
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol., 16 November 2021
Sec. Biomaterials
Volume 9 - 2021 | https://doi.org/10.3389/fbioe.2021.759303
This article is part of the Research TopicBiomaterials with the regulation of reactive oxygen/nitrogen species for biomedical applicationsView all 21 articles
Microbial infections caused by wearing contact lenses has become a major health problem, so the design and development of antibacterial contact lenses has attracted widespread attention. To safely and effectively inhibit bacterial adhesion of contact lenses, we have facilely prepared epigallocatechin gallate (EGCG) loaded starch hydrogel/contact lens composites by in-situ free radical polymerization of the mixture containing 2-hydroxylethyl methacrylate, methacrylic acid and ethylene glycol dimethacrylate. The adequate transmittance of the resulting contact lenses was characterized by ultraviolet-visible spectrophotometry, and their satisfactory stability was examined using differential scanning calorimetry and thermogravimetric analysis. Whereafter, cytotoxicity and degradation experiments were performed to investigate the biocompatibility and degradability of the contact lenses. The results showed the nontoxicity and good degradability of the composites. Besides, the capacity of the contact lenses for in vitro release of EGCG was also evaluated, and the results showed that the EGCG in these contact lenses can be sustainably released for at least 14 days. Further bacterial adhesion assay suggested that the EGCG loaded starch hydrogel/contact lenses could significantly reduce the adhesion of Pseudomonas aeruginosa compared to the control. The EGCG loaded starch hydrogel/contact lens composites provide a potential intervention strategy for preventing ocular microbial infections and inhibiting bacterial keratitis.
There are more than 140 million contact lenses wearers all over the world (Stapleton et al., 2007). The contact lenses have been widely utilized in the vision correction of nearsightedness (myopia), far-sightedness (hyperopia), presbyopia and astigmatism. However, it can also cause several ocular discomforts, such as neovascularization, acute red eye and corneal abrasion (Kates and Tuli, 2021). More seriously, bacterial keratitis was easily induced during wearing or storage of contact lenses, especially in the case of corneal abrasion (Liu et al., 2020) due to the bacterial adhesion and biofilm formation on their surface. Pseudomonas aeruginosa (P. aeruginosa), an opportunistic Gram-negative pathogen, is the most common cause for the contact lens-associated bacterial keratitis (Hilliam et al., 2020; Spernovasilis et al., 2020). Biofilm growth of P. aeruginosa can enhance the tolerance to antibiotic agents and host immune responses, which involves regulation in its quorum sensing and expression of virulence genes (Liu et al., 2020). The resulting P. aeruginosa keratitis can lead to permanent vision loss or even eyeball removal without the prompt treatment (Vazirani et al., 2015; Fernandes et al., 2016). Therefore, it is of significance to develop the highly biocompatible and antimicrobial contact lenses for effective prevention of contact lens-associated bacterial keratitis (Seggio et al., 2019; Fleiszig et al., 2020; Meretoudi et al., 2021).
Due to the complex ocular surface structure and strong resistance to foreign body transport, the drug often stays on the ocular surface for a short time and has low bioavailability, resulting in unsatisfactory therapeutic effects (Gaudana et al., 2010). With the development of material technology, it is possible to drugs deliver to the ocular surface continuously using drug-loaded contact lenses as delivery carriers. With the limited tear exchange rate, the contact lenses can greatly increase the residence time of the drug on the ocular surface and improve the bioavailability (Xu et al., 2018).
Although drug-loaded contact lenses have broad application prospects, the safety and biocompatibility are worrisome. Edible polysaccharide starch has been widely used in the fields of pharmaceutical and biomedical engineering due to its wide sources, low cost, good biocompatibility and degradability (Pandey et al., 2015). It has been reported that the hydrophobic starch can be used as delivery carrier, and the drug can also be chemically bonded to the starch backbone for sustained release, which indicates that starch is an ideal and safe drug delivery system (Pandey et al., 2015; Kou et al., 2020).
EGCG, a member of the polyphenol family, is the main biologically active ingredient in green tea (Ouyang et al., 2020). With a wide range of biological activities including antioxidant, anti-tumor, anti-inflammatory, anti-fibrosis, anti-microbe, antivirus, anti-obesity, EGCG has been used in the prevention and treatment of bacterial infection, cancer and obesity research (Cui et al., 2012; Kwak et al., 2016; Suzuki et al., 2016; Basu et al., 2018; Crous-Masó et al., 2018). In recent years, it has been reported that EGCG can protect lens epithelial cells from UV damage, prevent tryptophan oxidation of γ-crystallin in cataract in the presence of H2O2, slow down retinal degeneration, and relieve dry eye inflammation in rabbits and mice (Heo et al., 2008; Peng et al., 2010; Lee et al., 2011; Chaudhury et al., 2015; Tseng et al., 2016; Perdices et al., 2020) due to its antioxidant, anti-inflammatory and anti-apoptotic activities. However, the antibacterial activity of EGCG has been little studied in ophthalmic drug delivery systems.
In this study, we firstly reported the preparation of EGCG loaded starch hydrogel/contact lens composites by in-situ free radical polymerization of the mixture containing 2-hydroxylethyl methacrylate, methacrylic acid, ethylene glycol dimethacrylate, AIBN, starch and EGCG. The resulting composites were assessed in their physical characterizations, in vitro EGCG release, in vitro biocompatibility and in vitro degradability. Then bacterial adhesion inhibition effects were further evaluated by bacterial adhesion assay (Figure 1).
