
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Bioeng. Biotechnol. , 02 November 2022
Sec. Bioprocess Engineering
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.1008760
This article is part of the Research Topic Algal Biomass and Biofuels View all 11 articles
Editorial on the Research Topic
Algal biomass and biofuels
Algal technology is one of the promising areas of research that can alleviate several ongoing global challenges such as global warming, food, and fuel scarcity, and the growing demand for biopharmaceutical products for humans and animals. Algae bio sequester CO2 from the environment during photosynthesis and produce biomass. Algal biomass is a promising feedstock for extracting biofuels and commercially important biopharmaceutical products. The scope of this Research Topic is to collect high-quality research articles that can address the challenges associated with algal technology spanning over three broad domains: algal cultivation for biomass, biofuels production, and extraction of commercially valuable biomolecules (Figure 1). The process of algal biomass cultivation should be scalable, cost-effective, environment-friendly, and sustainable. The algal cultivation in photobioreactors has advantages over open ponds because of easy control of physio-chemical parameters and preventing contamination. In addition to these attributes, biomass should contain energy-dense or value-added biomolecules. The energy-dense biomolecules should be extracted and converted to biofuels efficiently, and the process should be benign to the environment. Several algal species are known to synthesize high-value secondary metabolites (SMs), such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and astaxanthin. However, selecting the most promising strain and enhancing their yield is a top priority.
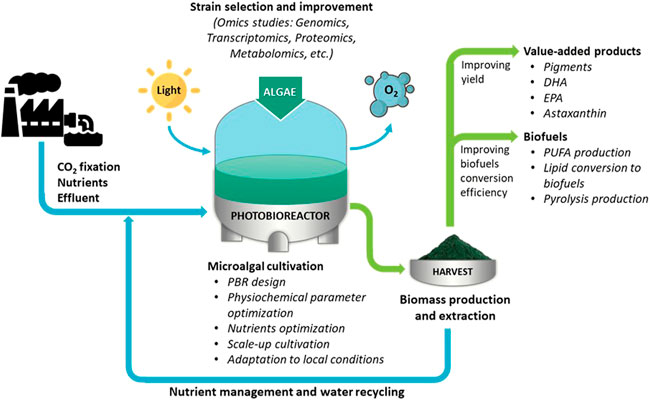
FIGURE 1. The challenges of algal technology for algal cultivation, biofuels production, and utilization of algal biomass to extract value-added products. PBR, photobioreactor; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PUFA, polyunsaturated fatty acids.
This Research Topic contains ten high-quality articles, which include nine original research articles and one review paper. Fifty two authors belonging to eight countries: India, China, Malaysia, Saudi Arabia, Australia, the United Kingdom, Belgium, and Canada, have participated in this Research Topic. Furthermore, some articles also show inter-country and inter-continental research collaborations.
The isolation of indigenous microalgal species, random mutation of them, and exploring psychrotolerant (low temperature) microalga for cold climate countries are some of the suggested strategies for sustainably improving the algal biomass productivity. Further, the algal cultivation process can be cost-effective and environment-friendly when applying a better nutrients management strategy and recycling spent water several times. Three original research articles are focused on microalgal cultivation for improving biomass production and CO2 fixation efficiency in a photobioreactor. Schoeters et al. cultivated the psychrotolerant snow alga Chloromonas typhlos at colder ambient temperatures in a 350 L pilot scale photobioreactor. Their primary purpose was to assess its biomass productivity and CO2 fixation efficiency. The study by Paquette et al. on cyanobacteria consortium at high pH and alkalinity revealed that spent cultivation medium could be reused at least five times by proper nutrient management without significantly inhibiting the biomass productivity. Farooq conducted a similar study of nutrient optimization (nitrogen and carbon dioxide) and water recycling during cultivation but used Parachlorella kessleri HY-6 as a model microalga. The author found the inhibitory effect of organics accumulated in the spent media for the subsequent cultivation and therefore applied activated carbon to remove the organics and improve the water recyclability. The water recycling strategy circumvents the water-intensive algal cultivation process, especially in arid countries.
