
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol., 03 January 2024
Sec. Biosensors and Biomolecular Electronics
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1285945
 Takayuki Ogasawara1,2*
Takayuki Ogasawara1,2* Masahiko Mukaino2,3
Masahiko Mukaino2,3 Kenichi Matsunaga4
Kenichi Matsunaga4 Yoshitaka Wada2
Yoshitaka Wada2 Takuya Suzuki5
Takuya Suzuki5 Yasushi Aoshima5
Yasushi Aoshima5 Shotaro Furuzawa5
Shotaro Furuzawa5 Yuji Kono2,5
Yuji Kono2,5 Eiichi Saitoh2
Eiichi Saitoh2 Masumi Yamaguchi1
Masumi Yamaguchi1 Yohei Otaka2
Yohei Otaka2 Shingo Tsukada1
Shingo Tsukada1Background: The importance of being physically active and avoiding staying in bed has been recognized in stroke rehabilitation. However, studies have pointed out that stroke patients admitted to rehabilitation units often spend most of their day immobile and inactive, with limited opportunities for activity outside their bedrooms. To address this issue, it is necessary to record the duration of stroke patients staying in their bedrooms, but it is impractical for medical providers to do this manually during their daily work of providing care. Although an automated approach using wearable devices and access points is more practical, implementing these access points into medical facilities is costly. However, when combined with machine learning, predicting the duration of stroke patients staying in their bedrooms is possible with reduced cost. We assessed using machine learning to estimate bedroom-stay duration using activity data recorded with wearable devices.
Method: We recruited 99 stroke hemiparesis inpatients and conducted 343 measurements. Data on electrocardiograms and chest acceleration were measured using a wearable device, and the location name of the access point that detected the signal of the device was recorded. We first investigated the correlation between bedroom-stay duration measured from the access point as the objective variable and activity data measured with a wearable device and demographic information as explanatory variables. To evaluate the duration predictability, we then compared machine-learning models commonly used in medical studies.
Results: We conducted 228 measurements that surpassed a 90% data-acquisition rate using Bluetooth Low Energy. Among the explanatory variables, the period spent reclining and sitting/standing were correlated with bedroom-stay duration (Spearman’s rank correlation coefficient (R) of 0.56 and −0.52, p < 0.001). Interestingly, the sum of the motor and cognitive categories of the functional independence measure, clinical indicators of the abilities of stroke patients, lacked correlation. The correlation between the actual bedroom-stay duration and predicted one using machine-learning models resulted in an R of 0.72 and p < 0.001, suggesting the possibility of predicting bedroom-stay duration from activity data and demographics.
Conclusion: Wearable devices, coupled with machine learning, can predict the duration of patients staying in their bedrooms. Once trained, the machine-learning model can predict without continuously tracking the actual location, enabling more cost-effective and privacy-centric future measurements.
Several studies associated with stroke rehabilitation have reported a positive correlation between the duration of daily rehabilitation therapy and functional improvements in daily activities (Langhorne et al., 1996; Kwakkel et al., 2004; Yagi et al., 2017). It has also been expected that providing additional opportunities for physical activity, such as self-training and avoiding prolonged bed rest, supports better outcomes for patients (Patel et al., 2012; Tijsen et al., 2019). However, studies have shown that stroke patients admitted to rehabilitation units often spend a majority of their day in or near their beds (West and Bernhardt, 2013; Chouliara et al., 2021). One study highlighted that during most of their time in the bedroom, patients are either inactive or engaged in passive activities such as talking, reading, or watching television (West and Bernhardt, 2013). Such healthcare design can potentially impact patient outcomes or contribute to disuse syndrome (Li et al., 2020). Given the anticipated rise in global stroke-related deaths, advancing stroke rehabilitation to support better outcomes is a pressing issue globally (Fan et al., 2023). Wearable devices offer promising benefits for monitoring the activity of stroke patients (Rosenberger et al., 2016). They are increasingly used in medical studies in real-world settings due to their cost-effectiveness and small size. However, the complexity of impairment and disability in stroke patients poses challenges, especially regarding the detection accuracy of activity (Storti et al., 2008; Carroll et al., 2012). An alternative approach involves quantitatively assessing patient inactivity by recording the duration of stroke patients staying in their bedroom (West and Bernhardt, 2012). However, researchers conducted these observations manually, which may be impractical for healthcare providers to adopt in their routine care.
Automatically measuring the duration of stroke patients staying in their bedrooms using wireless access points that receive signals from patient-worn beacons or wearable devices will be useful. However, implementing access points in indoor environments, such as hospitals, requires a significant budget to cover expansive areas. To balance the cost-effectiveness of wearable devices with the accurate location-identification capabilities of access points, we focused on using machine-learning models, which are promising for the medical applications for their prediction and classification tasks, especially when paired with Internet of Things (IoT) devices (Kumar et al., 2023; Lai et al., 2023). Once trained via supervised learning using data from wearable devices, these models can potentially predict the duration of stroke patients staying in their bedrooms without continuous reliance on the actual location identified with access points. Predicting bedroom-stay duration may also support the planning of future care programs to obtain better outcomes.
Thus, our objective was to evaluate the performance of predicting the duration of stroke patients staying in their bedrooms using wearable-derived data. We also investigated the correlation between these estimates, demographic information, and patient-activity features measured with wearable devices.
While the Global Positioning System or cellular wireless networks in wearable devices or smartphones is commonly used to identify location, it is often ineffective in indoor environments where electromagnetic shields, such as walls or doors, obstruct satellite or wireless signals (Hao et al., 2020). Attention has thus turned to indoor-positioning technologies, such as pedestrian dead reckoning (PDR), Wi-Fi, Bluetooth Low Energy (BLE), and radio frequency identification (RFID) (Wu et al., 2019; Alhomayani and Mahoor, 2020). We focused on long-term patient monitoring to measure the duration of stroke patients staying in their bedrooms for more than 24 h. Given that gyroscopes, essential for reliable PDR methods for estimating indoor location using only sensors (Hou and Bergmann, 2020), consume more power than other inertial sensors such as accelerometers, PDR is challenging for long-term monitoring. Hence, we opted for indoor-location tracking using BLE. BLE offers a balance between Wi-Fi and RFID, which is promising for reliable indoor-location tracking due to its wider wireless coverage than RFID and relatively lower power consumption than Wi-Fi, which is practical for clinical applications (Givehchian et al., 2022; Shang and Wang, 2022). There have been several reports on location-identification systems using BLE in hospitals (Rozum et al., 2019; Shipkovenski et al., 2020; Wichmann, 2022; Hadian et al., 2023), and in particular, systems that record patient locations covering their entire life space in hospitals in real-world settings have been developed (Abubeker and Baskar, 2023; Samama and Patarot, 2023). However, neither the automated recording of the duration of inpatients staying in their bedrooms nor its prediction has been demonstrated. To address this issue, we installed a system that simultaneously records both the activity and location of patients in hospital buildings. This system comprises a wearable device that sends BLE signals and access points installed on the ceilings of patients’ life spaces such as bedrooms, training rooms, and common spaces, enabling the automated recording of duration. This paper is the first study to explore the potential for machine learning to predict the duration of stroke patients staying in their bedrooms using activity data measured with a wearable device. To clarify the novelty of the present study, Table 1 summarizes the approaches of location-identification studies conducted in hospitals.
This paper is structured as follows. Section 2 presents participant details and the system designed to measure their locations and activities using BLE access points and wearable devices. This section also describes the activity features measured with wearable devices, machine-learning models, and statistical tests we used. Section 3 presents the results of activity measurements and location identifications and the evaluation of the prediction performance of the duration of stroke patients’ stay in their bedrooms. Section 4 discusses this study’s potential benefits and its limitations. Finally, Section 5 concludes the study.
