- 1Transfusion Medicine and Regeneration Medicine, Azienda Ospedaliera SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy
- 2Department of Plastic and Reconstructive Surgery, Azienda Ospedaliera SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy
- 3Neuro-Rehabilitation Unit, Rehabilitation Department, Azienda Ospedaliera Nazionale SS Antonio e Biagio e Cesare Arrigo-Alessandria, Alessandria, Italy
Background: Biological dressings with non-transfusion blood components are among the treatments available for pressure ulcers (PUs). Biological dressings contain active concentrated pro-regenerative molecules that can modify and switch off local inflammatory pathways. This re-establishes the physiological homing, which results in healing. In our study, we used a biological component obtained by ultrafiltration of plasma-platelet concentrate: protein-enriched filtered platelet-rich plasma (PEFPRP) with a higher platelet and higher plasma protein concentration. We tested whether treatment with PEFPRP could improve healing in advanced-stage pressure ulcers with a large surface area. All the patients in this study had a surgical indication but were not able to undergo surgery for various reasons.
Materials and methods: Ten patients with severe neurological disability and advanced-stage sacral pressure ulcers were treated with allogenic PEFPRP. The mean lesion surface area at T0 was 13.4 cm2 ( ± 9.8 SD). PEFPRP was derived from allogenic plasma-platelet apheresis that had been pre-ultrafiltered with a ProSmart™ filter (Medica, Italy) to obtain a concentration after filtration of the plasma protein (12–16 g/dL) and platelet (1–1.2 x 106 microL).
Results and Conclusion: All cases showed a reduction in the surface area of the pressure ulcer and in the Pressure Ulcer Scale for Healing (PUSH) score. The mean reduction values at week 6 were as follows: −52% for surface area and −21% for PUSH. Rapid wound healing is fundamental to avoid infections and improve patients’ quality of life. This blood component builds new tissue by creating a new extracellular matrix. This, in turn, promotes rapid restoration of the three-dimensional structure of the tissue necessary for healing deeper wounds. PEFPRP shrinks the PU and improves its morphological features (reducing undermining and boosting granulation tissue). PEFPRP also promotes tissue restoration, obtaining an optimal scar. It is a safe and feasible treatment, and these preliminary results support the use of PEFPRP in the treatment of pressure ulcers. PEFPRP dressings could be integrated in the standard treatment of advanced-stage PU.
1 Introduction
Pressure ulcers (PUs) are localized skin injuries usually found on bony prominences. They may occur in the superficial skin or in the underlying tissue. PUs are caused by hypoxic damage due to pressure and/or shear stress on the skin. They represent a medical, social, and economic burden that significantly affects the patient’s condition and the rehabilitation programs. The treatment of PUs is expensive and requires multidisciplinary management (Hill et al., 2022). The most common risk factors for the development of PUs are extrinsic and intrinsic hypoxemia (e.g., poor mobilization, pressure or shear force, smoking, and COPD), changes in sensitivity, poor nutrition, urinary and fecal incontinence, and overall patient status (age, hematological diseases, infectious diseases, and cognitive deficiency). The NPUAP/EPUAP classification stages the severity of the lesion and defines the treatment for each stage (Jan et al., 2019). Nutritional status in subjects at risk and in those with PUs must be monitored; in fact, poorly nourished patients often show a major risk of developing PUs and a delay in the healing process (Saghaleini et al., 2018; Munoz and Posthauer, 2022). Patient mobilization is fundamental for the treatment and prevention of PUs; weight distribution and the use of high skin protection mattresses and cushions help improve comfort and hygiene (Shi et al., 2021). PU treatment consists of debridement, cleaning, treatment of underlying infection (if needed), and dressing. In clinical practice, we often use dressings containing hydrocolloid, hydrogel, calcium alginate, and foam. If these are not effective, and the PU worsens (with new formation of necrotic tissue, tunneling, and undermining), physical therapies (negative pressure and pulsed electrical stimulation) and surgery are other options (Moore and Patton, 2019). Biological dressing is also an available treatment for PUs, specifically dressings containing non-transfusion blood components (platelet gel) (Aprili et al., 2013). Chronic ulcers of different etiologies have in common a prolonged inflammatory response. It is possible to halt this process by providing platelet-derived growth factors (GFs) and plasma proteins to the ulcer to facilitate the tissue healing process (Qu et al., 2021; Singh et al., 2021). Ten patients with large surface-area PUs were treated with protein-enriched filtered platelet-rich plasma (PEFPRP) instead of surgery (Mazzucco et al., 2021). Surgery is the elective treatment for lesions at this stage, but where elective surgical treatment is not possible, whether on medical grounds or due to patient refusal, an alternative is needed.
