- 1State Key Laboratory of Oral Diseases and West China School of Stomatology and National Clinical Research Center for Oral Diseases, Sichuan University, Chengdu, China
- 2Fujian Key Laboratory of Oral Diseases and Fujian Provincial Engineering Research Center of Oral Biomaterial and Stomatological Key Laboratory of Fujian College and University, School of Stomatology, Fujian Medical University, Fuzhou, China
Smoking is considered a key risk factor for implant survival; however, how it interacts with the pathogens in peri-implant infections is not clear. Here, we identified that nicotine, the key component of cigarette smoking, can interact with Staphylococcus aureus and synergistically induce peri-implant infections in a rat osteolysis model. The nicotine–S. aureus combination group increased the gross bone pathology, osteolysis, periosteal reactions, and bone resorption compared to the nicotine or S. aureus single treated group (p < 0.05). Nicotine did not promote the proliferation of S. aureus both in vitro and in vivo, but it can significantly upregulate the expression of staphylococcal protein A (SpA), a key virulence factor of S. aureus. The nicotine–S. aureus combination also synergistically activated the expression of RANKL (receptor activator of nuclear factor-kappa B ligand, p < 0.05) to promote the development of peri-implant infections. The synergistic effects between nicotine and S. aureus infection can be a new target to reduce the peri-implant infections.
Introduction
The dental implant, as an osseointegration technique, has become one of the most common strategies for prosthetic rehabilitation (Amornvit P, 2014). The osseointegration technique has also been extended to orthopedics, limb amputees, total joint replacements, maxillofacial reconstruction, and orbital prostheses due to its superiority in mobility and biocompatibility (Hebert et al., 2017; Depypere et al., 2020; Lu et al., 2020). However, complications have become a big challenge for their clinical practice. Peri-implant infections, as one of the major inflammatory complications, can cause suppuration, revision surgery, and even removal of the prostheses (Albrektsson T, 1994; Annibali S, 2008; Lindhe et al., 2008; Lang et al., 2011; Romano et al., 2013; Zimmerli and Sendi, 2017; Schwarz et al., 2018; Carrasco-Garcia et al., 2019; Kordbacheh Changi et al., 2019; Rokaya et al., 2020). The dental peri-implant infection prevalence was about 22%, while the global implant infection risk in orthopedic surgeries was about 2–5% (Derks and Tomasi, 2015; Berglundh et al., 2018; Dreyer et al., 2018; Ahn et al., 2019; Weinstein et al., 2020). Implant infections may increase by 20 times in high-risk patients, such as populations with diabetes and immunosuppressed diseases. Peri-implant infections dramatically increased the medical costs and human sufferings (Seebach and Kubatzky, 2019; Depypere et al., 2020; Zhou et al., 2020).
Staphylococcus aureus is commonly recognized as the key pathogenic agent for the early failure of implants due to its ability to attach onto different titanium surfaces (Harris and Richards, 2004; Cortizo et al., 2012; Shrestha et al., 2012; Persson and Renvert, 2014; Zhao et al., 2014; Thomas; Thurnheer and Belibasakis, 2016; Oliveira et al., 2018; Sahrmann et al., 2020). Moreover, S. aureus can also invade and persist in human osteoblasts to cause cell death and osteolysis (Bost et al., 1999; Alexander and Hudson, 2001). The staphylococcal protein A (SpA) is an important virulence factor of S. aureus. SpA can be recognized by the host immune system to induce the differentiation of osteoclast and can interact with the tumor necrosis factor receptor superfamily, member 1A (TNFR-1) on the surface of the osteoblast to activate the expression of RANKL (receptor activator of nuclear factor-kappa B ligand), which then promotes osteoclastogenesis (Somayaji et al., 2008; Claro et al., 2011; Jin et al., 2013; Kamohara et al., 2020).
Smoking is another risk factor for various diseases including dental peri-implant diseases and bone fragility (Kasat and Ladda, 2012; Barao et al., 2015; Carvalho and Rossi, 2019; Kordbacheh Changi et al., 2019; Vignoletti et al., 2019; Anderson et al., 2020). Bain CA (1993) reported an implant failure rate of 11.28% in smokers but only 4.76% in nonsmokers. The peri-implant disease symptoms were all worse in cigarette smokers than that in nonsmokers (ALHarthi et al., 2018). Nicotine, among more than 4,000 harmful substances in cigarette, is one of the key components (Barao et al., 2015). Nicotine regulated bone formation through the RANK-RANKL-OPG (osteoprotegerin) system (Lappin et al., 2007; Tang et al., 2009). Rats with cigarette smoking exhibited higher RANKL/OPG ratio to promote osteoclast formation (Giorgetti et al., 2010). Nicotine could also induce the osteoclast differentiation and enhance the resorbing ability of osteoclasts through RANKL (Costa-Rodrigues et al., 2018). However, the interactions between nicotine and S. aureus infection are not clear. Therefore, we investigated the interaction between S. aureus and nicotine on the initiation of peri-implant infections and bone loss in a murine osteolysis model in this study, and their effects on osteoblasts were also evaluated.
Materials and Methods
Bacteria Strains and Cultivation
S. aureus ATCC 25923 was used in this study (Inoue et al., 2017; Ao et al., 2019). Strains were maintained on TSB plates (3% trypticase soy broth, 2% agar). Single colonies were subjected to a liquid TSB medium incubating at 37°C with 150 rpm agitation overnight. S. aureus cells were collected by centrifugation at 5,000 r/min, 4°C for 10 min. Then, the final S. aureus suspension was adjusted to the desired concentration in the TSB medium or in PBS. For the nicotine–S. aureus coculture experiment, optical densities of S. aureus–nicotine (nicotine concentration: 1 μM) (Costa-Rodrigues et al., 2018) or S. aureus alone were detected by using a spectrophotometer every half-hour, and growth curves were drawn. The S. aureus–nicotine combination and the single S. aureus overnight cultures were used for real-time quantitative polymerase chain reaction (RT-qPCR).
The Murine Model
All animal works were conducted in strict accordance with the guidelines of the Ethics Committee of West China School of Sichuan University, and the protocols were fully approved by this Agency (license number WCHSIRB-D-2020-415).
