
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Bioeng. Biotechnol., 28 May 2021
Sec. Synthetic Biology
Volume 9 - 2021 | https://doi.org/10.3389/fbioe.2021.656465
This article is part of the Research TopicEvolving Environmental Biotechnology with Synthetic and Systems BiologyView all 4 articles
 Lakshika Dissanayake1
Lakshika Dissanayake1 Lahiru N. Jayakody1,2*
Lahiru N. Jayakody1,2*Polyethylene terephthalate (PET) is globally the largest produced aromatic polyester with an annual production exceeding 50 million metric tons. PET can be mechanically and chemically recycled; however, the extra costs in chemical recycling are not justified when converting PET back to the original polymer, which leads to less than 30% of PET produced annually to be recycled. Hence, waste PET massively contributes to plastic pollution and damaging the terrestrial and aquatic ecosystems. The global energy and environmental concerns with PET highlight a clear need for technologies in PET “upcycling,” the creation of higher-value products from reclaimed PET. Several microbes that degrade PET and corresponding PET hydrolase enzymes have been successfully identified. The characterization and engineering of these enzymes to selectively depolymerize PET into original monomers such as terephthalic acid and ethylene glycol have been successful. Synthetic microbiology and metabolic engineering approaches enable the development of efficient microbial cell factories to convert PET-derived monomers into value-added products. In this mini-review, we present the recent progress of engineering microbes to produce higher-value chemical building blocks from waste PET using a wholly biological and a hybrid chemocatalytic–biological strategy. We also highlight the potent metabolic pathways to bio-upcycle PET into high-value biotransformed molecules. The new synthetic microbes will help establish the circular materials economy, alleviate the adverse energy and environmental impacts of PET, and provide market incentives for PET reclamation.
Plastic, a synthetic polymer, plays a vital role in modern life due to its versatility, advantageous material properties, and low production cost. It has been estimated that about 5–13 million tons of plastic is ended up in the ocean annually, and 5 trillion plastic particles are estimated to float in Earth’s oceans, which injures and kills marine life (Eriksen et al., 2014; Law et al., 2020). Although plastic is less prone to biodegradability, it can be partially fragmented to microplastic (5 mm to 1 μm) particularly by ultraviolet radiation, and microplastics have invaded not only terrestrial and marine ecosystem, but atmospheric ecosystems as well (Eriksen et al., 2014; de Sá et al., 2018; Allen et al., 2019). Microplastics enters the food chain, spreads toxins, and poses a potential threat to human health (Wang et al., 2018). The systematic presence of synthetic micro polymers is threatening to create a global-scale environmental crisis (Thompson et al., 2009; Jambeck et al., 2015; Geyer et al., 2017).
Polyethylene terephthalate (PET) is a thermoplastic polyester of terephthalic acid (TPA) and ethylene glycol (EG) monomers (Kim and Lee, 2012). The wide applicability of PET in various industries such as in packaging, textiles, electrical and electronics, and automotive industry is related to its properties such as high mechanical strength, light weight, electrical insulating properties, chemical inertness, and gas and moisture barrier properties (Webb et al., 2013). Global PET fiber and resin production was estimated to be around 77 million tons in 2015 (Fiber Economics Bureau, 2015; Plastic Insight, 2016). Mismanagement of PET waste contributes to the plastic pollution and demanding techno-economically feasible end-of-life or circular economy options for PET, including recycling post-consumer PET back to the original material (Hamid et al., 2018; Meys et al., 2020). Mechanical conversion of PET to the same use often results in PET polymer with poorer mechanical and structural properties, and thus, lower value by 33% (Awaja and Pavel, 2005). PET can be chemically recycled via full breakdown to monomers and repolymerized back to PET; however, chemical-based recycling costs of PET to remake the same polymer is not economically feasible (Rahimi and García, 2017; Vollmer et al., 2020). However, It has been predicted that recycled PET over virgin PET has remarkable energy and environmental impact on reducing greenhouse gas (GHG) emissions by 1.5 CO2-eq-ton/recycle PET and energy input over virgin PET by >20 MJ/ton (Rorrer et al., 2019).
The novel discovery of PET depolymerizing enzymes has transformed the field to develop a techno-economically feasible bio-based PET recycling process (Wei and Zimmermann, 2017; Tournier et al., 2020; Zimmermann, 2020). Researchers have uncovered novel PET hydrolases from the microorganism in plastic ecosystems (Plastisphere) and investigated them to establish bio-based PET recycling approaches (Mueller, 2006; Kawai et al., 2020). The CARBOIS, a green chemistry company, developed industrially applicable enzyme-based recycling technology to remake PET bottles with similar material properties only using recycled PET monomers (Tournier et al., 2020). Comprehensive review articles have been published on bio-based PET recycling techniques (Wei and Zimmermann, 2017; Blank et al., 2020; Ru et al., 2020; Wei et al., 2020; Kawai, 2021). With the advances in synthetic microbiology, the development of sustainable microbial-based “PET upcycling” toward a green route of the circular economy becomes attractive. Upcycling is achieved by adding value to the PET waste by providing a path for utilizing PET-derived compounds to manufacture high-value chemicals and materials (Kenny et al., 2008; Rorrer et al., 2019; Blank et al., 2020; Sohn et al., 2020). Microbial cell factories have been tailored to the deconstruction of PET in concert with the chemical processes (i.e., hybrid biochemical process). PET-derived monomers can be biotransformed into high-value platform chemicals and biomaterials, including bioplastic PET alternatives. It enables the creation of a circular material economy for PET (Sohn et al., 2020; Tiso et al., 2020). Hence, this mini-review highlights the current progress on microbial-based PET upcycling.
The conventional culture-dependent methods, cutting-edge multi-omics-based systems biology approaches, and molecular biology techniques enable researchers to identify novel PET-hydrolyzing enzymes from the plastisphere to cleave ester bonds of PET (Supplementary Table 1). Also, computational and machine learning approaches enable the researcher to discover novel potent PET enzymes from metagenomics databases (Danso et al., 2018; Furukawa et al., 2019; Subramanian et al., 2019). These enzymes belong to esterase, cutinase, and lipase families, and may evolve in a PET-rich environment (Roager and Sonnenschein, 2019; Alam et al., 2020; Maurya et al., 2020). The discovery of the bacterium Ideonella sakaiensis harboring hydrolyzing enzymes PETase and MHETase has revolutionized the field. These enzymes can completely degrade the synthetic polymer PET to its monomers TPA and EG at ambient temperature (Yoshida et al., 2016; Carr et al., 2020). Employing biological catalysis for commercial PET depolymerization is challenging due to the limited accessibility of polymer’s high crystalline ester linkages. Hence, researchers engineer the PET-hydrolyzing enzymes to enhance the activity by in-depth structure/activity relationships studies (Supplementary Table 2). For instance, (1) narrowing the binding cleft of PETase, an α/β-hydrolase fold enzyme via mutation of two active-site residues to conserved amino acids in cutinases (i.e., S238F/W159H), enhanced the crystalline PET degradation to convert PET to mono-(2-hydroxyethyl) terephthalate (MHET), and (2) the second enzyme MHETase, a specific lid-domain-containing esterase, hydrolyzes MHET to TPA and EG, but not bis-(2-hydroxyethyl) terephthalate (BHET) natively (Austin et al., 2018; Knott et al., 2020). Palm and coworkers successfully demonstrated structure-guided alterations of MHETase to active BHET by introducing three mutations to the lid domain of the MHETase (R411A/S419G/F424N) (Palm et al., 2019).
