies (Di Benedetto et al., 2021; Zhou et al., 2023). An alternative surgical technique for the repair of RCTs is patch augmentation, which can improve the strength of the tendon–bone junction and the healing and self-integration processes due to its ability to promote vascularization and cellular growth (Cai et al., 2018). In addition, patch augmentation may reduce the re-tear rate compared to surgical partial repair of massive RCTs with low-grade fat infiltration and pain scores (Mori et al., 2013; de Andrade et al., 2022). The overlap of tendon and bone by the patch is performed by open surgical techniques or arthroscopically. Patch augmentation can be biological (animal or human, such as extracellular matrix-based patches), synthetic, or biosynthetic (degradable or non-degradable) (Veronesi et al., 2020). Synthetic patches have shown good results, but they can induce an immune response as a foreign body reaction; they do not have the same mechanical properties as native tissue and, unlike biological patches, can negatively influence healing due to the stress shielding phenomenon. Instead, biological matrices can mimic the extracellular matrix microenvironment and, thus, promote cellular differentiation, growth, and tissue repair. However, the results of clinical studies have highlighted conflicting views on the benefits of this procedure, as tension overload may damage the tendon–bone interface if the patch has low elasticity or is too weak, and the healing process may be inadequate, leading to unpredictable clinical outcomes (Avanzi et al., 2019; Cobb et al., 2022).
From a translational perspective, using animal models to evaluate the efficacy of decellularized patches can provide important information on safety and efficacy although they do not provide a perfect representation of the clinical condition (Yang et al., 2022). For these reasons, the aim of this systematic review is to highlight the benefits obtained in preclinical studies on animal models of RCT surgery using the biological decellularized matrix augmentation technique to promote healing and reduce the rate of re-tears after surgery.
The present literature review involved a systematic search carried out according to the PRISMA statement in three electronic databases (PubMed, Scopus, and Web of Knowledge: www.pubmed.gov, www.scopus.com, and www.webofknowledge.com). The search was performed using the following keywords: “(acellular OR decellularized) AND (dermis OR graft OR dermal matrix OR scaffold OR patch OR biomaterial OR membrane) AND (shoulder OR rotator cuff).” The search was limited to papers published in the period from 1 January 2018 to 31 December 2023 and written in English.
The screening process and analysis were conducted separately by three independent observers (SB, GC, and MT) using the collaboration platform Rayyan (Ouzzani et al., 2016). First, the articles were screened based on title and abstract using the following inclusion criteria: papers investigating the efficacy of decellularized matrices from different sources for treating rotator cuff tears and using preclinical in vivo models. The exclusion criteria encompassed articles written in other languages, reviews, unavailable abstracts or full texts, editorials, technical notes or conference proceedings, in vitro and clinical studies, and publications lacking animal models, rotator cuff lesions, and decellularized patches. The reference lists of the included papers were screened to obtain further studies. Disagreements were resolved by discussion, and where resolution was not possible, the fourth and fifth reviewers were consulted (MC and LM).
The protocol was registered at inception in the PROSPERO register (record no. CRD42023468716).
The papers’ main characteristics were extracted by GC and MT based on the animal model, species, strain, number, sex, lesion’s site and dimensions, surgical procedure and treatments, decellularized patch used with the source and tissue, decellularization protocol, main tests with selected experimental times, main findings, and the first author’s name with the year of publication. Data were checked for accuracy and completeness by a third author (S.B.), and disagreements were resolved by discussion, and where resolution was not possible, the fourth and fifth reviewers were consulted (M.C. and L.M.). The included papers were grouped according to the animal species.
A quality assessment of the in vivo studies was performed using the SYRCLE tool for animals, which comprises a 10-item checklist (Hooijmans et al., 2014). A low, high, or unclear risk of bias was scored if items were reported, not reported, or unclearly reported, respectively. The assessment was performed by two independent authors (GC and SB). Any disagreement was resolved by consensus with a third reviewer (MT).
The initial literature search using the above keywords was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and yielded the following results: 121 articles were retrieved from PubMed (www.pubmed.gov), 111 from Web of Knowledge (www.webofknowledge.com), and 55 from Scopus (www.scopus.com). The resulting references were uploaded to the Rayyan platform, where each author blindly evaluated the inclusion/exclusion criteria. Duplicate articles were then identified and removed (n = 134). Excluded articles were reviews (n = 25), articles without full text (n = 2), editorial comment or proceedings (n = 7), technical notes (n = 16), letters to the editor (n = 1), book chapter (n = 2), clinical trials (n = 37), in vitro preclinical studies (n = 3), and articles not including decellularized patches (n = 7), preclinical models (n = 16), or rotator cuff tears (n = 24). A list of excluded articles is provided in Supplementary Table S1. No articles were retrieved from the reference lists. Finally, 13 in vivo preclinical studies were included in this systematic review (Figure 1). The included papers were grouped and discussed according to animal species.
Data were extracted per animal species: rats are reported in Table 1, rabbits in Table 2, sheep in Table 3, and dogs in Table 4. Most of the studies used rabbits (6/13 papers, 46%), followed by rats (4/13, 31%), dogs (2/13, 15%), and sheep (1/13, 8%).
Three studies out of the four used Sprague–Dawley rats (Ide and Tokunaga, 2018; Chen et al., 2020; Shim et al., 2022), and one utilized Wistar rats. Thangarajah et al. (2018) and Chen et al. (2020) produced a unilateral detachment of the supraspinatus tendon (SST), while Ide and Tokunaga (2018) created bilateral defects of the SSTs (3 × 5 mm). All research groups performed the augmentation technique for tendon-to-bone repair with or without scaffold: in two cases, the commercially available human dermal matrix GraftJacket™, alone (Ide and Tokunaga, 2018) or loaded with mesenchymal stem cells (MSCs) in fibrin glue (Thangarajah et al., 2018), has been used in the experimental group compared to the unoperated or defect-untreated control group; in one article, acellular xenogenic rabbit fibrocartilage from pubic symphysis loaded with recombinant C-terminus stromal cell-derived factor 1α (C-SDF), chemokine (C-X-C motif) receptor 4 (CXCR4+), and synovium-derived MSCs (SMSCs) has been utilized to repair SST detachment (Chen et al., 2020). Shim et al. (2022) and Thangarajah et al. (2018) repaired the SST lesion by applying a decellularized patch derived from bovine pericardium loaded with autologous MSCs.
