- 1Chinese-German Joint Laboratory for Natural Product Research, College of Biological Science and Engineering, Shaanxi University of Technology, Hanzhong, China
- 2Centre of Molecular and Environmental Biology, University of Minho, Department of Biology, Braga, Portugal
- 3Department of Biomedical Sciences, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
- 4Department of Microbiology, PSG College of Arts & Science, Bharathiar University, Coimbatore, India
Egg, a highly nutritious food, contains high-quality proteins, vitamins, and minerals. This food has been reported for its potential pharmacological properties, including antibacterial, anti-cancer, anti-inflammatory, angiotensin-converting enzyme (ACE) inhibition, immunomodulatory effects, and use in tissue engineering applications. The significance of eggs and their components in disease prevention and treatment is worth more attention. Eggs not only have been known as a “functional food” to combat diseases and facilitate the promotion of optimal health, but also have numerous industrial applications. The current review focuses on different perceptions and non-food applications of eggs, including cosmetics. The versatility of eggs from an industrial perspective makes them a potential candidate for further exploration of several novel components.
Systematic Review Registration: [website], identifier [registration number].
Highlights
1. Biologically active ingredients of hen eggs are widely used in medicine/veterinary medicine.
2. Egg ingredients have been widely used in bio-industries.
3. Transgenetic henscan produce eggs with large number of specific proteins/peptides of need.
4. Nonfood uses of hen eggs needs to be highlighted in egg research and egg industry.
Introduction
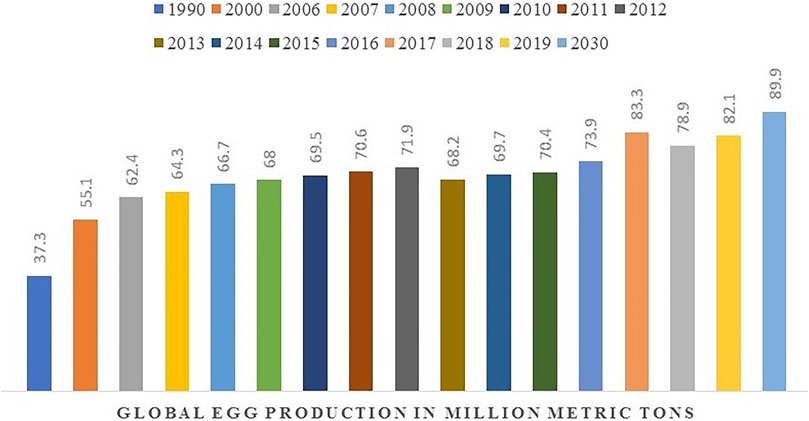
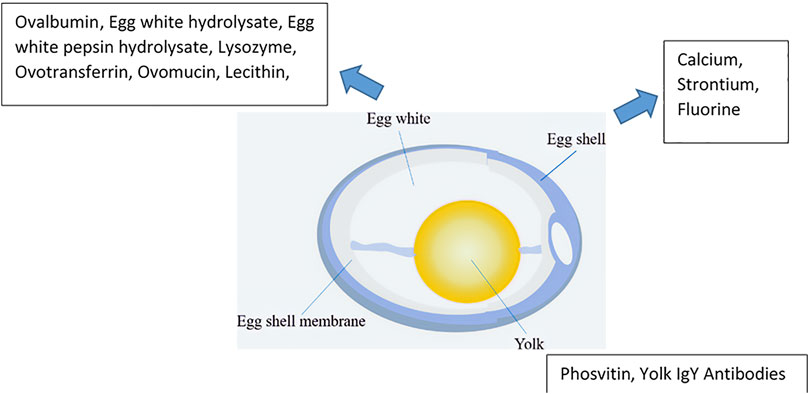
The consumption of eggs, particularly hen eggs, has a long history alongside the development of human civilization. The global egg production was 73.8 million tons in 2016 and is expected to reach 89.9 million tons in 2030, with an average annual increase of 1.6% (Conway, 2012; Portal Statistics, 2018) (Figure 1). The principal components of eggs include the eggshell (ES), egg white (EW), the yolk, and the eggshell membrane (ESM) (Figure 2).
In addition to food consumption, increasing attention has been given to exploring the unique biological values and functions of eggs and their comprehensive applications as a non-food resource in different industrial sectors. This review aimed to summarize and analyze the cost-effectiveness of this essential food product that can make the academic communities and industries explore its potential, its bioactive components, and the potential use of its industrial waste for recycling and development into profitable non-food products (Abeyrathne et al., 2013). In addition, an overall understanding of its bioactive components and their potential in large-scale development for applications in biotechnology, medicine, pharmaceuticals, cosmeceuticals, and nutraceuticals may give rise to future developments in the egg industry (Anton et al., 2006). Extensive studies have been carried out to identify and characterize the biologically active components of hen’s eggs, apart from the products already being produced by industries for various biomedical applications (Kovacs-Nolan and Mine, 2004), for human and veterinary medicine, and other potential applications in non-biological industries (Bhat et al., 2015).
Bioactive Egg Compounds in Human Medicine
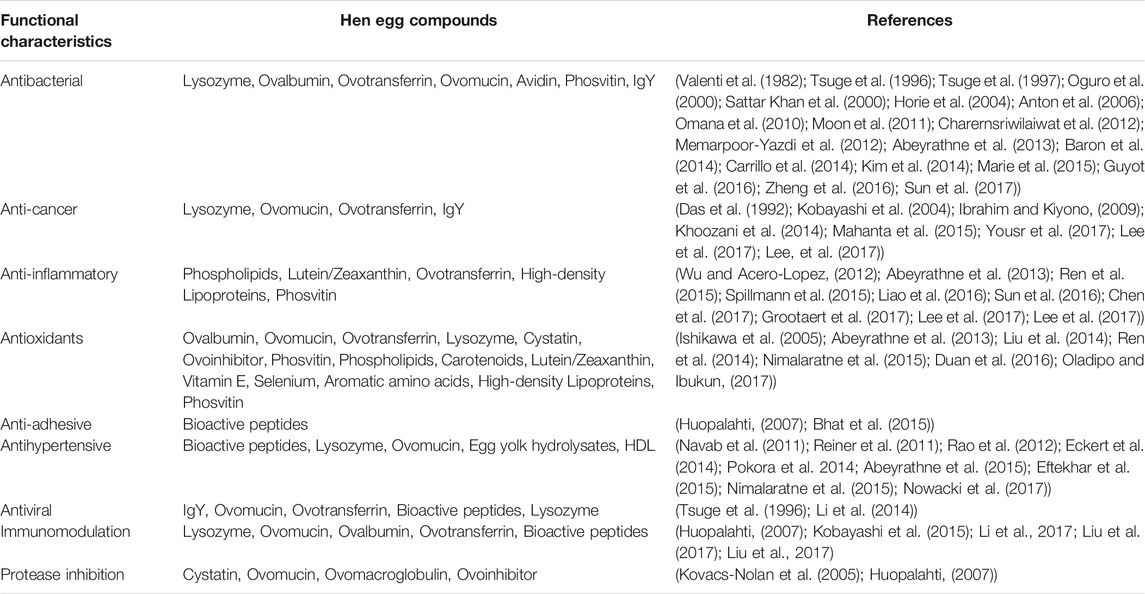
Chicken eggs harbor numerous active biological ingredients and have the potential to serve as raw materials in various biomedical sectors and industries (Table 1; Figure 3).
Ovalbumin for the Treatment of Egg Allergy
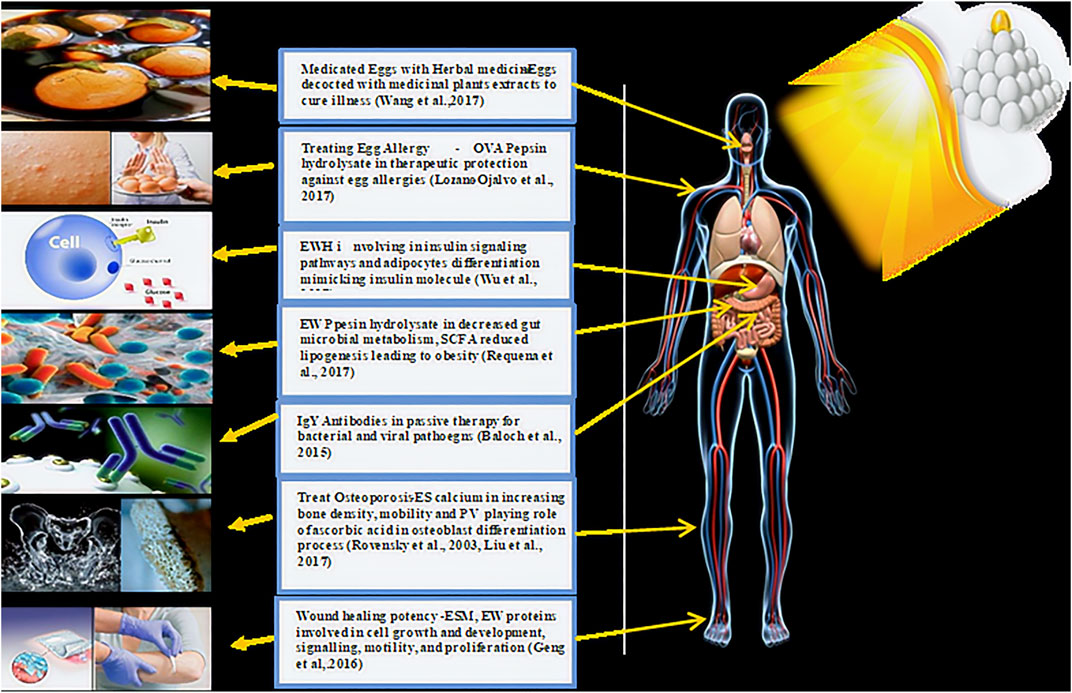
Allergies to eggs are the second most common food allergy, next only to milk, which could be countered with oral immunotherapy using peptides to avoid atopic reactions and prolonged treatment regimes. Ovalbumin (OVA), a 45-kDa phosphorylated glycoprotein, is responsible for the antigenic and allergenic properties of eggs, comprising 54% w/v of the total egg white proteins. The amino acid sequences that belong to the serpin family of proteinase inhibitors are composed of three genes, X, Y, and OVA (Huopalahti, 2007). It has been reported that the ability of OVA pepsin hydrolysate with intact OVA to treat egg allergies in BALB/c mice expressed better therapeutic protection against egg allergies by inducing regulatory cells (Tregs) and upregulating the expression of TGF-β, IL-10, IL-17, Foxp3, and RORγt in the intestinal tissues (Lozano-Ojalvo et al., 2017). Thus, oral formulations containing OVA pepsin hydrolysates can be employed in the future for infants and children to reduce the risk of egg allergies.
