
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 08 September 2022
Sec. Nanobiotechnology
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.988607
This article is part of the Research Topic Capping Agents Encapsulated Nanoparticles in Plant Biotechnology View all 5 articles
 Maaz Ahmad
1,2
Maaz Ahmad
1,2
 Ahmad Ali
2
Ahmad Ali
2
 Zahid Ullah
2
Zahid Ullah
2 Hassan Sher2
Hassan Sher2
 Dong-Qin Dai
1*
Dong-Qin Dai
1*
 Mohammad Ali
3
Mohammad Ali
3
 Javed Iqbal
4
Javed Iqbal
4 Muhammad Zahoor5
Muhammad Zahoor5
 Iftikhar Ali
2,6*
Iftikhar Ali
2,6*Nanomaterials are gaining tremendous potential as emerging antimicrobials in the quest to find resistance-free alternatives of chemical pesticides. In this study, stable silver nanoparticles were synthesized using the aqueous extract of medicinal plant species Polygonatum geminiflorum , and their morphological features were evaluated by transmission electron microscopy, X-ray diffraction spectroscopy and energy dispersive X-ray analysis. In vitro Antifungal activity of the synthesized silver nanoparticles (AgNPs) and P. geminiflorum extract (PE) either alone or in combination (PE-AgNPs) against Fusarium oxysporum was evaluated using disc-diffusion and well-diffusion methods. In planta assay of the same treatments against Fusarium wilt diseases of tomato was evaluated by foliar spray method. Moreover, plant extract was evaluated for the quantitative investigation of antioxidant activity, phenolics and flavonoids by spectroscopic and HPLC techniques. Phytochemical analysis indicated the presence of total phenolic and flavonoid contents as 48.32 mg ± 1.54 mg GAE/g and 57.08 mg ± 1.36 mg QE/g, respectively. The DPPH radical scavenging of leaf extract was found to be 88.23% ± 0.87%. Besides, the HPLC phenolic profile showed the presence of 15 bioactive phenolic compounds. Characterization of nanoparticles revealed the size ranging from 8 nm to 34 nm with average crystallite size of 27 nm. The FTIR analysis revealed important functional groups that were responsible for the reduction and stabilization of AgNPs. In the in vitro assays, 100 μg/ml of AgNPs and AgNPs-PE strongly inhibited Fusarium oxysporum. The same treatments tested against Fusarium sprayed on tomato plants in controlled environment exhibited nearly 100% plant survival with no observable phytotoxicity. These finding provide a simple baseline to control Fusarium wilt using silver nano bio-control agents without affecting the crop health.
All over the world, agricultural crops are severely affected by phytopathogenic microbes which cause a variety of diseases and lead to reduction in their overall productivity (Savary et al., 2012; Rafique et al., 2017; Saratale et al., 2018). Disruption of the productivity of important crops due to pathogenic diseases is a serious threat to the global food security (Savary et al., 2012). Different fungi, bacteria and viruses produce toxic substances that are harmful to the health of beneficial life forms, thereby compromising the quality and quantity of crops (Bashir et al., 2018; Gao et al., 2018). Conventionally, synthetic chemicals have been used to control these pathogenic diseases; however, their frequent administration is hazardous for non-targeted organisms (Gardener and Fravel, 2002; Hajek and Eilenberg, 2018). Therefore, the introduction of novel and targeted treatments is needed as a safer and alternative strategy to control plant pathogens (Leon-Buitimea et al., 2020; Avila-Quezada et al., 2021).
The field of nanotechnology, ensuing from the coupling of material science and biotechnology, is focused to develop novel agents for the effective control of phytopathogens (Zhang and Webster, 2009; Saxena et al., 2012). Nanoparticles have tremendous importance due to their various applications in the fields like medicine, biology, chemistry, material science and physics (Albrecht et al., 2006; Thiruvengadam et al., 2006). Chemical and physical methods used for the synthesis of nanoparticles are costly and produce substances that are toxic to the health of living organisms (Yaqoob et al., 202). In contrast, biological synthesis is a simple and viable method that utilizes plants, microorganisms, polysaccharides and enzymes as synthesis substrates (Hebbalalu et al., 2013; Khan et al., 2022).
As nano factories, green plants provide relatively modest, environment-friendly and a faster way for nanoparticles synthesis on large scale (Abou El-Nour et al., 2010). Plant secondary products act as reducing and stabilizing agents to transform metal salts into stable nanoparticles (Kuppusamy et al., 2016). At low concentrations, green synthesized silver nanoparticles have documented antimicrobial activities against plant and human pathogens with no cytotoxicity (Jeong et al., 2005; Sharma et al., 2009; Alam et al., 2018).
Polygonatum Mill is a genus of rhizomatous, perennial, monocot herbs in the family Asparagaceae. The genus is represented by about 75 species in the world, distributed primarily in the temperate and alpine regions of the northern hemisphere, extending into mountains in the subtropical region as well (Chase et al., 2016; Floden and Schilling, 2018). Polygonatum species are characterized by sympodial rhizomes, and opposite to verticillate leaves and fleshy berries (Wang et al., 2022). The subterranean rhizome in many species of the genus have been utilized in traditional as well as modern medicine for the cure of several diseases. Polygonatum species are used as antidiabetic, as coolant, immunostimulatory, to treat respiratory problems (Jiao et al., 2018). Some species (P. cyrtonema, P. sibiricum) are also listed in Chinese Pharmacopoea. Recently it has been found that some species of Polygonatum are rich in proteins and nutrients and can become potential future grain crop (Si and Zhu, 2021. The genus Polygonatum includes important medicinal plants which are utilized for treating several human ailments such as diabetes, vertigo, ringworm and pulmonary problems, and exhibit hepatoprotective and antioxidant activities (Son et al., 1990; Zhao et al., 2018). Moreover, the wide use of Polygonatum species in various traditional systems of medicines as antidiabetic, aphrodisiac, antituberculant, tonic, diuretic etc., has previously been reported (Khan et al., 2013; Suyal et al., 2020; Sharma et al., 2021).
P. geminiflorum Decne. locally known as “Peramole” (Pashto), is an important rhizomatous perennial herb inhabiting temperate coniferous forests, and alpine zone in northern Pakistan’s Western Himalayan and Hindukush Mountain region, extending westward to Afghanistan (Chase et al., 2016). This species is closely related to P. verticillatum and is known to people by the same vernacular names “Noore-Alam and Peramole”. The rhizome of P. geminiflorum as well as P. verticillatum is crushed and fried in wheat flour and given to lactating mothers for increasing milk (Rahman et al., 2022). Khan and Khatoon, (2008) have reported that the local people in northern Pakistan use P. geminiflorum for the treatment of uterine tumor, menstrual abnormalities, gout, and skin diseases. Moreover, Khan and Rauf, (2015) have comprehensively described the phytochemical constituents of different species in the genus, and the associated antimibacterial, antifungal and antioxidant activities. This study has also reported that P. geminiflorum is rich in phenolics, saponins, alkaloides and phytoharmones, and therefore is a high value medicinal plant, like the most commonly explored P. vericillatum. Till date, no published report is available on the synthesis of AgNPs using P. geminiflorum. Therefore, the present study was aimed to synthesize biologically stable silver nanoparticles using aqueous extract from P. geminiflorum and to test the synthesized nanoparticles against Fusarium wilt disease of tomato in vitro and in planta.
For phytochemical analysis, methanolic extract was prepared by dissolving 50 g shade-dried powdered leaves of P. geminiflorum in 100 ml methanol (95%), followed by incubation at 28°C for 24 h. After incubation the solution was filtered, evaporated and 15 g final mass of crude extract was obtained which was then used for different phytochemicals tests.