Ti rods of 1.5 mm diameter and 20 mm height were used in this study. Forty Sprague Dawley (female, 12 weeks old) rats were firstly divided into two groups: Ti rods with PBS and Ti rods inoculated with S. aureus (concentration: 108 CFU/ml) (Zhou et al., 2020). Ti rods with PBS and Ti rods with S. aureus were implanted into the left femurs of the rats as previously described (Diefenbeck et al., 2016; Nie et al., 2017). Then, ten randomly assigned rats from each group were subcutaneously injected with nicotine (2 mg/kg) once a day (Milovanovic et al., 2018; Ullrich et al., 2020), and the other ten were injected with PBS as control. There were four groups in total: PBS as the control group, S. aureus group, nicotine group, and S. aureus-nicotine combination group. X-ray analysis was performed on each rat, and radiographic scores were calculated one day or three weeks after surgery (n = 5) (Zhou et al., 2020). The rats were sacrificed three weeks after surgery, and the femurs were collected for gross pathology scoring and colony-forming unit (CFU) counts of bacteria dwelling in Ti rods as well as in bone tissues (n = 5) (Yang et al., 2016). Five femurs of each group were scanned with microcomputed tomography (micro-CT).
Microcomputed Tomography
Rats were sacrificed three weeks after implantation, and the femurs were collected, fixed in 4% buffered formaldehyde, and scanned with a high-resolution micro-CT (SCANCO Medical AG, μCT 50, Brüttisellen, Switzerland) at a voxel size of 20 μm. The shafts of the femurs were chosen as the “region of interest” (ROI). Three-dimensional high-resolution (3D) images were obtained. Mean trabecular thickness (Tb.Th) and bone volume/total volume (BV/TV) were analyzed.
Cell Lines and Cultivation
MC3T3-E1 preosteoblastic cell line was obtained from the State Key Laboratory of Oral Diseases. Cells were cultured in Minimum Essential Medium α (α-MEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin–streptomycin at 37°C in the presence of 5% CO2. For the coculture experiment, S. aureus or the nicotine-treated S. aureus were treated as described previously (Kavanagh et al., 2018). For the nicotine and S. aureus–nicotine combination group, the culture medium was supplemented with 1 μM nicotine. Cells were harvested for RT-qPCR (Widaa et al., 2012; Zheng et al., 2019).
Relative Quantification of Differentially Expressed Genes by RT-qPCR
The relative expressions of spa and RANKL were examined. Total RNA from S. aureus treated with or without nicotine (concentration: 1 μM) (Costa-Rodrigues et al., 2018) and MC3T3-E1 cells treated with or without nicotine and/or S. aureus was extracted with 1 ml TRIZol reagent (Invitrogen, United States) followed by the manufacturer’s instructions. cDNA was synthesized according to the One Step RNA PCR kit (Takara Inc.) protocols. The RT-qPCR were then proceeded following the SYBR® PremixEx TaqTM kit (Takara Inc.) two-step strategy: 1) 95°C for 30 s; 2) 40 PCR cycles (95°C for 5 s, a gene-specific annealing temperature for 30 s). All primer sequences used are listed in Table 1(Zheng et al., 2019), and 16S rDNA and β-actin were chosen as the reference genes. RT-qPCRs were run on LightCycler 480 II (Roche, Basel, Switzerland). The gene expression level relative to the calibrator was expressed as 2−ΔΔCT.
Results
Nicotine and S. aureus Synergistically Increased the Gross Bone Pathology
A gross bone pathology assessment was performed to evaluate the clinical symptoms. Clinical symptoms of pyogenic infections such as abscess and shaft widening could be seen in S. aureus and its combination with nicotine groups after three weeks as pointed out by white arrows (Figure 1A), while the diaphysis was infected only in the nicotine–S. aureus combination group (Figure 1A). Gross bone pathology scores indicated that the average scores from both the S. aureus and nicotine–S. aureus combination groups significantly increased and the nicotine–S. aureus combination group caused the severest symptoms (Figure 1B). However, there was no significant difference between the nicotine and control groups (Figure 1B).
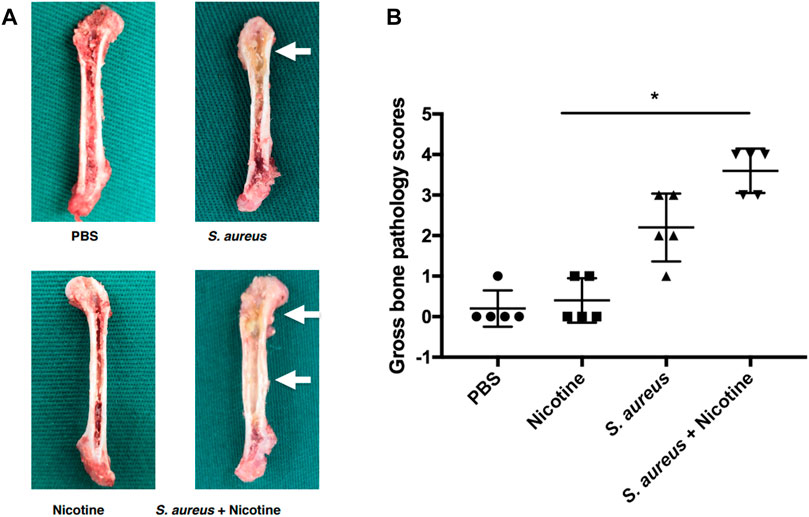
FIGURE 1. Nicotine and S. aureus synergistically increased the gross bone pathology. (A) Gross appearance of the rat femurs three weeks after implantation. Abscess and widening of the shaft are pointed out with white arrows. (B) Gross bone pathology scores of rat femurs. *p < 0.05.
Nicotine and S. aureus Synergistically Induced the Osteolysis and Periosteal Reactions
To further determine the synergistic effect of nicotine and S. aureus, X-ray analysis was performed for the radiographical evaluation at day one and week three. The S. aureus group and the nicotine–S. aureus combination group both exhibited obvious symptoms of osteolysis and periosteal reactions after three weeks as indicated by white arrows (Figure 2A). There was a large scale of bone resorption and sequestrum formation in the nicotine–S. aureus combination group, while little bone infection was observed in the nicotine group (Figure 2A) indicating the synergism between nicotine and S. aureus. There was no significant difference in the radiographical scores among the groups at one day after surgery, but the radiographical scores of the S. aureus and the nicotine–S. aureus combination groups were significantly higher after three weeks (Figure 2B).
