- 1Department of Botany, University of Kerala, Thiruvananthapuram, Kerala, India
- 2School of Chemical Engineering, College of Engineering, University of Tehran, Tehran, Iran
- 3Department of Civil and Environmental Engineering, Western University, London, ON, Canada
- 4Department of Marine Biology, Faculty of Marine Science and Oceanography, Khorramshahr University of Marine Science and Technology, Khorramshahr, Iran
- 5Regulatory Systems Biology Lab, Center for Nuclear Energy in Agriculture, University of São Paulo, Piracicaba, Brazil
- 6Centro de Investigación en Biotecnología y Nanotecnología, Facultad de Ciencias Químicas, Universidad Autónoma de Nuevo León, Parque de Investigación e Innovación Tecnológica, Apodaca, Nuevo León, Mexico
- 7Facultad de Ciencias Químicas, Universidad Autónoma de Nuevo León, Universidad Autonoma de Nuevo Leon (UANL), Av Universidad s/n, CD. Universitaria, San Nicolás de los Garza, Nuevo León, Mexico
The burgeoning human population has resulted in an augmented demand for raw materials and energy sources, which in turn has led to a deleterious environmental impact marked by elevated greenhouse gas (GHG) emissions, acidification of water bodies, and escalating global temperatures. Therefore, it is imperative that modern society develop sustainable technologies to avert future environmental degradation and generate alternative bioproduct-producing technologies. A promising approach to tackling this challenge involves utilizing natural microbial consortia or designing synthetic communities of microorganisms as a foundation to develop diverse and sustainable applications for bioproduct production, wastewater treatment, GHG emission reduction, energy crisis alleviation, and soil fertility enhancement. Microalgae, which are photosynthetic microorganisms that inhabit aquatic environments and exhibit a high capacity for CO2 fixation, are particularly appealing in this context. They can convert light energy and atmospheric CO2 or industrial flue gases into valuable biomass and organic chemicals, thereby contributing to GHG emission reduction. To date, most microalgae cultivation studies have focused on monoculture systems. However, maintaining a microalgae monoculture system can be challenging due to contamination by other microorganisms (e.g., yeasts, fungi, bacteria, and other microalgae species), which can lead to low productivity, culture collapse, and low-quality biomass. Co-culture systems, which produce robust microorganism consortia or communities, present a compelling strategy for addressing contamination problems. In recent years, research and development of innovative co-cultivation techniques have substantially increased. Nevertheless, many microalgae co-culturing technologies remain in the developmental phase and have yet to be scaled and commercialized. Accordingly, this review presents a thorough literature review of research conducted in the last few decades, exploring the advantages and disadvantages of microalgae co-cultivation systems that involve microalgae-bacteria, microalgae-fungi, and microalgae-microalgae/algae systems. The manuscript also addresses diverse uses of co-culture systems, and growing methods, and includes one of the most exciting research areas in co-culturing systems, which are omic studies that elucidate different interaction mechanisms among microbial communities. Finally, the manuscript discusses the economic viability, future challenges, and prospects of microalgal co-cultivation methods.
1 Introduction
The escalating human population’s increased demand for raw materials and energy sources is predicted to have a deleterious environmental impact characterized by elevated greenhouse gas (GHG) emissions. This trend is expected to continue in the near future (Barati et al., 2021a), given the ongoing process of industrialization, economic growth, and energy consumption (Masson-Delmotte et al., 2021). Consequently, to counterbalance these environmental threats and generate alternative bioproduct-producing technologies, sustainable methodologies are no longer an option but a necessity. One promising approach involves utilizing microbial consortiums or communities as a platform for developing diverse, sustainable applications that can outperform current wastewater treatment technologies, reduce GHG emissions, alleviate the energy crisis, and improve soil fertility (Das et al., 2021). The inclusion of microalgae species in such consortia addresses an essential aspect of circular economy and bioeconomy strategies: the generation of high-value compounds derived from the photosynthetic metabolism of oxygenic microalgae species.
Microalgae are photosynthetic microorganisms inhabiting marine and/or freshwater ecosystems. They exhibit a remarkably high CO2 fixation capacity compared to any other land plant, while also producing oxygen (Barati et al., 2021b). They can convert light energy into biomass and organic chemicals (Moreno-Garcia et al., 2017) and can consume atmospheric CO2 or industrial flue gases under specific circumstances, thereby reducing GHG emissions while producing biomass. Furthermore, microalgae can consume nutrients available in wastewater and collaborate with bioremediation (Barati et al., 2021b). Culturing domestic strains is typically straightforward, easy to maintain, and does not compete for arable lands (Lakshmikandan et al., 2020). Moreover, several species can exhibit an extraordinary capacity to adapt to different environmental niches, facilitating the bioprospecting of a microalgae species suitable for a particular environmental condition or its adaptation to a cultivation process (Lam and Lee, 2012). The potential for biotechnological and commercial applications of microalgae biomass is vast. It has been used in animal and human nutrition, cosmetics, biofertilization, the dyes industry, and antioxidant and pharmaceutical compounds (Rizwan et al., 2018). Additionally, bio-oil from microalgae can be used for biofuel production, in agricultural applications, controlling ammonia and balancing pH drops caused by nitrifying bacteria in an aquaponic system (Addy et al., 2017). They can also benefit plant growth in hydroponic systems by providing oxygen for the plant and utilizing the CO2 produced by respiration and exudation of crop roots for their growth, thereby inhibiting anaerobiosis in the crop’s root system (Huo et al., 2020).
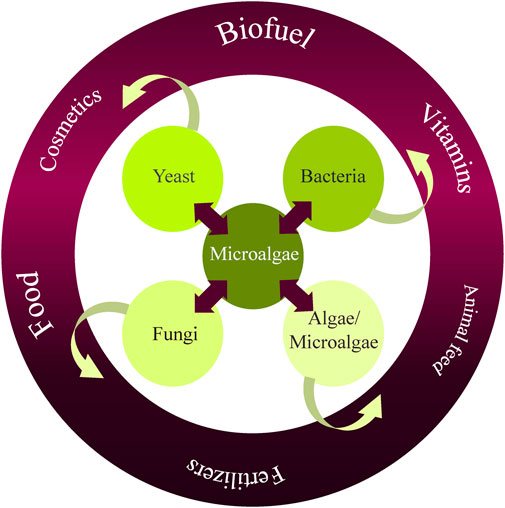
Most microalgae cultivation studies have been focused on monoculture systems. However, monoculture open cultivation systems pose significant challenges due to contamination by other microorganisms, such as yeasts, fungi, bacteria, and other microalgae species. These instances of contamination can lead to low productivity, culture collapse, low-quality biomass, and nutrient loss. Accordingly, recent attention has been drawn to co-culture systems and the potential advantages of developing specific, robust microorganism consortia. Due to microalgae’s metabolic adaptability and capacity for survival in diverse environmental conditions, co-culturing microalgae with other microorganisms may circumvent the constraints of monoculture in open systems (Rashid et al., 2019). Co-culturing microalgae in consortia, at both small and large scales, has been developed and is utilized in biomanufacturing, with proposed applications in the food, agronomic, pharmaceutical, nutraceutical, chemical, biofuel sectors, and other industries associated with bioremediation and nutrient recycling strategies (Figure 1).
This review presents a thorough examination of research conducted in the last few decades, exploring the advantages and disadvantages of microalgae co-cultivation systems, including microalgae-bacteria, microalgae-fungi, and microalgae-microalgae/algae systems. The manuscript also addresses diverse uses of co-culture systems and growing methodologies and includes one of the most exciting research areas in co-cultivation systems, specifically, omics analysis, capable of elucidating different interaction mechanisms among microbial communities. Finally, the manuscript discusses the economic viability, future challenges, and prospects of microalgal co-cultivation methods.
2 Microalgal co-cultivation systems
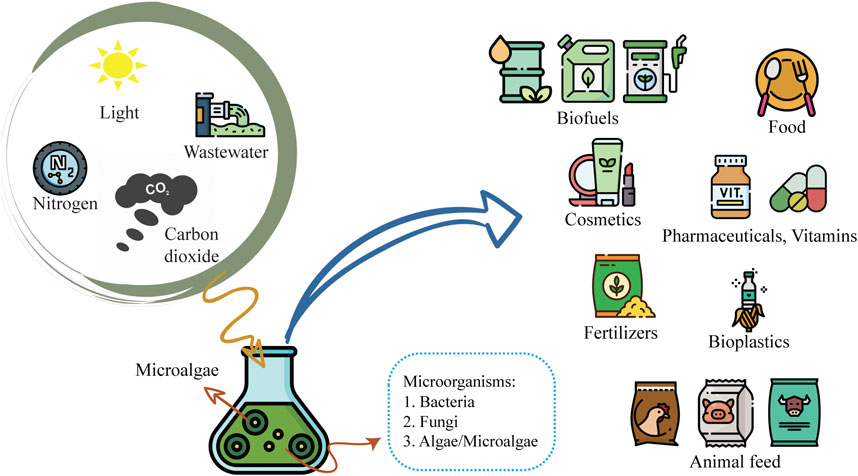
Microalgae cultivation systems are a promising intersection of biology and sustainable industrial practice, offering potential for diverse product generation and bioremediation. These systems can be broadly classified into two categories: open and closed. An open system primarily comprises artificial ponds that are highly influenced by environmental fluctuations and are particularly susceptible to contamination by non-beneficial microorganisms (Figure 2). In contrast, closed systems consist of various photo-bioreactors (PBRs) (Figure 3), which require greater investments in initial infrastructure but are typically less vulnerable to cross-kingdom and cross-species contamination and can result in higher productivity of special high-value compounds.
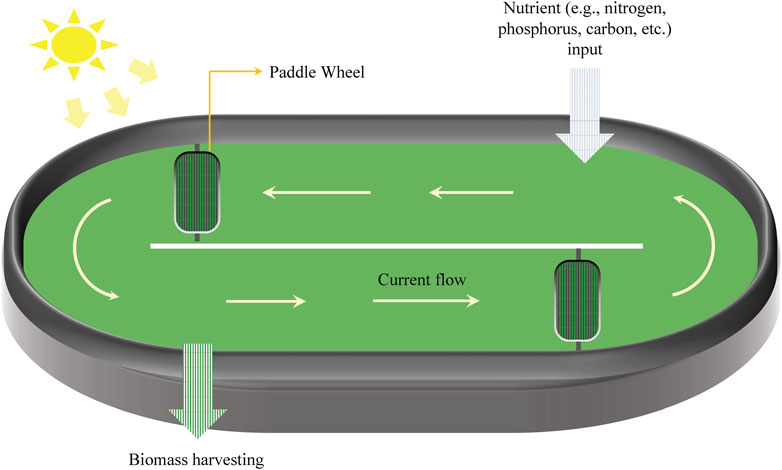
FIGURE 2. Schematic representation of the open pond system. The co-cultivation of microalgae in an open pond system is somewhat similar to the consortia in nature.
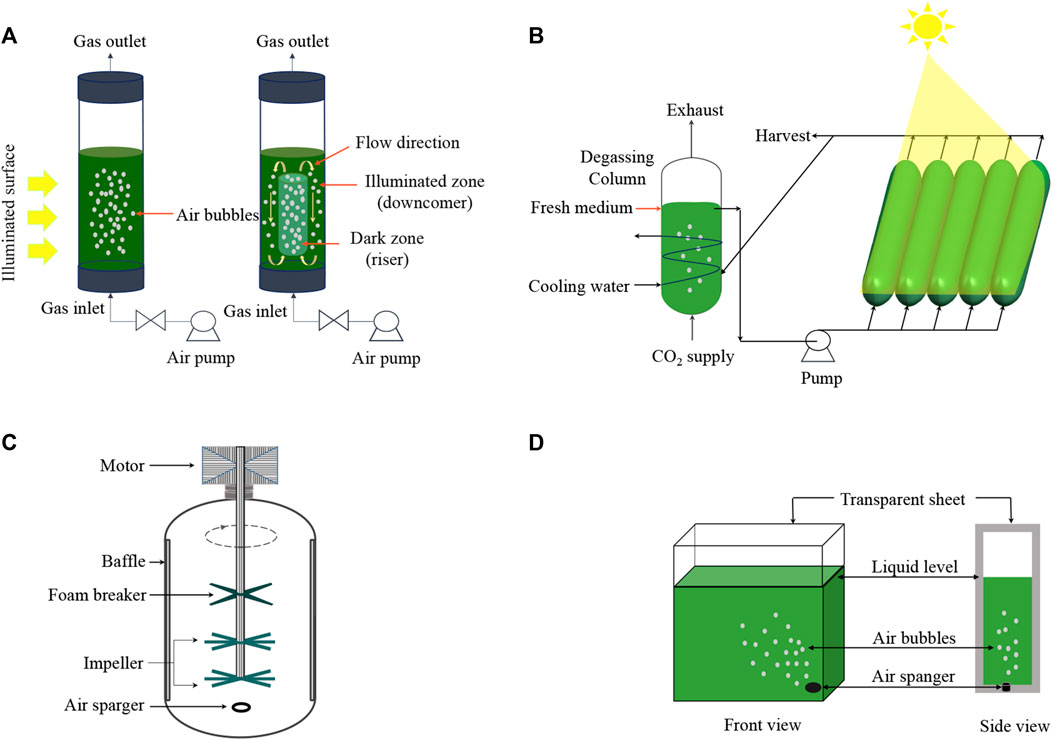
FIGURE 3. Types of photobioreactors for microalgae consortium. Vertical tube PBR (A): Bubble column (left) and Airlift column (right); Horizontal tube PBR (B); Stirred tank mechanism (C); Flat panel PBR (D).
Co-cultivation systems, an intersection of biology and sustainable industrial practice, hold promise for diverse product generation and bioremediation. The construction of these systems, whether open or closed, hinges on a clear understanding of the elements involved. Within these systems, we primarily encounter two types of cultures: axenic cultures, which host a single species, and non-axenic cultures, which are consortia of multiple microorganisms. The choice between these cultures depends on the goals of the co-culture, whether they be production, substrate consumption, or biomass accumulation. An essential consideration is whether survival of all constituent species is vital.
Designing these co-cultures involves applying principles of evolution: natural selection acting upon a diverse population (‘top-down’) or hand-picking strains (‘bottom-up’). Both approaches carry potential, dependent on the circumstances and the goals set. Biological interactions are as complex as they are vital. The range of possible interactions include from mutualism and commensalism to predation, parasitism, amensalism, and competition. A co-culture system may host several of these interactions simultaneously, even with just two species. Understanding these interactions is a prerequisite for effective system design.
Co-cultures come in various forms - suspended, flocculation, biofilms, and membranes, each with implications for reactor design and operational strategies. Another pivotal factor is illumination management. This is especially important for algae-based co-cultures, because light directly influences the productivity of photosynthetic microorganisms.
While creating minor adjustments to the co-culturing system can disrupt its balance, it is vital to establish new ways to design vessels, tanks, or reactors to anticipate culture behavior and facilitate future optimization. Identifying the composition of the extracellular chemical milieu, including metabolites, peptides, or proteins secreted by species within the consortium, is a significant step towards building consortium production (Lakshmikandan et al., 2021). However, we face challenges tracking molecular transfers between microbes and boosting the large-scale application of these technologies. One of the primary obstacles to further implementing microalgal biomass as an economically feasible feedstock is to achieve high biomass production coupled with the high yield of desired metabolites, cost-effective dewatering and harvesting of biomass, and a green and efficient procedure for product extraction (Kumar et al., 2020). Despite numerous attempts to increase microalgal productivity through nutritional, environmental, and physiological alteration-based cultivation, commercial success has been modest (Pierobon et al., 2018). Therefore, it is highly desirable to improve our understanding of the limitations and advantages of the current strategies of microalgae co-cultivation and the incorporation of recent knowledge generated through omics analysis into the perception and development of future co-cultivation systems towards increasing their applicability, especially in sustainable applications.
Next, we examine the primary methods currently employed for microalgae co-cultivation, highlighting the potential contributions of omics analysis data and recent knowledge acquired about microalgae metabolism. These findings may complement and enhance novel co-cultivation strategies.
3 Microalgae-bacteria co-cultivation
Establishing any type of association between microorganisms in the same cultivation system requires a deep understanding of their metabolic needs. The ideal situation for maintaining a biological consortium is where two or more species involved benefit from the interaction. This can be achieved in some cases where the products of one species’ metabolism can be metabolized by the other, and vice versa, to boost both growth and the desired biotechnological goal (e.g., bioremediation, chemical degradation, bioproduct synthesis, etc.).
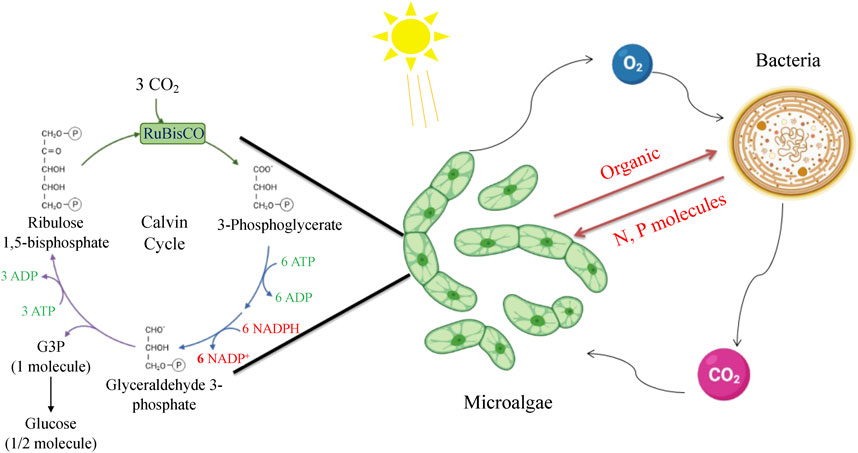
Co-cultivation systems of microalgae and bacteria can be established for specific combinations of species where complex nutrient cycling patterns can be obtained, satisfying the needs of each organism through interactions with other members of the culture, resulting in either a symbiotic interaction or synergetic association, which are at least partially advantageous for both species in the microorganism community. The selection and optimization of this system for long-term sustainable applications require an understanding of the microorganism community and its structure, which is usually dynamic during processes that use waste or residue materials. This type of co-culture has been proposed for sustainable energy production, bioremediation (mainly for wastewater treatment), food, pharmaceutical, and medical industries (Padmaperuma et al., 2018; Yao et al., 2019). Currently, the application of microalgae-bacteria consortia has shown several advantages, such as bacteria stimulating the growth of microalgae by producing growth-promoting substances, vitamins, and cofactors (Dao et al., 2018), microalgae producing O2 through photosynthesis, which oxygenic bacteria can use, and in return, bacteria producing CO2 which can be fixed by microalgae photosynthetically (Makut et al., 2020), and microalgae secreting several complex molecules that may serve as a source of carbon and nitrogen for bacterial growth (Guo and Tong, 2014).
Microalgae species have the capability to fix atmospheric carbon dioxide through photosynthesis, which is then assimilated into organic compounds via the Calvin–Benson–Bassham (CBB) cycle, a crucial part of their primary carbon metabolism. In addition, the microalgae’s respiration produces oxygen, which can benefit heterotrophic bacteria. Additionally, microalgae can release dissolved organic matter (DOM), dissolved organic carbon (DOC), dissolved organic nitrogen (Cieplik et al., 2018), and dissolved organic phosphorous (DOP), which serve as nutrient sources for bacterial growth. Bacterial re-mineralization of these organic nutrients into inorganic forms can further promote microalgae growth (Amin et al., 2012; Borowitzka and Moheimani, 2013; Thompson and Zehr, 2013). Apart from nutrient cycling, microalgae and bacteria have a beneficial interaction via the exchange of molecules such as siderophores, which enhance iron supply to microalgae during growth (Vraspir and Butler, 2009). Microalgae and bacteria can also synthesize different vitamins, including B12, B1, and B7. This are essential for microalgal growth, establishing another instance of mutually beneficial interaction between the two microbial communities (Yong et al., 2014).
