- 1Department of Pharmacy, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Key Laboratory of Biomedical Information Engineering of the Ministry of Education, School of Life Science and Technology, Xi'an Jiaotong University, Xi'an, China
- 3Interventional Department, The First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou, China
- 4Department of Technology, Stem Cell Medicine Engineering & Technology Research Center of Inner Mongolia, Huhhot, China
- 5Department of Blood, The People's Hospital of Xing'an League, Ulanhot, China
The usage of animal serum may ultimately prevent the application of ex vivo cultured mesenchymal stromal cells (MSCs) in a clinical setting due to safety concerns and batch-to-batch variability. Increasing regulatory pressure to limit use of animal serum has been issued and serum-free, xeno-free, and chemically defined media (S&XFM-CD) is encouraged to replace serum-containing media (SCM) in the stem cell preparation process. We previously developed a S&XFM-CD for the expansion of umbilical cord-derived MSCs (UCMSCs). Different culture conditions affect the function of MSCs, which may further affect the therapeutic efficiency and mechanisms of action. In this study, we compared the therapeutic effect and mechanism of UCMSCs in S&XFM-CD (UCMSCS&XFM−CD) in experimental colitis with those in SCM (UCMSCSCM). UCMSCS&XFM−CD exhibited better therapeutic effects than UCMSCSCM by body weight, disease activity index, and histological colitis score. UCMSCS&XFM−CD or UCMSCSCM migrated to the inflammation site of injured colon, but exhibited low levels of recruitment and persistence. Systemic depletion of endogenous macrophages impaired the therapeutic effects of UCMSCSCM and UCMSCS&XFM−CD. Furthermore, UCMSCS&XFM−CD more markedly promoted intestinal macrophage polarisation from M1 to M2 phenotype to produce higher levels of IL-10 and lower levels of TNF-α in colon tissue than UCMSCSCM, while a higher level of IL-4 was produced in UCMSCSCM-treated group. UCMSCS&XFM−CD cocultured with RAW264.7 cells in a transwell system promoted the release of TSG-6 and IL-6, whereas UCMSCSCM increased PGE2 levels. Taken together, we demonstrated that UCMSCs in S&XFM-CD exhibited improved therapeutic effects with altered cytokine secretion in an experimental acute colitis model.
Introduction
Inflammatory bowel disease (IBD) is a group of intestinal non-specific inflammatory diseases that mainly includes ulcerative colitis and Crohn's disease. Traditional methods and drugs for IBD treatment frequently cause serious side effects and promote treatment resistance. Therefore, exploring alternative treatment options is urgently required in the clinic (Verstockt et al., 2018). Mesenchymal stromal cells (MSCs) have demonstrated great potential as a feasible and effective strategy in experimental models of IBD (Conklin et al., 2017; Ciccocioppo and Corazza, 2018). However, several preclinical studies have shown that only a low percentage of implanted MSCs can home to the injured tissue and survive in vivo, suggesting that the therapeutic action is unlikely to be due to replacement of diseased tissue (Wang et al., 2016; Lopez-Santalla et al., 2017). Indeed, we (Ma et al., 2019b) and others (Barnhoorn et al., 2018; Markovic et al., 2018) have demonstrated that the immunosuppressive characteristics of MSCs provide the theoretical grounds for MSCs therapy in experimental IBD models. Recently, MSCs have also been reported to recruit macrophages to alleviate experimental colitis (Liu et al., 2015). Further research shows that the administration of MSCs ameliorates colitis by decreasing the number of total and M1 macrophages (Park et al., 2018) or increasing the percentage of M2 macrophages in the colon (Song et al., 2017b).
Despite an increasing number of studies showing the benefit of MSCs in preclinical IBD models (Markovic et al., 2018), MSCs involved in these studies are cultured in a medium supplemented with foetal bovine serum (FBS), which has predominantly been used for clinical-grade manufacturing of MSCs (Phinney et al., 2019). FBS is an animal-derived product and is associated with several problematic issues. For example, FBS bears serious safety concerns of transmitting unknown viruses, mycoplasma, prions, or adventitious zoonotic agents. It has been reported that 20–50% of FBS in the market is virus-positive (van der Valk et al., 2018). In addition, FBS could potentially induce undesirable immunologic reactions. Early studies have shown that MSCs grown in FBS-supplemented medium carry a certain amount of FBS proteins (7–30 mg/100 million MSCs) (Jeffrey et al., 2004), which potentially trigger undesirable immunologic reactions (Owens et al., 2016). Furthermore, the exact composition of FBS is unknown and some of these components may be harmful to MSCs growth and cause an unstable transcriptional profile in MSCs (Shahdadfar et al., 2005). Finally, FBS has seasonal and geographical lot-to-lot variability, which could ultimately lead to variability of MSC characteristics and limit the reproducibility of MSC products.
The usage of FBS may ultimately prevent the application of ex vivo cultured MSCs in a clinical setting. Increasing regulatory pressure to limit the use of FBS in cell culture products has been issued (van der Valk et al., 2018). According to the guidelines for quality control and preclinical studies of stem cell preparation in China, animal serum should be avoided as much as possible and serum-free, xeno-free, and chemically defined media (S&XFM-CD) is encouraged to replace serum-containing media (SCM) in the stem cell preparation process. Notably, some studies show that several commercially available S&XFM-CD allow for isolation and expansion of MSCs (Corotchi et al., 2013; Simoes et al., 2013; Devito et al., 2014; Swamynathan et al., 2014; Badraiq et al., 2015; Ma et al., 2019a). However, no studies have yet evaluated the effects of MSCs cultured in S&XFM-CD in ulcerative colitis. We have previously developed a S&XFM-CD for the culture of MSCs derived from umbilical cord (UCMSCs) that contains hormones, nutrients, minerals, and growth factors (see Patent No. CN. ZL201210350602.0 and Wu et al., 2016). Moreover, we further confirmed the immunosuppressive effect of UCMSCs in S&XFM-CD on experimental colitis (Ma et al., 2019b). Growing evidence supports that different culture conditions affect the function of cells (Liu et al., 2018; Yoshida et al., 2018; Kong et al., 2019), which may further affect the therapeutic efficiency and mechanisms of action. We reason that S&XFM-CD might impact the therapeutic mechanisms and effects of UCMSCs on IBD. Thus, our study aimed to assess the therapeutic efficacy of UCMSCs in S&XFM-CD in the treatment of IBD and examine its therapeutic mechanisms.
Materials and Methods
Ethics Statement
This study was approved by the Ethics Committee of the Beijing Friendship Hospital affiliated with the Capital Medical University (authorization no. 17-2031). All protocols for collecting and processing human umbilical cord samples were approved by the Ethics Committee of Beijing Friendship Hospital affiliated with the Capital Medical University (authorization no. 2017-P2-179-02) with informed maternal consent.
Preparation of UCMSCs
Umbilical cord samples were collected from healthy full-term pregnant women (age range: 23–31 years, mean: 26 years). UCMSCs were isolated and cultured as described previously (Mu et al., 2018). Briefly, the umbilical vessels were manually removed. Wharton's jelly was minced and digested with an enzyme cocktail at 37°C for 60 min. The digested mixture was passed through a 70 μM mesh and plated in S&XFM-CD or SCM (10% FBS-supplemented medium) at 37°C and 5% CO2. The formulation of S&XFM-CD including basal medium and xeno-free defined supplement was showed in Supplementary Table 1, and S&XFM-CD was prepared as described previously (Wu et al., 2016). UCMSCs were selected by adherence to plastic culture plates after 5 days, and passaged at a density of 3,000 cells/cm2 when reached 90% confluence. UCMSCs from seven independent donors (n = 7) at passage 5 were used for the subsequent experiments in this study.
Colitis Induction and Treatment
Acute colitis was induced in male C57BL/6 mice aged 6–8 weeks with 2.5% dextran sulphate sodium (DSS, MP Biochemicals, China) in drinking water for 7 consecutive days (Fuenzalida et al., 2016) unless the application of humane endpoint (severe bleeding) was needed. We intraperitoneally injected 1 × 106 UCMSCs in S&XFM-CD or SCM (UCMSCS&XFM−CD and UCMSCSCM, respectively) in 100 μL phosphate buffer saline (PBS) into each mouse and monitored their body weight daily. Mice receiving DSS-free water were used as controls (naive). Each experiment was repeated with UCMSCs obtained from different donors, and seven mice were analysed in each experimental group. The disease activity index (DAI) was calculated by combined assessment of weight loss, stool consistency, and bleeding severity (Supplementary Table 2). At the indicated time points, mice were sacrificed and the colon was collected. The entire colon was surgically separated from the cecum to the anus and the colon length was measured.
