
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 26 May 2023
Sec. Biomaterials
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1175345
This article is part of the Research Topic Functionally-adapted Biomaterials for Diagnosis, Treatment and Prevention View all 5 articles
Objective: Developing a simple, rapid, reliable, sensitive, and cost-effective method for prenatal detection of fetomaternal haemorrhage by combining multi-aperture silk membrane with enzyme-linked immunosorbent assay (ELISA), which does not require any complicated instruments and can be visually colored, so as to provide a new method for clinical detection of fetomaternal haemorrhage.
Methods: As a carrier, a chemically treated silk membrane was used to immobilize anti-A/anti-B antibody reagent. PBS washed slowly after vertically dropping red blood cells. After adding biotin-labeled anti-A/anti-B antibody reagent, PBS is slowly washed, enzyme-labeled avidin is added, and TMB is used for color development after washing.
Results: When there were both anti-A and anti-B fetal erythrocytes in pregnant women’s peripheral blood, the final color was dark brown. When there are no anti-A and anti-B fetal red blood cells in pregnant women’s peripheral blood, the final color development results do not change, which corresponds to the color of chemically treated silk membrane.
Conclusion: The new enzyme-linked immunosorbent assay (ELISA) based on a silk membrane can distinguish fetal red blood cells from maternal red blood cells prenatally and can be used for prenatal detection of fetomaternal haemorrhage.
Two or three out of every 10,000 pregnant women are reported to have FMH (fetomaternal haemorrhage) (Tao et al., 2022). The essence of detecting FMH is the quantitative detection of fetal red blood cells in the peripheral blood of pregnant women (Troia et al., 2019). Prenatal detection of maternal hemorrhage is the foundation for preventing newborn hemolytic disease and administering immunoglobulin therapy (Christino Luiz et al., 2019; Cormack et al., 2019; Flynn et al., 2022).
FMH refers to the entry of fetal red blood cells into the mother’s body as a result of a compromised placental barrier. Pregnant women produce specific antibodies against incompatible fetal red blood cell antigens, which act on fetal red blood cells and result in their hemolysis (Simoes et al., 2022) (Figure 1). It may lead to fetal anemia, jaundice, edema, and even death (Carr et al., 2022). There are numerous causes of placenta rupture, but the most common are trauma and intrauterine death (Davis, 2019; Hsia et al., 2019; Hookins and Vatsayan, 2020; Monteiro et al., 2021). In the United States, 40% of pregnant women die from trauma, according to statistics (Krywko et al., 2022). In addition, if the fetus survives during pregnancy, mothers with incompatible blood types can produce specific IgG antibodies against fetal red blood cells, which can cause hemolysis in fetal red blood cells via the placenta. Nonetheless, if the fetus survives, the newborn may still develop hemolytic disease or pass away after a few months (Akorsu et al., 2019; Song et al., 2021). Common antibodies that can easily cause fetal bleeding include anti-A, anti-B, anti-D, anti-Kell, anti-E, and anti-C antibodies (Pal and Williams, 2015; Erhabor et al., 2020; Minuk et al., 2020; Tneh et al., 2021). 10% of fetal deaths are attributed to maternal bleeding, according to statistical evidence. Therefore, early detection of FMH and treatment with immunoglobulin can reduce fetal mortality (Glazebrook et al., 2020).
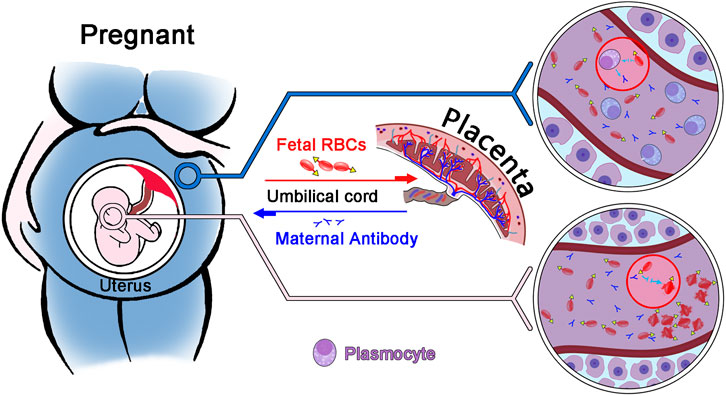
FIGURE 1. Diagram illustrating the principle of fetomaternal haemorrhage. Through the placenta, fetal red blood cells enter the mother, and the mother produces an IgG antibody against fetal red blood cells. This antibody enters the fetus through the placenta, causing red blood cell hemolysis in the fetus.
Indicators for the detection of FMH consist primarily of fetal red blood cell antigen, fetal hemoglobin, and gene detection (Athiel et al., 2020; Zhang et al., 2021). Fetal red blood cell antibody is utilized to identify fetal red blood cells based on the difference between fetal and maternal blood types. Currently, the most common methods for detecting fetal red blood cells in the mother’s blood are the rosette test (Kim and Makar, 2012) and the microcolumn gel test (Agaylan et al., 2007; Ben-Haroush et al., 2007). Nevertheless, the sensitivity of the rosette test is low, and the microcolumn gel technique cannot be used as a routine clinical detection method due to its complex operation and high material cost. Therefore, it is crucial to establish a method that is sensitive, inexpensive, simple, and quick.