Egg Components in Improving Osteoporosis
The eggshell is a rich source of calcium in addition to strontium, fluorine, and other minerals, and is a potential industrial raw material for use in applications such as bone metabolism. The positive effects of calcium on bone and cartilage in experiments involving piglets, female rats, and postmenopausal women, are evidenced by a reduction in pain, osteoporation, increased bone density, and mobility (Rovenský et al., 2003). Shell hydroxyapatite mimicking human bones and teeth can be used to prepare bio-composite materials for human soft tissues, bone implantations, dental fixtures in various forms of powders, and porous blocks (Quina et al., 2016). In addition, eggshell nano-additives can be employed in soft drinks to prevent tooth erosion (Khoozani et al., 2014).
Egg yolk phosvitin, a highly phosphorylated protein naturally found in nature, plays a vital role in the osteoblast differentiation process, similar to ascorbic acid. Real-time PCR analysis of cultured mouse osteoblastic MC3T3-E1 cells treated with ascorbic acid and phosvitin revealed a similar expression of osteogenic gene markers, including collagen type I, osteocalcin, runt-related transcription factor 2, and bone morphogenetic protein-2 (Liu et al., 2017). Phosvitin can effectively play the role of ascorbic acid in the osteoblast differentiation process when the former is unavailable, with immediate applications for individuals who are susceptible to bone loss, providing alternative treatment options for patients with osteoporosis. Industries currently manufacturing artificial bone and dental fixtures can replace them with a natural biological material containing eggshell waste to rapidly improve bone structure formation.
Insulin Mimetic Property and Metabolic Syndrome
Insulin resistance and inflammation in the adipose tissue increase the risk of metabolic syndrome. Egg white hydrolysate (EWH) exhibits antioxidant, anti-inflammatory, and antihypertensive properties by inhibiting the activity of the angiotensin-converting enzyme (ACE) in the renin-angiotensin system (RAS), which is also involved in insulin signaling pathways and adipocyte differentiation (Martinez et al., 2019). EWH displays insulin-mimetic and sensitizing effects, and a previous study showed the effects of these hydrolysates on insulin signaling in adipocytes (Jahandideh et al., 2017). The high incidence of cardiovascular diseases (CVD) and diabetes have led to huge market demand for insulin (and insulin-like) molecules. In summary, the analysis of the insulin-mimetic property of EWH may aid in the effective prophylaxis and management of metabolic syndrome in the future.
Obesity Control Concerning the Gut Microbiota
EW pepsin hydrolysates could reduce short-chain fatty acids in association with the gut microbiota to reduce the incidence of obesity-associated complications and dyslipidemia. A reduction in the microbial load in the feces along with high concentrations of short-chain fatty acids (SCFA), lactate, fecal lactate, and ammonium were observed in obese rats treated with 750 mg/kg body weight of EW pepsin hydrolysate in drinking water for 12 days in comparison to the control. The reduction in microbial load (including Lactobacillus, Enterococcus, and Clostridium leptum), in turn, diminished microbial metabolism as evidenced by a decrease in SCFA levels, which was attributed to the antioxidant and anti-inflammatory properties of EW during lipogenesis (Requena et al., 2017).
Wound Healing Potency of Egg Compounds
The use of ESM to improve biological and biodegradable matrices has gained attention as a new material for use in biological dressings in the wound regeneration process in split-thickness skin graft donors (Huang et al., 2013), and in nerve regeneration enhancement in the sciatic nerves of rats (Farjah et al., 2013). ESM, with its biodegradability due to proteoglycans, has been successfully used to treat non-healing wounds and burns (Mogoşanu and Grumezescu, 2014). Manufacturing wound dressings from massive amounts of ESM industrial waste may have the market potential for solutions involving chronic wound healing. Similarly, EW proteins displayed proliferative bioactivity, are involved in cell migration, and have rapid wound healing properties (Lee et al., 2013; Geng et al., 2016). A total of 33 proteins have been identified via LC-MS/MS in EW, most of which play important roles in cell growth and development, signaling, motility, and proliferation. The bioactivity of these candidate molecules suggests that EW contains essential compounds that contribute to the growth of an embryo before fertilization (Lee et al., 2013).
IgY Antibodies in Human Medicine
Egg yolk antibody (IgY), as a possible substitute for mammalian antibodies, has been used for the diagnosis, prevention, and treatment of infections caused by bacteria and viruses (Kovacs-Nolan and Mine, 2004; Spillner et al., 2012; Baloch et al., 2014; Thu et al., 2017). The use of IgY in human medicine has gained interest in passive immunotherapeutic clinical conditions, including colitis, influenza, and bacterial and fungal infections (such as those caused by Clostridium botulinum, Staphylococcus aureus, Candida albicans, Helicobacter pylori, and Pseudomonas aeruginosa) (Horie et al., 2004; Kovacs-Nolan and Mine, 2012). For instance, IgY has been incorporated into toothpaste and mouthwash to reduce the levels of oral Streptococcus mutans (Chi et al., 2004). At present, many mature IgY drugs have already entered the market, including Helicobacter pylori NCT02721355 and Pseudomonas aeruginosa NCT01455675 (Leiva et al., 2020). Clinical trials involving IgY showed encouraging results, catapulting products with mono-specific or mixed IgY formulations into the novel nutraceuticals or health supplements market for use in prophylaxis against several diseases. With the rising consumer demand towards natural products for health concerns, IgY is an emerging pharmaceutical preparation from a functional food that can be used soon (Huopalahti, 2007; Thu et al., 2017).
Anti-Cancer Effects of Egg Compounds
Lysozyme was the first protein from hen eggs with its sequence known. It can prevent bacterial carcinogenesis and improve the effects of anti-cancer drugs and induce rapid recovery from immune suppression (Zheng et al., 2016). Treatment with a preparation containing self-assembled nanostructured lysozyme particles showed a tumor inhibition rate of 31.53 and a survival extension of 4–5 days in different murine tumor models (Das et al., 1992), as well as an inhibition of breast cancer cell migration (Mahanta et al., 2015). An egg yolk gel filtration fraction (EYGF-33) from EW pepsin and pancreatin hydrolysate displayed anti-proliferative activity in human colon cancer cells (Caco-2), inhibiting cell viability without affecting human colon epithelial normal cells (HCECs) after treatment for 48 h. The viability of cancer cells was suppressed by the initiation of apoptosis upon administration of EW hydrolysates (Yousr et al., 2017). Ovotransferrin (OVT) safeguards the developing embryo with its antioxidant, antimicrobial, anti-cancer, and strong metal chelation properties (Valenti et al., 2012). Functional peptides obtained from OVT hydrolysis possess strong anti-cancer effects against colon and breast cancer cells (Ibrahim and Kiyono, 2009). Peptides from Pramod 278p (OTH-P) and thermolysin (OTH-T) hydrolysis showed anti-cancer activity in human stomach adenocarcinoma cells with <20% cytotoxicity against MRC-5 cells (human normal lung fibroblasts) (Lee et al., 2017; Lee et al., 2017).
Biologically active peptides from OVT and lysozyme can be employed in cancer treatments, including for colon and breast cancer, since they have reduced cytotoxicity against normal cells as well as interfere with cancer cell progression.
Antioxidant Activity of Egg Bioactive Peptides
Various compounds obtained from eggs, including albumin, OVT hydrolysates, and phosvitin complexes exhibit antioxidant and anti-inflammatory properties. The peptide (NTDGSTDYGILQINSR) produced by pepsin, via trypsin hydrolysis, or lysozyme from albumen showed antioxidant and antimicrobial activity against both Gram-positive bacteria (Leuconostoc mesenteroides) and Gram-negative bacteria (Escherichia coli) (Memarpoor-Yazdi et al., 2012). Even with a limited number of studies exploring the use of antioxidants from animal sources, evidence has shown that the antioxidant property of egg proteins decreases after cooking, while gastrointestinal tract (GIT) digestion increases the antioxidant potential of hydrolyzed peptides obtained from egg proteins. The antioxidant activity of minute amounts of lutein and zeaxanthin contributes to ocular health improvement by preventing macular degeneration and the development of cataracts (Handelman et al., 1999). Cystatin, an inhibitor of cysteine proteinases with antibacterial properties, can also modulate NO• synthesis and protect brain neurons from oxidative damage, demonstrating its potential activity as an antioxidant (Nimalaratne and Wu, 2015).
ACE Inhibition and Drug Candidates Against Cardiovascular Diseases and Oxidative Stress
Hypertension and its associated complications can be minimized by the inhibition of ACE in the RAS. ACE inhibitory peptides (Met-Lys-Arg, Arg-Gly-Tyr, and Val-Ala-Trp) from the enzymatic hydrolysis of HEW lysozyme exhibited high inhibitory activity (Rao et al., 2012), but more comprehensive studies on the mechanism of their absorption from the intestinal walls to the bloodstream need to be conducted to determine their pharmacologic potential.
ACE inhibitory peptides from the multienzyme hydrolysate of ovomucoid (OVM) with pepsin, trypsin, and alcalase can lower the blood pressure (Abeyrathne et al., 2015). A pentapeptide from OVM pepsin hydrolysate, Trp-Asn-Trp-Ala-Asp (WNWAD), has an extraordinary oxygen radical absorption capacity in HEK-293 cells (Abeyrathne et al., 2015).
High-density lipoprotein (HDL) is associated with an increased risk of CVD, as evidenced by epidemiological studies, and protects against atherosclerotic CVD (Reiner et al., 2011) by promoting reverse cholesterol transport (Navab et al., 2011). A decreased incidence of atherosclerotic plaques with increased plasma HDL levels and reverse cholesterol transport from tissues to the liver have been observed from experimental studies on rats, suggesting that HDL is an effective anti-atherosclerotic agent for high-risk populations of patients with CVD (Eftekhar et al., 2015).
Lecithin esterified from fatty acids significantly reduces blood pressure and lowers the serum levels of inflammatory factors. Serum levels of oxidative stress markers such as nitro-tyrosine and heart rate were lowered by approximately 30%–34% in both hypertensive and normotensive animals. The above attributes prove the potential of lecithin as a candidate molecule for the prevention and therapy of cardiovascular diseases (Nowacki et al., 2017). Interestingly, eggs have been used in decoctions for medicinal plant extracts primarily to obtain food and health benefits in some ethnomedicinal practices (Xin et al., 2014; Wang et al., 2017).