UCMSCs Labelling and Imaging
UCMSCs were labelled with the fluorescent dye CM-Dil (Life Technologies, USA) according to the manufacturer's instructions before transplantation. Briefly, UCMSCS&XFM−CD or UCMSCSCM were incubated (37°C, 5 min; 4°C, 15 min) with 2 μg/mL CM-DiI, washed twice with PBS, and injected intraperitoneally into mice on day 0. Mice were sacrificed on day 3 and 10 and 5-μm-thick colon cryosections were made to investigate cell migration in vivo. The labelled UCMSCs were observed by fluorescence microscopy.
In vivo Depletion of Macrophages
Mice were fed by drinking water with 2.5% DSS for 7 consecutive days to establish the model as described above, and received 200 μL of dichloromethylene diphosphonate (Cl2MDP) liposomes (FormuMax Scientific, Northern California, USA) via intravenous injection once every three days (Hunter et al., 2010) and 24 h prior to and following intraperitoneally injection of UCMSCS&XFM−CD, UCMSCSCM, or PBS.
Histological Evaluation
The colon tissues were fixed in 4% paraformaldehyde, serially dehydrated, and embedded in paraffin. The 5-μm-thick sections were collected and stained with haematoxylin and eosin (H&E) for light microscopy. Histological score was calculated by 2 blinded trained pathologists with a combined evaluation of epithelial damage, loss of crypts, and infiltration of inflammatory cells (Supplementary Table 3).
Flow Cytometry
The colon tissues were digested with 0.1% collagenase type 1 and 0.05% trypsin (Sigma-Aldrich, USA) for 30 min at 37°C. The cell suspensions were passed through a 70 μm cell strainer, collected, and incubated with CD45-PE-cy5, F4/80-FITC, CD86-PE, and CD206-PE (Santa Cruz Biotechnology, USA). Then, the cells were washed and analysed using flow cytometry with a FACS Calibur (BD Biosciences, USA). The gate was set on the CD45+ population, and surface markers were further analysed in this gate using Flowjo program (Tree Star, Ashland, OR, USA).
MSCs/Macrophage Co-cultures
3.5 × 105 RAW264.7 cells (American Type Tissue Collection, Manassas, USA) were seeded in the upper chamber of the transwell insert, followed by lipopolysaccharide (LPS, 100 ng/mL) and interferon-γ (IFN-γ, 10 ng/mL) treatment for 12 h and then cocultured with 3.5 × 104 UCMSCSCM or UCMSCS&XFM−CD, which were seeded in the lower chamber for 24 h as described previously (Song et al., 2017b). UCMSCs from seven independent donors were used in this study (n = 7), and each sample is repeated three times.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from colon tissue, RAW264.7 cells, and UCMSCs using TRIzol reagent (Invitrogen, USA) and reversely transcribed to cDNA with the QuantiTect Reverse Transcription Kit (Qiagen, Germany). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using a Platinum SYBR Green PCR Mix (Invitrogen, USA) and a 7700 Sequence Detector (Applied Biosystems, USA). The PCR cycling conditions were 94°C for 3 min, 40 cycles at 94°C for 30 s, 62°C for 30 s, and 72°C for 30 s. The primers are shown in Supplementary Table 4. The mRNA expressions of each gene were analysed and normalised to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCt method.
Cytokine and Indoleamine 2,3-Dioxygenase Activity Assay
Secreted protein levels of prostaglandin E2 (PGE2), interleukin (IL)-6, and tumour necrosis factor-α-induced protein 6 (TSG-6) were determined in co-cultured supernatants using specific cytokine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, USA) as per manufacturer's instructions. Indoleamine 2,3-dioxygenase (IDO) activity, determined as kynurenine concentration, was detected using a spectrophotometric assay as described previously (Vasandan et al., 2016). Briefly, 30% trichloroacetic acid was added to the collected supernatants, and centrifuged at 8,000 g for 5 min. 85 μL of the supernatant was transferred to 96-well plates, and 85 μL of 1% Ehrlich reagent was added and incubated for 10 min. The absorbance was measured at 490 nm.
Statistical Analysis
The data were represented as mean ± standard deviation (SD). A Student's t-test was applied to calculate the differences between two groups while a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test was applied for comparison among multiple groups. P < 0.05 was considered statistically significant as indicated in each case (*indicates P < 0.05, **indicates P < 0.01 and ***indicates P < 0.001). Statistical calculations were performed with SPSS 17.0.
Results
UCMSCS&XFM-CD Was More Effective Than UCMSCSCM in Alleviating DSS-Induced Colitis
To assess the therapeutic effect of UCMSCS&XFM−CD and UCMSCSCM, acute colitis was induced by DSS. UCMSCS&XFM−CD or UCMSCSCM were injected intraperitoneally and mice were sacrificed on day 10 (Figure 1A). UCMSCS&XFM−CD exhibited a rapid recovery of weight loss on days 5–10 compared with PBS and more rapid recovery on days 9 and 10 compared with UCMSCSCM (Figure 1B). The administration of UCMSCSCM or UCMSCS&XFM−CD showed lower DAI scores on day 10 compared with PBS-treated mice and UCMSCS&XFM−CD administration maintained lower DAI scores compared with UCMSCSCM (Figure 1C). We next measured the colon length on day 10 and found that the colon lengths were significantly increased in UCMSCSCM- or UCMSCS&XFM−CD-treated groups compared with PBS treatment, but no significant differences were observed between UCMSCs-treated groups (Figure 1D). In addition, UCMSCS&XFM−CD was more effective in the amelioration of colon damage than UCMSCSCM as indicated by H&E staining and histopathological scoring (Figures 1E,F). These results suggest that both UCMSCSCM and UCMSCS&XFM−CD alleviated DSS-induced colitis, of which the latter was more effective.
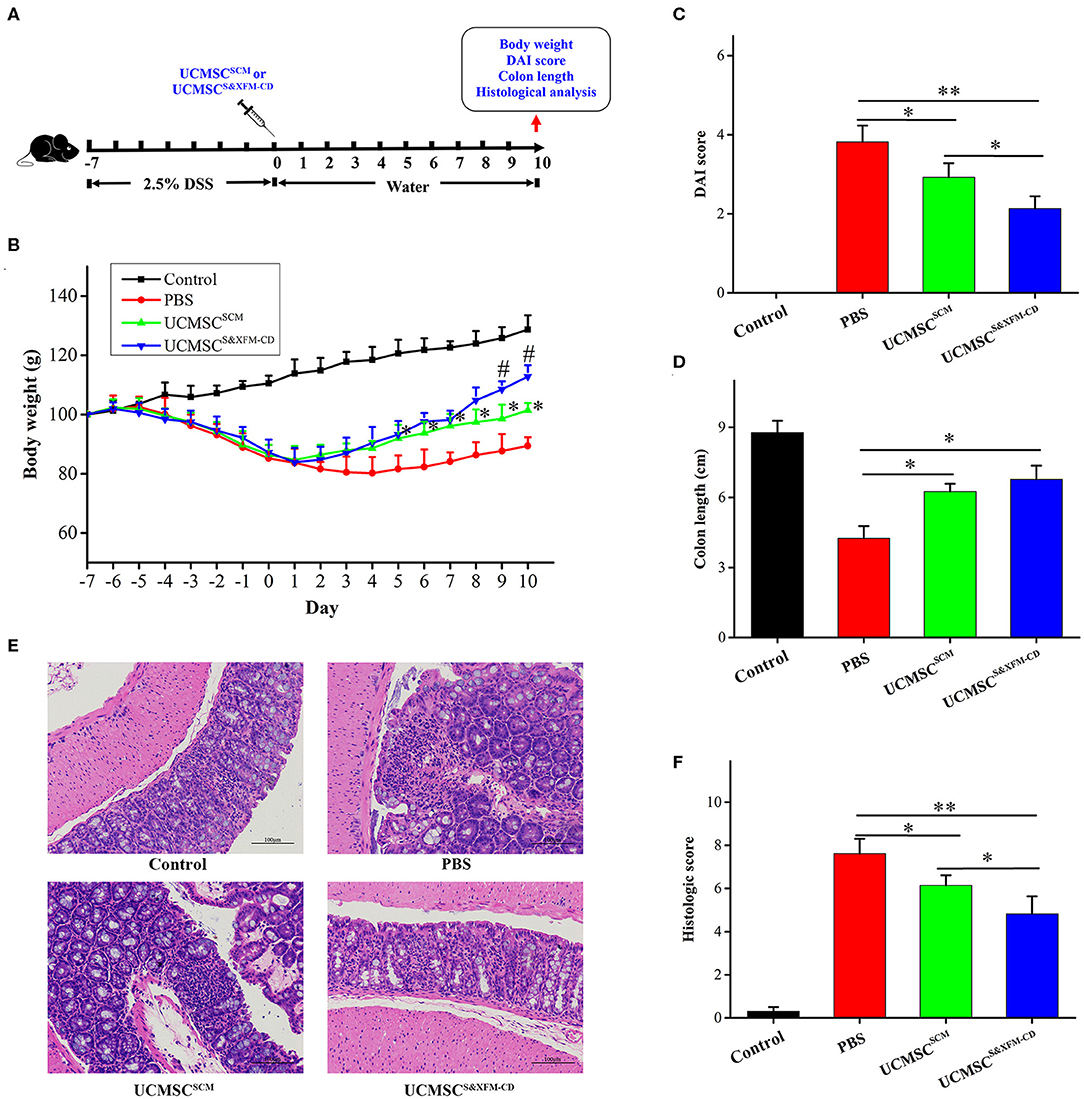
Figure 1. UCMSCS&XFM−CD exhibited better therapeutic effects in a mouse model of acute colitis than UCMSCSCM. (A) Experimental design of DSS-induced acute colitis. UCMSCS&XFM−CD or UCMSCSCM were injected intraperitoneally and mice were sacrificed on day 10. (B) Body weight loss over time (n = 7 mice/group, each mouse was transplanted with UCMSCs from a different donor). Significance was analysed using one-way ANOVA followed by Tukey's multiple comparisons test for multiple group comparisons. *p < 0.05 vs. PBS group, #P < 0.05 vs. UCMSCSCM. (C) DAI scores on day 10. (D) Disease-related shortening of the colon. (E) Representative images of H&E stain of colons on day 10. Scale bars = 100 μm. (F) Histological scores on day 10 (n = 5 mice/group, each mouse was transplanted with UCMSCs from a different donor). Significance was analysed using one-way ANOVA followed by Tukey's multiple comparisons test for multiple group comparisons. *p < 0.05 and **p < 0.01.