ELISA based on silk membrane developed in this experiment has the following benefits: high sensitivity, low cost, simplicity, rapidity, no instrument required, and visual visualization of chromogenic results. As a natural protein polymer with non-toxicity, non-irritation, biodegradability, biocompatibility, and mechanical properties, natural silk is indispensable not only in the textile industry but also in biomedicine (Wang et al., 2019; Jaleel et al., 2020; Patel et al., 2020; Rodriguez et al., 2020). Silk can be used as a material for sutures, biological scaffolds, and tissue engineering transplants (Feng et al., 2020; Yonesi et al., 2021). In addition to the aforementioned properties, silk is produced by domestic silkworms, making it a readily available and inexpensive material (Ni et al., 2019). Silk is composed of a fibrous protein (fibroin) and a glue protein (sericin) (Song et al., 2020). Silk protein is extracted from the cocoon of the silkworm and is immunogenic (Wang et al., 2021). As a globulin on the surface of silk fibroin, sericin has excellent water-solubility (Wray et al., 2011). Degumming can therefore be used to eliminate sericin, as it does not alter its biocompatibility, immunogenicity, or other properties (Lopez Barreiro et al., 2019).
In this experiment, ELISA developed by silk membrane is simple, rapid, inexpensive, harmless to the health of the fetus and mother, does not require any instruments or equipment, and the results are straightforward to observe. This technique is one type of immunosensor analysis, which combines antigen, antibody, and analyte of interest. In addition to the immune activity and mechanical properties of natural silk, its three-dimensional, multilayered pore structure is also utilized. This structure allows red blood cells with an average diameter of 7.2 microns to pass through without obstruction.
The experimental silk film was acquired at the Suzhou market. Anti-silk membrane monoclonal antibody and cross-linked antibody were from the Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences. And their preparation methods were consistent with those of Wang’s article. The antibodies’ specificity, affinity, and purity have been confirmed. From Shanghai Hematology, anti-A and anti-B reagents were acquired. Biotin-avidin was purchased from Beijing Biodragon Immunotechnologies Co.,Ltd. The blood sample came from China-Japan Union Hospital of Jilin University and has been approved by the hospital’s Ethics Committee (approval number:20,220,425,011). It is stored at 4°C. Freeze dryer (ALPHA1-4/LD plus) was purchased from Beijing Boli Instrument Co., Ltd.
The natural silk membrane was cut into a disc (with an aperture of 10 mm), soaked in hot water, and the fluffy filaments and the bottom layer of membrane (which is difficult for red blood cells to penetrate) were removed to produce a carrier with the same thickness and aperture. Soak with mouse anti-silk membrane antibody (2H3) followed by cross-linked antibody (sheep anti-mouse IgG immunoglobulin). Specific affinity was then employed to attach the two antibodies to the surface of the silk membrane. Then, anti-A antibody and anti-B antibody were coupled to form a silk membrane capable of recognizing type A red blood cell antigen and type B red blood cell antigen. The non-A-type and non-B-type red blood cells cannot be combined with the chemically treated silk membrane, but they will fall off and separate from the membrane after PBS washing, whereas the red blood cell antigen that has been specifically combined with the silk membrane surface will not fall off. In addition, when precipitated TMB was added to a solution containing biotin-avidin coupled with anti-A/anti-B antibody, distinct hues will be observed. Figure 2 illustrates experimental steps of the silk membrane-based ELISA.
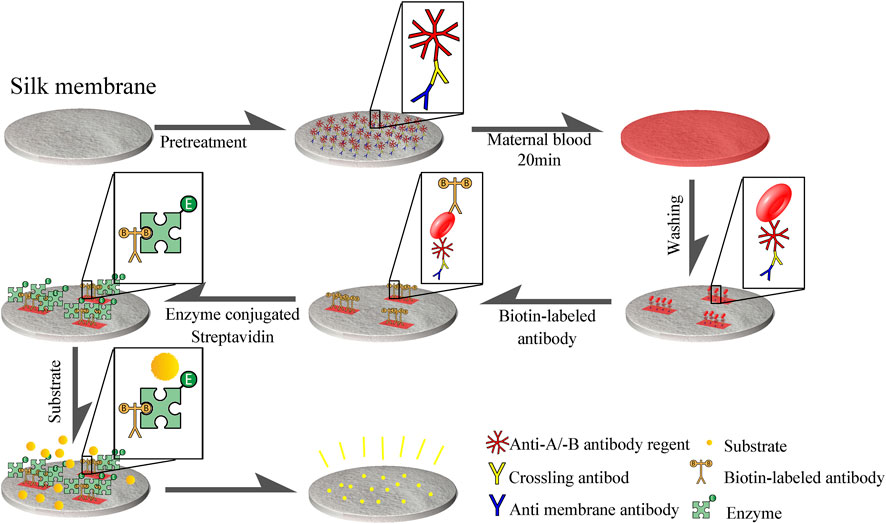
FIGURE 2. Procedure for detecting FMH using ELISA based a silk membrane. Disk-shaped silk membrane was treated with mouse anti-silk membrane antibody, cross-linked antibody (sheep anti-mouse IgG immunoglobulin), and anti -A/anti -B antibody reagent to form an immune complex structure consisting of anti-silk antibody, cross-linked antibody, and anti -A/anti -B antibody reagent that was connected to silk membrane. Red blood cell antigen that can be coupled with anti-A and anti-B antibodies will be attached to a silk membrane, and then biotin-biotin will be employed.
Under an electron microscope, the cross-sections and surfaces of untreated natural silk membrane and chemically treated silk membrane were observed.
The natural silk membrane was formed into a 10-mm-diameter disc using a 10-mm-diameter hole punch, soaked in hot water to remove wax and magazines, and the loose silk fibers were removed by hand. Soak the treated silk film in a chemical treatment solution for 2 h in a water bath at 56°C. Vacuum twice. Soak at 56°C for 2 h. The PBST was cleaned thrice. Soak overnight at 4 °C with 100 g/mL anti-silk membrane antibody. The PBST was cleaned thrice. 5% skim milk powder was sealed for 2 h at 37°C. The PBST was cleaned thrice. The silk membrane was then soaked overnight at 4°C in 200 g/mL of cross-linked antibody. Wash PBS three times, each time gently absorbing water with filter paper, and dry.