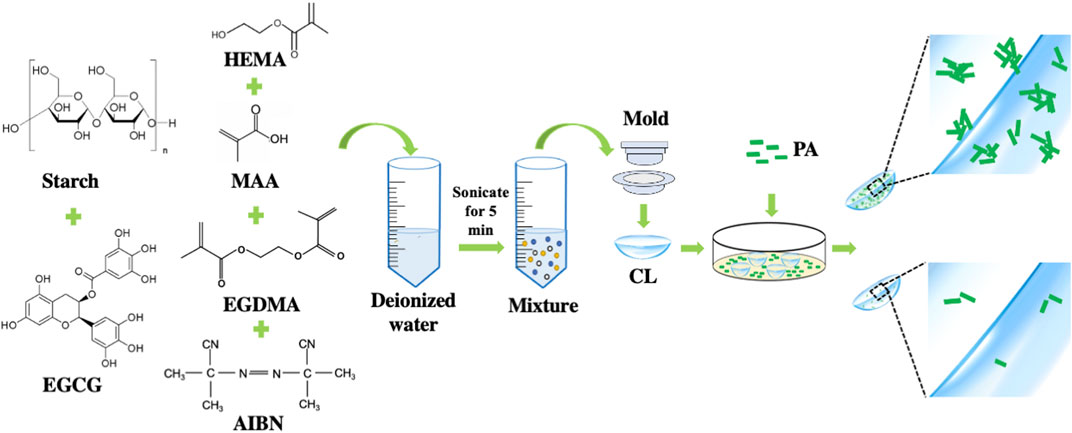
FIGURE 1. Schematic preparation of epigallocatechin gallate loaded starch hydrogel/contact lens composites.
2-Hydroxylethyl methacrylate (HEMA), Ipomoea batatas starch and EGCG were obtained from Macklin (Shanghai, China). Methacrylic acid (MAA) and ethylene glycol dimethacrylate (EGDMA) were obtained from ThermoFisher (United States). 2,2′-azobis (2-methylpropionitrile) (AIBN) was obtained from Sigma-Aldrich (United States) and deionized water was used in the study.
Different amounts of starch solution (33.3%, w%), EGCG (0, 0.3, 0.6 and 1%, w%), HEMA/MAA (70:30, 70 wt%), EGDMA (0.45 wt%), and AIBN (0.15 wt%) were mixed into the deionized water. The mixture was sonicated for 5 min, transferred into the contact lens molds, placed in a water-bath at 65°C for 30 h, and then boiled for 5 min to remove the unreacted monomers, affording contact lenses CL-0, CL-1, CL-2, CL-3. Conventional hydrogel contact lenses were prepared according to the same method mentioned above except the absence of starch (CL-1′, CL-2′, CL-3’) or both starch and EGCG (CL).
The optical transmittances of the contact lenses were measured with an ultraviolet-visible spectrophotometer (SpectraMax M2, Molecular Devices, MD, United States) at 50 nm intervals in the wavelength range of 250–800 nm. The contact lenses were removed from the molds, dried, and the dry weight (Wd) was recorded. Then immerse the contact lenses in deionized water to assess their expansion performance. Take out the lenses at regular intervals and drain the lenses surface completely with filter paper, and record their wet weight (Wt).
Differential scanning calorimetry (DSC) detection and thermogravimetric analysis (TGA) were performed through the synchronous thermal analyzer (TA DSC-TGA Q600, United States). The experiment was performed in the following conditions: nitrogen purity, 99.999%; temperature, 20–600°C; sample weight, 5–10 mg. Fourier transform infrared spectrum analysis, X-ray photoelectron spectroscopy, and other characterization tests were carried out for the contact lenses with or without starch.
The contact lenses were immersed into 4 ml of deionized water. At a predetermined time point, 1 ml of the release medium was taken out and replaced with an equal amount of fresh deionized water. NanoDrop One (Thermo, United States) was used to determine the content of EGCG at the wavelength of 278 nm. Then the cumulative release curve of EGCG with time was plotted by GraphPad Prism 9. The experiment was repeated three times.
The cytotoxicity of EGCG loaded starch hydrogel/contact lens composites was evaluated based on the effects of contact lenses extracts on the proliferation of human corneal epithelial cells (HCECs, Seoul, Korea) through cell counting kit-8 (CCK-8) assay. 2.5 and 5 mg of different type contact lenses were respectively dipped in 10 ml DMEM/F12 (Sigma-Aldrich) containing 10% (v/v) fetal bovine serum (Sigma-Aldrich) and 1% (v/v) antibiotic/antimycotics (Sigma-Aldrich) in 37°C, 5% CO2 incubator for 24 h to obtain contact lenses extracts with concentrations of 1:4 and 1:2 (Li et al., 2020). While the HCECs were plated into a 96-well plate at 2000/well and cultured in standard culture medium (DMEM/F12, 10% FBS, 1% antibiotic/antimycotics) for 24 h in 37°C, 5% CO2 incubator. Then, the culture medium of all the wells was taken out and different group cells were cultured with the prepared contact lens extracts along with fresh standard culture medium, respectively. After culturing in 37°C, 5% CO2 incubator for 1, 3, 5 and 7 days, CCK-8 detection reagent (Dojindo Laboratories Kumamoto, Japan) was added to measure the absorbance of the cells at 450 nm wavelength with SpectraMax M2 (Molecular Devices, United States).
