- 1Department of Engineering Mechanics, Dalian University of Technology, Dalian, China
- 2DUT-BSU Joint Institute, Dalian University of Technology, Dalian, China
- 3Xi’an Aerospace Propulsion Institute, Xi’an, China
- 4School of Engineering, Cardiff University, Cardiff, United Kingdom
- 5Department of Orthopedics, Dalian Municipal Central Hospital Affiliated of Dalian University of Technology, Dalian, China
- 6State Key Laboratory of Structural Analysis for Industrial Equipment, Dalian University of Technology, Dalian, China
The design of bionic bone scaffolds to mimic the behaviors of native bone tissue is crucial in clinical application, but such design is very challenging due to the complex behaviors of native bone tissues. In the present study, bionic bone scaffolds with the anisotropic mechanical properties similar to those of native bone tissues were successfully designed using a novel self-learning convolutional neural network (CNN) framework. The anisotropic mechanical property of bone was first calculated from the CT images of bone tissues. The CNN model constructed was trained and validated using the predictions from the heterogonous finite element (FE) models. The CNN model was then used to design the scaffold with the elasticity matrix matched to that of the replaced bone tissues. For the comparison, the bone scaffold was also designed using the conventional method. The results showed that the mechanical properties of scaffolds designed using the CNN model are closer to those of native bone tissues. In conclusion, the self-learning CNN framework can be used to design the anisotropic bone scaffolds and has a great potential in the clinical application.
1 Introduction
Every year, millions of bone replacement surgeries have to be performed worldwide to fix the large bone defects (Dang et al., 2018). In these surgeries, the autograft and allograft are the techniques widely used in the clinic, but in autograft there is the issue associated with the lack of bone supply and in allograft there are issues, such as the disease transmission (Finkemeier, 2002; Laurencin et al., 2006). Because of these, the bone scaffold has emerged as a promising method for fixing large bone defects. However, there are still many issues to be solved, such as the stress shielding. The structural design is one of the main approaches to solve these challenges but it is still in its early stage and requires extensive research.
In the structural design of bone scaffolds, the design target is to have a bionic scaffold which can mimic the behaviors of the defected native bone tissues in all aspects including the geometrical features, the mechanical and biological functions, etc. However, it should be noted that geometrically the native bone possesses irregular shapes and mechanically the bone is anisotropic in different scales, which poses a big challenge in the bionic design of bone scaffolds. In the past, many efforts have been made to achieve the bionic design of bone scaffolds. For example, the microstructure of bone scaffolds has been evolved from the periodic regular lattices to the triply periodic minimal surface (TPMS) based structures and further to the irregular, non-periodic structures (Vijayavenkataraman et al., 2018; Huo et al., 2021). The periodic regular lattices (e.g., the cube, the hexagon) are widely used in the early stage of the design of bone scaffold (Bucklen et al., 2008). The TPMS based scaffold is one of the main types of structures widely used nowadays because of the bionic features of the TPMS, such as a mean curvature of zero. Most recently, the focus of the scaffold design has been put on the anisotropic behaviors of TPMS scaffolds (Ataee et al., 2018; Bonatti and Mohr, 2019; Kang et al., 2020; Peng et al., 2021). For example, Peng et al. (2021) has managed to increase the range of scaffold anisotropy by modifying the geometrical parameters of Gyroid cellular structure. In addition, the techniques such as grading and hybrid design, etc. are used to design the scaffolds with anisotropic mechanical properties (Liu et al., 2018; Al-Ketan et al., 2019; Chen et al., 2019). However, because the TPMS based scaffolds are based on the periodic units and additionally the number of design variables in the TPMS scaffolds is limited, the design space for the anisotropic mechanical properties of the TPMS scaffolds is limited. Designing the irregular and non-periodic bone scaffolds using some advanced mathematical algorithms (e.g., the Voronoi algorithm) (Gómez et al., 2016; Wang et al., 2018) is one of the strategies to achieve a larger design space for the anisotropic mechanical properties, but high complexities are involved in the advanced mathematical algorithms which hinders its development and application. Therefore, a novel and efficient approach for designing the scaffolds with controllable anisotropy is still highly needed.
In recent years, the machine learning has been evolved as a novel and fast-growing technique, which has been successfully applied in many fields, e.g., the accurate prediction of musculoskeletal force (Rane et al., 2019), the automatic tracking of joint kinematics (Burton et al., 2021), etc. In the design of porous materials, the machine learning based technique has also been widely explored in the recent years. For example, Zheng et al. (2021) has managed the inverse design of auxetic metamaterials using deep learning; Gu et al. (2018) has managed the design of bioinspired hierarchical composite using machine learning; a deep-learning based model was proposed by Tan et al. (2020) for the efficient design of microstructural materials. The advantage of the machine learning technique is that once the machine learning model is well trained and validated, it can serve as an efficient surrogate model for generating the real-time outputs from new inputs. Additionally, the machine learning based technique is able to deal with structural design involving a large number of design variables, which is extremely crucial in the design of porous scaffolds, because the design space for the scaffold anisotropy can be easily expanded by adding more design variables. Therefore, the machine learning technique has the great potential in designing the fully bionic bone scaffolds. Nevertheless, to the best of our knowledge, the design of bone scaffolds with anisotropic mechanical properties using the machine learning technique has not been fully elaborated. The aim of the present study was to design bionic bone scaffolds with the mechanical properties similar to those of native bone tissues using the emerging machine learning technique.
This paper is organized in the following scheme. The calculation of the elasticity matrix of native bone tissue and the details on the self-learning convolutional neural network based design framework are illustrated in Section 2. In Section 3, the performance of the developed framework is demonstrated using a two-dimensional (2D) bone sample. In Section 4, the predictive accuracy of the machine learning based model and the design results are discussed and conclusions are drawn in the end.
