
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 20 January 2023
Sec. Tissue Engineering and Regenerative Medicine
Volume 11 - 2023 | https://doi.org/10.3389/fbioe.2023.1061622
 Sunita Brimmer1,2,3
Sunita Brimmer1,2,3 Pengfei Ji1,2,3
Pengfei Ji1,2,3 Aditya K. Birla1,2
Aditya K. Birla1,2 Sundeep G. Keswani1,2,4,5
Sundeep G. Keswani1,2,4,5 Christopher A. Caldarone2,3,4,5†
Christopher A. Caldarone2,3,4,5† Ravi K. Birla1,2,3,4,5*†
Ravi K. Birla1,2,3,4,5*†The field of biological pumps is a subset of cardiac tissue engineering and focused on the development of tubular grafts that are designed generate intraluminal pressure. In the simplest embodiment, biological pumps are tubular grafts with contractile cardiomyocytes on the external surface. The rationale for biological pumps is a transition from planar 3D cardiac patches to functional biological pumps, on the way to complete bioartificial hearts. Biological pumps also have applications as a standalone device, for example, to support the Fontan circulation in pediatric patients. In recent years, there has been a lot of progress in the field of biological pumps, with innovative fabrication technologies. Examples include the use of cell sheet engineering, self-organized heart muscle, bioprinting and in vivo bio chambers for vascularization. Several materials have been tested for biological pumps and included resected aortic segments from rodents, type I collagen, and fibrin hydrogel, to name a few. Multiple bioreactors have been tested to condition biological pumps and replicate the complex in vivo environment during controlled in vitro culture. The purpose of this article is to provide an overview of the field of the biological pumps, outlining progress in the field over the past several years. In particular, different fabrication methods, biomaterial platforms for tubular grafts and examples of bioreactors will be presented. In addition, we present an overview of some of the challenges that need to be overcome for the field of biological pumps to move forward.
There is a chronic shortage of donor hearts in both the adult and pediatric population, with significant challenges in both patient populations (Evers et al., 2011; Alyaydin et al., 2020; Jiritano et al., 2020; Lin and Fang, 2020; Malik et al., 2020). In both the adult and pediatric population, the quality of life is comprised in heart transplant recipients, due to the need to be on lifelong immunosuppression in both the adult and pediatric patient populations (Asleh et al., 2018; Gale et al., 2019; Heeney et al., 2019; Hussain et al., 2021; Mylonas et al., 2022; Nassetta et al., 2022). In the pediatric population, in addition to the challenges with lifelong immunosuppression, there are very unique challenges associated with retransplantation; there is a high risk for retransplant of the heart after a decade of initial transplant due to growth of the pediatric patient (Greenleaf et al., 2016). While the burden of a single transplant on a pediatric patient is challenging, confounding this problem with the need for a retransplant to match the metabolic needs of the growing patients, which makes this situation even more challenging.
The holy grail in the field of cardiac tissue engineering is the ability to bioengineer a complete bioartificial heart (Birla and Williams, 2020). The ability to bioengineer transplantable hearts that are not rejected by the patient will be a life option for thousands of patients across the globe annually (Virani et al., 2021). The human heart is a very complex organ (Docherty, 2005) and there are numerous challenges in accomplishing this milestone. To tackle this challenge, there are many different and parallel strategies being tested. The field of cardiac tissue engineering has been broadly divided into two categories. The first approach, and the more ambitious, is focused on strategies to bioengineer a fully functional transplantable heart (Ott et al., 2008; Lu et al., 2013; Yasui et al., 2014; Hogan et al., 2015; Tao et al., 2015; Noor et al., 2019). This approach is the most ideal from a clinical standpoint and once successful, will lead to an off the shelf option for heart transplant patients. Clearly, this approach is challenging, and many scientific and technological milestones must be met before accomplishing this goal. The approaches for whole heart bioengineering have also been very diverse, ranging from the use of acellular matrices (Ott et al., 2008; Lu et al., 2013) to the use of recent advance in bioprinting methods (Noor et al., 2019).
The second approach is focused on bioengineer components of the hearts, divided based on function to include contractile tissue which include heart muscle (Hogan et al., 2015; Tao et al., 2017; Morrissette-McAlmon et al., 2018; Pena et al., 2018; Rodrigues et al., 2018; Rosellini et al., 2018; Tao et al., 2018; Tijore et al., 2018; Turnbull et al., 2018; Valarmathi et al., 2018; Vozzi et al., 2018; Wu and Guo, 2018; Abbasgholizadeh et al., 2020; Birla, 2020a), ventricles (Yildirim et al., 2007; Lee et al., 2008; Patel and Birla, 2016; Patel et al., 2016; Patel et al., 2017; Li et al., 2018; MacQueen et al., 2018; Patel and Birla, 2018; Lee et al., 2019; Birla, 2020b) and the biological pumps (Mohamed et al., 2015), the electrical tissue to include the Purkinje networks (Tracy et al., 2020) and the vasculature (Marsano et al., 2013; Mehrabi et al., 2020). There are numerous examples in the recent literature that show different technologies that have been adapted for the use in bioengineering cardiac tissue with excellent review articles covering recent state of the art (Williams and Birla, 2020). There are numerous reasons for bioengineering cardiovascular tissue. First, individual components can be assembled to form functional transplantable hearts, a parallel streety to bioengineering whole hearts. Second, in cases of acute myocardial dysfunction, where a heart transplant is not needed, bioengineered heart muscle tissue could be used to support myocardial dysfunction. Such a therapeutic strategy can be used in combination with currently used beta blockers and angiotensin converting enzyme (Piacentini et al., 2007) inhibitors. Third, bioengineer cardiovascular tissue can be developed and used for high throughput drug screening and/or cardiotoxicity testing. Forth, the lesions learnt in fabricating functional cardiovascular tissue can be translated to whole heart bioengineering.
While there are two major categories in the field of cardiac tissue engineering, as described earlier, there are other avenues that are being pursued. For example, there has been much interest in developing spheroids and other organoid models that can be used for high throughput drug screening (Hribar et al., 2015; Behroodi et al., 2020; Feng et al., 2020; Hong and Song, 2021). These spheroids and organoids can be viewed as a small conglomerate of cells, that have been fabricated in a 3D configuration. The diameter of the spheroids is in the range of a couple hundred microns and contains a couple hundred cells per spheroid. These spheroids are being designed as potential tools for high throughput screening for cardiotoxicity testing as they can maintained in a 96-well or 384-well configuration (Hribar et al., 2015; Behroodi et al., 2020; Feng et al., 2020; Hong and Song, 2021).
The field of cardiac tissue engineering is vast and expanding and only a brief introduction has been presented in this article; an overview of the field of cardiac tissue engineering has been the focus of several recent review articles (Boroumand et al., 2021; Gisone et al., 2022; Roacho-Perez et al., 2022; Zhuang et al., 2022). In this article, we review recent advances in one specific aspect of the field, biological pumps. In recent years, there have been several excellent review articles in the field of biological pumps, though most of them have been very narrow in focus and covering cell sheet engineering methods (Sekine et al., 2012; Haraguchi et al., 2014; Sekine et al., 2016; Kobayashi et al., 2019). While cell sheet engineering has been a very important development and one that has moved the field of forward, recent work in this space has expanded to cover many different fabrication technologies. The purpose of this review article is to provide an overview of the field of biological pumps, to include cell sheet engineering and other recent methods in the field.
The concept of a biological pump is not one that is obvious. This is in comparison to other areas in the field of cardiac tissue engineering, for example, like cardiac patches, that are easier to understand, have an extensive publication record (Cho et al., 2022) and a clear clinical application; for example, bioengineered cardiac patches are designed to increase the lost functional of heart muscle tissue after acute myocardial infarction (Jackman et al., 2015; Jackman et al., 2018). However, there are not very many publications related to biological pumps and the clinical applications are not obvious. Prior to a discussion of biological pumps, a brief overview of cardiac patches will provide the necessary framework. Cardiac patches are planer 3D tissue constructs fabricated by culturing contractile cardiomyocytes within a biologically active hydrogel or another biomaterial, Figure 2A. Functional integration at the host-material results coupling of the cardiomyocytes with the functional sites on the biomaterial fibers and support formation of functional 3D heart muscle. These tissue patches are contractile, and the functional performance is measured by quantification of the twitch force of contraction. The objective is to fabricate 3D cardiac patches that are similar in form and function to mammalian heart muscle tissue. Most of the field, if not all, is devoted to developing new technologies to bridge the gap between bioengineered and mammalian heart muscle tissue (Cho et al., 2022).
The transition from a 3D planar patch to a bioartificial heart is one that is faced with challenges, and not a simple jump from the former to the later. The transition is also not a linear, with several developmental milestones along the pathway. Several enabling technologies are critical to move this field forward, including advancements in valve bioengineering, development of vasculature and electrical conduction system and the fabrication of the complex geometry of left and right atrium and ventricles. The transition from 3D cardiac patches to whole hearts also represents a transition in functional metrics, from twitch force of contraction to left ventricle pressure, or in simpler terms, from contraction to intraluminal pressure.
The concept of biological pumps was developed to facilitate the transition from 3D cardiac patches to bioartificial hearts and provide an intermediate step in the developmental platform. The early thinking was to develop technology “along the way” or “during transition” that can provide insight into the building blocks required for whole heart bioengineering. The initial concept was to develop a 3D tissue construct that can transition from contractility as the key performance metric to intraluminal pressure, more indicative of the functional metrics necessary for whole hearts. During the initial stages of development, biological pumps were developed as a tool to understand the alignment and orientation of contractile cardiomyocytes with 3D scaffolds in a tubular configuration and the subsequent requirements to support “pressure generation” as opposed to contractility measures in 3D patches.
The field of biological pumps initially evolved to support the transition from planar 3D patches to pulsatile pumps and as a transition to whole hearts. However, as the field of biological pumps developed, it became clear there was many applications for biological pumps as a standalone technology. Biological pumps are now being developed as Fontan pumps to provide active support during the Fontan circulation, as biological assist devices and for high throughput cardiotoxicity testing, all of which are discussed here.