TPC was determined according to the Folin-Ciocalteu’s colorimetric method as discussed by Singleton and Rossi, (1965) using calibration curve of standard gallic acid. Briefly, 5 mg of crude extract was added to dH2O (10 ml), and the solution was heated in a water bath for 30 min and then filtered into a vial. About 600 µl of the filtrate was taken and mixed with 100 µl of Folin-Ciocalteu’s reagent, followed by addition of Na2CO3 (sodium carbonate, 7%). The mixture was kept at room temperature for 90 min in dark. After the reaction mixture turned blue, the absorbance was recorded at 760 nm.
TFC determination was carried out by Aluminum chloride (AlCl3) colorimetric method as described in Chang et al. (2002) using standard quercetin curve. Approximately 5 mg of crude extract was added to dH2O (10 ml), the solution was heated in a water bath for 30 min, filtered and five different dilutions (62.5 μg/ml, 125 μg/ml, 250 μg/ml, 500 μg/ml and 1,000 μg/ml) were prepared. From each dilution, 100 µl of extract was thoroughly mixed with 500 µl dH2O and 100 µl of NaNO3 (5%), 150 µl of AlCl3 (10%) and 200 µl of sodium hydroxide (1 M). The absorbance of reaction mixtures was recorded at 510 nm by using UV-VIS double beam spectrophotometer.
The antioxidant assay was determined as DPPH radical scavenging by following the method of Goyal et al. (2010) using standard ascorbic acid.
Determination of the phenolics in leaf extract was accomplished using HPLC Agilent 1,260 system equipped with UV detector, following Zeb, (2015) with minor modifications. Approximately, 1 g dried sample was mixed with 50% methanol (v/v; 20 ml) and the solution was placed in hot water bath at 50°C for 1 h. The solution was filtered 2 times and poured into HPLC vials for the detection of phenolic compounds. For the separation of components, ZORBAX Eclipse C18 (4.6 mm × 250 mm, 5 Micron) column was used and the identification of compounds was carried out by the comparison of retention times with available standards and those reported in literature.
The biosynthesis of silver nanoparticles from P. geminiflorum leaf extract was carried out using the protocols of Ali et al. (2016). Briefly, plant aqueous leaf extract (20 mg/ml) was prepared by heating 2 g shade-dried and powdered leaves of P. geminiflorum in 100 ml distilled water until boiled. The prepared extract was cooled down at room temperature and filtered three times using Whatman No. 1 filter paper. Distilled water was added to adjust the final volume as 100 ml. The stock extract was then diluted to 5 mg/ml by adding distilled water. The extract was stored at 4°C until used for nanoparticles synthesis.
For silver nanoparticles synthesis, 5 ml of the plant aqueous extract (5 mg/ml) was mixed with 5 ml of AgNO3 (4 mM) solution in a test tube. The reaction mixture was exposed to sunlight for 15 min and the color change was monitored following the reaction of both reactants. The mixture was incubated at room temperature and spectral readings were recorded at various time points until consistency in surface plasmon resonance was achieved after 48 h. The synthesized AgNPs were purified by following the protocol of Arif et al. (2022). Briefly, the samples were centrifuged at 15,000 rpm, the supernatants were discarded, and the pellets were dissolved in deionized distilled water by using ultrasonic sonicator. The process of centrifugation and washing was repeated three times until purified AgNPs were obtained. The purified nanoparticles were kept at room temperature for drying which were later subjected to various characterization techniques. To prepare plant extract-encapsulated silver nanoparticles (AgNPs-PE), appropriate volumes of 5 mg/L plant extract was added to dried AgNPs in falcon tubes (15 ml).
Characterization of the synthesized AgNPs was accomplished using different physical techniques, as has been previously reported (Gopinath and Velusamy, 2013; Ali et al., 2016).
The UV-visible spectral analysis was recorded to find out the characteristic peak for silver nanoparticles. The spectral range of 300 nm–600 nm was used to monitor the surface plasmon resonance. For this purpose, the Multiskan™ Sky Microplate Spectrophotometer (MAN0018930) was used.
The FTIR characterization were performed using Thermo-Nicolet 6,700 FTIR Spectrometer (Madison, WI, United States) with spectrum ranging from 4,000 cm−1 to 400 cm−1. The functional groups were detected using Ge crystal in ATR reflection mode. The functional groups responsible for the formation of stable nanoparticles were identified by comparing observed FTIR peaks with IR spectrum table.
The TEM analysis of the biosynthesized AgNPs were performed on JEOL JEM-101 system. Different magnification lens was employed for exploration of the shape, size and morphology of prepared AgNPs. Further, SAED (selected area electron diffraction) determined the crystalline nature of AgNPs.
For EDX study, scanning electron microscope (JSM5910, JEOL, Japan) equipped with energy dispersive x-ray system was used. The EDX characterization determined the elemental composition for the synthesized silver nanoparticles.
The XRD analysis of biosynthesized AgNPs were performed through JDX-3432, JEOL, Japan. The average crystallite size for the prepared nanoparticles were calculated using Debye–Scherrer equation which is assessed by:
The in vitro antifungal activity of AgNPs was performed against F. oxysporum using well-diffusion and disc-diffusion methods following Gopinath and Velusamy, (2013), with certain modifications. Briefly, the confirmed fungal strain F. oxysporum was taken from the plant pathogens facility at the Centre for Plant Sciences and Biodiversity, University of Swat, Pakistan. The strain was cultured and maintained on PDA plates. For well diffusion, four wells of equal size were made on PDA plates and each well was loaded with 100 µl of either AgNPs (100 μg/ml), plant extract (5 mg/ml) or AgNPs-PE (100 μg/ml). The same volume of distilled water was taken as control. The cultures were incubated at 28°C and the zone of inhibition (mm) around the wells was recorded after 4 days of culture. Similarly, disc-diffusion method was carried out by employing the same treatments and concentrations as for well-diffusion method. The F. oxysporum was cultured and four discs (each poured with 20 µl solution) were placed on PDA plate. The inhibition zone was measured after incubation at 28°C for 4 days (Table 1).
The in planta experiment against F. oxysporum was performed following the protocol of Ali et al. (2015) with some modifications. Briefly, the culture of F. ozysporum were grown overnight in potato dextrose broth (PDB) and a final concentration of 1.5 × 104 conidia ml−1 was adjusted. Further, the seeds of Solanum lycopersicon were sown in a greenhouse upheld at 24°C ± 5°C and a photoperiod of 14-h day/10-h night. The 18 days old seedlings were transferred to pots and kept under the same temperature and photoperiod. After 10 days, the plants were sprayed until excess with AgNPs (100 μg/ml), plant extract (5 mg/ml) and AgNPs-PE (100 μg/ml). Commercial fungicide (bromuconazole 100 μg/ml) was taken as positive control and water as negative control. Each treatment was replicated three times and each replication was consisted of nine plants placed in plastic pots. After 24 h, each treated plant was drenched with 30 ml of F. oxysporum (concentration of 1.5 × 104 conidia ml−1) PDB culture. After inoculation the treated plants were observed for one week and the disease spontaneity or inhibition was recorded in terms of percent plant survival. The obtained data was statistically analyzed using student’s t test to find out the significance of difference between the treatments for the percent healthy plants.