In this section, we detail the participants recruited for this study, devices integrated into the experimental system, signal processing of sensor data to compute features related to patient activity, machine-learning models used to estimate the duration of stroke patients staying in their bedrooms using these features, and statistical tests we conducted to verify significance.
We enrolled 99 hemiparetic stroke inpatients from Fujita Health University (FHU) Hospital. Written informed consent was obtained from all participants. We measured data on electrocardiograms (ECGs) and chest acceleration using a wearable device. The locations or names of the rooms where BLE access points detected the device’s signals emitted from the trunk of the participant, were recorded as well. Each measurement was conducted for 2 days. Patients underwent multiple measurement sessions with their consent; in such instances, these 2-day measurements were repeated biweekly. A total of 343 measurements were conducted. We also documented participants’ demographic information such as age, height, weight, and sex, as well as the motor and cognitive categories of the functional independence measure (mFIM and cFIM), which are clinical scales to assess the degree of independence of daily activities and communication (Keith et al., 1987). The mFIM and cFIM were assessed by rehabilitation professionals. On the basis of the data-acquisition rate, explained in a later section, 284 measurements involving 85 patients were selected and analyzed.
Figure 1 illustrates the configuration of the experimental system installed at FHU Hospital. The system comprises smart clothing with a transmitter that sends BLE signals and BLE access points. This clothing was worn by the patients, and BLE access points were positioned on the ceilings of each patient’s bedroom, the training room, and common areas for comprehensive coverage (Matsunaga et al., 2019). The “hitoe” transmitter 01 (NTT DOCOMO Inc., Tokyo) was used as the wearable device (Nakata et al., 2022). The smart clothing (Toray Industries, Inc., Tokyo) was designed to be skin-tight, ensuring the transmitter maintains close contact with the patient’s body; the clothing was changed daily. This device is equipped with an ECG and a three-axis acceleration sensor. The ECG and acceleration sensor have sampling rates of 1 kHz and 25 Hz, respectively (Tsukada et al., 2019; Ogasawara et al., 2021). To transfer the data measured with the wearable device to a server (PRIMERGY TX1320 M4, Fujitsu Ltd., Tokyo), we used a wireless gateway (OpenBlocks IoT BX0, Plat'Home Co., Ltd., Tokyo) as the BLE access point. These access points were networked with the server through the local area network (LAN) of FHU Hospital. We custom-developed the applications operating on the BLE access points and server using Python 3.9. We used PostgreSQL 13.6 to set up a database on the server, where the measurement data were stored.
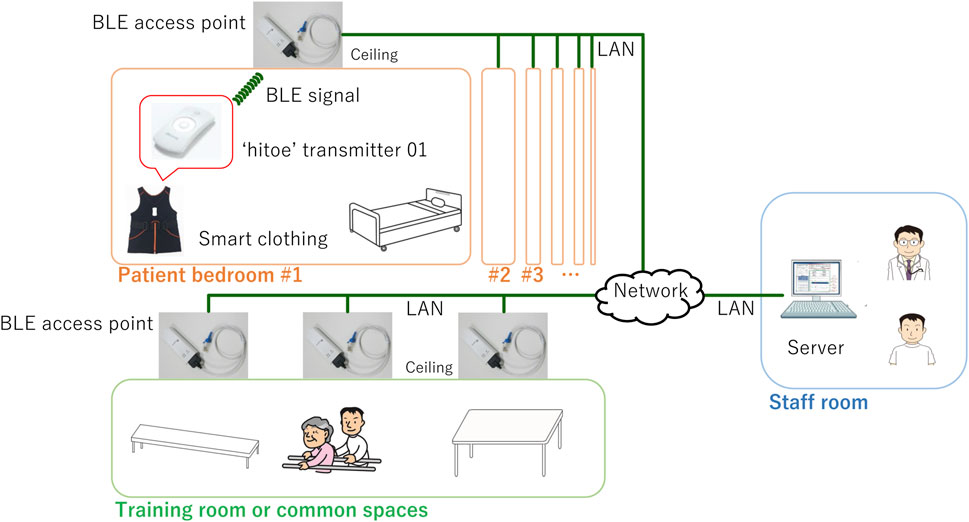
FIGURE 1. Configuration of experimental system. Wearable device, integrated into smart clothing, is worn by patient and equipped to send BLE signals. This device is used to continuously measure ECG and acceleration data of patient. BLE access points are installed throughout rehabilitation wards of FHU Hospital. Specifically, these access points are positioned on ceiling of each patient’s bedroom, training room, and common areas. These access points are designed to receive signals transmitted by wearable device worn by patient. Collected measurement data and location name are then transmitted and stored on server, which is located in staff room within hospital.
Figure 2 illustrates the placement of BLE access points in the rehabilitation wards. The FHU rehabilitation wards are located across two buildings: one building is dedicated to rehabilitation training activities, and the other is focused on the daily-life rehabilitation of inpatients. BLE signals, being generally weaker than Wi-Fi or cellular signals, present a unique challenge. Previous research indicates that BLE-signal strength attenuates significantly when a wearable device moves several meters from the access point or to an adjacent room separated by walls or doors (Faragher and Harle, 2015). This significant attenuation can lead to disconnection in wireless communication. To address this, we implemented the following two rules when deploying the access points within the buildings (Matsunaga et al., 2019). One is overlapping coverage. We aimed to keep the distance between neighboring BLE access points at about 5 m. This rule was intended to create overlapping zones of wireless coverage, ensuring that communication remains stable and continuous. Even in the spacious training areas, which lack substantial electromagnetic shielding, distances between access points were kept at less than 10 m. The other rule is dedicated bedroom coverage. We placed at least one access point in each patient’s bedroom. This rule was necessary due to the bedrooms in this hospital being electromagnetically shielded by metal-containing firewalls, which further dampen the already weak BLE signals. When an access point detects a BLE signal from a patient’s wearable device, it establishes a communication link with that device. All access points continuously monitor the received-signal-strength indicator of BLE advertise packets. If a patient moves and one access point loses the signal, another nearby access point is programmed to swiftly re-establish the connection with the wearable device to continue data acquisition. The data-acquisition rate between the BLE access points and wearable devices was found to be nearly identical to that with conventional monitoring approaches that use smartphones to connect with wearable devices (Matsunaga et al., 2019).

FIGURE 2. Location of BLE access points in rehabilitation wards. Approximately 50 BLE access points were distributed across two buildings to provide comprehensive coverage.
Table 2 lists the features of stroke-patient activity calculated from the measurement data acquired with a wearable device. The relationships between these features and the motor function of stroke patients have been explored (Arad et al., 2002; Kanai et al., 2018; Nathoo et al., 2018; Murayama et al., 2020; Mukaino et al., 2022). The experimental system described above automatically calculates these features using ECG and acceleration data. We briefly summarize this calculation process. The heart rate was calculated every minute using R-R (R-wave peak to R-wave peak) intervals detected in the ECG in the transmitter (Matsuura et al., 2022). Percent heart rate reserve (%HRR) is a well-known indicator that correlates with the amount of activity or exercise in stroke rehabilitation (Nathoo et al., 2018). The %HRR was calculated from heart rate and age in this study (Matsuura et al., 2019). Patient activity was classified into three categories: reclining, sitting/standing, and walking. This classification began with calculating the angle or declination of the acceleration sensor relative to the direction of gravitational acceleration in the sagittal plane of the patient’s trunk (Fortune et al., 2014). Using this calculated angle and a predetermined threshold, patients’ postures were initially categorized as either reclining or sitting/standing. To further delineate patient activity, the wearable device was designed to recognize periods when the patient was walking. This recognition was achieved by detecting walking steps, which were inferred from the norm of the acceleration using a rules-based algorithm (Ogasawara et al., 2016). Specifically, this allowed the system to differentiate between walking and non-walking states when the initial posture was identified as sitting/standing. This posture-classification method has been validated through studies involving both healthy individuals and clinical patients (Rauen et al., 2018; Ogasawara et al., 2023). In addition to posture classifications, moving standard averaging of trunk acceleration (MSDA) is used as an indicator of the physical-activity intensity of stroke patients (Mukaino et al., 2022).
