
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 19 August 2020
Sec. Nanobiotechnology
Volume 8 - 2020 | https://doi.org/10.3389/fbioe.2020.01011
This article is part of the Research Topic Network-Oriented Approaches to Anticancer Drug Response View all 8 articles
The tumor microenvironment (TME) presents a challenging barrier for effective nanotherapy-mediated drug delivery to solid tumors. In particular for tumors less vascularized than the surrounding normal tissue, as in liver metastases, the structure of the organ itself conjures with cancer-specific behavior to impair drug transport and uptake by cancer cells. Cells and elements in the TME of hypovascularized tumors play a key role in the process of delivery and retention of anti-cancer therapeutics by nanocarriers. This brief review describes the drug transport challenges and how they are being addressed with advanced in vitro 3D tissue models as well as with in silico mathematical modeling. This modeling complements network-oriented techniques, which seek to interpret intra-cellular relevant pathways and signal transduction within cells and with their surrounding microenvironment. With a concerted effort integrating experimental observations with computational analyses spanning from the molecular- to the tissue-scale, the goal of effective nanotherapy customized to patient tumor-specific conditions may be finally realized.
In order for drug molecules to elicit a pharmacological response, the molecules must arrive in sufficient quantities to the tissue of interest and bind to the molecular target activating or inhibiting particular pathways. To achieve therapeutic responses in solid tumors, drug molecules need to overcome various barriers at different physical scales. The tumor microenvironment (TME) includes several scales: (a) molecular (nano-) scale, including up- and down-regulation of various proteins that can signal for tumor growth or drug-efflux mechanisms; (b) nano- to micro- scale, which incorporates gradients of cell nutrients and oxygen, growth factors, and other means of cell-to-cell communication; (c) micro-scale, in which interactions between cells occur in the acellular stroma compartment of the tumor; (d) micro to macro scale, which incorporates the organ architecture, blood supply, lymphatics, and other physiological factors. While these barriers span several orders of magnitude, they are intricately linked and cross-communicate. As an example, the architecturally/anatomically irregular and functionally impaired tumor neovasculature (micro to macro scale) is characterized by reduced oxygen tension, oscillating flow, constricted blood vessels, and other abnormal features (Jain, 2003; Folkman, 2007; Chung et al., 2010; Dewhirst and Secomb, 2017). Consequently, the TME becomes heterogeneous in terms of gradients of solutes and nutrients (nano- to micro- scale) as well as differences in pH and cell viability due to hypoxia (micro-scale). Hypoxia promotes recruitment of immune cells to the tissue, while prompting release of cytokines and chemokines (molecular (nano-) scale) that affect cell-to-cell interactions (micro-scale).
The heterogeneous TME has a significant effect on therapeutic outcomes. First, TME heterogeneity and three-dimensionality represent a significant barrier to systemically administered therapeutics, including nanotherapeutics. As a result, in vitro efficiencies of anti-cancer drugs (especially those shown in 2D cultures) do not correlate well with potencies observed in vivo, as has been shown in several studies (Agiostratidou et al., 2001; Fruehauf, 2002). The results highlight discrepancies in positive predictive values between clinical efficacies and in vitro therapy selection (50–70%) and negative predictive accuracy (∼90%), demonstrating that enhanced potency of drugs in 2D cultures largely disregards the barriers in the heterogeneous TME. Second, cells in the TME such as endothelial cells, macrophages and other cells of the immune system, and fibroblasts/myofibroblasts actively interact with the tumor cells in most solid tumors and affect cancer cell proliferation, survival, polarity and invasive capacity (Williams et al., 2016; Guo and Deng, 2018; Sarode et al., 2020).
Multiple studies have demonstrated that while the normal cellular microenvironment can inhibit or even prevent the growth of tumor cells, the changes that happen in the TME synergistically support tumor growth. Tumors shape their microenvironment promoting the growth not only malignant cells, but also non-malignant TME or stromal cells. There are many mechanisms that still need to be elucidated in the tumor-stroma interactions, although the importance of an altered TME in the process of tumorigenesis is no longer questioned. Numerous successful cancer therapies targeting the TME have been approved or are being developed, highlighting the importance of the cells and tissue neighboring malignant cells for tumor survival and invasion. As an example, tumor macrophages can be polarized to be tumor-growth supportive (M2 phenotype) or inhibiting (M1 phenotype) (Pollard, 2008; Ruffell et al., 2012; Ruffell and Coussens, 2015; Williams et al., 2016; Kielbassa et al., 2019). The M1/M2 ratio has been shown to be a strong prognostic factor in a variety of solid tumors including liver metastasis (Cui et al., 2013; He et al., 2013; Zhang et al., 2014; Yuan et al., 2017).
The TME is dependent not only on the origin and the characteristics of tumor cells, but also on the anatomy and physiology of the organ to which tumor cells disseminate. We discuss the liver as one particular example to illustrate this complexity (van den Eynden et al., 2013) and the challenges it poses to drug delivery. Metastatic lesions represent the most common malignancy in the liver and are up to 40 times more frequent in clinical practice than primary liver tumors (Rummeny and Marchal, 1997). The liver is a highly vascularized organ that has a dense network of capillaries, sinusoids, efficiently providing oxygen and soluble nutrients to the innermost cells in the organ. Two physiological factors have been linked to the high incidence of the liver being an organ of choice for distant metastasis: (Chung et al., 2010) increased likelihood of invasion due to dual blood supply from systemic and portal circulation; (Dewhirst and Secomb, 2017) presence of fenestrations in the liver sinusoids that allow for tumor cell invasion from the circulation.
Uniquely, incipient liver metastases preserve the stromal structure of the liver and do not rely on angiogenesis for survival (Stessels et al., 2004). This vascularization pattern, in which tumor cells primarily use existing vasculature in surrounding parenchyma, is unconventional, compared to most solid tumors (Pezzella et al., 1997; Danet et al., 2003a, b; Namasivayam et al., 2007), and significantly limits diffusive transport into the lesions Thus, the most frequent tumor types with liver metastasis, including breast, colon, lung, and gastric carcinomas create hypovascular lesions usually showing perilesional enhancement (Namasivayam et al., 2007; Hazhirkarzar et al., 2020). This characteristic significantly impairs the delivery of systemically administered therapeutics to tumors in the liver.
For instance, it has been shown that breast cancer and pancreatic ductal adenocarcinoma liver metastases are characterized by poor permeation of molecules and are clinically observed as hypo-attenuating spots, which intravenously injected contrast agents do not permeate (Liu et al., 2003). Impaired diffusion is an important factor limiting adequate concentration of therapeutics, and could explain why chemotherapy fails to cure unresectable liver lesions. Poor permeation is especially acute with high molecular weight (HMW) molecules, such as m99Tc microaggregated albumin (Daly et al., 1985).
Recent data from in vivo studies in breast cancer liver tumors with low vascularization patterns and high macrophage (Mϕ) content confirm that these lesions lack efficient perfusion. In intravital microscopy (IVM) studies, transport of HMW molecules (fluorescent dextrans) into tumor lesions was impeded compared to healthy tissue (Figure 1). When a HMW drug nAb-PTX, possessing similar hydrodynamic diameter as 40 KDa dextran (∼12 nm), was packaged in a solid multistage porous silicon NV (MSV) (Godin et al., 2010a, b, 2011, 2012; Tanaka et al., 2010; Tasciotti et al., 2011; Srinivasan et al., 2013; Yokoi et al., 2013; Jaganathan et al., 2014; Tanei et al., 2016), the nanotherapeutic was taken up by Mϕ in the proximity of breast cancer liver metastases, thus enabling higher drug concentrations in the lesions and dramatically improving nAb-PTX therapeutic efficacy and animal survival (Figure 2).
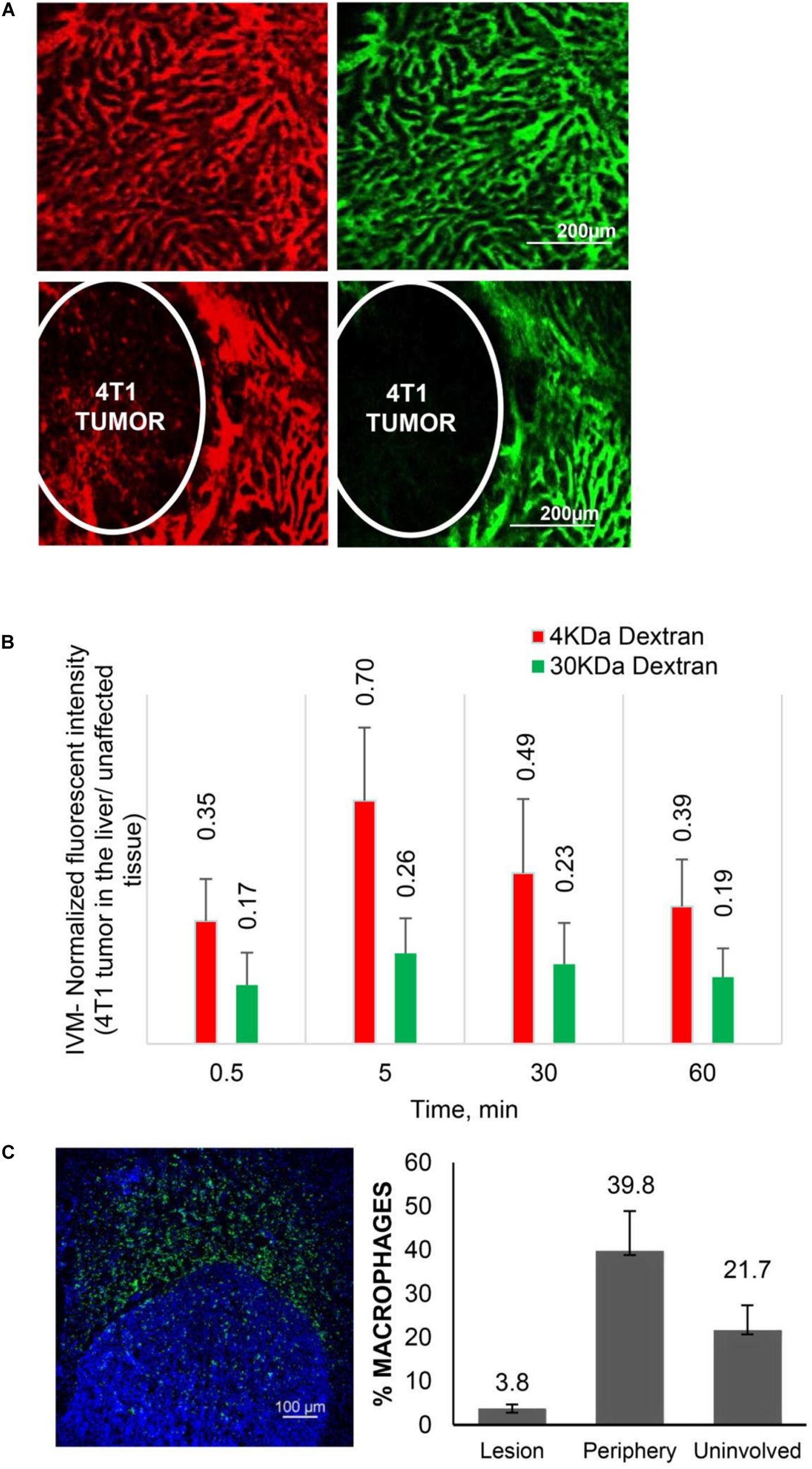
Figure 1. Transport characteristics in breast cancer liver metastases. (A) Perfusion and diffusion of 3 KDa (red) and 40 KDa (green) fluorescent dextrans by IVM in normal liver (upper panels) and 4T1 breast cancer liver metastases after iv injection. (B) Fluorescent intensities of the dextrans in breast cancer liver metastases normalized to unaffected liver. (C) Distribution of Mϕ (F4/80 antibody, green) in breast cancer liver metastases. Number of Mϕ in the lesion, the periphery (40–50 micron from the tumor border) and unaffected liver is normalized to cell number detected by DAPI staining. Reprinted with permission from Tanei et al. (2016).
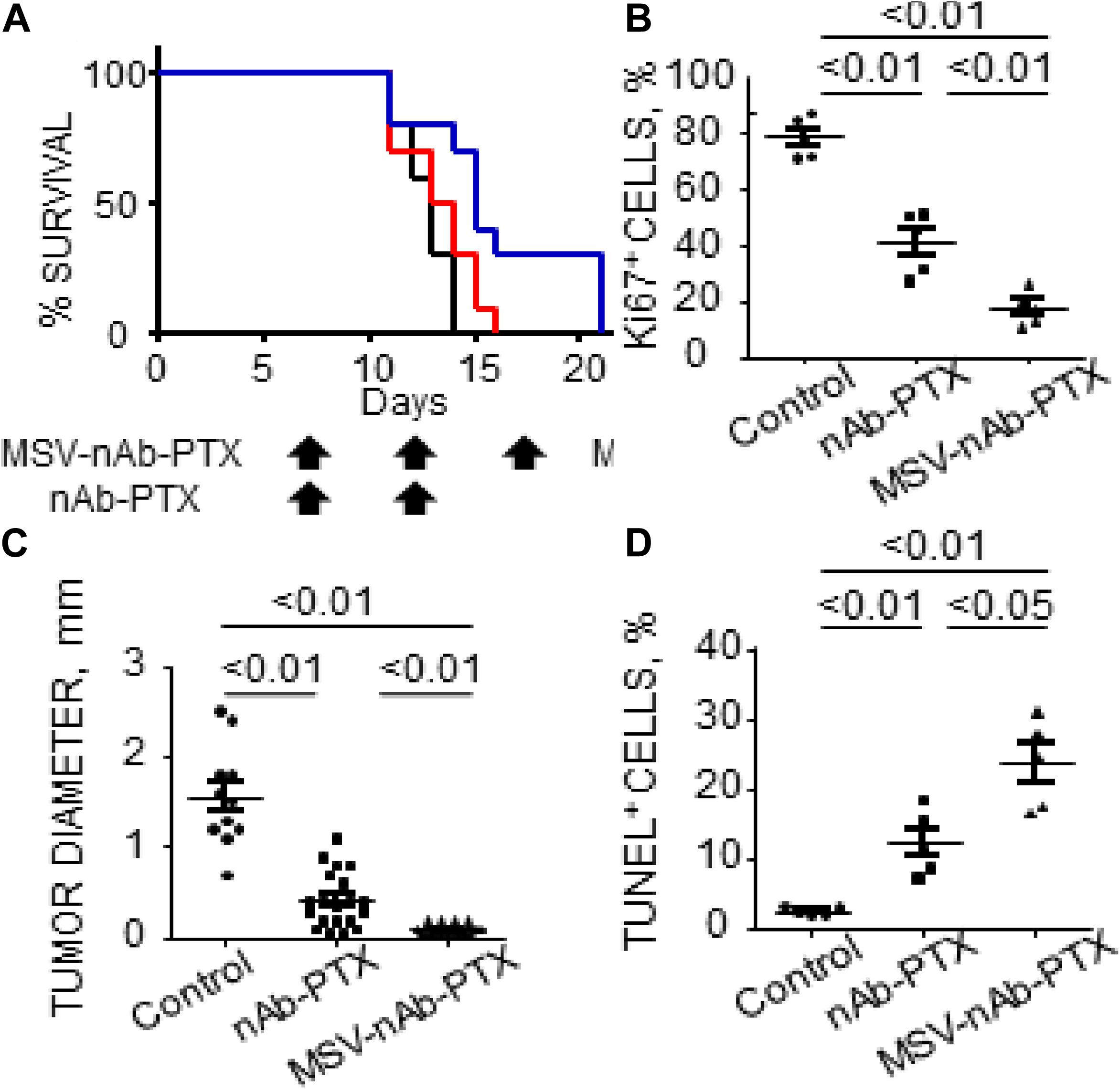
Figure 2. Therapeutic efficacy and survival of mice bearing breast cancer liver metastases following IV administration of MSV-nAb-PTX, or nAb-PTX. (A) Kaplan–Meier survival curves. Mice were injected intrasplenically with cancer cells and therapy was initiated 1 week later. Therapy was administered every 5 days until animals were moribund (Log-rank test for MSV-nAb-PTX vs. nAb-PTX is P < 0.05 for 4T1 and P < 0.01 for 3LL models, respectively). (B) Quantitative analysis of proliferating Ki67-positive cancer cells. (C) Quantification of tumor diameters in mice liver treated with the systems. (D) Apoptotic TUNEL-positive cancer cells. Reprinted with permission from Tanei et al. (2016).