3.1 Microalgae-bacteria co-cultivation methods
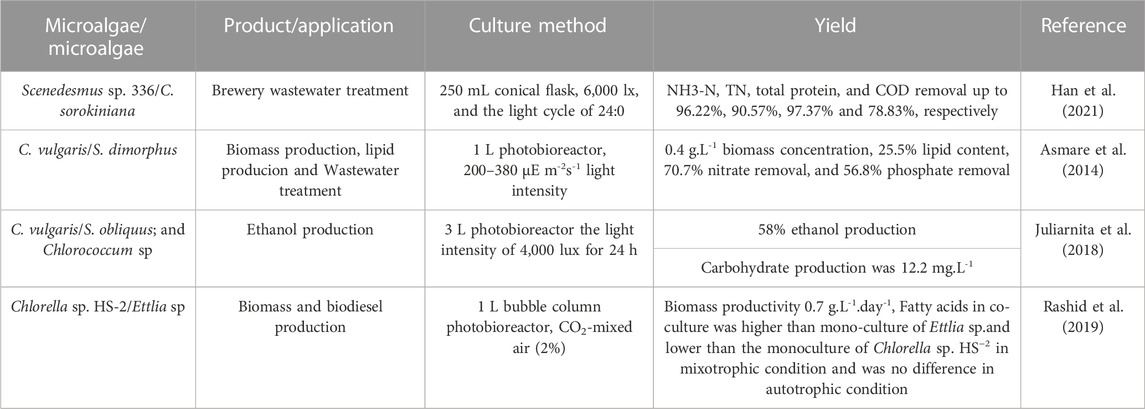
There are two major types of interactions that can be seen in co-cultures: mutualism and commensalism. In a mutualistic relationship, both organisms gain benefits from the interaction. On the other hand, commensalism refers to a relationship where one organism benefits, and the other is unaffected. These relationships can be complex, with outcomes dependent on the specific organisms and environmental conditions involved. Both of these microalgae-bacteria interactions in co-cultivation have been widely used for various applications through several methods, including direct mixing, pelletization and flocculation, encapsulation, biofilm formation, cell droplets synthesis, membrane separation, dialysis tube system, and agar. Each of these methods has its own advantages and drawbacks, such as scalability, ease of implementation, and yield potential, which should be considered when selecting the appropriate method for a particular application. A summary of these methods is presented in Table 1.
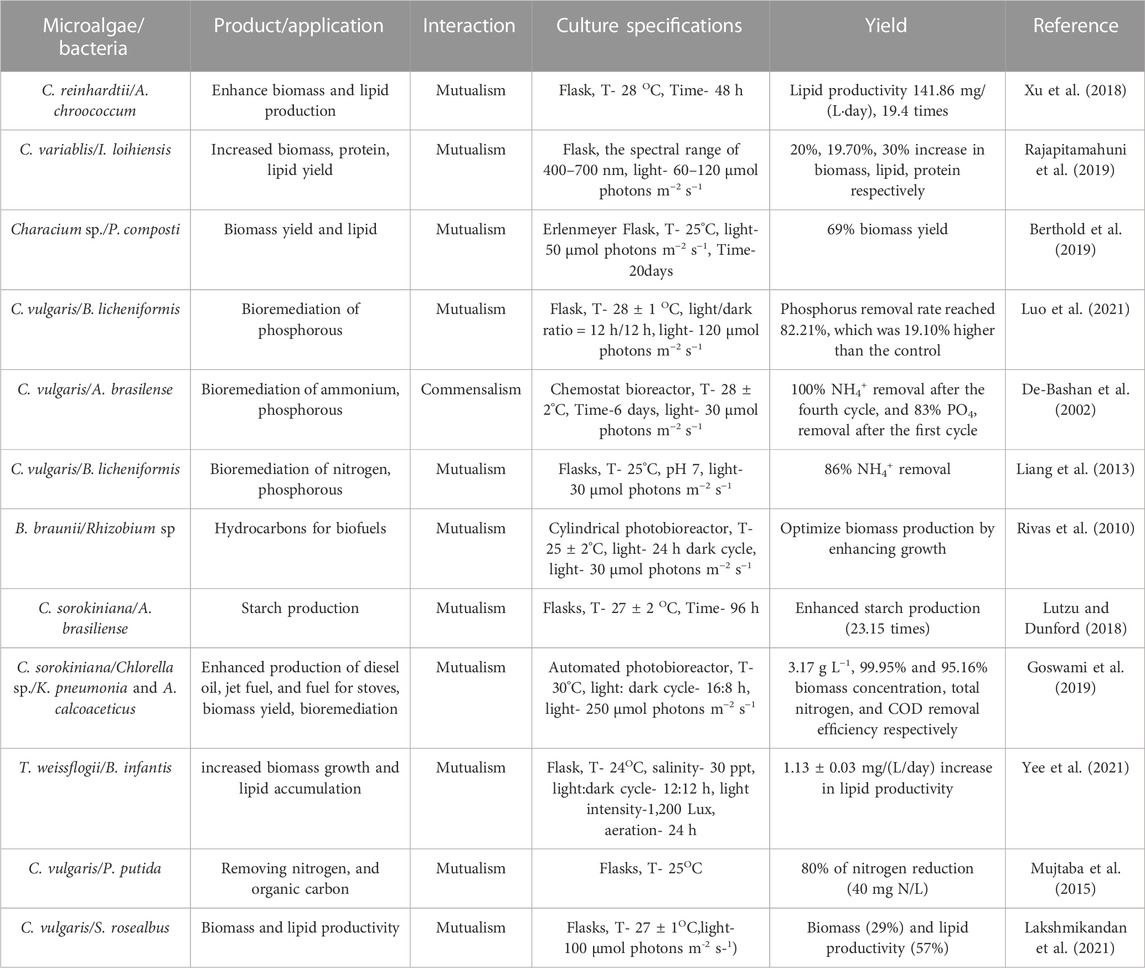
TABLE 1. Microalgae-bacterial co-culture systems used for bioremediation, biomass, and lipid productions (T-temperature).
3.1.1 Direct mixing
Direct mixing is a commonly used method for establishing a consortium between microalgae and bacteria. This method involves the physical contact between the two organisms, leading to the exchange of signaling molecules and metabolites, as well as the competition for nutrients in an uncontrolled manner. Direct mixing is often employed for the cultivation of microorganisms for bioremediation, hydrogen, and biofuel production, and is known to lead to better outcomes in terms of bioactive compound production when compared to monocultures (Tran et al., 2010). An example of its potential can be seen in a study that observed a mutualistic relationship between the dinoflagellate Prorocentrum minimum and the bacterium Dinoroseobacter shibae. The study showed that direct mixing can lead to a symbiotic relationship where both organisms equally benefit (Wang et al., 2014). However, generalizations from these findings should be taken with caution, as every co-culturing situation has its unique characteristics.
3.1.2. Pelletization and flocculation
Establishing specific consortia of microorganisms can be achieved by exploiting the flocculating properties of one or both partner cells, which can be induced by compounds released by one member of the consortium. This process results in the formation of pellets or aggregates of cells, which can optimize symbiosis and improve settling ability, especially of microalgae species (Powell and Hill, 2013). For example, a pH-dependent and reversible aggregation process was observed in Bacillus sp. with Nannochloropsis oceanica, which retained its ability to aggregate after fixation with paraformaldehyde, allowing for cell reuse (Powell and Hill, 2013). Co-cultivation of Chlorella vulgaris with different species of bacteria has been shown to cause microalgal flocculation, resulting in improved biomass yield and metabolite production (Lee et al., 2013). Similarly, consortia between Halomonas sp. and Micrococcus sp., Staphylococcus sp., or Pseudomonas sp. have also been reported to result in microalgal flocculation (Okaiyeto et al., 2013).
3.1.3 Biofilms
Biofilm-based co-cultivation of microalgae and bacteria has gained recent attention due to its potential for high efficiency in collecting and dewatering cell suspensions. It is considered an alternative method for producing biomass that overcomes the drawbacks of suspended cultivation systems, while meeting the requirements for generating biomass for biofuel coupled with wastewater treatment (Kesaano and Sims, 2014; Berner et al., 2015). Most microalgal biofilms are composed of bacteria and microalgae, which are typically inexpensive, accessible, and reusable. Biofilm-based cultivation provides the microalgae with more exposure to light than when suspended in a liquid medium (Zhang et al., 2018), which improves biomass productivity, shortens hydraulic retention times (Boelee et al., 2014), makes it easier to control the cell growth area, and appears to have high efficacy in wastewater treatment (Ma et al., 2018). The improved biomass and pigment synthesis of aquatic photosynthetic microalgae biofilms were reported in the presence of Bacillus stratosphericus (Miranda et al., 2017). Despite these potential advantages, the complexities of biofilm interactions and management must not be overlooked.
3.1.4 Encapsulation
In this method, one microorganism is immobilized in beads and co-cultured with the other microorganisms in a liquid medium, increasing the chances of biomass reuse. Co-culturing of Synechococcus sp. (cyanobacterium) beads with Chlamydomonas reinhardtii (microalgae) improved the growth and lipids production of the microalgae and increased the possibility of recycling the beads (Magdouli et al., 2016). Co-immobilization and co-encapsulation of microalgae and bacteria in alginate beads prevent the ingress of external microbiota and the release of immobilized microorganisms into wastewater, making them suitable for bioremediation processes to reduce ammonium and phosphorous from wastewater. However, the growth suppression is limited by the native wastewater bacterial community (Covarrubias et al., 2012). The microalgae Haematococcus pluvialis is widely studied in encapsulation systems for food applications. Cyst cells of H. pluvialis generated under unfavorable environmental conditions contain a substantial amount of astaxanthin, a powerful antioxidant. Due to its sensitivity to light, oxygen, and high temperatures, astaxanthin extraction has been studied using encapsulation techniques (Sarada et al., 2006; Zhao T. et al., 2019).
3.1.5 Cell droplets
The cell droplets technique, although not commonly used in reactor settings, has gained attention for its ability to facilitate the culturing of microorganisms that are difficult to cultivate under laboratory conditions. This method involves developing aqueous two-phase systems with polymers to create water-in-water emulsion systems that promote multi-organism aggregation, allowing individual cells to represent single batches of cultivation. Symbiotic microorganisms have been successfully isolated using this technique. In a previous report, an aqueous two-phase system was used to trap bacterial colonies within magnetic dextran phases and suspend them as cell droplets to algal colonies in a polyethylene glycol phase, resulting in improved communication between the droplet colonies (Byun et al., 2013). This method has been described as high-throughput screening of cell-to-cell interactions (HiSCI) in algae growth-supporting bacteria.
3.1.6 Membrane separation (vessel chambers)
The membrane separation technique involves culturing microorganisms in separate compartments of a vessel, connected by a semipermeable membrane that allows the diffusion of metabolites between the chambers. This method has been used to study ecological systems such as predator-prey and phytoplankton communities (Paul et al., 2013). For example, the co-culture of D. shibae and Thalassiosira pseudonana separated by a membrane resulted in high metabolite diffusion rates, with the bacterium products influencing the metabolic profile of T. pseudonana cells and enhancing their amino acid content (Paul et al., 2013). However, the success of this method is dependent on the nature of the exchanged molecules and the positive or negative effects of allelopathic interactions (impacts induced by the products released in the medium) on cell growth. The interaction of Oocystis marsonii with Microcystis aeruginosa through membrane diffusion has been shown to inhibit the allelopathic activity of the bacteria on the green algae, compared to the direct mixing method (Dunker et al., 2017).
3.1.7 Dialysis tube
Even though the dialysis system operates under a concept similar to the membrane separation system, it is worth including as a separate co-culturing method since it offers a different level of control over the exchanges between the microorganisms. It is a co-culturing method in which molecules and ions produced by one microorganism can selectively move through a dialysis membrane to the culture medium of the other microorganism, and vice versa. The properties of the dialysis membrane, such as its semi-permeability and molecular weight cut-offs, determine the size of the molecules that can be transferred or exchanged between the microorganisms. In this method, the guest strain of the microorganism is placed in a dialysis bag and suspended in a large vessel containing the host strain of the microorganism in a free liquid medium. Dialysis-mediated co-culture has been used to explore new interspecies allelopathic interactions with methods such as biochemical analyses, proteomics, and metabolomics. For example, the co-culture of M. aeruginosa with C. vulgaris through dialysis mediated a negative inhibition of microalgae growth by releasing linoleic acid, while the nitric oxide from C. vulgaris stimulated the positive feedback mechanism of linoleic acid production by M. aeruginosa (Song et al., 2017).
3.1.8 Agar
Agar systems offer a method of spatially separating and co-culturing microorganisms on a porous solid agar support with different compositions, such as potato dextrose and LB-agar (Barka et al., 2002). While this might resemble the process of flocculation, it is essential to differentiate that the cell aggregation in agar systems happens due to the solid medium’s properties rather than the induced properties of the microorganisms. The concentration of agar used may vary depending on the nature of the microorganisms and the purpose of the study. Co-culturing Pseudomonas diminuta and Pseudomonas vesicularis on agar plates with Scenedesmus bicellularis and Chlorella sp. Revealed that the rate of diffusion for info-chemicals depends on the agar’s volume, porosity, and composition (Mouget et al., 1995). The extracellular metabolites in a large pool of culture medium with a very low concentration are difficult to isolate, identify, quantify, and reproduce.
In conclusion, these methods indeed have overlapping features, but it is the nuanced differences and the contexts in which they are used that create their individual identities. From the simplicity and cost-effectiveness of direct mixing to the unique control mechanisms of encapsulation and dialysis tube systems, each method has its own specific applications and benefits.
3.1.9 Applications, advances, challenges, and future prospective in microalgae-bacteria co-cultivation
Microalgae have been extensively studied for co-culturing with growth-promoting bacteria. This is due to their ability to produce extracellular chemicals and act as a potential and ecologically sound alternative to current carbon sequestration techniques for CO2 mitigation. Although most microalgae lack the mechanism for fixing nitrogen and phosphorus, they compensate by providing fixed organic carbon to the bacteria (Kim et al., 2014) (Figure 4). This cooperative interaction has proved to be an effective co-culturing method for microalgae and bacteria, with increasing densities of bacteria and microalgae expected to strengthen the beneficial relationship. In the context of CO2 bio-mitigation applications, microalgae-bacteria consortia can supply O2 and organic compounds for bacterial consumption through photosynthesis (C3 Calvin cycle and C4 pathways), while bacteria can produce CO2 and inorganic substances to support microalgal growth (Liu et al., 2017). By utilizing this mutually beneficial interaction of CO2 and O2, the capital expenses for the oxygenation of activated sludge tanks and the risk of thermal decomposition can be significantly decreased (Acién et al., 2016). Furthermore, bacteria can produce micronutrient metabolites such as vitamin B12, phytohormones, thiamine derivatives, and siderophores to speed up microalgal metabolism and biomass growth (Ramanan et al., 2016). The interactions between microalgae and heterotrophic bacteria also occur in oligotrophic conditions, particularly through macronutrient-mediated interactions.
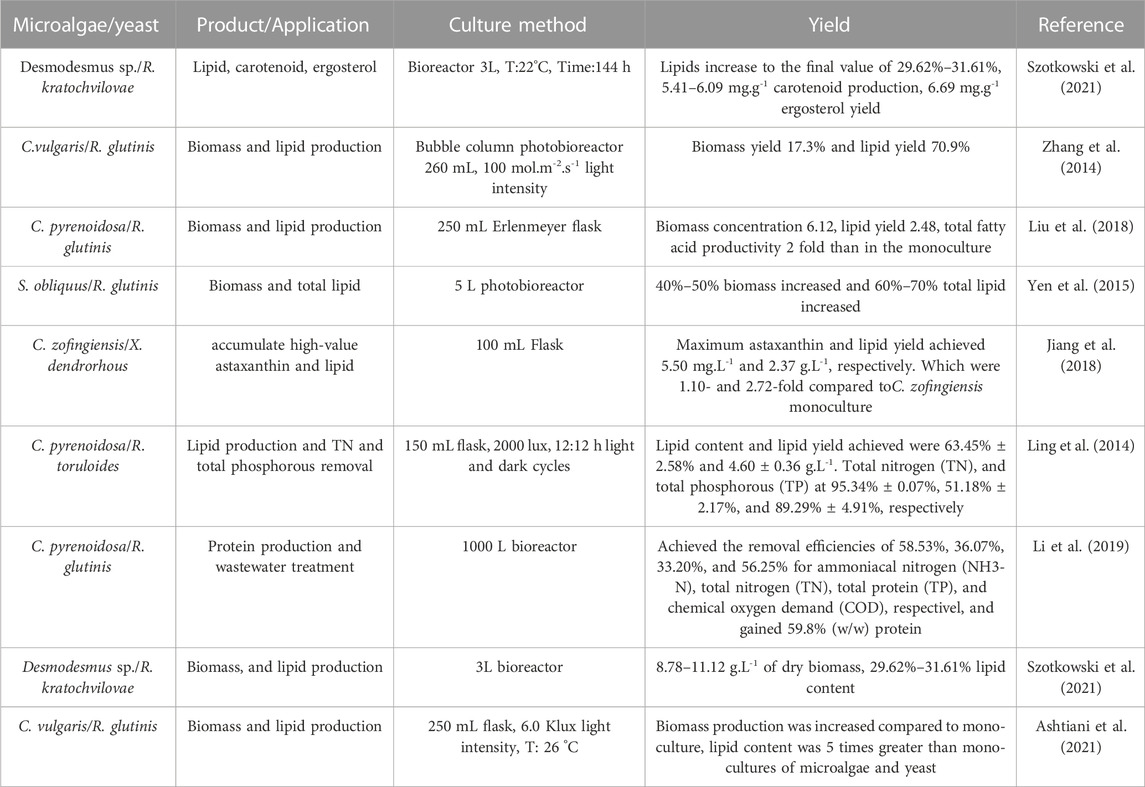
The use of microalgae-bacteria symbiosis for bioremediation offers several advantages over conventional methods, such as its ability to withstand a variety of environmental conditions, the stability of the partnership, metabolite and nutrient exchange, and protection against invading species (Subashchandrabose et al., 2011). For example, the co-cultivation of C. vulgaris with activated sludge bacteria improved nutrient and dissolved oxygen performance and facilitated microalgal harvesting (Medina and Neis, 2007). The remediation of synthetic wastewater was also enhanced by the co-cultivation of photosynthetic bacterium R. sphaeroides and green algae C. sorokiniana (Ogbonna et al., 2000). The interactions between microalgae and bacteria have been found to enhance microalgal biomass production and nutrient removal (Sültemeyer, 1998). Careful selection of co-culture members is important for efficient nutrient removal from wastewater and enhanced algal biomass production. Table 1 provides an overview of microalgae-bacterial co-culture systems used for bioremediation, biomass, and lipid production.
In biofuel production, microalgae-bacteria co-cultures have demonstrated improved efficiency compared to monocultures. Co-cultures have been successfully used to convert microalgae biomass into various types of biofuels, including biodiesel, biohydrogen, bioethanol, biomethanol, biobutanol, and biomethane, using lipids, carbohydrates, and proteins (Owolabi et al., 2012). Biodiesel is a promising renewable energy source that can significantly reduce emissions of unburned hydrocarbons and carbon monoxide, without the production of sulfur and aromatic byproducts (Mondal et al., 2017). Microalgae can produce organic matter, such as triglycerides (TAGs), from CO2 and water, which can be used as precursors for biodiesel production (Scott et al., 2010). TAGs are usually over-accumulated and stored in specialized lipid bodies present in the cytosol of the cells (Mata et al., 2010). Some species of Pseudomonas in association with Chlorella sp., have been efficiently utilized for biodiesel production (Bell et al., 2016).
Moreover, microalgae have the potential to be a renewable and carbon-neutral source of energy through the production of hydrogen fuel, which can be converted into electricity for various applications. Bacterial fermentation produces CO2, which promotes microalgal growth; on the other hand, oxygen is synthesized during microalgal photosynthesis, promoting bacterial growth. However, due to the production of oxygen and the presence of hydrogen, which make an explosive gas combination, the removal of oxygen from the media, through physical or chemical methods, is a critical step in achieving efficient H2 photoproduction (Fakhimi et al., 2020). Among the various H2 photoproduction systems studied for yield and sustainability, Chlamydomonas in consortia with different bacterial species such as Pseudomonas, Bacillus, Clostridium, Bradyrhizobium japonicum, Rhizobium etli, and Escherichia coli have been extensively researched (Xu et al., 2016; Ban et al., 2018). Microalgae-bacteria consortia, specifically the Chlamydomonas-bacteria system, have shown potential for enhancing algal H2 production through starch accumulation and metabolite exchange secretion. This process involves the release of electron donors such as acetate, ethanol, formate, and glycerol into the medium, which can be used by bacteria to synthesize H2 (Xu et al., 2017; Carbonell et al., 2018). Additionally, Lactobacillus amylovorus can hydrolyze starch to lactic acid from algal biomass, which can be used for photo-H2 production in Rhodobacter capsulate, Rhodobacter sphaeroides, Rhodobium marinum, and Rhodospirillum rubrum (Kawaguchi et al., 2001). Some bacteria can also synthesize H2 through fermentative pathways. Certain bacteria, such as Clostridium, have a PFOR-H2 production pathway that can synthesize acetic acid as an end-product. Similarly, E. coli uses FPL-H2 production pathways to obtain acetic acid and ethanol as end products (Oh et al., 2011).