Role of Egg Components in Veterinary Medicine
Effective Cryopreservation of Spermatozoa
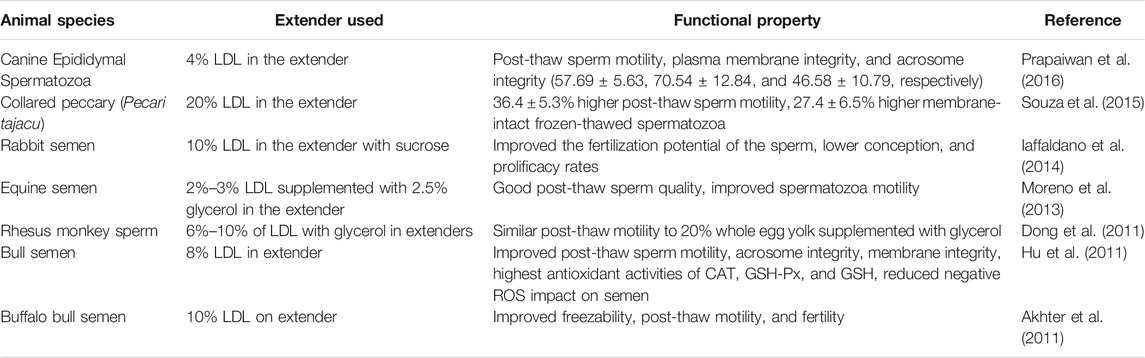
Cryopreservation of veterinary spermatozoa can improve the mobility and stability of sperm as well as prevent loss of activity upon continuous freeze-thawing for prolonged durations (Kampschmidt et al., 1953). Replacement of the whole yolk with low-density lipoproteins (LDL) in extenders has gained much attention in recent years, providing higher plasma membrane integrity (Table 2). However, it has been noticed that the cryoprotective property of LDL greatly depends on the sperm characteristics obtained from different animal species as well as the percentage of LDL used to effectively replace whole egg yolk in conventional sperm preservation methods. Commercially available ready-to-use extenders with predefined standardized LDL concentrations specific to different animal species can be manufactured to improve semen quality and ease of use for consumers.
Poultry Feed Supplements
ESM contains numerous proteins and peptides, including collagens, laminin, agrin, keratin, clusterin peptides, and defensins, which have cryoprotective and chaperone-like functions as well as antimicrobial and immunomodulatory properties that are crucial for embryo development. ESM has emerged as an efficient feed supplement to post-hatchery chicks to improve immunity and egg-laying performance (Makkar et al., 2015). The protection of embryos upon treatment with ESM is attributed to the antimicrobial nature of lysozyme, keratin peptides, ovocalyxin, ovotransferrin, ovalbumin Y, ovostatin, ovomucoid, and ovoglycan, since a bacterial LPS-induced inflammatory response and a reduced level of stress markers such as plasma corticosterone levels were transferred to feed chicks (Rath et al., 2016). ES powder, a rich source of calcium, can improve the laying performance of birds (Cordeiro and Hincke, 2011). Consequently, ESM and ES obtained as discarded products from industrial egg waste can be recycled in a more efficient way to improve the quality of chicks from poultry, in a cyclic process that can be adopted by poultry manufacturing sectors to obtain diverse products with health benefits.
Novel Alternative Drug Delivery Systems
Ovomucoid as an Alternative Drug Delivery System
Existing drug delivery systems can be improved for more effective drug release at targeted sites with fewer side effects by using biocompatible materials such as proteins, nanoparticles, chitosan, and alginate. The mucoadhesive property of OVM can be exploited for the sustained release of drugs in the biological system through the mucus layer, which highly depends on mucus pH, viscoelasticity, mucin-to-water ratio, ion content, and turnover time of the luminous and adherent mucus layers (Abeyrathne et al., 2015). A drug delivery system containing OVM should be dealt with more specifically because of its ability to release drugs before replacement with a new mucous layer because the turnover time differs in each biological system.
The drug release kinetics of riboflavin, brilliant blue, and ciprofloxacin loaded onto OVM particles suspended in PBS, simulated intestine tract fluid (SIF), and simulated gastric tract fluid (SGF) showed that negatively charged ovomucin particles were better than the usual chitosan, alginate, and polyacrylic acid in establishing maximum detachment force and maximum viscoelasticity properties. Previous evidence showed that the system was suitable for heat-labile drugs because OVM is water-soluble and that the drawback of OVM degradation in saline environments can be rectified by the preparation of polyelectrolyte hydrogels to serve as the carrier for heat-labile pharmaceuticals (Akbari and Wu, 2017). In addition, cross-linked nanogels of egg yolk LDL prepared with N-hydroxysuccinimide proved to be an effective carrier for the delivery of curcumin encapsulated into nanogels, presenting good stability in both fasting and fed gastrointestinal conditions against digestive enzymes and aggregation under acidic conditions. Cross-linking provided a sustained slow release of curcumin from the nanogel with better performance than non-cross-linked ones (Zhou et al., 2018).
Lecithin Organogels for Applications in Topical Drug Delivery
Wrinkles, uneven skin tone, black spots, lines, and pigmentation are symptoms of skin aging that result from environmental pollution, poor skincare, and exposure to ultraviolet light. Cosmeceutical drug preparations can delay the process of aging and reduce skin damage, but a better delivery system is needed to penetrate the dermal layer more effectively. Lecithin organogels and phospholipids, in conjunction with suitable additives, are promising topical drug delivery vehicles (Date et al., 2011; Elnaggar et al., 2014). Lecithin from purified egg yolk is superior to other topical drug delivery systems by serving as an organic medium to improve the penetration of less permeable drugs into the dermal layer of the skin. The hydrophilic and lipophilic properties of lecithin make it a suitable carrier for most anti-aging drugs, and its natural biocompatibility makes it a safe drug delivery tool for use in products in the skin cosmeceutical industry (Raut et al., 2012).
Biomaterial Fabrication in Tissue Engineering
Albumin in Bioactive Glasses in the Grafting Process
Bioactive glass, which is composed of SiO2-CaO-MgO-P2O5 (Kokubo 1990) (97), is an important class of bioceramics (Peitl et al., 2012) and is used as a graft material to aid in new bone formation.
Albumin, the key protein in the human extracellular protein matrix, is involved in bioactive glass adsorption with implants as it surrounds the foreign bodies exposed in the bloodstream. The bioactive glass system SiO2-CaO-MgO-P2O5 immersed in simulated human plasma with calcium-phosphate (Ca-P) with or without albumin during a 7- and 14-day immersion period, as studied via XRD and AFM analyses, reported the formation of amorphous octa-Ca-P precipitates on the surface of albumin. On the other hand, a thin, easily detachable layer was observed in the glass immersed in albumin-free SBF, suggesting that post-adsorption of albumin to the bioactive glass surface acts as a bridge between Ca2+ and PO3- ions, thus forming a connection between the glass and the Ca-P layer (Paiva et al., 2007; Peitl et al., 2012). The bone and tissue grafting process can be made feasible by the inclusion of albumin to stabilize the connection and support the stability of the layer of new bone tissues formed.
Applications of Advanced Materials Design for Tissue Engineering
The highly crisscrossed linkage of protein fibers in the ESM, similar to egg white albumin, initiates the biomineralization of eggshell formation and plays a major role in protecting the developing embryo from microbial pathogens. ESM is often disposed of as industrial waste from egg processing factories despite recent attention towards the isolation of bioactive compounds from it (Intharapat et al., 2012; Baláž, 2014). Because of their ultrastructure, biomineralization in organisms proceeds through the properties of organic–inorganic composites, which can retain water and their intrinsic biodegradability, as well as microcapsules that facilitate nutrient delivery (Cordeiro and Hincke, 2011; Guru and Dash, 2014). The presence of collagen, hyaluronic acid, fibronectin, osteopontin, and calcium carbonate in the ESM makes it a template for cells in tissue as stable mechanical support to adsorb and interact with growth factors and signaling molecules to form fully functional tissues during regeneration of skin, bone, cartilage, and nerves. Therefore, ESM serves as a potential biomaterial for various tissue engineering applications (Sah and Rath, 2016).
OVM hydrogels have been prepared previously, utilizing their unique foaming property in preparing scaffolds of various porosities, along with gelatin, to maintain their pore size and stability (Carpena et al., 2017). OVM scaffolds subcutaneously implanted in rats showed good adhesion of rat bone marrow mesenchymal stem cells with a lower incidence of fibrosis, vascularization, and activation of alternate macrophages. In addition, there is evidence for the satisfactory formation of blood vessels in mesenchymal stem cells with minimal macrophage infiltration and hence eliciting a weaker immunological response.
A biocompatible, non-toxic material with high elasticity, tensile strength, and tunable mechanical properties can be used for the fabrication of scaffolds in tissue engineering (You et al., 2017). The preparation of implants, scaffolds, and hydrogels with bioactive compounds from egg sources possessing tunable properties with enhanced tissue regeneration activity shows promise for tissue engineering applications.
Development of Biosensors and Detection Systems
ESM in Electrical Devices and Engineering Applications
The highly unique crisscrossed micro- and nanostructures of ESM make it a functional platform for the preparation and fabrication of materials in electrical devices, including sensors for environmental monitoring and biomedical engineering applications (Park and Kim, 2017). Given that the carbonized ESM has a good electric capacity, the adsorption property of the carbonized ESM can effectively allow the removal of on-site pollutants using fluorescent dyes (Shao et al., 2011; Xiong et al., 2012). Both native and functionalized forms of ESM have been used to develop biosensors for the detection of specific biomolecules from different samples (Ray and Roy, 2016). High amounts of amino acids and glycoproteins present in the ES and ESM make them efficient in cell adhesion and viability for applications in biomedical engineering. Since the commercial availability of ESM is currently very limited, it can be procured as supplementary material for the further study of shell membranes.
Alternative to Antibodies in Toxin Detection Systems
The development of a highly accurate, rapid, and sensitive toxin detection platform is crucial for monitoring the consequences of the release of harmful toxins by microbial pathogens. Lectin is a biomolecule found in both plants and animals and plays a dual role with beneficial and harmful benefits when used as a biological sensor. Lectins possess a high affinity towards synthetic glycan ligands and antibodies that are currently employed in available toxin detection systems. Affinity nanoprobes functionalized with OVA with higher sensitivity and specificity for the detection of multiple lectins (ConA, BanLec, and ricin) in a single system have been developed (Selvaprakash and Chen, 2018). Lectin interactions with glycan ligands can be selectively released from nanoprobes by adding sugars such as mannose, glucose, and β-galactose as releasing agents. The replacement of antibodies with OVA probes for multi-lectins proves to be a cost-effective alternative with much higher sensitivity and specificity for the molecules of interest. Antibodies from mammalian sources can be avoided to an extent by replacing them with egg OVA in toxin detection systems, thus favoring the manufacture of portable kits for applications that need rapid processing.
IgY Antibodies in Immunoassays
The presence of trace amounts of microbial toxins, hazardous chemicals, pollutants, toxic metals, and xenobiotic compounds in the environment and food samples is a major concern to human health, as prolonged exposure results in devastating long-term health issues. The presence of antibiotics poses a threat to multidrug-resistant microbial strains and superbugs. Minute levels of antibiotic residues in complex matrices raise the challenge of developing an appropriate analytical method for detection. IgY antibody-based enzyme-linked immunosorbent assays have been developed for the detection of antibiotics such as kanamycin and gentamicin from animal-derived food samples (Li et al., 2016). A similar antibody immunoassay platform was developed for the screening of a vital enzyme, CYP2E1 inhibitor/enhancer, from herbal medicines (Jiang et al., 2016). This approach made IgY-based systems detect microbial toxins, hazardous chemicals, pollutants, toxic metals, and foreign biological compounds in a wide variety of samples.