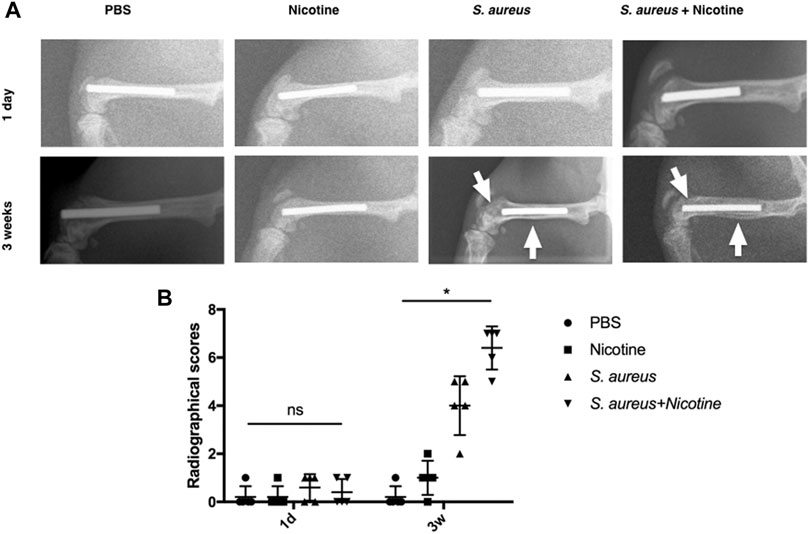
FIGURE 2. Nicotine and S. aureus synergistically induced the osteolysis and periosteal reactions. (A) X-ray images of rat femurs with Ti rod implants at one day or three weeks after surgery. White arrows indicate osteolytic lesions and cortical bone destruction. (B) Radiographical scores of X-ray images. *p < 0.05. ns: p ≧ 0.05.
Nicotine and S. aureus Synergistically Promoted the Bone Resorption
High-resolution micro-CT assessment was conducted to qualitatively and quantitatively analyze the bone tissues of rat femurs three weeks after implantation. 3D images indicated strong osteolysis and cortical bone absorption in the S. aureus and nicotine–S. aureus combination groups, while few bone infection signs were observed in the nicotine group (Figure 3A). The S. aureus group also showed reduced BV/TV and Tb.Th scores, but there was no significant difference between the nicotine and control groups (Figure 3B). The BV/TV and Tb.Th significantly decreased in the nicotine–S. aureus combination groups compared with the other three groups (Figure 3B) indicating their synergism on the bone resorption.
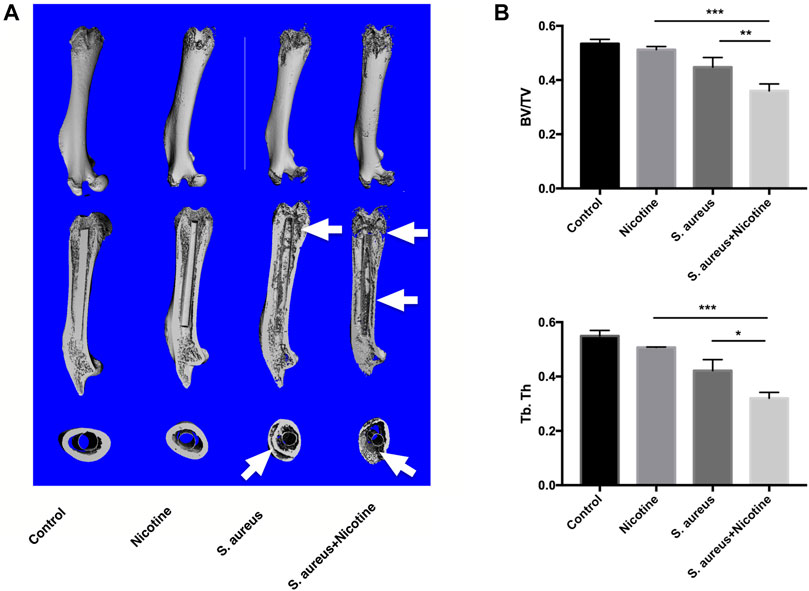
FIGURE 3. Nicotine and S. aureus synergistically promoted the bone resorption. (A) 3D micro-CT images of rat femurs at three weeks after surgery. Osteolytic lesions and cortical bone destruction are pointed out by white arrows. (B) BV/TV and Tb.Th of the selected regions evaluated by micro-CT. *p < 0.05. **p < 0.01. ***p < 0.001.
Nicotine Showed No Impact on the Proliferation of S. aureus
We further evaluated whether the synergistic effect is achieved through nicotine’s impact on the proliferation of S. aureus. The CFU counts of bacteria dwelling in the bone tissues and Ti rods were calculated. There was no significant difference in the bacteria load between the S. aureus and nicotine–S. aureus combination groups (Figure 4A) indicating that nicotine at this concentration did not affect the proliferation of S. aureus. To verify if nicotine could influence the proliferation of S. aureus in vitro, we detected the optical density of the S. aureus and nicotine–S. aureus combination groups every half-hour and the growth curves were drawn. As shown in Figure 4B, no significant difference was observed between these two groups.
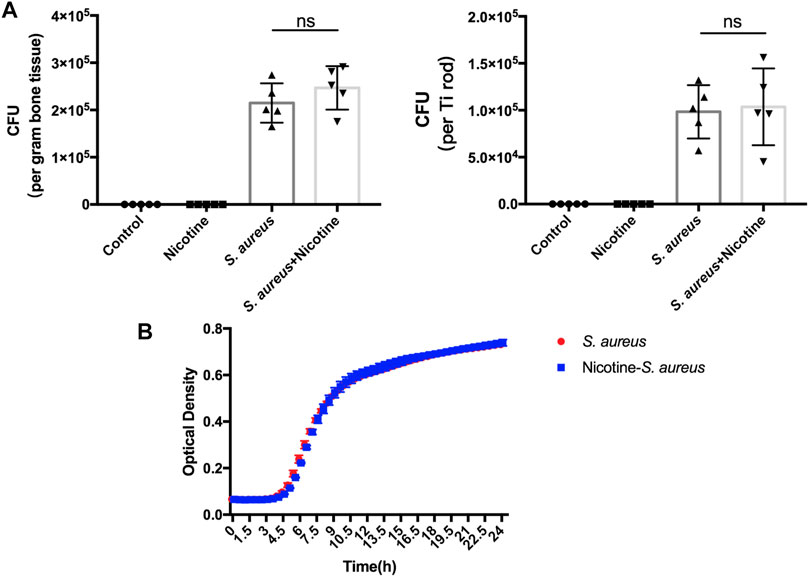
FIGURE 4. Nicotine showed no impact on the proliferation of S. aureus. (A) Quantitative analysis of S. aureus dwelling in Ti rods or in the bone tissue. (B) Growth curves of S. aureus and nicotine–S. aureus. ns: p ≧ 0.05.
