
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Bioeng. Biotechnol. , 07 June 2023
Sec. Biosafety and Biosecurity
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1211789
This article is part of the Research Topic Policy and Regulation in Bioengineering and Biotechnology View all 12 articles
 Tlou Samuel Masehela1*
Tlou Samuel Masehela1* Eugenia Barros2
Eugenia Barros2The advances in the field of biotechnology (and bioengineering) over the past decades has allowed the precise development of new products across the agricultural, environmental, and pharmaceutical sectors. This has led to the need to evaluate the relevance and applicability of existing policies and frameworks that regulate the current transgenic technologies. On the African continent, there are delays in the development and implementation of biosafety policies and regulations. Most African countries formulate their policies, regulations, and frameworks by following The Convention on Biological Diversity’s (CBD) guidelines. Although the CBD documents are continually evolving, this happens at a slower pace. It is becoming increasingly important for countries to deal swiftly with the advances in biotechnology in a manner that balances the regulatory complexities, while safeguarding the net gains for human health, the environment, and the economy. For the African countries, some of these net gains are similar, while concerns and perceived risks associated with the adoption and use of the technology are also common. Furthermore, the challenges relating to capacity, knowledge, and skills to address some of the regulatory complexities. In this article we explore the advancement of some African countries in the development and implementation of various biosafety policies and detail the challenges and constraints faced by those countries that are lagging behind. We conclude by outlining identified opportunities for neighbouring and regional countries to assist one another and work in a more organised and coordinated approach towards developing, implementing, and strengthening their respective biosafety policies, regulations, and frameworks.
The field of biotechnology has overtime been recognised to be rapid in terms of new improvements and advancements towards supporting innovation across the different fields of research and development (Barragán-Ocaña, 2020; Ma, 2021). The significant potential for their applications cuts across many fields and disciplines, with the major ones being agriculture and health (medicine). In these two fields, biotechnology has presented to the human population several useful products by using enzymes, microbes, proteins, and various metabolic machinery of plants and animals (Masson et al., 2001; Khan, 2014; Pham, 2018).
The biggest impact of biotechnology has been in the field of agriculture mainly because of the need for more sustainable food production to feed the ever-increasing world population (Giller et al., 2021). Working with agricultural farmers, scientists have developed biotechnology tools to complement conventional crop improvement methodologies to produce genetically modified crops (GMOs). These crops are better adapted to grow in different environments, to be more resistant to agricultural biotic and abiotic stresses, to be better protected against pests and to have improved nutritional quality (Tran et al., 2010; Abdallah et al., 2014; Kamthan et al., 2016). The latest plant-breeding technology tool that has the potential to revolutionize agriculture is the development of genome edited crops. If the African continent is to benefit from these biotechnology developments there is an urgent need for discussion, debate, and harmonization of guidelines across the continent.
The adoption, application, and use, of biotechnology has not always been positive, as it has been marked with various concerns and controversies (Bauer, 2002). The debates on this subject comes mainly from the public and goes as far back as the early introductions of Genetically Modified (GM) products (Hielscher et al., 2016). In their early years, Genetically Modified crops, and foods, were to a large degree met with different perceptions and a strong level of mistrust–especially those based on personal or religious beliefs (Phillips, 2008). In most instances, the discussions and perceptions remain highly emotional, and focused on the potential economic, environmental, human health and social risks (Carr & Levidow, 2000; Goyal & Gurtoo, 2011; Lucht, 2015). Although, the trend on concerns varies across the continents, common issues are centred around ethical standards of practice, the morality and unpredictable results that come with different gene manipulations and experiments (Deane-Drummond et al., 2001). In some instances, questions are raised around the impacts on small-scale farmers and communities when it comes to seed rights and the socio-economic implications (direct/indirect), issuing of patents, and the equitable sharing of some of the proceeds from the biological resources and genetic material derived from regions/countries (Masehela et al., 2021). Furthermore, arguments remain that the GM technology depicts and promotes a particular narrative around a solution towards the global food crisis focusing on crops and traits (Stone & Glover, 2011; Stone & Glover, 2017). At the same time, others argue that a lot of the debates and criticism of the technology discredits various benefits already achieved with its application and use (Klümper & Qaim, 2014; Smyth, 2020).
The dawn of GMOs on the African continent has forever been marked by the hesitancy to accept, emanating from unfavourable policies and a wide array of public opinions (Gbadegesin et al., 2022). Besides the general lack of knowledge base, education and awareness of the technology and its application to the public (Gastrow et al., 2018), undecisive political attitude to GMOs has also been noted to have added more confusion and indirectly increased mistrust within the technology space (de Cheveigné et al., 2002). It is for this reason that there has been calls for care-based approach to ethics and politics so that social, economic, and ethical considerations are strategically incorporated into biotechnology governance and regulatory assessments (Wickson et al., 2017). For the African continent, this is important given that public trust is critical for the technology’s success and its benefits to be realised. However, this does not mean that the longstanding concerns, implications, and questions around safety should be forgotten (Trump et al., 2022). We know now that the world has begun embracing New Breeding Technologies (NBTs), spearheaded by the likes of CRISPR-Cas9 and other gene editing techniques (de Graeff et al., 2019). Already, we are seeing several concerns and oppositions to these technologies across the world (Helliwell et al., 2017), and since the African continent has not fully advanced from its GMOs challenges and drawbacks, it might be difficult to advance to the new politics and governance of these new technologies.
Countries and governments across the globe have set up regulatory agencies (bodies and committees) that will have oversight and make decisions regarding the validity of the research, development and the safety in the application of the technology and its derived products (McLean et al., 2012; Komen et al., 2020; Turnbull et al., 2021). However, the level in which the various regulations, biosafety frameworks and policy instruments are designed, implemented, enforced, and monitored differs depending on the country/government needs (Cantley, 2007). The focus areas are to a large extend guided, shaped and controlled, by country priorities, political influences and leadership, and the economic elements. For those countries that are signatories and party to the Cartegena Protocol on Biosafety to the Convention on Biological Diversity (CBD), the treaty was and remains instrumental in providing guidance and governance on the movements of living modified organisms (LMOs) resulting from modern biotechnology (Glass, 2000). Subsequent, several supplementary protocols and agreements have been put in place, recognising that with the rapid advances in the field of biotechnology; there is a need to protect biological diversity from the potential risks posed by living modified organisms (Shibata, 2014). At the same time, these key protocols have had their own shortcomings as they have not fully kept up with the fast developments within the biotechnology space and this is evident with the lack of clear definitions and guidance in fields such as Synthetic Biology (Hokanson, 2019; Groenewald, 2021). Although this can be viewed as a drawback, it should not undermine the substantial work done over the years through the various committees, expert and working groups [e.g., Ad Hoc Technical Expert Group (AHTEG)] and online forums of the CBD.