Evidence with other types of hypo-perfused tumors supports these data. The composition of the stroma in preclinical models of orthotopic primary pancreatic based on L3.6pL cells were analyzed in Yokoi et al. (2013). Profound differences in the cellular elements of pancreatic stroma as a result of different treatments were observed (Figure 3, Borsoi et al., 2015). Mice were treated with a combination of gemcitabine and nAb-PTX encapsulated or nAb-PTX packed into an engineered MSV (Tasciotti et al., 2011; Yokoi et al., 2013; Borsoi et al., 2015, 2017). These data demonstrate that cellular elements in the TME of poorly perfused tumors dramatically change during disease progression and in response to therapy.
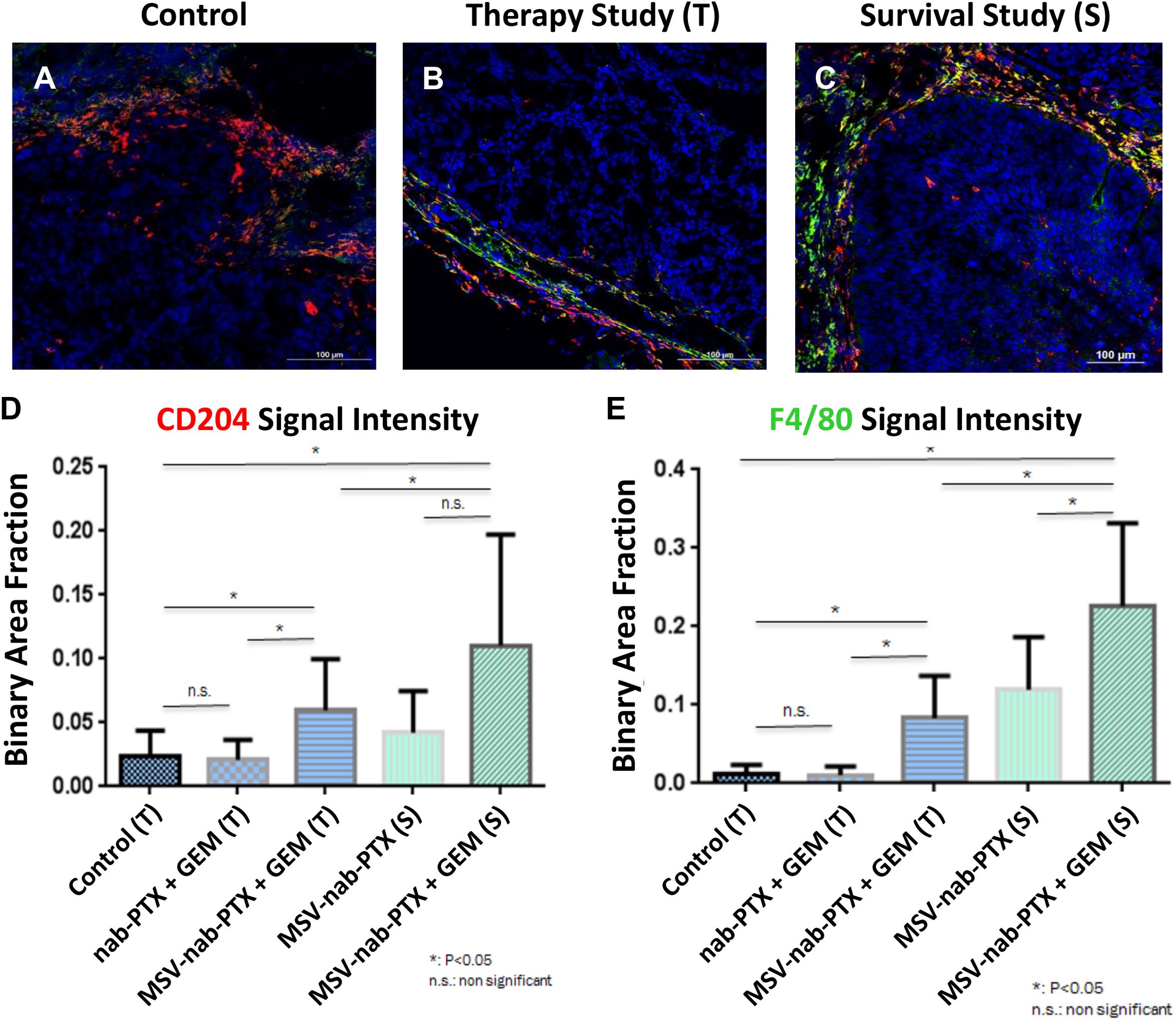
Figure 3. Changes in stroma composition of hypo-perfused pancreatic ductal adenocarcinoma as a result of therapy with gemcitabine (GEM) and MSV-nab-PTX. M2 macrophages (CD204, red) and M1 + M2 macrophages (F4/80, green) were visualized using confocal microscopy. (A) Untreated mice; (B) mice treated 2×; (C) mice treated 12×; (D) M1 macrophages quantification; (E) M1 + M2 macrophages quantification [data from Borsoi et al. (2015)].
In vitro approaches have been a traditional stronghold for anti-cancer therapeutic screening, with the goal of maximizing predictive potency while closely mimicking tumor physiology and cell-cell interactions (Zoli et al., 2001; Breslin and O’Driscoll, 2016). Importantly, 3D in vitro models complement in vivo rodent models and overcome their limitations, such as high cost, long latency and ethical minimization of the use of animals. Furthermore, microenvironments that recapitulate more precisely human tumor physiology can be created in vitro, enabling evaluation of the effect of various factors on the mechanisms of tumor initiation, progression and response to therapy. The addition of the third spatial dimension to in vitro models has introduced improved cell-cell interaction setting up transport limitations to oxygen, nutrients and potential therapeutics, thereby increasing the utility for therapeutic screening (Griffith and Swartz, 2006). Here, we briefly review considerations in evaluating drug efficacy in terms of adopting the appropriate 3D in vitro/ex vivo models, and consider integrated engineering examples of therapeutic efficiency directed toward hypovascularized lesions such as liver metastases.
A majority of therapeutics suffer from high attrition rates as they move through the oncologic discovery pipeline, due to a lack of translation from the preclinical to the clinical stage (Hay et al., 2014; Wong et al., 2019). Preliminary considerations while identifying the most appropriate in vitro model draw a balance between the complex physiological relevance (cellular complexity, molecular pathology) of 3D models and their ability to scale up for drug screening. While cellular simplicity may be sufficient for evaluating key molecular targets for targeted therapy, models that include elements of tumor stroma have to be considered for more nuanced drug delivery approaches. For example, a nanoparticle-based approach targeting collagenase in hypovascular pancreatic ductal adenocarcinoma had the goal of demonstrating increased drug penetration after nanoparticle treatment (Zinger et al., 2019). Similarly, novel nano-immunotherapies in development target phagocytic cells like macrophages or antigen presenting cells within the TME (Caster et al., 2019).
Another important consideration in preclinical screening is how therapeutic efficacy is defined. Specific to 3D models, IC50 alone may not be the most suitable metric to evaluate therapeutic efficiency in isolation (Zoli et al., 2001). Traditional plate-reader based viability/metabolic activity assays are not designed to discern individual contributions of cellular compartments within tumoroid/organoid models (Horman et al., 2015). However, a combination of IC50 with imaging methodologies that evaluate drug uptake, molecular read outs and morphometric analysis may offer more information of the dynamics of the therapeutic process (Raghavan et al., 2015, 2016; Stock et al., 2016; Santo et al., 2017). Such high content imaging approaches can reliably provide morphological and cellular dynamics information that can be correlated with therapeutic outcomes (Garvey et al., 2016; Ahonen et al., 2017; Bulin et al., 2017).
A fundamental consideration in preclinical in vitro models remains the methodologies utilized to create the actual models – engineering approaches can often dictate resultant drug sensitivity. This section briefly describes some of the most popular approaches utilized currently to manufacture 3D in vitro/ex vivo tumor models, with examples of how they have been utilized in drug delivery to hypovascular lesions such as liver metastases and pancreatic ductal adenocarcinoma.
Spheroids represent the most frequently used 3D cancer model in preclinical research. Most cancer cell lines will grow and organize as avascular 3D structures called spheroids, either aided by scaffolds, hydrogels, magnetic levitations, extracellular matrix (ECM) gels or in scaffold-free suspension or liquid overlay cultures. The organoid/tumoroid model has been demonstrated as a promising tool to recapitulate patient response in colorectal (Sato et al., 2011), ovarian (Kopper et al., 2019), pancreatic (Boj et al., 2015; Ware et al., 2016a, b), breast (Jaganathan et al., 2014; Leonard and Godin, 2016), and gastrointestinal cancers (Aberle et al., 2018). Spheroids and organoids derived from cancer stem cells/tumor-initiating cells have been described to capture inter-patient heterogeneity, with the opportunity to rapidly relate phenotype to genotype, and high fidelity drug sensitivity (Sachs and Clevers, 2014; Raghavan et al., 2017). In regards to 3D models of hypovascularized tumors (e.g., liver metastasis of breast, lung and colorectal tumors, pancreatic, etc.) tumor spheroids recapitulate the nutrient and therapeutic supply patterns (mainly from the surrounding tissue).
The biggest advantage of the spheroid/organoid approach is to be able to deconstruct the cellular and environmental elements of the TME, and reverse engineer them as appropriate, to study specific targeting strategies. For example, avascular spheroids of breast cancer cells surrounded by macrophages were constructed using magnetic levitation and bioprinting to recapitulate the microenvironment of liver metastasis (Leonard and Godin, 2016; Leonard et al., 2016, 2020). In another study, hepatocellular carcinoma (HCC) spheroids were created by combining alginate microbead technology with decellularized liver matrix-derived ECM, to encapsulate HCC cells (Sun et al., 2018). To model the anchorage-independent aggregation of pre-metastatic ovarian cancer cells within malignant ascites, spheroids containing ovarian cancer stem cells and macrophages were utilized (Raghavan et al., 2019). Pancreatic tumor-rich spheroids were designed for drug screening with various human pancreatic cancer cells and human stellate cells using a combination of polymer matrix and hanging drop techniques (Ware et al., 2016b).
The establishment of these explant cultures/sheet models relies on precision-cut slicing mechanisms, and access to primary patient-derived tumors. These models have the advantage of preserving complete tissue architecture, as well as, the cellular heterogeneity of the tumor, and can virtually be derived from any accessible surgical tumor specimen. The challenges of preservation and proliferation, however, still remain. Using explant cultures, anticancer drug screening has been demonstrated in rectal cancer liver metastases, which could be replicated in rodent xenograft models (Zhang et al., 2020). Tumor slices have also been utilized to test nanoparticle-based drug delivery of oligonucleotides to ovarian cancer and gliomas, and non-small cell lung cancer (Dong et al., 2011; Ewe et al., 2017).
The use of naturally derived scaffolds for solid organs has been well developed for regenerative medicine (Baptista et al., 2009), and has made inroads into use in anticancer therapeutics (Hinderer et al., 2016). The most advantageous aspect of the use of natural ECM-based bio-scaffolds is the ability to retain bioactive molecules like growth factors and other signaling compounds embedded within the scaffolding structure, providing cues to tumor cells that are seeded on them. Metastatic colorectal cancer cells and native HCC cells grown on human decellularized liver scaffolds demonstrated less efficacy to chemotherapeutic regimens that were used at standard 2D determined IC50 (Hussein et al., 2016; D’Angelo et al., 2020). This implies the retaining of bioactivity in decellularized scaffolds, which speaks not only to their utility in re-engineering the architectural and scaffolding components of the TME, but also highlights that the metastatic TME triggers a physiologically more resistant cancer phenotype. Recently, decellularized liver scaffolds have been manufactured by immersion decellularization of chick embryos, which bypasses the slow whole-organ decellularization approach. A dramatic reduction in doxorubicin efficiency against triple negative breast cancer liver metastases was demonstrated (Guller et al., 2020). Importantly, the speed at which the matrices can be produced using this approach lends itself high throughput amenable for anticancer screening. It should be noted that decellularized tissue scaffolds can be combined with other techniques for 3D tumor growth, as described above.
Microfluidic approaches have been implemented to generate tumor spheroids, relying on perfusion and microwells. These models are mainly used for tumors with enhanced degree of angiogenesis and vascularization as compared to the surrounding tissue. The utility of microfluidic approaches in the study of cancer drug delivery is well reviewed (Unger et al., 2014; Ozcelikkale et al., 2017). The advantage of using microfluidic approaches is the inclusion of dynamics of the TME (Agastin et al., 2011; Kim et al., 2012). Microfluidic models of pancreatic ductal adenocarcinoma demonstrated transcriptome level similarity to patient-derived pancreatic stellate cells (Gioeli et al., 2019). To model liver metastasis, gelatin methacryloyl was combined with decellularized liver matrix components including bioactive factors derived from liver matrix, in a dynamic culture system to assess drug-dose responses to acetaminophen and sorafenib (Lu et al., 2018). In a similar metastasis-on-a-chip model of kidney cancer metastasized to the liver, NP-conjugated 5-fluorouracil was shown to be more efficacious than free drug (Wang et al., 2020).
Bioprinting is an emerging engineering strategy to develop 3D tumor models, where 3D printing is exploited to deposit cells and biomaterials in tissue-like structures. The biggest limitation of bioprinting approaches has been the maintenance of cellular viability through the simultaneous layer-by-layer assembly process of cells and bio-inks (Albritton and Miller, 2017). Early attempts using bio-inks like gelatin, alginate and/or fibrinogen demonstrated that heterotypic cell interactions can be maintained in bioprinted tumors, which result in them being more chemoresistant (Zhao et al., 2014; Dai et al., 2016; Zhang et al., 2016; Zhou et al., 2016). This technique has been interestingly utilized to create metastasis models at the tumor-vasculature interface (Meng et al., 2019), as well as, mimicking organ specific metastasis using decellularized liver matrix ECM as a bioink (Lee et al., 2017). This technology has potential for combining organoid technology with rapid production, potentially rendering it high-throughput amenable for personalized medicine and therapeutic screening applications.