Total phenolic content of the aqueous and methanolic extract of P. geminiflorum is given in Table 2, which was estimated using the regression equation of standard gallic acid. The TPC for both extracts were found 42.27 mg ± 1.73 mg and 48.29 mg ± 1.54 mg GAE/g, respectively. Regarding, total flavonoid contents, the highest TFC was found in the dilution of 1,000 μg/ml that is presented in Figure 1. Further, results regarding DPPH free radical scavenging capability of P. geminiflorum methanolic leaf extract revealed the highest scavenging percent for the concentration of 1,000 μg/ml. The obtained results were compared with standard ascorbic acid and the data of DPPH assay is presented in Figure 1. The HPLC analysis revealed fifteen bioactive phenolic compounds in the leaf extract of P. geminiflorum (Figure 2). The most prominent possible compounds identified were P-coumaric acid derivative, ellagic acid, p-hydroxy benzoic acid, Vanillic acid, rutin and quercetin-3-malonylglucoside (Figure 2; Table 3).
The reaction mixture was turned brown after mixing the equal volume of AgNO3 (4 mM) with plant extract (5 mg/ml) at room temperature. Plant extract reduced the AgNO3 solution to Ag ions and capped the Ag+ with important secondary constituents. The silver ions in the presence of plant secondary constituents were stabilized into Ag nanoparticles. The appearance of brown color was due to silver ions reduction which is a general characteristic for the AgNPs biosynthesis. No color change was observed after 24 h (Figure 3A).
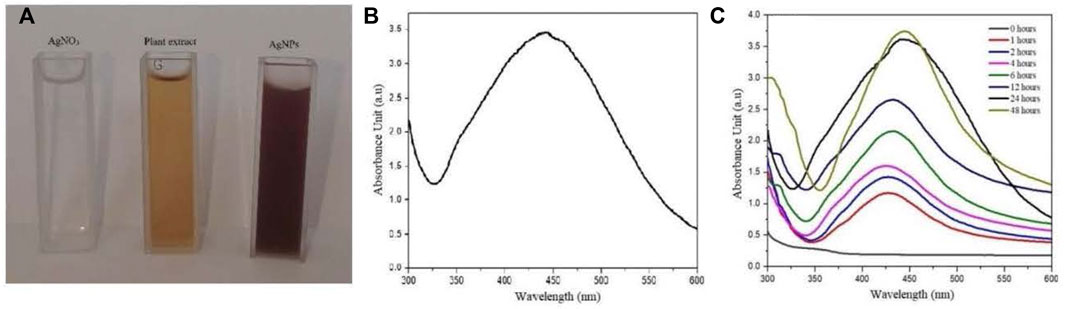
FIGURE 3. (A) Color change of reaction mixture, (B) and (C) UV-Visible absorbance spectrum of the biosynthesized AgNPs.
The UV-Vis spectral analysis showed an increase spectrum between 380 nm and 500 nm where the highest absorbance peak was recorded at 440 nm. Moreover, the solution was observed under UV-Vis spectra at different time interval for 30 days showing no momentous change in the absorbance spectrum after 48 h (Figures 3B,C).
FTIR analysis of the plant extract identify different peaks at specific wavenumber indicating the occurrence of various functional groups. Briefly, broad peaks at 3,499 cm−1 and 3,200 cm−1 was due to the O-H stretching of alcohol. A medium peak at 3,086 cm−1 was found due to the C-H stretching of alkene. A weak band at 2,569 cm−1 was detected for the S-H stretching of thiol. At 2,276 cm−1 a strong broad band was observed for the N = C = O stretching of isocyanate. A strong peak at 1809 cm−1 was due to the C = O stretching of acid halide. A medium peak at 1,635 was present due the C = C stretching of alkene. Similarly, the FTIR analysis of AgNPs also revealed various peaks for specific functional groups. Concisely, a weak broad peak at 3,017.6 cm−1 and 2,904 cm−1 was detected for the O-H stretching of alcohol. A weak peak at 2,595.2 cm−1 was observed for the S-H stretching of thiol and at 2,276.8 cm−1 a strong broad band was detected due to N = C = O stretching of isocyanate. A medium band at 1,635.2 cm−1 was observed due to C = C stretching of conjugated alkene. These groups were probably detected due to the capping layers of the plant secondary metabolites and resulted with stable formation of AgNPs (Figure 4).
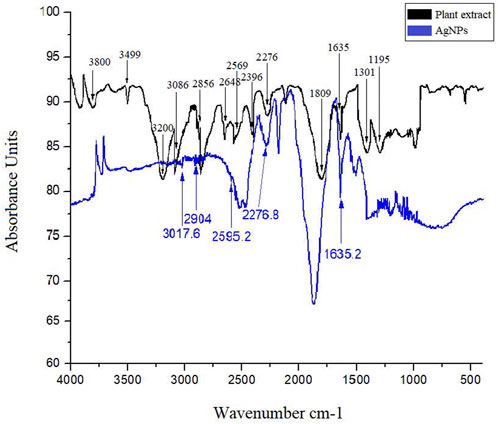
FIGURE 4. FTIR spectrum of plant extract and AgNPs showing bands for various functional groups responsible for stabilization of silver nanoparticles.
The size and shape details of the biosynthesized AgNPs were analyzed with TEM. The TEM micrographs showed different shapes for AgNPs but maximum particles were round or spherical in shape. It can be clearly seen in TEM images that the size of the biosynthesized AgNPs ranged from 8 nm to 34 nm and most of them were monodispersed. The TEM observations showed that due to presence of capping agents the prepared AgNPs were not in straight contact even inside aggregates. Further, Brags reflections rings were recorded in selected area electron diffraction (SAED) study that were corresponding to the crystalline nature of AgNPs (Figure 5A). Histogram showing size distribution of the synthesized AgNPs is shown in Figure 5B.
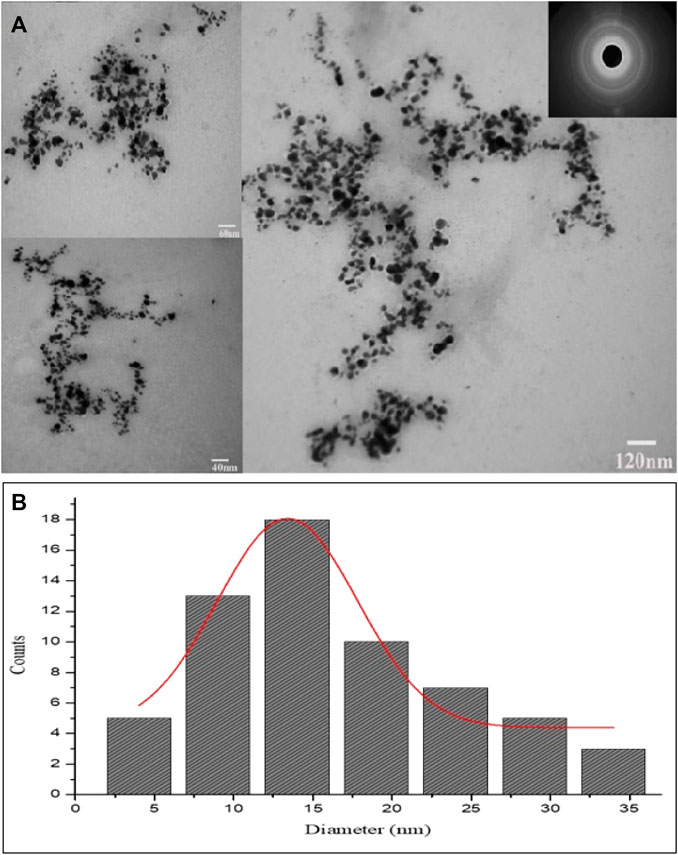
FIGURE 5. (A) TEM images and SAED pattern of the green synthesized AgNPs. (B) Histogram showing size distribution of the synthesized AgNPs.
