
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 25 March 2022
Sec. Biomaterials
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.856398
This article is part of the Research Topic Biodegradable Polymers for Biomedical Applications View all 13 articles
 Yiting Ze
1,2
†
Yiting Ze
1,2
† Yanxi Li1,2
†
Yanxi Li1,2
†
 Linyang Huang
1,2
Linyang Huang
1,2 Yixin Shi1,2
Yixin Shi1,2
 Peiran Li
1,2
Peiran Li
1,2 Ping Gong1,2
Ping Gong1,2 Jie Lin1,2*
Jie Lin1,2* Yang Yao1,2*
Yang Yao1,2*Mature vasculature is important for the survival of bioengineered tissue constructs, both in vivo and in vitro; however, the fabrication of fully vascularized tissue constructs remains a great challenge in tissue engineering. Indirect three-dimensional (3D) bioprinting refers to a 3D printing technique that can rapidly fabricate scaffolds with controllable internal pores, cavities, and channels through the use of sacrificial molds. It has attracted much attention in recent years owing to its ability to create complex vascular network-like channels through thick tissue constructs while maintaining endothelial cell activity. Biodegradable materials play a crucial role in tissue engineering. Scaffolds made of biodegradable materials act as temporary templates, interact with cells, integrate with native tissues, and affect the results of tissue remodeling. Biodegradable ink selection, especially the choice of scaffold and sacrificial materials in indirect 3D bioprinting, has been the focus of several recent studies. The major objective of this review is to summarize the basic characteristics of biodegradable materials commonly used in indirect 3D bioprinting for vascularization, and to address recent advances in applying this technique to the vascularization of different tissues. Furthermore, the review describes how indirect 3D bioprinting creates blood vessels and vascularized tissue constructs by introducing the methodology and biodegradable ink selection. With the continuous improvement of biodegradable materials in the future, indirect 3D bioprinting will make further contributions to the development of this field.
At present, only a few tissue-engineered products, such as skin (Ryssel et al., 2008) and cartilage (Makris et al., 2015), have achieved clinical success. For organs and tissues with more complex structure, such as the heart, liver, or spleen, there is still a long way to go (Berthiaume et al., 2011; Datta et al., 2017). An immature vascular system is one of the most important reasons for the failure of these products (Duan, 2017; Richards et al., 2017). In recent years, the rise of 3D bioprinting technology has enabled outstanding contributions to be made towards solving the problem of vasculature fabrication, thus extending the potential application of artificial tissues (Zhu et al., 2016; Alonzo et al., 2019; Hann et al., 2019). Indirect 3D bioprinting increasingly attracts research attention because of its superior capabilities with respect to vascularization (Wang Z. et al., 2019). Biodegradable materials are a crucial part of tissue engineering. They are typically designed to promote new tissue generation by serving as temporary templates and providing physical or chemical signals for cells (Asghari et al., 2017). Biodegradable materials also have interesting applications and new challenges in indirect 3D bioprinting for vascularization. In this review, we describe the printing methods, selection of biodegradable inks, and applications of indirect 3D bioprinting for blood vessels and vascularized tissue constructs; furthermore, we point out the existing challenges and trends for future development.
Vasculature is an essential part of the human body. Mature vasculature provides continuous perfusion, transporting nutrients to and removing metabolic wastes from cells, thus maintaining high cell viability and normal tissue function (Leong et al., 2013; Kim et al., 2016; Hann et al., 2019). For nonvascular tissues, the diffusion range of oxygen and nutrients is generally 100–200 μm (Carmeliet and Jain, 2000; Kaully et al., 2010). This means that, for successful in vivo implantation of engineered tissue constructs any larger in scale than this limit, it is necessary to ensure sufficient vasculature throughout the construct and good integration with the host vascular system (Ko et al., 2007). However, the vascularization of thick tissues has always been a major challenge and a research hotspot in tissue engineering.
3D bioprinting technology, also referred to as additive manufacturing, uses special 3D printers and bioinks containing cellular/bioactive components to fabricate scaffolds that imitate living tissues, in a layer-by-layer deposition approach with the help of computer-aided design (CAD) (Datta et al., 2017; Abdollahi et al., 2019). Compared with other tissue fabrication methods, 3D bioprinting stands out because of its convenience of customization, precise multi-dimensional control, and ability to fabricate 3D biostructures with suitable mechanical properties. In particular, its accurate control of complex and delicate structures within constructs, combined with the adoption of bioinks, make 3D bioprinting a powerful and efficient tool to address the problem of vascularization by creating a vasculogenic (Laschke and Menger, 2012; Balaji et al., 2013) (generating new blood vessels from endothelial cells or vascular progenitor cells) and angiogenic (Patel-Hett and D'Amore, 2011; Bae et al., 2012; Rouwkema and Khademhosseini, 2016) (germination or remodeling of existing vessels) environment for blood vessel formation. Most studies focus on two aspects: the fabrication of vascular grafts or vascular networks in thick constructs; however, these studies share one goal: to manufacture bionic vasculature that can work stably and continuously both in vitro and in vivo.
Although technological progress has helped the progress of vascularization in bioengineered constructs, major challenges remain (Novosel et al., 2011; Nazeer et al., 2021). The first challenge is the stable and delicate printing of microvascular networks. The diameters of capillaries, small vessels (small arteries and small veins), and small arteries with a three-layered structure are about 5–10 μm, 10–200 μm, and 30 μm, respectively; but the resolution of most 3D bioprinting is at the 100 μm level or above (Norotte et al., 2009; Hasan et al., 2014), making it difficult to print smaller diameter channels or the complex three-layered structure (adventitia, intima, and media) of blood vessels. Furthermore, the tiny hollow structure is unstable and prone to deformation or collapse owing to several factors, such as poor strength of the material, curing expansion of the bioink, or improper external extrusive force. In addition, microvascular networks should have complex multi-level branching structures. At present, the complexity of printed microvasculature hardly matches that of native vasculature (Robu et al., 2019).
The second challenge is the integration of allogeneic and autologous vascular networks. Effective integration with host circulation is the precondition of effective blood perfusion after implantation (Reis et al., 2016). Studies have shown that in implant tissue engineering, compared to the disordered vascular network formed by randomly distributed cells in culture, ordered vascular structure showed faster integration with the host and more stable perfusion, for which 3D bioprinting has the advantage (Vacanti, 2012; Baranski et al., 2013). However, the physiological blood vessel structure is so subtle that it is still difficult to use 3D bioprinting to fabricate smooth and regularly arranged microchannels at the micron level. Furthermore, most experiments are conducted in vitro. To date, no in vivo experiment has shown that, even with good integration with the host, thick tissue constructs remain viable for long or that necrosis will not occur after implantation.
Thirdly, it is difficult to ensure complex but stable blood perfusion in thick tissue constructs. Good blood perfusion is a necessary condition for engineered tissue survival in vivo. Currently, many strategies depend on the ingrowth of host vessels to achieve graft blood perfusion (Cheng et al., 2011; White et al., 2014; Kang et al., 2015), which is very slow (<1 mm per day) (Zhang et al., 2020), to preserve cell viability within thick tissue grafts (Sooppan et al., 2016). In order to accelerate the process, prevascularization is widely applied. Prevascularization refers to the generation of preformed microvasculature before tissue construct implantation (Laschke and Menger, 2016). It relies on seeding or encapsulating endothelial cells (ECs) in the construct in vitro. However, the process of cell infiltration and growth in vitro is uncertain, and the resultant vasculature is usually inhomogeneous (Leong et al., 2013). When implanted, immature vasculature results in insufficient blood perfusion and induces core necrosis in constructs (Asakawa et al., 2010), causing the failure of thick constructs to survive (Rouwkema et al., 2008).
Nowadays, many 3D bioprinting methods have been developed to mimic vasculature, including extrusion-, laser-based systems, electrospinning, stacking of micropatterns or modules, and cell sheet techniques (Yamamura et al., 2007; Kolesky et al., 2014; Sarker M. et al., 2018; Sarker M. D. et al., 2018). New methods are constantly being explored to solve the abovementioned problems, and indirect 3D bioprinting may cast light on vascularization in tissue engineering.
Instead of directly simulating and manufacturing the target constructs, indirect 3D bioprinting fabricates a sacrificial mold or template (negative model) and fills it with a second material. After selective dissolution, the sacrificial mold or template is removed while the construct (patrix) formed by the second material is preserved (Lee et al., 2013; Van Hoorick et al., 2015). Through this approach, pores, cavities, and channels can be precisely made throughout thick constructs (Lee et al., 2008; Schumacher et al., 2010). Owing to its specific manner of printing, other names for indirect 3D printing include lost-mold technique, indirect rapid prototyping, indirect additive manufacturing, and indirect solid free-form fabrication (Chu et al., 2001; Schumacher et al., 2010; Van Hoorick et al., 2015; Houben et al., 2016; Houben et al., 2017).
Currently, indirect 3D bioprinting has made progress in both hard and soft tissue engineering systems. Some specific tissue constructs such as functional nerve guide conduits, human knee meniscal scaffolds, sized vascular grafts, can be successfully produced in laboratories as proof of concept (Schöneberg et al., 2018; Huang et al., 2021; P B et al., 2022). At the same time, with the assistance of sacrificial molds, materials that are traditionally difficult to print can be utilized to manufacture fine structures. For example, ceramic materials play a vital role in bone tissue engineering, but its additive manufacturing is considered challenging. Chawich et al. creatively utilized a sacrificial honeycomb mold of polylactic acid (PLA), and they ultimately produced customizable stable Si-based 3D non-oxide cellular ceramic structures (El Chawich et al., 2022). Another team produced stiffness memory nanohybrid scaffolds from poly(urea-urethane) (PUU) solution which exhibits outstanding performance in long-term implantable cardiovascular devices but can not be printed directly (Wu et al., 2018). Of all the breakthroughs, the greatest concern is the potential for this technique to address problems where vascularization is a key issue.
Generally, indirect 3D bioprinting for blood vessels and vascularized tissue constructs includes two general aspects: channel fabrication and vascularization. Channel fabrication can be further divided into three steps: sacrificial mold fabrication, patrix fabrication, and sacrificial mold removal. Manufacture of the negative mold is designed to simulate the shape and extension direction of the vascular network in a solid columnar form. In patrix fabrication, the patrix is built around the sacrificial mold to form the final scaffold or construct. Afterwards, special steps are performed to remove the sacrificial mold, leaving isometric hollow channels inside the scaffold or construct. The detailed production process and precautions of each step have been described in excellent reviews (Lee and Yeong, 2016; Houben et al., 2017; Zhu et al., 2021). Figure 1 provides a brief overview of the printing process. Here we mainly introduce the techniques frequently used and bioink selection.
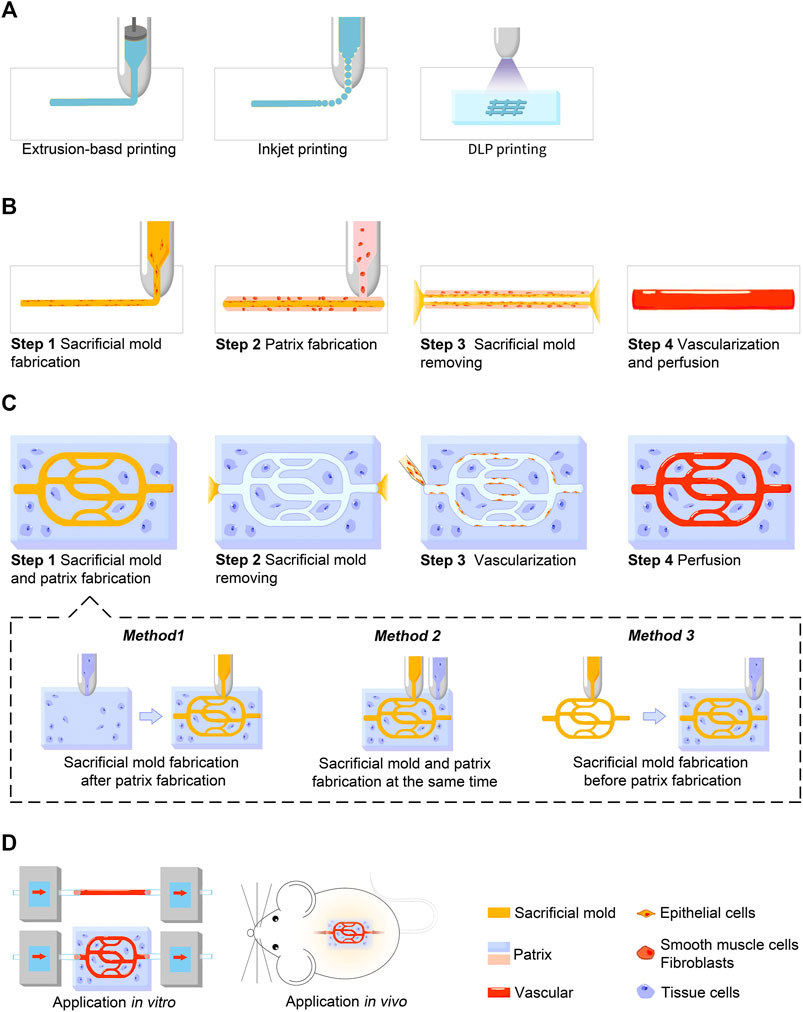
FIGURE 1. Schematic illustration showing the indirect 3D bioprinting process for blood vessels and vascularized tissue constructs. (A) Three major techniques used in indirect 3D bioprinting for both sacrificial mold and patrix fabrication, including extrusion-based printing, inkjet-based printing, and DLP printing. (B) Process of blood vessel fabrication. (C) Process of vascularized tissue construct fabrication. Sacrificial mold and patrix fabrication can be further divided into three methods according to the sequence of fabrication in step 1. (D) Applications in vitro or in vivo. Blood vessel grafts constructed by indirect 3D bioprinting are currently used for studies in vitro, while vascularized tissue constructs are used for both studies in vitro and animal experiments in vivo.