Nicotine Upregulated the Virulence of S. aureus and Synergistically Activated the RANKL Pathway
To explore the synergistic mechanisms, we examined the relative expression of spa from the nicotine–S. aureus combination and S. aureus groups. Interestingly, the relative expression of spa was significantly upregulated in the nicotine–S. aureus combination group compared with S. aureus alone (Figure 5A). We then tested if nicotine and S. aureus could synergistically promote RANKL expression. S. aureus alone increased the RANKL expression, but there was no significant difference between the nicotine and control groups (Figure 5B). The relative expression was significantly upregulated in the nicotine–S. aureus combination group compared with the other three groups (Figure 5B) indicating their synergism on the activation of the RANKL pathway.
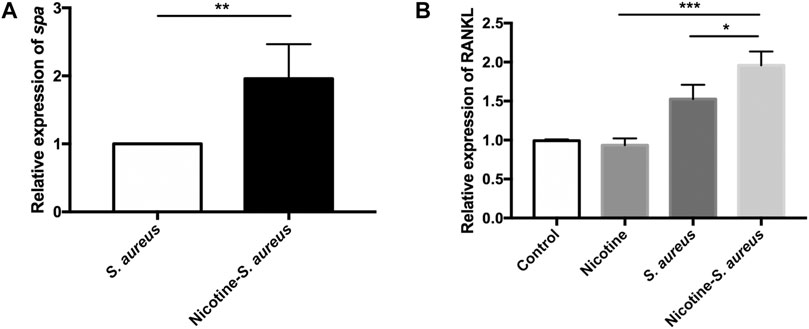
FIGURE 5. Nicotine upregulated the virulence of S. aureus and synergistically activated the RANKL pathway. (A) Relative expression of spa in S. aureus or nicotine-treated S. aureus measured by RT-qPCR. All gene expression levels were normalized by 16s rRNA gene expression. (B) Relative expression of nuclear factor-kappa B ligand (RANKL) in MC3T3-E1 in the presence of nicotine, S. aureus, or both, measured by RT-qPCR. All gene expression levels were normalized by β-actin gene expression. *p < 0.05. **p < 0.01. ***p < 0.001.
Discussion
Peri-implant diseases have been recognized as a global health challenge in dental prosthetic rehabilitation, orthopedics, joint replacements, etc. The microbial biofilm accumulation has been considered as the essential initiator of peri-implant diseases (Lindhe et al., 2008; Oliveira et al., 2018), but the mechanisms for peri-implant infection progress are still not fully characterized because of the complex interactions between different risk factors.
Peri-implant infections are characterized by osteolysis and bone loss. Osteoclasts play an essential role in this process as they are the only bone-resorbing cells (Albrektsson T, 1994; Kadoya et al., 1997; Annibali S, 2008; Uehara et al., 2018; Rokaya et al., 2020). The bone resorption is proved to be decided by the activity and survival of osteoclasts (Goodman and Gallo, 2019). The RANK-RANKL-OPG pathway is critical for osteoclast maturation (Xing et al., 2012). Briefly, RANKL expressed by osteoblasts activated the RANK on the surface of osteoclast precursors and then promoted the activation of NF-κB (nuclear factor-kappa B) to regulate the osteoclast differentiation, activation, and survival (Altaf and Revell, 2013; Park et al., 2017). According to previous studies, nicotine and S. aureus could both participate in this pathway to some extent leading to osteoclast activation (Somayaji et al., 2008; Giorgetti et al., 2010; Claro et al., 2011; Jin et al., 2013; Costa-Rodrigues et al., 2018; Kamohara et al., 2020). In our study, we verified the interactions between nicotine and S. aureus infection through a murine model. Their synergistic effects on peri-implant infections and the activation of RANKL were observed (Figures 1–3, 5).
The impacts of nicotine on bone resorption are controversial. Most scholars believed that cigarette smoking had a negative influence on bone healing while nicotine alone did not have such effect (Stefani et al., 2002; Cesar-Neto et al., 2003; Giorgetti et al., 2010; Zhu et al., 2020). However, nicotine administrated alone was reported to induce the osteoclastogenesis in another study (Ullrich et al., 2020). In our study, nicotine administrated alone induced few osteolysis and no significant difference was observed between the nicotine and control groups. However, it synergized with S. aureus infections to promote the gross bone pathology, osteolysis, periosteal reactions, and bone resorption (Figures 1–3). Our results also showed that nicotine had no impact on the proliferation of S. aureus both in vivo and in vitro (Figure 4) suggesting that the synergistic effect was not a result of the bacteria burden. Costa-Rodrigues et al. (2018) found that nicotine could induce osteoclast differentiation and enhance the resorbing ability of osteoclasts through the RANKL pathway. The virulence factor SpA of S. aureus could also interact with TNFR-1 on the osteoblast surface to promote the expression of RANKL, so we evaluated whether nicotine could facilitate spa expression to activate the RANKL pathway. We found that nicotine significantly upregulated the expression of spa in S. aureus and then significantly activated the RANKL pathway (Figure 5). Here, we identified another way nicotine participated in promoting osteoclastogenesis and bone resorption. To further investigate the detailed mechanisms, the transcriptome, proteome, and metabolome will be studied in the future.
The effects of nicotine on host cells may be dose dependent. An in vitro study examined the direct effect of nicotine on RAW264.7 cells and bone marrow cells, and the results demonstrated that 10-5 M to 10-3 M nicotine reduced the bone resorption by suppressing V-ATPase d2, cathepsin K and MMP-9 expression, and actin reorganization (Tanaka et al., 2013). In our study, we used 1 μM nicotine to treat MC3T3-E1 cells as suggested previously (Costa-Rodrigues et al., 2018). This dosage of nicotine was representative of the concentrations observed in the plasma and saliva of smokers. We found that this dosage of nicotine was able to synergize with S. aureus to activate RANKL expressions. Another study employed 2 mg/ml of nicotine to treat S. aureus and found that the biofilm mass was promoted (Shi et al., 2019). However, the biofilm contained increased numbers of dead S. aureus cells and the agr-dependent virulence of S. aureus was significantly reduced (Shi et al., 2019). This concentration of nicotine was higher than that in serum and was cytocidal to host cells and S. aureus cells in our previous results. We believe that the nicotine level is important for the investigation of the correlations among nicotine, microbiota, and the host.