Researchers developed PET hydrolyzing enzymes acting near or above the glass transition temperature, Tg of PET (67–81°C) maximizes the PET polyester chain mobility and ester bond hydrolyzing reaction. To increase the thermal stability of TfCut2, binding sites of Ca2+ and Mg2+ are identified as potential targets for engineering (Then et al., 2015). Introduction of a disulfide bridge to substitute TfCut2 Ca-binding site increased thermal stability and activity against PET (Then et al., 2016). Thermostability of leaf-branch compost cutinase (LCC) is improved to 94.5°C by replacing the divalent metal site with a disulfide bridge (D238C/S283C). The loss of enzyme activity is restored by introducing additional mutation of residue in contact with the PET substrates (F243I) (Tournier et al., 2020). The engineered enzymes enable industrially relevant PET recycling to manufacture PET bottles with similar material properties using recovered TPA. Indeed, they achieved a 90% conversion of pre-treated post-consumer PET in less than 10 h, with a mean productivity of 16.7 g TPA L–1 h–1 with a yield of 27.9 g TPA g enzyme–1, and demonstrate the green route of the circular economy. In concert with computation studies, protein engineering shows the potential to develop efficient PET-hydrolyzing enzymes with improved crystalline PET activity, expanded substrate specificity, alleviated product inhibitions, and thermostability (Cui et al., 2021).
A dual enzyme system consisting of a polyester hydrolase, TfCut2 or LCC, and a carboxylesterase TfCa from Thermobifida fusca KW3 has been tested to overcome the inhibition of MHET on PET degradation (Barth et al., 2016). With the addition of TfCa, the total products reported an increase of 91% with TfCut2 and 104% increase with LCC. The results indicated the successful use of TfCa as a secondary biocatalyst to improve PET degradation by increased hydrolysis of MHET. Similarly, the synergistic effect on PETase and MHETase enhances the PET degradation activity, and a higher MHETase load further improved the degradation rate. Furthermore, MHETase and PETase’s chimeric enzymes (MHETase C terminus connected to PETase via flexible glycine-serine linkers) outperformed the degradation rate of unlinked PETase and MHETase (Knott et al., 2020). Pichia pastoris has shown potential as a better expression host for PET-hydrolyzing enzymes such as LCC and Thc_Cut1 (Gamerith et al., 2017; Shirke et al., 2018). The engineered thermophilic Clostridium thermocellum expressing the thermophilic PET hydrolase LCC enables the selective degradation of PET at 60°C and outperformed whole-cell-based PET biodegradation systems that employ mesophilic bacteria or microalgae. A recent study reported that expression of BhrPETase in Bacillus subtilis showed higher activity on amorphous PET compared to LCC and PETase, and this is the most thermostable PET hydrolase reported to date (Xi et al., 2021).
The selection of an appropriate host strain is necessary to obtain the active enzymes, and codon optimization of genes is vital to produce accurately folded soluble protein (Angov, 2011). It has been shown that TfCut2 expressed in B. subtilis is more efficient and thermostable than when expressed in Escherichia coli, and the enzymes can be used to depolymerize post-consumer PET food packaging effectively (Wei et al., 2019). Additionally, B. subtilis successfully employs LCC and IsPETase, methylotrophic yeast, P. pastoris for LCC, and cutinase from Thermobifida cellulosilytica (Gamerith et al., 2017; Shirke et al., 2018; Xi et al., 2021). Notably, for the commercial production of PET-hydrolyzing enzymes, the strains that allow secretory high-level expression should be used to avoid additional costly purification steps (Su et al., 2013). Since enzyme expression and purification add extra cost to the process, researchers developed the whole-cell microbial catalysts to degrade the PET by heterologous expression of PET-hydrolyzing enzymes (Samak et al., 2020). A promising strategy to overcome the problem of PET waste in marine environments where an engineered photosynthetic marine microalgae Phaeodactylum tricornutum with the ability to produce and secrete an improved PETase into the culture medium has been developed (Moog et al., 2019). Seo and coworkers successfully fused the PETase with E. coli SRP-dependent signal peptides to enable the secretion of PETase via a sec-dependent secretion system and demonstrated the PET degradation activity by engineered E. coli (Seo et al., 2019). Expressing PETase on yeast’s cell surface shows new insights into developing whole-cell eukaryotic systems for efficient degradation of highly crystalline PET (Chen et al., 2020). The cell surface display of bacterial PETase on P. pastoris showed a 36-fold turnover rate compared to purified PETase, showing a promising approach toward whole-cell biocatalysts for efficient biodegradation of PET. In sum, biorecycling enables the researcher to obtain the original monomers of PET to upcycle into value-added chemicals and materials via synthetic biocatalysts.