The EPUAP 2019 guidelines suggest treatment with platelet gel for advanced-stage PUs (grade of recommendation: B1); the rational biological use of topical blood components is to modify and switch off the local inflammatory response by providing concentrates of regenerating active molecules (GFs, anti-inflammatory interleukins, fibrin, and extracellular matrix proteins) (Olczyk et al., 2014). This would lead to healing of the lesion through the stimulation of cellular physiological homing and angiogenesis (Anitua et al., 2012; Collins et al., 2021). For this study, we used PEFPRP obtained from the ultrafiltration of allogenic blood components (plasma-platelet apheresis) that contains five times the platelet concentration compared to the baseline, that is not aggressive on platelets that preserve their structure and function, and that has a high plasma protein concentration (approximately two times the usual concentration). PEFPRP collects and concentrates platelets and all the proteins present in the plasma and released by platelets (exosomal component and other), ensuring the complete and total collection of all PRP molecules that are normally lost during centrifugation and preparation by other methods (Dhurat and Sukesh, 2014; Everts et al., 2020). The purpose was to provide GFs derived from concentrated platelets to stimulate cells and plasma proteins to regenerate the extracellular matrix. Allogenic blood components were chosen (Van der Bijl et al., 2019; Liao et al., 2020; Akbarzadeh et al., 2021; Saputro et al., 2022); we collected the plasma-platelet apheresis from the donor to have more products available for treatment (large surface lesions) and have a more standardized equivalent product for all the patients enrolled in the study (treatment period 8 weeks). In addition, the PEFPRP was ready for use, without having to collect blood from debilitated patients for each treatment. The purpose of this study was to determine the safety and feasibility of treating PUs with PEFPRP as an alternative to plastic surgery. In PEFPRP, there are 30 upregulated proteins that are involved in cytoskeleton organization, regulation of proteolysis, and cellular response to cytokine stimulus. These proteins were classified by their function according to keywords related to the healing process of damaged tissues and corresponding to pro-inflammation, anti-inflammation, wound healing, clot stabilization, and anti-microbial and other plasma components such as complement system, cell–matrix adhesion, and immunoglobulins (Piccin et al., 2017; Cecerska-Heryć et al., 2022). The clot stabilization and cell–matrix adhesion proteins (fibrinogen, fibrin, fibronectin, and thrombin) contribute to a three-dimensional environment that is a crucial condition for driving cell–cell and protein–protein interactions and achieving tissue regeneration and healing. PEFPRP is a new topical hemocomponent with multiple functions since it provides molecules stimulating biological mechanisms of resident cells and reconstruction of the extracellular matrix.
Our previous studies had shown that patients with chronic non-healing wounds showed a substantial improvement when treated with the PLT gel in an average time of 10 weeks (Mazzucco et al., 2004); one of the purposes of the study was to evaluate if the treatment with PEFPRP improved the healing time frame (8 weeks) (Wallace et al., 2023) even on large wounds. Eight weeks was considered a sufficient time to assess reduction in wound size and/or possible complications. Reduction in lesion area, with particular attention to physical and morphological characteristics such as exudate and basal tissue (epithelial or granulation tissue, slough, and necrosis), was the principal indicator of the study output.
2 Materials and methods
For this prospective study, 10 patients (limited number due to difficulties in enrollment because of restrictive inclusion criteria including non-acceptance/non-eligibility to the surgical approach) were enrolled in the Neurorehabilitation Department of the Physical Medicine and Rehabilitation—Hospital SS. Antonio e Biagio di Alessandria (Piedmont); the patients had large pressure ulcers, which were already treated for at least 6 weeks with conventional dressings without result. The study took 1 year for enrollment (the study was postponed due to COVID-19).