The Fontan circulation is particularly relevant during the surgical management of congenital patients with hypoplastic left heart syndrome (HLHS) (Hoashi et al., 2020; Chowdhury et al., 2022). HLHS is a condition in which neonates are born with a missing or underdeveloped left ventricle (Roeleveld et al., 2018; Bejjani et al., 2021; Birla et al., 2022; Jacobs, 2022). This condition is fatal is not aggressively managed at the time of birth. Patients with HLHS are managed through a series of three very complex timed surgeries. The Norwood operation is typically performed 1–2 weeks after birth, the Glenn operation is typically performed 4–6 months after birth and the Fontan operation is performed 3–4 years after birth. During the Fontan operation, the inferior vena cava is separate from the heart and connected to the pulmonary artery using an inert Gore-Tex conduit (Roeleveld et al., 2018; Arunamata et al., 2020; Danton, 2021; King et al., 2022). This conduit only serves to provide a length of inert tubing to support blood flow and does not have a contractile function (Hoashi et al., 2020; Daley and d'Udekem, 2021; Lee et al., 2007; Ochiai et al., 2009; Perez-Caballero et al., 2022). However, replacing this inert conduct with a pulsatile biological pump will add additional functionality; the pulsatile activity of the pump can be used to support the flow of blood through the interior vena cava to the pulmonary artery.
A second potential application of biological pumps is a biological left ventricular assist device (LVADs). LVADs are mechanical pumps that are used to support failing hearts by pumping blood directly from the left ventricle to the aorta. These devices are used extensively as a bridge to transplantation and also as destination therapy, for long-term applications of LVADs to support failing hearts. However, LVADs have been faced with challenges. The interface between the LVAD and host heart is non-functional, as LVADs are mechanical devices with no biological components and do not functionally integrate with the host myocardium. In addition, while LVADs are used the patients has to be on anticoagulation therapy, with a unique set of challenges. A biological pump, once it meets the functional requirements, will be able to alleviate the challenges. Biological pumps can be used as biological LVAD and provide the functional support needed, and also provide a biological interface with the host tissue.
Biological pumps can also be used a functional in vitro assay for cardiotoxicity testing. These pumps can be fabricated and maintained in a 6-well configuration and be exposed to different pharmacological agents and the subsequent effect on intraluminal pressure assessed. This will provide a significant advantage over current animal models used to conduct these test as biological pumps can be maintained in culture for extended periods of time compared to hours when rodent hearts are used. In addition, as these biological pumps are fabricated using human iPSC-CMs, all ethical challenges related to animal testing is removed.
These applications are very important, and each can have a very significant implications that can impact the quality of care for heart failure patients. While the field of biological pumps has evolved significantly over the past decade, there are very few review articles highlighting this work, the focus of this article. This article will provide an overview of the current state of the art in biological pumps and provide insight into the scientific challenges that need to be overcome to move this field forward.
The goal of biological pumps is to support left ventricle output from the human heart and must satisfy rigorous functional performance metrics. At minimum, any biological pump should generate intraluminal pressures in excess of 120 mmHg. In addition, the inner surface of the biological pump must be lined with endothelial cells while the outer layers of cardiomyocytes (CMs) must be thick and vascularized. The outer layer of musculature must be fabricated using billions of mature patient specific induced pluripotent stem cell derived cardiomyocytes (iPSC-CMs). The biological pump must withstand millions of repetitive contractions and therefore, has to be fabricated using mechanically strong and yet compliant materials. Unidirectional valves must be incorporated at both ends to support unidirectional blood flow. Synchronous contractions of the biological pumps with the host heart are also another challenge, one that will require both sensing the host contractions and functioning in a synchronized manner. While all these requirements are necessary for a biological pump that can be used in the human body, no such pump has been described in the published literature. However, in the next few sections, we provide an overview of recent advances in biological pumps, which can be viewed as intermediate development steps, on the way to the ideal biological pump.
In this section, we describe a generic process to bioengineer biological pumps, Figure 1, while in a subsequent section, we describe published methods based on this process. Table 1 provides an overview of some of the recent methods to fabricate biological pumps.
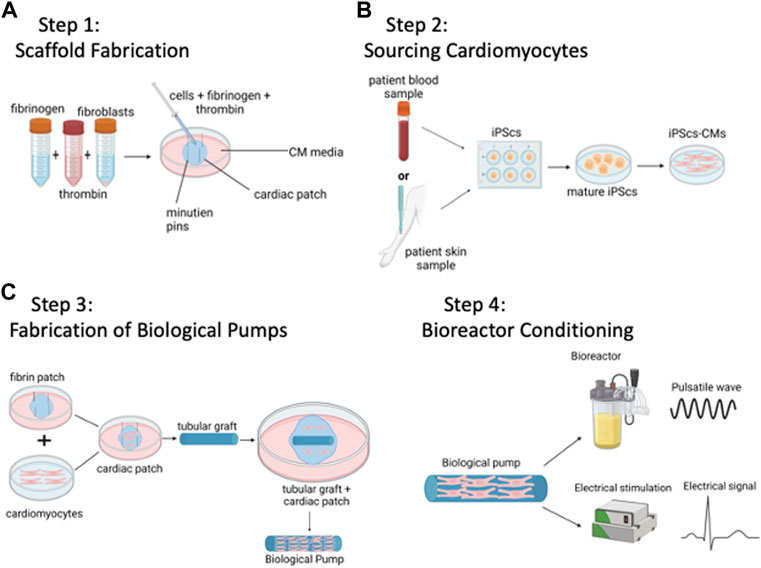
FIGURE 1. Method to Bioengineer Biological Pumps—(A) Step 1: Scaffold Fabrication. (B) Step 2: Sourcing Cardiomyocytes. (C) Step 3: Fabrication of Biological Pumps. (D) Step 4: Bioreactor Conditioning. Created with BioRender.com.
The goal is to fabricate a tubular scaffold that will serve as the template for the biological pump, Figure 1A. As with any material-based application, there are both biological and mechanical design constraints. Biologically, any tubular graft must be able to support the attachment, proliferation, and function of cardiomyocytes. Mechanically, the tubular graft must be able to withstand repetitive pulsatile contractions from the functional cardiomyocytes, in other words, functional as a pump without mechanical failure. Examples of materials have used are acellular vascular grafts from large animals, e.g., goat carotid arteries (Mohamed et al., 2015), or tubular grafts that have been bioengineered from polymeric scaffolds, with chitosan being one example (Evers et al., 2011). Generating a tubular scaffold is only one of several configurations that have been tested; another common configuration has been that of a left ventricle (Michas et al., 2022). In this case, the tubular scaffold is replaced with a cone shaped left ventricle, and again, populated with contractile cardiomyocytes. In this case, the goal is to bioengineer a functional human left ventricle, rather than a biological pump. In both cases, the applications are similar, and both biological pumps and left ventricles are designed to recapitulate the pumping function of the heart.
The functional machinery or the powerhouse of the biological pumps, cardiomyocytes provide the repetitive and rhythmic contractions that drive the biological pumps, Figure 1B. During the early development of the field, CMs were isolated from neonatal ventricular rat cardiomyocytes (NVRMs) and used for initial proof of concept studies (Evers et al., 2011). These cells still prove to be an excellent choice for model development and optimization studies. The main advantages of using NVRMs are the ease of availability, low cost and the standard protocols for isolation and culture. This provides a significant advantage in the development of biological pumps as a large supply of cardiomyocytes is required to optimize the contractile platform. Recent studies have been focused on CMs derived from induced pluripotent stem cells, iPSC-CMs (Yildirim et al., 2007; Lee et al., 2008; Patel and Birla, 2016; Patel et al., 2016; Patel et al., 2017; Li et al., 2018; MacQueen et al., 2018; Patel and Birla, 2018; Lee et al., 2019; Tsuruyama et al., 2019). These methods lead to the development of patient specific therapies and/or technologies, often referred to as personalized medicine, and offer significant advantages in terms of patient specificity (Zhang et al., 2009; Hattori and Fukuda, 2012; Lian et al., 2013). However, challenges in working with iPSC-CMs include high costs, and technical expertise required. However, both iPSC-CMs and NVRMs continue to be instrumental in the field of biological pumps. One strategy that has been successfully adopted in this field is the use of NVRMs during early stages of model development, followed by iPSC-CMs once the models are developed to provide patient specific solutions.
The next step is coupling contractile CMs with the tubular scaffold to form functional biological pumps, Figure 1C. This is a challenging task as the number of CMs, seeding density, proportion of different cell types (CMs + fibroblasts) are important parameters that need to be optimized. In addition, cell viability, functional integration at the cell-material interface, intercellular connectivity and formation of electromechanical junctions are important functional parameters that also need measured. Examples of cellularization strategies used to populate tubular grafts with cells include direction injection of isolated CMs (Blan and Birla, 2008), placement of bioengineered heart muscle on the outer (Evers et al., 2011). In addition, a novel 2-stage cellularization strategy has been described (Patel et al., 2016), which consists of direct cell injection followed by anchoring of bioengineered heart muscle on the outer of the graft (Patel et al., 2016).
Bioreactors are custom devices to simulate in vivo physiology during in vitro culture (Birla et al., 2007). The human heart is a complex organ with a myriad of signals that regulate function and physiology; these signals are essentials for cardiomyocytes to maintain differentiated phenotype and for regulation of cardiac output. Biological pumps need to be cultured in custom bioreactors to deliver coupled electromechanical stimulation of iPSC-CMs or NVRMs (Kashiwagi et al., 1998; Akhyari et al., 2002; Campbell and Chandra, 2006; Birla et al., 2007; Birla et al., 2008a; Clause et al., 2009; Salazar et al., 2015a; Salazar et al., 2015b; Abilez et al., 2018; Kaur et al., 2020). In addition, continuous media flow is important to meet the metabolic demands of the biological pumps (Kashiwagi et al., 1998; Akhyari et al., 2002; Campbell and Chandra, 2006; Khait et al., 2008a; Hecker et al., 2008; Clause et al., 2009; Hecker et al., 2009; Abilez et al., 2018; Kaur et al., 2020).
The two cell sources that have been used are primary cardiomyocytes isolated from NVRMs (Khait et al., 2008b) and iPSC-CMs (Zhang et al., 2009; Hattori and Fukuda, 2012; Lian et al., 2013; Tohyama and Fukuda, 2017), Figure 2. The field of biological pumps, and cardiac tissue engineering, started with the use of NVRMs and gradually transitioned to the use of iPSC-CMS. At the time of the inception of the field, iPSC-CMs were not discovered and therefore, not an option for studies; NVRMs were the most practical option during the early stages of the field to support the development of biological pumps. Advantages of NVRMs included ease of availability, low cost, and mature phenotype (Khait et al., 2008b), all of which resulted in outstanding results during the early stages in the development of biological pumps, Figure 2A. NVRMS were particularly advantageous to support the optimization of biological pumps; these studies often required an abundance of cells at high frequency interval, to test the effect of different variables on the formation and function of biological pumps. In addition, the isolation of NVRMS was optimized with standard protocols for isolation and culture (Khait et al., 2008b). These optimization studies were essential to the development of the field and NVRMs provided an excellent tool to support these studies.
