- 1Hunan Key Laboratory of Biomedical Nanomaterials and Devices, Hunan University of Technology, Zhuzhou, China
- 2Zhuzhou Hospital Affiliated to Xiangya School of Medicine, Department of Pathology, Central South University, Zhuzhou, China
- 3College of Food Science and Engineering, Central South University of Forestry and Technology, Changsha, China
The global burden of foodborne disease is enormous and foodborne pathogens are the leading cause of human illnesses. The detection of foodborne pathogenic bacteria has become a research hotspot in recent years. Rapid detection methods based on immunoassay, molecular biology, microfluidic chip, metabolism, biosensor, and mass spectrometry have developed rapidly and become the main methods for the detection of foodborne pathogens. This study reviewed a variety of rapid detection methods in recent years. The research advances are introduced based on the above technical methods for the rapid detection of foodborne pathogenic bacteria. The study also discusses the limitations of existing methods and their advantages and future development direction, to form an overall understanding of the detection methods, and for point-of-care testing (POCT) applications to accurately and rapidly diagnose and control diseases.
Introduction
Foodborne pathogens continue to cause many intestinal diseases in humans around the world, causing a huge health and economic burden (Ling et al., 2019; Akter et al., 2021; Prata et al., 2021; Qiu et al., 2021). Figures from the World Health Organization (WHO) estimate that about 2 billion people die each year from diarrhea or disease caused by contaminated food, and 30% of them are children under 5 years of age (Scallan Walter et al., 2020; Belina et al., 2021; Van Puyvelde et al., 2021). The United States has one of the safest food supplies in the world, yet one in four people gets sick from foodborne diseases every year (Hoffmann and Walter, 2020; Hoffmann et al., 2021; Ge et al., 2022). The frequency and importance of these foodborne diseases depend on interactions between foodborne pathogens, hosts, food, and the environment (Ishaq et al., 2021; Jahan et al., 2021; Saravanan et al., 2021; Zarkani and Schikora, 2021). Bacterial foodborne illnesses are caused by infections with bacterium, such as Salmonella, Campylobacter spp., Escherichia coli, Shigella, Vibrio, Listeria monocytogenes (LM) and Clostridium botulinum, and Clostridium perfringens (Liu et al., 2019a; Xiao et al., 2019; Christopher et al., 2021; Fuochi et al., 2021; Kim et al., 2021a; Darbandi et al., 2022; Oyejobi et al., 2022). Viruses commonly reported are Norovirus and Hepatitis A, while examples of parasites involved are Cryptosporidium spp, Giardia lamblia, Trichinella spiralis, Cyclospora spp, Toxoplasma canis, and Entamoeba histolytica (Stryinski et al., 2020; Bozkurt et al., 2021; Lee and Yoon, 2021; Segeritz et al., 2021; Patel et al., 2022). Typical symptoms of foodborne illness include abdominal pain, diarrhea, vomiting, nausea, fever, difficulty breathing, and even death in severe cases (Abebe et al., 2020; Aik et al., 2020; Myintzaw et al., 2021; Sun et al., 2021; Janekrongtham et al., 2022). These symptoms are caused by ingested pathogens, such as foodborne infections (Salmonellosis, Listeriosis, etc.) (Gallo et al., 2020; Jang et al., 2021), or by microbial toxins produced in the host, such as toxic infections (C. perfringens food poisoning, etc.) (Rajkovic et al., 2020; Sharma et al., 2021). In the case of foodborne poisoning, toxins produced by pathogens in food cause symptoms (C. botulinum food poisoning, etc.) (Augustin et al., 2020; Walter et al., 2021). Poultry, ground meat, seafood, milk and dairy products, fruits, and vegetables have been blamed for most of the outbreaks (Leon Madrazo and Segura Campos, 2020; Visciano and Schirone, 2021; Singha et al., 2022).
Because foodborne pathogens pose a great threat to public health, it is therefore important to detect these pathogens (Dumen et al., 2020; Teffo and Tabit, 2020; Du et al., 2021a; Mi et al., 2021). Traditional methods for food pathogen detection mainly include plate separation, chemical analysis, and immunoassay (Wang et al., 2020a; Wang et al., 2020b; Han et al., 2021; Weng et al., 2021). However, these methods have more or less shortcomings, such as cumbersome steps, long detection cycle, high cost, and high requirements for a professional level of operators (Zhang et al., 2020a; Vidyadharani et al., 2021; Xie et al., 2021a; Nassarawa et al., 2022). Therefore, it is urgent to develop simple, sensitive, rapid, and low-cost methods for the detection of pathogenic bacteria in complex food samples.
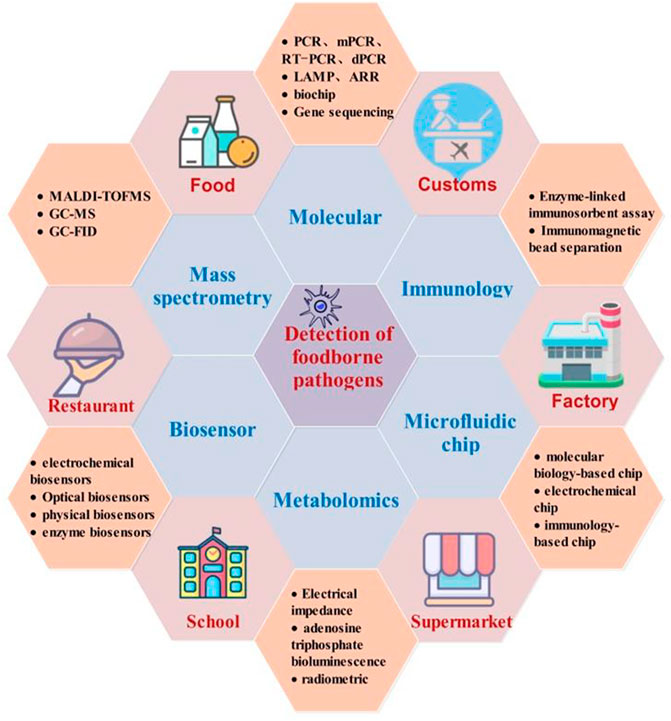
Point-of-care testing (POCT) technology is a rapidly developing foodborne pathogen detection method in recent years that has advantages, such as simple operation, rapid operation, portability, and automation (Huang et al., 2018; Xu et al., 2021). In this study, applications of POCT in the detection of foodborne pathogens based on biomolecules, immunoassay, gene sequencing, microfluidic, metabolism, biosensor, mass spectrometry, and related technologies in recent years have been reviewed (Figure 1). The principle, advantages, and disadvantages of each method and its application status are described. The existing problems and future development of rapid detection methods are also discussed. This study provides a reference for the development of rapid detection technology for foodborne pathogens and has certain significance for research on various disciplines and food safety supervision.
Immunological detection techniques
From its birth to its current development, the wide application of immunological detection technology determines its dominant position in the fields of biological science, food science, and clinical medicine. Its principle is based on antibody–antigen interaction, that is, the binding of specific antibodies (polyclonal antibody or monoclonal antibody) to their specific antigens (Jayan et al., 2020; Mishra et al., 2020; Wang et al., 2020c; Pires et al., 2021; Sohrabi et al., 2022).
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) is a sensitive and specific analytical biochemical method that can be used for the detection and quantitative or qualitative analysis of analytes without requiring sophisticated or expensive equipment (Ferone et al., 2020; Nath et al., 2020; Rani et al., 2021; Kotsiri et al., 2022). At present, although traditional ELISA is widely used in scientific research and testing institutions and is the ideal method for the detection of viruses and antibodies (Leva-Bueno et al., 2020; Navarro et al., 2020; Xiao et al., 2021), this traditional method is time-consuming and also requires skilled operation techniques and sophisticated instruments (Luo et al., 2020a; Razmi et al., 2020; Huang et al., 2022). So the researchers changed the traditional approach.
Zuo et al. (2021) developed an indirect ELISA for testing the broad spectrum of anti-NoV antibodies (Figure 2A). The entire process of testing the spectrum of unknown antibodies required 2 h for completion. The intra-assay and inter-assay coefficients of variation were less than 10%. He et al. (2020) developed a sandwich-ELISA, which could detect 6 CFU/ml of Salmonella enteritidis (S. enteritidis) in milk after 10 h of enrichment. Zhao et al. (2020) developed a wax-printed paper-based enzyme-linked immunosorbent assay (P-ELISA) based on microfluidic paper-based analytical devices, with the whole operation time being less than 3 h and only needing 5 μl of samples for detection. The limit of detection for E. coli O157:H7 (E. coli O157:H7) reached 104 CFU/ml.
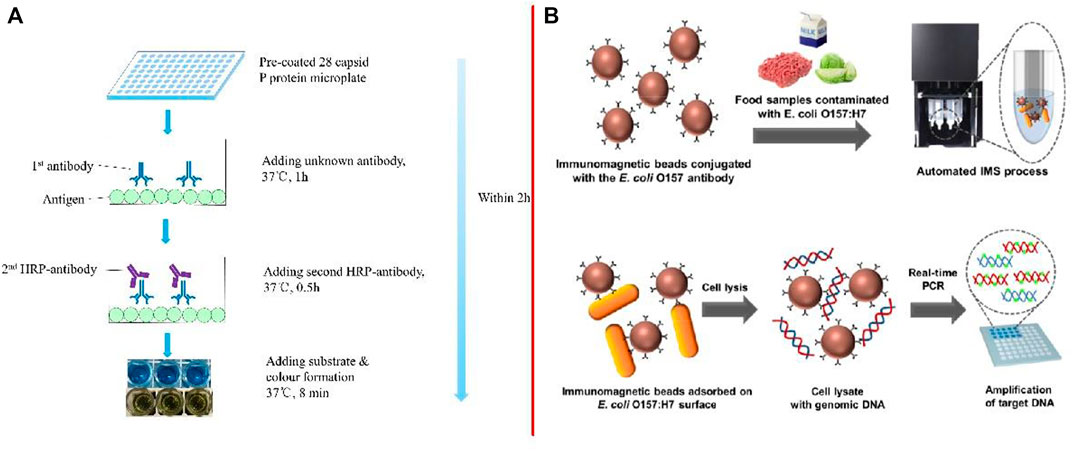
FIGURE 2. (A) Step-by-step schematic representation of ELISA (Zuo et al., 2021); (B) Schematic illustration of the detection method for E. coli O157:H7 using the automated IMS device combined with real-time PCR (Park et al., 2020).
Immunomagnetic separation technology
Immunomagnetic separation (IMS) is an effective pre-concentration technique for food samples that can quickly and selectively separate and concentrate target bacteria from complex food substrates (Liu et al., 2019c; Fang et al., 2021a; Nadar et al., 2021). The main principle is the surface of superparamagnetic particles after chemical modification, combined with target bacteria-specific active protein made of immunomagnetic bead separation (IMBS), and then the antibodies on IMBS will specifically identify and capture the target bacteria in the samples to be tested, which leads to the formation of IMBS-target bacteria complex (Ma et al., 2012; Jiang et al., 2013; Shen et al., 2021a; Zhang et al., 2021a). Finally, the complex is rapidly separated from other impurities in the sample by the force of a magnetic field, so as to achieve the efficient and accurate concentration of the target microorganism (Li et al., 2015; Wang and Lin, 2020; Wang et al., 2020d; Hou et al., 2020; Tang et al., 2020).
IMS technology has the characteristics such as strong specificity, high sensitivity, and fast separation speed (Pissuwan et al., 2020; Zhang et al., 2020b; Qi et al., 2022) and can be combined with a variety of other technologies (Yao et al., 2020; Zhai et al., 2021; Zhao et al., 2021a; Dester and Alocilja, 2022), such as ELISA, chemiluminescence immunoassay (CLIA), flow cytometry (FCM), immunochromatography (ICA), polymerase chain reaction (PCR), and other detection methods, to make the detection process more rapid and efficient (Yang et al., 2014a; He et al., 2017; Li et al., 2017; Lin et al., 2020; Nguyen and Kim, 2020; Sourri et al., 2022).
Moreover, Park et al. (2020) described the development of an automated IMS device combined with real-time PCR for detecting foodborne bacteria (Figure 2B). Target bacteria in the range of 101–102 colony-forming units per mg or g of sample can be detected in food samples, such as milk, ground beef, and cabbage, by using the proposed approach. Lv et al. (2021) developed an IMS technique by combining improved propidium monoazide and droplet digital PCR to detect the pathogenic viable but non-culturable Cronobacter sakazakii (C. sakazakii). The detection limit for this method in a background of powdered infant formula (PIF) was 5.6 copies/g. Jiang et al. (2020) first detected Vibrio parahaemolyticus (V. parahaemolyticus) in oysters by recombinant enzyme polymerase amplification (RPA) and side-flow (LF) combined with IMS. The method effectively combined sample preparation, amplification, and detection on one platform and could also detect V. parahaemolyticus within 15 min.
The IMS technology also has some limitations, such as the selected antigen target should have strong specificity, can specifically enrich the target bacteria, and avoid the enrichment of miscellaneous bacteria (Wang et al., 2020e; Zhai et al., 2020). Therefore, in order to rapidly develop in the field of foodborne pathogenic bacteria detection, specific antibodies from pathogenic bacteria must be screened.
Other immunological techniques
In addition to the above two immunological detection techniques, immuno chromatography (IC), immunodiffusion, immunofluorescence, Western blot, and Latex agglutination have all been applied in the detection of foodborne pathogens (Zhou et al., 2017; Li et al., 2018; Wu et al., 2020a; Morales-Pablos et al., 2020; Gao et al., 2021; Lopes-Luz et al., 2021; Wangman et al., 2021; Zhao et al., 2021b). Immunology technology has good specificity, high efficiency, low testing cost, and does not need the advantages of large instrument, but when the influenza virus contains competitive target bacteria in food material is very likely a false-positive result, the sensitivity is not high, these factors limit the immunology technology widely application in detection of foodborne pathogenic bacteria (Wang and Park, 2020; Zhao and Wu, 2020; Yan et al., 2021).
Molecular biology detection technology
Molecular biology detection technology is based on nucleic acid, through the detection of specific target pathogens DNA or RNA for detection purposes (Xi et al., 2014; Liu et al., 2017a; Foddai and Grant, 2020; Zheng and Tan, 2020; Kim and Oh, 2021). It is achieved by hybridizing the target sequence with complementary probes or primers (Yang et al., 2014b; Yang et al., 2017; Chen et al., 2020a; Wachiralurpan et al., 2020).
Temperature-changing amplification technology
Variable temperature amplification is a method based on PCR technology. Conventional PCR can only detect one pathogen at a time, but there are many pathogenic bacteria in food (Kim and Kim, 2021). Therefore, based on conventional PCR, dozens of different types of PCR methods were derived, mainly including multiple polymerase chain reaction (mPCR), real-time quantitative polymerase chain reaction (qPCR), and digital polymerase chain reaction technology (dPCR) (Tang et al., 2018; Mou et al., 2019a; Lei et al., 2020a; Cardoso et al., 2020; Huang et al., 2021a).
mPCR is based on traditional PCR, whereby multiple pairs of specific primers and templates are added into the same PCR reaction system (primers are specifically bound to corresponding templates) to amplify multiple DNA fragments with different sequences (Zhang et al., 2020c; Hossain et al., 2021; Bonny et al., 2022; Liu et al., 2022). Multiple DNA fragments amplified in the same reaction system can simultaneously detect multiple foodborne pathogens, reduce the number of experimental operations, shorten the detection time, and save reagents (Ma et al., 2020a; Yang et al., 2020a). He et al. (2022) developed a detection system based on magnetic separation, mPCR, and capillary electrophoresis (CE) technologies for the simultaneous detection of four foodborne pathogens. The detection limit for bacterial DNA reached 10−5–10−7 ng/μl and in the analysis of mocked food samples, the assay showed good sensitivity for bacterial detection ranging from 101 to 105 CFU/ml with excellent specificity.
mPCR is suitable for the detection of multiple foodborne pathogens with the same symptoms or easily contaminating the same food, which can standardize the detection of microorganisms in food (Du et al., 2020a; Ripolles-Avila et al., 2020). However, because multiple pairs of primers are amplified in the same system, each pair of primers affects each other, so the amplification effect and the actual number of amplified fragments in the actual operation of mPCR are often not satisfactory (Luo et al., 2020; Chen et al., 2021).
qPCR operates by adding fluorescent-labeled probes or fluorescent substances into the PCR system and monitors the accumulation of fluorescence signals in the whole PCR amplification process in real-time through the instrument. Finally, the method for quantitative analysis of samples with unknown concentrations was carried out through standard curves (Fu et al., 2020; Baoutina and Bhat, 2021; Wan et al., 2021). Moreover, Chen et al. (2017) presented a new POCT system based on magnetic nanoparticles that enable sample in-answer out (SIAO) automated real-time testing for pathogens (Figure 3A). Real-time PCR by the two methods (TaqMan-based probe and SYBR green dye) in the SIAO system was achievable by the manual method with comparable results. Xie and Liu. (2021) developed a double TaqMan real-time fluorescence quantitative PCR (DRT-PCR) method for their simultaneous enumeration within two kinds of powdered infant foods (PIFs). The dRT-PCR could quantify as low as 102 and 101 CFU/ml C. sakazakii and Staphylococcus aureus (S. aureus) in both pure cultures and spiked PIFs.
