- 1Department of Chemistry, GC University Lahore, Lahore, Pakistan
- 2Division of Science and Technology, University of Education Lahore, Lahore, Pakistan
- 3Department of Chemistry, Faculty of Sciences, University of Central Punjab, Lahore, Pakistan
- 4University of South Wales, South Wales, NSW, Australia
- 5King Saud Medical City, Al Riyadh, Saudi Arabia
- 6King Saud University, Riyadh, Saudi Arabia
- 7University of Sahiwal, Sahiwal, Pakistan
In this study, ZIF-67-based mixed matrix membrane was synthesized with a solution casting method using tetrahydrofuran as the solvent. The as-synthesized ZIF-67 was characterized using PXRD, TGA, ATR-FTIR, and BET analysis for the surface area measurements. The minimum 3 wt% loading of ZIF-67 was incorporated within a hydrophobic polymer to evaluate the CO2 adsorption performance of ZIF-67. The stability of ZIF-67 in pure water and inorganic solvents was investigated. The maximum CO2 adsorption of the ZIF-67 mixed-matrix membrane (MMM) was 0.5 mmol/g at 273 K, which is higher than that of the pure polymer. The fabricated ZIF-67-based mixed-matrix membrane showed higher CO2 capture even at lower MOF loading using THF. The current study highly recommends the combination of hydrophobic polysulfone and a water-stable ZIF-67 for CO2 capture from wet flue gases.
Introduction
The burning of fossil fuels to meet the rising energy demand is the primary source of anthropogenic carbon dioxide (CO2) emissions in the atmosphere, which is a major contributor to greenhouse gas (GHG) emissions and global warming (Habib et al., 2020). The concentration of atmospheric CO2 has steadily increased during the last century, rising from 280 parts per million in the 1800s to more than 389 parts per million in 2019 and 400 parts per million in 2020 (Al-Rowaili et al., 2021). Adsorption processes based on porous solids (e.g., carbon materials) are becoming a great alternative to real technology based on absorption processes, utilizing basic solutions in terms of cost and environmental impact (Casco et al., 2014). Membrane-based gas separation techniques, on the other hand, provide an economically and technologically viable alternative for CCS (carbon, capture, and storage) due to their high energy efficiency, cheap capital cost, low carbon footprint, and simple and continuous operation mode (Cheng et al., 2019). In comparison to inorganic and molecular sieve materials, polymeric membranes have drawbacks such as lower polymer/particle interface compatibility. As a result, mixed-matrix membranes (MMMs) with a polymer matrix and an inorganic substance as the dispersion phase were developed. To improve polymer separation capabilities, various particles such as metal oxide nanoparticles and inorganic molecular sieves were introduced to the polymer matrix (Dorosti and Alizadehdakhel, 2018).
One of the most significant problems is that the fillers do not have adequate interfacial compatibility with the polymer matrix. MMMs frequently underperform their projected separation performance behavior due to poor adhesion between the polymer matrix and the fillers (Ishaq et al., 2019). Metal organic frameworks (MOFs) are made up of organic linkers, and the metal center functions as a filler in membrane preparation. MOFs have changeable pore/aperture size, high surface area, and specific adsorption affinity. As a result, MOF-based membranes have high potential for gas separation application (Yuan et al., 2017).
ZIFs (zeolitic imidazolate frameworks), a new type of metal organic frameworks (MOFs) with outstanding chemical and thermal stability, are being tested for a variety of applications such as gas adsorption and catalysis, etc. (Qian et al., 2012). The introduction of ZIFs into polymeric membranes improves the efficiency of gas separation processes. The Co-N bond in ZIF-67 is stronger than the Zn-N bond in ZIF-8, and the smaller pore size of ZIF-67 makes it a more suitable material for gas separation application (Gupta and Murthy, 2022). Zhang et al. (2012a) established successful separation output with C3H6-C3H8 by utilizing a zeolitic imidazolate framework. They found it excellent for C3H6/C3H8 separation due to effective pore size (3.4 Ao) of ZIF-8 (Zhang et al., 2012b).