The three main techniques are extrusion-based, inkjet-based, and light-assisted 3D printing (Vijayavenkataraman et al., 2018). Each technique has advantages and disadvantages, and raise different requirements for bioinks. In extrusion-based printing, the most common technique, 3D structures are built layer by layer using a nozzle to dispense continuous filament (Štumberger and Vihar, 2018; Yang et al., 2020). This technique can achieve resolutions of >100 μm, uniform cell distribution, and moderate printing speed (Placone and Engler, 2018). The technique is compatible with various materials with a viscosity range of 30 mPa s to 6 × 107 mPa s (Murphy and Atala, 2014). Furthermore, its cell-friendly printing environment allows it to be used for printing scaffolds with high cell density (Skardal et al., 2015). One promising method is coaxial printing, also referred to as coaxial extrusion. Its key features are the concentric and multi-layered nozzles that enable concentric multi-material deposition. With the use of sacrificial materials, this technique allows synchronous manufacturing of the sacrificial mold and scaffold, simplifying the production process (Kjar et al., 2021). However, for extrusion-based printing, the shear stress produced during printing is an important cause of cell death and, because the nozzle size is inversely proportional to extrusion pressure, small nozzles used to improve resolution may have a greater negative impact on cell viability (Kyle et al., 2017).
Inkjet-based printing is defined as dispensing bioink through small orifices via piezoelectric, thermal, or electrostatic actuators, and accurately positioning a very small amount of the bioink on a substrate (Calvert, 2007; Datta et al., 2017). This technique can reach high resolutions of 10–100 μm with short printing times (Poellmann et al., 2011; Gudapati et al., 2016). Different inkjet printing techniques require different material viscosities. For thermal, piezoelectric, and electrostatic inkjet printing the ink viscosity should be relatively low, because the nozzle diameter is usually small and is prone to clogging (Li et al., 2020). However, for electrohydrodynamic jet bioprinting which produces droplets through an electric field, bioink with a high viscosity of over 2000 mPa s can be printed (Workman et al., 2014). Nozzle clogging, small nozzle aperture indirectly leading to cell viability damage, and low-viscosity bioink resulting in poor shape fidelity present challenges to broadening the range of implementation (Chahal et al., 2012; Malda et al., 2013; Zhu et al., 2017).
Light-assisted printing includes digital light processing (DLP)-based and laser-based printing. DLP printing is realized by dynamically projecting an entire computer-generated optical mask into a photosensitive prepolymer solution to induce photopolymerization, while laser-based printing uses a bottom-up approach (drop-by-drop) to build constructs (Zhu et al., 2021). DLP printing is now attracting greater attention as this technique provides precise control of scaffold structures and features and high printing speed (Mandrycky et al., 2016). Nevertheless, the selection of biomaterials is limited to photosensitive polymers and additional chemical modifications are required for most biomaterials (Yue et al., 2015). Moreover, the properties of printing complex patterns directly make DLP more frequently used in direct printing. Therefore, light-assisted printing has only been used in a few cases of indirect 3D printing (Thomas et al., 2020).
Another technique called embedded extrusion bioprinting involves printing a cell-containing hydrogel in a supporting bath, which serves as a sacrificial printing environment (Rocca et al., 2018). As atypical indirect 3D bioprinting, this technique does not impose any requirements for the shape of the sacrificial mold but instead skips this step and directly prints the male mold. The advantage is that low-viscosity hydrogels can be used to construct complex draping and hollow structures with the support of the sacrificial supporting bath via free-form bioprinting, which is difficult to achieve in air. Limitations are that shape fidelity of the complex pore structure is low, resolution is currently at the millimeter level, and the removal of the sacrificial supporting bath is relatively cumbersome (Afghah et al., 2020).
Table 1 summarizes the characteristics of the common techniques covered in this review.
Vascularization is essential for engineered tissue grafts to achieve biological function and can be realized by seeding ECs into hollow scaffold channels (Bae et al., 2012; Massa et al., 2017). After migration, survival, proliferation, and differentiation of ECs, a monolayer of cells is ultimately formed on the surface of the channels, mimicking the physiological vessel wall. Usually, growth factors and other bioactive factors are perfused along with ECs to regulate cell behavior or promote cell differentiation (Auger et al., 2013). The generation of new blood vessels involves complex effects of a variety of cells, factors released by platelets, extracellular matrix, and angiogenic and anti-angiogenic factors. Vascular endothelial growth factor (VEGF) and fibroblast growth factor b (FGFb) are the most effective angiogenic growth factors and are commonly used in the making of angiogenic biomaterials (Sun et al., 2011; Matsui and Tabata, 2012). Research reports successful seeding of human umbilical vein endothelial cells (HUVECs) into microchannels after mold sacrifice, and a uniform HUVEC layer formation coating the channels. It was demonstrated that the HUVEC layer could, to some extent, act as a barrier to protect scaffold cells from harmful substances (Massa et al., 2017). However, a potential problem is that the channel diameter may affect cell inoculation. Narrow channels will become blocked by ECs, hindering perfusion, while diameters too large will require more time for cells to cover the channel surface. Studies have shown that ideal channel diameter ranges from 280 to 1,270 μm (Zhang et al., 2016).
Another elegant vascularization strategy of indirect 3D bioprinting is to encapsulate ECs in the sacrificial ink to realize synchronous distribution of ECs in the process of blood vessel (sacrificial channel) fabrication. This approach reduces the manufacturing process and can realize precise control of cell distribution (Thomas et al., 2020). Owing to direct contact with cells and bioactive factors, biocompatibility and biodegradability of materials are particularly important. Studies have demonstrated that cells show increased proliferation and proliferation in hydrogels with faster degradation rates (Patterson and Hubbell, 2010). A further study suggests that biodegradable materials can promote angiogenesis through temporarily controlled delivery of siRNAs (Nelson et al., 2014). Application of biodegradable materials may better promote vascularization.
Biodegradable materials are the main scaffold materials in the field of tissue engineering, which have a broad range of applications in medicine and pharmacy because of good biosafety, reducing inflammation, ability to mimic the extracellular matrix (ECM), and enzymatic degradation in vivo (Asghari et al., 2017). Eventually these scaffolds are replaced by host tissues (Silva et al., 2020). Biodegradable materials are generally classified into synthetic materials and natural materials. Synthetic materials such as polyglycolic acid (PGA), polycaprolactone (PCL), PLA, poly (N-isopropylacrylamide), and their copolymers have already been widely used in tissue engineering. Advantages are that the key properties of these materials as scaffolds can be artificially controlled, such as degradation rate and some specific mechanical properties, including stiffness and elasticity; however, their biosafety is comparatively poor. Natural materials, such as chitin, alginate, collagen, and gelatin, are polymers produced by biological systems. Natural materials have many advantages and disadvantages. The most prominent advantage is their similarity to host tissues, including the ability to communicate with biological systems, enzymatic degradation, metabolic compatibility, and low inflammatory response (Asghari et al., 2017). However, owing to the poor strength of natural materials, scaffolds often collapse before finishing their task. In addition, they generally show slow and inhomogeneous degradation in vivo, and are inconsistent with host tissue regeneration rate.
In the field of indirect 3D printing, biodegradable polymers play vital roles in fabricating vascular or vascularized tissue: 1) As scaffold materials, they allow endothelial cells and a variety of angiogenic factors to exist, as well as providing a flexible environment for complex angiogenesis. 2) As sacrificial materials, compared with non-degradable ones, the damage to cell activity when manufacturing or removing the sacrificial molds is much lower. 3) Since the biodegradable materials can degrade in vivo, the use of them as sacrificial material helps to skip the processing step of sacrificial mold removal in vitro, thus shortening the manufacturing time. 4) In theory, by regulating and matching the degradation rate of scaffolds and sacrificial materials, the removal of sacrificial channels and channel endothelialization can be completed in vivo before the scaffolds completely degrade, thus realizing the direct in vivo application of constructs.
In the following section, we mainly enumerate the commonly used biodegradable polymers in indirect 3D bioprinting for blood vessels and vascularized tissue constructs. Table 2 summarizes the characteristics, combinations, and current applications of different scaffold and sacrificial materials.
Scaffold materials, whose purpose is to simulate the ECM and provide support for tissue regeneration in the human body, should have good biosafety, biocompatibility, biodegradability, and mechanical properties that are favorable for cell growth, proliferation, and migration (Lee A. Y. et al., 2014). Ideally, they should also be able to promote angiogenesis (Do et al., 2015; Xu et al., 2018). After crosslinking, scaffold materials need a certain degree of stiffness to maintain structural integrity during removal of sacrificial materials, and to support the flow of perfusion (Skylar-Scott et al., 2019). Controllable degradation rate consistent with the growth and repair rate of host tissue in vivo is also necessary (Malda et al., 2013; Serbo and Gerecht, 2013).
To date, various natural biodegradable polymers have been used for scaffold bioprinting, such as gelatin (Chen et al., 2012), fibrin (Schöneberg et al., 2018), and alginate (Jia et al., 2014); they are collectively referred to as hydrogels. They show excellent human ECM features and allow cell encapsulation (Malda et al., 2013), but they have all been deficient in some respect. Uncontrolled degradation and poor mechanical properties are the main problems, and they are also the research focus of material modification. The simplest and most used method is blending modification. In this approach, the hydrogel ratio is controlled to prevent excessive polymer concentration or dilution, which may have an adverse effect on cell behavior or mechanical properties (Malda et al., 2013; Duarte Campos et al., 2016; Zou et al., 2020).
Gelatin is one of the most widely used scaffold materials extracted from collagen, the main component of natural human ECM. Gelatin is composed of 85–92% protein, water, and mineral salts, and is highly susceptible to several proteases (Bello et al., 2020). Its excellent biocompatibility and similarity with collagen have made it the preferred material for the assembly of scaffolds. However, as a biomaterial ink, gelatin does have several drawbacks; these include low viscosity and yield stress, as well as relatively long crosslinking time, which leads to poor shape retention and structural collapse, and is the main obstacle to creating high resolution 3D pore or microchannel structures (O’Bryan et al., 2017). These disadvantages can be improved by making gelatin composites, or applying sacrificial materials to support the scaffold hydrogel before crosslinking (Moroni et al., 2018). In addition, the degradation rate of large solid gelatin in vivo is relatively slow (Daly et al., 2018). Large numbers of gelatin residues within thick tissues remain a challenge. Solutions may include lowering the degree of metacrylation and the macromer concentration (Nichol et al., 2010; Chen et al., 2012). Furthermore, by creating microchannels within the hydrogels to enhance host interaction, the degradation of gelatin can be enhanced (Daly et al., 2018). This is possibly because of the increased invasion by host immune cells, such as macrophages, which is vital for degrading gelatin hydrogels (Kim et al., 2014).
Gelatin-methacryloyl (GelMA) is the most commonly used material among gelatin derivatives and composites. GelMA is a photopolymerizable hydrogel made of gelatin derivatized with methacrylamide side groups (Van Den Bulcke et al., 2000). It has high biosafety compared with several gelatin-based hydrogels formed by chemical crosslinking methods, such as ones fabricated using glutaraldehyde or transglutimase derived from bacteria (Benton et al., 2009). GelMA is sensitive to matrix metalloproteinases and can be degraded by cells (Nichol et al., 2010). By adjusting the rate of polymerization and ratio of methacrylic acid, GelMA can maintain relatively good shape with adjustable mechanical properties (Benton et al., 2009). Research has shown that ECs as well as endothelial colony-forming cells can undergo active angiogenesis or vasculogenesis in GelMA hydrogels either in vivo or in vitro (Chen et al., 2012; Massa et al., 2017).
Fibrin scaffolds have a wide range of applications in tissue engineering, especially in bone tissue engineering. Fibrin is a natural biopolymer produced by thrombin cleavage of fibrinogen, and serves as a temporary scaffold for tissue healing in physiologic processes (Noori et al., 2017). It also plays an important role in specific receptor-mediated interactions with cells because of its ability to bind different types of proteins and growth factors, including FGF and VEGF that promote angiogenesis (Breen et al., 2009; Litvinov and Weisel, 2017). Fibrin hydrogel can be easily remodeled by ECs, which is favorable for fast angiogenesis. Nevertheless, similar to gelatin, fibrin rapidly degrades, and has poor mechanical stability, durability, and shape fidelity (Calderon et al., 2017). To overcome these problems, fibrin composites and mimics have been developed in 3D bioprinting. For example, by combining fibrin and gelatin, the stiffness of the hydrogel increased, and lower water loss on compression was observed (Schöneberg et al., 2018). Another attempt at a fibrin composite is called ELP-RGD, composed of ELP (elastin-like protein) hydrogel along with a cell adhesion RGD amino acid sequence derived from fibronectin. ELP hydrogel contains elastin (a kind of fibrin) -like repeat units alternating with biologically active domains (Madl et al., 2017). As a scaffold material, ELP-RGD has adjustable stiffness, is readily hydrolyzable with protease, and is able to promote matrix remodeling as well as cell proliferation (Chung et al., 2012). It has been demonstrated to be suitable for on-chip platforms with vascular-like networks (Duarte Campos et al., 2020).