Patients selected for treatment were all candidates for reconstructive surgery but not feasible because some were at risk of surgical complications or were non-compliant (refusal of surgery). The mean age of the patients was 65 years (SD ± 9.67). The study was conducted in accordance with the Helsinki Declaration and was approved by the local ethics committee (prot.ASO19.07CE 2019). The subjects were given a detailed explanation about the study and signed three informed consent forms (one to use allogenic blood components, one for the data collection, and one for the privacy policy). The study enrollment included a medical examination carried out by a specialist in physical medicine and rehabilitation, an evaluation of the PU, and staging (EPUAP) carried out by a plastic surgeon. Each patient showed advanced-stage sacral PUs (8 subjects: stage IV and 2 subjects: stage III). The patients presented with neurological disability with different etiologies: three severe brain injuries, two spinal cord injuries, and five critical illness polyneuropathies. Admission assessment scales were issued by the rehabilitation specialist. The mean Barthel Index (BI) was 3.2 ( ± 3.8 SD—BI from 0 to 20), and the mean Functional Independence Measure (FIM) was 45.4 ( ± 21.7 SD—FIM from 18 to 126). At the beginning of the study, the patients were clinically stable (mean hemoglobin 11.7 mg/dL ± 1, 5 SD—mean WBC 7862/mm3 +/− 2379 SD) and had negative SARS-CoV-2 swabs.
2.1 Nutritional support and high skin protection surfaces
The nutritional status was evaluated during hospitalization. We monitored the serum protein and albumin levels, and nutritional counseling was given to ensure adequate protein intake (in accordance with EPUAP guidelines). Optimal skin protection mattresses and cushions were provided to reduce pressure and shear stress on the skin.
2.2 Preparation and treatment with protein-enriched filtered platelet-rich plasma (PEFPRP)
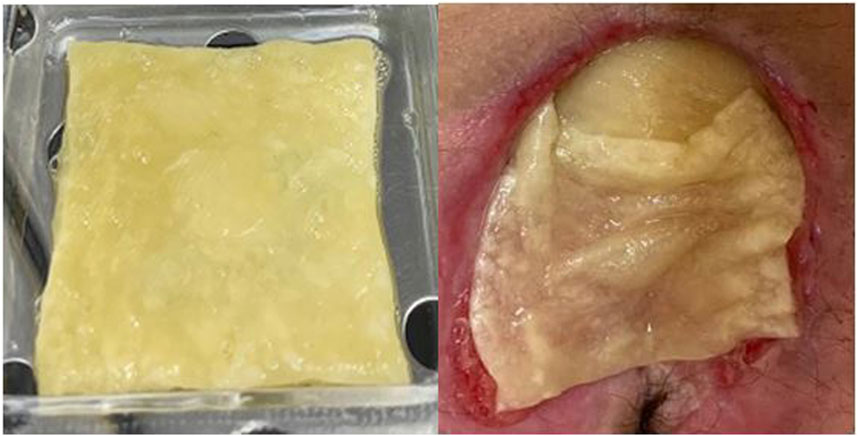
PEFPRP was derived from plasma-platelet apheresis collected at the Blood Transfusion Center, Alessandria Hospital, Italy. This blood component was obtained according to standard transfusion procedures (DM 2 November 2015—Italian Law). The platelet concentrate was standardized at a concentration of 1 × 106/microL with plasma that had been prefiltered using a ProtSmart™ filter (Medica SpA, Medolla, Italy). The plasma protein concentration after filtration was between 12 and 16 g/dL. The PEFPRP obtained was divided into 8-mL samples and stored in blood bank refrigerators at a temperature of −40°C. Type I collagen and hyaluronic acid (Fidia Farmaceutici S.p.A,, Italy) matrices were used to spread and stabilize the product over the lesion. The biological dressing with PEFPRP was applied to infection-free lesions aiming to cover most of the PU surface (Figure 1) and was reapplied every 3 days, and the treatment lasted for 8 weeks (56 days).
2.3 Surface evaluation and statistical analysis
Before PEFPRP treatment, all the PUs were surgically debrided to remove necrotic tissue. After this procedure, they were staged. The bed of the PUs was evaluated for local bacterial infection (GRAM+ and GRAM-) with fluorescent light technology (MolecuLight Smith and Nephew, Canada). Photographs were taken to provide evidence of changes on the lesion surface; the size of the lesion was measured with a digital program (ImageJ.exe). The data were analyzed with Minitab. The Pressure Ulcer Scale for Healing (PUSH tool—NPUAP) was used for assessment and monitoring. PUSH is a widely used tool developed by the National Pressure Ulcer Advisory Panel (NPUAP) that grades pressure ulcers based on wound size, wound bed tissue type, and exudate amount. PUSH and the surface area were measured weekly until the end of the treatment. The data obtained were analyzed using descriptive statistics, and the results were expressed as sums or percentages (Thomas et al., 1997; Stotts et al., 2001; Pressure Ulcer Scale for Healing PUSH, 2013; Choi et al., 2016; Rennie et al., 2019).
