
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 04 June 2024
Sec. Tissue Engineering and Regenerative Medicine
Volume 12 - 2024 | https://doi.org/10.3389/fbioe.2024.1337269
This article is part of the Research Topic Clinical Translation & Commercialisation of Advanced Therapy Medicinal Products, Volume II View all 3 articles
 Yijian Ying1
Yijian Ying1 Kaiwen Cai2
Kaiwen Cai2 Xiongxiong Cai1
Xiongxiong Cai1 Kai Zhang2
Kai Zhang2 Rongzhang Qiu1
Rongzhang Qiu1 Hangtian Hu1
Hangtian Hu1 Guoqiang Jiang2
Guoqiang Jiang2 Kefeng Luo2*
Kefeng Luo2*Objective: To investigate the technical feasibility of applying a simple suture guide device to close the annulus fibrosus (AF) of the intervertebral discs (IVD).
Methods: 30 sheep functional discal units (FDUs) were obtained and subjected to mock discectomy. Mock sutures were performed using 3–0 non-absorbable sutures under a novel AF suture device following a suture procedure. The FDUs were compressed under axial loading at 1.8 mm/min and evaluated for Failure load (N).
Results: The failure loads of the hand stitching group (Group H) and suture device stitching group (Group S) were significantly higher than those of the control group (Group C) (p = 0.033; p < 0.001).
Conclusion: This study provides reasonable reasons to believe that the simple suture guide device described here is technically feasible for AF defect closure. It thus constitutes an encouraging proof of concept for the proposed device; however, it does not constitute a complete demonstration of the device’s feasibility in the clinical setting considering that the annulus closure operation is performed ex vivo on functional spinal units, as opposed to within an environment that mimics the clinical setting. To this end, confirmatory experiments will be conducted such as more multiaxial or dynamic mechanical testing, and notably performing the surgery on sheep models instead of on ex vivo functional spinal units.
Lumbar disc herniation (LDH) is one of the leading causes of low back pain (LBP) (Morlion, 2013; Risbud and Shapiro, 2014; Hartvigsen et al., 2018). LBP dramatically reduces the quality of patients’ lives, as well as posing a significant socio-economic burden (Hoy et al., 2014; Dowdell et al., 2017).
The recurrence rates have been reported to be 0%–15% for conventional nucleus pulposus (NP) removal surgery (Moliterno et al., 2010; Casal-Moro et al., 2011; Matsumoto et al., 2013; Gibson et al., 2017; Zhang et al., 2018) and 0.8%–11% for microendoscopic or foraminoscopic procedures (Choi et al., 2015; Yin et al., 2018; Park et al., 2019), both of which have high recurrence rates. Surgical treatment relieves nerve root compression by eliminating herniated tissue. However, low cellularity, non-vascularity (Vernon-Roberts et al., 2007), and poor regenerative capacity (Bailey et al., 2013) of the AF lead to a high incidence of post-surgical reherniation (Andersson, 1999; Bailey et al., 2013), potentially causing adjacent vertebral degeneration (Häkkinen et al., 2007). Patients with significant AF defects (≥6 mm) after lumbar discectomy were found to be at higher risk of symptomatic recurrence and reoperation (Miller et al., 2018). In summary, the recurrence rate of LDH is high and dramatically reduces the postoperative quality of life of surgical patients. Effective closure of AF breaks is essential.
However, Not all herniation procedures require AF closure, and the application scenario for the suture technique is in patients who are considered to have a high recurrence rate in the preoperative evaluation (e.g., young and middle-aged manual workers, patients with abundant disc content and a large intervertebral space, and patients with intervertebral space instability). Regarding whether reherniation occurs after AF closure, Suh et al. found no recurrence at 3 years after AF closure in 19 patients (Suh et al., 2015). Of course, there is also a risk of failure in AF closure. Gauthen et al. studied 254 surgical patients with LDH and found that the 2-year recurrence rate was 21% in patients without AF sutures, 10% with 1-stitch sutures, and decreased to 5% with 2-stitch sutures (Gauthen, 2005).