Alginate is a natural polysaccharide extracted from alginic acid. The long polysaccharide chains provide it with pliability and gelling adeptness, and it undergoes hydrolytic cleavage under acidic conditions or enzymatic degradation by lyase (Pawar and Edgar, 2012; Rastogi and Kandasubramanian, 2019). Despite its mechanical instability and poor cell attachment, alginate is widely used as a hydrogel because of its low cost, good biosafety, and its ability to be rapidly but reversibly crosslinked by Ca2+ under mild conditions (Jia et al., 2016; Rastogi and Kandasubramanian, 2019). However, one important limitation of alginate application in vivo is the low degradation rate and unpredictable degradation process owing to the lack of alginate degrading enzyme in the human body (Reakasame and Boccaccini, 2018). Moreover, alginate shows relatively poor cell adhesion and infiltration (Balakrishnan et al., 2014). Measures like modification or mixing in additives, such as nanomaterials, peptides, and growth factors, are useful to regulate rheological properties, promote cell adhesion, or guide cell differentiation (Lee and Mooney, 2012; Piras and Smith, 2020). For example, by oxidative modification, more reactive sites are provided to the structure, accelerating the alginate’s biodegradability (Liang et al., 2011). Ino et al. combined sodium alginate with sacrificial molds of sugar structures, and by soaking the structure in a CaCl2 solution they achieved simultaneous dissolution of the mold and formation of calcium alginate hydrogel, thus simplifying and hastening the manufacturing process (Ino et al., 2020).
Silk fibroin (SF), a silk-derived protein-based material approved by the FDA (Perrone et al., 2014), has been used to make clinical sutures for many years. In recent decades, because of new processing techniques and further understanding of its properties, SF has attracted great interest in bone and cartilage engineering (Liu et al., 2013). Compared with the abovementioned materials, native silk fibers have excellent mechanical properties including good strength and toughness (Kundu et al., 2013; M et al., 2017). Other advantages of SF include good biosafety, good biocompatibility, controllable biodegradability and bone induction, and low immunogenicity (Wenk et al., 2009; Kundu et al., 2013). The host immune system has been shown to play a significant role in the degradation of SF scaffolds. Macrophages mediate the process, suggesting that SF is also bioresorbable (Wang et al., 2008). It is worth noting that SF remains strong during degradation, which is its unique advantage in tissue engineering (Kundu et al., 2013). Studies were carried out to produce vascularized scaffolds via SF. In combination with indirect 3D bioprinting, a layered SF-bioactive glass composite scaffold with excellent compressive strength, flexibility, and 10–50 μm micropores has been fabricated (Bidgoli et al., 2019). The scaffold comprises hierarchically micro and sub-micro pores, which are important features for promoting cell migration, differentiation, bone formation, and angiogenesis (Qi et al., 2018); results showed that the scaffold enhanced cell adhesion and cell proliferation (Bidgoli et al., 2019). However, drawbacks of SF such as lack of biological activity, general poor performance under humid conditions, and the difficulty of transportation and long-term storage may limit its further application. These limitations are potential future research directions.
Other scaffold materials commonly used in indirect 3D bioprinting but that are not yet, or rarely, used for vasculature fabrication include polyethylene glycol (PEG), hyaluronic acid (HA), PLA, PCL, poly (l-lactide-co-ɛ-caprolactone) (PLCL), and poly(lactic-co-glycolic) acid (PLGA) (Park et al., 2014; Houben et al., 2016; Aljohani et al., 2018; Im et al., 2018). Inorganic substances are more frequently used for osteochondral tissue fabrication because of their excellent mechanical properties. Future studies on these materials can be performed in the field of indirect 3D bioprinting for tissue vascularization.
Ideal sacrificial materials should have good fluidity for free molding, rapid solidification to save printing time, a low expansion rate, and appropriate mechanical strength to achieve good shape fidelity. During the process of printing, there should be no adverse reactions with the scaffold material that result in deformation of the scaffold structure. If working in combination with scaffold materials containing cells or other bioactive factors, sacrificial materials should be chosen to ensure non-toxic and non-stimulatory conditions. Also, the conditions for their state transformation and removal should be mild and easy to achieve, preserving the shape and properties of the scaffold. When exposed to living cells in vivo or bioactive components, cytotoxicity, biocompatibility, and whether the removal conditions are inconducive are usually considered first.
Currently the commonly used sacrificial materials that come closest to meeting the conditions described earlier are carbohydrate glass (Miller et al., 2012), Pluronics (Afghah et al., 2020), and PVA (Zou et al., 2020). Carbohydrate glass and Pluronics are nonbiodegradable materials. Carbohydrate glass is a simple glass consisting of a mixture of carbohydrates, including glucose, sucrose, and dextran, and was one of the first materials applied to indirect 3D bioprinting as a sacrificial biomaterial ink (Miller et al., 2012). The synthetic glass shows sufficient mechanical stiffness to maintain its shape in air, as well as rapid dissolution to accelerate the process. Results demonstrated that it can be compatible with a variety of natural or synthetic hydrogel materials, such as agarose, alginate, fibrin, and Matrigel, adapting well to their different properties and means of crosslinking (Miller et al., 2012). Currently, vessels with diameters ranging from 150 μm to 1 mm and smooth in-plane junctions can be achieved with this sacrificial ink (Pollet et al., 2019). Pluronics are a class of amphiphilic tri-block copolymers popular in drug and clinical applications. Because they have the characteristics of solubilizer, emulsifier, and stabilizer, they are often used as excipients in pharmaceutical preparations (Jarak et al., 2020). The most frequently used Pluronic in tissue engineering is Pluronic F127, which can rapidly dissolve in aqueous media or biological fluids. Its sol-gel transition at room temperature and convenient removal attracts attention as a sacrificial ink. Channels with a diameter as small as 150 μm can now be printed with Pluronic F127 (Homan et al., 2016). However, both carbohydrate glass and Pluronic F127 show cytotoxicity when dissolved, which is one of the most prominent shortcomings (Miller et al., 2012; Deng et al., 2020). Besides, Pluronic F127 liquifies at low temperatures, making it difficult to use with some scaffold materials that require these temperatures during casting, such as collagen and matrix gelatin (Ding and Chang, 2018; Hu et al., 2018).
As mentioned above, nonbiodegradable materials usually exhibit certain cytotoxicity and their removal is relatively cumbersome. As a result, researchers have investigated biodegradable materials, which show higher biosafety and are expected to achieve direct application in vivo.
PVA, a commonly used biodegradable sacrificial material, has satisfactory biocompatibility and similar functions to natural tissues, including high water content, high elasticity, and low interfacial tension with biological fluids (Teodorescu et al., 2019). It shows resistance to protein absorption, which is important for bone formation (Kim et al., 2018). The ease of printability allows it to be used for repeatable fabrication of complex vascular patterns (Hu et al., 2018). In terms of mechanical properties, PVA has good strength and stiffness at 25°C (Charron et al., 2019) and fits well with different biodegradable natural polymers, such as gelatin and silk (Mohanty et al., 2016). Tocchio et al. used PVA successfully to make sacrificial templates with characteristic sizes of 100–500 μm in multi-branch structures, which helped to further simulate the complex environment of cell growth, and showed great potential for production of large-sized vascularized scaffolds that would meet clinical needs (Tocchio et al., 2015). Compared to other sacrificial materials, the stable chemical properties, convenience of preservation, and low cost of PVA are huge advantages for its use in industrial production. However, owing to the lack of bioactive components, PVA tends to be resistant to protein absorption and cell adhesion, which limits its further application outside bone tissue engineering (Schmedlen et al., 2002). Furthermore, if PVA filaments are too large in diameter (>500 μm reported), they are likely to deform as they cannot support their own weight (Hernández-Córdova et al., 2016). More in-depth research is needed in the future.
HA-based enzymatically degradable photoink was developed by different research teams (Zhu et al., 2017; Thomas et al., 2020). HA is a linear polysaccharide. It is an essential component of the ECM and has vital effects on many cellular responses, including cellular signaling, wound repair, morphogenesis, and angiogenesis (Burdick and Prestwich, 2011). In the abovementioned two experiments, HA was chemically modified to achieve photopolymerization and mixed with gelatin to form the photoink. The hydrogels could be digested with hyaluronidase to achieve fast (within hours) and collaborative (not limited by graft size) degradation. Channels ranging from 50 to 720 µm have been successfully fabricated and achieved vascularization. However, the enzymolysis process may reduce EC activity, and because the enzyme is encapsulated in the bioink, degradation occurs at the same time as printing, which exerts a certain amount of time pressure (Zhu et al., 2017; Thomas et al., 2020).
Other research teams have also produced alternate solutions; for example, agarose was used as a sacrificial ink (Massa et al., 2017). It shows good rheological properties and printability similar to Pluronics when combined with alginate (López-Marcial et al., 2018). Currently, agarose has been shown capable of fabricating 100–1,000 μm diameter pipes and forming a smooth channel surface for cell inoculation (Massa et al., 2017). Another example is gelatin, that has been used as a sacrificial material and is removed by warming (Skylar-Scott et al., 2019). Channels over 400 μm in diameter could be printed with high fidelity. Another team used alginate and CaCl2 to create ultrafine fibers with a size range of 150–200 μm, and clear, interconnected microchannel structures were observed through the hydrogel as a result (Hammer et al., 2014). It is worth mentioning that some of the removal processes of these biodegradable sacrificial materials are physical processes. The utilization of their biodegradable properties needs to be further explored, as they show great potential to facilitate simplified fabrication processes and direct in vivo application of tissue constructs.
When 3D printing techniques print with cells, the printing ink is also referred to as bioink. It includes cells, biomaterials that serve as a cell-delivery medium, and biological factors (Groll et al., 2018). The existence of cells and biological factors require higher biosafety of the materials to maintain good bioactivity of the bioinks as discussed before. Also, to prevent cells from excessive shear forces, low viscosity fluid is required. Viscosity and rheological properties greatly influence the printability of the bioinks, and they are mainly determined by the molecular weight and concentration of polymer in solution (Schwab et al., 2020). Gels with shear-thinning properties or solutions containing hydrogel precursors are preferred. In current studies of indirect 3D bioprinting for vascular systems, HUVECs, smooth muscle cells (SMCs), and fibroblasts have been used, and the cell viability is generally over 80% with high cell density (Table 3). Still, more efforts should be put on developing optimized bioinks in future investigations.
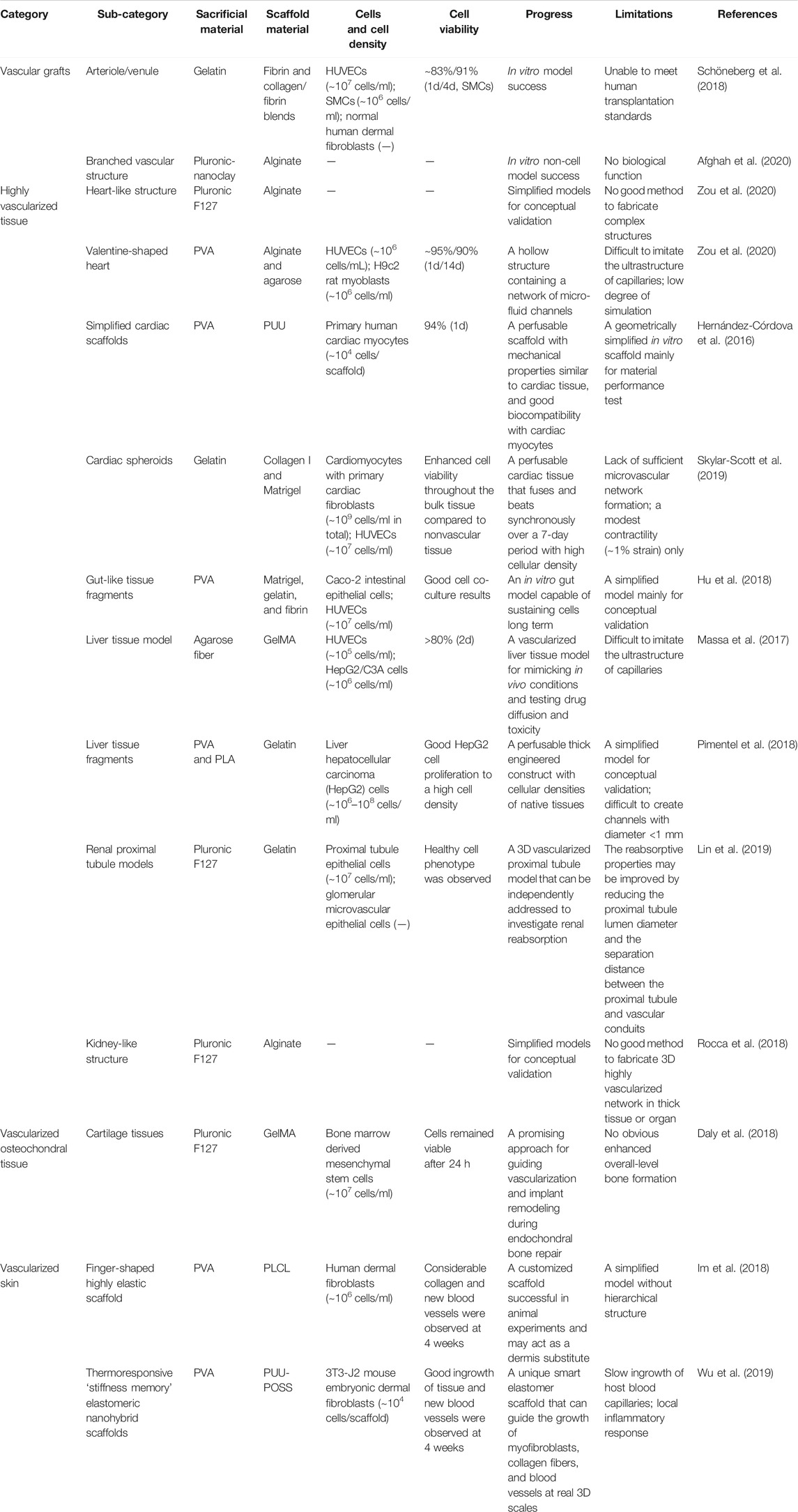
TABLE 3. Summary of indirect 3D bioprinting applications and bioink selection for different tissue vascularization covered in this review.