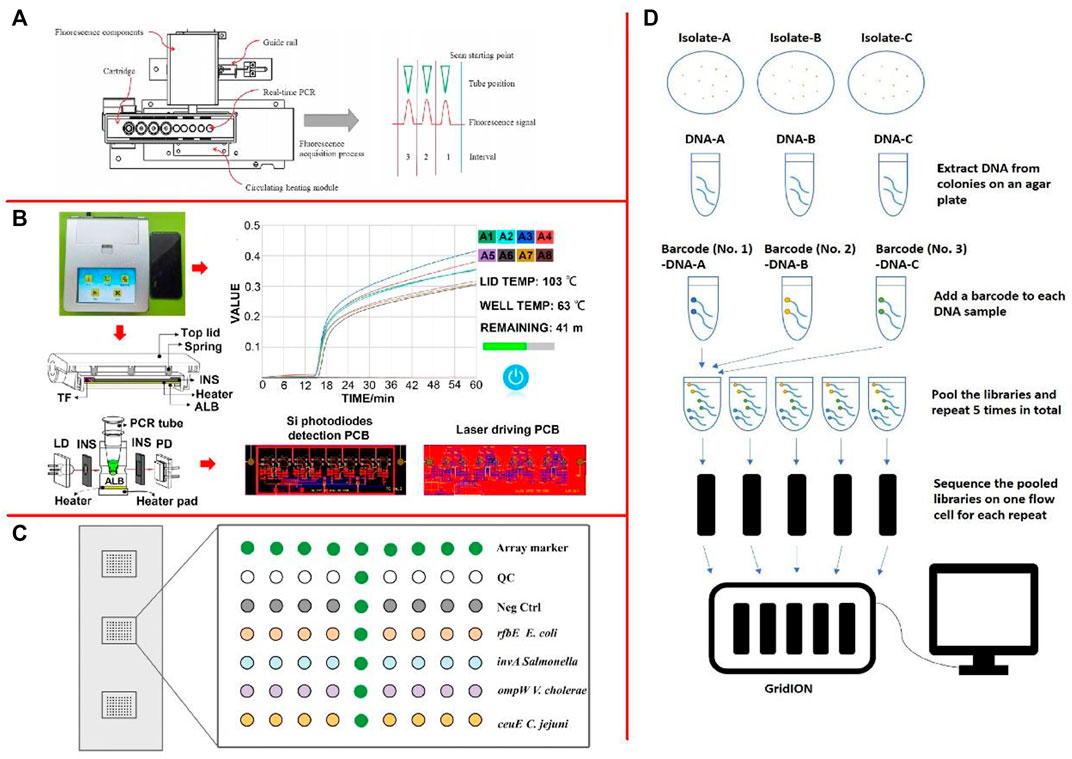
FIGURE 3. (A) Positions of the fluorescence detection module and the Peltier device, and the principle of fluorescence acquisition (Chen et al., 2017); (B) The design of the diagnostic device (Chen et al., 2022); (C) Layout of oligonucleotide probes on the detection microarray (Liu et al., 2017b); (D) An example of the workflow of multiplex ONT sequencing library preparation and sequencing (Wu et al., 2021).
Compared with ordinary PCR, the nucleic acid amplification of qPCR technology is completed in a closed system, and no electrophoresis analysis is required after the amplification, which not only reduces the chances for sample contamination, but also avoids false-positive results caused by contamination and also shortens the detection time (Obande and Singh, 2020; Kim et al., 2021b). However, the experimental cost of real-time quantitative PCR is high, and the equipment required is expensive, which also requires the operator to have a high level of professional technology (Nunez-Bajo et al., 2020; Wang et al., 2021a).
dPCR does not require the establishment of a standard curve and concentration comparison by Ct value (the number of cycles that PCR fluorescence signal goes through when it reaches a set threshold) and is considered an absolute quantitative method (Grudlewska-Buda et al., 2020; Lei et al., 2021; Plante et al., 2021). Droplet digital PCR (ddPCR) is a new method that disperses a single target DNA molecule into multiple separated droplets, detects each droplet one by one after PCR amplification, and accurately quantifies DNA copy number (Lei et al., 2020b; Iwu et al., 2020). Du et al. (2021b) developed an effective pretreatment method based on an in-situ enrichment culture with an immunomagnetic separation step, combined with ddPCR technology to achieve rapid detection of trace Salmonella in milk, which allowed detecting as low as 10−1 CFU/ml level of Salmonella. ddPCR has the advantages of high sensitivity, high accuracy, high tolerance, and absolute quantification and has been widely used in rare mutation detection and gene expression detection in complex samples (Salipante and Jerome, 2020; Yang et al., 2020b).
Constant temperature amplification technology
At present, constant temperature amplification techniques applied to foodborne pathogenic microorganisms mainly include loop-mediated isothermal amplification (LAMP), recombinant enzyme-mediated nucleic acid amplification (RAA), RPA, and nucleic acid sequence-dependent amplification (NASBA) (Chen et al., 2018; Khan et al., 2019; Safenkova et al., 2020; He et al., 2021a; Hoang et al., 2021; Tian et al., 2022).
LAMP is a mature isothermal nucleic acid amplification technique in which the target sequence was amplified with two or three sets of primers at a constant temperature of 60–65°C (Prasannakumar et al., 2020; Wang et al., 2020f; Zhang et al., 2021b). Typically, four different primers are used to amplify six different regions on the target gene, which increases specificity (Xie et al., 2022). Another pair of “cyclic primers” can further accelerate the reaction. In addition to replication activity, polymerases with high chain displacement activity are required for amplification (Garafutdinov et al., 2020; Padzil et al., 2022). Liu et al. (2022) developed a LAMP method for LM detection using SYTO9 staining and image processing techniques. The detection limit of LM was 6 copies/μl. Chen et al. (2022) developed a novel Enter cytozoon hepatopenaei (EHP) field rapid detection device (size 18.8 × 16.7 × 6.6 cm3) based on magnesium pyrophosphate precipitation and LAMP (Figure 3B). The detection limit for EHP was 0.1 fg/μl. Moreover, Jin et al. (2020) developed a LAMP-based microdevice for performing high-throughput visual detection. The approach was able to perform simultaneous identification of six foodborne pathogens within 1 h. LAMP is an isothermal amplification technique with high practical value and detection efficiency, but it also has obvious disadvantages, such as false-positive results after the addition of ring primers (Yu et al., 2021a). Although the frequency of false-positive can be reduced by various methods, it is still impossible to avoid the high requirement and difficulty in primer design.
RAA is a technique for nucleic acid amplification using recombinant enzyme, single-chain binding protein, and DNA polymerase under isothermal conditions (optimal temperature 37°C) (Feng et al., 2022a; Hou et al., 2022; Zhang et al., 2022). The established RAA method can effectively shorten the detection time and does not require temperature change during nucleic acid amplification (Aman et al., 2020; Teklemariam et al., 2020; Li et al., 2021a). Li et al. (2021b) introduced the transcleavage activity of CRISPR/Cas12a into an electrochemical biosensor (ECRISPR), combined with RAA, to establish a cost-effective, specific, and ultrasensitive method, namely, RAA-based E-CRISPR. Under optimized conditions, the RAA-based E-CRISPR can detect as low as 0.68 aM of genomic DNA and 26 CFU/ml of LM in pure cultures. Xie et al. (2021b) proposed a modified propidium monoazide (PMAxx) dye combined with RAA for the rapid and real-time detection of viable S. aureus. The detection limit for viable S. aureus was 102 CFU/ml under 3 h enrichment and 101 CFU/ml under 6 h enrichment in artificially contaminated milk, respectively. Zhou et al. (2022) reported a novel CRISPR/Cas12a-based fluorescence enhanced lateral flow biosensor (LFB) in conjunction with functionalized quantum dots, combined with RAA, to establish low-cost, simple, and sensitive detection of S. aureus, namely, CRISPR/Cas-recombinase-assisted amplification-based LFB (CRA-LFB). The limit of detection was as low as 75 aM of genomic DNA, and 5.4 × 102 CFU/ml of S. aureus in pure cultures were detected. RAA has a great development advantage due to its lower requirements on environmental temperature, operating skills, and experimental equipment (Mu et al., 2021).
Biochip technology
Biochip technology was started in the 1980s. It is a micro biochemical analysis system of molecular microarray. It uses mechanical arm sampling technology or microelectronic lithography technology to construct up to tens of thousands of different probes on the surface of a certain volume of the solid carrier to detect a variety of biological components (Li et al., 2013; Azizipour et al., 2020; Aladese and Jeong, 2021; Tahir et al., 2021). The biochip technology has the advantages such as diversification, high throughput, short detection time, and portability. At present, gene chip, protein chip technology, and liquid chip are widely used in the detection of foodborne pathogens (Pos et al., 2020; Qian et al., 2022).
Gene chip is the first developed and earliest researched and developed technology in biochip technology (Zeng et al., 2014; Chen et al., 2020b; Kumar et al., 2020; Ali et al., 2021). The sequencing principle for the gene chip is the hybridization sequencing method, by which hybridization with a group of nucleic acid probes with a known sequence of target nucleotide, and with a known sequence for nucleic acid sequencing are fixed on the surface of a substrate, (Zhang et al., 2020d; Hariharan and Prasannath, 2021; Taguchi et al., 2021). Liu et al. (2017b) developed a magnetic nanoparticle-enhanced oligonucleotide microarray assay for rapid and sensitive identification of E. coli O157:H7, Salmonella enterica, Vibrio cholerae, and Campylobacter jejuni (C. jejuni) in food (Figure 3C). In comparison with conventional single-stranded target preparation methods, this magnetic nanoparticles-based method yielded up to 15-fold increase in the hybridization signal and achieved 1 similar to 2 orders of magnitude enhancement on the limit of detection. Sarengaowa et al. (2020) developed an in situ-synthesized gene chip for the detection of foodborne pathogens on fresh-cut fruits and vegetables. The detection limit for the five target pathogens on fresh-cut cantaloupe and lettuce was approximately 3 log CFU/g without culturing and with a detection time of 24 h. Shen et al. (2020) performed the genome-wide DNA microarray analysis using S. typhimurium incubated with 0.001% epsilonpolylysine in 0.1% Bacto Soytone at 30°C for 2 h.
The high degree of automation of gene chip technology can analyze a large number of samples at one time, and the data are objective and reliable (Jia et al., 2021a). But the cost is high, with low detection sensitivity, poor repeatability, and narrow analysis scope (Zaczek-Moczydlowska et al., 2021).
Protein chip technology is a kind of protein microarray, which is different from gene chip to realize binding based on the principle of base complementary pairing. It uses the interaction between proteins, such as the reaction between antigen and antibody, enzyme and substrate, for detection (Khan et al., 2021; Zhou et al., 2021; Hang et al., 2022). With the continuous development and improved protein microarray technology, the technology has been gradually applied to the detection of foodborne pathogens. Liu et al. (2019c) constructed a protein chip to screen for antibody titers present in test sera raised against whole C. jejuni cells with over 1,400 individually purified GST-tagged C. jejuni proteins, representing over 86% of the proteome. These results indicated that the unbiased chip-based screen can reveal the full repertoire of host antibodies against microbial proteomes. Protein microarray technology is an emerging technology with bright development prospects, but there are still some problems in maintaining protein activity, protein fixation methods, and detection sensitivity (Xia et al., 2021), which need further research and optimization.
Liquid chips, also known as microsphere suspension chips, began in the mid-1990s, with their diverse fluorescent encoded microspheres, up to 100 different probes can be crosslinked by different ways of binding and hybridization (Jia et al., 2021b). Qualitative and quantitative detection of microsphere fluorescence coding and molecular fluorescence intensity by two different laser beams is a new generation of high-throughput molecular detection technology platform following DNA chip and protein microarray (Han et al., 2020; Li et al., 2020; Yin et al., 2020). Pang et al. (2019) established a microsphere-based suspension array (MSA) for the detection and identification of 55 V. parahaemolyticus K-serogroups based on CPSgc-specific genes. This system was then used to examine 845 publicly available V. parahaemolyticus genomes. Shi et al. (2019) established a rapid and accurate method based on mPCR combined with suspension array flexible sequence-tagged (xTAG) technology to simultaneously detect S. typhimurium, Brucella spp., Bacillus cereus, and Shigella spp. in raw milk. The results showed that the detection of milk samples demonstrated 100% specificity.
Gene sequencing
In molecular biology, DNA sequence analysis is the basis for further research and modification of target genes (Mou et al., 2019b; Uelze et al., 2020; Hu et al., 2021). At present, there are two types of mainstream technologies for sequencing: 1) Traditional sequencing technology, or first-generation sequencing technology, is represented by the Sanger sequencing method (Segerman, 2020; Kaprou et al., 2021). 2) The next-generation sequencing (NGS) technology developed in recent years is also known as high-throughput sequencing technology, and its sequencing principles include simultaneous sequencing and single-molecule sequencing (Reuter et al., 2015; Lu et al., 2020; Van Poelvoorde et al., 2020). Specifically, synthetization sequencing (also known as the second-generation sequencing technology) is represented by Roche’s 454 technology, Illumina’s Solexa, Hiseq technology, and ABI’s Solid technology (Lewis et al., 2020; Shen et al., 2021b). Single-molecule sequencing (also known as third-generation sequencing technology) is based on Helicos Bioscience’s HeliScope genetic analysis system, Pacific Biosciences’ PacBio RS single-molecule real-time sequencing system, and Oxfold Nanopore Examples include Technologies’ GridION and MinION (Yu et al., 2020; Gunther et al., 2021; Van Reckem et al., 2021). Single-cell sequencing technology is to sequence each individual cell through high-throughput sequencing technology to obtain the genetic information of each individual cell (Davey and Valdivia, 2020; Peng et al., 2020a).
The principle for the Sanger sequencing method is to randomly cut genomic DNA into small fragments, and then many small fragments of DNA are cloned into plasmid vectors and transformed into E. coli. Finally, the cultured E. coli extracts plasmid for sequencing, and each sequencing reaction is completed in a reaction system for only a few microliters (Efimochkina and Sheveleva, 2022). Syromyatnikov et al. (2020) used high-throughput sequencing and Sanger sequencing of individual bacterial colonies to analyze the microbial content of commercially available butter brands. We identified a total of 94 amplicon sequence variants corresponding to different microbial taxa. Sanger sequencing technology is relatively common in small bacterial genome sequencing, plasmid sequencing, and other research fields, with its accuracy, precision target, and low throughput (Maguire et al., 2021). However, in large-scale sequencing tasks, Sanger sequencing technology has defects of low throughput, slow speed, and high cost, thus promoting high-throughput sequencing technology (Sheka et al., 2021).
High-throughput sequencing technology is a revolutionary improvement on traditional sequencing technology in history, which can simultaneously determine the molecular sequence of millions or even tens of millions of DNA (Cassotta et al., 2020; Chelliah et al., 2022). Muriuki et al. (2021) used 454 pyrosequencing, Illumina high-throughput sequencing of 16S rRNA gene in the analysis of total community DNA extracted from samples using the phenol-chloroform method. Uncultured Candidatus Koribacter and Candidatus Solibacter were also detected in the food samples. There was a significant difference in the microbial community structure among the sample types (p < 0.1). Gutierrez et al. (2021) sequenced 62 cases of Shiga toxin-producing E. coli (STEC) isolated from Chile using MiSeq Illumina. The results indicated that there may be local emerging STEC with unique features, nevertheless, no molecular markers were detected. Wu et al. (2021) evaluated the serotype prediction accuracy of using whole-genome sequencing (WGS) data from multiplex ONT sequencing (Figure 3D). This study demonstrated that accurate serotype prediction results could be obtained when multiplexing five or less Salmonella isolates with an average of 6 h of multiplex ONT sequencing, where each multiplexed isolate received at least 50× depth of genome coverage of sequencing data after demultiplexing.
The advantage of second-generation sequencing technology is that the cost is greatly reduced and the flux is greatly improved compared with the first generation, but the disadvantage is that the PCR process introduced will increase the sequencing error rate to a certain extent, and has a systematic bias, and the read length is relatively short (Kaavya et al., 2021). The third-generation sequencing technology is developed to solve the shortcomings of the second generation. Its fundamental feature is single-molecule sequencing, which does not require any PCR process, in order to effectively avoid system errors caused by PCR bias, while improving the read length, and maintaining the advantages of the second-generation technology of high throughput and low cost (Quijada et al., 2020).
Microfluidic detection technology
Microfluidics provides a powerful tool for testing applications with its advantages of portability, miniaturization, automation, multi-channel sample testing, minimization of hazardous material handling, and cost savings (Ragab and El-Kimary, 2021; Su et al., 2021; Tseng et al., 2021). In addition, all analytical processes, including sample preparation, reaction, separation, and detection are integrated into a microfluidic chip for field test applications (Fu et al., 2021; Xie et al., 2021c). Biosensors that use a variety of technologies combined with microfluidic chips to detect foodborne pathogens have been widely reported (Ali et al., 2020; Kaya et al., 2021). Many new microfluidic chips have been successfully developed for the detection of foodborne pathogens. At present, according to the detection principle, the microfluidic detection chips are mainly divided into three categories: molecular biology-based microfluidic detection chips, immunology-based microfluidic detection chips, and electrochemical microfluidic detection chips.
Xue et al. (2021) developed a microfluidic biosensor for rapid and sensitive detection of Salmonella using manganese dioxide nanoflowers (MnO2 NFs), a microfluidic chip with a convergence-divergence spiral micromixer, and a smartphone app with a saturation calculation algorithm (Figure 4A). This biosensor was able to detect Salmonella from 4.4 × 101 to 4.4 × 106 CFU/ml in 45 min with a detection limit of 44 CFU/ml. Zhang et al. (2021b) have created an embedded paper-based microchip based on LAMP which can rapidly and sensitively detect foodborne pathogens (Figure 4B). The detection limit for Salmonella spp. in the sample measured by the microchip was approximately 12 CFU/ml. Song et al. (2020) developed a microfluidic platform to detect S. aureus by fluorescence labeling method and a self-made microfluidic chip, which has immune spheres were used to study the effect of capturing S. aureus (Figure 4C). Results showed that our platform can detect S. aureus at an injection rate of 5 μl/min reacted for 4 min and the detection limit of bacteria was 1.5 × 101 CFU/mul. Nguyen et al. (2020) proposed an integrated smartphone-based genetic analyzer. The LAMP mixture for Eriochrome Black T (EBT) colorimetric detection was injected into the LAMP chip to identify the E. coli O157:H7 (Figure 4D). The limit-of-detection (LOD) reached up to 101 copies/μl. Moreover, Asgari et al. (2022) developed a sensitive Surface-enhanced Raman spectroscopy (SERS)-based microfluidic simmunosensor to separate and detect E. coli O157:H7 in romaine lettuce. The limit of detection for E. coli O157:H7 in romaine lettuce was found to be 0.5 CFU/ml. Wang et al. (2022) demonstrated an ultrasensitive and simple microfluidic immunosensor for a point-of-care test of S. aureus based on stir bar enrichment and DNAzyme-assisted click reaction. The detection limit was 3 CFU/ml. Jiang et al. (2021) designed a thread-based microfluidic electrochemical aptasensor, fabricated and tested by using label-free aptamer immunosensing technology for rapid and highly sensitive detection of V. parahaemolyticus in seafood. The proposed aptasensor has a dynamic detection range of 10–106 CFU/ml, with a detection limit of 5.74 CFU/ml.