In addition to S. aureus, numerous studies have also found that Gram-negative pathogens could play some roles in peri-implant diseases, such as Veillonella sp. spirochetes, Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia (Lafaurie et al., 2017; Teles, 2017; Sahrmann et al., 2020; Kotsakis and Olmedo, 2021). It has been recognized that smokers are more susceptible to P. gingivalis than nonsmokers and that nicotine may impact P. gingivalis’s inflammatory effect (Baek et al., 2012; Cogo et al., 2012; Kashiwagi et al., 2012). The interactions between nicotine and other microbial species or multispecies are also important in the development of peri-implant diseases, and we will investigate that in the future.
In conclusion, our results indicated that nicotine and S. aureus can synergistically induce peri-implant infections. Nicotine upregulated the virulence gene spa in S. aureus to increase the RANKL expression in osteoblast precursors. Our results highlighted that targeting the interaction between nicotine and S. aureus was a practical way to reduce the peri-implant infections, especially in smokers.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of West China School of Sichuan University.
Author Contributions
Conceptualization, YH, BR, and LC; methodology, YH, WZ, BR, and LC; writing—original draft preparation, YH; writing—review and editing, YH, WZ, CZ, YZ, QG, XH, BY, BR, and LC; supervision, BR and LC; and funding acquisition, BR and LC. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grants 82071106 (LC), 81870778 (BR), 81600858 (BR), and 81870759 (LC), Applied Basic Research Program of Sichuan Province, 2020YJ0227 (BR), the Youth Grant of Science and Technology Department of Sichuan Province, China, 2017JQ0028 (LC), and Innovative Research Team Program of Sichuan Province (LC) Fund of State Key Laboratory of Oral Diseases (SKLOD201913) (BR), SKLOD202113 (LC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We extremely appreciate Dr. Ning Ji for his kind help on the murine experiments.
References
Ahn, D.-H., Kim, H.-J., Joo, J.-Y., and Lee, J.-Y. (2019). Prevalence and Risk Factors of Peri-Implant Mucositis and Peri-Implantitis after at Least 7 Years of Loading. J. Periodontal Implant Sci. 49 (6), 397–405. doi:10.5051/jpis.2019.49.6.397
Albrektsson T, I. F. (1994).Consensus Report: Implant Therapy. In Proceedings of the 1st European Workshop on Periodontology. Berlin, Germany: Quintessence.
Alexander, E. H., and Hudson, M. C. (2001). Factors Influencing the Internalization of Staphylococcus aureus and Impacts on the Course of Infections in Humans. Appl. Microbiol. Biotechnol. 56 (3-4), 361–366. doi:10.1007/s002530100703
ALHarthi, S. S., BinShabaib, M. S., Ahmed, H. B., Mehmood, A., Khan, J., and Javed, F. (2018). Comparison of Peri-Implant Clinical and Radiographic Inflammatory Parameters Among Cigarette and Waterpipe (Narghile) Smokers and Never-Smokers. J. Periodontol. 89 (2), 213–218. doi:10.1902/jop.2017.170358
Altaf, H., and Revell, P. A. (2013). Evidence for Active Antigen Presentation by Monocyte/macrophages in Response to Stimulation with Particles: the Expression of NFκB Transcription Factors and Costimulatory Molecules. Inflammopharmacol 21 (4), 279–290. doi:10.1007/s10787-013-0170-z
Amornvit, P., Bajracharya, S., Rokaya, D., Keawcharoen, K., and Supavanich, W. (2014). Management of Obstructive Sleep Apnea with Implant Retained Mandibular Advancement Device. World J. Dent 5 (03), 184–189. doi:10.5005/jp-journals-10015-1285
Anderson, N., Lords, A., Laux, R., Woodall, W., and Abubakr, N. H. (2020). Retrospective Analysis of the Risk Factors of Peri-Implantitis. J. Contemp. Dent Pract. 21 (12), 1350–1353. doi:10.5005/jp-journals-10024-2973
Annibali S, R. M., La Monaca, G., Tonoli, F., and Cristalli, M. P. (2008). Local Complications in Implant Surgery: Prevention and Management. Oral Implantol. 1, 21–33.
Ao, H., Yang, S., Nie, B. e., Fan, Q., Zhang, Q., Zong, J., et al. (2019). Improved Antibacterial Properties of Collagen I/hyaluronic Acid/quaternized Chitosan Multilayer Modified Titanium Coatings with Both Contact-Killing and Release-Killing Functions. J. Mater. Chem. B 7 (11), 1951–1961. doi:10.1039/c8tb02425a
Baek, O., Zhu, W., Kim, H. C., and Lee, S.-W. (2012). Effects of Nicotine on the Growth and Protein Expression of Porphyromonas Gingivalis. J. Microbiol. 50 (1), 143–148. doi:10.1007/s12275-012-1212-8
Bain, C. A., and Moy, P. K. (1993). The Association between the Failure of Dental Implants and Cigarette Smoking. Int. J. Oral Maxillofac. Implants 8, 609–615.
Barão, V. A. R., Ricomini-Filho, A. P., Faverani, L. P., Del Bel Cury, A. A., Sukotjo, C., Monteiro, D. R., et al. (2015). The Role of Nicotine, Cotinine and Caffeine on the Electrochemical Behavior and Bacterial Colonization to Cp-Ti. Mater. Sci. Eng. C 56, 114–124. doi:10.1016/j.msec.2015.06.026
Berglundh, T., Armitage, G., Araujo, M. G., Avila-Ortiz, G., Blanco, J., Camargo, P. M., et al. (2018). Peri-implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 89 (Suppl. 1), S313–S318. doi:10.1002/JPER.17-0739
Bost, K. L., Ramp, W. K., Nicholson, N. C., Bento, J. L., Marriott, I., and Hudson, M. C. (1999). Staphylococcus aureusInfection of Mouse or Human Osteoblasts Induces High Levels of Interleukin‐6 and Interleukin‐12 Production. J. Infect. Dis. 180 (6), 1912–1920. doi:10.1086/315138
Carrasco-Garcia, A., Castellanos-Cosano, L., Corcuera-Flores, J., Rodriguez-Perez, A., Torres-Lagares, D., and Machuca-Portillo, G. (2019). Influence of Marginal Bone Loss on Peri-Implantitis: Systematic Review of Literature. J. Clin. Exp. Dent 11 (11), e1045–e1071. doi:10.4317/jced.56202
Carvalho, J. P., and Rossi, V. (2019). Effect of Smoking in Peri-Implant Diseases: Literature Review. EC Dental Sci. 18 (8), 1720–1724.