HRP-labeled sheep anti-mouse IgM and HRP-labeled sheep anti-mouse IgG were incubated at 37°C for 1 h with silk membrane coupled with anti-silk membrane antibody and silk membrane coupled with cross-linked antibody, and then evenly mixed every 15 min. The PBST was washed thoroughly five times. TMB was used to evaluate the coupling of anti-silk membrane antibody and cross-linked antibody.
The red blood cells were vertically dripped onto the chemically treated silk membrane, the red blood cell distribution on the front and back of the membrane was observed, and the membrane’s permeability was compared to commercially available NC membrane and natural untreated silk membrane.
Biotin-coupled anti-A and anti-B antibodies were prepared and stored in accordance with the biotin labeling kit’s instructions (Beijing Biodragon Immunotechnologies Co., Ltd.).
0.1%, 0.3%, 0.5%, and 1% of fetal red blood cells were mixed with adult red blood cells (the ratio of fetal red blood cells to mixed red blood cells composed of fetal red blood cells and adult red blood cells). 10 L of mixed red blood cells were dripped vertically onto the front of a silk membrane, left to stand for 2 min, and then vertically washed with PBST until the negative control displayed untreated color (positive control only added fetal red blood cells, negative control only added adult red blood cells). Adding biotin-coupled anti-A and anti-B antibodies, washing, adding enzyme-labeled avidin, washing, and then treating in the dark for 2 min with TMB. Observe the outcomes. In this experiment, the biotin-streptomycin-treated silk film was simultaneously analyzed with ImageJ software, and the results were quantified. The data were then plotted using GraphPad Prism 9.
While detecting fetal red blood cells and adult red blood cells by silk membrane-based ELSA, flow cytometry detected the same portion of fetal red blood cells and adult red blood cells simultaneously. The detection method for FMH using anti-fetal red blood cell antibodies is consistent with that in Kim’s article. Using a parallel control of fetal red blood cells and adult red blood cells by a silk membrane-based ELSA and flow cytometry, the accuracy of negative and positive photographs was confirmed, and the silk membrane-based ELSA was successfully validated to detect FMH.
In addition, the above method is compared to the traditional K-B test to determine their sensitivity. During the K-B test, 10,000 cells were observed under a microscope and the number of fetal red blood cells was counted in strict accordance with the AABB technical manual.
Under an electron microscope, it is evident that the silk membrane consists of multiple layers of porous membrane that are interwoven with individual fibers. A structure with multiple layers can absorb more carriers and allow red blood cells to permeate the silk membrane. Moreover, compared to a single layer, the multi-layer structure can absorb more red blood cells, increase the adsorption space of antigen-antibody immune complexes, and improve visual results (Figure 3).
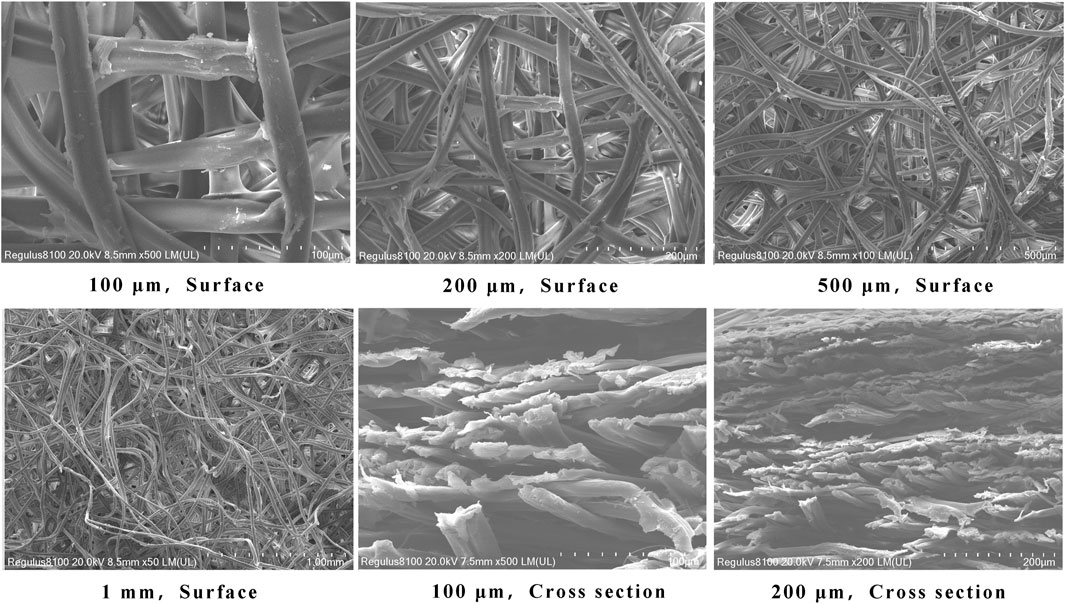
FIGURE 3. Electron microscopy of the morphology of a silk membrane. 3a∼3 days are top views of silk membrane at various magnifications, whereas 3e and 3f are cross sections of silk membrane at various magnifications.
Anti-silk membrane antibody and cross-linked antibody are antibodies of the mouse that can react with HRP labeled anti-mouse globulin. After the anti-silk membrane antibody or cross-linked antibody is fixed to the silk membrane, the color of the silk membrane becomes blue after the Goat anti-mouse-globulin and the color developer are added; However, the color of the silk film without anti-silk film antibody or cross-linked antibody was not changed even if Goat anti-mouse-globulin and chromogenic agent were added. Figure 4 shows that the antibodies were successfully coupled to the silk membrane. It indicates that this experimental step has been successfully established.