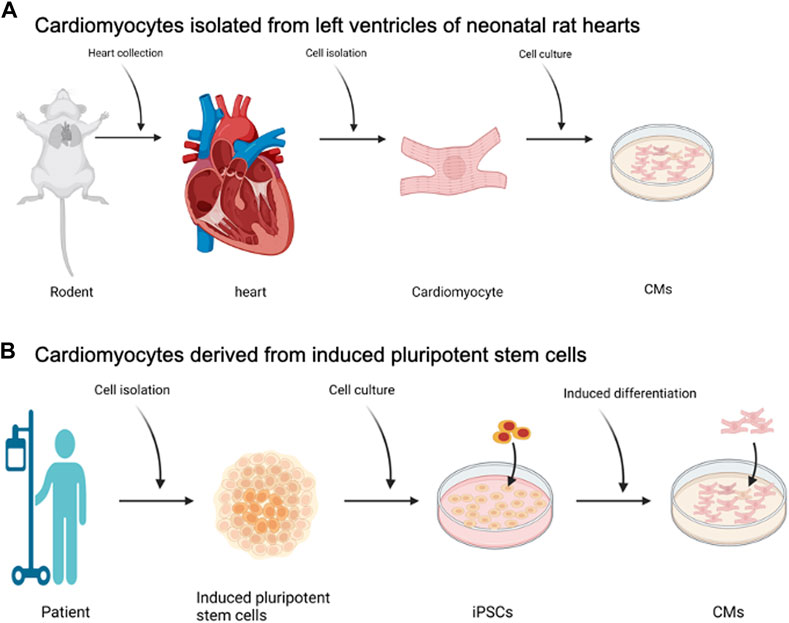
FIGURE 2. Cell Sourcing for Biological Pumps—(A) Cardiomyocytes isolated from left ventricles of neonatal rat hearts, known as NVRMs. (B) Cardiomyocytes derived from induced pluripotent stem cells. Created with BioRender.com.
The advent of iPSC-CMs created a shift away from NVRMs, Figure 2B. The rationale to do so was the patient derived specificity of iPSC-CMs and the translational potential of these cells (Zhang et al., 2009; Hattori and Fukuda, 2012; Lian et al., 2013; Tohyama and Fukuda, 2017). The primary advantage of iPSC-CMs for the fabrication of biological pumps was to develop personalized solutions that are patient centric. For example, a simple blood draw can be used to isolate peripheral blood mononuclear cells (PBMCs) which are then used to generate iPSCs and iPSC-CMs. This way, the iPSC-CMs are patient centric and specific to the patient. These patient centric iPSC-CMs can then be used to fabricate biological pumps. The key advantage of the biological pumps is the patient centric nature, which means that the functional performance of the biological pump will replicate the pathophysiology of the patient. As a simple example, iPSC-CMs and subsequently biological pumps, can be generated from pediatric patients with hypoplastic left heart syndrome (HLHS), a condition in which a patient is born with a missing or underdeveloped left ventricle. When compared against wild type controls, biological pumps fabricated from HLHS patients can provide insight into changes in the functional performance of these hearts.
The use of iPSC-CMs to fabricate biological pumps is the most practical approach; however, it is not without drawbacks. The use of iPSC-CMs comes with very high cost, highly specialized skills to generate and maintain in culture and the infrequent availability of these cells. These challenges are further confounded by the lack of maturity of iPSC-CMS, which require specialized bioreactors for electromechanical stimulation to support a mature phenotype (Ronaldson-Bouchard et al., 2018).
In addition to electromechanical stimulation, chemical conditioning and changes in the microenvironment have shown to play a key role in driving the maturation phenotype of iPSC-CMs (Huang et al., 2020; DePalma et al., 2021). In most cases, the phenotype of iPSC-CMs still remains immature and much needs to be done prior to generating mature iPSC-CMs that can be used to bioengineer highly functional biology pumps. While NVRMs have been used frequently in the development of biological pumps, these cells are also derived from a neonatal source, and the CMs are less mature then CMs derived from adult sources. Nonetheless, while the immature phenotype of both iPSC-CMs and NVRMs remains a challenge in the field, the former is the clear choice for potential clinical applications while the latter are preferred for initial model development and validation studies.
Working with iPSC-CMs presents itself with another challenge, related to the ability to generate large number of cells at a low cost. With NVRMs, production of a large number of cells is generally not a challenge and this produces a significant advantage to justify the use of these cells. This is particularly true in the case of tissue engineering studies, like the fabrication of biological pumps, which requires hundreds of millions of cells per study. Production of hundreds of millions of iPSC-CMs is yet not feasible in most labs, although recent advances in bioreactors have been developed to push the field in this direction (Hamad et al., 2019). In addition, there have been several recent developments that aim to make iPSC technology more affordable and assess by reducing the cost and frequency of media (Kuo et al., 2020) and simplifying the process to generate iPSC-CMs (Lian et al., 2012). In addition, the utilization of iPSC-CMs for production of biological pumps that can be used clinically will require regulatory challenges and a complex FDA approval process. While these challenges are not specific to biological pumps and are universal to the field of iPSCs as a whole, many scientific, technological and regulatory roadblocks need to be overcome to move the field forward.
The field has shifted towards iPSC-CMS and for the most part, abandoned the use of NVRMs. However, given the current state of the field of biological pumps, where pressure generation in these devices has been reported to be under 5 mmHg, there is still an abundance of rigorous optimization studies required. These studies may be better supported using NVRMs, due to the many advantages described earlier. Progress in the field based on NVRMs can then be translated to iPSC-CMs, providing all the advantages associated with these cells, including personalized medicine.
The goal is to fabricate a tubular graft that can be populated with contractile CMs on the outer surface of the graft, Figure 3. There are two main categories of tubular grafts that have been used to fabricate biological pumps, the first based resected samples from rodents, Figure 3A (Sekine et al., 2006) and second, tubular grafts that have been fabricated using molds (Figure 3B) or bioprinting (Figure 3C) (Birla et al., 2008b; Bliley et al., 2022). The result in all cases has been the same, the production of tubular grafts that can support the formation of functional biological pumps.
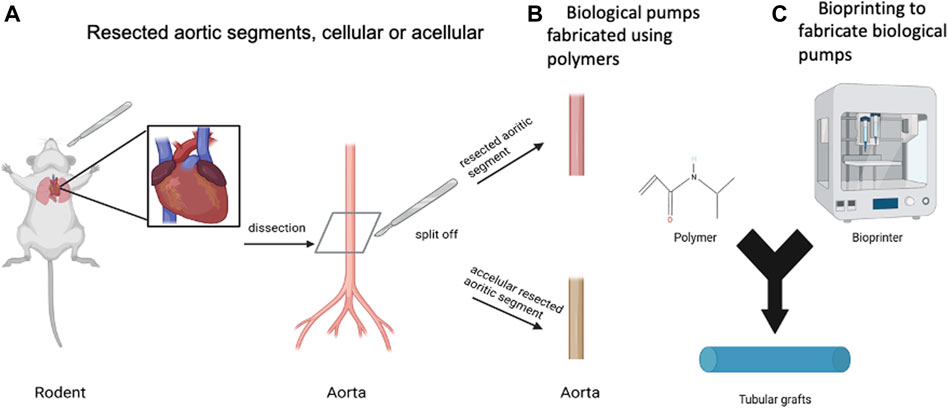
FIGURE 3. Biomaterials for Biological Pumps—(A) Resected aortic segments, cellular or acellular. (B) Biological pumps fabricated using polymers. (C) Bioprinting to fabricate biological pumps. Created with BioRender.com.
The primary function of the tubular graft has been to support the repetitive contractions of the CM layer on the outer surface of the graft. In addition to this mechanical role, the tubular graft also has a biological role, to interface with the CMs to support integrin mediated connectivity between the cells and the matrix. This biological role, though critical, has not been demonstrated in any published studies, thereby reducing the role of the tubular graft to support repetitive contractions resulting from the CMs.
There have been examples on the use of resected rat thoracic aorta from rats (Sekine et al., 2006), acellular rat aorta (Birla et al., 2008b), acellular goat carotid arteries (Mohamed et al., 2015), type I collagen (Bliley et al., 2022) and chitosan (Birla et al., 2008b), all of which were effective in generating biological pumps. In most cases, if not all, the studies have been focused on proof of concept and designed to demonstrate functional biological pumps, thought the intra-luminal pressures were always reported to be low. At such low pressures, it is difficult ascertain differences in the performance of the tubular graft, along with relative differences, which will become more apparent as high-performance pumps are generated. Suffice to say, that many different materials have been used to generate biological pumps, all of which have proven to be effective.
While different methods have been used to bioengineer biological pumps, the most common method has been to first fabricate a cohesive monolayer of contracting CMs, and second, physically anchor this cell monolayer to the outer surface of a tubular graft (Birla et al., 2008b; Seta et al., 2017). This approach has been central to several studies and has proven effective in generating functional pumps. While very simple, this approach has worked and continues to be the mainstay of the field. The challenge has been in the development of a cohesive cell monolayer, as well as anchoring to the surface of the tubular graft. Another advantage of this approach is the ability to anchor multiple layers of cell monolayers on the surface of the tubular grafts, thereby providing a mechanism to increase the function of biological pumps.
More recent studies have focused on bioprinting to fabricate biological pumps, with contracting cardiomyocytes suspended in a type I collagen matrix (Bliley et al., 2022). The primary advantage of bioprinting is the ability to program the STL file, the detailed instructions on how to print the biological pump, and load this onto the bioprinter; the machine does the rest. While an interesting approach, bioprinting is still a niche area limited to a few specialized labs.
As the field of biological pumps has progressed, more recent efforts have focused on the use of micro-fluidics to develop “on-the-chip” models of these pumps (Michas et al., 2022). This innovative approach relies on recapitulating the functional characteristics of biological pumps, which include the pressure-volume relationship. However, the goal is to recreate the functional characteristics in a compact chip configuration, rather than recapitulating the anatomy of the biological pump. The smaller footprint allows for the development of high-throughput screening systems, while maintaining the functionality of biological pumps.
In the previous section, we discussed a general process to bioengineer biological pumps. While this flowchart provides a broad overview of steps that are required to bioengineer biological pumps, not all investigators follow this process, with deviations in the process scheme. Due to the early stage in technology development for biological pumps, many different fabrication methods have been adopted, each with unique advantages and specific challenges. In this section, we provide an overview of many of the methods that have been used to bioengineer biological pumps, Figure 4. This section is designed to provide a survey of current methods to bioengineer biological pumps, and not meant to be an exhaustive list.
