
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 01 December 2022
Sec. Biomechanics
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.1030458
 Tian Han1,2,3,4†
Tian Han1,2,3,4† Wanru Shi1,2,3,4†
Wanru Shi1,2,3,4† Yingjun Chen1,2,3,4
Yingjun Chen1,2,3,4 Yang Shen1,2,3,4
Yang Shen1,2,3,4 Ye Xu1,2,3,4*
Ye Xu1,2,3,4* Xingtao Zhou1,2,3,4*
Xingtao Zhou1,2,3,4*Purpose: To develop predictive models for the intraocular pressure (IOP) of patients undergoing small incision lenticule extraction (SMILE) procedures, measured with a noncontact tonometer (NCT), Goldmann applanation tonometry (GAT), and an ocular response analyzer (ORA).
Methods: In this prospective study, a total of 104 eyes (−6.23 ± 2.06 diopters) of 52 patients (24.38 ± 4.76 years) undergoing SMILE procedures were included. The intraocular pressure was measured (IOPNCT with NCT, IOPGAT with GAT, and IOPcc and IOPg with ORA) before surgery and at postoperative 6 months. Information on age, preoperative and attempted spherical equivalent (SE), ablation depth, preoperative values and postoperative changes in central corneal thickness (CCT), K1, K2, Km, corneal hysteresis (CH) and corneal resistance factor (CRF) values was collected in order to predict IOPs.
Results: All surgeries were uneventful. At postoperative 6 months, the efficacy and safety index were 1.04 ± 0.15 and 1.08 ± 0.18, respectively. Significant decreases were detected in postoperative IOPNCT, IOPGAT, IOPcc, and IOPg compared to preoperative values (all p < 0.001). No relationship was found between any IOP and ablation depth, attempted SE, and preoperative SE, as well as CCTdifference (all p > 0.05). Predictive models for IOPs were constructed to predict preoperative values, and R2 values were 67.5% (IOPNCT), 64.5% (IOPGAT), 78.7% (IOPcc), and 82.0% (IOPg). The prediction band of IOPNCT and IOPGAT was 7.4–15.1 mmHg and 8–16 mmHg, respectively.
Conclusion: Predictive models for IOP measurements after SMILE procedures can be helpful in clinical practice.
In clinical practice, it is important to monitor the intraocular pressure (IOP), especially for patients undergoing refractive surgeries. IOP management after refractive surgeries calls for the avoidance of steroid-induced hypertension and an accurate diagnosis of glaucoma, since myopia is a risk factor for open-angle glaucoma (Marcus et al., 2011).
Factors such as central corneal thickness (CCT), corneal curvature, and corneal biomechanics affect IOP measurements (Okafor and Brandt, 2015). Both Goldmann applanation tonometry (GAT), a standard measurement for normal corneas, and noncontact tonometer (NCT), a commonly used instrument, can underestimate the IOP after refractive surgeries due to the corneal tissue being removed during surgeries (Ehlers et al., 1975; Ito et al., 2012; Schallhorn et al., 2015). The ocular response analyzer (ORA) provides Goldmann IOP (IOPg), corneal-compensated IOP (IOPcc) (Moreno-Montanes et al., 2008), and corneal biomechanics: corneal hysteresis (CH) and corneal resistance factor (CRF) (Medeiros and Weinreb, 2006). Corneal biomechanics may be helpful in predictive IOP models (Li et al., 2016).
There are many studies on IOP among patients undergoing laser-assisted in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) (Chang and Stulting, 2005; Kohlhaas et al., 2006; Yang et al., 2006; Pepose et al., 2007; Johannesson et al., 2012; Han et al., 2013; Schallhorn et al., 2015; Sales-Sanz et al., 2016), while limited literature reports exist on predictive models of IOP changes after small incision lenticule extraction (SMILE) (Li et al., 2016; Shen et al., 2016). As a flapless procedure utilizing a small incision, SMILE preserves the integrity of the corneal tissue (including Bowman’s layer) and serves a better corneal biomechanics than LASIK (Wang et al., 2022), although it does not preserve suspect corneas before iatrogenic ectasia (Bao et al., 2022; Xin et al., 2022). The impact of SMILE on the corneal structure prompts changes in the IOP that might differ from other refractive surgeries. In addition, IOP changes should be focused for the fact that the number of SMILE surgeries has currently reached 5 million globally.
