
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 02 June 2023
Sec. Biomechanics
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1181117
Background: Patients with unilateral post-LASIK keratectasia (KE) have clinical ectasia in one eye but not in the fellow eye. As serious complications, these cases are rarely reported but are worth investigating. This study aimed to explore the characteristics of unilateral KE and the accuracy of corneal tomographic and biomechanical parameters to detect KE and distinguish fellow eyes from control eyes.
Methods: The study analyzed 23 KE eyes, 23 KE fellow eyes, and 48 normal eyes from age- and sex-matched patients who had undergone LASIK. The Kruskal–Wallis test and further paired comparisons were performed to compare the clinical measurements of the three groups. The receiver operating characteristic curve was used to evaluate the ability to distinguish KE and fellow eyes from the control eyes. Binary logistic regression with the forward stepwise method was performed to produce a combined index, and the DeLong test was used to compare the discriminability difference of the parameters.
Results: Males accounted for 69.6% of patients with unilateral KE. The duration between corneal surgery and the onset of ectasia ranged from 4 months to 18 years, with a median time of 10 years. The KE fellow eye had a higher posterior evaluation (PE) value than the control eyes (5 vs. 2, p = 0.035). Diagnostic tests showed that PE, posterior radius of curvature (3 mm), anterior evaluation (FE), and Corvis biomechanical index–laser vision correction (CBI-LVC) were sensitive indicators for distinguishing KE in the control eyes. The ability of PE to detect the KE fellow eye from the control eye was 0.745 (0.628 and 0.841), with 73.91% sensitivity and 68.75% specificity at a cut-off value of 3. The ability of a combined index, constructed using PE and FE, to distinguish fellow eyes of KE from controls was 0.831 (0.723 and 0.909), which was higher than that of PE and FE individually (p < 0.05).
Conclusion: The fellow eyes of patients with unilateral KE had significantly higher PE values than control eyes, and a combination of PE and FE enhanced this differentiation in a Chinese population. More attention should be paid to the long-term follow-up of patients after LASIK and to be wary of the occurrence of early KE.
Laser-assisted in situ keratomileusis (LASIK) is an effective refractive surgery performed worldwide (Sandoval et al., 2016). On average, 1,608,880 corneal refractive surgery procedures were conducted per year between 1991 and 2015 (Moshirfar et al., 2021). With an increasing number of surgeries being performed, surgical complications, which are often irreversible, have attracted attention in recent years (Bohac et al., 2018). Post-LASIK keratectasia (KE) after corneal refractive surgery is a serious complication that was first reported by Seiler et al. (1998). Previous studies have reported that the incidence of KE after LASIK ranged from 0.033% to 0.9%, with a postoperative follow-up period of 2–10 years (Pallikaris et al., 2001; Randleman et al., 2003; Moshirfar et al., 2014; Santhiago et al., 2016; Bohac et al., 2018; Ambrosio, 2019). Similar to primary keratoconus (KC), KE is characterized by thinning and bulging of the cornea, loss of visual acuity, and irregular clinical astigmatism (Ambrosio et al., 2010). Global consensus stated that true unilateral KC is nonexistent, and the fellow normal eye of asymmetric patients may develop KC (Gomes et al., 2015). The justification is related to KC being considered a genetic-related condition (Santodomingo-Rubido et al., 2022). Furthermore, several studies have reported that the corneal shape and biomechanical parameters of the KC fellow eye were different from KC and normal control eyes (Bae et al., 2014; Degirmenci et al., 2019; Koc et al., 2019). Therefore, it is worth investigating whether the same phenomenon exists in patients with unilateral KE who underwent bilateral corneal refractive surgery.
Unilateral KE, iatrogenic secondary corneal ectasia, could occur due to a purely mechanical process (Ambrosio, 2019; Salomão et al., 2021). While the prevalence is relatively low, the incidence of KE is increasing in clinical practice (Bohac et al., 2018). The characteristics of patients with unilateral KE have important research value, but case studies are rarely reported. Although slit-lamp examination has traditionally been used to diagnose KE, corneal tomographic and biomechanical parameters, which are important for the early diagnosis of corneal diseases, are gradually being used to identify KE. Ueki et al. (2018) and Yang et al. (2020) reported that central corneal thickness exhibited no significant difference between normal post-LASIK eyes and KE eyes, but the radius and deflection amplitude at the highest concavity were significantly different. In addition, our previous study showed that KE eyes exhibited a lower stiffness parameter at first applanation value than did normal LASIK eyes and higher values of maximum inverse radius, deformation amplitude (DA) ratio max (2 mm), pachy slope, DA ratio max (1 mm), and integrated radius (Yang et al., 2020). However, research on the fellow eyes of patients with unilateral KE is limited, and the accuracy of corneal tomographic and biomechanical parameters to distinguish clinical KE and fellow eyes from control eyes has not been reported. Thus, the current study aimed to investigate the characteristics of unilateral KE in a Chinese population and further explore the ability of corneal tomographic and biomechanical parameters to distinguish KE and fellow eyes from control eyes to provide a reference for diagnosing KE early.