One of the major challenges for countries/parties has been that of taking on the guidance documents, training manuals and other supplementary materials for further development in line with their country needs (Pertry et al., 2014). Often, this failure is attributed to the lack of political will, lack of financial resources, relevant expertise, knowledge and experience in the respective policy and framework areas (Kameri-Mbote, 2002; Falkner & Gupta, 2004). This is particularly true for the African continent and remains a great challenge for most countries–in turn, lack of progression when it comes to exploring the potential applications of biotechnology and its associated bioengineering tools (Makinde et al., 2009). In this article, we explore: 1) the relevance and applicability of agricultural biotechnology to the African continent; 2) review and outline African countries that have made good strides in developing relevant biosafety protocols towards regulating the use of the technology; 3) explore some of the drawbacks of progress or reluctance in formulating and implementing biosafety protocols; and 4) propose or put forward an approach that could benefit the continent towards achieving various components of their frameworks, policy and biosafety protocols for guidance when considering the adoption and use of biotechnology–and bioengineering tools/options.
Biotechnology has a strong significance for the African continent in terms of contribution towards solving and/or offering options in mitigating a multitude of problems in both the agriculture and health sectors. Several studies do recognise the massive potential that biotechnology has to offer to the continent when it comes to improving agricultural production (Juma, 2015), improving economic growth, contributing to food and nutrition security (Binswanger-Mkhize et al., 2010; Kedir & Kararach, 2019), strengthening scientific capacity and advancement, providing alternative solutions to waste management, and improving health as well pharmaceutical options in the medicinal field (Bediako, 2022). The 2009 publication by the New Partnerships for Africa’s Development (NEPAD), outlines challenges facing the African continent on biotechnology and biosafety (Makinde et al., 2009). Among others, the report highlighted the financial challenges, the lack/loss of trained technical expertise; slow development of the biotechnology sector; inadequate Intellectual Property Rights infrastructure; lack of political will and government leadership. Today, these shortcomings remain prevalent and are evident in the lack of progress in biotechnology policy advancement and/or development of national biosafety frameworks (NBFs) across the continent.
Without these laws, regulations, guidelines, or policies related to biotechnology, it remains difficult to carry out or conduct any biotechnology related activities in the respective countries. Paarlberg (2009) indicates that one of the major constrains exploring new technologies in agriculture for Africa, stems from the lack of formulation–subsequently, implementation of relevant policies and regulations that would be geared towards agricultural advancement through science. In fact, they specifically cite the disapprovals on modern agricultural biotechnology because of inadequate policy frameworks to support its update. Similarly, Egwang (2001) and Bediako (2022), demonstrates that biotechnology has the potential to transform the health and the economies of most African countries, and that for this to be realised, African governments must create enabling environments through positive policies and the availability of resources.
African countries continue to face challenges when it comes to food production and medicinal needs (Pinstrup-Andersen & Watson, 2011). Countries find it difficult to provided adequate healthcare (and products/medicines), while farmers find it difficult to control and manage agricultural pests. At the same time, multilevel approaches are needed to overcome these challenges that are further exacerbated by increasing environmental, economic, and social challenges. Moreover, biotechnology has moved far beyond the basic principles of GMOs, offering some of the most powerful technological tools as options for mitigating most challenges and constraints in both agriculture and medical fields. Wambugu (1999), Machuka (2001), Nitin et al. (2022), Mfutso-Bengo & Muula (2007) and Sammut (2021) outline some of the potential benefits that can be realised for the African continent in agriculture and medicine, respectively.
The regulatory landscape of genetically modified products in Africa is still very diverse and harmonization of its regulatory processes has not yet been archived. There are many obstacles facing the commercial release of GM crops and they include biosafety factors, public and farmer acceptance as well as, political will and support (Akinbo et al., 2021). The 55 member states of the African union have developed specific regulatory agencies to approve seed regulation and variety regulation of crops produced by conventional methodologies under the Seed Act in addition to a National Biosafety Authority (NBA) that regulates crops developed using biotechnological approaches, like GMOs. Under the Seed Act regulation many African countries require approval by the National Performance Trial Committee (NPTC) and the National Variety Release Committee (NVRC) for the release and commercialization of conventionally derived seeds. Regarding the environmental release and commercialization of GMOs, African countries are at different levels of adoption of GM crops and only a few have approved the commercial release of crops for farmer adoption. To consider a joint and co-ordinated regulatory guideline for the continent one needs to understand where they are at, what regulations are in place and where the regulatory process could be fast tracked. An outline of the process used by Kenya, Nigeria, Eswatini, Ethiopia, Ghana, Malawi, Mozambique, Sudan and South Africa is summarized in Table 1 (Akinbo et al., 2021). These countries have commercialized GM crops (e.g., Bt cotton) but have their own specific Seed Laws and Regulations, and follow different steps some of which maybe more laborious resulting in a fast or slow approval of GM crops.