The in vitro degradation of starch in the EGCG loaded starch hydrogel/contact lens composites was evaluated by Bacillus alpha-amylase (Solarbio, Beijing, China, pH 5.5–7.5, T 50–70°C). Briefly, the contact lenses were immersed in iodine solution for 2 min, and the color of contact lenses was observed by slit lamp microscope. The contact lenses were rinsed gently with deionized water to remove residual iodine solution, and then soaked in active alpha-amylase solution for enzymatic hydrolysis at 50°C for 24 h (0.1 mg/ml and 0.01 mg/ml, 10 ml). At estimated time points, the colorless lenses were taken out and softly rinsed with deionized water to remove residual amylase solution. After addition of iodine solution, the contact lenses were further photographed to detect the color reaction of starch and iodine.
P. aeruginosa ATCC 19660 inoculum was cultured on LB agar medium at 37°C, while planktonic culture was grown with an initial optical density (OD) of ∼0.02 and shaking in an incubator at 37°C for 24 h (Li et al., 2020). The bacteria were washed with PBS and then serially diluted to 106 CFU/ml. The contact lenses were softly washed with PBS in 24 pore culture plate, and added in 1 ml prepared bacterial suspension, then incubated at 37°C and 120 rpm for 24 h. After incubation, the cocultures of the contact lenses and bacteria were photographed and the number of bacteria left on the lenses was counted. In brief, contact lenses were gently rinsed 3 times with PBS to remove loosely adhered bacteria, then grinded to detect the number of adhered bacteria by plate counting (Salvagni et al., 2020).
Statistical analysis was performed using Kruskal–Wallis test with Statistical Package for Social Sciences software (version 24.0). All the data were obtained from at least three independent experiments. The descriptive statistics were presented as the mean ± SD. p < 0.05 was considered a statistically significant difference.
Microbial infection is a common problem associated with contact lenses, and poor sanitary conditions and overnight wearing contact lenses are the main risk factors for microbial keratitis (Cope et al., 2015; Cope et al., 2017). Long-term contact between cornea and contact lenses will affect the nutrient exchange of normal corneal epithelial cells, causing ocular discomfort or serious complications (Forte et al., 2010; Lim et al., 2018). Hypoxia environment of ocular surface can destruct the integrity of extracellular matrix proteins, promote bacterial adhesion, and provide a good condition for opportunistic pathogens to invade the cornea (Forte et al., 2010). In recent years, with the development of drug delivery, the design and exploitation of antimicrobial contact lenses had attracted extensive attention.
In-situ free radical polymerization is a common method for preparation of therapeutic contact lenses by addition of bioactive ingredients. In this study, antibacterial contact lenses were facilely prepared by thermally initiated polymerization of the mixture containing starch, EGCG and conventional hydrogel components. Starch significantly improves the sustained release performance of conventional hydrogel contact lenses without changing the basic physical properties.
The contact lenses were prepared according to specific ratio of different ingredients as reported previously (Dursch et al., 2014). In short, different amounts of EGCG (0, 0.3, 0.6 and 1%, w/w), hydrogel solution and AIBN were added to the starch solution and prepared into CL-0, CL-1, CL-2, CL-3 using specific contact lenses models, while CL was prepared as negative control.
The addition of starch was confirmed through element analysis as shown in Figure 2. The initial carbon and oxygen content in CL was 69.08 and 30.92%, respectively. After adding starch, the carbon content increased to 76.58% and the oxygen content reduced to 23.42%, indicating the successful preparation of starch hydrogel/contact lenses. Studies have confirmed that the natural polymers are non-toxic and biodegradable and can bind non-specifically or specifically to proteins and cells, which are the ideal materials for hydrogel-based sustained-release systems or cellular carriers (Kirchhof et al., 2015). As a kind of natural polysaccharide with wide sources and good biocompatibility, starch is widely used in pharmaceutical and biomedical engineering fields, which was selected as the component of the contact lenses in present study (Pandey et al., 2015; Hadisi et al., 2018; Kou et al., 2020). Based on our data, the addition of starch to the hydrogel material does not affect the basic properties of contact lenses such as transparency, optical transmittance, swelling, thermal stability and thermoplasticity.
The optical transmittance data of contact lenses with different treatments are shown in Figure 3. Compared with the negative control CL, there was no significant difference in transmittance between CL-0 and CL group, while the optical transmittance of starch hydrogel/contact lenses (CL-0) decreased slightly (Figure 3), indicating that the addition of starch had little effect on transmittance. In EGCG group, with the addition of EGCG, the color of contact lenses became yellowish and the optical transmittance gradually decreased (CL-1, 84.00 ± 0.72%; CL-2, 77.70 ± 0.90%; CL-3, 54.37 ± 0.53%; Supplementary Figure S1, Figure 3). Furthermore, swelling test results confirmed that the swelling rate of CL-0 group (21.92 ± 1.30%) was higher than that of CL group (18.88 ± 2.10%) after immersion in deionized water for 24 h, (Figure 4A). It was indicated that starch improved the swelling ratio of contact lenses to a certain extent.
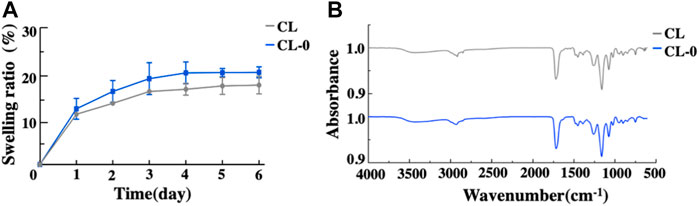
FIGURE 4. Swelling ratio (A) and Fourier transform infrared spectroscopy analysis of the contact lenses (B).