Annulus closure devices (ACDs) such as Xclosure, Barricaid annular closure devices, etc., have been launched, and the clinical outcomes are encouraging. However, several devices are only available for open surgery and cannot be inserted endoscopically, while others are small enough but expensive and not easily available. Therefore, we developed a novel simple suture guide device, which can be applied endoscopically while being relatively low-cost.
This study was approved by the Ethics Committee of The Affiliated Hospital of Medical School of Ningbo University (KY20201112).
In this study, we utilised an ACD fabricated by our research group (Figure 1). The ACD is divided into the grip, the needle body and the puncture head. The handle and needle body are made of 304 stainless steel with a length of 200 mm. The diameter of the internal sewing channel is 0.75 mm, and the diameter of the wire pick-up pliers channel is 2.8 mm. The length of the matching wire pick-up pliers is 280 mm.
1) Placement of suture lines: 3–0 suture silk thread (length >400 mm) is moistened and then sucked into the suture channel of the ACD with a negative pressure suction device. Reserve the thread length of 10–15 mm at the tip of the puncture needle.
2) Placement of ACD: Insert the ACD through the working channel of the microendoscopic so that the puncture needle is located in the scope view.
3) Puncture: Puncture the tip into the edge of one side of the broken AF under direct vision and push the ACD to enable the puncture needle and suture to enter the disc.
4) Clip and pull the suture line: Grasp the suture line with the wire pick-up pliers and pull it out. Exit the ACD, allowing both ends of the suture line to lie outside.
5) Repeat on the contralateral side: The ACD reload the lines and repeats the above suturing operation on the contralateral edge of AF.
6) Knot: Knot the middle two lines, pull the outer two lines to bury the knot into the disc, then knot the outer two lines, and use a long push knotter to push the knot in and tighten it.
7) Adjustment: If the AF rupture is too large or the effect of a single suture is unsatisfactory, the above suture steps might repeat to achieve multiple sutures (Figure 2; Figure 3).

Figure 2. Schematic diagram of the suturing procedure. (A) puncture; (B,C) clip and pull the suture line; (D) repeat on the contralateral side; (E,F) knot.
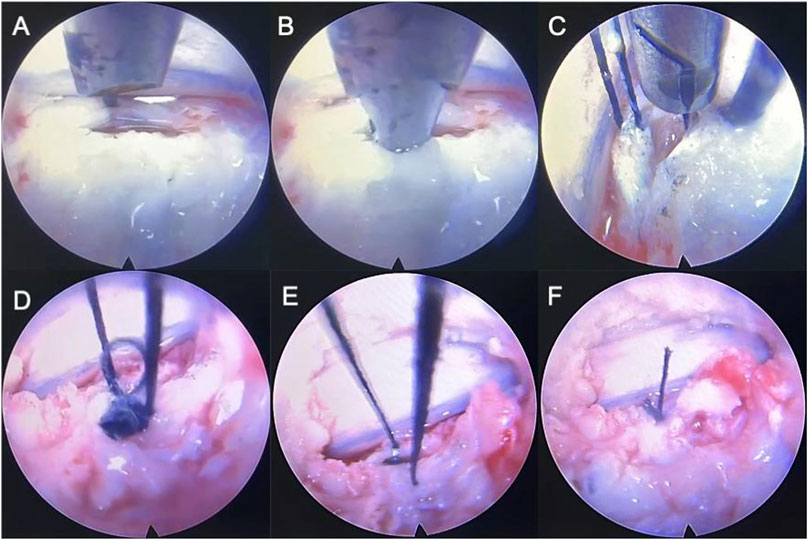
Figure 3. Simulation of a fully endoscopic suture of sheep IVD model. (A) puncture; (B) clip and pull the suture line; (C) repeat on the contralateral side; (D,E), (F) knot the middle two lines, pull the outer two lines to bury the knot into the disc, then knot the outer two lines, and use a long push knotter to push the knot in and tighten it.
