
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol., 17 June 2022
Sec. Biomechanics
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.886579
This article is part of the Research TopicBone Integrity in Patients with Osteoporosis: Evaluation of Fracture Risk and Influence of Pharmacological Treatments and Mechanical AspectsView all 9 articles
Denosumab (Dmab) treatment against postmenopausal osteoporosis (PMO) has proven very efficient in increasing bone mineral density (BMD) and reducing the risk of bone fractures. However, concerns have been recently raised regarding safety when drug treatment is discontinued. Mechanistic pharmacokinetic-pharmacodynamic (PK-PD) models are the most sophisticated tools to develop patient specific drug treatments of PMO to restore bone mass. However, only a few PK-PD models have addressed the effect of Dmab drug holidays on changes in BMD. We showed that using a standard bone cell population model (BCPM) of bone remodelling it is not possible to account for the spike in osteoclast numbers observed after Dmab discontinuation. We show that inclusion of a variable osteoclast precursor pool in BCPMs is essential to predict the experimentally observed rapid rise in osteoclast numbers and the associated increases in bone resorption. This new model also showed that Dmab withdrawal leads to a rapid increase of damage in the bone matrix, which in turn decreases the local safety factor for fatigue failure. Our simulation results show that changes in BMD strongly depend on Dmab concentration in the central compartment. Consequently, bone weight (BW) might play an important factor in calculating effective Dmab doses. The currently clinically prescribed constant Dmab dose of 60 mg injected every 6 months is less effective in increasing BMD for patients with high BW (2.5% for 80 kg in contrast to 8% for 60 kg after 6 years of treatment). However, bone loss observed 24 months after Dmab withdrawal is less pronounced in patients with high BW (3.5% for 80kg and 8.5% for 60 kg). Finally, we studied how to safely discontinue Dmab treatment by exploring several transitional and combined drug treatment strategies. Our simulation results indicate that using transitional reduced Dmab doses are not effective in reducing rapid bone loss. However, we identify that use of a bisphosphonate (BP) is highly effective in avoiding rapid bone loss and increase in bone tissue damage compared to abrupt withdrawal of Dmab. Furthermore, the final values of BMD and damage were not sensitive to the time of administration of the BP.
Osteoporosis (OP) is a systemic skeletal disorder which is characterized by low bone mineral density (BMD) and a deteriorated bone microarchitecture which results in reduced bone strength, ultimately leading to an increase in fragility fractures (Lorentzon and Cummings, 2015). The age-dependent decrease in BMD is a strong indicator of the increase in fracture risk seen in the aging population (Kanis and Kanis, 1994; Felsenberg et al., 2002).
Currently, anti-resorptive agents are most widely used to treat osteoporosis (Anastasilakis et al., 2018). The most potent anti-resorptive agent is denosumab (Dmab) (Cummings et al., 2009), a monoclonal antibody to the receptor activator of nuclear factor-kB ligand (RANKL). RANKL is a key regulator of bone resorption by its effect on osteoclast development, function and survival. While Dmab has been shown to be effective in increasing BMD and reduce risk of fracture at major skeletal sites (Cummings et al., 2009; Papapoulos et al., 2015), a recent review on the effects of Dmab discontinuation (Anastasilakis et al., 2021) indicates that drug withdrawal leads to a rapid, significant increase in the concentrations of bone turnover markers (BTMs), in most cases to above pre-treatment baseline levels (Bone et al., 2011; Zanchetta et al., 2018).
It was also found that discontinuation of Dmab is typically associated with a decline in BMD throughout the skeleton. Indeed, the rate of BMD loss observed in patients who had stopped Dmab therapy (and did not receive any subsequent osteoporosis medication) was as high as 5–11% at all sites during the first year off-treatment (Miller et al., 2008; McClung et al., 2017; Popp et al., 2018; Zanchetta et al., 2018). Bone et al. showed that treatment of postmenopausal osteoporosis (PMO) with Dmab for 24 months subsequently followed for another 24 months off-treatment resulted in BMD loss at all skeletal sites that was evident at 6 months after the last injection (Bone et al., 2011). It was noted that BMD loss is bone site specific, with greatest BMD loss in the lumbar spine (LS) occurring at a mean of 18 months off-treatment, while both total hip (TH) and 1/3 radius (R) BMD continued to decline up to a mean of 30 months after the last injection (Bone et al., 2011).
It is well known that mechanistic pharmacokinetics-pharmacodynamics (PK-PD) models of OP treatments can be used to identify optimal treatment regimes. We and others have previously investigated the effect of Dmab dosing patterns on changes in BTMs, bone cell numbers and BMD (Marathe et al., 2011; Peterson and Riggs, 2012; Scheiner et al., 2014; Martínez-Reina and Pivonka, 2019). Based on these studies it was identified that inclusion of bone mineralisation is an essential model feature in order to predict BMD gains during Dmab treatment (Martínez-Reina and Pivonka, 2019). Recently, we also addressed the experimental finding of increased bone resorption after Dmab discontinuation using PK-PD modelling (Martínez-Reina et al., 2021a). Surprisingly, the bone cell population model (BCPM) of bone remodelling was not able to predict the rapid increase of active osteoclast numbers and the associated loss in BMD after Dmab drug holiday (Anastasilakis et al., 2021). Here we address the question on which model features need to be added to current state-of-the-art BCPMs in order to predict the effect of Dmab drug holiday on bone biomarkers. Given that in a clinical context it is essential to be able to switch drug treatments without detrimental effects to patients, such a model might be able to identify Dmab dosing regimens which minimise negative effects of drug holidays and/or change to a different drug treatment regimen.
As reviewed in Anastasilakis et al. (Anastasilakis et al., 2021), discontinuation of Dmab treatment is associated with a 3 to 5-fold higher risk for vertebral, major osteoporotic, and hip fractures (Lyu et al., 2020; Tripto-Shkolnik et al., 2020). This has been attributed to a given unopposed fracture risk similar to placebo-controlled trials. It was found that the off-treatment fracture risk among patients who had received Dmab was not different compared to the placebo group (Brown et al., 2013). However, amongst those discontinuing Dmab treatment a significant increase in multiple vertebral fractures were identified (Cummings et al., 2018). The fractures in this setting are typically clinical, occurring a few months after the effect of the last Dmab injection has been depleted (Anastasilakis et al., 2017) and are often described as rebound associated vertebral fractures (RAVFs). The review by Anastasilakis et al. summarizes RAVFs from these case reports starting from 2016 (see (Anastasilakis et al., 2021), Table 1).
In healthy subjects, a major purpose of bone remodelling is to remove old and damaged bone tissue (through the action of osteoclasts) and replace it with newly formed bone (laid down by osteoblasts) (Manolagas, 2018). This process is tightly regulated by osteocytes, i.e., terminally differentiated osteoblastic cells embedded in the bone matrix (Manolagas and Parfitt, 2013). Osteocytes regulate bone formation through expression of Wnt inhibitors including sclerostin (SOST) and Dickkopf-1 (DKK-1) (Schaffler et al., 2014; Tu et al., 2015), and bone resorption through expression of receptor activator of nuclear factor kappa-B ligand (RANKL) (Nakashima et al., 2011; Xiong et al., 2015) and its decoy receptor osteoprotegerin (OPG) (Udagawa et al., 2000). Other factors such as TGF-β, which is released from the bone matrix during osteoclastic bone resorption, regulate coupling between osteoclastic and osteoblastic cell populations (Matsuo and Irie, 2008).
Dmab binds RANKL with high affinity, and so prevents its binding to RANK receptors expressed on the surface of osteoclastic cells. Hence, Dmab suppresses osteoclast recruitment, maturation, function and survival, and significantly decreases bone resorption and associated bone loss (Kostenuik et al., 2009). As a bone anti-resorptive agent, its effect on osteoblasts is largely indirect through coupling of resorption and formation within the remodelling process.
In recent years, several investigators started with development of in-silico models of the action of Dmab in PMO with the aim to optimise and customise drug dosing. The majority of these models are based on mechanistic PK-PD models of drug treatments in osteoporosis (Lemaire et al., 2004; Pivonka et al., 2008; Scheiner et al., 2013; Martínez-Reina and Pivonka, 2019). A major finding of these models was that in order to be able to accurately model the effects of anti-resorptive drugs on BMD it is essential to take into account bone mineralisation as a physiological process (Scheiner et al., 2014; Martínez-Reina and Pivonka, 2019) together with accumulation of microcracks due to increased brittleness of the bone matrix (Martínez-Reina et al., 2021a; Martínez-Reina et al., 2021b).
As described above none of these BCPMs was able to reproduce the spike in bone resorption markers and associated rapid bone loss after Dmab discontinuation. Based on proposed molecular and cellular mechanisms for explaining this behaviour we analysed model structures of common BCPMs and identified that all current models are based on the assumption of a constant osteoclast precursor cell pool. This finding leads us to our major hypothesis for explaining effects of Dmab discontinuation:
• Overshoot in the active osteoclast population after removing remodelling suppression is linked to a variable osteoclast precursor pool;
• A secondary hypothesis is that differentiation of the uncommitted osteoclasts into the osteoclast precursors is regulated by RANKL.