In recordings lasting more than 24 h, one of the challenges encountered is data loss. This loss can occur due to various factors including equipment failure, limitations in data-collection capabilities, and human error (Cismondi et al., 2013; Lai et al., 2020). To mitigate the impact of missing data, we implemented a data-imputation technique that is based on averaging (Ogasawara et al., 2021; Ogasawara et al., 2023). This technique generates a time series of features of stroke-patient activity over a 24-h period with reduced data loss by calculating the averages and ratios of these features at the same time of day during several measurement-session days.
We introduce a framework for estimating the bedroom-stay durations of inpatients by using the features of stroke-patient activity calculated from data measured with wearable devices in conjunction with machine-learning methodologies. The notation
Figure 3 illustrates the training and validation processes undertaken with machine-learning models. The objective variable to be estimated in this study was the duration of stroke patients staying in their bedrooms identified with BLE access points. The explanatory variables in the models include activity data measured with a wearable device, averaged over 24 h, and demographic information of the patients. We used a range of machine-learning models for comparison, including both established and newer ones. We used random forest (RF) (Dey et al., 2020), gradient boosting (GB) (Xu et al., 2022), and support vector machine (SVM) (Xiang et al., 2020), which are frequently used models in medical studies. These models were implemented using the scikit-learn library in Python 3. We also used a deep-neural-network (DNN) specifically designed for high-performance prediction of tabular data and implemented with the pytorch-tabular library (Joseph, 2021) as well as evaluated ensembles of these models.
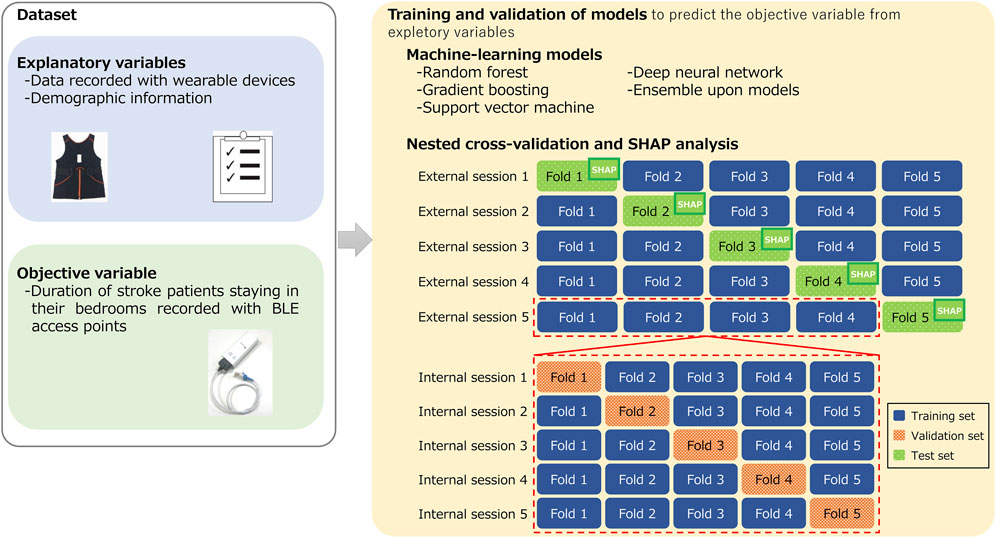
FIGURE 3. Training and validation processes undertaken with machine-learning models to estimate duration of stroke patients staying in their bedrooms.
To validate these machine-learning models, we used a nested cross-validation (CV), also known as double CV. This is a common approach to obtain robust prediction models and is effective at avoiding overfitting, which results in biased training outcomes (Cawley and Talbot, 2010; Vabalas et al., 2019). Nested CV consists of internal and external validation processes. The internal process aims to optimize the models’ hyperparameters. During this internal process, the set of hyperparameters that yield the lowest prediction errors are identified. With these optimal hyperparameters in place, the training and evaluation of the models are conducted in the external validation process using different test samples that were not involved in the internal process. CV provides a participant-independent estimate of performance for new or previously unseen participants because training and test dataset were different. In our study, samples were shuffled randomly at the starting of both internal and external CV. Both CVs were conducted with five folds. For hyperparameter optimization in the internal CV, we used AutoML from the Optuna library in Python 3 (Akiba et al., 2019).
To interpret the estimation mechanisms of the trained models, we used SHapley Additive exPlanations (SHAP) (Lundberg and Lee, 2017). SHAP is a unified framework for interpreting model predictions and enables us to investigate the contribution of each feature to the trained models. We conducted an overall feature summary analysis on the basis of SHAP.
To explore the relationships between the features calculated from the data recorded with the wearable device or demographics and the duration of stroke patients staying in their bedrooms, we conducted a statistical analysis. Initially, we conducted a Shapiro-Wilk test to assess the normality of the data distribution. If the normality of at least one of the paired values was not confirmed, a correlation analysis was carried out using Spearman’s rank correlation coefficient R (Schober and Vetter, 2020).
This section first presents the results of a preliminary analysis then the demographic information of the participants involved in this study. The correlation relationships between the features of stroke-patient activity and demographics and the duration of the stroke patients staying in their bedrooms are also detailed. Finally, the evaluation of machine-learning models for estimating the duration of a patient staying in their bedroom is discussed.
This section presents the assessment of BLE-access-point performance. Specifically, Section 3.1.1 assesses the performance of location identification using the BLE access points, and Section 3.1.2 assesses the data-acquisition rate of the BLE access points.
To confirm the location-identification performance of the BLE access points, we assessed the duration for an access point to establish a connection with a wearable device when a user enters a room where the BLE access point is located as well as the duration for the connection to be terminated when a user exits the room (refer to Supplementary Figure S1). The results indicate that the average duration required to establish a connection with four participants was 8.82 s, and the average duration for disconnection was 1.03 s. The results suggest the sufficient specificity in the location-identification capabilities of the BLE access points because these durations are on the scale of seconds and fast enough for location data with a data-rate of 1 min. For further details, refer to Supplementary Method S1 and Results S1.
Figure 4 illustrates the data-acquisition rate of participants’ location data using the BLE access points during nighttime hours (21:30 to 6:00). Nearly all patients are expected to remain in their bedrooms in this period. A 100% data-acquisition rate is achieved when there is no data loss between the wearable device and a BLE access point placed in rehabilitation wards. We set a 90% data-acquisition rate as the threshold for further analysis. Of the 343 measurements, 284 (or 82.8%) exceeded this threshold. The average data-acquisition rate among these 284 measurements was 98.0% ± 2.23%. We conducted correlation analysis and trained machine-learning models using these 284 measurements.
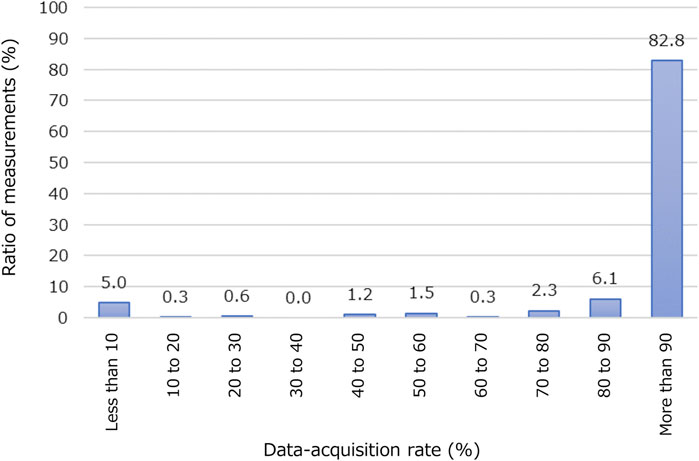
FIGURE 4. Acquisition rate of participants’ location data using BLE access points. Horizontal axis represents percentage of period when data of location information was successfully acquired at night (from 21:30 to 6:00). Vertical axis represents ratio of number of measurements in each class to total number of measurements (343 in this study).