Three studies out of the four reported the sex of animals: Thangarajah et al. (2018) used female rats, whereas Chen et al. (2020) and Ide and Tokunaga (2018) used male rats. In addition, the animal’s average age varied between 8 and 22 weeks (Ide and Tokunaga, 2018; Shim et al., 2022). Experimental times ranged between 2 weeks and 12 months.
Macroscopic analyses, radiological investigations, and biomechanical tests were performed to assess the efficacy of the tested decellularized patches. Macroscopic observations were necessary to qualitatively evaluate the tendon repair at tendon-to-bone insertion: GraftJacket™ insertion at the tendon interface was visible (Thangarajah et al., 2018) in comparison with the decellularized bovine pericardial patch, in which there was continuity between the repaired scaffold-tendon and the bone.
Thangarajah et al. (2018), Chen et al. (2020), and Shim et al. (2022) performed magnetic resonance imaging (MRI) and micro-CT to measure histomorphometric parameters, such as bone mineral density (BMD), bone volume (BV/TV), and trabecular thickness (Tb.Th). BMD was decreased in the GraftJacket™ group compared to non-operated controls (Thangarajah et al., 2018); however, there was an increase in BMD and abovementioned histomorphometric parameters at 8 weeks after the acellular rabbit fibrocartilage implantation compared to controls (Chen et al., 2020).
Finally, biomechanical analyses conducted by Chen et al. (2020) and Shim et al. (2022) showed higher load-to-failure and stiffness in experimental groups compared to untreated control groups; in contrast, these biomechanical parameters were significantly lower in treated animals compared to unoperated healthy controls (Ide and Tokunaga, 2018).
All studies used New Zealand White rabbits; only three papers out of six reported the sex (male) or age (28–32 weeks old) (de Lima Santos et al., 2020; He et al., 2021; Santos et al., 2022). Most surgeries (67%) involved the creation of defects of 5 mm (de Lima Santos et al., 2020; Yuan et al., 2022a; Yuan et al., 2022b; Santos et al., 2022). Only He et al. (2021) performed a bilateral IST detachment. One group performed a two-step surgery to obtain bilateral chronic and retracted RCTs (Yildiz et al., 2019). Treatments comprised the use of decellularized patches in comparison with the contralateral side used as an untreated control. de Lima Santos et al. (2020) and Santos et al. (2022) investigated allograft decellularized tendon scaffold (DTS) from rabbit gastrocnemius muscle tendons; Yuan et al. (2022a) and Yuan et al. (2022b) repaired the created defects of SSTs and ISTs with decellularized umbilical cord Wharton jelly (DUCWJ) alone or conjugated with kartogenin (KGN) using the bridging technique and augmentation technique, respectively. He et al. (2021) used a demineralized cortical bone (hDCB) coated with the extracellular matrix (ECM) for IST detachment and rabbit tendon-derived stem cells (TDSCs) for enthesis healing; Yildiz et al. (2019) realized superior capsule reconstruction by grafting an acellular human dermal graft (HDG) in the right shoulders in comparison with an autologous tensor fascia lata (TFL) in the left shoulders.
Experimental times ranged between 2 weeks and 3 months. All studies performed macroscopic analyses for tendon repair and healing assessments; De Lima Santos et al. and Yuan et al. observed an increasing integration of DTS and DUCWJ, respectively, at each time point. Yuan et al. (2022b), Santos et al. (2022), He et al. (2021), and Yildiz et al. (2019) showed a complete enthesis healing with hDCB-TDSCs-ECM and HDG/TFL. Moreover, He et al. (2021) performed micro-CT for BV/TV and trabecular thickness (Tb.Th) measurements at the tendon-to-bone insertion, evidencing an increase in these parameters in the hDCB-ECM group compared to untreated controls.
Biomechanical tests were conducted by most of the research groups (67%): all experimental groups exhibited higher tensile stress (He et al., 2021) and strength values (Yildiz et al., 2019; He et al., 2021; Yuan et al., 2022b; Yuan et al., 2022a), failure loads (Yuan et al., 2022a; Yuan et al., 2022b), and cyclical loading values (Yildiz et al., 2019) with increasing experimental times in comparison to control groups, except for one study in which decellularized patches failed to load, evidencing lower biomechanical competence than the untreated control group (Yildiz et al., 2019).
Two studies out of 13 (15%) used canine models: Smith et al. (2020) performed half-thickness resection of the articular portion of SST (3.7 x 3–4 mm) in 2–3-year-old purpose-bred dogs, whereas Chen et al. (2022) executed a unilateral IST detachment from the insertion at the humerus in 8-month-old beagle dogs. The SST defect was left untreated in the control group or treated with different commercial matrices: amnion matrix cord scaffold (AM from Arthrex, Inc.), decellularized human dermal allograft (ArthroFLEX (AF) from Arthrex, Inc./LifeNet Health, Virginia Beach, VA, United States), and bovine collagen patch (rotation medical patch, RMP, from Smith & Nephew, London, United Kingdom) (Smith et al., 2020). Chen et al. (2022) developed a collagen-binding peptide (CBP)-growth factor (GF)-decellularized enthesis matrix (O-BDEM) loaded with or without urine-derived stem cells (USCs) (Smith et al., 2020). Only one reported the sex of animals, with experimental times ranging between 3 and 6 months (Smith et al., 2020; Chen et al., 2022).
Both research groups performed MRI and biomechanical testing to, respectively, assess the repaired area (Smith et al., 2020) and tendon and muscle conditions (Smith et al., 2020) by measuring tendon tensile loading (Smith et al., 2020), failure load (Chen et al., 2022), and stiffness (Smith et al., 2020; Chen et al., 2022). MRI in Smith et al. (2020) evidenced the bridging of the SST defect in AM, AF, and RMP groups, but hyper-intense peritendon, intramuscular, intra-articular fluid, and impingement of biceps were found in all groups at 3 months, and impingement of biceps was increased at 6 months, especially in the AF group. In the work of Chen et al. (2022), a better regeneration of IST was observed in the experimental group compared to the control group. In addition, Smith et al. (2020) and Chen et al. (2022) performed ultrasonography and micro-CT investigations, respectively: AM-treated and AF-treated SSTs were thinner but showed more organized tendon fiber alignment than the other groups at 6 months (Johnson et al., 2020); bone parameters BV/TV, Tb.Th, and Tb.N have improved in the treated group after patch augmentation in comparison with untreated controls (Chen et al., 2022).