When evaluating MOFs for adsorption applications, their behavior in the presence of water is a critical consideration. Water vapor is present in many industrial streams and must be taken into consideration when choosing adsorbents for adsorption separation and purification systems (Burtch et al., 2014).
S. Meshkat et al. prepared a mixed matrix membrane using the solvent evaporation method with different loadings of ZIF-67 up to 5 wt. % with Pebax polymer for CO2/N2 gas separation application (Zhang et al., 2012b; Meshkat et al., 2020). N. Gupta et al. studied the effect of different wt.% loadings of ZIF-67 with polysulfone for extraction of NaNO3 and Na2SO4 from synthetic solutions using the phase inversion method (Gupta and Murthy, 2022). S. Feng et al. fabricated a mixed-matrix membrane incorporated with morphological regulated ZIF-67 nanosheets up to 5 wt.% loadings with Pebax using the solvent evaporation method for CO2/N2 separation application (Feng et al., 2020). Another scientific report suggested Pebax as a copolymer due to its thermoplastic elastomer property, with PA recognized for mechanical strengthening and PE for flexible gas transport (Barbi et al., 2003; Car et al., 2008). Hägg et al. (Nafisi and Hägg, 2014) reported that the permeability of CH4, N2, and CO2 was elevated by using ZIF-8 nanoparticles in Pebax polymer and fabricated dual sheet MMM. Furthermore, the selectivity parameter for CO2/NO2 decreased from 33.8 to 32.3 of the Pebax membrane in comparison with ZIF-8 loaded MMMs. Another study adapted IL@ZIF-8 socked with activated filler in methanol and BMIIm, finally blended with Pebax polymer. The 15 wt% MMM can efficiently raise the selectivity of CO2-CH4 and CO2-N2 by 92 % and 74%, respectively, whereas CO2 permeability by 45% (Li et al., 2016). Rodrigue et al. fabricated Pebax-based ZIF-67 and reported a 130% (162 barrer) increase in CO2 permeability. Another investigation on the fabricated ZIF-67 NS/Pebax reported enhanced CO2-N2 selectivity to 73.2 barrer and permeability of CO2 to 139.4 barrer, whereas DnBMCl Pebax 1,657 with ZIF8 association delivered high selectivities and permeabilities for CO2/CH4, CO2/H2, and CO2/N2 (Jomekian et al., 2017; Meshkat et al., 2020).
In this study, as far as our knowledge, there are no studies on ZIF- 67 based MMM using THF as a solvent. In this work, we synthesized a mixed-matrix membrane based on water stable ZIF-67 and hydrophobic polymer using the solvent evaporation method to check the CO2 adsorption performance of lower MOFs. In this work, ZIF-67 was made at room temperature, and a ZIF-67-based mixed matrix membrane was developed. The prepared ZIF-67 mixed-matrix membrane is highly stable after exposure to organic solvents and pure water. The CO2 adsorption isotherms were measured to evaluate the performance prepared membrane.
Materials
Polysulfone (PS) average Mw ∼35,000 g/mol, density (1.24 g/ml at 25°C) by Sigma Aldrich and cobalt nitratehexahydrate (Co(NO3)2∙6H2O, 98%),2-methylimidazole (C4H6N2, 99%) were obtained from Sigma Aldrich. All solvents, such as methanol (CH3OH) and tetrahydrofuran (C4H8O), were analytical grade. All chemicals and glassy polymer were used without any processing. Pure gas CO2 was used for gas sorption analyses.
Synthesis of ZIF-67
In a typical synthesis of ZIF-67 with some changes in the previously reported method, 1.436 g of Co (NO3)2·6H2O and 3.244 g 2-MeIm were weighed and separately dissolved in 100 ml of methanol. The metal solution was slowly poured into ligand solution under stirring at room temperature until the solution turned into dark purple in color. Then, stirring was carried out at room temperature for 3 h and the solution was allowed to stand without stirring at room temperature for 24 h. Afterward, the product was collected using centrifugation at 5000 rpm for 15 min. The washing was carried out three times with methanol and one time with chloroform to remove the dissolved impurities. The product was dried at 120°C in a vacuum oven for 3 days (Gupta and Murthy, 2022).