The potential of microalgae-bacteria symbiosis for bioethanol production from polysaccharides such as starch, cellulose, and sugars through fermentation has also gained attention. Microalgae species like Chlorella, Dunaliella, and Scenedesmus have been identified as feedstock for bioethanol production due to their high starch content (Özçimen et al., 2015). Starch granules make up to 40% of most microalgae species’ dry weight, and bacteria can ferment these to produce ethanol (Ramanan et al., 2016). Marine microalgae’s starch-containing biomass can be saccharified to produce ethanol using amylase from the bacterium Pseudoalterimonas undina (Matsumoto et al., 2003). Efficient bioethanol production has been demonstrated by enzymatic hydrolysis of C. reinhardtii using amylase from the marine bacterium Bacillus licheniformis, followed by fermentation with Saccharomyces cerevisiae (de Farias Silva and Bertucco, 2016).
Microalgae provide trace elements like iron, cobalt, and zinc that can fulfill the nutrient requirements for bacteria, whose biomass can be converted into biomethane and biogas by anaerobic digestion (Grobbelaar et al., 2004). The metabolic activities of anaerobic bacteria can impact the proportion of proteins, carbohydrates, and lipids in the biomass (Illman et al., 2000), and the methanogenic potential of microalgae can be influenced by the protease resistance of their cell walls (Angelidaki and Sanders, 2004). The lysis of microalgal cell walls in Botryococcus braunii and Nannochloropsis gaditana can be achieved by endoglucanase activities of various cellulolytic bacterial species (Muñoz et al., 2014). Bio-augmentation of C. vulgaris biomass with a cellulolytic and hydrogenogenic bacterium, Clostridium thermocellum, can improve the degradation efficiency, leading to higher levels of methane and hydrogen production and increasing the overall biogas yield (Lü et al., 2013).
Microalgae biomass is composed of carbohydrates, proteins, lipids, and organic and inorganic molecules that can be converted into various products through enzymatic, chemical, or microbial conversions (Mutanda et al., 2020). Microalgae are rich in poly-unsaturated fatty acids (PUFAs), such as arachidonic acid, docosahexaenoic acid, linolenic acid, and eicosapentaenoic acid, which have potential applications as dietary supplements for humans and animals (Benemann, 1990; Radmer, 1996). The β-carotene from the microalgae Dunaliella salina is used as an antioxidant supplement in humans and animals (Spolaore et al., 2006), while microalgal biomass from Chlorella, Dunaliella, Isochrysis, Nannochloropsis, Nitzschia, Pavlova, Phaeodactylum, Scenedesmus, Skeletonema, Spirulina, Tetraselmis, and Thalassiosira have been used as feed for mollusks, crustaceans, and fish. Additionally, microalgal biomass can be used to produce fertilizers (Metting and Pyne, 1986), exopolysaccharides for medical and pharmaceutical purposes (Zhang et al., 2019), as well as biodegradable plastics, bioflocculants, bioactive compounds, cosmetics, and polysaccharides (Olaizola, 2003; Pulz and Gross, 2004).
The current drawbacks of microalgae-bacteria co-cultivation include its high cost and low sustainability due to challenges associated with its disposal. However, recent research suggests that the use of biomaterials with advanced harvesting methods can facilitate the repurposing of residual organic matter for the production of biofuels, biomolecules, and animal feed, thus promoting a more sustainable bio-economy (Stiles et al., 2018). Microalgae-bacteria symbiosis has gained attention as a cost-efficient method for wastewater treatment and bioremediation, as it enables successful removal of pollutants, sequestration of greenhouse gases, flocs production, and elimination of pathogens (Barati et al., 2021a; Barati et al., 2021b; Saravanan et al., 2021).
4 Microalgae fungi co-cultivation
Microalgae-fungi co-cultivation despite being a more recent method in comparison to microalgae-bacteria co-culture, offers promising outcomes for microalgae biomass separation via co-pelletization into fungal pellets. This process utilizes filamentous fungi-based flocculation, an approach that is cost-effective and environmentally-friendly, given its lack of chemical reliance, ability to yield various floc sizes, and wide applicability in biomass processing (Xie et al., 2013; Rosero-Chasoy et al., 2021). The interplay between fungi and microalgae within a shared ecological niche involves competition for nutrients and space, with the release of extracellular enzymes that hold potential for the production of high-value products (Sandland et al., 2007; Bertrand et al., 2014). Antagonistic (where fungi benefit from the host), mutualistic (where both organisms benefit from one another), and parasitic (where fungi benefits from other microorganisms) relationships often typify the dynamics between fungi and other microorganisms (Yu et al., 2021). The process of fungal pellet formation involves the germination of embryonic mycelium germinates from the fungal spore branch (fungal hyphae) leading to visible pellets. When growth conditions deteriorate, the hyphae begin self-decomposition and can aggregate in submerged culture (Espinosa-Ortiz et al., 2016). Pellet formation is dependent on electrostatic interactions, hydrophobicity, and the specific interactions of spore wall components, influenced by the medium composition and physicochemical properties of fungi (Grimm et al., 2005; Zhang and Zhang, 2016). Microalgae-fungi co-cultivation under optimal conditions can lead to the formation of fungi-microalgae pellets, facilitating microalgae harvesting through simple filtration, a fundamental principle behind this method. The fungal spore-assisted (FSA) or fungal pellet-assisted (FPA) methods are commonly employed for microalgal harvesting from the co-culture of microalgae with fungal spores or pre-cultured fungal pellets (Chen et al., 2018; Yang et al., 2019). Microalgae cells can bind to fungal cells, possibly via a charge-neutralization mechanism (Zhang and Hu, 2012; Wrede et al., 2014). The negative charge on the algae surface at neutral pH due to the presence of proton-active carboxylic, phosphoric, phosphodiester, hydroxyl, and amine functional groups, can be neutralized by the positive charge on fungi, acting as a cationic flocculant towards microalgae (Wrede et al., 2014). The mutual benefits of co-cultivation of microalgae and fungi, particularly yeast, has been evidenced in biodiesel production, wastewater treatment, chemical production, and aquaculture feed applications. For instance, yeast in a co-culture system can generate carbon dioxide for microalgae biosynthesis, while microalgae provide oxygen for yeast respiration. The efficiency of resource utilization and reduction of carbon emissions into the atmosphere, however, are offset by challenges in biomass production cost and energy-efficiency (Rakesh and karthikeyan, 2019).
4.1 Microalgae-fungi co-cultivation methods
Co-cultivation techniques can vary, each with distinct benefits and limitations. These methods include direct mixing, encapsulation, pelletization and flocculation, biofilms, and solid-liquid interfaces. Notably, it is important to understand the nuances between seemingly similar techniques, such as membranes and dialysis, or agar and flocculation, each having specific applications and effects on co-cultivation. While these methods can generally improve efficiency compared to monocultures, it is critical to validate this claim with empirical evidence from specific applications.
4.1.1 Direct mixing
This method, whereby microalgae and fungi are co-cultivated resulting in direct interaction and exchange of signaling molecules within the same environment (Brenner et al., 2008), is common to many co-cultures. It has been utilized to explore the physical and biochemical interactions, yield parameters, and metabolite production between fungi and algae (Oh et al., 2007). For example, in the co-culture of Chlorella and Aspergillus, microalgae biomass yield, lipid content, and cellular oil exhibited improvements (Yang et al., 2019). Similarly, the consortium of Mucor circinelloides and C. vulgaris showed improved biomass yield, lipids, and saturated and unsaturated fatty acids that can be utilized for biodiesel production (Zorn et al., 2020). However, the effectiveness of this method significantly depends on the selection of microorganisms and their interactions. For example, establishing a balanced co-culture of Scenedesmus obliquus and Candida tropicalis, by altering the population density to algae: fungi (2:1), improved algal biomass production (Wang et al., 2015). The significance of inoculation ratios/population density was later confirmed by a study using a consortium of Chlorella pyrenoidosa and Rhodotorula glutinis, where a higher ratio of algae: fungi (3:1) was found to be ideal for achieving the highest biomass concentration and lipid productivity as well as enhancing nutrient removal from wastewater and protein productivity (Li et al., 2019). Direct mixing of the microalgae Chlorella sp. and S. cerevisiae, showed an increase in biomass and lipid productivity of Chlorella sp. and enhanced the carbon bio-fixation compared to their mono-culture (Shu et al., 2013). Furthermore, co-cultivation of S. obliquus and R. glutinis showed a significant increase in the biomass and lipid productivity of S. obliquus (Yen et al., 2015). This method, though commonly used, does not apply to all co-cultures and variations exist based on the organisms and environment used.
4.1.2 Encapsulation
This method, where microorganisms are immobilized through gel entrapment, usually involves natural polysaccharides such as alginates and agar (Kitcha and Cheirsilp, 2014). This is distinct from pelletization and flocculation, as encapsulation focuses on entrapment within a gel matrix. For instance, co-capsulation of the yeast Trichosporonoides spathulata and the microalgae C. vulgaris in alginate gel beads not only simplifies the harvesting process but also maintained growth and lipid production of C. vulgaris at levels comparable to those of free cells (Kitcha and Cheirsilp, 2014).
4.1.3 Pelletization and flocculation
Co-cultivation of microalgae with fungi can lead to natural pelletization and flocculation. For example, co-cultivation of Chlorella protothecoides and Tetraselmissuecica with fungal strains isolated from compost, straws, and soil resulted in higher biomass, lipid productivity, and bioremediation efficacy compared to monocultures (Muradov et al., 2015). Similar co-cultures of C. vulgaris and two species of Aspergillus sp. Xhibited similar results (Zhou et al., 2012). Pleurotus ostreatus, an edible fungus strain, has been developed to increase the efficiency of microalgae harvesting for feed or food production in a low-cost manner (Luo et al., 2019). They found that pellets of Pleurotusostreatus co-cultured with Chlorella sp. at 100 rpm agitation and low pH showed better harvesting efficiency than pellets cultured under 0 rpm and 150 rpm agitation. In heterotrophic co-culture conditions, the co-culture of Aspergillus niger and C. vulgaris presented lower flocculation efficiency compared to autotrophic conditions (Zhang and Hu, 2012). The pelletization and flocculation efficiency of the consortium can be influenced by the co-cultured microorganisms and the carbon source used in the system (Gultom et al., 2014; Luo et al., 2019). The optimal culture conditions for pelletization and flocculation can vary depending on the system. For instance, co-cultivation of filamentous fungus (A. niger) and microalgae (C. vulgaris) to produce cell pellets was evaluated under various concentrations of organic carbon sources (glucose, glycerol, and sodium acetate), and the optimal culture conditions for reaching >90% cell harvest efficiency were found to be 2 g L-1 glucose as an organic carbon supply for fungal growth and the formation of cell pellets (Gultom et al., 2014). However, the concentration of the flocculant and its binding strength were proven ineffective at higher microalgae biomass concentrations, resulting in variations in pellet morphology. A co-culturing ratio of 1:300 (fungi: microalgae) improved the harvested efficiency by over 90% (Gultom et al., 2014). The co-pelletization of autotrophic microalgae C. vulgaris by precultured Aspergillus oryzae pellets promoted biomass production (99.23%), lipid (33.97%), and biofuel production (Chu et al., 2021). Although charge neutralization was not the main mechanism involved in fungi-algae aggregations, changes in functional groups on cell surfaces and secreted metabolites in the medium could be mainly responsible for inducing the bioflocculation process.
4.1.4 Biofilms
In the field of bioremediation and bioprocessing applications such as biomass harvesting, mycoalgae biofilms, resembling lichen structures on a supporting polymer matrix, have been gaining interest. The concept of mycoalgae biofilms has stemmed from previous knowledge of fungi and algae interactions (Rajendran and Hu, 2016; Rajendran et al., 2017). Biofilms formed by microalgae in the presence of non-photosynthetic cohabitants, such as Acremonium sp. and Aspergillus sp., have shown enhanced biomass growth and photosynthetic efficiency. These improvements have been evidenced by fingerprint profiles of the isolated photosynthetic components (Miranda et al., 2017).
4.1.5 Solid-liquid interface
In a novel approach, a photobioreactor system was used to enhance biomass and lipid productivity of microalgae and yeast in a co-culture (Santos et al., 2013). This system is unique to the co-culture of Rhodosporidium toruloides and C. protothecoides and involves the heterotrophic growth of the former and autotrophic of the latter in separate vertical-alveolar-panel (VAP) photobioreactors. These photobioreactors are connected via the gas phase to enable the exchange of O2 produced by microalgae and CO2 produced by yeast. Unlike industrial flue gas, which is often associated with toxicity, the CO2 produced by yeast was not toxic to the microalgae. The system resulted in a 94% increase in biomass productivity and an 87% increase in lipid productivity of C. protothecoides compared to normal cultivation conditions. The uniqueness of this method underlies its distinction from other methods like encapsulation and biofilms, hence it is not universally applicable.
While the aforementioned co-culture methods share similarities, such as the co-cultivation of microorganisms, they each have unique characteristics and applications that differentiate them from each other. Also, while microalgae-bacteria co-cultures have demonstrated improved efficiency in biofuel production compared to monocultures in certain cases, it is important to note that these results can vary significantly based on the organisms used, the environmental conditions, and the specific method employed.
4.1.6 Applications, advances, challenges, and future prospectives of microalgae-fungi co-cultivation
The use of fungi in facilitating microalgae harvesting and wastewater treatment has attracted considerable recognition recently, primarily because of its cost-effectiveness and high efficacy (Leng et al., 2021). Microalgae and fungi can form co-pellets, aiding microalgae harvesting through electrostatic neutralization, protein surface interaction, and exopolysaccharide adhesion, as a result of the co-culture process (Serra et al., 2008; Leng et al., 2021). The interplay of heterotrophic or mixotrophic relationships within this system culminates in elevated Chemical Oxygen Demand (COD) elimination. Through RuBiSCO or similar enzymes, CO2 molecules diffuse into microalgae cells and undergo the Calvin-Benson-Bassham cycle (CBB), synthesizing oxygen and other organic matter for metabolic purposes (Gonçalves et al., 2017). The process results in enhanced growth and development of both partners due to the gas exchange, which subsequently reduces the carbon content in the wastewater (Wang Y. et al., 2016). Fungal secretions of extracellular enzymes and the pellet structures they form with microalgae aid in capturing suspended solids (Wang Y. et al., 2016). The microalgae-fungi consortium effectively rids treated wastewater, activated sludge, and biogas slurry of COD and nutrients, outperforming monoculture (Wang et al., 2017; Yang et al., 2019). The co-culture of microalgae Chlorella sp. and yeast S. cerevisiae aerated by 1% CO2 demonstrated an increased CO2 bio-fixation rate of 64.75 mg.L-1. h-1, leading to a significant boost in cell density (Xmax = 73.7%), and maximum oil production (Pmax = 93.3%) compared to Chlorella sp. Monoculture (Shu et al., 2013). Similarly, the co-cultivation of C. protothecoides and yeast Rhodosporidium toruloides in a VAP photobioreactor had a CO2 bio-fixation rate of 29 mg.L-1. h-1, nearly twice that of the control cultivation in a VAP photobioreactor (Santos et al., 2013).
The joint cultivation of microalgae and fungi outperforms mono-microalgae and mono-fungi systems in phosphorus removal (Yang et al., 2019). For instance, the co-culture of C. vulgaris and Ganoderma lucidum demonstrated a higher efficiency in phosphorus removal from wastewater (Zhou et al., 2012). The pH reduction from the microalgae/fungi co-culture and fungi´s enzyme secretions facilitate the degradation of precipitated PO4-P and promote phosphorus assimilation (Zhang et al., 2020). Nitrogen removal is also considerably enhanced when filamentous fungi are co-cultured with microalgae (Zhou et al., 2012). For example, the combination of fungi and microalgae in municipal water resulted in a 100% removal efficiency of NH4-N within a day (Salih, 2011). The nitrogen exchange in co-culture systems between fungi and microalgae was established with isotopic labeling experiments (Du et al., 2019). Co-cultivating microalgae Scenedesmus sp. and wild yeast (1:1 v/v) achieved high nutrient removal rates (96% nitrate, 100% total ammonia nitrogen, and 93% orthophosphate) (Walls et al., 2019). The microalgae/fungi cell wall comprises functional groups such as cellulose, hemicellulose, protein, and other polymers with excellent adsorption properties, electrostatic interactions, ion exchange, and chelation/complexation, making them useful for eutrophication of heavy metals, drug, and antibiotic removal (Leng et al., 2021). The co-culture pellet of microalgae/fungi proved more efficient in absorbing arsenic and gold than monocultures (Bodin et al., 2016; Shen and Chirwa, 2020). Additionally, the fungi-assisted microalgae harvesting process shows promise in the removal of a wide range of pesticides in wastewater (Hultberg and Bodin, 2018).
The combined use of microalgae and fungi has considerable potential for the industrial production of biofuels, including biodiesel, bioethanol, biomethane, and biohydrogen (Prajapati et al., 2016). Lipids extracted from S. obliquus and Cunninghamella chinulata pellets have been shown to improve the fuel properties to meet international standards (Srinuanpan et al., 2018). Also, flocculation of N. oceanic with oleaginous fungi Mortierella elongata enhances the yield of poly-unsaturated fatty acids (PUFAs) (Du et al., 2018). However, the composition and yield production of lipids and fatty acids can vary significantly depending on the fungi and microalgae strains combined in the consortium. The classes of lipids generated through these consortia could be modulated, and fatty acid composition could be tailored and optimized by co-culture parameters, which is beneficial for biodiesel production (Leng et al., 2021) (Table 2).
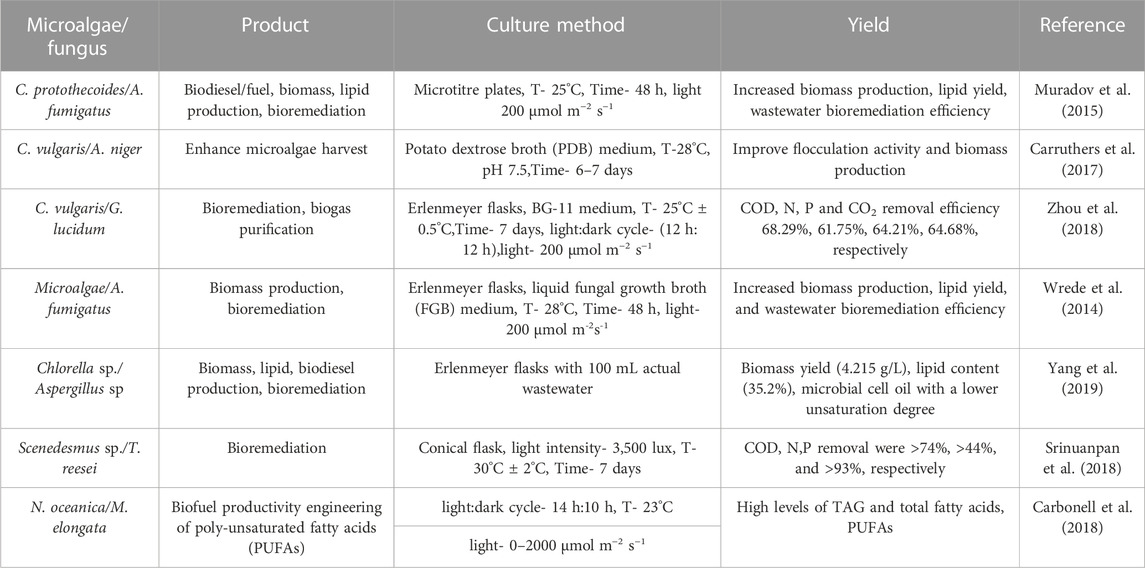
TABLE 2. Microalgae-fungal co-culture systems used for bioremediation, biomass, and lipid productions (T-temperature).