3 Results
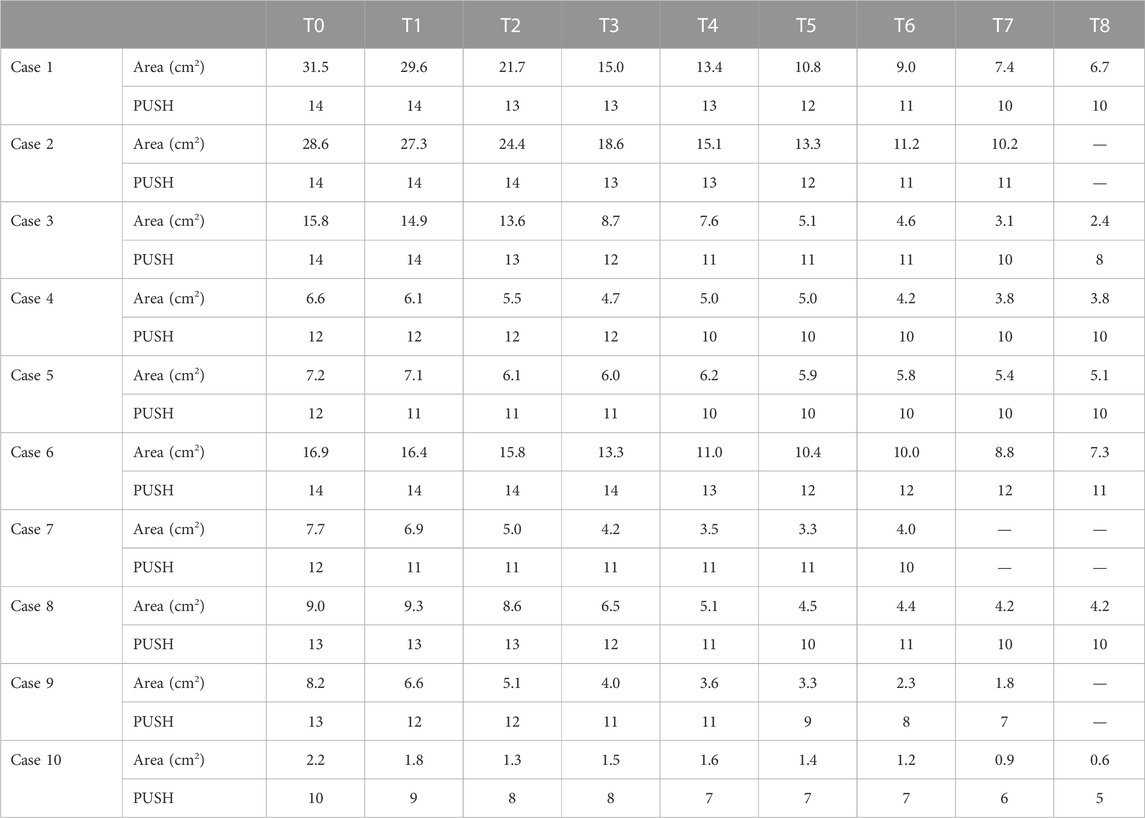
Of the 10 patients evaluated, 7 were treated for 8 weeks (56 days), 2 for 7 weeks (49 days), and 1 for 6 weeks (42 days). At T0, the mean lesion superficial area was 13.4 cm2 ( ± 9.8 SD), and the mean PUSH was 12.8 ( ± 1.3 SD). All cases showed a reduction in the PU surface area (Figure 2) and PUSH (Figure 3). The mean reduction at week 6 was −52% for the surface area and −21% for PUSH. For the statistical analysis, we considered that consistency in our sample was obtained at Week 6. In the patients treated for 7 or 8 weeks, further reductions in surface area and PUSH were obtained (mean reduction in wound surface area: 13.4–3 cm2). None of the patients suffered any side effects. At T6, the mean lesion surface area was 5.7 cm2 ( ± 3.3 SD), and the mean PUSH was 10.1 ( ± 1.5 SD). At discharge from the neurorehabilitation department, the assessment scales showed a mean Barthel Index score of 11.8 ± SD 6.2 and a mean FIM of 89.3 ± 33.2 (Table 1; Figure 4).