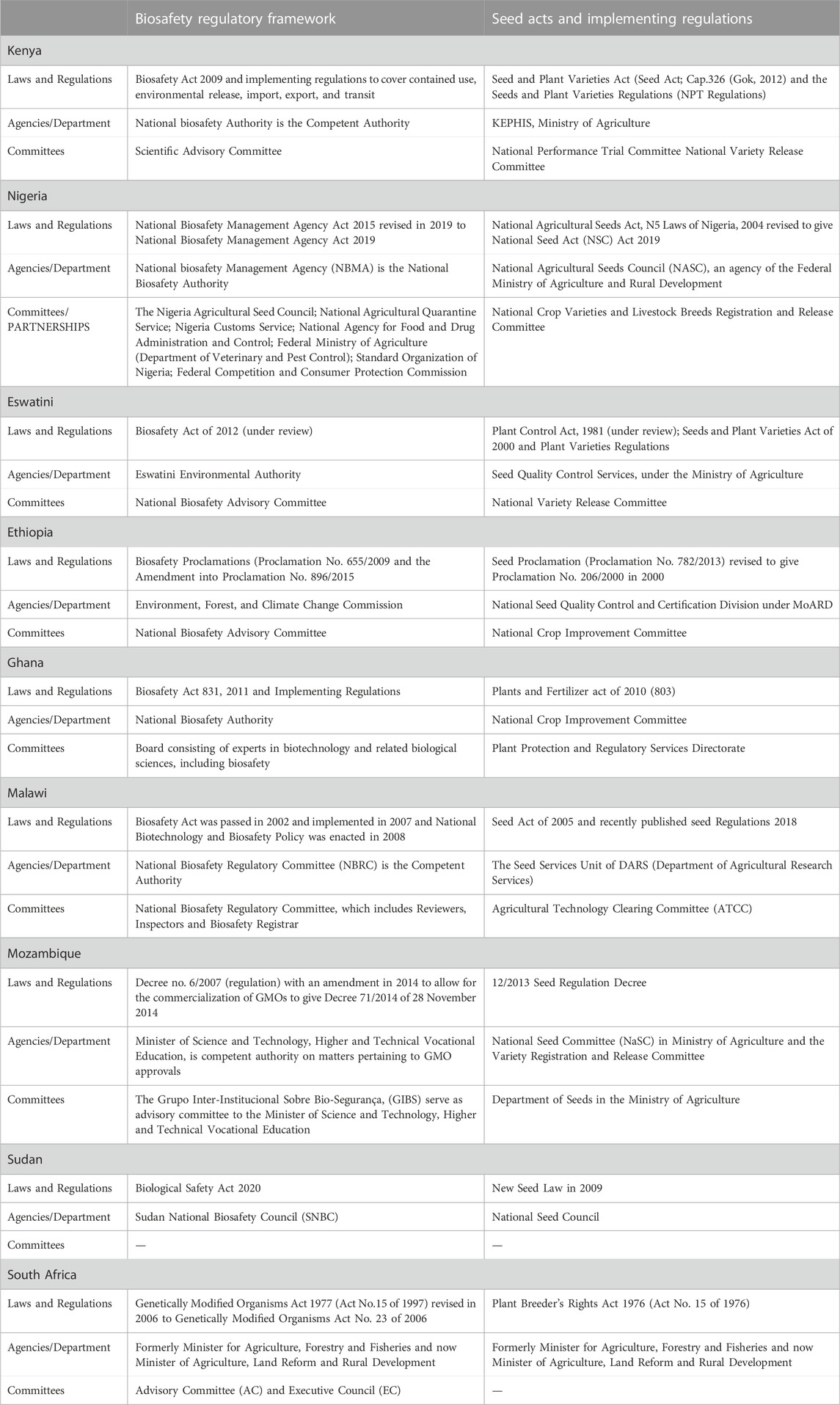
TABLE 1. Regulatory processes adopted by different African countries (adopted and modified from Akinbo et al., 2021).
With the rapid advances in biotechnology, it is crucial for African countries to work together and try to harmonize their science-based regulatory guidelines to be ready for the release and approval of products developed using CRISPR/Cas9-mediated genome editing. CRISPR-Cas9-based genome editing has become the most prevalent genetic engineering approach to develop improved crop varieties in addition to conventional technologies due to its simplicity, precision, and accuracy (Arora & Narula, 2017; Montecillo et al., 2020). Genome editing technologies enable the targeted manipulation of plant genomes and therefore it speeds up the breeding processes enabling breeders to address urgent goals with greater precision (Ceasar et al., 2016; Rao & Wang, 2021). Although globally there is not yet a definite consensus on how to regulate genome editing products, some countries have opted to regulate genome-edited crops based on the presence/absence of foreign DNA integration. So, genome-edited crops that do not have any foreign gene and the edited gene is not harmful to other plants and its safety attributes are comparable to its conventionally bred crops, does not require regulatory evaluation. Likewise, genome-edited foods whose safety attributes are comparable to those produced by conventionally bred crops, do not require regulatory evaluation.
Here, we are not suggesting or advocating that the African continent take a limited oversight on gene edited products, but rather explore paths towards homogeneity within the regulatory space of these new technology-based products, in line with their country specific needs and economical advancements. We also note that the scope of the technology and its applications will continue to advance, and the flexibility to accommodate these future developments will be of great importance. Therefore, bringing into the spotlight the need for effective risk management, responsible governance, and a robust approach to regulatory coherence.
To date, Nigeria was the first African country to develop biosafety guidelines through the National Biosafety Management Agency (NBMA 2020) to regulate genome editing products followed by Kenya. Both countries have adopted a case-by-case biosafety regulations for genome-edited products. As a result, when the genetic manipulation process requires the use of recombinant DNA sequences or the genome-edited product has a novel combination of genetic material, the product will be regulated as a GMO. But if the genetic changes do not include foreign DNA and thus introduces genetic changes that are comparable to conventional breeding outcomes, the product will be treated as a non-GMO and are therefore exempt from GMO regulations. South Africa has adopted the approach that gene-edited products should be treated as GMOs and as such to be regulated as GMOs (DALRRD Public Notice, 2021). Since the CRISPR/Cas9 technology was discovered, many African countries have been using it in the improvement of the major staple food crops (Tripathi et al., 2022). Currently, Burkina Faso, Egypt, Ethiopia, Ghana, Kenya, South Africa, and Uganda are the only African countries with active projects that involve the use of gene editing techniques (Gakpo, 2021; Karembu, 2021; Sprink et al., 2022).
Over the years, there has been various suggestions on how African countries can better approach processes of product development, deployment, and commercialization of biotech products (Makinde et al., 2009; Glover et al., 2018; Akinbo et al., 2021). Most common in these suggestions, is the regulatory process by legislative means that needs to be agile, proactive towards advancing tools and mechanisms of biotechnology, and overall harmonisation of the various steps within the evaluation and decision-making processes. The development of biosafety legislation across African countries, has not seen much improvement or progress since 2016. However, the efforts of NEPAD in establishing the African Biosafety Network of Expertise (ABNE) Programme in 2009, has contributed immensely to assisting African countries to develop functional biosafety systems, followed by the implementation of the Cartagena Protocol on Biosafety. At regional level, both Economic Community of West African States (ECOWAS) and Common Market for Eastern and Southern Africa (COMESA) have made commendable efforts towards development and harmonization of biosafety regulations for their members (Akinbo et al., 2021). The envisaged action plans on biotechnology and biosafety are mainly geared towards increased investment and promoting economic trade opportunities in the region. The AUDA-NEPAD (African Union Development Agency–New Partnership for Africa Development), transformed in July 2018, has also initiated the establishment of the Integrated Vector Management (IVM) Programme to strengthen or build regulatory capacities to enable scientists to explore genetic engineering for potential novel vector control tools on the continent (Savadogo, 2022). According to NEPAD, one of the key IVM Programme objectives includes bringing together biosafety regulators and health-related regulators to ensure safe development and potential deployment of Genetically Based Vector Control innovative tools.