In this paper, we extend our recent BCPM with respect to introducing osteoclast precursor cells as an additional cell pool of the osteoclastic linage. We first show that depending on the choice of RANKL regulation of the differentiation rates of uncommitted osteoclasts and osteoclast precursors this extended model can either represent the original model with a constant pool of precursors or represent new model features. We then test the hypothesis that introducing an additional cell pool for modelling the differentiation of osteoclastic cells is able to capture the following experimental observations after Dmab discontinuation: 1) BMD loss is dependent on the duration of Dmab treatment and 2) BMD loss is site specific, i.e. 18 months at the lumbar spine and 30 months at the hip.
This paper is organised as follows: In Section 2 we provide a detailed description of the mechanistic PK − PD model. The comparison of simulation results and experimentally observed changes in BMD are reported in Section 3, together with parametric studies of essential model parameters. The results are discussed in detail with respect to the clinical bone biology literature in Section 4. Conclusions and outlook to future work is presented in Section 5.
Following the approach taken by Martin et al. (Martin et al., 2019), the bone remodelling process can be described as cell balance equations. The bone cell types (i.e. state variables) considered in the current model are: osteoblast precursor cells (Obp), active osteoblasts (Oba), osteoclast precursor cells (Ocp), active osteoclasts (Oca) and osteocytes (Ot). The cell pools of uncommitted progenitor cells of both lineages (Obu, Ocu) were assumed constant:
where
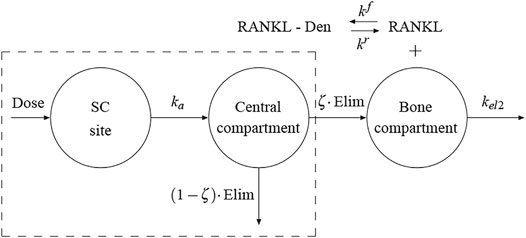
FIGURE 1. Schematic representation of the proposed PK model. The framed box represents the 1-compartment PK model proposed by Marathe et al. (Marathe et al., 2011). The bone compartment has been added here and the flux of Dmab from the central compartment to the bone compartment represents the production term in the competitive binding reactions between RANKL, OPG and Dmab.
Equation 5 establishes that the population of osteocytes varies proportional to the bone matrix fraction fbm, given that the density of osteocytes is constant within the bone matrix, η, is assumed constant, as done in (Martin et al., 2019). Finally, the variation of bone matrix fraction is obtained through the balance between resorbed and formed tissue:
where kres and kform are, respectively, the rates of bone resorption and osteoid formation (see Supplementary Table S1).
The RANK-RANKL-OPG signalling pathway controls the differentiation of uncommitted osteoclast progenitors and osteoclasts maturation, respectively through
where the binding of Dmab to RANKL is considered in the last term of the bracket of Eq. 9;
where
where
Following (Martínez-Reina et al., 2021b), the RANKL production due to PMO,
where
Equations 11–13 can be substituted in Eq. 9 to work out the free RANKL concentration, i.e. [RANKL]. Then, the activator functions in Eqs. 3, 4 have the same structure:
and different constants:
Several pharmacokinetic (PK) models of Dmab have been proposed including one- and two-compartment models (Dua et al., 2015). In recent papers (Martínez-Reina and Pivonka, 2019; Martínez-Reina et al., 2021a) we have used a one-compartment model with Michaelis-Menten kinetics in order to characterise the serum Dmab PK profiles, with the constants of that model fitted from a clinical study by Marathe et al. (Marathe et al., 2011). This model is framed in a dashed box in Figure 1. A first-order rate process (ka) governs the absorption of the drug (Dose) from the subcutaneous (SC) injection site into the central compartment ([Dmab]CC), being Vc/F the volume of the central compartment adjusted for bioavailability. The drug elimination from the central compartment is described by a combination of a linear first-order process (kel1) and a non-linear saturation process (Vmax, Km):
In Eq. 15 Dose is given in ng per kg of body weight and then [Dmab]CC is calculated in ng/ml and subsequently converted into pmol/l, through the molecular weight of Dmab MDmab = 149 kDa (Amgen). Supplementary Table S1 summarises the PK model parameters of the one-compartment model adjusted by Marathe et al. (Marathe et al., 2011).
In previous models (Martínez-Reina and Pivonka, 2019; Martínez-Reina et al., 2021a) we have assumed that a fraction of the Dmab present in the central compartment was available in the bone compartment to compete with RANK to bind to RANKL. However, this availability actually implied a reversible exchange of Dmab between both compartments. Given the affinity of Dmab for RANKL, which is expressed by osteoblasts precursors within the bone compartment, a flux from the central compartment to the bone compartment seems more plausible than a reversible exchange. To this end, we have added the bone compartment to the PK model (see Figure 1). The term in square brackets in Eq. 15 represents the elimination from the central compartment in the model of Marathe et al. We have assumed that only a fraction (1 − ζ) of this term is actually eliminated via urine and the rest, ζ, is the flux of Dmab into the bone compartment. In turn, the latter fraction can be considered as
This production rate can be replaced in the expression that gives the concentration of ligands in competitive binding reactions (see Supplementary Material), i.e.:
This expression can be used in Eq. 9 to give the concentration of free RANKL. All the parameters are known from previous works (Martínez-Reina and Pivonka, 2019), except for
Targeted bone remodelling theories hypothesise that one of the major functions of bone remodelling is to remove microcracks from bone matrix, so avoiding an excessive accumulation of the latter, which could result in macroscopic failure (Parfitt, 2002). The accumulation of microcracks in a particular volume of material is addressed here using a Continuum Damage Mechanics approach (Lemaitre and Chaboche, 1990). This theory introduces a damage variable, d, which is linked to the density of microcracks in a volume of material and to the loss of stiffness through Eq. 18. This variable is such that d ∈ [0, 1], with d = 0 corresponding to an undamaged state and d = 1 to a local fracture or failure situation:
where C and C0 are, respectively, the stiffness tensors of damaged and undamaged bone (Lemaitre and Chaboche, 1990). In the isotropic damage theory, Eq. 18 can be rewritten in terms of the respective Young’s moduli, E and E0, as E = (1 − d) E0 (Pattin et al., 1996; Zioupos and Currey, 1998) (see Eq. 38).
A balance of microdamage is considered through the accumulation due to fatigue loading and the removal due to bone remodelling, as osteoclasts resorb the damaged tissue, while the osteoid deposited by osteoblasts is initially intact. The evolution law for damage can be expressed as:
where
Damage accumulation is evaluated following the procedure described in (Martínez-Reina et al., 2008; Martínez-Reina et al., 2009). This procedure makes use of the results of the experimental fatigue tests performed by Pattin et al. (Pattin et al., 1996), who provided the evolution of damage with the strain level and the number of cycles. This evolution was mathematically modelled by García-Aznar et al. (García-Aznar et al., 2005) to yield the following differential equation under tensile stresses:
where
where Kf([Ca]) is a function of the mineral content which will be defined next. The experimental tests perfomed by Pattin et al. (Pattin et al., 1996) included an estimation of fatigue life, Nf, which was related to the deformation by the following expression:
where Kf was assumed constant and equal to 1.445 ⋅ 1053 in tension. Martínez-Reina et al. (Martínez-Reina et al., 2008) introduced a correction in Kf to consider the degradation of the fatigue properties with the increase in mineral content. A life Nf = 107 cycles was assigned to the fatigue limit, which is usually assumed to occur for a given fraction of the ultimate tensile strain, ɛu/β, where the parameter β depends on the type of material (Juvinall, 1967) and β = 2 was assumed for bone (Martínez-Reina et al., 2008) with good results. So, Kf was obtained from Eq. 23 as:
where the ultimate tensile strain depends on the calcium concentration of bone matrix, [Ca], as Currey (Currey, 2004) showed. The following regression was fitted in (Martínez-Reina et al., 2008) from the experimental results presented by Currey (Currey, 2004):
where ɛu is expressed in μɛ and the concentration [Ca] is expressed in mg of calcium per g of bone matrix. This concentration is directly related to the ash fraction, α, which will be defined in the next section. More precisely, the relation [Ca] = 398.8 α was assumed, based on the molecular weigths of hydroxyapatite and type I collagen (Martínez-Reina et al., 2008).
The mineralisation model used in this work is based on that presented in (Martínez-Reina et al., 2008) and implemented in a model which is similar to the present one (Martínez-Reina and Pivonka, 2019). That model was a mixture of differential and recursive equations, difficult to implement in a system of ODEs. For this reason, it has been simplified to yield an explicit set of differential equations, explained next.