Table 3 lists the demographics of the participants in this study. The sample size related to age, sex, height, and weight, corresponds to the number of patients (99 and 85). The sample size for other demographics corresponds to the number of measurements (343 and 284) because we repeated 2-day measurements biweekly for an identical patient, and these values often changed drastically.
Table 4 shows the correlation relationships between the features of patient activity or demographic information and the duration of the patients staying in their bedrooms. We used R because a normal distribution was not confirmed with the duration of the patients staying in their bedrooms in the Shapiro-Wilk test. There were correlation relationships between the period spent reclining and the patients’ stay in their bedrooms (R = 0.56) and between the period spent sitting or standing and the time spent in their bedrooms (R = −0.52). Both relationships were statistically significant (p < 0.001). Interestingly, neither mFIM nor cFIM, clinical indicators of the motor or cognitive abilities of stroke patients, showed any correlation (R = −0.04 and 0.04). These results suggest the importance of monitoring patients’ activities using wearable devices. Figure 5 presents examples of correlation plots. Compared with the plot of mFIM, for which correlation was not confirmed, those of the period of reclining and sitting/standing displayed observable positive or negative slopes in distribution.
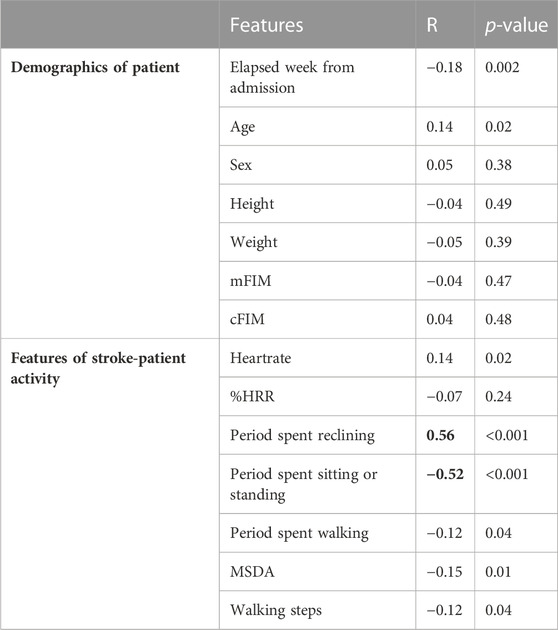
TABLE 4. Correlation relationships between features of stroke-patient activity or demographic information and duration of stroke patients staying in their bedrooms. The correlation relationships with statistical significance are highlighted in bold.
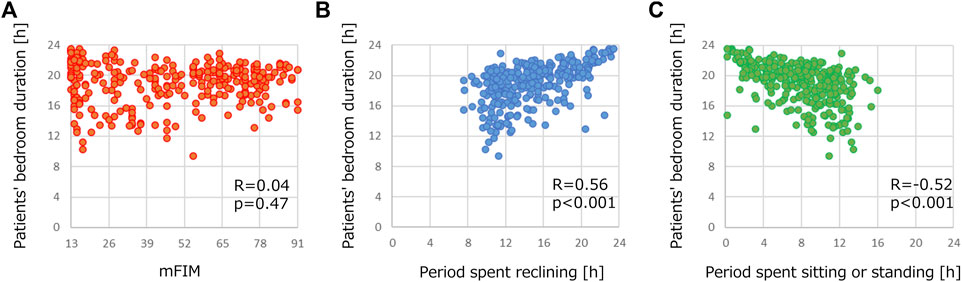
FIGURE 5. Examples of correlation plots of demographics in Table 3 and duration of stroke patients staying in their bedrooms. Correlation plots between patients’ bedroom duration and (A) mFIM, (B) period spent reclining and (C) period spent sitting or standing.
Figure 6 shows the correlation relationships between the actual and estimated durations of the patients staying in their bedrooms. The vertical axis represents the estimated duration identified with the BLE access points, and the horizontal axis represents the duration actual using machine-learning models. All results were statistically significant with p-values less than 0.001. Among the models in Figures 6A–D, R ranged from 0.51 to 0.69, with GB and RF proving the most successful in estimation (R = 0.69). Even the lowest R resulting from the DNN was close to the best R among features shown in the previous section (R = 0.51). When machine-learning models were ensembled, R further improved. Although R was 0.70 when all four models were ensembled (Figure 6E), excluding the DNN, R increased to 0.72 (Figure 6F). The results in Figure 6 support the validity of using machine learning to estimate the duration of stroke patients staying in their bedrooms.
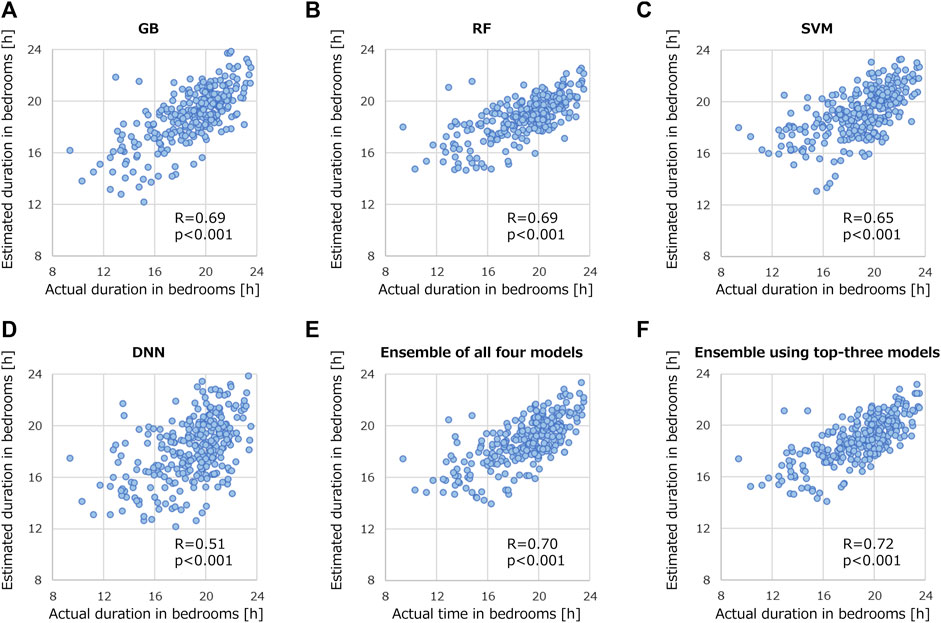
FIGURE 6. Correlation relationships between actual and estimated duration of stroke patients staying in their bedrooms. Validated models are (A) GB, (B) RF, (C) SVM, (D) DNN, (E) ensemble of all four models, and (F) ensemble using top-three models.
Figure 7 presents the results of the SHAP analysis for the four machine-learning models. The horizontal axis of the plot displays the SHAP values, which quantify the contribution of each feature to the prediction performance of a model. These SHAP values are normalized and presented as a percentage of the total contribution of all features. In all the models except the DNN, the period spent reclining emerged as the feature with the most substantial contribution to the predictions regarding the duration of stroke patients staying in their bedrooms. Other notable contributors across the models were the periods spent sitting/standing, mFIM, and cFIM. Interestingly, in the DNN, the number of walking steps showed the highest contribution, differing from the other models.
