
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Bioeng. Biotechnol. , 13 December 2021
Sec. Biomaterials
Volume 9 - 2021 | https://doi.org/10.3389/fbioe.2021.811229
This article is part of the Research Topic Biomaterials In Asia View all 36 articles
Pseudoaneurysms of the common iliac artery caused by Brucellosis are exceedingly uncommon. Infected common iliac artery pseudoaneurysms, particularly those caused by brucellosis, are more difficult to diagnose and cure than general pseudoaneurysms. The risk of mortality is significantly high in this condition. Nonsurgical treatment of a brucellosis-induced common iliac artery pseudoaneurysm is futile, and it should be operated on as soon as feasible. Long-term and multi-course antibacterial therapy with combination antibiotics is required. For the treatment of Brucella-infected pseudoaneurysms, endovascular surgery can be both effective and safe.
Brucellosis is a zoonotic infection caused by bacteria of the genus Brucella (Wang et al., 2017; Amjadi et al., 2019; Bagheri Nejad et al., 2020; Iqbal et al., 2020). Fever, hyperhidrosis, fatigue, joint soreness, and lymphadenopathy (Jia et al., 2017; Liang et al., 2018; Alkahtani et al., 2020) are some of the clinical manifestations of the condition. Infection is spread mostly through infected animals and their products (Zhang et al., 2018; Willems et al., 2021). Brucella infection in humans is typically transmitted by direct contact with the skin and mucous membranes (Haque et al., 2011). However, on occasion, food-borne diseases are transferred through the digestive tract and infections are transmitted through the respiratory tract via inhalation of contaminated droplets and dust; humans are generally susceptible to Brucella. Additionally, brucellosis can have a detrimental effect on the respiratory, circulatory, digestive, and nervous systems. Endocarditis and pericarditis are the most prevalent disease symptoms of the circulatory system, while pseudoaneurysms involving the left common iliac artery are extremely rare. A pseudoaneurysm involving the common iliac artery that is infected is unstable. In medicine, it is frequently referred to as an “untimed bomb” implanted in the body that is likely to “explode” at any time, referring to aneurysm rupture and hemorrhage. Severe bleeding can result in hemorrhagic shock and lead to death. Herein, we report a rare instance of pseudoaneurysm affecting the left common iliac artery due to brucellosis and detail the treatment of this patient.
A 67-year-old man, a local farmer specializing in sheep and swine production, was admitted to the Department of Vascular Surgery of the First Bethune Hospital of Jilin University in July 2019 after experiencing back pain for 3 days. For 1 month, the patient developed a fever with a maximum temperature of 39°C, which was alleviated with non-steroidal anti-inflammatory medicines (NSAIDs). The course of the disease was accompanied by fatigue and hyperhidrosis, but there was no joint discomfort. Medical and family history were unremarkable. The skin and sclera of the patient were not yellowish, nor were his abdominal muscles tense, tender, or creating rebound pain. The patient’s liver and spleen were not palpable beneath the ribs, nor was the entire abdomen. Percussion and auscultation in the abdomen were normal. Physical examination revealed a temperature of 36.3°C, heart rate of 78/min, respiratory rate of 18/min, and blood pressure of 146/80 mmHg. Laboratory examination showed the following