Bone is made up of a solid bone matrix and pores filled with marrow. A certain representative volume element, VRVE, can be divided into the bone matrix volume, Vbm, and the volume of pores, Vp. The bone matrix volume is divided into inorganic (mineral), organic (mainly collagen) and water phases, designated as Vm, Vo and Vw, respectively:
The composition of bone matrix is defined in terms of the volume fractions of the three phases as:
Thus, the following condition holds
The mineral content is usually measured by the so-called ash fraction, the ratio between mass of mineral (or ash mass) and dry mass (the sum of inorganic and organic mass):
where Eq. 27 have been used and ρi are the corresponding densities of the three phases, being ρm = 3.2 g/cm3 (Currey, 2004) and ρo = 1.41 g/cm3 (Hernandez et al., 2001a). The tissue density is then given by:
where ρw = 1 g/cm3 and Eqs. 27, 28 have been used to derive the right-hand side of Eq. 30.
Osteoid, the tissue laid by osteoblasts, contains only the organic phase and no mineral. Mineral accumulates in bone matrix afterwards, during the mineralisation process, which consists of three phases: 1) an initial phase, called mineralisation lag time, that lasts from 6 to 22 days (Eriksen et al., 1990; Need et al., 2007) during which no deposition of mineral occurs; 2) a primary phase, which is very quick (it takes a few days to reach the 70% of the maximum mineral content (Hernandez et al., 2001b)), and 3) a secondary phase, when mineral is added at a decreasing rate (Parfitt, 1983), as the tissue becomes saturated with mineral. Mineral accumulates in bone matrix by displacing water (Hernandez et al., 2001a). Thus, the volume fraction of organic phase is approximately constant during the mineralisation process and fixed here at vo = 3/7 (Martin, 1984); while the variations of mineral and water volume fractions would hold Δvm = −Δvw. So, the mineralisation process is accounted for through the temporal variation of vm. As
The first term corresponds to the variation of the mineral content due to mineralisation or resorption and it would be equal to the variation of mineral content if Vbm were constant. The second term is due to the variation of porosity. To understand this term it must be noted that the mineral content decreases when osteoid is deposited, since osteoid does not contain any mineral and it only contributes to increase the bone matrix volume, so reducing the concentration of mineral. Both terms will be analysed separately:
In a previous model (Martínez-Reina et al., 2008; Martínez-Reina and Pivonka, 2019) the variation of vm due to mineralisation was defined in a piecewise manner, considering the three phases separately: with no variation of vm during the mineralisation lag time, a linear increase during the primary phase and an exponential increase during the secondary phase. Here, this procedure will be simplified by assuming that it is governed by a saturation model:
leading to an exponential solution which approximates rather well the global response of the previous model for a value of the constant K = 0.007, with a fast initial mineralisation rate (primary phase) that slows down in the mid-long-term (secondary phase). The maximum mineral content
The variation of mineral content due to resorption is similar to the damage repair term in the damage model (see Eq. 20). It must be taken into account that mineral is dissolved by osteoclasts and so removed from the bone matrix as damage was previously assumed. Thus, the amount of mineral removed by resorption is proportional to the mineral content of the tissue being resorbed, vm, and to the proportion of tissue being resorbed within the bone matrix,
Using Eqs. 32-34 Eq. 31 can be rewritten as:
where it has been used that
Once vm is updated, the ash fraction can be derived from Eq. 29 and the tissue density from Eq. 30. Then, the apparent density is given by:
Bone was assumed to be an isotropic material with a Poisson’s ratio ν = 0.3 and a Young’s modulus given in MPa by the following expresions:
These are based on the correlations experimentally obtained by Jacobs (Jacobs, 1994), which were multiplied by the factor (1 − d) to consider microstructural damage as usually done in Continuum Damage Mechanics (Lemaitre and Chaboche, 1990).
The main effect of the treatment is the bone density gain (BDG), which is measured with respect to baseline, i.e. at the beginning of treatment, when bone apparent density is ρ0:
The monthly time derivative of apparent density (MTDAD) is defined in Eq. 40 and used to measure the intensity of bone loss and, more precisely, the spike after Dmab discontinuation, as bone loss is accelerated a few months after the last Dmab injection, as compared to the disease state before treatment:
Finally, the fracture risk is evaluated by assessing the critical load σcrit(t), i.e. the overload that would lead to local fracture (d = 1) in a few days for a given bone. This overload would be given by the present model as a function of the porosity, mineral content, bone turnover rate and current damage level. So, for a given instant t, the overload is calculated by integrating Eq. 19 and using the following algorithm:
1. Extract the results fbm(t), d(t), Oca(t) and α(t) for the considered instant t.
2. Assume fbm(t), Oca(t) and α(t) as constants and consider d(t) = d0 as the initial condition for the integration of Eq. 19. This equation is integrated over time τ > t.
3. Evaluate the apparent density, ρ(t), from fbm(t) and α(t) by using Eqs. 29, 30, and 37.
4. Consider the Young’s modulus from Eq. 38,
5. Start with an estimation of the critical load σ = 0.
6. Assuming a uniaxial stress state, calculate the maximum tensile strain:
7. Integrate Eq. 19 over time τ in the domain τ ∈ [t, t + 10] days. This is done with the help of Eqs. 20 and 21, which, in turn, need Eqs. 22 to 25.
8. If d reaches failure (d = 0.99 in practical terms) before 10 days, establish σcrit(t) = σ and exit. Else, increment σ and go back to step 6 to commence a new iteration.
To compare the deterioration of bone mechanical properties with the disease and after discontinuation of the treatment, the critical load at each time point was compared to the critical load at the onset of disease giving the following safety factor:
The new PK-PD model proposed for Dmab (see Figure 1) was adjusted first to obtain
where the superscripts model and clin refer to the in-silico and clinical results, respectively; the subscript i stands for lumbar spine and hip and the subscript j refers to the time points for which the results by Bone et al. (Bone et al., 2011) were available and could be compared (see Figure 2). This error was computed for all the points of the aforementioned grid and the subregion where the error was lower was refined to search for a global minimum of E, obtained for the pair of values
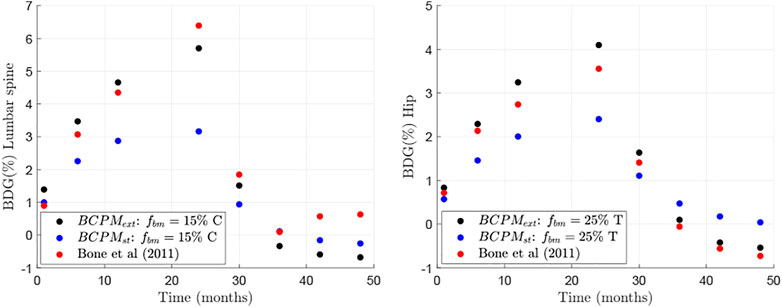
FIGURE 2. Comparison between clinical results (from Bone et al. (Bone et al., 2011)) and in-silico results of changes in BMD with respect to baseline for two different bone sites: lumbar spine (left) and hip (right).
Next we investigate the cause of the BDG rebound behaviour, which is due to the underlying changes of bone cell numbers in the BCPM. Figure 3 shows the temporal evolution of osteoclasts and osteoblasts populations for the case of the hip during Dmab treatment, normalised by the cell numbers at the beginning of the treatment. The models BCPMst (dashed) and BCPMext (solid) are compared.
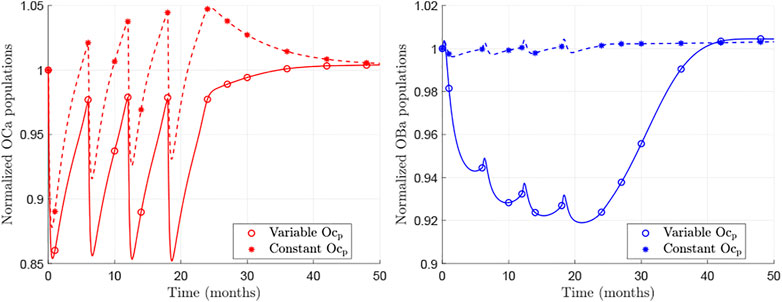
FIGURE 3. Normalised evolution of active osteoclasts (left) and active osteoblasts (right) obtained with BCPMext (solid) and BCPMst (dashed). The lines represent the continuous evolutions predicted by the models and the asterisks mark the values at those time points analysed in the clinical study by Bone et al. (Bone et al., 2011).
Focusing on the results of BCPMext, one can see that the predicted population of osteoclasts falls with Dmab injections, as the drug suppresses the effect of RANKL, but as the concentration of Dmab decreases, a rebound in osteoclastic numbers is observed. When the treatment is discontinued, this rebound is prolonged and pre-treatment values are reached.
The osteoblasts population is also reduced and a rebound is observed as well. The changes in osteoblast numbers follow those of osteoclastic cells though slower and deferred in time. Consequently, the rebound is noticeable some months after stopping the Dmab treatment.
On the other hand, the behaviour predicted by the BCPMst is significantly different: no rebound in osteoclastic cell numbers can be observed. On the contrary, a decrease after discontinuation of the treatment can be seen, and there are no evident changes in osteoblastic cell numbers.
