- 1California Medical Innovations Institute Inc, San Diego, CA, United States
- 2Cook Research Incorporated, West Lafayette, IN, United States
- 3Cook Medical, Bloomington, IN, United States
Current leading managements for diverticular disease cannot prevent the recurrence of diverticulitis, bleeding and/or other complications. There is an immediate need for developing new minimal invasive therapeutic strategies to prevent and treat this disease. Through a biomechanical analysis of porcine colon with diverticular lesions, we proposed a novel adhesive patch concept aiming at mechanical reconstruction of the diseased colon wall. This study aims to evaluate the surgical feasibility (safety and efficacy) of pulmonary visceral pleura (PVP) patch therapy using a pig model of diverticulosis. Six female Yucatan miniature pigs underwent collagenase injection (CI) for the development of diverticular lesions. The lesions in each animal either received patch implantation (treated group, n = 40 for 6 pigs) or left intact (untreated group, n = 44 for 6 pigs). The normal colonic wall in each animal received patch implantation at two spots to serve as control (n = 12 for 6 pigs). After 3 months of observation, the performance and safety of the patch treatment were evaluated through macroscopic and histological examination. We found that 95% of pouch-like herniation of the mucosa was prevented from the colon wall with the treatment. The pouch diameter was significantly reduced in the treated group as compared to the untreated group (p < 0.001). The patch application caused a significant increase in the levels of collagen of the colon tissue as compared to the untreated and control groups (p < 0.001). No difference was found in the lymphocyte and macrophage inflammatory infiltrate between the groups. Our results suggest that patch treatment efficiently inhibits the diverticular pouch deformation and promotes the healing of the colon wall with a normal inflammatory response, which may minimize the risk of diverticulosis reoccurrence and complications over time.
Introduction
Diverticular disease is highly prevalent in Western countries, but its incidence has been gradually increasing in the entire world over the past decade (Fong et al., 2011; Alatise et al., 2012; Isohata et al., 2021). The risk of acquiring the disease rises uniformly with age, with approximately 50% of people aged more than 60 years being affected in developed countries (Peery et al., 2016; Strate and Morris, 2019). Although most people with colonic diverticulosis remain asymptomatic, about 10%–25% of patients will have an attack of diverticulitis during their lifetime (Tursi et al., 2015; Reitano et al., 2023). This disease is becoming the leading cause of lower gastrointestinal bleeding in the United States (∼30% of the cases) (Ghassemi and Jensen, 2013), and one of the most common reasons for elective colon resection (Hawkins et al., 2020). Although fiber, antibiotics and probiotics seem to be effective in treating symptomatic and uncomplicated patients (Elisei and Tursi, 2016; Tochigi et al., 2017), the overall recurrence rate following the conservative treatments was reported to be as high as 28% (Perrone et al., 2021). Resection of the infected portion of the colon is currently the mainstay of therapy for chronic complications of diverticular disease. Elective colon resection, however, is related to a high rate of anastomotic bleeding or leakage or other postoperative complications (Kirchhoff et al., 2010; Wu et al., 2017). Specifically, post-surgery development of diverticula still occurred in certain patients (Giulio et al., 2022; Waser et al., 2023). Hence, there is a substantial desire for developing new therapeutic strategies to reduce the chance of recurrence of diverticulosis and its complications.
Utilization of clinically relevant large animal models of diverticulosis is essential for advancing our understanding of diverticulosis pathogenesis and developing new therapies, especially with minimally invasive surgical interventions (Patel et al., 2018). It is well known that collagen is the most abundant protein in mammals and serves to maintain the structural integrity of connective tissues (Lord et al., 1977). By creating a weakness in the colon wall with collagen fiber degradation, we have developed the first large animal model to replicate the pathology of diverticulosis in swine (Guo et al., 2019). Based on the biomechanical analysis of porcine colon with diverticular lesions (Patel et al., 2020), we proposed an adhesive patch concept aiming at mechanical reconstruction of the diseased colon wall. The major rationale is that an increase in local stress will cause progressive dilation and growth of colon tissue that can result in diverticulum while reinforcement of the tissue with an external patch will reduce the stress on the diverticulum and allow for reverse remodeling and healing of the colon tissue. Our group recently identified that pulmonary visceral pleura (PVP) from bovine may be a suitable biomaterial for the patch treatment of diverticulosis due to its beneficial mechanical and structural features (Lu et al., 2019; Lu et al., 2022). We hypothesize that application of a PVP patch would reduce the deformation of diverticula pouch and mitigate the natural progression of diverticulosis over time. The goal of this study is to evaluate the surgical feasibility (safety and efficacy) of adhesive PVP patch therapy using our pig model of diverticulosis.
Materials and methods
Experimental animals
Six female Yucatan miniature pigs at ages 2–8 years old were used in the study. All pigs underwent collagenase injection (CI) for 3 months followed by a 3-month patch treatment. Of the 84 diverticular lesions developed in 6 pigs at 3 months following CI, 40 were randomly selected to receive adhesive patch implantation as the treated group for 3 months, and the remaining 44 were left intact as the untreated group for 3 months. For each pig, the normal colonic wall (without diverticular lesions) also received patch treatment at 2 regions to serve as control (n = 12 for 6 pigs). All animal experiments were performed in accordance with national and local ethical guidelines, including the Institute of Laboratory Animal Research guidelines, Public Health Service policy, the Animal Welfare Act, as approved by Institutional Animal Care and Use Committee at California Medical Innovations Institute, San Diego.