results: C-reactive protein, 8.19 mg/L; erythrocyte sedimentation rate, 29 mm/1 h; white blood cell count, 5.95 × 109/L; and hemoglobin, 127 g/L. All other laboratory tests were within normal limits. The Brucella tube agglutination test showed a titer of 1:400, and a solid and accurate diagnosis was made by isolating Brucella in the blood culture. We found pseudoaneurysm formation using computed tomography angiography (CTA) (Figures 1A,B). Accordingly, the patient was diagnosed with a brucellosis-related left common iliac artery pseudoaneurysm. The patient had no pseudoaneurysms elsewhere. To our knowledge, no previous report of a left common iliac artery pseudoaneurysm owing to brucellosis has been reported. Nonsurgical treatment of a common iliac artery pseudoaneurysm caused by brucellosis is ineffective, and it should be operated on as soon as possible. However, the patient underwent surgery while being infected systemically and then faced the possibility of reoperation and death following implant infection. Hence, a long-term and multi-course antibiotic treatment with combined antibacterial agents was required. Our patient was first treated for 6 weeks with doxycycline and rifampicin while preparing for emergency surgery at any time. After 6 weeks, the Brucella tube agglutination test was positive, and the blood culture of Brucella was negative. It represents a previous infection with Brucella. Endovascular aneurysm repair (EVAR) was performed under general anesthesia after the patient’s infection was controlled to prevent mortality and further complications from the left common iliac artery pseudoaneurysm. A TAG-covered stent graft (W. L. Gore & Associates, Inc., Flagstaff, AE, United States) was used to isolate the pseudoaneurysm. A 6F sheath (Terumo Corporation, Japan) was retrogradely inserted into the left common femoral artery intraoperatively. On digital subtraction angiography with a pigtail catheter, a pseudoaneurysm was identified (Figure 2A). Then, with the help of a Lunderquist® Extra-Stiff Wire Guide (Cook Medical Inc., Denmark), a 22F sheath (W. L. Gore & Associates, Inc., Flagstaff, AE, United States) was introduced and a 26 mm × 12 mm × 18 cm TAG-coated stent-graft and a 16 mm × 12 mm × 12 cm endoprosthesis contralateral leg (W. L. Gore & Associates, Inc., Flagstaff, AE, United States) were implanted to isolate the pseudoaneurysm. In the final angiography, no endoleaks were identified. The contrast agent went smoothly through the left common iliac artery without leakage (Figure 2B). The anti-brucellosis medication was maintained following surgery due to possible recurrence, and the patient’s painful symptoms progressively subsided after 3 days. After surgery, the patient was discharged from the hospital. There were no reports of relapse in the 1-month, 6-months, 1-year, and 2-years follow-up assessments. The patient had no other endovascular complications.
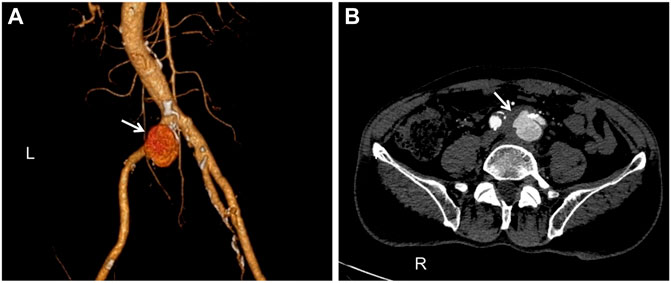
FIGURE 1. Computed tomography angiography (CTA): (A). The pseudoaneurysm has a maximum diameter of 33 mm and is placed near the commencement of the left common iliac artery (white arrow). (B). A breach is visible in the arterial wall, and there is an overflow of contrast agents (white arrow).
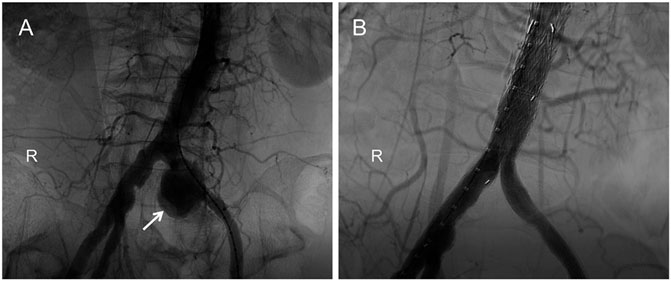
FIGURE 2. Digital subtraction angiography (DSA): (A). The pseudoaneurysm was observed by contrast-medium overflow (white arrow). (B). Postoperatively, angiography showed no contrast-agent spillage in the left common iliac artery.