In this prospective study, we developed predictive models for four IOPs among patients undergoing SMILE procedures.
Patients who underwent SMILE procedures at the Refractive Surgery Center of the Department of Ophthalmology, Eye and ENT Hospital of Fudan University, were enrolled in this prospective study. This study was registered in the Chinese Clinical Trial Registry (ChiCTRONRC13003114), followed the tenets of the Declaration of Helsinki, and was approved by the ethics committee of the Eye and ENT Hospital of Fudan University. Informed consent was obtained from all participants.
Inclusion criteria included an age of 18–48 years, myopia with a spherical equivalent (SE) less than 12.50 diopters (D), corrected distance visual acuity (CDVA) of 20/20 or better, a stable refraction for 2 years, IOPGAT between 10 and 21 mmHg, and no use of any kind of contact lenses within the previous 2 weeks. Patients with any systemic diseases, any history of ocular surgery or trauma, or any history of ocular disease except myopia or astigmatism were excluded.
Except for normal preoperative examinations, the IOPNCT of all patients was measured using a noncontact tonometer (NCT) (TX-F; Canon, Tokyo, Japan), IOPGAT by Goldmann applanation tonometry (GAT) (Haag-Streit, Bern, Switzerland), and IOPcc and IOPg using an ocular response analyzer (ORA) (Reichert, Inc., Depew, NY, United States) between 9 and 12 o’clock in the morning. In all cases, each IOP was measured three times by experienced clinicians.
The same surgeon (XZ) performed all SMILE procedures. The 500-kHz VisuMax femtosecond laser system (Carl Zeiss Meditec, Jena, Germany) was used with a pulse energy of 130 nJ. The lenticule diameter was set between 6.00 and 6.80 mm; the cap diameter was set to 7.5 mm at a 120 μm depth. A 90-degree single side cut with a length of 2.0 mm was created during the procedure.
The postoperative follow-up was set at 6 months. At postoperative 1 month, all patients were told to stop medication such as steroids, which can affect the IOP. Routine examinations such as uncorrected distance visual acuity (UDVA), CDVA, and manifest refraction were performed. Information on CCT, K1, K2, and Km was collected by rotating Scheimpflug camera imaging (Pentacam; Oculus GmbH, Wetzlar, Germany).
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, Version 22) (IBM, Armonk, NY, United States) and Statistical Analysis Software (SAS Version 9.4, SAS Institute, Cary, NC, United States). Data were reported as the mean ± standard deviation. A generalized linear mixed model (GLMM) was used to build predictive models of IOPdifference and to detect the difference between the four IOPs with the inter-eye correlation. From the model constructed using the GLMM, the goodness-of-fit statistic R2 was calculated using a least-square method. For all tests, p < 0.05 was defined as statistically significant.
A total of 104 eyes of 52 patients (30 female) were enrolled in the study. The demographic characteristics are shown in Table 1. All surgical procedures and postoperative follow-ups were uneventful. Complications, including severe dry eye, haze, edema, or infection were not observed. At postoperative 6 months, the efficacy and safety index were 1.04 ± 0.15 and 1.08 ± 0.18, respectively.
The IOP values are shown in Figure 1. Except for IOPNCT and IOPcc (p = 0.631) and IOPGAT and IOPg (p = 0.382), other preoperative IOPs were different from each other (all p < 0.05). Post-operation, each IOP was different from the other three IOPs (all p < 0.001), except for IOPNCT and IOPGAT (p = 0.095).
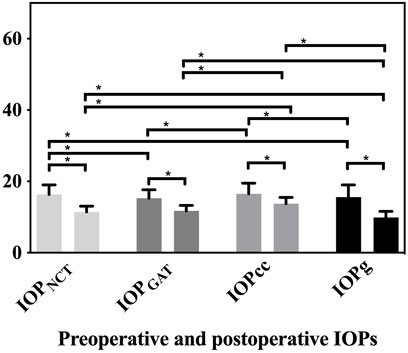
FIGURE 1. Preoperative and postoperative values of IOPNCT, IOPGAT, IOPg, and IOPcc. Significant decreases in all IOPs were detected compared to preoperative values (all p < 0.001). Except for IOPNCT and IOPcc (p = 0.631) and IOPGAT and IOPg (p = 0.382), all other preoperative IOPs differed from each other (all p < 0.05) (IOPNCT and IOPGAT p = 0.005, IOPNCT and IOPg p = 0.049, IOPGAT and IOPcc p = 0.001, and IOPcc and IOPg p = 0.015). After the surgery, except for IOPNCT and IOPGAT (p = 0.095), each postoperative IOP was different from other the three IOPs (all p < 0.001).