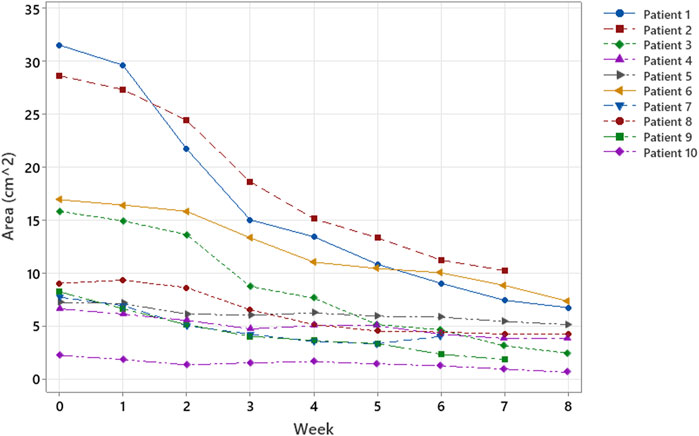
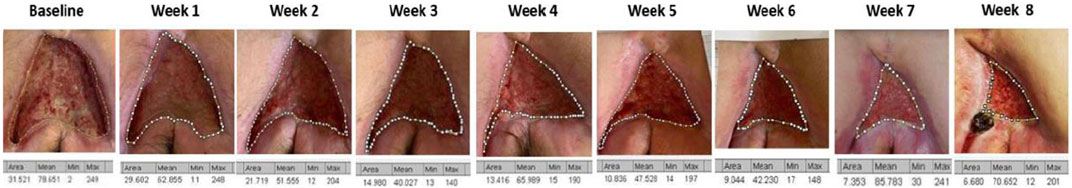
FIGURE 2. Trend of the surfaces of the areas under investigation over time. All wound areas reduced; four patients had a more abrupt reduction in the first 3–4 weeks.
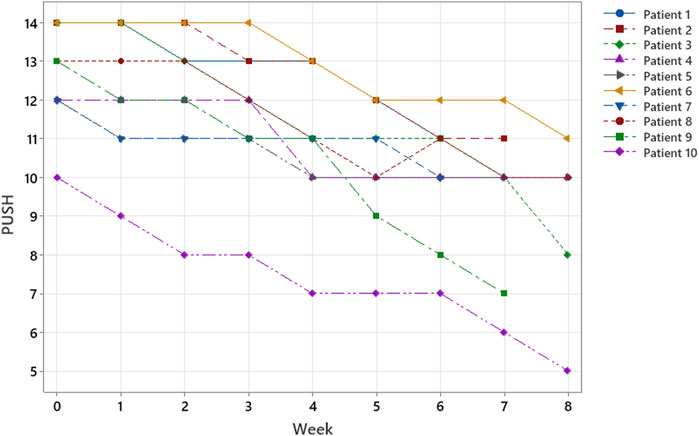
FIGURE 3. According to ulcer classification (surface, exudate, and wound tissue type)—PUSH SCORE; within 8 weeks, all patients responded to treatment, and three patients healed in only 6–7 weeks.
4 Discussion
Timing in PU healing is fundamental to avoid infections and improve the patient’s quality of life. It has been known for many years that regenerative medicine protocols involving treatment with blood components can stimulate and accelerate wound healing (Greppi et al., 2011). The etiology of PU is varied, but local hypoxic damage plays a fundamental role in its development (NPUAP). Ischemia and hypoxia due to various causes reduce the supply of nutrients to the tissues, both locally and systemically. Providing concentrates of biomolecules derived from donors to the site of the lesion can boost biological pathways that have become blocked by in situ catabolites and inflammatory molecules. Previous studies have demonstrated that advanced wound therapy using local applications of PRP is a promising alternative to standard saline dressings in PU healing; in young patients, there is a significant improvement in the histopathological condition of the ulcer after 5 weeks (Singh et al., 2014; Jharwal Rajesh et al., 2023). Conceptually, PEFPRP is a new product derived from the ultrafiltration of platelets and plasma concentrates. It is rich not only in platelets—whose key components for the regeneration or replacement of tissue are GFs (VEGF, EGF, and PDGF-bb)—but also in plasma proteins, which play a role in dermal regeneration. This characteristic promotes the rebuilding of the extracellular matrix, which is the 3D scaffold for cells. This new environment is proangiogenic for resident stem cells and induces the development of endothelial sprouts, their transformation into vessels, and the final maturation of the capillary network into granulation tissue.