César-Neto, J. B., Duarte, P. M., Sallum, E. A., Barbieri, D., Moreno, H., and Nociti, F. H. (2003). A Comparative Study on the Effect of Nicotine Administration and Cigarette Smoke Inhalation on Bone Healing Around Titanium Implants. J. Periodontol. 74 (10), 1454–1459. doi:10.1902/jop.2003.74.10.1454
Claro, T., Widaa, A., O'Seaghdha, M., Miajlovic, H., Foster, T. J., O'Brien, F. J., et al. (2011). Staphylococcus aureus Protein A Binds to Osteoblasts and Triggers Signals that Weaken Bone in Osteomyelitis. PLoS One 6 (4), e18748. doi:10.1371/journal.pone.0018748
Cogo, K., de Andrade, A., Labate, C. A., Bergamaschi, C. C., Berto, L. A., Franco, G. C. N., et al. (2012). Proteomic Analysis ofPorphyromonas Gingivalisexposed to Nicotine and Cotinine. J. Periodontal Res. 47 (6), 766–775. doi:10.1111/j.1600-0765.2012.01494.x
Cortizo, M. C., Oberti, T. G., Cortizo, M. S., Cortizo, A. M., and Fernández Lorenzo de Mele, M. A. (2012). Chlorhexidine Delivery System from Titanium/polybenzyl Acrylate Coating: Evaluation of Cytotoxicity and Early Bacterial Adhesion. J. Dentistry 40 (4), 329–337. doi:10.1016/j.jdent.2012.01.008
Costa-Rodrigues, J., Rocha, I., and Fernandes, M. H. (2018). Complex Osteoclastogenic Inductive Effects of Nicotine over Hydroxyapatite. J. Cel Physiol 233 (2), 1029–1040. doi:10.1002/jcp.25956
Depypere, M., Morgenstern, M., Kuehl, R., Senneville, E., Moriarty, T. F., Obremskey, W. T., et al. (2020). Pathogenesis and Management of Fracture-Related Infection. Clin. Microbiol. Infect. 26 (5), 572–578. doi:10.1016/j.cmi.2019.08.006
Derks, J., and Tomasi, C. (2015). Peri-implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 42 (Suppl. 16), S158–S171. doi:10.1111/jcpe.12334
Diefenbeck, M., Schrader, C., Gras, F., Mückley, T., Schmidt, J., Zankovych, S., et al. (2016). Gentamicin Coating of Plasma Chemical Oxidized Titanium alloy Prevents Implant-Related Osteomyelitis in Rats. Biomaterials 101, 156–164. doi:10.1016/j.biomaterials.2016.05.039
Dreyer, H., Grischke, J., Tiede, C., Eberhard, J., Schweitzer, A., Toikkanen, S. E., et al. (2018). Epidemiology and Risk Factors of Peri-Implantitis: A Systematic Review. J. Periodont Res. 53 (5), 657–681. doi:10.1111/jre.12562
Giorgetti, A. P. O., César Neto, J. B., Ruiz, K. G. S., Casati, M. Z., Sallum, E. A., and Nociti, F. H. (2010). Cigarette Smoke Inhalation Modulates Gene Expression in Sites of Bone Healing: a Study in Rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 110 (4), 447–452. doi:10.1016/j.tripleo.2010.02.029
Goodman, S. B., and Gallo, J. (2019). Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. Jcm 8 (12), 2091. doi:10.3390/jcm8122091
Harris, L. G., and Richards, R. G. (2004). Staphylococcus aureus Adhesion to Different Treated Titanium Surfaces. J. Mater. Sci. Mater. Med. 15 (4), 311–314. doi:10.1023/b:jmsm.0000021093.84680.bb
Hebert, J. S., Rehani, M., and Stiegelmar, R. (2017). Osseointegration for Lower-Limb Amputation. JBJS Rev. 5 (10), e10. doi:10.2106/JBJS.RVW.17.00037
Inoue, D., Kabata, T., Ohtani, K., Kajino, Y., Shirai, T., and Tsuchiya, H. (2017). Inhibition of Biofilm Formation on Iodine-Supported Titanium Implants. Int. Orthopaedics (Sicot) 41 (6), 1093–1099. doi:10.1007/s00264-017-3477-3
Jin, T., Zhu, Y. l., Li, J., Shi, J., He, X. q., Ding, J., et al. (2013). Staphylococcal Protein A, Panton-Valentine Leukocidin and Coagulase Aggravate the Bone Loss and Bone Destruction in Osteomyelitis. Cell Physiol Biochem 32 (2), 322–333. doi:10.1159/000354440
Kadoya, Y., Revell, P. A., Kobayashi, A., al-Saffar, N., Scott, G., and Freeman, M. A. R. (1997). Wear Particulate Species and Bone Loss in Failed Total Joint Arthroplasties. Clin. Orthopaedics Relat. Res. 340, 118–129. doi:10.1097/00003086-199707000-00016
Kamohara, A., Hirata, H., Xu, X., Shiraki, M., Yamada, S., Zhang, J.-Q., et al. (2020). IgG Immune Complexes with Staphylococcus aureus Protein A Enhance Osteoclast Differentiation and Bone Resorption by Stimulating Fc Receptors and TLR2. Int. Immunol. 32 (2), 89–104. doi:10.1093/intimm/dxz063
Kasat, V., and Ladda, R. (2012). Smoking and Dental Implants. J. Int. Soc. Prevent Communit Dent 2 (2), 38–41. doi:10.4103/2231-0762.109358
Kashiwagi, Y., Yanagita, M., Kojima, Y., Shimabukuro, Y., and Murakami, S. (2012). Nicotine Up-Regulates IL-8 Expression in Human Gingival Epithelial Cells Following Stimulation with IL-1β or P. Gingivalis Lipopolysaccharide via Nicotinic Acetylcholine Receptor Signalling. Arch. Oral Biol. 57 (5), 483–490. doi:10.1016/j.archoralbio.2011.10.007
Kavanagh, N., O’Brien, F. J., and Kerrigan, S. W. (2018). Staphylococcus aureus Protein A Causes Osteoblasts to Hyper-Mineralise in a 3D Extra-cellular Matrix Environment. PLoS One 13 (6), e0198837. doi:10.1371/journal.pone.0198837
Kordbacheh Changi, K., Finkelstein, J., and Papapanou, P. N. (2019). Peri‐implantitis Prevalence, Incidence Rate, and Risk Factors: A Study of Electronic Health Records at a U.S. Dental School. Clin. Oral Impl Res. 30 (4), 306–314. doi:10.1111/clr.13416
Kotsakis, G. A., and Olmedo, D. G. (2021). Peri‐implantitis Is Not Periodontitis: Scientific Discoveries Shed Light on Microbiome‐biomaterial Interactions that May Determine Disease Phenotype. Periodontol. 2000 86 (1), 231–240. doi:10.1111/prd.12372