Nowadays, only a few tissues with less stringent vascular structures, such as cartilage and the cornea, have achieved good clinical outcomes (Rouwkema et al., 2010; Seifu and Mequanint, 2012; Pimentel et al., 2018). Advanced techniques and suitable biomaterials are essential for the development of functional engineered tissues. Indirect 3D bioprinting and the related biodegradable inks provide new opportunities for commercial development. The process has been applied to manufacturing various tissues and has achieved corresponding results. In general, our appraisal can be divided into vascular grafts and vascularized tissue; the latter includes highly vascularized tissue, vascularized osteochondral tissue, and vascularized skin, as shown in Table 3.
Most vascular regions treated during surgery are larger than 1 mm in diameter, which, theoretically, both direct and indirect 3D bioprinting can achieve. Biomaterial selection requirements for tissue-engineered vascular grafts are low immunogenicity, good mechanical properties, and similarity to native tissue characteristics (Liu et al., 2018). Compared with non-degradable polymers, biodegradable natural polymers (such as decellularized tissue scaffolds and fibrin) have relatively poor mechanical properties, but they have lower antigenicity and can better simulate natural tissue structures (Aper et al., 2016). Meanwhile, biodegradable synthetic polymers (such as PGA and PCL) can have adjustable mechanical properties and degradation rate, and they are considered the ideal biomaterials for tissue-engineered vascular grafts (Liu et al., 2018). Afghah et al. used embedded extrusion bioprinting with a composite Pluronic-nanoclay support-bath and biocompatible alginate to create a branched vascular structure with diameters of several millimeters. This vascular mold showed good mechanical properties and preservation of shape fidelity after removal from the support-bath, but its biological functions have not yet been verified (Afghah et al., 2020). While Schöneberg et al. used the indirect bioprinting technique to fabricate biofunctional multi-layered blood vessel models in vitro, which direct printing cannot yet achieve (Schöneberg et al., 2018). They used three different degradable hydrogels with three different cell types to simulate and reconstruct the adventitia (fibroblast matrix), medial layer (elastic SMC), and intima (endothelium), and successfully replicate the three-layered natural vascular channel structure with a wall thickness of up to 425 μm, and diameters of around 1 mm (Figure 2). These bioinks provide a friendly living environment for cells. Currently, engineered vascular grafts do not meet human transplantation standards, and most of them are used for in vitro experiments, such as drug prescreening or preliminary concept verification.
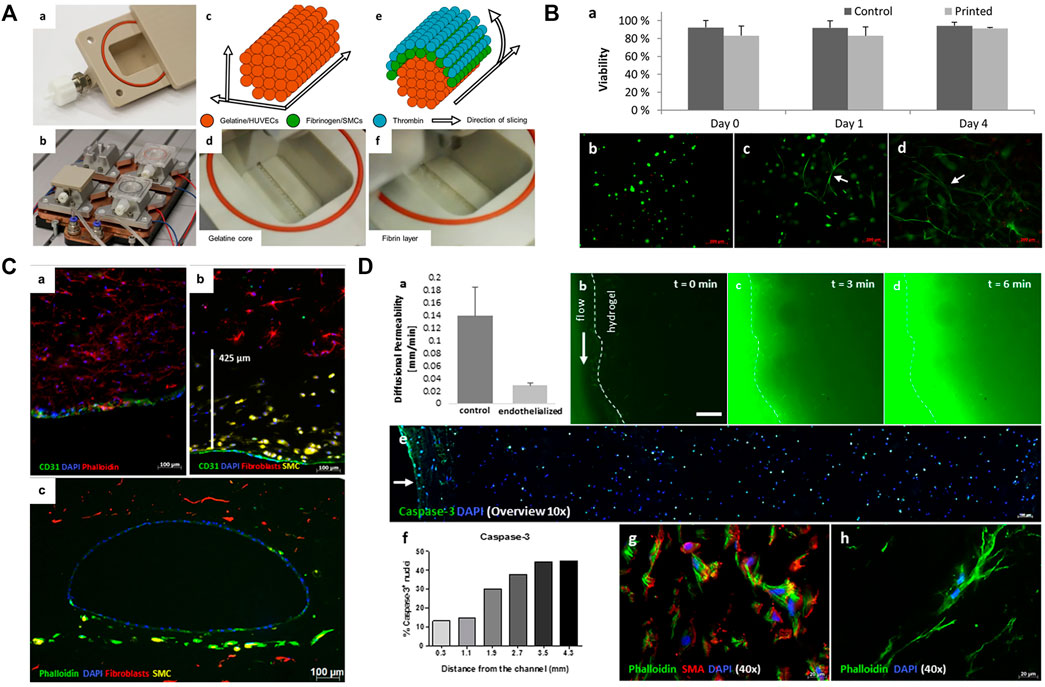
FIGURE 2. Printing of blood vessels with multi-layered structure. (A) Schematic diagram showing the printing procedure in a bioreactor. The printing includes a gelatin core and a surrounding fibrin layer. (B) Results showed that cell viability was not affected after the printing process. (C) Fluorescence micrographs show the homogenous distribution and good combination of ECs, SMCs, and fibroblasts. (D) Permeability testing and cell viability evaluation. Adapted with permission (Schöneberg et al., 2018). Copyright 2018, Nature Publishing Group.
Currently, most studies are in the conceptual validation phase and, to date, there are no good methods or compatible bioinks to fabricate a 3D functional, highly vascularized network in thick tissue or organ constructs, which limits the development of tissue engineering (Duan, 2017).
For highly vascularized tissue, such as liver, kidney, and heart, more precise microvessels are required, ranging from a few microns to millimeters. However, the current techniques have difficulty achieving this accuracy and resolution. Zou et al. created a valentine-shaped synthetic heart with a simplified aorta by using indirect 3D bioprinting and biodegradable alginate (Zou et al., 2020). They demonstrated no collapse of the scaffold structure and 90% cell viability. Nevertheless, the constructed microchannels are still at the level of hundreds of microns, and it remains difficult to emulate the ultrastructure of capillaries. An in vitro liver model (GelMA loaded with HepG2/C3A) was developed to test drug toxicity (Massa et al., 2017), and 3D human heart- and kidney-like objects, composed of dozens of alginate layers, were produced by embedded extrusion bioprinting (Rocca et al., 2018); however, these models were about 2 cm3 in size, with very simple vascular structure, and were used only for concept validation. Lin et al. used indirect 3D bioprinting to fabricate adjacent open cavities (representing proximal renal tubules and parallel blood vessels) embedded in the permeable ECM. After endothelialization and epithelization, the selective reabsorption and vectorial transmission of solute were realized through external circulation devices, which favor the further study of tissue-engineered kidneys (Lin et al., 2019).
There are also some special needs for biomaterials. Highly vascularized tissues usually consist of large amounts of active cells, which require the biomaterials to be sufficiently friendly to cells and cell migration, growth, and proliferation, and should facilitate substance exchange (Xie et al., 2020). Usually, softer biomaterials are needed; indeed, one important requirement for cardiac scaffolds is that it should not provide resistance to muscle contraction during systole while providing mechanical support to resist the tensile stress during diastole (Hernández-Córdova et al., 2016). This requires the elastic modulus of the biomaterial to match that of the myocardial reported interval (7.9–1,200 kPa) (Courtney et al., 2006; Chan et al., 2013; Hernández-Córdova et al., 2016). Alginate, gelatin, fibrin, and collagen can be used for cardiac scaffolds (Alonzo et al., 2019). In an indirect 3D bioprinting case, PUU was developed as a scaffold material that had suitable elastic modulus. They also used PVA as sacrificial material, and finally created a cardiac scaffold containing 300–500 µm channels. The biomaterial showed good biocompatibility with cardiomyocytes (Hernández-Córdova et al., 2016). Meanwhile, to verify the feasibility of multicellular tissue printing, Skylar-Scott et al. reported an embedded indirect 3D bioprinting method to create perfusable vascular channels in ECM solution with organ building blocks (OBBs) composed of thousands of patient-specific-induced pluripotent stem cell–derived organoids, as shown in Figure 3. As an example, they then fabricated a cardiac tissue with physiological functions over a 7-day period with high cellular density, showing the huge prospects of indirect 3D bioprinting (Skylar-Scott et al., 2019).
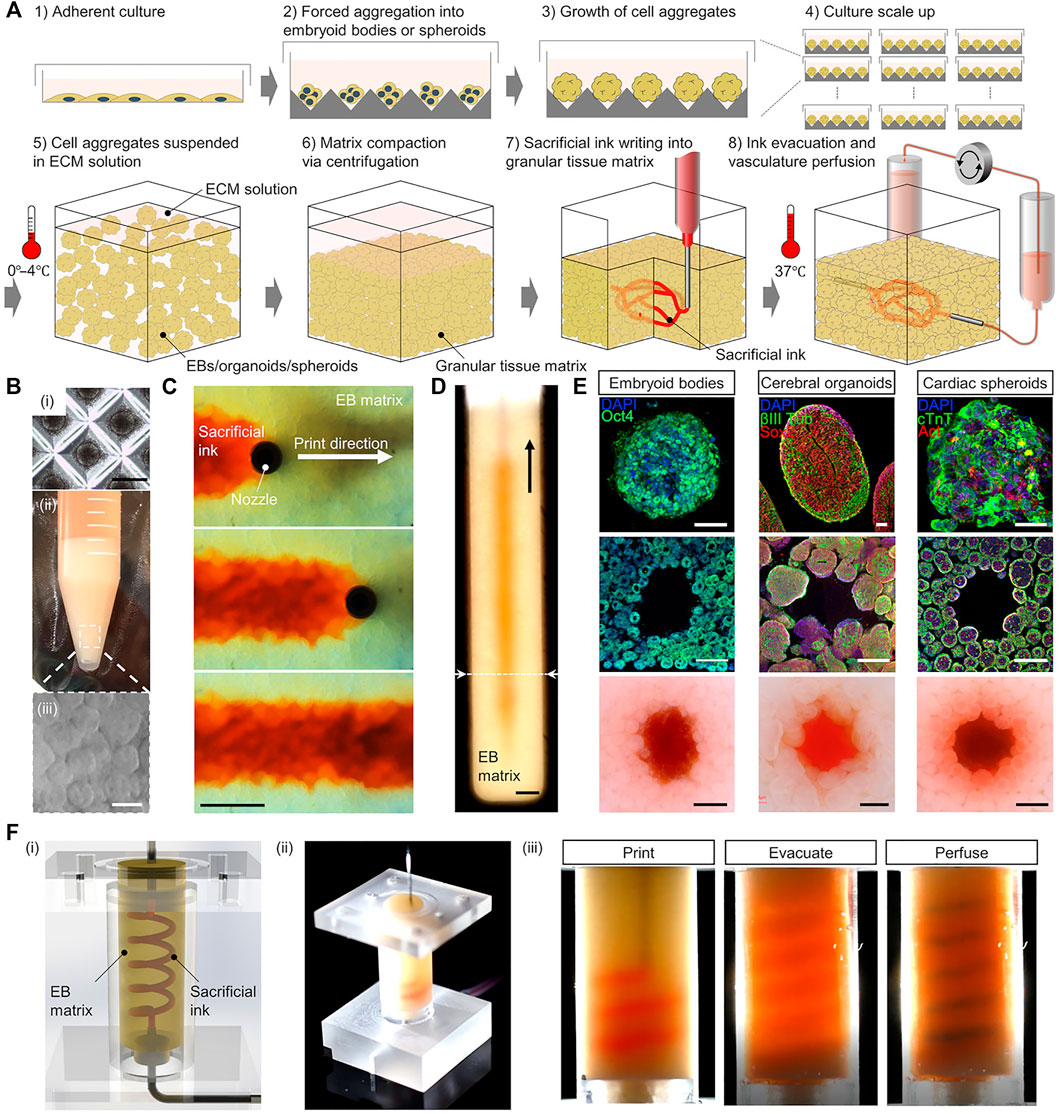
FIGURE 3. Printing of highly vascularized tissues with high cell density. (A) Schematic diagram of the indirect 3D bioprinting workflow. (B) Organ building block (OBB) tissue matrix formation. (C,D) Sacrificial ink writing within an embryoid body (EB) matrix. (E) Examples of different OBB-based matrices. (F) Fabrication of a helical vascular structure in an EB matrix. Reproduced with permission (Skylar-Scott et al., 2019). Copyright 2019, American Association for the Advancement of Science (AAAS).
The 3D printing technique is relatively mature in the field of osteochondral tissue engineering and has been used in the fabrication of long bones, mandibles, cheekbones, human finger bones, and other structures (Hollister et al., 2005; Lee et al., 2005; Wang et al., 2009). Biomaterials for osteochondral tissue engineering emphasize mechanical properties (Daly et al., 2017). For the design and production of vascularized bone tissue engineering scaffolds, pore-forming agents are usually added to form pores of specific sizes (>300 μm) to promote angiogenesis (Karageorgiou and Kaplan, 2005; Wang J.-Q. et al., 2019; Gonzalez-Fernandez et al., 2019). Uniform channels of controlled size that simulate the natural morphology of bone tissue, including Volkman’s and Haversian canals for better osteogenesis, are more and more created in molds via indirect 3D bioprinting (Houben et al., 2016; Houben et al., 2017). However, few studies have focused on the analysis of angiogenesis and new bioink development.