Patients with unilateral KE who were referred to Henan Eye Hospital between April 2018 and January 2023 were recruited for this retrospective analysis. The diagnostic criteria for unilateral KE were as follows: 1) eyes that underwent bilateral LASIK for myopia and myopic astigmatism; 2) KE eyes that have the presence of certain signs on corneal tomography (such as displacement of the corneal apex, a decrease in pachymetry, an asymmetric tomographic pattern, and posterior elevation (PE) values above 16) or with abnormal indications of slit-lamp examination (central corneal thinning, conical protrusion, Vogt’s striae, Munson’s signs, or Fleischer’s ring) (Chan et al., 2018; Yang et al., 2020), while the KE fellow eyes have no aforementioned signs. The inclusion criteria for the control eyes were as follows: 1) eyes that underwent corneal refractive surgery at least 1 year prior; 2) eyes with corrected distance visual acuity with logMAR ≤0.1; 3) eyes with no detectable disease; and 4) eyes that were age- and sex-matched. In contrast, eyes in contact with rigid contact lenses within the past 4 weeks and soft contact lenses within the past 2 weeks and patients with severe uncontrolled diabetes, other eye conditions (e.g., cataract and glaucoma), and a history of previous eye surgeries (except refractive surgeries) were excluded. Overall, 23 patients with unilateral KE (23 KE eyes and 23 fellow eyes) and 48 control eyes were included in the analysis. The study was approved by the Institutional Review Board of Henan Eye Hospital [ethical approval number HNEECKY-2019 (5)], and written informed consent was obtained from all the participants.
All patients underwent the following clinical examinations (Yang et al., 2021): autorefraction (Topcon KR-800), a standard logarithmic visual acuity chart to obtain corrected distance visual acuity, slit-lamp examination (Vogt’s striae, Fleischer’s ring, Munson’s sign, and corneal scarring), and corneal tomography and biomechanical parameter. Experienced operators conducted the measurements between 9:00 and 17:00.
Corneal tomography parameters were measured using Pentacam HR (Oculus, Wetzlar, Germany), which uses a rotating high-resolution camera to analyze the eye to assess the anterior and posterior surfaces of the cornea (de Luis Eguileor et al., 2018) (Hwang et al., 2018). The findings with a high-quality factor were recorded for each eye, and the following parameters were analyzed: (1) the central 3.0 mm of the anterior and posterior corneal surfaces in terms of flat, steep, and mean keratometries; (2) the maximum keratometry of the anterior corneal surface; (3) the corneal thickness at the pachy apex, the center of the pupil, and the thinnest point of the cornea; (4) topometric screening indices containing the index of surface variation, index of vertical asymmetry, keratoconus index (KI), central KI (CKI), index of height asymmetry, and index of height decentration; (5) the thinnest corneal point (anterior evaluation [FE] and PE values); and (6) Belin–Ambrósio display indices (D). In addition, the flap thickness was measured manually, and N1–N4 and N6–N23 patients were measured through CASIA SS-1000, and the N5 patient was measured through Visante OCT (Zeiss).
Corneal biomechanics were obtained using Corneal Visualization Scheimpflug Technology (Corvis-ST, Oculus, Wetzlar, Germany), which is a non-contact tonometer. Decompensation of biomechanical properties is the initiating element of ectasia progression (Vinciguerra et al., 2016a; Vinciguerra et al., 2017). The instrument uses Scheimpflug images of the anterior segment at a rate of 4,330 frames/s and can obtain corneal biomechanical parameters through three phases: first applanation, the highest concavity, and second applanation (Jedzierowska and Koprowski, 2019; Yang et al., 2019). The applanation time, velocity, radius (calculated during the concave phase of the deformation response) (Vinciguerra et al., 2016b), DA, deflection length, deflection amplitude (DLA), deflection area (DLAr), and delta arc length were recorded. In addition, intraocular pressure, biomechanical corrected intraocular pressure, central corneal thickness, peak distance, radius, and whole eye movement are presented. New parameters, such as DA ratio max (1 mm), DA ratio max (2 mm)27, pachy slope, max inverse radius, integrated radius, Ambrósio’s relational thickness horizontal profile, and stiffness parameter at first applanation were added using the updated software (software number 1.5r1902) (Vinciguerra et al., 2016a), and Corvis biomechanical index–laser vision correction (CBI-LVC) was calculated on the basis of a logistic regression formula (Vinciguerra et al., 2021).
The quantitative data of the patients are presented as the median (M) and range (P25 and P75). The Kruskal–Wallis test was used to compare the differences among the control, KE, and fellow eyes, and further paired comparisons of the least significant difference were performed. The receiver operating characteristic curve was used to evaluate the ability of the parameters to distinguish clinical KE and the fellow eye from control eyes, and the area under the receiver operating characteristic curve (AUC) and 95% confidence interval (CI) were recorded. The combined model was constructed to improve the ability to detect the KE fellow eye, which used binary logistic regression with the forward stepwise method (p < 0.1 for retention in the model). The DeLong test was used to compare the differences in discriminability of the parameters. All data were analyzed using SPSS 23.0 and MedCalc 15.2.2, and p < 0.05 (two-tailed) was considered a statistically significant difference.
Table 1 shows the basic data from 23 patients with unilateral KE after corneal refractive surgery. The median age of KE diagnosis was 32 years, ranging from 18 to 38 years, and males accounted for 69.6% of all patients. The duration between corneal surgery and the onset of KE ranged from 4 months to 18 years, with a median of 10 years. Data related to the flap thickness were measured manually, and the flap thickness of the corresponding measurement positions was indicated by arrows in Supplementary Figure S1. No statistically significant differences in age, sex, and duration were found between the unilateral KE and control eyes (all p > 0.05).
Corneal tomographic and biomechanical parameters were compared among the control, KE, and fellow eyes (Supplementary Tables S1, S2). Significant differences in clinical measurements were found between the KE and control eyes (all p < 0.05). Compared with the control eyes, KE fellow eyes had a higher PE value (5 vs. 2, p = 0.035, Figure 1A). At p < 0.1 level, the KE fellow eye had higher FE values (−3 vs. −6, p = 0.062, Figure 1B) and A1DLAr (0.15 vs. 0.12, p = 0.073, Figure 1C) values than the control eyes.