2 Materials and methods
2.1 Calculation of the elasticity matrices for the native bone and bone scaffold
In the present study, a 2D bone example is presented to demonstrate the application of the machine learning based method in the design of the anisotropic porous bone scaffold. The anisotropic bone scaffold to be designed is to replace the defected bone tissues and an ideal scaffold should possess the mechanical properties similar to those of the replaced bone tissue. Therefore, the first step in the scaffold design is to work out the mechanical properties of bone tissue, which are calculated from the CT images of native human bone tissue. The CT images used were acquired in the previous studies (Lu et al., 2015). Briefly, thirty-five cadavers were harvested from the female patients with a mean age of 81.3 ± 7.2 year-old (range: 65 to 90 year-old). The spinal segments of T11/T12/L1 were dissected and the specimens were scanned in the frozen state using the HR-pQCT scanner (XtremeCT, Scanco Medical AG, Bruettisellen, Switzerland) operated at 59.4 kV, 900.0 μAs with an image voxel size of 82.0 × 82.0 × 82.0 μm3. In the present study, only the cancellous fraction was used and thus the volumes of interest covering only the cancellous bone were cropped out from the CT images of the spinal segments (Figure 1A).
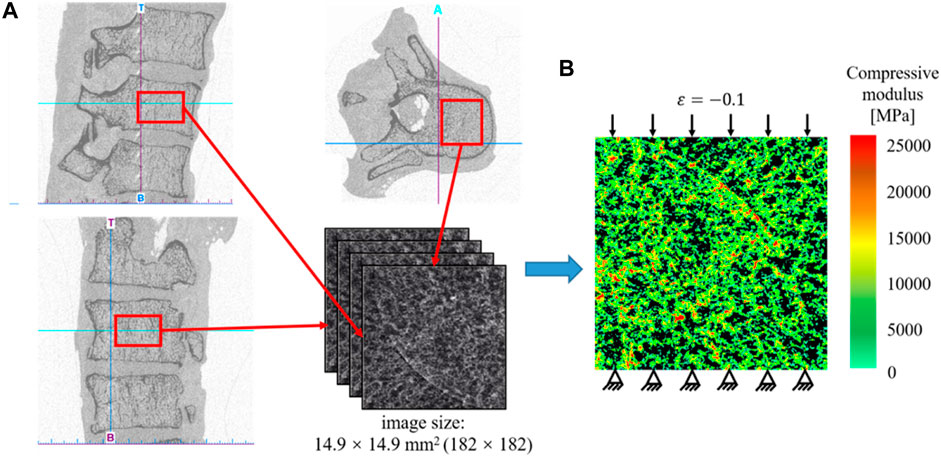
FIGURE 1. (A) Extraction of the CT data of the human cancellous bone. (B) Establishment of the heterogeneous finite element model for calculating the effective elastic mechanical modulus of bone tissue.
Because the native bone tissue exhibits the anisotropic mechanical properties, the homogenized elasticity matrix of the bone was calculated to describe the anisotropic mechanical properties of the native bone tissue, which were calculated from the finite element (FE) analysis as follows (Figure 1). First, the heterogeneous FE models of the cancellous fraction were generated using the standard method previously developed (Lu et al., 2019a). Briefly, the cancellous fraction (Figure 1A) with the dimension of 14.9 × 14.9 mm2 was cropped out from the HR-pQCT images of human vertebral body. The grayscale image was first smoothed using a Gaussian filter (sigma = 1.2, support = 2.0) to reduce the influence of image noise. Then, the grayscale values were converted to vBMD values based on the linear calibration equation provided by the HR-pQCT scanner. The vBMD values were further converted into bone ash density according to the relationship of
The plane stress problem is assumed and the following constitutive model is used to describe the anisotropic mechanical behavior of the bone tissue (Xiao et al., 2021):
where
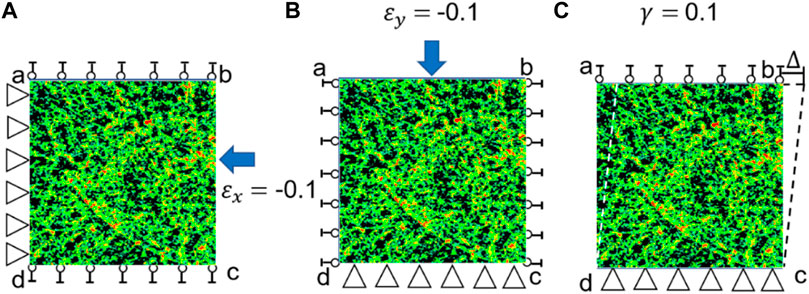
FIGURE 2. Three loading scenarios used for calculating the elasticity matrix of bone tissue. (A) Uniaxial compression in the x direction (
First, the strain in the x direction is set to −0.1 while the other two strains are set to zeros, i.e.,
Second, the strain in the y direction is set to −0.1 and other two strains are set to zeros, i.e.,
Third, the shear strain is set to 0.1 and other two strains are set to zeros, i.e.,
It should be noted that the same loading scenarios (Figure 3) and procedure described above was used to calculate the elasticity matrix of the bone scaffold (also meshed using PLANE182) in the subsequent analysis.
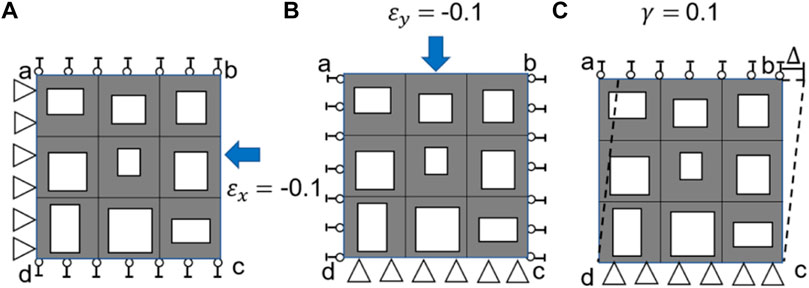
FIGURE 3. Three loading scenarios used for calculating the elasticity matrix of bone scaffold, (A) uniaxial loading in the x direction, (B) uniaxial loading in the y direction and (C) shear loading in the x-y plane.