Nanoparticles have been used clinically for tumor therapy since the early 1990s. Currently available nanotherapies improve safety and efficacy of chemotherapies. As an example, Doxyl®, the first nano-drug, liposomal doxorubicin, was introduced to reduce the cardiotoxicity of doxorubicin, leveraging the differences in biodistribution of free drug vs. liposomal entity (Barenholz, 2012). Since then, a few dozens of nanotherapeutics have been approved for cancer therapy. Many other nanomedicine-based approaches are under investigation worldwide. Nanotherapy offers the possibility to improve metastatic disease treatment by increasing the concentrations and attaining controlled release of therapeutics in distant lesions (Sanga et al., 2007; Farokhzad Omid and Langer, 2009; Misra et al., 2010; Bourzac, 2012; Parhi et al., 2012; Zamboni et al., 2012; Bertrand et al., 2014; Grodzinski and Farrell, 2014; Fernandes et al., 2015). Nanovectors (NV) can favorably change pharmacokinetics of drugs in plasma and in tissues, prolonging circulation time and enhancing delivery to tumors (Bae et al., 2005, 2007; Bae and Kataoka, 2009; Maniwa et al., 2010; Ponta and Bae, 2014; Khawar et al., 2015; Reichel et al., 2015, 2017; Curtis et al., 2016b; Khalid et al., 2016).
Generally, systemically administered nanotherapies first flow in the circulation and either attach to the elements in the TME (e.g., receptors overexpressed in the tumor-associated endothelium and stroma elements) or extravasate through the gaps in the leaky and disorganized tumor neovasculature. A key mechanism related to the extravasation of nanotherapies and macromolecules in the tumor is called the Enhanced Permeation and Retention (EPR) effect (Greish, 2010). Factors that contribute to the EPR effect include: enhanced vascular permeability and angiogenesis, which sustain rapid growth of tumor on one side, and impaired lymphatic drainage, on the other side (Maeda et al., 2003; Nakamura et al., 2015).
Another mechanism for tumor targeting proposed specifically for nanotherapies includes their interaction with immune cells, such as macrophages, which are abundant in the TME. Macrophages are professional phagocytes and, as such, efficiently take up particles from the circulation (Gustafson et al., 2015). These immune cells can thus serve as a cellular depot for therapeutics in the TME (Choi et al., 2007; Leonard et al., 2016; Tanei et al., 2016; Hu et al., 2019). Additionally, nanotherapies can affect the polarization and the function of the immune cells, reversing their phenotype from pro-tumorigenic M2 to anti-tumorigenic M1 (Ponzoni et al., 2018; Hu et al., 2019; Reichel et al., 2019).
Clinical benefits from nanotherapy remain controversial because in vivo efficacy varies from tumor to tumor (Kyle et al., 2007; Vicent et al., 2009; Cukierman and Khan, 2010; Fang et al., 2011; Bhatia et al., 2012; Mehta et al., 2012; Cho et al., 2013; Crist et al., 2013; Prabhakar et al., 2013; Venditto and Szoka, 2013; Dawidczyk et al., 2014; Danhier, 2016). Further, it is well known that organ physiology and microenvironment significantly impact the efficacy of cancer therapy (Curtis and Frieboes, 2016). As an example, while well-documented in primary tumors, the EPR effect does not usually apply to organs enriched with blood vessels, such as the liver. In the liver, tumor lesions are less vascularized than the organ itself (e.g., they appear as “white spots” on a “red bed”). Thus, management of cancer metastasized to different organs should ideally account for these variations, providing personalized therapy based on the location of the metastasis.
A number of HMW-based therapeutic strategies has been clinically approved and proposed for the therapy of advanced breast cancer, pancreatic ductal denocarcinoma and other tumors, including albumin-bound drug conjugates, such as nanoalbumin bound paclitaxel, nAb-PTX or Abraxane® (Blomstrand et al., 2019; Li and Kwon, 2019; Untch et al., 2019), monoclonal antibodies (mAb), such as anti-HER2 mAb trastuzumab (Figueroa-Magalhaes et al., 2014) or anti-EGFR mAb, cetuximab (Huang and Buchsbaum, 2009), and genetic materials, including siRNA, mRNA, and aptamers (Kang et al., 2015; Ngamcherdtrakul and Yantasee, 2019). In liver metastatic lesions with low vascularization patterns, these potent therapeutics are unable to be transported deeply enough into the lesions prior to their clearance from circulation. Thus, new approaches to enhance their accumulation in liver metastases are necessary.
The complexity of the TME coupled with cellular plasticity may preclude any one particular therapeutic from fully succeeding in eradicating a tumor. Yet, historically, the search for the one “silver bullet” that could cure patients has garnered much attention in research and in clinical medicine (e.g., Zempolich and Dubeau, 1998; Koontz, 2017). More recently, approaches that consider information from different physical scales and perspectives, and that are able to integrate the associated information into a coherent picture, have been pursued. These approaches include the combination of experimentation with mathematical modeling and computational simulation (Frieboes et al., 2006, 2012, 2019; Sanga et al., 2007; Sinek et al., 2007; Brocato et al., 2018; Dogra et al., 2019).
Newer approaches to cancer therapy involving molecular profiling represent a promising avenue, especially as they seek to elucidate the role of the microenvironment in the evolution of acquired drug resistance. In particular, the development of gene analysis tools has offered the opportunity to more quickly and cheaply assess variations in the genetic make-up of tumors (Heath et al., 2016; Miryala et al., 2018). Yet the interpretation of these data for clinical application is non-trivial. Although the detection of genetic variation in individual patient tumors is considered crucial for the success of personalized medicine, for the most part it remains unclear how this variation translates to tumor-scale phenotype. With the exception of a few well-studied genes [e.g., BRCA1 and BRCA2 in breast cancer (Grimmett et al., 2018)], most of the genetic information elucidated from these analyses has yet to be meaningfully interpreted.
A major reason for the challenge to link the molecular to the tissue scale is that the growth and treatment response of many cancers do not solely depend on variation at the genetic scale but rather on the combination of characteristics at multiple scales, including genetic, cellular, and tissue conditions such as the tumor and organ microenvironment. A purely empirical approach would be insurmountable to determine optimal drug therapy, due to the many variables involved. For this task, which requires a systems-level perspective, mathematical modeling and computational simulation are ideally suited. Network-oriented approaches have sought to “connect the dots,” so to speak, to make sense of intra-cellular relevant pathways as well as signal transduction within cells and with their surrounding microenvironment (Kreeger and Lauffenburger, 2010; Bachmann et al., 2012; Lecca and Re, 2017). In addition to the effort to model the molecular-scale, the integration of experimental and computational modeling to enable realistic, predictive evaluation of tumor behavior during therapy has been pursued. The application of mathematical modeling can help to bridge the associated physical scales (from molecules to tissue), and thus lead to improved interpretation of particular tumor characteristics and how they might influence the drug transport in the tumor, and consequently, the drug response.
Mathematical modeling complements network-oriented techniques (e.g., principal network analysis) and data-based approaches (e.g., statistical and machine learning) by providing for mechanistic insight of tissue behavior in time as well as space. This would be considered important to evaluate the effects of the microenvironment on tumor response to therapy. In particular when evaluating omics strategies, recent work strongly suggests that the best predictive results are obtained via studies accounting for multiple types of datasets (Cho et al., 2014; Ge et al., 2015; Li et al., 2016; Ren et al., 2016; Dong et al., 2017), as a single approach may not suffice for personalized cancer patient treatment. The interdependence of the datasets indicates an integrated approach that links genes to phenotype (Su et al., 2011; Zhang et al., 2013; Bolger et al., 2014; Minton et al., 2015; Murakami et al., 2015). For example, metabolomics provides insight into outcomes of transcription changes, which reflect differential functionality of specific metabolites influencing the tumor response. Attempts to predict therapy response based on metabolomics analysis only (Tian et al., 2018) or gene expression (Geeleher and Cox, 2014) yield results typically relying on statistical analyses that do not necessarily represent any particular tumor (Peng et al., 2018; Mucaki et al., 2019). Ideally, a mathematical-based framework would be capable of recreating particular patients’ tumors for in silico prediction of behavior in time and space prior to treatment, incorporating omics data for patient customization, and thus move toward the goal of predictive personalized treatment.
The ability to predict personalized drug response would help to obviate unnecessary, high-cost and high-morbidity treatments, allowing more efficient patient management. Although a number of theoretical models of tumor drug response have been developed in recent years (e.g., Byrne et al., 2006; Ribba et al., 2006; Stamatakos et al., 2006; Enderling et al., 2007; Roeder and Glauche, 2008; Frieboes et al., 2009; Hinow et al., 2009; Sinek et al., 2009; Gevertz, 2011), few have focused on a multiscale integration of molecular data to evaluate cancer treatment response. By extracting mathematical model parameter values from tumor-specific cell proliferation, apoptosis, and molecular characteristics, and simulating the effects of nanocarrier and drug transport and retention in tissue, it may become possible to predict the drug response customized for individual patients, beyond what would have been expected from sole consideration of any one of these parameters or the intrinsic resistance of the cancer cells themselves.
Mathematical modeling and computational analysis are actively being pursued in several aspects of oncology to personalize and improve therapeutic outcomes (e.g., Ibrahim-Hashim et al., 2017). In particular, tissue structure and transport in liver (Rani et al., 2006; Hoehme et al., 2007; Campbell et al., 2008; Hoehme et al., 2010, 2017, 2018; Holzhutter et al., 2012; Drasdo et al., 2014; Dutta-Moscato et al., 2014; Lettmann and Hardtke-Wolenski, 2014; Schliess et al., 2014; Siggers et al., 2014; Bethge et al., 2015; Ricken et al., 2015; Schwen et al., 2015; Nishii et al., 2016; Sluka et al., 2016; White et al., 2016; Friedman and Hao, 2017; Hudson et al., 2017, 2019; Meyer et al., 2017; Fu et al., 2018; Mahlbacher et al., 2018; Clendenon et al., 2019; Van Liedekerke et al., 2020) as well as pancreas (Haeno et al., 2012; Louzoun et al., 2014; Ng and Frieboes, 2017, 2018; Roy and Finley, 2017; Yamamoto et al., 2017; Chen et al., 2020; Dogra et al., 2020b) have been modeled. While numerous studies have simulated tumor growth and angiogenesis [see recent reviews and related work (Cristini et al., 2008; Edelman et al., 2010; Lowengrub et al., 2010; Osborne et al., 2010; Rejniak and McCawley, 2010; Vineis et al., 2010; Andasari et al., 2011; Chaplain, 2011; Deisboeck et al., 2011; Frieboes et al., 2011; Michor et al., 2011; Rejniak and Anderson, 2011; Swanson et al., 2011)] including metastatic conditions (Campbell et al., 2008; Haeno et al., 2012; Bethge et al., 2015; Hudson et al., 2017, 2019), as well as pharmacokinetics/pharmacodynamics (Lee et al., 2013; Pascal et al., 2013a, b; Wang et al., 2015, 2016; Barbolosi et al., 2016; Enriquez-Navas et al., 2016; Curtis et al., 2018) and drug discovery (Wang and Deisboeck, 2014; Zhang and Brusic, 2014), few have focused on HMW-based therapeutics. To address this need, models have been proposed which, coupled with experimentally measured parameters, have paved the way for more realistic multiscale modeling of cancer nanotherapy (Dogra et al., 2019), integrating spatial scales nm to cm- and temporal scales from sub-sec to weeks (as summarized in Table 1).
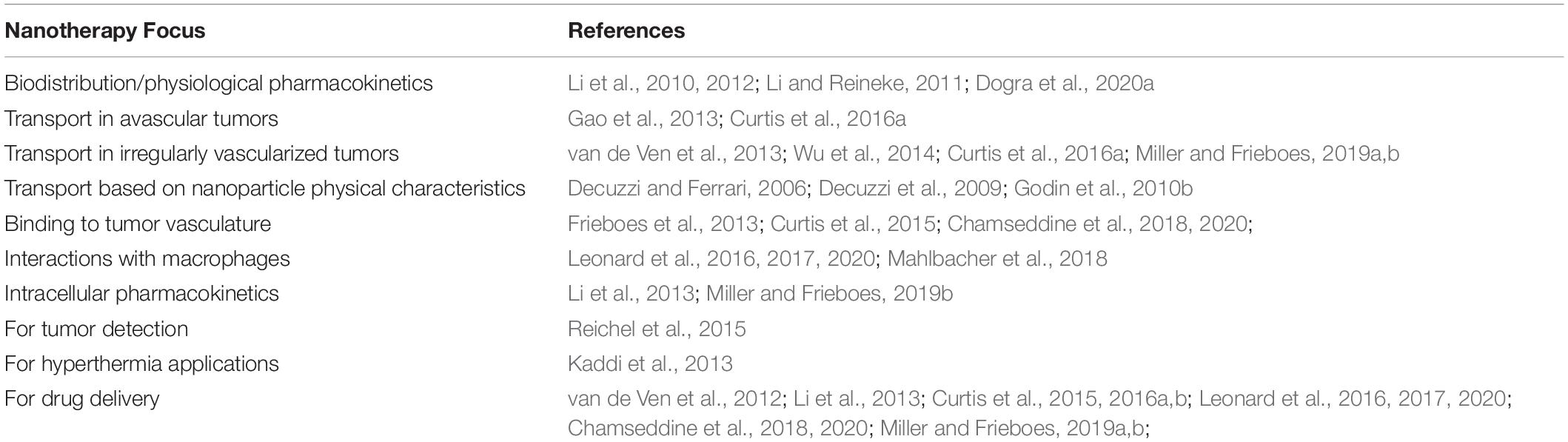
Table 1. Overview of recent mathematical modeling to study nanoparticle delivery and efficacy in tumors.
Traditional pharmacokinetics and pharmacodynamics (PK/PD) correlate efficacy of small molecule drugs with time-dependent changes in average bulk drug concentration at the site of action. However, it has been shown that these PK/PD methods often fail to predict efficacy of drug-loaded NV that control drug concentrations in vascular, interstitial and intracellular spaces of tumor (Ponta and Bae, 2014; Ponta et al., 2015; Curtis et al., 2016b). In addition, traditional analyses frequently simplify or rule out tumor physiology changing over time (Khawar et al., 2015; Khalid et al., 2016). To overcome these issues, computational modeling that integrates key parameters influencing NV-based drug delivery into tumor tissue to realistically predict in vivo efficacy of nanotherapy has been proposed.
Physiologically based pharmacokinetic (PBPK) models have been utilized to evaluate drug efficacy, including nanoparticle-delivered drugs (Li et al., 2010). These models evaluate the absorption, distribution, metabolism, and excretion of small molecules such as drugs or nanoparticles by organizing tissues and organs as distinct compartments linked via mass transport. In Li et al. (2013), PBPK modeling was applied to evaluate the intracellular pharmacokinetics of paclitaxel delivered by nanoparticles, with the model parameters set from experimental data with human breast cancer MCF7 cells in vitro. The results revealed that the simulated intracellular pharmacokinetics corresponded with relevant parameters, including nanoparticle PK, drug-release kinetics, and drug dose.
The effect of irregular vascularization on nanoparticle transport and drug release in tumor tissue has been evaluated in several studies (van de Ven et al., 2012; Li et al., 2013; Curtis et al., 2015, 2016a,b; Leonard et al., 2016, 2017, 2020; Chamseddine et al., 2018, 2020; Miller and Frieboes, 2019a, b). The role of macrophages in nanotherapeutic transport and effect on hypo-vascularized tumor lesions such as breast cancer liver metastases was evaluated in Leonard et al. (2016, 2017). In particular, the response due to repetitive therapy with MSV-nAb-PTX and nAb-PTX, showed that encapsulation of the drug in multi-stage vectors (MSV-nAb-PTX) could maximize the tumor regression (Figure 4).