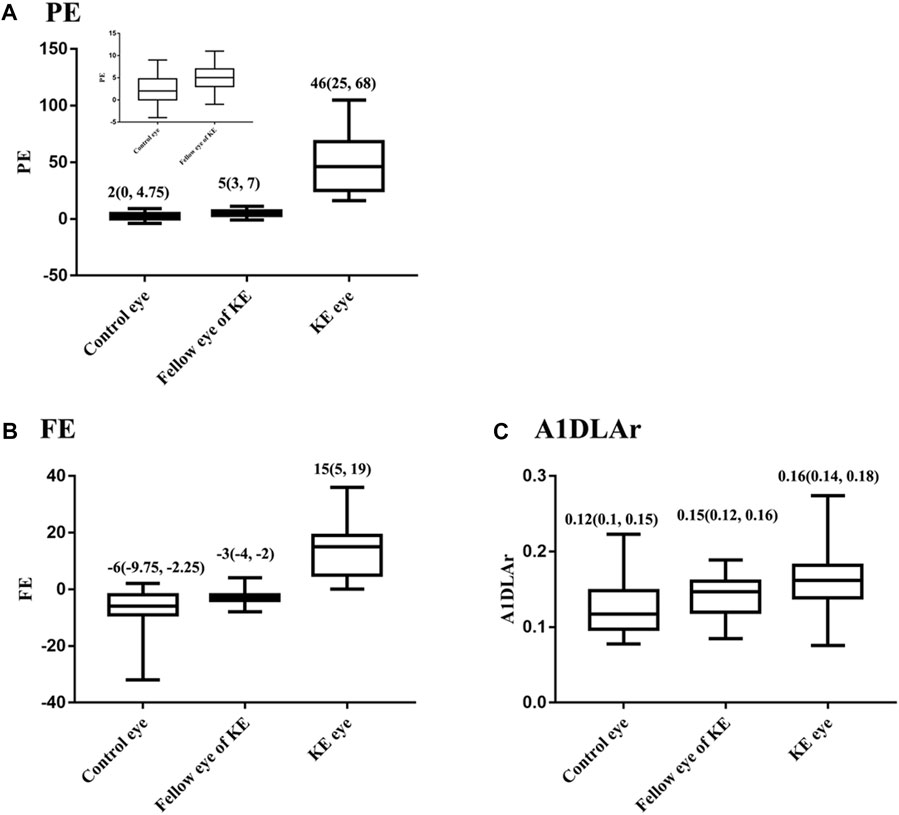
FIGURE 1. Distribution of PE, FE and among control, KE and the fellow eyes, M (P25, P75). (A) PE, (B) FE, (C) A1DLAr.
Supplementary Table S3 shows the AUC of corneal tomographic parameters for distinguishing the KE eye from the control eye. Similar to PE, the posterior radius of curvature (PRC) (3 mm) correctly diagnosed KE eyes at a criterion value of 5.94 (Figures 2A, B). In addition, FE had a high Youden value in distinguishing KE from control eyes, with 100% sensitivity and 93.75% specificity at a cut-off value of −1 (Figure 2C). Among the corneal biomechanical parameters, the CBI-LVC was a relatively good parameter for detecting KE (0.983, 95% CI: 0.917-0.999), with 95.65% sensitivity and 100% specificity (Figure 2D; Supplementary Table S4).
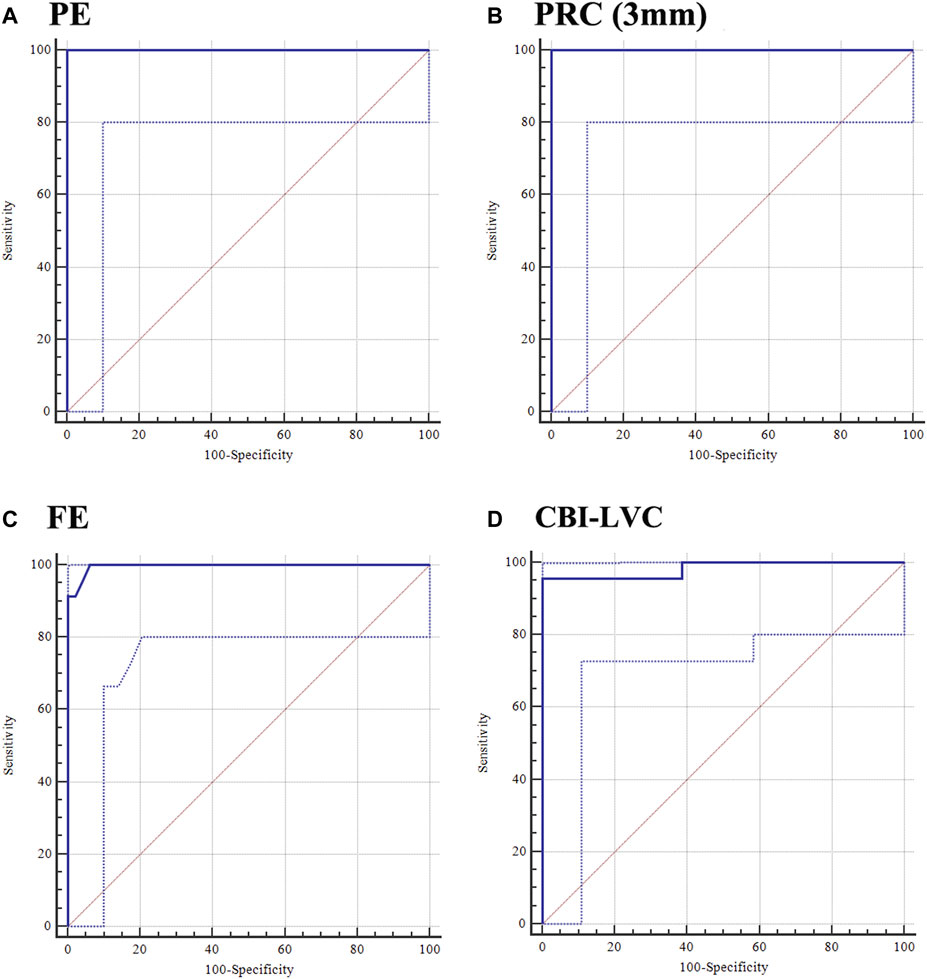
FIGURE 2. Ability of PE, PRC (3 mm), FE, and CBI-LVC in distinguishing the KE eye from the control eye. (A) PE; (B) PRC (3 mm); (C) FE; and (D) CBI-LVC.