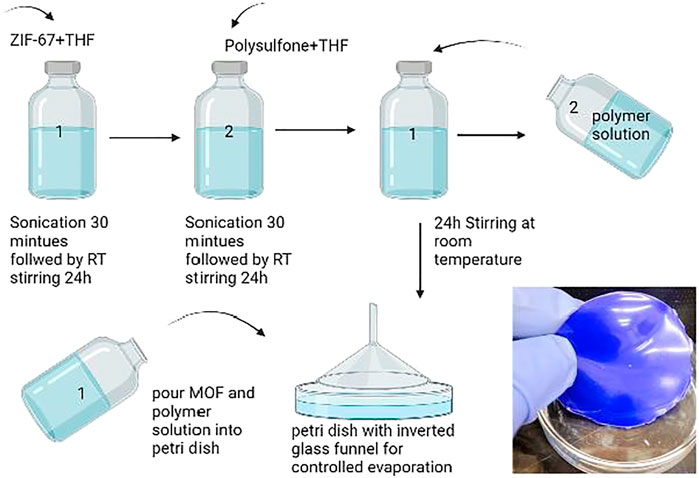
Membrane Preparation
For the preparation of 3% ZIF-67 mixed-matrix membrane, 0.03 g of ZIF-67 was weighed in a 20-ml vial, and then 67 ml of THF was added into the vial. The solution was sonicated for 30 min followed by stirring for 24 h at room temperature. For polymer solution preparation, 0.97 g of polysulfone was taken in a 20-ml vial and then 67 ml of THF added to the vial, allowing it to stir for 24 h at room temperature. Then, the entire polymer solution was added into MOF solution and sonicated for 30 min followed by 24 h stirring at RT. After 24 h, sonication is carried out for 30 min before casting solution. The solution was poured into a glass petri dish and allowed to evaporate at room temperature under an inverted funnel. The inverted funnel was used for controlled evaporation of the solvent.
Characterization Techniques
Powder X-Ray Diffraction (PXR)
The XRD diffraction study of the sample was characterized by X-ray diffraction (Powder XRD, Bruker, D8). All the samples were analyzed with scan range 2θ from 3° to 45°, step size 0.02°, and time per step 0.2 s.
Thermogravimetric Analysis (TGA)
The thermal resistance of all prepared samples was checked by using a thermo gravimetric analyzer TA Q500 (TA instruments, DE, United States). The samples were heated from 25°C to 590°C, while keeping a heating rate of 5°C/min under an N2 environment.
Fourier Transform Infrared Spectroscopy (FT-IR) with Attenuated Total Reflectance (ATR)
The functional groups present in all fabricated MMMs were identified using an Agilent Cary 630 Fourier Transform Infrared Spectroscopy (FT-IR) with Attenuated Total Reflectance (ATR) crystal. All membrane samples were placed under the ATR-FTIR holder, and absorbance data were collected.
Gas Sorption Analyses
The gas sorption analyses for CO2 (99.998%) were conducted on virgin Polysulfone, ZIF-67, and ZIF-67 membrane at 273 K using an Accelerated Surface Area & Porosimetry System (ASAP) 2,460 supplied by Micromeritics Instruments Inc. After cutting the membranes into small pieces, the fabricated membrane was activated by being subjected to vacuum at 70°C for 24 h. The N2 77 K isotherm was used to evaluate the surface area (Langmuir and Brunauer–Emmett–Teller (BET) surface areas, etc.) of ZIF-67, while CO2 adsorption isotherms at 273 K were employed to evaluate the CO2 uptake performance of pure and mixed-matrix membrane based on ZIF-67.
Results and Discussion
The results of different characterization techniques, that is, XRD, ATR -FTIR, TGA, and gas sorption analysis of ZIF-67-based mixed-matrix membrane are described below.
Powder X-Ray Diffraction
XRD is a non-destructive technique. This technique is used to check the purity of the material, the impact of filler on polymer chain arrangement in the MMM, and the crystallinity of the MOF after incorporating it into the polymer.