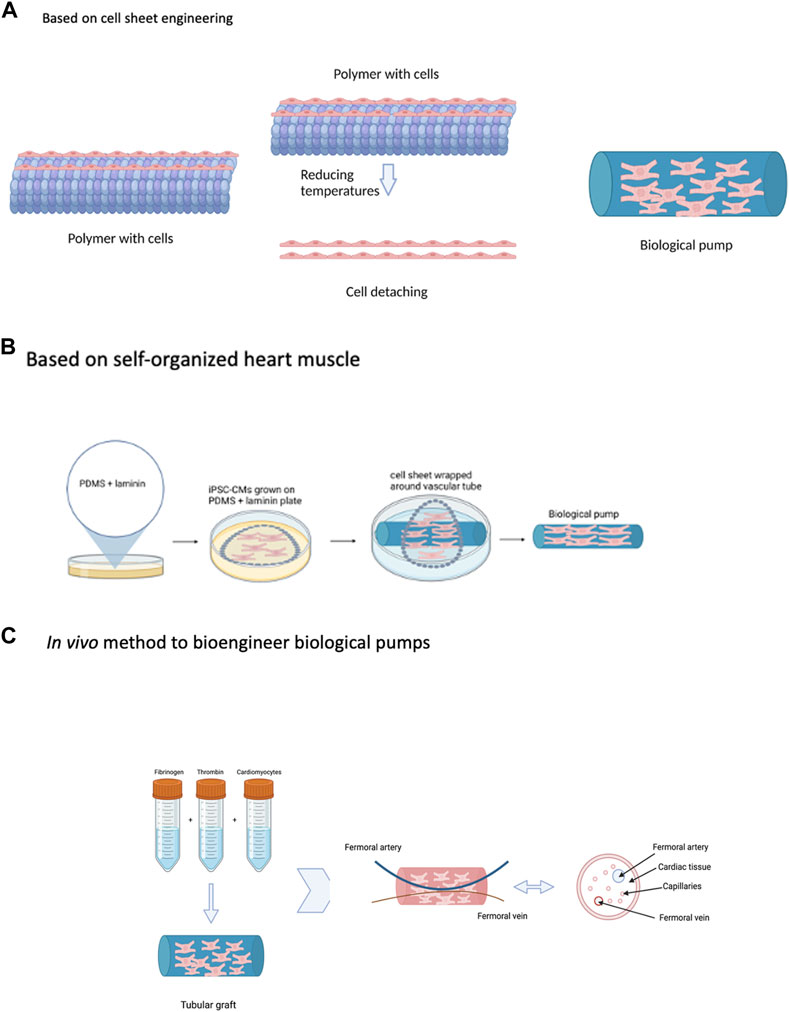
FIGURE 4. Strategies to Bioengineer Biological Pumps—(A) Based on cell sheet engineering. (B) Based on self-organized heart muscle. (C) In vivo method to bioengineer biological pumps. Created with BioRender.com.
This is a novel method to bioengineer pumps and one that has been very influential in the field, Figure 4A (Haraguchi et al., 2014; Sekine et al., 2016; Kobayashi et al., 2019). The central component of this method is the fabrication of sheets of contractile cardiomyocytes, a single layer thick using a novel temperature responsive culture surface (Kobayashi et al., 2019). In this method, tissue culture polystyrene is modified with a thin layer of a temperature response polymer poly(N-isopropylacrylamide) (PIPAAm) (Kobayashi et al., 2019). This modification changes the hydrophobicity of the culture surface as a function of temperature. At 37oC, the temperature at which cells are maintained in an incubator, the PIPAAm coated tissue culture surface is hydrophilic and supports the culture and attachment of cardiomyocytes (Kobayashi et al., 2019). Reduction in the culture temperature to 20oC changes the surface properties of the PIPAAm from hydrophilic to hydrophobic, resulting in detachment of the cell monolayer (Kobayashi et al., 2019). The unique property of cell sheet engineering, is that the entire cell monolayer detaches as a continuous sheet, maintaining intercellular connectivity. In addition, individual sheets can be stacked to form multi-layer heart muscle tissue, with thickness of two and even three cell layers (Kobayashi et al., 2019). Applied to the fabrication of biological pumps, cell sheets were first generated using primary cardiomyocytes isolated from neonatal rat hearts and cultured on the surface of temperature responsive polymers (Sekine et al., 2006). The resulting cell sheets were physically wrapped around the external surface of thoracic aorta resected from adult rats; a total of 6 individual sheets were layered around the tubular graft (Sekine et al., 2006). Once fabricated, the biological pump was transplanted in place of the abdominal aorta, also in adult rats, for a period of 4 weeks (Sekine et al., 2006). Upon explantation, the biological pumps generated intraluminal pressures more than 5 mmHg (Sekine et al., 2006). The in vivo environment produced the physiological environment, in terms of pulsatile conditioning and circulating growth factors and cytokines, to support the maturation of biological pumps. This work was extended to and biological pumps were fabricated using iPSC-CMS, a significant advancement over the use of animal derived cells (Tsuruyama et al., 2019).
The primary advantage of cell sheet engineering to fabricate biological pumps is the remarkable simplicity of the approach, leading to a highly effective and functional pump. The system has been tested and validated with both animal derived cardiomyocytes and iPSC-CMs, providing flexibility in cell sourcing. The ability to generate single layer cell sheets, and cell sheets with two, three and even more layers, provides remarkable customization.
This method is based on a novel method to bioengineer 3D heart muscle tissue, one that was developed based on scaffold free technology, Figure 4B (Baar et al., 2005). In the previous method described, cell sheets were fabricated by culturing primary cardiomyocytes on temperature sensitive surfaces; these cell sheets were formed in the absence of any external scaffolding material and served as the scaffold for pump formation. Self-organized heart muscle is based on the same concept, one that makes use of scaffold free methods to bioengineer heart muscle tissue and then make use of this heart muscle tissue as the substrate to bioengineer functional pumps (Birla et al., 2008b). In the case of self-organized heart muscle, the concentration of the adhesion protein, in this case laminin, was used as the guide to tissue formation (Baar et al., 2005). The tissue culture surface is first coated with PDMS, a hydrophobic surface which does not support cell adhesion and growth (Baar et al., 2005). The PDMS surface is then coated with a fixed concentration of laminin, an adhesion protein used to support the culture and growth of primary cardiomyocytes (Baar et al., 2005). An optimized density of primary cardiomyocytes is seeded on the surface of the PDMS/laminin coated plates (Baar et al., 2005). The cardiomyocytes attach to the surface laminin and form a cohesive cell monolayer (Baar et al., 2005). During the next several days in culture, the surface laminin gradually degrades and exposes the cardiomyocytes to the underlying hydrophobic PDMS surface (Baar et al., 2005). This results in detachment of the cell monolayer from the culture surface and subsequent delamination towards the center of the tissue culture plate (Baar et al., 2005). During the delamination process, a pre-fabricated tubular graft is placed in the center and subsequent attached of the delaminating cell monolayer to the tubular graft results in the formation of a biological pump (Birla et al., 2008b). The self-organized heart muscle forms a continuous monolayer of contracting cardiomyocytes surrounding the external surface of the tubular graft (Birla et al., 2008b).
The novelty of this approach is the use of the self-organized heart muscle as a substrate for the fabrication of pumps; the absence of scaffolding material for heart muscle formation resulted in continuous contractions of isolated cardiomyocytes that are transmitted to the underlying tubular graft. This approach has been further extended with biological pumps fabricated using primary smooth muscle cells and skeletal muscle as well (Evers et al., 2011). The use of smooth muscle cells results in high endurance pumps while the use of skeletal muscle cells resulted in high performance pumps.
While cell sheet engineering and self-organization methods are similar, as they both rely on scaffold free technology, there are subtle differences between the two. In the case of cell sheet engineering, changes in temperature are used to guide the formation of cell sheets, while in the case of self-organization, changes in the contraction of the surface adhesion protein are the driving force. The result of both is the same, to create a physiological environment that supports the formation of a cohesive monolayer of contracting cardiomyocytes. From a standpoint of terminology, cell sheet engineering and self-organization has been retained based on the terminology used in the publications that describe these methods.
Cell sheet engineering and self-organization are two very distinct methods to fabricate biological pumps, as discussed before. The functional performance of biological pumps is similar, based on the magnitude of the intraluminal pressure and both methods leading to low pressures. The results are similar for both methods, and this is due to the infancy of both these technologies. The distinction between the two cannot be ascertained at this early stage, though after further optimization, differences may become more apparent based on cellular organization and electrical activity.
In this method, an STL file was first created using one of many available software platforms (Bliley et al., 2022); an STL file is a 3D rendering of the object that will eventually be printed, in this case a biological pump. The STL file consists of a set of instructions that guide the mechanical components of the bioprinter and the precise movement of the printhead in the x, y and z-direction. The bioprinter itself, is the central component of the process and there are now many commercially available options, though in this case an in-house custom bioprinter was used (Bliley et al., 2022). Most commercial bioprinters, including the custom bioprinter used in this study are extrusion based, which means the bioink formulation is extruded from the printhead using an external force; in the case of most commercial bioprinters, an external air pressure is used, while in this study, a rotating mechanical screw was utilized (Bliley et al., 2022). The bioink itself is another central component of the process, which consists of a mixture of the biomaterial and the cells and for this study, the bioink consisted of a mixture of type I collagen and iPSC-CMs (Bliley et al., 2022). The final component of this system was the support bath, consisting of microparticles of gelatin (Bliley et al., 2022); the support bath is used as a platform to print into and once the print has been completed, is washed away by an elevation in temperature. The purpose of the support bath was to hold the individual fibers in place during the printing process and increase print resolution. Using iPSC-CMs suspended in type I collagen as the bioprinting, loaded onto a custom fabricated bioprinter, with gelatin microparticles as the support matrix, biological pumps were fabricated (Bliley et al., 2022).
Bioprinting is an interesting strategy to bioengineer biological pumps, as it provides precise control over the geometry of the printed object, offering high resolution. Limitations of this approach are the need for specialized equipment and a high degree of training and technical skill.
All three methods described earlier are based on in vitro technologies, which means that all aspects of the process take place in vitro. One of the limitations of all these approaches is the lack of a vasculature, which places an upper limit on the thickness of the cardiomyocyte layer. To overcome this limitation, a very novel method was developed to incorporate vasculature in the cell layer of the biological pump, Figure 4C (Birla et al., 2005); the most important aspect of this method is the use of an in vivo biochamber to support the fabrication of biological pumps. In other words, the entire process for the fabrication of biological pumps was conducted in vivo; the primary advantage of this was the ability to incorporate a vasculature into the cell layer of the biological pump. In addition, this method was designed in such a way that circulating cytokines and growth factors from the host tissue was utilized to support the development, maturation, and performance of the biological pump (Birla et al., 2005).