Fourier transform infrared spectroscopy analysis showed that the corresponding functional groups of starch hydrogel contact lens did not change significantly. In addition, the characteristic peak of starch infrared absorption spectrum didn’t appear due to the low starch content (Figure 4B). Figure 5A showed the similar TGA curves of CL and CL-0 groups. It was clearly found that both samples could maintain 90% of weight even the temperature rose to 300°C. Furthermore, the DSC melting curve of CL group was similar as that of CL-0. The addition of starch has no significant effect on the material fusibility (Figure 5B). These results confirmed the good thermal stability and thermoplasticity of EGCG loaded starch hydrogel/contact lens composites.
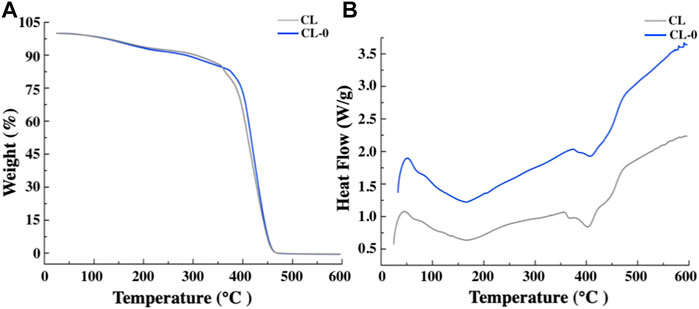
FIGURE 5. Thermogravimetric analysis (A) and Differential scanning calorimetry test of the contact lenses (B).
Biosafety is a great challenge in the development of functional contact lenses. Table 1 lists the research representatives on antimicrobial contact lenses recently, showing that a variety of antimicrobial components are loaded on different types of contact lenses. These contact lenses mainly bind variable antibacterial materials to carriers such as hydrogels through in-situ synthesis, coating or covalently attachment. Although functional contact lenses containing antibiotics, non-steroidal anti-inflammatory drugs or silver ions have significant antibacterial properties, they could cause certain adverse reactions, such as the emergence of drug-resistant strains, eye irritation, and biocompatibility problems of metal ions (Silva et al., 2018; Xiao et al., 2018; Hoyo et al., 2019; Nahum et al., 2019; Khan and Lee, 2020; Salvagni et al., 2020). In this study, cytotoxicity test was investigated to evaluate the cytocompatibility of EGCG-loaded starch hydrogel/contact lenses. After different types of contact lenses were immersed in standard medium for 24 h, the cytotoxicity of contact lenses extracts against HCECs were detected. Based on absorbance data of the solution at 450 nm wavelength, it was showed that contact lens CL-0, CL-1, CL-2 and CL-3 extracts had no inhibitory effect on the proliferation of HCECs compared with untreated HCECs and CL group (Figure 6). There was no significant difference in the absorbance between ECGC groups. Those results demonstrated that EGCG loaded starch hydrogel/contact lens composite exhibited no obvious cytotoxicity, which makes it possible to be applied in the medical field in the future.
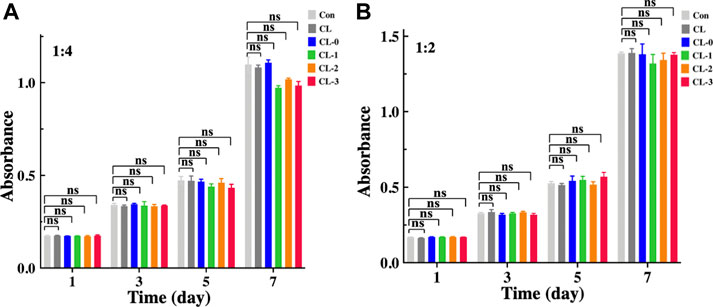
FIGURE 6. Cytotoxicity and proliferation activities of the contact lenses on human corneal epithelial cells (HCECs). Cell counting kit-8 analysis of HCECs seeded in the contact lenses extracts with a concentration of 1:4 (A) and 1:2 (B), n = 3.
In order to evaluate the degradability of materials, an in vitro alpha-amylase degradability detection of the composites was performed. The EGCG starch hydrogel/contact lens composite (CL-1) was stained with iodine solution and showed dark blue spots on the surface. After incubation with active alpha-amylase solution, the amounts of blue spots on the surface decreased with the increase of enzymatic hydrolysis time. The blue spots on the lenses disappeared after 12 h of enzymatic hydrolysis using the 0.1 mg/ml alpha-amylase solution, while there were still a few blue spots on the lenses after 24 h of enzymatic hydrolysis using the 0.01 mg/ml alpha-amylase solution (Figure 7). Above all, it was suggested that the EGCG starch hydrogel/contact lens composites had good degradability.