2.2 Design setting for the bone scaffold
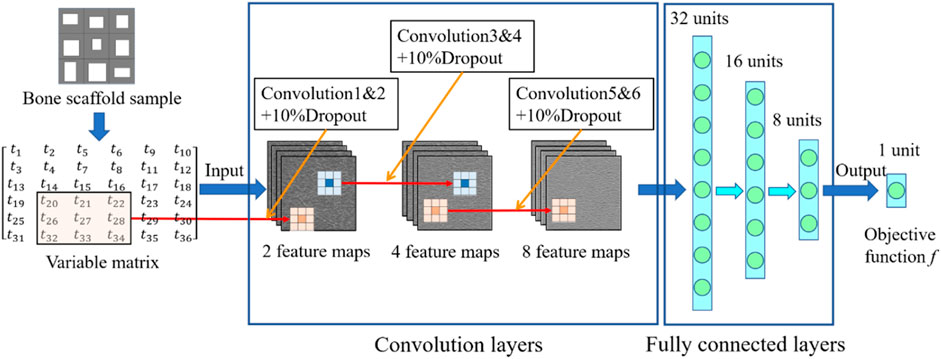
In the present study, the 2D bone scaffold with the anisotropic mechanical properties was intended to be designed. The porous bone scaffold with 3 × 3 cells was used for the demonstration (Figure 4). Nevertheless, the readers can also use the same framework to design the scaffolds with more cells. The dimension of the scaffold was set to 18.0 × 18.0 mm2, which is the size similar to the defected human vertebral part. In each cell of the scaffold, the four dimensional parameters, i.e., the four thicknesses, were set as the independent design variables. Therefore, there are 36 independent design variables (
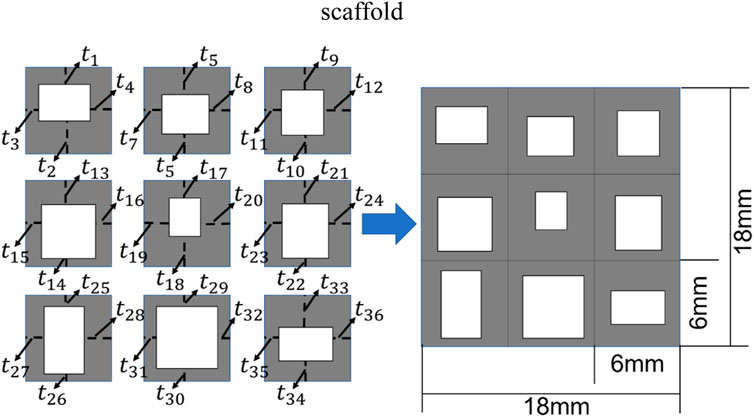
FIGURE 4. The scheme for designing the bone scaffold using the convolutional neural network (CNN) model (36 independent design variables).
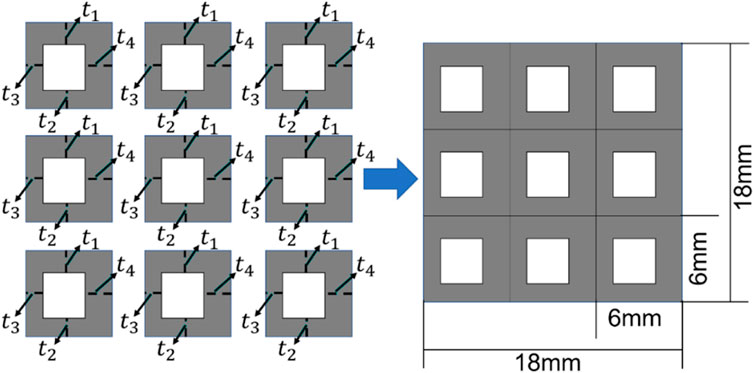
FIGURE 5. The scheme for designing the bone scaffold using the conventional framework (periodic cell and four independent design variables in each cell).
Considering the minimal structural thickness, which can be produced by the additive manufacturing (e.g., the selective laser melting), is approximately 0.2 mm, the minimal dimension of the design variable was set to 0.2 mm and the thickness of the scaffold was increased or decreased by 0.2 mm in the iterations. The scaffold designed was intended to replace the defected bone tissues. Therefore, to make the scaffold clinically relevant and to increase the computational efficiency, three discrete values, i.e., 0.2, 0.4 and 0.6 mm, were set as the design ranges for both the 36 design variables in the CNN method and the four design variables in the conventional design framework. Because of the constraint on the structural dimensions, the porosity of the scaffold is consequently constrained.
Regarding the characterization of the mechanical property of the bone scaffold, because all the dimensional parameters were set as the independent design, the scaffold designed can exhibit the anisotropic mechanical behavior and thus the constitutive relation presented Eq. 1 was used to describe the mechanical properties of scaffold. In the present study, the Ti-6Al-4V was considered as the base material for producing the porous scaffold. Therefore, in the FE models of 2D bone scaffolds, the Young’s modulus of the solid part was set to 113.8 GPa and the Poisson’s ratio was set to 0.34 (Niinomi, 1998).