Brucellosis is a zoonotic disease that is frequently transmitted from animals to humans, but is only rarely passed between humans (Colomba et al., 2012). Vascular pathological symptoms of brucellosis are infrequent (Willems et al., 2021). After bacterial colonization of the arterial wall (Amirghofran et al., 2011), the artery’s structure is damaged, which may result in life-threatening bleeding. Once ruptured, it can also be wrapped by surrounding soft tissues, resulting in the formation of pseudoaneurysms. Pseudoaneurysms produced by brucellosis are uncommon in clinical practice. Diagnosing and treating infected common iliac artery pseudoaneurysms, particularly those produced by Brucella, is more challenging than general pseudoaneurysms (Chiyoya et al., 2019). The incidence of positive blood cultures (particularly following antibiotic therapy) is clinically insignificant, and markers such as erythrocyte sedimentation rate, C-reactive protein (al-Kassab et al., 1991), and procalcitonin are not specific for infected pseudoaneurysms (Kayaaslan et al., 2016; Liu and Zhao, 2017). The signs and symptoms of the patient were not specific (Harman et al., 2004). Patients in some cases were asymptomatic. Owing to the fact that Brucella reproduces primarily in the cells of the human reticuloendothelial system, it is difficult to treat and relapses often. If pseudoaneurysms are not treated promptly, they might rupture at any time. As a result, it must be operated upon as soon as possible. Traditionally, aneurysmectomy, local debridement, and graft replacement have been used to treat this condition (Chakfé et al., 2020). Under general anesthesia, the patient had a major incision to reveal and excise the pseudoaneurysm and replace it with an artificial blood vessel. Pseudoaneurysms lack a full wall, making surgery challenging, posing a high risk of rupture, resulting in significant surgical stress and a protracted postoperative recovery time. While endovascular repair involves minimal trauma, has a low complication rate, and allows for rapid postoperative recovery (Willems et al., 2021), it has stringent criteria for correct stent placement and sealing. Primary treatment, in our opinion, should always be the first option in endovascular surgery. Because endovascular repair does not require anatomical or arterial incision, it significantly streamlines the surgery process. This method results in less bleeding and trauma; is well tolerated by the patient and is safe and effective; results in fewer problems; has a quicker recovery; and has other benefits that traditional surgery cannot match (Goodney et al., 2011). The primary advantage of endovascular repair for an infected left common iliac artery pseudoaneurysm is that it minimizes the risk of infection spreading. If endovascular repair is unsuccessful, conventional surgery to remove the iliac artery and rebuild arterial access can be undertaken. However, endograft infection and difficulty with secondary intervention are common issues for both intracavitary and open conventional surgery. Infected endografts are very difficult to treat, and often have poor prognosis (Laohapensang et al., 2017). Therefore, surgical therapy should begin as soon as the existing infection is managed. As a result, antibiotics must be used in combination. The World Health Organization recommends a 6-weeks course of doxycycline (100 mg, bid) and rifampicin (600–900 mg/d) (Fillmore and Valentine, 2003). Reasonable and standardized application of antibiotics can play a key role in the effective control of Brucella, thereby reducing the possibility of pseudoaneurysms in other blood vessels. Once the pseudoaneurysm appears, it should be treated as soon as possible to avoid aggravation. Therefore, regular physical examinations are necessary. To assess if a patient can wait for surgery following infection management, we consider two factors: 1) repeat CT to detect whether the pseudoaneurysm body has increased further; and 2) determine whether the patient’s symptoms such as back pain and fever have deteriorated. Following a repeat CT examination, our patient’s pseudoaneurysm body did not expand, and the patient’s symptoms progressively improved with anti-infective medication. In preparation for emergency surgery, we make every effort to complete an adequate antibiotic course. No bacterial growth was seen in blood cultures. After the patient’s infection was managed, EVAR was conducted. Antibiotic therapy was continued for 6 weeks following surgery. We recommend CT examination every 6 months. Although endovascular therapy has established itself as a safe and successful alternative to open surgery in the treatment of pseudoaneurysms, there is still a dearth of experience in this area. Simultaneously, medication therapy for brucellosis is crucial. Thus, we presented this case report to increase awareness of the condition, decrease the rate of missed diagnoses and misdiagnoses, and promote active and successful treatment and improve prognosis. Therefore, in individuals with a history of contact with cattle and sheep, long-term unexplained fever, and back and abdominal pain, Brucella-infection induced common iliac artery pseudoaneurysm should be considered in the differential diagnosis, and timely blood culture, antibody testing, and CTA, as well as other related checks should be performed to avoid life-threatening delays in treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the First Bethune Hospital of Jilin University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QW evaluated the patient and initiated the case report and reviewed the literature and drafted the manuscript; LT, ZC, QW, and PZ consulted the relevant literature and contributed to the diagnosis and treatment of the patient; YQ and PZ were responsible for formulating the patient’s treatment plan and revising the manuscript. All authors issued final approval for the version to be submitted.