At postoperative 6 months, significant decreases in IOPNCT (4.88 ± 2.69 mmHg, 95% CI: 4.33–5.42 mmHg), IOPGAT (3.58 ± 2.57 mmHg, 95% CI: 3.03–4.13 mmHg), IOPcc (2.75 ± 3.16 mmHg, 95% CI: 2.21–3.30 mmHg), and IOPg (5.72 ± 3.38 mmHg, 95% CI: 5.17–6.27 mmHg) were detected compared to preoperative values (all p < 0.001). All changes in IOPs differed from those of the other three IOPs (all p < 0.01) (Figure 2).
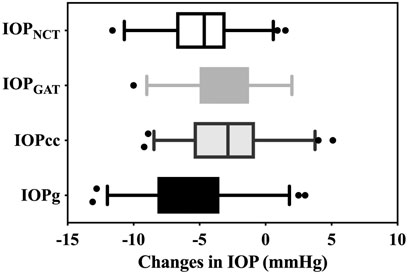
FIGURE 2. Decreases in IOPNCT, IOPGAT, IOPg, and IOPcc. Each of the changes in IOP differed from the other three IOPs (all p < 0.01) (IOPNCT and IOPg (p = 0.005), IOPGAT and IOPcc (p = 0.006), and other p < 0.001)
Information on age, preoperative and attempted SE, ablation depth, preoperative values, and postoperative changes in the central corneal thickness (CCT), K1, K2, Km, CH, and CRF values were collected in order to predict IOPs. Significant parameters in predictive models for IOPs are shown in Table 2 (all p < 0.05).
Predictive models are given in Table 3, and R2 values were 67.5% (IOPNCT), 64.5% (IOPGAT), 78.7% (IOPcc), and 82.0% (IOPg).
The preoperative IOP was the only significant parameter in the predictive models for IOPNCT and IOPGAT (the scatterplots between IOP changes and CCT changes, ablation depth, and attempted SE are shown in Figure 3). Thus, considering that the normal range of IOP (10–21 mmHg) is well-known, it is possible to calculate the confidence band and prediction band of the postoperative IOPNCT and IOPGAT (Lenhoff et al., 1999). Table 4 and Table 5 show the estimated confidence band and prediction band of postoperative IOPNCT and IOPGAT with preoperative IOP set as an integral.
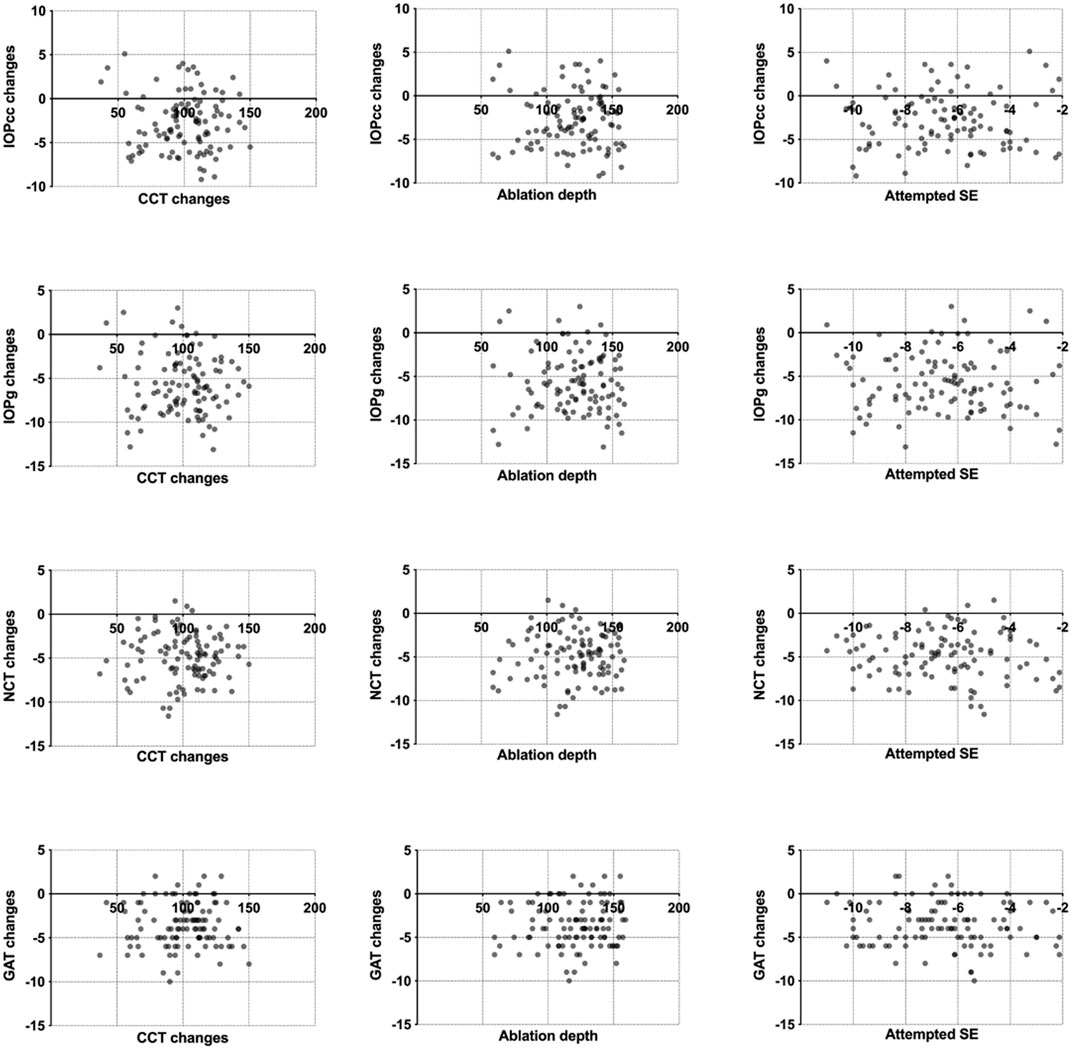
FIGURE 3. Scatterplots between IOPNCT, IOPGAT, IOPg and IOPcc, and CCT changes, ablation depth, and attempted SE.