For endochondral bone repair, current techniques enable in vivo angiogenesis around cartilage models (Thompson et al., 2016), but the internal regions of the models remain nonvascular (Mesallati et al., 2015). Daly et al. constructed a microchanneled cartilage template using sacrificial Pluronic ink. After in vivo cultivation, they found that, compared to solid templates, channeled templates showed better vascularization, more degradation of cartilage precursor hydrogel in the core region, less ectopic bone formation, and better integration with the host tissue, which are all clinically important (Daly et al., 2018). However, more evidence is needed to demonstrate a difference in total bone formation between the channeled and solid templates for endochondral bone repair.
Creating man-made skin grafts for wounds and burn healing is the primary purpose of skin tissue engineering (Adams and Ramsey, 2005). Tissue-engineered skin grafts should be non-toxic, have low inflammatory response, allow water vapor transmission, and act as a barrier. They are also expected to quickly adhere to the wound surface, have controllable degradation, and promote angiogenesis (MacNeil, 2007). Biodegradable, non-toxic, non-immunogenic, and non-inflammatory biomaterials with low risk of disease transmission and easy access are ideal skin substitutes (Vig et al., 2017). To date, indirect 3D bioprinting has not been widely applied in vascularized skin tissue fabrication. Wu et al. created a thermoresponsive stiffness memory elastomer nanohybrid scaffold via indirect 3D bioprinting and found that the scaffold promoted fibroblast proliferation in vitro and angiogenesis in vivo (Wu et al., 2019). Another team found that by combining bioactive peptide hydrogels with scaffolds having finger-shaped pores created via indirect 3D bioprinting, more vessels and more collagen I and III formed in the scaffolds (Im et al., 2018). Most studies that focused on tissue design have been based on simplified skin models, while few models consider controlled porosity, biodegradable material selection, and cell distribution (Lee V. et al., 2014; Yan et al., 2018). It is important to mention that for the reconstruction of simple epidermis or thin dermis, vascularization is not necessary.
As described in this paper, tissue vascularization has always been a critical issue in tissue engineering and is key to the application and survival of engineered tissue constructs in vivo. Many accomplishments have demonstrated the feasibility of indirect 3D bioprinting for manufacturing blood vessels and vascularized tissue constructs, and most experiments show that indirect 3D bioprinting has advantages in channel structure design and construction. On the other hand, the selection of biodegradable inks is an important aspect of indirect 3D bioprinting for successful 3D tissue construct fabrication and in vitro/vivo application. Theoretically, indirect 3D bioprinting allows a wider range of materials to be used, since the use of sacrificial materials lowers the mechanical performance requirements for scaffold materials. However, this introduces new requirements for the sacrificial materials. At present, owing to the lack of satisfactory biodegradable materials, further in vivo applications are limited. A large number of studies are investigating advanced sacrificial/scaffold bioinks, which assists in the assembly of biodegradable, biosafe, bioactive, and more bionic structures at higher resolution. Efforts are now transitioning from theoretical verification to tissue and organ model construction. We expect that with the future continuous development of biodegradable materials, the use of indirect 3D bioprinting will continue to increase and will contribute to the field of tissue engineering.
JL and YY conceived and coordinated this project. YZ and YL wrote this article. LH and YS collected and summarized literatures. PL edited pictures in this paper. PG revised this paper. All authors contributed to manuscript revision, read and approved the submitted version.
We thank the financial support from the Sichuan University-Luzhou City Cooperaton Program (No.2021CDLZ-8), the Horizontal Project of Sichuan University (No. 21H0441), and the College Students’ Innovative Entrepreneurial Training Plan Program in Sichuan University (No.20221220L).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YD declared a shared affiliation, with no collaboration, with all of the authors to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdollahi, S., Boktor, J., and Hibino, N. (2019). Bioprinting of Freestanding Vascular Grafts and the Regulatory Considerations for Additively Manufactured Vascular Prostheses. Translational Res. 211, 123–138. doi:10.1016/j.trsl.2019.05.005
Adams, D. C., and Ramsey, M. L. (2005). Grafts in Dermatologic Surgery: Review and Update on Full- and Split-Thickness Skin Grafts, Free Cartilage Grafts, and Composite Grafts. Dermatol. Surg. 31 (8 Pt 2), 1055–1067. doi:10.1111/j.1524-4725.2005.31831
Afghah, F., Altunbek, M., Dikyol, C., and Koc, B. (2020). Preparation and Characterization of Nanoclay-Hydrogel Composite Support-bath for Bioprinting of Complex Structures. Sci. Rep. 10 (1), 5257. doi:10.1038/s41598-020-61606-x
Aljohani, W., Ullah, M. W., Zhang, X., and Yang, G. (2018). Bioprinting and its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromolecules 107 (Pt A), 261–275. doi:10.1016/j.ijbiomac.2017.08.171
Alonzo, M., AnilKumar, S., Roman, B., Tasnim, N., and Joddar, B. (2019). 3D Bioprinting of Cardiac Tissue and Cardiac Stem Cell Therapy. Translational Res. 211, 64–83. doi:10.1016/j.trsl.2019.04.004
Aper, T., Wilhelmi, M., Gebhardt, C., Hoeffler, K., Benecke, N., Hilfiker, A., et al. (2016). Novel Method for the Generation of Tissue-Engineered Vascular Grafts Based on a Highly Compacted Fibrin Matrix. Acta Biomater. 29, 21–32. doi:10.1016/j.actbio.2015.10.012
Asakawa, N., Shimizu, T., Tsuda, Y., Sekiya, S., Sasagawa, T., Yamato, M., et al. (2010). Pre-vascularization of In Vitro Three-Dimensional Tissues Created by Cell Sheet Engineering. Biomaterials 31 (14), 3903–3909. doi:10.1016/j.biomaterials.2010.01.105
Asghari, F., Samiei, M., Adibkia, K., Akbarzadeh, A., and Davaran, S. (2017). Biodegradable and Biocompatible Polymers for Tissue Engineering Application: a Review. Artif. Cell Nanomedicine, Biotechnol. 45 (2), 185–192. doi:10.3109/21691401.2016.1146731
Auger, F. A., Gibot, L., and Lacroix, D. (2013). The Pivotal Role of Vascularization in Tissue Engineering. Annu. Rev. Biomed. Eng. 15, 177–200. doi:10.1146/annurev-bioeng-071812-152428
Bae, H., Puranik, A. S., Gauvin, R., Edalat, F., Carrillo-Conde, B., Peppas, N. A., et al. (2012). Building Vascular Networks. Sci. Transl. Med. 4 (160), 160ps123. doi:10.1126/scitranslmed.3003688
Balaji, S., King, A., Crombleholme, T. M., and Keswani, S. G. (2013). The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing. Adv. Wound Care 2 (6), 283–295. doi:10.1089/wound.2012.0398
Balakrishnan, B., Joshi, N., Jayakrishnan, A., and Banerjee, R. (2014). Self-crosslinked Oxidized Alginate/gelatin Hydrogel as Injectable, Adhesive Biomimetic Scaffolds for Cartilage Regeneration. Acta Biomater. 10 (8), 3650–3663. doi:10.1016/j.actbio.2014.04.031
Baranski, J. D., Chaturvedi, R. R., Stevens, K. R., Eyckmans, J., Carvalho, B., Solorzano, R. D., et al. (2013). Geometric Control of Vascular Networks to Enhance Engineered Tissue Integration and Function. Proc. Natl. Acad. Sci. U.S.A. 110 (19), 7586–7591. doi:10.1073/pnas.1217796110
Bello, A. B., Kim, D., Kim, D., Park, H., and Lee, S.-H. (2020). Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. B: Rev. 26 (2), 164–180. doi:10.1089/ten.TEB.2019.0256
Benton, J. A., DeForest, C. A., Vivekanandan, V., and Anseth, K. S. (2009). Photocrosslinking of Gelatin Macromers to Synthesize Porous Hydrogels that Promote Valvular Interstitial Cell Function. Tissue Eng. A 15 (11), 3221–3230. doi:10.1089/ten.TEA.2008.0545
Bertassoni, L. E., Cecconi, M., Manoharan, V., Nikkhah, M., Hjortnaes, J., Cristino, A. L., et al. (2014). Hydrogel Bioprinted Microchannel Networks for Vascularization of Tissue Engineering Constructs. Lab. Chip 14 (13), 2202–2211. doi:10.1039/c4lc00030g
Berthiaume, F., Maguire, T. J., and Yarmush, M. L. (2011). Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2, 403–430. doi:10.1146/annurev-chembioeng-061010-114257
Bidgoli, M. R., Alemzadeh, I., Tamjid, E., Khafaji, M., and Vossoughi, M. (2019). Fabrication of Hierarchically Porous Silk Fibroin-Bioactive Glass Composite Scaffold via Indirect 3D Printing: Effect of Particle Size on Physico-Mechanical Properties and In Vitro Cellular Behavior. Mater. Sci. Eng. C 103, 109688. doi:10.1016/j.msec.2019.04.067
Breen, A., O'Brien, T., and Pandit, A. (2009). Fibrin as a Delivery System for Therapeutic Drugs and Biomolecules. Tissue Eng. Part B: Rev. 15 (2), 201–214. doi:10.1089/ten.TEB.2008.0527
Burdick, J. A., and Prestwich, G. D. (2011). Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 23 (12), H41–H56. doi:10.1002/adma.201003963
Calderon, G. A., Thai, P., Hsu, C. W., Grigoryan, B., Gibson, S. M., Dickinson, M. E., et al. (2017). Tubulogenesis of Co-cultured Human iPS-Derived Endothelial Cells and Human Mesenchymal Stem Cells in Fibrin and Gelatin Methacrylate Gels. Biomater. Sci. 5 (8), 1652–1660. doi:10.1039/c7bm00223h
Carmeliet, P., and Jain, R. K. (2000). Angiogenesis in Cancer and Other Diseases. Nature 407 (6801), 249–257. doi:10.1038/35025220
Chahal, D., Ahmadi, A., and Cheung, K. C. (2012). Improving Piezoelectric Cell Printing Accuracy and Reliability through Neutral Buoyancy of Suspensions. Biotechnol. Bioeng. 109 (11), 2932–2940. doi:10.1002/bit.24562
Chan, V., Raman, R., Cvetkovic, C., and Bashir, R. (2013). Enabling Microscale and Nanoscale Approaches for Bioengineered Cardiac Tissue. ACS Nano 7 (3), 1830–1837. doi:10.1021/nn401098c
Charron, P. N., Braddish, T. A., and Oldinski, R. A. (2019). PVA-gelatin Hydrogels Formed Using Combined Theta-Gel and Cryo-Gel Fabrication Techniques. J. Mech. Behav. Biomed. Mater. 92, 90–96. doi:10.1016/j.jmbbm.2019.01.002
Chen, Y.-C., Lin, R.-Z., Qi, H., Yang, Y., Bae, H., Melero-Martin, J. M., et al. (2012). Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 22 (10), 2027–2039. doi:10.1002/adfm.201101662
Cheng, G., Liao, S., Kit Wong, H., Lacorre, D. A., di Tomaso, E., Au, P., et al. (2011). Engineered Blood Vessel Networks Connect to Host Vasculature via Wrapping-And-Tapping Anastomosis. Blood 118 (17), 4740–4749. doi:10.1182/blood-2011-02-338426
Chiesa, I., De Maria, C., Lapomarda, A., Fortunato, G. M., Montemurro, F., Di Gesù, R., et al. (2020). Endothelial Cells Support Osteogenesis in an In Vitro Vascularized Bone Model Developed by 3D Bioprinting. Biofabrication 12 (2), 025013. doi:10.1088/1758-5090/ab6a1d
Chu, T.-M. G., Halloran, J. W., Hollister, S. J., and Feinberg, S. E. (2001). Hydroxyapatite Implants with Designed Internal Architecture. J. Mater. Sci. Mater. Med. 12 (6), 471–478. doi:10.1023/a:1011203226053