The elemental composition for the prepared AgNPs were determined using energy dispersive x-ray spectroscopy. The major peaks for Ag, Cl, C, O and S elements were found at EDX spectra having the weight percentage 77.85, 15.44, 4.92, 1.50 and 0.30 respectively. The high energy peak spectrum between 3 KeV and 4 KeV was noticed which is particular for Ag element (Figure 6A). Similarly, the XRD study were performed over 2θ diffraction angle ranging from 10° to 80° that showed four different Bragg’s reflections. The XRD diffraction peaks AgNPs were located at 38.30, 44.35, 64.40, and 77.55 equivalent to the silver crystal planes of (111), (200), (220), and (311) respectively. The average crystallite size of AgNPs found according to Debye–Scherrer equation was 27 nm (Figure 6B).
The AgNPs (100 μg/ml), plant extract (5 mg/ml) and AgNPs-PE (100 μg/ml) strongly inhibited the growth of F. oxysporum in the well and disc diffusion methods. The AgNPs-PE exhibited highest inhibition, followed by AgNPs and plant extract while no inhibition in response to water (control) was observed. In well diffusion method, the AgNPs-PE, AgNPs and plant extract inhibited the growth by 18 mm
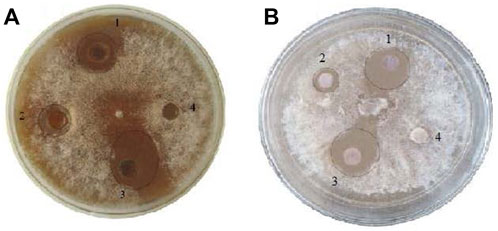
FIGURE 7. In vitro well diffusion. (A) and disc diffusion, (B) methods. AgNPs treated, 1 plant extract treated, 2 AgNPs-PE combined treated, 3 and water as control 4.
To investigate the in planta antifungal activities against the Fusarium wilt of tomato, the pot grown plants were treated with AgNPs (100 μg/ml), plant extract (5 mg/ml), AgNPs-PE (100 μg/ml), fungicide bromuconazole (100 μg/ml) as positive control and water as negative control. After inoculation, the control plants treated with water started yellowing of the leaves, followed by wilting and stunting of growth. However, the plants treated with fungicide inhibited the pathogen and the plants showed optimum growth. Similarly, no wilting symptoms were observed in response to both concentrations (100 µg) of AgNPs. However, the plants treated with plant extract showed partial onset of wilting symptoms. Overall, the AgNPs-PE completely inhibited the F. oxysporum growth and the plants were healthy and showed maximum growth. The percent plant survival after inoculation of F. oxysporum in response to different treatment is shown in Figure 8. The obtained results of in planta experiment were correlated with the results of in vitro experiment and both showed comparable effects. Moreover, the effects of different treatments on root and shoot length, and on biomass has been presented in Table 4.
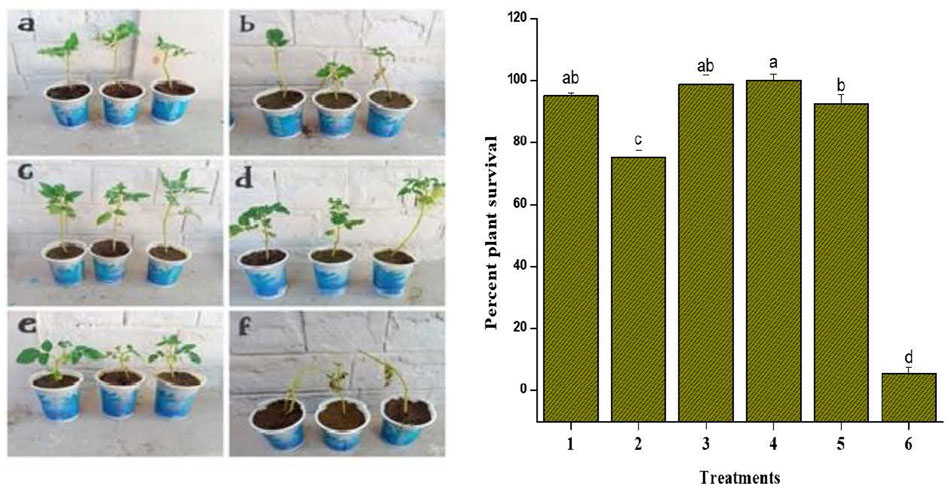
FIGURE 8. In planta treatments against F. oxysporum. Bar graph showing plant survival percent after the inoculation of F. oxysporum. Treatments included: 1 AgNPs (100 μg/ml); 2 plant extract (5 mg/ml); 3 AgNPs-PE (AgNPs 100 μg/ml + PE 5 mg/ml); 4 fungicide (bromuconazole 100 μg/ml); 5 AgNPs (50 μg/ml); 6 Control. Different letters are representing statistically significant differences after performing Tukey HSD test.
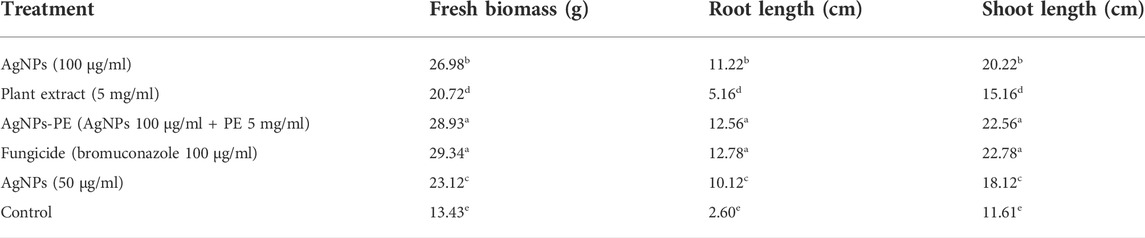
TABLE 4. Effect of different treatments on the growth parameters of tomato during in planta experiment against F. oxysporum. Different letters are showing statistical differences among treatments at p < 0.05 after performing Tukey HSD test.
The overuse of synthetic pesticides leads to disease resistance in microbes which is becoming a major hazard to health of beneficial life forms including human (Rudramurthy et al., 2016). These resistant microbes cause various diseases and reduce the yield and quality of crops (Fletcher et al., 2006). An example of such diseases is Fusarium wilt of tomato which negatively affect the plant growth and productivity (Prajapat et al., 2013; Özkara et al., 2016. However, due to their non-hazardous nature, silver nanoparticles have been used as antimicrobial agents against disease causing microbes (Burdusel et al., 2018; Liao et al., 2019). Several reports have shown the effectiveness of green synthesized AgNPs against a variety of microbes due to the combined effects of plant secondary metabolites and Ag metal (Kim et al., 2007; Choudhury et al., 2016; Durán et al., 2016; Marslin et al., 2018). Moreover, plant based synthesis of silver nanoparticles is economically feasible, efficient against pathogens and non-toxic (Liaqat et al., 2022; Ahmed and Mustafa, 2020).