Figure 4 compares further the predictions of the two models with respect to the evolution of apparent density (top left), MTDAD (top right), damage level (bottom left) and the safety factor (bottom right), which are presented for the case fbm = 25% subjected to uniaxial tension and under a treatment 60Q6 beginning 10 years after menopause and discontinued after 5 years of treatment. It can be seen that menopause produces an immediate and pronounced bone loss (apparent density falls and MTDAD is negative) after menopause, when RANKL levels increase. Bone mass gain is evident after the Dmab injection, when the increase of RANKL levels is counteracted by the drug. Later, a series of cycles occur during the treatment and bone mass gain slows down as Dmab is cleared away from blood to lead again to bone loss at the end of each treatment cycle.
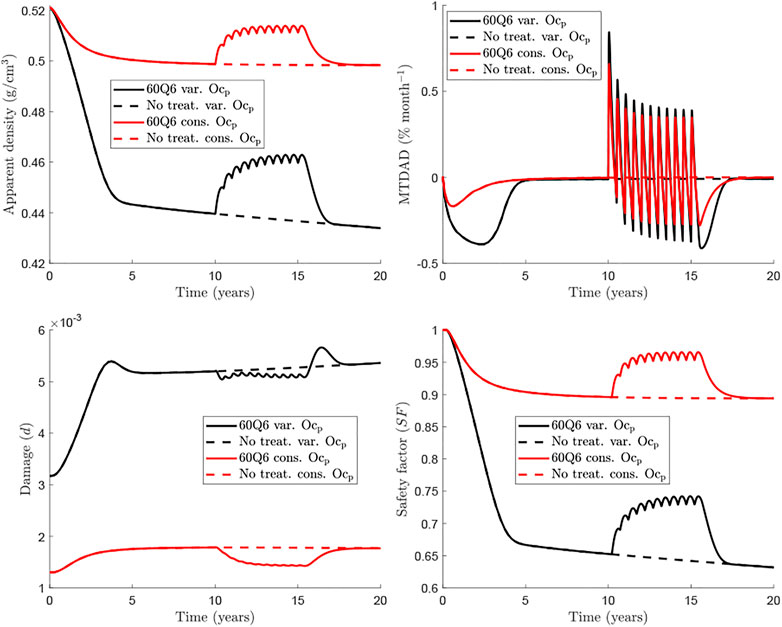
FIGURE 4. Comparison of BCPMext (black) and BCPMst (red) models for the femoral neck (fbm =25%). The treatment 60Q6 (solid) is compared with the no treatment case (dashed) for each model. The evolution of apparent density (top left), MTDAD (top right), damage (bottom left) and safety factor (bottom right) are represented.
This global behaviour is seen both in BCPMext and BCPMst, but there is a clear difference between them, which is more evident in MTDAD: bone loss predicted after discontinuation by BCPMext (black line) is more pronounced and prolonged than that predicted by BCPMst (red line). The risk of treatment discontinuation is quite evident looking at the evolution of damage, which presents a peak some months after the last Dmab injection, a peak which is only predicted by BCPMext.
The safety factor is a variable that measures how far is the tissue to local failure and is mainly influenced by the damage level and the magnitude of strain. The latter is, in turn, influenced by the stiffness and thus by the apparent density. In summary, the safety factor is controlled by the apparent density and the damage level. However, in view of the results, it appears that the former has more influence, for such values of damage and SF shows a similar trend to apparent density though it decreases by 35% in 20 years while apparent density decreases only by 16% in the case shown in Figure 4.
Currently, Dmab is administered at a constant dose of 60 mg every 6 months. Consequently, dose is not adjusted for the patient’s body weight (BW) though it influences the concentration of drug present in the central compartment. Marathe et al. (Marathe et al., 2011) showed a strong influence of BW on Dmab efficacy. For this reason, a BW = 60 kg has been taken as the reference in the previous simulations. In the following, we analyse the effect of BW on the response of bone to the usual Dmab treatment 60Q6 (see Figure 5). It can be seen that the rebound in osteoclastic cell numbers seems more pronounced for lighter patients (see both apparent density and MTDAD plot, Figure 5). Furthermore, the increment in damage after discontinuation follows a similar trend. Nonetheless, bone density gain and consequently the safety factor, are clearly lower for heavier patients, for whom the dose of 60 mg appears to be insufficient.
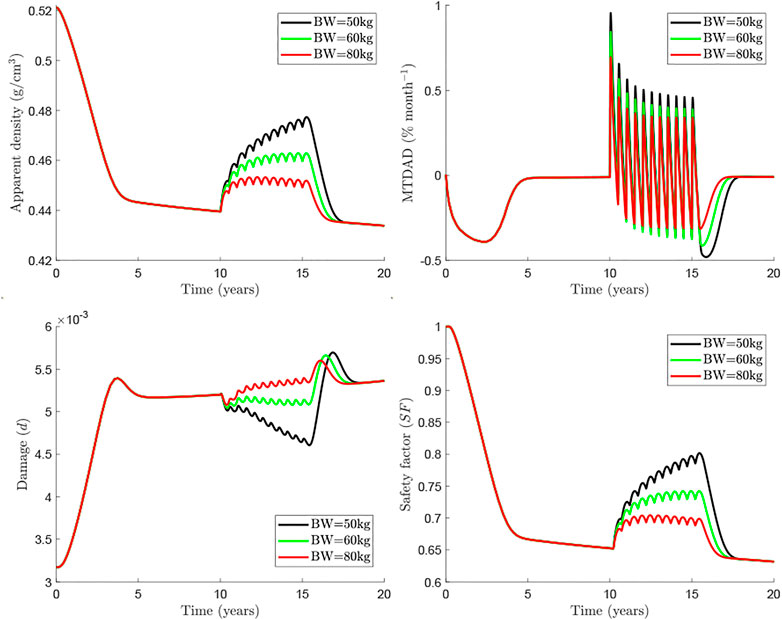
FIGURE 5. Influence of body weight in the treatment 60Q6 commencing 10 years after menopause and discontinued after 8 years.
An alternative for heavier patients could be an adjustment of the dosage, either with a greater dose or with a more frequent injection. The rationale for this option is based on the clinical results obtained by Marathe et al. (Marathe et al., 2011) on the temporal evolution of Dmab concentration in the central compartment. These authors observed a sharp drop around 5 months after the injection of 1 mg/kg of BW. This could justify the more frequent dosage in heavier patients, so to limit the bone loss rebound seen at the end of each injection cycle. Figure 6 compares the treatment 60Q5 for a patient of BW = 80 kg, which yields a quite similar response to that of the WHO-approved 60Q6 for BW = 60 kg.
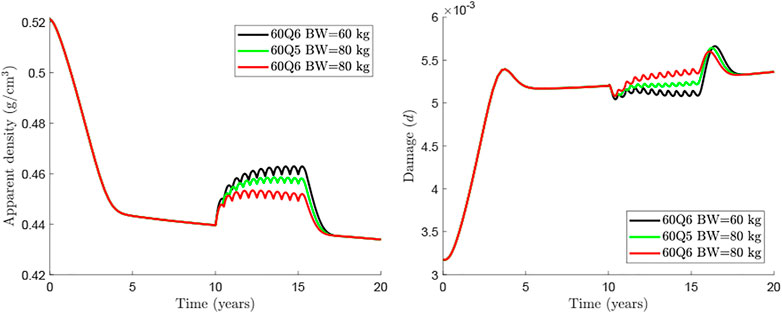
FIGURE 6. Comparison of treatments 60Q6 and 60Q5 commencing 10 years after menopause and discontinued after 8 years for different body weights.
Given that an abrupt withdrawal of Dmab treatment leads to rapid bone loss and increases bone fracture risk, an important question remains to be answered: “How can one safely discontinue Dmab treatment”? In this section we perform in-silico simulations to address this question.
One strategy would be to define a time period where Dmab is reduced to zero dose and then create a transition from the original dose (60 mg) to 0 mg by incrementally reducing the dose. Here, we simulate this transitional treatment by maintaining the frequency at 6 months and progressively decreasing the Dmab dose in the last 5 injections to 50, 40, 30, 20 and 10 mg. Figure 7 compares the 60Q6 treatment (black line) with the decreasing dose treatment (green line). It can be seen that there is no significant difference between the finally attained values for apparent bone density and damage. However, there is some difference on how quick bone is lost with the decreasing dose treatment showing a slower bone loss compared to an abrupt withdrawal. Similar, the damage variable increases more rapidly with the abrupt withdrawal compared with the decreasing dose strategy.