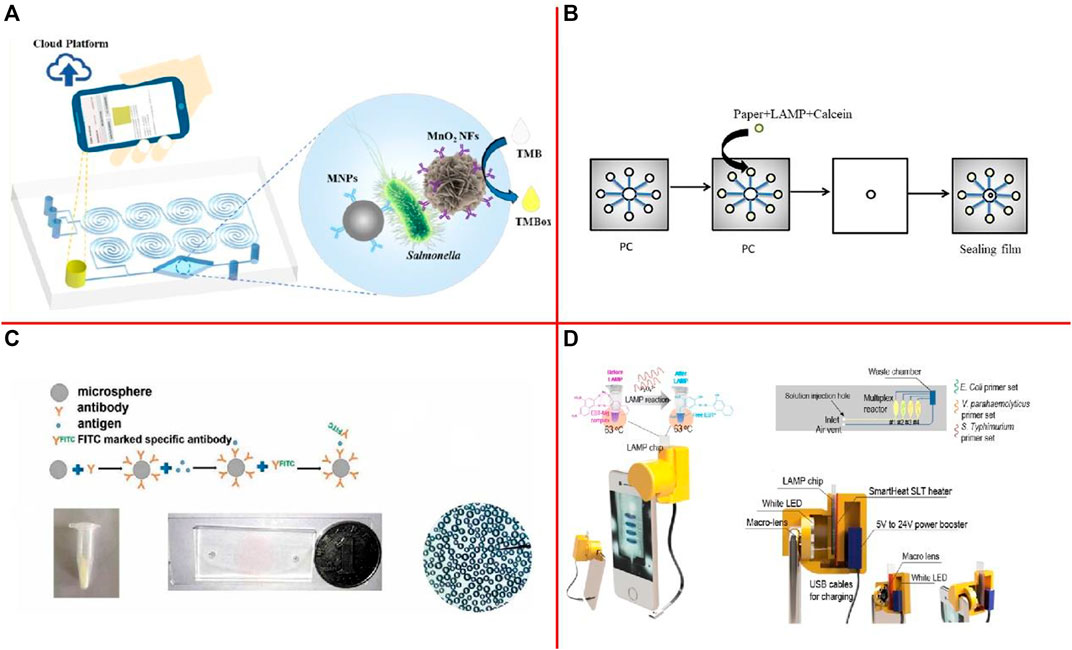
FIGURE 4. (A) Schematic of this Colorimetric Biosensor for Detection of Salmonella (Xue et al., 2021); (B) Making the process of the microchip (Zhang et al., 2021b); (C) The whole process of a microfluidic platform for detection of S. aureus by fluorescence labeling (Song et al., 2020); (D) Schematics of the i-Gene (Nguyen et al., 2020).
At present, microfluidic technology has shown great potential in environmental monitoring, food science, drug screening, disease diagnosis, and other fields, but there is still a long way to go to the market application. Therefore, the development of new materials and new processing methods is an important means to promote the development of microfluidic technology (Tsougeni et al., 2020).
Metabolic detection techniques
Metabolic detection is a common technique for the detection of foodborne pathogens (Castle et al., 2021). Its principle is to use various techniques to detect the variation characteristics of the amount and type of primary metabolites or secondary metabolites produced by different pathogenic bacteria in a specific cultural environment, to identify the pathogenic bacteria (Duarte-Sierra et al., 2020; Subjakova et al., 2021). According to different detection technologies, it can be divided into electrical impedance technology, radiometric technology adenosine triphosphate (ATP) bioluminescence technology, microcalorimeter technology, etc. (Mobed et al., 2020; McCuskey et al., 2022).
Electrical impedance technology
According to different metabolic activities of microorganisms in the growth process, electrical impedance technology is used to detect and identify microorganisms. Wang et al. (2020g) developed a sensitive electrochemical aptasensor using aptamer coated gold interdigitated microelectrode for targeted capture and impedance measurement, and antibody-modified nickel nanowires (NiNWs) for target separation and impedance amplification. This electrochemical aptasensor was able to quantitatively detect Salmonella ranging from 102 to 106 CFU/ml in 2 h, with a detection limit of 80 CFU/ml. Wang et al. (2021b) developed a novel impedance immunosensor based on a metal-organic framework (Mn-MOF-74) to rapidly and sensitively detect LM in milk. The recoveries for L.m cells at concentration between 1.0 × 100 and 1.0 × 104 CFU/ml were 90.2%–101.7% in water and 88.5%–96.2% in milk. Wang et al. (2021c) developed a bacteria-imprinted conductive poly (3-thiopheneacetic acid) (BICP) film-based impedimetric sensor for the rapid, sensitive, and label-free detection of S. aureus.
Biosensor detection technology
Biosensor is an analysis device consisting of a biosensor and transducer. It mainly uses antigens (antibodies), various sensitive enzymes, alkaloids, and gene sequences to detect microorganisms (Shiba 2006; Wang et al., 2013; Liu et al., 2017c; Ma et al., 2020b; Guo et al., 2021; Das and Mishra, 2022). When the sample to be tested reacts with the above substances, biological interactions will occur, which can then be converted into measurable electrical signals by signal transducers, which can be read and detected by signal amplifiers (Lai et al., 2018a; Lai et al., 2018b; Huang et al., 2021). According to different working principles, biosensors can be divided into optical biosensors, electrochemical biosensors, enzyme biosensors, physical biosensors, mechanical biosensors, and so on (Liu et al., 2018; Liu et al., 2019d; He et al., 2019; Ahovan et al., 2020; Naresh and Lee, 2021; Yu et al., 2021). Biosensors commonly used for rapid detection of foodborne pathogens include; optical biosensors and electrochemical biosensors (Tian et al., 2019; Wu et al., 2020b; Du et al., 2020b; Magesa et al., 2020; Mei et al., 2022).
Optical biosensors are widely used in the detection of foodborne pathogens due to their rapid detection and high sensitivity (Nie et al., 2014; Sun et al., 2021a; Wei et al., 2021). At present, the main optical sensing technologies include chemiluminescence, colorimetry, fluorescence, and surface plasmon resonance (Luan et al., 2020).
Quintela et al. (2019) developed a novel approach for simultaneous optical detection of various Salmonella spp. strains in contaminated complex matrices by utilizing oligonucleotide-functionalized AuNPs as a sensitive optical biosensing platform in combination with an efficient sample pooling and IMS system that ensure the detection of viable cells (Figure 5A). The results showed that the highly sensitive assay toward its target with a superior detection limit of <10 CFU/ml or g and 100% specificity. Srivastava et al. (2021) developed a nanophotonic structure with electric control-based photocatalytic nanocomposite to realize label-free optical detection of foodborne pathogens. The fabricated biosensor is capable of detecting E. coli bacteria concentrations of 5,000 CFU/ml. Angelopoulou et al. (2021) presented an optical biosensor for the detection of S. typhimurium lipopolysaccharide (LPS) and Salmonella bacteria in drinking water, based on white light reflectance spectroscopy (Figure 5B). The total assay duration was 15 min, while the achieved detection limits were 4 ng/ml for LPS and 320 CFU/ml for bacteria.
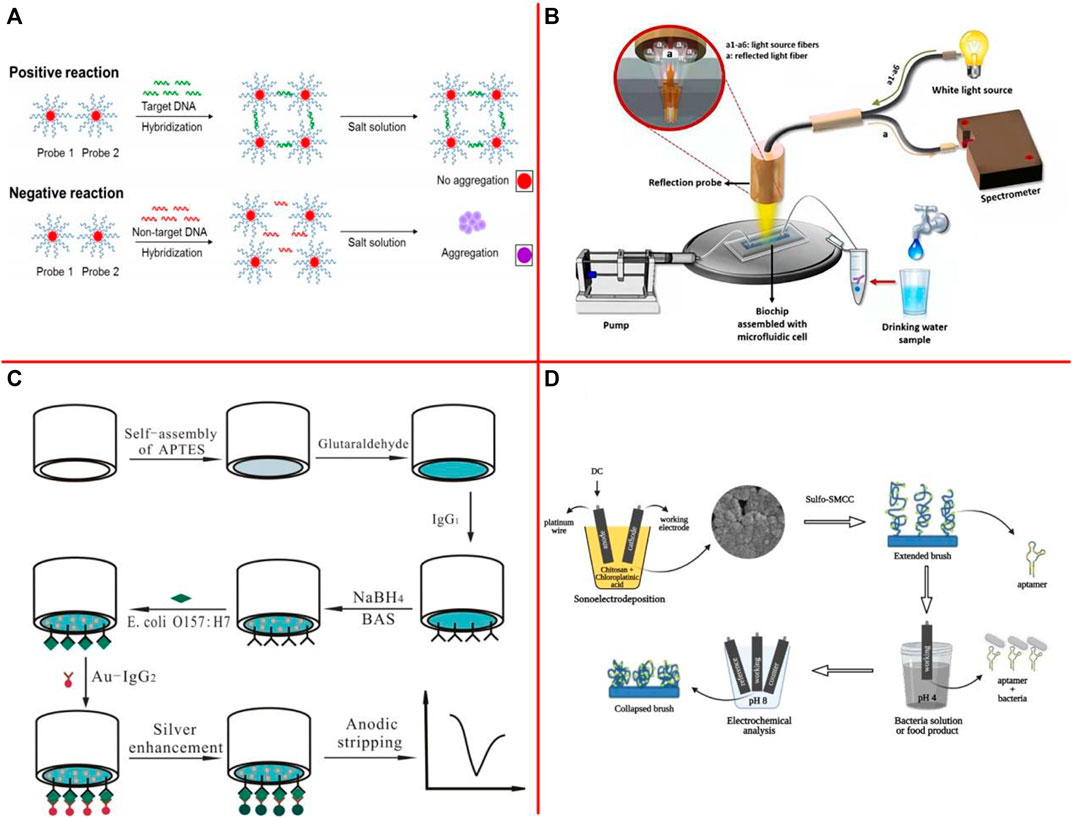
FIGURE 5. (A) The novel principles behind AuNPs optical biosensing and the schematic representation of DNA sandwich hybridization targeting ttrRSBCA locus of Salmonella spp. (Quintela et al., 2019); (B) Illustration of the white light reflectance spectroscopy (WLRS) optical setup and sensing and sensing principle (Angelopoulou et al., 2021); (C) Schematic diagram of electrochemical detection of E. coli O157:H7 by gold nanoparticle-catalyzed silver enhancement at porous pseudo-carbon paste electrode (Xu et al., 2012); (D) Fabrication, biofunctionalization, and sensing scheme of CHI/Pt aptasensor (Oliveira et al., 2021).
The electrochemical biosensor uses the electrode as a signal converter, and the target analyte performs an electrochemical reaction on the electrode interface, which causes the change of current, potential, impedance, or conductivity on the sensor surface (Deng et al., 2013a; Deng et al., 2013b; Upasham et al., 2021; Vidic and Manzano, 2021). Xu et al. (2012) described the fabrication of three different electrodes based on functional porous pseudo-carbon paste electrodes (PPCPEs) (Figure 5C). A linear relationship between the anodic stripping peak current and the concentration of E. coli O157:H7 from 1.0 × 103 to 1.0 × 107 cells/ml and a limit of detection as low as 8.0 × 102 cells/ml were obtained when PPCPE-CHO was used. The target analyte can be quantified by monitoring the change of these signals. Oliveira et al. (2021) developed a label-free and rapid electrochemical biosensor for LM detection using a new one-step simultaneous sonoelectrodeposition of platinum and chitosan (CHI/Pt) to create a biomimetic nanostructure that actuates under pH changes (Figure 5D). Actuation led to improved LM detection with a low limit of detection (33 CFU/10 ml in chicken broth). Feng et al. (2022b) constructed an electrochemical immunosensor for Salmonella detection by using a Fe3O4@graphene modified electrode. Under optimized experimental conditions, a good linear relationship was achieved in the Salmonella concentration range of 2.4 × 102 to 2.4 × 107 CFU/ml, and the limit of detection for the immunosensor was 2.4 × 102 CFU/ml.
Biosensor technology detection of microorganisms has rapid, sensitive, simple operation and low requirement for operating personnel, but biological sensors are used to identify biological molecules of the original life is relatively short, with high-cost production, so the use of biosensor technology is limited by some, and most is still in the development stage (Shen et al., 2021c).
Mass spectrometry
With the development of mass spectrometry, mass spectrometry (MS) is a new detection method of pathogenic bacteria, that has been developed gradually (Fang et al., 2021b). MS is a non-biochemical instrumental analysis method, which takes the characteristics of bacteria or their proteins as the research object and realizes the identification and detection of target bacteria by analyzing the characteristic ions generated after ionization.
Feucherolles et al. (2022) combined Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) protein mass spectra with a prediction approach as an antimicrobial resistance (AMR) screening tool for relevant foodborne pathogens, such as Campylobacter coli and C. jejuni. A maximum sensitivity and precision of 92.3% and 81.2%, respectively, were reached. Moreover, Li et al. (2022) presented a novel strategy using mass tag-mediated surface engineering for simultaneous detection of multiple bacteria by MALDI-TOF MS. This strategy converted the detection of bacteria to the analysis of mass tags, allowing simultaneous detection of multiple bacteria and avoiding the dependence of microbial mass spectra databases. Dias et al. (2022) used gas chromatography-mass spectrometry (GC-MS) and gas chromatography-flame ionization detector (GC-FID) to determine the antibacterial activity of three essential oils (EOs) and their main components against foodborne pathogens and spoilage foods.
Among many detection technologies, the foodborne pathogenic bacteria MS has the highest detection rate at present, with fast detection speed and convenient operation, but there are still many problems in the actual detection process (Mangmee et al., 2020). In the detection process, it is necessary to improve the sensitivity and stability of foodborne pathogenic bacteria MS technology, so it is necessary to constantly debug spray voltage, flow rate, capillary temperature, and other issues to achieve the optimal state.
Conclusion
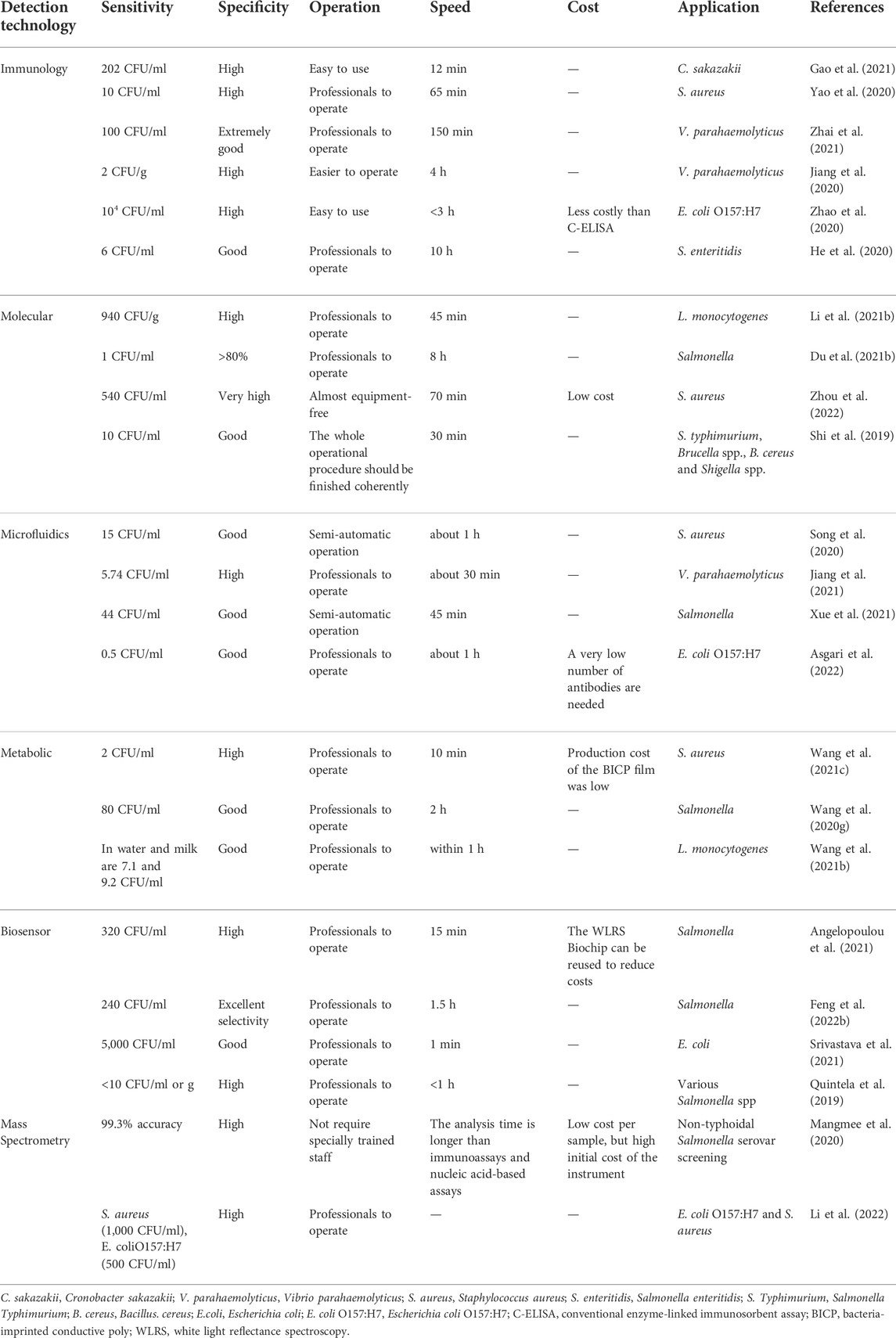
In recent years, many methods for the detection of foodborne pathogens have been developed to address food safety and public health issues, especially with the increased consumption of fresh food and food with short shelf life. Rapid detection technologies are becoming more marketable (Freitas et al., 2020; Ezzatpanah et al., 2022). For example, many scholars have combined POCT technologies such as molecular immunology, bio-molecular, biosensor, and microfluidics to provide new approaches for rapid, low-cost, highly sensitive, and highly specific detection methods for foodborne pathogens (Cimafonte et al., 2020; Wang et al., 2021d; Zhang et al., 2021c). The POCT technology provides simple, fast, and sensitive platforms for the detection of foodborne pathogens and will become a powerful multi-functional tool for food safety, biological threat detection, and environmental monitoring. However, there are still shortcomings that require researchers to continuously improve the existing detection technologies (He et al., 2021b; Wagner et al., 2021). The sensitivity, specificity, ease of operation and detection speed, cost, and application of the techniques for detecting foodborne pathogens are given in Table 1.