Biomechanical parameters were improved in the experimental group compared to controls in Chen et al.’s (2022) study, while no significant differences were observed between experimental groups in the work of Smith et al. (2020). Moreover, macroscopic analyses were performed to examine the gap healing rate and SST integrity: in one study, no significant differences were observed between groups (Chen et al., 2022), whereas in the other study, SSTs were partially to fully intact in all groups at each time point (Smith et al., 2020).
Smith et al. (2020) analyzed the lameness grade and the level of forelimb function at trot, assessed arthroscopically the articular cartilage, biceps tendon impingement, synovium, SSTs, and lateral glenohumeral ligament, and used comfortable shoulder range of motion (CROM) and visual analog scale (VAS) pain scores to compare groups: all treatment groups exhibited significantly lower CROM and higher VAS pain scores at each time point, especially DB and RMP groups compared to controls, and a most severe synovitis, fibrosis, and biceps tendon impingement in the DB group at 3 months and least severe in AF and AM groups at 6 months after surgery.
Rambouillet cross sheep, 2–3 years old, were adopted as a preclinical model of chronic rotator cuff (RC) degeneration by Credille et al. This group realized a chronic model of full-thickness IST degeneration in the right shoulder and reconstructed the tear after 6 weeks of surgery by augmentation with a biphasic interpositional decellularized trabecular bone allograft.
A qualitative macroscopic examination was conducted on the front limbs, all major organs, scar tissue, tendon retraction, and muscle atrophy. Gross pathology evidenced a thickening of ISTs and their covering with fibrotic scar tissue after 6 weeks of tendon degeneration (Credille et al., 2023).
The risk of bias was assessed using the SYRCLE tool, and it is shown in Figure 2. The risk of bias resulted high for most items, such as items 1 (sequence generation), 3 (allocation concealment), 4 (random housing), 5 (blinding), and 6 (random outcome assessment), with respective frequencies of 100%, 92%, 100%, 92%, and 92%. There was a low risk of bias for items 2 (baseline characteristics), 8 (incomplete outcome data), 9 (selective outcome reporting), and 10 (other sources of bias) with frequencies of 69%, 77%, 100%, and 85%, respectively. Item 7 (blinding) resulted in an unclear risk of bias. Raw data are reported in Supplementary Table S1.
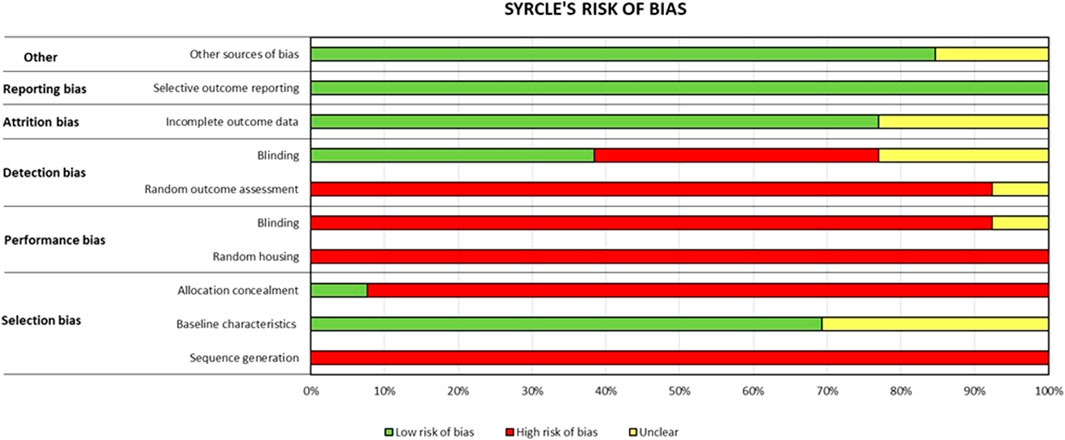
FIGURE 2. Risk of bias assessment of each in vivo paper by applying the SYRCLE tool (de Andrade et al., 2022). The frequency % of each item is reported as high risk of bias (red bar), low risk of bias (green bar), and unclear risk of bias (yellow bar).
The aim of this systematic review is to provide an overview of the literature on the efficacy of biological patches applied using the augmentation technique for RCT treatment. Decellularized patches for RC augmentation are biological (human or animal) acellular matrices (autograft, allograft, and xenograft) used for their ability to induce native tissue growth, promote RC healing, and provide biomechanical support (Zumstein et al., 2017; Cobb et al., 2022).
Our review identifies a small number of papers investigating the efficacy of decellularized patches in animal models of RCTs. This important aspect is related to the anatomical and biomechanical differences that exist between the human and animal scapulohumeral joints: in humans, the scapulohumeral joint is characterized by the most varied and extensive movements compared to other quadrupedal mammals, in which the arm does not detach from the trunk or detaches very little and in which abduction, adduction, circumduction, and rotation movements are very limited. There are relatively few in vivo studies of RC repair in the bibliography. This is because only non-human primates, albeit with ethical and legal limitations, have the same biomechanical characteristics as humans. Therefore, the in vivo RC repair model is not truly translational to what happens in humans. As evidenced, spontaneous shoulder injuries are very rare in veterinary medicine.
Another concern is related to the evaluation of efficacy; in fact, unlike clinical studies, where MRI and ultrasound imaging investigations are routinely used to assess the healing or re-tear rate, in in vivo studies, there is no gold standard for the outcome. Only three studies (two in dogs and one in rats) performed MRI and/or ultrasound evaluations, while in the remaining studies, different macroscopic, histological, and histomorphometric scoring systems and measurements were applied. For this reason, comparing studies is difficult. Despite the requirement for sample harvest, these analyses allow for the determination of a variety of aspects related to the biological response of tissues to decellularized patches (Maglio et al., 2020), such as the formation of new fibrocartilage tissue at the insertion site, enthesis maturation, implant degradation, organization, and orientation of newly deposited collagen fibers, as well as the presence of inflammatory infiltrates or the formation of new blood vessels. In addition, the evaluation of the expression levels of genes such as COL1, COL2, COL3, ACAN, and TNC after RNA extraction from tissues may provide additional useful information on RC healing before and after treatment (Yuan et al., 2022a; Yuan et al., 2022b; Pagani et al., 2023). No gait analysis is performed, except for a paper assessing the degree of lameness and functionality of the forelimbs (Smith et al., 2020); this test is performed for locomotion analysis and allows longitudinal repeated measurements, sequentially or at different time points, without the need to restrain the animal, thus ethically complying with the principles of animal reduction and refinement. Pain scales are widely used in clinical practice to quantify pain severity, such as the VAS for the subjective measurement of pain experienced by patients.