Chung, C., Anderson, E., Pera, R. R., Pruitt, B. L., and Heilshorn, S. C. (2012). Hydrogel Crosslinking Density Regulates Temporal Contractility of Human Embryonic Stem Cell-Derived Cardiomyocytes in 3D Cultures. Soft Matter 8 (39), 10141–10148. doi:10.1039/c2sm26082d
Courtney, T., Sacks, M., Stankus, J., Guan, J., and Wagner, W. (2006). Design and Analysis of Tissue Engineering Scaffolds that Mimic Soft Tissue Mechanical Anisotropy. Biomaterials 27 (19), 3631–3638. doi:10.1016/j.biomaterials.2006.02.024
Daly, A. C., Freeman, F. E., Gonzalez-Fernandez, T., Critchley, S. E., Nulty, J., and Kelly, D. J. (2017). 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 6 (22), 1700298. doi:10.1002/adhm.201700298
Daly, A. C., Pitacco, P., Nulty, J., Cunniffe, G. M., and Kelly, D. J. (2018). 3D Printed Microchannel Networks to Direct Vascularisation during Endochondral Bone Repair. Biomaterials 162, 34–46. doi:10.1016/j.biomaterials.2018.01.057
Datta, P., Ayan, B., and Ozbolat, I. T. (2017). Bioprinting for Vascular and Vascularized Tissue Biofabrication. Acta Biomater. 51, 1–20. doi:10.1016/j.actbio.2017.01.035
Deng, Q., Huang, S., Wen, J., Jiao, Y., Su, X., Shi, G., et al. (2020). PF-127 Hydrogel Plus Sodium Ascorbyl Phosphate Improves Wharton's Jelly Mesenchymal Stem Cell-Mediated Skin Wound Healing in Mice. Stem Cell Res Ther 11 (1), 143. doi:10.1186/s13287-020-01638-2
Ding, H., and Chang, R. C. (2018). Printability Study of Bioprinted Tubular Structures Using Liquid Hydrogel Precursors in a Support Bath. Appl. Sci. 8. doi:10.3390/app8030403
Do, A.-V., Khorsand, B., Geary, S. M., and Salem, A. K. (2015). 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 4 (12), 1742–1762. doi:10.1002/adhm.201500168
Duan, B. (2017). State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 45 (1), 195–209. doi:10.1007/s10439-016-1607-5
Duarte Campos, D. F., Blaeser, A., Buellesbach, K., Sen, K. S., Xun, W., Tillmann, W., et al. (2016). Bioprinting Organotypic Hydrogels with Improved Mesenchymal Stem Cell Remodeling and Mineralization Properties for Bone Tissue Engineering. Adv. Healthc. Mater. 5 (11), 1336–1345. doi:10.1002/adhm.201501033
Duarte Campos, D. F., Lindsay, C. D., Roth, J. G., LeSavage, B. L., Seymour, A. J., Krajina, B. A., et al. (2020). Bioprinting Cell- and Spheroid-Laden Protein-Engineered Hydrogels as Tissue-On-Chip Platforms. Front. Bioeng. Biotechnol. 8, 374. doi:10.3389/fbioe.2020.00374
El Chawich, G., El Hayek, J., Rouessac, V., Cot, D., Rebière, B., Habchi, R., et al. (2022). Design and Manufacturing of Si-Based Non-oxide Cellular Ceramic Structures through Indirect 3D Printing. Materials 15 (2), 471. doi:10.3390/ma15020471
Gonzalez-Fernandez, T., Rathan, S., Hobbs, C., Pitacco, P., Freeman, F. E., Cunniffe, G. M., et al. (2019). Pore-forming Bioinks to Enable Spatio-Temporally Defined Gene Delivery in Bioprinted Tissues. J. Controlled Release 301, 13–27. doi:10.1016/j.jconrel.2019.03.006
Groll, J., Burdick, J. A., Cho, D.-W., Derby, B., Gelinsky, M., Heilshorn, S. C., et al. (2018). A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 11 (1), 013001. doi:10.1088/1758-5090/aaec52
Gudapati, H., Dey, M., and Ozbolat, I. (2016). A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 102, 20–42. doi:10.1016/j.biomaterials.2016.06.012
Hammer, J., Han, L.-H., Tong, X., and Yang, F. (2014). A Facile Method to Fabricate Hydrogels with Microchannel-like Porosity for Tissue Engineering. Tissue Eng. C: Methods 20 (2), 169–176. doi:10.1089/ten.TEC.2013.0176
Hann, S. Y., Cui, H., Esworthy, T., Miao, S., Zhou, X., Lee, S.-j., et al. (2019). Recent Advances in 3D Printing: Vascular Network for Tissue and Organ Regeneration. Translational Res. 211, 46–63. doi:10.1016/j.trsl.2019.04.002
Hasan, A., Paul, A., Vrana, N. E., Zhao, X., Memic, A., Hwang, Y.-S., et al. (2014). Microfluidic Techniques for Development of 3D Vascularized Tissue. Biomaterials 35 (26), 7308–7325. doi:10.1016/j.biomaterials.2014.04.091
Hernández‐Córdova, R., Mathew, D. A., Balint, R., Carrillo‐Escalante, H. J., Cervantes‐Uc, J. M., Hidalgo‐Bastida, L. A., et al. (2016). Indirect Three‐dimensional Printing: A Method for Fabricating Polyurethane‐urea Based Cardiac Scaffolds. J. Biomed. Mater. Res. 104 (8), 1912–1921. doi:10.1002/jbm.a.35721
Hollister, S., Lin, C., Saito, E., Lin, C., Schek, R., Taboas, J., et al. (2005). Engineering Craniofacial Scaffolds. Orthod. Craniofac. Res. 8 (3), 162–173. doi:10.1111/j.1601-6343.2005.00329.x
Homan, K. A., Kolesky, D. B., Skylar-Scott, M. A., Herrmann, J., Obuobi, H., Moisan, A., et al. (2016). Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 6, 34845. doi:10.1038/srep34845
Houben, A., Pien, N., Lu, X., Bisi, F., Van Hoorick, J., Boone, M. N., et al. (2016). Indirect Solid Freeform Fabrication of an Initiator-free Photocrosslinkable Hydrogel Precursor for the Creation of Porous Scaffolds. Macromol. Biosci. 16 (12), 1883–1894. doi:10.1002/mabi.201600289
Houben, A., Van Hoorick, J., Van Erps, J., Thienpont, H., Van Vlierberghe, S., and Dubruel, P. (2017). Indirect Rapid Prototyping: Opening up Unprecedented Opportunities in Scaffold Design and Applications. Ann. Biomed. Eng. 45 (1), 58–83. doi:10.1007/s10439-016-1610-x
Hu, M., Dailamy, A., Lei, X. Y., Parekh, U., McDonald, D., Kumar, A., et al. (2018). Facile Engineering of Long‐Term Culturable Ex Vivo Vascularized Tissues Using Biologically Derived Matrices. Adv. Healthc. Mater. 7 (23), 1800845. doi:10.1002/adhm.201800845
Huang, Y., Wu, W., Liu, H., Chen, Y., Li, B., Gou, Z., et al. (2021). 3D Printing of Functional Nerve Guide Conduits. Burns Trauma 9, tkab011. doi:10.1093/burnst/tkab011
Im, H., Kim, S. H., Kim, S. H., and Jung, Y. (2018). Skin Regeneration with a Scaffold of Predefined Shape and Bioactive Peptide Hydrogels. Tissue Eng. Part A 24 (19-20), 1518–1530. doi:10.1089/ten.TEA.2017.0489
Ino, K., Fukuda, M. T., Hiramoto, K., Taira, N., Nashimoto, Y., and Shiku, H. (2020). Fabrication of Three-Dimensional Calcium Alginate Hydrogels Using Sacrificial Templates of Sugar. J. Biosci. Bioeng. 130 (5), 539–544. doi:10.1016/j.jbiosc.2020.06.014
Jarak, I., Varela, C. L., Tavares da Silva, E., Roleira, F. F. M., Veiga, F., and Figueiras, A. (2020). Pluronic-based Nanovehicles: Recent Advances in Anticancer Therapeutic Applications. Eur. J. Med. Chem. 206, 112526. doi:10.1016/j.ejmech.2020.112526
Ji, S., Almeida, E., and Guvendiren, M. (2019). 3D Bioprinting of Complex Channels within Cell-Laden Hydrogels. Acta Biomater. 95, 214–224. doi:10.1016/j.actbio.2019.02.038
Jia, J., Richards, D. J., Pollard, S., Tan, Y., Rodriguez, J., Visconti, R. P., et al. (2014). Engineering Alginate as Bioink for Bioprinting. Acta Biomater. 10 (10), 4323–4331. doi:10.1016/j.actbio.2014.06.034
Jia, W., Gungor-Ozkerim, P. S., Zhang, Y. S., Yue, K., Zhu, K., Liu, W., et al. (2016). Direct 3D Bioprinting of Perfusable Vascular Constructs Using a Blend Bioink. Biomaterials 106, 58–68. doi:10.1016/j.biomaterials.2016.07.038
Kang, H.-W., and Cho, D.-W. (2012). Development of an Indirect Stereolithography Technology for Scaffold Fabrication with a Wide Range of Biomaterial Selectivity. Tissue Eng. Part C: Methods 18 (9), 719–729. doi:10.1089/ten.tec.2011.0621
Kang, Y., Mochizuki, N., Khademhosseini, A., Fukuda, J., and Yang, Y. (2015). Engineering a Vascularized Collagen-β-Tricalcium Phosphate Graft Using an Electrochemical Approach. Acta Biomater. 11, 449–458. doi:10.1016/j.actbio.2014.09.035
Karageorgiou, V., and Kaplan, D. (2005). Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 26 (27), 5474–5491. doi:10.1016/j.biomaterials.2005.02.002
Kaully, T., Kaufman-Francis, K., Lesman, A., and Levenberg, S. (2010). Vascularization: The Conduit to Viable Engineered Tissues. Tissue Eng. Part. B Rev. 15 (2), 159–169. doi:10.1089/ten.teb.2008.0193
Khattak, S. F., Bhatia, S. R., and Roberts, S. C. (2005). Pluronic F127 as a Cell Encapsulation Material: Utilization of Membrane-Stabilizing Agents. Tissue Eng. 11 (5-6), 974–983. doi:10.1089/ten.2005.11.974
Kim, H., Yang, G. H., Choi, C. H., Cho, Y. S., and Kim, G. (2018). Gelatin/PVA Scaffolds Fabricated Using a 3D-Printing Process Employed with a Low-Temperature Plate for Hard Tissue Regeneration: Fabrication and Characterizations. Int. J. Biol. Macromolecules 120 (Pt A), 119–127. doi:10.1016/j.ijbiomac.2018.07.159
Kim, J. J., Hou, L., and Huang, N. F. (2016). Vascularization of Three-Dimensional Engineered Tissues for Regenerative Medicine Applications. Acta Biomater. 41, 17–26. doi:10.1016/j.actbio.2016.06.001
Kim, Y.-H., Furuya, H., and Tabata, Y. (2014). Enhancement of Bone Regeneration by Dual Release of a Macrophage Recruitment Agent and Platelet-Rich Plasma from Gelatin Hydrogels. Biomaterials 35 (1), 214–224. doi:10.1016/j.biomaterials.2013.09.103
Kjar, A., McFarland, B., Mecham, K., Harward, N., and Huang, Y. (2021). Engineering of Tissue Constructs Using Coaxial Bioprinting. Bioactive Mater. 6 (2), 460–471. doi:10.1016/j.bioactmat.2020.08.020
Ko, H., Milthorpe, B. K., Milthorpe, B., and McFarland, C. (2007). Engineering Thick Tissues - the Vascularisation Problem. eCM 14, 1–19. doi:10.22203/ecm.v014a01
Kolesky, D. B., Truby, R. L., Gladman, A. S., Busbee, T. A., Homan, K. A., and Lewis, J. A. (2014). 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 26 (19), 3124–3130. doi:10.1002/adma.201305506
Kundu, B., Rajkhowa, R., Kundu, S. C., and Wang, X. (2013). Silk Fibroin Biomaterials for Tissue Regenerations. Adv. Drug Deliv. Rev. 65 (4), 457–470. doi:10.1016/j.addr.2012.09.043
Kyle, S., Jessop, Z. M., Al-Sabah, A., and Whitaker, I. S. (2017). 'Printability' of Candidate Biomaterials for Extrusion Based 3D Printing: State-Of-The-Art. Adv. Healthc. Mater. 6 (16), 1700264. doi:10.1002/adhm.201700264
Laschke, M. W., and Menger, M. D. (2016). Prevascularization in Tissue Engineering: Current Concepts and Future Directions. Biotechnol. Adv. 34 (2), 112–121. doi:10.1016/j.biotechadv.2015.12.004
Laschke, M. W., and Menger, M. D. (2012). Vascularization in Tissue Engineering: Angiogenesis versus Inosculation. Eur. Surg. Res. 48 (2), 85–92. doi:10.1159/000336876
Lee, A. Y., Mahler, N., Best, C., Lee, Y.-U., and Breuer, C. K. (2014a). Regenerative Implants for Cardiovascular Tissue Engineering. Translational Res. 163 (4), 321–341. doi:10.1016/j.trsl.2014.01.014
Lee, J.-Y., Choi, B., Wu, B., and Lee, M. (2013). Customized Biomimetic Scaffolds Created by Indirect Three-Dimensional Printing for Tissue Engineering. Biofabrication 5 (4), 045003. doi:10.1088/1758-5082/5/4/045003
Lee, J. M., and Yeong, W. Y. (2016). Design and Printing Strategies in 3D Bioprinting of Cell-Hydrogels: A Review. Adv. Healthc. Mater. 5 (22), 2856–2865. doi:10.1002/adhm.201600435
Lee, K. Y., and Mooney, D. J. (2012). Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 37 (1), 106–126. doi:10.1016/j.progpolymsci.2011.06.003
Lee, M., Dunn, J. C. Y., and Wu, B. M. (2005). Scaffold Fabrication by Indirect Three-Dimensional Printing. Biomaterials 26 (20), 4281–4289. doi:10.1016/j.biomaterials.2004.10.040