Collagenase injection
Collagenase injection (CI)-induced diverticulosis in swine was described in detail in our previous publication (Guo et al., 2019). In brief, pigs were fasted for 12 h prior to surgery. Pigs were pre-anesthetized with TKX (Telazol 10 mg/kg, Ketamine 5 mg/kg, and Xylazine 5 mg/kg, i.m.). Pigs were then anesthetized with 2%–3% isoflurane inhalation. A laparotomy (midline incision) was performed, and the descending colon was exposed gently. Collagenase (10u/μL, 200 µL) was injected into the colonic wall at 10–15 spots along the descending colon (the length of the treated colon segment is around 15–20 cm). The injected spots started to bleed due to collagen digestion in the colon 20 min following the injection. Electrocautery was then applied to stop bleeding. After the colon was inspected for bleeding, the abdominal incision was closed.
PVP patch preparation
The PVP was peeled from fresh bovine lungs (provided by a local slaughterhouse) with hydro-dissection as described in our previous publication (Lu et al., 2021). The PVP patch was then fixed in 0.65% glutaraldehyde for overnight and stored in 0.25% glutaraldehyde. All patches were sterilized with a solution containing 2.05 g/L NaOH, 10.83 g/L PO4H2K, 200 mL/L Alcohol, 40 mL/L 25% glutaraldehyde, 110 mL/L 4% formaldehyde at 37°C for 24 h and then thoroughly rinsed in saline before in vivo implantation.
Adhesive PVP treatment
Pigs were fasted for 12 h prior to the procedure at 3 months following CI. Under anesthesia with TKX and followed by 2%–3% isoflurane inhalation, a laparotomy (midline incision) was performed. The descending colon was exposed gently, and diverticular pouch-like lesions (up to 20 mm in maximum diameter) were found and measured in the CI-treated colon segment (Figure 1A). On a normal colon spot (no diverticular lesions), an 18-gauge catheter was inserted into the colon lumen. Since air in the colon was released from the catheter, the intracolonic pressure was dropped, and the diverticular pouch was deflated. The PVP patch was trimmed to a size slightly larger than the lesion. Histoacryl (a tissue adhesive, around 200–300 µL) was applied evenly over the surface of the diverticular lesions. The PVP patch was then placed on top of the histoacryl layer to cover the lesion area. After the adhesive was allowed to harden for 30–60 s, the patch was firmly attached to the colon and prevented the diverticular pouch from protruding out of the colon wall (Figure 1B). The abdominal incision was closed after inspection.
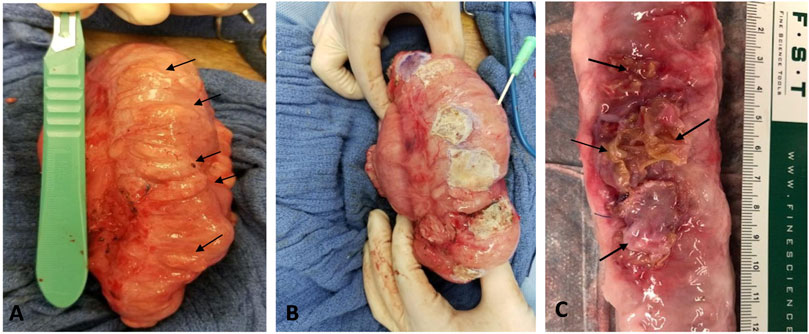
FIGURE 1. (A) In vivo image of diverticular lesions (arrows) following 3 months of collagen injection. (B) In vivo image of the lesions covered with adhesive PVP patch during the surgical patch implantation. A catheter was inserted into lumen to deflate the colon before the patch placement. (C) Ex vivo image of treated spots (arrows) after 3 months of treatment.
Terminal study
At the end of 3-month patch treatment, animal was anesthetized, and the abdomen was opened. The descending colon was exposed and macroscopically inspected for the appearance of PVP patch, changes of diverticular lesions (e.g., extent of pouch protrusion), signs of focal inflammation (e.g., erosions, erythema, edema and hyperemia) and presence of complications (e.g., bleeding, colonic rigidity, stenosis and adhesion). Autonomic intestinal peristalsis in treated colon segments was also observed and recorded. The animal was then euthanized with a saturated solution of potassium chloride (120 mL) injection through the jugular vein to arrest the heart under deep anesthesia. The descending colon was harvested for histological analysis.
Histological assessment
The histological assessment of patch treatment included three aspects: 1) Dimensional changes of diverticular lesions (by measuring pouch opening size); 2) Morphological changes of the colon wall; 3) Colonic inflammatory response. The colon tissue was fixed with 4% paraformaldehyde for 48 h. The tissue was embedded in paraffin and sectioned at 4 µm thickness. Section was then processed with standard Hematoxylin and Eosin (H&E) and Trichrome staining for comprehensive overview. To exhibit the microscopical changes of the full-thickness colon wall structure, multiphoton microscope (MPM, Zeiss LSM 710 NLO) was used to scan the representative frozen tissue sections. Briefly, the paraformaldehyde-fixed colon tissue was embedded in OCT medium and frozen sectioned at 7 um thickness. The sections were incubated with standard fluorescent dyes (Phalloidin, Alexa 488 and Hoechst 33342) and imaged by MPM. For visualization and quantitation of collagen, the colon section was stained with Picro Sirius red. Six randomly selected microscopic fields (10X) on the diseased site were imaged for each section. The quantitative estimation of collagen content was conducted by ImageJ software (NIH) (Chen et al., 2017). The severity of inflammatory response was assessed by a semi-quantitation of lymphocytes and macrophages count. An immunofluorescence detection of lymphocyte with an anti-CD3 antibody and macrophage with an anti-CD68 antibody was performed, and the average score for cell count of the randomly chosen five microscopic fields (40X) on each section was calculated for CD3 and CD68. The inflammatory cells infiltration was graded according to a scale of 0–3 as listed in Table 1.