The ability of PE to detect KE fellow eyes from control eyes was 0.745 (0.628 and 0.841), with 73.91% sensitivity and 68.75% specificity at a cut-off value of 3 (Table 2, Figure 3A). Furthermore, the AUCs (95% CIs) of FE (cut-off = −6, Figure 3B) and A1DLAr (cut off = 0.124) for diagnosing KE fellow eyes were 0.722 (0.603 and 0.822) and 0.687 (0.562 and 0.795), respectively.
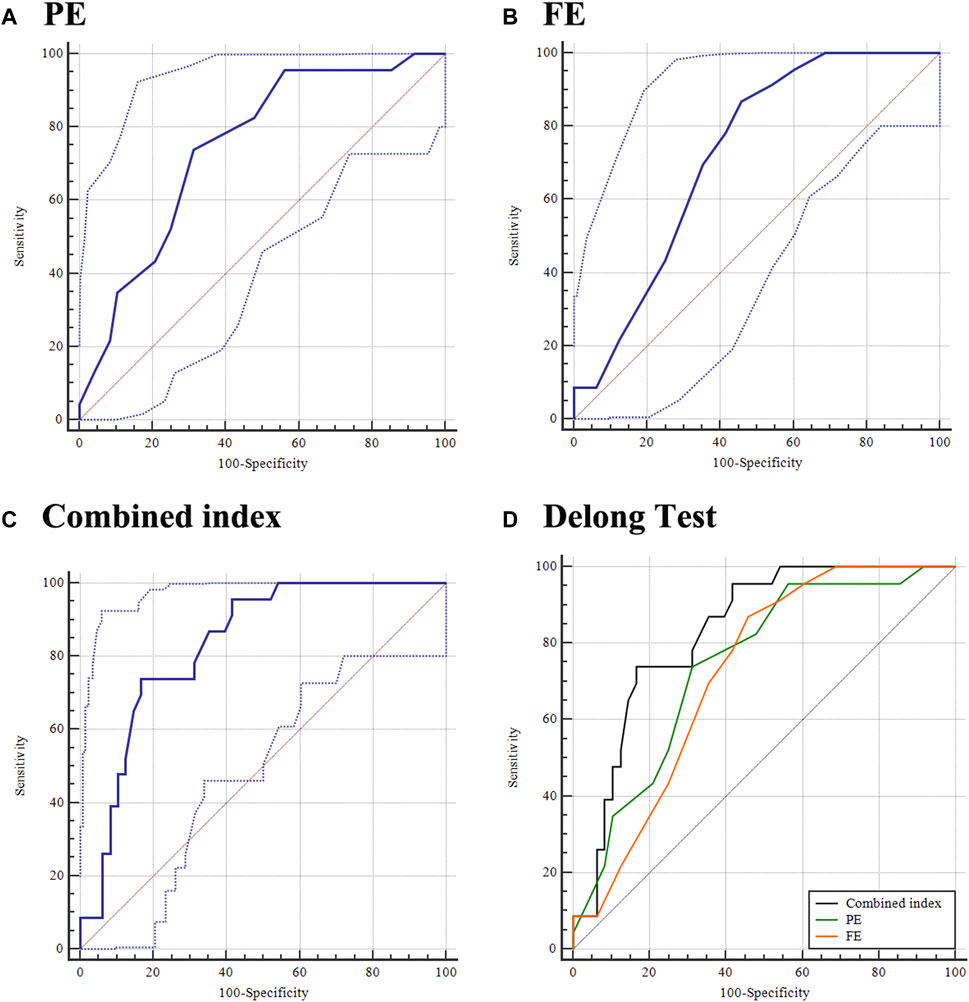
FIGURE 3. Ability of PE, FE, and combined index in distinguishing the fellow eye of KE from the control eye. (A) PE; (B) FE; (C) combined index; and (D) DeLong test.
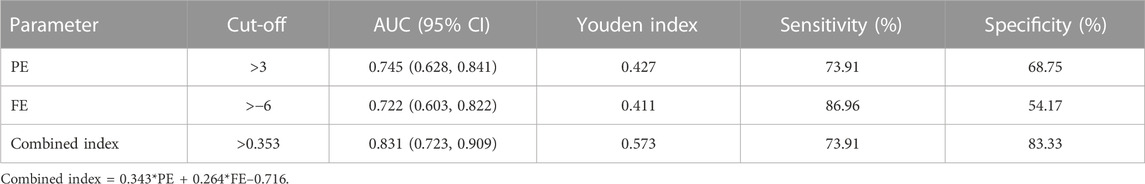
TABLE 2. Ability of corneal tomographic parameters in distinguishing the fellow eye of the KE eye from control eyes.