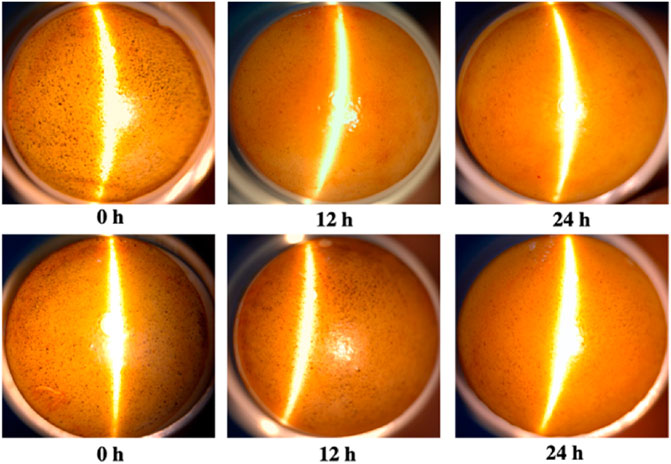
FIGURE 7. In vitro degradation of epigallocatechin gallate loaded starch hydrogel/contact lens composites by alpha-amylase. The concentration of active alpha-amylase was 0.1 mg/ml (A) and 0.01 mg/ml (B).
EGCG is the main biologically active ingredient in tea, which has a wide range of antioxidant, anti-tumor, anti-inflammatory, anti-fibrosis, anti-microbe, antivirus, anti-obesity and other activities (Cui et al., 2012; Kwak et al., 2016; Suzuki et al., 2016; Basu et al., 2018; Crous-Masó et al., 2018; Ouyang et al., 2020). In view of the safety and eligible biocompatibility of contact lens extracts against HCECs, we further tested the EGCG release of those contact lenses. After being immersed in deionized water, the cumulative release amount of EGCG from the EGCG loaded starch hydrogel/contact lenses was detected at different time points (Figure 8). The results exhibited that the EGCG in these contact lenses (CL-1, CL-2, CL-3) can be sustainably released for at least 14 days. The durations are obviously longer than those (less than 4 days) in conventional hydrogel contact lenses (Figure 8A). With the increase of EGCG amount in contact lenses, the cumulative release of EGCG continuously increased from 82.79 ± 5.77 to 143.97 ± 5.77 ug (Figure 8B).
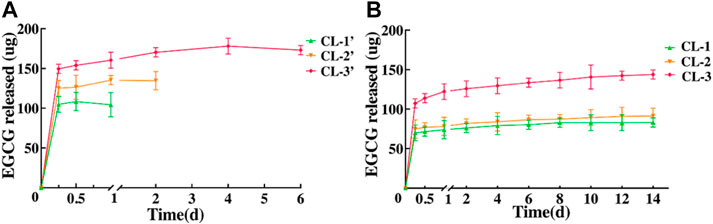
FIGURE 8. In vitro EGCG release from conventional hydrogel contact lenses (A) and starch hydrogel/contact lenses (B).
It has been reported that drug delivery systems with good sustained release properties, such as peptide-functionalized contact lenses, have a sustained-release time of approximately 7 days (Salvagni et al., 2020). In this study, the sustained-release capacity of EGCG loaded starch hydrogel/contact lens composites were greatly improved. It was indicated that the properties of drug delivery of the contact lenses in this study were effectively improved compared with the commercial CL (Table 1). Extending the drug release time of lenses plays an important role in the long-term inhibition of bacterial adhesion, which is beneficial for prolonging the safe wearing time of contact lenses in the future.
Many in vitro studies have shown that P. aeruginosa had the strongest adhesion to silicone hydrogel and hydrogel contact lenses, reaching the maximum adhesion of bacterial within 1 h, and forming biofilm within 24 h (Dutta et al., 2012). The formation of biofilms relies on the bacterial quorum sensing system to sense and regulate the diffusion signal molecules related to the bacterial population density and the expression of virulence factors, thus providing an ecosystem for bacteria to enhance their virulence and drug resistance (Jimenez et al., 2012).
To examine the antimicrobial activity of those contact lenses, bacterial adhesion assay against P. aeruginosa ATCC 19660 was performed. After 24 h of incubation with P. aeruginosa, the amounts of bacteria adhered onto lenses were quantified. Compared with CL and CL-0, the coculture liquid of CL-1, CL-2 and CL-3 was more clearly and the amount of bacterial adhesion was significantly reduced (p < 0.05) (Supplementary Figure S2). Compared with CL and CL-0, the amounts of bacterial adhesion on CL-1, CL-2, CL-3 are significantly reduced (p < 0.05). With the increase of EGCG content, the amount of bacterial adhesion on contact lenses decreased gradually (CL-1, 4531 ± 4100; CL-2, 3967 ± 2517; CL-3, 1967 ± 751) (Figure 9). These results indicated that the composites could effectively inhibit the adhesion of P. aeruginosa, which provides the possibility to reduce the incidence of contact lens induced P. aeruginosa keratitis in the future.
In summary, the EGCG loaded starch hydrogel/contact lens composites have been successfully prepared by in-situ free radical polymerization of the mixture containing HEMA, MAA, EGDMA, starch and EGCG. The resulting composites exhibited favorable biocompatibility and superior drug release ability for EGCG at least 14 days, which processed enhanced sustained release capacity of EGCG than conventional hydrogel contact lens (4 days). Hence, they can effectively inhibit the adhesion of P. aeruginosa on the lenses, which is beneficial for prolonging the safe wearing of contact lenses and provides the potential for the clinical therapy of bacterial keratitis in the future. Furthermore, the study enriches the research scope of functional contact lenses, and various natural products with specific activities can be introduced in the preparation of therapeutic contact lenses for treatment of other ophthalmological diseases.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
LZ: write manuscript and do the experiment; HW: do statistical analysis and material synthesis; CF: consult literature and do cell experiment; FS: design the experiments, review and edit the manuscript; XD: design and fund experiment.