Given that the study has some limitations, that is, the low number of subjects enrolled and non-homogeneity in terms of age, comorbidity, and lesion site, this approach was chosen to overcome the conditions of the unsuitability of the patients for surgical treatment and the need to induce rapid stimulation for closure of the large lesion. Our results show a mean reduction of 52% in lesion area and 21% in PUSH after 6 weeks of treatment (Table 1). All cases showed improvement, although two showed only minimal improvement and a slight reduction in the surface area. Even the evidence of no worsening is a positive result, considering that these are patients with tissue hypoxia of various etiologies. Choosing this treatment over surgery has the advantage that it spares the patient’s anatomy. Surgical transfer of muscle flaps from the gluteus maximus or hamstrings invariably causes loss of body function, such as loss of strength in lower limb extension, which can lead to gait deficiency in ambulant patients (Kuo et al., 2014). It must be said that PU is more common in people with mobility deficiencies, such as spinal cord injury (SCI) patients who are wheelchair-bound. For this population, the availability of effective conservative treatment options is highly beneficial. Muscle tissue is limited and should be spared unless there is no other option available. Furthermore, surgery for sacral PU requires at least 30 days of bed rest in the prone position to allow the wound to heal. This requires increased healthcare assistance and longer hospitalization times, as well as the suspension of ongoing physiotherapeutic rehabilitation programs. Avoiding surgery also prevents possible surgical complications (hemorrhage, wound infection, and hematoma) and discomfort (local pain and prolonged immobilization) (Sameem et al., 2012). It is also clear that choosing a biological treatment to treat these wounds will reduce costs for the healthcare system (Demarre et al., 2015; Hajhosseini et al., 2020). However, this regenerative treatment is not always applicable; with advanced-stage PU and extensive destruction of the underlying tissue, the possibility of achieving proper healing without healthy tissue grafting is unlikely. Treatment with PEFPRP requires a lot of time and compliance with care to achieve partial or total closure of the injury, but it is a matter of evidence that this product stimulates the formation of new tissue, ensuring also a better rooting of a possible graft to permanently close the lesion. A good option might be to integrate both treatments, e.g., reducing the PU using biological components and then performing surgery on the resulting smaller lesion. PEFPRP could boost wound healing, and after a fixed timing of biological treatment, PU treatment could be reconsidered in favor of other therapies (e.g., standard dressing or surgery).
In conclusion, the combination of concentrated plasma proteins and platelet proteins may facilitate tissue regeneration, demonstrating that PEFPRP is a safe and feasible treatment for PU. Moreover, PEFPRP reduces the size of the PU and improves its morphological features: e.g., it quickly reduces undermining and improves physical features, promoting the formation of granulation tissue. PEFPRP dressings could be integrated into the care of advanced-stage PU as a possible choice of natural biological treatment. These dressings could be used as conservative treatment alone or together with reconstructive surgery, though further studies are needed to confirm the efficacy of the latter.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LM: Investigation, Writing–original draft. VB: Investigation, Writing–original draft. EZ: Investigation, Writing–review and editing. MD: Investigation, Writing–review and editing. MM: Investigation, Writing–review and editing. FP: Supervision, Writing–review and editing. IV: Investigation, Writing–original draft. LP: Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank their colleagues in their respective workplaces for scientific and technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akbarzadeh, S., McKenzie, M. B., Rahman, M. M., and Cleland, H. (2021). Allogenic platelet-rich plasma: is it safe and effective for wound repair? Eur. Surg. Res. 62 (1), 1–9. doi:10.1159/000514223
Anitua, E., Alkhraisat, M. H., and Orive, G. (2012). Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control Release 157 (1), 29–38. doi:10.1016/j.jconrel.2011.07.004