With the advancement of science and technology, artificial intelligence, gene editing, nanotechnology, and other cutting-edge disciplines, the integration of these technologies and POCT technology in the rapid detection of foodborne pathogens will also become the future development trend (He et al., 2018; Chen et al., 2020c; Ding et al., 2020; Peng et al., 2020b; Yang et al., 2020c; Gong et al., 2021; Xiao et al., 2022). The following is the prospect of POCT technology in the future development trend: 1) the detection index is gradually transformed from biochemical and immunity to nucleic acid molecules, and from single to multiple indicators. 2) The devices are more miniaturized and integrated by the development of micro-nano fabrication and 3D printing and new materials technology. More and more functions can be integrated into a small device, such as integrate sample extraction and detection into a chip our cassette, etc. 3) The devices have the features of higher sensitivity and quicker time with the application of novel CRISPR method and gold nanoparticles and so on. 4) The devices will be more convenient and lower cost for the use of smart mobile phones, lateral flow dipsticks, or paper chips. Moreover, with the advance of cloud computing, Internet of things technology, devices make more intelligence.
The future detection technology for foodborne pathogenic bacteria will focus toward the integration of a variety of detection technologies, making flux detection faster, with higher sensitivity and repeatability, faster time, and lower cost. The future will also include wide promotion and standardization direction for these technologies, including production, processing, distribution, and sale of the whole production chain of food safety regulations.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China (No. 61901168), Hunan Provincial Natural Science Foundation of China (No. 2022JJ30229), China Postdoctoral Science Foundation (No. 2018M630498), and Education Department of Hunan Province (No. 21B0526).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abebe, E., Gugsa, G., and Ahmed, M. (2020). Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 1–19. doi:10.1155/2020/4674235
Ahovan, Z. A., Hashemi, A., De Plano, L. M., Gholipourmalekabadi, M., and Seifalian, A. (2020). Bacteriophage based biosensors: Trends, outcomes and challenges. Nanomaterials 10, 501. doi:10.3390/nano10030501
Aik, J., Turner, R. M., Kirk, M. D., Heywood, A. E., and Newall, A. T. (2020). Evaluating food safety management systems in Singapore: A controlled interrupted time-series analysis of foodborne disease outbreak reports. Food control. 117, 107324. doi:10.1016/j.foodcont.2020.107324
Akter, R., Rahman, M. H., Bhattacharya, T., Kaushik, D., Mittal, V., Parashar, J., et al. (2021). Novel coronavirus pathogen in humans and animals: An overview on its social impact, economic impact, and potential treatments. Environ. Sci. Pollut. Res. 28, 68071–68089. doi:10.1007/s11356-021-16809-8
Aladese, A. D., and Jeong, H.-H. (2021). Recent developments in 3D printing of droplet-based microfluidics. BioChip J. 15, 313–333. doi:10.1007/s13206-021-00032-1
Ali, A. A., Altemimi, A. B., Alhelfi, N., and Ibrahim, S. A. (2020). Application of biosensors for detection of pathogenic food bacteria: A review. Biosens. (Basel). 10, 58. doi:10.3390/bios10060058
Ali, Q., Ahmar, S., Sohail, M. A., Kamran, M., Ali, M., Saleem, M. H., et al. (2021). Research advances and applications of biosensing technology for the diagnosis of pathogens in sustainable agriculture. Environ. Sci. Pollut. Res. 28, 9002–9019. doi:10.1007/s11356-021-12419-6
Aman, R., Mahas, A., and Mahfouz, M. (2020). Nucleic acid detection using CRISPR/cas biosensing technologies. ACS Synth. Biol. 9, 1226–1233. doi:10.1021/acssynbio.9b00507
Angelopoulou, M., Tzialla, K., Voulgari, A., Dikeoulia, M., Raptis, I., Kakabakos, S. E., et al. (2021). Rapid detection of Salmonella typhimurium in drinking water by a white light reflectance spectroscopy immunosensor. Sensors 21, 2683. doi:10.3390/s21082683
Asgari, S., Dhital, R., Aghvami, S. A., Mustapha, A., Zhang, Y., Lin, M., et al. (2022). Separation and detection of E. coli O157:H7 using a SERS-based microfluidic immunosensor. Microchim. Acta 189, 111. doi:10.1007/s00604-022-05187-8
Augustin, J.-C., Kooh, P., Bayeux, T., Guillier, L., Meyer, T., Jourdan-Da Silva, N., et al. (2020). Contribution of foods and poor food-handling practices to the burden of foodborne infectious diseases in France. Foods 9 (11), 1644. doi:10.3390/foods9111644
Azizipour, N., Avazpour, R., Rosenzweig, D. H., Sawan, M., and Ajji, A. (2020). Evolution of biochip technology: A review from lab-on-a-chip to organ-on-a-chip. Micromachines 11, 599. doi:10.3390/mi11060599
Baoutina, A., and Bhat, S. (2021). Novel design of nucleic acid standards for hydrolysis probe-based PCR with melting analysis. Gene Ther. doi:10.1038/s41434-021-00288-0
Belina, D., Hailu, Y., Gobena, T., Hald, T., and Njage, P. M. K. (2021). Prevalence and epidemiological distribution of selected foodborne pathogens in human and different environmental samples in Ethiopia: A systematic review and meta-analysis. One Health Outlook 3 (1), 19. doi:10.1186/s42522-021-00048-5
Bonny, S. Q., Hossain, M. A. M., Uddin, S. M. K., Pulingam, T., Sagadevan, S., Johan, M. R., et al. (2022). Current trends in polymerase chain reaction based detection of three major human pathogenic vibrios. Crit. Rev. Food Sci. Nutr. 62, 1317–1335. doi:10.1080/10408398.2020.1841728
Bozkurt, H., Kim-Yen, P.-T., van Ogtrop, F., Bell, T., and McConchie, R. (2021). Outbreaks, occurrence, and control of norovirus and hepatitis a virus contamination in berries: A review. Crit. Rev. Food Sci. Nutr. 61, 116–138. doi:10.1080/10408398.2020.1719383
Cardoso, G. V. F., Lima, J. S., de Oliveira, A. C. d. S., da Silva, J. B., Roos, T. B., de Moraes, C. M., et al. (2020). SYBR green qPCR technique for the detection of trypanosoma cruzi in acai pulp. Foodborne Pathog. Dis. 17, 466–469. doi:10.1089/fpd.2019.2745
Cassotta, M., Forbes-Hernandez, T. Y., Calderon Iglesias, R., Ruiz, R., Elexpuru Zabaleta, M., Giampieri, F., et al. (2020). Links between nutrition, infectious diseases, and microbiota: Emerging technologies and opportunities for human-focused research. Nutrients 12, 1827. doi:10.3390/nu12061827
Castle, L. M., Schuh, D. A., Reynolds, E. E., and Furst, A. L. (2021). Electrochemical sensors to detect bacterial foodborne pathogens. ACS Sens. 6, 1717–1730. doi:10.1021/acssensors.1c00481
Chelliah, R., Banan-MwineDaliri, E., Khan, I., Wei, S., Elahi, F., Yeon, S.-J., et al. (2022). A review on the application of bioinformatics tools in food microbiome studies. Brief. Bioinform. 23, bbac007. doi:10.1093/bib/bbac007
Chen, H., Wu, Y., Chen, Z., Hu, Z., Fang, Y., Liao, P., et al. (2017). Performance evaluation of a novel sample in-answer out (SIAO) system based on magnetic nanoparticles. J. Biomed. Nanotechnol. 13, 1619–1630. doi:10.1166/jbn.2017.2478
Chen, S., Li, J., Yang, L., and Zhong, C. (2020b). Research on healthcare and external medicine based on nano biochip technology. Int. J. Nanotechnol. 17, 106. doi:10.1504/ijnt.2020.110710
Chen, Y., Qian, C., Liu, C., Shen, H., Wang, Z., Ping, J., et al. (2020a). Nucleic acid amplification free biosensors for pathogen detection. Biosens. Bioelectron. X. 153, 112049. doi:10.1016/j.bios.2020.112049
Chen, Y., Wang, Z., Shi, Q., Huang, S., Yu, T., Zhang, L., et al. (2021). Multiplex PCR method for simultaneous detection of five pathogenic bacteria closely related to foodborne diseases. 3 Biotech. 11, 219. doi:10.1007/s13205-021-02759-y
Chen, Z., Xiao, C., Qiu, H., Tan, X., Jin, L., He, Y., et al. (2020c). Recent advances of artificial intelligence in cardiovascular disease. J. Biomed. Nanotechnol. 16, 1065–1081. doi:10.1166/jbn.2020.2955
Chen, Z., Yang, T., Yang, H., Li, T., Nie, L., Mou, X., et al. (2018). A portable multi-channel turbidity system for rapid detection of pathogens by loop-mediated isothermal amplification. J. Biomed. Nanotechnol. 14, 198–205. doi:10.1166/jbn.2018.2524
Chen, Z., Zhao, K., He, Z., Luo, X., Qin, Z., Tan, Y., et al. (2022). Development and evaluation of a thermostatic nucleic acid testing device based on magnesium pyrophosphate precipitation for detecting Enterocytozoon hepatopenaei. Chin. Chem. Lett. 33, 4053–4056. doi:10.1016/j.cclet.2022.01.072
Christopher, A., Sarkar, D., and Shetty, K. (2021). Elicitation of stress-induced phenolic metabolites for antimicrobial applications against foodborne human bacterial pathogens. Antibiot. (Basel). 10 (2), 109. doi:10.3390/antibiotics10020109
Cimafonte, M., Fulgione, A., Gaglione, R., Papaianni, M., Capparelli, R., Arciello, A., et al. (2020). Screen printed based impedimetric immunosensor for rapid detection of Escherichia coli in drinking water. Sensors 20, 274. doi:10.3390/s20010274
Darbandi, A., Asadi, A., Ari, M. M., Ohadi, E., Talebi, M., Zadeh, M. H., et al. (2022). Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 36, e24093. doi:10.1002/jcla.24093
Das, J., and Mishra, H. N. (2022). Recent advances in sensors for detecting food pathogens, contaminants, and toxins: A review. Eur. Food Res. Technol. 248, 1125–1148. doi:10.1007/s00217-021-03951-3
Davey, L., and Valdivia, R. H. (2020). Bacterial genetics and molecular pathogenesis in the age of high throughput DNA sequencing. Curr. Opin. Microbiol. 54, 59–66. doi:10.1016/j.mib.2020.01.007
Deng, Y., Wang, W., Ma, C., and Li, Z. (2013a). Fabrication of an electrochemical biosensor array for simultaneous detection of L-glutamate and acetylcholine. J. Biomed. Nanotechnol. 9, 1378–1382. doi:10.1166/jbn.2013.1633
Deng, Y., Wang, W., Zhang, L., Lu, Z., Li, S., Xu, L., et al. (2013b). Preparation and electrochemical behavior of L-glutamate electrochemical biosensor. J. Biomed. Nanotechnol. 9, 318–321. doi:10.1166/jbn.2013.1487
Dester, E., and Alocilja, E. (2022). Current methods for extraction and concentration of foodborne bacteria with glycan-coated magnetic nanoparticles: A review. Biosens. (Basel). 12 (2), 112. doi:10.3390/bios12020112
Dias, A. L. B., Fernandes, C. C., de Souza, J. H., Martins, C. H. G., Moreira, F. F., Crotti, A. E. M., et al. (2022). Antibacterial activity of essential oils from Brazilian plants and their major constituents against foodborne pathogens and spoilage bacteria. J. Essent. Oil Res. 34, 195–202. doi:10.1080/10412905.2022.2032424
Ding, Z., Wang, Y., Zhou, Q., Ding, Z., Liu, J., He, Q., et al. (2020). Microstructure, wettability, corrosion resistance and antibacterial property of Cu-MTa2O5 multilayer composite coatings with different Cu incorporation contents. Biomolecules 10, 68. doi:10.3390/biom10010068
Du, H., Li, Z., Wang, Y., Yang, Q., and Wu, W. (2020b). Nanomaterial-based optical biosensors for the detection of foodborne bacteria. Food Rev. Int. 38, 655–684. doi:10.1080/87559129.2020.1740733
Du, H., Wang, X., Yang, Q., and Wu, W. (2021a). Quantum dot: Lightning invisible foodborne pathogens. Trends Food Sci. Technol. 110, 1–12. doi:10.1016/j.tifs.2021.01.065
Du, J., Wu, S., Niu, L., Li, J., Zhao, D., Bai, Y., et al. (2020a). A gold nanoparticles-assisted multiplex PCR assay for simultaneous detection of Salmonella typhimurium, Listeria monocytogenes and Escherichia coli O157:H7. Anal. Methods 12, 212–217. doi:10.1039/c9ay02282a
Du, M. H., Li, J. W., Liu, Q. J., Wang, Y. F., Chen, E. N., Kang, F. Y., et al. (2021b). Rapid detection of trace Salmonella in milk using an effective pretreatment combined with droplet digital polymerase chain reaction. Microbiol. Res. 251, 126838. doi:10.1016/j.micres.2021.126838
Duarte-Sierra, A., Tiznado-Hernandez, M. E., Jha, D. K., Janmeja, N., and Arul, J. (2020). Abiotic stress hormesis: An approach to maintain quality, extend storability, and enhance phytochemicals on fresh produce during postharvest. Compr. Rev. Food Sci. Food Saf. 19, 3659–3682. doi:10.1111/1541-4337.12628
Dumen, E., Ekici, G., Ergin, S., and Bayrakal, G. M. (2020). Presence of foodborne pathogens in seafood and risk ranking for pathogens. Foodborne Pathog. Dis. 17, 541–546. doi:10.1089/fpd.2019.2753
Efimochkina, N. R., and Sheveleva, S. A. (2022). Prospective molecular methods for sequencing microorganisms in the system of assessment and control of food safety. Problems Nutr. 91, 37–52. doi:10.33029/0042-8833-2022-91-1-37-52
Ezzatpanah, H., Gomez-Lopez, V. M., Koutchma, T., Lavafpour, F., Moerman, F., Mohammadi, M., et al. (2022). New food safety challenges of viral contamination from a global perspective: Conventional, emerging, and novel methods of viral control. Compr. Rev. Food Sci. Food Saf. 21, 904–941. doi:10.1111/1541-4337.12909
Fang, S., Liu, S., Song, J., Huang, Q., and Xiang, Z. (2021b). Recognition of pathogens in food matrixes based on the untargeted in vivo microbial metabolite profiling via a novel SPME/GC×GC-QTOFMS approach. Food Res. Int. 142, 110213. doi:10.1016/j.foodres.2021.110213
Fang, Y., Liu, H., Wang, Y., Su, X., Jin, L., Wu, Y., et al. (2021a). Fast and accurate control strategy for portable nucleic acid detection (PNAD) system based on magnetic nanoparticles. J. Biomed. Nanotechnol. 17, 407–415. doi:10.1166/jbn.2021.3028
Feng, K., Li, T., Ye, C., Gao, X., Yue, X., Ding, S., et al. (2022b). A novel electrochemical immunosensor based on Fe3O4@graphene nanocomposite modified glassy carbon electrode for rapid detection of Salmonella in milk. J. Dairy Sci. 105, 2108–2118. doi:10.3168/jds.2021-21121
Feng, Z.-S., Li, J.-Y., Zhang, J.-Y., Li, F.-Y., Guan, H.-X., Zhang, R.-Q., et al. (2022a). Development and evaluation of a sensitive recombinase aided amplification assay for rapid detection of Vibrio parahaemolyticus. J. Microbiol. Methods 193, 106404. doi:10.1016/j.mimet.2021.106404
Ferone, M., Gowen, A., Fanning, S., and Scannell, A. G. M. (2020). Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 19, 3106–3129. doi:10.1111/1541-4337.12618
Feucherolles, M., Nennig, M., Becker, S. L., Martiny, D., Losch, S., Penny, C., et al. (2022). Combination of MALDI-TOF mass spectrometry and machine learning for rapid antimicrobial resistance screening: The case of Campylobacter spp. Front. Microbiol. 12, 804484. doi:10.3389/fmicb.2021.804484
Foddai, A. C. G., and Grant, I. R. (2020). Methods for detection of viable foodborne pathogens: Current state-of-art and future prospects. Appl. Microbiol. Biotechnol. 104, 4281–4288. doi:10.1007/s00253-020-10542-x
Freitas, J., Vaz-Pires, P., and Camara, J. S. (2020). From aquaculture production to consumption: Freshness, safety, traceability and authentication, the four pillars of quality, 518, 734857. doi:10.1016/j.aquaculture.2019.734857Aquaculture
Fu, L., Qian, Y., Zhou, J., Zheng, L., and Wang, Y. (2020). Fluorescence-based quantitative platform for ultrasensitive food allergen detection: From immunoassays to DNA sensors. Compr. Rev. Food Sci. Food Saf. 19, 3343–3364. doi:10.1111/1541-4337.12641
Fu, X., Sun, J., Liang, R., Guo, H., Wang, L., Sun, X., et al. (2021). Application progress of microfluidics-integrated biosensing platforms in the detection of foodborne pathogens. Trends Food Sci. Technol. 116, 115–129. doi:10.1016/j.tifs.2021.07.006
Fuochi, V., Emma, R., and Furneri, P. M. (2021). Bacteriocins, A natural weapon against bacterial contamination for greater safety and preservation of food: A review. Curr. Pharm. Biotechnol. 22, 216–231. doi:10.2174/1389201021666200704145427