Further logistic regression analysis included PE and FE in a combined model, in which the coefficients of PE and FE were 0.343 and 0.264, respectively (Supplementary Table S5, all p < 0.05). The AUC (95% CI) of the combined index for distinguishing KE fellow eyes from control eyes was 0.831 (0.723 and 0.909), with 73.91% sensitivity and 83.33% at a cut-off value of 0.353 (Figure 3C). Further AUC pairwise comparisons are presented in Figure 3D, in which the combined index had a higher detection ability than the individual diagnosis ability of PE (AUC difference, 0.086; 95% CI: 0.007–0.164; p = 0.032) and FE (AUC difference: 0.109; 95% CI: 0.007–0.211; p = 0.037).
The assessment of corneal shape and biomechanical parameters plays a vital role in evaluating eye conditions, and assessing the parameters of KE and fellow eyes helps diagnose early KE so as to avoid further vision loss (Sedaghat et al., 2018; Hocaoglu et al., 2020). The current study showed that PE was different between the KE fellow eyes and control eyes, and the combined index of PE and FE improved the ability to differentiate between the KE fellow and control eyes.
KE does not remain biomechanically stable during the postoperative period; however, KE eyes exhibit progressive central or inferior corneal steepening associated with stromal thinning, similar to that noted in KC eyes (Ueki et al., 2018; He et al., 2020). A previous study reported that central corneal thickness in normal post-LASIK and KE eyes decreased, which is related to the removal of corneal tissue, and softening of tissue would be expected because of structural alterations caused by severing tension-bearing lamellae (Lee et al., 2017; Ueki et al., 2018). Biomechanical instability of the cornea is linked to the development of KE, and unilateral secondary corneal ectasia can occur due to a purely mechanical process (Gomes et al., 2015; Bohac et al., 2018; Salomão et al., 2021). Several studies have reported the potential risk factors for developing KE, which include high myopia, thin cornea, other preoperatively suspicious KC symptoms, a high percentage of tissue altered, and low specific residual stromal bed thickness (Randleman et al., 2003; Santhiago et al., 2016). The preoperative data of the five patients were re-evaluated, revealing no abnormal tomographic map findings (Supplementary Table S6). Due to the long duration time from surgery to diagnose ectasia, the preoperative data and surgical data (level of correction, ablation depth, flap thickness, etc.) of other patients cannot be accurately obtained, which limits the assessment of factors for KE occurrence. In addition, age, eye rubbing, allergy, atopy, family history, and stromal hydration are related to the occurrence of KE (Randleman et al., 2003; Rabinowitz, 2006; Randleman et al., 2008; Santhiago et al., 2016). Deep research is warranted into the occurrence of KE. The present study found that the duration between corneal surgery and the onset of ectasia ranged from 4 months to 18 years, with a median time of 10 years, which is consistent with the results of several reviews (Randleman et al., 2003; Bohac et al., 2018). Males accounted for 69.6% in the current retrospective study, while a recent review reported that there was no difference between the frequency of ectasia in male and female patients (Moshirfar et al., 2021),. The basic characteristics of unilateral KE indicate the importance of follow-up of asymptomatic clinical signs, and an in-depth analysis of KE is necessary.
The corneal tomographic and biomechanical parameters were significantly different in KE vs. control eyes and KE vs. KE fellow eyes, which helped clinicians diagnose KE effectively. Among corneal tomographic parameters, PE and PRC (3 mm) are posterior evaluation parameters that have been demonstrated to be effective in differentiating KE from the control eyes. The changes in early ecstatic conditions typically occur on the posterior corneal surface before anterior changes (de Sanctis et al., 2008; Duncan et al., 2016). PRC (3 mm) is the posterior radius of curvature taken from the 3 mm zone centered on the thinnest point (Duncan et al., 2016). PE is corneal height data that uses a conventional best-fit sphere (BFS) as the reference surface (in µm), and posterior corneal elevation difference values were taken as the differential changes in corneal elevation of the thinnest points between the BFS and the enhanced BFS (de Sanctis et al., 2008; Huseynli et al., 2018). Similar to PE, FE is the elevation of the thinnest point from the 8-mm anterior corneal height data (Huseynli et al., 2018). The present study indicated that KE eyes had a higher FE value than control eyes, indicating that FE could effectively discriminate KE eyes from control eyes, and the elevation differences between a standard BFS and the enhanced reference surface were highly significant quantitatively in separating ecstatic changes from normal (Huseynli et al., 2018; Imbornoni et al., 2018; Santodomingo-Rubido et al., 2022). CBI-LVC, calculated on the basis of six dynamic corneal response parameters, had a good ability to discriminate KE from control eyes in the current analysis, which is consistent with the previous study (Vinciguerra et al., 2021). In addition, A1DLA, as a measured value in the direction parallel to the air pulse, was higher in KE eyes than that in control eyes, which is consistent with several previous studies (Ueki et al., 2018; Yang et al., 2020). The ability to distinguish KE eyes from control eyes was relatively good among the corneal biomechanical parameters, which should attract the attention of physicians for clinical applications.