NVRMs were suspended in a 3D fibrin gel and placed in the lumen of a silicone tubing, which was then implanted in the groin region of recipient rats (Birla et al., 2005). At the time of implantation, the femoral artery and vein of the host were placed within the silicone tubing, thereby creating a biochamber (Birla et al., 2005). The purpose of the femoral artery and vein was to provide a source for vascularization from the host to the transplanted tissue, provide a source of circulating cytokines and growth factors to promote tissue development and provide a source of pulsatile blood flow to condition the implanted tissue. The implanted tissue was harvested after three weeks, after which time, a biological pump had formed (Birla et al., 2005). The femoral artery and vein were retained as a part of the transplanted tissue, and the transplanted tissue was shown to be vascularized and the intraluminal pressure was reported to be in excess of 2 mmHg (Birla et al., 2005).
The method described in this study is very innovative as it took advantage of an in vivo physiological environment to support the formation of a highly functional biological pump. This was also the first time that biological pumps were fabricated using a completely in vivo system and was a significant advancement in the field. While this novel in vivo system provided a platform for vascularization of biological pumps, it does have limitations. Clearly, an in vivo approach will be used primarily for model development and validation studies and not for any work for patient use. In addition, while the in vivo approach described here does take advantage of a complex myriad of signals observed physiologically, these signals are difficult to separate out; as such, the effect of individual compounds on the functional performance of biological pumps cannot be discerned.
Biological pumps represent a very important field and one where the full potential has yet to be realized. As a standalone device, or as a part of a larger system designed to simulate components of the heart, biological pumps serve an integral component of cardiovascular tissue engineering and represent a cornerstone in the field. While much progress has been made in the field, the functional performance of biological pumps remains low, reported to be less than 5 mmHg; future research needs to target this challenge.
The field has progressed mostly based on iPSC-CMs, while some of the earlier work was conducted using NVRMs. For the field to move forward, a dual strategy needs to be adopted, one which includes the use of NVRMs, as these cells provide a valuable tool for model development and validation. In addition to this, simultaneous work needs to be conducted in generating mature iPSC-CMs, a challenge that is not specific to biological pumps, but the field of stem cell engineering. While progress has been made, using a variety of techniques, including the use of electromechanical stimulation, these methods still do not produce cells with a high degree of maturity and not in the numbers required for the fabrication of biological pumps. In addition, the use of specialized equipment, which is often very expensive, excludes most researchers from using this technology. Furthermore, chamber specific iPSC-CMs exhibit very different functional metrics, for example, with ventricular CMs exhibiting higher forces than atrial CMs (Kane and Terracciano, 2017). Biological pumps fabricated from chamber specific iPSC-CMs are expected to generate very different functional metrics, correlating with the functional metrics of the CMs. This provides a powerful tool to use chamber specific biological pumps to interrogate developmental cues during cardiogenesis and to recapitulate differential functional response of different regions of the heart (ventricle vs. atria).
The scaffolds that have been tested to fabricate biological pumps have been limited; resected aortas, either acellular or with cells, or even the use of chitosan as a scaffold, have not proven to be ideal scaffolds for biological pumps. An ideal scaffold would be one that functionally interacts with the outer cardiomyocytes via integrin mediated signaling and that can withstand repetitive contractions over millions of cycles. These criteria have not been met with any of the published scaffolds and is a major challenge that needs to be addressed.
The development of bioreactors is essential for the culture of biological pumps, which are currently maintained in a static environment. Bioreactors are devices designed to simulate the complex in vivo milieu during in vitro culture and in the case of biological pumps, will consists of coupled electromechanical stimulation. Due to the complexities in bioreactor development and design, only a select few labs can implement this technology in the process development.
SB prepared the section on Fabrication Methods for Biological Pumps, PJ prepared the section on Biomaterials for Biological Pumps, AB prepared the introduction and the section on what exactly are biological pumps? RB prepared the section on Methodology to Bioengineer Cell Based Biological Pumps and Published Methods to Fabricate Biological Pumps, CC prepared the section on Summary and Future Perspective, SK prepared the section on Cell Sourcing for Biological Pumps. SB and PJ prepared the Figures. All authors have supported editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbasgholizadeh, R., Islas, J. F., Navran, S., Potaman, V. N., Schwartz, R. J., and Birla, R. K. (2020). A highly conductive 3D cardiac patch fabricated using cardiac myocytes reprogrammed from human adipogenic mesenchymal stem cells. Cardiovasc Eng. Technol. 11 (2), 205–218. Epub 2020/01/10PubMed PMID: 31916039. doi:10.1007/s13239-019-00451-0
Abilez, O. J., Tzatzalos, E., Yang, H., Zhao, M. T., Jung, G., Zollner, A. M., et al. (2018). Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells 36 (2), 265–277. Epub 2017/11/01PubMed PMID: 29086457; PMCID: PMC5785460. doi:10.1002/stem.2732
Akhyari, P., Fedak, P. W., Weisel, R. D., Lee, T. Y., Verma, S., Mickle, D. A., et al. (2002). Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation 106, I137–I142. Epub 2002/10/02. PubMed PMID: 12354723. doi:10.1161/01.cir.0000032893.55215.fc
Alyaydin, E., Welp, H., Reinecke, H., and Tuleta, I. (2020). Predisposing factors for late mortality in heart transplant patients. Cardiol. J. 28, 746–757. Epub 2020/02/14. doi:10.5603/CJ.a2020.0011
Arunamata, A., Tacy, T. A., Kache, S., Mainwaring, R. D., Ma, M., Maeda, K., et al. (2020). Recent outcomes of the extracardiac Fontan procedure in patients with hypoplastic left heart syndrome. Ann. Pediatr. Cardiol. 13 (3), 186–193. Epub 20200629PubMed PMID: 32863652; PMCID: PMC7437630. doi:10.4103/apc.APC_5_20
Asleh, R., Briasoulis, A., Kremers, W. K., Adigun, R., Boilson, B. A., Pereira, N. L., et al. (2018). Long-term sirolimus for primary immunosuppression in heart transplant recipients. J. Am. Coll. Cardiol. 71 (6), 636–650. PubMed PMID: 29420960. doi:10.1016/j.jacc.2017.12.005
Baar, K., Birla, R., Boluyt, M. O., Borschel, G. H., Arruda, E. M., and Dennis, R. G. (2005). Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 19 (2), 1–21. Epub 2004/12/03PubMed PMID: 15574489. doi:10.1096/fj.04-2034fje
Behroodi, E., Latifi, H., Bagheri, Z., Ermis, E., Roshani, S., and Salehi Moghaddam, M. (2020). A combined 3D printing/CNC micro-milling method to fabricate a large-scale microfluidic device with the small size 3D architectures: An application for tumor spheroid production. Sci. Rep. 10 (1), 22171. Epub 20201217PubMed PMID: 33335148; PMCID: PMC7747638. doi:10.1038/s41598-020-79015-5
Bejjani, A. T., Wary, N., and Gu, M. (2021). Hypoplastic left heart syndrome (HLHS): Molecular pathogenesis and emerging drug targets for cardiac repair and regeneration. Expert Opin. Ther. Targets 25 (8), 621–632. Epub 20210915PubMed PMID: 34488532. doi:10.1080/14728222.2021.1978069
Birla, R. K., and Williams, S. K. (2020). 3D bioprinting and its potential impact on cardiac failure treatment: An industry perspective. Apl. Bioeng. 4 (1), 010903. Epub 2020/02/26. doi:10.1063/1.5128371
Birla, R. K., Borschel, G. H., Dennis, R. G., and Brown, D. L. (2005). Myocardial engineering in vivo: Formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Eng. 11 (5-6), 803–813. Epub 2005/07/07PubMed PMID: 15998220. doi:10.1089/ten.2005.11.803
Birla, R. K., Huang, Y. C., and Dennis, R. G. (2007). Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng. 13 (9), 2239–2248. Epub 2007/06/26PubMed PMID: 17590151. doi:10.1089/ten.2006.0359
Birla, R. K., Huang, Y. C., and Dennis, R. G. (2008). Effect of streptomycin on the active force of bioengineered heart muscle in response to controlled stretch. Vitro Cell Dev. Biol. Anim. 44 (7), 253–260. Epub 2008/06/24PubMed PMID: 18568374. doi:10.1007/s11626-008-9114-0
Birla, R. K., Dow, D. E., Huang, Y. C., Migneco, F., Khait, L., Borschel, G. H., et al. (2008). Methodology for the formation of functional, cell-based cardiac pressure generation constructs in vitro. Vitro Cell Dev. Biol. Anim. 44 (8-9), 340–350. Epub 2008/05/22PubMed PMID: 18493826. doi:10.1007/s11626-008-9098-9
Birla, R. K., Dhawan, V., Dow, D. E., Huang, Y. C., and Brown, D. L. (2009). Cardiac cells implanted into a cylindrical, vascularized chamber in vivo: Pressure generation and morphology. Biotechnol. Lett. 31 (2), 191–201. Epub 2008/10/16PubMed PMID: 18854950. doi:10.1007/s10529-008-9859-2
Birla, A. K., Brimmer, S., Short, W. D., Olutoye, O. O., Shar, J. A., Lalwani, S., et al. (2022). Current state of the art in hypoplastic left heart syndrome. Front. Cardiovasc. Med. 9, 878266. doi:10.3389/fcvm.2022.878266
Birla, R. K. (2020). A methodological nine-step process to bioengineer heart muscle tissue. Tissue Cell 67, 101425. Epub 2020/08/28PubMed PMID: 32853859. doi:10.1016/j.tice.2020.101425
Birla, R. K. (2020). Current state of the art in ventricle tissue engineering. Front. Cardiovasc Med. 7, 591581. Epub 2020/11/27PubMed PMID: 33240941; PMCID: PMC7669614. doi:10.3389/fcvm.2020.591581
Blan, N. R., and Birla, R. K. (2008). Design and fabrication of heart muscle using scaffold-based tissue engineering. J. Biomed. Mater Res. A 86 (1), 195–208. Epub 2007/11/01PubMed PMID: 17972281. doi:10.1002/jbm.a.31642
Bliley, J., Tashman, J., Stang, M., Coffin, B., Shiwarski, D., Lee, A., et al. (2022). FRESH 3D bioprinting a contractile heart tube using human stem cell-derived cardiomyocytes. Biofabrication 14 (2). Epub 20220316PubMed PMID: 35213846. doi:10.1088/1758-5090/ac58be
Boroumand, S., Haeri, A., Nazeri, N., and Rabbani, S. (2021). Review insights in cardiac tissue engineering: Cells, scaffolds, and pharmacological agents. Iran. J. Pharm. Res. 20 (4), 467–496. PubMed PMID: 35194460. doi:10.22037/IJPR.2021.114730.15012
Campbell, K. B., and Chandra, M. (2006). Functions of stretch activation in heart muscle. J. Gen. Physiol. 127 (2), 89–94. Epub 2006/02/01PubMed PMID: 16446501. doi:10.1085/jgp.200509483
Cho, S., Discher, D. E., Leong, K. W., Vunjak-Novakovic, G., and Wu, J. C. (2022). Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat. Methods 19, 1064–1071. Epub 20220905PubMed PMID: 36064773. doi:10.1038/s41592-022-01591-3
Chowdhury, U. K., George, N., Sankhyan, L. K., Pradeep, D., Chittimuri, C., Chauhan, A., et al. (2022). Fontan failure: Phenotypes, evaluation, management, and future directions. Cardiol. Young 32 (10), 1554–1563. Epub 20220622PubMed PMID: 35730178. doi:10.1017/S1047951122001433
Clause, K. C., Tinney, J. P., Liu, L. J., Keller, B. B., and Tobita, K. (2009). Engineered early embryonic cardiac tissue increases cardiomyocyte proliferation by cyclic mechanical stretch via p38-MAP kinase phosphorylation. Tissue Eng. Part A 15 (6), 1373–1380. Epub 2009/02/07PubMed PMID: 19196150. doi:10.1089/ten.tea.2008.0169
Daley, M., and d'Udekem, Y. (2021). The optimal Fontan operation: Lateral tunnel or extracardiac conduit? J. Thorac. Cardiovasc Surg. 162 (6), 1825–1834. Epub 20201228PubMed PMID: 33581907. doi:10.1016/j.jtcvs.2020.11.179
Danton, M. H. D. (2021). Right ventricular remodelling in hypoplastic left heart syndrome following Fontan completion. Eur. J. Cardiothorac. Surg. 61 (1), 43–44. PubMed PMID: 34109371. doi:10.1093/ejcts/ezab267
DePalma, S. J., Davidson, C. D., Stis, A. E., Helms, A. S., and Baker, B. M. (2021). Microenvironmental determinants of organized iPSC-cardiomyocyte tissues on synthetic fibrous matrices. Biomater. Sci. 9 (1), 93–107. PubMed PMID: 33325920. doi:10.1039/d0bm01247e
Docherty, B. (2005). The heart: Part one--the anatomy. Nurs. Times 101 (30), 28–29. Epub 2005/08/12. PubMed PMID: 16092283.