To find out the absence and presence of crystallinity of ZIF-67 in the MMM, XRD was performed in the 2θ range of 2°–45° Figure 1B. All diffraction peaks of as-synthesized ZIF-67 observed in Figure 1A matched well with their corresponding simulated pattern in literature (Kachhadiya and Murthy, 2021). This result indicates that the crystalline structure of ZIF-67 is well-maintained and no impurities are observed. The sharp and intense peak at 2θ position of 7.33° in Figure 1A proved the high crystalline nature of as-synthesized ZIF-67. The intensive peak in Figure 1A was observed at 2θ position of 7.33° attributed to (110) crystal plane, which is higher than other peaks (Mostafazadeh et al., 2018). In case of 3 wt.% ZIF-67 MMM, a very small peak is observed at 2θ position of 7.33ᵒ that confirms the presence of ZIF-67 in the membrane. However, the less intense peak observed in 3 wt.% MMM is due to the small wt.% content of ZIF-67 in polysulfone. The characteristic peak for polysulfone appeared in 2θ position of 18.49°.
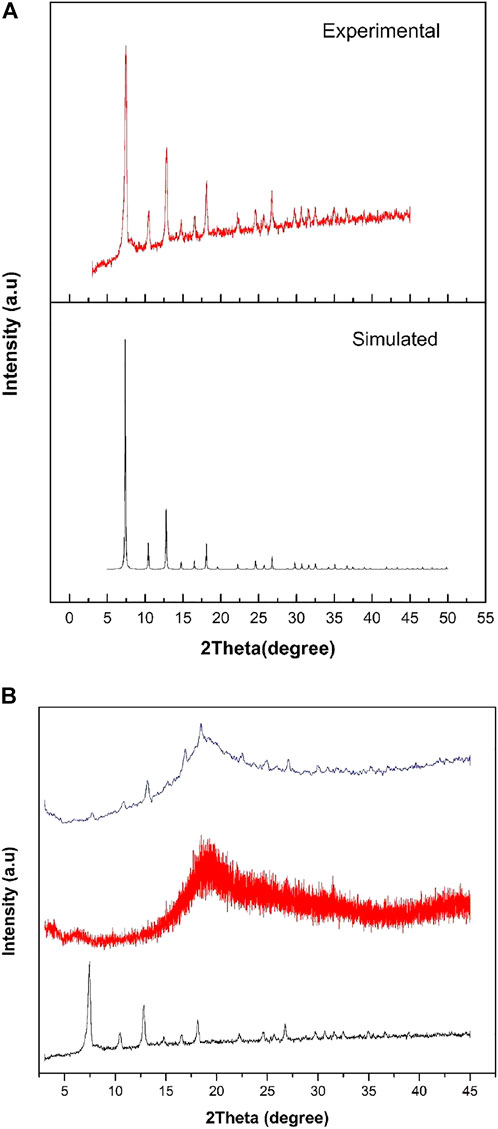
FIGURE 1. (A) PXRD pattern of simulated (Black) and experimental (red) ZIF-67 and (B) PXRD of ZIF-67(black), 3 wt% ZIF-67 MMM (blue) and pure polysulfone (red).