This work was supported by the Academic Promotion Program of Shandong First Medical University (2019ZL001); Qingdao Science and Technology Project (19-6-1-24-nsh); National Natural Science Foundation of China (82000852).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.759303/full#supplementary-material
Basu, A., Masek, E., and Ebersole, J. (2018). Dietary Polyphenols and Periodontitis-A Mini-Review of Literature. Molecules 23, 1786. doi:10.3390/molecules23071786
Bin Sahadan, M. Y., Tong, W. Y., Tan, W. N., Leong, C. R., Bin Misri, M. N., Chan, M., et al. (2019). Phomopsidione Nanoparticles Coated Contact Lenses Reduce Microbial Keratitis Causing Pathogens. Exp. Eye Res. 178, 10–14. doi:10.1016/j.exer.2018.09.011
Casciaro, B., Dutta, D., Loffredo, M. R., Marcheggiani, S., McDermott, A. M., Willcox, M. D., et al. (2018). Esculentin‐1a Derived Peptides killPseudomonas Aeruginosabiofilm on Soft Contact Lenses and Retain Antibacterial Activity upon Immobilization to the Lens Surface. Pept. Sci. 110, e23074. doi:10.1002/bip.23074
Chaudhury, S., Ghosh, I., Saha, G., and Dasgupta, S. (2015). EGCG Prevents Tryptophan Oxidation of Cataractous Ocular Lens Human γ-crystallin in Presence of H2O2. Int. J. Biol. Macromolecules 77, 287–292. doi:10.1016/j.ijbiomac.2015.03.040
Cope, J. R., Collier, S. A., Nethercut, H., Jones, J. M., Yates, K., and Yoder, J. S. (2017). Risk Behaviors for Contact Lens-Related Eye Infections Among Adults and Adolescents - United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 66, 841–845. doi:10.15585/mmwr.mm6632a2
Cope, J. R., Collier, S. A., Rao, M. M., Chalmers, R., Mitchell, G. L., Richdale, K., et al. (2015). Contact Lens Wearer Demographics and Risk Behaviors for Contact Lens-Related Eye Infections - United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 64, 865–870. doi:10.15585/mmwr.mm6432a2
Crous-Masó, J., Palomeras, S., Relat, J., Camó, C., Martínez-Garza, Ú., Planas, M., et al. (2018). (−)-Epigallocatechin 3-Gallate Synthetic Analogues Inhibit Fatty Acid Synthase and Show Anticancer Activity in Triple Negative Breast CancerEpigallocatechin 3-gallate Synthetic Analogues Inhibit Fatty Acid Synthase and Show Anticancer Activity in Triple Negative Breast Cancer. Molecules 23, 1160. doi:10.3390/molecules23051160
Cui, Y., Oh, Y. J., Lim, J., Youn, M., Lee, I., Pak, H. K., et al. (2012). AFM Study of the Differential Inhibitory Effects of the green tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Gram-Positive and Gram-Negative Bacteria. Food Microbiol. 29, 80–87. doi:10.1016/j.fm.2011.08.019
Dursch, T. J., Taylor, N. O., Liu, D. E., Wu, R. Y., Prausnitz, J. M., and Radke, C. J. (2014). Water-soluble Drug Partitioning and Adsorption in HEMA/MAA Hydrogels. Biomaterials 35, 620–629. doi:10.1016/j.biomaterials.2013.09.109
Dutta, D., Cole, N., and Willcox, M. (2012). Factors Influencing Bacterial Adhesion to Contact Lenses. Mol. Vis. 18, 14–21.
Fernandes, M., Vira, D., Medikonda, R., and Kumar, N. (2016). Extensively and Pan-Drug Resistant Pseudomonas aeruginosa Keratitis: Clinical Features, Risk Factors, and Outcome. Graefes Arch. Clin. Exp. Ophthalmol. 254, 315–322. doi:10.1007/s00417-015-3208-7
Fleiszig, S. M. J., Kroken, A. R., Nieto, V., Grosser, M. R., Wan, S. J., Metruccio, M. M. E., et al. (2020). Contact Lens-Related Corneal Infection: Intrinsic Resistance and its Compromise. Prog. Retin. Eye Res. 76, 100804. doi:10.1016/j.preteyeres.2019.100804
Forte, R., Cennamo, G., Del Prete, S., Cesarano, I., and Del Prete, A. (2010). Scanning Electron Microscopy of Corneal Epithelium in Soft Contact Lens Wearers. Cornea 29, 732–736. doi:10.1097/ico.0b013e3181c32f1a
Gaudana, R., Ananthula, H. K., Parenky, A., and Mitra, A. K. (2010). Ocular Drug Delivery. AAPS J. 12, 348–360. doi:10.1208/s12248-010-9183-3
Guo, Y., Qian, S., Wang, L., Zeng, J., Miao, R., Meng, Y., et al. (2020). Reversible Antibiotic Loading and pH-Responsive Release from Polymer Brushes on Contact Lenses for Therapy and Prevention of Corneal Infections. J. Mater. Chem. B 8, 10087–10092. doi:10.1039/d0tb01508c