Only Smith et al. (2020) assessed pain severity using validated CROM and VAS scales in dogs although many human acute pain scales, such as the composite scale (CS), numerical rating scale (NRS), and simple descriptive scale (SDS), are adopted for animal species to establish appropriate pain therapy by ensuring animal welfare (Bufalari et al., 2007; Hielm-Björkman, 2013; Bianchi et al., 2023). Moreover, the Grimace scale is used to evaluate pain in different animal models (https://www.nc3rs.org.uk/3rs-resources/grimace-scales) (Häger et al., 2017); the use of these scales is desired and has to be improved even to accomplish one of the 3R principles in terms of refinement (Russell and Burch, 1959). The review shows that the rabbit is the most used animal model for this type of study, followed by the rat. The former is mainly used to understand the mechanisms underlying the muscle changes associated with RCTs, while the latter is used to better understand the potential mechanisms of RC injury due to the anatomical analogy with the SST tendon (Edelstein et al., 2011). Small animal models have many advantages, including ease of management, but they also have limitations in terms of clinical translation and surgical procedures. Larger animal models, such as sheep and dogs, have been used to overcome these problems, albeit to a lesser extent. Smith et al. and Chen et al. used a canine model for their studies due to the reliability of performance and biological regenerative patterns (Yang et al., 2022). However, the use of canine models is limited in Europe due to ethical and legal concerns (Martini et al., 2001). Instead, Credille et al. used sheep because the ovine shoulder girdle is similar to the human IST and SST and is a commonly used model for orthopedic research (Martini et al., 2001).
None of the included studies analyzed sex differences because they did not test the efficacy of the patches in both sexes simultaneously; the research groups either used only male animals (Ide and Tokunaga, 2018; Chen et al., 2020; Chen et al., 2022; de Lima Santos et al., 2020; He et al., 2021; Santos et al., 2022) or, to a lesser extent, female animals (Thangarajah et al., 2018; Credille et al., 2023). It is now well established that sex differences, mainly due to hormonal variations and levels, affect musculoskeletal pathologies, including tendons (Contartese et al., 2020; Tschon et al., 2021; Mondini Trissino Da Lodi et al., 2022; Salamanna et al., 2022; Salamanna et al., 2023). Clinical studies involving both men and women have shown a difference in prevalence (Razmjou et al., 2016; Sabo et al., 2021) on the extent of shoulder pathology, repair, and healing, with a higher rate of re-tear in women than in men (Collin et al., 2015; Razmjou et al., 2016).
Regarding the surgically induced injury, two types of surgery have been performed: unilateral or bilateral tendon detachment (Thangarajah et al., 2018; Chen et al., 2020; Chen et al., 2022; He et al., 2021) and the creation of tendon–bone defects (Ide and Tokunaga, 2018; Yildiz et al., 2019; de Lima Santos et al., 2020; Smith et al., 2020; Yuan et al., 2022a; Yuan et al., 2022b; Santos et al., 2022; Shim et al., 2022). In rats and rabbits, the average size of the induced tear is 5 mm in length (Ide and Tokunaga, 2018; Yuan et al., 2022a; Yuan et al., 2022b; Santos et al., 2022), whereas in dogs it is 7.5 ± 1.0 mm (Smith et al., 2020). In rabbits, several studies find that RCTs > 5 mm in diameter could not heal spontaneously, whereas no specific determinations of lesion size as critical have been reported for larger animal models (Sabo et al., 2021).
As reported in the literature, these sizes of RCTs are compatible with partial or full-thickness moderate and large-to-massive rotator cuff injuries seen in humans (Liu et al., 2011; Onay, 2013; Ditsios et al., 2014; Redler et al., 2014; Kataoka et al., 2018; Kwon et al., 2018; Zhao et al., 2022).
In humans, pathologies affecting RC are usually chronic lesions associated with myotendinous retraction, atrophy, and fatty infiltration of the muscles. In in vivo preclinical testing, there are difficulties in reproducing these degenerative alterations (Sevivas et al., 2015). Several animal models have been widely used to emphasize the etiology or pathogenesis of RC disorders and assess innovative experimental approaches. Tendon detachment in the rat leads to degenerative changes such as tendon degeneration, inflammation, and muscle atrophy comparable to those seen in the clinical setting. Moreover, in this model, Thangarajah et al. (2017) found a reduction in BMD at the enthesis that could be helpful for innovative bone fixation device evaluation. On the other hand, in a study of rat SST detachment, Barton et al. (2005) demonstrated the spontaneous healing of the tendon. In addition, unilateral or bilateral detachment has also been used as a surgical technique to create RCTs although it has been shown that the amount of scar tissue formed during the injury repair process leads to permanent gait impairment and interferes with the healing process itself (Theodossiou and Schiele, 2019). So far, the defect creation could overcome some of the limitations of the tendon detachment, leading to tears of different sizes, mimicking large-to-massive RC defects, as in the clinical setting. In addition, animal models of RC defects allow for the implantation of experimental scaffolds, such as in cases of decellularized soft membranes. Almost all authors realized an acute model of RC injury, as tendon injuries/defects are treated immediately in the same surgical session.
Thangarajah et al. (2018), Yildiz et al. (2019), Shim et al. (2022), and Credille et al. (2023) realized a chronic model of RCTs, which is a condition more like human tendinopathy. It consists of a two-step surgery to induce the RC defects and treat them within a timeframe ranging from 3 weeks to 8 weeks after the first surgery. The article by Sengupta (2013) correlated the age of rats and rabbits to that of humans and claimed that 1 month of life of a rat and rabbit equals 3 and 1 human year, respectively, since they undergo very rapid growth in the early life stages (Sengupta and Dutta, 2020). According to Abdou et al. (2019), 4 weeks from the first to the second surgery is the suitable timeframe to make a chronic RCT rabbit model. It should also be considered that, in contrast to the arthroscopic minimally invasive approach in the human clinical setting, all animal surgeries are performed in an open setting (Modi et al., 2022).