Production of industrial chemicals, both natural and non-natural, using renewable biomass feedstock via synthetic microbes has been well-established (Chubukov et al., 2016; Cravens et al., 2019; Lee et al., 2019). In the same vein, microbes can be engineered to valorize plastic feedstock, including the PET-derived TPA and EG. Developing advanced and efficient engineered microorganisms to convert and upcycle plastic waste, including PET, is an exciting opportunity for synthetic microbiologists and metabolic engineers. Hence, it is vital to identify the major metabolic and catabolic routes of EG and TPA to develop the microbial chassis for PET upcycling. Both C2 and aromatic metabolic and catabolic pathways are key targets to develop PET upcycle strategies (Kenny et al., 2008). Several microbes capable of EG metabolism has been studied, and among them, Pseudomonas is an extensively studied organism. The EG metabolic pathway of Pseudomonas putida KT2440 was mapped via comprehensive omics-based systems biology approaches, adaptive laboratory evolution (ALE), and metabolic engineering approaches (Blank et al., 2008; Mückschel et al., 2012; Wehrmann et al., 2017; Franden et al., 2018). Researchers further engineered Pseudomonas putida KT2440 for efficient utilization of EG by expression of the entire gcl operon and glycolate oxidase (glcDEF) operon to overcome the problem of toxic intermediates, glycolaldehyde and glyoxal or knocking out the regulator glcR (Franden et al., 2018; Li et al., 2019). Unlike EG, TPA does not freely diffuse via the microbial cell membrane and require a specific TPA transporters. Several TPA transporters and metabolic pathways have been identified and characterized, including the microbes that natively degrade and catabolize PET (Hara et al., 2007; Yoshida et al., 2016; Salvador de Lara et al., 2019; Pardo et al., 2020). Generally, TPA is converted to protocatechuate (PCA) via 1,6-dihydroxycyclohexa-2,4-diene-dicarboxylate (DCD). TphA1A2A3, which is a dioxygenase, catalyzes the conversion of TPA to DCD, and dehydrogenase TphB catalyzes the conversion of DCD to PCA (Frazee et al., 1993; Wang et al., 1995; Maruyama et al., 2004; Kasai et al., 2009; Salvador de Lara et al., 2019). Tph genes in the analysis of databases have revealed similar genetic organization in few organisms belonging to genus Comamonas, Ideonella, Ramlibacter, Pseudomonas, and Rhodococcus (Choi et al., 2005; Sasoh et al., 2006; Salvador de Lara et al., 2019; Ru et al., 2020). Since we can map the major metabolic and catabolic routes of EG and TPA, we present the overview of potential systematic metabolic engineering routes to efficiently convert PET-derived TPA and EG into high-value chemicals, enabling PET upcycling (Figure 1).
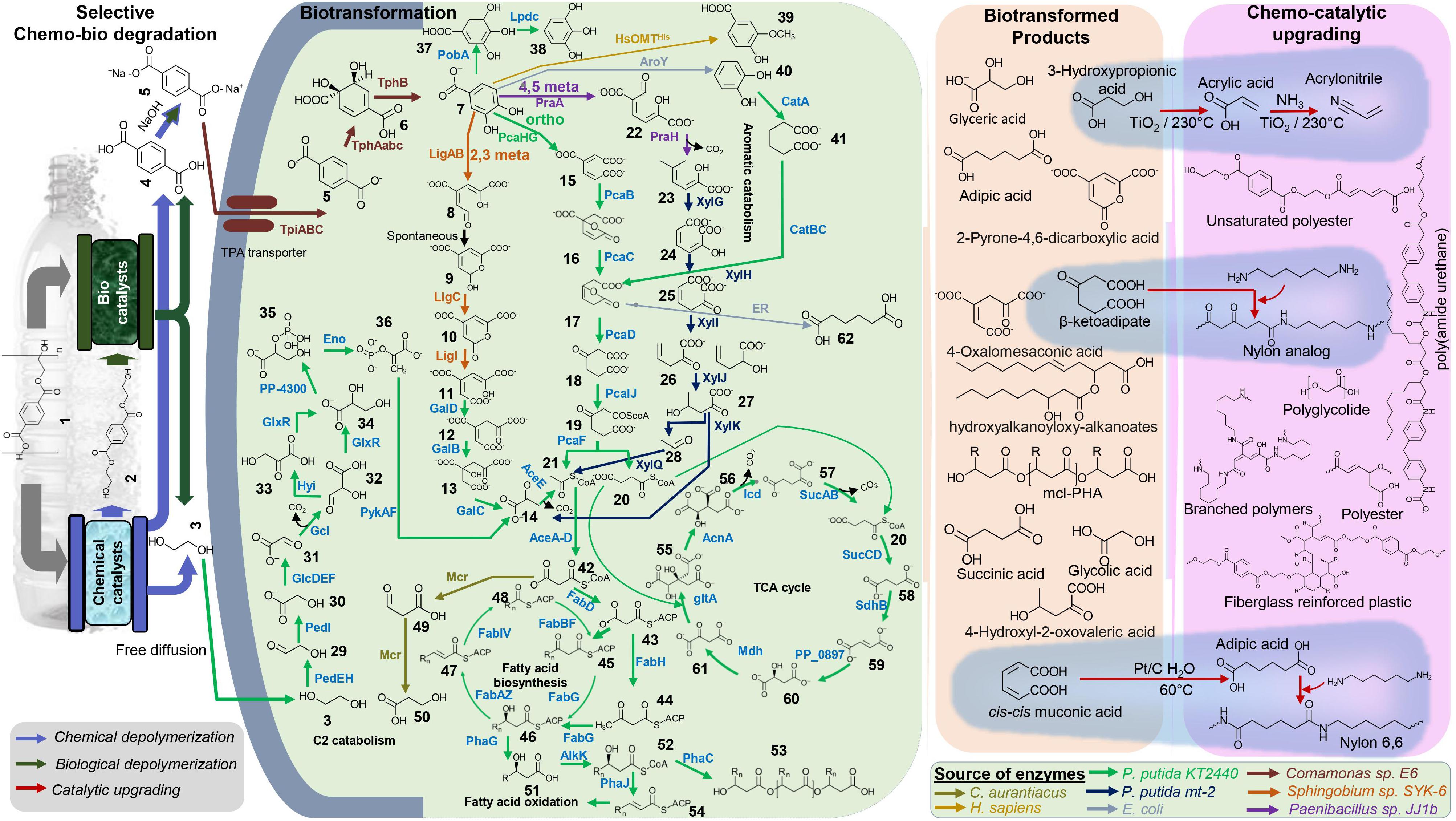
Figure 1. Overview of hybrid biochemical upcycling of PET. The potential bacterial biofunneling pathways of selectively degraded PET products, TPA, and EG into various economically valuable compounds and materials are highlighted. Examples of high-value biotransformed chemicals and chemocatalytic upgrading of primary chemicals to performance advanced material are presented (highlighted with blue background). Detail of all the chemicals and enzymes can be found in Supplementary Tables 3 and 4.