Biomass production and lipid yield from microalgae/fungi co-culture are higher than the mono-culture, and the exchange of gases and nutrients, enhances individual metabolic activity, enabling the consortium to accumulate nutrients from the surroundings more effectively (Piercey-Normore and Athukorala, 2017). Fungi can use carbon resources stored in microalgal cell walls by using various extracellular enzymes such as fat hydrolase and cellulolytic enzymes to directly synthesize free fatty acids for cell growth and proliferation (Lennen and Pfleger, 2013). Additionally, fungi can convert the adsorbed fatty acids into Triacylglycerides and accumulate them into lipid bodies (Ji and Ledesma-Amaro, 2020). As the microalgae biomass reaches a specific concentration, the shading effect usually restricts autotrophic microalgae from getting sunlight. The microalgae cells, fixed in pellets in the microalgae/fungi consortium, can facilitate light transmission, promote the overall growth of microalgae, and significantly increase the algal biomass yield (Prajapati et al., 2016). Although microalgae/fungi co-culture biomass shows considerable potential for producing value-added products, biomass harvesting, and separation can be challenging due to the hydrolyzation of microalgae cell walls through the interaction between microalgae and fungi. This situation is not ideal for separately recovering microalgae or fungi. Moreover, biomass obtained from wastewater, whether fungal or bacteria-assisted harvesting, should be carefully implemented in applications such as food and feed supplements and cosmetics production.
Co-culture of microalgae and yeast is a promising technology for lipid production due to the mutual benefits conferred by the co-culture of the two microorganisms (Magdouli et al., 2016). Cheirslip et al. demonstrated that the growth and lipid productivity of R. glutinis increased when co-cultivated with microalgae C. vulgaris in industrial waste (Cheirsilp et al., 2011). In another study, the growth of S. obliquus was increased by 30.3% in a co-culture with C. tropicalis, and its lipid content and productivity were enhanced compared to the microalgae monoculture system (Wang R. et al., 2016). A study on a co-cultivation system of Chlorella sp. KKY-S2 and yeast Torulaspora maleeae Y30 reached a maximum lipid yield of 1.339 g.L-1 after 5 days in the co-culture system, which was higher than the lipid yield (0.969 g.L-1) achieved by using atmospheric CO2 after 6 days of cultivation (Puangbut and Leesing, 2012). The highest biomass and lipid content of a co-culture system of yeast Trichosporonoides spathulate and microalgae C. vulgaris in a photobioreactor under optimum conditions reached 12.2 g.L-1 and 47%, respectively (Kitcha and Cheirsilp, 2014). Co-culture of oleaginous yeast T. maleeae Y30 and T. globose YU5/2 with microalgae Chlorella sp. Resulted in higher growth and biomass concentration than their monocultures (Puangbut and Leesing, 2012). The monoculture of T. maleeae, T. globose, and Chlorella sp. Showed a biomass concentration and lipid yield of 8.267 g.L-1 and 0.920 g.L-1, 8.333 g.L-1 and 1.141 g.L-1, and 1.933 g.L-1 and 0.052 g.L-1, respectively. The biomass concentration and lipid yield of co-cultivation of T. maleeae and T. globose with Chlorella sp. were 8.733 g.L-1, 1.564 g.L-1, 8.010 g.L-1, and 2.424 g.L-1, respectively (Papone et al., 2012). Various methods used for biomass, lipid, and protein production and wastewater treatment by microalgae-yeast co-cultivation are mentioned in Table 3.
Yeast and microalgae can mutually benefit each other in a co-culture system by using the O2 generated by microalgae and the CO2 and organic acids produced by yeast. However, some of these organic acids can inhibit yeast growth. Yeast can also provide microalgae with simple sugars obtained by breaking down complex sugars like low-cost agricultural waste, reducing the total cost of the final product (Rakesh and karthikeyan, 2019).
Both yeast and microalgae are promising feedstocks for biodiesel production due to their high lipid content, but the high operational cost and low lipid productivity make current industrial biodiesel production using microalgae or yeast impossible (Meng et al., 2009). Various strategies, including nutrient starvation, multi-stage cultivation, genetic engineering, and co-cultivation, have been implemented to address these issues (Arora et al., 2019; Esakkimuthu et al., 2020). However, nutrient starvation leads to a significant reduction in biomass production, markedly reducing microalgae’s lipid productivity. Additionally, genetic engineering requires extensive knowledge about the compartmentalization of photosynthesis, TAG synthesis and regulation, and the connection between TAG synthesis (Esakkimuthu et al., 2019).
A mixed-culture system of Chlorella sp. and S. cerevisiae exhibited significant improvements in growth and lipid accumulation with a 128.1% and 165.2% increase, respectively, compared to their respective monocultures. Moreover, the CO2 removal rate was enhanced by 195% compared to the Chlorella sp. Monoculture (Shu et al., 2013). Similarly, a mixed-culture of Isochrysis galbana and Ambrosiozyma cicatricosa showed higher growth rates and biomass concentration than their monocultures (Cai et al., 2007). The co-cultivation of yeast R. glutinis and microalga Spirulina platensis demonstrated a lipid yield of 467 mg.L-1, which was 3.18 times higher than yeast monoculture and 3.92 times higher than microalgae monoculture (Xue et al., 2010). These findings demonstrate that the co-culture of microalgae and yeast can lead to improved growth and lipid accumulation, highlighting its potential for sustainable biofuel production.
In wastewater treatment, the presence of high levels of organic suspended solids (COD>5 g.L-1) and turbidity can impede microalgae growth and limit its performance. However, this issue can be addressed by co-cultivating microalgae with heterotrophic microorganisms like yeast. For instance, co-cultivating R. glutinis and S. obliquus led to a 40%–50% increase in suspended organic solids removal from domestic wastewater (Li et al., 2019). Yeast can thrive in a medium with high COD concentrations (ranging from 15 to 50 g.L-1) but may not efficiently remove nitrogen and phosphorous. Therefore, co-cultivating microalgae and yeast can lead to the efficient removal of COD, total organic carbon (TOC), nitrogen, and phosphorous from wastewater (Ling et al., 2014). Additionally, the co-cultivation of C. vulgaris and Yarrowia lipolytica in a liquid digestate from the yeast industry demonstrated better growth and nutrient removal compared to monoculture systems of each microorganism (Yang et al., 2019).
The emergence of the microalgae-fungi consortium is a promising strategy for bioremediation, microalgae biomass, lipids, and biofuel production. However, this technology is still in its infancy, and upscaling it for industrial applications poses significant challenges. The choice of microalgae and fungi species for the co-culture, and the conditions of co-culture, such as light intensity, carbon source, and agitation, can greatly influence the entire process, thereby impacting its scalability. To optimize the large-scale cultivation of the microalgae/fungi consortium, additional research using advanced metabolomics or proteomics techniques is required. These techniques could aid in the selection of suitable strains and identification of the underlying mechanisms of interactions between microalgae, fungi, and other microbes in the consortium. The three-way interaction among these microorganisms remains unclear and should be taken into account to achieve the success of the overall process.
It is crucial to continue exploring the synergistic interactions between microalgae and fungi, and to further investigate the potential of these co-cultures for large-scale applications. Despite the present hurdles, the potential benefits of such systems for environmental remediation, renewable energy production, and the sustainable management of resources are substantial. With continued research and development, the prospects for fully realizing the potential of microalgae-fungi consortium are promising.
5 Microalgae-microalgae co-cultivation
The majority of existing commercial microalgae cultivations prioritize large-scale growth of single species over co-cultivation of microalgae. However, microalgae monocultures, particularly those in open pond systems which offer greater economic feasibility, are at risk of contamination from other algae, pathogens, and grazers. Contamination control methods, such as pesticide application require a comprehensive understanding of the pests and pose both environmental risks and substantial costs. Co-cultivation of microalgae mitigates these challenges, offering advantages for large-scale microalgae cultivation, including increased stability, resource utilization efficiency, and enhanced biomass, and lipid productivity (Novoveská et al., 2016).
5.1 Microalgae-microalgae co-cultivation methods
5.1.1 Direct mixing
As a fundamental method in mixed-culture systems, direct mixing demonstrates considerable potential. The mixed cultivation of C. sorokiniana and Euglena gracilis, for instance, under photoautotrophic, mixotrophic, and heterotrophic conditions, resulted in notable enhancements to the growth rate compared to monoculture. A total yield of 51.73×105 cells/mg glucose and 67.64 ×105 cells/mg glucose was achieved in the co-culture of the 2 cell strains (Friday et al., 2010).
The co-culture of two selected algae strains, C. vulgaris and Pseudokirchneriella subcapitata, resulted in increased production of chlorellin, mainly composed of a C18 fatty acid mixture (DellaGreca et al., 2010). Recycling a portion of the harvested biomass to a co-culture system of microalgae promoted the rapid settling of dominant microalgae species, thus increasing biomass productivity and harvesting of the culture by 35% and 25%, respectively (Park et al., 2011). Employing gravity recycling using Pediastrum boryanum (a rapidly settleable alga) in the pilot-scale HRAPc system (high-rate algal ponds) for treating domestic wastewater dominated with Dictyosphaerium sp. (a poorly settleable alga), improved harvesting and biomass yield (Park and Craggs, 2014). Recycling of either solid or liquid portions had a similar effect on harvest, which may be attributed to the presence of EPSs in the liquid portion of the culture, enhanced cell concentration and efficiency in solar consumption by increasing algal residence time, and/or increased overall growth rate of microalgae due to shifts in the relative proportions of algal growth stage (Park and Craggs, 2014). In another experiment, Park and Craggs studied the effect of different recycling rates (1%, 2.5%, 5%, 10%, 25%, and 50%) on the growth and settling ability of co-cultivating of microalgae in a high-rate algal pond (HRAPs) for treating domestic wastewater. Their results showed that recycling 10% of daily harvested biomass increased biomass productivity and settling ability by 10% and 40%, respectively, contributing to enhancing the harvesting of the culture Pediastrum boryanum because of its morphology, improving its concentration by 30% in the medium (Park and Craggs, 2014).
Injection of CO2 into microalgae co-culture systems can provide various benefits, such as preserving the microalgae culture from high pH inhibitory effects, enhancing the availability of orthophosphate and ammonia for microalgae utilization, increasing the carbon availability and C:N ratio in wastewater, enhancing the microalgae cells concentration, and increasing the lipid content while improving the lipid profile of the microalgae (Mehrabadi et al., 2017). Optimization of CO2 injection and medium pH can also increase the dominance of the suitable microalgae strain for harvesting (Park et al., 2013). For instance, a study performed on co-culturing S. obliquus and C. vulgaris in a flat plate photobioreactor for urban wastewater treatment reported that CO2 addition and biomass recycling increased biomass productivity, the dominance of larger microalgae (S. obliquus), and gravity sedimentation by 314%, 38%, and 85%, respectively (Fallahi et al., 2021). Furthermore, Mehrabadi et al. reported that the optimum CO2 concentration of 10% maximizes biomass productivity of high-rate algal mesocosms (HRAM) leading to a 50% increase compared to the control without CO2 injection. CO2 injection also enhanced culture harvesting, with 0.5% CO2 HRAM biomass increasing mean 1-h harvest efficiency about five times compared to 2% CO2 HRAM biomass, and nearly twice that of 5% CO2 HRAM. Micractinium sp. was the dominant species in both 5, and 10% CO2 HRAM biomasses (Mehrabadi et al., 2017). Sun et al. reported that recycling microalgae biomass (2% and 10%) also increased biomass recovery in an HRAP system from 75% to 89% without recycling to 92%–94% with recycling (Gutiérrez et al., 2016).
5.1.2 Encapsulation
Encapsulation offers an innovative approach for achieving high biomass concentrations and immobilizing microalgal cells. The encapsulation of microalgal cells within hollow polymer shells of rhombohedral shape offers a promising strategy for microbial-cell immobilization and high-biomass-concentration applications. The encapsulation is made possible by embedding microalgae in CaCO3 crystals, layer-by-layer (LbL) coating of polyelectrolytes, and removal of sacrificial crystals. Embedding microalgae in CaCO3 crystals involves a two-step process consisting of heterogeneous crystal nucleation on the cell surface and subsequent cell embedment by crystal growth. This approach enables micrometer-sized microalgae to be perfectly coated in calcite crystals without altering their rhombohedral shape, owing to the favorable surface properties of the microalgal cells for calcite crystal growth (Kim et al., 2018). The surfaces of these microcapsules can be further coated with gold nanoparticles, Fe3O4 magnetic nanoparticles, and carbon nanotubes (CNTs), which provide additional functionalities such as light-triggered discharge, magnetic separation, and enhanced mechanical and electrical strength, respectively. This technology represents an innovative and versatile platform for a wide range of bioapplications requiring the immobilization of microbial cells (Kim et al., 2018). A high-throughput screening study of algal community combinations was conducted using microfluidic technology to generate millions of parallel, nanoliter-scale mixed cultures for biomass accumulation estimation trials. The study revealed that combining different algal species could result in either positive or negative interactions leading to increased or reduced biomass production, respectively. For instance, Ankistrodesmus falcatus and Chlorella sorokiniana, and C. sorokiniana and Selenastrum minutum had improved performance, while Selenastrum capricornutum and Scenedesmus ecornis showed reduced productivity when co-cultured (Carruthers et al., 2017). The interaction between microbial populations can result in enhanced productivity and decreased community invasibility, which are favorable traits for scalable bioproduction systems. Microfluidic devices may be essential for the efficient and cost-effective discovery of such synergistic communities through rapid, high-throughput screening of microbial combinations (Carruthers et al., 2017).
5.1.3 Biofilms
Microalgae biofilms offer a promising alternative for large-scale microalgae cultivation. For instance, a consortium of Chlorella, Nitzschia, and Scenedesmus species grown on mesh-type materials in an open pond showed improved biomass production and system efficiency. The mesh-type substrates linked to microalgae can also remove residual treated wastewater directly, further enhancing system efficiency. In addition, a simple and cost-effective dewatering method using natural sunlight was successfully implemented for algal biomass, instead of using a freeze-drying method, making it a feasible technique for bulk biodiesel synthesis (Lee et al., 2014). Biochar, a carbonaceous solid support, was investigated as a growth substrate for Klebsormidium flaccidum and Anabaena cylindrica biofilms cultured on BG11 media. After 20 days of incubation under a 16:8 (light/dark) photoperiod, the dry biomass, total carbon, and nitrogen contents of the cultures with and without biochar were compared, revealing an 80% increase in A. cylindrica growth with the inclusion of biochar (Kholssi et al., 2018).
The productivity and cost-efficiency of algal biofuel production can be enhanced by a mixotrophic microalgae biofilm composed of C. vulgaris and Scenedesmus dimorphus. The mixotrophic microalgae biofilm outperforms autotrophic microalgae biofilms in terms of biomass yield, feedstock quality, lipid accumulation, and ash content; producing 2–3 times higher biomass yield, 2–10 times higher lipid accumulation, and 40%–60% lower ash content (Roostaei et al., 2018). Moreover, the growth activities of microalgae biofilms and productivity of mixotrophic biofilms are significantly influenced by cell-surface properties such as hydrophobicity and roughness and they are substantially correlated in particular with surface hydrophobicity (Roostaei et al., 2018).
5.1.4 Advances, challenges, and future prospective
Co-culture systems of diverse microalgae species have exhibited superior stability, a higher rate of biomass and lipid production, and improved biofuel properties compared to monocultures. Microalgal lipids extracted from these co-cultures present a larger quantity of short-chain unsaturated fatty acids, leading to enhanced biofuel characteristics including iodine number, cetane number, octane number, heating value, and kinematic viscosity (Ishika et al., 2017; Das et al., 2021).
Mixed microalgae cultures also show promise in various wastewater treatment systems due to their capacity for bio-flocculation and efficient biomass harvesting. Qin et al. reported chemical oxygen demand (COD) removal rates of 57%–63% and total phosphorous removal rates of 91%–96% in a microalgae co-culture system, which were higher than those of a monoculture system of Chlorella sp. (45% COD, and 87% total phosphorous) (Qin et al., 2016). These cultures are also cost-effective, less labor-intensive, and resistant to contamination, making them an attractive choice for wastewater treatment. In binary microalgae cultures, enhanced cell-cell interactions result in the production of more extracellular polymeric substances (EPS) as a metabolic strategy to adapt to unfavorable conditions, such as nutrient deprivation. However, excessive EPS accumulation can inhibit mass transfer and nutrient fixation, hindering microorganisms from utilizing dissolved CO2. Thus, a thoughtful selection of microalgae species for co-cultivation is critical for the success of large-scale applications, ensuring productive wastewater treatment and bioenergy production (Ray et al., 2021). Despite improved biomass and lipid yields in microalgae consortia, further exploration is required to fully understand the symbiotic mechanisms at play and optimize productivity. The incorporation of omics resources and genetic engineering techniques, including gene transformation procedures, mutagenesis, and genome-editing tools in co-cultivation studies, promises to unravel the intricate metabolic pathways that microalgal cells undergo (Kuo et al., 2022).
5.1.5 Application of microalgae-microalgae co-cultivation
Co-cultivation of microalgae species offers exciting prospects for environmental pollutant removal and renewable energy production. The mutualistic interactions among microalgae enhance nutrient removal capacity and facilitate adaptation to varying environmental conditions in the wastewater treatment system (Han et al., 2021). For instance, Prathima Devi et al. achieved a biomass concentration of 0.98 mg.L-1. d-1 by heterotrophic co-cultivation of microalgae collected from an Indian lake in domestic wastewater (Devi et al., 2012). Similarly, in a study by Taskan et al. the organic matter (OM) and nutrient removal efficiencies were investigated by co-cultivating microalgae in a slaughterhouse wastewater treatment photobioreactor. The study reported 70.2%, 96.2%, and 89.6% removal efficiencies for total nitrogen (TN), total phosphorous (TP), and total organic carbon (TOC), respectively (Taşkan, 2016).
Microalgae co-cultivation also provides a potential solution to the high operational costs associated with biodiesel production (Zhao et al., 2014). Recent studies have underscored the feasibility of co-cultivation techniques for biodiesel production at lower costs. For example, a co-culture of Chlorella sp. and Monoraphidium sp., showed significant increases in total biomass productivity (62 mg.L-1. d-1), total lipid content (47.72%), and lipid productivity (29.52 mg.L-1. d-1) compared to monoculture systems (Zhao et al., 2014). An overview of different methods employed for microalgae biomass, lipid production, and wastewater treatment is provided in Table 4.
6 In-depth analysis of omics studies and consortia: Merging recent trends with a roadmap to microalgae co-cultivation optimization for sustainable applications
Emerging strategies such as photo-bioreactor configuration have been proposed for wastewater treatment, utilizing anoxic/aerobic-algal/bacterial consortia to facilitate the rapid settling of algal/bacterial populations and the removal of nitrogen, organic, and inorganic carbon, with effective biomass recycling (Alcántara et al., 2015). However, the chemical treatments and biomass recovery processes associated with this approach are still expensive. To mitigate these costs, a nutrient remediation and recovery method was suggested that involves a synergistic co-culture of eukaryotic and prokaryotic microorganisms, which maximizes biomass production while minimizing associated expenses (Wicker and Bhatnagar, 2020). Significant progress has been made in omics analysis and metabolic engineering of microalgae and bacterial strains, allowing for the construction (Crozet et al., 2018) and standardization of new pathways in model microbes (Mishra et al., 2019). These methodologies represent an important milestone towards harnessing the potential of photoautotrophic/bacterial co-cultures, particularly with respect to understanding the molecular mechanisms underlying co-cultivation systems and ensuring their stability and productivity.