Lafaurie, G. I., Sabogal, M. A., Castillo, D. M., Rincón, M. V., Gómez, L. A., Lesmes, Y. A., et al. (2017). Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 88 (10), 1066–1089. doi:10.1902/jop.2017.170123
Lang, N. P., Berglundh, T., and Working Group 4 of Seventh European Workshop on Periodontology, (2011). Periimplant Diseases: where Are We Now? - Consensus of the Seventh European Workshop on Periodontology Periimplant Diseases: where Are We Now?--Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 38 (Suppl. 11), 178–181. doi:10.1111/j.1600-051X.2010.01674.x
Lappin, D. F., Sherrabeh, S., Jenkins, W. M. M., and Macpherson, L. M. D. (2007). Effect of Smoking on Serum RANKL and OPG in Sex, Age and Clinically Matched Supportive-Therapy Periodontitis Patients. J. Clin. Periodontol. 34 (4), 271–277. doi:10.1111/j.1600-051X.2007.01048.x
Lindhe, J., Meyle, J., and Group, D. o. E. W. o. P. (2008). Peri-implant Diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 35 (8 Suppl. l), 282–285. doi:10.1111/j.1600-051X.2008.01283.x
Lu, S., Vien, B. S., Russ, M., Fitzgerald, M., and Chiu, W. K. (2020). Non-radiative Healing Assessment Techniques for Fractured Long Bones and Osseointegrated Implant. Biomed. Eng. Lett. 10 (1), 63–81. doi:10.1007/s13534-019-00120-0
Milovanovic, P., Stojanovic, M., Antonijevic, D., Cirovic, A., Radenkovic, M., and Djuric, M. (2018). "Dangerous Duo": Chronic Nicotine Exposure Intensifies Diabetes Mellitus-Related Deterioration in Bone Microstructure - an Experimental Study in Rats. Life Sci. 212, 102–108. doi:10.1016/j.lfs.2018.09.044
Nie, B. e., Ao, H., Long, T., Zhou, J., Tang, T., and Yue, B. (2017). Immobilizing Bacitracin on Titanium for Prophylaxis of Infections and for Improving Osteoinductivity: An In Vivo Study. Colloids Surf. B: Biointerfaces 150, 183–191. doi:10.1016/j.colsurfb.2016.11.034
Oliveira, W. F., Silva, P. M. S., Silva, R. C. S., Silva, G. M. M., Machado, G., Coelho, L. C. B. B., et al. (2018). Staphylococcus aureus and Staphylococcus Epidermidis Infections on Implants. J. Hosp. Infect. 98 (2), 111–117. doi:10.1016/j.jhin.2017.11.008
Park, J. H., Lee, N. K., and Lee, S. Y. (2017). Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cell 40 (10), 706–713. doi:10.14348/molcells.2017.0225
Persson, G. R., and Renvert, S. (2014). Cluster of Bacteria Associated with Peri-Implantitis. Clin. Implant Dentistry Relat. Res. 16 (6), 783–793. doi:10.1111/cid.12052
Rokaya, D., Srimaneepong, V., Wisitrasameewon, W., Humagain, M., and Thunyakitpisal, P. (2020). Peri-implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur. J. Dent 14 (4), 672–682. doi:10.1055/s-0040-1715779
Romanò, C. L., Toscano, M., Romanò, D., and Drago, L. (2013). Antibiofilm Agents and Implant-Related Infections in Orthopaedics: where Are We? J. Chemother. 25 (2), 67–80. doi:10.1179/1973947812Y.0000000045
Sahrmann, P., Gilli, F., Wiedemeier, D. B., Attin, T., Schmidlin, P. R., and Karygianni, L. (2020). The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 8 (5), 661. doi:10.3390/microorganisms8050661
Schwarz, F., Derks, J., Monje, A., and Wang, H.-L. (2018). Peri-implantitis. J. Clin. Periodontol. 45 (Suppl. 20), S246–S266. doi:10.1111/jcpe.12954
Seebach, E., and Kubatzky, K. F. (2019). Chronic Implant-Related Bone Infections-Can Immune Modulation Be a Therapeutic Strategy? Front. Immunol. 10, 1724. doi:10.3389/fimmu.2019.01724
Shi, L., Wu, Y., Yang, C., Ma, Y., Zhang, Q.-z., Huang, W., et al. (2019). Effect of Nicotine on Staphylococcus aureus Biofilm Formation and Virulence Factors. Sci. Rep. 9 (1), 20243. doi:10.1038/s41598-019-56627-0
Shrestha, B., Theerathavaj, M. L. S., Thaweboon, S., and Thaweboon, B. (2012). In Vitro antimicrobial Effects of Grape Seed Extract on Peri-Implantitis Microflora in Craniofacial Implants. Asian Pac. J. Trop. Biomed. 2 (10), 822–825. doi:10.1016/S2221-1691(12)60236-6
Somayaji, S. N., Ritchie, S., Sahraei, M., Marriott, I., and Hudson, M. C. (2008). Staphylococcus aureus Induces Expression of Receptor Activator of NF-Κb Ligand and Prostaglandin E 2 in Infected Murine Osteoblasts. Infect. Immun. 76 (11), 5120–5126. doi:10.1128/IAI.00228-08
Stefani, C. M., Filho, G. R. N., Sallum, E. A., Toledo, S. D., Sallum, A. W., and Nociti, F. H. (2002). Influence of Nicotine Administration on Different Implant Surfaces: a Histometric Study in Rabbits. J. Periodontol. 73 (2), 206–212. doi:10.1902/jop.2002.73.2.206
Tanaka, H., Tanabe, N., Kawato, T., Nakai, K., Kariya, T., Matsumoto, S., et al. (2013). Nicotine Affects Bone Resorption and Suppresses the Expression of Cathepsin K, MMP-9 and Vacuolar-type H+-ATPase D2 and Actin Organization in Osteoclasts. PLoS One 8 (3), e59402. doi:10.1371/journal.pone.0059402
Tang, T. H., Fitzsimmons, T. R., and Bartold, P. M. (2009). Effect of Smoking on Concentrations of Receptor Activator of Nuclear Factor κ B Ligand and Osteoprotegerin in Human Gingival Crevicular Fluid. J. Clin. Periodontol. 36 (9), 713–718. doi:10.1111/j.1600-051X.2009.01444.x
Teles, F. R. F. (2017). The Microbiome of Peri-Implantitis: Is it Unique? Compend. Contin. Educ. Dent 38 (8 Suppl. l), 22–25.