In the present report, P. geminiflorum leaf extract was investigated for phytochemicals and antioxidant activity along with antifungal properties of the biosynthesized AgNPs. Plant secondary metabolites including terpenoids, flavonoids, phenolics etc. may act as reducing and stabilizing agents in green synthesizing nanoparticles; however, complexity about the exact mechanism of synthesis exists (Marslin et al., 2018). This may be attributed to the synergism of these biomolecules in reduction of metal ions and variable phytochemical profiles of different plant extracts leading to uncertainty about a generalized mechanism. The potential role of these biomolecules in synthesis of nanoparticles has been extensively discussed by Mustapha et al. (2022) and Siddiqi et al. (2018). Jain and Mehata, (2017) and Pradeep et al. (2021) have recently documented reduction of silver ions through standard phenolic compounds. Still, other metabolites like terpenes have also been documented to exhibit dual role i.e. capping as well as reducing agents (Mashwani et al., 2016). TPC in methanolic extract (48.32 mg ± 1.54 mg GAE/g) and aqueous extract (42.30 mg ± 1.73 mg GAE/g) was found to be comparable. Moreover, the highest total flavonoid content found in 1,000 μg/ml was 57.08 mg ± 1.36 mg QE/g which was compared with previous studies (Gupta et al., 2018; Nazir et al., 2020). Highest DPPH radical scavenging activity of 88.23% ± 0.87% was exhibited by the methanolic extract. Moreover, a total of fifteen phenolic compounds were found in the leaf extract with respect to standards and reported data. The results were compared with previous studies on HPLC based phytochemical investigations of Pistacia integerrima, Pisum sativum L. Ziziphus oxyphylla, Grewia optiva (Nazir et al., 2020; Sharifi-Rad et al., 2020). Results depicted rich phytochemical profile and significant antioxidant properties of the investigated plant extract which suggest its probable involvement as the major reducing compounds of silver ions. The solution turned brown due to reduction of silver ions in reaction mixture which indicated the formation of silver nanoparticles, and the result was compared with previously reported studies on green nanoparticles synthesis (Ali et al., 2016; Arif et al., 2022). Moreover, a characteristic surface plasmon resonance peak at 440 nm during UV-Vis analysis was observed which further confirmed the synthesis of nanoparticles. The obtained UV-Vis spectral data was compared with previous studies which showed similar pattern for silver nanoparticles (Ali et al., 2015; Marslin et al., 2018; Ahmed and Mustafa, 2020).
The FTIR spectra identified various functional groups responsible for the synthesis process of AgNPs. These functional groups were likely responsible for synthesis of and providing capping layers to Ag nanoparticles. Therefore, the synthesized AgNPs were more stable and non-toxic as previously been discovered by Arif et al. (2022). The TEM analysis revealed spherical or round shaped monodispersed AgNPs that were not in direct contact because of the capping layers of secondary metabolites. The SAED analysis showed the synthesized particles as of crystalline nature. The EDX study showed an intense peak of Ag metal with a weight of 77.85%. The TEM and EDX based results about the physical characteristics of nanoparticles were positively correlated with previous studies (Khan et al., 2020).
The XRD analysis revealed the planes of 111, 200, 220, and 311 at diffraction peaks of 38.30, 44.35, 64.40, and 77.55, respectively. Moreover, the Debye–Scherrer equation showed the average size of 27 nm and the obtained results were compared to the previous literature studies (Bindhu et al., 2020; Ghojavand et al., 2020).
The prepared AgNPs showed substantial antifungal activity against F. oxysporum. The in vitro experiment resulted in significant inhibition potency of AgNPs-PE against the tested fungus. The plant extract has important secondary constituents that coat and increase the antimicrobial effects of Ag metal. Further, antifungal activities of the AgNPs-PE, AgNPs (100 μg/ml), plant extract and fungicide were compared using in planta experiment. The in planta experiment revealed efficiency of the AgNPs-PE with the highest percent survival of plants. However, the AgNPs (50 μg/ml) showed pronounced antifungal activity because no symptoms of leaf yellowing (chlorosis) was observed throughout the experiment. The obtained results of in vitro and in planta experiments are in general agreement and much promising when correlated with the previously reported studies on antimicrobial activities of silver nanoparticles (Jo et al., 2009; Ali et al., 2015; Gopinath et al., 2017; Some et al., 2018; Alam et al., 2019; Haroon et al., 2019; Santiago et al., 2019; Vanti et al., 2019; Renuka et al., 2020; Rizwan et al., 2020; Tariq et al., 2021). The green AgNPs in the presence of plant capping agents did not allow particles to aggregate and increases its long-term stability (Spagnoletti et al., 2021). The plant extract carries out dual function i.e., perform the reduction of silver ion and stabilization of AgNPs in the reaction mixture (Sunkar and Nachiyar, 2012). The AgNPs-PE showed the highest activity because of its increased antifungal property due to the presence of plant secondary chemicals.
Green synthesized nanoparticles are considered advantageous because of its non-toxicity, environment friendly nature, are more economical and sustainable (Alsammarraie et al., 2018; Ying et al., 2022). At the same time, less availability of raw materials and high homogeneity in particle size of the final product may affect its quality (Turunc et al., 2017; Zhang et al., 2020). Both in vitro and in planta results showed significant inhibition of the F. oxysporum which may be attributed to capping of AgNPs by plant secondary constituents that increased its antifungal potential (Mashwani et al., 2016; Marslin et al., 2018; Vanti et al., 2019; Leon-Buitimea et al., 2020) (8, 52, 60,and 74). Our findings regarding the synthesis and antifungal activity suggested that green AgNPs as a novel drug can be used on large industrial scale in order to control the growth of F. oxysporum.
The antimicrobial mechanism of metal nanoparticles against plant pathogens has been excellently reviewed (Ali et al., 2020). Although the exact mechanism of action of nanoparticles against microbes is not clear, various mechanisms are thought to be involved. The antimicrobial activities of AgNPs could be the result of a loss of replication activity that inactivates the cellular proteins and enzymes of the pathogens (Feng et al., 2000; Yamanaka et al., 2005). Previously, AgNPs have shown to arrest mycelial growth of Fusarium oxysporum (Akpinar et al., 2021), Fusarium graminearum (Jian et al., 2022) and Phytophthora spp. (Ali et al., 2015). A recent study on the molecular level inhibition of Fusarium graminearum in response to AgNPs has shown to induce the expression of azole-related ATP-binding cassette (ABC) transporters and generation of reactive oxygen species, and thus compromise the development, cell structure, cellular energy utilization, and metabolic pathways of this fungus (Jian et al., 2022). Other studies show that nanoparticles penetrate the cell wall and cell membrane and disrupt the cell integrity (Mikhailova, 2020). Some reports suggest nanoparticles induced damage to DNA RNA and proteins, leakage of cellular contents and ultimately death of cells (Kumari et al., 2019; Zhou et al., 2021). Moreover, the application of AgNPs on tomato seedlings has demonstrated to stimulate the antioxidant potential in hydroponics (Noori et al., 2020) which could be considered to enhance the antimicrobial action of AgNPs.
In the present study, we found P. geminiflorum an excellent biological substrate for AgNPs synthesis, most probably due to its rich medicinal phytochemical profile. The plant species showed major medicinal secondary metabolites in qualitative and quantitative analysis. Moreover, the antioxidant activity of the leaf extract was found to be linked with medicinally important secondary metabolites. The AgNPs (100 μg/ml) and the AgNPs-PE (100 μg/ml) inhibited the growth of F. oxysporum substantially in the in vitro experiment. Further, the in planta application of AgNPs alone and combined with plant extract prevented the wilting disease of tomato caused by F. oxysporum. Therefore, the antifungal silver nanoparticles synthesized in the current study could be effectively used against F. oxysporum as alternatives to hazardous synthetic pesticides. However, further studies are needed to evaluate the antifungal potency of AgNPs alone and in combination with P. geminiflorum in the field conditions.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
AA and MoA designed the research. MaA conducted the research. Interpretation of the results was done by IA and ZU. The manuscript draft was prepared by AA, HS, D-QD, JI, MZ and IA.