Egg Components on Industrial Applications
Latent Source of Keratin Sulfate
Corneal keratin sulfate plays a major role in ocular inflammation, corneal injury, and keratitis, and is a major component of ocular medications to restore normal visual conditions (Pomin, 2015). Cartilage keratin sulfate (KS) maintains the hydration of tissues, making them resistant to physical stress (Hayashi et al., 2011). Fully sulfated KS disaccharide has been proposed as a potent drug for the treatment of chronic obstructive pulmonary disease (Shirato et al., 2013). KS deficiency is attributed to the early-phase pathogenesis of amyotrophic lateral sclerosis, making it a possible therapeutic agent for amyotrophic lateral sclerosis (ALS) (Hirano et al., 2013). Previously, a highly potent KS molecule was prepared from bovine corneas but was eventually withdrawn due to an outbreak of bovine spongiform encephalopathy. The pharmaceutical industry has explored the potential of KS to increase demand and identified EW as a potential source of keratin sulfate, hyaluronic acid, chondroitin sulfate, and heparin sulfate for pharmaceutical preparations (Fu et al., 2016).
Industrial Role of Lecithin and Phospholipids
Phospholipids in eggs can improve serum lipid metabolism, prevent aging and arteriosclerosis, and improve lipid metabolism in the liver. In addition, these molecules can promote the absorption of fat-soluble vitamins by inhibiting cholesterol and neutralizing fat, which can be used as parenteral lipid emulsions and drug delivery systems (Hartmann and Wilhelmson, 2001; Sahebkar, 2013; Blesso, 2015). The pharmaceutical industry uses phospholipids as wetting agents, emulsifiers, and builders or components of mesophases, such as liposomes, micelles, and mixed micelles (van Hoogevest and Wendel, 2014). Lecithin, a mixture of phospholipids, is mainly extracted from eggs and vegetables, including soybeans, sunflower, rapeseed, and cottonseed. As a natural emulsifier, lecithin can be applied in the food industry at an estimated world market demand of 150,000–170,000 tons, next to its applications as cosmetics and lubricants. Its molecular structure, with dual hydrophilic and lipophilic groups, makes it a surface-active molecule contributing to emulsification, anti-spattering, wetting, anti-staling, dough-conditioning, and antioxidant functions in various foods. Hence, lecithin has emerged as an important ingredient in food products because of its emulsifying properties (Cui and Decker, 2016).
Lecitihin, a polyunsaturated phosphatidylcholine that is a functional and structural component of all biological membranes, acts as the rate-limiting step in the activation of membrane enzymes such as superoxide dismutase (Hanin et al., 1990). Ineffective activation of these antioxidant enzymes leads to increased damage to membranes by reactive oxygen species (Hanin et al., 1990). In addition, lecithin increases bile secretion, prevents stagnation in the bladder, and consequently decreases lithogenicity (Herron and Fernandez, 2004; Miranda et al., 2015). It also acts as an emulsifier in enteral formulas to reduce the incidence of diarrhea in rats (Akashi et al., 2017). Formulations containing egg yolk lecithin, medium-chain triglycerides, and dietary fiber for their potential use in lipid absorption have also been studied in rats with short bowel syndrome. The results showed no difference in the average particle size of egg yolk lecithin emulsifier upon the addition of artificial gastric juice, increased serum triglyceride concentrations, and improvement in fecal consistency and bowel movement frequency. The enteral formula promoted lipid adsorption by preventing the destruction of emulsified substances by gastric acid and improving diarrhea. Replacing conventional oral formula with lecithin as an emulsifier may reduce the incidence of diarrhea, thereby reducing medical costs due to hospitalizations.
Egg Components in Various Industrial Sectors
ES and ESM as Emerging Vital Constituents in Multiple Industries
In addition to the efficient capability of ES waste to eliminate dyes from industrial effluents, its notable roles in other industries extend to its use as a catalyst in industrial processes such as biodiesel synthesis, synthesis of dimethyl carbonate (DMC), synthesis of hydrogen/syngas, wastewater treatment plants, and pollutant removal or immobilization in liquid, soil, or gaseous emissions (Quina et al., 2016; Laca et al., 2017). Interestingly, calcium carbonate purified from ES waste has been recently utilized as a building material along with cement and mortar, enhancing the strength of buildings and other improvements in the construction industry. The brightness and smoothness of writing materials such as papers can be improved by including ESW in the paper manufacturing process, as well as in the paint and dye manufacturing industries (Kirboga and Öner, 2013). Both ES and ESM are used as substrates for the enzymatic production of alkaline protease from Bacillus altitudinis GVC1 along with maltose as an additional carbon source, increasing the enzyme production by up to 13%, proving their potential in increasing production capacity in microbial enzyme manufacturing industries (Nagamalli et al., 2017).
Egg Components in Cosmeceutical Preparations
ES and ESM proteins are arranged in a perfect matrix by the fibrous protein collagen and play a crucial role in tissue structures by supporting them in place between the shell and the membrane. This property of collagen is exploited in preparing skincare cosmeceutical products to foil skin wrinkles and to improve the elasticity and thickness of the skin (Cordeiro and Hincke, 2011). Cosmeceutical preparations containing natural ingredients such as lecithin and phospholipids as a substitute for synthetic emulsifiers and functional additives helps fulfill the growing demand for natural cosmetic formulations. Lecithin and phospholipids obtained from eggs possess excellent hydration, soothing properties, and applications in cosmetic creams, lotions, and gels, providing satisfactory moisturizing effects with negligible irritability as they are biocompatible to human skin.
Role of Egg Components in Environmental Protection
Elimination of Toxic Chemicals From Water Resources
Most populations generate tons of ES waste; industries also emit huge volumes of industrial effluents into water bodies despite strict regulations, drastically affecting the food chain. Both issues involve environmental protection and can be solved using egg-based products. In addition to materials such as peat, plant by-products, and activated charcoal used for the removal of toxic chemicals, ES can also adsorb organic industrial dyes and pigments, such as direct red 80, acid blue 25, methylene blue, brilliant green, and malachite green (Podstawczyk et al., 2014) from textile industrial effluents. The biosorption mechanism of harmful textile dyes by ES lies in the physical adsorption of the dyes to the cell wall of dead cells and the associated functional groups that constitute the ES. This, combined with micro-precipitation, reduces the concentration of chlorinated phenols, fluorides, phosphate, and other organic pollutants in water bodies worldwide (Guru and Dash, 2014; Quina et al., 2016). The presence of calcium carbonate and the protein acid mucopolysaccharides with distinguished pore structures in the shell aid the development of nanomaterials for the removal of toxic heavy metals, including lead, copper, and cadmium, from the environment (Chumlong et al., 2007). Thus, large quantities of ESW can be utilized in a more appropriate way to save organisms thriving in water bodies by eliminating toxic chemicals and industrial dyes.
Enriching Plant Growth and Development
Soil nutrient availability, along with the physical parameters (pH and salinity), has a great influence on plant growth. When soil pH decreases and becomes highly acidic (pH < 4.5), a decline in calcium carbonate level occurs, leading to a loss of bioaccessibility to the plant, interfering with its growth and development. The addition of ESW as compost or co-compost material has been shown to reverse the calcium carbonate loss, thereby increasing the capacity of the soil to improve the growth and yield of plants (Soares et al., 2015). Compost materials, including ESW, can be manufactured to enhance horticulture activities more efficiently.
Next-Generation Transgenic and Enriched Eggs
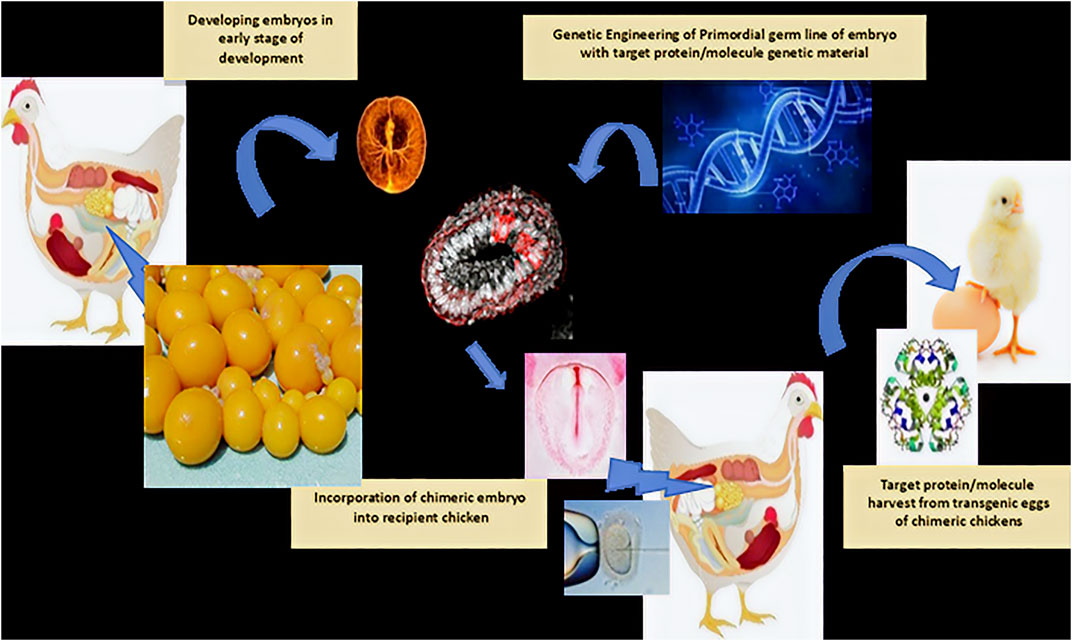
Eggs can be enriched with specific molecules, such as omega-3 fatty acids, vitamins, minerals, docosahexaenoic acid (DHA), selenium, to have lower cholesterol, or become pigmented eggs, among others (Laudadio et al., 2015; Singh et al., 2012). Herb-enriched eggs have been developed by feeding hens with herbs containing active compounds such as allicin, betaine, eugenol, lumiflavin, lutein, taurine, and sulforaphane, which showed a significant reduction in triglyceride levels, increased levels of HDL cholesterol, improved immunity, and increased hematocrit levels, as tested in human volunteers (Zaheer, 2015). Furthermore, functionally modified eggs through genetic manipulation of the chicken immune system to produce a specific protein, peptide, or molecule of pharmaceutical interest can allow its natural retention in the system and later harvest, such as insulin molecules or antibody-enriched eggs (Figure 4) (Sang, 1994; June Byun et al., 2017; Bednarczyk et al., 2018; Ching et al., 2018).
Despite the identification of novel peptides with unique biomedical properties, large-scale production is still being continuously improved to achieve a sustainable turnover for industries. Industrial-scale production of bioactive peptides is feasible with different techniques involving supercritical fluid technology, magnetic particles for separation of specific egg compounds by immune magnetic separation (Huopalahti, 2007), and enzymatic hydrolysis of egg white albumin and yolk followed by ultrafiltration to obtain low-molecular-weight peptides combined with chromatographic techniques (Nimalaratne et al., 2015). Egg components, including lysozyme, avidin, and IgY antibodies, are currently under industrial production using standardized purification protocols that can be extended to identify other bioactive peptides.