Obtain healthy, similarly sized sheep lumbar vertebrae, amputate the posterior column structures, and excise excess paravertebral soft tissues. The vertebral body is cut transversely in the middle of the vertebral body with a chainsaw and divided into separate functional discal units (FDU). The FDU consisted of the lower vertebral body of the previous vertebral body, the IVD, and the upper vertebral body of the next vertebral body. The AF was incised in the anterolateral aspect of the FDU and divided into the Control group (Group C) (incised discs with no suturing), Hand stitching group (Group H) and Suture device stitching group (Group S) with 10 in each group for a total of 30.
Following completion of the suture operation, the FDU was placed in a mould to fix the upper and lower vertebrae with dental powder to ensure uniform force. Record the load-displacement curves, failure load readings (peak readings) and NP leakage (Figure 4).
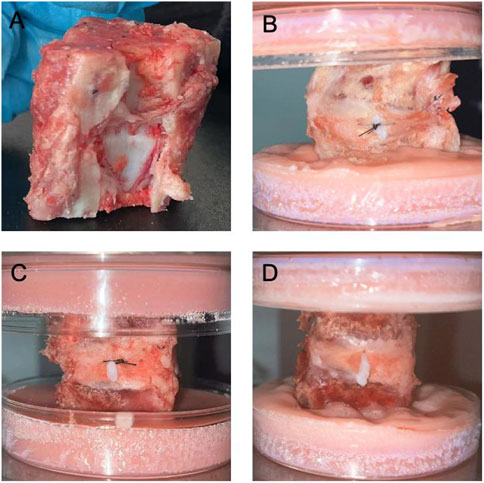
Figure 4. Sheep-isolated IVD models and NPs leakage after compression in respective groups. (A). Functional Discal Unit (FDU); (B). Suture device stitching group (Group S); (C). Hand stitching group (Group H); (D). Control group (Group C).
Statistical analyses were performed with SPSS Version 26 (IBM Corporation, Armonk, NY). Kruskal Wallis test was performed to compare the failure load of each group, and p < 0.05 was considered statistically significant.
This study included 30 FDU models. The difference in failure load between Group H and Group C was statistically significant (p = 0.033); Group S and Group C had a statistically significant difference in failure load (p < 0.001) (Table 1; Table 2).
With further research, closure of the AF notch is increasingly recognised as a valuable method of preventing IVD herniation after discectomy (Klassen et al., 2017; Thomé et al., 2018; Ardeshiri et al., 2019). AF repair aims to preserve the remaining NP tissue, minimise the recurrence of IVD herniation, and maintain the water content and pressurisation of the NP tissue. An annular suture reduces early postoperative recurrence, maintains the intervertebral space’s height, reduces the nerve root’s mechanical and inflammatory irritation, and promotes scar healing in AF (Parker et al., 2016; Li et al., 2020).
Previous AF closure strategies include mesh-like devices, sutures (Heuer et al., 2008), and patch and plug-like implants (Bron et al., 2010; Chik et al., 2013). However, these are not effective in closing AF defects. The ideal AF repair solution is tissue-engineered materials to promote AF regeneration (Du et al., 2020; Shamsah et al., 2020; Wang et al., 2020), but there are several drawbacks: 1) it is currently stuck in the experimental stage; 2) the clinical application faces various safety and ethical issues; 3) the practical application is still pending. The ACDs that have been applied in clinical practice are The Xclose Tissue Repair System, The AnchorKnot® suture-passing device, The Barricaid® Annular Closure Device, Beijing 2020 Medical Science and Technology’s Disposable Fibre Loop Suture Device (EFIT-I-II-III-IV-V, ELAS-A, SMILE, STAR). The Xclose Tissue Repair System decreased the risk of re-herniation and re-operation, favouring the short-term outcome of the patient (2 years) with no additional increase in surgical risk. However, the symptoms of back and leg pain caused by the surgery were significant (Bailey et al., 2013; Choy et al., 2018). Suturing the AF with The AnchorKnot® suture-passing device significantly reduced the rate of re-herniation; however, it was not effective in maintaining the volume of the IVDs (Bateman et al., 2016). The Barricaid® Annular Closure Device restores IVD height, reduces pain and decreases re-herniation rates without increasing the risk of epidural haematoma (Bailey et al., 2013; Klassen et al., 2017). Nevertheless, it carries risks of device prolapse, inflammation and osteophyte formation (Parker et al., 2016; Strenge et al., 2019; Miller et al., 2020). Our ACD features the following advantages: the device is designed to be utilised in minimally invasive total endoscopic spine surgery with no foreign body residue in the body except for the suture lines; Convenient and affordable, suitable for areas where healthcare costs are controlled, or other ACDs are not available.