This research was supported by the National Natural Science Foundation of China (No. NSFC 31760013) and High-Level Talent Recruitment Plan of Yunnan Provinces Young Talents Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abou El-Nour, K. M., Eftaiha, A. A., Al-Warthan, A., and Ammar, R. A. (2010). Synthesis and applications of silver nanoparticles. Arab. J. Chem. 3 (3), 135–140. doi:10.1016/j.arabjc.2010.04.008
Ahmed, R. H., and Mustafa, D. E. (2020). Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett. 10 (1), 1–14. doi:10.1007/s40089-019-00291-9
Akpinar, I., Unal, M., and Sar, T. (2021). Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 3 (4), 506–509. doi:10.1007/s42452-021-04524-5
Alam, M. T., Rauf, M. A., Siddiqui, G. A., Owais, M., and Naeem, A. (2018). Green synthesis of silver nanoparticles, its characterization, and chaperone-like activity in the aggregation inhibition of α-chymotrypsinogen A. Int. J. Biol. Macromol. 120, 2381–2389. doi:10.1016/j.ijbiomac.2018.09.006
Alam, T., Khan, R. A. A., Ali, A., Sher, H., Ullah, Z., and Ali, M. (2019). Biogenic synthesis of iron oxide nanoparticles via Skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Mater. Sci. Eng. C 98, 101–108. doi:10.1016/j.msec.2018.12.117
Albrecht, M. A., Evans, C. W., and Raston, C. L. (2006). Green chemistry and the health implications of nanoparticles. Green Chem. 8 (5), 417–432. doi:10.1039/b517131h
Ali, M., Ahmed, T., Wu, W., Hossain, A., Hafeez, R., Islam Masum, M., et al. (2020). Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 10 (6), 1146. doi:10.3390/nano10061146
Ali, M., Kim, B., Belfield, K. D., Norman, D., Brennan, M., and Ali, G. S. (2016). Green synthesis and characterization of silver nanoparticles using Artemisia absinthium aqueous extract—A comprehensive study. Mater. Sci. Eng. C 58, 359–365. doi:10.1016/j.msec.2015.08.045
Ali, M., Kim, B., Belfield, K. D., Norman, D., Brennan, M., and Ali, G. S. (2015). Inhibition of Phytophthora parasitica and P. capsici by silver nanoparticles synthesized using aqueous extract of Artemisia absinthium. Phytopathology 105 (9), 1183–1190. doi:10.1094/phyto-01-15-0006-r
Alsammarraie, F. K., Wang, W., Zhou, P., Mustapha, A., and Lin, M. (2018). Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surfaces B Biointerfaces 171, 398–405. doi:10.1016/j.colsurfb.2018.07.059
Arif, M., Ullah, R., Ahmad, M., Ali, A., Ullah, Z., Ali, M., et al. (2022). Green synthesis of silver nanoparticles using Euphorbia wallichii leaf extract: Its antibacterial action against citrus canker causal agent and antioxidant potential. Molecules 27 (11), 3525. doi:10.3390/molecules27113525
Avila-Quezada, G. D., Golinska, P., and Rai, M. (2021). Engineered nanomaterials in plant diseases: Can we combat phytopathogens? Appl. Microbiol. Biotechnol. 1-13, 117–129. doi:10.1007/s00253-021-11725-w
Bashir, M. R., Atiq, M., Sajid, M., Mohsan, M., Abbas, W., Alam, M. W., et al. (2018). Antifungal exploitation of fungicides against Fusarium oxysporum f. sp. capsici causing Fusarium wilt of chilli pepper in Pakistan. Environ. Sci. Pollut. Res. 25 (7), 6797–6801. doi:10.1007/s11356-017-1032-9
Bindhu, M. R., Umadevi, M., Esmail, G. A., Al-Dhabi, N. A., and Arasu, M. V. (2020). Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B Biol. 205, 111836. doi:10.1016/j.jphotobiol.2020.111836
Burdușel, A. C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., and Andronescu, E. (2018). Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 8 (9), 681. doi:10.3390/nano8090681
Chang, C. C., Yang, M. H., Wen, H. M., and Chern, J. C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food. Drug. Anal. 10 (3), 178–182. doi:10.38212/2224-6614.2748
Chase, M. W., Christenhusz, M. J. M., Fay, M. F., Byng, J. W., Judd, W. S., Soltis, D. E., et al. (2016). An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: Apg IV. Bot. J. Linn. Soc. 181 (1), 1–20. doi:10.1111/boj.12385
Choudhury, R., Majumder, M., Roy, D. N., Basumallick, S., and Misra, T. K. (2016). Phytotoxicity of Ag nanoparticles prepared by biogenic and chemical methods. Int. Nano Lett. 6 (3), 153–159. doi:10.1007/s40089-016-0181-z
Durán, N., Durán, M., De Jesus, M. B., Seabra, A. B., Fávaro, W. J., and Nakazato, G. (2016). Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine Nanotechnol. Biol. Med. 12 (3), 789–799. doi:10.1016/j.nano.2015.11.016
Feng, Q. L., Wu, J., Chen, G. Q., Cui, F. Z., Kim, T. N., and Kim, J. O. (2000). A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mat. Res. 52 (4), 662–668. doi:10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3
Fletcher, J., Bender, C., Budowle, B., Cobb, W. T., Gold, S. E., Ishimaru, C. A., et al. (2006). Plant pathogen forensics: Capabilities, needs, and recommendations. Microbiol. Mol. Biol. Rev. 70 (2), 450–471. doi:10.1128/mmbr.00022-05
Floden, A., and Schilling, E. E. (2018). Using phylogenomics to reconstruct phylogenetic relationships within tribe Polygonateae (Asparagaceae), with a special focus on Polygonatum. Mol. Phylogenet. Evol. 129, 202–213. doi:10.1016/j.ympev.2018.08.017
Gao, H., Niu, J., and Li, S. (2018). Impacts of wheat powdery mildew on grain yield & quality and its prevention and control methods. Am. J. Agric. For. 6, 141–147. doi:10.11648/j.ajaf.20180605.14
Gardener, B. B. M., and Fravel, D. R. (2002). Biological control of plant pathogens: Research, commercialization, and application in the USA. Plant Health Prog. 3 (1), 17. doi:10.1094/php-2002-0510-01-rv
Ghojavand, S., Madani, M., and Karimi, J. (2020). Green synthesis, characterization and antifungal activity of silver nanoparticles using stems and flowers of felty germander. J. Inorg. Organomet. Polym. Mat. 30 (8), 2987–2997. doi:10.1007/s10904-020-01449-1
Gopinath, V., Priyadarshini, S., Loke, M. F., Arunkumar, J., Marsili, E., MubarakAli, D., et al. (2017). Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 10 (8), 1107–1117. doi:10.1016/j.arabjc.2015.11.011
Gopinath, V., and Velusamy, P. (2013). Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 106, 170–174. doi:10.1016/j.saa.2012.12.087
Goyal, A. K., Middha, S. K., and Sen, A. (2010). Evaluation of the DPPH radical scavenging activity, total phenols and antioxidant activities in Indian wild Bambusa vulgaris" Vittata" methanolic leaf extract. J. Nat. Pharm. 1 (1), 40–45. doi:10.4103/2229-5119.73586
Gupta, N., Upadhyaya, C. P., Singh, A., Abd-Elsalam, K. A., and Prasad, R. (2018). “Applications of silver nanoparticles in plant protection,” in Nanobiotechnol. Appl. Plant prot. (Cham: Springer).
Hajek, A., and Eilenberg, J. (2018). “Microbial antagonists combating plant pathogens and plant parasitic nematodes,” in Natural enemies: An introduction to biological control (Cambridge: Cambridge University Press), 308–324.