Thermal Gravimetric Analyses and Surface Area Measurement
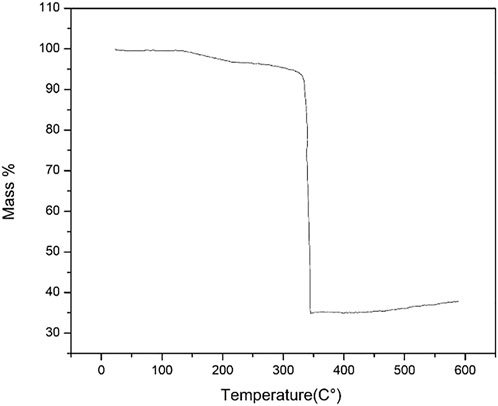
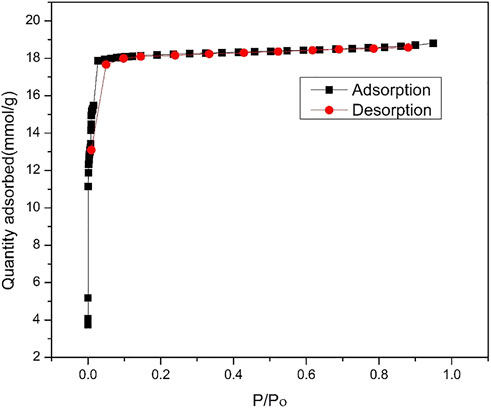
The thermal stability of ZIF-67 was checked and shown in Figure 2 is 350°C with a minimum 3% mass loss that is in complete agreement with that of previously reported work. The initial mass percent loss is due to the removal of methanol. This stability of MOF is still considered better for MOF in terms of real membrane-based applications for gas separation (Qian et al., 2012). The N2 adsorption–desorption isotherm in Figure 3 reveals a reversible type 1 isotherm that is a characteristic of the microporous material. A plateau appears after certain uptake of gas at lower pressure, indicating that a multilayer is formed, and no more pores left for gas uptake. The sharp uptake of gas at higher pressure regions could be due to physiosorbed liquid N2. The BET area of as-synthesized ZIF-67 crystals is 1,696 m2/g higher than that of ZIF-67 synthesized using water as a solvent (Guo et al., 2016).
Stability Testing
The water stability testing of ZIF-67 was carried out using different organic solvents and liquid water. The ZIF67 powder was immersed in ethanol, Liq. H2O, and THF for 24 h at room temperature. The PXRD pattern was checked after 24 h, as shown in Figure 4. It can be clearly observed from Figure 4 that there is no change in the crystal structure pattern of ZIF-67 after exposure to organic solvent and water. This result suggested that ZIF-67 is highly stable under pure water and organic solvents and can be utilized in future membrane-based applications for gas separation under humid conditions.
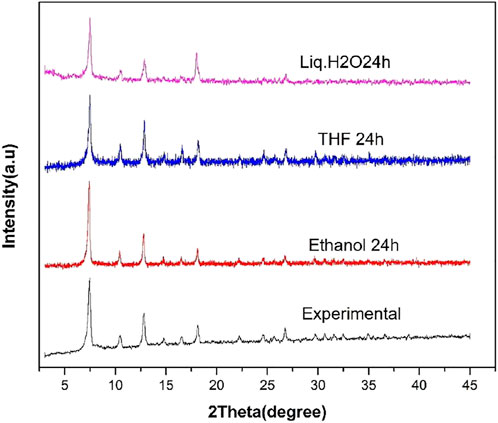
FIGURE 4. PXRD of ZIF-67 after soaking in ethanol (red), THF (blue), and liquid H2O (purple) for 24 h.
ATR-FTIR
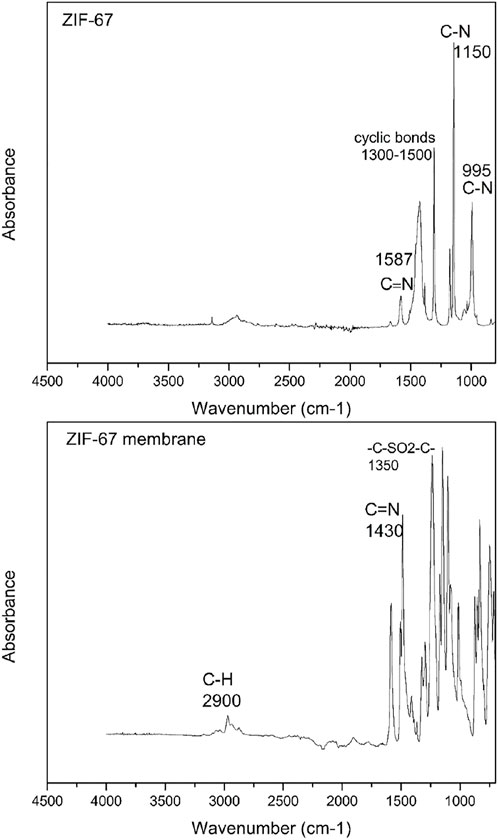
The ATR-FTIR spectra of ZIF-67- and ZIF-67-based mixed-matrix membrane in the wavenumber range of 700 cm−1 to 4500 cm−1are presented in Figure 5. The spectrum of as-synthesized ZIF-67 indicates the stretching and bending vibration of C-N at 995 cm−1 and 1150 cm−1. The bands observed at 3,000 cm−1 and 2900 cm−1 attribute the presence of aromatic and aliphatic C-H in the imidazole ring in case of ZIF67 (Yang et al., 2012). The incorporation of ZIF-67 within the polymer was successful, as shown in Fig (Dorosti and Alizadehdakhel, 2018). There were no new peaks observed in the ZIF-67 membrane which is the confirmation of no new chemical interaction occurred. The characteristic peak for polysulfone (-C -SO2-C-) was seen clearly at 1,350 cm−1 in the ZIF-67 membrane in Figure 5. The major peaks for 2 -methylimidazole (2-MeI) was observed in the ZIF-67 membrane in Figure 5 from 700 cm−1 to 1400 cm−1 due to the stretching and bending vibration of the imidazole functional group.