Our study demonstrated the technical feasibility of a novel ACD for repairing AF. In the sheep IVD ex vivo experiments, the mean failure load of Group H and Group S were significantly greater than those of Group C (p = 0.033, p < 0.001). However, the mean failure load of Group H was less than that of Group S. The reason is that the Group H suture uses a rounded needle, which requires a shallow needle insertion as it will not be able to return the needle if inserted too deeply. With the long tip of the ACD, the Group S suture will entail deeper penetration of the needle, which will suture more AF tissue and increase AF strength.
There are some limitations in our study. 1) we only performed simple biomechanical assessments. 2) the reason for utilising sheep IVDs in this study is that they are biomechanically similar to human IVDs and have been utilised as an in vivo or in vitro model of IVD degeneration (Hoogendoorn et al., 2008; Paul et al., 2013). Nevertheless, there are still differences, such as the sheep IVD containing significantly less NP than the human IVD. In the pressure test, many models failed to cause NP leakage when the pressure reached the maximum range of the instrument owing to the low NP content. 3) we applied the single-axis load, failing to fully correspond to the normal situation. The reason is that torsion, shear and buckling also occur during the usual walk (Smit et al., 1997; Smit, 2002). And uniaxial compression is unlikely to cause herniation under physiological or even supraphysiological loading conditions (Berger-Roscher et al., 2017). Our next research needs to refine the multiaxial mechanical testing. On the other hand, we utilised continuous rather than cyclic loading at a specific amplitude and frequency, which is at variance with the IVD pressures of daily human behaviour. 4) the simple suture apparatus features a complicated suturing procedure requiring a high-level learning curve.
This study provides reasonable reasons to believe that the simple suture guide device described here is technically feasible for AF defect closure. It thus constitutes an encouraging proof of concept for the proposed device; however, it does not constitute a complete demonstration of the device’s feasibility in the clinical setting considering that the annulus closure operation is performed ex vivo on functional spinal units, as opposed to within an environment that mimics the clinical setting. To this end, confirmatory experiments will be conducted such as more multiaxial or dynamic mechanical testing, and notably performing the surgery on sheep models instead of on ex vivo functional spinal units.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The animal study was approved by The Ethics Committee of The Affiliated Hospital of Medical School of Ningbo University. The study was conducted in accordance with the local legislation and institutional requirements.
YY: Conceptualization, Data curation, Investigation, Methodology, Software, Writing–original draft. KC: Methodology, Supervision, Writing–original draft. XC: Data curation, Investigation, Methodology, Software, Writing–original draft. KZ: Data curation, Methodology, Writing–original draft. RQ: Data curation, Writing–original draft. HH: Data curation, Writing–original draft. GJ: Methodology, Supervision, Writing–review and editing. KL: Formal Analysis, Funding acquisition, Project administration, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Medical and Health Research Project of Zhejiang Province (Grant No: 2021427071).