Summary and Future Prospects
Various egg components, including lysozyme, avidin, IgY, lecithin, and bioactive peptides, display anti-cancer, antihypertensive, anti-inflammatory, and antimicrobial activities, and have great industrial opportunities in the pharmaceutical sector. In recent years, the cosmeceutical industry has taken advantage of the beneficial effects of egg yolk lecithin to expand its use in many skincare products. Meanwhile, the wastes generated from the egg processing industries can be more efficiently utilized by other textile and dye manufacturing industries. For instance, recycling egg waste for the removal of toxic pollutants from industrial effluents is a synergistic process between the two industries, decreasing the environmental impact of the waste generated. The future of the egg industry lies in generating functional eggs by enriching them with specific compounds or preparing transgenic eggs via genetic manipulation of chickens to fulfill the need for the production of specific proteins in the eggs to treat various diseases. Recent advancements in the large-scale purification of compounds from eggs using newer methods such as supercritical technology and the separation of bioactive peptides via magnetic separation made it feasible for industrial preparations. Eggs harboring biologically and industrially important peptides encourage the exploration for other efficient bioactive peptides in the future, not only by the scientific community but also by the industrial sector.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the China Natural Science Foundation (grant number 31873006); the Incubation Project on State Key Laboratory of Biological Resources and Ecological Environment of Qinba Areas (grant number SLGPT2019KF04-04); the Science and Technology Innovation Talent Support Program for Colleges and Universities in Henan Province, China (grant number 18HASTIT035); and the FCT I.P, the Ministério da Ciência Tecnologia e Ensino Superior (MCTES), the European Regional Development Fund (ERDF) (grant number POCI-01-0145-FEDER-007569). DST INSPIRE Fellowship to Brindha Chelliappan by DST, Govt. of India.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Dr. XZ is grateful for the Distinguished Professorship awarded by Shaanxi University of Technology that provides an excellent environment in which to conduct research. Dr. Zhang also thanks the University of Guelph for providing an adjunct faculty position.
References
Abeyrathne, E. D. N. S., Lee, H. Y., and Ahn, D. U. (2013). Egg white Proteins and Their Potential Use in Food Processing or as Nutraceutical and Pharmaceutical Agents-A Review. Poult. Sci. 92 (12), 3292–3299. doi:10.3382/ps.2013-03391
Abeyrathne, E. D. N. S., Lee, H. Y., Jo, C., Suh, J. W., and Ahn, D. U. (2015). Enzymatic Hydrolysis of Ovomucoid and the Functional Properties of its Hydrolysates. Poult. Sci. 94 (9), 2280–2287. doi:10.3382/ps/pev196
Akashi, T., Muto, A., Takahashi, Y., and Nishiyama, H. (2017). Enteral Formula Containing Egg Yolk Lecithin Improves Diarrhea. J. Oleo Sci. 66 (9), 1017–1027. doi:10.5650/jos.ess17007
Akbari, A., and Wu, J. (2017). Ovomucin Nanoparticles: Promising Carriers for Mucosal Delivery of Drugs and Bioactive Compounds. Drug Deliv. Transl. Res. 7 (4), 598–607. doi:10.1007/s13346-017-0406-3
Akhter, S., Ansari, M. S., Rakha, B. A., Andrabi, S. M. H., Khalid, M., and Ullah, N. (2011). Effect of Low Density Lipoproteins in Extender on Freezability and Fertility of buffalo (Bubalus Bubalis) Bull Semen. Theriogenology 76 (4), 759–764. doi:10.1016/j.theriogenology.2011.04.009
Anton, M., Nau, F., and Nys, Y. (2006). Bioactive Egg Components and Their Potential Uses. World Poult. Sci J 62, 237–244. doi:10.1017/S004393390600105X10.1079/wps2005105
Baláž, M. (2014). Eggshell Membrane Biomaterial as a Platform for Applications in Materials Science. Acta Biomater. 10 (9), 3827–3843. doi:10.1016/j.actbio.2014.03.020
Baloch, A. R., Zhang, X.-Y., and Schade, R. (2014). IgY Technology in Aquaculture - a Review. Rev. Aquacult 7, 153–160. doi:10.1111/raq.12059
Baron, F., Jan, S., Gonnet, F., Pasco, M., Jardin, J., Giudici, B., et al. (2014). Ovotransferrin Plays a Major Role in the strong Bactericidal Effect of Egg white against the Bacillus Cereus Group. J. Food Prot. 77 (6), 955–962. doi:10.4315/0362-028x.Jfp-13-473
Bednarczyk, M., Kozłowska, I., Łakota, P., Szczerba, A., Stadnicka, K., and Kuwana, T. (2018). Generation of Transgenic Chickens by the Non-viral, Cell-Based Method: Effectiveness of Some Elements of This Strategy. J. Appl. Genet. 59 (1), 81–89. doi:10.1007/s13353-018-0429-6
Bhat, Z. F., Kumar, S., and Bhat, H. F. (2015). Bioactive Peptides from Egg: A Review. Nutr. Food Sci. 45, 190–212. doi:10.1108/NFS-10-2014-0088
Blesso, C. (2015). Egg Phospholipids and Cardiovascular Health. Nutrients 7 (4), 2731–2747. doi:10.3390/nu7042731
Carpena, N. T., Abueva, C. D. G., Padalhin, A. R., and Lee, B.-T. (2017). Evaluation of Egg white Ovomucin-Based Porous Scaffold as an Implantable Biomaterial for Tissue Engineering. J. Biomed. Mater. Res. 105 (7), 2107–2117. doi:10.1002/jbm.b.33750
Carrillo, W., García-ruiz, A., Recio, I., and Moreno-Arribas, M. V. (2014). Antibacterial Activity of Hen Egg white Lysozyme Modified by Heat and Enzymatic Treatments against Oenological Lactic Acid Bacteria and Acetic Acid Bacteria. J. Food Prot. 77 (10), 1732–1739. doi:10.4315/0362-028x.Jfp-14-009
Charernsriwilaiwat, N., Opanasopit, P., Rojanarata, T., and Ngawhirunpat, T. (2012). Lysozyme-loaded, Electrospun Chitosan-Based Nanofiber Mats for Wound Healing. Int. J. Pharmaceutics 427 (2), 379–384. doi:10.1016/j.ijpharm.2012.02.010
Chen, S., Jiang, H., Peng, H., Wu, X., and Fang, J. (2017). The Utility of Ovotransferrin and Ovotransferrin-Derived Peptides as Possible Candidates in the Clinical Treatment of Cardiovascular Diseases. Oxidative Med. Cell Longevity 2017, 1–6. doi:10.1155/2017/6504518
Chi, Z. B., Gao, Y. X., Pan, Y., Zhang, B., and Feng, X. P. (2004). The Inhibitive Effect of IgY Toothpaste against Oral Streptococcus Mutans. Shanghai Kou Qiang Yi Xue 13 (4), 256–258. doi:10.3969/j.issn.1006-7248.2004.04.005
Ching, K. H., Collarini, E. J., Abdiche, Y. N., Bedinger, D., Pedersen, D., Izquierdo, S., et al. (2018). Chickens with Humanized Immunoglobulin Genes Generate Antibodies with High Affinity and Broad Epitope Coverage to Conserved Targets. MAbs 10 (1), 71–80. doi:10.1080/19420862.2017.1386825
Chumlong, A., Wanvisa, K., Acharaporn, K., Pokethitiyook, P., and Patra, P. (2007). Removal of lead from Battery Manufacturing Wastewater by Egg Shell. Songklanakarin J. Sci. Techn. 29, 857–868.
Conway, A. (2012). China Remains World’s Top Egg Producer in 2012. Illinois, United States: WATT Global media. Available at: https://www.wattagnet.com/articles/14095-china-remains-world-s-top-egg-producer-in-2012 (Accessed February 19, 2018).
Cui, L., and Decker, E. A. (2016). Phospholipids in Foods: Prooxidants or Antioxidants? J. Sci. Food Agric. 96 (1), 18–31. doi:10.1002/jsfa.7320
Das, S., Banerjee, S., and Gupta, J. D. (1992). Experimental Evaluation of Preventive and Therapeutic Potentials of Lysozyme. Chemotherapy 38 (5), 350–357. doi:10.1159/000239025
Date, A. A., Nagarsenker, M. S., Patere, S., Dhawan, V., Gude, R. P., Hassan, P. A., et al. (2011). Lecithin-based Novel Cationic Nanocarriers (Leciplex) II: Improving Therapeutic Efficacy of Quercetin on Oral Administration. Mol. Pharmaceutics 8 (3), 716–726. doi:10.1021/mp100305h
Dong, Q.-X., Rodenburg, S. E., Hill, D., and Vandevoort, C. A. (2011). The Role of Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) in Comparison with Whole Egg Yolk for Sperm Cryopreservation in Rhesus Monkeys. Asian J. Androl. 13 (3), 459–464. doi:10.1038/aja.2010.145
Duan, X., Li, M., Ma, H., Xu, X., Jin, Z., and Liu, X. (2016). Physicochemical Properties and Antioxidant Potential of Phosvitin-Resveratrol Complexes in Emulsion System. Food Chem. 206, 102–109. doi:10.1016/j.foodchem.2016.03.055
Eckert, E., Zambrowicz, A., Pokora, M., Setner, B., Dąbrowska, A., Szołtysik, M., et al. (2014). Egg-yolk Protein By-Product as a Source of ACE-Inhibitory Peptides Obtained with Using Unconventional Proteinase from Asian Pumpkin (Cucurbita Ficifolia). J. Proteomics 110, 107–116. doi:10.1016/j.jprot.2014.08.003
Eftekhar, S., Parsaei, H., Keshavarzi, Z., Yazdi, A. T., Hadjzadeh, M. A., Rajabzadeh, A., et al. (2015). The Prevention and Treatment Effects of Egg Yolk High Density Lipoprotein on the Formation of Atherosclerosis Plaque in Rabbits. Iran J. Basic Med. Sci. 18 (4), 343–349.
Elnaggar, Y. S. R., El-Refaie, W. M., El-Massik, M. A., and Abdallah, O. Y. (2014). Lecithin-based Nanostructured Gels for Skin Delivery: an Update on State of Art and Recent Applications. J. Controlled Release 180, 10–24. doi:10.1016/j.jconrel.2014.02.004
Farjah, G. H., Heshmatian, B., Karimipour, M., and Saberi, A. (2013). Using Eggshell Membrane as Nerve Guide Channels in Peripheral Nerve Regeneration. Iran J. Basic Med. Sci. 16 (8), 901–905.