A decade ago, the PET-derived TPA upcycling (obtained from pyrolysis fractionation) into biodegradable plastic polyhydroxyalkanoate (PHA) by P. putida (GO16), P. putida (GO19), and Pseudomonas frederiksbergensis (GO23) has been successfully demonstrated (Kenny et al., 2008). Synthetic microbe-based biological or integrated biological and chemical reactions could be used to produce high-value building blocks, monomers, and fine chemicals obtained by recycled TPA, and EG can then be supplied to the chemical industry. Several models and non-model microbial systems have been developed to upcycle the PET using advanced synthetic microbiology tools in recent years. For instance, EG can be converted to medium chain length polyhydroxyalkanoates (mcl-PHA) using the engineered P. putida KT2440, PHAs widely used in many applications, including biomedicine and biodegradable plastic alternative (Franden et al., 2018; Rodriguez-Contreras, 2019). Furthermore, chemical catalytic upgrading could be adopted to convert the PHA into valuable fully deoxygenated hydrocarbon jet (C8–C16)- or diesel (C8–C21)-grade fuels (Linger et al., 2014). Of note, the PHA production of P. putida KT2440 could be enhanced by additional strain engineering strategy such as knocking out PHA depolymerase gene phaZ and β-oxidation genes fadBA1 and fadBA2 and overexpressing phaG, alkK, phaC1, and phaC2 to increase carbon flux into mcl-PHA biosynthesis (Salvachúa et al., 2020). A recent study demonstrated that the hybrid, enzymatic hydrolysis and microbial bioconversion process enables the simultaneous funneling of the PET-derived EG and TPA into PHA and hydroxyalkanoyloxy-alkanoate (HAA), respectively. The evolved strain of Pseudomonas sp. GO16 can use both EG and TPA that were used as a biocatalyst and engineered to produce extracellular HAA. The obtained HAA can be converted into a novel biodegradable biopolymer poly(amide urethane) via a chemical catalytic process (Tiso et al., 2020). Kang and coworkers designed a chemo-microbial hybrid process to produce of 2-pyrone-4,6-dicarboxylic acid (PDC), a promising bioplastic monomer from PET-derived TPA (Kang et al., 2020). Engineered E. coli consortia were used to produce PDC. One strain was developed by expressing tphAabc and tphB genes from Comamonas sp. E6 to convert TPA into protocatechuic acid (PCA) via 3,4-dihydroxy-cyclohexa-1,5-diene-1,4-dicarboxylic acid (DCD), and the second strain was designed to convert PCA into PDC via 4-carboxy-2-hydroxymuconate semialdehyde by expressing using ligABC genes from Sphingobium sp. SYK-6. Another study on upcycling PET degradation monomers describes directing TPA toward the production of high-value aromatics PCA, gallic acid (GA), pyrogallol (PG), catechol (CA), muconic acid (MA), vanillic acid (VA), and EG toward glycolic acid (GLA) (Kim et al., 2019). They engineered E. coli strains to harbor relevant metabolic pathways to funnel TPA into the desired product using single or combined reactions of hydroxylation, decarboxylation, oxidative ring cleavage, and methylation. For instance, the above-discussed Comamonas sp. E6 genes were expressed to enable TPA conversion to PCA, and expression of PobA from P. putida KT2440 enables hydroxylation of PCA to GA. EG was converted to GLA by EG-fermenting Gluconobacter oxydans. Notably, they implemented a tandem reaction approach to improve the production using a double bio-catalytic system. For example, the strain expressing TphAabc and TphB from Comamonas sp. E6 was used to convert TPA into PCA, and the strain expressing O-methyltransferase, HsOMTHis, from Homo sapiens was used to convert PCA into VA.
Recently, Rorrer and coworkers demonstrated PET upcycling by incorporating renewably sourced, bio-derived compounds (e.g., muconic acid and acrylic acid) with partially deconstructed PET (i.e., BHET) to manufacture fiberglass-reinforced plastics (Rorrer et al., 2019). P. putida KT2440 was engendered to produce innovative chemicals from aromatic catabolism and enables the production of high-performance novel polymers and materials (Johnson et al., 2019). The resulting materials exhibit improved properties relative to the petroleum-based standard (Rorrer et al., 2017; Johnson et al., 2019). For example, as shown in Figure 1, those chemicals could be produced using PET-derived TPA and EG via synthetic microbes.
For instance, β-ketoadipic acid can be obtained from TPA by knocking out the pcaIJ in the engineered TPA catabolizing strain (e.g., tpiABC, tpaAabc, and tph). β-ketoadipic acid can be reacted with hexamethyl diamine (HMDA) to produce a polyamide analogous to nylon 6,6. The polymer showed increased melting temperature and crystallinity and reduced water uptake relative to petroleum-based nylon 6,6 (Sudarsan et al., 2016). It is also possible to funnel EG into 3-hydroxypropionic, and it can be catalytically converted into a high-value acrylic, acrylonitrile, to produce performance advanced polymers and materials (Matsakas et al., 2018). The proposed hybrid biochemical upcycle concept (Figure 1) will enable the end-of-life approach to the PET waste. We anticipated economic incentive from the proposed pathway to produce advanced chemical building blocks. It will remarkably lower the GHG and the energy usage for the production of monomers relative to the fossil-fuel-based production: e.g., upcycling PET into fiberglass-reinforced materials reduces GHG by 40% and energy by 57% (Rorrer et al., 2019).
Notably, the titer, yield, and rate (TYR) of monomers’ bioproduction from PET-derived substrates (Figure 1) need to be improved via metabolic engineering and process design approaches to enable commercial production. Development of in silico computational and machine learning programs to assist the design–build–test–learn cycle (high throughput screening of enzymes and design metabolic pathways) enables rational engineering of commercially applicable superior microbial biocatalyst to upcycle PET. ALE enables the strain to optimize further the engineered genome and fine-tuning of the desired metabolic pathway (Kim et al., 2013; Lee et al., 2014; Oh et al., 2016). We could deploy ALE to improve the PET conversion TYR of the engineered strain.
The waste PET may carry toxic compounds such as emerging contaminants (ECs) and poly organic pollutants (POPs); thus, the process requires a priori detoxification steps. For instance, we could use efficient chemical-based metal-organic frameworks or enzyme-based laccase or peroxidase to detoxify the PET-associated ECs and POPs (Pi et al., 2018; Mishra et al., 2019; Morsi et al., 2020). Indeed, laccase can be expressed on PET upcycling microbes to enable in situ detoxification (Chen et al., 2016). Given that most of the substrates, intermediates, and targeted products are toxic to the host microbes, engineering multiple toxicity tolerance mechanisms will be necessary. For instance, overcoming the aldehyde tolerance and acid tolerance during EG metabolism could be achieved by alleviating the metabolic bottlenecks and engineering the protein quality control machineries (Franden et al., 2018; Jayakody et al., 2018; Guan and Liu, 2020).
One exciting area of study is adapting the architecture of cellulosomes to develop a multi-enzyme complex capable of efficiently degrading PET. The advanced synthetic biology techniques enable the formulation of large cellulosomes and facilitate superior activity toward the recalcitrant cellulose (Anandharaj et al., 2020). The same concept could be adopted to tailor microbial cell factories to the degradation of high-crystalline PET via developing PETsome (Supplementary Figure 1). It is vital to discover the component that would act as a PET binding domain, analogous to the cellulose-binding domain (Ribitsch et al., 2013; Weber et al., 2019). An efficient cell surface expressing system for bacteria has recently been developed (Chen et al., 2019; Dvořák et al., 2020). Together, those approaches can be implemented to design a consolidated bioprocessing system, a microbial system that can efficiently degrade and upcycle PET into advanced chemicals simultaneously. It will be beneficial to overcome techno-economic challenges such as end-product toxicity on degradation enzymes and the overall operating costs.