The delay in the acceptance of GM crops in the African continent indicate that the introduction of similar or more advanced technologies, their envisaged benefits, their safety reservations/challenges and the associated safety guidelines should be addressed in a more transparent and coordinated manner to avoid a similar reaction towards NBT crops, that have already been adopted in some parts of the global north. So, policymakers should be given science-based information that would enable decision making in terms of biosafety, based on each country’s sovereign policies aiming at achieving the safe approval of GM crops and NBT/genome edited crops in the region, that would be environmentally and human safe and enable them to benefit from the advances in biotechnology (Akinbo et al., 2021). In the sections below, we identify areas where regions and the continent can work together, in a well-coordinated manner through a consultative approach towards advancing their biosafety regulations and biotechnology regulatory frameworks and policies.
Across the four recognised African regions, the challenges and needs in terms of the economic advancement, addressing poverty, hunger, health and education are the same if not similar. The needs are in line with the African Union’s goals and priorities of Agenda 2063, whereby goal 3, 5 and 7, are specific to healthy and well-nourished citizens, modern agriculture for increased productivity and production, as well as environmentally sustainable and climate resilient economies and communities, respectfully (African Union Agenda, 2063, 2015). Furthermore, the Agenda 2063 links the various goals to the various Sustainable Development Goals (SDGs), an indication that the continent is geared towards realising a better and more sustainable future for all.
In this article, we have already demonstrated how biotechnology can help improve some of the current conditions for the African continent in the agriculture sector. Already, these regions address some of the political and economic challenges and conflicts they face through their joint regional committees, and the same should be done when it comes to other areas that are not necessarily political. Already, the AU-NEPAD Africa’s Science and Technology Consolidated Plan of Action (CPA) was adopted in 2005, reaffirming the continent’s collective action for using technological innovations (Makinde et al., 2009). The CPA work has been coordinated through the different centres, namely, 1) North African Biosciences Network (NABNet); 2) West African Biosciences Network (WABNet); 3) Southern African Network for Biosciences (SANBio) and 4) Biosciences eastern and central Africa Network (BecNet). Each of these centres (nodes) has its own focus area of work depending on the region’s needs aligned with various technological development and advancements. However, not much is known about these networks and what work they do or what their annual targets are in terms of their plans, focus work area and scope. Therefore, the goals of these networks need to be well communicated and coordinated across the regions so that those willing to get involved know how to do so. Also, there needs to be strong partnerships with various stakeholders and multidisciplinary teams to ensure efficiency and that all projects are implemented in a coherent manner.
Horizon scanning has been an effective tool to help adequately prepare for any future activities or for the anticipation of new challenges. If performed consistently, it can assist towards identifying the areas of needs, gaps, and there could be plans formulated towards addressing any of these. Also, horizon scanning is an effective tool for bringing different skills set and knowledge (expertise) in different subject areas together, to not only unpack common challenges, but to also find viable and sustainable solutions. Within the regions, initiatives such as the African Scientists Directory, administered by the Academy of Science of South Africa (Mark, 2020), can be used to bring different experts across the fields of biosafety and biotechnology together to work through any challenges or to plan ahead for Africa’s needs and challenges. Through such initiatives, capacity building can also be fast tracked by encouraging knowledge sharing and exchange of programs with the various institutions of higher education. However, it is important that participation in all of these forums and initiatives include all countries to make sure that no one is left behind.
As already indicated, the African continent like many countries in the world is still grappling with the major areas of concern around the adoption and use of biotechnology. The major areas of concern remain, but not limited to the unintended harmful effects, environmental and food safety as well as ethical consideration. The social attitudes (and cultural aspects) also play a big role as they contribute to the public trust in the various processes governing the regulation and approval of GMOs on the continent. As a result, there remains strong doubts and to some degree prevalent acts of rebellion on any new form of biotechnology. Several studies have shown how the public is less aware and/or educated on the use and application of the technology across the continent (Zerbe, 2008; Clark et al., 2014; Gastrow et al., 2018). In some instances, it is also the general lack of understanding when it comes to the nature of genetic modification, its related techniques, and subsequent products (Marris, 2001; Aerni, 2013). It is also of note that even when such educational initiatives are put in place, there remains a greater degree of no interest, lack of participation or outright ignorance (Ahteensuu. 2012). Therefore, it remains an individual’s choice on how to receive and use the information at their disposal in the communication and debates related to the technology.
Other contributing factors relates to how the lack of transparency from governments is perceived by the public also contributes towards the erosion of trust on the newly deployed technologies. For example, the recent decision by the Kenyan government to lift a 10-year ban on GMOs brought about intense public opinion and debates (Oloo, 2022; The East African, 2022). Furthermore, it sparked fears that the country will be exposed to the control of seeds by multinational corporations, while biodiversity will continue to be at risk from GM crop cultivation. Also, the regulatory capacity was brought into question, with most activist groups and Non-Governmental Organizations (NGOs) believing that the country lacks the right approach to make the correct decisions on GMOs (Langat, 2022). Here, we witness once again the lack in proactiveness by regulatory authorities to take the public into their confidence in the decision taken on GMOs and addressing concerns on perceived risks. At the same time, we must acknowledge that it can also be difficult or close to impossible to try and convince the public to accept the decision on GMOs. However, it comes back to education and awareness, and the efforts to communicate transparently and in time, while allowing for a public participation process to take place. When such matters are debated vigorously in one country, it is bound to trickle to neighbouring countries and the region, making it difficult to manage any new ventures with the fear of the same (similar) setbacks. It is therefore important that the education and awareness on perceived risks associated with biotechnology be driven at regional level, with the help of experts in the field and the networks already established in the regions to deal with research and development of biotechnology.