Aprili, G., Gandini, G., Guaschino, R., Mazzucco, L., Salvaneschi, L., Vaglio, S., et al. (2013). SIMTI recommendations on blood components for non-transfusional use. Blood Transfus. 11, 611–622. doi:10.2450/2013.0118-13
Cecerska-Heryć, E., Goszka, M., Serwin, N., Roszak, M., Grygorcewicz, B., Heryć, R., et al. (2022). Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 64, 84–94. doi:10.1016/j.cytogfr.2021.11.003
Choi, E. P., Chin, W. Y., Wan, E. Y., and Lam, C. L. (2016). Evaluation of the internal and external responsiveness of the Pressure Ulcer Scale for Healing (PUSH) tool for assessing acute and chronic wounds. J. Adv. Nurs. 72 (5), 1134–1143. doi:10.1111/jan.12898
Collins, T., Alexander, D., and Barkatali, B. (2021). Platelet-rich plasma: a narrative review. EFORT Open Rev. 6 (4), 225–235. doi:10.1302/2058-5241.6.200017
Demarre, L., Van Lancker, A., Van Hecke, A., Verhaeghe, S., Grypdonck, M., Lemey, J., et al. (2015). The cost of prevention and treatment of pressure ulcers: a systematic review. Int. J. Nurs. Stud. 52, 1754–1774. doi:10.1016/j.ijnurstu.2015.06.006
Dhurat, R., and Sukesh, M. (2014). Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J. Cutan. Aesthet. Surg. 7 (4), 189–197. doi:10.4103/0974-2077.150734
Everts, P., Onishi, K., Jayaram, P., Lana, J. F., and Mautner, K. (2020). Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 21 (20), 7794. doi:10.3390/ijms21207794
Greppi, N., Mazzucco, L., Galetti, G., Bona, F., Petrillo, E., Smacchia, C., et al. (2011). Treatment of recalcitrant ulcers with allogeneic platelet gel from pooled platelets in aged hypomobile patients. Biologicals 39 (2), 73–80. doi:10.1016/j.biologicals.2011.01.002
Hajhosseini, B., Longaker, M. T., and Gurtner, G. C. (2020). Pressure injury. Ann. Surg. 271 (4), 671–679. doi:10.1097/SLA.0000000000003567
Hill, J. E., Edney, S., Hamer, O., Williams, A., and Harris, C. (2022). Interventions for the treatment and prevention of pressure ulcers. Br. J. Community Nurs. 27 (6), S28–S36. doi:10.12968/bjcn.2022.27.sup6.s28
Jan, K., Cuddigan, J., Carville, K., Balzer, K., Berlowitz, D., Law, S., et al. (2019). Prevention and treatment of pressure ulcers/injuries: the protocol for the second update of the international Clinical Practice Guideline 2019. J. Tissue Viability 28 (2), 51–58. doi:10.1016/j.jtv.2019.01.001
Jharwal Rajesh, K., Om, P., Mithlesh, G., and Bhargav, S. (2023). A randomized control study of autologous platelet rich plasma and normal saline dressing in management of pressure ulcer in spinal cord injury patients. Eur. J. Mol. Clin. Med. 10 (1), 888–900.
Kuo, P.-J., Chew, K.-Y., Kuo, Y.-R., and Lin, P.-Y. (2014). Comparison of outcomes of pressure sore reconstructions among perforator flaps, perforator-based rotation fasciocutaneous flaps, and musculocutaneous flaps. Microsurgery 34 (7), 547–553. doi:10.1002/micr.22257
Liao, X., Liang, J. X., Li, S. H., Huang, S., Yan, J. X., Xiao, L. L., et al. (2020). Allogeneic platelet-rich plasma therapy as an effective and safe adjuvant method for chronic wounds. Surg. Res. 246, 284–291. doi:10.1016/j.jss.2019.09.019
Mazzucco, L., Balbo, V., Martinotti, S., Ranzato, E., Patrone, M., Manfredi, M., et al. (2021). Protein-enriched Platelet- Rich Plasma (PEFPRP) a new product for tissue regeneration developed through the ultrafiltration of PRP- Preclinical Study. Frontiers 1 (1), 1–6. doi:10.11648/j.frontiers.20210101.11
Mazzucco, L., Medici, D., Serra, M., Panizza, R., Rivara, G., Orecchia, S., et al. (2004). The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion 44 (7), 1013–1018. doi:10.1111/j.1537-2995.2004.03366.x
Moore, Z. E., and Patton, D. (2019). Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst. Rev. 1 (1), CD006471. doi:10.1002/14651858.cd006471.pub4
Munoz, N., and Posthauer, M. E. (2022). Nutrition strategies for pressure injury management: implementing the 2019 International Clinical Practice Guideline. Nutr. Clin. Pract. 37 (3), 567–582. doi:10.1002/ncp.10762
Olczyk, P., Mencner, Ł., and Komosinska-Vassev, K. (2014). The role of the extracellular matrix components in cutaneous wound healing. Biomed. Res. Int. 2014, 1–8. doi:10.1155/2014/747584
Piccin, A., Di Pierro, A. M., Canzian, L., Primerano, M., Corvetta, D., Negri, G., et al. (2017). Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 15 (4), 333–340. doi:10.2450/2016.0038-16
Pressure Ulcer Scale for Healing (Push), (2013). PUSH tool version 3.0:9/15/98 national pressure ulcer advisory Panel. New York, NY, USA: Pressure Ulcer Scale for Healing PUSH.