In summary, PET upcycling via synthetic microbial biocatalyst or hybrid biochemical approaches has shown great promise to sustainable large-scale solutions for PET waste management in terms of end-of-life to PET. It is essential to perform a comprehensive life cycle and techno-economic analysis to identify the upcycle process’ industrial and environmental feasibility using the engineered biocatalyst. We envision that innovative synthetic microbiology and metabolic engineering approaches may enable the microbial biocatalyst to reach the commercial scale from laboratory bioreactor to upcycle PET, create a circular material economy, and help protect our environment from PET waste.
LJ outlined the manuscript. LD surveyed the literature. LJ and LD drafted the article. Both authors read and approved the final version of the manuscript prior to its submission.
This work received new faculty startup funding from the Office of the Vice Chancellor for Research and the Fermentation Science Institute, Southern Illinois University Carbondale.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Scott D. Hamilton-Brehm for proofreading this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.656465/full#supplementary-material
Alam, I., Aalismail, N., Martin, C., Kamau, A., Guzmán-Vega, F. J., Jamil, T., et al. (2020). Rapid evolution of plastic-degrading enzymes prevalent in the global ocean. bioRxiv [Preprint]. 285692. doi: 10.1101/2020.09.07.285692
Allen, S., Allen, D., Phoenix, V. R., Roux, G. L., Jiménez, D. P., Simonneau, A., et al. (2019). Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 12, 339–344. doi: 10.1038/s41561-019-0335-5
Anandharaj, M., Lin, Y. J., Rani, R. P., Nadendla, E. K., Ho, M. C., Huang, C. C., et al. (2020). Constructing a yeast to express the largest cellulosome complex on the cell surface. Proc. Nat. Acad. Sci. 117:2385. doi: 10.1073/pnas.1916529117
Angov, E. (2011). Codon usage: nature’s roadmap to expression and folding of proteins. Biotechnol. J. 6, 650–659. doi: 10.1002/biot.201000332
Austin, H. P., Allen, M. D., Donohoe, B. S., Rorrer, N. A., Kearns, F. L., Silveira, R. L., et al. (2018). Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. U. S. A. 115, E4350–E4357. doi: 10.1073/pnas.1718804115
Awaja, F., and Pavel, D. (2005). Recycling of PET. Eur. Polym. J. 41, 1453–1477. doi: 10.1016/j.eurpolymj.2005.02.005
Barth, M., Honak, A., Oeser, T., Wei, R., Belisário-Ferrari, M. R., Then, J., et al. (2016). A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol. J. 11, 1082–1087. doi: 10.1002/biot.201600008
Blank, L. M., Ionidis, G., Ebert, B. E., Bühler, B., and Schmid, A. (2008). Metabolic response of Pseudomonas putida during redox biocatalysis in the presence of a second octanol phase. FEBS J. 275, 5173–5190. doi: 10.1111/j.1742-4658.2008.06648.x
Blank, L. M., Narancic, T., Mampel, J., Tiso, T., and O’connor, K. (2020). Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 62, 212–219. doi: 10.1016/j.copbio.2019.11.011
Carr, C. M., Clarke, D. J., and Dobson, A. D. W. (2020). Microbial polyethylene terephthalate hydrolases: current and future perspectives. Front. Microbiol. 11:571265. doi: 10.3389/fmicb.2020.571265
Chen, T., Wang, K., Chi, X., Zhou, L., Li, J., Liu, L., et al. (2019). Construction of a bacterial surface display system based on outer membrane protein F. Microb. Cell Fact. 18:70. doi: 10.1186/s12934-019-1120-2
Chen, Y., Stemple, B., Kumar, M., and Wei, N. (2016). Cell surface display fungal laccase as a renewable biocatalyst for degradation of persistent micropollutants bisphenol A and sulfamethoxazole. Environ. Sci. Technol. 50, 8799–8808. doi: 10.1021/acs.est.6b01641
Chen, Z., Wang, Y., Cheng, Y., Wang, X., Tong, S., Yang, H., et al. (2020). Efficient biodegradation of highly crystallized polyethylene terephthalate through cell surface display of bacterial PETase. Sci. Total Environ. 709:136138. doi: 10.1016/j.scitotenv.2019.136138
Choi, K. Y., Kim, D., Sul, W. J., Chae, J.-C., Zylstra, G. J., Kim, Y. M., et al. (2005). Molecular and biochemical analysis of phthalate and terephthalate degradation by Rhodococcus sp. strain DK17. FEMS Microbiol. Lett. 252, 207–213. doi: 10.1016/j.femsle.2005.08.045
Chubukov, V., Mukhopadhyay, A., Petzold, C. J., Keasling, J. D., and Martín, H. G. (2016). Synthetic and systems biology for microbial production of commodity chemicals. NPJ Syst. Biol. Appl. 2:16009. doi: 10.1038/npjsba.2016.9
Cravens, A., Payne, J., and Smolke, C. D. (2019). Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 10:2142. doi: 10.1038/s41467-019-09848-w
Cui, Y., Chen, Y., Liu, X., Dong, S., Tian, Y. E., Qiao, Y., et al. (2021). Computational Redesign of a PETase for Plastic Biodegradation under Ambient Condition by the GRAPE Strategy. ACS Catal. 11, 1340–1350. doi: 10.1021/acscatal.0c05126
Danso, D., Schmeisser, C., Chow, J., Zimmermann, W., Wei, R., Leggewie, C., et al. (2018). New insights into the function and global distribution of polyethylene terephthalate (PET)-degrading bacteria and enzymes in marine and terrestrial metagenomes. Appl. Environ. Microbiol. 84, e02773–17. doi: 10.1128/AEM.02773-17
de Sá, L. C., Oliveira, M., Ribeiro, F., Rocha, T. L., and Futter, M. N. (2018). Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci. Total Environ. 645, 1029–1039. doi: 10.1016/j.scitotenv.2018.07.207
Dvořák, P., Bayer, E. A., and De Lorenzo, V. (2020). Surface display of designer protein scaffolds on genome-reduced strains of Pseudomonas putida. bioRxiv doi: 10.1101/2020.05.13.093500
Eriksen, M., Lebreton, L. C. M., Carson, H. S., Thiel, M., Moore, C. J., Borerro, J. C., et al. (2014). Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One 9:e111913. doi: 10.1371/journal.pone.0111913