According to global consensus, KC is progressive (Gomes et al., 2015). Even if one eye is not initially affected, the contralateral normal eye in most patients may eventually get affected (Degirmenci et al., 2019). Furthermore, several studies have reported that the characteristics of topographic, tomographic, and biomechanical parameters are significantly different between KC fellow eyes and normal eyes (Bae et al., 2014; Hashemi et al., 2016; Degirmenci et al., 2019). As a special type of corneal ectasia, unilateral KE was reported in the clinic; identifying differences between the KE fellow eyes and post-LASIK eyes helps detect early KE. In the present study, only PE was significantly different between KE fellow eyes and control eyes. FE and A1DLAr, which indicate the deflection area at A1, seemed to differ between the KE fellow eyes and control eyes at a wider statistical level. The results indicate that changes in the posterior cornea may occur without concurrent anterior changes, and early ectasia may have posterior progression despite a normal anterior surface (Huseynli et al., 2018; Imbornoni et al., 2018). Although the A1DLAr was not included in the final logistic regression, our previous studies found that the A1DLAr value in KE eyes was higher than that in post-LASIK control eyes, indicating that a higher value of A1DLAr suggests an increased risk of corneal ectasia (Yang et al., 2020). Further diagnostic tests showed that PE and FE had moderate AUC values in diagnosing the KE fellow eye individually, and the combination of PE and FE could effectively improve diagnostic capability. These findings further demonstrated that the differences between the elevation values, BFS, and enhanced reference surface were highly predictive in screening for ecstatic disease (Huseynli et al., 2018; Imbornoni et al., 2018). Notably, the D value was not different between the control and KE fellow eyes, which is a comprehensive display that incorporates anterior and posterior elevation, BFS, enhanced reference surface, and corneal pachymetric map into a normative database. This may be related to the variety of parameters and should be brought to the attention of clinicians. The prevalence of KE is theoretically low, although several researchers have suggested that these values are an underestimate (Santhiago et al., 2016; Bohac et al., 2018). Early diagnosis of KE is limited, especially for the KE fellow eyes of patients with unilateral KE. Overall, more attention should be paid to KE in clinical applications.
The present study showed that the combined index of PE and FE could effectively discriminate the KE fellow eye from control eyes, providing a reference for exploring the detection of corneal ectasia. However, this study has some limitations. First, the current study recruited 23 patients with unilateral KE, which is a relatively limited sample size and might fail to reflect statistical discrepancies. Considering the limited prevalence of KE and the paucity of published reports, the present study provides a reference for clinical applications and future investigations. Second, participants were recruited when they were diagnosed with ectasia at our clinic, and data on preoperative examinations and surgical procedures that might have been performed in different hospitals were lacking. More preoperative information and risk factors of KE patients need to be collected in later research. Thus, the detailed mechanism of KE has not yet been evaluated, and further studies are necessary. Finally, the study was conducted at one center, in which the ethnicity and the heterogeneity of populations were not evaluated, and extrapolation of the results requires further multicenter collaborative studies.
In conclusion, the fellow eyes of patients with unilateral KE had significantly higher PE values than control eyes, and a combination of PE and FE improved the ability to distinguish KE fellow eyes from control eyes in a Chinese population. This provides a reference for diagnosing KE early. Multicenter studies with larger sample sizes are warranted to investigate the characteristics of patients with unilateral KE and screen for early diagnostic indicators of KE.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Henan Eye Hospital [Ethical Approval number HNEECKY-2019 (5)]. The patients/participants provided their written informed consent to participate in this study.
SR conceived and designed the study. KY and QF analyzed the data and took responsibility for the integrity and accuracy of the information. LX, YG, and CP contributed to the reagents/materials/analysis tools. KY, QF, and SR drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China (No. 81200664), Henan Provincial Medical Science Building Key Program (Nos. SBGJ202002028 and SBGJ202102051), Henan Provincial Medical Science and Technology Joint Program (LHGJ20210080), Henan Young Health Science and Technology Innovation Outstanding Program (No. YXKC2020023), Henan Provincial Science and Technology Research Project (Nos. 222102310599 and 222102310307), Special Program for Basic Research of Henan Eye Hospital (No. 20JCZD003), and Youth Special Program for Basic Research of Henan Eye Hospital (Nos. 21JCQN006 and 21JCQN008). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. All relevant data are included in the paper and its supporting information files. Contact SR (c2hlbmd3ZWlyZW4xOTg0QDE2My5jb20= and eXNnenoyMDE4QDE2My5jb20=) for additional information.
The authors would like to thank all the participants of this study and Editage (www.editage.cn) for English language editing assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2023.1181117/full#supplementary-material
AUC, area under the receiver operating characteristic curve; BFS, best-fit sphere; CKI, central keratoconus index; CI, confidence interval; CBI-LVC, Corvis biomechanical index–laser vision correction; DLA, deflection amplitude; DLAr, deflection area; DA, deformation amplitude; FE, anterior evaluation; KC, keratoconus; KE, post-LASIK keratectasia; LASIK, laser-assisted in situ keratomileusis; and PE, posterior elevation.