Gallo, M., Ferrara, L., Calogero, A., Montesano, D., and Naviglio, D. (2020). Relationships between food and diseases: What to know to ensure food safety. Food Res. Int. 137, 109414. doi:10.1016/j.foodres.2020.109414
Gao, S., Wu, J., Wang, H., Hu, S., and Meng, L. (2021). Highly sensitive detection of Cronobacter sakazakii based on immunochromatography coupled with surface-enhanced Raman scattering. J. Dairy Sci. 104, 2748–2757. doi:10.3168/jds.2020-18915
Garafutdinov, R. R., Sakhabutdinova, A. R., Kupryushkin, M. S., and Pyshnyi, D. V. (2020). Prevention of DNA multimerization using phosphoryl guanidine primers during isothermal amplification with Bst exo- DNA polymerase. Biochimie 168, 259–267. doi:10.1016/j.biochi.2019.11.013
Ge, H., Wang, Y., and Zhao, X. (2022). Research on the drug resistance mechanism of foodborne pathogens. Microb. Pathog. 162, 105306. doi:10.1016/j.micpath.2021.105306
Gong, L., Zhao, L., Tan, M., Pan, T., He, H., Wang, Y., et al. (2021). Two-Photon fluorescent nanomaterials and their applications in biomedicine. J. Biomed. Nanotechnol. 17, 509–528. doi:10.1166/jbn.2021.3052
Grudlewska-Buda, K., Skowron, K., and Gospodarek-Komkowska, E. (2020). Comparison of the intensity of biofilm formation by Listeria monocytogenes using classical culture-based method and digital droplet PCR. Amb. Express 10, 75. doi:10.1186/s13568-020-01007-5
Gunther, N. W., Kanrar, S., and Uhlich, G. (2021). Complete genomic sequences of Campylobacter coli strains isolated from poultry sold in Pennsylvania farmers' markets. Microbiol. Resour. Announc. 10, e00015-21. doi:10.1128/mra.00015-21
Guo, W., Zhang, C., Ma, T., Liu, X., Chen, Z., Li, S., et al. (2021). Advances in aptamer screening and aptasensors' detection of heavy metal ions. J. Nanobiotechnology 19, 166. doi:10.1186/s12951-021-00914-4
Gutierrez, S., Diaz, L., Reyes-Jara, A., Yang, X., Meng, J., Gonzalez-Escalona, N., et al. (2021). Whole-genome phylogenetic analysis reveals a wide diversity of non-O157 STEC isolated from ground beef and cattle feces. Front. Microbiol. 11, 622663. doi:10.3389/fmicb.2020.622663
Han, A., Hao, S., Yang, Y., Li, X., Luo, X., Fang, G., et al. (2020). Perspective on recent developments of nanomaterial based fluorescent sensors: Applications in safety and quality control of food and beverages. J. Food Drug Anal. 28, 487–508. doi:10.38212/2224-6614.1270
Han, X., Liu, Y., Yin, J., Yue, M., and Mu, Y. (2021). Microfluidic devices for multiplexed detection of foodborne pathogens. Food Res. Int. 143, 110246. doi:10.1016/j.foodres.2021.110246
Hang, Y., Boryczka, J., and Wu, N. (2022). Visible-light and near-infrared fluorescence and surface-enhanced Raman scattering point-of-care sensing and bio-imaging: A review. Chem. Soc. Rev. 51, 329–375. doi:10.1039/c9cs00621d
Hariharan, G., and Prasannath, K. (2021). Recent advances in molecular diagnostics of fungal plant pathogens: A mini review. Front. Cell. Infect. Microbiol. 10, 600234. doi:10.3389/fcimb.2020.600234
He, L., Yang, H., Xiao, P., Singh, R., He, N., Liu, B., et al. (2017). Highly selective, sensitive and rapid detection of Escherichia coli O157: H7 using duplex PCR and magnetic nanoparticle-based chemiluminescence assay. J. Biomed. Nanotechnol. 13, 1243–1252. doi:10.1166/jbn.2017.2422
He, Q., Liu, J., Liu, X., Li, G., Deng, P., Liang, J., et al. (2018). Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surfaces B Biointerfaces 172, 565–572. doi:10.1016/j.colsurfb.2018.09.005
He, Q., Tian, Y., Wu, Y., Liu, J., Li, G., Deng, P., et al. (2019). Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles-coated reduced graphene oxide nanocomposite. Biomolecules 9, 176. doi:10.3390/biom9050176
He, S., Fong, K., Wang, S., and Shi, X. (2021b). Ethanol adaptation in foodborne bacterial pathogens. Crit. Rev. Food Sci. Nutr. 61, 777–787. doi:10.1080/10408398.2020.1746628
He, S., Huang, Y., Ma, Y., Yu, H., Pang, B., Liu, X., et al. (2022). Detection of four foodborne pathogens based on magnetic separation multiplex PCR and capillary electrophoresis. Biotechnol. J. 17, 2100335. doi:10.1002/biot.202100335
He, Y., Ren, Y., Guo, B., Yang, Y., Ji, Y., Zhang, D., et al. (2020). Development of a specific nanobody and its application in rapid and selective determination of Salmonella enteritidis in milk. Food Chem. x. 310, 125942. doi:10.1016/j.foodchem.2019.125942
He, Z., Tong, Z., Tan, B., He, X., Zhang, T., Guo, Y., et al. (2021a). Rapid detection of DNA methylation with a novel real-time fluorescence recombinase-aided amplification assay. J. Biomed. Nanotechnol. 17, 1364–1370. doi:10.1166/jbn.2021.3111
Hoang, T. X., Phan, L. M. T., Vo, T. A. T., and Cho, S. (2021). Advanced signal-amplification strategies for paper-based analytical devices: A comprehensive review. Biomedicines 9, 540. doi:10.3390/biomedicines9050540
Hoffmann, S., Ashton, L., and Ahn, J.-W. (2021). Food safety: A policy history and introduction to avenues for economic research. Appl. Econ. Perspect. Policy 43, 680–700. doi:10.1002/aepp.13158
Hoffmann, S., and Walter, E. S. (2020). Acute complications and sequelae from foodborne infections: Informing priorities for cost of foodborne illness estimates. Foodborne Pathog. Dis. 17, 172–177. doi:10.1089/fpd.2019.2664
Hossain, M. A. M., Uddin, S. M. K., Sultana, S., Wahab, Y. A., Sagadevan, S., Johan, M. R., et al. (2021). Authentication of Halal and Kosher meat and meat products: Analytical approaches, current progresses and future prospects. Crit. Rev. Food Sci. Nutr. 62, 285–310. doi:10.1080/10408398.2020.1814691
Hou, L., Li, D., Zhang, N., Zhao, J., Zhao, Y., Sun, X., et al. (2022). Development of an isothermal recombinase-aided amplification assay for the rapid and visualized detection of Klebsiella pneumoniae. J. Sci. Food Agric. 102, 3879–3886. doi:10.1002/jsfa.11737
Hou, Y., Tang, W., Qi, W., Guo, X., and Lin, J. (2020). An ultrasensitive biosensor for fast detection of Salmonella using 3D magnetic grid separation and urease catalysis. Biosens. Bioelectron. X. 157, 112160. doi:10.1016/j.bios.2020.112160
Hu, L., Cao, G., Brown, E. W., Allard, M. W., Ma, L. M., Zhang, G., et al. (2021). Whole genome sequencing and protein structure analyses of target genes for the detection of Salmonella. Sci. Rep. 11, 20887. doi:10.1038/s41598-021-00224-7
Huang, F., Zhang, Y., Lin, J., and Liu, Y. (2021b). Biosensors coupled with signal amplification technology for the detection of pathogenic bacteria: A review. Biosens. (Basel). 11, 190. doi:10.3390/bios11060190
Huang, L., Zhang, D., Jiao, L., Su, E., and He, N. (2018). A new quality control method for lateral flow assay. Chin. Chem. Lett. 29, 1853–1856. doi:10.1016/j.cclet.2018.11.028
Huang, T., Shi, Y., Zhang, J., Han, Q., Xia, X.-S., Zhang, A. M., et al. (2021a). Rapid and simultaneous detection of five, viable, foodborne pathogenic bacteria by photoinduced PMAxx-coupled multiplex PCR in fresh juice. Foodborne Pathog. Dis. 18, 640–646. doi:10.1089/fpd.2020.2909
Huang, Y., Mu, X., Wang, J., Wang, Y., Xie, J., Ying, R., et al. (2022). The recent development of nanozymes for food quality and safety detection. J. Mat. Chem. B 10, 1359–1368. doi:10.1039/d1tb02667d
Ishaq, A. R., Manzoor, M., Hussain, A., Altaf, J., Rehman, S. U., Javed, Z., et al. (2021). Prospect of microbial food borne diseases in Pakistan: A review. Braz. J. Biol. 81, 940–953. doi:10.1590/1519-6984.232466
Iwu, C. D., Korsten, L., and Okoh, A. I. (2020). The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 9, e1035. doi:10.1002/mbo3.1035
Jahan, N. A., Lindsey, L. L., and Larsen, P. A. (2021). The role of peridomestic rodents as reservoirs for zoonotic foodborne pathogens. Vector-Borne Zoonotic Dis. 21, 133–148. doi:10.1089/vbz.2020.2640
Janekrongtham, C., Dejburum, P., Sujinpram, S., Rattanathumsakul, T., and Swaddiwudhipong, W. (2022). Outbreak of seafood-related food poisoning from undetectable Vibrio parahaemolyticus-like pathogen, Chiang Mai Province, Thailand, December 2020. Trop. Med. Int. Health 27, 92–98. doi:10.1111/tmi.13700
Jang, H.-J., Kim, H.-J., Park, J.-i., Yu, S.-N., Park, B. B., Ha, G.-J., et al. (2021). Comparative analysis of detection methods for food-borne pathogens in fresh-cut agricultural materials. J. Life Sci. 31 (1), 10–16. doi:10.5352/jls.2021.31.1.10
Jayan, H., Pu, H., and Sun, D.-W. (2020). Recent development in rapid detection techniques for microorganism activities in food matrices using bio-recognition: A review. Trends Food Sci. Technol. 95, 233–246. doi:10.1016/j.tifs.2019.11.007
Jia, X.-X., Li, S., Han, D.-P., Chen, R.-p., Yao, Z.-Y., Ning, B.-A., et al. (2021a). Development and perspectives of rapid detection technology in food and environment. Crit. Rev. Food Sci. Nutr. 62, 4706–4725. doi:10.1080/10408398.2021.1878101
Jia, X.-X., Yao, Z.-Y., Gao, Z.-X., and Fan, Z.-C. (2021b). The role of suspension array technology in rapid detection of foodborne pollutants: Applications and future challenges. Crit. Rev. Anal. Chem., 1–14. doi:10.1080/10408347.2021.1882833
Jiang, H., Sun, Z., Guo, Q., and Weng, X. (2021). Microfluidic thread-based electrochemical aptasensor for rapid detection of Vibrio parahaemolyticus. Biosens. Bioelectron. X. 182, 113191. doi:10.1016/j.bios.2021.113191
Jiang, H., Zeng, X., Xi, Z., Liu, M., Li, C., Li, Z., et al. (2013). Improvement on controllable fabrication of streptavidin-modified three-layer core-shell Fe3O4@SiO2@Au magnetic nanocomposites with low fluorescence background. J. Biomed. Nanotechnol. 9, 674–684. doi:10.1166/jbn.2013.1575
Jiang, W., Ren, Y., Han, X., Xue, J., Shan, T., Chen, Z., et al. (2020). Recombinase polymerase amplification-lateral flow (RPA-LF) assay combined with immunomagnetic separation for rapid visual detection of Vibrio parahaemolyticus in raw oysters. Anal. Bioanal. Chem. 412, 2903–2914. doi:10.1007/s00216-020-02532-9
Jin, Z. J., Ding, G. T., Li, G. Y., Yang, G. X., Han, Y. H., Hao, N., et al. (2020). Rapid detection of foodborne bacterial pathogens using visual high-throughput microfluidic chip. J. Chem. Technol. Biotechnol. 95, 1460–1466. doi:10.1002/jctb.6331
Kaavya, R., Pandiselvam, R., Abdullah, S., Sruthi, N. U., Jayanath, Y., Ashokkumar, C., et al. (2021). Emerging non-thermal technologies for decontamination of Salmonella in food. Trends Food Sci. Technol. 112, 400–418. doi:10.1016/j.tifs.2021.04.011
Kaprou, G. D., Bergspica, I., Alexa, E. A., Alvarez-Ordonez, A., and Prieto, M. (2021). Rapid Methods for Antimicrobial Resistance Diagnostics, 10, 209. doi:10.3390/antibiotics10020209Antibiotics-Basel
Kaya, H. O., Cetin, A. E., Azimzadeh, M., and Topkaya, S. N. (2021). Pathogen detection with electrochemical biosensors: Advantages, challenges and future perspectives. J. Electroanal. Chem. (Lausanne). 882, 114989. doi:10.1016/j.jelechem.2021.114989
Khan, H., Khan, A., Liu, Y., Wang, S., Bibi, S., Xu, H., et al. (2019). CRISPR-Cas13a mediated nanosystem for attomolar detection of canine parvovirus type 2. Chin. Chem. Lett. 30, 2201–2204. doi:10.1016/j.cclet.2019.10.032
Khan, S., Burciu, B., Filipe, C. D. M., Li, Y., Dellinger, K., Didar, T. F., et al. (2021). DNAzyme-based biosensors: Immobilization strategies, applications, and future prospective. Acs Nano 15, 13943–13969. doi:10.1021/acsnano.1c04327
Kim, J.-H., and Oh, S.-W. (2021). Pretreatment methods for nucleic acid-based rapid detection of pathogens in food: A review. Food control. 121, 107575. doi:10.1016/j.foodcont.2020.107575
Kim, J. M., Park, J. S., Yoon, T. H., Park, J., and Park, K. S. (2021b). Nucleic acid lateral flow assay for simultaneous detection of hygiene indicator bacteria. Anal. Bioanal. Chem. 413, 5003–5011. doi:10.1007/s00216-021-03462-w
Kim, S.-O., and Kim, S.-S. (2021). Bacterial pathogen detection by conventional culture-based and recent alternative (polymerase chain reaction, isothermal amplification, enzyme linked immunosorbent assay, bacteriophage amplification, and gold nanoparticle aggregation) methods in food samples: A review. J. Food Saf. 41, e12870. doi:10.1111/jfs.12870
Kim, S. Y., Bang, H. W., and Choi, Y. S. (2021a). Distribution of pathogen resources by the national culture collection for pathogens in South Korea from 2015 to 2019. Biopreserv. Biobank. 19 (6), 511–524. doi:10.1089/bio.2020.0147
Kotsiri, Z., Vidic, J., and Vantarakis, A. (2022). Applications of biosensors for bacteria and virus detection in food and water-A systematic review. J. Environ. Sci. 111, 367–379. doi:10.1016/j.jes.2021.04.009
Kumar, H., Kuca, K., Bhatia, S. K., Saini, K., Kaushal, A., Verma, R., et al. (2020). Applications of nanotechnology in sensor-based detection of foodborne pathogens. Sensors 20, 1966. doi:10.3390/s20071966
Lai, Y., Deng, Y., Yang, G., Li, S., Zhang, C., Liu, X., et al. (2018a). Molecular imprinting polymers electrochemical sensor based on AuNPs/PTh modified GCE for highly sensitive detection of carcinomaembryonic antigen. J. Biomed. Nanotechnol. 14, 1688–1694. doi:10.1166/jbn.2018.2617
Lai, Y., Wang, L., Liu, Y., Yang, G., Tang, C., Deng, Y., et al. (2018b). Immunosensors based on nanomaterials for detection of tumor markers. J. Biomed. Nanotechnol. 14, 44–65. doi:10.1166/jbn.2018.2505
Lee, H., and Yoon, Y. (2021). Etiological agents implicated in foodborne illness world wide. Food Sci. Anim. Resour. 41, 1–7. doi:10.5851/kosfa.2020.e75
Lei, S., Chen, S., and Zhong, Q. (2021). Digital PCR for accurate quantification of pathogens: Principles, applications, challenges and future prospects. Int. J. Biol. Macromol. 184, 750–759. doi:10.1016/j.ijbiomac.2021.06.132
Lei, S., Gu, X., Xue, W., Rong, Z., Wang, Z., Chen, S., et al. (2020a). A 4-plex droplet digital PCR method for simultaneous quantification and differentiation of pathogenic and non-pathogenicVibrio parahaemolyticusBased on single intact cells. Front. Microbiol. 11, 1727. doi:10.3389/fmicb.2020.01727
Lei, S., Gu, X., Zhong, Q., Duan, L., and Zhou, A. (2020b). Absolute quantification of Vibrio parahaemolyticus by multiplex droplet digital PCR for simultaneous detection of tlh, tdh and ureR based on single intact cell. Food control. 114, 107207. doi:10.1016/j.foodcont.2020.107207
Leon Madrazo, A., and Segura Campos, M. R. (2020). Review of antimicrobial peptides as promoters of food safety: Limitations and possibilities within the food industry. J. Food Saf. 40 (6), e12854. doi:10.1111/jfs.12854
Leva-Bueno, J., Peyman, S. A., and Millner, P. A. (2020). A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 209, 343–362. doi:10.1007/s00430-020-00668-0
Lewis, E., Hudson, J. A., Cook, N., Barnes, J. D., and Haynes, E. (2020). Next-generation sequencing as a screening tool for foodborne pathogens in fresh produce. J. Microbiol. Methods 171, 105840. doi:10.1016/j.mimet.2020.105840
Li, F., Wang, Z., Huang, Y., Xu, H., He, L., Deng, Y., et al. (2015). Delivery of PUMA apoptosis gene using polyethyleneimine-SMCC-TAT/DNA nanoparticles: Biophysical characterization and in vitro transfection into malignant melanoma cells. J. Biomed. Nanotechnol. 11, 1776–1782. doi:10.1166/jbn.2015.2151
Li, F., Ye, Q., Chen, M., Xiang, X., Zhang, J., Pang, R., et al. (2021a). Cas12aFDet: A CRISPR/cas12a-based fluorescence platform for sensitive and specific detection of Listeria monocytogenes serotype 4c. Anal. Chim. Acta X. 1151, 338248. doi:10.1016/j.aca.2021.338248