UCMSCS&XFM-CD and UCMSCSCM Exhibited Low Levels of Recruitment and Persistence in the Injured Site
To determine whether UCMSCS&XFM−CD or UCMSCSCM could migrate to the injured site of the colon with acute colitis, 1 × 106 UCMSCS&XFM−CD or UCMSCSCM labelled with CM-DiI were injected intraperitoneally and detected in the colon on days 3 and 10 (Figure 2A). As shown in Figure 2B, CM-Dil could be detected in the inflamed colon of acute colitis mice at 3 days post-cell transplantation, but no fluorescence was found in the colon of normal mice. Moreover, no fluorescence was detected in the colon of acute colitis or normal mice at day 10 (Figure 2C). These results suggest that intraperitoneally injected UCMSCS&XFM−CD or UCMSCSCM could migrate to the inflammation site of the injured colon, but both UCMSCs exhibited low levels of recruitment and persistence.
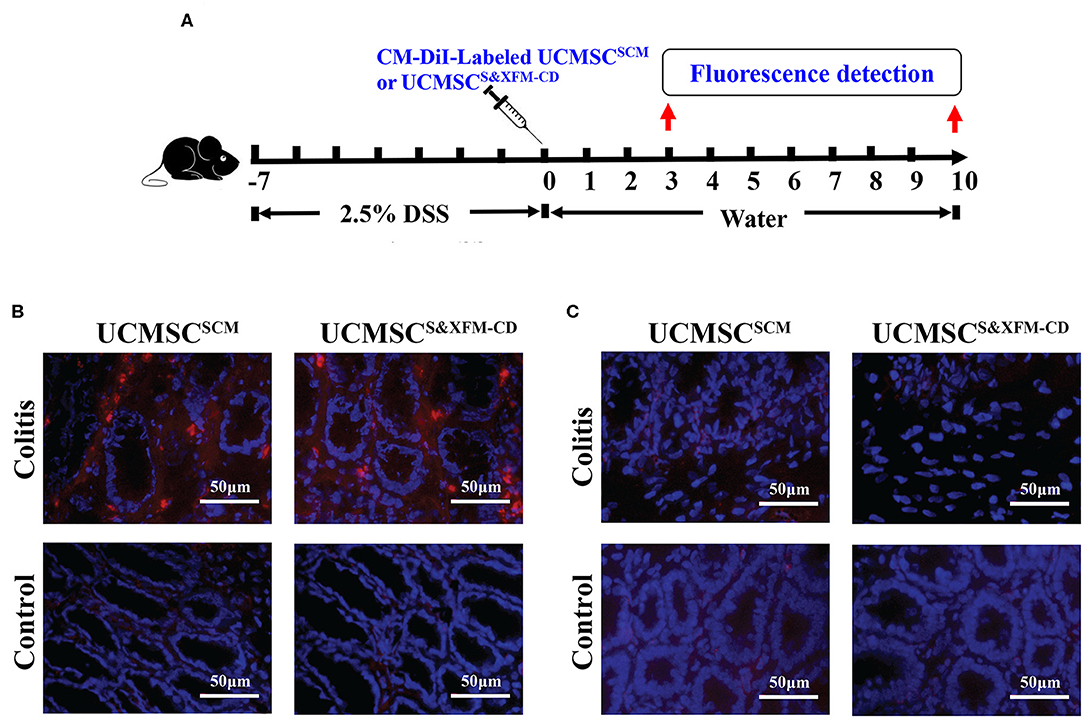
Figure 2. UCMSCS&XFM−CD and UCMSCSCM exhibited low levels of recruitment and persistence in the injured colon of acute colitis mouse model. (A) Experimental design for determination of UCMSCs migration to the injured site of the colon. UCMSCS&XFM−CD or UCMSCSCM labelled with CM-DiI were injected intraperitoneally and detected in the colon on days 3 and 10 acute colitis by fluorescence evaluation (n = 5 mice/group, each mouse was transplanted with UCMSCs from a different donor). Mice receiving DSS-free water (without colitis) were used as controls. (B) Representative images of colonic fluorescence on day 3. UCMSCS&XFM−CD or UCMSCSCM (red) migrated to the inflamed colon of colitis animals on day 3, but not in the non-inflamed colon of control mice. Nucleuses were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). (C) Representative images of colonic fluorescence on day 10. No labelled UCMSCS&XFM−CD or UCMSCSCM were observed in both colitis and control animals. Scale bars = 50 μm.
Depletion of Macrophages Impaired the Therapeutic Effects of UCMSCSCM and UCMSCS&XFM-CD
To investigate whether the macrophages were involved in the role of UCMSCs in different media, we intravenously injected Cl2MDP liposomes once every 3 days and 24 h prior to and following intraperitoneally injection of UCMSCS&XFM−CD or UCMSCSCM (Figure 3A). As expected, intravenous injection of Cl2MDP liposomes markedly reduced macrophage proportions in blood, spleen, and colon (Figures 3B,C). Cl2MDP itself did not aggravate colitis as we observed no significant difference in DAI and histopathological scoring between the PBS-treated group and the PBS and Cl2MDP-treated group (Figures 3D–F). However, additional Cl2MDP liposomes attenuated the benefits of UCMSCS&XFM−CD or UCMSCSCM (Figures 3D–F). Furthermore, UCMSCS&XFM−CD or UCMSCSCM showed no further therapeutic effect when administered in conjunction with Cl2MDP (Figures 3D–F). Collectively, our results suggest that depletion of macrophages impaired the benefits of UCMSCS&XFM−CD or UCMSCSCM, indicating that the therapeutic effects of MSCs depend on macrophages.