Thurnheer, T., and Belibasakis, G. N. (2016). Incorporation of Staphylococci into Titanium‐grown Biofilms: an In Vitro "submucosal" Biofilm Model for Peri‐implantitis. Clin. Oral Impl. Res. 27 (7), 890–895. doi:10.1111/clr.12715
Uehara, S., Udagawa, N., and Kobayashi, Y. (2018). Non-canonical Wnt Signals Regulate Cytoskeletal Remodeling in Osteoclasts. Cell. Mol. Life Sci. 75 (20), 3683–3692. doi:10.1007/s00018-018-2881-1
Ullrich, N., Schröder, A., Bauer, M., Spanier, G., Jantsch, J., Deschner, J., et al. (2020). The Role of HIF-1α in Nicotine-Induced Root and Bone Resorption during Orthodontic Tooth Movement. Eur. J. Orthod. 12. doi:10.1093/ejo/cjaa057
Vignoletti, F., Di Domenico, G. L., Di Martino, M., Montero, E., and de Sanctis, M. (2019). Prevalence and Risk Indicators of Peri‐implantitis in a Sample of university‐based Dental Patients in Italy: A Cross‐sectional Study. J. Clin. Periodontol. 46 (5), 597–605. doi:10.1111/jcpe.13111
Weinstein, T., Clauser, T., Del Fabbro, M., Deflorian, M., Parenti, A., Taschieri, S., et al. (2020). Prevalence of Peri-Implantitis: A Multi-Centered Cross-Sectional Study on 248 Patients. Dentistry J. 8 (3), 80. doi:10.3390/dj8030080
Widaa, A., Claro, T., Foster, T. J., O’Brien, F. J., and Kerrigan, S. W. (2012). Staphylococcus aureus Protein A Plays a Critical Role in Mediating Bone Destruction and Bone Loss in Osteomyelitis. PLoS One 7 (7), e40586. doi:10.1371/journal.pone.0040586
Xing, L., Xiu, Y., and Boyce, B. F. (2012). Osteoclast Fusion and Regulation by RANKL-dependent and Independent Factors. Wjo 3 (12), 212–222. doi:10.5312/wjo.v3.i12.212
Yang, Y., Ao, H., Wang, Y., Lin, W., Yang, S., Zhang, S., et al. (2016). Cytocompatibility with Osteogenic Cells and Enhanced In Vivo Anti-infection Potential of Quaternized Chitosan-Loaded Titania Nanotubes. Bone Res. 4, 16027. doi:10.1038/boneres.2016.27
Zhao, B., van der Mei, H. C., Subbiahdoss, G., de Vries, J., Rustema-Abbing, M., Kuijer, R., et al. (2014). Soft Tissue Integration versus Early Biofilm Formation on Different Dental Implant Materials. Dental Mater. 30 (7), 716–727. doi:10.1016/j.dental.2014.04.001
Zheng, L., Yang, L., Zhao, X., Long, N., Li, P., and Wang, Y. (2019). Effect of Risperidone on Proliferation and Apoptosis of MC3T3-E1 Cells. Braz. J. Med. Biol. Res. 52 (3), e8098. doi:10.1590/1414-431X20188098
Zhou, W., Peng, X., Ma, Y., Hu, Y., Wu, Y., Lan, F., et al. (2020). Two-staged Time-dependent Materials for the Prevention of Implant-Related Infections. Acta Biomater. 101, 128–140. doi:10.1016/j.actbio.2019.10.023
Zhu, S., Häussling, V., Aspera-Werz, R. H., Chen, T., Braun, B., Weng, W., et al. (2020). Bisphosphonates Reduce Smoking-Induced Osteoporotic-like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-culture Model. Ijms 22 (1), 53. doi:10.3390/ijms22010053
Keywords: peri-implant infection, Staphylococcus aureus, nicotine, synergistic effect, RANKL
Citation: Hu Y, Zhou W, Zhu C, Zhou Y, Guo Q, Huang X, Yang B, Ren B and Cheng L (2021) The Synergistic Effect of Nicotine and Staphylococcus aureus on Peri-Implant Infections. Front. Bioeng. Biotechnol. 9:658380. doi: 10.3389/fbioe.2021.658380
Received: 04 February 2021; Accepted: 30 July 2021;
Published: 13 September 2021.
Edited by:
Nihal Engin Vrana, Sparta Medical, FranceReviewed by:
Ika Dewi Ana, Gadjah Mada University, IndonesiaFrancois Malherbe, Swinburne University of Technology, Australia
Copyright © 2021 Hu, Zhou, Zhu, Zhou, Guo, Huang, Yang, Ren and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Ren, cmVuYmlhb0BzY3UuZWR1LmNu; Lei Cheng, Y2hlbmdsZWlAc2N1LmVkdS5jbg==
 Yao Hu
Yao Hu Wen Zhou
Wen Zhou Chengguang Zhu
Chengguang Zhu Yujie Zhou
Yujie Zhou Qiang Guo
Qiang Guo Xiaoyu Huang
Xiaoyu Huang Bina Yang
Bina Yang Biao Ren
Biao Ren Lei Cheng
Lei Cheng