Gas Sorption Analyses
The CO2 adsorption isotherm of pure polysulfone, ZIF-67, and 3 wt% ZIF-67-MMM was collected at temperature 273 K and pressure up to 1.2 bar to check the performance of lower MOF loading in polysulfone using THF as the solvent as shown in Figure 6. The maximum CO2 adsorption of ZIF-67 at 1bar is 1.47 mmol/g, which is in accordance with previously reported results (Yang et al., 2012), while CO2 uptake for 3 wt% ZIF-67-Polysulfone composite is 0.5 mmol/g higher than that of pure polysulfone uptake, that is, 0.3 mmol/g. The ZIF-67 maximum capacity for CO2 uptake was retained in the membrane even at minimum wt.% loading of MOF. This gas sorption results strongly recommend the use of ZIF-67 MMM as a competent candidate to capture CO2 from wet flue gases.
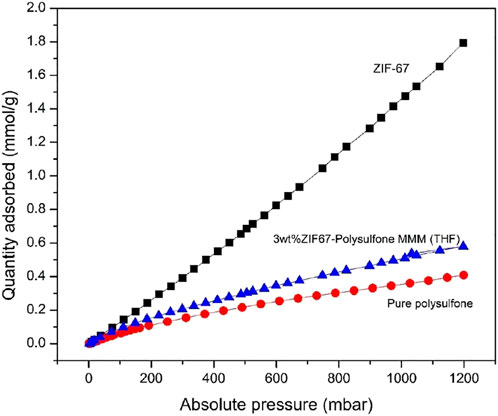
FIGURE 6. Gas adsorption isotherm of pure polysulfone (Red), ZIF-67 (black), and ZIF-67 MMM (blue) at 273 K.
Conclusion
In summary, a mixed-matrix membrane with lower MOF loading has been prepared with the solution casting method using THF as the solvent. The fabricated 3 wt.% ZIF-67 membrane showed higher CO2 uptake than the pure polymer. The prepared ZIF-67/polysulfonemixed matrix membrane was stable, flexible, and free standing. The as-synthesized ZIF-67 was highly stable in pure water and organic solvents. The combination of a hydrophobic polymer and a hydrophobic MOF was proved compatible with each other to fabricate a mixed-matrix membrane. This study provides an opportunity to utilize ZIF-67 MOF as competent material for CO2 capture from wet flue gases.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
SS: Writing—original draft, AA: reviewing and editing, MN: methodology, ZA: data curation, AMA: funding of this project, AA: supervision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Authors are grateful to the Researchers Supporting Project No. (RSP-2021/1), King Saud University, Riyadh, Saudi Arabia.