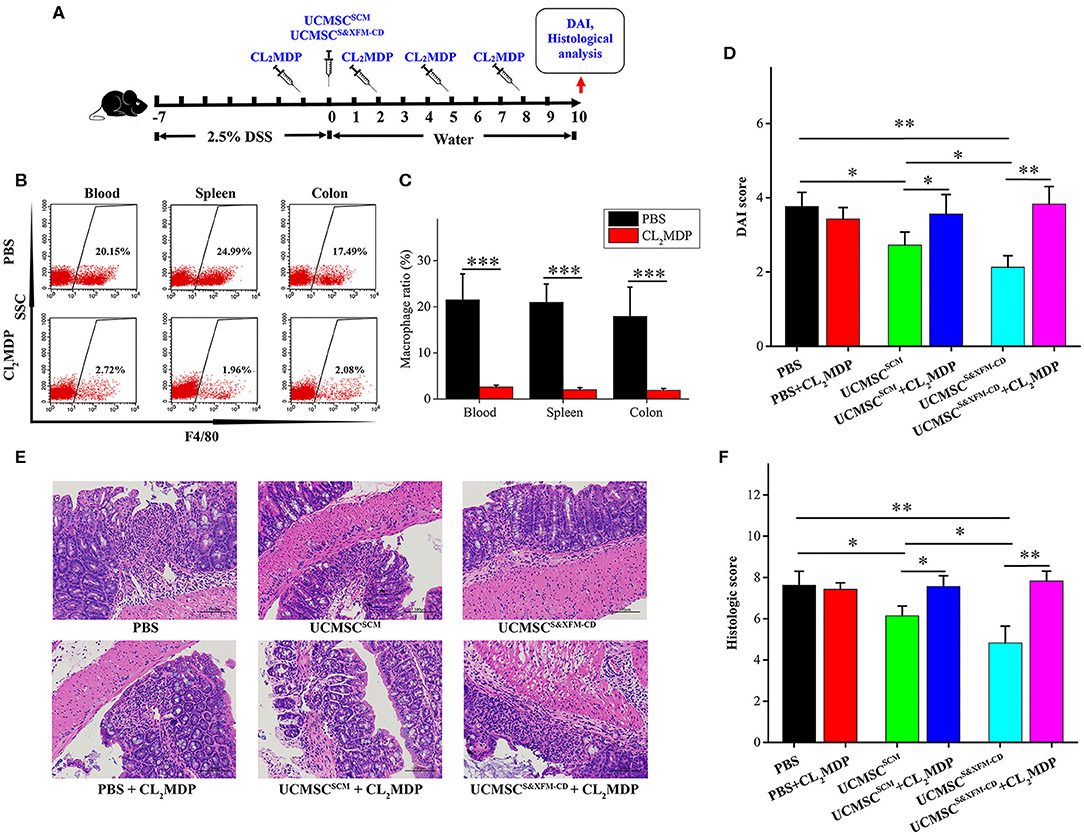
Figure 3. Systemic depletion of endogenous macrophages impaired the therapeutic effects of UCMSCSCM and UCMSCS&XFM−CD treatment. (A) Macrophage depletion protocol using Cl2MDP liposomes in DSS-induced acute colitis. UCMSCS&XFM−CD or UCMSCSCM were injected intraperitoneally and mice were sacrificed on day 10. (B) Representative flow cytometry plots and (C) proportions of F4/80+ macrophages in the spleen, blood, and colon from Cl2MDP- and PBS-treated animals on day 3 (n = 5 mice/group, each mouse was transplanted with UCMSCs from a different donor). Significance was analysed using Student's t-test for two group's comparison. ***P < 0.001. (D) DAI scores on day 10 (n = 5 mice/group, each mouse was transplanted with UCMSCs from a different donor). Significance was analysed using Student's t-test for two group's comparison. *p < 0.05 and **p < 0.01. (E) Representative images of H&E stain of colons. Scale bars = 100 μm. (F) Histological scores on day 10 (n = 5 mice/group, each mouse was transplanted with UCMSCs from a different donor). Significance was analysed using Student's t-test for two group's comparison. *p < 0.05 and **p < 0.01.
UCMSCS&XFM-CD Promoted More Intestinal Macrophage Polarization From M1 to M2 Phenotype Than UCMSCSCM in vivo
To further investigate whether administering UCMSCSCM or UCMSCS&XFM−CD affect the infiltration and polarization of macrophages in the injured area of the colon with acute colitis, 1 × 106 UCMSCS&XFM−CD or UCMSCSCM were injected intraperitoneally and the ratio of CD45+, F4/80+, CD86+, and CD206+ cells was analysed by flow cytometry and qRT-PCR on days 3 and 10 (Figure 4A). Flow cytometric analysis revealed that the proportion of F4/80+ macrophages was markedly increased on day 3 in PBS-treated colitis mice compared with normal mice, but was not changed after UCMSCSCM or UCMSCS&XFM−CD treatment (Figures 4B,C). However, further analysis showed that the proportion of CD86+ cells representing M1 macrophages was dramatically decreased on day 3 in UCMSCSCM-treated mice, which was further exacerbated by UCMSCS&XFM−CD treatment (Figures 4D,E). Conversely, the proportion of CD206+ cells representing M2 macrophages was significantly increased in UCMSCSCM-treated group and UCMSCS&XFM−CD treatment further promoted this effect (Figures 4F,G). Furthermore, qRT-PCR analysis showed that the expression of M1-related genes, such as tumour necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and inducible nitric oxide synthase (iNOS) in colon tissue were significantly reduced on day 3, whereas M2-related genes, such as IL-4, IL-10, and arginase-1 (Arg-1) were increased in UCMSCSCM- or UCMSCS&XFM−CD-treated groups. Similarly, UCMSCS&XFM−CD treated group showed higher IL-10 but lower IL-4 and TNF-α levels than UCMSCSCM (Figure 4H). We observed similar trends on day 10 (data not shown). Collectively, these data demonstrate that UCMSCS&XFM−CD markedly promoted intestinal macrophage polarisation from M1 to M2 phenotype to produce different inflammatory factors in colon tissue compared with UCMSCSCM.
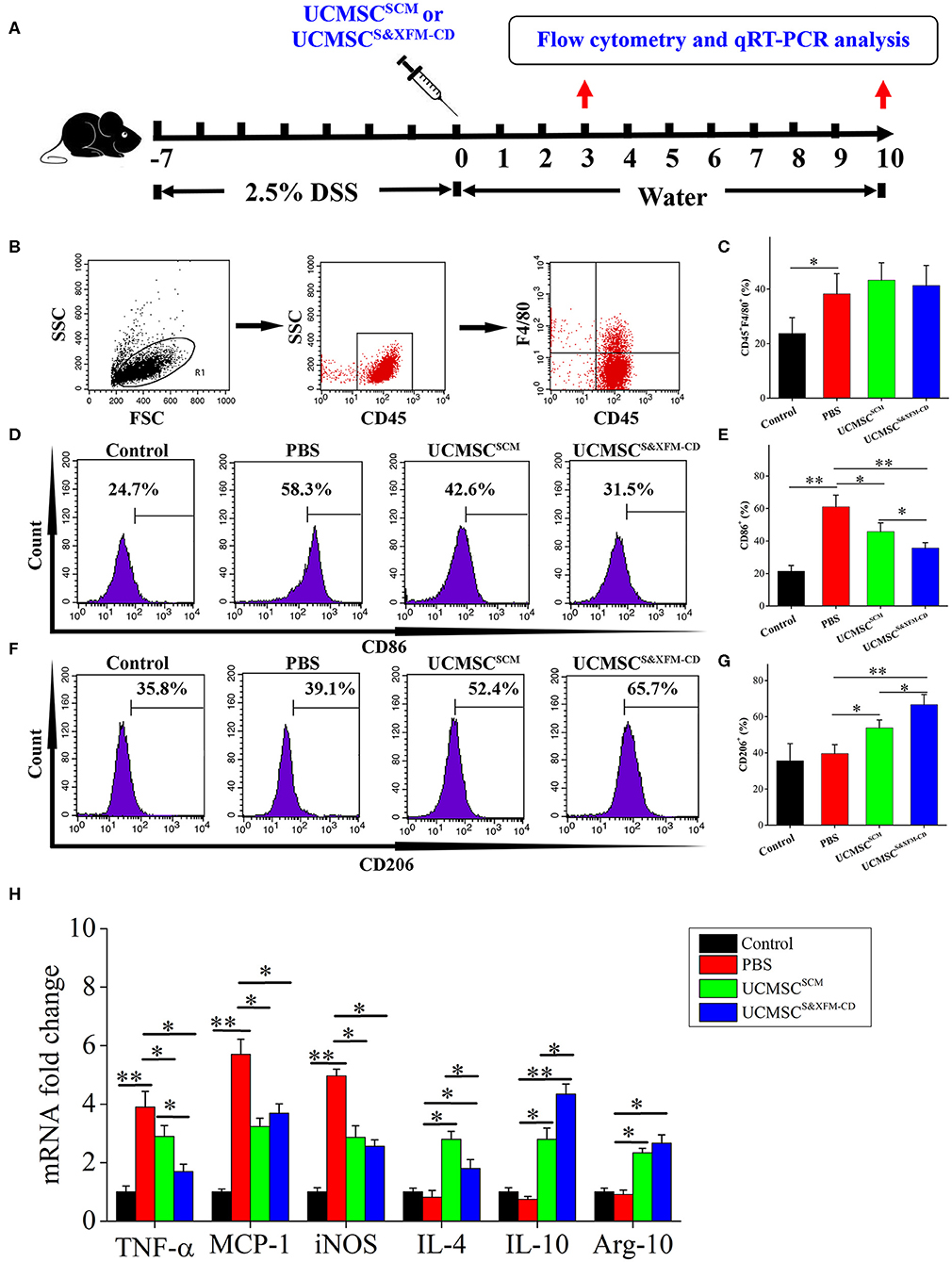
Figure 4. UCMSCS&XFM−CD more markedly promoted intestinal macrophage polarization from M1 to M2 phenotype than UCMSCSCM. (A) Experimental design for the infiltration and polarization of macrophages in the colon. Acute colitis was induced and UCMSCS&XFM−CD or UCMSCSCM were injected intraperitoneally and the ratio of CD45+, F4/80+, CD86+, and CD206+ cells was analysed by flow cytometry and qRT-PCR on days 3 and 10. (B) Colonic macrophage gating strategy. Proportions of (C) F4/80+, (D) CD86+ M1, (E) CD86+, (F) CD206+ M2, and (G) CD206+ macrophages in the colon on day 3 (n = 7 mice/group, each mouse was transplanted with UCMSCs from a different donor). (H) qRT-PCR analysis of gene expression in colon on day 3 (n = 7 mice/group, each mouse was transplanted with UCMSCs from a different donor). Significance was analysed using one-way ANOVA followed by Tukey's multiple comparisons test for multiple group comparisons. *p < 0.05 and **p < 0.01.