Fu, L., Sun, X., He, W., Cai, C., Onishi, A., Zhang, F., et al. (2016). Keratan Sulfate Glycosaminoglycan from Chicken Egg white. Glycobiology 26 (7), 693–700. doi:10.1093/glycob/cww017
Geng, F., Huang, X., and Ma, M. (2016). Hen Egg white Ovomacroglobulin Promotes Fibroblast Migration via Mediating Cell Adhesion and Cytoskeleton. J. Sci. Food Agric. 96 (9), 3188–3194. doi:10.1002/jsfa.7498
Grootaert, C., Matthijs, B., Voorspoels, S., Possemiers, S., Smagghe, G., and Van Camp, J. (2017). Egg-derived Bioactive Peptides with ACE-Inhibitory Properties: a Literature Update. Food Funct. 8 (11), 3847–3855. doi:10.1039/c7fo00839b
Guru, P. S., and Dash, S. (2014). Sorption on Eggshell Waste-A Review on Ultrastructure, Biomineralization and Other Applications. Adv. Colloid Interf. Sci. 209, 49–67. doi:10.1016/j.cis.2013.12.013
Guyot, N., Labas, V., Harichaux, G., Chessé, M., Poirier, J.-C., Nys, Y., et al. (2016). Proteomic Analysis of Egg white Heparin-Binding Proteins: towards the Identification of Natural Antibacterial Molecules. Sci. Rep. 6, 27974. doi:10.1038/srep27974
Handelman, G. J., Nightingale, Z. D., Lichtenstein, A. H., Schaefer, E. J., and Blumberg, J. B. (1999). Lutein and Zeaxanthin Concentrations in Plasma after Dietary Supplementation with Egg Yolk. Am. J. Clin. Nutr. 70 (2), 247–251. doi:10.1093/ajcn.70.2.247
Hanin, I. (1990). “Chapter 3 Microorganisms as Sources of Phospholipids,” in Book Lecithin-Technological, Biological and Therapeutic Aspects (New York, US: Plenum Press).
Hartmann, C., and Wilhelmson, M. (2001). The Hen's Egg Yolk: A Source of Biologically Active Substances. World's Poult. Sci. J. 57, 13–28. doi:10.1079/WPS20010003
Hayashi, M., Kadomatsu, K., Kojima, T., and Ishiguro, N. (2011). Keratan Sulfate and Related Murine Glycosylation Can Suppress Murine Cartilage Damage In Vitro and In Vivo. Biochem. Biophysical Res. Commun. 409 (4), 732–737. doi:10.1016/j.bbrc.2011.05.077
Herron, K. L., and Fernandez, M. L. (2004). Are the Current Dietary Guidelines Regarding Egg Consumption Appropriate? J. Nutr. 134 (1), 187–190. doi:10.1093/jn/134.1.187
Hirano, K., Ohgomori, T., Kobayashi, K., Tanaka, F., Matsumoto, T., Natori, T., et al. (2013). Ablation of Keratan Sulfate Accelerates Early Phase Pathogenesis of ALS. PLoS One 8 (6), e66969. doi:10.1371/journal.pone.0066969
Horie, K., Horie, N., Abdou, A. M., Yang, J.-O., Yun, S.-S., Chun, H.-N., et al. (2004). Suppressive Effect of Functional Drinking Yogurt Containing Specific Egg Yolk Immunoglobulin on Helicobacter pylori in Humans. J. Dairy Sci. 87 (12), 4073–4079. doi:10.3168/jds.S0022-0302(04)73549-3
Hu, J.-H., Jiang, Z.-L., Lv, R.-K., Li, Q.-W., Zhang, S.-S., Zan, L.-S., et al. (2011). The Advantages of Low-Density Lipoproteins in the Cryopreservation of Bull Semen. Cryobiology 62 (1), 83–87. doi:10.1016/j.cryobiol.2010.12.007
Huang, W.-Y., Davidge, S. T., and Wu, J. (2013). Bioactive Natural Constituents from Food Sources-Potential Use in Hypertension Prevention and Treatment. Crit. Rev. Food Sci. Nutr. 53 (6), 615–630. doi:10.1080/10408398.2010.550071
Huopalahti, R. (2007). in Book Bioactive Egg Compounds. Editors R. Huopalahti, R. López-Fandiño, M. Anton, and R. Schade (Berlin, Germany: Springer publication.
Iaffaldano, N., Di Iorio, M., Rosato, M. P., and Manchisi, A. (2014). Cryopreservation of Rabbit Semen Using Non-permeable Cryoprotectants: Effectiveness of Different Concentrations of Low-Density Lipoproteins (LDL) from Egg Yolk versus Egg Yolk or Sucrose. Anim. Reprod. Sci. 151 (3-4), 220–228. doi:10.1016/j.anireprosci.2014.10.020
Ibrahim, H. R., and Kiyono, T. (2009). Novel Anticancer Activity of the Autocleaved Ovotransferrin against Human colon and Breast Cancer Cells. J. Agric. Food Chem. 57 (23), 11383–11390. doi:10.1021/jf902638e
Intharapat, P., Kongnoo, A., and Kateungngan, K. (2012). The Potential of Chicken Eggshell Waste as a Bio-Filler Filled Epoxidized Natural Rubber (ENR) Composite and its Properties. J. Polym. Environ. 21, 245–258. doi:10.1007/s10924-012-0475-9
Ishikawa, S.-I., Ohtsuki, S., Tomita, K., Arihara, K., and Itoh, M. (2005). Protective Effect of Egg Yolk Phosvitin against Ultraviolet- Light-Induced Lipid Peroxidation in the Presence of Iron Ions. Bter 105 (1-3), 249–256. doi:10.1385/bter:105:1-3:249
Jahandideh, F., Chakrabarti, S., Davidge, S. T., and Wu, J. (2017). Egg white Hydrolysate Shows Insulin Mimetic and Sensitizing Effects in 3T3-F442a Pre-adipocytes. PLoS One 12 (10), e0185653. doi:10.1371/journal.pone.0185653
Jiang, Z., Jiang, X., Li, C., Xue, H., and Zhang, X. (2016). Development of an IgY Antibody-Based Immunoassay for the Screening of the CYP2E1 Inhibitor/Enhancer from Herbal Medicines. Front. Pharmacol. 7, 502. doi:10.3389/fphar.2016.00502
June Byun, S., Yuk, S.-S., Jang, Y.-J., Choi, H., Jeon, M.-H., Erdene-Ochir, T., et al. (2017). Transgenic Chickens Expressing the 3D8 Single Chain Variable Fragment Protein Suppress Avian Influenza Transmission. Sci. Rep. 7 (1), 5938. doi:10.1038/s41598-017-05270-8
Kampschmidt, R. F., Mayer, D. T., and Herman, H. A. (1953). Lipid and Lipoprotein Constituents of Egg Yolk in the Resistance and Storage of Bull Spermatozoa. J. Dairy Sci. 36, 733–742. doi:10.3168/jds.S0022-0302(53)91553-7
Khoozani, N. E., Bahrololoom, M., and Bagheri, R. (2014). Modification of a Soft Drink by Adding Calcium Carbonate Nanoparticles to Prevent Tooth Erosion. Shiraz University of Medical Sciences: Iran.
Kim, H.-J., Zhang, K., Moore, L., and Ho, D. (2014). Diamond Nanogel-Embedded Contact Lenses Mediate Lysozyme-dependent Therapeutic Release. ACS Nano 8 (3), 2998–3005. doi:10.1021/nn5002968
Kirboga, S., and Öner, M. (2013). Application of Experimental Design for the Precipitation of Calcium Carbonate in the Presence of Biopolymer. Powder Techn. 249, 95–104. doi:10.1016/j.powtec.2013.07.015
Kobayashi, K., Hattori, M., Hara-Kudo, Y., Okubo, T., Yamamoto, S., Takita, T., et al. (2004). Glycopeptide Derived from Hen Egg Ovomucin Has the Ability to Bind Enterohemorrhagic Escherichia coli O157:H7. J. Agric. Food Chem. 52 (18), 5740–5746. doi:10.1021/jf0353335
Kobayashi, Y., Rupa, P., Kovacs-Nolan, J., Turner, P. V., Matsui, T., and Mine, Y. (2015). Oral Administration of Hen Egg white Ovotransferrin Attenuates the Development of Colitis Induced by Dextran Sodium Sulfate in Mice. J. Agric. Food Chem. 63 (5), 1532–1539. doi:10.1021/jf505248n
Kokubo, T. (1990). Surface Chemistry of Bioactive Glass-Ceramics. J. Non-Crystalline Sol. 120, 138–151. doi:10.1016/0022-3093(90)90199-V
Kovacs-Nolan, J., and Mine, Y. (2004). Avian Egg Antibodies: Basic and Potential Applications. Avian Poul. Biolog. Rev. 15, 25–46. doi:10.3184/147020604783637462
Kovacs-Nolan, J., and Mine, Y. (2012). Egg Yolk Antibodies for Passive Immunity. Annu. Rev. Food Sci. Technol. 3, 163–182. doi:10.1146/annurev-food-022811-101137
Kovacs-Nolan, J., Phillips, M., and Mine, Y. (2005). Advances in the Value of Eggs and Egg Components for Human Health. J. Agric. Food Chem. 53 (22), 8421–8431. doi:10.1021/jf050964f
Laca, A., Laca, A., and Díaz, M. (2017). Eggshell Waste as Catalyst: A Review. J. Environ. Manage. 197, 351–359. doi:10.1016/j.jenvman.2017.03.088
Laudadio, V., Lorusso, V., Lastella, N., Lastella, N. M. B., Dhama, K., Karthik, K., et al. (2015). Enhancement of Nutraceutical Value of Table Eggs through Poultry Feeding Strategies. Int. J. Pharmacol. 11, 201–212. doi:10.3923/ijp.2015.201.212
Lee, A., Molloy, M. P., Baker, M. S., and Kapur, A. (2013). Tandem Ion Exchange Fractionation of Chicken Egg white Reveals the Presence of Proliferative Bioactivity. J. Agric. Food Chem. 61 (17), 4079–4088. doi:10.1021/jf305276c
Lee, D., Bamdad, F., Khey, K., and Sunwoo, H. H. (2017). Antioxidant and Anti-inflammatory Properties of Chicken Egg Vitelline Membrane Hydrolysates. Poult. Sci. 96 (9), 3510–3516. doi:10.3382/ps/pex125
Lee, J. H., Moon, S. H., Kim, H. S., Park, E., Ahn, D. U., and Paik, H.-D. (2017). Antioxidant and Anticancer Effects of Functional Peptides from Ovotransferrin Hydrolysates. J. Sci. Food Agric. 97 (14), 4857–4864. doi:10.1002/jsfa.8356
Leiva, C. L., Gallardo, M. J., Casanova, N., Terzolo, H., and Chacana, P. (2020). IgY-technology (Egg Yolk Antibodies) in Human Medicine: A Review of Patents and Clinical Trials. Int. Immunopharmacology 81, 106269. doi:10.1016/j.intimp.2020.106269
Li, C., Geng, F., Huang, X., Ma, M., and Zhang, X. (2014). Phosvitin Phosphorus Is Involved in Chicken Embryo Bone Formation through Dephosphorylation. Poult. Sci. 93 (12), 3065–3072. doi:10.3382/ps.2014-04098
Li, C. H., Bai, Y. L., Selvaprakash, K., Mong, K. K. T., and Chen, Y. C. (2017). Selective Detection of Shiga-Like Toxin 1 From Complex Samples Using Pigeon Ovalbumin Functionalized Gold Nanoparticles as Affinity Probes. J. Agri. Food Chemist. 65 (21), 4359–4365.