Changes in corneal structures influence the evaluation of intraocular pressure, and both changes in the corneal structure and IOP evaluation differ after various corneal refractive surgeries (Chang and Stulting, 2005; Kohlhaas et al., 2006; Yang et al., 2006; Pepose et al., 2007; Johannesson et al., 2012; Han et al., 2013; Shen et al., 2014; Schallhorn et al., 2015; Sales-Sanz et al., 2016). Because a lenticule is extracted through a 2-mm incision during a SMILE procedure, the integrity of the corneal tissue (including Bowman’s layer) and corneal biomechanics are highly preserved. Thus, predictive models for IOP evaluation after a SMILE procedure is worthy of discussion.
In this study, significant decreases exist in all four IOP measurements compared to preoperative values. Previous studies on other refractive surgeries also found that IOP values decreased (Chang and Stulting, 2005; Kohlhaas et al., 2006; Yang et al., 2006; Pepose et al., 2007; Johannesson et al., 2012; Han et al., 2013; Schallhorn et al., 2015; Sales-Sanz et al., 2016). IOP value changes in this study are similar to those in previous studies on SMILE (Li et al., 2016; Shen et al., 2016; Hosny et al., 2017; Chen et al., 2018; Chen et al., 2020). These results suggest an underestimation of IOP measurement after refractive surgeries.
Ablation depth commonly plays an important role in predictive models after refractive surgeries, like LASIK and PRK. It is interesting to note that ablation depth fails to contribute to the predictive models in this study. Our results were similar to those of other studies on changes in IOP after SMILE, in that ablation depth, preoperative SE, attempted SE, CCT changes, differences between preoperative and postoperative K1, K2, and Km parameters were not parameters in all predictive models (Li et al., 2016; Shen et al., 2016; Hosny et al., 2017). The unusual phenomenon might be attributed to distinctive corneal structure changes after SMILE procedures. The integrity of the corneal tissue and the small incision leads to these changes in IOP after SMILE compared to changes after other refractive surgeries.