Three original research articles describe the biofuels potential of microalgae. Jain et al. isolated several indigenous marine microalgae such as Nannochloropsis oculate, Chlorella sp., and Planophila sp., from the coast of the Arabian Sea. They explored their potential for polyunsaturated fatty acids (PUFA) synthesis, especially α-Linolenic acid. Similarly, Chen et al. isolated indigenous Euglena gracilis 815 and evaluated it as a new candidate for biodiesel production. The microalgal biomass of Gonium pectoral was a potential feedstock for pyrolysis. The study also assessed the CO2 biofixation potential along with pyrolytic kinetics of G. pectoral using kinetics modeling and an artificial neural network Altriki et al.
Four manuscripts containing three original research articles and one review article investigate the potential of microalgal biomass for secondary metabolites (SMs) production. The review summarized different genetic engineering studies targeting the improvement of microalgae for secondary metabolite production in microalgae (Sreenikethanam et al.). Three original research articles describe the production of SMs such as DHA, EPA, and astaxanthin from microalgae: Schizochytrium sp. (Zeng et al.), Nannochloropsis oculata (Razali et al.), and Chromochloris zofingiensis (Chen et al.), respectively. The mutagenesis on Schizochytrium sp. and N. oculate and nutrient optimization on C. zofingiensis enhanced the synthesis of SMs. The study on Schizochytrium sp., N. oculata, and C. zofingiensis was supported by metabolome, proteome, and bioprocess-based analysis, respectively. The atmospheric and room-temperature plasma (ARTP) mutagenesis combined with screening by iodoacetic acid and dehydroepiandrosterone enhanced the DHA synthesis in Schizochytrium sp. (Zeng et al.). The primary metabolites quantified using LC/MS correlated with the increased growth rate and DHA biosynthesis. The mutant strain produced the highest reported DHA concentration and productivity of 41.4 g L−1 and 430.7 mg L−1 h−1, respectively, at the end of 96 h fermentation. Similarly, the random mutagenesis coupled with the chemical (Ethyl methane sulfonate)-inhibitor-based selection method improved the EPA synthesis in the marine microalga, N. oculata (Razali et al.). The study also revealed the presence of alternative pathways for EPA synthesis when analyzed using label-free quantitative proteomics for differential protein expression. Based on Fatty acid methyl ester (FAME) data, the most suitable mutant strain had enhanced EPA content (1.7-fold) and EPA concentration (1.4-fold) compared to the wild type. Further, an effective two-stage heterotrophic cultivation of the unicellular green microalga, C. zofingiensis, enabled the synthesis of ultrahigh biomass and astaxanthin production (Chen et al.). C. zofingiensis was cultivated on a laboratory scale (7.5 L) and later scaled up in the 500 L fermenter. The combination of suitable concentrations of phytohormones gibberellic Acid-3 (GA3), C/N ratio, and NaCl enhanced the biomass and the astaxanthin synthesis. The highest biomass and astaxanthin yields were 235.4 g L−1 and 0.318 g L−1 (0.144% of DW), respectively. The astaxanthin yield was 5.4-fold more significant than the highest reported value in popular microalga, Haematococcus pluvialis. In this way, articles communicated in this Research Topic show significant improvement in the scientific knowledge base and contribute to the ongoing challenges of the commercialization of algal technology.
KK: writing, review, editing, and conceptualization. WK: figure drawing, and review. NK: writing and review. All authors contributed to the article and approved the submitted version.
The author KK would like to thank the CSIR–Director, Indian Institute of Integrative Medicine (IIIM), Jammu for providing the platform to continue this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: photobioreactor, cultivation, spent media, fatty acids, secondary metabolites (SMs)
Citation: Kumar K, Khetkorn W and Khanna N (2022) Editorial: Algal biomass and biofuels. Front. Bioeng. Biotechnol. 10:1008760. doi: 10.3389/fbioe.2022.1008760
Received: 31 August 2022; Accepted: 17 October 2022;
Published: 02 November 2022.
Edited and reviewed by:
Manfred Zinn, HES-SO Valais-Wallis, SwitzerlandCopyright © 2022 Kumar, Khetkorn and Khanna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanhaiya Kumar, ay5rdW1hcjIyMkBpaWltLnJlcy5pbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.