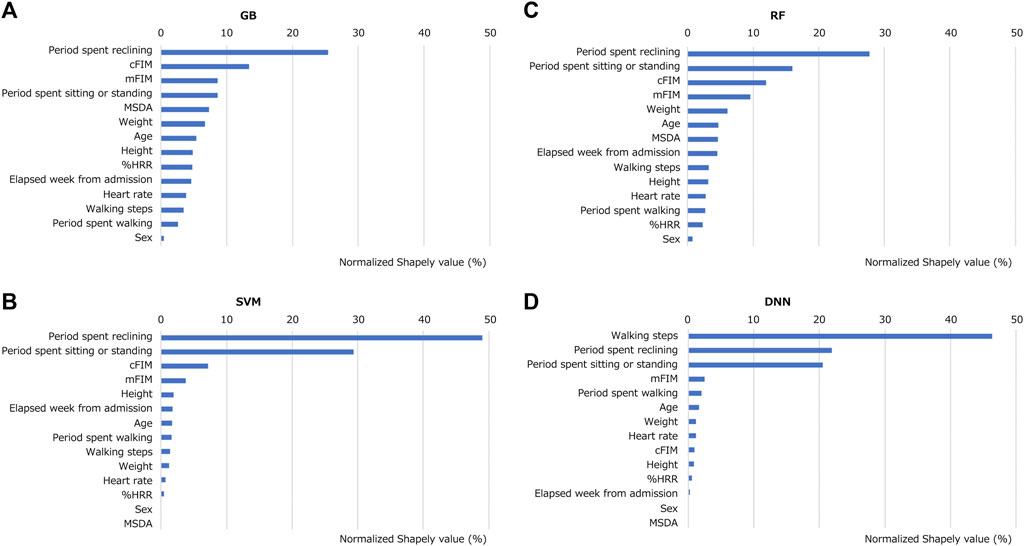
FIGURE 7. Contribution of each feature for trained models in predicting duration of stroke patients staying in their bedrooms. SHAP analysis was performed to the models of (A) GB, (B) SVM, (C) RF and (D) DNN.
We conducted another investigation into how the recording time-window impacts the prediction performance of the models. Figure 8 presents the prediction performances of GB using data measured during different segments of the day: morning (6:00 to 13:00), afternoon (13:00 to 18:00), evening (18:00 to 21:30), and lights-out period (21:30 to 6:00). Using only data recorded in the morning or afternoon (Figures 8A,B) yielded notable correlations: R of 0.64 and 0.61, respectively. However, these correlations fell short compared with that using record during 24 h R = 0.69 (Figure 6A). Predictions based on data recorded in the evening or lights-out period were even less successful, as shown in Figures 8C,D. When the time window was expanded to encompass both morning and afternoon (Figure 8E), R peaked at 0.68. In contrast, R remained low on the basis of data recorded both in the evening and lights-out period (Figure 8F). These results suggest that data collection only in daytime is helpful, and a broader recording window results in developing better models to predict the duration of stroke patients staying in their bedrooms.
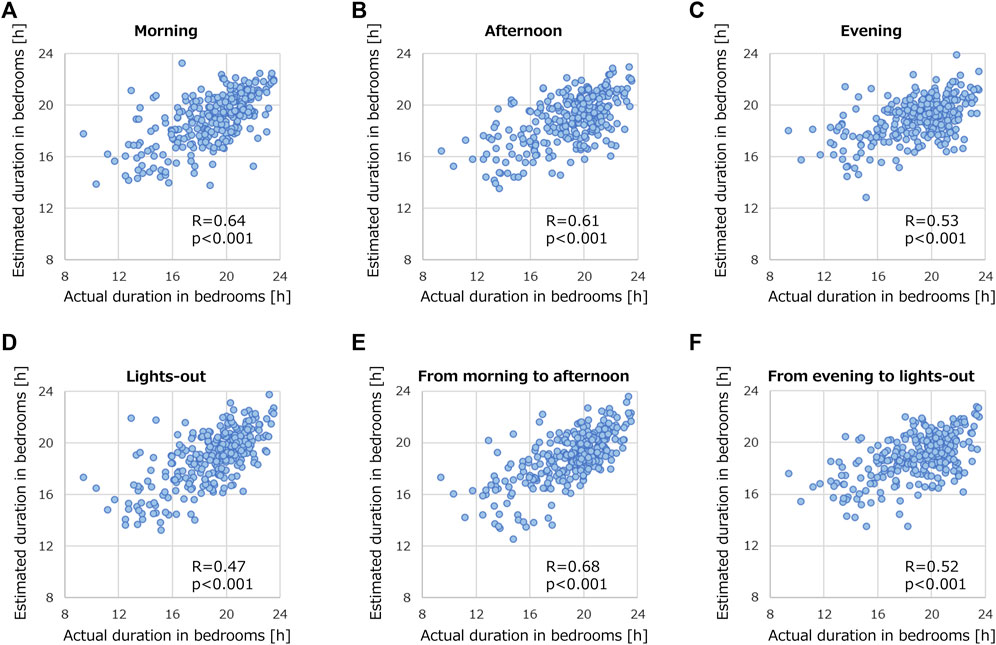
FIGURE 8. Correlation relationships between actual and estimated duration of stroke patients staying in their bedrooms by using recorded data. The data was segmented into various time periods: (A) morning (6:00 to 13:00), (B) afternoon (13:00 to 18:00), (C) evening (18:00 to 21:30), (D) lights-out (21:30 to 6:00), (E) morning to afternoon (6:00 to 18:00), and (F) evening to lights-out (18:00 to 6:00). GB was employed for this analysis.
Our study advanced prior research in two ways. We first demonstrated the automatic recording of the duration of stroke patients staying in their bedrooms by implementing a location-identification system with BLE signals. We then suggested the possibility of predicting the duration using wearable-derived data. We now discuss the potential advantages and limitations of predicting the duration of stroke patients staying in their bedrooms using a wearable device and machine learning, which will lead to future work.
We investigated features that could be related to the duration of a patient staying in their bedroom. As indicated in Table 4, significant correlations were suggested between the period spent reclining and those spent sitting/standing. In contrast, demographic information showed no significant relationship. Thus, the results suggest the importance of activity measurement with a wearable device. Those features indicating posture were important valuables for the machine-learning models, as shown in Figure 7, and were calculated from acceleration data. An acceleration-sensor module is generally power-efficient and cost-effective, making it suitable for long-term activity monitoring.
Among the clinical scales for rehabilitation, mFIM and cFIM demonstrated a lack of strong correlation with the bedroom stay duration. They are used to evaluate independence in terms of need for assistance with daily activities and communication scored by rehabilitation professionals. Previous studies have suggested that many stroke patients often spend a majority of their day in or near their beds regardless of their FIM values (West and Bernhardt, 2013; Chouliara et al., 2021). The results from our study support the tendency. However, mFIM and cFIM exhibited significant contributions in the SHAP analysis in tree-structured models, such as GB and RF. This finding suggests that these machine-learning models might assign importance to mFIM and cFIM not linearly related. In the tree-structured models, for example, these non-correlating indicators might prove valuable in deeper branches for estimation.
Some machine-learning models yielded higher R of 0.69, as shown in Figures 6A,B, compared with the best R of 0.56 derived from the patient-activity features presented in Table 4. This suggests the potential usefulness of machine-learning models to predict the duration of patients staying in their bedrooms. It also suggests the possibility of conducting more cost-effective and privacy-conscious measurements in the future, as once the model has been trained with supervised learning, it can make predictions using only the explanatory variables without further recording and storing the patients’ actual location history.
The technique used to combine prediction models demonstrated enhanced prediction performance, as shown in Figure 6. This is consistent with theoretical studies that indicate an appropriately constructed ensemble model typically offers a reduced squared error compared with individual predictive models (Krogh and Vedelsby, 1994; Ganaie et al., 2022). We experimentally applied the theory in activity monitoring. To construct an appropriate ensemble model, we eliminated the poor-prediction model, as shown in Figure 6F. The ensemble technique we used was quite basic, averaging the predicted values of the models. This suggests the potential for enhanced performance by adding more sophisticated techniques, such as attention models (Singh et al., 2023), in future research.