This study was financially supported by the Natural Science Foundation of Jilin Province, China: No. 20200201353JC, No. 20210204157YY, and No. 20210101276JC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
al-Kassab, A. S., Nur, M. A., and Malik, J. M. (1991). Evaluation of Serum C-Reactive Protein in the Diagnosis of Arthritic and Non-arthritic Brucellosis. J. Trop. Med. Hyg. 94, 92–96.
Alkahtani, A. M., Assiry, M. M., Chandramoorthy, H. C., Al-Hakami, A. M., and Hamid, M. E. (2020). Sero-prevalence and Risk Factors of Brucellosis Among Suspected Febrile Patients Attending a Referral Hospital in Southern Saudi Arabia (2014-2018). BMC Infect. Dis. 20, 26. doi:10.1186/s12879-020-4763-z
Amirghofran, A. A., Karimi, A., Emaminia, A., Sharifkazemi, M. B., and Salaminia, S. (2011). Brucellosis Relapse Causing Prosthetic Valve Endocarditis and Aortic Root Infective Pseudoaneurysm. Ann. Thorac. Surg. 92, e77–e79. doi:10.1016/j.athoracsur.2011.03.144
Amjadi, O., Rafiei, A., Mardani, M., Zafari, P., and Zarifian, A. (2019). A Review of the Immunopathogenesis of Brucellosis. Infect. Dis. 51, 321–333. doi:10.1080/23744235.2019.1568545
Bagheri Nejad, R., Krecek, R. C., Khalaf, O. H., Hailat, N., and Arenas-Gamboa, A. M. (2020). Brucellosis in the Middle East: Current Situation and a Pathway Forward. Plos Negl. Trop. Dis. 14, e0008071. doi:10.1371/journal.pntd.0008071
Chakfé, N., Diener, H., Lejay, A., Assadian, O., Berard, X., Caillon, J., et al. (2020). Editor's Choice - European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Vascular Graft and Endograft Infections. Eur. J. Vasc. Endovascular Surg. 59, 339–384. doi:10.1016/j.ejvs.2019.10.016
Chiyoya, M., Kondo, N., Taniguchi, S., and Fukuda, I. (2019). Endovascular Treatment for a Perforated Superficial Femoral Vein Graft for an Infected Abdominal Aortic Aneurysm. Ann. Vasc. Dis. 12, 91–94. doi:10.3400/avd.cr.18-00095
Colomba, C., Siracusa, L., Rubino, R., Trizzino, M., Scarlata, F., Imburgia, C., et al. (2012). A Case ofBrucellaEndocarditis in Association with Subclavian Artery Thrombosis. Case Rep. Infect. Dis. 2012, 1–3. doi:10.1155/2012/581489
Fillmore, A. J., and Valentine, R. J. (2003). Surgical Mortality in Patients with Infected Aortic Aneurysms. J. Am. Coll. Surgeons 196, 435–441. doi:10.1016/s1072-7515(02)01607-1
Goodney, P. P., Travis, L., Lucas, F. L., Fillinger, M. F., Goodman, D. C., Cronenwett, J. L., et al. (2011). Survival after Open versus Endovascular Thoracic Aortic Aneurysm Repair in an Observational Study of the Medicare Population. Circulation 124, 2661–2669. doi:10.1161/circulationaha.111.033944
Haque, N., Bari, M. S., Hossain, M. A., Muhammad, N., Ahmed, S., Rahman, A., et al. (2011). An Overview of Brucellosis. Mymensingh Med. J. 20, 742–747.