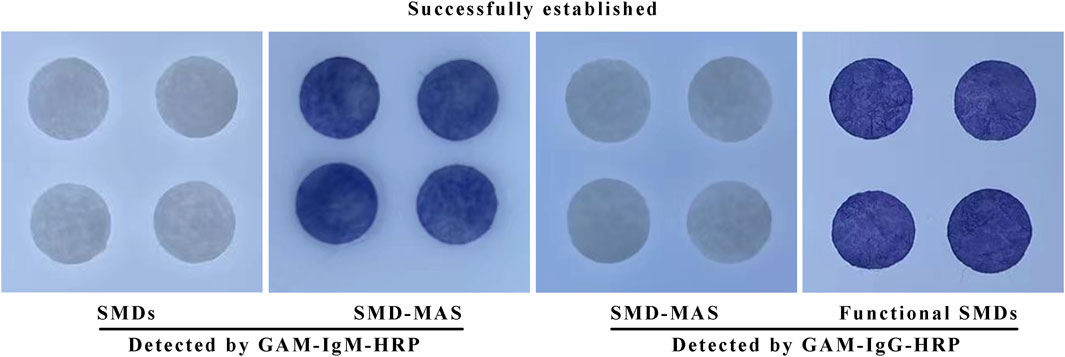
FIGURE 4. The results of the coupling of anti-silk membrane antibody and cross-linked antibody to silk membrane using GAM—HRP.
Figure 5 demonstrates that the chemically treated silk membrane can fully permeate both PBS and red blood cells, and that red blood cells can cover both the front and back of the membrane. However, natural silk membrane that has not been treated is impermeable to PBS and red blood cells, whereas NC membrane can pass through PBS but not red blood cells. Therefore, the chemical treatment of silk film in this experiment is beneficial to the experiment’s progression.
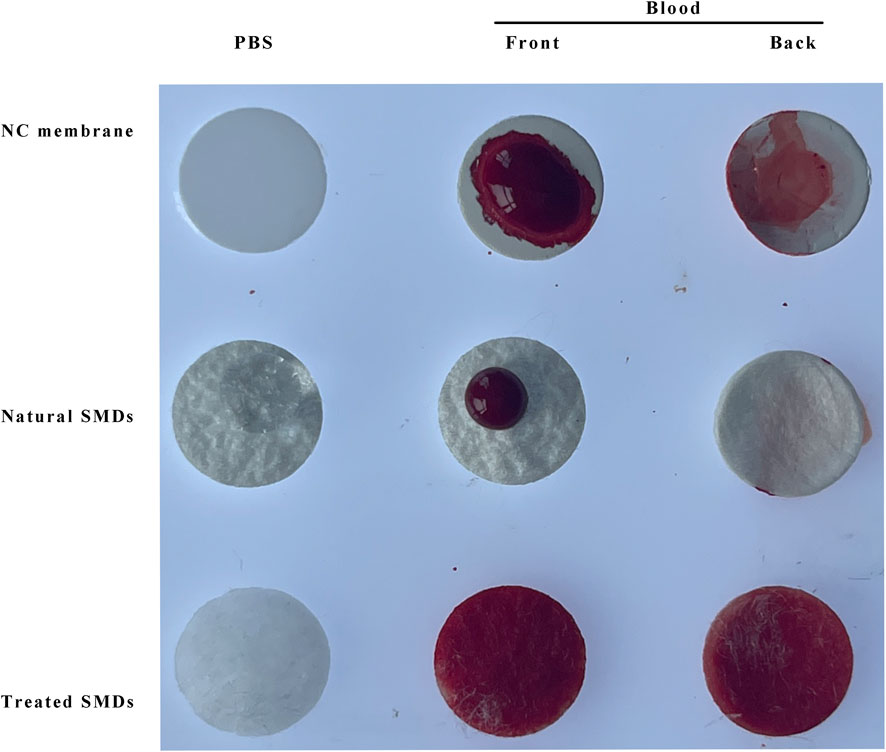
FIGURE 5. Permeability comparison of NC membrane, natural silk membrane, and chemically treated silk membrane. SMD: silk cocoon membrane disc; MAS: Mouse anti-silk proteins; GAM: goat anti-mouse.
Using a novel technique and flow cytometry, identical fetal and adult red blood cells were identified. The silk membrane in Figure 6 was blue and yellow for the negative result and brown for the positive result. At this time, flow cytometry results indicated that the fluorescence intensity of positive results was greater than that of negative results. This demonstrated that the ELISA based on silk membrane has been successfully established and it was reliable and accurate for the detection of FMH.
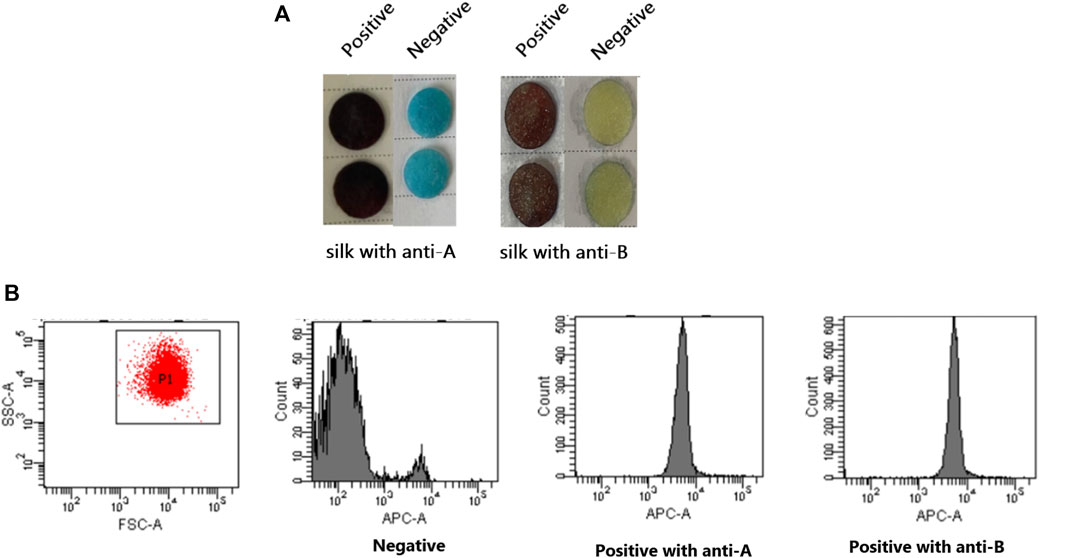
FIGURE 6. Confirmation of the successful establishment of an ELISA based on a silk membrane to detect FMH by flow cytometry. (A): The negative and positive FMH results detected by ELISA based a silk membrane. (B): The negative and positive FMH results detected by flow cytometry.