UCMSCSCM and UCMSCS&XFM-CD Polarised Macrophages From M1 to M2 Phenotype Through Different Cytokine Secretion in vitro
To further better understand the differences in the molecular mechanisms between UCMSCSCM and UCMSCS&XFM−CD on macrophage polarisation, we established a transwell-based coculture system (Figure 5A). UCMSCS&XFM−CD and UCMSCSCM significantly inhibited mRNA upregulation of the M1 marker CD86 and pro-inflammatory factors including TNF-α, MCP-1, and iNOS in the RAW264.7 cells (Figure 5B). Notably, the inhibitory effect of UCMSCS&XFM−CD on TNF-α expression was much greater than UCMSCSCM. Conversely, UCMSCS&XFM−CD and UCMSCSCM caused upregulation of the mRNA levels of the M2 markers CD206 and Arg-1 and anti-inflammatory factors, such as IL-4 and IL-10 (Figure 5B). Similarly, UCMSCS&XFM−CD was more capable of upregulating IL-10, but showed decreased IL-4 expression compared with UCMSCSCM (Figure 5B). We also investigated the expression profile of the UCMSCs and found that UCMSCSCM promoted M2 macrophage polarisation by increasing the mRNA expression of TSG-6, PGE2, IL-6, and IDO. Moreover, the mRNA expression levels of TSG-6 and IL-6 in UCMSCS&XFM−CD were significantly higher, although the level of PGE2 was significantly lower than that in UCMSCSCM (Figures 5C–E). In addition, IDO expression did not differ between the two UCMSCs (Figure 5F). Similar trends were observed at the protein level of TSG-6, IL-6, PGE2, and IDO by ELISA analysis (Figures 5G–J). Taken together, these data indicate that UCMSCSCM and UCMSCS&XFM−CD significantly polarised macrophages from M1 to M2 phenotype through secretion of different cytokine profiles in vitro.
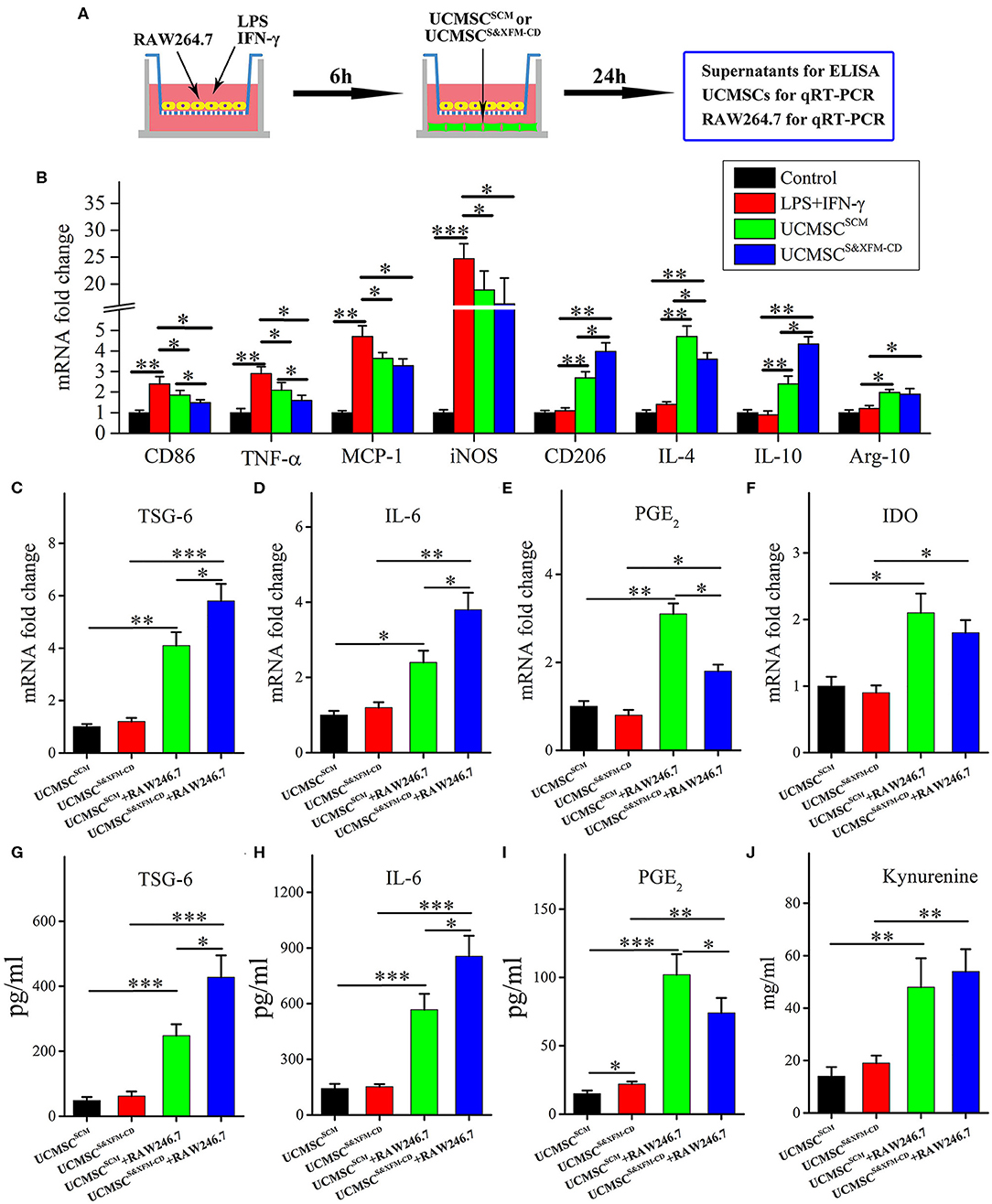
Figure 5. UCMSCSCM and UCMSCS&XFM−CD polarized macrophages from M1 to M2 phenotype in vitro through different mechanisms. (A) Schematic depiction of the cell coculture system. RAW264.7 cells were seeded in the upper chamber of transwell insert and cocultured with UCMSCSCM or UCMSCS&XFM−CD in the lower chamber. (B) mRNA levels of M1- and M2-related markers, pro-inflammatory factors, and anti-inflammatory factors in RAW264.7 cells by qRT-PCR (n = 7 independent experiments). (C–F) mRNA and (G–I) protein levels of TSG-6, IL-6, PGE2, and IDO in UCMSCs. (J) IDO activity, determined as kynurenine concentration in UCMSCs (n = 7 independent experiments). Significance was analysed using one-way ANOVA followed by Tukey's multiple comparisons test for multiple group comparisons. *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
In this study, our data showed that UCMSCS&XFM−CD exhibited better therapeutic efficacy in an acute colitis mouse model compared with UCMSCSCM. It has been reported that UCMSCs can be manipulated in vitro by preconditioning in SCM and deconditioning in serum-free medium, leading to enhanced immunosuppressive and therapeutic effects on IBD (Yang et al., 2018). Although the components of serum-free media in the previous studies are different from ours, the reported results are consistent with our findings, which indicates that S&XFM-CD enhances the effectiveness of UCMSCs for the treatment of IBD and may represent an attractive alternative to FBS for culturing clinical-grade UCMSCs.