Harman, M., Irmak, H., Arslan, H., Arslan, n., and Kayan, M. (2004). Popliteal Artery Pseudoaneurysm: a Rare Complication of Brucellosis. J. Clin. Ultrasound 32, 33–36. doi:10.1002/jcu.10217
Iqbal, M., Fatmi, Z., and Khan, M. (2020). Brucellosis in Pakistan: a Neglected Zoonotic Disease. J. Pak Med. Assoc. 70, 1625–1626. doi:10.5455/jpma.24139
Jia, B., Zhang, F., Lu, Y., Zhang, W., Li, J., Zhang, Y., et al. (2017). The Clinical Features of 590 Patients with Brucellosis in Xinjiang, China with the Emphasis on the Treatment of Complications. Plos Negl. Trop. Dis. 11, e0005577. doi:10.1371/journal.pntd.0005577
Kayaaslan, B., Bastug, A., Aydin, E., Akinci, E., But, A., Aslaner, H., et al. (2016). A Long-Term Survey of Brucellosis: Is There Any Marker to Predict the Complicated Cases? Infect. Dis. 48, 215–221. doi:10.3109/23744235.2015.1107187
Laohapensang, K., Arworn, S., Orrapin, S., Reanpang, T., and Orrapin, S. (2017). Management of the Infected Aortic Endograft. Semin. Vasc. Surg. 30, 91–94. doi:10.1053/j.semvascsurg.2017.11.001
Liang, C., Wei, W., Liang, X. W., Wang, L. J., Peng, L., and De, E. J. (2018). Occupational Characteristics and Clinical Manifestations of 245 Cases of Occupational Brucellosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 36, 755–758. doi:10.3760/cma.j.issn.1001-9391.2018.10.009
Liu, J., and Zhao, X. (2017). Clinical Features and Serum Profile of Inflammatory Biomarkers in Patients with Brucellosis. J. Infect. Dev. Ctries 11, 840–846. doi:10.3855/jidc.8872
Wang, S., Wang, Q., Liu, H., Sun, S., Sun, X., Zhang, Y., et al. (2017). Endovascular Treatment of Thoracic Aortic Pseudoaneurysm Due to Brucellosis: a Rare Case Report. BMC Infect. Dis. 17, 387. doi:10.1186/s12879-017-2485-7
Willems, S. A., Buntinx, M., Gelinck, L. B. S., van Schaik, J., and Eefting, D. (2021). Ruptured Aneurysm of the Common Iliac Artery Caused by Brucella Melitensis: A Case Report. EJVES Vasc. Forum 52, 26–29. doi:10.1016/j.ejvsvf.2021.06.011
Keywords: brucellosis, common iliac artery, pseudoaneurysm, endovascular surgery, case report
Citation: Wang Q, Tang L, Qin Y, Wang Q, Zhang P and Cheng Z (2021) Case Report: A Pseudoaneurysm Involving the Left Common Iliac Artery Secondary to Brucellosis: A Rare Case Report. Front. Bioeng. Biotechnol. 9:811229. doi: 10.3389/fbioe.2021.811229
Received: 08 November 2021; Accepted: 30 November 2021;
Published: 13 December 2021.
Edited by:
Mingqiang Li, Sun Yat-sen University, ChinaReviewed by:
Zilun Li, The First Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2021 Wang, Tang, Qin, Wang, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zhang, el9waW5nQGpsdS5lZHUuY24=; Zhihua Cheng, Y2hlbmd6aEBqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.