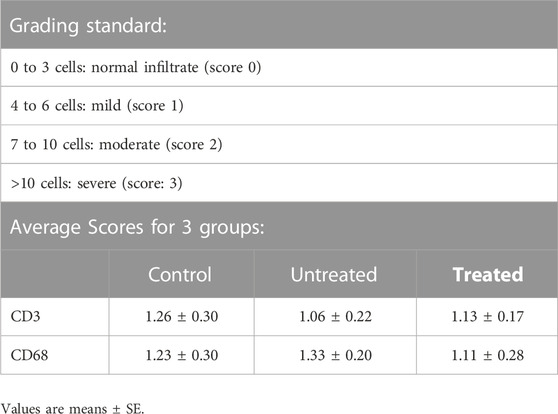
TABLE 1. The grading standard and scores of lymphocyte (CD3) and macrophage (CD68) inflammatory infiltrate in control, untreated and treated groups after 3 months of treatment on magnification (×40).
Statistical analysis
Data was expressed as mean ± SD or mean ± SE as specified. The significance of the differences between treated and untreated groups was evaluated by X2-test or One-way ANOVA. The results were considered statistically significant when p < 0.05 (2-tailed). Statistical analysis was performed using SigmaStat (Version 4.0, Systat Software Inc, California, USA).
Results
All experimental pigs subjected to CI and patch treatment survived until the end of the study. After the initial surgical procedure, pigs were monitored daily for appetite, general body condition, behavior, mobility and defecation until termination. All animals showed tolerance to the surgical interventions throughout the follow-up period and maintained their weights with no significant change before and after patch treatment (110 ± 11.6 kg vs. 115 ± 12.9 kg). No animal developed signs of infection, bleeding, constipation, bowel obstruction, or other postoperative complications.
Three months following CI, a total of 84 lesions (defined as mucosa herniation ≥ 2 mm aborally from the colon wall) were identified macroscopically from all 6 pigs (Figure 1A). Upon termination after 3 month-patch treatment for 40 lesions, we found that around 95% (38/40) of the PVP patches remained adhered to the original application sites and prevented mucosa protrusion (diverticular pouches) as shown in Figure 1C. Partial or complete implant dislocation/detachment occurred in 2 treated lesions (5%) causing persistent mucosa protrusion. For those untreated lesions (n = 44), all diverticular pouches persisted throughout the study. The patch significantly reduced the rate of pouch protrusion compared to the untreated sites (38/40 vs. 0/44, X2 test, p < 0.001). To assess the dimensional changes of the lesions in response to the treatment, the diameter of the diverticular pouch (defined as the distance of muscle separation) was measured using H&E histological images as schematically shown in Figure 2. The pouch diameter was significantly decreased in the treated lesions as compared to the untreated lesions (11.1 ± 2.6 mm vs. 6.6 ± 1.8 mm, p < 0.001).
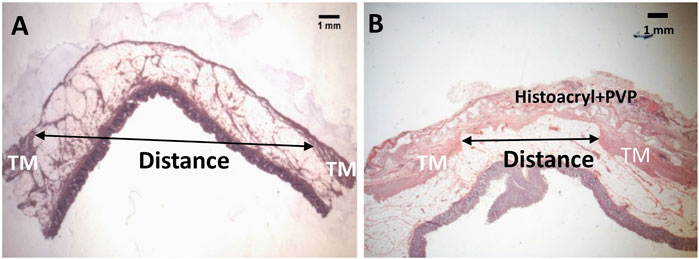
FIGURE 2. A schematic outline of the measurement of diameter for a diverticulum (A) and an adhesive patch-treated diverticulum (B) with H&E staining. TM: tunic muscularis.
During the surgical patch implantation, we noticed that 3 animals (50%) had focal intra-abdominal adhesion at 1-2 spots between the large intestine caused by CI-induced diverticula formation. Those adhesions are regarded as mild because they were dissectible with dull dissection. Three months after patch treatment, no remarkable changes in the levels and locations of the adhesions were seen and no new focal adhesion was formed. During the terminal study, we also observed an autonomic intestinal peristalsis in treated colon segments with no visible signs of rigidity and stenosis in all 6 pigs.
The morphology of the diverticular lesions with patch treatment was assessed by Trichrome staining (Figure 3). Although some of the implant materials were washed out during the histological process, the area of application was clearly detectable under a microscope. The diseased colon exhibited focal loss of tunic muscularis which was replaced by the remnant adhesive and PVP patches in combination with varying degrees of connective tissue, cellular debris and scattered lymphocytes and multinucleated macrophages. Multiphoton microscope images of tissue sections clearly displayed that tissue repair on the treated lesions was exuberant with increased collagen formation and appearance of aberrant bundles of smooth muscle cells (SMCs) (Figure 4). With Picro Sirius red staining, deposition of collagen fibers was seen within the submucosa layer of the colon wall (pictures are not shown). Quantitative analysis found that the collagen content was significantly higher in the treated group than that in the untreated and control groups (p < 0.01, Figure 5). The increased collagen content was also found in the untreated group when compared to the control group (p < 0.01, Figure 5).