Some studies show that intraperitoneally injected MSCs disseminate to heart, lung, liver, spleen, and kidney, but do not migrate to the colon (Song et al., 2017b). However, other studies have confirmed that intraperitoneal but not intravenous MSCs could migrate to the inflammatory site of the injured colon (Castelo-Branco et al., 2012; Lee et al., 2018; Lopez-Santalla et al., 2018). Our data showed that CM-Dil-labeled UCMSCSCM and UCMSCS&XFM−CD were only transiently recruited to the injured colon and both UCMSCs exhibited low levels of recruitment and persistence in experimental colitis. Due to the sensitivity of fluorescence imaging, a low number of MSCs may remain undetected. Therefore, we tracked colon colonization by MSCs using qRT-PCR as described previously (Song et al., 2017b). The presence of UCMSCs existence was detected at day 3, but gradually decreased over time and both UCMSCSCM and UCMSCS&XFM−CD were no longer present in the inflamed colon after day 3 (data not shown). The loss of colonic MSCs may be attributed to many causes, such as washout, cell death, or even rejection by the innate immune system. Although MSCs were initially proposed for as a therapeutic tool based on their differentiation capability, the lack of cell engraftment or differentiation at the site of injury has led to suggestions that MSCs may exert their therapeutic effects mainly through paracrine signalling (Shi et al., 2018). In this study, the intraperitoneal injection of UCMSCS&XFM−CD or UCMSCSCM significantly ameliorated the severity of experimental colitis. Our previous study also shows the therapeutic effect of exosomes from UCMSCs in S&XFM-CD on experimental colitis (Ma et al., 2019b), thus we postulate that the therapeutic benefits of intraperitoneally injected UCMSCSCM or UCMSCS&XFM−CD stem from immunomodulatory mechanisms and are mediated by indirect paracrine factors rather than direct cell-to-cell interactions. This hypothesis is consistent with the current view that MSCs exerted its benefit via its paracrine effect (Khubutiya et al., 2014).
Macrophages play a critical role in the modulation of colon inflammation after IBD (Isidro and Appleyard, 2016). Therefore, we investigated the role of macrophages in UCMSCSCM or UCMSCS&XFM−CD therapy. Our data showed that the depletion of macrophages abolished the beneficial effects of both UCMSCSCM and UCMSCS&XFM−CD transplantation in acute colitis, consistent with results in animal models of other diseases including allergic asthma (Mathias et al., 2013), myocardial infarction (Ben-Mordechai et al., 2013; Wang et al., 2015), and liver injury (Ghanem et al., 2019). Thus, we confirmed the hypothesis that the protective effects of UCMSCs on acute colitis are mediated by macrophages independent of culture conditions.
MSCs polarize macrophages from pro-inflammatory M1 to anti-inflammatory M2 to exert an immunosuppressive and therapeutic effect (Zheng et al., 2015; Mao et al., 2017). Therefore, we examined the potential effects of UCMSCSCM or UCMSCS&XFM−CD on macrophage quantity and subpopulations in vivo. Our results suggested that UCMSCSCM or UCMSCS&XFM−CD increased the proportion of total macrophages in the colon, which was consistent with some studies (Liu et al., 2015), although others show macrophage suppression or no significant changes in macrophage populations (Simovic Markovic et al., 2016; Song et al., 2017b; Park et al., 2018). We speculate that the inconsistencies may be due to different detection times or methods. Interestingly, recent studies have shown that macrophage proportions are not altered when exosomes from MSCs are administrated at the same time using the same methods (Liu et al., 2019). This indicates that there are functional differences between exosomes and MSCs. Our analysis showed that UCMSCSCM or UCMSCS&XFM−CD polarised macrophages from proinflammatory M1 to anti-inflammatory M2 macrophages, which dampen intestinal inflammation. Similar findings have been reported previously (Song et al., 2017a; Liu et al., 2019), although other studies have shown contradicting conclusions (Song et al., 2017b; Park et al., 2018). One study shows that the administration of MSCs ameliorates colitis by decreasing the proportion of M1 macrophages population, but found no significant change in M2 macrophages (Park et al., 2018) and conversely another study demonstrates that this effect is caused by an increase in the percentage of M2 macrophages without affecting M1 macrophages (Song et al., 2017b). These discrepancies may be related to different times of cell transplantation. We observed that MSCs cultured in S&XFM-CD further enhanced macrophage polarization from proinflammatory M1 to anti-inflammatory M2 macrophages and was consistent with previous reports by Yoshida and colleagues who used a different serum-free medium (Yoshida et al., 2018). It has been reported that the immunological paradigm of M1/M2 dichotomy following macrophage polarisation is unclear in humans due to key differences in macrophage biology between human and mouse (Na et al., 2019). There is a continuum between M1-like and M2-like macrophages where boundaries are still unclear. A current challenge in the study of macrophage phenotypes is that some markers used to identify M1 and M2 macrophages in mice cannot be directly applied to human subsets (Watanabe et al., 2019). For example, iNOS and Arg1 are well-established markers for mouse M1 and M2 macrophages, respectively, but their significance in the human subsets has not been defined. This limitation likely explains emerging literature in which the data collected in mice and humans reveal macrophage phenotypes that are inconsistent with the M1/M2 paradigm (Hine and Loke, 2019).
Understanding the molecular mechanisms involved in the crosstalk between MSCs and macrophages will contribute to the optimal use of MSCs in clinical practice. Our data demonstrated that UCMSCS&XFM−CD more markedly promoted intestinal macrophage polarization from M1 to M2 phenotype to produce higher levels of IL-10 and lower levels of TNF-α in colon tissue compared with UCMSCSCM, similar to previous reports (Simovic Markovic et al., 2016; Song et al., 2017b; Liu et al., 2019). To our surprise, UCMSCS&XFM−CD treatment also lowered the expression of IL-4. This suggests that UCMSCs may be involved in multiple inflammatory processes and that UCMSCs in different media employ other pathways. MSC-mediated macrophage polarization has been demonstrated in various inflammatory diseases and is regulated by several mediators secreted by MSCs, such as PGE2 (Ylostalo et al., 2012; Vasandan et al., 2016; Park et al., 2018), IL-6 (Deng et al., 2015; Xie et al., 2015), TSG-6 (Shin et al., 2016; Di et al., 2017; Song et al., 2017b; Yoshida et al., 2018), and IDO (Francois et al., 2012; Lee et al., 2016). We confirmed that UCMSCS&XFM−CD cocultured with RAW264.7 cells in a transwell system promoted M2 macrophage polarization by decreasing the release of PGE2 and increasing TSG-6 and IL-6. PGE2 and TSG-6 patterns were in line with data described by Yoshida et al. (2018), but the increase in IL-6 was inconsistent, which may be related to the different components of the serum-free medium. Taken together, these data further indicate that UCMSCs in different media mediate their therapeutic effects through different cellular mechanisms in an acute colitis mouse model. In our case, UCMSCS&XFM−CD moderated inflammation mainly through TSG-6 and IL-6-dependent mechanisms while UCMSCSCM primarily utilised the PGE2 pathway (Figure 6).
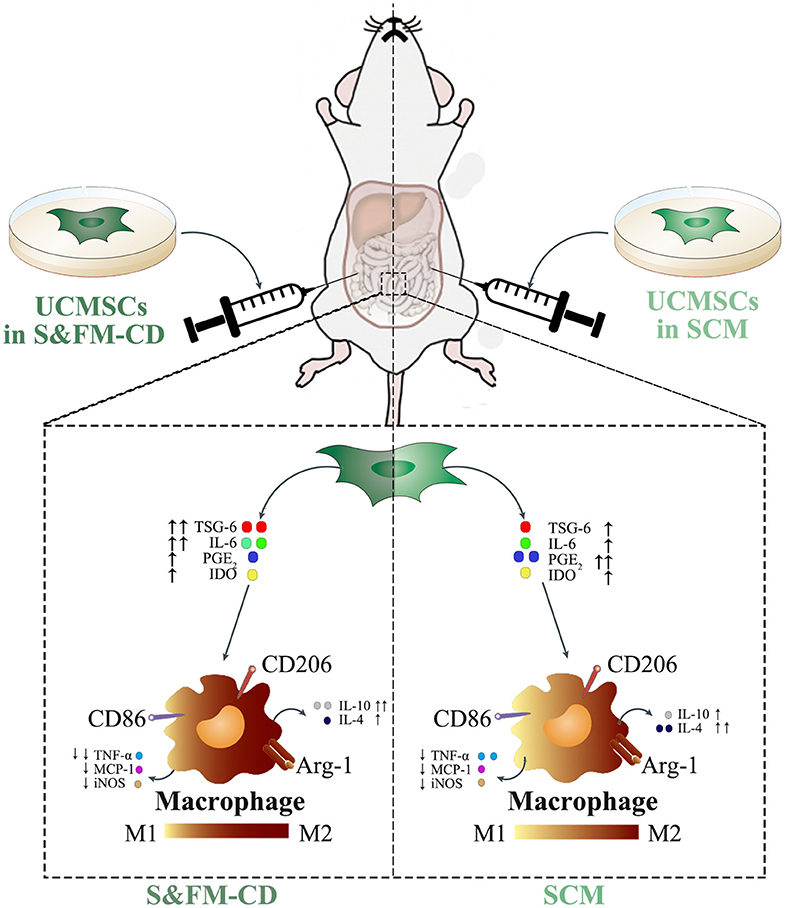
Figure 6. UCMSCSCM and UCMSCS&XFM−CD dampened DSS-induced acute colitis though polarisation of macrophages from M1 to M2 phenotype, mediated via different mechanisms. In response to the proinflammatory environment, UCMSCSCM or UCMSCS&XFM−CD secreted immunomodulatory mediators including PGE2, TSG-6, IL-6, and IDO. These mediators inhibited the polarisation of M1 macrophages and the production of pro-inflammatory cytokines (TNF-α, MCP-1, and iNOS). In addition, they promoted the polarisation of M2 macrophages and the production of anti-inflammatory cytokines (IL-4 and IL-10), which lead to the attenuation of DSS-induced colon inflammation and injury. However, culture condition altered the mechanisms employed by the UCMSCs. While UCMSCSCM produced PGE2 and increased the release of IL-4 to promote intestinal macrophage polarization to an M2 phenotype, UCMSCS&XFM−CD secreted TSG-6 and IL-6 to achieve this more effectively and further increased IL-10 and reduced TNF-α compared with UCMSCSCM. This culminated in the improved therapeutic effects of UCMSCS&XFM−CD in DSS-induced colitis.