Li, F., Ye, Q. H., Chen, M. T., Zhou, B. Q., Zhang, J. M., Pang, R., et al. (2021b). An ultrasensitive CRISPR/Cas12a based electrochemical biosensor for Listeria monocytogenes detection. Biosens. Bioelectron. X. 179, 113073. doi:10.1016/j.bios.2021.113073
Li, N., Zhang, W., Lin, J., Xing, G., Li, H., Lin, J.-M., et al. (2022). A specific mass-tag approach for detection of foodborne pathogens using MALDI-TOF mass spectrometry. Anal. Chem. 94, 3963–3969. doi:10.1021/acs.analchem.1c05069
Li, S., Liu, H., Deng, Y., Lin, L., and He, N. (2013). Development of a magnetic nanoparticles microarray for simultaneous and simple detection of foodborne pathogens. J. Biomed. Nanotechnol. 9, 1254–1260. doi:10.1166/jbn.2013.1610
Li, T., Yi, H., Liu, Y., Wang, Z., Liu, S., He, N., et al. (2018). One-step synthesis of DNA templated water-soluble Au-Ag bimetallic nanoclusters for ratiometric fluorescence detection of DNA. J. Biomed. Nanotechnol. 14, 150–160. doi:10.1166/jbn.2018.2491
Li, Y., Huang, T.-Y., Ye, C., Chen, L., Liang, Y., Wang, K., et al. (2020). Formation and control of the viable but non-culturable state of foodborne pathogen Escherichia coli O157:H7. Front. Microbiol. 11, 1202. doi:10.3389/fmicb.2020.01202
Li, Z., Wang, J., Yang, H., Chen, S., Ma, G., Zhang, X., et al. (2017). Ultrasensitive detection of gastric cancer plasma MicroRNAs via magnetic beads-based chemiluminescent assay. J. Biomed. Nanotechnol. 13, 1272–1280. doi:10.1166/jbn.2017.2426
Lin, L., Zheng, Q., Lin, J., Yuk, H.-G., and Guo, L. (2020). Immuno- and nucleic acid-based current technique for Salmonella detection in food. Eur. Food Res. Technol. 246, 373–395. doi:10.1007/s00217-019-03423-9
Ling, Y. Z., Zhu, Y. F., Fan, H. H., Zha, H. L., Yang, M., Wu, L., et al. (2019). Rapid method for detection of Staphylococcus aureus in feces. J. Biomed. Nanotechnol. 15, 1290–1298. doi:10.1166/jbn.2019.2781
Liu, F., Zhang, C., Wang, Y., and Chen, G. (2022). A review of the current and emerging detection methods of marine harmful microalgae. Sci. Total Environ. 815, 152913. doi:10.1016/j.scitotenv.2022.152913
Liu, H., Dong, H., Chen, Z., Lin, L., Chen, H., Li, S., et al. (2017b). Magnetic nanoparticles enhanced microarray detection of multiple foodborne pathogens. J. Biomed. Nanotechnol. 13, 1333–1343. doi:10.1166/jbn.2017.2418
Liu, J., Dong, S., He, Q., Yang, S., Xie, M., Deng, P., et al. (2019a). Facile preparation of Fe3O4/C nanocomposite and its application for cost-effective and sensitive detection of tryptophan. Biomolecules 9 (6), 245. doi:10.3390/biom9060245
Liu, J., Parrish, J. R., Hines, J., Mansfield, L., and Finley, R. L. (2019c). A proteome-wide screen of Campylobacter jejuni using protein microarrays identifies novel and conformational antigens. PLoS One 14, e0210351. doi:10.1371/journal.pone.0210351
Liu, M., Yu, X., Chen, Z., Yang, T., Yang, D., Liu, Q., et al. (2017a). Aptamer selection and applications for breast cancer diagnostics and therapy. J. Nanobiotechnology 15, 81. doi:10.1186/s12951-017-0311-4
Liu, S., He, X., Zhang, T., Zhao, K., Xiao, C., Tong, Z., et al. (2022). Highly sensitive smartphone-based detection of Listeria monocytogenes using SYTO9. Chin. Chem. Lett. 33 (4), 1933–1935. doi:10.1016/j.cclet.2021.11.051
Liu, Y., Deng, Y., Li, T., Chen, Z., Chen, H., Li, S., et al. (2018). Aptamer-based electrochemical biosensor for mercury ions detection using AuNPs-modified glass carbon electrode. J. Biomed. Nanotechnol. 14, 2156–2161. doi:10.1166/jbn.2018.2655
Liu, Y., Lai, Y., Yang, G., Tang, C., Deng, Y., Li, S., et al. (2017c). Cd-aptamer electrochemical biosensor based on AuNPs/CS modified glass carbon electrode. J. Biomed. Nanotechnol. 13, 1253–1259. doi:10.1166/jbn.2017.2424
Liu, Y., Li, T., Ling, C., Chen, Z., Deng, Y., He, N., et al. (2019b). Electrochemical sensor for Cd2+ and Pb2+ detection based on nano-porous pseudo carbon paste electrode. Chin. Chem. Lett. 30, 2211–2215. doi:10.1016/j.cclet.2019.05.020
Liu, Y., Li, T., Ling, C., Wang, Z., Jin, L., Zhao, Y., et al. (2019d). A simple visual method for DNA detection based on the formation of gold nanoparticles. Chin. Chem. Lett. 30, 2359–2362. doi:10.1016/j.cclet.2019.10.033
Lopes-Luz, L., Mendonca, M., Fogaca, M. B., Kipnis, A., Bhunia, A. K., Buhrer-Sekula, S., et al. (2021). Listeria monocytogenes: Review of pathogenesis and virulence determinants-targeted immunological assays. Crit. Rev. Microbiol. 47, 647–666. doi:10.1080/1040841x.2021.1911930
Lu, I. N., Muller, C. P., and He, F. Q. (2020). Applying next-generation sequencing to unravel the mutational landscape in viral quasispecies. Virus Res. 283, 197963. doi:10.1016/j.virusres.2020.197963
Luan, Y., Wang, N., Li, C., Guo, X., and Lu, A. (2020). Advances in the application of aptamer biosensors to the detection of aminoglycoside antibiotics. Antibiot. (Basel). 9, 787. doi:10.3390/antibiotics9110787
Luo, F., Li, Z., Dai, G., Lu, Y., He, P., Wang, Q., et al. (2020b). Simultaneous detection of different bacteria by microchip electrophoresis combined with universal primer-duplex polymerase chain reaction. J. Chromatogr. A 1615, 460734. doi:10.1016/j.chroma.2019.460734
Luo, K., Kim, H.-Y., Oh, M.-H., and Kim, Y.-R. (2020a). Paper-based lateral flow strip assay for the detection of foodborne pathogens: Principles, applications, technological challenges and opportunities. Crit. Rev. Food Sci. Nutr. 60, 157–170. doi:10.1080/10408398.2018.1516623
Lv, X., Wang, L., Zhang, J., He, X., Shi, L., Zhao, L., et al. (2021). Quantitative detection of trace VBNC Cronobacter sakazakii by immunomagnetic separation in combination with PMAxx-ddPCR in dairy products. Food Microbiol. 99, 103831. doi:10.1016/j.fm.2021.103831
Ma, B., Li, J., Chen, K., Yu, X., Sun, C., Zhang, M., et al. (2020a). Multiplex recombinase polymerase amplification assay for the simultaneous detection of three foodborne pathogens in seafood. Foods 9, 278. doi:10.3390/foods9030278
Ma, C., Li, C., He, N., Wang, F., Ma, N., Zhang, L., et al. (2012). Preparation and characterization of monodisperse core-shell Fe3O4 @ SiO2 microspheres and its application for magnetic separation of nucleic acids from E. coli BL21. J. Biomed. Nanotechnol. 8, 1000–1005. doi:10.1166/jbn.2012.1454
Ma, T., Huang, H., Guo, W., Zhang, C., Chen, Z., Li, S., et al. (2020b). Recent progress in Black phosphorus sensors. J. Biomed. Nanotechnol. 16, 1045–1064. doi:10.1166/jbn.2020.2963
Magesa, F., Wu, Y., Dong, S., Tian, Y., Li, G., Vianney, J. M., et al. (2020). Electrochemical sensing fabricated with Ta2O5 nanoparticle-electrochemically reduced graphene oxide nanocomposite for the detection of oxytetracycline. Biomolecules 10, 110. doi:10.3390/biom10010110
Maguire, M., Kase, J. A., Roberson, D., Muruvanda, T., Brown, E. W., Allard, M., et al. (2021). Precision long-read metagenomics sequencing for food safety by detection and assembly of Shiga toxin-producing Escherichia coli in irrigation water. PLoS One 16, e0245172. doi:10.1371/journal.pone.0245172
Mangmee, S., Reamtong, O., Kalambaheti, T., Roytrakul, S., and Sonthayanon, P. (2020). MALDI-TOF mass spectrometry typing for predominant serovars of non-typhoidal Salmonella in a Thai broiler industry. Food control. 113, 107188. doi:10.1016/j.foodcont.2020.107188
McCuskey, S. R., Chatsirisupachai, J., Zeglio, E., Parlak, O., Panoy, P., Herland, A., et al. (2022). Current progress of interfacing organic semiconducting materials with bacteria. Chem. Rev. 122, 4791–4825. doi:10.1021/acs.chemrev.1c00487
Mei, Y., He, C., Zeng, W., Luo, Y., Liu, C., Yang, M., et al. (2022). Electrochemical biosensors for foodborne pathogens detection based on carbon nanomaterials: Recent advances and challenges. Food bioproc. Tech. 15, 498–513. doi:10.1007/s11947-022-02759-7
Mi, F., Guan, M., Hu, C., Peng, F., Sun, S., Wang, X., et al. (2021). Application of lectin-based biosensor technology in the detection of foodborne pathogenic bacteria: A review. Analyst 146, 429–443. doi:10.1039/d0an01459a
Mishra, A., Tyagi, M., Pilloton, R., Jain, S., and Narang, J. (2020). Evolving techniques for the detection of Listeria monocytogenes: Underlining the electrochemical approach. Int. J. Environ. Anal. Chem. 100, 507–523. doi:10.1080/03067319.2019.1674502
Mobed, A., Hasanzadeh, M., Ahmadalipour, A., and Fakhari, A. (2020). Recent advances in the biosensing of neurotransmitters: Material and method overviews towards the biomedical analysis of psychiatric disorders. Anal. Methods 12, 557–575. doi:10.1039/c9ay02390a
Morales-Pablos, M. I., Mejia-Sanchez, P., Diaz-Aparicio, E., Palomares-Resendiz, E. G., Gutierrez-Hernandez, J. L., Reyna-Granados, J. R., et al. (2020). Risk factors associated with the seroprevalence of paratuberculosis in sheep flocks in the hot-arid region of Sonora, Mexico. Trop. Anim. Health Prod. 52, 1357–1363. doi:10.1007/s11250-019-02139-y
Mou, X., Chen, Z., Li, T., Liu, M., Liu, Y., Ali, Z., et al. (2019a). A highly sensitive strategy for low-abundance hepatitis B virus detection via one-step nested polymerase chain reaction, chemiluminescence technology and magnetic separation. J. Biomed. Nanotechnol. 15, 1832–1838. doi:10.1166/jbn.2019.2802
Mou, X., Sheng, D., Chen, Z., Liu, M., Liu, Y., Deng, Y., et al. (2019b). In-situ mutation detection by magnetic beads-probe based on single base extension and its application in genotyping of hepatitis B virus pre-C region 1896nt locus single nucleotide polymorphisms. J. Biomed. Nanotechnol. 15, 2393–2400. doi:10.1166/jbn.2019.2862
Mu, D., Zhou, D., Xie, G., Liu, J., Xiong, Q., Feng, X., et al. (2021). The fluorescent probe-based recombinase-aided amplification for rapid detection of Escherichia coli O157:H7. Mol. Cell. Probes 60, 101777. doi:10.1016/j.mcp.2021.101777
Muriuki, S. W., Rengan, M. S., and Budambula, N. L. M. (2021). Prokaryotic diversity and potentially pathogenic bacteria in vended foods and environmental samples. Ann. Microbiol. 71, 27. doi:10.1186/s13213-021-01640-w
Myintzaw, P., Jaiswal, A. K., and Jaiswal, S. (2021). A review on campylobacteriosis associated with poultry meat consumption. Food Rev. Int., 1–15. doi:10.1080/87559129.2021.1942487
Nadar, S. S., Kelkar, R. K., Pise, P. V., Patil, N. P., Patil, S. P., Chaubal-Durve, N. S., et al. (2021). The untapped potential of magnetic nanoparticles for forensic investigations: A comprehensive review. Talanta 230, 122297. doi:10.1016/j.talanta.2021.122297
Naresh, V., and Lee, N. (2021). A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 21, 1109. doi:10.3390/s21041109
Nassarawa, S. S., Luo, Z., and Lu, Y. (2022). Conventional and emerging techniques for detection of foodborne pathogens in horticulture crops: A leap to food safety. Food bioproc. Tech. 21, 1248–1267. doi:10.1007/s11947-021-02730-y
Nath, P., Kabir, A., Khoubafarin Doust, S., Kreais, Z. J., and Ray, A. (2020). Detection of bacterial and viral pathogens using photonic point-of-care devices. Diagnostics 10 (10), 841. doi:10.3390/diagnostics10100841
Navarro, A., Barcena, C., Pozo, P., Diez-Guerrier, A., Martinez, I., Polo, C., et al. (2020). Liver transudate, a potential alternative to detect anti-hepatitis E virus antibodies in pigs and wild boars (Sus scrofa). Microorganisms 8 (3), 450. doi:10.3390/microorganisms8030450
Nguyen, H. V., Nguyen, V. D., Liu, F., and Seo, T. S. (2020). An integrated smartphone-based genetic analyzer for qualitative and quantitative pathogen detection. Acs Omega 5, 22208–22214. doi:10.1021/acsomega.0c02317
Nguyen, Q. H., and Kim, M. I. (2020). Nanomaterial-mediated paper-based biosensors for colorimetric pathogen detection. TrAC Trends Anal. Chem. 132, 116038. doi:10.1016/j.trac.2020.116038
Nie, L., Liu, F., Ma, P., and Xiao, X. (2014). Applications of gold nanoparticles in optical biosensors. J. Biomed. Nanotechnol. 10, 2700–2721. doi:10.1166/jbn.2014.1987
Nunez-Bajo, E., Collins, A. S. P., Kasimatis, M., Cotur, Y., Asfour, T., Tanriverdi, U., et al. (2020). Disposable silicon-based all-in-one micro-qPCR for rapid on-site detection of pathogens. Nat. Commun. 11, 6176. doi:10.1038/s41467-020-19911-6
Obande, G. A., and Singh, K. K. B. (2020). Current and future perspectives on isothermal nucleic acid amplification technologies for diagnosing infections. Infect. Drug Resist. 13, 455–483. doi:10.2147/idr.S217571
Oliveira, D. A., Althawab, S., McLamore, E. S., and Gomes, C. L. (2021). One-step fabrication of stimuli-responsive chitosan-platinum brushes for Listeria monocytogenes detection. Biosens. (Basel). 11, 511. doi:10.3390/bios11120511
Oyejobi, G. K., Sule, W. F., Akinde, S. B., Khan, F. M., and Ogolla, F. (2022). Multidrug-resistant enteric bacteria in Nigeria and potential use of bacteriophages as biocontrol. Sci. Total Environ. 824, 153842. doi:10.1016/j.scitotenv.2022.153842
Padzil, F., Mariatulqabtiah, A. R., Tan, W. S., Ho, K. L., Isa, N. M., Lau, H. Y., et al. (2022). Loop-mediated isothermal amplification (LAMP) as a promising point-of-care diagnostic strategy in avian virus research. Anim. (Basel). 12, 76. doi:10.3390/ani12010076
Pang, Y., Guo, X., Tian, X., Liu, F. X., Wang, L., Wu, J. L., et al. (2019). Developing a novel molecular serotyping system based on capsular polysaccharide synthesis gene clusters of Vibrio parahaemolyticus. Int. J. Food Microbiol. 309, 108332. doi:10.1016/j.ijfoodmicro.2019.108332
Park, J. Y., Lim, M. C., Park, K., Ok, G., Chang, H. J., Lee, N. A., et al. (2020). Detection of E. coli O157:H7 in food using automated immunomagnetic separation combined with real-time PCR. Processes 8 (8), 908. doi:10.3390/pr8080908
Patel, A., Jenkins, M., Rhoden, K., and Barnes, A. N. (2022). A systematic review of zoonotic enteric parasites carried by flies, cockroaches, and dung beetles. Pathogens 11 (1), 90. doi:10.3390/pathogens11010090
Peng, L.-H., Zhou, L.-Q., Chen, X., and Piao, X. (2020b). A computational study of potential miRNA-disease association inference based on ensemble learning and kernel ridge regression. Front. Bioeng. Biotechnol. 8, 40. doi:10.3389/fbioe.2020.00040
Peng, L., Tian, X., Tian, G., Xu, J., Huang, X., Weng, Y., et al. (2020a). Single-cell RNA-seq clustering: Datasets, models, and algorithms. RNA Biol. 17, 765–783. doi:10.1080/15476286.2020.1728961
Pires, N. M. M., Dong, T., Yang, Z., and da Silva, L. F. B. A. (2021). Recent methods and biosensors for foodborne pathogen detection in fish: Progress and future prospects to sustainable aquaculture systems. Crit. Rev. Food Sci. Nutr. 61, 1852–1876. doi:10.1080/10408398.2020.1767032
Pissuwan, D., Gazzana, C., Mongkolsuk, S., and Cortie, M. B. (2020). Single and multiple detections of foodborne pathogens by gold nanoparticle assays. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12 (1), e1584. doi:10.1002/wnan.1584
Plante, D., Barrera, J. A. B., Lord, M., Iugovaz, I., and Nasheri, N. (2021). Development of an RNA extraction protocol for norovirus from raw oysters and detection by qRT-PCR and droplet-digital RT-PCR. Foods 10, 1804. doi:10.3390/foods10081804
Pos, Z., Pos, O., Styk, J., Mocova, A., Strieskova, L., Budis, J., et al. (2020). Technical and methodological aspects of cell-free nucleic acids analyzes. Int. J. Mol. Sci. 21, 8634. doi:10.3390/ijms21228634
Prasannakumar, M. K., Parivallal, P. B., Manjunatha, C., Mahesh, H. B., Pramesh, D., Narayan, K. S., et al. (2020). Correction to: Loop-mediated isothermal amplification assay for pre-symptomatic stage detection of Xanthomonas axonopodis pv. punicae infection in pomegranate. Australas. Plant Pathol. 49, 475–476. doi:10.1007/s13313-020-00724-6