A similar or higher R2 value for a predictive model of IOPNCT was reported in other studies. In a study on IOP changes after LASIK in 133,752 myopic eyes, the R2 value is 45% (Schallhorn et al., 2015). A much higher R2 value (91%) was achieved in Yang et al. (2006)’s predictive model for IOPNCT after LASIK. In our opinion, there is room for improvement in the predictive models. Although only preoperative IOPNCT and IOPGAT played a significant role in the predictive models, it seems that there should be more parameters involved, such as corneal biomechanics (Xin et al., 2022).
However, since only one variable predicts postoperative IOPNCT and IOPGAT, it is convenient to apply the predictive models in clinical practice by calculating the estimated confidence band and prediction band. The confidence band and prediction band can be easily understood as the mean and evaluation range of a postoperative IOP value when the preoperative IOP is a known point. For example, if the preoperative IOPNCT of an eye that underwent a SMILE procedure was 15 mmHg, then the 95% CI of the mean postoperative IOPNCT would be 10.9–11.6 mmHg, and the 95% CI of the postoperative IOPNCT range would be 8.2–14.3 mmHg. In addition, the application of all predictive models was limited to patients of SMILE procedures with normal IOPs. Thus, for these eyes, as shown in Table 3, Table 4, and Table 5, the 95% CI of the postoperative IOPNCT and IOPGAT range was 7.4–15.1 mmHg and 8–16 mmHg, respectively. In practice, if the postoperative IOPNCT of an eye that underwent a SMILE procedure was 18 mmHg, hypertension would be highly suspected.
Although there was no difference between IOPGAT and IOPg at pre-operation, the decrease in IOPGAT was smaller than in IOPg after SMILE. This suggests that IOPGAT is more stable than IOPg, which is consistent with a study on IOP changes after LASIK (Pepose et al., 2007). The decrease in IOPcc was the smallest among all four IOPs, supporting previous observations that IOPcc is less affected by changes in the corneal structure (Medeiros and Weinreb, 2006; Li et al., 2016). In this study, predictive models explained 67.5% of IOPNCT variance, 64.5% of IOPGAT variance, 78.7% of IOPcc variance, and 82.0% of IOPg variance. R2 values of IOPcc (78.7%) and IOPg (82.0%) were much higher than those of IOPNCT (67.5%) and IOPGAT (64.5%). We speculate that this might relate to the corneal biomechanics, which were present in both predictive models of IOPg and IOPcc.
To obtain the most accurate IOP measurements, all measurements were performed between 9 and 12 o’clock in the morning, avoiding IOP fluctuation (Kohlhaas et al., 2006). Moreover, considering that the flap depth affects IOP measurements, all cap depths were set to 120 μm (Lin et al., 2016; Liu et al., 2018). In addition, effective refractive outcomes are the basis for developing predictive models. In this study, the efficacy and safety index were 1.04 ± 0.15 and 1.08 ± 0.18, respectively. The outcomes were consistent with previous studies (Li et al., 2016).
Limited sample size is a potential limitation to this study. A larger sample size would be more convincing.
In conclusion, predictive models for IOP measurements after SMILE procedures can be helpful in clinical practice. It is worthwhile to enhance these models in further studies.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Eye and ENT Hospital of Fudan University. The patients/participants provided their written informed consent to participate in this study.
Conception and design: TH, WS, YC, YS, YX, and XZ. Data collection: TH, WS, YC, and YS. Analysis and interpretation: TH and WS. Funding acquisition: TH and XZ. Overall responsibility: TH, WS, YC, YS, YX, and XZ.