Ambrosio, R., Dawson, D. G., Salomao, M., Guerra, F. P., Caiado, A. L., and Belin, M. W. (2010). Corneal ectasia after LASIK despite low preoperative risk: Tomographic and biomechanical findings in the unoperated, stable, fellow eye. J. Refract. Surg. 26, 906–911. doi:10.3928/1081597x-20100428-02
Ambrosio, R. (2019). Post-LASIK ectasia: Twenty years of a conundrum. Seminars Ophthalmol. 34, 66–68. doi:10.1080/08820538.2019.1569075
Bae, G. H., Kim, J. R., Kim, C. H., Lim, D. H., Chung, E. S., and Chung, T. Y. (2014). Corneal topographic and tomographic analysis of fellow eyes in unilateral keratoconus patients using Pentacam. Am. J. Ophthalmol. 157, 103–109.e1. doi:10.1016/j.ajo.2013.08.014
Bohac, M., Koncarevic, M., Pasalic, A., Biscevic, A., Merlak, M., Gabric, N., et al. (2018). Incidence and clinical characteristics of post LASIK ectasia: A review of over 30,000 LASIK cases. Seminars Ophthalmol. 33, 869–877. doi:10.1080/08820538.2018.1539183
Chan, T. C., Wang, Y. M., Yu, M., and Jhanji, V. (2018). Comparison of corneal dynamic parameters and tomographic measurements using Scheimpflug imaging in keratoconus. Br. J. Ophthalmol. 102, 42–47. doi:10.1136/bjophthalmol-2017-310355
de Luis Eguileor, B., Escudero Argaluza, J., Pijoan Zubizarreta, J. I., Santamaria Carro, A., and Etxebarria Ecenarro, J. (2018). Evaluation of the reliability and repeatability of Scheimpflug system measurement in keratoconus. Cornea 37, 177–181. doi:10.1097/ico.0000000000001373
de Sanctis, U., Loiacono, C., Richiardi, L., Turco, D., Mutani, B., and Grignolo, F. M. (2008). Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology 115, 1534–1539. doi:10.1016/j.ophtha.2008.02.020
Degirmenci, C., Palamar, M., Ismayilova, N., Egrilmez, S., and Yagci, A. (2019). Topographic evaluation of unilateral keratoconus patients. Turkish J. Ophthalmol. 49, 117–122. doi:10.4274/tjo.galenos.2018.90958
Duncan, J. K., Belin, M. W., and Borgstrom, M. (2016). Assessing progression of keratoconus: Novel tomographic determinants. Eye Vis. Lond. Engl. 3, 6. doi:10.1186/s40662-016-0038-6
Gomes, J. A., Tan, D., Rapuano, C. J., Belin, M. W., Ambrósio, R., Guell, J. L., et al. (2015). Global consensus on keratoconus and ectatic diseases. Cornea 34, 359–369. doi:10.1097/ico.0000000000000408
Hashemi, H., Beiranvand, A., Yekta, A., Maleki, A., Yazdani, N., and Khabazkhoob, M. (2016). Pentacam top indices for diagnosing subclinical and definite keratoconus. J. Curr. Ophthalmol. 28, 21–26. doi:10.1016/j.joco.2016.01.009
He, C., Joergensen, J. S., Knorz, M. C., McKay, K. N., and Zhang, F. (2020). Three-step treatment of keratoconus and post-LASIK ectasia: Implantation of ICRS, corneal cross-linking, and implantation of toric posterior chamber phakic IOLs. J. Refract. Surg. 36, 104–109. doi:10.3928/1081597x-20191217-01
Hocaoglu, M., Kara, C., Sen, E. M., and Ozturk, F. (2020). Relationships between corneal biomechanics and the structural and functional parameters of glaucoma damage. Arq. Bras. Oftalmol. 83, 132–140. doi:10.5935/0004-2749.20200019
Huseynli, S., Salgado-Borges, J., and Alio, J. L. (2018). Comparative evaluation of Scheimpflug tomography parameters between thin non-keratoconic, subclinical keratoconic, and mild keratoconic corneas. Ophthalmic & physiological Opt. J. Br. Coll. Ophthalmic Opt. (Optometrists) 28, 521–534. doi:10.1177/1120672118760146
Hwang, E. S., Perez-Straziota, C. E., Kim, S. W., Santhiago, M. R., and Randleman, J. B. (2018). Distinguishing highly asymmetric keratoconus eyes using combined Scheimpflug and spectral-domain OCT analysis. Ophthalmology 125, 1862–1871. doi:10.1016/j.ophtha.2018.06.020
Imbornoni, L. M., McGhee, C. N. J., and Belin, M. W. (2018). Evolution of keratoconus: From diagnosis to therapeutics. Klin. Monatsblatter fur Augenheilkd. 235, 680–688. doi:10.1055/s-0044-100617
Jedzierowska, M., and Koprowski, R. (2019). Novel dynamic corneal response parameters in a practice use: A critical review. Biomed. Eng. online 18, 17. doi:10.1186/s12938-019-0636-3
Koc, M., Aydemir, E., Tekin, K., Inanc, M., Kosekahya, P., and Kiziltoprak, H. (2019). Biomechanical analysis of subclinical keratoconus with normal topographic, topometric, and tomographic findings. J. Refract. Surg. 35, 247–252. doi:10.3928/1081597x-20190226-01
Lee, H., Roberts, C. J., Kim, T. I., Ambrosio, R., Elsheikh, A., and Yong Kang, D. S. (2017). Changes in biomechanically corrected intraocular pressure and dynamic corneal response parameters before and after transepithelial photorefractive keratectomy and femtosecond laser-assisted laser in situ keratomileusis. J. cataract Refract. Surg. 43, 1495–1503. doi:10.1016/j.jcrs.2017.08.019
Moshirfar, M., Smedley, J. G., Muthappan, V., Jarsted, A., and Ostler, E. M. (2014). Rate of ectasia and incidence of irregular topography in patients with unidentified preoperative risk factors undergoing femtosecond laser-assisted LASIK. Clin. Ophthalmol. Auckl. NZ) 8, 35–42. doi:10.2147/opth.s53370