Prata, J. C., da Costa, J. P., Lopes, I., Andrady, A. L., Duarte, A. C., Rocha-Santos, T., et al. (2021). A One Health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ. 777, 146094. doi:10.1016/j.scitotenv.2021.146094
Qi, W., Wang, L., Rong, N., Huo, X., Li, Y., Liao, M., et al. (2022). A lab-on-a-tube biosensor for automatic detection of foodborne bacteria using rotated Halbach magnetic separation and Raspberry Pi imaging. Talanta 239, 123095. doi:10.1016/j.talanta.2021.123095
Qian, S., Chen, Y., Xu, X., Peng, C., Wang, X., Wu, H., et al. (2022). Advances in amplification-free detection of nucleic acid: CRISPR/Cas system as a powerful tool. Anal. Biochem. 643, 114593. doi:10.1016/j.ab.2022.114593
Qiu, H., Zhang, Y., Li, Z., Jiang, P., Guo, S., He, Y., et al. (2021). Donepezil ameliorates pulmonary arterial hypertension by inhibiting M2-macrophage activation. Front. Cardiovasc. Med. 8, 639541. doi:10.3389/fcvm.2021.639541
Quijada, N. M., Hernandez, M., and Rodriguez-Lazaro, D. (2020). High-throughput sequencing and food microbiology. Adv. Food Nutr. Res. 91, 275–300. doi:10.1016/bs.afnr.2019.10.003
Quintela, I. A., de los Reyes, B. G., Lin, C. S., and Wu, V. C. H. (2019). Simultaneous colorimetric detection of a variety of Salmonella spp. in food and environmental samples by optical biosensing using oligonucleotide-gold nanoparticles. Front. Microbiol. 10, 1138. doi:10.3389/fmicb.2019.01138
Ragab, M. A. A., and El-Kimary, E. I. (2021). Recent advances and applications of microfluidic capillary electrophoresis: A comprehensive review (2017-mid 2019). Crit. Rev. Anal. Chem. 51, 709–741. doi:10.1080/10408347.2020.1765729
Rajkovic, A., Jovanovic, J., Monteiro, S., Decleer, M., Andjelkovic, M., Foubert, A., et al. (2020). Detection of toxins involved in foodborne diseases caused by Gram-positive bacteria. Compr. Rev. Food Sci. Food Saf. 19, 1605–1657. doi:10.1111/1541-4337.12571
Rani, A., Ravindran, V. B., Surapaneni, A., Mantri, N., and Ball, A. S. (2021). Review: Trends in point-of-care diagnosis for Escherichia coli O157:H7 in food and water. Int. J. Food Microbiol. 349, 109233. doi:10.1016/j.ijfoodmicro.2021.109233
Razmi, N., Hasanzadeh, M., Willander, M., and Nur, O. (2020). Recent progress on the electrochemical biosensing of Escherichia coli O157:H7: Material and methods overview. Biosens. (Basel). 10, 54. doi:10.3390/bios10050054
Reuter, J. A., Spacek, D. V., and Snyder, M. P. (2015). High-throughput sequencing technologies. Mol. Cell 58, 586–597. doi:10.1016/j.molcel.2015.05.004
Ripolles-Avila, C., Martinez-Garcia, M., Capellas, M., Yuste, J., Fung, D. Y. C., Rodriguez-Jerez, J.-J., et al. (2020). From hazard analysis to risk control using rapid methods in microbiology: A practical approach for the food industry. Compr. Rev. Food Sci. Food Saf. 19, 1877–1907. doi:10.1111/1541-4337.12592
Safenkova, I. V., Ivanov, A. V., Slutskaya, E. S., Samokhvalov, A. V., Zherdev, A. V., Dzantiev, B. B., et al. (2020). Key significance of DNA-target size in lateral flow assay coupled with recombinase polymerase amplification. Anal. Chim. Acta X. 1102, 109–118. doi:10.1016/j.aca.2019.12.048
Salipante, S. J., and Jerome, K. R. (2020). Digital PCR-an emerging technology with broad applications in microbiology. Clin. Chem. 66, 117–123. doi:10.1373/clinchem.2019.304048
Saravanan, A., Kumar, P. S., Hemavathy, R. V., Jeevanantham, S., Kamalesh, R., Sneha, S., et al. (2021). Methods of detection of food-borne pathogens: A review. Environ. Chem. Lett. 19, 189–207. doi:10.1007/s10311-020-01072-z
Sarengaowa, H. W., Feng, K., Jiang, A., Xiu, Z., Lao, Y., Li, Y., et al. (2020). An in situ-synthesized gene chip for the detection of food-borne pathogens on fresh-cut cantaloupe and lettuce. Front. Microbiol. 10, 3089. doi:10.3389/fmicb.2019.03089
Scallan Walter, E. J., McLean, H. Q., and Griffin, P. M. (2020). Hospital discharge data underascertain enteric bacterial infections among children. Foodborne Pathog. Dis. 17, 530–532. doi:10.1089/fpd.2019.2773
Segeritz, L., Anders, O., Middelhoff, T. L., Winterfeld, D. T., Maksimov, P., Schares, G., et al. (2021). New insights into gastrointestinal and pulmonary parasitofauna of wild eurasian lynx (Lynx lynx) in the harz mountains of Germany. Pathogens 10 (12), 1650. doi:10.3390/pathogens10121650
Segerman, B. (2020). The most frequently used sequencing technologies and assembly methods in different time segments of the bacterial surveillance and RefSeq genome databases. Front. Cell. Infect. Microbiol. 10, 527102. doi:10.3389/fcimb.2020.527102
Sharma, P. C., Sharma, D., Sharma, A., Bhagat, M., Ola, M., Thakur, V. K., et al. (2021). Recent advances in microbial toxin-related strategies to combat cancer. Semin. Cancer Biol. doi:10.1016/j.semcancer.2021.07.007
Sheka, D., Alabi, N., and Gordon, P. M. K. (2021). Oxford nanopore sequencing in clinical microbiology and infection diagnostics. Brief. Bioinform. 22, bbaa403. doi:10.1093/bib/bbaa403
Shen, C., Islam, M. T., Masuda, Y., Honjoh, K.-i., and Miyamoto, T. (2020). Transcriptional changes involved in inhibition of biofilm formation by epsilon-polylysine in Salmonella Typhimurium. Appl. Microbiol. Biotechnol. 104, 5427–5436. doi:10.1007/s00253-020-10575-2
Shen, Y. F., Xu, L. Z., and Li, Y. B. (2021c). Biosensors for rapid detection of Salmonella in food: A review. Compr. Rev. Food Sci. Food Saf. 20, 149–197. doi:10.1111/1541-4337.12662
Shen, Y., Nie, J., Kuang, L., Zhang, J., and Li, H. (2021b). DNA sequencing, genomes and genetic markers of microbes on fruits and vegetables. Microb. Biotechnol. 14, 323–362. doi:10.1111/1751-7915.13560
Shen, Y., Zhang, Y., Gao, Z. F., Ye, Y., Wu, Q., Chen, H.-Y., et al. (2021a). Recent advances in nanotechnology for simultaneous detection of multiple pathogenic bacteria. Nano Today 38, 101121. doi:10.1016/j.nantod.2021.101121
Shi, Y. X., Wang, Y., Tian, Y. F., Liu, W. S., Zhu, W. H., Sun, C. B., et al. (2019). Establishment of a method for the simultaneous detection of four foodborne pathogens using high-throughput suspension array xTAG technology. Int. J. Food Sci. Technol. 54, 2578–2585. doi:10.1111/ijfs.14169
Shiba, K. (2006). Functionalization of carbon nanomaterials by evolutionary molecular engineering: Potential application in drug delivery systems. J. Drug Target. 14, 512–518. doi:10.1080/10611860600845033
Singha, S., Thomas, R., Viswakarma, J. N., and Gupta, V. K. (2022). Foodborne illnesses of Escherichia coli O157origin and its control measures. J. Food Sci. Technol. doi:10.1007/s13197-022-05381-9
Sohrabi, H., Majidi, M. R., Khaki, P., Jahanban-Esfahlan, A., de la Guardia, M., Mokhtarzadeh, A., et al. (2022). State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf. 21, 1868–1912. doi:10.1111/1541-4337.12913
Song, B., Wang, J. S., Yan, Z. J., Liu, Z. J., Pan, X. X., Zhang, Y. B., et al. (2020). Microfluidics for the rapid detection of Staphylococcus aureus using antibody-coated microspheres. Bioengineered 11, 1137–1145. doi:10.1080/21655979.2020.1831362
Sourri, P., Tassou, C. C., Nychas, G.-J. E., and Panagou, E. Z. (2022). Fruit juice spoilage by alicyclobacillus: Detection and control methods-A comprehensive review. Foods 11 (5), 747. doi:10.3390/foods11050747
Srivastava, S., Singh, L., Kaushik, V., Rajput, S., Jain, S., Pal, M. K., et al. (2021). Electrically controlled nanophotonic slot structure based on photocatalytic nanocomposite for optical detection of foodborne pathogens. J. Light. Technol. 39, 6670–6677. doi:10.1109/jlt.2021.3104409
Stryinski, R., Lopienska-Biernat, E., and Carrera, M. (2020). Proteomic insights into the biology of the most important foodborne parasites in europe. Foods 9 (10), 1403. doi:10.3390/foods9101403
Su, W., Liang, D., and Tan, M. (2021). Microfluidic strategies for sample separation and rapid detection of food allergens. Trends Food Sci. Technol. 110, 213–225. doi:10.1016/j.tifs.2021.02.004
Subjakova, V., Oravczova, V., Tatarko, M., and Hianik, T. (2021). Advances in electrochemical aptasensors and immunosensors for detection of bacterial pathogens in food. Electrochim. Acta 389, 138724. doi:10.1016/j.electacta.2021.138724
Sun, F., Zhang, J., Yang, Q., and Wu, W. (2021a). Quantum dot biosensor combined with antibody and aptamer for tracing food-borne pathogens. Food Qual. Saf. 5, fyab019. doi:10.1093/fqsafe/fyab019
Sun, L., Zhang, H., Chen, J., Chen, L., Qi, X., Zhang, R., et al. (2021b). Epidemiology of foodborne disease outbreaks caused by nontyphoidal Salmonella in zhejiang Province, China, 2010-2019. Foodborne Pathog. Dis. 18 (12), 880–886. doi:10.1089/fpd.2021.0006
Syromyatnikov, M. Y., Kokina, A. V., Solodskikh, S. A., Panevina, A. V., Popov, E. S., Popov, V. N., et al. (2020). High-throughput 16S rRNA gene sequencing of butter microbiota reveals a variety of opportunistic pathogens. Foods 9, 608. doi:10.3390/foods9050608
Taguchi, T., Ishikawa, M., Ichikawa, M., Tadenuma, T., Hirakawa, Y., Yoshino, T., et al. (2021). Amplification-free detection of bacterial genes using a signaling probe-based DNA microarray. Biosens. Bioelectron. X. 194, 113659. doi:10.1016/j.bios.2021.113659
Tahir, U., Shim, Y. B., Kamran, M. A., Kim, D.-I., and Jeong, M. Y. (2021). Nanofabrication techniques: Challenges and future prospects. J. Nanosci. Nanotechnol. 21, 4981–5013. doi:10.1166/jnn.2021.19327
Tang, C., He, Z., Liu, H., Xu, Y., Huang, H., Yang, G., et al. (2020). Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnology 18, 62. doi:10.1186/s12951-020-00613-6
Tang, Y., Ali, Z., Dai, J., Liu, X., Wu, Y., Chen, Z., et al. (2018). Single-nucleotide polymorphism genotyping of exoS in Pseudomonas aeruginosa using dual-color fluorescence hybridization and magnetic separation. J. Biomed. Nanotechnol. 14, 206–214. doi:10.1166/jbn.2018.2525
Teffo, L. A., and Tabit, F. T. (2020). An assessment of the food safety knowledge and attitudes of food handlers in hospitals. BMC Public Health 20 (1), 311. doi:10.1186/s12889-020-8430-5
Teklemariam, A. D., Samaddar, M., Alharbi, M. G., Al-Hindi, R. R., and Bhunia, A. K. (2020). Biosensor and molecular-based methods for the detection of human coronaviruses: A review. Mol. Cell. Probes 54, 101662. doi:10.1016/j.mcp.2020.101662
Tian, Y., Deng, P., Wu, Y., Ding, Z., Li, G., Liu, J., et al. (2019). A simple and efficient molecularly imprinted electrochemical sensor for the selective determination of tryptophan. Biomolecules 9, 294. doi:10.3390/biom9070294
Tian, Y., Liu, T., Liu, C., Xu, Q., and Liu, Q. (2022). Pathogen detection strategy based on CRISPR. Microchem. J. 174, 107036. doi:10.1016/j.microc.2021.107036
Tseng, C.-C., Kung, C.-T., Chen, R.-F., Tsai, M.-H., Chao, H.-R., Wang, Y.-N., et al. (2021). Recent advances in microfluidic paper-based assay devices for diagnosis of human diseases using saliva, tears and sweat samples. Sensors Actuators B Chem. 342, 130078. doi:10.1016/j.snb.2021.130078
Tsougeni, K., Kaprou, G., Loukas, C. M., Papadakis, G., Hamiot, A., Eck, M., et al. (2020). Lab-on-Chip platform and protocol for rapid foodborne pathogen detection comprising on-chip cell capture, lysis, DNA amplification and surface-acoustic-wave detection. Sensors Actuators B Chem. 320, 128345. doi:10.1016/j.snb.2020.128345
Uelze, L., Gruetzke, J., Borowiak, M., Hammerl, J. A., Juraschek, K., Deneke, C., et al. (2020). Typing methods based on whole genome sequencing data. One Health Outlook 2, 3. doi:10.1186/s42522-020-0010-1
Upasham, S., Banga, I. K., Jagannath, B., Paul, A., Lin, K.-C., Muthukumar, S., et al. (2021). Electrochemical impedimetric biosensors, featuring the use of Room Temperature Ionic Liquids (RTILs): Special focus on non-faradaic sensing. Biosens. Bioelectron. X. 177, 112940. doi:10.1016/j.bios.2020.112940
Van Poelvoorde, L. A., Saelens, X., Thomas, I., and Roosens, N. H. (2020). Next-generation sequencing: An eye-opener for the surveillance of antiviral resistance in influenza. Trends Biotechnol. 38, 360–367. doi:10.1016/j.tibtech.2019.09.009
Van Puyvelde, L., Aissa, A., Panda, S. K., De Borggraeve, W. M., Mukazayire, M. J., Luyten, W., et al. (2021). Bioassay-guided isolation of antibacterial compounds from the leaves of Tetradenia riparia with potential bactericidal effects on food-borne pathogens. J. Ethnopharmacol. 273, 113956. doi:10.1016/j.jep.2021.113956
Van Reckem, E., De Vuyst, L., Weckx, S., and Leroy, F. (2021). Next-generation sequencing to enhance the taxonomic resolution of the microbiological analysis of meat and meat-derived products. Curr. Opin. Food Sci. 37, 58–65. doi:10.1016/j.cofs.2020.09.004
Vidic, J., and Manzano, M. (2021). Electrochemical biosensors for rapid pathogen detection. Curr. Opin. Electrochem. 29, 100750. doi:10.1016/j.coelec.2021.100750
Vidyadharani, G., Vijaya Bhavadharani, H. K., Sathishnath, P., Ramanathan, S., Sariga, P., Sandhya, A., et al. (2021). Present and pioneer methods of early detection of food borne pathogens. J. Food Sci. Technol. 59, 2087–2107. doi:10.1007/s13197-021-05130-4
Visciano, P., and Schirone, M. (2021). Food frauds: Global incidents and misleading situations. Trends Food Sci. Technol. 114, 424–442. doi:10.1016/j.tifs.2021.06.010
Wachiralurpan, S., Chansiri, K., and Lieberzeit, P. A. (2020). Direct detection of Listeria monocytogenes DNA amplification products with quartz crystal microbalances at elevated temperatures. Sensors Actuators B Chem. 308, 127678. doi:10.1016/j.snb.2020.127678
Wagner, E., Fagerlund, A., Langsrud, S., Moretro, T., Jensen, M. R., Moen, B., et al. (2021). Surveillance of Listeria monocytogenes: Early detection, population dynamics, and quasimetagenomic sequencing during selective enrichment. Appl. Environ. Microbiol. 87, e0177421. doi:10.1128/aem.01774-21
Walter, E. J. S., Griffin, P. M., Bruce, B. B., and Hoekstra, R. M. (2021). Estimating the number of illnesses caused by agents transmitted commonly through food: A scoping review. Foodborne Pathog. Dis. 18, 841–858. doi:10.1089/fpd.2021.0038
Wan, J., Zheng, L., Kong, L., Lu, Z., Tao, Y., Feng, Z., et al. (2021). Development of a rapid detection method for real-time fluorescent quantitative PCR of Salmonella spp. and Salmonella Enteritidis in ready-to-eat fruits and vegetables. LWT 149, 111837. doi:10.1016/j.lwt.2021.111837
Wang, B., and Park, B. (2020). Immunoassay biosensing of foodborne pathogens with surface plasmon resonance imaging: A review. J. Agric. Food Chem. 68, 12927–12939. doi:10.1021/acs.jafc.0c02295
Wang, D., Lian, F., Yao, S., Liu, Y., Wang, J., Song, X., et al. (2020e). Simultaneous detection of three foodborne pathogens based on immunomagnetic nanoparticles and fluorescent quantum dots. Acs Omega 5, 23070–23080. doi:10.1021/acsomega.0c02833
Wang, J., Tan, L., Bi, W., Shen, H., Li, D., Yu, Z., et al. (2022). Ultrasensitive microfluidic immunosensor with stir bar enrichment for point-of-care test of Staphylococcus aureus in foods triggered by DNAzyme-assisted click reaction. Food Chem. x. 378, 132093. doi:10.1016/j.foodchem.2022.132093
Wang, K., Wang, Z., Zeng, H., Luo, X., and Yang, T. (2020a). Advances in portable visual detection of pathogenic bacteria. ACS Appl. Bio Mat. 3, 7291–7305. doi:10.1021/acsabm.0c00984
Wang, L.-X., Fu, J.-J., Zhou, Y., Chen, G., Fang, C., Lu, Z. S., et al. (2020f). On-chip RT-LAMP and colorimetric detection of the prostate cancer 3 biomarker with an integrated thermal and imaging box. Talanta 208, 120407. doi:10.1016/j.talanta.2019.120407