Figure 7 demonstrates that when the positive control and the negative control are established, the color changes of 0.1% fetal red blood cells and 0.3% fetal red blood cells are comparable, as are the color changes of 0.5% fetal red blood cells and 1% fetal red blood cells. Nonetheless, 0.5%–1% of fetal red blood cell test results are darker than 0.1%–0.3% of fetal red blood cell test results, and the range of FMH can be determined based on color. Moreover, each experiment was conducted four times with consistent results. Figure 11 illustrated the error bar. Additionally, this experiment quantified the experimental outcomes in greater detail. Using ImageJ software, the silk film was separated into “blue, green, and red” color channels and then inverted (see Figures 8–10). Then, the brightness data of inverted images in three distinct color channels was measured individually. GraphPad Prism 9 was used to manage the data (Figure 11). The optimal outcomes are as follows: the value of silk membrane treated with fetal red blood cells > the value of silk membrane treated with 1% fetal RBCs > the value of silk membrane treated with 0.5% fetal RBCs > the value of silk membrane treated with 0.3% fetal RBCs > the value of silk membrane treated with 0.1% fetal RBCs > the value of silk membrane treated with adult red blood cells. Compared to Figure 11, the silk film treated with anti-A produces superior results in the blue color channel (Figure 11A), while the silk film treated with anti-B produces superior results in the green color channel (Figure 11D). Consequently, ELISA based on a silk membrane can quantify with high sensitivity, high repeatability, and high dependability. Figure 12 shows the results of K-B test to detect different proportions of FMH. 12(A) is the result chart (positive control) of detecting fetal red blood cells by K-B test, which shows that the result of detecting fetal red blood cells by K-B test is red under the microscope; 12(B) is the result chart (negative control) of adult red blood cells detected by K-B test, which shows that adult red blood cells detected by K-B test are ghostly under the microscope. 12(C) is the result of detecting different proportions of fetal red blood cells in mixed blood by K-B test. Comparing Figure 7 with Figure 12, both K-B test and enzyme-linked immunosorbent assay based on silk membrane can detect 0.1% of fetal red blood cells, which can meet the needs of clinical detection of FMH sensitivity. In addition, ELISA based on silk membrane can be quantified by software, which was simple and quick, whereas the K-B experiment was subjective and requires the detection of 10,000 cells, which was time-consuming. This experiment replaced the blue silk film and yellow silk film used in the new method with white silk film and conducted parallel experiments to determine whether the color of the silk film affected the experimental outcomes. It was discovered that the silk film was colored similarly in both the positive and negative results. The reason for this is that haemoglobin was a type of pigment that can combine with silk membrane, altering the results of the experiment. This colored silk membrane eliminated the interference of haemoglobin and other pigments. Figure 13 displayed the outcomes.

FIGURE 13. The negative and positive control results of an ELISA with a white silk membrane as basement membrane.
Figure 7 demonstrates that ELISA based on a silk membrane developed in this experiment can detect the situation in which the fetal blood type is A or B and the maternal blood type is O. By using the anti-A/anti-B antibody attached to the silk membrane in this experiment, we can identify all instances in which the fetus has a different blood type than the mother. This experiment developed a novel method for prenatal detection of FMH, but it cannot determine the fetus’ blood type. Fortunately, the blood type of the fetus can be predicted based on the blood types of the father and mother, and the observation of the results is straightforward, so the experimental procedure will not be complicated. Table 1 displays the fetal blood types for which the father’s and mother’s blood types are known, as well as the test results for different blood types using new technology. When the mother’s blood type is AB or the same as that of the fetus, it is not necessary to test for fetal bleeding. When the mother’s blood type is A, a positive test result for 0.1% fetal bleeding of anti-B silk membrane has clinical significance; when the mother’s blood type is B, a positive test result for 0.1% fetal bleeding of anti-A silk membrane has clinical significance.
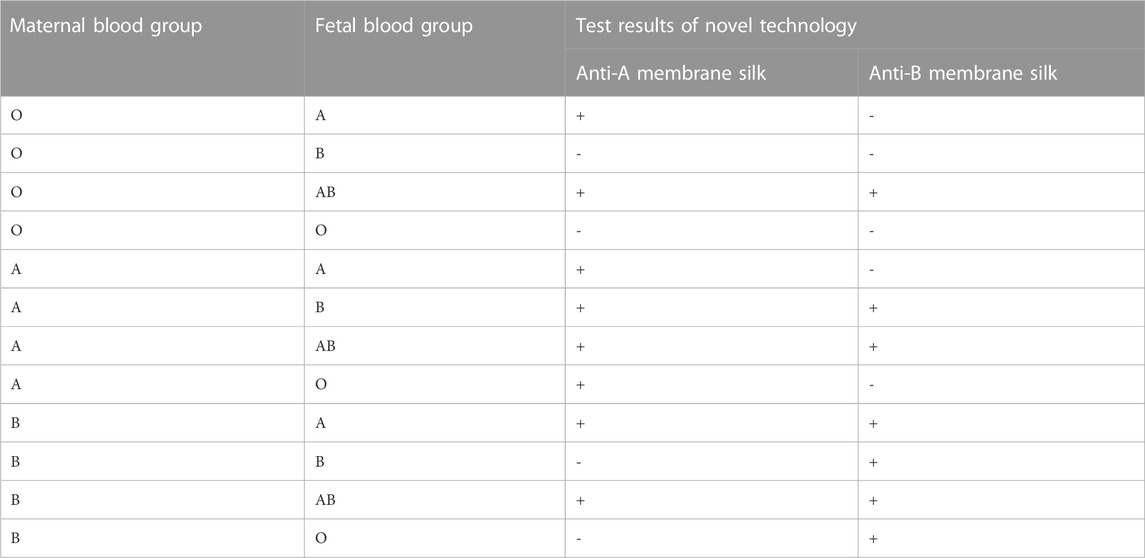
TABLE 1. Summary of the types of FMH detected by ELISA based on silk membrane (Silk membrane can be separated into anti-A and anti-B subtypes based on the conjugated antibody; +: positive result; -: negative result).