The African continent faces many challenges, yet the resources required to address many of the challenges are never adequate, especially in those countries that need them the most. This has over the years contributed to the growing gap between country advancements in many areas. While some countries continue to do well in the markets and other elements of trade and development, other countries continue to lag behind. Although the urgency to address certain challenges will vary from country to country, there are those that are common within the agriculture, environment and health sectors that affect countries similarly if not equally. Also, the impacts thereafter often means that countries end up assisting each other or relying on one another for certain services and/or aid. Therefore, through the use of tools such as the horizon scanning process, countries and regions can begin to narrow down on what needs to be done or achieved first, followed by a phased in plan and strategies of common interest and how to achieve them. The knowledge and expertise through the expert’s consultation would be critical for identifying the skills sets and resources needed to achieve the identified goals or priority areas. Central to this process, would be to identify the lead institutions or networks–per region, to champion the process. Here, various oversight, monitoring and reporting mechanisms would need to be in place for all reporting purposes and to account for any activities within the programs.
The emerging and advancing biotechnology tools and methods have led to the regulatory authorities having to rethink the long-adopted approach of process-based regulations, previously developed for the GMO technology. In recent times, countries such as Argentina, Australia, Brazil, China, Japan, the United States, Nigeria, have taken the product-based approach (Lloyd et al., 2022). In both instances, the case-by-case basis evaluation in line with the CBD guidelines remains applicable. The debate is still out there in terms of the pros’ versus cons’ on the two regulatory approaches, but with the view that when it comes to CRISPR/Cas9-mediated (based) genome editing, there needs to be less regulatory burden as this hampers innovation; and this technology only modifies existing genetic material of the desired plant/animal (Lassoued et al., 2021). Therefore, the argument is that the same or similar regulations for GMOs, should not be subjected to genome edited products. For majority of the African countries (if not all), these new technologies are tried and licensed to foreign multinational companies and countries also remain importers of the “final product(s)”, derived through the new technologies.
As indicated, only seven (7) countries on the continent currently make use of the gene editing technology in various areas of research and development (Gakpo, 2021; Karembu, 2021; Sprink et al., 2022). Therefore, countries might remain net importers of GE derived products, making it difficult for them to apply the process-based risk analysis and regulations. Also, with the reality of the situation of porous borders between countries on the African continent where there is movement of people (including farmers), legally or illegally, may result in the exchange of seeds and food products where they are not approved or regulated formally. On the African continent, communities and small holder farmers have relied on informal seed systems for decades (Almekinders et al., 1994; Jones et al., 2001). This has served as a reliable and most important seed source of traditional food crops (Hlatshwayo et al., 2021). Furthermore, seed exchanges are central to the some of the traditional norms, are central to food sovereignty and strengthen social as well as cultural value systems among communities (van Niekerk & Wynberg, 2017). In addition, informal seed exchanges are not always restricted to or between farmers, as the practice can extend across villages or different regions (Pratap & Gupta, 2020).
Although the exchange of GM seeds or those developed using the technology is not established on the continent, it has been recorded that farmers do save GM derived seeds in South Africa (Masehela & Gouse, 2021). This makes it critical for countries to develop, finalise and implement their regulatory frameworks, and the process versus product regulatory approach will no doubt be central to deliberations involving the adoption and use of new technologies. As a result, countries and regions will need to engage in a more joint and coordinated manner to formulate their respective approaches in this regard, knowing very well that the option not to regulate, does not mean you will not have to deal with the product being present in the country.
While the field of biotechnology suffers from its own politics, the politics of governance–per country also needs to be decisive and favourable for research and development to thrive. It has been shown that government policies and positive political commitment to the biotechnology industry can have influence on how various investments are channelled for funding (Zarrilli, 2007). Africa also suffers from the formulation of many frameworks, action plans and the establishment of “working groups or committees”. Often, these groups come up with great regional approach and policy documents, which are signed off and endorsed by countries and regions, but hardly get implemented or reviewed for the effectiveness in terms of implementation. In some instances, no feedback is ever shared or given in terms of any progress or achievements. As a result, this adds to the frustrations in every attempt to fully implement biosafety regimes across the continent. Furthermore, managing public expectations becomes difficult as the overall public confidence and acceptance of biotechnology is pinned against the much-desired transparency and political goodwill.
Currently, there is a strong regional approach towards issues of trade (import/export) across the continent through the Inter Africa Trade discussions and policy developments, under the African Continental Free Trade Area (AfCFTA). These discussions also cover, to a large extent, country specific and regional orientated needs, challenges, and priorities. It is at this level that the biotechnology developments and advancements also need to take place, if they are to be taken seriously through any political agenda of the continent. Ultimately, harmonizing regulations and standards for biotechnology products, facilitating trade and economies is necessary for the advancement and adoption of new technologies in Africa.
We are not the first authors to identify challenges in the acceptance and adoption of GMOs in the African continent. Also, pointing out that this currently impacts on how the new and emerging technologies are being view in the public domain. While the development and implementation of various biosafety regulations and policies remain a challenge for many African countries, a few have made good strides and have also started utilizing new technologies such as genome editing. This is because they realise the potential to harness the products that will benefit the countries towards addressing several challenges relating to, among others, economic growth and trade, the impacts associated with climate change, hunger and nutrition, crop diseases and pests, as well as health and pharmaceutical needs. All these developments cannot be successful if there is limited involvement of African scientists, regulators and policymakers in the development and harmonization of regulations and policies that favours the adoption and use of new and emerging technologies. It is for these reasons that we put forward a few consultative and collaborative based approaches that the countries, regions and continent must consider if they are to fully give the technology and its various developmental stages a chance on the African continent. Central to this proposal is the political will, commitment, and action. Ultimately, the scientists, regulators and policymakers need to come together and openly discuss how they view the impact of these technologies, address any reservations that potentially may cause delays in the implementation of regulatory frameworks and policies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
TM and EB were both responsible for the conception and design of the article. TM wrote the outline approach to the article, with inputs and guidance from EB. Both TM and EB had dedicated sections to write for the manuscript. TM carried out the final revisions and edits, while EB carried out the final proof reading for the submitted version. All authors contributed to the article and approved the submitted version.