Research studies that focus on physiological characteristics within consortia have become increasingly important in the context of implementing cell-to-cell communication. These studies utilize advanced techniques such as microscopy, mass spectrometry, quorum sensing, as well as molecular and genetic engineering to gain a better understanding of consortia interactions (Brenner et al., 2008). Genomic approaches are also utilized to identify species composition, genetic variability, and to compare different species within the consortia (Gou et al., 2020). However, these techniques have limitations in that they require specific genomic libraries and are unable to isolate low-abundant species in natural or synthetic consortia. High-throughput proteomic and enzymatic studies have also been employed to improve the understanding of the relationships between genetic and biochemical information, as well as regulatory mechanisms in consortia (VerBerkmoes et al., 2009). While omics approaches are highly sensitive, they are also expensive and require skilled professionals for sample preparation, data analysis, and problem-solving. However, this information is essential for better understanding microbial interactions, optimizing the use of available substrates, increasing productivity, and addressing cultivation optimization problems. Genome-editing approaches offer promising potential for generating more efficient microalgae/bacteria consortia in the future. Currently, microbial consortia systems face challenges in the development and consolidation of computational and mathematical assistance for co-culture realization, which strongly affects the total costs and required time for large-scale productions, a critical criterion for the synthesis and commercialization of bioproducts (Scognamiglio et al., 2021). An open-access database that provides relevant metadata about tested consortia, including descriptions of strains, growth dynamics, biomolecules released, data related to bioprocess conditions in bioreactors, and possible metabolic and omics outcomes, would undoubtedly contribute to improving the current scenario and expanding the applications of microalgae-bacteria consortia in biotechnological applications. In addition, meta-secretomics analysis has proven beneficial in identifying total surface-bound proteins and secretions in consortia. Through the use of reliable and reproducible metabolomics techniques, qualitative and quantitative data can be achieved regarding the metabolites produced by the consortia (Adav et al., 2012).
Moreover, the cultivation of microalgae consortia with bacteria, fungi, or other microalgae relies on the appropriate provision of nutrient, light, and water conditions for optimal biomass yield. By analyzing the intricate dynamics and interactions of these consortia through the lens of omics techniques, we can classify them as either microalgae-assistant or microalgae-dominant. Omic studies influence significantly our understanding of nutrient uptake and CO2 assimilation across different microalgae species (Van Den Hende et al., 2012). Some microbial species are able to tolerate high concentrations of supplemented CO2, usually ranging from 14 to 100 percent of dissolved gas, while growth inhibition occurs once the supplied CO2 concentration exceeds the maximum cellular capacity (Salih, 2011). High CO2 tolerant freshwater microalgae strains, in their natural habitats, can survive in a CO2-rich environment (up to 30% CO2) with better biomass yield and CO2 bio-fixation rate, although decreased carotenoid content was reported at the highest CO2 level (Swarnalatha et al., 2015). Omic insights therefore can play a pivotal role in the modulation of growth conditions and the improvement of biomass yield.
In the complex microcosm of carbon utilization, microalgae have shown a remarkable capability to metabolize various sources, including alcohol, sucrose, and glucose, in addition to CO2 (Tan et al., 2018).
Omics techniques unravel the mechanistic interplay involved in this process, such as the triggering of the Carbon Concentrating Mechanism in aquatic environments. Omics reveal that overexpression of diverse Carbonic anhydrase enzymes is responsible for improving CO2 uptake, ultimately favoring the high photosynthetic efficiency of the cells (Kuken et al., 2018). Omics have also provided insights into the effect of high CO2 conditions and the modulation of protein secretion in microalgae in flue gas concentrations (>3%) (Baba et al., 2011), indicating the occurrence of vegetative and gametic forms of microalgae when bacteria respiration exceeds the mentioned CO2 air level or co-cultivation systems have higher CO2 supplements. Insights from omics analysis highlight the importance of sufficient carbón supply in the form of salts (Acién et al., 2017). or CO2 rich air for maintaining high productivity in biomass yield (Cho et al., 2011).
The omics techniques further underscore the potential of different organic carbon sources, like non-edible lignocellulosic biomass, agricultural waste, and glycerol from biodiesel production, for cost-effective cultivation. (Patel et al., 2016). Furthermore, omics have revealed how the manipulation of carbon-to-nitrogen (C/N) ratio can induce nitrogen starvation and subsequently increase the lipid content of microalgae (Liu et al., 2008). In a co-culture system of C. pyrenoidosa and R. glutinis in a BBM medium, an increase in C/N ratio from 16 to 64 led to an increase in biomass (from 2.92 to 6.12 g.L-1) and lipid content (from 25% to 40.55%) (Liu et al., 2018). The optimal biomass concentration and lipid productivity (90 mg.L-1. day-1) in the co-culture of C. pyrenoidosa and R. glutinis was attained at a ratio of 3:1, with total fatty acid (TFA) content twice that of a monoculture. Optimal conditions for the co-cultivation of microalgae and yeast R. glutinis have been shown to be at a C/N ratio of 64, resulting in a biomass concentration of 6.12 and a lipid yield of 2.48 (Liu et al., 2018). Omics studies have revealed that nitrogen deprivation can significantly affect the primary and secondary metabolism of microalgal cells, leading to reduced protein synthesis, photosynthetic capacity, and cell growth while increasing the production of neutral lipids (Schmollinger et al., 2014).
Omics technologies have also shed light on the role of stress conditions, such as salt stress, and nitrogen levels in lipids accumulation (Mastrobuoni et al., 2012). The identification and selection of suitable microalgae and fungi strains that can withstand these stress conditions while maintaining productivity are crucial (de Oliveira Magalhães et al., 2022). Therefore, for the co-cultivation of microalgae consortia for synthesizing secondary metabolites, it is essential to ensure a proper nitrogen source supply (>0.35 mM) (Prajapati et al., 2016). For instance, R. glutinis is known for its ability to produce lipid and β-carotene, while C. tropicalis is an oleaginous yeast that can grow on various inexpensive agricultural raw materials (Liu et al., 2018). The use of consolidated bioprocessing (CBP), which employs an ethanologenic and a cellulolytic strain for bioethanol production, is another approach to co-cultivation (Park et al., 2012). The co-culture of C. vulgaris and R. glutinis improved C. vulgaris lipid content to 20.8% using 20 g.L-1 glucose, while a co-culture of S. obliquus and R. glutinis enhanced the lipid content of S. obliquus to 24% (Arora et al., 2019). The use of omic techniques in co-cultivation systems comprising R. glutinis and S. platensis has provided valuable insights that has led to increased biomass and lipid accumulation (Magdouli et al., 2016). Selection of an appropriate medium for microalgae co-cultivation is crucial, with deionized water and artificial ocean water being the most commonly used media (Chia et al., 2019). Artificial media offer greater flexibility in terms of nutrient composition and can be optimized for microalgae cultivation. However, the quest for a sustainable culture medium, aided by omics studies, that can provide nutrients to all consortia members while minimizing pollutant production during cultivation continues (Chia et al., 2021).
Omics technologies can provide key insights into how the availability and quality of light sources play a crucial role in achieving optimal biomass production in microalgae co-cultivation, under photoautotrophic and mixotrophic conditions. By understanding the genome, transcriptome, proteome, and metabolome of the microbes involved, we can understand how degree of light penetration is a major factor that influences the efficiency and quality of the consortium (González-Fernández et al., 2011). Additionally, omics allow us to study and understand the significant impact on microalgae-bacteria/fungi co-cultures grown under distinct conditions, such as duration, intensity, and potential limitations of light. As microalgal cells become more densely packed, they can cast shadows on each other and impede light penetration, creating light-limited conditions. In such situations, microalgae can thrive in mixotrophic or heterotrophic modes, depending on the presence of organic carbon sources. Therefore, supplementing organic carbon sources, such as acetate or sugars, instead of improving the light source, is a critical alternative for such growth (Tandon and Jin, 2017). Furthermore, light intensity can influence the interaction between microalgae and bacteria or fungi. Higher light intensity has been found to enhance microalgal growth and thus promote increased biomass yield (Zhai et al., 2017). On the other hand, increased light intensity favored microalgae growth while limiting the nitrification process in the microalgae-bacteria culture. In contrast, a non-graduated temperature increase (up to 32°C) under light intensities (up to 55 µE) promoted the proliferation of the nitrifying bacteria and nitrite and nitrate accumulation (Gonzalez-Camejo et al., 2018). The optimization of light supply is a critical factor in the growth and productivity of photosynthetic microorganisms. While light is the primary energy source for photosynthesis, excessive light levels, coupled with inappropriate temperature or high oxygen concentrations, can negatively impact the photosynthesis process, leading to reduced growth rates (Fernández et al., 2001). Most microalgae perform photosynthesis at 100 to 500 µE, but optimal productivity is achieved at constant irradiance levels in the range of 50–100 µE (Vejrazka et al., 2011). Saturation points are reached at irradiance levels higher than 300 µE in several strains, resulting in photoinhibition (Acién et al., 2017). The impact of light quality and intensity on microalgae metabolism has been studied experimentally and through modeling, demonstrating that even moderate light intensities can alter the set of metabolites produced by cells, leading to reduced availability of nuclear transcripts, proteins, and metabolites, with little to no change in plastid transcripts (Mettler et al., 2014). These findings suggest that microalgae products synthesized at the plastids may be resistant to fluctuations in light intensity during co-cultivation.
Microalgae growth is not only dependent on light intensity but also on wavelength and photoperiod (Iasimone et al., 2018). As light is essential for metabolic activity under photo and mixotrophic conditions, this culture parameter significantly affects their growth (Schulze et al., 2014). The effect of light on cell growth metabolism varies among different species or strains. In a microalgae consortium composed of C. vulgaris, P. subcapitata, M. aeruginosa, and Synechocystis salina, the optimum daily irradiance of light on nutrients uptake and growth was found to be 208 µE (Gonçalves et al., 2016). Therefore, in general terms, light irradiance levels should be maintained between 50 and 200 µE to avoid photoinhibition. However, the behavior of microalgae under different light conditions and the optimal light intensity and irradiance can be selected based on models of cell growth simulations under different light sources. Additionally, the possible type of metabolites produced by the strains under specific light conditions provided to the consortia can be observed and coordinated with the simulation models (Chang et al., 2011).
The supply of essential nutrients like nitrogen, phosphorus, and trace metals is critical for the growth of both microalgae and bacteria in a consortium (Markou et al., 2014). The bacteria present in the consortia are known to facilitate the breakdown of various nitrogen-containing compounds, which aid in the proliferation of microalgae (Zhou et al., 2013). For instance, the co-cultivation of the bacteria Azotobacter vinelandii with microalgae strains Neochloris sp. and Scenedesmus sp. has been found to result in a commensal relationship where the bacteria provide nitrogen for microalgae growth (Delgadillo-Mirquez et al., 2016).
Omics studies illustrate how nitrogen forms and trace metals play a crucial role in physiological processes like anti-oxidative defense and nutrient uptake (Oliveira et al., 2020). The presence and concentration of nitrogen and phosphorus in the culture medium are critical as they are fundamental building blocks for enzymes and nucleic acid synthesis (Arora et al., 2019). The nutrient constraint has a significant impact on the chemical compositions of microalgae. An optimal growing medium for microalgae must contain carbon (C), nitrogen (N), phosphorus (P), and iron (Fe), although this may vary depending on the specific species. Carbon is an essential component of all biomolecules synthesized by microalgae, including carbohydrates, proteins, nucleic acids, vitamins, and lipids, and is thus necessary in high concentrations (Richmond, 2008). Certain species of mixotrophic microalgae can also use organic compounds such as sugars, acids, and alcohols as carbon sources. Nitrogen plays a vital role in the formation of structural and functional proteins and is a significant component in the production of proteins, nucleic acids, vitamins, and photosynthetic pigments (Richmond, 2008). Nitrogen, a crucial nutrient for microalgae growth, can be supplied in various forms, including inorganic forms such as NO3, NO2, NO, NH4+, or organically via urea or amino acids (Perez-Garcia et al., 2011). Among the nitrogen sources, ammonium is the preferred nitrogen source for two microalgae species, C. vulgaris and Pseudokirchneriella subcapitata, due to its easier assimilation and less energy requirement (Jia and Yuan, 2016). When microalgae are nitrogen-limited, it can affect the synthesis of antioxidants, as found in Phaeodactylum tricornutum, Tetraselmis suecica, and C. vulgaris, where the content of chlorophyll a in biomass was lower under nitrogen-limited condition (Goiris et al., 2015). Along with macronutrients, microalgae growth medium should contain essential micronutrients such as Mg, S, Na, Cl, Ca, Fe, Mo, Mn, Zn, Cu, B, and Co, with an emphasis on magnesium (Mg), sulphur (S), and iron (Fe) (Markou and Georgakakis, 2011).
Fungi and microalgae exhibit opposite zeta potentials, which is the charge developed at the interface of a solid and liquid medium. When the pH is lower, the zeta potential of the co-culture system tends towards neutrality, facilitating electrostatic neutralization and fast fungal spore aggregation. Therefore, slightly acidic environments have shown to be more effective for bio-flocculation (Zhang and Zhang, 2016). However, the efficiency of microalgae-fungi flocculation depends on several other factors, including the carbon source, light, and the combination of microorganisms in the culture (Gultom et al., 2014). The optimal pH selection should be based on the specific applications and harvesting methods since different species exhibit varied metabolic responses to pH changes, leading to varying flocculation efficiency (Prajapati et al., 2016). While some studies have reported that alkaline conditions were more suitable for flocculation than neutral or acidic ones (Leng et al., 2021), others found little effect of pH on flocculation efficiency (Li et al., 2017; Pei et al., 2021). For the co-cultivation of microalgae and yeast, a pH of 5 was reported as optimal (Liu et al., 2015; Arora et al., 2019). The composition and buffering capacity of the culture medium, the dissolved CO2 amount, temperature, and the metabolic activity of cells can influence the pH of the culture medium (Singh and Dhar, 2011). The pH tolerance of microalgae culture media is species-specific and can significantly impact growth rates (Zhu, 2015). Optimal pH levels for most microalgae cultures fall within the neutral to slightly alkaline range (pH 7.0–10.0), with some species having an optimal pH as low as 3.0 (Lu et al., 2014). However, beyond these pH ranges, the yield of microalgae is significantly reduced. In the absence of carbonate ion, Dunaliella bardawil and Chlorella ellipsoidea have been shown to grow at pH levels exceeding 10.0 (Khalil et al., 2010). Aside from pH, other factors such as temperature, agitation, and initial inoculum concentration can impact the composition and stability of a microalgae-based consortium.
Temperature constitutes a paramount environmental factor modulating the proliferation rate, cellular dimensions, biochemical composition, and nutritional requisites of microalgae. Microalgae assimilate thermal energy originating from luminous sources, engendering an elevation in the internal temperature milieu of the culture. Consequently, in the context of extensive outdoor cultivation systems, it is crucial to address both photonic irradiance and the concomitant thermal factors (Deb et al., 2017). The optimal thermal range for microalgal cultivation lies between 20 and 35°C, with particular mesophilic species displaying tolerance to temperatures approaching 40°C. The microalgae consortia of P. tricormutum, Tetraselmis gracilis, Chaetoceros sp., and Minutocellus polymorphus exhibited the best growth rates within the temperature range of 11°C–36°C, as reported in various studies (Sigaud and Aidar, 1993). Microalgal cultivation is significantly impacted by seasonal changes, which cause temperature fluctuations during the day/night cycle. Small-scale reactors, which operate in relatively cooler ambient temperatures, do not require temperature control, as the input of heat through radiation is balanced by the output through circulation. However, large-scale outdoor reactors experience high levels of solar radiation, necessitating the use of different heat control devices to maintain the optimal temperature (Huesemann et al., 2013).
When utilizing fungi pellets for flocculating microalgae in microalgae/fungi co-cultivation, it is widely accepted that a higher concentration of fungi (higher inoculation ratio to microalgae) is favorable for efficient harvesting (Liu et al., 2008). However, when fungal spores are directly mixed with a microalgal solution, increasing spore concentration may not always improve flocculation efficiency (Liu et al., 2008). At excessively high spore concentrations, microalgae-fungal pellets cannot form, leading to low harvesting rates. The initial inoculum concentration of bacteria/fungi/microalgae plays a critical role in the formation of fungi-microalgae pellets and the growth of microalgae-bacteria, which further affects biomass yield and metabolite production (Yang et al., 2019). This may be due to the interaction between fungal hyphae during the early growth stages, which prevents pellet formation under high initial inoculum concentrations (Zhao Y. et al., 2019). Additionally, the size of the fungal spore inoculation is linked to the pH of the medium, which indirectly influences flocculation efficiency (Zhao et al., 2013). In the co-cultivation of Chlorella sp. and S. cerevisiae, the effect of different inoculum ratios of microalgae on yeast (1:2, 1:1, 2:1) has a significant impact on the final biomass, oil production, and specific production yield (Yp/x). The best results were obtained at a ratio of 2:1 for all studied parameters, but with only a slight increase compared to the 1:1 inoculum ratio (Shu et al., 2013). In the co-culture of C. pyrenoidosa and R. glutinis in piggery wastewater, the optimal ratio of yeast to algae was found to be 3:1, resulting in 36.07% TN (total nitrogen) removal, 58.53% ammonium nitrogen removal, 33.20% TP (total phosphate) removal, and 56.25% COD (chemical oxygen demand) removal after 6 days (Li et al., 2019). The highest biomass concentration and photosynthesis activity for co-cultivating S. obliquus and C. Tropicalis were achieved at a microalga to yeast ratio of 3:1 (Wang R. et al., 2016).
Proper agitation is a critical factor in the morphology of fungi, which plays a significant role in the harvesting efficiency of the co-culture system (Porcel et al., 2005). Certain studies have reported that low agitation speed facilitates the attachment of microalgae to fungi, overcoming electrostatic repulsion (Choi, 2015; Bhattacharya et al., 2017). The co-cultivation of C. vulgaris and R. glutinis reported 150 rpm as the optimum agitating speed, with further increase having no significant effect on biomass and lipid production (Cheirsilp et al., 2011). Photobioreactor design is another crucial parameter to consider in a co-culture system. Higher biomass density than monocultures greatly influences mass transfer rates, thereby impacting growth. Designing a cultivation system with improved mass transfer efficiency, ease of scale-up, and lower costs is thus essential, with nanoparticles offering a promising solution to this end (Magdouli et al., 2016).
We therefore argue that the use of omic technologies in the optimization of microalgae co-cultivation can provide critical insights that can significantly enhance sustainable applications. Omics provide valuable insights into the influence of various factors such as carbon and nitrogen sources, light conditions, and temperature on the microalgae consortia’s performance. It helps unravel the metabolic interactions within the consortia and identify the key parameters for successful co-cultivation. Thus, omics research plays a pivotal role in improving microalgae co-cultivation for sustainable applications. More extensive use of these techniques in conjunction with experimentation will allow for the refinement of the cultivation conditions and contribute to the optimization of microalgae co-cultivation, ultimately contributing to the attainment of sustainability goals.
7 Conclusion
Co-cultivation systems incorporating microalgae have risen to prominence as a resource platform for an array of biotechnological applications, which span the spectrum from biodiesel production to wastewater treatment. These systems have a remarkable ability to thrive on inexpensive feedstocks such as cellulosic biomass, thereby reducing operational costs substantially. Yet, to translate microalgae cultivation into a large-scale operation, we need a more profound understanding of cultivation methods, metabolite recovery and purification from co-culture systems, and strategies to bolster culture resilience. Resilience, in this context, refers to the ability of the co-culture system to withstand changes in environmental conditions and recover from disturbances. The resilience of co-cultivation systems is essential for sustainable large-scale operations.
Future research should focus on optimizing the selection of cost-effective feedstock and harvesting methods, and on the evaluation of varying operating conditions. These include factors such as pH, temperature, light intensity, nutrients, and carbon availability that govern the successful operation of these systems. These steps will be crucial to achieve the economic feasibility of large-scale production of high-value microalgae products. While the significance of microalgae cannot be understated, it is equally important to acknowledge the critical role played by the associated organisms in the co-culture system. The success of these co-cultivation systems hinges not only on the algal components but also on the symbiotic relationship established with other microbial members. Their contributions are fundamental in enhancing the productivity and overall functionality of these systems.
Advancements in the field of omics have opened up new horizons for microalgae cell analysis and present promising prospects for co-cultivation systems. Continuous research efforts in this domain can potentially unveil intricate details about the interactions within these co-cultures, contributing substantially to our comprehensive understanding of these systems. To conclude, the sustainable synthesis of diverse products at an industrial scale lies in the successful implementation of microalgae co-cultivation. This vision, however, can only be realized through an in-depth understanding of the co-culture system as a whole, focused optimization of operating conditions, and leveraging the advancements in omics technology.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
FW thanks FAPESP for financial support (grant #2016/06601-4, São Paulo Research Foundation), CAPES (#001), CNPq, and University of São Paulo, PRP for financial support. JM-R thanks Paicyt 2021–2022, Paicyt 2022–2023, Science Grants from the Universidad Autónoma de Nuevo León; CONACyT Grants Fronteras de la Ciencia 1502, Infraestructura Grant 279957, Apoyos a la Ciencia de Frontera Grant 316869 and Ciencia de Frontera CF-2023-I-1327.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acién, F., Molina, E., Reis, A., Torzillo, G., Zittelli, G. C., Sepúlveda, C., et al. (2017). “Photobioreactors for the production of microalgae,” in Microalgae-based biofuels and bioproducts (Elsevier), 1–44.