This study was supported in part by the National Natural Science Foundation of China for Young Scholars (Grant No. 82000929), the Shanghai Sailing Program (Grant No. 20YF1405000), the National Natural Science Foundation of China (Grant No. 81770955), the Project of Shanghai Science and Technology (Grant No. 20410710100), the Clinical Research Plan of SHDC (SHDC2020CR1043B), the Project of Shanghai Xuhui District Science and Technology (2020-015), the Project of Shanghai Xuhui District Science and Technology (XHLHGG202104), the Shanghai Engineering Research Center of Laser and Autostereoscopic 3D for Vision Care (20DZ2255000), and the construction of a 3D digital intelligent prevention and control platform for the whole life cycle of highly myopic patients in the Yangtze River Delta (21002411600).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bao, F., Lopes, B. T., Zheng, X., Ji, Y., Wang, J., and Elsheikh, A. (2022). Corneal biomechanics losses caused by refractive surgery. Curr. Eye Res., 1–7. doi:10.1080/02713683.2022.2103569
Chang, D. H., and Stulting, R. D. (2005). Change in intraocular pressure measurements after LASIK the effect of the refractive correction and the lamellar flap. Ophthalmology 112 (6), 1009–1016. doi:10.1016/j.ophtha.2004.12.033
Chen, K. J., Joda, A., Vinciguerra, R., Eliasy, A., Sefat, S. M. M., Kook, D., et al. (2018). Clinical evaluation of a new correction algorithm for dynamic Scheimpflug analyzer tonometry before and after laser in situ keratomileusis and small-incision lenticule extraction. J. Cataract. Refract. Surg. 44 (5), 581–588. doi:10.1016/j.jcrs.2018.01.023
Chen, S., Lopes, B. T., Huang, W., Zheng, X., Wang, J., Zhu, R., et al. (2020). Effectiveness of 4 tonometers in measuring IOP after femtosecond laser-assisted LASIK, SMILE, and transepithelial photorefractive keratectomy. J. Cataract. Refract. Surg. 46 (7), 967–974. doi:10.1097/j.jcrs.0000000000000204
Ehlers, N., Hansen, F. K., and Aasved, H. (1975). Biometric correlations of corneal thickness. Acta Ophthalmol. 53 (4), 652–659. doi:10.1111/j.1755-3768.1975.tb01784.x
Han, K. E., Kim, H., Kim, N. R., Jun, I., Kim, E. K., and Kim, T. I. (2013). Comparison of intraocular pressures after myopic laser-assisted subepithelial keratectomy: Tonometry-pachymetry, Goldmann applanation tonometry, dynamic contour tonometry, and noncontact tonometry. J. Cataract. Refract. Surg. 39 (6), 888–897. doi:10.1016/j.jcrs.2013.01.035
Hosny, M., Aboalazayem, F., El Shiwy, H., and Salem, M. (2017). Comparison of different intraocular pressure measurement techniques in normal eyes and post small incision lenticule extraction. Clin. Ophthalmol. 11, 1309–1314. doi:10.2147/OPTH.S132578
Ito, K., Tawara, A., Kubota, T., and Harada, Y. (2012). IOP measured by dynamic contour tonometry correlates with IOP measured by Goldmann applanation tonometry and non-contact tonometry in Japanese individuals. J. Glaucoma 21 (1), 35–40. doi:10.1097/IJG.0b013e31820275b4
Johannesson, G., Hallberg, P., Eklund, A., Koskela, T., and Linden, C. (2012). Change in intraocular pressure measurement 2 years after myopic laser-assisted subepithelial keratectomy. J. Cataract. Refract. Surg. 38 (9), 1637–1642. doi:10.1016/j.jcrs.2012.04.033
Kohlhaas, M., Spoerl, E., Boehm, A. G., and Pollack, K. (2006). A correction formula for the real intraocular pressure after LASIK for the correction of myopic astigmatism. J. Refract. Surg. 22 (3), 263–267. doi:10.3928/1081-597x-20060301-11
Lenhoff, M. W., Santner, T. J., Otis, J. C., Peterson, M. G., Williams, B. J., and Backus, S. I. (1999). Bootstrap prediction and confidence bands: A superior statistical method for analysis of gait data. Gait Posture 9 (1), 10–17. doi:10.1016/s0966-6362(98)00043-5
Li, H., Wang, Y., Dou, R., Wei, P., Zhang, J., Zhao, W., et al. (2016). Intraocular pressure changes and relationship with corneal biomechanics after SMILE and FS-LASIK. Invest. Ophthalmol. Vis. Sci. 57 (10), 4180–4186. doi:10.1167/iovs.16-19615