Author EB is the director of EB Biosciences and Consulting (Pty) Ltd. EB is a consultant in the field of biotechnology and in the risk assessment space, and serves in the Advisory Committee (AC) for Genetically Modified Organisms in the Department of Agriculture, Land Reform and Rural Development (DALRRD). EB has not produced any commercial products or patents.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, N. A., Moses, V., and Prakash, C. S. (2014). The impact of possible climate changes on developing countries: The needs for plants tolerant to abiotic stresses. GM crops food 5 (2), 77–80. doi:10.4161/gmcr.32208
Aerni, P. (2013). Resistance to agricultural biotechnology: The importance of distinguishing between weak and strong public attitudes. Biotechnol. J. 8 (10), 1129–1132. doi:10.1002/biot.201300188
Ahteensuu, M. (2012). Assumptions of the deficit model type of thinking: Ignorance, attitudes, and science communication in the debate on genetic engineering in agriculture. J. Agric. Environ. ethics 25, 295–313. doi:10.1007/s10806-011-9311-9
Akinbo, O., Obukosia, S., Ouedraogo, J., Sinebo, W., Savadogo, M., Timpo, S., et al. (2021). Commercial release of genetically modified crops in Africa: Interface between biosafety regulatory systems and varietal release systems. Front. Plant Sci. 12, 605937. doi:10.3389/fpls.2021.605937
Almekinders, C. J., Louwaars, N. P., and De Bruijn, G. H. (1994). Local seed systems and their importance for an improved seed supply in developing countries. Euphytica 78, 207–216. doi:10.1007/bf00027519
Arora, L., and Narula, A. (2017). Gene editing and crop improvement using CRISPR-Cas9 system. Front. Plant Sci. 8, 1932. doi:10.3389/fpls.2017.01932
Barragán-Ocaña, A., Silva-Borjas, P., Olmos-Peña, S., and Polanco-Olguín, M. (2020). Biotechnology and bioprocesses: Their contribution to sustainability. Processes 8 (4), 436. doi:10.3390/pr8040436
Bauer, M. W. (2002). Controversial medical and agri-food biotechnology: A cultivation analysis. Public Underst. Sci. 11 (2), 93–111. doi:10.1088/0963-6625/11/2/301
Bediako, Y. (2022). African biotech holds the key to transforming not just the health of African people, but our economies as well. Online at: https://speakingofmedicine.plos.org/2022/11/29/african-biotech-holds-the-key-to-transforming-not-just-the-health-of-african-people-but-our-economies-as-well/.
Binswanger-Mkhize, H. P., McCalla, A. F., and Patel, P. (2010). Structural transformation and African agriculture. Glob. J. Emerg. Mark. Econ. 2 (2), 113–152. doi:10.1177/097491011000200203
Cantley, M. (2007). An overview of regulatory tools and frameworks for modern biotechnology: A focus on agro-food.
Carr, S., and Levidow, L. (2000). Exploring the links between science, risk, uncertainty, and ethics in regulatory controversies about genetically modified crops. J. Agric. Environ. ethics 12, 29–39. doi:10.1023/a:1009595924500
Ceasar, S. A., Rajan, V., Prykhozhij, S. V., Berman, J. N., and Ignacimuthu, S. (2016). Insert, remove or replace: A highly advanced genome editing system using CRISPR/cas9. Biochimica Biophysica Acta (BBA)-Molecular Cell Res. 1863 (9), 2333–2344. doi:10.1016/j.bbamcr.2016.06.009
Clark, N., Mugabe, J., and Smith, J. (2014). Governing agricultural biotechnology in Africa building public confidence and capacity for policy-making.
DALRRD Public Notice (2021). Notice SA's regulatory approach for NBT's 2021. Online at: http://www.old.dalrrd.gov.za/Branches/Agricultural-Production-Health-Food-Safety/Genetic-Resources/Biosafety/Notifications.
de Cheveigné, S., Daniel, B., and Galloux, J. C. (2002). Les biotechnologies en débat: Pour une démocratie scientifique. Balland, 253.
de Graeff, N., Jongsma, K. R., Johnston, J., Hartley, S., and Bredenoord, A. L. (2019). The ethics of genome editing in non-human animals: A systematic review of reasons reported in the academic literature. Philosophical Trans. R. Soc. B 374 (1772), 20180106. doi:10.1098/rstb.2018.0106
Deane-Drummond, C., Grove-White, R., and Szerszynski, B. (2001). Genetically modified theology: The religious dimensions of public concerns about agricultural biotechnology. Stud. Christian Ethics 14 (2), 23–41. doi:10.1177/095394680101400203
Egwang, T. G. (2001). Biotechnology issues in Africa. Electron. J. Biotechnol. 4 (3), 23–24. doi:10.2225/vol4-issue3-fulltext-12
Falkner, R., and Gupta, A. (2004). Implementing the biosafety protocol: Key challenges. Royal Institute of International Affairs.
Gakpo, J. O. (2021). Ghana scientist turns to gene editing to improve sweet potato crop. Online at: https://allianceforscience.org/blog/2021/02/ghana-scientist-turns-to-gene-editing-to-improve-sweet-potato-crop/.
Gastrow, M., Roberts, B., Reddy, V., and Ismail, S. (2018). Public perceptions of biotechnology in South Africa. South Afr. J. Sci. 114 (1-2), 9. doi:10.17159/sajs.2018/20170276
Gbadegesin, L. A., Ayeni, E. A., Tettey, C. K., Uyanga, V. A., Aluko, O. O., Ahiakpa, J. K., et al. (2022). GMOs in Africa: Status, adoption and public acceptance. Food Control.109193.
Giller, K. E., Delaune, T., Silva, J. V., Descheemaeker, K., van de Ven, G., Schut, A. G., et al. (2021). The future of farming: Who will produce our food? Food Secur. 13 (5), 1073–1099. doi:10.1007/s12571-021-01184-6
Glass, J. A. (2000). The merits of ratifying and implementing the Cartagena Protocol on Biosafety. Nw. J. Int'l L. Bus. 21, 491.
Glover, B., Akinbo, O., Savadogo, M., Timpo, S., Lemgo, G., Sinebo, W., et al. (2018)., 12. BioMed Central, 11–28. doi:10.1186/s12919-018-0108-yStrengthening regulatory capacity for gene drives in Africa: Leveraging NEPAD’s experience in establishing regulatory systems for medicines and GM crops in AfricaBMC Proc., No. 8
Goyal, P., and Gurtoo, S. (2011). Factors influencing public perception: Genetically modified organisms. GMO Biosaf. Res. 2. doi:10.5376/gmo.2011.02.0001
Groenewald, H. (2021). “Chapter 5: The future: Induced genetic variation technologies, GMOs and responsible governance,” in Masehela. et al. 2021, an initial assessment of impacts on biodiversity from GMOs released into the environment in South Africa (Pretoria: South African National Biodiversity Institute, an entity of the Department of Forestry, Fisheries and the Environment), 84–91.