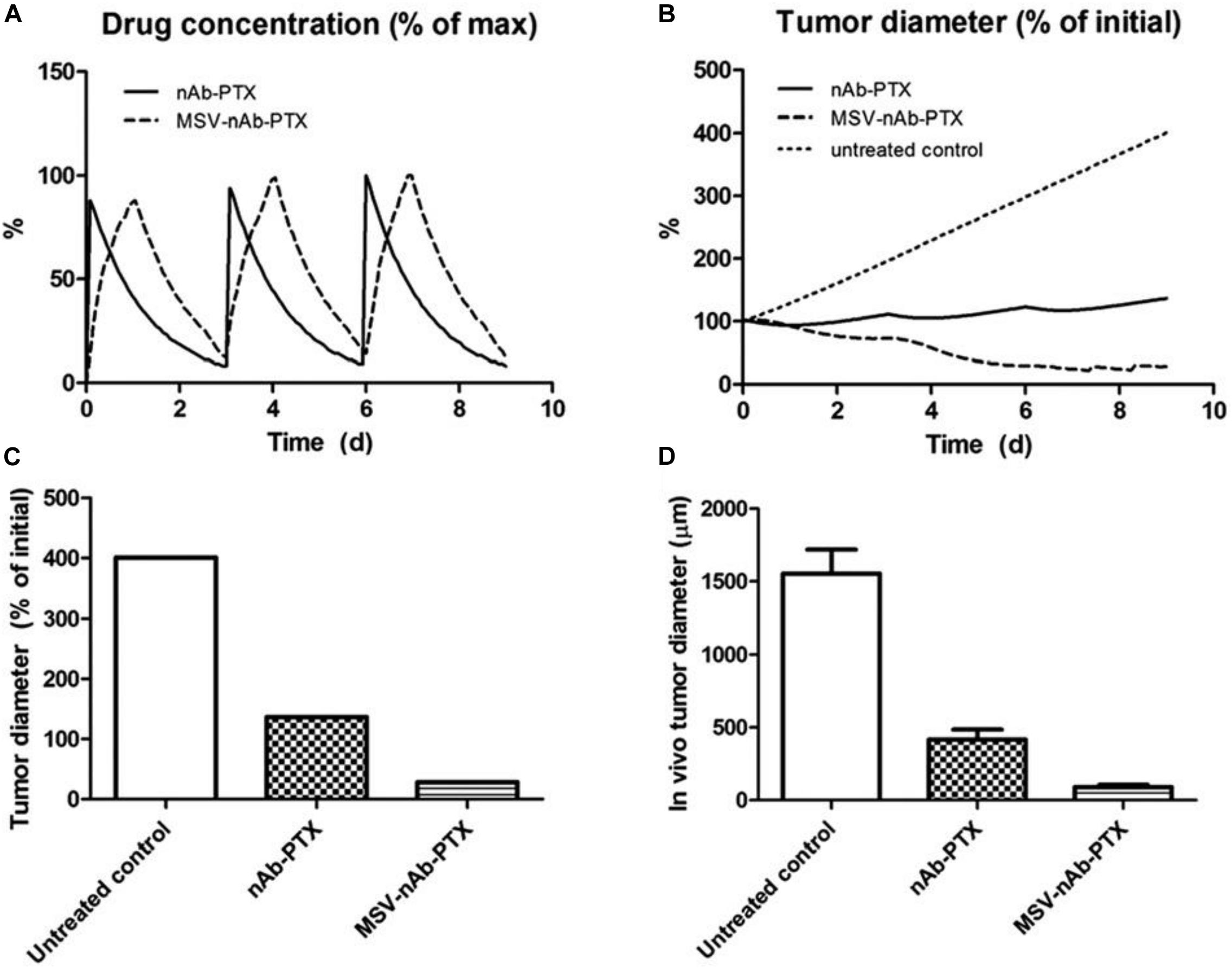
Figure 4. Effect of repeated therapy on simulated breast cancer liver metastsis lesions over 9 day, showing (A) drug (as% of maximum blood levels) and (B) tumor effect (as% of initial lesion diameter) after nAb-PTX and MSV-nAb-PTX injection. In all cases, therapy is initiated at 0, 3, and 6 day. (C) Simulated tumor diameter after three treatments as % of initial tumor. (D) Comparable results from in vivo tumor after three treatments as reported in Tanei et al. (2016). The longer-acting and spatially focused drug release with macrophages achieves a more pronounced regression over the course of therapy than with bolus injection. Reprinted with permission from Leonard et al. (2016).
Shifting macrophage polarization from an anti-inflammatory and tumorigenic M2 phenotype to a pro-inflammatory and anti-cancerous M1 phenotype has recently garnered increased focus (Pyonteck et al., 2013; Leonard et al., 2017; Tariq et al., 2017; Poh and Ernst, 2018), with some promising results (Pyonteck et al., 2013). This shift would ideally be combined with standard therapies. Computational modeling has predicted that the tumor response depends non-linearly on the M1:M2 ratio (Leonard et al., 2017). To explore this further, mathematical modeling was recently employed to analyze the effects of the nanotherapy while simulating manipulation of the macrophage phenotype via a hypothetical immunotherapy affecting macrophage polarization (Leonard et al., 2020). Although the role of macrophages in cancer therapy has been evaluated in the past via mathematical modeling (Mahlbacher et al., 2019), the effect of varying macrophage phenotypes on nanotherapy response has not been extensively explored. The simulations indicated that the M2-tumor interaction may have a dual role in the response to MSV-nab-PTX, initially promoting tumor death and subsequently aiding tumor regrowth.
To test this model-derived hypothesis, CRISPR technology was employed in the laboratory to achieve a stable polarization of macrophages and avoid their repolarization in the dynamically changing TME. The experiments showed that the response to MSV-nab-PTX was non-uniform with respect to the M1:M2 ratio. For 72 h exposure, an M1:M2 ratio of 1500:500 reached lower viability than 2000:0 with only M1, demonstrating that the M2 subtype increases the therapeutic efficacy. Similarly, the ratio of 0:2000 with only M2 had lower viability than the 500:1500 ratio. The tumor response to MSV-nab-PTX loaded macrophages predicted by the computational model is in Figure 5. Figure 5A shows a general trend of decreased tumor size when the M1:M2 ratio increases. Simulating the inactivation of M2 macrophages to gauge the M1-only effect (Figure 5B) while maintaining the same number of activated M1 shows that the response is significantly less than when the M2 are active, even for a high M1:M2 ratio of 3.8:1. Hence, a dual action of the M2 macrophages is forecast by the model. Since PTX is a cell-cycle inhibitor, M2 macrophages synergistically augment the drug effect during treatment by promoting cell proliferation, and support tumor recovery after the treatment. By simulating repeated treatment cycles with MSV-nab-PTX (Figures 5C,D), the model showed that the presence of both macrophage subtypes significantly supports tumor regression.
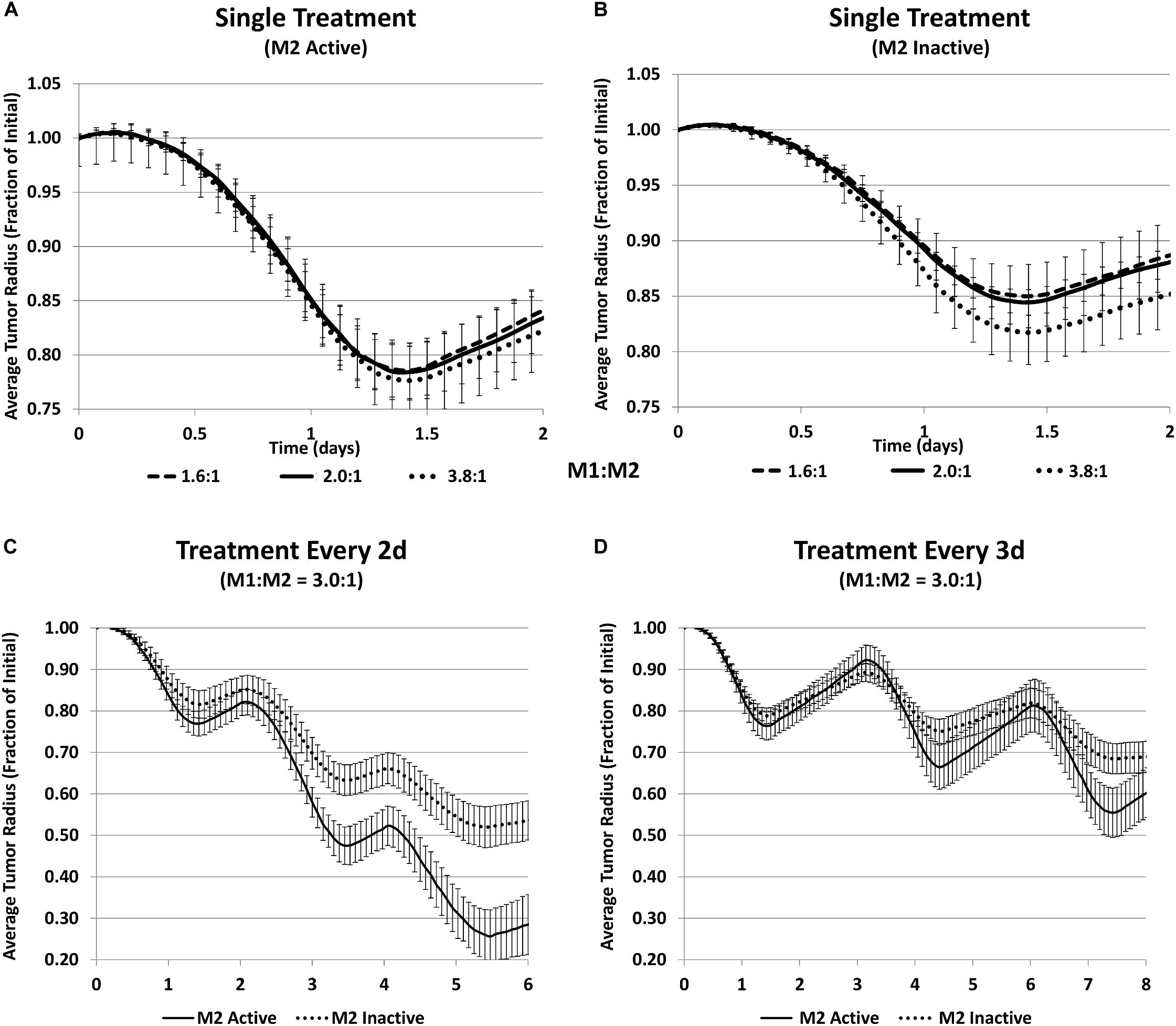
Figure 5. Simulation of tumor progression for untreated and MSV-nAb-PTX treated cases including various combinations of macrophage polarizations simulated average tumor radius (n = 5, mean +/− SD) over time when treated with MSV-nab-PTX-loaded macrophages. (A) Single treatment with both M1 and M2 subtypes active for three different M1:M2 ratios; (B) single treatment with only M1 active for three different M1:M2 ratios; (C) treated every 2d with M1:M2 of 3.0:1; (D) treated every 3d with M1:M2 of 3.0:1. Reprinted with permission from Leonard et al. (2020).
These modeling results suggest that immunotherapy strategies primarily dependent on raising the M1:M2 ratio may be less effective than protocols that establish an M1:M2 ratio that maximizes tumor regression during chemotherapeutic exposure, and then tilts this ratio in favor of the M1 macrophages during the tumor recovery phase in order to leverage their cytotoxic effect.
While molecular targets in tumors are currently clinically evaluated and considered in determining therapeutic strategies, the physical and physiological barriers in the TME may not be taken into account. As discussed in this review, in many instances, the resistance to therapy can have physical or physiological origins. In the case of tumor metastasis to the liver or other hypovascularized lesions, the tumor lesion blood supply represents a critical limiting factor. The ability to retain the drug in the proximity of tumor cells, for example, by anchoring it to the cells of the TME, could bring significant therapeutic advantage. Modeling therapeutic responses and the efficiency of advanced tools, such as nanomedicines, for enhancing these responses would be of prime interest to improve outcomes.
Three-dimensional tumor models are being designed and utilized to bridge the gap between 2D cell cultures and the gold-standard animal models. While for some purposes, such as drug delivery from vasculature to the tumor mass in hypovascular tumors, simple, high-throughput and highly reproducible spheroids can be used, organoids may represent an advantageous systems when TME-tumor cell interactions are important to consider (e.g., in the case of immunotherapy evaluation). With the advent of bioprinting technologies and the ability to recreate complex heterotypic cellular interactions within 3D models, it is expected that the use of 3D tumor models in anticancer therapeutic screening will be significantly expanded. Combined with novel nanotherapy-based targeting strategies and integrated with computational predictions of NP behavior within the TME, preclinical testing in 3D tissue models could benefit from in vitro-ex vivo/in silico approaches in the oncologic drug discovery pipeline.
Nanotherapy-tumor interactions are expected to depend non-linearly (i.e., non-additively) on nanotherapy and tumor tissue-specific conditions, including vascularization, hypoxia, and other microenvironment characteristics affecting tumor response. We have illustrated in this review that the analysis of such interactions could benefit from mathematical modeling that provides a capability for system analysis. In order to leverage the power of these models, their parameters need to be based on biologically relevant data, including clinical information. Recent advances in computational power may enable simulations with enhanced biology to more fully capture the complexity of the TME, including fibroblast cells and extra-cellular matrix components. These models could then be integrated with network-oriented approaches to fully link the molecular- to the tissue-scale. Ideally, nanotherapy candidates would be evaluated prior to treatment by informing the parameters of such models with patient tumor-specific characteristics, as can be observed via analysis of biopsy samples, imaging, and omics information. The models could then be used to determine protocols for optimal tumor response.
All authors jointly wrote and revised the manuscript, and approved the final version.
BG acknowledges partial support by Cancer Prevention and Research Institute of Texas (CPRIT RP150611). BG and SR acknowledge partial support by the Houston Methodist Cancer Center and the Dr. and Mrs. Alan Kaplan Gynecologic Cancer Research Fund. HF acknowledges partial support by the National Institutes of Health/National Cancer Institute grant R15CA203605. SR acknowledges support from the Texas A&M Engineering Experiment Station and the Texas A&M University Department of Biomedical Engineering.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aberle, M. R., Burkhart, R. A., Tiriac, H., Olde Damink, S. W. M., Dejong, C. H. C., Tuveson, D. A., et al. (2018). Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br. J. Surg. 105, e48–e60. doi: 10.1002/bjs.10726
Agastin, S., Giang, U.-B. T., Geng, Y., DeLouise, L. A., and King, M. R. (2011). Continuously perfused microbubble array for 3D tumor spheroid model. Biomicrofluidics 5:024110. doi: 10.1063/1.3596530
Agiostratidou, G., Sgouros, I., Galani, E., Voulgari, A., Chondrogianni, N., Samantas, E., et al. (2001). Correlation of in vitro cytotoxicity and clinical response to chemotherapy in ovarian and breast cancer patients. Anticancer Res. 21, 455–459.
Ahonen, I., Akerfelt, M., Toriseva, M., Oswald, E., Schuler, J., and Nees, M. (2017). A high-content image analysis approach for quantitative measurements of chemosensitivity in patient-derived tumor microtissues. Sci. Rep. 7:6600.