Lee, M., Wu, B. M., and Dunn, J. C. Y. (2008). Effect of Scaffold Architecture and Pore Size on Smooth Muscle Cell Growth. J. Biomed. Mater. Res. 87A (4), 1010–1016. doi:10.1002/jbm.a.31816
Lee, V., Singh, G., Trasatti, J. P., Bjornsson, C., Xu, X., Tran, T. N., et al. (2014b). Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C: Methods 20 (6), 473–484. doi:10.1089/ten.TEC.2013.0335
Leong, M. F., Toh, J. K. C., Du, C., Narayanan, K., Lu, H. F., Lim, T. C., et al. (2013). Patterned Prevascularised Tissue Constructs by Assembly of Polyelectrolyte Hydrogel Fibres. Nat. Commun. 4. doi:10.1038/ncomms3353
Li, X., Liu, B., Pei, B., Chen, J., Zhou, D., Peng, J., et al. (2020). Inkjet Bioprinting of Biomaterials. Chem. Rev. 120 (19), 10793–10833. doi:10.1021/acs.chemrev.0c00008
Liang, Y., Liu, W., Han, B., Yang, C., Ma, Q., Song, F., et al. (2011). An In Situ Formed Biodegradable Hydrogel for Reconstruction of the Corneal Endothelium. Colloids Surf. B: Biointerfaces 82 (1), 1–7. doi:10.1016/j.colsurfb.2010.07.043
Lin, N. Y. C., Homan, K. A., Robinson, S. S., Kolesky, D. B., Duarte, N., Moisan, A., et al. (2019). Renal Reabsorption in 3D Vascularized Proximal Tubule Models. Proc. Natl. Acad. Sci. U.S.A. 116 (12), 5399–5404. doi:10.1073/pnas.1815208116
Litvinov, R. I., and Weisel, J. W. (2017). Fibrin Mechanical Properties and Their Structural Origins. Matrix Biol. 60-61, 110–123. doi:10.1016/j.matbio.2016.08.003
Liu, C. Z., Xia, Z. D., Han, Z. W., Hulley, P. A., Triffitt, J. T., and Czernuszka, J. T. (2008). Novel 3D Collagen Scaffolds Fabricated by Indirect Printing Technique for Tissue Engineering. J. Biomed. Mater. Res. 85B (2), 519–528. doi:10.1002/jbm.b.30975
Liu, M. J. J., Chou, S. M., Chua, C. K., Tay, B. C. M., and Ng, B. K. (2013). The Development of Silk Fibroin Scaffolds Using an Indirect Rapid Prototyping Approach: Morphological Analysis and Cell Growth Monitoring by Spectral-Domain Optical Coherence Tomography. Med. Eng. Phys. 35 (2), 253–262. doi:10.1016/j.medengphy.2011.09.029
Liu, R. H., Ong, C. S., Fukunishi, T., Ong, K., and Hibino, N. (2018). Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B: Rev. 24 (2), 133–143. doi:10.1089/ten.TEB.2017.0350
López-Marcial, G. R., Zeng, A. Y., Osuna, C., Dennis, J., García, J. M., and O’Connell, G. D. (2018). Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 4 (10), 3610–3616. doi:10.1021/acsbiomaterials.8b00903
M, J. C., Reardon, P. J., Konwarh, R., Knowles, J. C., and Mandal, B. B. (2017). Mimicking Hierarchical Complexity of the Osteochondral Interface Using Electrospun Silk-Bioactive Glass Composites. ACS Appl. Mater. Inter. 9 (9), 8000–8013. doi:10.1021/acsami.6b16590
MacNeil, S. (2007). Progress and Opportunities for Tissue-Engineered Skin. Nature 445 (7130), 874–880. doi:10.1038/nature05664
Madl, C. M., LeSavage, B. L., Dewi, R. E., Dinh, C. B., Stowers, R. S., Khariton, M., et al. (2017). Maintenance of Neural Progenitor Cell Stemness in 3D Hydrogels Requires Matrix Remodelling. Nat. Mater 16 (12), 1233–1242. doi:10.1038/nmat5020
Makris, E. A., Gomoll, A. H., Malizos, K. N., Hu, J. C., and Athanasiou, K. A. (2015). Repair and Tissue Engineering Techniques for Articular Cartilage. Nat. Rev. Rheumatol. 11 (1), 21–34. doi:10.1038/nrrheum.2014.157
Malda, J., Visser, J., Melchels, F. P., Jüngst, T., Hennink, W. E., Dhert, W. J. A., et al. (2013). 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 25 (36), 5011–5028. doi:10.1002/adma.201302042
Mandrycky, C., Wang, Z., Kim, K., and Kim, D.-H. (2016). 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 34 (4), 422–434. doi:10.1016/j.biotechadv.2015.12.011
Massa, S., Sakr, M. A., Seo, J., Bandaru, P., Arneri, A., Bersini, S., et al. (2017). Bioprinted 3D Vascularized Tissue Model for Drug Toxicity Analysis. Biomicrofluidics 11 (4), 044109. doi:10.1063/1.4994708
Matsui, M., and Tabata, Y. (2012). Enhanced Angiogenesis by Multiple Release of Platelet-Rich Plasma Contents and Basic Fibroblast Growth Factor from Gelatin Hydrogels. Acta Biomater. 8 (5), 1792–1801. doi:10.1016/j.actbio.2012.01.016
Mesallati, T., Sheehy, E. J., Sheehy, E., Vinardell, T., Buckley, C., and Kelly, D. (2015). Tissue Engineering Scaled-Up, Anatomically Shaped Osteochondral Constructs for Joint Resurfacing. eCM 30, 163–186. ; discussion 185-166. doi:10.22203/ecm.v030a12
Miller, J. S., Stevens, K. R., Yang, M. T., Baker, B. M., Nguyen, D.-H. T., Cohen, D. M., et al. (2012). Rapid Casting of Patterned Vascular Networks for Perfusable Engineered Three-Dimensional Tissues. Nat. Mater 11 (9), 768–774. doi:10.1038/nmat3357
Mohanty, S., Sanger, K., Heiskanen, A., Trifol, J., Szabo, P., Dufva, M., et al. (2016). Fabrication of Scalable Tissue Engineering Scaffolds with Dual-Pore Microarchitecture by Combining 3D Printing and Particle Leaching. Mater. Sci. Eng. C 61, 180–189. doi:10.1016/j.msec.2015.12.032
Moroni, L., Boland, T., Burdick, J. A., De Maria, C., Derby, B., Forgacs, G., et al. (2018). Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 36 (4), 384–402. doi:10.1016/j.tibtech.2017.10.015
Murphy, S. V., and Atala, A. (2014). 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 32 (8), 773–785. doi:10.1038/nbt.2958
Nazeer, M. A., Karaoglu, I. C., Ozer, O., Albayrak, C., and Kizilel, S. (2021). Neovascularization of Engineered Tissues for Clinical Translation: Where We Are, where We Should Be? APL Bioeng. 5 (2), 021503. doi:10.1063/5.0044027
Nelson, C. E., Kim, A. J., Adolph, E. J., Gupta, M. K., Yu, F., Hocking, K. M., et al. (2014). Tunable Delivery of siRNA from a Biodegradable Scaffold to Promote Angiogenesis In Vivo. Adv. Mater. 26 (4), 607506–607614. doi:10.1002/adma.201303520
Nichol, J. W., Koshy, S. T., Bae, H., Hwang, C. M., Yamanlar, S., and Khademhosseini, A. (2010). Cell-laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 31 (21), 5536–5544. doi:10.1016/j.biomaterials.2010.03.064
Noori, A., Ashrafi, S. J., Vaez-Ghaemi, R., Hatamian-Zaremi, A., and Webster, T. J. (2017). A Review of Fibrin and Fibrin Composites for Bone Tissue Engineering. Ijn 12, 4937–4961. doi:10.2147/ijn.S124671
Norotte, C., Marga, F. S., Niklason, L. E., and Forgacs, G. (2009). Scaffold-free Vascular Tissue Engineering Using Bioprinting. Biomaterials 30 (30), 5910–5917. doi:10.1016/j.biomaterials.2009.06.034
Novosel, E. C., Kleinhans, C., and Kluger, P. J. (2011). Vascularization Is the Key challenge in Tissue Engineering. Adv. Drug Deliv. Rev. 63 (4-5), 300–311. doi:10.1016/j.addr.2011.03.004
O’Bryan, C. S., Bhattacharjee, T., Niemi, S. R., Balachandar, S., Baldwin, N., Ellison, S. T., et al. (2017). Three-dimensional Printing with Sacrificial Materials for Soft Matter Manufacturing. MRS Bull. 42 (8), 571–577. doi:10.1557/mrs.2017.167
P B, S., S, G., J, P., Muthusamy, S., R, N., Krishnakumar, G. S., et al. (2022). Tricomposite Gelatin-Carboxymethylcellulose-Alginate Bioink for Direct and Indirect 3D Printing of Human Knee Meniscal Scaffold. Int. J. Biol. Macromolecules 195, 179–189. doi:10.1016/j.ijbiomac.2021.11.184
Park, J. H., Jung, J. W., Kang, H.-W., and Cho, D.-W. (2014). Indirect Three-Dimensional Printing of Synthetic Polymer Scaffold Based on thermal Molding Process. Biofabrication 6 (2), 025003. doi:10.1088/1758-5082/6/2/025003
Patel-Hett, S., and DAmore, P. A. (2011). Signal Transduction in Vasculogenesis and Developmental Angiogenesis. Int. J. Dev. Biol. 55 (4-5), 353–363. doi:10.1387/ijdb.103213sp
Patterson, J., and Hubbell, J. A. (2010). Enhanced Proteolytic Degradation of Molecularly Engineered PEG Hydrogels in Response to MMP-1 and MMP-2. Biomaterials 31 (30), 7836–7845. doi:10.1016/j.biomaterials.2010.06.061
Pawar, S. N., and Edgar, K. J. (2012). Alginate Derivatization: a Review of Chemistry, Properties and Applications. Biomaterials 33 (11), 3279–3305. doi:10.1016/j.biomaterials.2012.01.007
Perrone, G. S., Leisk, G. G., Lo, T. J., Moreau, J. E., Haas, D. S., Papenburg, B. J., et al. (2014). The Use of Silk-Based Devices for Fracture Fixation. Nat. Commun. 5, 3385. doi:10.1038/ncomms4385
Pimentel C., R., Ko, S. K., Caviglia, C., Wolff, A., Emnéus, J., Keller, S. S., et al. (2018). Three-dimensional Fabrication of Thick and Densely Populated Soft Constructs with Complex and Actively Perfused Channel Network. Acta Biomater. 65, 174–184. doi:10.1016/j.actbio.2017.10.047
Piras, C. C., and Smith, D. K. (2020). Multicomponent Polysaccharide Alginate-Based Bioinks. J. Mater. Chem. B 8 (36), 8171–8188. doi:10.1039/d0tb01005g
Placone, J. K., and Engler, A. J. (2018). Recent Advances in Extrusion‐Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 7 (8), 1701161. doi:10.1002/adhm.201701161
Poellmann, M. J., Barton, K. L., Mishra, S., and Johnson, A. J. W. (2011). Patterned Hydrogel Substrates for Cell Culture with Electrohydrodynamic Jet Printing. Macromol. Biosci. 11 (9), 1164–1168. doi:10.1002/mabi.201100004
Pollet, A. M. A. O., Homburg, E. F. G. A., Cardinaels, R., and den Toonder, J. M. J. (2019). 3D Sugar Printing of Networks Mimicking the Vasculature. Micromachines 11 (1), 43. doi:10.3390/mi11010043
Qi, X., Wang, H., Zhang, Y., Pang, L., Xiao, W., Jia, W., et al. (2018). Mesoporous Bioactive Glass-Coated 3D Printed Borosilicate Bioactive Glass Scaffolds for Improving Repair of Bone Defects. Int. J. Biol. Sci. 14 (4), 471–484. doi:10.7150/ijbs.23872
Rastogi, P., and Kandasubramanian, B. (2019). Review of Alginate-Based Hydrogel Bioprinting for Application in Tissue Engineering. Biofabrication 11 (4), 042001. doi:10.1088/1758-5090/ab331e
Reakasame, S., and Boccaccini, A. R. (2018). Oxidized Alginate-Based Hydrogels for Tissue Engineering Applications: A Review. Biomacromolecules 19 (1), 3–21. doi:10.1021/acs.biomac.7b01331
Reis, L. A., Chiu, L. L. Y., Feric, N., Fu, L., and Radisic, M. (2016). Biomaterials in Myocardial Tissue Engineering. J. Tissue Eng. Regen. Med. 10 (1), 11–28. doi:10.1002/term.1944
Richards, D., Jia, J., Yost, M., Markwald, R., and Mei, Y. (2017). 3D Bioprinting for Vascularized Tissue Fabrication. Ann. Biomed. Eng. 45 (1), 132–147. doi:10.1007/s10439-016-1653-z
Robu, A., Mironov, V., and Neagu, A. (2019). Using Sacrificial Cell Spheroids for the Bioprinting of Perfusable 3D Tissue and Organ Constructs: A Computational Study. Comput. Math. Methods Med. 2019, 1–9. doi:10.1155/2019/7853586
Rocca, M., Fragasso, A., Liu, W., Heinrich, M. A., and Zhang, Y. S. (2018). Embedded Multimaterial Extrusion Bioprinting. SLAS TECHNOLOGY: Translating Life Sci. Innovation 23 (2), 154–163. doi:10.1177/2472630317742071
Rouwkema, J., and Khademhosseini, A. (2016). Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 34 (9), 733–745. doi:10.1016/j.tibtech.2016.03.002
Rouwkema, J., Koopman, B. F. J. M., Blitterswijk, C. A. V., Dhert, W. J. A., and Malda, J. (2009). Supply of Nutrients to Cells in Engineered Tissues. Biotechnol. Genet. Eng. Rev. 26, 163–178. doi:10.5661/bger-26-163
Rouwkema, J., Rivron, N. C., and van Blitterswijk, C. A. (2008). Vascularization in Tissue Engineering. Trends Biotechnol. 26 (8), 434–441. doi:10.1016/j.tibtech.2008.04.009