Acién, F. G., Gómez-Serrano, C., Morales-Amaral, MdM., Fernández-Sevilla, J. M., and Molina-Grima, E. (2016). Wastewater treatment using microalgae: how realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 100, 9013–9022. doi:10.1007/s00253-016-7835-7
Adav, S. S., Ravindran, A., Cheow, E. S. H., and Sze, S. K. (2012). Quantitative proteomic analysis of secretome of microbial consortium during saw dust utilization. J. proteomics 75, 5590–5603. doi:10.1016/j.jprot.2012.08.011
Addy, M. M., Kabir, F., Zhang, R., Lu, Q., Deng, X., Current, D., et al. (2017). Co-cultivation of microalgae in aquaponic systems. Bioresour. Technol. 245, 27–34. doi:10.1016/j.biortech.2017.08.151
Alcántara, C., Domínguez, J. M., García, D., Blanco, S., Pérez, R., García-Encina, P. A., et al. (2015). Evaluation of wastewater treatment in a novel anoxic–aerobic algal–bacterial photobioreactor with biomass recycling through carbon and nitrogen mass balances. Bioresour. Technol. 191, 173–186. doi:10.1016/j.biortech.2015.04.125
Amin, S. A., Parker, M. S., and Armbrust, E. V. (2012). Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 76, 667–684. doi:10.1128/mmbr.00007-12
Angelidaki, I., and Sanders, W. (2004). Assessment of the anaerobic biodegradability of macropollutants. Re/Views Environ. Sci. Bio/Technology 3, 117–129. doi:10.1007/s11157-004-2502-3
Arora, N., Patel, A., Mehtani, J., Pruthi, P. A., Pruthi, V., and Poluri, K. M. (2019). Co-Culturing of oleaginous microalgae and yeast: paradigm shift towards enhanced lipid productivity. Environ. Sci. Pollut. Res. 26, 16952–16973. doi:10.1007/s11356-019-05138-6
Ashtiani, F-R., Jalili, H., Rahaie, M., Sedighi, M., and Amrane, A. (2021). Effect of mixed culture of yeast and microalgae on acetyl-CoA carboxylase and Glycerol-3-phosphate acyltransferase expression. J. Biosci. Bioeng. 131, 364–372. doi:10.1016/j.jbiosc.2020.11.006
Asmare, A. M., Demessie, B. A., and Murthy, G. S. (2014). Investigation of microalgae Co-cultures for nutrient recovery and algal BiomassProduction from dairy manure. Appl. Eng. Agric. 30, 335–342. doi:10.13031/aea.30.10151
Baba, M., Suzuki, I., and Shiraiwa, Y. (2011). Proteomic analysis of high-CO(2)-inducible extracellular proteins in the unicellular green alga, Chlamydomonas reinhardtii. Plant Cell Physiol. 52, 1302–1314. doi:10.1093/pcp/pcr078
Ban, S., Lin, W., Wu, F., and Luo, J. (2018). Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 251, 350–357. doi:10.1016/j.biortech.2017.12.072
Barati, B., Zafar, F. F., Rupani, P. F., and Wang, S. (2021b). Bacterial pretreatment of microalgae and the potential of novel nature hydrolytic sources. Environ. Technol. Innovation 21, 101362. doi:10.1016/j.eti.2021.101362
Barati, B., Zeng, K., Baeyens, J., Wang, S., Addy, M., Gan, S-Y., et al. (2021a). Recent progress in genetically modified microalgae for enhanced carbon dioxide sequestration. Biomass Bioenergy 145, 105927. doi:10.1016/j.biombioe.2020.105927
Barka, E. A., Gognies, S., Nowak, J., Audran, J-C., and Belarbi, A. (2002). Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol. Control 24, 135–142. doi:10.1016/s1049-9644(02)00034-8
Bell, T. A., Prithiviraj, B., Wahlen, B. D., Fields, M. W., and Peyton, B. M. (2016). A lipid-accumulating alga maintains growth in outdoor, alkaliphilic raceway pond with mixed microbial communities. Front. Microbiol. 6, 1480. doi:10.3389/fmicb.2015.01480
Benemann, J. (1990). Microalgae products and production: an overview. Microalgae Prod. Prod. Overv. 31, 247–256.
Berner, F., Heimann, K., and Sheehan, M. (2015). Microalgal biofilms for biomass production. J. Appl. Phycol. 27, 1793–1804. doi:10.1007/s10811-014-0489-x
Berthold, D. E., Shetty, K. G., Jayachandran, K., Laughinghouse, I. V. H. D., and Gantar, M. (2019). Enhancing algal biomass and lipid production through bacterial co-culture. Biomass bioenergy 122, 280–289. doi:10.1016/j.biombioe.2019.01.033
Bertrand, S., Bohni, N., Schnee, S., Schumpp, O., Gindro, K., and Wolfender, J-L. (2014). Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 32, 1180–1204. doi:10.1016/j.biotechadv.2014.03.001
Bhattacharya, A., Mathur, M., Kumar, P., Prajapati, S. K., and Malik, A. (2017). A rapid method for fungal assisted algal flocculation: critical parameters and mechanism insights. Algal Res. 21, 42–51. doi:10.1016/j.algal.2016.10.022
Bodin, H., Daneshvar, A., Gros, M., and Hultberg, M. (2016). Effects of biopellets composed of microalgae and fungi on pharmaceuticals present at environmentally relevant levels in water. Ecol. Eng. 91, 169–172. doi:10.1016/j.ecoleng.2016.02.007
Boelee, N., Janssen, M., Temmink, H., Taparavičiūtė, L., Khiewwijit, R., Jánoska, Á., et al. (2014). The effect of harvesting on biomass production and nutrient removal in phototrophic biofilm reactors for effluent polishing. J. Appl. Phycol. 26, 1439–1452. doi:10.1007/s10811-013-0178-1
Brenner, K., You, L., and Arnold, F. H. (2008). Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 26, 483–489. doi:10.1016/j.tibtech.2008.05.004
Byun, C. K., Hwang, H., Choi, W. S., Yaguchi, T., Park, J., Kim, D., et al. (2013). Productive chemical interaction between a bacterial microcolony couple is enhanced by periodic relocation. J. Am. Chem. Soc. 135, 2242–2247. doi:10.1021/ja3094923
Cai, S., Hu, C., and Du, S. (2007). Comparisons of growth and biochemical composition between mixed culture of alga and yeast and monocultures. J. Biosci. Bioeng. 104, 391–397. doi:10.1263/jbb.104.391
Carbonell, P., Jervis, A. J., Robinson, C. J., Yan, C., Dunstan, M., Swainston, N., et al. (2018). An automated Design-Build-Test-Learn pipeline for enhanced microbial production of fine chemicals. Commun. Biol. 1, 66–10. doi:10.1038/s42003-018-0076-9
Carruthers, D. N., Byun, C. K., Cardinale, B. J., and Lin, X. N. (2017). Demonstration of transgressive overyielding of algal mixed cultures in microdroplets. Integr. Biol. 9, 687–694. doi:10.1039/c6ib00241b
Chang, R. L., Ghamsari, L., Manichaikul, A., Hom, E. F., Balaji, S., Fu, W., et al. (2011). Metabolic network reconstruction of Chlamydomonas offers insight into light-driven algal metabolism. Mol. Syst. Biol. 7, 518. doi:10.1038/msb.2011.52
Cheirsilp, B., Suwannarat, W., and Niyomdecha, R. (2011). Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. New Biotechnol. 28, 362–368. doi:10.1016/j.nbt.2011.01.004
Chen, J., Leng, L., Ye, C., Lu, Q., Addy, M., Wang, J., et al. (2018). A comparative study between fungal pellet-and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour. Technol. 259, 181–190. doi:10.1016/j.biortech.2018.03.040
Chia, S. R., Chew, K. W., Leong, H. Y., Ho, S-H., Munawaroh, H. S. H., and Show, P. L. (2021). CO2 mitigation and phycoremediation of industrial flue gas and wastewater via microalgae-bacteria consortium: possibilities and challenges. Chem. Eng. J. 425, 131436. doi:10.1016/j.cej.2021.131436
Chia, S. R., Chew, K. W., Show, P. L., Sivakumar, M., Ling, T. C., and Tao, Y. (2019). Isolation of protein from Chlorella sorokiniana CY1 using liquid biphasic flotation assisted with sonication through sugaring-out effect. J. Oceanol. Limnol. 37, 898–908. doi:10.1007/s00343-019-8246-2
Cho, S., Luong, T. T., Lee, D., Oh, Y-K., and Lee, T. (2011). Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour. Technol. 102, 8639–8645. doi:10.1016/j.biortech.2011.03.037
Choi, H. J. (2015). Effect of eggshells for the harvesting of microalgae species. Biotechnol. Biotechnol. Equip. 29, 666–672. doi:10.1080/13102818.2015.1031177
Chu, R., Li, S., Yin, Z., Hu, D., Zhang, L., Xiang, M., et al. (2021). A fungal immobilization technique for efficient harvesting of oleaginous microalgae: key parameter optimization, mechanism exploration and spent medium recycling. Sci. Total Environ. 790, 148174. doi:10.1016/j.scitotenv.2021.148174
Cieplik, F., Deng, D., Crielaard, W., Buchalla, W., Hellwig, E., Al-Ahmad, A., et al. (2018). Antimicrobial photodynamic therapy–what we know and what we don’t. Crit. Rev. Microbiol. 44, 571–589. doi:10.1080/1040841x.2018.1467876
Covarrubias, S. A., de-Bashan, L. E., Moreno, M., and Bashan, Y. (2012). Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 93, 2669–2680. doi:10.1007/s00253-011-3585-8
Crozet, P., Navarro, F. J., Willmund, F., Mehrshahi, P., Bakowski, K., Lauersen, K. J., et al. (2018). Birth of a photosynthetic chassis: A MoClo toolkit enabling synthetic biology in the microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 7, 2074–2086. doi:10.1021/acssynbio.8b00251
Dao, G-H., Wu, G-X., Wang, X-X., Zhang, T-Y., Zhan, X-M., and Hu, H-Y. (2018). Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res. 33, 345–351. doi:10.1016/j.algal.2018.06.006
Das, P. K., Rani, J., Rawat, S., and Kumar, S. (2021). Microalgal Co-cultivation for biofuel production and bioremediation: current status and benefits. BioEnergy Res. 15, 1–26. doi:10.1007/s12155-021-10254-8
de Farias Silva, C. E., and Bertucco, A. (2016). Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochem. 51, 1833–1842. doi:10.1016/j.procbio.2016.02.016
de Oliveira Magalhães, L., Nunes de Mello, F., and Vischi Winck, F. (2022). Characterization of the nuclear proteome of Chlamydomonas in response to salt stress. Phycology 2, 280–296. doi:10.3390/phycology2030015
De-Bashan, L. E., Moreno, M., Hernandez, J-P., and Bashan, Y. (2002). Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res. 36, 2941–2948. doi:10.1016/s0043-1354(01)00522-x
Deb, U. K., Shahriar, M., Bhowmik, J., and Chowdury, M. (2017). The effect of irradiance related temperature on microalgae growth in a tubular photo bioreactor for cleaner energy. Am. J. Comput. Math. 7, 371–384. doi:10.4236/ajcm.2017.73026
Delgadillo-Mirquez, L., Lopes, F., Taidi, B., and Pareau, D. (2016). Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 11, 18–26. doi:10.1016/j.btre.2016.04.003
DellaGreca, M., Zarrelli, A., Fergola, P., Cerasuolo, M., Pollio, A., and Pinto, G. (2010). Fatty acids released by Chlorella vulgaris and their role in interference with Pseudokirchneriella subcapitata: experiments and modelling. J. Chem. Ecol. 36, 339–349. doi:10.1007/s10886-010-9753-y
Devi, M. P., Subhash, G. V., and Mohan, S. V. (2012). Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: effect of nutrient supplementation. Renew. energy 43, 276–283. doi:10.1016/j.renene.2011.11.021
Du, Z-Y., Alvaro, J., Hyden, B., Zienkiewicz, K., Benning, N., Zienkiewicz, A., et al. (2018). Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol. biofuels 11, 174. doi:10.1186/s13068-018-1172-2
Du, Z-Y., Zienkiewicz, K., Pol, N. V., Ostrom, N. E., Benning, C., and Bonito, G. M. (2019). Algal-fungal symbiosis leads to photosynthetic mycelium. Elife 8, e47815. doi:10.7554/eLife.47815
Dunker, S., Althammer, J., Pohnert, G., and Wilhelm, C. (2017). A fateful meeting of two phytoplankton species—Chemical vs. cell-cell-interactions in co-cultures of the green algae Oocystis marsonii and the cyanobacterium Microcystis aeruginosa. Microb. Ecol. 74, 22–32. doi:10.1007/s00248-016-0927-1
Esakkimuthu, S., Krishnamurthy, V., Wang, S., Abomohra, A. E., Shanmugam, S., Ramakrishnan, S. G., et al. (2019). Simultaneous induction of biomass and lipid production in Tetradesmus obliquus BPL16 through polysorbate supplementation. Renew. Energy 140, 807–815. doi:10.1016/j.renene.2019.03.104
Esakkimuthu, S., Krishnamurthy, V., Wang, S., Hu, X., Swaminathan, K., and Abomohra, A. E. (2020). Application of p-coumaric acid for extraordinary lipid production in tetradesmus obliquus: A sustainable approach towards enhanced biodiesel production. Renew. Energy 157, 368–376. doi:10.1016/j.renene.2020.05.005
Espinosa-Ortiz, E. J., Rene, E. R., Pakshirajan, K., van Hullebusch, E. D., and Lens, P. N. (2016). Fungal pelleted reactors in wastewater treatment: applications and perspectives. Chem. Eng. J. 283, 553–571. doi:10.1016/j.cej.2015.07.068
Fakhimi, N., Gonzalez-Ballester, D., Fernández, E., Galván, A., and Dubini, A. (2020). Algae-bacteria consortia as a strategy to enhance H2 production. Cells 9, 1353. doi:10.3390/cells9061353
Fallahi, A., Hajinajaf, N., Tavakoli, O., and Mehrabadi, A. (2021). Effects of simultaneous CO<sub>2</sub> addition and biomass recycling on growth characteristics of microalgal mixed culture. J. Chem. Technol. Biotechnol. 96, 3398–3407. doi:10.1002/jctb.6896
Fernández, F. A., Sevilla, J. F., Pérez, J. S., Grima, E. M., and Chisti, Y. (2001). Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: assessment of design and performance. Chem. Eng. Sci. 56, 2721–2732. doi:10.1016/s0009-2509(00)00521-2
Friday, E. T., Rapheal, E., Nwalo, F., and Ayodele, S. (2010). Mixed cultivation of Euglena gracilis and Chlorella sorokiniana: A production method of algae biomass on a large scale. J. Appl. Biosci. 35, 2225–2234.
Goiris, K., Van Colen, W., Wilches, I., León-Tamariz, F., De Cooman, L., and Muylaert, K. (2015). Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 7, 51–57. doi:10.1016/j.algal.2014.12.002
Gonçalves, A. L., Pires, J. C., and Simões, M. (2017). A review on the use of microalgal consortia for wastewater treatment. Algal Res. 24, 403–415. doi:10.1016/j.algal.2016.11.008
Gonçalves, A. L., Pires, J. C., and Simoes, M. (2016). The effects of light and temperature on microalgal growth and nutrient removal: an experimental and mathematical approach. RSC Adv. 6, 22896–22907. doi:10.1039/c5ra26117a
Gonzalez-Camejo, J., Barat, R., Pachés, M., Murgui, M., Seco, A., and Ferrer, J. (2018). Wastewater nutrient removal in a mixed microalgae–bacteria culture: effect of light and temperature on the microalgae–bacteria competition. Environ. Technol. 39, 503–515. doi:10.1080/09593330.2017.1305001
González-Fernández, C., Molinuevo-Salces, B., and García-González, M. C. (2011). Nitrogen transformations under different conditions in open ponds by means of microalgae–bacteria consortium treating pig slurry. Bioresour. Technol. 102, 960–966. doi:10.1016/j.biortech.2010.09.052
Goswami, G., Makut, B. B., and Das, D. (2019). Sustainable production of bio-crude oil via hydrothermal liquefaction of symbiotically grown biomass of microalgae-bacteria coupled with effective wastewater treatment. Sci. Rep. 9, 15016. doi:10.1038/s41598-019-51315-5
Gou, J-Y., Suo, S-Z., Shao, K-Z., Zhao, Q., Yao, D., Li, H-P., et al. (2020). Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L). World J. Microbiol. Biotechnol. 36, 86–12. doi:10.1007/s11274-020-02863-w
Grimm, L., Kelly, S., Völkerding, I., Krull, R., and Hempel, D. (2005). Influence of mechanical stress and surface interaction on the aggregation of Aspergillus niger conidia. Biotechnol. Bioeng. 92, 879–888. doi:10.1002/bit.20666
Grobbelaar, J. U. (2004). “Algal nutrition: mineral nutrition,” in Handbook of microalgal culture: biotechnology and applied phycology. Editors A. Richmond, and Q. Hu (John Wiley and Sons), 97–115.
Gultom, S. O., Zamalloa, C., and Hu, B. (2014). Microalgae harvest through fungal pelletization—Co-culture of Chlorella vulgaris and Aspergillus niger. Energies 7, 4417–4429. doi:10.3390/en7074417
Guo, Z., and Tong, Y. W. (2014). The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J. Appl. Phycol. 26, 1483–1492. doi:10.1007/s10811-013-0186-1
Gutiérrez, R., Ferrer, I., González-Molina, A., Salvadó, H., García, J., and Uggetti, E. (2016). Microalgae recycling improves biomass recovery from wastewater treatment high rate algal ponds. Water Res. 106, 539–549. doi:10.1016/j.watres.2016.10.039
Han, X., Hu, X., Yin, Q., Li, S., and Song, C. (2021). Intensification of brewery wastewater purification integrated with CO2 fixation via microalgae co-cultivation. J. Environ. Chem. Eng. 9, 105710. doi:10.1016/j.jece.2021.105710
Huesemann, M. H., Van Wagenen, J., Miller, T., Chavis, A., Hobbs, S., and Crowe, B. (2013). A screening model to predict microalgae biomass growth in photobioreactors and raceway ponds. Biotechnol. Bioeng. 110, 1583–1594. doi:10.1002/bit.24814
Hultberg, M., and Bodin, H. (2018). Effects of fungal-assisted algal harvesting through biopellet formation on pesticides in water. Biodegradation 29, 557–565. doi:10.1007/s10532-018-9852-y
Huo, S., Liu, J., Addy, M., Chen, P., Necas, D., Cheng, P., et al. (2020). The influence of microalgae on vegetable production and nutrient removal in greenhouse hydroponics. J. Clean. Prod. 243, 118563. doi:10.1016/j.jclepro.2019.118563
Iasimone, F., Panico, A., De Felice, V., Fantasma, F., Iorizzi, M., and Pirozzi, F. (2018). Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: biomass production, lipids accumulation and settleability characteristics. J. Environ. Manag. 223, 1078–1085. doi:10.1016/j.jenvman.2018.07.024
Illman, A., Scragg, A., and Shales, S. (2000). Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 27, 631–635. doi:10.1016/s0141-0229(00)00266-0
Ishika, T., Moheimani, N. R., and Bahri, P. A. (2017). Sustainable saline microalgae co-cultivation for biofuel production: A critical review. Renew. Sustain. energy Rev. 78, 356–368. doi:10.1016/j.rser.2017.04.110
Ji, X-J., and Ledesma-Amaro, R. (2020). Microbial lipid biotechnology to produce polyunsaturated fatty acids. Trends Biotechnol. 38, 832–834. doi:10.1016/j.tibtech.2020.02.003
Jia, H., and Yuan, Q. (2016). Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2, 1275089. doi:10.1080/23311843.2016.1275089
Jiang, X., Liu, L., Chen, J., and Wei, D. (2018). Effects of Xanthophyllomyces dendrorhous on cell growth, lipid, and astaxanthin production of Chromochloris zofingiensis by mixed culture strategy. J. Appl. Phycol. 30, 3009–3015. doi:10.1007/s10811-018-1553-8
Juliarnita, I. G. A., Hadisoebroto, R., and Rinanti, A. (2018). “Bioethanol production from mixed culture microalgae biomass with temperature hydrolysis variation,” in MATEC web of conferences (EDP Sciences).13010.