In this experiment, the chemically treated silk membrane was combined with the cascade amplification enzyme-linked immunosorbent assay, and a new prenatal diagnosis method for FMH was developed, which was simple, fast, reliable, low-cost, easy to obtain, did not harm the health of the fetus or mother, and was anticipated to be applied to routine clinical detection. Silk membrane, as the matrix material of ELISA, not only capitalized on its excellent performance, but also took advantage of its pores’ ability to permeate red blood cells. In addition, the multilayer pore structure of natural silk membrane provided ample binding space for antigen and antibody. The experimental outcomes were in agreement with Wang’s article (Wanga et al., 2020). In contrast, the NC membrane on the market is incapable of penetrating red blood cells when compared to the treated silk membrane. Consequently, grinding natural silk film into paper resembling NC film facilitates the detection of FMH in future experiments. In addition, vacuuming degums the aperture and makes it more transparent. Anti-A and anti-B antibodies that bind specifically to fetal red blood cells in an ELISA utilizing a silk membrane are IgM blood group antibodies. The antibody can directly agglutinate with blood group antigen to form an immune complex that is bound to the pore diameter of the silk membrane. If anti-D antibody is attached to a silk membrane to combine with D antigen or if anti-fetal hemoglobin antibody is used to capture fetal hemoglobin, it must be linked to anti-human globulin or enzyme because anti-D antibody and anti-fetal hemoglobin antibody are IgG antibodies (Lemaitre et al., 2020; Ayenew, 2021).
This experiment has some limitations. This experiment merely has demonstrated that ELISA based on a silk membrane can detect whether the fetus’ blood type is A or B and the mother’s blood type is O. By using the anti-A/anti-B antibody attached to the silk membrane in this experiment, it is possible to identify all instances in which the fetus has a different blood type than the mother. The theoretical test results for various maternal and fetal blood types are presented in Table 1. Moreover, the clinical samples of FMH were not used for the experiment, mainly because FMH is a rare disease. However, in this experiment, different proportions of fetal red blood cells and adult red blood cells are used to simulate the real state of FMH, and the new enzyme-linked immunosorbant assay (ELISA) based on a silk membrane established in this experiment can detect 0.1% of FMH, which has the same sensitivity as K-B test, and is suitable for clinical sensitivity analysis of FMH detection.
In the experiment, the cross-sections were prepared by shearing off, which can be by immersed in liquid nitrogen, and later mannualy breaking. In future, the silk membrane in this study may be replaced by electrospun fibrous membrane, which can be more sensitive due to the smaller diameters of nanofibers, large surface and big porosity (Ge et al., 2023; Yu et al., 2023). This experiment replicates Wang’s idea of distinguishing anti -A antibody, anti -B antibody, and anti -D antibody by using different colored membranes, and uses different colored silk membranes to distinguish before detection in order to prevent detection errors caused by the inability to differentiate silk membranes treated with anti -A or anti -B antibodies. For instance, the silk membrane treated with anti-A antibody is blue and is used to detect erythrocyte antigen of type A; the silk membrane treated with anti-B antibody is yellow and is used to detect red blood cell antigen of type B. Obviously, it will be studied further in the future to use white silk films instead of different colors for experiments, so as to reduce color interference with test results.