Fiber Economics Bureau. (2015). 2015 World Directory of Manufactured Fiber Producers [Online]. URL: http://www.fibersource.com/f-info/FiberProduction.pdf
Franden, M. A., Jayakody, L. N., Li, W. J., Wagner, N. J., Cleveland, N. S., Michener, W. E., et al. (2018). Engineering Pseudomonas putida KT2440 for efficient ethylene glycol utilization. Metab. Eng. 48, 197–207. doi: 10.1016/j.ymben.2018.06.003
Frazee, R. W., Livingston, D. M., Laporte, D. C., and Lipscomb, J. D. (1993). Cloning, sequencing, and expression of the Pseudomonas putida protocatechuate 3,4-dioxygenase genes. J. Bacteriol. 175:6194–202. doi: 10.1128/jb.175.19.6194-6202.1993
Furukawa, M., Kawakami, N., Tomizawa, A., and Miyamoto, K. (2019). Efficient degradation of poly(ethylene terephthalate) with Thermobifida fusca cutinase exhibiting improved catalytic activity generated using mutagenesis and additive-based approaches. Sci. Rep. 9:16038. doi: 10.1038/s41598-019-52379-z
Gamerith, C., Vastano, M., Ghorbanpour, S. M., Zitzenbacher, S., Ribitsch, D., Zumstein, M. T., et al. (2017). Enzymatic degradation of aromatic and aliphatic polyesters by P. pastoris expressed cutinase 1 from Thermobifida cellulosilytica. Front. Microbiol. 8:938. doi: 10.3389/fmicb.2017.00938
Geyer, R., Jambeck, J. R., and Law, K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv. 3:e1700782. doi: 10.1126/sciadv.1700782
Guan, N., and Liu, L. (2020). Microbial response to acid stress: mechanisms and applications. Appl. Microbiol. Biotechnol. 104, 51–65. doi: 10.1007/s00253-019-10226-1
Hamid, F. S., Bhatti, M. S., Anuar, N., Anuar, N., Mohan, P., and Periathamby, A. (2018). Worldwide distribution and abundance of microplastic: how dire is the situation? Waste Manag. Res. 36, 873–897. doi: 10.1177/0734242X18785730
Hara, H., Eltis, L. D., Davies, J. E., and Mohn, W. W. (2007). Transcriptomic analysis reveals a bifurcated terephthalate degradation pathway in Rhodococcus sp. strain RHA1. J. Bacteriol. 189:1641–1647. doi: 10.1128/JB.01322-06
Jambeck, J. R., Geyer, R., Wilcox, C., Siegler, T. R., Perryman, M., Andrady, A., et al. (2015). Plastic waste inputs from land into the ocean. Science 347, 768–771. doi: 10.1126/science.1260352
Jayakody, L. N., Johnson, C. W., Whitham, J. M., Giannone, R. J., Black, B. A., Cleveland, N. S., et al. (2018). Thermochemical wastewater valorization via enhanced microbial toxicity tolerance. Energy Environ. Sci. 11, 1625–1638. doi: 10.1039/C8EE00460A
Johnson, C. W., Salvachúa, D., Rorrer, N. A., Black, B. A., Vardon, D. R., and St. John, P. C. (2019). Innovative chemicals and materials from bacterial aromatic catabolic pathways. Joule 3, 1523–1537. doi: 10.1016/j.joule.2019.05.011
Kang, M. J., Kim, H. T., Lee, M. W., Kim, K. A., Khang, T. U., Song, H. M., et al. (2020). A chemo-microbial hybrid process for the production of 2-pyrone-4,6-dicarboxylic acid as a promising bioplastic monomer from PET waste. Green Chem. 22, 3461–3469. doi: 10.1039/D0GC00007H
Kasai, D., Fujinami, T., Abe, T., Mase, K., Katayama, Y., Fukuda, M., et al. (2009). Uncovering the protocatechuate 2,3-cleavage pathway genes. J. Bacteriol. 191, 6758–6768. doi: 10.1128/JB.00840-09
Kawai, F. (2021). The current state of research on PET hydrolyzing enzymes available for biorecycling. Catalysts 11:206. doi: 10.3390/catal11020206
Kawai, F., Kawabata, T., and Oda, M. (2020). Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustain. Chem. Eng. 8, 8894–8908. doi: 10.1021/acssuschemeng.0c01638
Kenny, S. T., Runic, J. N., Kaminsky, W., Woods, T., Babu, R. P., Keely, C. M., et al. (2008). Up-cycling of PET (Polyethylene Terephthalate) to the biodegradable plastic PHA (Polyhydroxyalkanoate). Environ. Sci. Technol. 42, 7696–7701. doi: 10.1021/es801010e
Kim, D. J., and Lee, K. T. (2012). Determination of monomers and oligomers in polyethylene terephthalate trays and bottles for food use by using high performance liquid chromatography-electrospray ionization-mass spectrometry. Polym. Test. 31, 490–499. doi: 10.1016/j.polymertesting.2012.02.001
Kim, H. T., Kim, J. K., Cha, H. G., Kang, M. J., Lee, H. S., Khang, T. U., et al. (2019). Biological valorization of poly(ethylene terephthalate) monomers for upcycling waste PET. ACS Sustain. Chem. Eng. 7, 19396–19406. doi: 10.1021/acssuschemeng.9b03908
Kim, S. R., Skerker, J. M., Kang, W., Lesmana, A., Wei, N., Arkin, A. P., et al. (2013). Rational and evolutionary engineering approaches uncover a small set of genetic changes efficient for rapid xylose fermentation in Saccharomyces cerevisiae. PLoS One 8:e57048. doi: 10.1371/journal.pone.0057048
Knott, B. C., Erickson, E., Allen, M. D., Gado, J. E., Graham, R., Kearns, F. L., et al. (2020). Characterization and engineering of a two-enzyme system for plastics depolymerization. Proc.Natl. Acad. Sci. U. S. A. 117, 25476–25485 doi: 10.1073/pnas.2006753117
Law, K. L., Starr, N., Siegler, T. R., Jambeck, J. R., Mallos, N. J., and Leonard, G. H. (2020). The United States’ contribution of plastic waste to land and ocean. Sci. Adv. 6:eabd0288. doi: 10.1126/sciadv.abd0288
Lee, S.-M., Jellison, T., and Alper, H. S. (2014). Systematic and evolutionary engineering of a xylose isomerase-based pathway in Saccharomyces cerevisiae for efficient conversion yields. Biotechnol. Biofuels 7, 122–122. doi: 10.1186/s13068-014-0122-x
Lee, S. Y., Kim, H. U., Chae, T. U., Cho, J. S., Kim, J. W., Shin, J. H., et al. (2019). A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2, 18–33. doi: 10.1038/s41929-018-0212-4
Li, W. J., Jayakody, L. N., Franden, M. A., Wehrmann, M., Daun, T., Hauer, B., et al. (2019). Laboratory evolution reveals the metabolic and regulatory basis of ethylene glycol metabolism by Pseudomonas putida KT2440. Environ. Microbiol. 21, 3669–3682. doi: 10.1111/1462-2920.14703