Kawaguchi, H., Hashimoto, K., Hirata, K., and Miyamoto, K. (2001). H2 production from algal biomass by a mixed culture of Rhodobium marinum A-501 and Lactobacillus amylovorus. J. Biosci. Bioeng. 91, 277–282. doi:10.1016/s1389-1723(01)80134-1
Kesaano, M., and Sims, R. C. (2014). Algal biofilm based technology for wastewater treatment. Algal Res. 5, 231–240. doi:10.1016/j.algal.2014.02.003
Khalil, Z. I., Asker, M., El-Sayed, S., and Kobbia, I. A. (2010). Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J. Microbiol. Biotechnol. 26, 1225–1231. doi:10.1007/s11274-009-0292-z
Kholssi, R., Marks, E. A., Montero, O., Maté, A. P., Debdoubi, A., and Rad, C. (2018). The growth of filamentous microalgae is increased on biochar solid supports. Biocatal. Agric. Biotechnol. 13, 182–185. doi:10.1016/j.bcab.2017.12.011
Kim, B-H., Ramanan, R., Cho, D-H., Oh, H-M., and Kim, H-S. (2014). Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenergy 69, 95–105. doi:10.1016/j.biombioe.2014.07.015
Kim, M., Choi, M. G., Ra, H. W., Park, S. B., Kim, Y-J., and Lee, K. (2018). Encapsulation of multiple microalgal cells via a combination of biomimetic mineralization and LbL coating. Materials 11, 296. doi:10.3390/ma11020296
Kitcha, S., and Cheirsilp, B. (2014). Enhanced lipid production by co-cultivation and co-encapsulation of oleaginous yeast Trichosporonoides spathulata with microalgae in alginate gel beads. Appl. Biochem. Biotechnol. 173, 522–534. doi:10.1007/s12010-014-0859-5
Kuken, A., Sommer, F., Yaneva-Roder, L., Mackinder, L. C., Hohne, M., Geimer, S., et al. (2018). Effects of microcompartmentation on flux distribution and metabolic pools in Chlamydomonas reinhardtii chloroplasts. Elife 7, e37960. doi:10.7554/elife.37960
Kumar, G., Shekh, A., Jakhu, S., Sharma, Y., Kapoor, R., and Sharma, T. R. (2020). Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Front. Bioeng. Biotechnol. 8, 914. doi:10.3389/fbioe.2020.00914
Kuo, E. Y., Yang, R. Y., Chin, Y. Y., Chien, Y. L., Chen, Y. C., Wei, C. Y., et al. (2022). Multi-omics approaches and genetic engineering of metabolism for improved biorefinery and wastewater treatment in microalgae. Biotechnol. J. 17, 2100603. doi:10.1002/biot.202100603
Lakshmikandan, M., Murugesan, A., Wang, S., Abomohra, A. E-F., Jovita, P. A., and Kiruthiga, S. (2020). Sustainable biomass production under CO2 conditions and effective wet microalgae lipid extraction for biodiesel production. J. Clean. Prod. 247, 119398. doi:10.1016/j.jclepro.2019.119398
Lakshmikandan, M., Wang, S., Murugesan, A., Saravanakumar, M., and Selvakumar, G. (2021). Co-cultivation of Streptomyces and microalgal cells as an efficient system for biodiesel production and bioflocculation formation. Bioresour. Technol. 332, 125118. doi:10.1016/j.biortech.2021.125118
Lam, M. K., and Lee, K. T. (2012). Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 30, 673–690. doi:10.1016/j.biotechadv.2011.11.008
Lee, J., Cho, D-H., Ramanan, R., Kim, B-H., Oh, H-M., and Kim, H-S. (2013). Microalgae-associated bacteria play a key role in the flocculation of Chlorella vulgaris. Bioresour. Technol. 131, 195–201. doi:10.1016/j.biortech.2012.11.130
Lee, S-H., Oh, H-M., Jo, B-H., Lee, S-A., Shin, S-Y., Kim, H-S., et al. (2014). Higher biomass productivity of microalgae in an attached growth system, using wastewater. J. Microbiol. Biotechnol. 24, 1566–1573. doi:10.4014/jmb.1406.06057
Leng, L., Li, W., Chen, J., Leng, S., Chen, J., Wei, L., et al. (2021). Co-Culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresour. Technol. 330, 125008. doi:10.1016/j.biortech.2021.125008
Lennen, R. M., and Pfleger, B. F. (2013). Microbial production of fatty acid-derived fuels and chemicals. Curr. Opin. Biotechnol. 24, 1044–1053. doi:10.1016/j.copbio.2013.02.028
Li, H., Zhong, Y., Lu, Q., Zhang, X., Wang, Q., Liu, H., et al. (2019). Co-cultivation of Rhodotorula glutinis and Chlorella pyrenoidosa to improve nutrient removal and protein content by their synergistic relationship. RSC Adv. 9, 14331–14342. doi:10.1039/c9ra01884k
Li, Y., Xu, Y., Liu, L., Li, P., Yan, Y., Chen, T., et al. (2017). Flocculation mechanism of Aspergillus niger on harvesting of Chlorella vulgaris biomass. Algal Res. 25, 402–412. doi:10.1016/j.algal.2017.06.001
Liang, Z., Liu, Y., Ge, F., Xu, Y., Tao, N., Peng, F., et al. (2013). Efficiency assessment and pH effect in removing nitrogen and phosphorus by algae-bacteria combined system of Chlorella vulgaris and Bacillus licheniformis. Chemosphere 92, 1383–1389. doi:10.1016/j.chemosphere.2013.05.014
Ling, J., Nip, S., Cheok, W. L., de Toledo, R. A., and Shim, H. (2014). Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour. Technol. 173, 132–139. doi:10.1016/j.biortech.2014.09.047
Liu, J., Wu, Y., Wu, C., Muylaert, K., Vyverman, W., Yu, H-Q., et al. (2017). Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresour. Technol. 241, 1127–1137. doi:10.1016/j.biortech.2017.06.054
Liu, L., Chen, J., Lim, P-E., and Wei, D. (2018). Dual-species cultivation of microalgae and yeast for enhanced biomass and microbial lipid production. J. Appl. Phycol. 30, 2997–3007. doi:10.1007/s10811-018-1526-y
Liu, X., Jia, B., Sun, X., Ai, J., Wang, L., Wang, C., et al. (2015). Effect of initial pH on growth characteristics and fermentation properties of Saccharomyces cerevisiae. J. food Sci. 80, M800–M808. doi:10.1111/1750-3841.12813
Liu, Y., Liao, W., and Chen, S. (2008). Co-production of lactic acid and chitin using a pelletized filamentous fungus Rhizopus oryzae cultured on cull potatoes and glucose. J. Appl. Microbiol. 105, 1521–1528. doi:10.1111/j.1365-2672.2008.03913.x
Lu, D., Tabil, L. G., Wang, D., Wang, G., and Emami, S. (2014). Experimental trials to make wheat straw pellets with wood residue and binders. Biomass Bioenergy 69, 287–296. doi:10.1016/j.biombioe.2014.07.029
Lü, F., Ji, J., Shao, L., and He, P. (2013). Bacterial bioaugmentation for improving methane and hydrogen production from microalgae. Biotechnol. biofuels 6, 92–11. doi:10.1186/1754-6834-6-92
Luo, S., Wu, X., Jiang, H., Yu, M., Liu, Y., Min, A., et al. (2019). Edible fungi-assisted harvesting system for efficient microalgae bio-flocculation. Bioresour. Technol. 282, 325–330. doi:10.1016/j.biortech.2019.03.033
Luo, X., Zhang, H., and Zhang, J. (2021). The influence of a static magnetic field on a Chlorella vulgaris-Bacillus licheniformis consortium and its sewage treatment effect. J. Environ. Manag. 295, 112969. doi:10.1016/j.jenvman.2021.112969
Lutzu, G. A., and Dunford, N. T. (2018). Interactions of microalgae and other microorganisms for enhanced production of high-value compounds. Front. Bioscience—Landmark 23, 1487–1504. doi:10.2741/4656
Ma, L., Wang, F., Yu, Y., Liu, J., and Wu, Y. (2018). Cu removal and response mechanisms of periphytic biofilms in a tubular bioreactor. Bioresour. Technol. 248, 61–67. doi:10.1016/j.biortech.2017.07.014
Magdouli, S., Brar, S. K., and Blais, J-F. (2016). Co-Culture for lipid production: advances and challenges. Biomass Bioenergy 92, 20–30. doi:10.1016/j.biombioe.2016.06.003
Makut, B. B., Goswami, G., and Das, D. (2020). Evaluation of bio-crude oil through hydrothermal liquefaction of microalgae-bacteria consortium grown in open pond using wastewater. Biomass Convers. Biorefinery 12, 2567–2581. doi:10.1007/s13399-020-00795-x
Markou, G., and Georgakakis, D. (2011). Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: A review. Appl. Energy 88, 3389–3401. doi:10.1016/j.apenergy.2010.12.042
Markou, G., Vandamme, D., and Muylaert, K. (2014). Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res. 65, 186–202. doi:10.1016/j.watres.2014.07.025
Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S. L., Péan, C., Berger, S., et al. (2021). Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press.
Mastrobuoni, G., Irgang, S., Pietzke, M., Assmus, H. E., Wenzel, M., Schulze, W. X., et al. (2012). Proteome dynamics and early salt stress response of the photosynthetic organism Chlamydomonas reinhardtii. BMC Genomics 13, 215. doi:10.1186/1471-2164-13-215
Mata, T. M., Martins, A. A., and Caetano, N. S. (2010). Microalgae for biodiesel production and other applications: A review. Renew. Sustain. energy Rev. 14, 217–232. doi:10.1016/j.rser.2009.07.020
Matsumoto, M., Yokouchi, H., Suzuki, N., Ohata, H., and Matsunaga, T. (2003). Saccharification of marine microalgae using marine bacteria for ethanol production. Appl. Biochem. Biotechnol. 105, 247–254. doi:10.1385/abab:105:1-3:247
Medina, M., and Neis, U. (2007). Symbiotic algal bacterial wastewater treatment: effect of food to microorganism ratio and hydraulic retention time on the process performance. Water Sci. Technol. 55, 165–171. doi:10.2166/wst.2007.351
Mehrabadi, A., Farid, M. M., and Craggs, R. (2017). Effect of CO2 addition on biomass energy yield in wastewater treatment high rate algal mesocosms. Algal Res. 22, 93–103. doi:10.1016/j.algal.2016.12.010
Meng, X., Yang, J., Xu, X., Zhang, L., Nie, Q., and Xian, M. (2009). Biodiesel production from oleaginous microorganisms. Renew. energy 34, 1–5. doi:10.1016/j.renene.2008.04.014
Metting, B., and Pyne, J. W. (1986). Biologically active compounds from microalgae. Enzyme Microb. Technol. 8, 386–394. doi:10.1016/0141-0229(86)90144-4
Mettler, T., Muhlhaus, T., Hemme, D., Schottler, M. A., Rupprecht, J., Idoine, A., et al. (2014). Systems analysis of the response of photosynthesis, metabolism, and growth to an increase in irradiance in the photosynthetic model organism Chlamydomonas reinhardtii. Plant Cell 26, 2310–2350. doi:10.1105/tpc.114.124537
Miranda, A. F., Ramkumar, N., Andriotis, C., Höltkemeier, T., Yasmin, A., Rochfort, S., et al. (2017). Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol. Biofuels 10, 120–123. doi:10.1186/s13068-017-0798-9
Mishra, A., Medhi, K., Malaviya, P., and Thakur, I. S. (2019). Omics approaches for microalgal applications: prospects and challenges. Bioresour. Technol. 291, 121890. doi:10.1016/j.biortech.2019.121890
Mondal, M., Goswami, S., Ghosh, A., Oinam, G., Tiwari, O., Das, P., et al. (2017). Production of biodiesel from microalgae through biological carbon capture: A review. 3 Biotech. 7, 99–21. doi:10.1007/s13205-017-0727-4
Moreno-Garcia, L., Adjallé, K., Barnabé, S., and Raghavan, G. (2017). Microalgae biomass production for a biorefinery system: recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 76, 493–506. doi:10.1016/j.rser.2017.03.024
Mouget, J-L., Dakhama, A., Lavoie, M. C., and de la Noüe, J. (1995). Algal growth enhancement by bacteria: is consumption of photosynthetic oxygen involved? FEMS Microbiol. Ecol. 18, 35–43. doi:10.1111/j.1574-6941.1995.tb00159.x
Mujtaba, G., Rizwan, M., and Lee, K. (2015). Simultaneous removal of inorganic nutrients and organic carbon by symbiotic co-culture of Chlorella vulgaris and Pseudomonas putida. Biotechnol. bioprocess Eng. 20, 1114–1122. doi:10.1007/s12257-015-0421-5
Muñoz, C., Hidalgo, C., Zapata, M., Jeison, D., Riquelme, C., and Rivas, M. (2014). Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Appl. Environ. Microbiol. 80, 4199–4206. doi:10.1128/aem.00827-14
Muradov, N., Taha, M., Miranda, A. F., Wrede, D., Kadali, K., Gujar, A., et al. (2015). Fungal-assisted algal flocculation: application in wastewater treatment and biofuel production. Biotechnol. biofuels 8, 24–23. doi:10.1186/s13068-015-0210-6
Mutanda, T., Naidoo, D., Bwapwa, J. K., and Anandraj, A. (2020). Biotechnological applications of microalgal oleaginous compounds: current trends on microalgal bioprocessing of products. Front. Energy Res. 8, 299. doi:10.3389/fenrg.2020.598803
Novoveská, L., Franks, D. T., Wulfers, T. A., and Henley, W. J. (2016). Stabilizing continuous mixed cultures of microalgae. Algal Res. 13, 126–133. doi:10.1016/j.algal.2015.11.021
Ogbonna, J. C., Yoshizawa, H., and Tanaka, H. (2000). Treatment of high strength organic wastewater by a mixed culture of photosynthetic microorganisms. J. Appl. Phycol. 12, 277–284. doi:10.1023/a:1008188311681
Oh, D-C., Kauffman, C. A., Jensen, P. R., and Fenical, W. (2007). Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 70, 515–520. doi:10.1021/np060381f
Oh, Y-K., Raj, S. M., Jung, G. Y., and Park, S. (2011). Current status of the metabolic engineering of microorganisms for biohydrogen production. Bioresour. Technol. 102, 8357–8367. doi:10.1016/j.biortech.2011.04.054
Okaiyeto, K., Nwodo, U. U., Mabinya, L. V., and Okoh, A. I. (2013). Characterization of a bioflocculant produced by a consortium of Halomonas sp. okoh and Micrococcus sp. leo. Int. J. Environ. Res. public health 10, 5097–5110. doi:10.3390/ijerph10105097
Olaizola, M. (2003). Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol. Eng. 20, 459–466. doi:10.1016/s1389-0344(03)00076-5
Oliveira, C. Y. B., Nader, C., Silva, M. F., Fracalossi, D. M., Gálvez, A. O., Lopes, R. G., et al. (2020). Integrated use of microalgal biomass of choricystis minor var. minor: A promising model for production of biodiesel and aquafeeds. Biomass Convers. Biorefinery 12, 1565–1573. doi:10.1007/s13399-020-01091-4
Owolabi, R., Adejumo, A., and Aderibigbe, A. (2012). Biodiesel: fuel for the future (a brief review). Int. J. Energy Eng. 2, 223–231. doi:10.5923/j.ijee.20120205.06
Özçimen, D., İnan, B., and Biernat, K. (2015). An overview of bioethanol production from algae. Biofuels-Status Perspective, 141–162. doi:10.5772/59305
Padmaperuma, G., Kapoore, R. V., Gilmour, D. J., and Vaidyanathan, S. (2018). Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 38, 690–703. doi:10.1080/07388551.2017.1390728
Papone, T., Kookkhunthod, S., and Leesing, R. (2012). Microbial oil production by monoculture and mixed cultures of microalgae and oleaginous yeasts using sugarcane juice as substrate. Int. J. Nutr. Food Eng. 6, 195–199. doi:10.5281/zenodo.1332532
Park, E. Y., Naruse, K., and Kato, T. (2012). One-pot bioethanol production from cellulose by co-culture of Acremonium cellulolyticus and Saccharomyces cerevisiae. Biotechnol. biofuels 5, 64–11. doi:10.1186/1754-6834-5-64
Park, J., and Craggs, R. (2014). Effect of algal recycling rate on the performance of Pediastrum boryanum dominated wastewater treatment high rate algal pond. Water Sci. Technol. 70, 1299–1306. doi:10.2166/wst.2014.369
Park, J., Craggs, R., and Shilton, A. (2013). Investigating why recycling gravity harvested algae increases harvestability and productivity in high rate algal ponds. Water Res. 47, 4904–4917. doi:10.1016/j.watres.2013.05.027
Park, J., Craggs, R., and Shilton, A. (2011). Recycling algae to improve species control and harvest efficiency from a high rate algal pond. Water Res. 45, 6637–6649. doi:10.1016/j.watres.2011.09.042
Patel, A., Arora, N., Sartaj, K., Pruthi, V., and Pruthi, P. A. (2016). Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew. Sustain. energy Rev. 62, 836–855. doi:10.1016/j.rser.2016.05.014
Paul, C., Mausz, M. A., and Pohnert, G. (2013). A co-culturing/metabolomics approach to investigate chemically mediated interactions of planktonic organisms reveals influence of bacteria on diatom metabolism. Metabolomics 9, 349–359. doi:10.1007/s11306-012-0453-1
Pei, X-Y., Ren, H-Y., and Liu, B-F. (2021). Flocculation performance and mechanism of fungal pellets on harvesting of microalgal biomass. Bioresour. Technol. 321, 124463. doi:10.1016/j.biortech.2020.124463
Perez-Garcia, O., Escalante, F. M., De-Bashan, L. E., and Bashan, Y. (2011). Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 45, 11–36. doi:10.1016/j.watres.2010.08.037
Piercey-Normore, M. D., and Athukorala, S. N. (2017). Interface between fungi and green algae in lichen associations. Botany 95, 1005–1014. doi:10.1139/cjb-2017-0037
Pierobon, S., Cheng, X., Graham, P., Nguyen, B., Karakolis, E., and Sinton, D. (2018). Emerging microalgae technology: A review. Sustain. Energy and Fuels 2, 13–38. doi:10.1039/c7se00236j
Porcel, E. R., López, J. C., Pérez, J. S., Sevilla, J. F., and Chisti, Y. (2005). Effects of pellet morphology on broth rheology in fermentations of Aspergillus terreus. Biochem. Eng. J. 26, 139–144. doi:10.1016/j.bej.2005.04.011
Powell, R. J., and Hill, R. T. (2013). Rapid aggregation of biofuel-producing algae by the bacterium Bacillus sp. strain RP1137. Appl. Environ. Microbiol. 79, 6093–6101. doi:10.1128/aem.01496-13
Prajapati, S. K., Bhattacharya, A., Kumar, P., Malik, A., and Vijay, V. K. (2016). A method for simultaneous bioflocculation and pretreatment of algal biomass targeting improved methane production. Green Chem. 18, 5230–5238. doi:10.1039/c6gc01483f
Puangbut, M., and Leesing, R. (2012). Integrated cultivation technique for microbial lipid production by photosynthetic microalgae and locally oleaginous yeast. Int. J. Agric. Biosyst. Eng. 6, 242–246.