Helliwell, R., Hartley, S., Pearce, W., and O'Neill, L. (2017). Why are NGO s sceptical of genome editing? NGOs’ opposition to agricultural biotechnologies is rooted in scepticism about the framing of problems and solutions, rather than just emotion and dogma. EMBO Rep. 18 (12), 2090–2093. doi:10.15252/embr.201744385
Hielscher, S., Pies, I., Valentinov, V., and Chatalova, L. (2016). Rationalizing the GMO debate: The ordonomic approach to addressing agricultural myths. Int. J. Environ. Res. public health 13 (5), 476. doi:10.3390/ijerph13050476
Hlatshwayo, S. I., Modi, A. T., Hlahla, S., Ngidi, M., and Mabhaudhi, T. (2021). Usefulness of seed systems for reviving smallholder agriculture: A South African perspective. Afr. J. Food, Agric. Nutr. Dev. 21 (2), 17581–17603. doi:10.18697/ajfand.97.19480
Hokanson, K. E. (2019). When policy meets practice: The dilemma for guidance on risk assessment under the Cartagena protocol on biosafety. Front. Bioeng. Biotechnol. 7, 82. doi:10.3389/fbioe.2019.00082
Jones, R. B., Audi, P. A., and Tripp, R. (2001). The role of informal seed systems in disseminating modern varieties. The example of pigeonpea from a semi-arid area of Kenya. Exp. Agric. 37 (4), 539–548. doi:10.1017/s0014479701000461
Kameri-Mbote, P. (2002). The development of biosafety regulation in Africa in the context of the Cartagena protocol: Legal and administrative issues. Rev. Eur. Comp. Int'l Envtl. L. 11, 62–73. doi:10.1111/1467-9388.00303
Kamthan, A., Chaudhuri, A., Kamthan, M., and Datta, A. (2016). Genetically modified (GM) crops: Milestones and new advances in crop improvement. Theor. Appl. Genet. 129, 1639–1655. doi:10.1007/s00122-016-2747-6
Karembu, M. (2021). Genome editing in Africa’s agriculture 2021: An early take-off. Nairobi, Kenya: International Service for the Acquisition of Agri-biotech Applications (ISAAA AfriCenter.
Kedir, A. M., and Kararach, G. (2019). “Rethinking agricultural transformation and food security in Africa,” in Hunger and malnutrition as major challenges of the 21st century, 373–410.
Klümper, W., and Qaim, M. (2014). A meta-analysis of the impacts of genetically modified crops. PloS one 9 (11), e111629. doi:10.1371/journal.pone.0111629
Komen, J., Tripathi, L., Mkoko, B., Ofosu, D. O., Oloka, H., and Wangari, D. (2020). Biosafety regulatory reviews and leeway to operate: Case studies from sub-sahara Africa. Front. Plant Sci. 11, 130. doi:10.3389/fpls.2020.00130
Langat, A. (2022). Kenya lifts ban on genetically modified foods despite strong opposition. Online at: https://www.devex.com/news/kenya-lifts-ban-on-genetically-modified-foods-despite-strong-opposition-104170#:∼:text=Kenya%20lifts%20ban%20on%20genetically%20modified%20foods%20despite%20strong%20opposition,-By%20Anthony%20Langat&text=Genetically%20modified%20foods%20have%20been,country%20facing%20runaway%20food%20shortages.
Lassoued, R., Phillips, P. W., Macall, D. M., Hesseln, H., and Smyth, S. J. (2021). Expert opinions on the regulation of plant genome editing. Plant Biotechnol. J. 19 (6), 1104–1109. doi:10.1111/pbi.13597
Lloyd, J. R., Beger, D., and Pillay, P. (2022). South Africa should rethink regulations on genetically modified plants. Online at: https://theconversation.com/south-africa-should-rethink-regulations-on-genetically-modified-plants-176254.
Lucht, J. M. (2015). Public acceptance of plant biotechnology and GM crops. Viruses 7 (8), 4254–4281. doi:10.3390/v7082819
Ma, Q. P. (2021). Technological breakthroughs and future business opportunities in education, health, and outer space, 112–132.Biotechnology: Recent developments, emerging trends, and implications for business
Machuka, J. (2001). Agricultural biotechnology for Africa. African scientists and farmers must feed their own people. Plant physiol. 126 (1), 16–19. doi:10.1104/pp.126.1.16
Makinde, D., Mumba, L., and Ambali, A. (2009). Status of biotechnology in Africa: Challenges and opportunities. Asian Biotechnol. Dev. Rev. 11 (3), 1–10.
Mark, P. (2020). African Scientists Directory fosters collaboration, counters populism. Online at: https://www.universityworldnews.com/post.php?story=20201027033045494.
Marris, C. (2001). Public views on GMOs: Deconstructing the myths. EMBO Rep. 2 (7), 545–548. doi:10.1093/embo-reports/kve142
Masehela, T. S., and Gouse, M. (2021). “Chapter 3: Status and trends of GMO trials and general releases in South Africa,” in An initial assessment of impacts on biodiversity from GMOs released into the environment in South Africa. (Pretoria: South African National Biodiversity Institute, an entity of the Department of Forestry, Fisheries and the Environment), 35–53.
Masehela, T. S., Rhodes, J. I., Groenewald, H., Poole, C. J., Van den Berg, J., Gouse, M., et al. (2021). An initial assessment of impacts on biodiversity from GMOs released into the environment in South Africa. Pretoria: South African National Biodiversity Institute, an entity of the Department of Forestry, Fisheries and the Environment.
Masson, P., Tonello, C., and Balny, C. (2001). High-pressure biotechnology in medicine and pharmaceutical science. J. Biomed. Biotechnol. 1 (2), 85–88. doi:10.1155/s1110724301000158
McLean, M., Foley, M. E., and Pehu, E. (2012). The status and impact of bio safety regulation in developing economies since ratification of the Cartagena protocol.
Mfutso-Bengo, J. M., and Muula, A. S. (2007). Potential benefits and harm of biotechnology in developing countries: The ethics and social dimensions. Afr. J. Med. Med. Sci. 36, 63–67.