Lin, M. Y., Chang, D. C., Shen, Y. D., Lin, Y. K., Lin, C. P., and Wang, I. J. (2016). Factors influencing intraocular pressure changes after laser in situ keratomileusis with flaps created by femtosecond laser or mechanical microkeratome. PLoS One 11 (1), e0147699. doi:10.1371/journal.pone.0147699
Liu, T., Yu, T., Liu, L., Chen, K., and Bai, J. (2018). Corneal cap thickness and its effect on visual acuity and corneal biomechanics in eyes undergoing small incision lenticule extraction. J. Ophthalmol. 2018, 1–7. doi:10.1155/2018/6040873
Marcus, M. W., de Vries, M. M., Junoy Montolio, F. G., and Jansonius, N. M. (2011). Myopia as a risk factor for open-angle glaucoma: A systematic review and meta-analysis. Ophthalmology 118 (10), 1989–1994.e2. doi:10.1016/j.ophtha.2011.03.012
Medeiros, F. A., and Weinreb, R. N. (2006). Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J. Glaucoma 15 (5), 364–370. doi:10.1097/01.ijg.0000212268.42606.97
Moreno-Montanes, J., Maldonado, M. J., Garcia, N., Mendiluce, L., Garcia-Gomez, P. J., and Segui-Gomez, M. (2008). Reproducibility and clinical relevance of the ocular response analyzer in nonoperated eyes: Corneal biomechanical and tonometric implications. Invest. Ophthalmol. Vis. Sci. 49 (3), 968–974. doi:10.1167/iovs.07-0280
Okafor, K. C., and Brandt, J. D. (2015). Measuring intraocular pressure. Curr. Opin. Ophthalmol. 26 (2), 103–109. doi:10.1097/ICU.0000000000000129
Pepose, J. S., Feigenbaum, S. K., Qazi, M. A., Sanderson, J. P., and Roberts, C. J. (2007). Changes in corneal biomechanics and intraocular pressure following LASIK using static, dynamic, and noncontact tonometry. Am. J. Ophthalmol. 143 (1), 39–47.e1. doi:10.1016/j.ajo.2006.09.036
Sales-Sanz, M., Arranz-Marquez, E., Pinero, D. P., Arruabarrena, C., Mikropoulos, D. G., and Teus, M. A. (2016). Effect of laser in situ keratomileusis on schiotz, Goldmann, and dynamic contour tonometric measurements. J. Glaucoma 25 (4), e419–e423. doi:10.1097/IJG.0000000000000338
Schallhorn, J. M., Schallhorn, S. C., and Ou, Y. (2015). Factors that influence intraocular pressure changes after myopic and hyperopic LASIK and photorefractive keratectomy: A large population study. Ophthalmology 122 (3), 471–479. doi:10.1016/j.ophtha.2014.09.033
Shen, Y., Chen, Z., Knorz, M. C., Li, M., Zhao, J., and Zhou, X. (2014). Comparison of corneal deformation parameters after SMILE, LASEK, and femtosecond laser-assisted LASIK. J. Refract. Surg. 30 (5), 310–318. doi:10.3928/1081597x-20140422-01
Shen, Y., Su, X., Liu, X., Miao, H., Fang, X., and Zhou, X. (2016). Changes in intraocular pressure values measured with noncontact tonometer (NCT), ocular response analyzer (ORA) and corvis scheimpflug technology tonometer (CST) in the early phase after small incision lenticule extraction (SMILE). BMC Ophthalmol. 16 (1), 205. doi:10.1186/s12886-016-0381-3
Wang, C., Li, X., Guo, Y., He, R., Guo, H., and Chen, W. (2022). Effects of laser in situ keratomileusis and small-incision lenticule extraction on corneal biomechanical behavior: A finite element analysis. Front. Bioeng. Biotechnol. 10, 855367. doi:10.3389/fbioe.2022.855367
Xin, Y., Lopes, B. T., Wang, J., Wu, J., Zhu, M., Jiang, M., et al. (2022). Biomechanical effects of tPRK, FS-LASIK, and SMILE on the cornea. Front. Bioeng. Biotechnol. 10, 834270. doi:10.3389/fbioe.2022.834270
Keywords: small incision lenticule extraction (SMILE), intraocular pressure (IOP), noncontact tonometer (NCT), Goldmann applanation tonometry (GAT), ocular response analyzer (ORA)
Citation: Han T, Shi W, Chen Y, Shen Y, Xu Y and Zhou X (2022) Predictive models for IOPs measured with NCT, GAT, and ORA among patients undergoing SMILE. Front. Bioeng. Biotechnol. 10:1030458. doi: 10.3389/fbioe.2022.1030458
Received: 29 August 2022; Accepted: 18 November 2022;
Published: 01 December 2022.
Edited by:
Sabine Kling, ETH Zürich, SwitzerlandReviewed by:
Robert Herber, University Hospital Carl Gustav Carus, GermanyCopyright © 2022 Han, Shi, Chen, Shen, Xu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Xu, amFuZXR5ZXllQGhvdG1haWwuY29t; Xingtao Zhou, ZG9jdHpob3V4aW5ndGFvQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.