Evers, R., Khait, L., and Birla, R. K. (2011). Fabrication of functional cardiac, skeletal, and smooth muscle pumps in vitro. Artif. Organs 35 (1), 69–74. Epub 2010/07/14PubMed PMID: 20618224. doi:10.1111/j.1525-1594.2010.01007.x
Feng, Y., Wang, B., Tian, Y., Chen, H., Liu, Y., Fan, H., et al. (2020). Active fluidic chip produced using 3D-printing for combinatorial therapeutic screening on liver tumor spheroid. Biosens. Bioelectron. 151, 111966. Epub 20191226PubMed PMID: 31999576. doi:10.1016/j.bios.2019.111966
Gale, S. E., Ravichandran, B., Ton, V. K., Pham, S., and Reed, B. N. (2019). Alemtuzumab induction versus conventional immunosuppression in heart transplant recipients. J. Cardiovasc Pharmacol. Ther. 24 (5), 435–441. PubMed PMID: 31035777. doi:10.1177/1074248419841635
Gisone, I., Cecchettini, A., Ceccherini, E., Persiani, E., Morales, M. A., and Vozzi, F. (2022). Cardiac tissue engineering: Multiple approaches and potential applications. Front. Bioeng. Biotechnol. 10, 980393. Epub 20221003PubMed PMID: 36263357; PMCID: PMC9574555. doi:10.3389/fbioe.2022.980393
Greenleaf, C. E., Urencio, J. M., Salazar, J. D., and Dodge-Khatami, A. (2016). Hypoplastic left heart syndrome: Current perspectives. Transl. Pediatr. 5 (3), 142–147. Epub 2016/10/07PubMed PMID: 27709095. doi:10.21037/tp.2016.05.04
Hamad, S., Derichsweiler, D., Papadopoulos, S., Nguemo, F., Saric, T., Sachinidis, A., et al. (2019). Generation of human induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer and scalable 3D suspension bioreactor cultures with reduced batch-to-batch variations. Theranostics 9 (24), 7222–7238. Epub 20190925PubMed PMID: 31695764. doi:10.7150/thno.32058
Haraguchi, Y., Shimizu, T., Matsuura, K., Sekine, H., Tanaka, N., Tadakuma, K., et al. (2014). Cell sheet technology for cardiac tissue engineering. Methods Mol. Biol. 1181, 139–155. PubMed PMID: 25070334. doi:10.1007/978-1-4939-1047-2_13
Hattori, F., and Fukuda, K. (2012). Strategies for replacing myocytes with induced pluripotent stem in clinical protocols. Transpl. Rev. Orl. 26 (3), 223–232. Epub 2011/12/17PubMed PMID: 22172453. doi:10.1016/j.trre.2011.09.003
Hecker, L., Khait, L., Radnoti, D., and Birla, R. (2008). Development of a microperfusion system for the culture of bioengineered heart muscle. ASAIO J. 54 (3), 284–294. Epub 2008/05/23PubMed PMID: 18496279. doi:10.1097/MAT.0b013e31817432dc
Hecker, L., Khait, L., Radnoti, D., and Birla, R. (2009). Novel bench-top perfusion system improves functional performance of bioengineered heart muscle. J. Biosci. Bioeng. 107 (2), 183–190. Epub 2009/02/17PubMed PMID: 19217558. doi:10.1016/j.jbiosc.2008.09.019
Heeney, S. A., Tjugum, S. L., Corkish, M. E., and Hollis, I. B. (2019). Safety and tolerability of high-intensity statin therapy in heart transplant patients receiving immunosuppression with tacrolimus. Clin. Transpl. 33 (1), e13454. PubMed PMID: 30485535. doi:10.1111/ctr.13454
Hoashi, T., Shimada, M., Imai, K., Komori, M., Kurosaki, K., Ohuchi, H., et al. (2020). Long-term therapeutic effect of Fontan conversion with an extracardiac conduit. Eur. J. Cardiothorac. Surg. 57 (5), 951–957. PubMed PMID: 31883324. doi:10.1093/ejcts/ezz355
Hogan, M., Mohamed, M., Tao, Z. W., Gutierrez, L., and Birla, R. (2015). Establishing the framework to support bioartificial heart fabrication using fibrin-based three-dimensional artificial heart muscle. Artif. Organs 39 (2), 165–171. Epub 2014/05/21PubMed PMID: 24841763. doi:10.1111/aor.12318
Hong, S., and Song, J. M. (2021). A 3D cell printing-fabricated HepG2 liver spheroid model for high-content in situ quantification of drug-induced liver toxicity. Biomater. Sci. 9 (17), 5939–5950. Epub 20210727PubMed PMID: 34318795. doi:10.1039/d1bm00749a
Hribar, K. C., Finlay, D., Ma, X., Qu, X., Ondeck, M. G., Chung, P. H., et al. (2015). Nonlinear 3D projection printing of concave hydrogel microstructures for long-term multicellular spheroid and embryoid body culture. Lab. Chip 15 (11), 2412–2418. Epub 20150422PubMed PMID: 25900329. doi:10.1039/c5lc00159e
Huang, C. Y., Peres Moreno Maia-Joca, R., Ong, C. S., Wilson, I., DiSilvestre, D., Tomaselli, G. F., et al. (2020). Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J. Mol. Cell Cardiol. 138, 1–11. Epub 20191023PubMed PMID: 31655038. doi:10.1016/j.yjmcc.2019.10.001
Hussain, T., Nassetta, K., O'Dwyer, L. C., Wilcox, J. E., and Badawy, S. M. (2021). Adherence to immunosuppression in adult heart transplant recipients: A systematic review. Transpl. Rev. Orl. 35 (4), 100651. Epub 20210920PubMed PMID: 34592641. doi:10.1016/j.trre.2021.100651
Jackman, C. P., Shadrin, I. Y., Carlson, A. L., and Bursac, N. (2015). Human cardiac tissue engineering: From pluripotent stem cells to heart repair. Curr. Opin. Chem. Eng. 7, 57–64. Epub 2015/01/20PubMed PMID: 25599018. doi:10.1016/j.coche.2014.11.004
Jackman, C. P., Ganapathi, A. M., Asfour, H., Qian, Y., Allen, B. W., Li, Y., et al. (2018). Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials 159, 48–58. Epub 2018/01/09PubMed PMID: 29309993. doi:10.1016/j.biomaterials.2018.01.002
Jacobs, M. L. (2022). Early history and evolution of surgical therapies for HLHS. World J. Pediatr. Congenit. Heart Surg. 13 (5), 556–558. PubMed PMID: 36053098. doi:10.1177/21501351221115633
Jiritano, F., Coco, V. L., Matteucci, M., Fina, D., Willers, A., and Lorusso, R. (2020). Temporary mechanical circulatory support in acute heart failure. Card. Fail Rev. 6, 1–7. Epub 2020/04/08. doi:10.15420/cfr.2019.02
Kane, C., and Terracciano, C. M. N. (2017). Concise review: Criteria for chamber-specific categorization of human cardiac myocytes derived from pluripotent stem cells. Stem Cells 35 (8), 1881–1897. Epub 20170627PubMed PMID: 28577296; PMCID: PMC5575566. doi:10.1002/stem.2649
Kashiwagi, Y., Haneda, T., Osaki, J., Miyata, S., and Kikuchi, K. (1998). Mechanical stretch activates a pathway linked to mevalonate metabolism in cultured neonatal rat heart cells. Hypertens. Res. 21 (2), 109–119. Epub 1998/07/14PubMed PMID: 9661807. doi:10.1291/hypres.21.109
Kaur, S., Shen, X., Power, A., and Ward, M. L. (2020). Stretch modulation of cardiac contractility: Importance of myocyte calcium during the slow force response. Biophys. Rev. 12 (1), 135–142. Epub 2020/01/16PubMed PMID: 31939110. doi:10.1007/s12551-020-00615-6
Khait, L., Hecker, L., Radnoti, D., and Birla, R. K. (2008). Micro-perfusion for cardiac tissue engineering: Development of a bench-top system for the culture of primary cardiac cells. Ann. Biomed. Eng. 36 (5), 713–725. Epub 2008/02/16PubMed PMID: 18274906. doi:10.1007/s10439-008-9459-2
Khait, L., Hecker, L., Blan, N. R., Coyan, G., Migneco, F., Huang, Y. C., et al. (2008). Getting to the heart of tissue engineering. J. Cardiovasc Transl. Res. 1 (1), 71–84. Epub 2008/03/01PubMed PMID: 20559960. doi:10.1007/s12265-007-9005-x
King, G., Buratto, E., Daley, M., Iyengar, A., Alphonso, N., Grigg, L., et al. (2022). Impact of aortic atresia after fontan operation in patients with hypoplastic left heart syndrome. Ann. Thorac. Surg. Epub 20220921PubMed PMID: 36152877. doi:10.1016/j.athoracsur.2022.09.018
Kobayashi, J., Kikuchi, A., Aoyagi, T., and Okano, T. (2019). Cell sheet tissue engineering: Cell sheet preparation, harvesting/manipulation, and transplantation. J. Biomed. Mater Res. A 107 (5), 955–967. Epub 20190221PubMed PMID: 30684395. doi:10.1002/jbm.a.36627
Kohne, M., Behrens, C. S., Studemann, T., Bibra, C. V., Querdel, E., Shibamiya, A., et al. (2022). A potential future fontan modification: Preliminary in vitro data of a pressure-generating tube from engineered heart tissue. Eur. J. Cardiothorac. Surg. 62 (2), ezac111. PubMed PMID: 35218664; PMCID: PMC9373941. doi:10.1093/ejcts/ezac111