In conclusion, this study found that intraperitoneal administration of UCMSCS&XFM−CD exhibited better therapeutic effects than UCMSCSCM for the treatment of IBD. Moreover, UCMSCS&XFM−CD more markedly dampened intestinal inflammation by enhancing macrophage polarisation from proinflammatory M1 to anti-inflammatory M2. Mechanistically, we observed that UCMSCS&XFM−CD and UCMSCSCM mediated their therapeutic effects through different pathways. In addition to these notable effects, S&XFM-CD was useful for culturing UCMSCs due to several advantages over SCM including safety, efficacy, consistency, and reproducibility. In conclusion, our results suggest that the usage of S&XFM-CD will accelerate the clinical translation of UCMSCs and strengthen the therapeutic potential of UCMSCs in the treatment of IBD.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to XW, c3RlbWNlbGxzQGZveG1haWwuY29t.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Beijing Friendship Hospital affiliated to Capital Medical University. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Ethics Committee of Beijing Friendship Hospital affiliated to Capital Medical University.
Author Contributions
XW: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. DW: collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. YM: provision of study material, collection and/or assembly of data, data analysis and interpretation. YZ: provision of study material, collection and/or assembly of data. ZM: conception and design, financial support, manuscript writing, and final approval of manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 81960252 and 81860157) and Natural Science Foundation of Inner Mongolia (No. 2019MS08047).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2020.00586/full#supplementary-material
References
Badraiq, H., Devito, L., and Ilic, D. (2015). Isolation and expansion of mesenchymal stromal/stem cells from umbilical cord under chemically defined conditions. Methods Mol. Biol. 1283, 65–71. doi: 10.1007/7651_2014_116
Barnhoorn, M., de Jonge-Muller, E., Molendijk, I., van Gulijk, M., Lebbink, O., Janson, S., et al. (2018). Endoscopic administration of mesenchymal stromal cells reduces inflammation in experimental colitis. Inflamm. Bowel Dis. 24, 1755–1767. doi: 10.1093/ibd/izy130
Ben-Mordechai, T., Holbova, R., Landa-Rouben, N., Harel-Adar, T., Feinberg, M. S., Abd Elrahman, I., et al. (2013). Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J. Am. Coll. Cardiol. 62, 1890–1901. doi: 10.1016/j.jacc.2013.07.057
Castelo-Branco, M. T., Soares, I. D., Lopes, D. V., Buongusto, F., Martinusso, C. A., do Rosario, A., et al. (2012). Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS ONE 7:e33360. doi: 10.1371/journal.pone.0033360
Ciccocioppo, R., and Corazza, G. R. (2018). Mesenchymal stromal cells: an opportunity to treat chronic inflammatory intestinal conditions. Cytotherapy 20, 1223–1226. doi: 10.1016/j.jcyt.2018.08.004
Conklin, L. S., Hanley, P. J., Galipeau, J., Barrett, J., and Bollard, C. M. (2017). Intravenous mesenchymal stromal cell therapy for inflammatory bowel disease: lessons from the acute graft versus host disease experience. Cytotherapy 19, 655–667. doi: 10.1016/j.jcyt.2017.03.006
Corotchi, M. C., Popa, M. A., Remes, A., Sima, L. E., Gussi, I., and Lupu Plesu, M. (2013). Isolation method and xeno-free culture conditions influence multipotent differentiation capacity of human Wharton's jelly-derived mesenchymal stem cells. Stem Cell Res. Ther. 4:81. doi: 10.1186/scrt232
Deng, W., Chen, W., Zhang, Z., Huang, S., Kong, W., Sun, Y., et al. (2015). Mesenchymal stem cells promote CD206 expression and phagocytic activity of macrophages through IL-6 in systemic lupus erythematosus. Clin. Immunol. 161, 209–216. doi: 10.1016/j.clim.2015.07.011
Devito, L., Badraiq, H., Galleu, A., Taheem, D. K., Codognotto, S., Siow, R., et al. (2014). Wharton's jelly mesenchymal stromal/stem cells derived under chemically defined animal product-free low oxygen conditions are rich in MSCA-1(+) subpopulation. Regen. Med. 9, 723–732. doi: 10.2217/rme.14.60
Di, G., Du, X., Qi, X., Zhao, X., Duan, H., Li, S., et al. (2017). Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6-dependent stem cell activation and macrophage switch. Invest. Ophthalmol. Vis. Sci. 58, 4344–4354. doi: 10.1167/iovs.17-21506
Francois, M., Romieu-Mourez, R., Li, M., and Galipeau, J. (2012). Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195. doi: 10.1038/mt.2011.189
Fuenzalida, P., Kurte, M., Fernandez-O'ryan, C., Ibanez, C., Gauthier-Abeliuk, M., Vega-Letter, A. M., et al. (2016). Toll-like receptor 3 pre-conditioning increases the therapeutic efficacy of umbilical cord mesenchymal stromal cells in a dextran sulfate sodium-induced colitis model. Cytotherapy 18, 630–641. doi: 10.1016/j.jcyt.2016.02.002
Ghanem, L. Y., Mansour, I. M., Abulata, N., Akl, M. M., Demerdash, Z. A., El Baz, H. G., et al. (2019). Liver macrophage depletion ameliorates the effect of mesenchymal stem cell transplantation in a murine model of injured liver. Sci. Rep. 9:35. doi: 10.1038/s41598-018-37184-4
Hine, A. M., and Loke, P. (2019). Intestinal macrophages in resolving inflammation. J. Immunol. 203, 593–599. doi: 10.4049/jimmunol.1900345
Hunter, M. M., Wang, A., Parhar, K. S., Johnston, M. J., Van Rooijen, N., Beck, P. L., et al. (2010). In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 138, 1395–1405. doi: 10.1053/j.gastro.2009.12.041
Isidro, R. A., and Appleyard, C. B. (2016). Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G59–73. doi: 10.1152/ajpgi.00123.2016
Jeffrey, L., Gregory, A. C., Singh, H., Tucker, A. H., Peister, A., Lynch, J. P., et al. (2004). Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol. Ther. 9, 747–756. doi: 10.1016/j.ymthe.2004.02.012
Khubutiya, M. S., Vagabov, A. V., Temnov, A. A., and Sklifas, A. N. (2014). Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy 16, 579–585. doi: 10.1016/j.jcyt.2013.07.017
Kong, C. M., Lin, H. D., Biswas, A., Bongso, A., and Fong, C. Y. (2019). Manufacturing of human Wharton's jelly stem cells for clinical use: selection of serum is important. Cytotherapy 21, 483–495. doi: 10.1016/j.jcyt.2019.02.008
Lee, B. C., Shin, N., Lee, J. Y., Kang, I., Kim, J. J., Lee, S. E., et al. (2018). MIS416 enhances therapeutic functions of human umbilical cord blood-derived mesenchymal stem cells against experimental colitis by modulating systemic immune Milieu. Front. Immunol. 9:1078. doi: 10.3389/fimmu.2018.01078
Lee, S., Zhang, Q. Z., Karabucak, B., and Le, A. D. (2016). DPSCs from inflamed pulp modulate macrophage function via the TNF-alpha/IDO axis. J. Dent. Res. 95, 1274–1281. doi: 10.1177/0022034516657817
Liu, H., Liang, Z., Wang, F., Zhou, C., Zheng, X., Hu, T., et al. (2019). Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 4:e131273. doi: 10.1172/jci.insight.131273