Montecillo, J. A. V., Chu, L. L., and Bae, H. (2020). CRISPR-Cas9 system for plant genome editing: Current approaches and emerging developments. Agronomy 10 (7), 1033. doi:10.3390/agronomy10071033
Nitin, K. S., Masehela, T. S., Chakravarthy, A. K., and Geerts, S. (2022). “Management of pests using genetic tools in Africa,” in Genetic methods and tools for managing crop pests (Singapore: Springer Nature Singapore), 303–326.
Oloo, B. O. (2022). Kenya has lifted its ban on genetically modified crops: The risks and opportunities. Online at: https://theconversation.com/kenya-has-lifted-its-ban-on-genetically-modified-crops-the-risks-and-opportunities-192636.
Paarlberg, R. (2009). Starved for science: How biotechnology is being kept out of Africa. Harvard University Press.
Pertry, I., Sabbadini, S., Goormachtig, S., Lokko, Y., Gheysen, G., Burssens, S., et al. (2014). Biosafety capacity building: Experiences and challenges from a distance learning approach. New Biotechnol. 31 (1), 64–68. doi:10.1016/j.nbt.2013.08.008
Pham, P. V. (2018). “Medical biotechnology: Techniques and applications,” in Omics technologies and bio-engineering (Academic Press), 449–469.
Phillips, T. (2008). Genetically modified organisms (GMOs): Transgenic crops and recombinant DNA technology. Nat. Educ. 1 (1), 213.
Pinstrup-Andersen, P., and Watson, D. D. (2011). Food policy for developing countries: The role of government in global, national, and local food systems. Cornell University Press.
A. Pratap, and S. Gupta (Editors) (2020). The beans and the peas: From orphan to mainstream crops (Woodhead Publishing).
Rao, M. J., and Wang, L. (2021). CRISPR/Cas9 technology for improving agronomic traits and future prospective in agriculture. Planta 254, 68–16. doi:10.1007/s00425-021-03716-y
Sammut, S. M. (2021). The role of the biotechnology industry in addressing health inequities in Africa: Strengthening the entire health care value chain. J. Commer. Biotechnol. 26 (4). doi:10.5912/jcb1008
Savadogo, M. (2022). Capacity strengthening for risk assessment of new biotechnologies in Africa – building on experiences with genetic biocontrol. Role of AUDA NEPAD. Side Event 4467, COP15, Montreal, Canada, December 9, 2022.
A. Shibata (Editor) (2014). International liability regime for biodiversity damage: The nagoya-kuala lumpur supplementary protocol (Routledge).
Smyth, S. J. (2020). The human health benefits from GM crops. Plant Biotechnol. J. 18 (4), 887–888. doi:10.1111/pbi.13261
Sprink, T., Wilhelm, R., and Hartung, F. (2022). Genome editing around the globe: An update on policies and perceptions. Plant Physiol. 190 (3), 1579–1587. doi:10.1093/plphys/kiac359
Stone, G. D., and Glover, D. (2017). Disembedding grain: Golden rice, the green revolution, and heirloom seeds in the Philippines. Agric. Hum. Values 34 (1), 87–102. doi:10.1007/s10460-016-9696-1
Stone, G. D., and Glover, D. (2011). Genetically modified crops and the ‘food crisis’: Discourse and material impacts. Dev. Pract. 21 (4-5), 509–516. doi:10.1080/09614524.2011.562876
The East African (2022). East Africa divided on GM foods as Kenya lifts ban. Online at: http://www.channelafrica.co.za/sabc/home/channelafrica/news/details?id=fb608914-7958-49d7-b6be-e5f9b8aac601&title=East%20Africa%20divided%20on%20GM%20foods%20as%20Kenya%20lifts%20ban.
Tran, L. S. P., Nishiyama, R., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2010). Potential utilization of NAC transcription factors to enhance abiotic stress tolerance in plants by biotechnological approach. Gm. crops 1 (1), 32–39. doi:10.4161/gmcr.1.1.10569
Tripathi, L., Dhugga, K. S., Ntui, V. O., Runo, S., Syombua, E. D., Muiruri, S., et al. (2022). Genome editing for sustainable agriculture in Africa. Front. Genome Ed. 4, 876697. doi:10.3389/fgeed.2022.876697
Trump, B., Cummings, C., Klasa, K., Galaitsi, S., and Linkov, I. (2022). Governing biotechnology to provide safety and security and address ethical, legal, and social implications. Front. Genet. 13, 1052371. doi:10.3389/fgene.2022.1052371
Turnbull, C., Lillemo, M., and Hvoslef-Eide, T. A. (2021). Global regulation of genetically modified crops amid the gene edited crop boom–a review. Front. Plant Sci. 12, 630396. doi:10.3389/fpls.2021.630396
van Niekerk, J., and Wynberg, R. (2017). Traditional seed and exchange systems cement social relations and provide a safety net: A case study from KwaZulu-natal, South Africa. Agroecol. Sustain. Food Syst. 41 (9-10), 1–25. doi:10.1080/21683565.2017.1359738
Wambugu, F. (1999). Why Africa needs agricultural biotech. Nature 400 (6739), 15–16. doi:10.1038/21771
Wickson, F., Preston, C., Binimelis, R., Herrero, A., Hartley, S., Wynberg, R., et al. (2017). Addressing socio-economic and ethical considerations in biotechnology governance: The potential of a new politics of care. Food ethics 1, 193–199. doi:10.1007/s41055-017-0014-4
Zarrilli, S. (2007). Global perspective on production of biotechnology-based bioenergy and major trends”. Rome: FAO Biotechnology and Bioenergy Seminars, FAO.
Keywords: Africa, biotechnology, biosafety, regulatory guidelines, policy, convention on biological diversity (CBD), genome editing, new breeding technologies (NBTs)
Citation: Masehela TS and Barros E (2023) The African continent should consider a harmonized consultative and collaborative effort towards coordinated policy and regulatory guidelines across the fields of biotechnology. Front. Bioeng. Biotechnol. 11:1211789. doi: 10.3389/fbioe.2023.1211789
Received: 25 April 2023; Accepted: 26 May 2023;
Published: 07 June 2023.
Edited by:
Andrea Wilcks, University of Copenhagen, DenmarkReviewed by:
Jason A. Delborne, North Carolina State University, United StatesCopyright © 2023 Masehela and Barros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tlou Samuel Masehela, dGxvdS5tYXNlaGVsYUBzYW5wYXJrcy5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.