Kubo, H., Shimizu, T., Yamato, M., Fujimoto, T., and Okano, T. (2007). Creation of myocardial tubes using cardiomyocyte sheets and an in vitro cell sheet-wrapping device. Biomaterials 28 (24), 3508–3516. Epub 20070418PubMed PMID: 17482255. doi:10.1016/j.biomaterials.2007.04.016
Kuo, H. H., Gao, X., DeKeyser, J. M., Fetterman, K. A., Pinheiro, E. A., Weddle, C. J., et al. (2020). Negligible-cost and weekend-free chemically defined human iPSC culture. Stem Cell Rep. 14 (2), 256–270. Epub 20200109PubMed PMID: 31928950. doi:10.1016/j.stemcr.2019.12.007
Lee, C., Lee, C. H., Hwang, S. W., Lim, H. G., Kim, S. J., Lee, J. Y., et al. (2007). Midterm follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur. J. Cardiothorac. Surg. 31 (6), 1008–1012. Epub 20070406PubMed PMID: 17419069. doi:10.1016/j.ejcts.2007.03.013
Lee, E. J., Kim, D. E., Azeloglu, E. U., and Costa, K. D. (2008). Engineered cardiac organoid chambers: Toward a functional biological model ventricle. Tissue Eng. Part A 14 (2), 215–225. Epub 2008/03/13PubMed PMID: 18333774. doi:10.1089/tea.2007.0351
Lee, A., Hudson, A. R., Shiwarski, D. J., Tashman, J. W., Hinton, T. J., Yerneni, S., et al. (2019). 3D bioprinting of collagen to rebuild components of the human heart. Science 365 (6452), 482–487. Epub 2019/08/03PubMed PMID: 31371612. doi:10.1126/science.aav9051
Li, R. A., Keung, W., Cashman, T. J., Backeris, P. C., Johnson, B. V., Bardot, E. S., et al. (2018). Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 163, 116–127. Epub 2018/02/21PubMed PMID: 29459321. doi:10.1016/j.biomaterials.2018.02.024
Lian, X., Hsiao, C., Wilson, G., Zhu, K., Hazeltine, L. B., Azarin, S. M., et al. (2012). Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. U. S. A. 109 (27), E1848–E1857. Epub 20120529PubMed PMID: 22645348; PMCID: PMC3390875. doi:10.1073/pnas.1200250109
Lian, X., Zhang, J., Azarin, S. M., Zhu, K., Hazeltine, L. B., Bao, X., et al. (2013). Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 8 (1), 162–175. Epub 2012/12/22PubMed PMID: 23257984. doi:10.1038/nprot.2012.150
Lin, X., and Fang, L. (2020). Pharmaceutical treatment for heart failure. Adv. Exp. Med. Biol. 1177, 269–295. Epub 2020/04/05PubMed PMID: 32246448. doi:10.1007/978-981-15-2517-9_7
Lu, T. Y., Lin, B., Kim, J., Sullivan, M., Tobita, K., Salama, G., et al. (2013). Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 4, 2307. Epub 2013/08/15PubMed PMID: 23942048. doi:10.1038/ncomms3307
MacQueen, L. A., Sheehy, S. P., Chantre, C. O., Zimmerman, J. F., Pasqualini, F. S., Liu, X., et al. (2018). A tissue-engineered scale model of the heart ventricle. Nat. Biomed. Eng. 2 (12), 930–941. Epub 2019/04/25PubMed PMID: 31015723. doi:10.1038/s41551-018-0271-5
Malik, A., Brito, D., and Chhabra, L. (2020). Congestive heart failure (CHF). Treasure Island (FL): StatPearls.
Marsano, A., Maidhof, R., Luo, J., Fujikara, K., Konofagou, E. E., Banfi, A., et al. (2013). The effect of controlled expression of VEGF by transduced myoblasts in a cardiac patch on vascularization in a mouse model of myocardial infarction. Biomaterials 34 (2), 393–401. Epub 2012/10/23PubMed PMID: 23083931. doi:10.1016/j.biomaterials.2012.09.038
Mehrabi, A., Baheiraei, N., Adabi, M., and Amirkhani, Z. (2020). Development of a novel electroactive cardiac patch based on carbon nanofibers and gelatin encouraging vascularization. Appl. Biochem. Biotechnol. 190 (3), 931–948. Epub 2019/10/18PubMed PMID: 31620995. doi:10.1007/s12010-019-03135-6
Michas, C., Karakan, M. C., Nautiyal, P., Seidman, J. G., Seidman, C. E., Agarwal, A., et al. (2022). Engineering a living cardiac pump on a chip using high-precision fabrication. Sci. Adv. 8 (16), eabm3791. Epub 20220422PubMed PMID: 35452278. doi:10.1126/sciadv.abm3791
Mohamed, M. A., Hogan, M. K., Patel, N. M., Tao, Z. W., Gutierrez, L., and Birla, R. K. (2015). Establishing the framework for tissue engineered heart pumps. Cardiovasc Eng. Technol. 6 (3), 220–229. Epub 2015/11/19PubMed PMID: 26577356. doi:10.1007/s13239-015-0211-4
Morrissette-McAlmon, J., Blazeski, A., Somers, S., Kostecki, G., Tung, L., and Grayson, W. L. (2018). Adipose-derived perivascular mesenchymal stromal/stem cells promote functional vascular tissue engineering for cardiac regenerative purposes. J. Tissue Eng. Regen. Med. 12 (2), e962–e972. Epub 2017/01/20PubMed PMID: 28103423. doi:10.1002/term.2418
Mylonas, K. S., Soukouli, I., Avgerinos, D. V., and Boletis, J. N. (2022). Current immunosuppression strategies in pediatric heart transplant. Immunotherapy. Epub 20220504. doi:10.2217/imt-2021-0352
Nassetta, K., Hussain, T., Gambetta, K., Le, K., O'Dwyer, L. C., and Badawy, S. M. (2022). A systematic review of adherence to immunosuppression among pediatric heart transplant patients. J. Cardiovasc Dev. Dis. 9 (5), 165. PubMed PMID: 35621876; PMCID: PMC9145350. doi:10.3390/jcdd9050165
Noor, N., Shapira, A., Edri, R., Gal, I., Wertheim, L., and Dvir, T. (2019). 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. (Weinh). 6 (11), 1900344. Epub 2019/06/11PubMed PMID: 31179230; PMCID: PMC6548966. doi:10.1002/advs.201900344
Ochiai, Y., Imoto, Y., Sakamoto, M., Kajiwara, T., Sese, A., Watanabe, M., et al. (2009). Mid-term follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur. J. Cardiothorac. Surg. 36 (1), 63–68. PubMed PMID: 19329333. doi:10.1016/j.ejcts.2009.02.013
Ott, H. C., Matthiesen, T. S., Goh, S. K., Black, L. D., Kren, S. M., Netoff, T. I., et al. (2008). Perfusion-decellularized matrix: Using nature's platform to engineer a bioartificial heart. Nat. Med. 14 (2), 213–221. Epub 2008/01/15PubMed PMID: 18193059. doi:10.1038/nm1684
Park, J., Anderson, C. W., Sewanan, L. R., Kural, M. H., Huang, Y., Luo, J., et al. (2020). Modular design of a tissue engineered pulsatile conduit using human induced pluripotent stem cell-derived cardiomyocytes. Acta Biomater. 102, 220–230. Epub 20191019PubMed PMID: 31634626. doi:10.1016/j.actbio.2019.10.019
Patel, N. M., and Birla, R. K. (2016). Pulsatile flow conditioning of three-dimensional bioengineered cardiac ventricle. Biofabrication 9 (1), 015003. Epub 2016/12/06PubMed PMID: 27917819. doi:10.1088/1758-5090/9/1/015003
Patel, N. M., and Birla, R. K. (2018). The bioengineered cardiac left ventricle. ASAIO J. 64 (1), 56–62. Epub 2017/09/02PubMed PMID: 28863042. doi:10.1097/MAT.0000000000000642
Patel, N. M., Yazdi, I. K., Tasciotti, E., and Birla, R. K. (2016). Optimizing cell seeding and retention in a three-dimensional bioengineered cardiac ventricle: The two-stage cellularization model. Biotechnol. Bioeng. 113 (10), 2275–2285. Epub 2016/04/14PubMed PMID: 27071026. doi:10.1002/bit.25992
Patel, N. M., Mohamed, M. A., Yazdi, I. K., Tasciotti, E., and Birla, R. K. (2017). The design and fabrication of a three-dimensional bioengineered open ventricle. J. Biomed. Mater Res. B Appl. Biomater. 105 (8), 2206–2217. Epub 2016/07/21PubMed PMID: 27438342. doi:10.1002/jbm.b.33742
Pena, B., Laughter, M., Jett, S., Rowland, T. J., Taylor, M. R. G., Mestroni, L., et al. (2018). Injectable hydrogels for cardiac tissue engineering. Macromol. Biosci. 18 (6), e1800079. Epub 2018/05/08PubMed PMID: 29733514. doi:10.1002/mabi.201800079
Perez-Caballero, R., Pardo, C. A., Marques Correia, P., and Ballesteros, F. (2022). Recurrent infective stenosis of Fontan conduit. Eur. J. Cardiothorac. Surg. 62 (1), ezab562. PubMed PMID: 35018407. doi:10.1093/ejcts/ezab562
Piacentini, G., Marino, B., and Digilio, M. C. (2007). Familial recurrence of discrete membranous subaortic stenosis. J. Thorac. Cardiovasc Surg. 134 (3), 818–819. ; author reply 9. Epub 2007/08/29PubMed PMID: 17723851. doi:10.1016/j.jtcvs.2007.01.095
Roacho-Perez, J. A., Garza-Trevino, E. N., Moncada-Saucedo, N. K., Carriquiry-Chequer, P. A., Valencia-Gomez, L. E., Matthews, E. R., et al. (2022). Artificial scaffolds in cardiac tissue engineering. Life (Basel) 12 (8). Epub 20220725PubMed PMID: 35892919. doi:10.3390/life12081117