Albritton, J. L., and Miller, J. S. (2017). 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis. Model. Mech. 10, 3–14. doi: 10.1242/dmm.025049
Andasari, V., Gerisch, A., Lolas, G., South, A. P., and Chaplain, M. A. (2011). Mathematical modeling of cancer cell invasion of tissue: biological insight from mathematical analysis and computational simulation. J. Math. Biol. 63, 141–171. doi: 10.1007/s00285-010-0369-1
Bachmann, J., Raue, A., Schilling, M., Becker, V., Timmer, J., and Klingmuller, U. (2012). Predictive mathematical models of cancer signalling pathways. J. Intern. Med. 271, 155–165. doi: 10.1111/j.1365-2796.2011.02492.x
Bae, Y., and Kataoka, K. (2009). Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 61, 768–784. doi: 10.1016/j.addr.2009.04.016
Bae, Y., Nishiyama, N., Fukushima, S., Koyama, H., Matsumura, Y., and Kataoka, K. (2005). Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem. 16, 122–130. doi: 10.1021/bc0498166
Bae, Y., Nishiyama, N., and Kataoka, K. (2007). In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjugate Chem. 18, 1131–1139. doi: 10.1021/bc060401p
Baptista, P. M., Orlando, G., Mirmalek-Sani, S. H., Siddiqui, M., Atala, A., and Soker, S. (2009). Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 6526–6529.
Barbolosi, D., Ciccolini, J., Lacarelle, B., Barlesi, F., and Andre, N. (2016). Computational oncology–mathematical modelling of drug regimens for precision medicine. Nat. Rev. Clin. Oncol. 13, 242–254. doi: 10.1038/nrclinonc.2015.204
Barenholz, Y. (2012). Doxil(R)–the first FDA-approved nano-drug: lessons learned. J. Control Release 160, 117–134. doi: 10.1016/j.jconrel.2012.03.020
Bertrand, N., Wu, J., Xu, X., Kamaly, N., and Farokhzad, O. C. (2014). Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Del. Rev. 66, 2–25. doi: 10.1016/j.addr.2013.11.009
Bethge, A., Schumacher, U., and Wedemann, G. (2015). Simulation of metastatic progression using a computer model including chemotherapy and radiation therapy. J. Biomed. Inform. 57, 74–87. doi: 10.1016/j.jbi.2015.07.011
Bhatia, S., Frangioni, J. V., Hoffman, R. M., Iafrate, A. J., and Polyak, K. (2012). The challenges posed by cancer heterogeneity. Nat. Biotechnol. 30, 604–610. doi: 10.1038/nbt.2294
Blomstrand, H., Scheibling, U., Bratthall, C., Green, H., and Elander, N. O. (2019). Real world evidence on gemcitabine and nab-paclitaxel combination chemotherapy in advanced pancreatic cancer. BMC Cancer 19:40.
Boj, S. F., Hwang, C.-I., Baker, L. A., Chio, I. I. C., Engle, D. D., Corbo, V., et al. (2015). Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338.
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Borsoi, C., Leonard, F., Lee, Y., Zaid, M., Elganainy, D., Alexander, J. F., et al. (2017). Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma. Cancer Lett. 403, 296–304. doi: 10.1016/j.canlet.2017.06.026
Borsoi, C., Yokoi, K., Leonard, F., Ferrari, M., and Godin, B. (2015). “Enhanced therapeutic efficacy of a combination of gemcitabine and albumin-bound paclitaxel in multistage nanovectors in pancreatic ductal adenocarcinoma: Evaluation of transport phenomena,” Proceedings AACR 106th Annual Meeting 2015 (Philadelphia, PA: Cancer Res 2015), 2833.
Breslin, S., and O’Driscoll, L. (2016). The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 7, 45745–45756. doi: 10.18632/oncotarget.9935
Brocato, T. A., Coker, E. N., Durfee, P. N., Lin, Y. S., Townson, J., Wyckoff, E. F., et al. (2018). Understanding the connection between nanoparticle uptake and cancer treatment efficacy using mathematical modeling. Sci. Rep. 8:7538.
Bulin, A. L., Broekgaarden, M., and Hasan, T. (2017). Comprehensive high-throughput image analysis for therapeutic efficacy of architecturally complex heterotypic organoids. Sci. Rep. 7:16645.
Byrne, H. M., Owen, M. R., Alarcon, T., Murphy, J., and Maini, P. K. (2006). Modelling the response of vascular tumours to chemotherapy: A multiscale approach. Math. Mod. Meth. Appl. S 16, 1219–1241. doi: 10.1142/s0218202506001522
Campbell, A., Sivakumaran, T., Davidson, M., Lock, M., and Wong, E. (2008). Mathematical modeling of liver metastases tumour growth and control with radiotherapy. Phys. Med. Biol. 53, 7225–7239. doi: 10.1088/0031-9155/53/24/015
Caster, J. M., Callaghan, C., Seyedin, S. N., Henderson, K., Sun, B., and Wang, A. Z. (2019). Optimizing Advances in Nanoparticle Delivery for Cancer Immunotherapy. Adv. Drug Deliv. Rev. 144, 3–15. doi: 10.1016/j.addr.2019.07.009
Chamseddine, I. M., Frieboes, H. B., and Kokkolaras, M. (2018). Design optimization of tumor vasculature-bound nanoparticles. Sci. Rep. 8:17768.
Chamseddine, I. M., Frieboes, H. B., and Kokkolaras, M. (2020). Multi-objective optimization of tumor response to drug release from vasculature-bound nanoparticles. Sci. Rep. 10:8294.
Chaplain, M. A. J. (2011). Multiscale mathematical modelling in biology and medicine. IMA J. Appl. Math. 76, 371–388. doi: 10.1093/imamat/hxr025
Chen, J., Weihs, D., and Vermolen, F. J. (2020). Computational modeling of therapy on pancreatic cancer in its early stages. Biomech. Model Mechanobiol. 19, 427–444. doi: 10.1007/s10237-019-01219-0
Cho, E. J., Holback, H., Liu, K. C., Abouelmagd, S. A., Park, J., and Yeo, Y. (2013). Nanoparticle characterization: state of the art. Challenges, and emerging technologies. Mol. Pharm. 10, 2093–2110. doi: 10.1021/mp300697h
Cho, K., Evans, B. S., Wood, B. M., Kumar, R., Erb, T. J., Warlick, B. P., et al. (2014). Integration of untargeted metabolomics with transcriptomics reveals active metabolic pathways. Metabolomics 11, 503–517. doi: 10.1007/s11306-014-0713-3
Choi, M. R., Stanton-Maxey, K. J., Stanley, J. K., Levin, C. S., Bardhan, R., Akin, D., et al. (2007). A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 7, 3759–3765. doi: 10.1021/nl072209h
Chung, A. S., Lee, J., and Ferrara, N. (2010). Targeting the tumour vasculature: insights from physiological angiogenesis. Nat. Rev. Cancer 10, 505–514. doi: 10.1038/nrc2868
Clendenon, S. G., Fu, X., Von Hoene, R. A., Clendenon, J. L., Sluka, J. P., Winfree, S., et al. (2019). Spatial temporal analysis of fieldwise flow in microvasculature. J. Vis. Exp. 153.
Crist, R. M., Grossman, J. H., Patri, A. K., Stern, S. T., Dobrovolskaia, M. A., Adiseshaiah, P. P., et al. (2013). Common pitfalls in nanotechnology: lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integr. Biol. 5, 66–73. doi: 10.1039/c2ib20117h
Cristini, V., Frieboes, H. B., Li, X., Lowengrub, J., Macklin, P., Sanga, S., et al. (2008). Nonlinear Modeling and Simulation of Tumor Growth. Selected Topics in Cancer Modeling. Boston: Birkhäuser.
Cui, Y. L., Li, H. K., Zhou, H. Y., Zhang, T., and Li, Q. (2013). Correlations of tumor-associated macrophage subtypes with liver metastases of colorectal cancer. Asian Pac. J. Cancer Prev. 14, 1003–1007. doi: 10.7314/apjcp.2013.14.2.1003
Cukierman, E., and Khan, D. R. (2010). The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem. Pharmacol. 80, 762–770. doi: 10.1016/j.bcp.2010.04.020
Curtis, L. T., England, C. G., Wu, M., Lowengrub, J., and Frieboes, H. B. (2016a). An interdisciplinary computational/experimental approach to evaluate drug-loaded gold nanoparticle tumor cytotoxicity. Nanomedicine 11, 197–216. doi: 10.2217/nnm.15.195
Curtis, L. T., and Frieboes, H. B. (2016). The tumor microenvironment as a barrier to cancer nanotherapy. Adv. Exp. Med. Biol. 936, 165–190. doi: 10.1007/978-3-319-42023-3_9
Curtis, L. T., Rychahou, P., Bae, Y., and Frieboes, H. B. (2016b). A computational/experimental assessment of antitumor activity of polymer nanoassemblies for ph-controlled drug delivery to primary and metastatic tumors. Pharm. Res. 33, 2552–2564.
Curtis, L. T., van Berkel, V. H., and Frieboes, H. B. (2018). Pharmacokinetic/pharmacodynamic modeling of combination-chemotherapy for lung cancer. J. Theor. Biol. 448, 38–52. doi: 10.1016/j.jtbi.2018.03.035
Curtis, L. T., Wu, M., Lowengrub, J., Decuzzi, P., and Frieboes, H. B. (2015). Computational modeling of tumor response to drug release from vasculature-bound nanoparticles. PLoS One 10:e0144888. doi: 10.1371/journal.pone.0144888
Dai, X., Ma, C., Lan, Q., and Xu, T. (2016). 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 8:045005. doi: 10.1088/1758-5090/8/4/045005
Daly, J. M., Butler, J., Kemeny, N., Yeh, S. D., Ridge, J. A., Botet, J., et al. (1985). Predicting tumor response in patients with colorectal hepatic metastases. Ann. Surg. 202, 384–393. doi: 10.1097/00000658-198509000-00017
Danet, I. M., Semelka, R. C., Leonardou, P., Braga, L., Vaidean, G., Woosley, J. T., et al. (2003a). Spectrum of MRI appearances of untreated metastases of the liver. AJR Am. J. Roentgenol. 181, 809–817. doi: 10.2214/ajr.181.3.1810809
Danet, I. M., Semelka, R. C., Nagase, L. L., Woosely, J. T., Leonardou, P., and Armao, D. (2003b). Liver metastases from pancreatic adenocarcinoma: MR imaging characteristics. J. Magn. Reson. Imaging 18, 181–188. doi: 10.1002/jmri.10337
D’Angelo, E., Natarajan, D., Sensi, F., Ajayi, O., Fassan, M., Mammano, E., et al. (2020). Patient-Derived Scaffolds of Colorectal Cancer Metastases as an Organotypic 3D Model of the Liver Metastatic Microenvironment. Cancers 12:364. doi: 10.3390/cancers12020364
Danhier, F. (2016). To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control Release 244, 108–121. doi: 10.1016/j.jconrel.2016.11.015
Dawidczyk, C. M., Kim, C., Park, J. H., Russell, L. M., Lee, K. H., Pomper, M. G., et al. (2014). State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J. Control Release 187, 133–144. doi: 10.1016/j.jconrel.2014.05.036
Decuzzi, P., and Ferrari, M. (2006). The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 27, 5307–5314. doi: 10.1016/j.biomaterials.2006.05.024
Decuzzi, P., Pasqualini, R., Arap, W., and Ferrari, M. (2009). Intravascular delivery of particulate systems: does geometry really matter? Pharm. Res. 26, 235–243. doi: 10.1007/s11095-008-9697-x
Deisboeck, T. S., Wang, Z., Macklin, P., and Cristini, V. (2011). Multiscale cancer modeling. Annu. Rev. Biomed. Eng. 13, 127–155.
Dewhirst, M. W., and Secomb, T. W. (2017). Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 17, 738–750. doi: 10.1038/nrc.2017.93
Dogra, P., Butner, J. D., Chuang, Y. L., Caserta, S., Goel, S., Brinker, C. J., et al. (2019). Mathematical modeling in cancer nanomedicine: a review. Biomed. Microdevices 21:40.
Dogra, P., Butner, J. D., Ruiz Ramirez, J., Chuang, Y. L., Noureddine, A., Jeffrey Brinker, C., et al. (2020a). A mathematical model to predict nanomedicine pharmacokinetics and tumor delivery. Comput. Struct. Biotechnol. J. 18, 518–531. doi: 10.1016/j.csbj.2020.02.014
Dogra, P., Ramírez, J. P., Peláez, M. J., Wang, Z., Cristini, V., Parasher, G., et al. (2020b). Mathematical modeling to address challenges in pancreatic cancer. Curr. Top. Med. Chem. 20, 367–376. doi: 10.2174/1568026620666200101095641
Dong, M., Philippi, C., Loretz, B., Nafee, N., Schaefer, U. F., Friedel, G., et al. (2011). Tissue slice model of human lung cancer to investigate telomerase inhibition by nanoparticle delivery of antisense 2’-O-methyl-RNA. Int. J. Pharm. 419, 33–42. doi: 10.1016/j.ijpharm.2011.07.009
Dong, Y., Hu, J., Fan, L., and Chen, Q. R. N. A. - (2017). Seq-based transcriptomic and metabolomic analysis reveal stress responses and programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Sci. Rep. 7:42659.
Drasdo, D., Hoehme, S., and Hengstler, J. G. (2014). How predictive quantitative modelling of tissue organisation can inform liver disease pathogenesis. J. Hepatol. 61, 951–956. doi: 10.1016/j.jhep.2014.06.013
Dutta-Moscato, J., Solovyev, A., Mi, Q., Nishikawa, T., Soto-Gutierrez, A., Fox, I. J., et al. (2014). A Multiscale Agent-Based in silico Model of Liver Fibrosis Progression. Front. Bioeng. Biotechnol. 2:18.
Edelman, L. B., Eddy, J. A., and Price, N. D. (2010). In silico models of cancer. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 438–459.
Enderling, H., Chaplain, M. A., Anderson, A. R., and Vaidya, J. S. (2007). A mathematical model of breast cancer development, local treatment and recurrence. J. Theor. Biol. 246, 245–259. doi: 10.1016/j.jtbi.2006.12.010
Enriquez-Navas, P. M., Kam, Y., Das, T., Hassan, S., Silva, A., Foroutan, P., et al. (2016). Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci. Transl. Med. 8:327ra24. doi: 10.1126/scitranslmed.aad7842
Ewe, A., Hobel, S., Heine, C., Merz, L., Kallendrusch, S., Bechmann, I., et al. (2017). Optimized polyethylenimine (PEI)-based nanoparticles for siRNA delivery, analyzed in vitro and in an ex vivo tumor tissue slice culture model. Drug Deliv. Transl. Res. 7, 206–216. doi: 10.1007/s13346-016-0306-y
Fang, J., Nakamura, H., and Maeda, H. (2011). The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 63, 136–151. doi: 10.1016/j.addr.2010.04.009
Farokhzad Omid, C., and Langer, R. (2009). Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20.
Fernandes, E., Ferreira, J. A., Andreia, P., Luís, L., Barroso, S., Sarmento, B., et al. (2015). New trends in guided nanotherapies for digestive cancers: A systematic review. J. Control. Release 209, 288–307. doi: 10.1016/j.jconrel.2015.05.003
Figueroa-Magalhaes, M. C., Jelovac, D., Connolly, R., and Wolff, A. C. (2014). Treatment of HER2-positive breast cancer. Breast 23, 128–136.
Folkman, J. (2007). Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 6, 273–286. doi: 10.1038/nrd2115
Frieboes, H. B., Chaplain, M. A., Thompson, A. M., Bearer, E. L., Lowengrub, J. S., and Cristini, V. (2011). Physical oncology: a bench-to-bedside quantitative and predictive approach. Cancer Res.arch 71, 298–302. doi: 10.1158/0008-5472.can-10-2676
Frieboes, H. B., Edgerton, M. E., Fruehauf, J. P., Rose, F. R., Worrall, L. K., Gatenby, R. A., et al. (2009). Prediction of drug response in breast cancer using integrative experimental/computational modeling. Cancer Res. 69, 4484–4492. doi: 10.1158/0008-5472.can-08-3740
Frieboes, H. B., Sinek, J. P., Nalcioglu, O., Fruehauf, J. P., and Cristini, V. (2006). Nanotechnology in Cancer Drug Therapy: A Biocomputational Approach. Biological and Biomedical Nanotechnology, Vol. 20p. New York, NY: Springer-Verlag, 435–460.