Moshirfar, M., Tukan, A. N., Bundogji, N., Liu, H. Y., McCabe, S. E., Ronquillo, Y. C., et al. (2021). Ectasia after corneal refractive surgery: A systematic review. Ophthalmol. Ther. 10, 753–776. doi:10.1007/s40123-021-00383-w
Pallikaris, I. G., Kymionis, G. D., and Astyrakakis, N. I. (2001). Corneal ectasia induced by laser in situ keratomileusis. J. cataract Refract. Surg. 27, 1796–1802. doi:10.1016/s0886-3350(01)01090-2
Rabinowitz, Y. S. (2006). Ectasia after laser in situ keratomileusis. Curr. Opin. Ophthalmol. 17, 421–426. doi:10.1097/01.icu.0000243015.51886.3a
Randleman, J. B., Dawson, D. G., Grossniklaus, H. E., McCarey, B. E., and Edelhauser, H. F. (2008). Depth-dependent cohesive tensile strength in human donor corneas: Implications for refractive surgery. J. Refract. Surg. 24, S85–S89. doi:10.3928/1081597X-20080101-15
Randleman, J. B., Russell, B., Ward, M. A., Thompson, K. P., and Stulting, R. D. (2003). Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology 110, 267–275. doi:10.1016/s0161-6420(02)01727-x
Salomão, M. Q., Hofling-Lima, A. L., Gomes Esporcatte, L. P., Correa, F. F., Lopes, B., Sena, N., et al. (2021). Ectatic diseases. Exp. eye Res. 202, 108347. doi:10.1016/j.exer.2020.108347
Sandoval, H. P., Donnenfeld, E. D., Kohnen, T., Lindstrom, R. L., Potvin, R., Tremblay, D. M., et al. (2016). Modern laser in situ keratomileusis outcomes. J. cataract Refract. Surg. 42, 1224–1234. doi:10.1016/j.jcrs.2016.07.012
Santhiago, M. R., Giacomin, N. T., Smadja, D., and Bechara, S. J. (2016). Ectasia risk factors in refractive surgery. Clin. Ophthalmol. Auckl. NZ) 10, 713–720. doi:10.2147/opth.s51313
Santodomingo-Rubido, J., Carracedo, G., Suzaki, A., Villa-Collar, C., Vincent, S. J., and Wolffsohn, J. S. (2022). Keratoconus: An updated review. Contact lens anterior eye J. Br. Contact Lens Assoc. 45, 101559. doi:10.1016/j.clae.2021.101559
Sedaghat, M. R., Momeni-Moghaddam, H., Ambrosio, R., Heidari, H. R., Maddah, N., Danesh, Z., et al. (2018). Diagnostic ability of corneal shape and biomechanical parameters for detecting frank keratoconus. Cornea 37, 1025–1034. doi:10.1097/ico.0000000000001639
Seiler, T., Koufala, K., and Richter, G. (1998). Iatrogenic keratectasia after laser in situ keratomileusis. J. Refract. Surg. 14, 312–317. doi:10.3928/1081-597x-19980501-15
Ueki, R., Maeda, N., Fuchihata, M., Asai, T., Koh, S., Fujimoto, H., et al. (2018). Evaluation of corneal biomechanics in patients with keratectasia following LASIK using dynamic Scheimpflug analyzer. Jpn. J. Ophthalmol. 62, 443–450. doi:10.1007/s10384-018-0594-5
Vinciguerra, R., Ambrósio, R., Elsheikh, A., Hafezi, F., Yong Kang, D. S., Kermani, O., et al. (2021). Detection of postlaser vision correction ectasia with a new combined biomechanical index. J. cataract Refract. Surg. 47, 1314–1318. doi:10.1097/j.jcrs.0000000000000629
Vinciguerra, R., Ambrosio, R., Elsheikh, A., Roberts, C. J., Lopes, B., Morenghi, E., et al. (2016). Detection of keratoconus with a new biomechanical index. J. Refract. Surg. 32, 803–810. doi:10.3928/1081597x-20160629-01
Vinciguerra, R., Ambrosio, R., Roberts, C. J., Azzolini, C., and Vinciguerra, P. (2017). Biomechanical characterization of subclinical keratoconus without topographic or tomographic abnormalities. J. Refract. Surg. 33, 399–407. doi:10.3928/1081597x-20170213-01
Vinciguerra, R., Elsheikh, A., Roberts, C. J., Ambrósio, R., Kang, D. S. Y., Lopes, B. T., et al. (2016). Influence of pachymetry and intraocular pressure on dynamic corneal response parameters in healthy patients. J. Refract. Surg. 32, 550–561. doi:10.3928/1081597x-20160524-01
Yang, K., Xu, L., Fan, Q., Gu, Y., Song, P., Zhang, B., et al. (2020). Evaluation of new Corvis ST parameters in normal, Post-LASIK, Post-LASIK keratectasia and keratoconus eyes. Sci. Rep. 10, 5676. doi:10.1038/s41598-020-62825-y
Yang, K., Xu, L., Fan, Q., Gu, Y., Zhang, B., Meng, F., et al. (2021). A hospital-based study on clinical data, demographic data and visual function of keratoconus patients in Central China. Sci. Rep. 11, 7559. doi:10.1038/s41598-021-87291-y
Keywords: fellow eye, corneal biomechanics, diagnostic test, unilateral post-LASIK keratectasia, corneal tomographic parameters
Citation: Yang K, Fan Q, Xu L, Gu Y, Pang C and Ren S (2023) Accuracy of tomographic and biomechanical parameters in detecting unilateral post-LASIK keratoectasia and fellow eyes. Front. Bioeng. Biotechnol. 11:1181117. doi: 10.3389/fbioe.2023.1181117
Received: 07 March 2023; Accepted: 22 May 2023;
Published: 02 June 2023.
Edited by:
Renato Ambrósio Jr., Rio de Janeiro State Federal University, BrazilReviewed by:
Louise Esporcatte, Federal University of São Paulo, BrazilCopyright © 2023 Yang, Fan, Xu, Gu, Pang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengwei Ren, c2hlbmd3ZWlyZW4xOTg0QDE2My5jb20=, eXNnenoyMDE4QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.