In the design of bone scaffolds, the differences in the six elastic constants calculated from the bone and scaffolds may be very large. The constant with a large difference will make a significantly large contribution to the design objective function, leading to the ignorance of the constants with small differences. It is revealed in the authors’ previous study that the contributions of the elastic constants
where
The relative differences between the elastic constants of the bone scaffold and those of the bone tissue were calculated using the following formula:
where
Then the objective function used in the optimization process was defined as:
where
2.3 Machine learning based framework for the optimization of bone scaffold
In the design of anisotropic bone scaffold, there are 36 independent design variables and each variable has three values to be chosen from. Therefore, there are
The training and cross-validation of the CNN model is shown in Figure 8. In the training of the CNN model, first, 10,000 bone scaffolds were randomly generated, 8,000 of which were used for the training of the CNN model (Figure 8A) and the remaining 2,000 were used for the cross-validation of the CNN model (Figure 8B). The elastic constants of bone scaffolds calculated from the FE analysis were served as the ground truths for the training and cross-validation. In the training process, the CNN model learned a valid representation describing the geometric features of the bone scaffolds. A loss function was defined to quantify the differences between the elastic constants predicted from the CNN model and those calculated from the FE analysis. The kernels and biases in the convolutional layers and weights in the fully connected layers were then adjusted using the backpropagation algorithm (Rubio et al., 2011). Iterative adjustments were made to minimize the loss function using a large datasets of bone scaffolds. In the present study, the mean absolute error (MAE) between the FE prediction and the CNN prediction was set as the objective (loss) function:
where
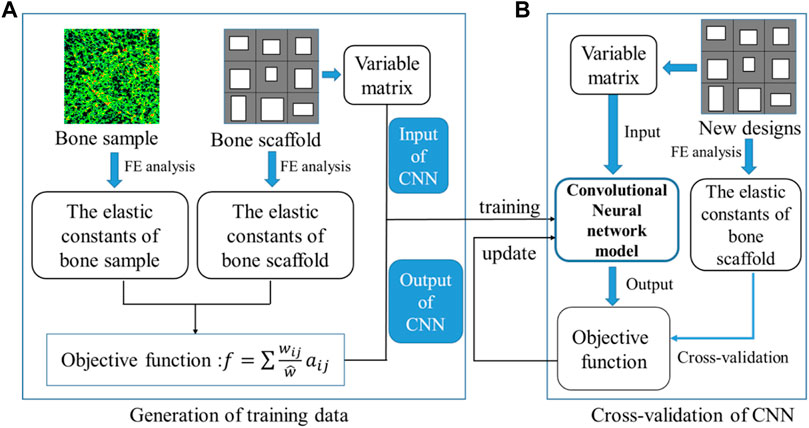
FIGURE 8. Training and cross-validation of the convolutional neural network model for predicting the elastic constants of bone scaffold, (A) generation of the training data and (B) cross-validation of the CNN model.
To assess the predictive power of the CNN model constructed, 500 new bone scaffolds were processed. The values of these bone scaffolds, calculated as the objective function for optimization, i.e., that presented in Eq. 8, were calculated using the trained CNN model and the FE method, respectively. The FE predictions were served as the ground truths and the predictive power of the CNN model was obtained by comparing the values obtained from the CNN and the FE models. The linear correlation analysis was performed between the CNN and FE predictions using the 500 data samples. Because the variance between the CNN and FE predictions increased with the amplitude of the values, the log transformed values were plotted and analyzed, in which the following transformation formula was used:
where
In summary, the CNN-based framework for designing the anisotropic scaffold is shown in Figure 9. It should be noted that a self-learning process was introduced into the scaffold design to accelerate the optimization process. The self-learning process consists of two parts: unsupervised learning (Figure 9A) and optimization variable (Figure 9B). Specifically, the process can be briefly explained as below: in the iterations, 10,000 new samples were generated from which the first 100 optimal designs were selected as the initial optimal samples. The process was repeated and the first 100 optimal designs were updated after each iteration, i.e., if the design is better than those in the 100 optimal designs and then it is used to replace the worst design in the 100 designs. Because some errors are presented in the CNN model and the MAE is the average prediction error between the prediction and the actual values, to avoid the exclusion of the optimal samples, all the designs with the error less than MAE remained in the iterations.
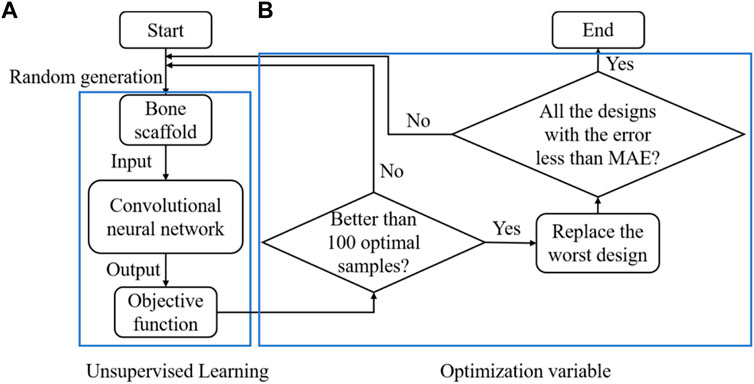
FIGURE 9. The design of the anisotropic scaffold using the self-learning CNN model, (A) unsupervised learning and (B) optimization variable.
Using the framework presented in Figure 9, the bone scaffold was designed to mimic the anisotropic mechanical behavior of a specific defected bone sample, which is one of the main design objectives in scaffold design (Gómez et al., 2016; Kang et al., 2020). To demonstrate the ability of the CNN based framework in designing the scaffolds for different bone samples and also to take into account the variances among different bones, 12 bone samples with different porosities and structures, obtained from the HR-pQCT images, were processed and the corresponding scaffolds were designed using the CNN and conventional methods. Statistical analysis was performed and the normality check for all the data samples was performed using the statistical program PASW statistics (SPSS Inc., Chicago, IL) and the probability of type I error was set to α = 0.05, i.e., p < 0.05 was considered normal distribution.
To quantify the differences between the mechanical properties of the bone scaffolds and those of the bone samples, the relative error (RE) was used, which is defined as below:
where
To enable the process of a large amount of bone scaffolds, all the pre-processing and post-processing were automated using the in-house developed Matlab (R2019, MathWorks, Natick, Massachusetts, United States) code and the FE analysis was performed using the Ansys (v18.0, ANSYS, Inc., Canonsburg, PA, United States). The CNN model was constructed using the Tensorflow 2.0 module in Python 3.7. The training process was conducted on a desktop computer setting to i7-8700 CPU, 32G RAM, and the Nvidia GTX1060. The batch size was set to 128 and the training was iterated for 200 epochs. The training process took approximately 2.0 h.
3 Results
3.1 Cross-validation and prediction power of the convolutional neural network model
The relationship between the mean absolute error (MAE) and the training iterations is shown in Figure 10. Because the initial values of the weights and biases are randomly assigned, the MAEs of the first a few iterations are high. However, after several iterations, the MAE descends rapidly and the MAE is below 1.0 after 120 training epochs. Therefore, no over-fitting is observed in the cross-validation of the CNN model developed. In the process of self-learning accelerated optimization, the changes of samples were recorded. It can be seen that this procedure ensured the errors in all the 100 optimal designs are less than MAE after some iterations (Figure 11). Afterwards, an optimal sample can be obtained from these 100 samples.