By using only the activity data recorded from morning to afternoon, we achieved a prediction performance (R = 0.68 in Figure 8E) that was closely comparable to using data from the entire day (R = 0.69 in Figure 6A). This suggests that the time during which activity is recorded plays a crucial role in predicting the duration of a patient staying in their bedroom. The possible reason could be that many patients tend to stay in their bedrooms from evening to the next morning, regardless of their physical condition. As wearable devices often require close body contact, which some patients find it uncomfortable, using such devices in the daytime might be a practical solution.
While using BLE access points for location tracking within the rehabilitation wards, we found that only 284 out of the 43 measurements (82.8%) achieved a data-acquisition rate exceeding 90%. This indicates that data loss remains a challenge that needs addressing, which is similar to other medical-sensor-network systems (Selvarajan et al., 2023). This data loss could be due to equipment malfunctions, such as battery depletion, interference from electromagnetic shields in the environment, and human errors. Specifically, BLE communication is prone to disruptions because of issues such as multipath fading and fluctuating conditions (Iannizzotto et al., 2023). Machine-learning techniques to impute missing health records have been proposed and could prove beneficial for studies that rely on location tracking (Getz et al., 2023).
The predictive capabilities of the machine-learning models we used, especially the DNN, still need to be addressed. The performance of the DNN (R = 0.51) was lower than those of the more common, well-established models of GB, RF and SVM. This is consistent with previous studies that the best performance with tabular data is often achieved with “shallow” models (Joseph, 2021). One potential explanation for this discrepancy might be the limited size of our dataset. Deep learning typically requires large datasets, often spanning hundreds or even thousands of examples. Fields, such as microarray studies or gene analyses, often have access to such voluminous data, but for many fields, accumulating sizable datasets remains a challenge (Park and Tamura, 2019; Lin and Tsai, 2020). Augmenting sensor data is also challenging, unlike using image data, where augmentation is a commonly used strategy to expand datasets. Thus, using an ensemble of models, as was done in this study, may be crucial with smaller datasets. Another alternative could be to transfer learning and self-supervised learning known to be tunable even with small datasets (Ebbehoj et al., 2022; Rani et al., 2023).
While we evaluated 14 features for our machine-learning models, there remain additional features yet to be explored. We could not include features related to heart rate variability (HRV) due to our experimental system’s data-transfer limitations. Given the advancements in recent studies on the capability to monitor HRV features, we anticipate their inclusion in future research (Ashokkumar et al., 2023). Assessing a patient’s in-bed status and their restlessness during rest are also important from a clinical standpoint. A broader range of sensors is essential to cover such features. Moving forward, it will be important to increase the number of sensor types and leverage sensor-fusion methodologies (Khadidos et al., 2023).
Data bias is also a limitation. All data for this study were collected in a single facility, which might not capture the variability of data from diverse demographics. To develop a more applicable model, data from multiple facilities would be crucial. However, the implementation of BLE access points across numerous locations may not be financially feasible for many hospitals due to hardware and installation costs. Given that the need for actual location data is temporal during the model-training phase, dozens of facilities would be sufficient. Follow-up studies at multiple facilities are needed to verify these possibilities.
We investigated the prediction of the duration of patients staying in their bedrooms using machine learning and wearable devices. We analyzed 284 measurements among 343, which exceeded the 90% data-acquisition rate at BLE access points. Correlation analysis suggested that a wearable device could provide indicators related to the duration of patients staying in their bedrooms, and those were the periods spent reclining and sitting/standing with an R of 0.56 and −0.52. Interestingly, mFIM or cFIM did not show any correlation. The machine-learning models RF, GB, SVM, DNN, and an ensemble of them were evaluated. The highest R among these models was 0.72, suggesting the possibility of predicting the duration of patients staying in their bedrooms from activity data and demographic information. In SHAP analysis, the machine-learning models showed high contribution to features with/without correlations, which were the period spent reclining and sitting/standing, mFIM, cFIM, and walking steps. Using only the activity data recorded from morning to afternoon, the prediction performance was R = 0.68, suggesting measurement during only the daytime may be useful. These results suggest the possibility of conducting more privacy-conscious measurements, as once the model has been trained with supervised learning, it can make predictions using only the explanatory variables without further recording and storing the patients’ actual location history.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Medical Ethics Committee of Fujita Health University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TO: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. MM: Data curation, Investigation, Methodology, Validation, Writing–original draft, Writing–review and editing. KM: Methodology, Software, Visualization, Writing–original draft, Writing–review and editing. YW: Investigation, Validation, Writing–review and editing. TS: Investigation, Writing–review and editing. YA: Investigation, Writing–review and editing. SF: Investigation, Writing–review and editing. YK: Investigation, Writing–review and editing. ES: Conceptualization, Supervision, Writing–review and editing, Methodology. MY: Software, Supervision, Writing–review and editing. YO: Methodology, Project administration, Supervision, Writing–review and editing. ST: Methodology, Project administration, Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Mr. Kenta Maruyama and Mr. Setsura Kato at NTT Basic Research Laboratories for their technical assistance in the data analysis and the nurses and therapists at Fujita Health University Hospital for their support in the experiments.
Authors TO, MY, ST, and KM were employed by the NTT Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2023.1285945/full#supplementary-material
Abubeker, K. M., and Baskar, S. (2023). A hand hygiene tracking system with LoRaWAN network for the abolition of hospital-acquired infections. IEEE Sensors J. 23 (7), 7608–7615. doi:10.1109/jsen.2023.3244582
Akiba, T., Sano, S., Yanase, T., Ohta, T., and Koyama, M. (2019). “Optuna: a next-generation hyperparameter optimization framework,” in Proceedings of the 25th ACM SIGKDD international conference on knowledge discovery and data mining, Anchorage AK USA, August 4 - 8, 2019, 2623–2631.
Alhomayani, F., and Mahoor, M. H. (2020). Deep learning methods for fingerprint-based indoor positioning: a review. J. Locat. Based Serv. 14 (3), 129–200. doi:10.1080/17489725.2020.1817582
Arad, M., Abboud, S., Radai, M. M., and Adunsky, A. (2002). Heart rate variability parameters correlate with functional independence measures in ischemic stroke patients. J. Electrocardiol. 35, 243–246. doi:10.1054/jelc.2002.37185
Ashokkumar, N., Pappa, C. K., Kumar, S., Srinivasan, D., and Vijay, M. M. (2023). “Certain investigation of optimization methods of sensor nodes in biomedical recording systems,” in 2023 9th International Conference on Advanced Computing and Communication Systems (ICACCS), 17-18 March 2023, 1175–1181.
Carroll, S. L., Greig, C. A., Lewis, S. J., McMurdo, M. E., Sniehotta, F. F., Johnston, M., et al. (2012). The use of pedometers in stroke survivors: are they feasible and how well do they detect steps? Archives Phys. Med. rehabilitation 93 (3), 466–470. doi:10.1016/j.apmr.2011.08.047
Cawley, G. C., and Talbot, N. L. (2010). On over-fitting in model selection and subsequent selection bias in performance evaluation. J. Mach. Learn. Res. 11, 2079–2107.