Li, C., He, J., Ren, H., Zhang, X., Du, E., and Li, X. (2016). Preparation of a Chicken scFv to Analyze Gentamicin Residue in Animal Derived Food Products. Anal. Chem. 88 (7), 4092–4098. doi:10.1021/acs.analchem.6b00426
Liao, W., Chakrabarti, S., Davidge, S. T., and Wu, J. (2016). Modulatory Effects of Egg White Ovotransferrin-Derived Tripeptide IRW (Ile-Arg-Trp) on Vascular Smooth Muscle Cells against Angiotensin II Stimulation. J. Agric. Food Chem. 64 (39), 7342–7347. doi:10.1021/acs.jafc.6b03513
Liu, L., Xu, M., Tu, Y., Du, H., Zhou, Y., and Zhu, G. (2017). Immunomodulatory Effect of Protease Hydrolysates from Ovotransferrin. Food Funct. 8 (4), 1452–1459. doi:10.1039/c6fo01669c
Liu, Q., Li, C., Geng, F., Huang, X., and Ma, M. (2017). Hen Egg Yolk Phosvitin Stimulates Osteoblast Differentiation in the Absence of Ascorbic Acid. J. Sci. Food Agric. 97 (13), 4532–4538. doi:10.1002/jsfa.8320
Liu, Z., Zhang, F., Li, L., Li, G., He, W., and Linhardt, R. J. (2014). Compositional Analysis and Structural Elucidation of Glycosaminoglycans in Chicken Eggs. Glycoconj J. 31 (8), 593–602. doi:10.1007/s10719-014-9557-3
Lozano-Ojalvo, D., Pérez-Rodríguez, L., Pablos-Tanarro, A., Molina, E., and López-Fandiño, R. (2017). Hydrolysed Ovalbumin Offers More Effective Preventive and Therapeutic protection against Egg Allergy Than the Intact Protein. Clin. Exp. Allergy 47 (10), 1342–1354. doi:10.1111/cea.12989
Mahanta, S., Paul, S., Srivastava, A., Pastor, A., Kundu, B., and Chaudhuri, T. K. (2015). Stable Self-Assembled Nanostructured Hen Egg white Lysozyme Exhibits strong Anti-proliferative Activity against Breast Cancer Cells. Colloids Surf. B: Biointerfaces 130, 237–245. doi:10.1016/j.colsurfb.2015.04.017
Makkar, S., Liyanage, R., Kannan, L., Packialakshmi, B., Lay, J. O., and Rath, N. C. (2015). Chicken Egg Shell Membrane Associated Proteins and Peptides. J. Agric. Food Chem. 63 (44), 9888–9898. doi:10.1021/acs.jafc.5b04266
Marie, P., Labas, V., Brionne, A., Harichaux, G., Hennequet-Antier, C., Rodriguez-Navarro, A. B., et al. (2015). Data Set for the Proteomic Inventory and Quantitative Analysis of Chicken Eggshell Matrix Proteins during the Primary Events of Eggshell Mineralization and the Active Growth Phase of Calcification. Data in Brief 4, 430–436. doi:10.1016/j.dib.2015.06.019
Martinez, C. S., Piagette, J. T., Escobar, A. G., Martín, A., Palacios, R., Peçanha, F. M., et al. (2019). Egg White Hydrolysate: A New Putative Agent to Prevent Vascular Dysfunction in Rats Following Long-Term Exposure to Aluminum. Food Chem. Toxicol. 133, 110799. doi:10.1016/j.fct.2019.110799
Memarpoor-Yazdi, M., Asoodeh, A., and Chamani, J. (2012). A Novel Antioxidant and Antimicrobial Peptide from Hen Egg white Lysozyme Hydrolysates. J. Funct. Foods 4, 278–286. doi:10.1016/j.jff.2011.12.004
Miranda, J., Anton, X., Redondo-Valbuena, C., Roca-Saavedra, P., Rodriguez, J., Lamas, A., et al. (2015). Egg and Egg-Derived Foods: Effects on Human Health and Use as Functional Foods. Nutrients 7 (1), 706–729. doi:10.3390/nu7010706
M.M. Cordeiro, C., and T. Hincke, M. (2011). Recent Patents on Eggshell: Shell and Membrane Applications. Fna 3 (1), 1–8. doi:10.2174/2212798411103010001
Mogoşanu, G. D., and Grumezescu, A. M. (2014). Natural and Synthetic Polymers for Wounds and burns Dressing. Int. J. Pharmaceutics 463 (2), 127–136. doi:10.1016/j.ijpharm.2013.12.015
Moon, S. H., Paik, H.-D., White, S., Daraba, A., Mendonca, A. F., and Ahn, D. U. (2011). Influence of Nisin and Selected Meat Additives on the Antimicrobial Effect of Ovotransferrin against Listeria Monocytogenes. Poult. Sci. 90 (11), 2584–2591. doi:10.3382/ps.2010-01275
Moreno, D., Bencharif, D., Amirat-Briand, L., Neira, A., Destrumelle, S., and Tainturier, D. (2013). Preliminary Results: The Advantages of Low-Density Lipoproteins for the Cryopreservation of Equine Semen. J. Equine Vet. Sci. 33, 1068–1075. doi:10.1016/j.jevs.2013.04.004
Nagamalli, H., Sitaraman, M., Kandalai, K. K., and Mudhole, G. R. (2017). Chicken Egg Shell as a Potential Substrate for Production of Alkaline Protease by Bacillus Altitudinis GVC11 and its Applications. 3 Biotech. 7 (3), 185. doi:10.1007/s13205-017-0801-y
Navab, M., Reddy, S. T., Van Lenten, B. J., and Fogelman, A. M. (2011). HDL and Cardiovascular Disease: Atherogenic and Atheroprotective Mechanisms. Nat. Rev. Cardiol. 8 (4), 222–232. doi:10.1038/nrcardio.2010.222
Nimalaratne, C., Bandara, N., and Wu, J. (2015). Purification and Characterization of Antioxidant Peptides from Enzymatically Hydrolyzed Chicken Egg white. Food Chem. 188, 467–472. doi:10.1016/j.foodchem.2015.05.014
Nimalaratne, C., and Wu, J. (2015). Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 7 (10), 8274–8293. doi:10.3390/nu7105394
Nowacki, D., Martynowicz, H., Skoczyńska, A., Wojakowska, A., Turczyn, B., Bobak, Ł., et al. (2017). Lecithin Derived from ω-3 PUFA Fortified Eggs Decreases Blood Pressure in Spontaneously Hypertensive Rats. Sci. Rep. 7 (1), 12373. doi:10.1038/s41598-017-12019-w
Oguro, T., Watanabe, K., Tani, H., Ohishi, H., and Ebina, T. (2000). Morphological Observations on Antitumor Activities of 70kDa Fragment in .ALPHA.-Subunit from Pronase-Treated Ovomucin in a Double Grafted Tumor System. Fstr 6, 179–185. doi:10.3136/fstr.6.179
Oladipo, G. O., and Ibukun, E. O. (2017). BioActivities ofCoturnix Japonica(quail) Egg Yolk and Albumen against Physiological Stress. Food Sci. Nutr. 5 (2), 334–343. doi:10.1002/fsn3.397
Omana, D. A., Wang, J., and Wu, J. (2010). Ovomucin - a Glycoprotein with Promising Potential. Trends Food Sci. Techn. 21 (9), 455–463. doi:10.1016/j.tifs.2010.07.001
Paiva, A. O., Costa, N., Cachinho, S. C. P., and Fernandes, M. H. V. (2007). Evaluation of the Influence of Albumin on the Mineralization of a Glass by Atomic Force Microscopy. J. Mater. Sci. Mater. Med. 18 (4), 599–604. doi:10.1007/s10856-007-2307-3
Peitl, O., Zanotto, E. D., Serbena, F. C., and Hench, L. L. (2012). Compositional and Microstructural Design of Highly Bioactive P2O5-Na2O-CaO-SiO2 Glass-Ceramics. Acta Biomater. 8 (1), 321–332. doi:10.1016/j.actbio.2011.10.014
Podstawczyk, D., Witek-Krowiak, A., Chojnacka, K., and Sadowski, Z. (2014). Biosorption of Malachite green by Eggshells: Mechanism Identification and Process Optimization. Bioresour. Techn. 160, 161–165. doi:10.1016/j.biortech.2014.01.015
Pokora, M., Zambrowicz, A., Dąbrowska, A., Eckert, E., Setner, B., Szołtysik, M., et al. (2014). An Attractive Way of Egg white Protein By-Product Use for Producing of Novel Anti-hypertensive Peptides. Food Chem. 151, 500–505. doi:10.1016/j.foodchem.2013.11.111
Pomin, V. H. (2015). Keratan Sulfate: an Up-To-Date Review. Int. J. Biol. Macromolecules 72, 282–289. doi:10.1016/j.ijbiomac.2014.08.029
Portal Statistics (2018). Global Egg Production from 1990 to 2019 (In 1,000 Metric Tons). United States: Statista Inc.. Available at: https://www.statista.com/statistics/263972/egg-production-worldwide-since-1990/(Accessed February 19, 2021).
Prapaiwan, N., Tharasanit, T., Punjachaipornpol, S., Yamtang, D., Roongsitthichai, A., Moonarmart, W., et al. (2016). Low-density Lipoprotein Improves Motility and Plasma Membrane Integrity of Cryopreserved Canine Epididymal Spermatozoa. Asian Australas. J. Anim. Sci. 29 (5), 646–651. doi:10.5713/ajas.15.0572
Quina, M. J., Soares, M. A. R., and Quinta-Ferreira, R. (2017). Applications of Industrial Eggshell as a Valuable Anthropogenic Resource. Resour. Conservation Recycling 123, 176–186. doi:10.1016/j.resconrec.2016.09.027
Rao, S.-Q., Ju, T., Sun, J., Su, Y.-J., Xu, R.-R., and Yang, Y.-J. (2012). Purification and Characterization of Angiotensin I-Converting Enzyme Inhibitory Peptides from Enzymatic Hydrolysate of Hen Egg white Lysozyme. Food Res. Int. 46, 127–134. doi:10.1016/j.foodres.2011.12.005
Rath, N. C., Liyanage, R., Makkar, S. K., and Lay, J. O. (2016). Protein Profiles of Hatchery Egg Shell Membrane. Proteome Sci. 15, 4. doi:10.1186/s12953-017-0112-6
Raut, S., Bhadoriya, S. S., Uplanchiwar, V., Mishra, V., Gahane, A., and Jain, S. K. (2012). Lecithin Organogel: A Unique Micellar System for the Delivery of Bioactive Agents in the Treatment of Skin Aging. Acta Pharmaceutica Sinica B 2, 8–15. doi:10.1016/j.apsb.2011.12.005
Ray, P. G., and Roy, S. (2016). Eggshell Membrane: A Natural Substrate for Immobilization and Detection of DNA. Mater. Sci. Eng. C 59, 404–410. doi:10.1016/j.msec.2015.10.034
Reiner, Z., Catapano, A., De Backer, G., Graham, I., Taskinen, M.-R., Wiklund, O., et al. (2011). ESC/EAS Guidelines for the Management of Dyslipidaemias. Revista española de cardiología 64. doi:10.1016/j.recesp.2011.09.014
Ren, J., Li, Q., Offengenden, M., and Wu, J. (2015). Preparation and Characterization of Phosphopeptides from Egg Yolk Phosvitin. J. Funct. Foods 18, 190–197. doi:10.1016/j.jff.2015.07.007
Ren, Y., Wu, H., Li, X., Lai, F., and Xiao, X. (2014). Purification and Characterization of High Antioxidant Peptides from Duck Egg white Protein Hydrolysates. Biochem. Biophysical Res. Commun. 452 (4), 888–894. doi:10.1016/j.bbrc.2014.08.116
Requena, T., Miguel, M., Garcés-Rimón, M., Martínez-Cuesta, M. C., López-Fandiño, R., and Peláez, C. (2017). Pepsin Egg white Hydrolysate Modulates Gut Microbiota in Zucker Obese Rats. Food Funct. 8 (1), 437–443. doi:10.1039/c6fo01571a
Rovenský, J., Stancíková, M., Masaryk, P., Svík, K., and Istok, R. (2003). Eggshell Calcium in the Prevention and Treatment of Osteoporosis. Int. J. Clin. Pharmacol. Res. 23 (2-3), 83–92.