We thank all the members who contributed to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Andersson, G. B. (1999). Epidemiological features of chronic low-back pain. Lancet (London, Engl.) 354 (9178), 581–585. doi:10.1016/s0140-6736(99)01312-4
Ardeshiri, A., Miller, L. E., and Thomé, C. (2019). Two-year real-world results of lumbar discectomy with bone-anchored annular closure in patients at high risk of reherniation. Eur. Spine J. 28 (11), 2572–2578. doi:10.1007/s00586-019-06036-8
Bailey, A., Araghi, A., Blumenthal, S., and Huffmon, G. V. (2013). Prospective, multicenter, randomized, controlled study of anular repair in lumbar discectomy: two-year follow-up. Spine 38 (14), 1161–1169. doi:10.1097/BRS.0b013e31828b2e2f
Bateman, A. H., Balkovec, C., Akens, M. K., Chan, A. H. W., Harrison, R. D., Oakden, W., et al. (2016). Closure of the annulus fibrosus of the intervertebral disc using a novel suture application device-in vivo porcine and ex vivo biomechanical evaluation. Spine J. 16 (7), 889–895. doi:10.1016/j.spinee.2016.03.005
Berger-Roscher, N., Casaroli, G., Rasche, V., Villa, T., Galbusera, F., and Wilke, H.-J. (2017). Influence of complex loading conditions on intervertebral disc failure. Spine 42 (2), E78–E85. doi:10.1097/BRS.0000000000001699
Bron, J. L., van der Veen, A. J., Helder, M. N., van Royen, B. J., and Smit, T. H. (2010). Biomechanical and in vivo evaluation of experimental closure devices of the annulus fibrosus designed for a goat nucleus replacement model. Eur. Spine J. 19 (8), 1347–1355. doi:10.1007/s00586-010-1384-z
Casal-Moro, R., Castro-Menéndez, M., Hernández-Blanco, M., Bravo-Ricoy, J. A., and Jorge-Barreiro, F. J. (2011). Long-term outcome after microendoscopic diskectomy for lumbar disk herniation: a prospective clinical study with a 5-year follow-up. Neurosurgery 68 (6), 1568–1575. doi:10.1227/NEU.0b013e31820cd16a
Chik, T. K., Ma, X. Y., Choy, T. H., Li, Y. Y., Diao, H. J., Teng, W. K., et al. (2013). Photochemically crosslinked collagen annulus plug: a potential solution solving the leakage problem of cell-based therapies for disc degeneration. Acta Biomater. 9 (9), 8128–8139. doi:10.1016/j.actbio.2013.05.034
Choi, K.-C., Lee, J.-H., Kim, J.-S., Sabal, L. A., Lee, S., Kim, H., et al. (2015). Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery 76 (4), 372–381. doi:10.1227/NEU.0000000000000628
Choy, W. J., Phan, K., Diwan, A. D., Ong, C. S., and Mobbs, R. J. (2018). Annular closure device for disc herniation: meta-analysis of clinical outcome and complications. BMC Musculoskelet. Disord. 19 (1), 290. doi:10.1186/s12891-018-2213-5
Dowdell, J., Erwin, M., Choma, T., Vaccaro, A., Iatridis, J., and Cho, S. K. (2017). Intervertebral disk degeneration and repair. Neurosurgery 80 (3S), S46–S54. doi:10.1093/neuros/nyw078
Du, J., Long, R. G., Nakai, T., Sakai, D., Benneker, L. M., Zhou, G., et al. (2020). Functional cell phenotype induction with TGF-β1 and collagen-polyurethane scaffold for annulus fibrosus rupture repair. Eur. Cells Mater. 39, 1–17. doi:10.22203/eCM.v039a01
Gibson, J. N. A., Subramanian, A. S., and Scott, C. E. H. (2017). A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur. Spine J. 26 (3), 847–856. doi:10.1007/s00586-016-4885-6