Haroon, M., Zaidi, A., Ahmed, B., Rizvi, A., Khan, M. S., and Musarrat, J. (2019). Effective inhibition of phytopathogenic microbes by eco-friendly leaf extract mediated silver nanoparticles (AgNPs). Indian J. Microbiol. 59 (3), 273–287. doi:10.1007/s12088-019-00801-5
Hebbalalu, D., Lalley, J., Nadagouda, M. N., and Varma, R. S. (2013). Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain. Chem. Eng. 1 (7), 703–712. doi:10.1021/sc4000362
Jain, S., and Mehata, M. S. (2017). Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 7, 15867. doi:10.1038/s41598-017-15724-8
Jeong, S. H., Yeo, S. Y., and Yi, S. C. (2005). The effect of filler particle size on the antibacterial properties of compounded polymer/silver fibers. J. Mat. Sci. 40 (20), 5407–5411. doi:10.1007/s10853-005-4339-8
Jian, Y., Chen, X., Ahmed, T., Shang, Q., Zhang, S., Ma, Z., et al. (2022). Toxicity and action mechanisms of silver nanoparticles against the mycotoxin-producing fungus Fusarium graminearum. J. Adv. Res. 38, 1–12. doi:10.1016/j.jare.2021.09.006
Jiao, J., Huang, W. L., Bai, Z. Q., Liu, F., Ma, C. D., Liang, Z., et al. (2018). DNA barcoding for the efficient and accurate identification of medicinal Polygonati rhizoma in China. PLoS One 13 (7), e0201015. doi:10.1371/journal.pone.0201015
Jo, Y. K., Kim, B. H., and Jung, G. (2009). Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 93 (10), 1037–1043. doi:10.1094/pdis-93-10-1037
Khan, H., and Rauf, A. (2015). Phytochemistry of genus Polygonatum: A review. Am. J. Biomed. Sci. 3 (2-1), 5–20. doi:10.11648/j.ajbls.s.2015030201.12
Khan, H., Saeed, M., Mehmood, M. H., Rehman, N. U., Muhammad, N., Haq, I. U., et al. (2013). Studies on tracheorelaxant and anti-inflammatory activities of rhizomes of Polygonatum verticillatum. BMC Complement. Altern. Med. 13 (1), 197–198. doi:10.1186/1472-6882-13-197
Khan, I., Zahoor, M., Zeb, A., and Ul Bari, W. (2020). Vitro antioxidant, antidiabetic, and anticholinesterase, and in vivo toxicological, hypoglycemic, and antilipidemic potentials of Ziziphus oxyphylla. Lat. Am. J. Pharm. 39 (1), 7–21.
Khan, S., Bibi, G., Dilbar, S., Iqbal, A., Ahmad, M., Ali, A., et al. (2022). Biosynthesis and characterization of iron oxide nanoparticles from Mentha spicata and screening its combating potential against Phytophthora infestans. Front. Plant Sci. 13, 1001499. doi:10.3389/fpls.2022.1001499
Khan, S. W., and Khatoon, S. (2008). Ethnobotanical studies on some useful herbs of Haramosh and Bugrote valleys in Gilgit, northern areas of Pakistan. P. J. Bot. 40 (1), 43–58.
Kim, J. S., Kuk, E., Yu, K. N., Kim, J. H., Park, S. J., Lee, H. J., et al. (2007). Antimicrobial effects of silver nanoparticles. Nanomedicine Nanotechnol. Biol. Med. 3 (1), 95–101. doi:10.1016/j.nano.2006.12.001
Kumari, M., Giri, V. P., Pandey, S., Kumar, M., Katiyar, R., Nautiyal, C. S., et al. (2019). An insight into the mechanism of antifungal activity of biogenic nanoparticles than their chemical counterparts. Pestic. Biochem. Physiol. 157, 45–52. doi:10.1016/j.pestbp.2019.03.005
Kuppusamy, P., Yusoff, M. M., Maniam, G. P., and Govindan, N. (2016). Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 24 (4), 473–484. doi:10.1016/j.jsps.2014.11.013
León-Buitimea, A., Garza-Cárdenas, C. R., Garza-Cervantes, J. A., Lerma-Escalera, J. A., and Morones-Ramírez, J. R. (2020). The demand for new antibiotics: Antimicrobial peptides, nanoparticles, and combinatorial therapies as future strategies in antibacterial agent design. Front. Microbiol. 24, 1669. doi:10.3389/fmicb.2020.01669
Liao, C., Li, Y., and Tjong, S. C. (2019). Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 20 (2), 449. doi:10.3390/ijms20020449
Liaqat, N., Jahan, N., Khalil-ur-Rahman, , , Anwar, T., and Qureshi, H. (2022). Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 10, 952006. doi:10.3389/fchem.2022.952006
Litchfield, J. J., and Wilcoxon, F. (1949). A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96 (2), 99–113.
Marslin, G., Siram, K., Maqbool, Q., Selvakesavan, R. K., Kruszka, D., Kachlicki, P., et al. (2018). Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 11 (6), 940. doi:10.3390/ma11060940
Mashwani, Z., Khan, M. A., Khan, T., and Nadhman, A. (2016). Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 234, 132–141. doi:10.1016/j.cis.2016.04.008
Mikhailova, E. O. (2020). Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 11 (4), 84. doi:10.3390/jfb11040084
Mustapha, T., Misni, N., Ithnin, N. R., Daskum, A. M., and Unyah, N. Z. (2022). A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 19 (2), 674. doi:10.3390/ijerph19020674
Nazir, N., Zahoor, M., and Nisar, M. (2020). A review on traditional uses and pharmacological importance of genus Elaeagnus species. Bot. Rev. 86 (3), 247–280. doi:10.1007/s12229-020-09226-y
Noori, A., Donnelly, T., Colbert, J., Cai, W., Newman, L. A., and White, J. C. (2020). Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: Physiological and molecular response. Int. J. Phytoremediation 22 (1), 40–51. doi:10.1080/15226514.2019.1634000
Ovais, M., Ayaz, M., Khalil, A. T., Shah, S. A., Jan, M. S., Raza, A., et al. (2018). HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Complement. Altern. Med. 18 (1), 1–13. doi:10.1186/s12906-017-2057-9
Özkara, A., Akyıl, D., and Konuk, M. (2016). “Pesticides, environmental pollution, and health,” in Environ health risk-hazard fact living spec (London, UK: IntechOpen). doi:10.5772/63094
Pradeep, M., Kruszka, D., Kachlicki, P., Mondal, D., and Franklin, G. (2021). Uncovering the phytochemical basis and the mechanism of plant extract-mediated eco-friendly synthesis of silver nanoparticles using ultra-performance liquid chromatography coupled with a photodiode array and high-resolution mass spectrometry. ACS Sustain. Chem. Eng. 10 (1), 562–571. doi:10.1021/acssuschemeng.1c06960
Prajapat, R., Marwal, A., and Jha, P. N. (2013). Erwinia carotovora associated with potato: A critical appraisal with respect to Indian perspective. Int. J. Curr. Microbiol. Appl. Sci. 2, 83–89.
Rafique, K., Rauf, C. A., Gul, A., Bux, H., Memon, R. A., Ali, A., et al. (2017). Evaluation of d-genome synthetic hexaploid wheats and advanced derivatives for powdery mildew resistance. P. J. Bot. 49 (2), 735–743.