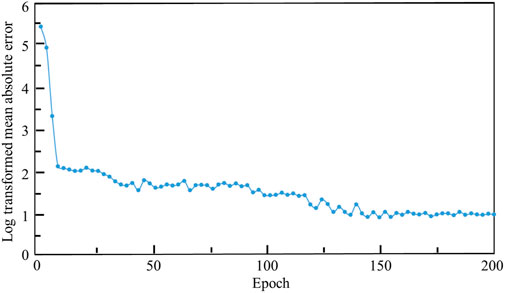
FIGURE 10. The relationship between the log transformed mean absolute error, where Y = ln (MAE)+1 is used, and the epoch.
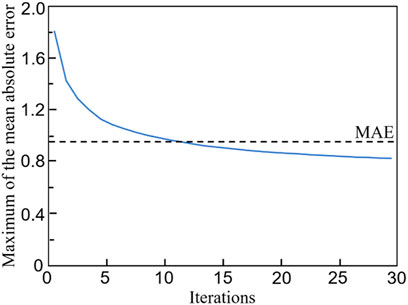
FIGURE 11. The evolution of the maximum mean absolute error (MAE) in 100 optimal samples with the number of iterations.
The linear correlation between the log transformed objective functions obtained from the CNN and FEM is shown in Figure 12A. A high coefficient of determination (R2) has been achieved, i.e. R2 = 0.95, implying a good prediction power of the CNN model. To further demonstrate the prediction power of the CNN model, Bland-Altman diagram of the objective function (f) between the FEM and CNN predictions is presented in Figure 12B, where the values in the x axis represent the mean objective functions obtained from the CNN and FEM predictions (both calculated using Eq. 8 and the values in the y axis represent the difference between the objective functions. It is shown in the figure that the mean difference, represented in the blue line, is very close to zero. Additionally, 493 out of 500 samples (98.6%) fall in the confidence interval from −1.96 standard deviation (SD) to +1.96 SD (corresponding to 6.28) and only seven samples are out of this interval, implying a high degree of agreement between the CNN and the FEM predictions.
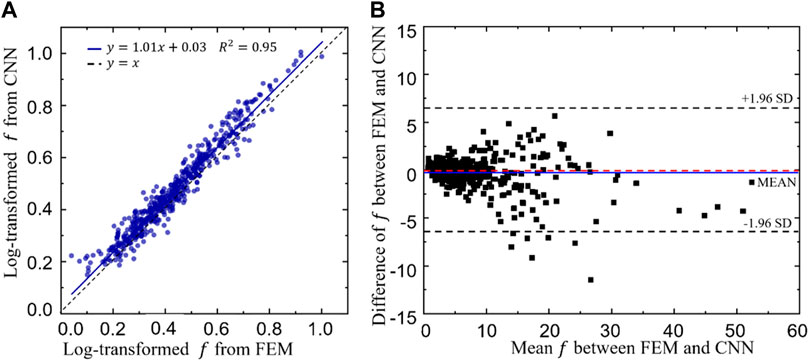
FIGURE 12. (A)The relationship between the log transformed objective functions predicted from the convolutional neural network (CNN) model and those calculated from the finite element method (FEM). (B) Bland-Altman diagram of the objective function (f) between the FEM and CNN predictions.
3.2 Design results from the self-learning convolutional neural network model
Twelve different bone samples are selected as the tissues to be replaced and the corresponding 12 bone scaffolds are designed using the self-learning CNN model developed. To assess the performance of the self-learning CNN model, the agreement between the elastic constants of the bone samples and those of the designed scaffolds was assessed using the Bland-Altman diagram (Figure 13), where the values in the x axis represent the mean elastic constants obtained from the bone samples and the bone scaffolds and the values in the y axis represent the differences between the elastic constants of the bone samples and those of bone scaffolds. It is shown in the figure that for the constants
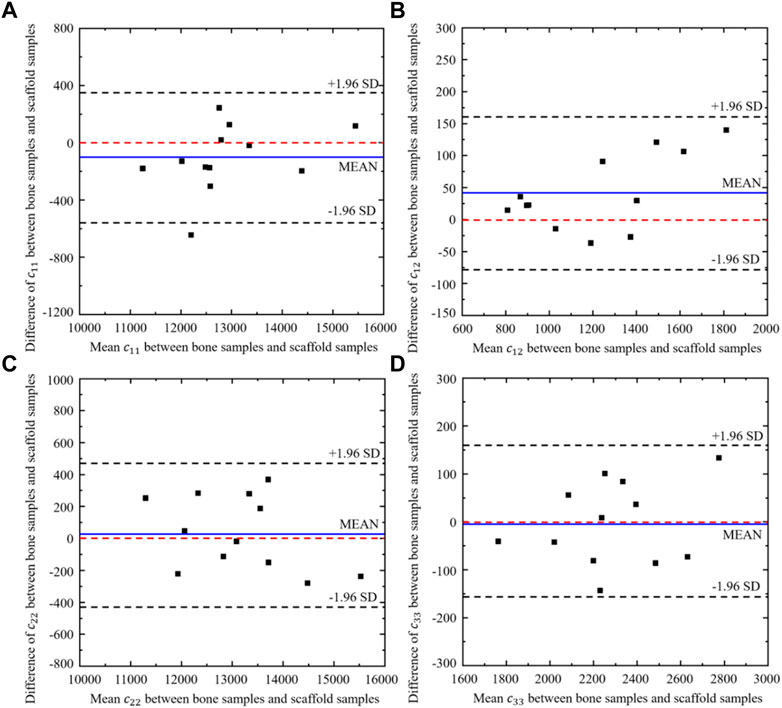
FIGURE 13. Bland-Altman diagram of elastic constants between the scaffold designed from CNN model and the corresponding bone samples (n = 12).