This experiment resulted in the development of new enzyme-linked immunosorbent assay (ELISA) based on a silk membrane that is simple, rapid, reliable, sensitive, inexpensive, and requires no complex equipment. It can visually develop color based on a silk membrane and can differentiate fetal red blood cells from maternal red blood cells prior to delivery, allowing it to be used for prenatal detection of FMH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of China-Japan Union Hospital of Jilin University (approval number: 20220425011). Written informed consent from the participant’s; legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
XL: conceptualization, methodology, investigation, writing—original draft, writing—review and editing, visualization. ML, HW, and SD: conceptualization, methodology, investigation, visualization. YL: project administration. TL: conceptualization, supervision, project administration, writing—original draft, writing—review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by Department of Science and Technology of Jilin Province under Grant [number20200708105YY]; Department of Finance of Jilin Province under Grant [number202201RK]; Department of Science and technology of Jilin Province under Grant [number20230508069RC].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agaylan, A., Meyer, O., Ahrens, N., Dudenhausen, J., Bombard, S., and Salama, A. (2007). A rapid gel agglutination test for the determination of fetomaternal haemorrhage. Transfus. Med. 17 (5), 395–398. doi:10.1111/j.1365-3148.2007.00763.x
Akorsu, E. E., Acquaye, J. K., Benneh, A. A., Oppong, S. A., and Olayemi, E. (2019). Fetomaternal hemorrhage among pregnant women in Accra, Ghana. Int. J. Gynaecol. Obstet. 146 (3), 333–338. doi:10.1002/ijgo.12890
Athiel, Y., Maisonneuve, E., Bleas, C., Maurice, P., Cortey, A., Toly-Ndour, C., et al. (2020). Reduced fetal movement during pregnancy: Is the Kleihauer-Betke test really useful? J. Gynecol. Obstet. Hum. Reprod. 49, 101748. doi:10.1016/j.jogoh.2020.101748
Ayenew, A. A. (2021). Prevalence of rhesus D-negative blood type and the challenges of rhesus D immunoprophylaxis among obstetric population in Ethiopia: A systematic review and meta-analysis. Matern. Health Neonatol. Perinatol. 7 (1), 8. doi:10.1186/s40748-021-00129-3
Ben-Haroush, A., Belkin, A., Chezar, J., Orlin, J., Hod, M., and Bar, J. (2007). Comparison of two techniques for the evaluation of fetomaternal hemorrhage in RhD-negative women: Gel agglutination and haemoglobin F determination by flow cytometry. Acta Obstet. Gynecol. Scand. 86 (7), 821–826. doi:10.1080/00016340701415509
Carr, N. R., Henry, E., Bahr, T. M., Ohls, R. K., Page, J. M., Ilstrup, S. J., et al. (2022). Fetomaternal hemorrhage: Evidence from a multihospital healthcare system that up to 40% of severe cases are missed. Transfusion 62 (1), 60–70. doi:10.1111/trf.16710
Christino Luiz, M. F., Baschat, A. A., Delp, C., and Miller, J. L. (2019). Massive fetomaternal hemorrhage remote from term: Favorable outcome after fetal resuscitation and conservative management. Fetal Diagn Ther. 45 (5), 361–364. doi:10.1159/000492750
Cormack, O. M., Guilfoyle, F., and Flynn, C. M. (2019). The prevalence of an elevated F cell population in a maternal and gynaecology cohort. Transfus. Med. 29 (5), 369–373. doi:10.1111/tme.12625
Davis, B. H. (2019). Enumeration of fetal red blood cells, hemoglobin-specific RBC cells, and F reticulocytes in human blood. Curr. Protoc. Cytom. 90 (1), e56. doi:10.1002/cpcy.56
Erhabor, O., Azachi, W. B., and Tosan, E. (2020). A case report of haemolytic disease of the foetus and newborn caused by Alloantibody D and Jka in a Rhesus D negative Nigerian woman: Justification for the implementation of universal access to prophylaxis and evidenced-based best practices. Hum. Antibodies 28 (3), 245–252. doi:10.3233/hab-200414
Feng, Y., Lin, J., Niu, L., Wang, Y., Cheng, Z., Sun, X., et al. (2020). High molecular weight silk fibroin prepared by papain degumming. Polym. (Basel) 12 (9), 2105. doi:10.3390/polym12092105
Flynn, A. N., Hoffman, E., Murphy, C., Jen, A., Schreiber, C. A., and Roe, A. H. (2022). Fetomaternal hemorrhage assessment in Rh-negative patients undergoing dilation and evacuation between 20 and 24 weeks' gestational age: A retrospective cohort study. Contraception 110, 27–29. doi:10.1016/j.contraception.2022.02.001
Ge, R., Ji, Y., Ding, Y., Huang, C., He, H., and Yu, D. G. (2023). Electrospun self-emulsifying core-shell nanofibers for effective delivery of paclitaxel. Front. Bioeng. Biotechnol. 11, 1112338. doi:10.3389/fbioe.2023.1112338
Glazebrook, B., Akers, C., Bielby, L., Bastin, K., Von Wielligh, K., and Daly, J. (2020). Quality audit of the guidelines for the use of RhD immunoglobulin in obstetrics: Are we getting it right? Aust. N. Z. J. Obstet. Gynaecol. 60 (4), 504–508. doi:10.1111/ajo.13177
Hookins, B., and Vatsayan, A. (2020). Intraplacental choriocarcinoma and fetomaternal haemorrhage and maternal disseminated intravascular coagulopathy in a term pregnancy: A case report. Case Rep. Womens Health 27, e00216. doi:10.1016/j.crwh.2020.e00216
Hsia, J. K., Schimmoeller, N. R., Cansino, C. D., Hou, M. Y., Jensen, H. M., and Creinin, M. D. (2019). Prospective quantification of fetomaternal hemorrhage with dilation and evacuation procedures. Contraception 99 (5), 281–284. doi:10.1016/j.contraception.2018.11.015
Jaleel, Z., Zhou, S., Martin-Moldes, Z., Baugh, L. M., Yeh, J., Dinjaski, N., et al. (2020). Expanding canonical spider silk properties through a DNA combinatorial approach. Mater. (Basel) 13 (16), 3596. doi:10.3390/ma13163596
Kim, Y. A., and Makar, R. S. (2012). Detection of fetomaternal hemorrhage. Am. J. Hematol. 87 (4), 417–423. doi:10.1002/ajh.22255
Krywko, D. M., Yarrarapu, S. N. S., and Shunkwiler, S. M. (2022). “Kleihauer betke test,” in StatPearls, StatPearls publishing copyright © 2022 (Treasure Island (FL): StatPearls Publishing LLC).
Lemaitre, J., Planche, L., and Ducarme, G. (2020). Systematic kleihauer-betke test after external cephalic version for breech presentation: Is it useful? J. Clin. Med. 9 (7), 2053. doi:10.3390/jcm9072053
Lopez Barreiro, D., Yeo, J., Tarakanova, A., Martin-Martinez, F. J., and Buehler, M. J. (2019). Multiscale modeling of silk and silk-based biomaterials-A review. Macromol. Biosci. 19 (3), e1800253. doi:10.1002/mabi.201800253
Minuk, L., Clarke, G., and Lieberman, L. (2020). Approach to red blood cell antibody testing during pregnancy: Answers to commonly asked questions. Can. Fam. Physician 66, 491–498.