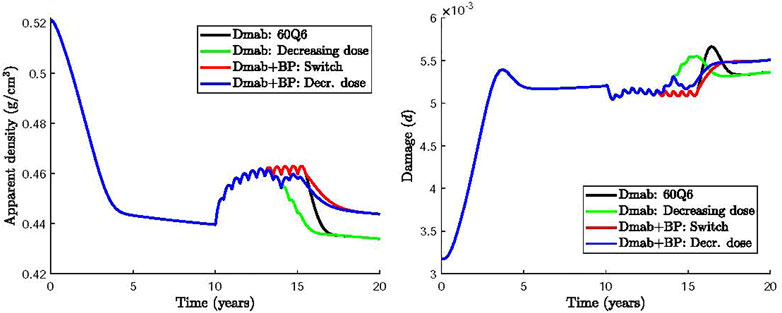
FIGURE 7. Effect of Dmab discontinuation strategies on bone apparent density and damage: 1) 60Q6 Dmab treatment discontinued after 5 years (black), 2) 60Q6 Dmab treatment with a decreasing dose (50, 40, 30, 20 and 10 mg) for the last 5 injections (green), 3) 60Q6 Dmab treatment switched to BP after 5 years (red), 4) 60Q6 Dmab treatment with a decreasing dose (50, 40, 30, 20 and 10 mg) for the last 5 injections and combined with BP from dose 30 mg onwards (blue).
Several clinical studies have investigated an alternative Dmab discontinuation strategy which uses a bisphosphonate (BP) prior to or at the time for Dmab withdrawal (Kendler et al., 2020; Burckhardt et al., 2021; Tsourdi et al., 2021). BP have been reported to induce apoptosis of active osteoclasts (Halasy-Nagy et al., 2001; Takagi et al., 2021). Here we simulate the effect of BP treatment by increasing the apoptosis rate of active osteoclasts,
Based on these clinical studies, two other alternative treatments were considered and compared in Figure 7: 1) the intake of BP 6 months after completing the 60Q6 constant dose Dmab treatment, as recommended by (Tsourdi et al., 2021) (Dmab + BP: Switch, red line); 2) the intake of BP 48 months after the Dmab treatment has commenced, or equivalently, 12 months after the Dmab dose was decreased following the pattern described above (i.e., 50, 40, 30, 20 and 10 mg for the last 5 injections) (Dmab + BP: Decr. dose, blue line).
It can be seen that the use of BP avoids the rapid bone loss and the increase of damage compared to the abrupt withdrawal of Dmab. Same values of apparent bone density and damage are obtained independent of the time of BP administration. The incremental reduction of Dmab does not appear to have a significant benefit compared to the instantaneous Dmab discontinuation.
The BDG results of the model presented in this work were compared to those of clinical studies investigating the effect of drug discontinuation of Dmab treatment (Bone et al., 2011) and demonstrated good agreement (Figure 2). The only exception was the part of the BDG results for the lumbar spine for the time period after Dmab discontinuation (see Figure 2 left). In this period, Bone et al. (Bone et al., 2011) obtained a decline in bone mass up to 1 year after discontinuation followed by an increase in bone mass, a trend that could not be explained by the current model. A plausible explanation for this increase could be that patients had started another PMO treatment, not considered in the simulations. In fact, Bone et al. (Bone et al., 2011) explicitly stated: “During the off-treatment phase, if the study investigator determined that the overall fracture risk of a participant required additional treatment for osteoporosis, they could treat the participant with an approved therapy for osteoporosis”. In any case, a strong rebound in bone loss after Dmab discontinuation is observed in both in-silico and clinical results, in agreement with other clinical results (Zanchetta et al., 2018).
The rebound in bone loss can be explained by bone cellular activities, which are reflected in the BTMs. Bone et al. (Bone et al., 2011) measured the serum C-terminal telopeptide of type 1 collagen (sCTXI) and the N-terminal propeptide of type 1 procollagen (PINP), which correlate, respectively, with the osteoclastic and osteoblastic activity. Some authors who have implemented similar BCPMs have proposed mathematical equations to relate the concentrations of osteoclasts and osteoblasts with BTMs (Marathe et al., 2011; Post et al., 2013; Lien et al., 2020). However, use of these formulae is problematic. Indeed, BCPMs are usually developed locally to model a bone-specific response which requires definition of selected RVE parameters for specific bone sites such as vertebrae or hip. These BCPMs allow to calculate local changes in BMD and bone cell numbers. On the other hand, the aforementioned BTMs are systemic quantities (i.e. not bone-site specific) that are measured in the serum or urine. They account for the response of the whole skeleton, reflecting systemic cellular activity. For that reason, the use of such mathematical formulations of BTMs has been dismissed in the current work. Comparison between the “systemic” clinical results of BTMs and the “bone-site specific” bone cell population results is provided in the next paragraph in a qualitative way.
Figure 3 (solid lines) shows the in-silico results of the new model, BCPMext. During the treatment osteoclast numbers oscillate, but on average decrease in concentration. After Dmab discontinuation the number of osteoclasts returns to pre-treatment levels. The clinical results obtained by Bone et al. (Bone et al., 2011) show a steep descent in sCTXI levels during the treatment, with oscillations that are smaller than those of the osteoclast concentration in the in-silico results and a rebound which reaches its maximum at 12 months after the last Dmab dose. BCPMext does not produce an overshoot (above baseline) in Oca population, but a recovery to baseline levels. However, BCPMext response is more similar to clinical results compared with the standard BCPMst, as the latter produces oscillations in Oca numbers whose peaks are too high and increase over time. Furthermore, after Dmab discontinuation BCPMst predicts a decline in osteoclastic activity (Figure 3, dashed lines) which is in contrast to the clinical data. In the next paragraph we analyse the mechanisms responsible for the different model responses.
Both models realistically capture the increase of Oca concentration at the end of each treatment cycle when Dmab levels decay. The main difference between the two models is that BCPMst considers a constant pool of Ocp, which is variable in BCPMext. In the latter model, the population of Ocp falls during the treatment, when the effect of RANKL is attenuated by Dmab. The lower concentration of precursor cells results in Oca values below those predicted by BCPMst (Figure 3, left). On the other hand, since the differentiation of Ocp into Oca was assumed to be inhibited (to a great extent in BCPMext compared to BCPMst) by Dmab, Ocp are accumulated at the end of each treatment cycle in BCPMext. Thus, after discontinuation both effects are superposed in BCPMext: an increased differentiation rate of Ocp into Oca, due to clearance of Dmab, and the high concentration of Ocp accumulated during the last treatment cycle.
McDonald et al. (McDonald et al., 2021) have recently shown that active osteoclasts do not only undergo apoptosis, but can also differentiate into another cell population. These authors stimulated the differentiation of osteoclasts in mice with soluble RANKL (sRANKL) and observed that osteoclast precursors underwent cell fusion to form active osteoclasts but also fissioned into mononucleated daughter cells, which they termed osteomorphs. RANKL inhibition through OPG resulted in the accumulation of osteomorphs and OPG withdrawal in the recycling of osteomorphs that fused to form functional osteoclasts. The role of Dmab is similar to that of OPG and it is likely that a similar phenomenon could occur during Dmab treatment of PMO. Although we have not modelled the above described mechanism, the variable pool of Ocp can partly account for the increase of osteoclast population in BCPMext.
The behaviour of osteoblasts (Figure 3 right, solid line) is similar and comparable to the clinical results, showing a decline in their activity during Dmab treatment and a recovery after discontinuation, which occurs more gradually than for osteoclasts. This behaviour is also seen in the clinical results (Bone et al., 2011): 9 months after the last Dmab dose sCTXI has increased by 30% over baseline levels, while the osteoblastic marker PINP has only returned to the baseline level.
This indicates that the increase in Oba population occurs delayed to that of Oca, as predicted by BCPMext. In the model, this delayed response is mediated via TGF-β, which is released from the bone matrix during bone resorption. TGF-β is known to upregulate differentiation of Obu into Obp and downregulate differentiation of Obp into Oba. This temporary imbalance between osteoblastic and osteoclastic activity in favour of the latter could also explain the rebound in loss of bone mass observed after Dmab discontinuation. However, the latter interpretation needs caution as one links systemic variables (i.e. BTMs) and bone-site specific variables (i.e. BMD). Besides, the lower Oca numbers predicted by BCPMext, on average would produce a lower release of TGF-β from bone matrix, with even lower numbers during Dmab treatment. This would result in a lower concentration of Obp and ultimately of Oba. On the contrary, the average concentration of TGF-β predicted by BCPMst would be higher and its effect on the populations of Obp and Oba less affected by its fluctuations, so yielding an approximately constant evolution of the number of osteoblasts, which is contrary to clinical results.
Our model also provides information on the rate of change in bone density measured by the monthly time derivative of apparent density (MTDAD) and the safety factor (SF) which is linked to how far a particular bone site is from failure. The evolution of apparent density exhibits a rapid decrease as shown by the evolution of MTDAD. After Dmab discontinuation MTDAD reaches values that are slightly greater than those predicted for the worst pre-treatment stages of the disease. Furthermore, the comparison between BCPMext and BCPMst (red vs black lines in Figure 4) reinforces the fact that traditional BCPM are not able to capture the spike of bone loss, as BCPMst predicts a gradual loss, with MTDAD being not as negative and as prolonged as predicted by BCPMext.