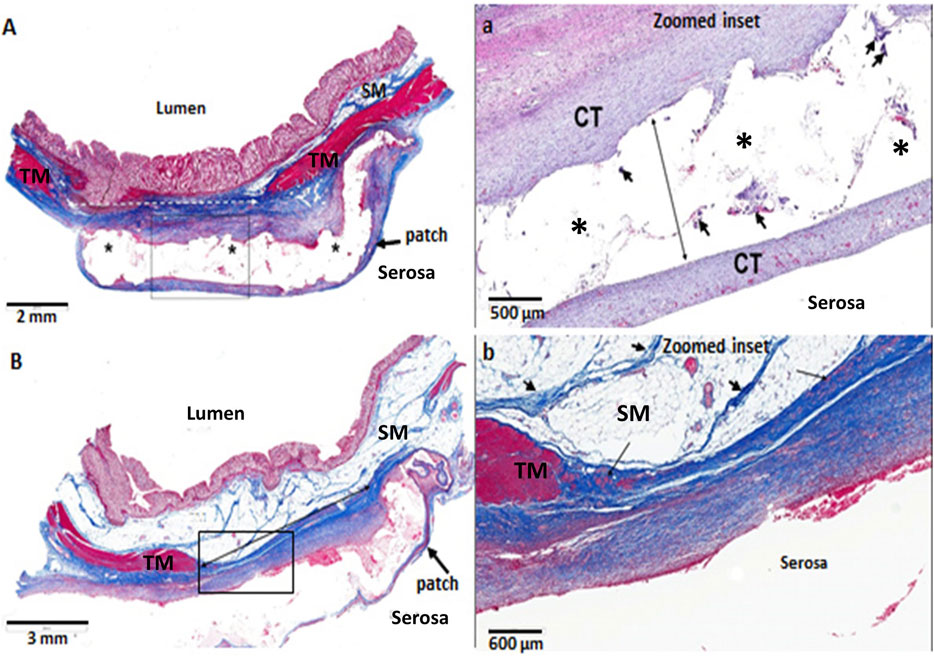
FIGURE 3. Histological images of diverticular lesions (A,B) following 3 months of adhesive PVP patch treatment (Trichrome staining). (A) The tunic muscularis (TM, red) within the injury area (dotted line) was replaced by connective tissue (blue) and covered with PVP patch (*). The submucosa (SM) is thinned and contains increased connective tissue. At higher magnification of the box in A (a), the clear space (* and long arrow) is where the treatment material was placed. The area contains a translucent adhesive, patch, minimal connective tissue and low numbers of multinucleated macrophages (arrowheads). The PVP patch is surrounded by mature connective tissue (CT). (B) The TM (red) within the injury area (solid line) was replaced by connective tissue (blue) and covered with PVP patch. At higher magnification of the box in B (b), connective tissue (blue) surrounds the serosal surface, which contains occasional clusters of smooth muscle cells (arrows). The SM is composed of normal fat and strands of fibrous connective tissue (arrowheads).
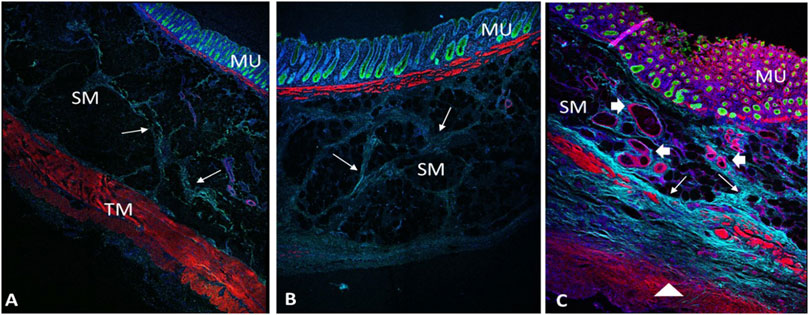
FIGURE 4. Multiphoton microscope images of control (A), untreated (B) and treated diverticulum (C) with Immunofluorescence staining. TM, tunic muscularis; SM, submucosa; MU, mucosa. Red, smooth muscle F-actin stained with Phalloidin; Green, mucosa stained with Alexa 488; Light blue, collagen autofluorescence; Dark blue, nuclei stained with Hoechst 33342. B, an untreated diverticulum shows lack of tunic muscularis layer with slight collagen deposition (arrows). C, a treated diverticulum shows absence of TM layer, but with newly growing disorganized muscle cells (triangle), more collagen deposition (arrows) and vessels regeneration (arrowheads).
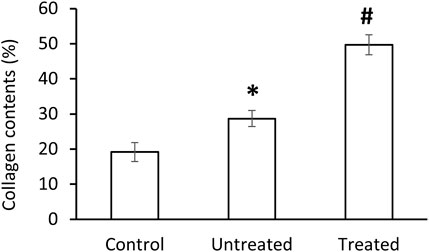
FIGURE 5. Collagen contents in control, untreated and treated groups. Data corresponds to mean ± SE. *p < 0.05, when compared to control group, #p < 0.05, when compared to untreated and control groups.
Figure 6 shows the histological images of CD3 and CD68 with fluorescence staining in control, untreated and treated diverticular lesions. CD3-positive lymphocytes and CD68-positive macrophages were mainly localized in the lamina propria and submucosal layer. The CD3 and CD68 infiltrated scores following 3 months of treatment are listed in Table 1. No significant differences in the inflammatory infiltrate of lymphocyte and macrophage were observed in the treated group as compared to the untreated and control groups.
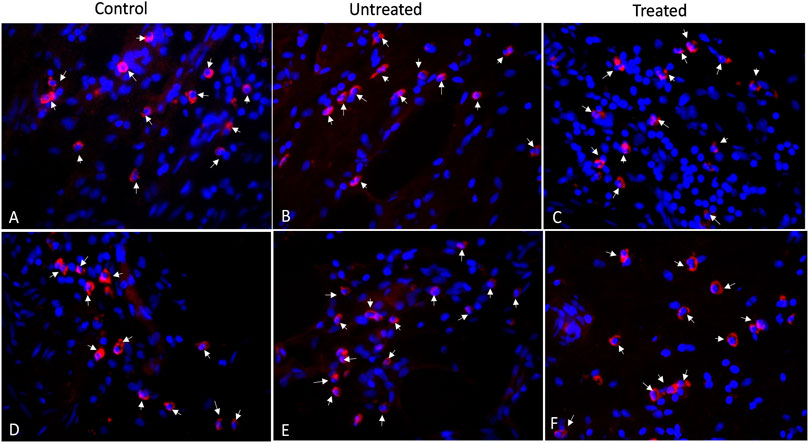
FIGURE 6. Immunofluorescence staining of CD3-positive lymphocytes (A–C) and CD68-positive macrophages (D–F) for control, untreated and treated diverticula (40X). CD3 and CD68 in red (arrows) and Nuclei in blue.