Linger, J. G., Vardon, D. R., Guarnieri, M. T., Karp, E. M., Hunsinger, G. B., Franden, M. A., et al. (2014). Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. U. S. A. 111, 12013–12018. doi: 10.1073/pnas.1410657111
Maruyama, K., Shibayama, T., Ichikawa, A., Sakou, Y., Yamada, S., and Sugisaki, H. (2004). Cloning and characterization of the genes encoding enzymes for the protocatechuate meta-degradation pathway of Pseudomonas ochraceae NGJ1. Biosci. Biotechnol. Biochem. 68, 1434–1441. doi: 10.1271/bbb.68.1434
Matsakas, L., Hrùzová, K., Rova, U., and Christakopoulos, P. (2018). Biological production of 3-hydroxypropionic acid: an update on the current status. Fermentation 4:13. doi: 10.3390/fermentation4010013
Maurya, A., Bhattacharya, A., and Khare, S. K. (2020). Enzymatic remediation of polyethylene terephthalate (PET)-based polymers for effective management of plastic wastes: an overview. Front. Bioeng. Biotechnol. 8:602325. doi: 10.3389/fbioe.2020.602325
Meys, R., Frick, F., Westhues, S., Sternberg, A., Klankermayer, J., and Bardow, A. (2020). Towards a circular economy for plastic packaging wastes – the environmental potential of chemical recycling. Resour. Conserv. Recycl. 162:105010. doi: 10.1016/j.resconrec.2020.105010
Mishra, A., Kumar, S., and Bhatnagar, A. (2019). “Chapter 7 - Potential of fungal laccase in decolorization of synthetic dyes,” in Microbial Wastewater Treatment, eds M. P. Shah and S. Rodriguez-Couto (Netherlands: Elsevier), 127–151. doi: 10.1016/b978-0-12-816809-7.00007-5
Moog, D., Schmitt, J., Senger, J., Zarzycki, J., Rexer, K. H., Linne, U., et al. (2019). Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb. Cell Fact. 18:171. doi: 10.1186/s12934-019-1220-z
Morsi, R., Bilal, M., Iqbal, H. M. N., and Ashraf, S. S. (2020). Laccases and peroxidases: the smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 714:136572. doi: 10.1016/j.scitotenv.2020.136572
Mückschel, B., Simon, O., Klebensberger, J., Graf, N., Rosche, B., Altenbuchner, J., et al. (2012). Ethylene glycol metabolism by Pseudomonas putida. Appl. Environ. Microbiol. 78, 8531–8539. doi: 10.1128/AEM.02062-12
Mueller, R. J. (2006). Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process Biochem. 41, 2124–2128. doi: 10.1016/j.procbio.2006.05.018
Oh, E. J., Skerker, J. M., Kim, S. R., Wei, N., Turner, T. L., Maurer, M. J., et al. (2016). Gene amplification on demand accelerates cellobiose utilization in engineered Saccharomyces cerevisiaei. Appl. Environ. Microbiol. 82:3631–3639. doi: 10.1128/AEM.00410-16
Palm, G. J., Reisky, L., Böttcher, D., Müller, H., Michels, E. A. P., and Walczak, M. C. (2019). Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat. Commun. 10:1717. doi: 10.1038/s41467-019-09326-3
Pardo, I., Jha, R. K., Bermel, R. E., Bratti, F., Gaddis, M., Mcintyre, E., et al. (2020). Gene amplification, laboratory evolution, and biosensor screening reveal MucK as a terephthalic acid transporter in Acinetobacter baylyi ADP1. Metab. Eng. 62, 260–274. doi: 10.1016/j.ymben.2020.09.009
Pi, Y., Li, X., Xia, Q., Wu, J., Li, Y., Xiao, J., et al. (2018). Adsorptive and photocatalytic removal of Persistent Organic Pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 337, 351–371. doi: 10.1016/j.cej.2017.12.092
Plastic Insight. (2016). Global PET Resin Production Capacity. [Online] URL: https://www.plasticsinsight.com/global-pet-resin-production-capacity/
Rahimi, A., and García, J. M. (2017). Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1:0046. doi: 10.1038/s41570-017-0046
Ribitsch, D., Yebra, A. O., Zitzenbacher, S., Wu, J., Nowitsch, S., Steinkellner, G., et al. (2013). Fusion of binding domains to thermobifida cellulosilytica cutinase to tune sorption characteristics and enhancing PET hydrolysis. Biomacromolecules 14, 1769–1776. doi: 10.1021/bm400140u
Roager, L., and Sonnenschein, E. C. (2019). Bacterial candidates for colonization and degradation of marine plastic debris. Environ. Sci. Technol. 53, 11636–11643. doi: 10.1021/acs.est.9b02212
Rodriguez-Contreras, A. (2019). Recent advances in the use of polyhydroyalkanoates in biomedicine. Bioengineering 6:82. doi: 10.3390/bioengineering6030082
Rorrer, N. A., Nicholson, S., Carpenter, A., Biddy, M. J., Grundl, N. J., and Beckham, G. T. (2019). Combining reclaimed PET with bio-based monomers enables plastics upcycling. Joule 3, 1006–1027. doi: 10.1016/j.joule.2019.01.018
Rorrer, N. A., Vardon, D. R., Dorgan, J. R., Gjersing, E. J., and Beckham, G. T. (2017). Biomass-derived monomers for performance-differentiated fiber reinforced polymer composites. Green Chem. 19, 2812–2825. doi: 10.1039/C7GC00320J
Ru, J., Huo, Y., and Yang, Y. (2020). Microbial degradation and valorization of plastic wastes. Front. Microbiol. 11:442. doi: 10.3389/fmicb.2020.00442
Salvachúa, D., Rydzak, T., Auwae, R., De Capite, A., Black, B. A., Bouvier, J. T., et al. (2020). Metabolic engineering of Pseudomonas putida for increased polyhydroxyalkanoate production from lignin. Microb. Biotechnol. 13, 290–298. doi: 10.1111/1751-7915.13481
Salvador de Lara, M., Abdulmutalib, U., Gonzalez, J., Kim, J., Smith, A. A., Faulon, J. L., et al. (2019). Microbial genes for a circular and sustainable bio-PET economy. Genes 10:373. doi: 10.3390/genes10050373
Samak, N. A., Jia, Y., Sharshar, M. M., Mu, T., Yang, M., Peh, S., et al. (2020). Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ. Int. 145:106144. doi: 10.1016/j.envint.2020.106144
Sasoh, M., Masai, E., Ishibashi, S., Hara, H., Kamimura, N., Miyauchi, K., et al. (2006). Characterization of the terephthalate degradation genes of Comamonas sp. strain E6. Appl. Environ. Microbiol. 72:1825–1832. doi: 10.1128/AEM.72.3.1825-1832.2006