Ryssel, H., Gazyakan, E., Germann, G., and Öhlbauer, M. (2008). The Use of MatriDerm in Early Excision and Simultaneous Autologous Skin Grafting in burns-A Pilot Study. Burns 34 (1), 93–97. doi:10.1016/j.burns.2007.01.018
Sarker, M. D., Naghieh, S., Sharma, N. K., and Chen, X. (2018b). 3D Biofabrication of Vascular Networks for Tissue Regeneration: A Report on Recent Advances. J. Pharm. Anal. 8 (5), 277–296. doi:10.1016/j.jpha.2018.08.005
Sarker, M., Izadifar, M., Schreyer, D., and Chen, X. (2018a). Influence of Ionic Crosslinkers (Ca2+/Ba2+/Zn2+) on the Mechanical and Biological Properties of 3D Bioplotted Hydrogel Scaffolds. J. Biomater. Sci. Polym. Edition 29 (10), 1126–1154. doi:10.1080/09205063.2018.1433420
Schmedlen, R. H., Masters, K. S., and West, J. L. (2002). Photocrosslinkable Polyvinyl Alcohol Hydrogels that Can Be Modified with Cell Adhesion Peptides for Use in Tissue Engineering. Biomaterials 23 (22), 4325–4332. doi:10.1016/s0142-9612(02)00177-1
Schöneberg, J., De Lorenzi, F., Theek, B., Blaeser, A., Rommel, D., Kuehne, A. J. C., et al. (2018). Engineering Biofunctional In Vitro Vessel Models Using a Multilayer Bioprinting Technique. Sci. Rep. 8 (1), 10430. doi:10.1038/s41598-018-28715-0
Schumacher, M., Uhl, F., Detsch, R., Deisinger, U., and Ziegler, G. (2010). Static and Dynamic Cultivation of Bone Marrow Stromal Cells on Biphasic Calcium Phosphate Scaffolds Derived from an Indirect Rapid Prototyping Technique. J. Mater. Sci. Mater. Med. 21 (11), 3039–3048. doi:10.1007/s10856-010-4153-y
Schwab, A., Levato, R., D’Este, M., Piluso, S., Eglin, D., and Malda, J. (2020). Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 120 (19), 11028–11055. doi:10.1021/acs.chemrev.0c00084
Seifu, D. G., and Mequanint, K. (2012). Fabrication of Vascular Tissue Engineering Scaffolds with Enhanced Oxygen Diffusivity and Cell Infiltration. j biomat tissue engng 2 (4), 280–291. doi:10.1166/jbt.2012.1061
Serbo, J. V., and Gerecht, S. (2013). Vascular Tissue Engineering: Biodegradable Scaffold Platforms to Promote Angiogenesis. Stem Cell Res Ther 4 (1). doi:10.1186/scrt156
Silva, M., Ferreira, F. N., Alves, N. M., and Paiva, M. C. (2020). Biodegradable Polymer Nanocomposites for Ligament/tendon Tissue Engineering. J. Nanobiotechnol 18 (1), 23. doi:10.1186/s12951-019-0556-1
Skardal, A., Devarasetty, M., Kang, H.-W., Mead, I., Bishop, C., Shupe, T., et al. (2015). A Hydrogel Bioink Toolkit for Mimicking Native Tissue Biochemical and Mechanical Properties in Bioprinted Tissue Constructs. Acta Biomater. 25, 24–34. doi:10.1016/j.actbio.2015.07.030
Skylar-Scott, M. A., Uzel, S. G. M., Nam, L. L., Ahrens, J. H., Truby, R. L., Damaraju, S., et al. (2019). Biomanufacturing of Organ-specific Tissues with High Cellular Density and Embedded Vascular Channels. Sci. Adv. 5 (9), eaaw2459. doi:10.1126/sciadv.aaw2459
Sooppan, R., Paulsen, S. J., Han, J., Ta, A. H., Dinh, P., Gaffey, A. C., et al. (2016). In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks. Tissue Eng. Part C: Methods 22 (1), 1–7. doi:10.1089/ten.TEC.2015.0239
Štumberger, G., and Vihar, B. (2018). Freeform Perfusable Microfluidics Embedded in Hydrogel Matrices. Materials 11 (12), 2529. doi:10.3390/ma11122529
Sun, G., Shen, Y.-I., Kusuma, S., Fox-Talbot, K., Steenbergen, C. J., and Gerecht, S. (2011). Functional Neovascularization of Biodegradable Dextran Hydrogels with Multiple Angiogenic Growth Factors. Biomaterials 32 (1), 95–106. doi:10.1016/j.biomaterials.2010.08.091
Teodorescu, M., Bercea, M., and Morariu, S. (2019). Biomaterials of PVA and PVP in Medical and Pharmaceutical Applications: Perspectives and Challenges. Biotechnol. Adv. 37 (1), 109–131. doi:10.1016/j.biotechadv.2018.11.008
Thomas, A., Orellano, I., Lam, T., Noichl, B., Geiger, M.-A., Amler, A.-K., et al. (2020). Vascular Bioprinting with Enzymatically Degradable Bioinks via Multi-Material Projection-Based Stereolithography. Acta Biomater. 117, 121–132. doi:10.1016/j.actbio.2020.09.033
Thompson, E. M., Matsiko, A., Kelly, D. J., Gleeson, J. P., and O'Brien, F. J. (2016). An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng. Part A 22 (5-6), 556–567. doi:10.1089/ten.TEA.2015.0457
Tocchio, A., Tamplenizza, M., Martello, F., Gerges, I., Rossi, E., Argentiere, S., et al. (2015). Versatile Fabrication of Vascularizable Scaffolds for Large Tissue Engineering in Bioreactor. Biomaterials 45, 124–131. doi:10.1016/j.biomaterials.2014.12.031
Vacanti, J. P. (2012). Tissue Engineering and the Road to Whole Organs. Br. J. Surg. 99 (4), 451–453. doi:10.1002/bjs.7819
Van Den Bulcke, A. I., Bogdanov, B., De Rooze, N., Schacht, E. H., Cornelissen, M., and Berghmans, H. (2000). Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 1 (1), 31–38. doi:10.1021/bm990017d
Van Hoorick, J., Declercq, H., De Muynck, A., Houben, A., Van Hoorebeke, L., Cornelissen, R., et al. (2015). Indirect Additive Manufacturing as an Elegant Tool for the Production of Self-Supporting Low Density Gelatin Scaffolds. J. Mater. Sci. Mater. Med. 26 (10), 247. doi:10.1007/s10856-015-5566-4
Vig, K., Chaudhari, A., Tripathi, S., Dixit, S., Sahu, R., Pillai, S., et al. (2017). Advances in Skin Regeneration Using Tissue Engineering. Ijms 18 (4), 789. doi:10.3390/ijms18040789
Vijayavenkataraman, S., Yan, W.-C., Lu, W. F., Wang, C.-H., and Fuh, J. Y. H. (2018). 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 132, 296–332. doi:10.1016/j.addr.2018.07.004
Wang, J.-Q., Jiang, B.-J., Guo, W.-J., and Zhao, Y.-M. (2019a). Indirect 3D Printing Technology for the Fabrication of Customised β-TCP/chitosan Scaffold with the Shape of Rabbit Radial Head-An In Vitro Study. J. Orthop. Surg. Res. 14 (1), 102. doi:10.1186/s13018-019-1136-7
Wang, P., Hu, J., and Ma, P. X. (2009). The Engineering of Patient-specific, Anatomically Shaped, Digits. Biomaterials 30 (14), 2735–2740. doi:10.1016/j.biomaterials.2009.01.037
Wang, Y., Rudym, D. D., Walsh, A., Abrahamsen, L., Kim, H.-J., Kim, H. S., et al. (2008). In Vivo degradation of Three-Dimensional Silk Fibroin Scaffolds. Biomaterials 29 (24-25), 3415–3428. doi:10.1016/j.biomaterials.2008.05.002
Wang, Z., Mithieux, S. M., and Weiss, A. S. (2019b). Fabrication Techniques for Vascular and Vascularized Tissue Engineering. Adv. Healthc. Mater. 8 (19), 1900742. doi:10.1002/adhm.201900742
Wenk, E., Meinel, A. J., Wildy, S., Merkle, H. P., and Meinel, L. (2009). Microporous Silk Fibroin Scaffolds Embedding PLGA Microparticles for Controlled Growth Factor Delivery in Tissue Engineering. Biomaterials 30 (13), 2571–2581. doi:10.1016/j.biomaterials.2008.12.073
White, S. M., Pittman, C. R., Hingorani, R., Arora, R., Esipova, T. V., Vinogradov, S. A., et al. (2014). Implanted Cell-Dense Prevascularized Tissues Develop Functional Vasculature that Supports Reoxygenation after Thrombosis. Tissue Eng. Part A 20 (17-18), 2316–2328. doi:10.1089/ten.TEA.2013.0311
Workman, V. L., Tezera, L. B., Elkington, P. T., and Jayasinghe, S. N. (2014). Controlled Generation of Microspheres Incorporating Extracellular Matrix Fibrils for Three-Dimensional Cell Culture. Adv. Funct. Mater. 24 (18), 2648–2657. doi:10.1002/adfm.201303891
Wu, L., Magaz, A., Maughan, E., Oliver, N., Darbyshire, A., Loizidou, M., et al. (2019). Cellular Responses to Thermoresponsive Stiffness Memory Elastomer Nanohybrid Scaffolds by 3D-TIPS. Acta Biomater. 85, 157–171. doi:10.1016/j.actbio.2018.12.019
Wu, L., Virdee, J., Maughan, E., Darbyshire, A., Jell, G., Loizidou, M., et al. (2018). Stiffness Memory Nanohybrid Scaffolds Generated by Indirect 3D Printing for Biologically Responsive Soft Implants. Acta Biomater. 80, 188–202. doi:10.1016/j.actbio.2018.09.016
Xie, R., Zheng, W., Guan, L., Ai, Y., and Liang, Q. (2020). Engineering of Hydrogel Materials with Perfusable Microchannels for Building Vascularized Tissues. Small 16 (15), 1902838. doi:10.1002/smll.201902838
Xu, Y., Hu, Y., Liu, C., Yao, H., Liu, B., and Mi, S. (2018). A Novel Strategy for Creating Tissue-Engineered Biomimetic Blood Vessels Using 3D Bioprinting Technology. Materials 11 (9), 1581. doi:10.3390/ma11091581
Yamamura, N., Sudo, R., Ikeda, M., and Tanishita, K. (2007). Effects of the Mechanical Properties of Collagen Gel on the In Vitro Formation of Microvessel Networks by Endothelial Cells. Tissue Eng. 13 (7), 1443–1453. doi:10.1089/ten.2006.0333
Yan, W.-C., Davoodi, P., Vijayavenkataraman, S., Tian, Y., Ng, W. C., Fuh, J. Y. H., et al. (2018). 3D Bioprinting of Skin Tissue: From Pre-processing to Final Product Evaluation. Adv. Drug Deliv. Rev. 132, 270–295. doi:10.1016/j.addr.2018.07.016
Yang, S., Tang, H., Feng, C., Shi, J., and Yang, J. (2020). The Research on Multi-Material 3D Vascularized Network Integrated Printing Technology. Micromachines 11 (3), 237. doi:10.3390/mi11030237
Yeong, W.-Y., Chua, C.-K., Leong, K.-F., Chandrasekaran, M., and Lee, M.-W. (2007). Comparison of Drying Methods in the Fabrication of Collagen Scaffold via Indirect Rapid Prototyping. J. Biomed. Mater. Res. 82B (1), 260–266. doi:10.1002/jbm.b.30729
Yue, K., Trujillo-de Santiago, G., Alvarez, M. M., Tamayol, A., Annabi, N., and Khademhosseini, A. (2015). Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 73, 254–271. doi:10.1016/j.biomaterials.2015.08.045
Zhang, G., Varkey, M., Wang, Z., Xie, B., Hou, R., and Atala, A. (2020). ECM Concentration and Cell‐mediated Traction Forces Play a Role in Vascular Network Assembly in 3D Bioprinted Tissue. Biotechnol. Bioeng. 117 (4), 1148–1158. doi:10.1002/bit.27250
Zhang, W., Zhang, Y. S., Bakht, S. M., Aleman, J., Shin, S. R., Yue, K., et al. (2016). Elastomeric Free-form Blood Vessels for Interconnecting Organs on Chip Systems. Lab. Chip 16 (9), 1579–1586. doi:10.1039/c6lc00001k
Zhu, J., Wang, Y., Zhong, L., Pan, F., and Wang, J. (2021). Advances in Tissue Engineering of Vasculature through Three‐dimensional Bioprinting. Dev. Dyn. 250 (12), 1717–1738. doi:10.1002/dvdy.385
Zhu, W., Ma, X., Gou, M., Mei, D., Zhang, K., and Chen, S. (2016). 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 40, 103–112. doi:10.1016/j.copbio.2016.03.014
Zhu, W., Qu, X., Zhu, J., Ma, X., Patel, S., Liu, J., et al. (2017). Direct 3D Bioprinting of Prevascularized Tissue Constructs with Complex Microarchitecture. Biomaterials 124, 106–115. doi:10.1016/j.biomaterials.2017.01.042
Keywords: biodegradable ink, indirect 3D bioprinting, vascularization, scaffold, tissue engineering
Citation: Ze Y, Li Y, Huang L, Shi Y, Li P, Gong P, Lin J and Yao Y (2022) Biodegradable Inks in Indirect Three-Dimensional Bioprinting for Tissue Vascularization. Front. Bioeng. Biotechnol. 10:856398. doi: 10.3389/fbioe.2022.856398
Received: 17 January 2022; Accepted: 09 March 2022;
Published: 25 March 2022.
Edited by:
Liqun Yang, China Medical University, ChinaReviewed by:
Qing Gao, Zhejiang University, ChinaCopyright © 2022 Ze, Li, Huang, Shi, Li, Gong, Lin and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Lin, ODQyMDQzNjJAcXEuY29t; Yang Yao, eWFveWFuZzk5OTlAMTI2LmNvbQ==
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.