Discussion
Except for colectomy to remove diseased colon permanently, current leading managements for diverticular disease including medication and endoscopic/laparoscopic surgery cannot prevent the occurrence/recurrence of diverticulitis, bleeding and/or other complications (Cirocchi et al., 2015; Yamada et al., 2015; Kaushik et al., 2016; Longchamp et al., 2021). Developing more effective minimally invasive approaches leading to less complications and lower diverticulosis recurrence are urgently needed both for patients and the healthcare system. After investigating the biomechanical behaviors of the colon with the presence of a pouch-like structure created in swine (Guo et al., 2019), our group are the first to verify that stress distribution may change in diverticula where a vicious cycle may occur during the progress of diverticulosis (Patel et al., 2020). These findings provide a theoretical basis for a new therapeutic strategy by reducing local stress in diverticulum and improving the mechanical strength of the weakened colon wall. Our patch design is technically simple and feasible since it only requires a currently available biological tissue material and tissue adhesive that can be delivered using traditional laparoscopic techniques.
As a requirement for the proposed treatment, the mechanical properties of a biocompatible patch material should be such that it is stiff enough to prevent the pouch protrusion but still compliant enough to permit the natural motility of the colon. Xenogeneic elastin-based biomaterials such as porcine PVP and small intestinal submucosa (SIS) have been used for the repair of gastrointestinal tissue in animal studies (Kajitani et al., 2000; Tan et al., 2014). The PVP was deliberately chosen for the study because it contains abundant elastin and collagen with larger proportion of elastin than that in the primarily collagen-based biomaterials such as swine SIS or bovine pericardium. This structural character provides more mechanical compliance to the PVP as shown in our uniaxial testing of PVP, SIS, and bovine pericardium, where we confirmed that the incremental moduli in stress-strain curves and relaxation moduli in the Maxwell-Weichert model of PVP were approximately one-tenth of SIS and pericardium (Lu et al., 2022). In another mechanical test, we observed similar burst pressure between the PVP-constructed tubular prosthesis and rat artery tissue (386 mmHg vs. 329 mmHg), indicating the potential of the PVP as an arterial graft (Lu et al., 2019). With an in vitro mechanical testing and computational simulation (data are not shown), we further demonstrated that PVP from bovine is the optimal material for colon tissue reinforcement with an ideal mechanical compliance showing no observable effect on colonic deformation or motility. In addition, our group found the bovine PVP, after processing with glutaraldehyde, to be degradable over a 12-month period with low cytotoxicity, low immune rejection, and low inflammation, exhibiting improved physical and physiological characteristics for surgical implantation in rat, swine and dog models (Lu et al., 2019; Lu et al., 2020; Lu et al., 2022).
Cyanoacrylate-based tissue adhesives have been used in suture-less surgery in medical and veterinary applications since the 1970s (Amiel et al., 1999; Esposito et al., 2004; Miyano et al., 2004). Previous studies also identified tissue adhesives as the most promising colonic anastomotic sealants (Al-Mubarak and Al-Haddab., 2013; Vakalopoulos et al., 2017a). Histoacryl (an FDA-approved topical skin adhesive) was selected for the proposed patch application as it has shown good local tolerance and biocompatibility validated by extensive toxicological and implantation studies in human and animals (Kukleta et al., 2012). A recent animal study applying histoacryl on the colon for 28 days found that histoacryl maintained a strong adhesive bond to the tissue and induced a limited local host reaction without toxic effects on the bowel (Vakalopoulos et al., 2017b).
In this report, we performed a proof-of-concept study of adhesive patch treatment using our pig model of diverticulosis (Guo et al., 2019). To the best of our knowledge, this is the first study to utilize the Xenogeneic (bovine) biocompatible material to repair diverticular defects in a large animal model. We found the PVP patch to be easy to handle and apply onto the surface of colon. The use of histoacryl generated a strong bond between the patch and the colon with no need for additional suture fixation. After 3 months of follow-up, we found that 95% of pouch-like mucosa herniation was prevented from the colon wall. Furthermore, histological examination revealed a significant decrease in the diameter of the diverticular pouch over the 3-month implantation as compared to the untreated group. These results indicate that adhesive patch treatment may prevent diverticulosis progression or recurrence and accordingly reduce the chance of complications.
The use of the patch was safe, and no adverse events were identified. All experimental animals maintained their weights within the range of 5% of their pre-implantation weights during the observation period of 3 months. After the patch implantation, we observed no signs of colon infections, bleeding, perforation, stenosis or obstruction. Although slight focal adhesion between the large bowels occurred during the formation of diverticula in 3 CI-injected pigs, the patch application did not increase the severity and amount of intra-abdominal adhesions. Moreover, autonomic bowel peristalsis was noted in vivo in the treated colon segments of all animals. Those macroscopic findings reveal that the presence of PVP patch caused no visible adverse effects and did not seem to affect the functional bowel movement. The responses of colonic mechanical properties and functional movements to patch therapy require further investigations.