Frieboes, H. B., Wu, M., Lowengrub, J., Decuzzi, P., and Cristini, V. (2013). A computational model for predicting nanoparticle accumulation in tumor vasculature. PLoS One 8:e56876. doi: 10.1371/journal.pone.0056876
Frieboes, H. B., Yokoi, K., Dave, B., Hussain, F., and Godin, B. (2012). Modeling the Tumor Microenvironment as a Biobarrier in Cancer Nanotherapeutics, Vol. 20. Singapore: Pan Stanford Publishing Pte. Ltd, 137–184.
Frieboes, H. B., Decuzzi, P., Sinek, J. P., Ferrari, M., and Cristini, V. (2019). Computational Modeling of Tumor Biobarriers: Implications for Delivery of Nano-Based Therapeutics, Vol. 20. New Jersey: World Scientific, 201–244.
Friedman, A., and Hao, W. (2017). Mathematical modeling of liver fibrosis. Math. Biosci. Eng. 14, 143–164. doi: 10.3934/mbe.2017010
Fruehauf, J. P. (2002). In vitro assay-assisted treatment selection for women with breast or ovarian cancer. Endocr. Relat. Cancer 9, 171–182. doi: 10.1677/erc.0.0090171
Fu, X., Sluka, J. P., Clendenon, S. G., Dunn, K. W., Wang, Z., Klaunig, J. E., et al. (2018). Modeling of xenobiotic transport and metabolism in virtual hepatic lobule models. PLoS One 13:e0198060. doi: 10.1371/journal.pone.0198060
Gao, Y., Li, M., Chen, B., Shen, Z., Guo, P., Wientjes, M. G., et al. (2013). Predictive models of diffusive nanoparticle transport in 3-dimensional tumor cell spheroids. AAPS J. 15, 816–831. doi: 10.1208/s12248-013-9478-2
Garvey, C. M., Spiller, E., Lindsay, D., Chiang, C. T., Choi, N. C., Agus, D. B., et al. (2016). A high-content image-based method for quantitatively studying context-dependent cell population dynamics. Sci. Rep. 6:29752.
Ge, Q., Zhang, Y., Hua, W. P., Wu, Y. C., Jin, X. X., Song, S. H., et al. (2015). Combination of transcriptomic and metabolomic analyses reveals a JAZ repressor in the jasmonate signaling pathway of Salvia miltiorrhiza. Sci Rep 5, 14048.
Geeleher, P., and Cox, N. J. (2014). S. HR. Clinical drug response can be predicted using baseline gene expression levels and in vitro drug sensitivity in cell lines. Genome Biol. 15:R47.
Gevertz, J. L. (2011). Computational modeling of tumor response to vascular-targeting therapies–part I: validation. Comput. Math. Methods Med. 2011:830515.
Gioeli, D., Snow, C. J., Simmers, M. B., Hoang, S. A., Figler, R. A., Allende, J. A., et al. (2019). Development of a multicellular pancreatic tumor microenvironment system using patient-derived tumor cells. Lab Chip 19, 1193–1204. doi: 10.1039/c8lc00755a
Godin, B., Chiappini, C., Srinivasan, S., Alexander, J. F., Yokoi, K., Ferrari, M., et al. (2012). Discoidal porous silicon particles: fabrication and biodistribution in breast cancer bearing mice. Adv. Funct. Mater. 22, 4225–4235. doi: 10.1002/adfm.201200869
Godin, B., Driessen, W. H., Proneth, B., Lee, S. Y., Srinivasan, S., Rumbaut, R., et al. (2010a). An integrated approach for the rational design of nanovectors for biomedical imaging and therapy. Adv. Genet. 69, 31–64. doi: 10.1016/s0065-2660(10)69009-8
Godin, B., Gu, J., Serda, R. E., Bhavane, R., Tasciotti, E., Chiappini, C., et al. (2010b). Tailoring the degradation kinetics of mesoporous silicon structures through PEGylation. J. Biomed. Mater. Res. A 94, 1236–1243.
Godin, B., Tasciotti, E., Liu, X., Serda, R. E., and Ferrari, M. (2011). Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc Chem. Res. 44, 979–989. doi: 10.1021/ar200077p
Greish, K. (2010). Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 624, 25–37. doi: 10.1007/978-1-60761-609-2_3
Griffith, L. G., and Swartz, M. A. (2006). Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7, 211–224. doi: 10.1038/nrm1858
Grimmett, C., Pickett, K., Shepherd, J., Welch, K., Recio-Saucedo, A., Streit, E., et al. (2018). Systematic review of the empirical investigation of resources to support decision-making regarding BRCA1 and BRCA2 genetic testing in women with breast cancer. Patient Educ. Couns. 101, 779–788. doi: 10.1016/j.pec.2017.11.016
Grodzinski, P., and Farrell, D. (2014). Future opportunities in cancer nanotechnology—NCI strategic workshop report. Cancer Res. 74, 1307–1310. doi: 10.1158/0008-5472.can-13-2787
Guller, A. E., Rozova, V. S., Kuschnerus, I., Khabir, Z., Nadort, A., Garcia-Bennett, A. E., et al. (2020). Tissue engineered model of hepatic breast cancer micrometastasis shows host-dependent colonization patterns and drug responses. bioRxiv [Preprint] doi: 10.1101/2020.01.08.898163v1
Guo, S., and Deng, C. X. (2018). Effect of stromal cells in tumor microenvironment on metastasis initiation. Int. J. Biol. Sci. 14, 2083–2093. doi: 10.7150/ijbs.25720
Gustafson, H. H., Holt-Casper, D., Grainger, D. W., and Ghandehari, H. (2015). Nanoparticle uptake: the phagocyte problem. Nano Today 10, 487–510. doi: 10.1016/j.nantod.2015.06.006
Haeno, H., Gonen, M., Davis, M. B., Herman, J. M., Iacobuzio-Donahue, C. A., and Michor, F. (2012). Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 148, 362–375. doi: 10.1016/j.cell.2011.11.060
Hay, M., Thomas, D. W., Craighead, J. L., Economides, C., and Rosenthal, J. (2014). Clinical development success rates for investigational drugs. Nat. Biotechnol. 32, 40–51. doi: 10.1038/nbt.2786
Hazhirkarzar, B., Khoshpouri, P., Shaghaghi, M., Ghasabeh, M. A., Pawlik, T. M., and Kamel, I. R. (2020). Current state of the art imaging approaches for colorectal liver metastasis. Hepatobiliary Surg. Nutr. 9, 35–48. doi: 10.21037/hbsn.2019.05.11
He, Y. F., Zhang, M. Y., Wu, X., Sun, X. J., Xu, T., He, Q. Z., et al. (2013). High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PLoS One 8:e79769. doi: 10.1371/journal.pone.0079769
Heath, J. R., Ribas, A., and Mischel, P. S. (2016). Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug Discov. 15, 204–216. doi: 10.1038/nrd.2015.16
Hinderer, S., Layland, S. L., and Schenke-Layland, K. (2016). ECM and ECM-like materials - Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 97, 260–269. doi: 10.1016/j.addr.2015.11.019
Hinow, P., Gerlee, P., McCawley, L. J., Quaranta, V., Ciobanu, M., Wang, S., et al. (2009). A spatial model of tumor-host interaction: application of chemotherapy. Math. Biosci. Eng. 6, 521–546. doi: 10.3934/mbe.2009.6.521
Hoehme, S., Bertaux, F., Weens, W., Grasl-Kraupp, B., Hengstler, J. G., and Drasdo, D. (2018). Model Prediction and Validation of an Order Mechanism Controlling the Spatiotemporal Phenotype of Early Hepatocellular Carcinoma. Bull. Math. Biol. 80, 1134–1171. doi: 10.1007/s11538-017-0375-1
Hoehme, S., Brulport, M., Bauer, A., Bedawy, E., Schormann, W., Hermes, M., et al. (2010). Prediction and validation of cell alignment along microvessels as order principle to restore tissue architecture in liver regeneration. Proc. Natl. Acad. Sci. U.S.A. 107, 10371–10376. doi: 10.1073/pnas.0909374107
Hoehme, S., Friebel, A., Hammad, S., Drasdo, D., and Hengstler, J. G. (2017). Creation of three-dimensional liver tissue models from experimental images for systems medicine. Methods Mol. Biol. 1506, 319–362. doi: 10.1007/978-1-4939-6506-9_22
Hoehme, S., Hengstler, J. G., Brulport, M., Schafer, M., Bauer, A., Gebhardt, R., et al. (2007). Mathematical modelling of liver regeneration after intoxication with CCl(4). Chem. Biol. Interact. 168, 74–93. doi: 10.1016/j.cbi.2007.01.010
Holzhutter, H. G., Drasdo, D., Preusser, T., Lippert, J., and Henney, A. M. (2012). The virtual liver: a multidisciplinary, multilevel challenge for systems biology. Wiley Interdiscip. Rev. Syst. Biol. Med. 4, 221–235. doi: 10.1002/wsbm.1158
Horman, S. R., Hogan, C., Delos Reyes, K., Lo, F., and Antczak, C. (2015). Challenges and opportunities toward enabling phenotypic screening of complex and 3D cell models. Future Med. Chem. 7, 513–525. doi: 10.4155/fmc.14.163
Hu, G., Guo, M., Xu, J., Wu, F., Fan, J., Huang, Q., et al. (2019). Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents Against Cancer and Inflammation. Front. Immunol. 10:1998.
Huang, Z. Q., and Buchsbaum, D. J. (2009). Monoclonal antibodies in the treatment of pancreatic cancer. Immunotherapy 1, 223–229.
Hudson, S. V., Dolin, C. E., Poole, L. G., Massey, V. L., Wilkey, D., Beier, J. I., et al. (2017). Modeling the kinetics of integrin receptor binding to hepatic extracellular matrix proteins. Sci. Rep. 7:12444.
Hudson, S. V., Miller, H. A., Mahlbacher, G. E., Saforo, D., Beverly, L. J., Arteel, G. E., et al. (2019). Computational/experimental evaluation of liver metastasis post hepatic injury: interactions with macrophages and transitional ECM. Sci. Rep. 9:15077.
Hussein, K. H., Park, K. M., Ghim, J. H., Yang, S. R., and Woo, H. M. (2016). Three dimensional culture of HepG2 liver cells on a rat decellularized liver matrix for pharmacological studies. J. Biomed. Mater. Res. B Appl. Biomater. 104, 263–273. doi: 10.1002/jbm.b.33384
Ibrahim-Hashim, A., Robertson-Tessi, M., Enriquez-Navas, P. M., Damaghi, M., Balagurunathan, Y., Wojtkowiak, J. W., et al. (2017). Defining cancer subpopulations by adaptive strategies rather than molecular properties provides novel insights into intratumoral evolution. Cancer Res. 77, 2242–2254. doi: 10.1158/0008-5472.can-16-2844
Jaganathan, H., Gage, J., Leonard, F., Srinivasan, S., Souza, G. R., Dave, B., et al. (2014). Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci. Rep. 4:6468.
Jain, R. K. (2003). Molecular regulation of vessel maturation. Nat. Med. 9, 685–693. doi: 10.1038/nm0603-685
Kaddi, C. D., Phan, J. H., and Wang, M. D. (2013). Computational nanomedicine: modeling of nanoparticle-mediated hyperthermal cancer therapy. Nanomedicine 8, 1323–1333. doi: 10.2217/nnm.13.117
Kang, S. A., Hasan, N., Mann, A. P., Zheng, W., Zhao, L., Morris, L., et al. (2015). Blocking the adhesion cascade at the premetastatic niche for prevention of breast cancer metastasis. Mol. Ther. 23, 1044–1054. doi: 10.1038/mt.2015.45
Khalid, A., Persano, S., Shen, H., Zhao, Y., Blanco, E., Ferrari, M., et al. (2016). Strategies for improving drug delivery: nanocarriers and microenvironmental priming. Expert. Opin. Drug Del. 14, 1–13.
Khawar, I. A., Kim, J. H., and Kuh, H.-J. (2015). Improving drug delivery to solid tumors: priming the tumor microenvironment. J. Control Release 201, 78–89. doi: 10.1016/j.jconrel.2014.12.018
Kielbassa, K., Vegna, S., Ramirez, C., and Akkari, L. (2019). Understanding the origin and diversity of macrophages to tailor their targeting in solid cancers. Front. Immunol. 10:2215.
Kim, C., Bang, J. H., Kim, Y. E., Lee, S. H., and Kang, J. Y. (2012). On-chip anticancer drug test of regular tumor spheroids formed in microwells by a distributive microchannel network. Lab Chip 12, 4135–4142.
Koontz, B. F. (2017). Stereotactic body radiation therapy for oligometastatic prostate cancer: the hunt for the silver bullet. Int. J. Radiat. Oncol. Biol. Phys. 99, 761–763. doi: 10.1016/j.ijrobp.2017.05.020
Kopper, O., de Witte, C. J., Lohmussaar, K., Valle-Inclan, J. E., Hami, N., Kester, L., et al. (2019). An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 25, 838–849. doi: 10.1038/s41591-019-0422-6
Kreeger, P. K., and Lauffenburger, D. A. (2010). Cancer systems biology: a network modeling perspective. Carcinogenesis 31, 2–8. doi: 10.1093/carcin/bgp261
Kyle, A. H., Huxham, L. A., Yeoman, D. M., and Minchinton, A. I. (2007). Limited tissue penetration of taxanes: A mechanism for resistance in solid tumors. Clin. Cancer Res. 13, 2804–2810. doi: 10.1158/1078-0432.ccr-06-1941
Lecca, P., and Re, A. (2017). Network-oriented approaches to anticancer drug response. Methods Mol. Biol. 1513, 101–117. doi: 10.1007/978-1-4939-6539-7_8
Lee, H., Han, W., Kim, H., Ha, D. H., Jang, J., Kim, B. S., et al. (2017). Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules 18, 1229–1237. doi: 10.1021/acs.biomac.6b01908
Lee, J. J., Huang, J., England, C. G., McNally, L. R., and Frieboes, H. B. (2013). Predictive modeling of in vivo response to gemcitabine in pancreatic cancer. PLoS Comput. Biol. 9:e1003231. doi: 10.1371/journal.pcbi.1003231
Leonard, F., Curtis, L. T., Hamed, A. R., Zhang, C., Chau, E., Sieving, D., et al. (2020). Nonlinear response to cancer nanotherapy due to macrophage interactions revealed by mathematical modeling and evaluated in a murine model via CRISPR-modulated macrophage polarization. Cancer Immunol. Immunother. 69, 731–744. doi: 10.1007/s00262-020-02504-z
Leonard, F., Curtis, L. T., Ware, M. J., Nosrat, T., Liu, X., Yokoi, K., et al. (2017). Macrophage polarization contributes to the anti-tumoral efficacy of mesoporous nanovectors loaded with albumin-bound paclitaxel. Front. Immunol. 8:693.