Hadisi, Z., Nourmohammadi, J., and Nassiri, S. M. (2018). The Antibacterial and Anti-inflammatory Investigation of Lawsonia Inermis-Gelatin-Starch Nano-Fibrous Dressing in Burn Wound. Int. J. Biol. Macromol 107, 2008–2019. doi:10.1016/j.ijbiomac.2017.10.061
Heo, J., Lee, B. R., and Koh, J.-W. (2008). Protective Effects of Epigallocatechin Gallate after UV Irradiation of Cultured Human Lens Epithelial Cells. Korean J. Ophthalmol. 22, 183–186. doi:10.3341/kjo.2008.22.3.183
Hilliam, Y., Kaye, S., and Winstanley, C. (2020). Pseudomonas aeruginosa and Microbial Keratitis. J. Med. Microbiol. 69, 3–13. doi:10.1099/jmm.0.001110
Hoyo, J., Ivanova, K., Guaus, E., and Tzanov, T. (2019). Multifunctional ZnO NPs-Chitosan-Gallic Acid Hybrid Nanocoating to Overcome Contact Lenses Associated Conditions and Discomfort. J. Colloid Interf. Sci. 543, 114–121. doi:10.1016/j.jcis.2019.02.043
Huang, J.-F., Zhong, J., Chen, G.-P., Lin, Z.-T., Deng, Y., Liu, Y.-L., et al. (2016). A Hydrogel-Based Hybrid Theranostic Contact Lens for Fungal Keratitis. ACS Nano 10, 6464–6473. doi:10.1021/acsnano.6b00601
Kates, M. M., and Tuli, S. (2021). Complications of Contact Lenses. JAMA 325 (18), 1912. doi:10.1001/jama.2020.20328
Khan, S. A., and Lee, C.-S. (2020). Recent Progress and Strategies to Develop Antimicrobial Contact Lenses and Lens Cases for Different Types of Microbial Keratitis. Acta Biomater. 113, 101–118. doi:10.1016/j.actbio.2020.06.039
Kirchhof, S., Goepferich, A. M., and Brandl, F. P. (2015). Hydrogels in Ophthalmic Applications. Eur. J. Pharmaceutics Biopharmaceutics 95 (Pt B), 227–238. doi:10.1016/j.ejpb.2015.05.016
Kou, Z., Dou, D., Lan, L., Zhang, J., Lan, P., Yu, Q., et al. (2020). Preparation, Characterization, and Performance Analysis of Starch-Based Nanomicelles. Int. J. Biol. Macromolecules 145, 655–662. doi:10.1016/j.ijbiomac.2019.12.220
Kwak, T. W., Park, S.-B., Kim, H.-J., Jeong, Y.-I., and Kang, D. H. (2016). Anticancer Activities of Epigallocatechin-3-Gallate against Cholangiocarcinoma Cells. Ott 10, 137–144. doi:10.2147/ott.s112364
Lee, H. S., Chauhan, S. K., Okanobo, A., Nallasamy, N., and Dana, R. (2011). Therapeutic Efficacy of Topical Epigallocatechin Gallate in Murine Dry Eye. Cornea 30, 1465–1472. doi:10.1097/ico.0b013e31821c9b5a
Li, H., Zhao, L., Wang, F., Wang, H., Dong, M., Liu, T., et al. (2020). Natural Cross-Linker-Stabilized Acellular Porcine Corneal Stroma for Lamellar Keratoplasty. Acta Biomater. 114, 270–284. doi:10.1016/j.actbio.2020.07.035
Li, J., Ma, X., Zhao, L., Li, Y., Zhou, Q., and Du, X. (2020). Extended Contact Lens Wear Promotes Corneal Norepinephrine Secretion and Pseudomonas aeruginosa Infection in Mice. Invest. Ophthalmol. Vis. Sci. 61, 17. doi:10.1167/iovs.61.4.17
Lim, C. H. L., Stapleton, F., and Mehta, J. S. (2018). Review of Contact Lens-Related Complications. Eye Contact Lens 44 (Suppl. 2), S1–S10. doi:10.1097/ICL.0000000000000481
Liu, G., Li, K., Wang, H., Ma, L., Yu, L., and Nie, Y. (2020). Stable Fabrication of Zwitterionic Coating Based on Copper-Phenolic Networks on Contact Lens with Improved Surface Wettability and Broad-Spectrum Antimicrobial Activity. ACS Appl. Mater. Inter. 12 (14), 16125–16136. doi:10.1021/acsami.0c02143
Meretoudi, A., Banti, C. N., Raptis, P. K., Papachristodoulou, C., Kourkoumelis, N., Ikiades, A. A., et al. (2021). Silver Nanoparticles from Oregano Leaves' Extracts as Antimicrobial Components for Non-infected Hydrogel Contact Lenses. Ijms 22, 3539. doi:10.3390/ijms22073539
Nadal Jimenez, P., Koch, G., Thompson, J. A., Xavier, K. B., Cool, R. H., and Quax, W. J. (2012). The Multiple Signaling Systems Regulating Virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 76, 46–65. doi:10.1128/mmbr.05007-11
Nahum, Y., Israeli, R., Mircus, G., Perelshtein, I., Ehrenberg, M., Gutfreund, S., et al. (2019). Antibacterial and Physical Properties of a Novel Sonochemical-Assisted Zn-CuO Contact Lens Nanocoating. Graefes Arch. Clin. Exp. Ophthalmol. 257, 95–100. doi:10.1007/s00417-018-4172-9
Ouyang, J., Zhu, K., Liu, Z., and Huang, J. (2020). Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid Med. Cel Longev 2020, 9723686. doi:10.1155/2020/9723686