Pulz, O., and Gross, W. (2004). Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 65, 635–648. doi:10.1007/s00253-004-1647-x
Qin, L., Wang, Z., Sun, Y., Shu, Q., Feng, P., Zhu, L., et al. (2016). Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ. Sci. Pollut. Res. 23, 8379–8387. doi:10.1007/s11356-015-6004-3
Radmer, R. J. (1996). Algal diversity and commercial algal products. Bioscience 46, 263–270. doi:10.2307/1312833
Rajapitamahuni, S., Bachani, P., Sardar, R. K., and Mishra, S. (2019). Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J. Appl. Phycol. 31, 29–39. doi:10.1007/s10811-018-1591-2
Rajendran, A., Fox, T., and Hu, B. (2017). Nutrient recovery from ethanol co-products by a novel mycoalgae biofilm: attached cultures of symbiotic fungi and algae. J. Chem. Technol. Biotechnol. 92, 1766–1776. doi:10.1002/jctb.5177
Rajendran, A., and Hu, B. (2016). Mycoalgae biofilm: development of a novel platform technology using algae and fungal cultures. Biotechnol. biofuels 9, 112–113. doi:10.1186/s13068-016-0533-y
Rakesh, S., and karthikeyan, S. (2019). Co-cultivation of microalgae with oleaginous yeast for economical biofuel production. J. Farm Sci. 32, 125–130. doi:10.13140/RG.2.2.10506.41928
Ramanan, R., Kim, B-H., Cho, D-H., Oh, H-M., and Kim, H-S. (2016). Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol. Adv. 34, 14–29. doi:10.1016/j.biotechadv.2015.12.003
Rashid, N., Ryu, A. J., Jeong, K. J., Lee, B., and Chang, Y-K. (2019). Co-cultivation of two freshwater microalgae species to improve biomass productivity and biodiesel production. Energy Convers. Manag. 196, 640–648. doi:10.1016/j.enconman.2019.05.106
Ray, A., Nayak, M., and Ghosh, A. (2021). A review on co-culturing of microalgae: A greener strategy towards sustainable biofuels production. Sci. Total Environ. 802, 149765. doi:10.1016/j.scitotenv.2021.149765
Richmond, A. (2008). Handbook of microalgal culture: biotechnology and applied phycology. John Wiley and Sons.
Rivas, M. O., Vargas, P., and Riquelme, C. E. (2010). Interactions of Botryococcus braunii cultures with bacterial biofilms. Microb. Ecol. 60, 628–635. doi:10.1007/s00248-010-9686-6
Rizwan, M., Mujtaba, G., Memon, S. A., Lee, K., and Rashid, N. (2018). Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 92, 394–404. doi:10.1016/j.rser.2018.04.034
Roostaei, J., Zhang, Y., Gopalakrishnan, K., and Ochocki, A. J. (2018). Mixotrophic microalgae biofilm: A novel algae cultivation strategy for improved productivity and cost-efficiency of biofuel feedstock production. Sci. Rep. 8, 12528. doi:10.1038/s41598-018-31016-1
Rosero-Chasoy, G., Rodríguez-Jasso, R. M., Aguilar, C. N., Buitrón, G., Chairez, I., and Ruiz, H. A. (2021). Microbial co-culturing strategies for the production high value compounds, a reliable framework towards sustainable biorefinery implementation–an overview. Bioresour. Technol. 321, 124458. doi:10.1016/j.biortech.2020.124458
Salih, F. M. (2011). Microalgae tolerance to high concentrations of carbon dioxide: A review. J. Environ. Prot. 2, 648–654. doi:10.4236/jep.2011.25074
Sandland, G. J., Rodgers, J. K., and Minchella, D. J. (2007). Interspecific antagonism and virulence in hosts exposed to two parasite species. J. Invertebr. Pathology 96, 43–47. doi:10.1016/j.jip.2007.02.005
Santos, C. A., Caldeira, M., da Silva, T. L., Novais, J., and Reis, A. (2013). Enhanced lipidic algae biomass production using gas transfer from a fermentative Rhodosporidium toruloides culture to an autotrophic Chlorella protothecoides culture. Bioresour. Technol. 138, 48–54. doi:10.1016/j.biortech.2013.03.135
Sarada, R., Vidhyavathi, R., Usha, D., and Ravishankar, G. (2006). An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agric. food Chem. 54, 7585–7588. doi:10.1021/jf060737t
Saravanan, A., Kumar, P. S., Varjani, S., Jeevanantham, S., Yaashikaa, P., Thamarai, P., et al. (2021). A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 271, 129540. doi:10.1016/j.chemosphere.2021.129540
Schmollinger, S., Muhlhaus, T., Boyle, N. R., Blaby, I. K., Casero, D., Mettler, T., et al. (2014). Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 26, 1410–1435. doi:10.1105/tpc.113.122523
Schulze, P. S., Barreira, L. A., Pereira, H. G., Perales, J. A., and Varela, J. C. (2014). Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 32, 422–430. doi:10.1016/j.tibtech.2014.06.001
Scognamiglio, V., Giardi, M. T., Zappi, D., Touloupakis, E., and Antonacci, A. (2021). Photoautotrophs–bacteria Co-cultures: advances, challenges and applications. Materials 14, 3027. doi:10.3390/ma14113027
Scott, S. A., Davey, M. P., Dennis, J. S., Horst, I., Howe, C. J., Lea-Smith, D. J., et al. (2010). Biodiesel from algae: challenges and prospects. Curr. Opin. Biotechnol. 21, 277–286. doi:10.1016/j.copbio.2010.03.005
Serra, T., Colomer, J., and Logan, B. E. (2008). Efficiency of different shear devices on flocculation. Water Res. 42, 1113–1121. doi:10.1016/j.watres.2007.08.027
Shen, N., and Chirwa, E. M. (2020). Live and lyophilized fungi-algae pellets as novel biosorbents for gold recovery: critical parameters, isotherm, kinetics and regeneration studies. Bioresour. Technol. 306, 123041. doi:10.1016/j.biortech.2020.123041
Shu, C-H., Tsai, C-C., Chen, K-Y., Liao, W-H., and Huang, H-C. (2013). Enhancing high quality oil accumulation and carbon dioxide fixation by a mixed culture of Chlorella sp. and Saccharomyces cerevisiae. J. Taiwan Inst. Chem. Eng. 44, 936–942. doi:10.1016/j.jtice.2013.04.001
Sigaud, T. C. S., and Aidar, E. (1993). Salinity and temperature effects on the growth and chlorophyll-α content of some planktonic aigae. Bol. do Inst. Ocean. 41, 95–103. doi:10.1590/s0373-55241993000100008
Singh, N. K., and Dhar, D. W. (2011). Microalgae as second generation biofuel. A review. Agron. Sustain. Dev. 31, 605–629. doi:10.1007/s13593-011-0018-0
Song, H., Lavoie, M., Fan, X., Tan, H., Liu, G., Xu, P., et al. (2017). Allelopathic interactions of linoleic acid and nitric oxide increase the competitive ability of Microcystis aeruginosa. ISME J. 11, 1865–1876. doi:10.1038/ismej.2017.45
Spolaore, P., Joannis-Cassan, C., Duran, E., and Isambert, A. (2006). Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87–96. doi:10.1263/jbb.101.87
Srinuanpan, S., Chawpraknoi, A., Chantarit, S., Cheirsilp, B., and Prasertsan, P. (2018). A rapid method for harvesting and immobilization of oleaginous microalgae using pellet-forming filamentous fungi and the application in phytoremediation of secondary effluent. Int. J. phytoremediation 20, 1017–1024. doi:10.1080/15226514.2018.1452187
Stiles, W. A., Styles, D., Chapman, S. P., Esteves, S., Bywater, A., Melville, L., et al. (2018). Using microalgae in the circular economy to valorise anaerobic digestate: challenges and opportunities. Bioresour. Technol. 267, 732–742. doi:10.1016/j.biortech.2018.07.100
Subashchandrabose, S. R., Ramakrishnan, B., Megharaj, M., Venkateswarlu, K., and Naidu, R. (2011). Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol. Adv. 29, 896–907. doi:10.1016/j.biotechadv.2011.07.009
Sültemeyer, D. (1998). Carbonic anhydrase in eukaryotic algae: characterization, regulation, and possible function during photosynthesis. Can. J. Bot. 76, 962–972. doi:10.1139/cjb-76-6-962
Swarnalatha, G., Hegde, N. S., Chauhan, V. S., and Sarada, R. (2015). The effect of carbon dioxide rich environment on carbonic anhydrase activity, growth and metabolite production in indigenous freshwater microalgae. Algal Res. 9, 151–159. doi:10.1016/j.algal.2015.02.014
Szotkowski, M., Holub, J., Šimanský, S., Hubačová, K., Sikorová, P., Mariničová, V., et al. (2021). Bioreactor Co-cultivation of high lipid and carotenoid producing yeast Rhodotorula kratochvilovae and several microalgae under stress. Microorganisms 9, 1160. doi:10.3390/microorganisms9061160
Tan, X-B., Zhao, X-C., Zhang, Y-L., Zhou, Y-Y., Yang, L-B., and Zhang, W-W. (2018). Enhanced lipid and biomass production using alcohol wastewater as carbon source for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour. Technol. 247, 784–793. doi:10.1016/j.biortech.2017.09.152
Tandon, P., and Jin, Q. (2017). Microalgae culture enhancement through key microbial approaches. Renew. Sustain. Energy Rev. 80, 1089–1099. doi:10.1016/j.rser.2017.05.260
Taşkan, E. (2016). Performance of mixed algae for treatment of slaughterhouse wastewater and microbial community analysis. Environ. Sci. Pollut. Res. 23, 20474–20482. doi:10.1007/s11356-016-7241-9
Thompson, A. W., and Zehr, J. P. (2013). Cellular interactions: lessons from the nitrogen-fixing cyanobacteria. J. Phycol. 49, 1024–1035. doi:10.1111/jpy.12117
Tran, H. T. M., Cheirsilp, B., Hodgson, B., and Umsakul, K. (2010). Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone–butanol–ethanol production from cassava starch. Biochem. Eng. J. 48, 260–267. doi:10.1016/j.bej.2009.11.001
Van Den Hende, S., Vervaeren, H., and Boon, N. (2012). Flue gas compounds and microalgae:(Bio-) chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 30, 1405–1424. doi:10.1016/j.biotechadv.2012.02.015
Vejrazka, C., Janssen, M., Streefland, M., and Wijffels, R. H. (2011). Photosynthetic efficiency of Chlamydomonas reinhardtii in flashing light. Biotechnol. Bioeng. 108, 2905–2913. doi:10.1002/bit.23270
VerBerkmoes, N. C., Denef, V. J., Hettich, R. L., and Banfield, J. F. (2009). Functional analysis of natural microbial consortia using community proteomics. Nat. Rev. Microbiol. 7, 196–205. doi:10.1038/nrmicro2080
Vraspir, J. M., and Butler, A. (2009). Chemistry of marine ligands and siderophores. Annu. Rev. Mar. Sci. 1, 43–63. doi:10.1146/annurev.marine.010908.163712
Walls, L. E., Velasquez-Orta, S. B., Romero-Frasca, E., Leary, P., Noguez, I. Y., and Ledesma, M. T. O. (2019). Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 151, 107319. doi:10.1016/j.bej.2019.107319
Wang, H., Tomasch, J., Jarek, M., and Wagner-Döbler, I. (2014). A dual-species co-cultivation system to study the interactions between Roseobacters and dinoflagellates. Front. Microbiol. 5, 311. doi:10.3389/fmicb.2014.00311
Wang, R., Tian, Y., Xue, S., Zhang, D., Zhang, Q., Wu, X., et al. (2016b). Enhanced microalgal biomass and lipid production via co-culture of Scenedesmus obliquus and Candida tropicalis in an autotrophic system. J. Chem. Technol. Biotechnol. 91, 1387–1396. doi:10.1002/jctb.4735
Wang, X., Gao, S., Zhang, Y., Zhao, Y., and Cao, W. (2017). Performance of different microalgae-based technologies in biogas slurry nutrient removal and biogas upgrading in response to various initial CO2 concentration and mixed light-emitting diode light wavelength treatments. J. Clean. Prod. 166, 408–416. doi:10.1016/j.jclepro.2017.08.071
Wang, Y., Ho, S-H., Cheng, C-L., Guo, W-Q., Nagarajan, D., Ren, N-Q., et al. (2016a). Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 222, 485–497. doi:10.1016/j.biortech.2016.09.106
Wang, Y., Yang, Y., Ma, F., Xuan, L., Xu, Y., Huo, H., et al. (2015). Optimization of C hlorella vulgaris and bioflocculant-producing bacteria co-culture: enhancing microalgae harvesting and lipid content. Lett. Appl. Microbiol. 60, 497–503. doi:10.1111/lam.12403
Wicker, R., and Bhatnagar, A. (2020). Application of Nordic microalgal-bacterial consortia for nutrient removal from wastewater. Chem. Eng. J. 398, 125567. doi:10.1016/j.cej.2020.125567
Wrede, D., Taha, M., Miranda, A. F., Kadali, K., Stevenson, T., Ball, A. S., et al. (2014). Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PloS one 9, e113497. doi:10.1371/journal.pone.0113497
Xie, S., Sun, S., Dai, S. Y., and Yuan, J. S. (2013). Efficient coagulation of microalgae in cultures with filamentous fungi. Algal Res. 2, 28–33. doi:10.1016/j.algal.2012.11.004
Xu, L., Cheng, X., and Wang, Q. (2018). Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with Azotobacter chroococcum. Front. plant Sci. 9, 741. doi:10.3389/fpls.2018.00741
Xu, L., Cheng, X., Wu, S., and Wang, Q. (2017). Co-cultivation of Chlamydomonas reinhardtii with Azotobacter chroococcum improved H 2 production. Biotechnol. Lett. 39, 731–738. doi:10.1007/s10529-017-2301-x
Xu, L., Li, D., Wang, Q., and Wu, S. (2016). Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Int. J. hydrogen energy 41, 9276–9283. doi:10.1016/j.ijhydene.2016.04.009
Xue, F., Miao, J., Zhang, X., and Tan, T. (2010). A new strategy for lipid production by mix cultivation of Spirulina platensis and Rhodotorula glutinis. Appl. Biochem. Biotechnol. 160, 498–503. doi:10.1007/s12010-008-8376-z
Yang, L., Li, H., and Wang, Q. (2019). A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 275, 35–43. doi:10.1016/j.biortech.2018.12.036
Yao, S., Lyu, S., An, Y., Lu, J., Gjermansen, C., and Schramm, A. (2019). Microalgae–bacteria symbiosis in microalgal growth and biofuel production: A review. J. Appl. Microbiol. 126, 359–368. doi:10.1111/jam.14095
Yee, C. S., Okomoda, V. T., Hashim, F., Waiho, K., Abdullah, S. R. S., Alamanjo, C., et al. (2021). Marine microalgae co-cultured with floc-forming bacterium: insight into growth and lipid productivity. PeerJ 9, e11217. doi:10.7717/peerj.11217
Yen, H-W., Chen, P-W., and Chen, L-J. (2015). The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour. Technol. 184, 148–152. doi:10.1016/j.biortech.2014.09.113
Yong, S. C., Roversi, P., Lillington, J., Rodriguez, F., Krehenbrink, M., Zeldin, O. B., et al. (2014). A complex iron-calcium cofactor catalyzing phosphotransfer chemistry. Science 345, 1170–1173. doi:10.1126/science.1254237
Yu, G., Sun, Y., Han, H., Yan, X., Wang, Y., Ge, X., et al. (2021). Coculture, an efficient biotechnology for mining the biosynthesis potential of macrofungi via interspecies interactions. Front. Microbiol. 12, 663924. doi:10.3389/fmicb.2021.663924
Zhai, J., Li, X., Li, W., Rahaman, M. H., Zhao, Y., Wei, B., et al. (2017). Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol. Eng. 108, 83–92. doi:10.1016/j.ecoleng.2017.07.023
Zhang, J., Feng, L., Ouyang, Y., Hu, R., Xu, H., and Wang, J. (2020). Phosphate-solubilizing bacteria and fungi in relation to phosphorus availability under different land uses for some latosols from Guangdong, China. Catena 195, 104686. doi:10.1016/j.catena.2020.104686
Zhang, J., and Hu, B. (2012). A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour. Technol. 114, 529–535. doi:10.1016/j.biortech.2012.03.054
Zhang, J., Liu, L., Ren, Y., and Chen, F. (2019). Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromol. 128, 761–767. doi:10.1016/j.ijbiomac.2019.02.009
Zhang, J., and Zhang, J. (2016). The filamentous fungal pellet and forces driving its formation. Crit. Rev. Biotechnol. 36, 1066–1077. doi:10.3109/07388551.2015.1084262
Zhang, Q., Li, X., Guo, D., Ye, T., Xiong, M., Zhu, L., et al. (2018). Operation of a vertical algal biofilm enhanced raceway pond for nutrient removal and microalgae-based byproducts production under different wastewater loadings. Bioresour. Technol. 253, 323–332. doi:10.1016/j.biortech.2018.01.014
Zhang, Z., Ji, H., Gong, G., Zhang, X., and Tan, T. (2014). Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement of biomass and lipid yields. Bioresour. Technol. 164, 93–99. doi:10.1016/j.biortech.2014.04.039
Zhao, P., Yu, X., Li, J., Tang, X., and Huang, Z. (2014). Enhancing lipid productivity by co-cultivation of Chlorella sp. U4341 and Monoraphidium sp. FXY-10. J. Biosci. Bioeng. 118, 72–77. doi:10.1016/j.jbiosc.2013.12.014
Zhao, T., Yan, X., Sun, L., Yang, T., Hu, X., He, Z., et al. (2019a). Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 91, 354–361. doi:10.1016/j.tifs.2019.07.014
Zhao, Y., Guo, G., Sun, S., Hu, C., and Liu, J. (2019b). Co-pelletization of microalgae and fungi for efficient nutrient purification and biogas upgrading. Bioresour. Technol. 289, 121656. doi:10.1016/j.biortech.2019.121656
Zhao, Y., Wang, J., Zhang, H., Yan, C., and Zhang, Y. (2013). Effects of various LED light wavelengths and intensities on microalgae-based simultaneous biogas upgrading and digestate nutrient reduction process. Bioresour. Technol. 136, 461–468. doi:10.1016/j.biortech.2013.03.051
Zhou, K., Zhang, Y., and Jia, X. (2018). Co-cultivation of fungal-microalgal strains in biogas slurry and biogas purification under different initial CO 2 concentrations. Sci. Rep. 8, 7786. doi:10.1038/s41598-018-26141-w
Zhou, W., Cheng, Y., Li, Y., Wan, Y., Liu, Y., Lin, X., et al. (2012). Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatment. Appl. Biochem. Biotechnol. 167, 214–228. doi:10.1007/s12010-012-9667-y
Zhou, Y., Schideman, L., Yu, G., and Zhang, Y. (2013). A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy and Environ. Sci. 6, 3765–3779. doi:10.1039/c3ee24241b
Zhu, L. (2015). Microalgal culture strategies for biofuel production: A review. Biofuels, Bioprod. Biorefining 9, 801–814. doi:10.1002/bbb.1576
Keywords: co-culturing systems, microalgae, bioprocess design, bioproduction, microbial consortia
Citation: Naseema Rasheed R, Pourbakhtiar A, Mehdizadeh Allaf M, Baharlooeian M, Rafiei N, Alishah Aratboni H, Morones-Ramirez JR and Winck FV (2023) Microalgal co-cultivation -recent methods, trends in omic-studies, applications, and future challenges. Front. Bioeng. Biotechnol. 11:1193424. doi: 10.3389/fbioe.2023.1193424
Received: 24 March 2023; Accepted: 08 September 2023;
Published: 20 September 2023.
Edited by:
Recep Kaan Dereli, University College Dublin, IrelandReviewed by:
Pengfei Cheng, Ningbo University, ChinaSvitlana Miros, University College Dublin, Ireland
Copyright © 2023 Naseema Rasheed, Pourbakhtiar, Mehdizadeh Allaf, Baharlooeian, Rafiei, Alishah Aratboni, Morones-Ramirez and Winck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose Ruben Morones-Ramirez, am9zZS5tb3JvbmVzcm1yQHVhbmwuZWR1Lm14; Flavia Vischi Winck, d2luY2tAY2VuYS51c3AuYnI=; Hossein Alishah Aratboni, YWxpc2hhaGFyYWRAZ21haWwuY29t
†These authors have contributed equally to this work
 Raseena Naseema Rasheed
Raseena Naseema Rasheed Asma Pourbakhtiar
Asma Pourbakhtiar Malihe Mehdizadeh Allaf
Malihe Mehdizadeh Allaf Maedeh Baharlooeian
Maedeh Baharlooeian Nahid Rafiei
Nahid Rafiei Hossein Alishah Aratboni
Hossein Alishah Aratboni Jose Ruben Morones-Ramirez
Jose Ruben Morones-Ramirez Flavia Vischi Winck
Flavia Vischi Winck