Leonard, F., Curtis, L. T., Yesantharao, P., Tanei, T., Alexander, J. F., Wu, M., et al. (2016). Enhanced performance of macrophage-encapsulated nanoparticle albumin-bound-paclitaxel in hypo-perfused cancer lesions. Nanoscale 8, 12544–12552. doi: 10.1039/c5nr07796f
Leonard, F., and Godin, B. (2016). 3D In Vitro Model for Breast Cancer Research Using Magnetic Levitation and Bioprinting Method. Methods Mol. Biol. 1406, 239–251. doi: 10.1007/978-1-4939-3444-7_21
Lettmann, K. A., and Hardtke-Wolenski, M. (2014). The importance of liver microcirculation in promoting autoimmune hepatitis via maintaining an inflammatory cytokine milieu–a mathematical model study. J. Theor. Biol. 348, 33–46. doi: 10.1016/j.jtbi.2014.01.016
Li, M., Al-Jamal, K. T., Kostarelos, K., and Reineke, J. (2010). Physiologically based pharmacokinetic modeling of nanoparticles. ACS Nano 4, 6303–6317.
Li, M., Czyszczon, E., and Reineke, J. (2013). Delineating intracellular pharmacokinetics of paclitaxel delivered by PLGA nanoparticles. Drug Deliv. Transl. Res. 3, 551–561. doi: 10.1007/s13346-013-0162-y
Li, M., Panagi, Z., Avgoustakis, K., and Reineke, J. (2012). Physiologically based pharmacokinetic modeling of PLGA nanoparticles with varied mPEG content. Int. J. Nanomed. 7, 1345–1356.
Li, M., and Reineke, J. (2011). Mathematical modelling of nanoparticle biodistribution: extrapolation among intravenous, oral and pulmonary administration routes. Int. J. Nano Biomater. 3, 222–223.
Li, S., Todor, A., and Luo, R. (2016). Blood transcriptomics and metabolomics for personalized medicine. Comput. Struct. Biotechnol. J. 14, 1–7. doi: 10.1016/j.csbj.2015.10.005
Li, X., and Kwon, H. (2019). Efficacy and safety of nanoparticle albumin-bound paclitaxel in elderly patients with metastatic breast cancer: a meta-analysis. J. Clin. Med. 8:1689. doi: 10.3390/jcm8101689
Liu, L. X., Zhang, W. H., and Jiang, H. C. (2003). Current treatment for liver metastases from colorectal cancer. World J. Gastroentero 9, 193–200.
Louzoun, Y., Xue, C., Lesinski, G. B., and Friedman, A. (2014). A mathematical model for pancreatic cancer growth and treatments. J. Theor. Biol. 351, 74–82. doi: 10.1016/j.jtbi.2014.02.028
Lowengrub, J. S., Frieboes, H. B., Jin, F., Chuang, Y. L., Li, X., Macklin, P., et al. (2010). Nonlinear modelling of cancer: bridging the gap between cells and tumours. Nonlinearity 23, R1–R9.
Lu, S., Cuzzucoli, F., Jiang, J., Liang, L. G., Wang, Y., Kong, M., et al. (2018). Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab Chip 18, 3379–3392. doi: 10.1039/c8lc00852c
Maeda, H., Fang, J., Inutsuka, T., and Kitamoto, Y. (2003). Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int. Immunopharmacol. 3, 319–328. doi: 10.1016/s1567-5769(02)00271-0
Mahlbacher, G., Curtis, L. T., Lowengrub, J., and Frieboes, H. B. (2018). Mathematical modeling of tumor-associated macrophage interactions with the cancer microenvironment. J. Immunother. Cancer 6:10.
Mahlbacher, G. E., Reihmer, K. C., and Frieboes, H. B. (2019). Mathematical modeling of tumor-immune cell interactions. J. Theor. Biol. 469, 47–60. doi: 10.1016/j.jtbi.2019.03.002
Maniwa, Y., Yoshimura, M., Hashimoto, S., Takata, M., and Nishio, W. (2010). Chemosensitivity of lung cancer: differences between the primary lesion and lymph node metastasis. Oncol. Lett. 1, 345–349. doi: 10.3892/ol_00000061
Mehta, G., Hsiao, A. Y., Ingram, M., Luker, G. D., and Takayama, S. (2012). Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 164, 192–204. doi: 10.1016/j.jconrel.2012.04.045
Meng, F., Meyer, C. M., Joung, D., Vallera, D. A., McAlpine, M. C., and Panoskaltsis-Mortari, A. (2019). 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater. 31:e1806899.
Meyer, K., Ostrenko, O., Bourantas, G., Morales-Navarrete, H., Porat-Shliom, N., Segovia-Miranda, F., et al. (2017). A predictive 3D multi-scale model of biliary fluid dynamics in the liver lobule. Cell Syst. 4:e9.
Michor, F., Liphardt, J., Ferrari, M., and Widom, J. (2011). What does physics have to do with cancer? Nat. Rev. Cancer 11, 657–670. doi: 10.1038/nrc3092
Miller, H. A., and Frieboes, H. B. (2019a). Evaluation of Drug-Loaded Gold Nanoparticle Cytotoxicity as a Function of Tumor Vasculature-Induced Tissue Heterogeneity. Ann. Biomed. Eng. 47, 257–271. doi: 10.1007/s10439-018-02146-4
Miller, H. A., and Frieboes, H. B. (2019b). Pharmacokinetic/Pharmacodynamics Modeling of Drug-Loaded PLGA Nanoparticles Targeting Heterogeneously Vascularized Tumor Tissue. Pharm. Res. 36:185.
Minton, D. R., Fu, L., Chen, Q., Robinson, B. D., Gross, S. S., Nanus, D. M., et al. (2015). Analyses of the transcriptome and metabolome demonstrate that HIF1alpha mediates altered tumor metabolism in clear cell renal cell carcinoma. PLoS One 10:e0120649. doi: 10.1371/journal.pone.0120649
Miryala, S. K., Anbarasu, A., and Ramaiah, S. (2018). Discerning molecular interactions: A comprehensive review on biomolecular interaction databases and network analysis tools. Gene 642, 84–94. doi: 10.1016/j.gene.2017.11.028
Misra, R., Acharya, S., and Sahoo, S. K. (2010). Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov. Today 15, 842–850. doi: 10.1016/j.drudis.2010.08.006
Mucaki, E. J., Zhao, J. Z. L., Lizotte, D. J., and Rogan, P. K. (2019). Predicting responses to platin chemotherapy agents with biochemically-inspired machine learning. Signal Transduct. Target Ther. 4:1.
Murakami, Y., Kubo, S., Tamori, A., Itami, S., Kawamura, E., Iwaisako, K., et al. (2015). Comprehensive analysis of transcriptome and metabolome analysis in Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Sci. Rep. 5:16294.
Nakamura, H., Fang, J., and Maeda, H. (2015). Development of next-generation macromolecular drugs based on the EPR effect: challenges and pitfalls. Expert. Opin. Drug Deliv. 12, 53–64. doi: 10.1517/17425247.2014.955011
Namasivayam, S., Martin, D. R., and Saini, S. (2007). Imaging of liver metastases: MRI. Cancer Imaging 7, 2–9. doi: 10.1102/1470-7330.2007.0002
Ng, C. F., and Frieboes, H. B. (2017). Model of vascular desmoplastic multispecies tumor growth. J. Theor. Biol. 430, 245–282. doi: 10.1016/j.jtbi.2017.05.013
Ng, C. F., and Frieboes, H. B. (2018). Simulation of multispecies desmoplastic cancer growth via a fully adaptive non-linear full multigrid algorithm. Front. Physiol. 9:821.
Ngamcherdtrakul, W., and Yantasee, W. (2019). siRNA therapeutics for breast cancer: recent efforts in targeting metastasis, drug resistance, and immune evasion. Transl. Res. 214, 105–120. doi: 10.1016/j.trsl.2019.08.005
Nishii, K., Reese, G., Moran, E. C., and Sparks, J. L. (2016). Multiscale computational model of fluid flow and matrix deformation in decellularized liver. J. Mech. Behav. Biomed. Mater. 57, 201–214. doi: 10.1016/j.jmbbm.2015.11.033
Osborne, J. M., Walter, A., Kershaw, S. K., Mirams, G. R., Fletcher, A. G., Pathmanathan, P., et al. (2010). A hybrid approach to multi-scale modelling of cancer. Philos. Trans. A Math. Phys. Eng. Sci. 368, 5013–5028.
Ozcelikkale, A., Moon, H. R., Linnes, M., and Han, B. (2017). In vitro microfluidic models of tumor microenvironment to screen transport of drugs and nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9:e1460. doi: 10.1002/wnan.1460
Parhi, P., Mohanty, C., and Sahoo, S. K. (2012). Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov. Today 17, 1044–1052. doi: 10.1016/j.drudis.2012.05.010
Pascal, J., Ashley, C. E., Wang, Z., Brocato, T. A., Butner, J. D., Carnes, E. C., et al. (2013a). Mechanistic modeling identifies drug-uptake history as predictor of tumor drug resistance and nano-carrier-mediated response. ACS Nano 7, 11174–11182. doi: 10.1021/nn4048974
Pascal, J., Bearer, E. L., Wang, Z., Koay, E. J., Curley, S. A., and Cristini, V. (2013b). Mechanistic patient-specific predictive correlation of tumor drug response with microenvironment and perfusion measurements. Proc. Natl. Acad. Sci. U.S.A. 110, 14266–14271. doi: 10.1073/pnas.1300619110
Peng, F., Liu, Y., He, C., Kong, Y., Ouyang, Q., Xie, X., et al. (2018). Prediction of platinum-based chemotherapy efficacy in lung cancer based on LC-MS metabolomics approach. J. Pharm. Biomed. Anal. 154, 95–101. doi: 10.1016/j.jpba.2018.02.051
Pezzella, F., Pastorino, U., Tagliabue, E., Andreola, S., Sozzi, G., Gasparini, G., et al. (1997). Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am. J. Pathol. 151, 1417–1423.
Poh, A. R., and Ernst, M. (2018). Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 8:49.
Pollard, J. W. (2008). Macrophages define the invasive microenvironment in breast cancer. J. Leukoc Biol. 84, 623–630. doi: 10.1189/jlb.1107762
Ponta, A., and Bae, Y. (2014). Tumor-preferential sustained drug release enhances antitumor activity of block copolymer micelles. J. Drug Target 22, 619–628. doi: 10.3109/1061186x.2014.910793
Ponta, A., Fugit, K. D., Anderson, B. D., and Bae, Y. (2015). Release, partitioning, and conjugation stability of doxorubicin in polymer micelles determined by mechanistic modeling. Pharm. Res. 32, 1752–1763. doi: 10.1007/s11095-014-1573-2
Ponzoni, M., Pastorino, F., Di Paolo, D., Perri, P., and Brignole, C. (2018). Targeting macrophages as a potential therapeutic intervention: impact on inflammatory diseases and cancer. Int. J. Mol. Sci. 19:1953. doi: 10.3390/ijms19071953
Prabhakar, U., Maeda, H., Jain, R. K., Sevick-Muraca, E. M., Zamboni, W., Farokhzad, O. C., et al. (2013). Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 73, 2412–2417. doi: 10.1158/0008-5472.can-12-4561
Pyonteck, S. M., Akkari, L., Schuhmacher, A. J., Bowman, R. L., Sevenich, L., Quail, D. F., et al. (2013). CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272. doi: 10.1038/nm.3337
Raghavan, S., Mehta, P., Horst, E. N., Ward, M. R., Rowley, K. R., and Mehta, G. (2016). Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget 7, 16948–16961. doi: 10.18632/oncotarget.7659
Raghavan, S., Mehta, P., Ward, M. R., Bregenzer, M. E., Fleck, E. M. A., Tan, L., et al. (2017). Personalized medicine-based approach to model patterns of chemoresistance and tumor recurrence using ovarian cancer stem cell spheroids. Clin Cancer Res 23, 6934–6945. doi: 10.1158/1078-0432.ccr-17-0133
Raghavan, S., Mehta, P., Xie, Y., Lei, Y. L., and Mehta, G. (2019). Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J. Immunother. Cancer 7:190.
Raghavan, S., Ward, M. R., Rowley, K. R., Wold, R. M., Takayama, S., Buckanovich, R. J., et al. (2015). Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays. Gynecol. Oncol. 138, 181–189. doi: 10.1016/j.ygyno.2015.04.014
Rani, H. P., Sheu, T. W., Chang, T. M., and Liang, P. C. (2006). Numerical investigation of non-Newtonian microcirculatory blood flow in hepatic lobule. J. Biomech. 39, 551–563. doi: 10.1016/j.jbiomech.2004.11.029
Reichel, D., Curtis, L. T., Ehlman, E., Evers, B. M., Rychahou, P., Frieboes, H. B., et al. (2017). Development of halofluorochromic polymer nanoassemblies for the potential detection of liver metastatic colorectal cancer tumors using experimental and computational approaches. Pharm. Res. 34, 2385–2402. doi: 10.1007/s11095-017-2245-9
Reichel, D., Rychahou, P., and Bae, Y. (2015). Polymer nanoassemblies with solvato- and halo-fluorochromic properties for real-time monitoring of drug release and pH-enhanced ex vivo imaging of metastatic tumors. Ther. Deliv. 6, 1221–1237. doi: 10.4155/tde.15.59
Reichel, D., Tripathi, M., and Perez, J. M. (2019). Biological effects of nanoparticles on macrophage polarization in the tumor microenvironment. Nanotheranostics 3, 66–88. doi: 10.7150/ntno.30052
Rejniak, K. A., and Anderson, A. R. (2011). Hybrid models of tumor growth. Wiley Interdiscip. Rev. Syst. Biol. Med. 3, 115–125. doi: 10.1002/wsbm.102
Rejniak, K. A., and McCawley, L. J. (2010). Current trends in mathematical modeling of tumor-microenvironment interactions: a survey of tools and applications. Exp. Biol. Med. 235, 411–423.
Ren, S., Shao, Y., Zhao, X., Hong, C. S., Wang, F., Lu, X., et al. (2016). Integration of metabolomics and transcriptomics reveals major metabolic pathways and potential biomarker involved in prostate cancer. Mol. Cell Proteomics 15, 154–163. doi: 10.1074/mcp.m115.052381
Ribba, B., Saut, O., Colin, T., Bresch, D., Grenier, E., and Boissel, J. P. (2006). A multiscale mathematical model of avascular tumor growth to investigate the therapeutic benefit of anti-invasive agents. J. Theor. Biol. 243, 532–541. doi: 10.1016/j.jtbi.2006.07.013
Ricken, T., Werner, D., Holzhutter, H. G., Konig, M., Dahmen, U., and Dirsch, O. (2015). Modeling function-perfusion behavior in liver lobules including tissue, blood, glucose, lactate and glycogen by use of a coupled two-scale PDE-ODE approach. Biomech. Model. Mechanobiol. 14, 515–536. doi: 10.1007/s10237-014-0619-z
Roeder, I., and Glauche, I. (2008). Pathogenesis, treatment effects, and resistance dynamics in chronic myeloid leukemia–insights from mathematical model analyses. J. Mol. Med. 86, 17–27. doi: 10.1007/s00109-007-0241-y
Roy, M., and Finley, S. D. (2017). Computational model predicts the effects of targeting cellular metabolism in pancreatic cancer. Front. Physiol. 8:217.