Qu, W., Wang, Z., Hunt, C., Morrow, A. S., Urtecho, M., Amin, M., et al. (2021). The effectiveness and safety of platelet-rich plasma for chronic wounds: a systematic review and meta-analysis. Mayo Clin. Proc. 96 (9), 2407–2417. doi:10.1016/j.mayocp.2021.01.030
Rennie, M. Y., Dunham, D., Lindvere-Teene, L., Raizman, R., Hill, R., and Linden, R. (2019). Understanding real-time fluorescence signals from bacteria and wound tissues observed with the MolecuLight i: XTM. Diagn. (Basel) 9 (1), 22. doi:10.3390/diagnostics9010022
Saghaleini, S. H., Dehghan, K., Shadvar, K., Sanaie, S., Mahmoodpoor, A., and Ostadi, Z. (2018). Pressure ulcer and nutrition. Indian J. Crit. Care Med. 22 (4), 283–289. doi:10.4103/ijccm.IJCCM_277_17
Sameem, M., Au, M., Wood, T., Farrokhyar, F., and Mahoney, J. (2012). A systematic review of complication and recurrence rates of musculocutaneous, fasciocutaneous, and perforator-based flaps for treatment of pressure sores. Plast. Reconstr. Surg. 130 (1), 67–77. doi:10.1097/PRS.0b013e318254b19f
Saputro, I. D., Rizaliyana, S., and Noverta, D. A. (2022). The effect of allogenic freeze-dried platelet-rich plasma in increasing the number of fibroblasts and neovascularization in wound healing. Med. Surg. (Lond) 73, 103217. doi:10.1016/j.amsu.2021.103217
Shi, C., Dumville, J. C., Cullum, N., Rhodes, S., McInnes, E., Goh, E. L., et al. (2021). Beds, overlays, and mattresses for preventing and treating pressure ulcers: an overview of Cochrane Reviews and network meta-analysis. Cochrane Database Syst. Rev. 8 (8), CD013761. doi:10.1002/14651858.CD013761.pub2
Singh, G., Borah, D., Khanna, G., and Jain, S. (2021). Efficacy of local autologous platelet-rich plasma in the treatment of pressure ulcer in spinal cord injury patients. Cureus 13 (10), e18668. doi:10.7759/cureus.18668
Singh, R., Rohilla, R. K., Dhayal, R. K., Sen, R., and Sehgal, P. K. (2014). Role of local application of autologous platelet-rich plasma in the management of pressure ulcers in spinal cord injury patients. Spinal Cord. 52 (11), 809–816. doi:10.1038/sc.2014.144
Stotts, N. A., Rodeheaver, G. T., Thomas, D. R., Frantz, R. A., Bartolucci, A. A., Sussman, C., et al. (2001). An instrument to measure healing in pressure ulcers: development and validation of the pressure ulcer scale for healing (PUSH). J. Gerontol. A Biol. Sci. Med. Sci. 56 (12), M795–M799. doi:10.1093/gerona/56.12.m795
Thomas, D. R., Rodeheaver, G. T., Bartolucci, A. A., Franz, R. A., Sussman, C., Ferrell, B. A., et al. (1997). Pressure ulcer scale for healing: derivation and validation of the PUSH tool. The PUSH Task Force. Adv. Wound Care 10 (5), 96–101.
Van der Bijl, I., Vlig, M., Middelkoop, E., and De Korte, D. (2019). Allogeneic platelet-rich plasma (PRP) is superior to platelets or plasma alone in stimulating fibroblast proliferation and migration, angiogenesis, and chemotaxis as relevant processes for wound healing. Transfusion 59, 3492–3500. doi:10.1111/trf.15535
Keywords: pressure ulcers, dressing, plasma proteins, protein-enriched filtered platelet-rich plasma (PEFPRP), neurologic patient
Citation: Mazzucco L, Balbo V, Zingarelli EM, Desilvestri M, Marchioni M, Perrero L, Pollis F and Varvello I (2024) Treatment of severe pressure ulcers with protein-enriched filtered platelet-rich plasma (PEFPRP): a possible management. Front. Bioeng. Biotechnol. 11:1279149. doi: 10.3389/fbioe.2023.1279149
Received: 17 August 2023; Accepted: 04 December 2023;
Published: 15 January 2024.
Edited by:
Tomoyuki kawase, Niigata University, JapanReviewed by:
Masako Fujioka-Kobayashi, Nippon Dental University, JapanMichael Clark, Welsh Wound Innovation Centre, United Kingdom
Copyright © 2024 Mazzucco, Balbo, Zingarelli, Desilvestri, Marchioni, Perrero, Pollis and Varvello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mazzucco, bG1henp1Y2NvQG9zcGVkYWxlLmFsLml0
 Laura Mazzucco
Laura Mazzucco Valeria Balbo1
Valeria Balbo1