Allogeneic and xenogeneic matrices are the most commonly used decellularized patches: half of the rat studies use a commercially available human dermal matrix, GraftJacket™ (Ide and Tokunaga, 2018; Thangarajah et al., 2018), which provides support and protection for ligaments and tendons, demonstrates improvements in pain, range of motion, and strength, and has been shown in various clinical trials to be effective in the treatment of irreparable RCTs with a low risk of re-tear (Wong et al., 2010; Barber et al., 2012; Sharma et al., 2018; Johnson et al., 2020; Modi et al., 2022). The decellularization protocol for this patch is not reported, as it is a registered trademark. Smith et al. (2020) compared the efficacy of three different commercial matrices for RC tendon defects in their study: amniotic membrane scaffold (AM), decellularized human dermal allograft (AF), and bovine collagen patch (RMP). AF shows the best results in terms of collagen fiber organization, patch integration at the insertion site, fibrosis, synovitis, biceps tendon impingement, and less severe pathology compared to the other matrices tested. As with GraftJacket™, the use of a commercial product ensures lower variability, greater reproducibility, and fewer adverse effects and may have a higher chance of success for RCT repair. In addition, AF is now being investigated in two clinical trials registered at https://clinicaltrials.gov/website. The other studies used different decellularized matrices derived from the bovine pericardium (Shim et al., 2022), rabbit pubic symphysis (Chen et al., 2020), rabbit gastrocnemius (de Lima Santos et al., 2020; Santos et al., 2022), human cortical or trabecular bone (He et al., 2021; Credille et al., 2023), human tensor fascia lata (Yildiz et al., 2019), human umbilical cord (Yuan et al., 2022b; Yuan et al., 2022a), and canine IST (Chen et al., 2022), as represented in Figure 3. Their main limitation is the high variability due to both a non-standardized decellularization protocol and the different animal models used, which do not help predict their success or failure rates in a clinical trial. None of these non-commercial patches are currently being investigated for the treatment of RCTs in clinical trials.
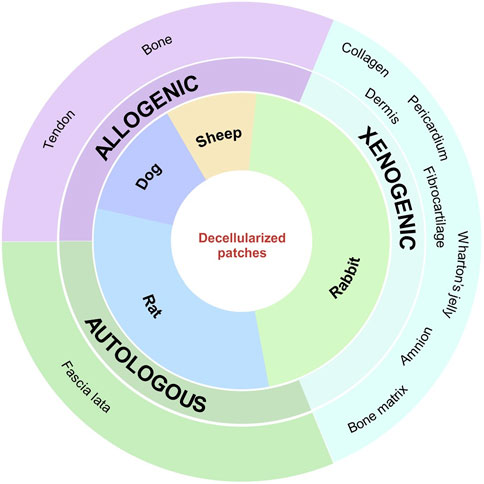
FIGURE 3. Image depicting the animal species used in the included articles, with the indication of the main types and sources of different acellular patches.
Regarding the quality of the included papers, the main limitations are related to the experimental design, as most papers do not report randomization of allocation and outcome measures, blinding, and allocation concealment, which increases the risk of bias in their studies. For example, less than half of the research teams performed an a priori analysis to determine the sample size (Thangarajah et al., 2018; Yildiz et al., 2019; de Lima Santos et al., 2020; Chen et al., 2022; Santos et al., 2022; Shim et al., 2022), although the use of a large sample size without an a priori analysis (Yuan et al., 2022a; Yuan et al., 2022b; Credille et al., 2023) could not ensure compliance with the ethical requirement of reduction (Russell and Burch, 1959).
This systematic review presents several limitations. First, we limit our search to the last 5 years although the search is performed strictly in accordance with the PRISMA guidelines, by registering our protocol in the public register PROSPERO at the beginning and by using an international tool to assess the risk of bias. The second drawback is related to the efficacy outcome measurement: our review of preclinical models shows that no gold standard technique is used, thus making studies inhomogenous and hampering the absolute recognition of the most suitable and effective decellularized patch. Inherently to the animal models, open surgery is mainly performed compared to arthroscopic procedures performed in the clinical scenario, and the animal posture is different than the human counterpart because in quadrupeds tendons are subjected to different forces and loads (Hast et al., 2014). When conducting a study aimed at investigating surgical and orthopedic problems, it is essential to carefully select an animal model that is as similar as possible to the human in terms of anatomy, affected area, and size. In this context, it is important to emphasize that no animal model, with the exception of non-human primates, can fully replicate the repair mechanisms and physiological conditions associated with human RC injury, healing, and regeneration due to the quadrupedal posture (Yang et al., 2022). Long-term follow-up is lacking for most of the reported augmentation options, and future studies with mid- and long-term follow-up are warranted.
Despite these limitations, this review provides a comprehensive overview of the use of acellular patches and their therapeutic potential in RC repair at the preclinical level, with the aim of expanding the strategies and matrices available to surgeons.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
GC: data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. MC: data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. SB: data curation, formal analysis, methodology, and writing–original draft. AR: formal analysis and writing–review and editing. LM: investigation, project administration, and writing–review and editing. GG: conceptualization, project administration, resources, and writing–review and editing. MT: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, writing–original draft, and writing–review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Ministry of Health (RF-2021-12372260) for the MODA project “Acellular matrix homologous from human dermis in combination with orthobiologic stimuli, subacromial bursa, and humeral bone marrow concentrate for augmentation of massive rotator cuff tears: therapeutic efficacy and improvements for the development of a cost-effective and ready-to-use product.”
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2024.1345343/full#supplementary-material
Abdou, M. A., Kim, G.-E., Kim, J., Kim, B.-H., Kim, Y.-K., Jeong, S.-E., et al. (2019). How long should we wait to create the goutallier stage 2 fatty infiltrations in the rabbit shoulder for repairable rotator cuff tear model? Biomed. Res. Int. 2019, 7387131–7387211. doi:10.1155/2019/7387131
Avanzi, P., Giudici, L. D., Capone, A., Cardoni, G., Lunardi, G., Foti, G., et al. (2019). Prospective randomized controlled trial for patch augmentation in rotator cuff repair: 24-month outcomes. J. Shoulder Elb. Surg. 28, 1918–1927. doi:10.1016/j.jse.2019.05.043
Barber, F. A., Burns, J. P., Deutsch, A., Labbé, M. R., and Litchfield, R. B. (2012). A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 28, 8–15. doi:10.1016/j.arthro.2011.06.038
Barton, E. R., Gimbel, J. A., Williams, G. R., and Soslowsky, L. J. (2005). Rat supraspinatus muscle atrophy after tendon detachment. J. Orthop. Res. 23, 259–265. doi:10.1016/j.orthres.2004.08.018
Bianchi, E., Leonardi, L., Breghi, G., and Melanie, P. (2023). LE SCALE DEL DOLORE COME AUSILIO NELL’INTERPRETAZIONE DELLO STATO ALGICO NEL CANE Annali della Facoltà di Medicina veterinaria, LVI/20. Avaliable at: http://www.biblio.vet.unipi.it/annali2003/267.pdf.