Seo, H., Kim, S., Son, H. F., Sagong, H. Y., Joo, S., and Kim, K. J. (2019). Production of extracellular PETase from Ideonella sakaiensis using sec-dependent signal peptides in E. coli. Biochem. Biophys. Res. Commun. 508, 250–255. doi: 10.1016/j.bbrc.2018.11.087
Shirke, A. N., White, C., Englaender, J. A., Zwarycz, A., Butterfoss, G. L., Linhardt, R. J., et al. (2018). Stabilizing leaf and branch compost cutinase (LCC) with glycosylation: mechanism and effect on PET hydrolysis. Biochemistry 57, 1190–1200. doi: 10.1021/acs.biochem.7b01189
Sohn, Y. J., Kim, H. T., Baritugo, K. A., Jo, S. Y., Song, H. M., Park, S. Y., et al. (2020). Recent advances in sustainable plastic upcycling and biopolymers. Biotechnol. J. 15:1900489. doi: 10.1002/biot.201900489
Su, L., Woodard, R. W., Chen, J., and Wu, J. (2013). Extracellular location of Thermobifida fusca cutinase expressed in Escherichia coli BL21(DE3) without mediation of a signal peptide. Appl. Environ. Microbiol. 79, 4192–4198. doi: 10.1128/AEM.00239-13
Subramanian, S. H. S., Balachandran, K. R. S., Rangamaran, V. R., and Gopal, D. (2019). RemeDB: tool for rapid prediction of enzymes involved in bioremediation from high-throughput metagenome data sets. J. Comput. Biol. 27, 1020–1029. doi: 10.1089/cmb.2019.0345
Sudarsan, S., Blank, L. M., Dietrich, A., Vielhauer, O., Takors, R., Schmid, A., et al. (2016). Dynamics of benzoate metabolism in Pseudomonas putida KT2440. Metab. Eng. Commun. 3, 97–110. doi: 10.1016/j.meteno.2016.03.005
Then, J., Wei, R., Oeser, T., Barth, M., Belisário-Ferrari, M. R., Schmidt, J., et al. (2015). Ca2+ and Mg2+ binding site engineering increases the degradation of polyethylene terephthalate films by polyester hydrolases from Thermobifida fusca. Biotechnol. J. 10, 592–598. doi: 10.1002/biot.201400620
Then, J., Wei, R., Oeser, T., Gerdts, A., Schmidt, J., Barth, M., et al. (2016). A disulfide bridge in the calcium binding site of a polyester hydrolase increases its thermal stability and activity against polyethylene terephthalate. FEBS Open Bio. 6, 425–432. doi: 10.1002/2211-5463.12053
Thompson, R., Swan, S., Moore, C., and Vom Saal, F. (2009). Our plastic age. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1973–1976. doi: 10.1098/rstb.2009.0054
Tiso, T., Narancic, T., Wei, R., Pollet, E., Beagan, N., Schröder, K., et al. (2020). Bio-upcycling of polyethylene terephthalate. bioRxiv [Preprint]. 993592. doi: 10.1101/2020.03.16.993592
Tournier, V., Topham, C. M., Gilles, A., David, B., Folgoas, C., Moya-Leclair, E., et al. (2020). An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580, 216–219. doi: 10.1038/s41586-020-2149-4
Vollmer, I., Jenks, M. J. F., Roelands, M. C. P., White, R. J., Van Harmelen, T., De Wild, P., et al. (2020). Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 59, 15402–15423. doi: 10.1002/anie.201915651
Wang, F., Wong, C. S., Chen, D., Lu, X., Wang, F., and Zeng, E. Y. (2018). Interaction of toxic chemicals with microplastics: a critical review. Water Res. 139, 208–219. doi: 10.1016/j.watres.2018.04.003
Wang, Y. Z., Zhou, Y., and Zylstra, G. J. (1995). Molecular analysis of isophthalate and terephthalate degradation by Comamonas testosteroni YZW-D. Environ. Health Perspect. 103, 9–12. doi: 10.1289/ehp.95103s49
Webb, H. K., Arnott, J., Crawford, R. J., and Ivanova, E. P. (2013). Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers 5, 1–18. doi: 10.3390/polym5010001
Weber, J., Petrović, D., Strodel, B., Smits, S. H. J., Kolkenbrock, S., Leggewie, C., et al. (2019). Interaction of carbohydrate-binding modules with poly(ethylene terephthalate). Appl. Microbiol. Biotechnol. 103, 4801–4812. doi: 10.1007/s00253-019-09760-9
Wehrmann, M., Billard, P., Martin-Meriadec, A., Zegeye, A., and Klebensberger, J. (2017). Functional role of lanthanides in enzymatic activity and transcriptional regulation of pyrroloquinoline quinone-dependent alcohol dehydrogenases in Pseudomonas putida KT2440. mBio 8:e00570–17. doi: 10.1128/mBio.00570-17
Wei, R., Breite, D., Song, C., Gräsing, D., Ploss, T., Hille, P., et al. (2019). Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 6:1900491. doi: 10.1002/advs.201900491
Wei, R., Tiso, T., Bertling, J., O’connor, K., Blank, L. M., and Bornscheuer, U. T. (2020). Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 3, 867–871. doi: 10.1038/s41929-020-00521-w
Wei, R., and Zimmermann, W. (2017). Biocatalysis as a green route for recycling the recalcitrant plastic polyethylene terephthalate. Microb. Biotechnol. 10, 1302–1307. doi: 10.1111/1751-7915.12714
Xi, X., Ni, K., Hao, H., Shang, Y., Zhao, B., and Qian, Z. (2021). Secretory expression in Bacillus subtilis and biochemical characterization of a highly thermostable polyethylene terephthalate hydrolase from bacterium HR29. Enzyme Microb. Technol. 143:109715. doi: 10.1016/j.enzmictec.2020.109715
Yoshida, S., Hiraga, K., Takehana, T., Taniguchi, I., Yamaji, H., Maeda, Y., et al. (2016). A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351:1196–1199. doi: 10.1126/science.aad6359
Keywords: synthetic microbes, polyethylene terephthalate, PET degradation, metabolic engineering, bio-upcycling
Citation: Dissanayake L and Jayakody LN (2021) Engineering Microbes to Bio-Upcycle Polyethylene Terephthalate. Front. Bioeng. Biotechnol. 9:656465. doi: 10.3389/fbioe.2021.656465
Received: 20 January 2021; Accepted: 12 April 2021;
Published: 28 May 2021.
Edited by:
Bin Cao, Nanyang Technological University, SingaporeReviewed by:
Lars M. Blank, RWTH Aachen University, GermanyCopyright © 2021 Dissanayake and Jayakody. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lahiru N. Jayakody, bGFoaXJ1LmpheWFrb2R5QHNpdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.