Microscopically, our histological examination showed that the damaged muscular layer on diverticulum was covered with the implanted material combined with growth of connective tissue. The representative multiphoton microscope imaging demonstrated that patch placement promoted repair of the damaged colon wall with newly growing muscle cells, collagen deposition and neovascularization in the muscle and serosa layers. Collagen, the main component of connective tissue, plays an important role in tissue repair and reconstruction (Krafts, 2010). Shogan et al. (2015) has reported that higher levels of collagen are associated with higher mechanical strength of intestine in a rat intestinal anastomoses model. In our study, 3 months of patch application resulted in a significantly increased levels of collagen as comparted to the untreated group, which possibly enhanced the mechanical strength of the diseased colon wall, and accordingly blocked the mucosa herniation. Lymphocytes and macrophages participate in the response of the adaptive immune system of the host (Vakalopoulos et al., 2017b). Although our assessment of the inflammatory reaction of the colon tissue to PVP patch displayed mild lymphocyte and macrophage infiltration, it was not significantly different from that of the untreated and control groups. This implies a normal histopathologic healing process of the colon wall following the treatment. Hence, we consider the PVP patch application a safe procedure. Longer observation time is necessary to evaluate the long-term behavior of the xenogeneic PVP application in swine.
With the rapid development of tissue engineering in regenerative medicine, developing biodegradable 3-dimensional scaffolds through the use of new biocompatible materials has been a growing research interest for tissue repair/regeneration (Choi et al., 2010; BaoLin and Ma, 2014). Synthetic prosthetic polymers, such as polyglycolic acid (PGA), polylactic acid (PLA), and polycaprolactone (PCL) were found to simulate the biologic and mechanical functions of the extracellular matrix (ECM), which could lead to tissue regrowing, remodeling, and response to injury (Timnak, et al., 2011; Sun et al., 2018). Moreover, composite scaffolds based on a combination of two or more materials (i.e., PCL-PHEA-PLA) have shown better mechanical strength and elasticity with a high level of cellular adhesion on fibers in animal studies (Lo Monte et al., 2012; Buscemi et al., 2017). These unique advantages are attractive as an alternative patch material for the treatment of injured colons that should be further explored in the future.
Limitations of study
The current study mainly focuses on the feasibility of patch therapy for diverticulosis with a short-term observation period of up to 12 weeks. Therefore, this study provides no information about long-term safety and effectiveness, which is critical to assess the advantages of the proposed approach on the reduction of complications over time. Although the pig model was created by replicating the pathological process of diverticulosis, the chronic response of the colon to the PVP patch in swine may be different from that in human due to species differences and co-morbidities. Another limitation is the lack of colon mechanical testing to assess the impact of patch on bowel function. It is possible that patch implantation may alter colon mechanical properties, without causing visible changes in colon functional movement during the 12-week follow-up. Despite those limitations, this work may open a new way for researchers and clinicians to explore an effective preventive treatment for diverticulosis via mechanical reconstruction of the diseased colon.
Conclusion
The adhesive PVP patch was used for the treatment of colon diverticulosis in a pig model. A 3-month patch implantation not only prevented mucosa herniation, but also accelerated tissue healing and strengthened colonic wall structure over the diverticular defects. The bovine PVP seems to be an excellent reinforcement biomaterial based on its efficacy, safety, stability, and ease of use. Our findings offer an alternative minimal invasive surgical procedure that may slow or even stop diverticulosis progression but maintain the anatomy and function of the colon. The current study demonstrated proof-of-concept using laparotomy, but future focus is needed to validate the feasibility of using traditional laparoscopy to deliver the PVP patch. Further studies are required to clarify the long-term benefits of this approach to minimize the risk of complications and recurrence of colonic diverticulosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee at California Medical Innovations Institute, San Diego.
Author contributions
Study design: XG, BP, SC, and GK; Animal study: XG, BP, and JN; Histology: LH and WV; Data collection and analysis: XG, LH, and JN; Manuscript preparation: XG, WV, and GK. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by 3DT Holdings and Cook Medical.
Conflict of interest
Author WV was employed by the company Cook Research Incorporated. Authors JN and SC were employed by the company Cook Medical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Mubarak, L., and Al-Haddab, M. (2013). Cutaneous wound closure materials: An overview and update. J. Cutan. Aesthet. Surg. 6 (4), 178–188. doi:10.4103/0974-2077.123395
Alatise, O. I., Arigbabu, A. O., Agbakwuru, E. A., Lawal, O. O., Ndububa, D. A., and Ojo, O. S. (2012). Spectrum of colonoscopy findings in Ile-Ife Nigeria. Niger. Postgrad. Med. J. 19, 219–224. doi:10.4103/1117-1936.169543
Amiel, G. E., Sukhotnik, I., Kawar, B., and Siplovich, L. (1999). Use of N-butyl-2-cyanoacrylate in elective surgical incisions—Longterm outcomes11No competing interests declared. J. Am. Coll. Surg. 189 (1), 21–25. doi:10.1016/S1072-7515(99)00068-X
BaoLin, G., and Ma, P. X. (2014). Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 57 (4), 490–500. doi:10.1007/s11426-014-5086-y
Buscemi, S., Palumbo, V. D., Maffongelli, A., Fazzotta, S., Palumbo, F. S., Licciardi, M., et al. (2017). Electrospun PHEA-PLA/PCL scaffold for vascular regeneration: A preliminary in vivo evaluation. Transpl. Proc. 49 (4), 716–721. doi:10.1016/j.transproceed.2017.02.017
Chen, Y., Yu, Q., and Xu, C. B. (2017). A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 10 (10), 14904–14910.
Choi, S. W., Zhang, Y., and Xia, Y. (2010). Three-dimensional scaffolds for tissue engineering: The importance of uniformity in pore size and structure. Langmuir 26 (24), 19001–19006. doi:10.1021/la104206h
Cirocchi, R., Grassi, V., Cavaliere, D., Renzi, C., Tabola, R., Poli, G., et al. (2015). New trends in acute management of colonic diverticular bleeding: A systematic review. Medicine 94 (44), e1710. doi:10.1097/MD.0000000000001710
Elisei, W., and Tursi, A. (2016). Recent advances in the treatment of colonic diverticular disease and prevention of acute diverticulitis. Ann. Gastroenterol. 29, 24–32.