Bufalari, A., Adami, C., Angeli, G., and Short, C. E. (2007). Pain assessment in animals. Vet. Res. Commun. 31, 55–58. doi:10.1007/s11259-007-0084-6
Cai, Y.-Z., Zhang, C., Jin, R.-L., Shen, T., Gu, P.-C., Lin, X.-J., et al. (2018). Arthroscopic rotator cuff repair with graft augmentation of 3-dimensional biological collagen for moderate to large tears: a randomized controlled study. Am. J. Sports Med. 46, 1424–1431. doi:10.1177/0363546518756978
Chen, C., Chen, Y., Li, M., Xiao, H., Shi, Q., Zhang, T., et al. (2020). Functional decellularized fibrocartilaginous matrix graft for rotator cuff enthesis regeneration: a novel technique to avoid in-vitro loading of cells. Biomaterials 250, 119996. doi:10.1016/j.biomaterials.2020.119996
Chen, C., Shi, Q., Li, M., Chen, Y., Zhang, T., Xu, Y., et al. (2022). Engineering an enthesis-like graft for rotator cuff repair: an approach to fabricate highly biomimetic scaffold capable of zone-specifically releasing stem cell differentiation inducers. Bioact. Mater 16, 451–471. doi:10.1016/j.bioactmat.2021.12.021
Cobb, T. E., Dimock, R. A. C., Memon, S. D., Consigliere, P., Ajami, S., Imam, M., et al. (2022). Rotator cuff repair with patch augmentation: what do we know? Arch. Bone Jt. Surg. 10, 833–846. doi:10.22038/ABJS.2022.61345.3012
Collin, P., Abdullah, A., Kherad, O., Gain, S., Denard, P. J., and Lädermann, A. (2015). Prospective evaluation of clinical and radiologic factors predicting return to activity within 6 months after arthroscopic rotator cuff repair. J. Shoulder Elb. Surg. 24, 439–445. doi:10.1016/j.jse.2014.08.014
Contartese, D., Tschon, M., De Mattei, M., and Fini, M. (2020). Sex specific determinants in osteoarthritis: a systematic review of preclinical studies. Int. J. Mol. Sci. 21, 3696. doi:10.3390/ijms21103696
Credille, K. T., Wang, Z. R. C., Horner, N. S., Regan, D. P., Gadomski, B. C., Easley, J. T., et al. (2023). Biphasic interpositional allograft for rotator cuff repair augmentation is safe in an ovine model. Arthroscopy S0749-8063 (23), 1983–1997. doi:10.1016/j.arthro.2023.03.018
Dang, A., and Davies, M. (2018). Rotator cuff disease: treatment options and considerations. Sports Med. Arthrosc. Rev. 26, 129–133. doi:10.1097/JSA.0000000000000207
de Andrade, A. L. L., Garcia, T. A., Brandão, H. D. S., Sardeli, A. V., Mouraria, G. G., and Belangero, W. D. (2022). Benefits of patch augmentation on rotator cuff repair: a systematic review and meta-analysis. Orthop. J. Sports Med. 10, 232596712110711. doi:10.1177/23259671211071146
de Lima Santos, A., da Silva, C. G., de Sá Barreto, L. S., Leite, K. R. M., Tamaoki, M. J. S., Ferreira, L. M., et al. (2020). A new decellularized tendon scaffold for rotator cuff tears – evaluation in rabbits. BMC Musculoskelet. Disord. 21, 689. doi:10.1186/s12891-020-03680-w
Di Benedetto, P., Mancuso, F., Tosolini, L., Buttironi, M. M., Beltrame, A., and Causero, A. (2021). Treatment options for massive rotator cuff tears: a narrative review. Acta Biomed. Atenei Parm. 92, e2021026. doi:10.23750/abm.v92iS3.11766
Ditsios, K., Boutsiadis, A., Kapoukranidou, D., Chatzisotiriou, A., Kalpidis, I., Albani, M., et al. (2014). Chronic massive rotator cuff tear in rats: in vivo evaluation of muscle force and three-dimensional histologic analysis. J. Shoulder Elb. Surg. 23, 1822–1830. doi:10.1016/j.jse.2014.04.016
Edelstein, L., Thomas, S. J., and Soslowsky, L. J. (2011). Rotator Cuff Tears: what have we learned from animal models? J. Musculoskelet. Neuronal Interact. 11, 150–162.