Rodrigues, I. C. P., Kaasi, A., Maciel Filho, R., Jardini, A. L., and Gabriel, L. P. (2018). Cardiac tissue engineering: Current state-of-the-art materials, cells and tissue formation. Einstein (Sao Paulo) 16 (3), eRB4538. Epub 2018/10/04PubMed PMID: 30281764; PMCID: PMC6178861. doi:10.1590/S1679-45082018RB4538
Roeleveld, P. P., Axelrod, D. M., Klugman, D., Jones, M. B., Chanani, N. K., Rossano, J. W., et al. (2018). Hypoplastic left heart syndrome: From fetus to fontan. Cardiol. Young 28 (11), 1275–1288. Epub 2018/09/19PubMed PMID: 30223915. doi:10.1017/S104795111800135X
Ronaldson-Bouchard, K., Ma, S. P., Yeager, K., Chen, T., Song, L., Sirabella, D., et al. (2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556 (7700), 239–243. Epub 2018/04/06PubMed PMID: 29618819; PMCID: PMC5895513. doi:10.1038/s41586-018-0016-3
Rosellini, E., Zhang, Y. S., Migliori, B., Barbani, N., Lazzeri, L., Shin, S. R., et al. (2018). Protein/polysaccharide-based scaffolds mimicking native extracellular matrix for cardiac tissue engineering applications. J. Biomed. Mater Res. A 106 (3), 769–781. Epub 2017/10/21PubMed PMID: 29052369; PMCID: PMC5845858. doi:10.1002/jbm.a.36272
Salazar, B. H., Reddy, A. K., Zewei, T., Madala, S., and Birla, R. K. (2015). 32-Channel system to measure the electrophysiological properties of bioengineered cardiac muscle. IEEE Trans. Biomed. Eng. 62 (6), 1614–1622. Epub 2015/02/11PubMed PMID: 25667345. doi:10.1109/TBME.2015.2399437
Salazar, B. H., Cashion, A. T., Dennis, R. G., and Birla, R. K. (2015). Development of a cyclic strain bioreactor for mechanical enhancement and assessment of bioengineered myocardial constructs. Cardiovasc Eng. Technol. 6 (4), 533–545. Epub 2015/11/19PubMed PMID: 26577484; PMCID: PMC4653094. doi:10.1007/s13239-015-0236-8
Sekine, H., Shimizu, T., Yang, J., Kobayashi, E., and Okano, T. (2006). Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation 114, I87–I93. PubMed PMID: 16820651. doi:10.1161/CIRCULATIONAHA.105.000273
Sekine, H., Shimizu, T., and Okano, T. (2012). Myocardial tissue engineering: Toward a bioartificial pump. Cell Tissue Res. 347 (3), 775–782. Epub 20111118PubMed PMID: 22095463. doi:10.1007/s00441-011-1267-6
Sekine, H., Shimizu, T., and Okano, T. (2016). “Cell sheet tissue engineering for heart failure,” in Etiology and morphogenesis of congenital heart disease: From gene function and cellular interaction to morphology. Tokyo: Springer. Editors T. Nakanishi, R. R. Markwald, H. S. Baldwin, B. B. Keller, D. Srivastava, and H. Yamagishi, 19–24.
Seta, H., Matsuura, K., Sekine, H., Yamazaki, K., and Shimizu, T. (2017). Tubular cardiac tissues derived from human induced pluripotent stem cells generate pulse pressure in vivo. Sci. Rep. 7, 45499. Epub 20170330PubMed PMID: 28358136. doi:10.1038/srep45499
Tao, Z. W., Mohamed, M., Hogan, M., Salazar, B., Patel, N. M., and Birla, R. K. (2015). Establishing the framework for fabrication of a bioartificial heart. ASAIO J. 61 (4), 429–436. Epub 2015/05/09PubMed PMID: 25955151. doi:10.1097/MAT.0000000000000233
Tao, Z. W., Mohamed, M., Hogan, M., Gutierrez, L., and Birla, R. K. (2017). Optimizing a spontaneously contracting heart tissue patch with rat neonatal cardiac cells on fibrin gel. J. Tissue Eng. Regen. Med. 11 (1), 153–163. Epub 2014/04/29PubMed PMID: 24771636. doi:10.1002/term.1895
Tao, Z. W., Mohamed, M., Jacot, J. G., and Birla, R. K. (2018). Bioengineering cardiac tissue constructs with adult rat cardiomyocytes. ASAIO J. 64 (5), e105–e14. Epub 2018/03/15. doi:10.1097/MAT.0000000000000765
Tijore, A., Irvine, S. A., Sarig, U., Mhaisalkar, P., Baisane, V., and Venkatraman, S. (2018). Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 10 (2), 025003. Epub 2017/12/14PubMed PMID: 29235444. doi:10.1088/1758-5090/aaa15d
Tohyama, S., and Fukuda, K. (2017). Safe and effective cardiac regenerative therapy with human-induced pluripotent stem cells: How should we prepare pure cardiac myocytes? Circ. Res. 120 (10), 1558–1560. Epub 2017/05/13PubMed PMID: 28495993. doi:10.1161/CIRCRESAHA.116.310328
Tracy, E., Gettler, B., Zakhari, J., Schwartz, R. J., Williams, S. K., and Birla, R. (2020). 3D bioprinting the cardiac Purkinje system using human adipogenic mesenchymal stem cell derived Purkinje cells. Cardiovasc Eng. Technol. 11, 587–604. doi:10.1007/s13239-020-00478-8
Tsuruyama, S., Matsuura, K., Sakaguchi, K., and Shimizu, T. (2019). Pulsatile tubular cardiac tissues fabricated by wrapping human iPS cells-derived cardiomyocyte sheets. Regen. Ther. 11, 297–305. Epub 20191001PubMed PMID: 31667209. doi:10.1016/j.reth.2019.09.001
Turnbull, I. C., Mayourian, J., Murphy, J. F., Stillitano, F., Ceholski, D. K., and Costa, K. D. (2018). Cardiac tissue engineering models of inherited and acquired cardiomyopathies. Methods Mol. Biol. 1816, 145–159. Epub 2018/07/11PubMed PMID: 29987817. doi:10.1007/978-1-4939-8597-5_11
Valarmathi, M. T., Fuseler, J. W., Potts, J. D., Davis, J. M., and Price, R. L. (2018). Functional tissue engineering: A prevascularized cardiac muscle construct for validating human mesenchymal stem cells engraftment potential in vitro. Tissue Eng. Part A 24 (1-2), 157–185. Epub 2017/05/02PubMed PMID: 28457188. doi:10.1089/ten.TEA.2016.0539
Virani, S. S., Alonso, A., Aparicio, H. J., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., et al. (2021). Heart disease and stroke statistics-2021 update: A report from the American heart association. Circulation 143 (8), e254–e743. Epub 20210127PubMed PMID: 33501848. doi:10.1161/CIR.0000000000000950
Vozzi, F., Logrand, F., Cabiati, M., Cicione, C., Boffito, M., Carmagnola, I., et al. (2018). Biomimetic engineering of the cardiac tissue through processing, functionalization, and biological characterization of polyester urethanes. Biomed. Mater 13 (5), 055006. Epub 2018/06/06PubMed PMID: 29869614. doi:10.1088/1748-605X/aaca5b
Williams, S. K., and Birla, R. K. (2020). Tissue engineering solutions to replace contractile function during pediatric heart surgery. Tissue Cell 67, 101452. Epub 2020/11/03PubMed PMID: 33137707. doi:10.1016/j.tice.2020.101452
Wu, Y., and Guo, L. (2018). Enhancement of intercellular electrical synchronization by conductive materials in cardiac tissue engineering. IEEE Trans. Biomed. Eng. 65 (2), 264–272. Epub 2017/10/21PubMed PMID: 29053443. doi:10.1109/TBME.2017.2764000
Yasui, H., Lee, J. K., Yoshida, A., Yokoyama, T., Nakanishi, H., Miwa, K., et al. (2014). Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials 35 (27), 7839–7850. Epub 2014/06/24PubMed PMID: 24952982. doi:10.1016/j.biomaterials.2014.05.080
Yildirim, Y., Naito, H., Didie, M., Karikkineth, B. C., Biermann, D., Eschenhagen, T., et al. (2007). Development of a biological ventricular assist device: Preliminary data from a small animal model. Circulation 116, I16–I23. Epub 2007/09/14PubMed PMID: 17846298. doi:10.1161/CIRCULATIONAHA.106.679688
Zhang, J., Wilson, G. F., Soerens, A. G., Koonce, C. H., Yu, J., Palecek, S. P., et al. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 104 (4), e30–e41. Epub 2009/02/14PubMed PMID: 19213953. doi:10.1161/CIRCRESAHA.108.192237
Keywords: tissue engineering, heart, pump, cardiomycoyte, bioreactor
Citation: Brimmer S, Ji P, Birla AK, Keswani SG, Caldarone CA and Birla RK (2023) Recent advances in biological pumps as a building block for bioartificial hearts. Front. Bioeng. Biotechnol. 11:1061622. doi: 10.3389/fbioe.2023.1061622
Received: 04 October 2022; Accepted: 04 January 2023;
Published: 20 January 2023.
Edited by:
George Alexander Truskey, Duke University, United StatesReviewed by:
Zhen Ma, Syracuse University, United StatesCopyright © 2023 Brimmer, Ji, Birla, Keswani, Caldarone and Birla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ravi K. Birla, cmF2aS5iaXJsYUBiY20uZWR1
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.