Another interesting feature predicted by the BCPMext is a spike in microstructural damage starting around 1 year after the last Dmab dose and lasting 2–3 years (Figure 4, damage variable plot). Again, standard BCPM are not able to predict this behaviour. We note that this spike corresponds to clinical observations of atypical fractures (AFs), occurring a few months after the effect of the last Dmab injection (Anastasilakis et al., 2017). For a 60 mg Dmab dose AFs occur about 8 months after the last injection (Marathe et al., 2011).
The risk of bone failure can be estimated by analysing the evolution of the safety factor SF (Figure 4). SF depends mainly on the damage accumulated in the bone matrix and the apparent bone density, but the latter seems to have a major influence, given that the evolutions of SF and apparent density are very similar. However, there is a key difference: the decrease in apparent density throughout the disease or after Dmab discontinuation is magnified in the safety factor. For instance, BCPMext predicted that SF has decreased by 35% at the beginning of the treatment (year 10), while apparent density has decreased only by 16%. After Dmab discontinuation SF falls, now by 10%; while apparent density decreases only by 5%. Again, BCPMext performs better than BCPMst in predicting the risk of failure after Dmab discontinuation. Thus, the former predicts a 10% drop in SF in about 1.5 years, while BCPMst predicts a more gradual drop, by 7.5% in 2.5 years.
The influence of patient’s BW and different Dmab doses were analysed respectively in Figures 5, 6. In general, if the Dmab dosage (mg/kg of BW) is increased, BMD gain is higher, but bone loss and the rebound of damage observed after Dmab withdrawal are also more pronounced. In other words, Dmab discontinuation is potentially more dangerous in patients with low BW. However, the benefits of the drug are more evident during the treatment and this is what should prevail, since the long-term response appears to be independent of BW. Thus, the in-silico results would indicate that the dosage 60Q6 could be suboptimal for an 80 kg patient given that bone gain is only moderate during the treatment and the lower rebound of damage after discontinuation would not justify its use since it is only slightly lower than in patients with low BW. On the other hand, decreasing the time between injections to 5 months (60Q5) has beneficial effects on bone density as Dmab is not totally depleted between injections (Marathe et al., 2011) and the spike of damage after Dmab withdrawal is not much higher than with 60Q6.
Motivated by the adverse effects of Dmab discontinuation for the dosage 60Q6 some alternative treatments were investigated. A gradual decrease in the dose during the last years of the treatment had no significant benefits as bone density loss is more pronounced and the spike of damage is only slightly reduced. On the other hand, administration of a different drug that promotes osteoclasts apoptosis, such as BP, can alleviate bone loss (Figure 7). Both switching from Dmab to BP (red line) or administering BP concurrently while the Dmab dose is being reduced (blue line), lead to significant reduction of BMD loss after Dmab withdrawal. The predicted values of microstructural damage are higher in the Dmab + BP cases, as the lower bone turnover rate is not able to repair bone matrix as effectively as in the Dmab cases. However, as mentioned above, apparent density has more impact on the safety factor than damage, at least for the levels reached in these simulations, and thus SF is eventually higher in the Dmab + BP cases. The evolution of SF is not shown in Figure 7 as it is almost identical to the evolution of apparent density though with different values. Thus, while SF falls by 37% in the Dmab cases after 20 years (10 years after the onset of the disease), it only falls by 33% in the Dmab + BP case. This would be in accordance with the retrospective study by Burckhardt et al. (Burckhardt et al., 2021) who found that a treatment with BP, especially after Dmab, had a protective effect. On the other hand, it was found that the final values of BMD and damage are not sensitive to the time of administration of BP.
We finally want to point out some limitations of our in-silico results. The effect of BP has been considered in the model in a simplistic way, by assuming that BP increases the apoptosis rate of osteoclasts by a fixed value that remains constant over time. In the future, a more detailed model describing the pharmacokinetics (PK) of BP must be developed. That PK model would be tailored to a particular BP such as alendronate. Other possible actions of BP on bone cells might need to be taken into account in this model. It has been reported that certain BP limit the resorbing capacity of osteoclasts (Halasy-Nagy et al., 2001; Takagi et al., 2021); and finally an important aspect which is specific to BP, the fact that it may remain trapped within bone matrix for long periods of time, until it is released through bone resorption. These aspects require further model refinements and are out of scope of the current paper.
The case assumed here to simulate the behaviour of the lumbar spine was a RVE of fbm = 15% subjected to uniaxial compression, while fbm = 25% and uniaxial tension was assumed for the hip. The latter would approximate the behaviour of the trabecular region of the femoral neck subjected to bending. Both assumptions constitute an important limitation of the study and are justified by the use of a RVE to implement the model. However, an organ level finite element (FE) model is needed to study bone strength and fracture risk in a more realistic manner.
In this paper, a previously published bone cell population model (BCPMst) has been extended (BCPMext) to simulate the rebound of bone loss observed after discontinuation of Dmab treatment in women with post-menopausal osteoporosis. The main difference between both models is that BCPMext considers a variable pool of osteoclast precursors, which were considered constant in the previous one. BCPMext predicts that these precursors accumulate at the end of each treatment cycle. Thus, once Dmab is depleted and RANKL is restored to pre-treatment values, the differentiation rate of precursors into mature osteoclasts tends to rise, as at the end of each treatment cycle, but the larger amount of available precursors enhance this phenomenon and that differentiation rate even exceeds pre-treatment values. This spike of bone loss induces a rebound in microstructural damage and these two factors together may be responsible for the atypical fractures reported in numerous clinical studies.
The in-silico results allow to draw the following conclusions:
• The accumulation of osteoclast precursors (or possibly osteomorphs) could explain the spike in bone loss after Dmab discontinuation.
• The adjustment of Dmab dose to body weight needs to be explored since the WHO-approved dose (60 mg injected every 6 months) could be insufficient for patients with high BW.
• Gradually decreasing the Dmab dose during the last years of treatment as an alternative to the abrupt discontinuation showed no clear benefits, but a transition to other drugs with different effects on bone turnover, such as BP that increase osteoclast apoptosis rate, might be a good strategy to reduce the fracture risk observed after Dmab discontinuation.
• The long-term values of BMD and damage are not sensitive to the time of administration of the BP in combined treatments.
These conclusions must be confirmed in future studies, both clinical and in-silico. The latter have the advantage that many combinations can be tested in a quick and systematic way, but they require the application of the BCPM in a FE model that allows considering different bone structural aspects not taken into account here. These aspects include more realistic loads, the influence of heterogeneity on bone density changes, microstructural damage accumulation and stress redistribution.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JM-R and PP conceived and planned the computational simulations. MM and JMR developed the mathematical model of bone adaptation. JC-G and JM-R carried out the computational simulations. JM-R and PP wrote the manuscript. All authors discussed the results, reviewed and commented on the manuscript.
Funding was provided by the “Fondo Europeo de Desarrollo Regional (FEDER)”, the “Consejería de Economía, Conocimiento, Empresas y Universidad de la Junta de Andalucía, dentro del Programa Operativo FEDER 2014-2020” and the “Universidad de Sevilla” to the project P18-RT-3611 entitled “Efecto combinado del ejercicio físico y el denosumab en el tratamiento de la osteoporosis. Diseño de un tratamiento farmacológico específico de paciente” for which this paper was prepared.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PP gratefully acknowledges support from the Australian Research Council (ARC ITTC, IC190100020).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2022.886579/full#supplementary-material
1In the damage model proposed by (Martínez-Reina et al., 2009), cracks were assumed to grow normal to the maximum strain direction and only under tensile strains, if ɛmax > 0. For example, in the case of uniaxial compression these strains are due to the Poisson's effect.