Chouliara, N., Fisher, R., Crosbie, B., Guo, B., Sprigg, N., and Walker, M. (2021). How do patients spend their time in stroke rehabilitation units in England? The REVIHR study. Disabil. rehabilitation 43 (16), 2312–2319. doi:10.1080/09638288.2019.1697764
Cismondi, F., Fialho, A. S., Vieira, S. M., Reti, S. R., Sousa, J. M., and Finkelstein, S. N. (2013). Missing data in medical databases: impute, delete or classify? Artif. Intell. anjaMed. 58 (1), 63–72. doi:10.1016/j.artmed.2013.01.003
Dey, S., Yoshida, T., and Schilling, A. F. (2020). Feasibility of training a random forest model with incomplete user-specific data for devising a control strategy for active biomimetic ankle. Front. Bioeng. Biotechnol. 8, 855. doi:10.3389/fbioe.2020.00855
Ebbehoj, A., Thunbo, M. Ø., Andersen, O. E., Glindtvad, M. V., and Hulman, A. (2022). Transfer learning for non-image data in clinical research: a scoping review. PLOS Digit. Health 1 (2), e0000014. doi:10.1371/journal.pdig.0000014
Fan, J., Li, X., Yu, X., Liu, Z., Jiang, Y., Fang, Y., et al. (2023). Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990–2030. Neurology 101 (2), e137–e150. doi:10.1212/wnl.0000000000207387
Faragher, R., and Harle, R. (2015). Location fingerprinting with bluetooth low energy beacons. IEEE J. Sel. Areas Commun. 33 (11), 2418–2428. doi:10.1109/jsac.2015.2430281
Fortune, E., Lugade, V. A., and Kaufman, K. R. (2014). Posture and movement classification: the comparison of tri-axial accelerometer numbers and anatomical placement. J. Biomech. Eng. 136, 051003. doi:10.1115/1.4026230
Ganaie, M. A., Hu, M., Malik, A. K., Tanveer, M., and Suganthan, P. N. (2022). Ensemble deep learning: a review. Eng. Appl. Artif. Intell. 115, 105151. doi:10.1016/j.engappai.2022.105151
Getz, K., Hubbard, R. A., and Linn, K. A. (2023). Performance of multiple imputation using modern machine learning methods in electronic health records data. Epidemiology 34 (2), 206–215. doi:10.1097/ede.0000000000001578
Givehchian, H., Bhaskar, N., Herrera, E. R., Soto, H. R. L., Dameff, C., Bharadia, D., et al. (2022). “Evaluating physical-layer ble location tracking attacks on mobile devices,” in 2022 IEEE Symposium on Security and Privacy (SP), San Francisco, CA, USA, May 22 2022 to May 26 2022, 1690–1704.
Hadian, K., Fernie, G., and Roshan Fekr, A. (2023). Development and evaluation of BLE-based room-level localization to improve hand hygiene performance estimation. J. Healthc. Eng. 2023, 1–15. doi:10.1155/2023/4258362
Hao, Z., Dang, J., Cai, W., and Duan, Y. (2020). A multi-floor location method based on multi-sensor and WiFi fingerprint fusion. IEEE Access 8, 223765–223781. doi:10.1109/access.2020.3039394
Hou, X., and Bergmann, J. (2020). Pedestrian dead reckoning with wearable sensors: a systematic review. IEEE Sensors J. 21 (1), 143–152. doi:10.1109/jsen.2020.3014955
Iannizzotto, G., Lo Bello, L., and Nucita, A. (2023). Improving BLE-based passive human sensing with deep learning. Sensors 23 (5), 2581. doi:10.3390/s23052581
Joseph, M. (2021). Pytorch tabular: a framework for deep learning with tabular data. arXiv preprint arXiv:2104.13638.
Kanai, M., Izawa, K. P., Kobayashi, M., Onishi, A., Kubo, H., Nozoe, M., et al. (2018). Effect of accelerometer-based feedback on physical activity in hospitalized patients with ischemic stroke: a randomized controlled trial. Clin. Rehabil. 32 (8), 1047–1056. doi:10.1177/0269215518755841
Keith, R. A., Granger, C. V., Hamilton, B. B., and Sherwin, F. S. (1987). The functional independence measure: a new tool for rehabilitation. Adv. Clin. Rehabil. 1, 6–18.
Khadidos, A. O., Khadidos, A. O., Selvarajan, S., and Mirza, O. M. (2023). TasLA: an innovative Tasmanian and Lichtenberg optimized attention deep convolution based data fusion model for IoMT smart healthcare. Alexandria Eng. J. 79, 337–353. doi:10.1016/j.aej.2023.08.010
Krogh, A., and Vedelsby, J. (1994). Neural network ensembles, cross validation, and active learning. Adv. neural Inf. Process. Syst. 7.
Kumar, A., Kumar, S. A., Dutt, V., Shitharth, S., and Tripathi, E. (2023). IoT based arrhythmia classification using the enhanced hunt optimization-based deep learning. Expert Syst., e13298. doi:10.1111/exsy.13298
Kwakkel, G., van Peppen, R., Wagenaar, R. C., Wood Dauphinee, S., Richards, C., Ashburn, A., et al. (2004). Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke 35, 2529–2539. doi:10.1161/01.STR.0000143153.76460.7d
Lai, D. K. H., Cheng, E. S. W., Lim, H. J., So, B. P. H., Lam, W. K., Cheung, D. S. K., et al. (2023). Computer-aided screening of aspiration risks in dysphagia with wearable technology: a Systematic Review and meta-analysis on test accuracy. Front. Bioeng. Biotechnol. 11, 1205009. doi:10.3389/fbioe.2023.1205009
Lai, X., Zhang, L., and Liu, X. (2020). Takagi-sugeno modeling of incomplete data for missing value imputation with the use of alternate learning. IEEE Access 8, 83633–83644. doi:10.1109/ACCESS.2020.2991669
Langhorne, P., Wagenaar, R., and Partridge, C. (1996). Physiotherapy after stroke: more is better? Physiother. Res. Int. 1, 75–88. doi:10.1002/pri.6120010204
Li, W., Yue, T., and Liu, Y. (2020). New understanding of the pathogenesis and treatment of stroke-related sarcopenia. Biomed. Pharmacother. 131, 110721. doi:10.1016/j.biopha.2020.110721
Lin, W. C., and Tsai, C. F. (2020). Missing value imputation: a review and analysis of the literature (2006–2017). Artif. Intell. Rev. 53 (2), 1487–1509. doi:10.1007/s10462-019-09709-4
Lundberg, S. M., and Lee, S. I. (2017). A unified approach to interpreting model predictions. Adv. neural Inf. Process. Syst. 30. doi:10.48550/arXiv.1705.07874
Matsunaga, K., Ogasawara, T., Kodate, J., Mukaino, M., and Saitoh, E. (2019). “On-site evaluation of rehabilitation patients monitoring system using distributed wireless gateways,” in 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Gremany, 23 – 27 July 2019, 3195–3198. doi:10.1109/EMBC.2019.8856963
Matsuura, H., Matsuura, M., Otaka, Y., Kagaya, H., Aoshima, Y., Suzuki, T., et al. (2019). Validity of simplified, calibration-less exercise intensity measurement using resting heart rate during sleep: a method-comparison study with respiratory gas analysis. BMC Sports Science, Medicine and Rehabilitation 11 (1), 1–8.
Matsuura, N., Kuwabara, K., and Ogasawara, T. (2022). “Lightweight heartbeat detection algorithm for consumer grade wearable ECG measurement devices and its implementation,” in 2022 44th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Glasgow, UK, July 11-15, 2022, 4299–4302.
Mukaino, M., Ogasawara, T., Matsuura, H., Aoshima, Y., Suzuki, T., Furuzawa, S., et al. (2022). Validity of trunk acceleration measurement with a chest-worn monitor for assessment of physical activity intensity. BMC Sports Sci. Med. Rehabilitation 14 (1), 104–111. doi:10.1186/s13102-022-00492-4
Murayama, I., Asai, T., Misu, S., Yamauchi, M., Miura, A., Ikemura, T., et al. (2020). Is increased “stay away from bed” time associated with improved clinical rehabilitation outcomes in Japanese rehabilitation hospitals? A prospective observational study and clinical practice. Aging Clin. Exp. Res. 32, 913–920. doi:10.1007/s40520-019-01269-5
Nakata, R., Tanaka, F., Sugawara, N., Kojima, Y., Takeuchi, T., Shiba, M., et al. (2022). Analysis of autonomic function during natural defecation in patients with irritable bowel syndrome using real-time recording with a wearable device. Plos one 17 (12), e0278922. doi:10.1371/journal.pone.0278922
Nathoo, C., Buren, S., El-Haddad, R., Feldman, K., Schroeder, E., Brooks, D., et al. (2018). Aerobic training in Canadian stroke rehabilitation programs. J. Neurologic Phys. Ther. 42 (4), 248–255. doi:10.1097/npt.0000000000000237
Ogasawara, T., Fukamachi, H., Aoyagi, K., Kumano, S., Togo, H., and Oka, K. (2021). Archery skill assessment using an acceleration sensor. IEEE Trans. Human-Machine Syst. 51 (3), 221–228. doi:10.1109/thms.2020.3046435
Ogasawara, T., Itoh, Y., Kuwabara, K., and Kasahara, R. (2016). Gait analysis using a wearable T-shirt type sensor. NTT Tech. Rev. 14 (4).