Guo, S., Zhu, Y., Song, G., and Jiang, C. (2020). Assessment of tendon retraction in large to massive rotator cuff tears: a modified Patte classification based on 2 coronal sections on preoperative magnetic resonance imaging with higher specificity on predicting reparability. Arthrosc. J. Arthrosc. Relat. Surg. 36, 2822–2830. doi:10.1016/j.arthro.2020.06.023
Häger, C., Biernot, S., Buettner, M., Glage, S., Keubler, L. M., Held, N., et al. (2017). The Sheep Grimace Scale as an indicator of post-operative distress and pain in laboratory sheep. PLoS One 12, e0175839. doi:10.1371/journal.pone.0175839
Hast, M. W., Zuskov, A., and Soslowsky, L. J. (2014). The role of animal models in tendon research. Bone & Jt. Res. 3, 193–202. doi:10.1302/2046-3758.36.2000281
He, S.-K., Ning, L.-J., Yao, X., Hu, R.-N., Cui, J., Zhang, Y., et al. (2021). Hierarchically demineralized cortical bone combined with stem cell–derived extracellular matrix for regeneration of the tendon-bone interface. Am. J. Sports Med. 49, 1323–1332. doi:10.1177/0363546521994511
Hielm-Björkman, A. (2013). “Recognition and assessment of chronic pain in dogs,” in Pain management in veterinary practice (John Wiley & Sons, Ltd), 227–237. doi:10.1002/9781118999196.ch22
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43. doi:10.1186/1471-2288-14-43
Ide, J., and Tokunaga, T. (2018). Rotator cuff tendon-to-bone healing at 12 months after patch grafting of acellular dermal matrix in an animal model. J. Orthop. Sci. 23, 207–212. doi:10.1016/j.jos.2017.11.018
Johnson, S. M., Cherry, J. V., Thomas, N., Jafri, M., Jariwala, A., and McLeod, G. G. (2020). Clinical outcomes and ultrasonographic viability of GraftJacket® augmented rotator cuff repair: a prospective follow-up study with mean follow-up of forty-one months. J. Clin. Orthop. Trauma 11, S372–S377. doi:10.1016/j.jcot.2019.09.001
Kataoka, T., Kokubu, T., Muto, T., Mifune, Y., Inui, A., Sakata, R., et al. (2018). Rotator cuff tear healing process with graft augmentation of fascia lata in a rabbit model. J. Orthop. Surg. Res. 13, 200. doi:10.1186/s13018-018-0900-4
Kuhn, J. E., Dunn, W. R., Sanders, R., An, Q., Baumgarten, K. M., Bishop, J. Y., et al. (2013). Effectiveness of physical therapy in treating atraumatic full thickness rotator cuff tears. A multicenter prospective cohort study. J. Shoulder Elb. Surg. 22, 1371–1379. doi:10.1016/j.jse.2013.01.026
Kwon, D. R., Park, G.-Y., and Lee, S. C. (2018). Treatment of full-thickness rotator cuff tendon tear using umbilical cord blood-derived mesenchymal stem cells and polydeoxyribonucleotides in a rabbit model. Stem Cells Int. 2018, 1–11. doi:10.1155/2018/7146384
Lippe, J., Spang, J. T., Leger, R. R., Arciero, R. A., Mazzocca, A. D., and Shea, K. P. (2012). Inter-rater agreement of the goutallier, Patte, and warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthrosc. J. Arthrosc. Relat. Surg. 28, 154–159. doi:10.1016/j.arthro.2011.07.016
Liu, X., Manzano, G., Kim, H. T., and Feeley, B. T. (2011). A rat model of massive rotator cuff tears. J. Orthop. Res. 29, 588–595. doi:10.1002/jor.21266
Maglio, M., Salamanna, F., Brogini, S., Borsari, V., Pagani, S., Nicoli Aldini, N., et al. (2020). Histological, histomorphometrical, and biomechanical studies of bone-implanted medical devices: hard resin embedding. Biomed. Res. Int. 2020, 1–13. doi:10.1155/2020/1804630
Martini, L., Fini, M., Giavaresi, G., and Giardino, R. (2001). Sheep model in orthopedic research: a literature review. Comp. Med. 51, 292–299.
McElvany, M. D., McGoldrick, E., Gee, A. O., Neradilek, M. B., and Matsen, F. A. (2015). Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am. J. Sports Med. 43, 491–500. doi:10.1177/0363546514529644
Modi, A., Haque, A., Deore, V., Singh, H. P., and Pandey, R. (2022). Interposition GraftJacket allografts for irreparable rotator cuff tears. Bone & Jt. J. 104-B, 91–96. doi:10.1302/0301-620X.104B1.BJJ-2021-0826.R1
Mondini Trissino Da Lodi, C., Salerno, M., Merli, G., Brama, P., Jenner, F., and Filardo, G. (2022). Tendinopathy: sex bias starts from the preclinical development of tendon treatments. A systematic review. Biol. Sex. Differ. 13, 44. doi:10.1186/s13293-022-00453-z
Mori, D., Funakoshi, N., and Yamashita, F. (2013). Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy 29, 1911–1921. doi:10.1016/j.arthro.2013.08.032
Onay, U. (2013). Comparison of repair techniques in small and medium-sized rotator cuff tears in cadaveric sheep shoulders. Acta Orthop. Traumatol. Turc 47, 179–183. doi:10.3944/AOTT.2013.2935
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A. (2016). Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5, 210. doi:10.1186/s13643-016-0384-4
Pagani, S., Maglio, M., Sicuro, L., Fini, M., Giavaresi, G., and Brogini, S. (2023). RNA extraction from cartilage: issues, methods, tips. Int. J. Mol. Sci. 24, 2120. doi:10.3390/ijms24032120
Razmjou, H., Lincoln, S., Macritchie, I., Richards, R. R., Medeiros, D., and Elmaraghy, A. (2016). Sex and gender disparity in pathology, disability, referral pattern, and wait time for surgery in workers with shoulder injury. BMC Musculoskelet. Disord. 17, 401. doi:10.1186/s12891-016-1257-7
Redler, L. H., Byram, I. R., Luchetti, T. J., Tsui, Y. L., Moen, T. C., Gardner, T. R., et al. (2014). Influence of rotator cuff tear size and repair technique on the creation and management of dog ear deformities in a transosseous-equivalent rotator cuff repair model. Orthop. J. Sports Med. 2, 232596711452925. doi:10.1177/2325967114529257
Russell, W. M. S., and Burch, R. L. (1959). The principles of humane experimental technique. Methuen.
Sabo, M. T., LeBlanc, J., and Hildebrand, K. A. (2021). Patient gender and rotator cuff surgery: are there differences in outcome? BMC Musculoskelet. Disord. 22, 838. doi:10.1186/s12891-021-04701-y
Salamanna, F., Contartese, D., Borsari, V., Pagani, S., Sartori, M., Tschon, M., et al. (2023). Gender-specific differences in human vertebral bone marrow clot. Int. J. Mol. Sci. 24, 11856. doi:10.3390/ijms241411856
Salamanna, F., Contartese, D., Tschon, M., Borsari, V., Griffoni, C., Gasbarrini, A., et al. (2022). Sex and gender determinants following spinal fusion surgery: a systematic review of clinical data. Front. Surg. 9, 983931. doi:10.3389/fsurg.2022.983931
Santos, A. D. L., Silva, C. G. D., Barreto, L. S. D. S., Tamaoki, M. J. S., Almeida, F. G. D., and Faloppa, F. (2022). Automated assessment of cell infiltration and removal in decellularized scaffolds - experimental study in rabbits. Rev. Bras. Ortop. (Sao Paulo) 57, 992–1000. doi:10.1055/s-0041-1739174
Sengupta, P. (2013). The laboratory rat: relating its age with human’s. Int. J. Prev. Med. 4, 624–630.