To further demonstrate the differences between bone scaffolds and bone samples, the relative errors in the four elastic constants, calculated using Eq. 10, are presented in Figure 14, where MAX and MIN represent the maximum and minimum values in the dataset, respectively.
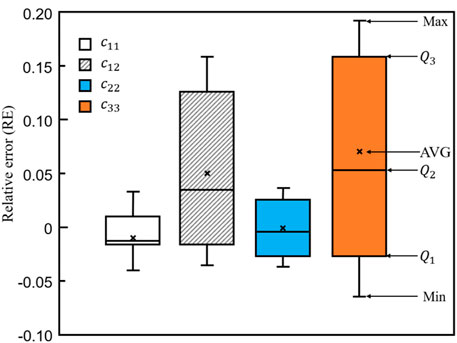
FIGURE 14. The relative errors in the four elastic constants designed by the self-learning CNN model.
3.3 Comparison of the results obtained from the convolutional neural network and conventional methods
To demonstrate the superior performance of the self-learning CNN model over the conventional method, the relative errors in the four elastic constants are presented in Figure 15. It is shown in the figure that for all the four constants, the average errors are closer to zero in the CNN group than those in the conventional method group. Furthermore, the relative errors from
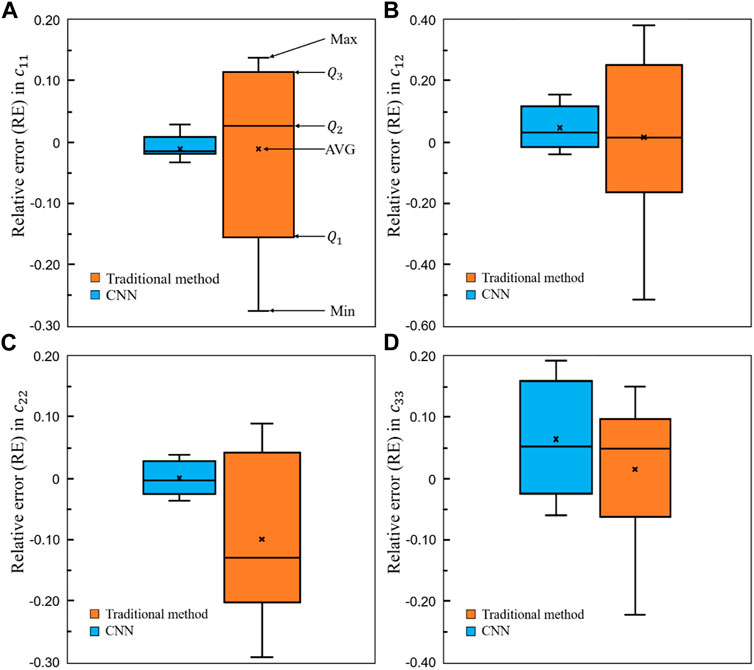
FIGURE 15. Comparison of the relative errors in the four elastic constants between the self-learning CNN and conventional methods (n = 12).
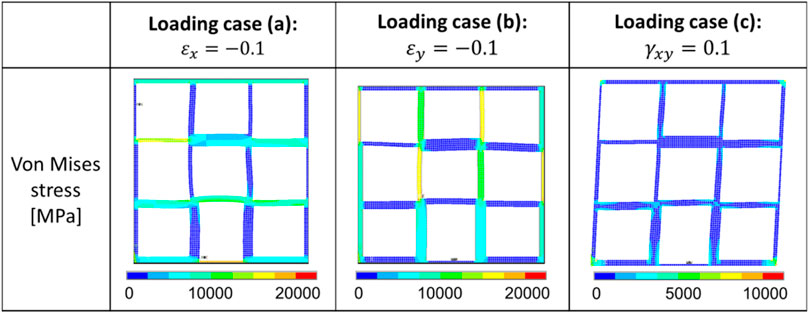
FIGURE 16. Distribution of the von Mises stress in one representative optimal scaffold under the three different loading cases.
4 Discussion
In the present study, a novel self-learning convolutional neural network (CNN) model was developed and its performance in designing the bone scaffolds with the anisotropic mechanical properties matched to the targeted bone tissue was demonstrated. The novelty of the present study lies in the novel design of anisotropic bone scaffolds using the emerging self-learning CNN technique.
The present study was motivated by the fact that it is crucial to design bone scaffolds with the anisotropic mechanical properties matched to those of the replaced defected bone tissues, but it is very challenging to realize this using the conventional design approaches because of the large amount of design variables involved. The emerging machine learning technique has the potential to solve the challenges. Indeed, it is revealed in the present study that the design and optimization of one anisotropic scaffold took approximately only 20 min excluding the time used in the training and cross-validation of the CNN model, which took approximately 2 days (including pre-processing of the 10,000 bone scaffolds, the model generation, the FE calculation, the post-processing, etc.). In contrast, it would take much more time to complete the design and optimization involving the same number of design variables, i.e., 36, using the conventional method, such as LSM. It should be noted that the more the independent design variables are involved in the design (e.g., in the 3D case), the more obvious efficient the self-learning CNN method is. The other advantage of the CNN technique is that because there is no constraint on the number of design variable, the design space can be largely expanded. As a consequence, new novel material designs can be made possible using the CNN model. Therefore, the CNN method has the great potential to be used as a novel and crucial tool in the design of 3D porous implants.
Regarding the ability of the self-learning CNN model in designing the scaffolds with anisotropic mechanical properties matched to those of the defected bone, a high ability has been achieved in the present study, which was assessed using the elasticity matrix. It should be noted that when evaluating the mechanical properties of bionic bone scaffolds, it is crucial to use the elasticity matrix of the structure, because the porous scaffolds are not isotropic. Nevertheless, in most previous studies (Dang et al., 2018; Zheng et al., 2021), the mechanical properties of scaffolds under just one or two loading scenarios are investigated and consequently their conclusions are limited to certain conditions.