Wang, L., Huo, X., Qi, W., Xia, Z., Li, Y., Lin, J., et al. (2020g). Rapid and sensitive detection of Salmonella Typhimurium using nickel nanowire bridge for electrochemical impedance amplification. Talanta 211, 120715. doi:10.1016/j.talanta.2020.120715
Wang, L., and Lin, J. (2020). Recent advances on magnetic nanobead based biosensors: From separation to detection. TrAC Trends Anal. Chem. 128, 115915. doi:10.1016/j.trac.2020.115915
Wang, L., Xue, L., Guo, R., Zheng, L., Wang, S., Yao, L., et al. (2020d). Combining impedance biosensor with immunomagnetic separation for rapid screening of Salmonella in poultry supply chains. Poult. Sci. 99, 1606–1614. doi:10.1016/j.psj.2019.12.007
Wang, M., Zhang, Y., Tian, F., Liu, X., Du, S., Ren, G., et al. (2021d). Overview of rapid detection methods for Salmonella in foods: Progress and challenges. Foods 10, 2402. doi:10.3390/foods10102402
Wang, N., Pan, G., Liu, P., Rong, S., Gao, Z., and Li, Q. (2021a). Advances and future perspective on detection technology of human norovirus. Pathogens 10, 1383. doi:10.3390/pathogens10111383
Wang, R., Wang, L., Yan, J., Luan, D., Sun, T., Wu, J., et al. (2021c). Rapid, sensitive and label-free detection of pathogenic bacteria using a bacteria-imprinted conducting polymer film-based electrochemical sensor. Talanta 226, 122135. doi:10.1016/j.talanta.2021.122135
Wang, S., Zhu, X., Meng, Q., Zheng, P., Zhang, J., He, Z., et al. (2021b). Gold interdigitated micro-immunosensor based on Mn-MOF-74 for the detection of Listeria monocytogens. Biosens. Bioelectron. X. 183, 113186. doi:10.1016/j.bios.2021.113186
Wang, W., Deng, Y., Li, S., Liu, H., Lu, Z., Zhang, L., et al. (2013). A novel acetylcholine bioensor and its electrochemical behavior. J. Biomed. Nanotechnol. 9, 736–740. doi:10.1166/jbn.2013.1577
Wang, Z., Cai, R., Gao, Z., Yuan, Y., and Yue, T. (2020c). Immunomagnetic separation: An effective pretreatment technology for isolation and enrichment in food microorganisms detection. Compr. Rev. Food Sci. Food Saf. 19, 3802–3824. doi:10.1111/1541-4337.12656
Wang, Z., Yao, X., Zhang, Y., Wang, R., Ji, Y., Sun, J., et al. (2020b). Functional nanozyme mediated multi-readout and label-free lateral flow immunoassay for rapid detection of Escherichia coli O157:H7. Food Chem. x. 329, 127224. doi:10.1016/j.foodchem.2020.127224
Wangman, P., Surasilp, T., Pengsuk, C., Sithigorngul, P., and Longyant, S. (2021). Development of a species‐specific monoclonal antibody for rapid detection and identification of foodborne pathogen Vibrio vulnificus. J. Food Saf. 41. doi:10.1111/jfs.12939
Wei, L., Wang, Z., Feng, C., Xianyu, Y., and Chen, Y. (2021). Direct transverse relaxation time biosensing strategy for detecting foodborne pathogens through enzyme-mediated sol-gel transition of hydrogels. Anal. Chem. 93, 6613–6619. doi:10.1021/acs.analchem.0c03968
Weng, X., Zhang, C., and Jiang, H. (2021). Advances in microfluidic nanobiosensors for the detection of foodborne pathogens. LWT 151, 112172. doi:10.1016/j.lwt.2021.112172
Wu, X., Luo, H., Xu, F., Ge, C., Li, S., Deng, X., et al. (2021). Evaluation of Salmonella serotype prediction with multiplex nanopore sequencing. Front. Microbiol. 12, 637771. doi:10.3389/fmicb.2021.637771
Wu, Y., Deng, P., Tian, Y., Ding, Z., Li, G., Liu, J., et al. (2020a). Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 131, 107393. doi:10.1016/j.bioelechem.2019.107393
Wu, Y., Deng, P., Tian, Y., Feng, J., Xiao, J., Li, J., et al. (2020b). Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnology 18, 112. doi:10.1186/s12951-020-00672-9
Xi, Z., Huang, R., Deng, Y., and He, N. (2014). Progress in selection and biomedical applications of aptamers. J. Biomed. Nanotechnol. 10, 3043–3062. doi:10.1166/jbn.2014.1979
Xia, J., Qiu, S., Zeng, H., Liu, C., and Liu, Q. (2021). A rapid detection of Escherichia coli O157 : H7 by competition visual antigen macroarray. J. Food Saf. 4141 (1), e12872. doi:10.1111/jfs.12872
Xiao, C., Guo, Y., Zhao, K., Liu, S., He, N., He, Y., et al. (2022). Prognostic value of machine learning in patients with acute myocardial infarction. J. Cardiovasc. Dev. Dis. 9, 56. doi:10.3390/jcdd9020056
Xiao, X., Hu, S., Lai, X., Peng, J., and Lai, W. (2021). Developmental trend of immunoassays for monitoring hazards in food samples: A review. Trends Food Sci. Technol. 111, 68–88. doi:10.1016/j.tifs.2021.02.045
Xiao, Z., Chen, H., Chen, H., Wu, L., Yang, G., Wu, Y., et al. (2019). Advanced diagnostic strategies for Clostridium difficile infection (CDI). J. Biomed. Nanotechnol. 15, 1113–1134. doi:10.1166/jbn.2019.2782
Xie, G. Y., Zhou, D. G., Zhao, G. J., Feng, X. Y., Aguilar, Z. P., and Xu, H. Y. (2021b). Recombinase aided amplification with photoreactive DNA-binding dye for rapid detection of viable Staphylococcus aureus. Lwt-Food Sci. Technol. 135, 110249. doi:10.1016/j.lwt.2020.110249
Xie, M., Chen, T., Xin, X., Cai, Z., Dong, C., Lei, B., et al. (2022). Multiplex detection of foodborne pathogens by real-time loop-mediated isothermal amplification on a digital microfluidic chip. Food control. 136, 108824. doi:10.1016/j.foodcont.2022.108824
Xie, W., Wei, S., Zheng, Z., Jiang, Y., and Yang, D. (2021a). Recognition of defective carrots based on deep learning and transfer learning. Food bioproc. Tech. 14, 1361–1374. doi:10.1007/s11947-021-02653-8
Xie, X. Q., and Liu, Z. (2021). Simultaneous enumeration of Cronobacter sakazakii and Staphylococcus aureus in powdered infant foods through duplex TaqMan real-time PCR. Int. Dairy J. 117, 105019. doi:10.1016/j.idairyj.2021.105019
Xie, Z., Pu, H., and Sun, D.-W. (2021c). Computer simulation of submicron fluid flows in microfluidic chips and their applications in food analysis. Compr. Rev. Food Sci. Food Saf. 20, 3818–3837. doi:10.1111/1541-4337.12766
Xu, L. J., Du, J. J., Deng, Y., and He, N. Y. (2012). Electrochemical detection of E. coli O157:H7 using porous pseudo-carbon paste electrode modified with carboxylic multi-walled carbon nanotubes, glutaraldehyde and 3-aminopropyltriethoxysilane. J. Biomed. Nanotechnol. 8, 1006–1011. doi:10.1166/jbn.2012.1456
Xu, Y., Wang, T., Chen, Z., Jin, L., Wu, Z., Yan, J., et al. (2021). The point-of-care-testing of nucleic acids by chip, cartridge and paper sensors. Chin. Chem. Lett. 32, 3675–3686. doi:10.1016/j.cclet.2021.06.025
Xue, L., Jin, N. N., Guo, R. Y., Wang, S. Y., Qi, W. Z., Liu, Y. J., et al. (2021). Microfluidic colorimetric biosensors based on MnO2 nanozymes and convergence-divergence spiral micromixers for rapid and sensitive detection of Salmonella. ACS Sens. 6, 2883–2892. doi:10.1021/acssensors.1c00292
Yan, J., Lu, Y., Xie, S., Tan, H., Tan, W., Li, N., et al. (2021). Highly fluorescent N-doped carbon quantum dots derived from bamboo stems for selective detection of Fe3+ ions in biological systems. J. Biomed. Nanotechnol. 17, 312–321. doi:10.1166/jbn.2021.3034
Yang, , J., Zhang, N., Lv, J., Zhu, P., Pan, X., Hu, J., et al. (2020b). Comparing the performance of conventional PCR, RTQ-PCR, and droplet digital PCR assays in detection of Shigella. Mol. Cell. Probes 51, 101531. doi:10.1016/j.mcp.2020.101531
Yang, G., Huang, H., Xiao, Z., Zhang, C., Guo, W., Ma, T., et al. (2020c). A novel strategy for liquid exfoliation of ultrathin Black phosphorus nanosheets. J. Biomed. Nanotechnol. 16, 548–552. doi:10.1166/jbn.2020.2909
Yang, H. W., Liang, W. B., Si, J., Li, Z. Y., and He, N. Y. (2014a). Long spacer arm-functionalized magnetic nanoparticle platform for enhanced chemiluminescent detection of hepatitis B virus. J. Biomed. Nanotechnol. 10, 3610–3619. doi:10.1166/jbn.2014.2047
Yang, H. W., Liu, M., Jiang, H. R., Zeng, Y., Jin, L., Luan, T., et al. (2017). Copy number variation analysis based on gold magnetic nanoparticles and fluorescence multiplex ligation-dependent probe amplification. J. Biomed. Nanotechnol. 13, 655–664. doi:10.1166/jbn.2017.2386
Yang, S., Guo, H., Wei, B., Zhu, S., Cai, Y., Jiang, P., et al. (2014b). Association of miR-502-binding site single nucleotide polymorphism in the 3-untranslated region of SET8 and TP53 codon 72 polymorphism with non-small cell lung cancer in Chinese population. Acta Biochimica Biophysica Sinica 46, 149–154. doi:10.1093/abbs/gmt138
Yang, Y., Chen, Y., Tang, H., Zong, N., and Jiang, X. (2020a). Microfluidics for biomedical analysis. Small Methods 4, 1900451. doi:10.1002/smtd.201900451
Yao, S., Li, J., Pang, B., Wang, X., Shi, Y., Song, X., et al. (2020). Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of gold nanoparticles. Microchim. Acta 187, 504. doi:10.1007/s00604-020-04473-7
Yin, M., Jing, C., Li, H., Deng, Q., and Wang, S. (2020). Surface chemistry modified upconversion nanoparticles as fluorescent sensor array for discrimination of foodborne pathogenic bacteria. J. Nanobiotechnology 18, 41. doi:10.1186/s12951-020-00596-4
Yu, H., Guo, W., Lu, X., Xu, H., Yang, Q., Tan, J., et al. (2021b). Reduced graphene oxide nanocomposite based electrochemical biosensors for monitoring foodborne pathogenic bacteria: A review. Food control. 127, 108117. doi:10.1016/j.foodcont.2021.108117
Yu, J., Hou, Q., Li, W., Huang, W., Mo, L., Yao, C., et al. (2020). Profiling of the viable bacterial and fungal microbiota in fermented feeds using single-molecule real-time sequencing. J. Anim. Sci. 98, skaa029. doi:10.1093/jas/skaa029
Yu, Y., Li, R., Ma, Z., Han, M., Zhang, S., Zhang, M., et al. (2021a). Development and evaluation of a novel loop mediated isothermal amplification coupled with TaqMan probe assay for detection of genetically modified organism with NOS terminator. Food Chem. x. 356, 129684. doi:10.1016/j.foodchem.2021.129684
Zaczek-Moczydlowska, M. A., Beizaei, A., Dillon, M., and Campbell, K. (2021). Current state-of-the-art diagnostics for Norovirus detection: Model approaches for point-of-care analysis. Trends Food Sci. Technol. 114, 684–695. doi:10.1016/j.tifs.2021.06.027
Zarkani, A. A., and Schikora, A. (2021). Mechanisms adopted by Salmonella to colonize plant hosts. Food Microbiol. 99, 103833. doi:10.1016/j.fm.2021.103833
Zeng, Y., Tan, X., Zhang, L., Jiang, N., and Cao, H. (2014). Identification and expression of fructose-1, 6-bisphosphate aldolase genes and their relations to oil content in developing seeds of tea oil tree (camellia oleifera). PLoS One 9, e107422. doi:10.1371/journal.pone.0107422
Zhai, Y., Meng, X., Li, L., Liu, Y., Xu, K., Zhao, C., et al. (2021). Rapid detection of Vibrio parahaemolyticus using magnetic nanobead-based immunoseparation and quantum dot-based immunofluorescence. RSC Adv. 11, 38638–38647. doi:10.1039/d1ra07580b
Zhai, Y., Zhao, C., Li, L., Xu, K., Wang, J., Song, X., et al. (2020). Production of phage display-derived peptide and the application for DetectingVibrio parahaemolyticusby combined PCR technology. Food Anal. Methods 13, 1906–1917. doi:10.1007/s12161-020-01800-9
Zhang, J., Wang, Y., and Lu, X. (2021c). Molecular imprinting technology for sensing foodborne pathogenic bacteria. Anal. Bioanal. Chem. 413, 4581–4598. doi:10.1007/s00216-020-03138-x
Zhang, K., Deng, R., Gao, H., Teng, X., and Li, J. (2020d). Lighting up single-nucleotide variation in situ in single cells and tissues. Chem. Soc. Rev. 49, 1932–1954. doi:10.1039/c9cs00438f
Zhang, L., Jiang, H., Zhu, Z., Liu, J., and Li, B. (2022). Integrating CRISPR/Cas within isothermal amplification for point-of-Care Assay of nucleic acid. Talanta 243, 123388. doi:10.1016/j.talanta.2022.123388
Zhang, M. M., Liu, J. F., Shen, Z. Q., Liu, Y. X., Song, Y., Liang, Y., et al. (2021b). A newly developed paper embedded microchip based on LAMP for rapid multiple detections of foodborne pathogens. BMC Microbiol. 21, 197. doi:10.1186/s12866-021-02223-0
Zhang, M., Ye, J., He, J.-s., Zhang, F., Ping, J., Qian, C., et al. (2020a). Visual detection for nucleic acid-based techniques as potential on-site detection methods. A review. Anal. Chim. Acta. 1099, 1–15. doi:10.1016/j.aca.2019.11.056
Zhang, R., Belwal, T., Li, L., Lin, X., Xu, Y., Luo, Z., et al. (2020b). Nanomaterial-based biosensors for sensing key foodborne pathogens: Advances from recent decades. Compr. Rev. Food Sci. Food Saf. 19, 1465–1487. doi:10.1111/1541-4337.12576
Zhang, Y., Qu, Q., Rao, M., Zhang, N., Zhao, Y., Tao, F., et al. (2020c). Simultaneous identification of animal-derived components in meats using high-throughput sequencing in combination with a custom-built mitochondrial genome database. Sci. Rep. 10, 8965. doi:10.1038/s41598-020-65724-4
Zhang, Y., Ren, F., Wang, G., Liao, T., Hao, Y., Zhang, H., et al. (2021a). Rapid and sensitive pathogen detection platform based on a lanthanide-labeled immunochromatographic strip test combined with immunomagnetic separation. Sensors Actuators B Chem. 329, 129273. doi:10.1016/j.snb.2020.129273
Zhao, , T., Ji, P., and Kumar, G. D. (2021b). Pre-harvest treatment for reduction of foodborne pathogens and microbial load on tomatoes. Food control. 119, 107469. doi:10.1016/j.foodcont.2020.107469
Zhao, L., Guo, J., Li, S., and Wang, J. (2021a). The development of thermal immunosensing for the detection of food-borne pathogens E. coli O157:H7 based on the novel substoichiometric photothermal conversion materials MoO3-x NPs. Sensors Actuators B Chem. 344, 130306. doi:10.1016/j.snb.2021.130306
Zhao, X., and Wu, C. (2020). Recent advances in peptide nucleic acids for rapid detection of foodborne pathogens. Food Anal. Methods 13, 1956–1972. doi:10.1007/s12161-020-01811-6
Zhao, Y., Zeng, D., Yan, C., Chen, W., Ren, J., Jiang, Y., et al. (2020). Rapid and accurate detection of Escherichia coli O157:H7 in beef using microfluidic wax-printed paper-based ELISA. Analyst 145, 3106–3115. doi:10.1039/d0an00224k
Zheng, X. T., and Tan, Y. N. (2020). Recent development of nucleic acid nanosensors to detect sequence-specific binding interactions: From metal ions, small molecules to proteins and pathogens. Sensors Int. 1, 100034. doi:10.1016/j.sintl.2020.100034
Zhou, B. Q., Ye, Q. H., Li, F., Xiang, X. R., Shang, Y. T., Wang, C. F., et al. (2022). CRISPR/Cas12a based fluorescence-enhanced lateral flow biosensor for detection of Staphylococcus aureus. Sensors Actuators B Chem. 351, 130906. doi:10.1016/j.snb.2021.130906
Zhou, L., Peng, Y., Wang, Q., and Lin, Q. (2017). An ESIPT-based two-photon fluorescent probe detection of hydrogen peroxide in live cells and tissues. J. Photochem. Photobiol. B Biol. 167, 264–268. doi:10.1016/j.jphotobiol.2017.01.011
Zhou, X., Pu, H., and Sun, D.-W. (2021). DNA functionalized metal and metal oxide nanoparticles: Principles and recent advances in food safety detection. Crit. Rev. Food Sci. Nutr. 61, 2277–2296. doi:10.1080/10408398.2020.1809343
Keywords: foodborne pathogens, rapid detection, immunoassay, molecular biology, POCT, microfluidic chip, biosensor
Citation: Liu S, Zhao K, Huang M, Zeng M, Deng Y, Li S, Chen H, Li W and Chen Z (2022) Research progress on detection techniques for point-of-care testing of foodborne pathogens. Front. Bioeng. Biotechnol. 10:958134. doi: 10.3389/fbioe.2022.958134
Received: 31 May 2022; Accepted: 30 June 2022;
Published: 08 August 2022.
Edited by:
Long Ma, Tianjin University of Science and Technology, ChinaReviewed by:
Zhirui Guo, Nanjing Medical University, ChinaTang Yongjun, Shenzhen Polytechnic, China
Wenbo Lu, Shanxi Normal University, China
Copyright © 2022 Liu, Zhao, Huang, Zeng, Deng, Li, Chen, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu Chen, Y2hlbnpodTIyMEAxNjMuY29t
 Sha Liu1
Sha Liu1 Hui Chen
Hui Chen Wen Li
Wen Li Zhu Chen
Zhu Chen