Häkkinen, A., Kiviranta, I., Neva, M. H., Kautiainen, H., and Ylinen, J. (2007). Reoperations after first lumbar disc herniation surgery; a special interest on residives during a 5-year follow-up. BMC Musculoskelet. Disord. 8, 2. doi:10.1186/1471-2474-8-2
Hartvigsen, J., Hancock, M. J., Kongsted, A., Louw, Q., Ferreira, M. L., Genevay, S., et al. (2018). What low back pain is and why we need to pay attention. Lancet (London, Engl.) 391 (10137), 2356–2367. doi:10.1016/S0140-6736(18)30480-X
Heuer, F., Ulrich, S., Claes, L., and Wilke, H.-J. (2008). Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J. Neurosurg. Spine 9 (3), 307–313. doi:10.3171/spi/2008/9/9/307
Hoogendoorn, R. J. W., Helder, M. N., Kroeze, R. J., Bank, R. A., Smit, T. H., and Wuisman, P. I. J. M. (2008). Reproducible long-term disc degeneration in a large animal model. Spine 33 (9), 949–954. doi:10.1097/BRS.0b013e31816c90f0
Hoy, D., March, L., Brooks, P., Blyth, F., Woolf, A., Bain, C., et al. (2014). The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann. Rheumatic Dis. 73 (6), 968–974. doi:10.1136/annrheumdis-2013-204428
Klassen, P. D., Bernstein, D. T., Köhler, H.-P., Arts, M. P., Weiner, B., Miller, L. E., et al. (2017). Bone-anchored annular closure following lumbar discectomy reduces risk of complications and reoperations within 90 days of discharge. J. Pain Res. 10, 2047–2055. doi:10.2147/JPR.S144500
Li, Z.-Z., Cao, Z., Zhao, H.-L., Shang, W.-L., and Hou, S.-X. (2020). A pilot study of full-endoscopic annulus fibrosus suture following lumbar discectomy: technique notes and one-year follow-up. Pain Physician 23 (5), E497–E506.
Matsumoto, M., Watanabe, K., Hosogane, N., Tsuji, T., Ishii, K., Nakamura, M., et al. (2013). Recurrence of lumbar disc herniation after microendoscopic discectomy. J. Neurological Surg. Part A, Central Eur. Neurosurg. 74 (4), 222–227. doi:10.1055/s-0032-1320031
Miller, L. E., Allen, R. T., Duhon, B., and Radcliff, K. E. (2020). Expert review with meta-analysis of randomized and nonrandomized controlled studies of Barricaid annular closure in patients at high risk for lumbar disc reherniation. Expert Rev. Med. Devices 17 (5), 461–469. doi:10.1080/17434440.2020.1745061
Miller, L. E., McGirt, M. J., Garfin, S. R., and Bono, C. M. (2018). Association of annular defect width after lumbar discectomy with risk of symptom recurrence and reoperation: systematic review and meta-analysis of comparative studies. Spine 43 (5), E308–E315. doi:10.1097/BRS.0000000000002501
Moliterno, J. A., Knopman, J., Parikh, K., Cohan, J. N., Huang, Q. D., Aaker, G. D., et al. (2010). Results and risk factors for recurrence following single-level tubular lumbar microdiscectomy. J. Neurosurg. Spine 12 (6), 680–686. doi:10.3171/2009.12.SPINE08843
Morlion, B. (2013). Chronic low back pain: pharmacological, interventional and surgical strategies. Nat. Rev. Neurol. 9 (8), 462–473. doi:10.1038/nrneurol.2013.130
Park, C. H., Park, E. S., Lee, S. H., Lee, K. K., Kwon, Y. K., Kang, M. S., et al. (2019). Risk factors for early recurrence after transforaminal endoscopic lumbar disc decompression. Pain Physician 22 (2), E133–E138.