Ruffell, B., Affara, N. I., and Coussens, L. M. (2012). Differential macrophage programming in the tumor microenvironment. Trends Immunol. 33, 119–126. doi: 10.1016/j.it.2011.12.001
Ruffell, B., and Coussens, L. M. (2015). Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–472. doi: 10.1016/j.ccell.2015.02.015
Rummeny, E. J., and Marchal, G. (1997). Liver imaging. Clinical applications and future perspectives. Acta Radiol. 38, 626–630. doi: 10.1080/02841859709172392
Sachs, N., and Clevers, H. (2014). Organoid cultures for the analysis of cancer phenotypes. Curr. Opin. Genet. Dev. 24, 68–73. doi: 10.1016/j.gde.2013.11.012
Sanga, S., Frieboes, H. B., Sinek, J. P., and Cristini, V. A. (2007). “Multiscale Approach for Computational Modeling of Biobarriers to Cancer Chemotherapy via Nanotechnology,” in Cancer Nanotechnology: Nanomaterials for Cancer Diagnosis And Therapy, eds H. S. Nalwa and T. J. Webster (Valencia, California: American Scientific), 1–21.
Santo, V. E., Rebelo, S. P., Estrada, M. F., Alves, P. M., Boghaert, E., and Brito, C. (2017). Drug screening in 3D in vitro tumor models: overcoming current pitfalls of efficacy read-outs. Biotechnol. J. 12:1600505. doi: 10.1002/biot.201600505
Sarode, P., Schaefer, M. B., Grimminger, F., Seeger, W., and Savai, R. (2020). Macrophage and tumor cell cross-talk is fundamental for lung tumor progression: we need to talk. Front. Oncol. 10:324.
Sato, T., Stange, D. E., Ferrante, M., Vries, R. G., Van Es, J. H., Van den Brink, S., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772. doi: 10.1053/j.gastro.2011.07.050
Schliess, F., Hoehme, S., Henkel, S. G., Ghallab, A., Driesch, D., Bottger, J., et al. (2014). Integrated metabolic spatial-temporal model for the prediction of ammonia detoxification during liver damage and regeneration. Hepatology 60, 2040–2051. doi: 10.1002/hep.27136
Schwen, L. O., Schenk, A., Kreutz, C., Timmer, J., Bartolome Rodriguez, M. M., Kuepfer, L., et al. (2015). Representative sinusoids for hepatic four-scale pharmacokinetics simulations. PLoS One 10:e0133653. doi: 10.1371/journal.pone.0133653
Siggers, J. H., Leungchavaphongse, K., Ho, C. H., and Repetto, R. (2014). Mathematical model of blood and interstitial flow and lymph production in the liver. Biomech. Model Mechanobiol. 13, 363–378. doi: 10.1007/s10237-013-0516-x
Sinek, J. P., Frieboes, H. B., Sivaraman, B., Sanga, S., and Cristini, V. (2007). Mathematical and Computational Modeling: Towards the Development and Application of Nanodevices for Drug Delivery. Nanotechnologies for the Life Sciences. Hoboken, NJ: Wiley-VCH Verlag GmbH & Co. KGaA.
Sinek, J. P., Sanga, S., Zheng, X., Frieboes, H. B., Ferrari, M., and Cristini, V. (2009). Predicting drug pharmacokinetics and effect in vascularized tumors using computer simulation. J. Math. Biol. 58, 485–510. doi: 10.1007/s00285-008-0214-y
Sluka, J. P., Fu, X., Swat, M., Belmonte, J. M., Cosmanescu, A., Clendenon, S. G., et al. (2016). A Liver-Centric Multiscale Modeling Framework for Xenobiotics. PLoS One 11:e0162428. doi: 10.1371/journal.pone.0162428
Srinivasan, S., Alexander, J. F., Driessen, W. H., Leonard, F., Ye, H., Liu, X., et al. (2013). Bacteriophage associated silicon particles: design and characterization of a novel theranostic vector with improved payload carrying potential. J. Mater. Chem. B Mater. Biol. Med. 1:10.1039/C3TB20595A.
Stamatakos, G. S., Antipas, V. P., and Uzunoglu, N. K. (2006). A spatiotemporal, patient individualized simulation model of solid tumor response to chemotherapy in vivo: the paradigm of glioblastoma multiforme treated by temozolomide. IEEE Trans. Biomed. Eng. 53, 1467–1477. doi: 10.1109/tbme.2006.873761
Stessels, F., Van Den Eynden, G., Van Der Auwera, I., Salgado, R., Van Den Heuvel, E., Harris, A. L., et al. (2004). Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br. J. Cancer 90, 1429–1436. doi: 10.1038/sj.bjc.6601727
Stock, K., Estrada, M. F., Vidic, S., Gjerde, K., Rudisch, A., Santo, V. E., et al. (2016). Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery. Sci. Rep. 6:28951.
Su, G., Burant, C. F., Beecher, C. W., Athey, B. D., and Meng, F. (2011). Integrated metabolome and transcriptome analysis of the NCI60 dataset. BMC Bioinformatics 12(Suppl. 1):S36.
Sun, D., Liu, Y., Wang, H., Deng, F., Zhang, Y., Zhao, S., et al. (2018). Novel decellularized liver matrix-alginate hybrid gel beads for the 3D culture of hepatocellular carcinoma cells. Int. J. Biol. Macromol. 109, 1154–1163. doi: 10.1016/j.ijbiomac.2017.11.103
Swanson, K. R., Rockne, R. C., Claridge, J., Chaplain, M. A., Alvord, E. C. Jr., and Anderson, A. R. (2011). Quantifying the role of angiogenesis in malignant progression of gliomas: in silico modeling integrates imaging and histology. Cancer Res. 71, 7366–7375. doi: 10.1158/0008-5472.can-11-1399
Tanaka, T., Godin, B., Bhavane, R., Nieves-Alicea, R., Gu, J., Liu, X., et al. (2010). In vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administrations in mice. Int. J. Pharm. 402, 190–197. doi: 10.1016/j.ijpharm.2010.09.015
Tanei, T., Leonard, F., Liu, X., Alexander, J. F., Saito, Y., Ferrari, M., et al. (2016). Redirecting transport of nanoparticle albumin-bound paclitaxel to macrophages enhances therapeutic efficacy against liver metastases. Cancer Res. 76, 429–439. doi: 10.1158/0008-5472.can-15-1576
Tariq, M., Zhang, J., Liang, G., Ding, L., He, Q., and Yang, B. (2017). Macrophage polarization: anti-cancer strategies to target tumor-associated macrophage in breast cancer. J. Cell Biochem. 118, 2484–2501. doi: 10.1002/jcb.25895
Tasciotti, E., Godin, B., Martinez, J. O., Chiappini, C., Bhavane, R., Liu, X., et al. (2011). Near-infrared imaging method for the in vivo assessment of the biodistribution of nanoporous silicon particles. Mol. Imaging 10, 56–68.
Tian, Y., Wang, Z., Liu, X., Duan, J., Feng, G., Yin, Y., et al. (2018). Prediction of chemotherapeutic efficacy in non-small cell lung cancer by serum metabolomic profiling. Clin. Cancer Res. 24, 2100–2109. doi: 10.1158/1078-0432.ccr-17-2855
Unger, C., Kramer, N., Walzl, A., Scherzer, M., Hengstschlager, M., and Dolznig, H. (2014). Modeling human carcinomas: physiologically relevant 3D models to improve anti-cancer drug development. Adv. Drug Deliv. Rev. 79-80, 50–67. doi: 10.1016/j.addr.2014.10.015
Untch, M., Jackisch, C., Schneeweiss, A., Schmatloch, S., Aktas, B., Denkert, C., et al. (2019). NAB-Paclitaxel Improves Disease-Free Survival in Early Breast Cancer: GBG 69-GeparSepto. J. Clin. Oncol. 37, 2226–2234. doi: 10.1200/jco.18.01842
van de Ven, A. L., Abdollahi, B., Martinez, C. J., Burey, L. A., Landis, M. D., Chang, J. C., et al. (2013). Modeling of nanotherapeutics delivery based on tumor perfusion. New J. Phys. 15:55004.
van de Ven, A. L., Wu, M., Lowengrub, J., McDougall, S. R., Chaplain, M. A., Cristini, V., et al. (2012). Integrated intravital microscopy and mathematical modeling to optimize nanotherapeutics delivery to tumors. AIP Adv. 2:11208.
van den Eynden, G. G., Majeed, A. W., Illemann, M., Vermeulen, P. B., Bird, N. C., Høyer-Hansen, G., et al. (2013). The multifaceted role of the microenvironment in liver metastasis: Biology and clinical implications. Cancer Res. 73, 2031–2043. doi: 10.1158/0008-5472.can-12-3931
Van Liedekerke, P., Neitsch, J., Johann, T., Warmt, E., Gonzalez-Valverde, I., Hoehme, S., et al. (2020). A quantitative high-resolution computational mechanics cell model for growing and regenerating tissues. Biomech. Model Mechanobiol. 19, 189–220. doi: 10.1007/s10237-019-01204-7
Venditto, V. J., and Szoka, F. C. (2013). Cancer nanomedicines: so many papers and so few drugs! Adv. Drug Del. Rev. 65, 80–88. doi: 10.1016/j.addr.2012.09.038
Vicent, M. J., Ringsdorf, H., and Duncan, R. (2009). Polymer therapeutics: Clinical applications and challenges for development. Adv. Drug Deliv. Rev. 61, 1117–1120. doi: 10.1016/j.addr.2009.08.001
Vineis, P., Schatzkin, A., and Potter, J. D. (2010). Models of carcinogenesis: an overview. Carcinogenesis 31, 1703–1709. doi: 10.1093/carcin/bgq087
Wang, Y., Wu, D., Wu, G., Wu, J., Lu, S., Lo, J., et al. (2020). Metastasis-on-a-chip mimicking the progression of kidney cancer in the liver for predicting treatment efficacy. Theranostics 10, 300–311. doi: 10.7150/thno.38736
Wang, Z., Butner, J. D., Cristini, V., and Deisboeck, T. S. (2015). Integrated PK-PD and agent-based modeling in oncology. J. Pharmacokinet. Pharmacodyn. 42, 179–189. doi: 10.1007/s10928-015-9403-7
Wang, Z., and Deisboeck, T. S. (2014). Mathematical modeling in cancer drug discovery. Drug Discov. Today 19, 145–150. doi: 10.1016/j.drudis.2013.06.015
Wang, Z., Kerketta, R., Chuang, Y. L., Dogra, P., Butner, J. D., Brocato, T. A., et al. (2016). Theory and experimental validation of a spatio-temporal model of chemotherapy transport to enhance tumor cell kill. PLoS Comput. Biol. 12:e1004969. doi: 10.1371/journal.pcbi.1004969
Ware, M. J., Colbert, K., Keshishian, V., Ho, J., Corr, S. J., Curley, S. A., et al. (2016a). Generation of Homogenous Three-Dimensional Pancreatic Cancer Cell Spheroids Using an Improved Hanging Drop Technique. Tissue Eng. Part C Methods 22, 312–321. doi: 10.1089/ten.tec.2015.0280
Ware, M. J., Keshishian, V., Law, J. J., Ho, J. C., Favela, C. A., Rees, P., et al. (2016b). Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials 108, 129–142. doi: 10.1016/j.biomaterials.2016.08.041
White, D., Coombe, D., Rezania, V., and Tuszynski, J. (2016). Building a 3D virtual liver: methods for simulating blood flow and hepatic clearance on 3d structures. PLoS One 11:e0162215. doi: 10.1371/journal.pone.0162215
Williams, C. B., Yeh, E. S., and Soloff, A. C. (2016). Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast. Cancer 2:15025.
Wong, C. H., Siah, K. W., and Lo, A. W. (2019). Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286. doi: 10.1093/biostatistics/kxx069
Wu, M., Frieboes, H. B., Chaplain, M. A., McDougall, S. R., Cristini, V., and Lowengrub, J. S. (2014). The effect of interstitial pressure on therapeutic agent transport: coupling with the tumor blood and lymphatic vascular systems. J. Theor. Biol. 355, 194–207. doi: 10.1016/j.jtbi.2014.04.012
Yamamoto, K. N., Yachida, S., Nakamura, A., Niida, A., Oshima, M., De, S., et al. (2017). Personalized management of pancreatic ductal adenocarcinoma patients through computational modeling. Cancer Res. 77, 3325–3335. doi: 10.1158/0008-5472.can-16-1208
Yokoi, K., Godin, B., Oborn, C. J., Alexander, J. F., Liu, X., Fidler, I. J., et al. (2013). Porous silicon nanocarriers for dual targeting tumor associated endothelial cells and macrophages in stroma of orthotopic human pancreatic cancers. Cancer Lett. 334, 319–327. doi: 10.1016/j.canlet.2012.09.001
Yuan, X., Zhang, J., Li, D., Mao, Y., Mo, F., Du, W., et al. (2017). Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 147, 181–187. doi: 10.1016/j.ygyno.2017.07.007
Zamboni, W. C., Torchilin, V., Patri, A. K., Hrkach, J., Stern, S., Lee, R., et al. (2012). Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin. Cancer Res. 18, 3229–3241. doi: 10.1158/1078-0432.ccr-11-2938
Zempolich, K. A., and Dubeau, L. (1998). Telomerase and cancer management: silver bullet or fool’s gold? Gynecol. Oncol. 68, 143–144. doi: 10.1006/gyno.1998.4948
Zhang, G., He, P., Tan, H., Budhu, A., Gaedcke, J., Ghadimi, B. M., et al. (2013). Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effects in human pancreatic cancer. Clin. Cancer Res. 19, 4983–4993. doi: 10.1158/1078-0432.ccr-13-0209
Zhang, M., He, Y., Sun, X., Li, Q., Wang, W., Zhao, A., et al. (2014). A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 7:19. doi: 10.1186/1757-2215-7-19
Zhang, P., and Brusic, V. (2014). Mathematical modeling for novel cancer drug discovery and development. Expert. Opin. Drug Discov. 9, 1133–1150. doi: 10.1517/17460441.2014.941351
Zhang, Y., Huang, W. J., Yang, Q. R., Zhang, H. D., Zhu, X. J., Zeng, M., et al. (2020). Cryopreserved biopsy tissues of rectal cancer liver metastasis for assessment of anticancer drug response in vitro and in vivo. Oncol. Rep. 43, 405–414.
Zhang, Y. S., Duchamp, M., Oklu, R., Ellisen, L. W., Langer, R., and Khademhosseini, A. (2016). Bioprinting the Cancer Microenvironment. ACS Biomater. Sci. Eng. 2, 1710–1721. doi: 10.1021/acsbiomaterials.6b00246
Zhao, Y., Yao, R., Ouyang, L., Ding, H., Zhang, T., Zhang, K., et al. (2014). Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6:035001. doi: 10.1088/1758-5082/6/3/035001
Zhou, X., Liu, C., Zhao, X., and Wang, X. (2016). A 3D bioprinting liver tumor model for drug screening. World J. Pharm. Pharm. Sci. 5, 196–213.
Zinger, A., Koren, L., Adir, O., Poley, M., Alyan, M., Yaari, Z., et al. (2019). Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS nano 13, 11008–11021. doi: 10.1021/acsnano.9b02395
Keywords: liver metastasis, nanotherapy, tumor microenvironment, macrophages, mathematical modeling, computational simulation
Citation: Frieboes HB, Raghavan S and Godin B (2020) Modeling of Nanotherapy Response as a Function of the Tumor Microenvironment: Focus on Liver Metastasis. Front. Bioeng. Biotechnol. 8:1011. doi: 10.3389/fbioe.2020.01011
Received: 16 June 2020; Accepted: 03 August 2020;
Published: 19 August 2020.
Edited by:
Paola Lecca, Free University of Bozen-Bolzano, ItalyReviewed by:
Katarzyna Anna Rejniak, Moffitt Cancer Center, United StatesCopyright © 2020 Frieboes, Raghavan and Godin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biana Godin, QkdvZGluQGhvdXN0b25tZXRob2Rpc3Qub3Jn; YmlhbmFnb2RpbnZAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.