Rahman, S. U., Ullah, Z., Ali, A., Aziz, M. A., Alam, N., Sher, H., et al. (2022). Traditional knowledge of medicinal flora among tribal communities of Buner Pakistan. Phytomedicine Plus 2 (3), 100277. doi:10.1016/j.phyplu.2022.100277
Renuka, R., Devi, K. R., Sivakami, M., Thilagavathi, T., Uthrakumar, R., and Kaviyarasu, K. (2020). Biosynthesis of silver nanoparticles using Phyllanthus emblica fruit extract for antimicrobial application. Biocatal. Agric. Biotechnol. 24, 101567. doi:10.1016/j.bcab.2020.101567
Rizwan, M., Amin, S., Malikovna, B. K., Rauf, A., Siddique, M., Ullah, K., et al. (2020). Green synthesis and antimicrobial potential of silver nanoparticles with Boerhavia procumbens extract. J. Pure Appl. Microbiol. 14 (2), 1437–1451. doi:10.22207/jpam.14.2.42
Rudramurthy, G. R., Swamy, M. K., Sinniah, U. R., and Ghasemzadeh, A. (2016). Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 21 (7), 836. doi:10.3390/molecules21070836
Santiago, T. R., Bonatto, C. C., Rossato, M., Lopes, C. A., Lopes, C. A. G., Mizubuti, E. S., et al. (2019). Green synthesis of silver nanoparticles using tomato leaf extract and their entrapment in chitosan nanoparticles to control bacterial wilt. J. Sci. Food Agric. 99 (9), 4248–4259. doi:10.1002/jsfa.9656
Saratale, R. G., Benelli, G., Kumar, G., Kim, D. S., and Saratale, G. D. (2018). Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 25 (11), 10392–10406. doi:10.1007/s11356-017-9581-5
Savary, S., Ficke, A., Aubertot, J. N., and Hollier, C. (2012). Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 4 (4), 519–537. doi:10.1007/s12571-012-0200-5
Saxena, A., Tripathi, R. M., Zafar, F., and Singh, P. (2012). Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mat. Lett. 67 (1), 91–94. doi:10.1016/j.matlet.2011.09.038
Sharifi-Rad, M., Epifano, F., Fiorito, S., and Álvarez-Suarez, J. M. (2020). Phytochemical analysis and biological investigation of Nepeta juncea Benth. different extracts. Plants 9 (5), 646. doi:10.3390/plants9050646
Sharma, S., Joshi, R., and Kumar, D. (2021). Metabolomics insights and bioprospection of Polygonatum verticillatum: An important dietary medicinal herb of alpine Himalaya. Food Res. Int. 148, 110619. doi:10.1016/j.foodres.2021.110619
Sharma, V. K., Yngard, R. A., and Lin, Y. (2009). Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 145 (1-2), 83–96. doi:10.1016/j.cis.2008.09.002
Si, J. P., and Zhu, A. G. (2021). Polygonati rhizoma—a new high-quality crop with great potential and not occupying farmland. Sci. Sin. Vitae. 51, 1477–1484. doi:10.1360/ssv-2020-0413
Siddiqi, K. S., Husen, A., and Rao, R. A. (2018). A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 16 (1), 14–28. doi:10.1186/s12951-018-0334-5
Singleton, V. L., and Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16 (3), 144–158.
Some, S., Sen, I. K., Mandal, A., Aslan, T., Ustun, Y., Yilmaz, E. Ş., et al. (2018). Biosynthesis of silver nanoparticles and their versatile antimicrobial properties. Mat. Res. Express 6 (1), 012001. doi:10.1088/2053-1591/aae23e
Son, K. H., Do, J. C., and Kang, S. S. (1990). Steroidal saponins from the rhizomes of Polygonatum sibiricum. J. Nat. Prod. 53 (2), 333–339. doi:10.1021/np50068a010
Spagnoletti, F. N., Kronberg, F., Spedalieri, C., Munarriz, E., and Giacometti, R. (2021). Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manage. 297, 113434. doi:10.1016/j.jenvman.2021.113434
Sunkar, S., and Nachiyar, C. V. (2012). Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian pac. J. Trop. Biomed. 2 (12), 953–959. doi:10.1016/s2221-1691(13)60006-4
Suyal, R., Bhatt, D., Rawal, R. S., and Tewari, L. M. (2020). Status of two threatened astavarga herbs, Polygonatum cirrhifolium and Malaxis muscifera, in west himalaya: Conservation implications. Proc. Natl. Acad. Sci. India Sect. B. Biol. Sci. 90 (3), 695–704. doi:10.1007/s40011-019-01144-3
Tariq, A., Shah, G. M., Zada, A., Ali, A., Shah, A. Z., and Fatima, I. (2021). Phytochemical analysis and in-vitro antibacterial and anti-fungal activity of Verbascum arianthum (Benth). Pure Appl. Biol. 10 (3), 797–806. doi:10.19045/bspab.2021.100082
Thiruvengadam, M., Rajakumar, G., and Chung, I. M. (2018). Nanotechnology: Current uses and future applications in the food industry. 3 Biotech. 8 (1), 74–13. doi:10.1007/s13205-018-1104-7
Turunc, E., Binzet, R., Gumus, I., Binzet, G., and Arslan, H. (2017). Green synthesis of silver and palladium nanoparticles using Lithodora hispidula (Sm.) Griseb.(Boraginaceae) and application to the electrocatalytic reduction of hydrogen peroxide. Mat. Chem. Phys. 202, 310–319. doi:10.1016/j.matchemphys.2017.09.032
Vanti, G. L., Nargund, V. B., Vanarchi, R., Kurjogi, M., Mulla, S. I., Tubaki, S., et al. (2019). Synthesis of Gossypium hirsutum‐derived silver nanoparticles and their antibacterial efficacy against plant pathogens. Appl. Organomet. Chem. 33 (1), e4630. doi:10.1002/aoc.4630
Wang, J., Qian, J., Jiang, Y., Chen, X., Zheng, B., Chen, S., et al. (2022). Comparative analysis of chloroplast genome and new insights into phylogenetic relationships of Polygonatum and tribe Polygonateae. Front. Plant Sci. 13, 882189. doi:10.3389/fpls.2022.882189
Yamanaka, M., Hara, K., and Kudo, J. (2005). Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 71 (11), 7589–7593. doi:10.1128/aem.71.11.7589-7593.2005
Yaqoob, A. A., Umar, K., and Ibrahim, M. N. M. (2020). Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 10 (5), 1369–1378. doi:10.1007/s13204-020-01318-w
Ying, S., Guan, Z., Ofoegbu, P. C., Clubb, P., Rico, C., He, F., et al. (2022). Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 26, 102336. doi:10.1016/j.eti.2022.102336
Zeb, A. (2015). Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). Springerplus 4 (1), 714–717. doi:10.1186/s40064-015-1514-5
Zhang, D., Ma, X. L., Gu, Y., Huang, H., and Zhang, G. W. (2020). Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front. Chem. 8, 799. doi:10.3389/fchem.2020.00799
Zhang, L., and Webster, T. J. (2009). Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 4 (1), 66–80. doi:10.1016/j.nantod.2008.10.014
Zhao, P., Zhao, C., Li, X., Gao, Q., Huang, L., Xiao, P., et al. (2018). The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 214, 274–291. doi:10.1016/j.jep.2017.12.006
Keywords: silver nanoparticles, antioxidants, phenolics, flavonoids, fusarium wilt, tomato
Citation: Ahmad M, Ali A, Ullah Z, Sher H, Dai D-Q, Ali M, Iqbal J, Zahoor M and Ali I (2022) Biosynthesized silver nanoparticles using Polygonatum geminiflorum efficiently control fusarium wilt disease of tomato. Front. Bioeng. Biotechnol. 10:988607. doi: 10.3389/fbioe.2022.988607
Received: 07 July 2022; Accepted: 22 August 2022;
Published: 08 September 2022.
Edited by:
Rabia Javed, Quaid-i-Azam University, PakistanReviewed by:
Murali M, University of Mysore, IndiaCopyright © 2022 Ahmad, Ali, Ullah, Sher, Dai, Ali, Iqbal, Zahoor and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iftikhar Ali, aWZ0aWtoYXJAZ2VuZXRpY3MuYWMuY24=; Dong-Qin Dai, Y2ljaWRhaWRvbmdxaW5AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.