Monteiro, S., Burling, M., and Doyle, H. (2021). Late diagnosis of intraplacental choriocarcinoma co-existing with fetomaternal haemorrhage causing fetal demise: A case report. Case Rep. Womens Health 31, e00341. doi:10.1016/j.crwh.2021.e00341
Ni, Y., Jiang, Y., Wang, K., Shao, Z., Chen, X., Sun, S., et al. (2019). Chondrocytes cultured in silk-based biomaterials maintain function and cell morphology. Int. J. Artif. Organs 42 (1), 31–41. doi:10.1177/0391398818806156
Pal, M., and Williams, B. (2015). Prevalence of maternal red cell alloimmunisation: A population study from queensland, Australia. Pathology 47 (2), 151–155. doi:10.1097/pat.0000000000000225
Patel, M., Dubey, D. K., and Singh, S. P. (2020). Phenomenological models of Bombyx mori silk fibroin and their mechanical behavior using molecular dynamics simulations. Mater Sci. Eng. C Mater Biol. Appl. 108, 110414. doi:10.1016/j.msec.2019.110414
Rodriguez, M., Kluge, J. A., Smoot, D., Kluge, M. A., Schmidt, D. F., Paetsch, C. R., et al. (2020). Fabricating mechanically improved silk-based vascular grafts by solution control of the gel-spinning process. Biomaterials 230, 119567. doi:10.1016/j.biomaterials.2019.119567
Simoes, M., Vale, G., Lacerda, C., Pais, P., and Pignatelli, D. (2022). Fetomaternal hemorrhage: A clue to intraplacental choriocarcinoma and neonatal malignancy. J. Matern. Fetal Neonatal Med. 35 (25), 6615–6617. doi:10.1080/14767058.2021.1918665
Song, S., Zhu, Y., Jorch, G., Zhang, X., Wu, Y., Chen, W., et al. (2021). A very preterm infant born to mother of mirror syndrome secondary to fetomaternal hemorrhage: A case report. BMC Pregnancy Childbirth 21 (1), 701. doi:10.1186/s12884-021-04179-5
Song, Y., Wang, H., Yue, F., Lv, Q., Cai, B., Dong, N., et al. (2020). Silk-based biomaterials for cardiac tissue engineering. Adv. Healthc. Mater 9, e2000735. doi:10.1002/adhm.202000735
Tao, E., Ye, D., Long, G., Hu, Y., Fu, Q., Yuan, T., et al. (2022). Severe neonatal anemia affected by massive fetomaternal hemorrhage: A single-center retrospective observational study. J. Matern. Fetal Neonatal Med. 35 (20), 3972–3978. doi:10.1080/14767058.2020.1845313
Tneh, S. Y., Baidya, S., and Daly, J. (2021). Clinical practices and outcomes of RhD immunoglobulin prophylaxis following large-volume fetomaternal haemorrhage in Queensland, Australia. Aust. N. Z. J. Obstet. Gynaecol. 61 (2), 205–212. doi:10.1111/ajo.13226
Troia, L., Al-Kouatly, H. B., McCurdy, R., Konchak, P. S., Weiner, S., and Berghella, V. (2019). The recurrence risk of fetomaternal hemorrhage. Fetal Diagn Ther. 45 (1), 1–12. doi:10.1159/000491788
Wang, F., Guo, C., Yang, Q., Li, C., Zhao, P., Xia, Q., et al. (2021). Protein composites from silkworm cocoons as versatile biomaterials. Acta Biomater. 121, 180–192. doi:10.1016/j.actbio.2020.11.037
Wang, Q., Han, G., Yan, S., and Zhang, Q. (2019). 3D printing of silk fibroin for biomedical applications. Mater. (Basel) 12 (3), 504. doi:10.3390/ma12030504
Wanga, H., Duan, S., Wang, M., Wei, S., Chen, Y., Chen, W., et al. (2020). Silk cocoon membrane-based immunosensing assay for red blood cell antigen typing. Sensors Actuators B Chem. 320, 128376. doi:10.1016/j.snb.2020.128376
Wray, L. S., Hu, X., Gallego, J., Georgakoudi, I., Omenetto, F. G., Schmidt, D., et al. (2011). Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater Res. B Appl. Biomater. 99 (1), 89–101. doi:10.1002/jbm.b.31875
Yonesi, M., Garcia-Nieto, M., Guinea, G. V., Panetsos, F., Perez-Rigueiro, J., and Gonzalez-Nieto, D. (2021). Silk fibroin: An ancient material for repairing the injured nervous system. Pharmaceutics 13 (3), 429. doi:10.3390/pharmaceutics13030429
Yu, D.-G., Li, Q., Song, W., Xu, L., Zhang, K., and Zhou, T. (2023). Advanced technique-based combination of innovation education and safety education in higher education. J. Chem. Educ. 100 (2), 507–516. doi:10.1021/acs.jchemed.2c00568
Keywords: silk cocoon membrane, enzyme-linked immunosorbent assay (ELISA), biotin-avidin, fetomaternal haemorrhage (FMH), fetal red blood cells (RBCs)
Citation: Li X, Li M, Duan S, Wang H, Li Y and Liu T (2023) A novel enzyme-linked immunostaining technique based on silk membrane for the prenatal detection of fetomaternal haemorrhage. Front. Bioeng. Biotechnol. 11:1175345. doi: 10.3389/fbioe.2023.1175345
Received: 27 February 2023; Accepted: 02 May 2023;
Published: 26 May 2023.
Edited by:
Andreas Nüssler, University of Tübingen, GermanyReviewed by:
Deng-Guang Yu, University of Shanghai for Science and Technology, ChinaCopyright © 2023 Li, Li, Duan, Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiemei Liu, bHRtQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.