Anastasilakis, A. D., Makras, P., Yavropoulou, M. P., Tabacco, G., Naciu, A. M., and Palermo, A. (2021). Denosumab Discontinuation and the Rebound Phenomenon: A Narrative Review. Jcm 10 (1), 152. doi:10.3390/jcm10010152
Anastasilakis, A. D., Polyzos, S. A., Makras, P., Aubry-Rozier, B., Kaouri, S., and Lamy, O. (2017). Clinical Features of 24 Patients with Rebound-Associated Vertebral Fractures after Denosumab Discontinuation: Systematic Review and Additional Cases. J. Bone Min. Res. 32 (6), 1291–1296. doi:10.1002/jbmr.3110
Anastasilakis, A. D., Polyzos, S. A., and Makras, P. (2018). Therapy of Endocrine Disease: Denosumab vs Bisphosphonates for the Treatment of Postmenopausal Osteoporosis. Eur. J. Endocrinol. 179 (1), R31–R45. doi:10.1530/EJE-18-0056
Bone, H. G., Bolognese, M. A., Yuen, C. K., Kendler, D. L., Miller, P. D., Yang, Y.-C., et al. (2011). Effects of Denosumab Treatment and Discontinuation on Bone Mineral Density and Bone Turnover Markers in Postmenopausal Women with Low Bone Mass. J. Clin. Endocrinol. Metabolism 96 (4), 972–980. doi:10.1210/jc.2010-1502
Brown, J. P., Roux, C., Törring, O., Ho, P. R., Beck Jensen, J. E., Gilchrist, N., et al. (2013). Discontinuation of Denosumab and Associated Fracture Incidence: Analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) Trial. J. Bone Min. Res. 28 (4), 746–752. doi:10.1002/jbmr.1808
Burckhardt, P., Faouzi, M., Buclin, T., and Lamy, O.The Swiss Denosumab Study Group (2021). Fractures after Denosumab Discontinuation: A Retrospective Study of 797 Cases. J. Bone Min. Res. 36 (9), 1717–1728. doi:10.1002/jbmr.4335
Cummings, S. R., Ferrari, S., Eastell, R., Gilchrist, N., Jensen, J.-E. B., McClung, M., et al. (2018). Vertebral Fractures after Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and its Extension. J. Bone Min. Res. 33 (2), 190–198. doi:10.1002/jbmr.3337
Cummings, S. R., Martin, J. S., McClung, M. R., Siris, E. S., Eastell, R., Reid, I. R., et al. (2009). Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 361 (8), 756–765. doi:10.1056/NEJMoa0809493
Currey, J. D. (2004). Tensile Yield in Compact Bone Is Determined by Strain, Post-yield Behaviour by Mineral Content. J. Biomechanics 37 (4), 549–556. doi:10.1016/j.jbiomech.2003.08.008
Dua, P., Hawkins, E., and van der Graaf, P. (2015). A Tutorial on Target-Mediated Drug Disposition (TMDD) Models. CPT Pharmacometrics Syst. Pharmacol. 4 (6), 324–337. URL: http://dx.doi.org/10.1002/psp4.41. doi:10.1002/psp4.41
Eriksen, E. F., Hodgson, S. F., Eastell, R., Riggs, B. L., Cedel, S. L., and O'Fallon, W. M. (1990). Cancellous Bone Remodeling in Type I (Postmenopausal) Osteoporosis: Quantitative Assessment of Rates of Formation, Resorption, and Bone Loss at Tissue and Cellular Levels. J. Bone Min. Res. 5 (4), 311–319. doi:10.1002/jbmr.5650050402
Felsenberg, D., Silman, A., Lunt, M., Armbrecht, G., Ismail, A., Finn, J., et al. (2002). Incidence of Vertebral Fracture in Europe: Results from the European Prospective Osteoporosis Study (EPOS). J. Bone Min. Res. 17 (4), 716–724. doi:10.1359/jbmr.2002.17.4.716
García-Aznar, J. M., Rueberg, T., and Doblaré, M. (2005). A Bone Remodelling Model Coupling Micro-damage Growth and Repair by 3D BMU-Activity. Biomech. Model. Mechanobiol. 4 (2-3), 147–167. doi:10.1007/s10237-005-0067-x
Halasy-Nagy, J. M., Rodan, G. A., and Reszka, A. A. (2001). Inhibition of Bone Resorption by Alendronate and Risedronate Does Not Require Osteoclast Apoptosis. Bone 29 (6), 553–559. doi:10.1016/s8756-3282(01)00615-9
Hernandez, C. J., Beaupré, G. S., Keller, T. S., and Carter, D. R. (2001a). The Influence of Bone Volume Fraction and Ash Fraction on Bone Strength and Modulus. Bone 29 (1), 74–78. doi:10.1016/s8756-3282(01)00467-7
Hernandez, C. J., Beaupré, G. S., and Carter, D. R. (2001b). A Model of Mechanobiologic and Metabolic Influences on Bone Adaptation. J. Rehabil. Res. Dev. 37 (2), 235–244.
Jacobs, C. (1994). Numerical Simulation of Bone Adaptation to Mechanical Loading (Stanford, California: Stanford University). Ph.D. thesis.
Juvinall, R. (1967). Engineering Considerations of Stress, Strain and Strength. New York, USA: McGraw-Hill.
Kanis, J. A., and Kanis, J. A. (1994). Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis: Synopsis of a WHO Report. Osteoporos. Int. 4 (6), 368–381. doi:10.1007/BF01622200
Kendler, D., Chines, A., Clark, P., Ebeling, P. R., McClung, M., Rhee, Y., et al. (2020). Bone Mineral Density after Transitioning from Denosumab to Alendronate. J. Clin. Endocrinol. Metab. 105 (3), e255–e264. doi:10.1210/clinem/dgz095
Kostenuik, P. J., Nguyen, H. Q., McCabe, J., Warmington, K. S., Kurahara, C., Sun, N., et al. (2009). Denosumab, a Fully Human Monoclonal Antibody to RANKL, Inhibits Bone Resorption and Increases BMD in Knock-In Mice that Express Chimeric (Murine/Human) RANKL*. J. Bone Mineral Res. 24 (2), 182–195. doi:10.1359/jbmr.081112
Lemaire, V., Tobin, F. L., Greller, L. D., Cho, C. R., and Suva, L. J. (2004). Modeling the Interactions between Osteoblast and Osteoclast Activities in Bone Remodeling. J. Theor. Biol. 229 (3), 293–309. URL: http://www.sciencedirect.com/science/article/pii/S0022519304001407. 10.1016/j.jtbi.2004.03.023. doi:10.1016/j.jtbi.2004.03.023
Lemaitre, J., and Chaboche, J. (1990). Mechanics of Solid Materials. Cambridge, UK: Cambridge University Press.
Lien, Y. T., Madrasi, K., Samant, S., Kim, M. J., Li, F., Li, L., et al. (2020). Establishment of a Disease‐Drug Trial Model for Postmenopausal Osteoporosis: A Zoledronic Acid Case Study. J. Clin. Pharmacol. 60 (S2), S86–S102. doi:10.1002/jcph.1748
Lorentzon, M., and Cummings, S. R. (2015). Osteoporosis: the Evolution of a Diagnosis. J. Intern Med. 277 (6), 650–661. doi:10.1111/joim.12369
Lyu, H., Yoshida, K., Zhao, S. S., Wei, J., Zeng, C., Tedeschi, S. K., et al. (2020). Delayed Denosumab Injections and Fracture Risk Among Patients with Osteoporosis. Ann. Intern. Med. 173 (7), 516–526. doi:10.7326/M20-0882
Manolagas, S. C., and Parfitt, A. M. (2013). For Whom the Bell Tolls: Distress Signals from Long-Lived Osteocytes and the Pathogenesis of Metabolic Bone Diseases. Bone 54 (2), 272–278. doi:10.1016/j.bone.2012.09.017
Manolagas, S. C. (2018). The Quest for Osteoporosis Mechanisms and Rational Therapies: How Far We've Come, How Much Further We Need to Go. J. Bone Min. Res. 33 (2), 371–385. doi:10.1002/jbmr.3400
Marathe, D. D., Marathe, A., and Mager, D. E. (2011). Integrated Model for Denosumab and Ibandronate Pharmacodynamics in Postmenopausal Women. Biopharm. Drug Dispos. 32 (8), 471–481. URL: http://dx.doi.org/10.1002/cnm.2584. doi:10.1002/bdd.770
Martínez-Reina, J., García-Aznar, J. M., Domínguez, J., and Doblaré, M. On the Role of Bone Damage in Calcium Homeostasis. J. Theor. Biol. 2008;254(3):704–712.doi:10.1016/j.jtbi.2008.06.007
Martin, M., Sansalone, V., Cooper, D. M. L., Forwood, M. R., and Pivonka, P. (2019). Mechanobiological Osteocyte Feedback Drives Mechanostat Regulation of Bone in a Multiscale Computational Model. Biomech. Model. Mechanobiol. 18 (5), 1475–1496. doi:10.1007/s10237-019-01158-w
Martin, R. B. (1984). Porosity and Specific Surface of Bone. Crit. Rev. Biomed. Eng. 10 (3), 179–222.