Ogasawara, T., Mukaino, M., Matsuura, H., Aoshima, Y., Suzuki, T., Togo, H., et al. (2023). Ensemble averaging for categorical variables: validation study of imputing lost data in 24-h recorded postures of inpatients. Front. Physiol. 14, 1094946. doi:10.3389/fphys.2023.1094946
Ogasawara, T., Mukaino, M., Otaka, Y., Matsuura, H., Aoshima, Y., Suzuki, T., et al. (2021). Validation of data imputation by ensemble averaging to quantify 24-h behavior using heart rate of stroke rehabilitation inpatients. J. Med. Biol. Eng. 41 (3), 322–330. doi:10.1007/s40846-021-00622-2
Park, K. S., and Tamura, T. (2019). Ubiquitous healthcare monitoring. Biomed. Eng. Lett. 9 (1), 1–2. doi:10.1007/s13534-019-00099-8
Patel, S., Park, H., Bonato, P., Chan, L., and Rodgers, M. (2012). A review of wearable sensors and systems with application in rehabilitation. J. neuroengineering rehabilitation 9 (1), 21–17. doi:10.1186/1743-0003-9-21
Rani, V., Nabi, S. T., Kumar, M., Mittal, A., and Kumar, K. (2023). Self-supervised learning: a succinct review. Archives Comput. Methods Eng. 30 (4), 2761–2775. doi:10.1007/s11831-023-09884-2
Rauen, K., Schaffrath, J., Pradhan, C., Schniepp, R., and Jahn, K. (2018). Accelerometric trunk sensors to detect changes of body positions in immobile patients. Sensors 18 (10), 3272. doi:10.3390/s18103272
Rosenberger, M. E., Buman, M. P., Haskell, W. L., McConnell, M. V., and Carstensen, L. L. (2016). Twenty-four hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med. Sci. sports Exerc. 48 (3), 457–465. doi:10.1249/mss.0000000000000778
Rozum, S., Kufa, J., and Polak, L. (2019). “Bluetooth low power portable indoor positioning system using simo approach,” in 2019 42nd International Conference on Telecommunications and Signal Processing (TSP), Budapest, Hungary, 1-3 July 2019, 228–231.
Samama, N., and Patarot, A. (2023). An IoT-based GeoData production system deployed in a hospital. Sensors 23 (4), 2086. doi:10.3390/s23042086
Schober, P., and Vetter, T. R. (2020). Correlation analysis in medical research. Anesth. Analgesia 130 (2), 332. doi:10.1213/ane.0000000000004578
Selvarajan, S., Manoharan, H., Iwendi, C., Alsowail, R. A., and Pandiaraj, S. (2023). A comparative recognition research on excretory organism in medical applications using artificial neural networks. Front. Bioeng. Biotechnol. 11, 1211143. doi:10.3389/fbioe.2023.1211143
Shang, S., and Wang, L. (2022). Overview of WiFi fingerprinting-based indoor positioning. IET Commun. 16 (7), 725–733. doi:10.1049/cmu2.12386
Shipkovenski, G., Kalushkov, T., Petkov, E., and Angelov, V. (2020). “A beacon-based indoor positioning system for location tracking of patients in a hospital,” in 2020 International Congress on Human-Computer Interaction, Optimization and Robotic Applications (HORA), Ankara, Turkey, 26 – 27 June 2020, 1–6.
Singh, S., Kumar, M., Kumar, A., Verma, B. K., and Shitharth, S. (2023). Pneumonia detection with QCSA network on chest X-ray. Sci. Rep. 13 (1), 9025. doi:10.1038/s41598-023-35922-x
Storti, K. L., Pettee, K. K., Brach, J. S., Talkowski, J. B., Richardson, C. R., and Kriska, A. M. (2008). Gait speed and step-count monitor accuracy in community-dwelling older adults. Med. Sci. sports Exerc. 40 (1), 59–64. doi:10.1249/mss.0b013e318158b504
Tijsen, L. M., Derksen, E. W., Achterberg, W. P., and Buijck, B. I. (2019). Challenging rehabilitation environment for older patients. Clin. interventions aging 14, 1451–1460. doi:10.2147/cia.s207863
Tsukada, Y. T., Tokita, M., Murata, H., Hirasawa, Y., Yodogawa, K., Iwasaki, Y. K., et al. (2019). Validation of wearable textile electrodes for ECG monitoring. Heart vessels 34, 1203–1211. doi:10.1007/s00380-019-01347-8
Vabalas, A., Gowen, E., Poliakoff, E., and Casson, A. J. (2019). Machine learning algorithm validation with a limited sample size. PloS one 14 (11), e0224365. doi:10.1371/journal.pone.0224365
West, T., and Bernhardt, J. (2012). Physical activity in hospitalised stroke patients. Stroke Res. Treat. 2012. doi:10.1155/2012/813765
West, T., and Bernhardt, J. (2013). Physical activity patterns of acute stroke patients managed in a rehabilitation focused stroke unit. BioMed Res. Int. 2013, 1-8. doi:10.1155/2013/438679
Wichmann, J. (2022). Indoor positioning systems in hospitals: a scoping review. Digit. Health 8, 205520762210816. doi:10.1177/20552076221081696
Wu, Y., Zhu, H. B., Du, Q. X., and Tang, S. M. (2019). A survey of the research status of pedestrian dead reckoning systems based on inertial sensors. Int. J. Automation Comput. 16, 65–83. doi:10.1007/s11633-018-1150-y
Xiang, Y., Wang, J., Tan, G., Wu, F. X., and Liu, J. (2020). Schizophrenia identification using multi-view graph measures of functional brain networks. Front. Bioeng. Biotechnol. 7, 479. doi:10.3389/fbioe.2019.00479
Xu, Z., Guo, K., Chu, W., Lou, J., and Chen, C. (2022). Performance of machine learning algorithms for predicting adverse outcomes in community-acquired pneumonia. Front. Bioeng. Biotechnol. 10, 903426. doi:10.3389/fbioe.2022.903426
Keywords: bedroom-stay duration, location tracking, rehabilitation, stroke, machine learning, wearable sensors
Citation: Ogasawara T, Mukaino M, Matsunaga K, Wada Y, Suzuki T, Aoshima Y, Furuzawa S, Kono Y, Saitoh E, Yamaguchi M, Otaka Y and Tsukada S (2024) Prediction of stroke patients’ bedroom-stay duration: machine-learning approach using wearable sensor data. Front. Bioeng. Biotechnol. 11:1285945. doi: 10.3389/fbioe.2023.1285945
Received: 30 August 2023; Accepted: 11 December 2023;
Published: 03 January 2024.
Edited by:
Guozhen Liu, The Chinese University of Hong Kong, ChinaCopyright © 2024 Ogasawara, Mukaino, Matsunaga, Wada, Suzuki, Aoshima, Furuzawa, Kono, Saitoh, Yamaguchi, Otaka and Tsukada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takayuki Ogasawara, dGFrYXl1a2kub2dhc2F3YXJhQG50dC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.