Sengupta, P., and Dutta, S. (2020). Mapping the age of laboratory rabbit strains to human. Int. J. Prev. Med. 11, 194. doi:10.4103/ijpvm.IJPVM_530_18
Sevivas, N., Serra, S. C., Portugal, R., Teixeira, F. G., Carvalho, M. M., Silva, N., et al. (2015). Animal model for chronic massive rotator cuff tear: behavioural and histologic analysis. Knee Surg. Sports Traumatol. Arthrosc. 23, 608–618. doi:10.1007/s00167-014-3441-3
Sharma, N., El Refaiy, A., and Sibly, T. F. (2018). Short-term results of rotator cuff repair using GraftJacket as an interpositional tissue-matched thickness graft. J. Orthop. 15, 732–735. doi:10.1016/j.jor.2018.05.037
Shim, I. K., Kang, M. S., Lee, E.-S., Choi, J. H., Lee, Y. N., and Koh, K. H. (2022). Decellularized bovine pericardial patch loaded with mesenchymal stromal cells enhance the mechanical strength and biological healing of large-to-massive rotator cuff tear in a rat model. Arthroscopy 38, 2987–3000. doi:10.1016/j.arthro.2022.06.004
Smith, M. J., Bozynski, C. C., Kuroki, K., Cook, C. R., Stoker, A. M., and Cook, J. L. (2020). Comparison of biologic scaffolds for augmentation of partial rotator cuff tears in a canine model. J. Shoulder Elb. Surg. 29, 1573–1583. doi:10.1016/j.jse.2019.11.028
Thangarajah, T., Henshaw, F., Sanghani-Kerai, A., Lambert, S. M., Pendegrass, C. J., and Blunn, G. W. (2017). Supraspinatus detachment causes musculotendinous degeneration and a reduction in bone mineral density at the enthesis in a rat model of chronic rotator cuff degeneration. Shoulder Elb. 9, 178–187. doi:10.1177/1758573217696450
Thangarajah, T., Sanghani-Kerai, A., Henshaw, F., Lambert, S. M., Pendegrass, C. J., and Blunn, G. W. (2018). Application of a demineralized cortical bone matrix and bone marrow-derived mesenchymal stem cells in a model of chronic rotator cuff degeneration. Am. J. Sports Med. 46, 98–108. doi:10.1177/0363546517727512
Theodossiou, S. K., and Schiele, N. R. (2019). Models of tendon development and injury. BMC Biomed. Eng. 1, 32. doi:10.1186/s42490-019-0029-5
Tschon, M., Contartese, D., Pagani, S., Borsari, V., and Fini, M. (2021). Gender and sex are key determinants in osteoarthritis not only confounding variables. A systematic review of clinical data. J. Clin. Med. 10, 3178. doi:10.3390/jcm10143178
Veronesi, F., Borsari, V., Contartese, D., Xian, J., Baldini, N., and Fini, M. (2020). The clinical strategies for tendon repair with biomaterials: a review on rotator cuff and Achilles tendons. J. Biomed. Mater. Res. Part B Appl. Biomaterials 108, 1826–1843. doi:10.1002/jbm.b.34525
Wong, I., Burns, J., and Snyder, S. (2010). Arthroscopic GraftJacket repair of rotator cuff tears. J. Shoulder Elb. Surg. 19, 104–109. doi:10.1016/j.jse.2009.12.017
Yang, J., Kang, Y., Zhao, W., Jiang, J., Jiang, Y., Zhao, B., et al. (2022). Evaluation of patches for rotator cuff repair: a systematic review and meta-analysis based on animal studies. Bioact. Mater 10, 474–491. doi:10.1016/j.bioactmat.2021.08.016
Yildiz, F., Bilsel, K., Pulatkan, A., Kapicioglu, M., Uzer, G., Çetindamar, T., et al. (2019). Comparison of two different superior capsule reconstruction methods in the treatment of chronic irreparable rotator cuff tears: a biomechanical and histologic study in rabbit models. J. Shoulder Elb. Surg. 28, 530–538. doi:10.1016/j.jse.2018.08.022
Yuan, Z., Cao, F., Gao, C., Yang, Z., Guo, Q., and Wang, Y. (2022a). Decellularized human umbilical cord Wharton jelly scaffold improves tendon regeneration in a rabbit rotator cuff tendon defect model. Am. J. Sports Med. 50, 371–383. doi:10.1177/03635465211055722
Yuan, Z., Li, H., He, S., Gao, C., Yang, Z., Xin, W., et al. (2022b). Kartogenin releasing decellularized umbilical cord Wharton’s jelly scaffold promotes rotator cuff fibrocartilaginous interface regeneration. Mater. Des. 218, 110710. doi:10.1016/j.matdes.2022.110710
Zhao, W., Yang, J., Kang, Y., Hu, K., Jiao, M., Zhao, B., et al. (2022). Animal models of rotator cuff injury and repair: a systematic review. Tissue Eng. Part B Rev. 28, 1258–1273. doi:10.1089/ten.teb.2022.0034
Zhou, T., Han, C., and Weng, X. (2023). Present situation and development prospects of the diagnosis and treatment of rotator cuff tears. Front. Surg. 10, 857821. doi:10.3389/fsurg.2023.857821
Keywords: animal models, decellularized biological patches, efficacy, rotator cuff lesions, systematic review
Citation: Codispoti G, Carniato M, Brogini S, Romanelli A, Martini L, Giavaresi G and Tschon M (2024) Decellularized biological matrices for the repair of rotator cuff lesions: a systematic review of preclinical in vivo studies. Front. Bioeng. Biotechnol. 12:1345343. doi: 10.3389/fbioe.2024.1345343
Received: 28 November 2023; Accepted: 11 January 2024;
Published: 01 February 2024.
Edited by:
Zhong Zheng, University of California, Los Angeles, United StatesReviewed by:
Xue Xu, Capital Medical University, ChinaCopyright © 2024 Codispoti, Carniato, Brogini, Romanelli, Martini, Giavaresi and Tschon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Brogini, c2lsdmlhLmJyb2dpbmlAaW9yLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.