The bionic scaffold with the mechanical properties matched to those of the native bone tissue is crucial in the clinical application, because when the scaffold is implanted in the human body, it has to be working together with the surrounding soft and hard tissues. If the mechanical properties of the implanted scaffold are too high, the stress shielding may occur and eventually affect the life expectance of the scaffold [ (Gómez et al., 2016), (Bloyer et al., 2007)]. If the mechanical properties of the implanted scaffold are too low, the scaffold may fail to afford the external loadings. Additionally, because of the daily activities of human body, all sorts of loading scenarios may occur in the bone (Huang et al., 2017) and consequently the anisotropic mechanical properties should be taken into account in the design of scaffold. Over the thousand years’ evolution, the native bone tissues have been optimized to be the best structures in daily activities. Therefore, the designed artificially bone scaffold should possess the mechanical properties similar to those of the native bone tissues. The present study has advanced the design of bone scaffold towards this goal and it is demonstrated that the bone scaffolds designed using the CNN model indeed possess the anisotropic mechanical properties very similar to those of the native bone tissue.
Some shortcomings in the present study should be noted. First, only one topology (i.e., rectangular) of the scaffold was investigated. The primary aim of the present study was to demonstrate the application of the CNN technique in the design of scaffolds with anisotropic mechanical properties. Nevertheless, in the future, the scaffolds with other topologies, such as sphere shape, etc. should also be investigated. Second, the simplified method (Figures 2, 3) for reconstructing the elastic matrices of bone and anisotropic scaffolds is used in the present study. More rigorous method such as periodic boundary condition should be incorporated in the future to have an accurate representation of the elastic matrix of the fully anisotropic structure. Third, due to the complexities in the design problem, only the 2D examples were presented and the plane stress scenario was assumed. In the current setting, there are 36 independent design variables and
In conclusion, 2D bone scaffolds with the anisotropic mechanical properties matched to those of the defected native bone tissue were successfully designed using a self-learning convolutional neural network model. It is revealed in the present study that not only the design space of the scaffolds can be expanded using the CNN method, but also the scaffolds designed by the CNN model possess the anisotropic mechanical properties better matched to those of the native bone tissue. Furthermore, the CNN model is efficient, because once the CNN model is well trained, it takes approximately only 20 min to complete the design of bone scaffold involving 36 independent design variables. Therefore, the CNN model developed possesses great potentials in the design of anisotropic bone scaffolds in the clinical setting.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YL, TG, ZY: conceptualization, methodology, formal analysis, investigation, writing—original draft. HZ: conceptualization, analysis, writing—review and editing. YaL, CW: resources, writing—review and; editing, supervision.
Funding
This work was supported by the National Natural Science Foundation of China (12072066, 12172083, U1908233), the Doctoral Scientific Research Foundation of Liaoning Province (2020-BS-280), the Natural Science Foundation of Dalian (21Z11019, 2021RQ025), the DUT-BSU grant (ICR 2103) and the Fundamental Research Funds for the Central Universities, China (DUT21TD105, DUT21LK21).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Ketan, O., Rashid, K., and Al-Rub, A. (2019). Multifunctional mechanical metamaterials based on triply periodic minimal surface lattices. Adv. Eng. Mat. 21 (10), 1900524. doi:10.1002/adem.201900524
Ataee, A., Li, Y., Fraser, D., Song, G., and Wen, C. (2018). Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 13, 345–354. doi:10.1016/j.matdes.2017.10.040
Bloyer, D. R., McNaney, J. M., Cannon, R. M., Saiz, E., Tomsia, A. P., and Ritchie, R. O. (2007). Stress-corrosion crack growth of Si-Na-K-Mg-Ca-P-O bioactive glasses in simulated human physiological environment. Biomaterials 28 (33), 4901–4911. doi:10.1016/j.biomaterials.2007.08.005
Bonatti, C., and Mohr, D. (2019). Smooth-shell metamaterials of cubic symmetry: Anisotropic elasticity, yield strength and specific energy absorption. Acta Mater. 164, 301–321. doi:10.1016/j.actamat.2018.10.034
Bucklen, B. S., Wettergreen, W. A., Yuksel, E., and Liebschner, M. A. K. (2008). Bone-derived CAD library for assembly of scaffolds in computer-aided tissue engineering. Virtual Phys. Prototyp. 3 (1), 13–23. doi:10.1080/17452750801911352
Burton, W. S., Myers, C. A., Jensen, A., Hamilton, L., Shelburne, K. B., Banks, S. A., et al. (2021). Automatic tracking of healthy joint kinematics from stereo-radiography sequences. Comput. Biol. Med. 139, 104945. doi:10.1016/j.compbiomed.2021.104945
Chen, Z., Xie, Y. M., Wu, X., Wang, Z., Li, Q., and Zhou, S. (2019). On hybrid cellular materials based on triply periodic minimal surfaces with extreme mechanical properties. Mater. Des. 183, 108109. doi:10.1016/j.matdes.2019.108109
Dang, M., Saunders, L., Niu, X., Fan, Y., and Ma, P. (2018). Biomimetic delivery of signals for bone tissue engineering. Bone Res. 6 (3), 25–216. doi:10.1038/s41413-018-0025-8
Davoodi, E., Montazerian, H., Mirhakimi, A. S., Zhianmanesh, M., Ibhadode, O., Shahabad, S. I., et al. (2021). Additively manufactured metallic biomaterials. Bioact. Mater. 15, 214–249. doi:10.1016/j.bioactmat.2021.12.027