Parker, S. L., Grahovac, G., Vukas, D., Vilendecic, M., Ledic, D., McGirt, M. J., et al. (2016). Effect of an annular closure device (Barricaid) on same-level recurrent disk herniation and disk height loss after primary lumbar discectomy: two-year results of a multicenter prospective cohort study. Clin. Spine Surg. 29 (10), 454–460. doi:10.1097/bsd.0b013e3182956ec5
Paul, C. P. L., Schoorl, T., Zuiderbaan, H. A., Zandieh Doulabi, B., van der Veen, A. J., van de Ven, P. M., et al. (2013). Dynamic and static overloading induce early degenerative processes in caprine lumbar intervertebral discs. PloS One 8 (4), e62411. doi:10.1371/journal.pone.0062411
Risbud, M. V., and Shapiro, I. M. (2014). Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 10 (1), 44–56. doi:10.1038/nrrheum.2013.160
Shamsah, A. H., Cartmell, S. H., Richardson, S. M., and Bosworth, L. A. (2020). Material characterization of PCL:PLLA electrospun fibers following six months degradation in vitro. Polymers 12 (3), 700. doi:10.3390/polym12030700
Smit, T. H. (2002). The use of a quadruped as an in vivo model for the study of the spine - biomechanical considerations. Eur. Spine J. 11 (2), 137–144. doi:10.1007/s005860100346
Smit, T. H., Odgaard, A., and Schneider, E. (1997). Structure and function of vertebral trabecular bone. Spine 22 (24), 2823–2833. doi:10.1097/00007632-199712150-00005
Strenge, K. B., DiPaola, C. P., Miller, L. E., Hill, C. P., and Whitmore, R. G. (2019). Multicenter study of lumbar discectomy with Barricaid annular closure device for prevention of lumbar disc reherniation in US patients: a historically controlled post-market study protocol. Medicine 98 (35), e16953. doi:10.1097/MD.0000000000016953
Suh, B.-G., Uh, J.-H., Park, S.-H., and Lee, G. W. (2015). Repair using conventional implant for ruptured annulus fibrosus after lumbar discectomy: surgical technique and case series. Asian Spine J. 9 (1), 14–21. doi:10.4184/asj.2015.9.1.14
Thomé, C., Klassen, P. D., Bouma, G. J., Kuršumović, A., Fandino, J., Barth, M., et al. (2018). Annular closure in lumbar microdiscectomy for prevention of reherniation: a randomized clinical trial. Spine J. 18 (12), 2278–2287. doi:10.1016/j.spinee.2018.05.003
Vernon-Roberts, B., Moore, R. J., and Fraser, R. D. (2007). The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine 32 (25), 2797–2804. doi:10.1097/BRS.0b013e31815b64d2
Wang, S., He, Y.-F., Ma, J., Yu, L., Wen, J.-K., and Ye, X.-J. (2020). Dynamic bioreactor culture for infiltration of bone mesenchymal stem cells within electrospun nanofibrous scaffolds for annulus fibrosus repair. Orthop. Surg. 12 (1), 304–311. doi:10.1111/os.12615
Yin, S., Du, H., Yang, W., Duan, C., Feng, C., and Tao, H. (2018). Prevalence of recurrent herniation following percutaneous endoscopic lumbar discectomy: a meta-analysis. Pain Physician 21 (4), 337–350.
Keywords: annulus fibrosus closure, suture, functional discal units, minimally invasive, discectomy
Citation: Ying Y, Cai K, Cai X, Zhang K, Qiu R, Hu H, Jiang G and Luo K (2024) Ex-vivo biomechanical evaluation of the application of a novel annulus closure device to closure of annulus fibrosus. Front. Bioeng. Biotechnol. 12:1337269. doi: 10.3389/fbioe.2024.1337269
Received: 12 November 2023; Accepted: 17 May 2024;
Published: 04 June 2024.
Edited by:
Alain A. Vertes, NxR Biotechnologies GmbH, SwitzerlandReviewed by:
Yingchao Han, Shanghai Jiao Tong University, ChinaCopyright © 2024 Ying, Cai, Cai, Zhang, Qiu, Hu, Jiang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kefeng Luo, ZHJsdW8yMDIyQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.