References
Abdelhameed, R. M., Abu-Elghait, M., and El-Shahat, M. (2020). Hybrid Three MOFs Composites (ZIF-67@ZIF-8@MIL-125-NH2): Enhancement the Biological and Visible‐light Photocatalytic Activity. J. Environ. Chem. Eng. 8 (5), 104107. doi:10.1016/j.jece.2020.104107
Al-Rowaili, F. N., Zahid, U., Onaizi, S., Khaled, M., Jamal, A., and Al-Mutairi, E. M. (2021). A Review for Metal-Organic Frameworks (MOFs) Utilization in Capture and Conversion of Carbon Dioxide into Valuable Products. J. CO2 Util. 53, 101715. doi:10.1016/j.jcou.2021.101715
Barbi, V., Funari, S. S., Gehrke, R., Scharnagl, N., and Stribeck, N. (2003). SAXS and the Gas Transport in Polyether-Block-Polyamide Copolymer Membranes. Macromolecules 36 (3), 749–758. doi:10.1021/ma0213403
Burtch, N. C., Jasuja, H., and Walton, K. S. (2014). Water Stability and Adsorption in Metal-Organic Frameworks. Chem. Rev. 114 (20), 10575–10612. doi:10.1021/cr5002589
Car, A., Stropnik, C., Yave, W., and Peinemann, K.-V. (2008). PEG Modified Poly(amide-B-Ethylene Oxide) Membranes for CO2 Separation. J. Membr. Sci. 307 (1), 88–95. doi:10.1016/j.memsci.2007.09.023
Casco, M. E., Martínez-Escandell, M., Silvestre-Albero, J., and Rodríguez-Reinoso, F. (2014). Effect of the Porous Structure in Carbon Materials for CO2 Capture at Atmospheric and High-Pressure. Carbon 67, 230–235. doi:10.1016/j.carbon.2013.09.086
Cheng, Y., Zhai, L., Ying, Y., Wang, Y., Liu, G., Dong, J., et al. (2019). Highly Efficient CO2 Capture by Mixed Matrix Membranes Containing Three-Dimensional Covalent Organic Framework Fillers. J. Mat. Chem. A 7 (9), 4549–4560. doi:10.1039/c8ta10333j
Dorosti, F., and Alizadehdakhel, A. (2018). Fabrication and Investigation of PEBAX/Fe-BTC, a High Permeable and CO2 Selective Mixed Matrix Membrane. Chem. Eng. Res. Des. 136, 119–128. doi:10.1016/j.cherd.2018.01.029
Feng, S., Bu, M., Pang, J., Fan, W., Fan, L., Zhao, H., et al. (2020). Hydrothermal Stable ZIF-67 Nanosheets via Morphology Regulation Strategy to Construct Mixed-Matrix Membrane for Gas Separation. J. Membr. Sci. 593, 117404. doi:10.1016/j.memsci.2019.117404
Guo, X., Xing, T., Lou, Y., and Chen, J. (2016). Controlling ZIF-67 Crystals Formation through Various Cobalt Sources in Aqueous Solution. J. Solid State Chem. 235 (3), 107–112. doi:10.1016/j.jssc.2015.12.021
Gupta, N., and Murthy, Z. V. P. (2022). Synthesis and Application of ZIF-67 on the Performance of Polysulfone Blend Membranes. Mater. Today Chem. 23, 100685. doi:10.1016/j.mtchem.2021.100685
Habib, N., Shamair, Z., Tara, N., Nizami, A. S., Akhtar, F. H., Ahmad, N. M., et al. (2020). Development of Highly Permeable and Selective Mixed Matrix Membranes Based on Pebax®1657 and NOTT-300 for CO2 Capture. Sep. Purif. Technol. 234, 116101. doi:10.1016/j.seppur.2019.116101
Ishaq, S., Tamime, R., Bilad, M. R., and Khan, A. L. (2019). Mixed Matrix Membranes Comprising of Polysulfone and Microporous Bio-MOF-1: Preparation and Gas Separation Properties. Sep. Purif. Technol. 210, 442–451. doi:10.1016/j.seppur.2018.08.031