Finkemeier, C. G. (2002). Bone-grafting and bone-graft substitutes. J. Bone Jt. Surgery-American Volume 84 (3), 454–464. doi:10.2106/00004623-200203000-00020
Gómez, S., Vlad, M. D., López, J., and Fernández, E. (2016). Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 42, 341–350. doi:10.1016/j.actbio.2016.06.032
Gu, G. X., Chen, C. T., Richmond, D. J., and Buehler, M. J. (2018). Bioinspired hierarchical composite design using machine learning: Simulation, additive manufacturing, and experiment. Mat. Horiz. 5 (5), 939–945. doi:10.1039/C8MH00653A
Huang, K. L., Marcora, E., Pimenova, A. A., Di-Narzo, A. F., Kapoor, M., Jin, S. C., et al. (2017). A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer's disease. Nat. Neurosci. 20 (8), 1052–1061. doi:10.1038/nn.4587
Huo, Y., Lu, Y., Meng, L., Meng, L., Wu, J., Gong, T., et al. (2021). A critical Review on the design, manufacturing and assessment of the bone scaffold for large bone defects. Front. Bioeng. Biotechnol. 9, 753715. doi:10.3389/fbioe.2021.753715
Huo, Y., Lyu, Y., Bosiakov, S., and Han, F. (2022). A critical Review of the design, manufacture, and evaluation of bone joint replacements for bone repair. Materials 15 (1), 153. doi:10.3390/ma15010153
Kang, J., Dong, E., Li, D., Dong, S., Zhang, C., and Wang, L. (2020). Anisotropy characteristics of microstructures for bone substitutes and porous implants with application of additive manufacturing in orthopaedic. Mater. Des. 191, 108608. doi:10.1016/j.matdes.2020.108608
Laurencin, C., Khan, Y., and El-Amin, S. F. (2006). Bone graft substitutes. Expert Rev. Med. Devices 3 (1), 49–57. doi:10.1586/17434440.3.1.49
Li, X., Liu, Z., Cui, S., Luo, C., Li, C., and Zhuang, Z. (2019). Predicting the effective mechanical property of heterogeneous materials by image based modeling and deep learning. Comput. Methods Appl. Mech. Eng. 347, 735–753. doi:10.1016/j.cma.2019.01.005
Liu, F., Mao, Z., Zhang, P., Zhang, D., Jiang, J., and Ma, Z. (2018). Functionally graded porous scaffolds in multiple patterns: New design method, physical and mechanical properties. Mater. Des. 160, 849–860. doi:10.1016/j.matdes.2018.09.053
Lu, Y., Krause, M., Bishop, N., Sellenschloh, K., Glüer, C. C., Püschel, K., et al. (2015). The role of patient-mode high-resolution peripheral quantitative computed tomography indices in the prediction of failure strength of the elderly women's thoracic vertebral body. Osteoporos. Int. 26 (1), 237–244. doi:10.1007/s00198-014-2846-7
Lu, Y., Zhu, Y., Krause, M., Huber, G., and Li, J. (2019a). Evaluation of the capability of the simulated dual energy X-ray absorptiometry-based two-dimensional finite element models for predicting vertebral failure loads. Med. Eng. Phys. 69, 43–49. doi:10.1016/j.medengphy.2019.05.007
Lu, Y., Zhao, W., Cui, Z., Zhu, H., and Wu, C. (2019b). The anisotropic elastic behavior of the widely-used triply-periodic minimal surface based scaffolds. J. Mech. Behav. Biomed. Mat. 99, 56–65. doi:10.1016/j.jmbbm.2019.07.012
Niinomi, M. (1998). Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 243 (1), 231–236. doi:10.1016/S0921-5093(97)00806-X
Peng, X., Huang, Q., Zhang, Y., Zhang, X., Shen, T., Shu, H., et al. (2021). Elastic response of anisotropic Gyroid cellular structures under compression: Parametric analysis. Mater. Des. 205, 109706. doi:10.1016/j.matdes.2021.109706
Rane, L., Ding, Z., and McGregor, A. H. (2019). Deep learning for musculoskeletal force prediction. Ann. Biomed. Eng. 47 (3), 778–789. doi:10.1007/s10439-018-02190-0
Rubio, J. J., Angelov, P., and Pacheco, J. (2011). Uniformly stable backpropagation algorithm to train a feedforward neural network. IEEE Trans. Neural Netw. 22 (3), 356–366. doi:10.1109/TNN.2010.2098481
Tan, R. K., Zhang, N. L., and Ye, W. (2020). A deep learning–based method for the design of microstructural materials. Struct. Multidiscipl. Optim. 61 (4), 1417–1438. doi:10.1007/s00158-019-02424-2
Vijayavenkataraman, S., Zhang, L., Zhang, S., Zhang, S., His, F., Jerry, Y., et al. (2018). Triply periodic minimal surfaces sheet scaffolds for tissue engineering applications: An optimization approach toward biomimetic scaffold design. ACS Appl. Bio Mat. 1 (2), 259–269. doi:10.1021/acsabm.8b00052
Wang, G., Shen, L., Zhao, J., Liang, H., Xie, D., Tian, Z., et al. (2018). Design and compressive behavior of controllable irregular porous scaffolds: Based on voronoi-tessellation and for additive manufacturing. ACS Biomater. Sci. Eng. 4 (2), 719–727. doi:10.1021/acsbiomaterials.7b00916
Xiao, P., Haque, E., Zhang, T., Dong, X. N., Huang, Y., and Wang, X. (2021). Can DXA image-based deep learning model predict the anisotropic elastic behavior of trabecular bone? J. Mech. Behav. Biomed. Mater. 124, 104834. doi:10.1016/j.jmbbm.2021.104834
Keywords: scafffold design, convolutional neural network, anisotropic property, bone tissue, finite element modeling
Citation: Lu Y, Gong T, Yang Z, Zhu H, Liu Y and Wu C (2022) Designing anisotropic porous bone scaffolds using a self-learning convolutional neural network model. Front. Bioeng. Biotechnol. 10:973275. doi: 10.3389/fbioe.2022.973275
Received: 20 June 2022; Accepted: 07 September 2022;
Published: 27 September 2022.
Edited by:
Zhen Luo, University of Technology Sydney, AustraliaReviewed by:
Yuhang Chen, Heriot-Watt University, United KingdomValentin Mateev, Technical University, Sofia, Bulgaria
Copyright © 2022 Lu, Gong, Yang, Zhu, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yadong Liu, ZG9jdG9yeWFkb25nQDE2My5jb20=
 Yongtao Lu
Yongtao Lu Tingxiang Gong
Tingxiang Gong Zhuoyue Yang3
Zhuoyue Yang3 Hanxing Zhu
Hanxing Zhu