Martínez-Reina, J., Calvo-Gallego, J. L., and Pivonka, P. (2021). Are Drug Holidays a Safe Option in Treatment of Osteoporosis? - Insights from an In Silico Mechanistic PK-PD Model of Denosumab Treatment of Postmenopausal Osteoporosis. J. Mech. Behav. Biomed. Mater. 113, 104140. doi:10.1016/j.jmbbm.2020.104140
Martínez-Reina, J., Calvo-Gallego, J. L., and Pivonka, P. (2021). Combined Effects of Exercise and Denosumab Treatment on Local Failure in Post-menopausal Osteoporosis-Insights from Bone Remodelling Simulations Accounting for Mineralisation and Damage. Front. Bioeng. Biotechnol. 9, 635056. doi:10.3389/fbioe.2021.635056
Martínez-Reina, J., García-Aznar, J. M., Domínguez, J., and Doblaré, M. (2009). A Bone Remodelling Model Including the Directional Activity of BMUs. Biomech. Model. Mechanobiol. 8 (2), 111–127. doi:10.1007/s10237-008-0122-5
Martínez-Reina, J., and Pivonka, P. (2019). Effects of Long-Term Treatment of Denosumab on Bone Mineral Density: Insights from an In-Silico Model of Bone Mineralization. Bone 125 (125), 87–95. URL: https://www.ncbi.nlm.nih.gov/pubmed/31055117. doi:10.1016/j.bone.2019.04.022
Matsuo, K., and Irie, N. (2008). Osteoclast-osteoblast Communication. Archives Biochem. Biophysics 473 (2), 201–209. doi:10.1016/j.abb.2008.03.027
McClung, M. R., Wagman, R. B., Miller, P. D., Wang, A., and Lewiecki, E. M. (2017). Observations Following Discontinuation of Long-Term Denosumab Therapy. Osteoporos. Int. 28 (5), 1723–1732. doi:10.1007/s00198-017-3919-1
McDonald, M. M., Khoo, W. H., Ng, P. Y., Xiao, Y., Zamerli, J., Thatcher, P., et al. (2021). Osteoclasts Recycle via Osteomorphs during RANKL-Stimulated Bone Resorption. Cell. 184 (5), 1330–1347. doi:10.1016/j.cell.2021.02.002
Miller, P. D., Bolognese, M. A., Lewiecki, E. M., McClung, M. R., Ding, B., Austin, M., et al. (2008). Effect of Denosumab on Bone Density and Turnover in Postmenopausal Women with Low Bone Mass after Long-Term Continued, Discontinued, and Restarting of Therapy: a Randomized Blinded Phase 2 Clinical Trial. Bone 43 (2), 222–229. doi:10.1016/j.bone.2008.04.007
Nakashima, T., Hayashi, M., Fukunaga, T., Kurata, K., Oh-Hora, M., Feng, J. Q., et al. (2011). Evidence for Osteocyte Regulation of Bone Homeostasis through RANKL Expression. Nat. Med. 17, 1231–1234. doi:10.1038/nm.2452
Need, A. G., Horowitz, M., Morris, H. A., Moore, R., and Nordin, C. (2007). Seasonal Change in Osteoid Thickness and Mineralization Lag Time in Ambulant Patients. J. Bone Min. Res. 22 (5), 757–761. doi:10.1359/jbmr.070203
Papapoulos, S., Lippuner, K., Roux, C., Lin, C. J. F., Kendler, D. L., Lewiecki, E. M., et al. (2015). The Effect of 8 or 5 Years of Denosumab Treatment in Postmenopausal Women with Osteoporosis: Results from the FREEDOM Extension Study. Osteoporos. Int. 26 (12), 2773–2783. doi:10.1007/s00198-015-3234-7
Parfitt, A. M. (2002). Targeted and Nontargeted Bone Remodeling: Relationship to Basic Multicellular Unit Origination and Progression. Bone 30 (1), 5–7. doi:10.1016/s8756-3282(01)00642-1
Pattin, C. A., Caler, W. E., and Carter, D. R. (1996). Cyclic Mechanical Property Degradation during Fatigue Loading of Cortical Bone. J. Biomechanics 29 (1), 69–79. doi:10.1016/0021-9290(94)00156-1
Peterson, M. C., and Riggs, M. M. (2010). A Physiologically Based Mathematical Model of Integrated Calcium Homeostasis and Bone Remodeling. Bone 46 (1), 49–63. doi:10.1016/j.bone.2009.08.053
Peterson, M., and Riggs, M. (2012). Predicting Nonlinear Changes in Bone Mineral Density over Time Using a Multiscale Systems Pharmacology Model. CPT Pharmacometrics Syst. Pharmacol. 1 (11), 14. doi:10.1038/psp.2012.15
Pivonka, P., Zimak, J., Smith, D. W., Gardiner, B. S., Dunstan, C. R., Sims, N. A., et al. (2008). Model Structure and Control of Bone Remodeling: A Theoretical Study. Bone 43 (2), 249–263. URL: http://dx.doi.org/10.1016/j.bone.2008.03.025. doi:10.1016/j.bone.2008.03.025
Popp, A. W., Varathan, N., Buffat, H., Senn, C., Perrelet, R., and Lippuner, K. (2018). Bone Mineral Density Changes after 1 Year of Denosumab Discontinuation in Postmenopausal Women with Long-Term Denosumab Treatment for Osteoporosis. Calcif. Tissue Int. 103 (1), 50–54. doi:10.1007/s00223-018-0394-4
Post, T. M., Schmidt, S., Peletier, L. A., de Greef, R., Kerbusch, T., and Danhof, M. (2013). Application of a Mechanism-Based Disease Systems Model for Osteoporosis to Clinical Data. J. Pharmacokinet. Pharmacodyn. 40 (2), 143–156. doi:10.1007/s10928-012-9294-9
Schaffler, M. B., Cheung, W.-Y., Majeska, R., and Kennedy, O. (2014). Osteocytes: Master Orchestrators of Bone. Calcif. Tissue Int. 94 (1), 5–24. doi:10.1007/s00223-013-9790-y
Scheiner, S., Pivonka, P., and Hellmich, C. (2013). Coupling Systems Biology with Multiscale Mechanics, for Computer Simulations of Bone Remodeling. Comput. Methods Appl. Mech. Eng. 254 (1), 181–196. URL: http://www.sciencedirect.com/science/article/pii/S0045782512003234. 10.1016/j.cma.2012.10.015. doi:10.1016/j.cma.2012.10.015
Scheiner, S., Pivonka, P., Smith, D. W., Dunstan, C. R., and Hellmich, C. (2014). Mathematical Modeling of Postmenopausal Osteoporosis and its Treatment by the Anti‐catabolic Drug Denosumab. Int. J. Numer. Meth. Biomed. Engng. 30 (1), 1–27. URL: http://dx.doi.org/10.1002/cnm.2584. doi:10.1002/cnm.2584
Takagi, Y., Inoue, S., Fujikawa, K., Matsuki-Fukushima, M., Mayahara, M., Matsuyama, K., et al. (2021). Effect of Nitrogen-Containing Bisphosphonates on Osteoclasts and Osteoclastogenesis: an Ultrastructural Study. Microscopy 70 (3), 302–307. doi:10.1093/jmicro/dfaa073
Tripto-Shkolnik, L., Fund, N., Rouach, V., Chodick, G., Shalev, V., and Goldshtein, I. (2020). Fracture Incidence after Denosumab Discontinuation: Real-World Data from a Large Healthcare Provider. Bone 130, 115150. doi:10.1016/j.bone.2019.115150
Tsourdi, E., Zillikens, M. C., Meier, C., Body, J.-J., Gonzalez Rodriguez, E., Anastasilakis, A. D., et al. (2021). Fracture Risk and Management of Discontinuation of Denosumab Therapy: A Systematic Review and Position Statement by ECTS. J. Clin. Endocrinol. Metab. 106 (1), 264–281. doi:10.1210/clinem/dgaa756
Tu, X., Delgado-Calle, J., Condon, K. W., Maycas, M., Zhang, H., Carlesso, N., et al. (2015). Osteocytes Mediate the Anabolic Actions of Canonical Wnt/β-Catenin Signaling in Bone. Proc. Natl. Acad. Sci. U.S.A. 112 (5), E478–E486. doi:10.1073/pnas.1409857112
Udagawa, N., Takahashi, N., Yasuda, H., Mizuno, A., Itoh, K., Ueno, Y., et al. (2000). Osteoprotegerin Produced by Osteoblasts Is an Important Regulator in Osteoclast Development and Function*. Endocrinology 141 (9), 3478–3484. doi:10.1210/endo.141.9.7634
Xiong, J., Piemontese, M., Onal, M., Campbell, J., Goellner, J. J., Dusevich, V., et al. (2015). Osteocytes, Not Osteoblasts or Lining Cells, Are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS ONE 10 (9), e0138189. doi:10.1371/journal.pone.0138189
Zanchetta, M. B., Boailchuk, J., Massari, F., Silveira, F., Bogado, C., and Zanchetta, J. R. Significant Bone Loss after Stopping Long-Term Denosumab Treatment: a Post FREEDOM Study. Osteoporos. Int. 2018;29(1):41–47. doi:10.1007/s00198-017-4242-6
Keywords: postmenopausal osteoporosis, bone remodelling, bone mineralisation, denosumab discontinuation, RANK-RANKL-OPG pathway, PK-PD modelling, osteoclast precursors
Citation: Martínez-Reina J, Calvo-Gallego JL, Martin M and Pivonka P (2022) Assessment of Strategies for Safe Drug Discontinuation and Transition of Denosumab Treatment in PMO—Insights From a Mechanistic PK/PD Model of Bone Turnover. Front. Bioeng. Biotechnol. 10:886579. doi: 10.3389/fbioe.2022.886579
Received: 28 February 2022; Accepted: 25 April 2022;
Published: 30 May 2022.
Edited by:
Andrea Malandrino, Universitat Politecnica de Catalunya, SpainReviewed by:
Maria Jose Gomez-Benito, University of Zaragoza, SpainCopyright © 2022 Martínez-Reina, Calvo-Gallego, Martin and Pivonka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javier Martínez-Reina, am1yZWluYUB1cy5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.