Sah, M. K., and Rath, S. N. (2016). Soluble Eggshell Membrane: A Natural Protein to Improve the Properties of Biomaterials Used for Tissue Engineering Applications. Mater. Sci. Eng. C 67, 807–821. doi:10.1016/j.msec.2016.05.005
Sahebkar, A. (2013). Fat Lowers Fat: Purified Phospholipids as Emerging Therapies for Dyslipidemia. Biochim. Biophys. Acta (Bba) - Mol. Cel Biol. Lipids 1831 (4), 887–893. doi:10.1016/j.bbalip.2013.01.013
Sang, H. (1994). Transgenic Chickens - Methods and Potential Applications. Trends Biotechnol. 12 (10), 415–420. doi:10.1016/0167-7799(94)90030-2
Sattar Khan, M. A., Nakamura, S., Ogawa, M., Akita, E., Azakami, H., and Kato, A. (2000). Bactericidal Action of Egg Yolk Phosvitin against Escherichia coli under Thermal Stress. J. Agric. Food Chem. 48 (5), 1503–1506. doi:10.1021/jf990700r
Selvaprakash, K., and Chen, Y.-C. (2018). Functionalized Gold Nanoparticles as Affinity Nanoprobes for Multiple Lectins. Colloids Surf. B: Biointerfaces 162, 60–68. doi:10.1016/j.colsurfb.2017.11.022
Shao, C., Yuan, B., Wang, H., Zhou, Q., Li, Y., Guan, Y., et al. (2011). Eggshell Membrane as a Multimodal Solid State Platform for Generating Fluorescent Metal Nanoclusters. J. Mater. Chem. 21, 2863–2866. doi:10.1039/C0JM04071A
Shirato, K., Gao, C., Ota, F., Angata, T., Shogomori, H., Ohtsubo, K., et al. (2013). Flagellin/Toll-like Receptor 5 Response Was Specifically Attenuated by Keratan Sulfate Disaccharide via Decreased EGFR Phosphorylation in normal Human Bronchial Epithelial Cells. Biochem. Biophysical Res. Commun. 435 (3), 460–465. doi:10.1016/j.bbrc.2013.05.009
Singh, V. P., Pathak, V., and Akhilesh, K. V. (2012). Modified or Enriched Eggs: A Smart Approach in Egg Industry: A Review. Am. J. Food Techn. 7, 266–277. doi:10.3923/ajft.2012.266.277
Soares, M. A. R., Quina, M. J., and Quinta-Ferreira, R. M. (2015). Immobilisation of lead and Zinc in Contaminated Soil Using Compost Derived from Industrial Eggshell. J. Environ. Manage. 164, 137–145. doi:10.1016/j.jenvman.2015.08.042
Souza, A. L. P., Lima, G. L., Peixoto, G. C. X., de Souza Castelo, T., Oliveira, M. G. C., de Paula, V. V., et al. (2015). Sperm Characteristics Following Freezing in Extenders Supplemented with Whole Egg Yolk and Different Concentrations of Low-Density Lipoproteins in the Collared Peccary (Pecari Tajacu). Reprod. Biol. 15 (4), 223–228. doi:10.1016/j.repbio.2015.10.006
Spillmann, F., Miteva, K., Pieske, B., Tschöpe, C., and Van Linthout, S. (2015). High-density Lipoproteins Reduce Endothelial-To-Mesenchymal Transition. Atvb 35 (8), 1774–1777. doi:10.1161/atvbaha.115.305887
Spillner, E., Braren, I., Greunke, K., Seismann, H., Blank, S., and du Plessis, D. (2012). Avian IgY Antibodies and Their Recombinant Equivalents in Research, Diagnostics and Therapy. Biologicals 40 (5), 313–322. doi:10.1016/j.biologicals.2012.05.003
Sun, X., Chakrabarti, S., Fang, J., Yin, Y., and Wu, J. (2016). Low-molecular-weight Fractions of Alcalase Hydrolyzed Egg Ovomucin Extract Exert Anti-inflammatory Activity in Human Dermal Fibroblasts through the Inhibition of Tumor Necrosis Factor-Mediated Nuclear Factor κB Pathway. Nutr. Res. 36 (7), 648–657. doi:10.1016/j.nutres.2016.03.006
Sun, X., Gänzle, M., and Wu, J. (2017). Identification and Characterization of Glycopeptides from Egg Protein Ovomucin with Anti-agglutinating Activity against Porcine K88 Enterotoxigenic Escherichia coli Strains. J. Agric. Food Chem. 65 (4), 777–783. doi:10.1021/acs.jafc.6b04299
Thu, H. M., Myat, T. W., Win, M. M., Thant, K. Z., Rahman, S., Umeda, K., et al. (2017). Chicken Egg Yolk Antibodies (IgY) for Prophylaxis and Treatment of Rotavirus Diarrhea in Human and Animal Neonates: A Concise Review. Korean J. Food Sci. Anim. Resour. 37 (1), 1–9. doi:10.5851/kosfa.2017.37.1.1
Tsuge, Y., Shimoyamada, M., and Watanabe, K. (1997). Bindings of Ovomucin to Newcastle Disease Virus and Anti-ovomucin Antibodies and its Heat Stability Based on Binding Abilities. J. Agric. Food Chem. 45, 4629–4634. doi:10.1021/jf970445f
Tsuge, Y., Shimoyamada, M., and Watanabe, K. (1996). Differences in Hemagglutination Inhibition Activity against Bovine Rotavirus and Hen Newcastle Disease Virus Based on the Subunits in Hen Egg white Ovomucin. Biosci. Biotechnol. Biochem. 60 (9), 1505–1506. doi:10.1271/bbb.60.1505
Valenti, L., Dongiovanni, P., and Fargion, S. (2012). Diagnostic and Therapeutic Implications of the Association between Ferritin Level and Severity of Nonalcoholic Fatty Liver Disease. Wjg 18 (29), 3782–3786. doi:10.3748/wjg.v18.i29.3782
Valenti, P., Antonini, G., Fanelli, M. R., Orsi, N., and Antonini, E. (1982). Antibacterial Activity of Matrix-Bound Ovotransferrin. Antimicrob. Agents Chemother. 21 (5), 840–841. doi:10.1128/aac.21.5.840
van Hoogevest, P., and Wendel, A. (2014). The Use of Natural and Synthetic Phospholipids as Pharmaceutical Excipients. Eur. J. Lipid Sci. Technol. 116 (9), 1088–1107. doi:10.1002/ejlt.201400219
Wang, T.-M., Fu, Y., Yu, W.-J., Chen, C., Di, X., Zhang, H., et al. (2017). Identification of Polar Constituents in the Decoction of Juglans Mandshurica and in the Medicated Egg Prepared with the Decoction by HPLC-Q-TOF MS2. Molecules 22 (9), 1452. doi:10.3390/molecules22091452
Wu, J., and Acero-Lopez, A. (2012). Ovotransferrin: Structure, Bioactivities, and Preparation. Food Res. Int. 46, 480–487. doi:10.1016/j.foodres.2011.07.012
Xin, N., Hasan, M., Li, W., and Li, Y. (2014). Juglans Mandshurica Maxim Extracts Exhibit Antitumor Activity on HeLa Cells In Vitro. Mol. Med. Rep. 9 (4), 1313–1318. doi:10.3892/mmr.2014.1979
Xiong, X., Li, Q., Lu, J.-W., Guo, Z.-X., Sun, Z.-H., and Yu, J. (2012). Fibrous Scaffolds Made by Co-electrospinning Soluble Eggshell Membrane Protein with Biodegradable Synthetic Polymers. J. Biomater. Sci. Polym. Edition ahead-of-print (9), 1–14. doi:10.1163/092050611x576981
You, R., Zhang, J., Gu, S., Zhou, Y., Li, X., Ye, D., and Xu, W. (2017). Regenerated egg white/silk fibroin composite films for biomedical applications. Materials Science and Engineering: C 79, 430–435. doi:10.1016/j.msec.2017.05.063
Yousr, M. N., Aloqbi, A. A., Omar, U. M., and Howell, N. K. (2017). Antiproliferative Activity of Egg Yolk Peptides in Human Colon Cancer Cells. Nutr. Cancer 69 (4), 674–681. doi:10.1080/01635581.2017.1295087
Zaheer, K. (2015). An Updated Review on Chicken Eggs: Production, Consumption, Management Aspects and Nutritional Benefits to Human Health. Fns 06, 1208–1220. doi:10.4236/fns.2015.613127
Zheng, K., Lu, M., Liu, Y., Chen, Q., Taccardi, N., Hüser, N., et al. (2016). Monodispersed Lysozyme-Functionalized Bioactive Glass Nanoparticles with Antibacterial and Anticancer Activities. Biomed. Mater. 11 (3), 035012. doi:10.1088/1748-6041/11/3/035012
Keywords: hen egg, biomedical resource, market potential, bioactive peptides, nonfood applications
Citation: Zhang X, Chelliappan B, S R and Antonysamy M (2021) Recent Advances in Applications of Bioactive Egg Compounds in Nonfood Sectors. Front. Bioeng. Biotechnol. 9:738993. doi: 10.3389/fbioe.2021.738993
Received: 09 July 2021; Accepted: 15 November 2021;
Published: 16 December 2021.
Edited by:
Jianxun Ding, Changchun Institute of Applied Chemistry (CAS), ChinaReviewed by:
Yuan Xue, Stony Brook University, United StatesXiaoran Li, Donghua University, China
Tatsuhiko Goto, Obihiro University of Agriculture and Veterinary Medicine, Japan
Copyright © 2021 Zhang, Chelliappan, S and Antonysamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoying Zhang, emhhbmdAYmlvLnVtaW5oby5wdA==
 Xiaoying Zhang
Xiaoying Zhang Brindha Chelliappan
Brindha Chelliappan Rajeswari S
Rajeswari S Michael Antonysamy4
Michael Antonysamy4