Jomekian, A., Bazooyar, B., Behbahani, R. M., Mohammadi, T., and Kargari, A. (2017). Ionic Liquid-Modified Pebax 1657 Membrane Filled by ZIF-8 Particles for Separation of CO2 from CH4, N2 and H2. J. Membr. Sci. 524, 652–662. doi:10.1016/j.memsci.2016.11.065
Kachhadiya, D. D., and Murthy, Z. V. P. (2021). Preparation and Characterization of ZIF-8 and ZIF-67 Incorporated Poly (Vinylidene Fluoride) Membranes for Pervaporative Separation of Methanol/water Mixtures. Mater. Today Chem. 22, 100591. doi:10.1016/j.mtchem.2021.100591
Li, H., Tuo, L., Yang, K., Jeong, H.-K., Dai, Y., He, G., et al. (2016). Simultaneous Enhancement of Mechanical Properties and CO2 Selectivity of ZIF-8 Mixed Matrix Membranes: Interfacial Toughening Effect of Ionic Liquid. J. Membr. Sci. 511, 130–142. doi:10.1016/j.memsci.2016.03.050
Meshkat, S., Kaliaguine, S., and Rodrigue, D. (2020). Comparison between ZIF-67 and ZIF-8 in Pebax® MH-1657 Mixed Matrix Membranes for CO2 Separation. Sep. Purif. Technol. 235, 116150. doi:10.1016/j.seppur.2019.116150
Mostafazadeh, N., Ghoreyshi, A. A., and Pirzadeh, K. (2018). Optimization of Solvothermally Synthesized ZIF-67 Metal Organic Framework and its Application for Cr (VI) Adsorption from Aqueous Solution. Iran. J. Chem. Eng. 15 (4), 27–47.
Nafisi, V., and Hägg, M.-B. (2014). Development of Dual Layer of ZIF-8/PEBAX-2533 Mixed Matrix Membrane for CO2 Capture. J. Membr. Sci. 459, 244–255. doi:10.1016/j.memsci.2014.02.002
Qian, J., Sun, F., and Qin, L. (2012). Hydrothermal Synthesis of Zeolitic Imidazolate Framework-67 (ZIF-67) Nanocrystals. Mater. Lett. 82, 220–223. doi:10.1016/j.matlet.2012.05.077
Yang, H., He, X.-W., Wang, F., Kang, Y., and Zhang, J. (2012). Doping Copper into ZIF-67 for Enhancing Gas Uptake Capacity and Visible-Light-Driven Photocatalytic Degradation of Organic Dye. J. Mat. Chem. 22 (41), 21849–21851. doi:10.1039/c2jm35602c
Yuan, J., Zhu, H., Sun, J., Mao, Y., Liu, G., and Jin, W. (2017). Novel ZIF-300 Mixed-Matrix Membranes for Efficient CO2 Capture. ACS Appl. Mat. Interfaces 9 (44), 38575–38583. doi:10.1021/acsami.7b12507
Zhang, C., Dai, Y., Johnson, J. R., Karvan, O., and Koros, W. J. (2012a). High Performance ZIF-8/6fda-DAM Mixed Matrix Membrane for Propylene/propane Separations. J. Membr. Sci. 389, 34–42. doi:10.1016/j.memsci.2011.10.003
Keywords: nanobiocatalysis, ZIF 67, CO2 emission, nanotechnology, adsorption
Citation: Saeed S, Bashir R, Rehman SU, Nazir MT, ALOthman ZA, Muteb Aljuwayid A, Abid A and Adnan A (2022) Synthesis and Characterization of ZIF-67 Mixed Matrix Nanobiocatalysis for CO2 Adsorption Performance. Front. Bioeng. Biotechnol. 10:891549. doi: 10.3389/fbioe.2022.891549
Received: 07 March 2022; Accepted: 21 April 2022;
Published: 05 September 2022.
Edited by:
Awais Ahmad, University of Córdoba, SpainReviewed by:
Mobashar Hassan, Jiangsu University, ChinaIkram Ahmad, University of Sahiwal, Pakistan
Moazma Akhbar, University of Faisalabad, Pakistan
Copyright © 2022 Saeed, Bashir, Rehman, Nazir, ALOthman, Muteb Aljuwayid, Abid and Adnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Adnan, YWhtYWRhZG5hbkBnY3UuZWR1LnBr
 Saira Saeed1
Saira Saeed1 M. Tariq Nazir
M. Tariq Nazir Zeid A. ALOthman
Zeid A. ALOthman