Esposito, C., Damiano, R., Settimi, A., De Marco, M., Maglio, P., and Centonze, A. (2004). Experience with the use of tissue adhesives in pediatric endoscopic surgery. Surg. Endosc. 18, 290–292. doi:10.1007/s00464-003-9032-x
Fong, S. S., Tan, E. Y., Foo, A., Sim, R., and Cheong, D. M. (2011). The changing trend of diverticular disease in a developing nation. Colorectal. Dis. 13, 312–316. doi:10.1111/j.1463-1318.2009.02121.x
Ghassemi, K. A., and Jensen, D. M. (2013). Lower GI bleeding: Epidemiology and management. Curr. Gastroenterol. Rep. 15, 333–336. doi:10.1007/s11894-013-0333-5
Giulio, M., Gaia, S., Andrea, C., Giacomo, C., Angela, P., Dario, M., et al. (2022). Recurrent diverticulitis after elective surgery. Int. J. Colorectal Dis. 37 (10), 2149–2155. doi:10.1007/s00384-022-04248-x
Guo, X., Patel, B., Ling, H., Ali-Dulaimi, H., Noblet, J., Chambers, S., et al. (2019). Novel swine model of colonic diverticulosis. Am. J. Physiol. Gastr. L. 317 (1), G51–G56. doi:10.1152/ajpgi.00408.2018
Hawkins, A. T., Wise, P. E., Chan, T., Lee, J. T., Glyn, T., Wood, V., et al. (2020). Diverticulitis: An update from the age old paradigm. Curr. Probl. Surg. 57 (10), 100862. doi:10.1016/j.cpsurg.2020.100862
Isohata, N., Nagata, K., Utano, K., Nozaki, R., Nozu, S., Kato, T., et al. (2021). Recent trends in the prevalence and distribution of colonic diverticula in Japan evaluated using computed tomography colonography. World J. Gastroenterol. 27 (27), 4441–4452. doi:10.3748/wjg.v27.i27.4441
Kajitani, M., Wadia, Y., Xie, H., Hinds, M. T., Shalaby, S. W., Swartz, K. R., et al. (2000). Use of a new elastin patch and glue for repair of a major duodenal injury. ASAIO. J. 46 (4), 409–414. doi:10.1097/00002480-200007000-00007
Kaushik, M., Bhullar, J. S., Bindroo, S., Singh, H., and Mittal, V. K. (2016). Minimally invasive management of complicated diverticular disease: Current status and review of literature. Dig. Dis. Sci. 61, 663–672. doi:10.1007/s10620-015-3924-1
Kirchhoff, P., Clavien, P. A., and Hahnloser, D. (2010). Complications in colorectal surgery: Risk factors and preventive strategies. Patient. Saf. Surg. 4, 5. doi:10.1186/1754-9493-4-5
Krafts, K. P. (2010). Tissue repair: The hidden drama. Organogenesis 6, 225–233. doi:10.4161/org.6.4.12555
Kukleta, J. F., Freytag, C., and Weber, M. (2012). Efficiency and safety of mesh fixation in laparoscopic inguinal hernia repair using n-butyl cyanoacrylate: Long-term biocompatibility in over 1,300 mesh fixations. Hernia 16, 153–162. doi:10.1007/s10029-011-0887-9
Lo Monte, A. I., Licciardi, M., Bellavia, M., Damiano, G., Palumbo, V. D., Palumbo, F. S., et al. (2012). Biocompatibility and biodegradability of electrospun PHEA-PLA scaffolds: Our preliminary experience in a murine animal model. Dig. J. Nanomat Biostruct 7, 841–851.
Longchamp, G., Abbassi, Z., Meyer, J., Toso, C., Buchs, N. C., and Ris, F. (2021). Surgical resection does not avoid the risk of diverticulitis recurrence-a systematic review of risk factors. Int. J. Colorectal Dis. 36 (2), 227–237. doi:10.1007/s00384-020-03762-0
Lord, M. G., Valies, 0., and Broughton, A. C. (1977). A morphologic study of the submucosa of the large intestine. Surg. Gyneco.l Obstet. 145, 55–60.