Pandey, S., Malviya, R., and Sharma, P. K. (2015). Applicability, Commercial Utility and Recent Patents on Starch and Starch Derivative as Pharmaceutical Drug Delivery Carrier. Recent Pat Drug Deliv. Formul 9, 249–256. doi:10.2174/1872211309666150724101454
Peng, P.-H., Chiou, L.-F., Chao, H.-M., Lin, S., Chen, C.-F., Liu, J.-H., et al. (2010). Effects of Epigallocatechin-3-Gallate on Rat Retinal Ganglion Cells after Optic Nerve Axotomy. Exp. Eye Res. 90, 528–534. doi:10.1016/j.exer.2010.01.007
Perdices, L., Fuentes-Broto, L., Segura, F., Cuenca, N., Orduna-Hospital, E., and Pinilla, I. (2020). Epigallocatechin Gallate Slows Retinal Degeneration, Reduces Oxidative Damage, and Modifies Circadian Rhythms in P23H Rats. Antioxidants 9, 718. doi:10.3390/antiox9080718
Salvagni, E., García, C., Manresa, À., Müller-Sánchez, C., Reina, M., Rodríguez-Abreu, C., et al. (2020). Short and Ultrashort Antimicrobial Peptides Anchored onto Soft Commercial Contact Lenses Inhibit Bacterial Adhesion. Colloids Surf. B: Biointerfaces 196, 111283. doi:10.1016/j.colsurfb.2020.111283
Seggio, M., Nostro, A., Ginestra, G., Quaglia, F., and Sortino, S. (2019). Contact Lenses Delivering Nitric Oxide under Daylight for Reduction of Bacterial Contamination. Ijms 20, 3735. doi:10.3390/ijms20153735
Shayani Rad, M., Khameneh, B., Sabeti, Z., Mohajeri, S. A., and Fazly Bazzaz, B. S. (2016). Antibacterial Activity of Silver Nanoparticle-Loaded Soft Contact Lens Materials: the Effect of Monomer Composition. Curr. Eye Res. 41, 1286–1293. doi:10.3109/02713683.2015.1123726
Silva, D., Sousa, H. C. d., Gil, M. H., Santos, L. F., Moutinho, G. M., Serro, A. P., et al. (2018). Antibacterial Layer-By-Layer Coatings to Control Drug Release from Soft Contact Lenses Material. Int. J. Pharmaceutics 553, 186–200. doi:10.1016/j.ijpharm.2018.10.041
Spernovasilis, N., Maraki, S., Kokorakis, E., Kofteridis, D., Tsilimbaris, M., Siganos, C., et al. (2020). Antimicrobial Susceptibility of Isolated Pathogens from Patients with Contact Lens-Related Bacterial Keratitis in crete, greece: a Ten-Year Analysis. Cont Lens Anterior Eye S1367-0484 (20), 30139–9. doi:10.1016/j.clae.2020.07.006
Stapleton, F., Keay, L., Jalbert, I., and Cole, N. (2007). The Epidemiology of Contact Lens Related Infiltrates. Optom. Vis. Sci. 84, 257–272. doi:10.1097/OPX0b013e3180485d5f
Suzuki, T., Pervin, M., Goto, S., Isemura, M., and Nakamura, Y. (2016). Beneficial Effects of tea and the green tea Catechin Epigallocatechin-3-Gallate on Obesity. Molecules 21, 1305. doi:10.3390/molecules21101305
Tseng, C.-L., Hung, Y.-J., Chen, Z.-Y., Fang, H.-W., and Chen, K.-H. (2016). Synergistic Effect of Artificial Tears Containing Epigallocatechin Gallate and Hyaluronic Acid for the Treatment of Rabbits with Dry Eye Syndrome. PloS one 11, e0157982. doi:10.1371/journal.pone.0157982
Ubani-Ukoma, U., Gibson, D., Schultz, G., Silva, B. O., and Chauhan, A. (2019). Evaluating the Potential of Drug Eluting Contact Lenses for Treatment of Bacterial Keratitis Using an Ex Vivo Corneal Model. Int. J. Pharmaceutics 565, 499–508. doi:10.1016/j.ijpharm.2019.05.031
Vazirani, J., Wurity, S., and Ali, M. H. (2015). Multidrug-Resistant Pseudomonas aeruginosa Keratitis. Ophthalmology 122, 2110–2114. doi:10.1016/j.ophtha.2015.06.007
Xiao, A., Dhand, C., Leung, C. M., Beuerman, R. W., Ramakrishna, S., and Lakshminarayanan, R. (2018). Strategies to Design Antimicrobial Contact Lenses and Contact Lens Cases. J. Mater. Chem. B 6, 2171–2186. doi:10.1039/c7tb03136j
Keywords: starch hydrogel, composite, contact lens, epigallocatechin gallate, antibacterial activity
Citation: Zhao L, Wang H, Feng C, Song F and Du X (2021) Preparation and Evaluation of Starch Hydrogel/Contact Lens Composites as Epigallocatechin Gallate Delivery Systems for Inhibition of Bacterial Adhesion. Front. Bioeng. Biotechnol. 9:759303. doi: 10.3389/fbioe.2021.759303
Received: 16 August 2021; Accepted: 28 October 2021;
Published: 16 November 2021.
Edited by:
Qihui Zhou, Qingdao University, ChinaReviewed by:
Lilong Gao, Qingdao University, ChinaCopyright © 2021 Zhao, Wang, Feng, Song and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangying Song, c29uZ2Zhbmd5aW5nX0AxMjYuY29t; Xianli Du, bGlsaWJlc3RldmVyQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.