Lu, X., Han, L., Golts, E., Baradarian, S., and Kassab, G. S. (2020). Homologous and heterologous assessment of a novel biomaterial for venous patch. J. Vasc. Surg. Venous. Lymphat. Disord. 8 (3), 458–469.e1. doi:10.1016/j.jvsv.2019.09.011
Lu, X., Han, L., Guo, X., Wang, M., Baradarian, S., Golts, E., et al. (2021). Novel biomaterial for artery patch in swine model with high-fat diet. Front. Bioeng. Biotechnol. 9, 679466. doi:10.3389/fbioe.2021.679466
Lu, X., Han, L., and Kassab, G. S. (2019). In vivo self-assembly of small diameter pulmonary visceral pleura artery graft. Acta. Biomater. 83, 265–276. doi:10.1016/j.actbio.2018.11.001
Lu, X., Han, L., and Kassab, G. S. (2022). Pulmonary visceral pleura biomaterial: Elastin- and collagen-based extracellular matrix. Front. Bioeng. Biotechnol. 10, 796076. doi:10.3389/fbioe.2022.796076
Miyano, G., Yamataka, A., Kato, Y., Tei, E., Lane, G. J., Kobayashi, H., et al. (2004). Laparoscopic injection of dermabond tissue adhesive for the repair of inguinal hernia: Short- and long-term follow-up. J. Pediatr. Surg. 39 (12), 1867–1870. doi:10.1016/j.jpedsurg.2004.08.018
Patel, B., Guo, X., Noblet, J., Chambers, S., and Kassab, G. S. (2020). Computational analysis of mechanical stress in colonic diverticulosis. Sci. Rep. 10 (1), 6014. doi:10.1038/s41598-020-63049-w
Patel, B., Guo, X., Noblet, J., Chambers, S., and Kassab, G. S. (2018). Animal models of diverticulosis: Review and recommendations. Di.g Di.s Sci. 63 (6), 1409–1418. doi:10.1007/s10620-018-5071-y
Peery, A. F., Keku, T. O., Martin, C. F., Eluri, S., Runge, T., Galanko, J. A., et al. (2016). Distribution and characteristics of colonic diverticula in a United States screening population. Clin. Gastroenterol. Hepatol. 14, 980–985.e1. doi:10.1016/j.cgh.2016.01.020
Perrone, G., Giuffrida, M., Bonati, E., Petracca, G. L., Tarasconi, A., Baiocchi, G., et al. (2021). Conservative management of complicated colonic diverticulitis in early and late elderly. Med. Kaunas. 58 (1), 29. doi:10.3390/medicina58010029
Reitano, E., Francone, E., Bona, E., Follenzi, A., and Gentilli, S. (2023). Gut microbiota association with diverticular disease pathogenesis and progression: A systematic review. Dig. Dis. Sci. 68 (3), 913–921. doi:10.1007/s10620-022-07600-x
Shogan, B. D., Belogortseva, N., Luong, P. M., Zaborin, A., Lax, S., Bethel, C., et al. (2015). Collagen degradation and MMP9 activation byEnterococcus faecaliscontribute to intestinal anastomotic leak. Sci. Transl. Med. 7, 286ra68. doi:10.1126/scitranslmed.3010658
Strate, L. L., and Morris, A. M. (2019). Epidemiology, pathophysiology, and treatment of diverticulitis. Gastroenterology 156 (5), 1282–1298.e1. doi:10.1053/j.gastro.2018.12.033
Sun, L., Gao, W., Fu, X., Shi, M., Xie, W., Zhang, W., et al. (2018). Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater. Sci. 6, 340–349. doi:10.1039/c7bm00545h
Tan, B., Wang, M., Chen, X., Hou, J., Chen, X., Wang, Y., et al. (2014). Tissue engineered esophagus by copper-small intestinal submucosa graft for esophageal repair in a canine model. Sci. China. Life. Sci. 57 (2), 248–255. doi:10.1007/s11427-013-4603-0
Timnak, A., Yousefi Gharebaghi, F., Shariati Pajoum, R., Bahrami, S. H., Javadian, S., Emami Sh, H., et al. (2011). Fabrication of nano-structured electrospun collagen scaffold intended for nerve tissue engineering. J. Mater Sci. Mater Med. 22 (6), 1555–1567. doi:10.1007/s10856-011-4316-5
Tochigi, T., Kosugi, C., Shuto, K., Mori, M., Hirano, A., and Koda, K. (2017). Management of complicated diverticulitis of the colon. Ann. Gastroenterol. Surg. 2 (1), 22–27. doi:10.1002/ags3.12035
Tursi, A., Papa, A., and Danese, S. (2015). Review article: The pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment. Pharmacol. Ther. 42 (6), 664–684. doi:10.1111/apt.13322
Vakalopoulos, K. A., Bos mans, J. W. A. M., van Barneveld, K. W. Y., Vogels, R. R. M., Boersema, G. S. A., Wu, Z., et al. (2017a). Impact of tissue adhesives on the prevention of anastomotic leakage of colonic anastomoses: An in vivo study. Int. J. Colorectal. Dis. 32 (7), 961–965. doi:10.1007/s00384-017-2834-4
Vakalopoulos, K. A., Wu, Z., Kroese, L. F., van der Horst, P. H., Lam, K. H., Dodou, D., et al. (2017b). Clinical, mechanical, and immunohistopathological effects of tissue adhesives on the colon: An in-vivo study: Effects of Tissue Adhesives on the Colon: An in Vivo Study. J. Biomed. Mater. Res. B. Appl. Biomater. 105 (4), 846–854. doi:10.1002/jbm.b.33621
Waser, A., Balaphas, A., Uhe, I., Toso, C., Buchs, N. C., Ris, F., et al. (2023). Incidence of diverticulitis recurrence after sigmoid colectomy: A retrospective cohort study from a tertiary center and systematic review. Int. J. Colorectal Dis. 38 (1), 157. doi:10.1007/s00384-023-04454-1
Wu, K-L., Lee, K. C., Liu, C-C., Chen, H-H., and Lu, C-C. (2017). Laparoscopic versus open surgery for diverticulitis: A systematic review and meta-analysis. Dig. Surg. 34, 203–215. doi:10.1159/000450683
Keywords: colon diverticula, PVP patch, treatment, safety, pig model
Citation: Guo X, Patel B, Han L, Van Alstine WG, Noblet JN, Chambers SD and Kassab GS (2023) Novel patch biomaterial treatment for colon diverticulosis in swine model. Front. Bioeng. Biotechnol. 11:1215362. doi: 10.3389/fbioe.2023.1215362
Received: 01 May 2023; Accepted: 18 July 2023;
Published: 28 July 2023.
Edited by:
Masoud Mozafari, University of Toronto, CanadaReviewed by:
Vincenzo Davide Palumbo, Euro-Mediterranean Institute of Science and Technology (IEMEST), ItalyFengyuan Zhao, Peking University Third Hospital, China
Xiaoyuan Li, Northeast Normal University, China
Copyright © 2023 Guo, Patel, Han, Van Alstine, Noblet, Chambers and Kassab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghassan S. Kassab, Z2thc3NhYkBjYWxtaTIub3Jn
 Xiaomei Guo
Xiaomei Guo Bhavesh Patel
Bhavesh Patel Ling Han1
Ling Han1 William G. Van Alstine
William G. Van Alstine Sean D. Chambers
Sean D. Chambers Ghassan S. Kassab
Ghassan S. Kassab