Liu, W., Zhang, S., Gu, S., Sang, L., and Dai, C. (2015). Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFbeta1. Cell. Physiol. Biochem. 35, 858–865. doi: 10.1159/000369743
Liu, Z., Screven, R., Boxer, L., Myers, M. J., and Devireddy, L. R. (2018). Characterization of canine adipose-derived mesenchymal stromal/stem cells in serum-free medium. Tissue Eng. C Methods 24, 399–411. doi: 10.1089/ten.TEC.2017.0409
Lopez-Santalla, M., Mancheno-Corvo, P., Escolano, A., Menta, R., DelaRosa, O., Abad, J. L., et al. (2017). Biodistribution and efficacy of human adipose-derived mesenchymal stem cells following intranodal administration in experimental colitis. Front. Immunol. 8:638. doi: 10.3389/fimmu.2017.00638
Lopez-Santalla, M., Mancheno-Corvo, P., Escolano, A., Menta, R., Delarosa, O., Redondo, J. M., et al. (2018). Comparative analysis between the in vivo biodistribution and therapeutic efficacy of adipose-derived mesenchymal stromal cells administered intraperitoneally in experimental colitis. Int. J. Mol. Sci. 19:1853. doi: 10.3390/ijms19071853
Ma, J., Wu, J., Han, L., Jiang, X., Yan, L., Hao, J., et al. (2019a). Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum-free condition. Stem Cell Res. Ther. 10:19. doi: 10.1186/s13287-018-1104-x
Ma, Z. J., Wang, Y. H., Li, Z. G., Wang, Y., Li, B. Y., Kang, H. Y., et al. (2019b). Immunosuppressive effect of exosomes from mesenchymal stromal cells in defined medium on experimental colitis. Int. J. Stem Cells 12, 440–448. doi: 10.15283/ijsc18139
Mao, F., Kang, J. J., Cai, X., Ding, N. F., Wu, Y. B., Yan, Y. M., et al. (2017). Crosstalk between mesenchymal stem cells and macrophages in inflammatory bowel disease and associated colorectal cancer. Contemp. Oncol. (Pozn). 21, 91–97. doi: 10.5114/wo.2017.68616
Markovic, B. S., Kanjevac, T., Harrell, C. R., Gazdic, M., Fellabaum, C., Arsenijevic, N., et al. (2018). Molecular and cellular mechanisms involved in mesenchymal stem cell-based therapy of inflammatory bowel diseases. Stem Cell Rev. 14, 153–165. doi: 10.1007/s12015-017-9789-2
Mathias, L. J., Khong, S. M., Spyroglou, L., Payne, N. L., Siatskas, C., Thorburn, A. N., et al. (2013). Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J. Immunol. 191, 5914–5924. doi: 10.4049/jimmunol.1300667
Mu, Y., Wu, X., and Hao, Z. (2018). Comparative evaluation of mesenchymal stromal cells from umbilical cord and amniotic membrane in xeno-free conditions. BMC Cell Biol. 19:27. doi: 10.1186/s12860-018-0178-8
Na, Y. R., Stakenborg, M., Seok, S. H., and Matteoli, G. (2019). Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16, 531–543. doi: 10.1038/s41575-019-0172-4
Owens, S. D., Kol, A., Walker, N. J., and Borjesson, D. L. (2016). Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in Horses. Stem Cells Int. 2016:5830103. doi: 10.1155/2016/5830103
Park, H. J., Kim, J., Saima, F. T., Rhee, K. J., Hwang, S., Kim, M. Y., et al. (2018). Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem. Biophys. Res. Commun. 498, 988–995. doi: 10.1016/j.bbrc.2018.03.096
Phinney, D. G., Galipeau, J., and Msc Committee of the International Society of Cell Gene Therapy (2019). Manufacturing mesenchymal stromal cells for clinical applications: a survey of good manufacturing practices at US academic centers. Cytotherapy 21, 782–792. doi: 10.1016/j.jcyt.2019.04.003
Shahdadfar, A., Fronsdal, K., Haug, T., Reinholt, F. P., and Brinchmann, J. E. (2005). In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 23, 1357–1366. doi: 10.1634/stemcells.2005-0094
Shi, Y., Wang, Y., Li, Q., Liu, K., Hou, J., Shao, C., et al. (2018). Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 14, 493–507. doi: 10.1038/s41581-018-0023-5
Shin, T. H., Kim, H. S., Kang, T. W., Lee, B. C., Lee, H. Y., Kim, Y. J., et al. (2016). Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 7:e2524. doi: 10.1038/cddis.2016.442
Simoes, I. N., Boura, J. S., dos Santos, F., Andrade, P. Z., Cardoso, C. M., Gimble, J. M., et al. (2013). Human mesenchymal stem cells from the umbilical cord matrix: successful isolation and ex vivo expansion using serum-/xeno-free culture media. Biotechnol. J. 8, 448–458. doi: 10.1002/biot.201200340
Simovic Markovic, B., Nikolic, A., Gazdic, M., Nurkovic, J., Djordjevic, I., Arsenijevic, N., et al. (2016). Pharmacological inhibition of Gal-3 in mesenchymal stem cells enhances their capacity to promote alternative activation of macrophages in dextran sulphate sodium-induced colitis. Stem Cells Int. 2016:2640746. doi: 10.1155/2016/2640746
Song, J. Y., Kang, H. J., Hong, J. S., Kim, C. J., Shim, J. Y., Lee, C. W., et al. (2017a). Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci. Rep. 7:9412. doi: 10.1038/s41598-017-09827-5
Song, W. J., Li, Q., Ryu, M. O., Ahn, J. O., Ha Bhang, D., Chan Jung, Y., et al. (2017b). TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in Mice. Sci. Rep. 7:5187. doi: 10.1038/s41598-017-04766-7
Swamynathan, P., Venugopal, P., Kannan, S., Thej, C., Kolkundar, U., Bhagwat, S., et al. (2014). Are serum-free and xeno-free culture conditions ideal for large scale clinical grade expansion of Wharton's jelly derived mesenchymal stem cells? A comparative study. Stem Cell Res. Ther. 5:88. doi: 10.1186/scrt477
van der Valk, J., Bieback, K., Buta, C., Cochrane, B., Dirks, W. G., Fu, J., et al. (2018). Fetal bovine serum (FBS): past–present–future. ALTEX 35, 99–118. doi: 10.14573/altex.1705101
Vasandan, A. B., Jahnavi, S., Shashank, C., Prasad, P., Kumar, A., and Prasanna, S. J. (2016). Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 6:38308. doi: 10.1038/srep38308
Verstockt, B., Ferrante, M., Vermeire, S., and Van Assche, G. (2018). New treatment options for inflammatory bowel diseases. J. Gastroenterol. 53, 585–590. doi: 10.1007/s00535-018-1449-z
Wang, M., Liang, C., Hu, H., Zhou, L., Xu, B., Wang, X., et al. (2016). Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci Rep. 6:30696. doi: 10.1038/srep30696
Wang, M., Zhang, G., Wang, Y., Liu, T., Zhang, Y., An, Y., et al. (2015). Crosstalk of mesenchymal stem cells and macrophages promotes cardiac muscle repair. Int. J. Biochem. Cell Biol. 58, 53–61. doi: 10.1016/j.biocel.2014.11.003
Watanabe, S., Alexander, M., Misharin, A. V., and Budinger, G. R. S. (2019). The role of macrophages in the resolution of inflammation. J. Clin. Invest. 129, 2619–2628. doi: 10.1172/JCI124615
Wu, X., Kang, H., Liu, X., Gao, J., Zhao, K., and Ma, Z. (2016). Serum and xeno-free, chemically defined, no-plate-coating-based culture system for mesenchymal stromal cells from the umbilical cord. Cell Prolif. 49, 579–588. doi: 10.1111/cpr.12279
Xie, Z., Hao, H., Tong, C., Cheng, Y., Liu, J., Pang, Y., et al. (2015). Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 34, 627–639. doi: 10.1002/stem.2238
Yang, F. Y., Chen, R., Zhang, X., Huang, B., Tsang, L. L., Li, X., et al. (2018). Preconditioning enhances the therapeutic effects of mesenchymal stem cells on colitis through PGE2-mediated T-cell modulation. Cell Transplant. 27, 1352–1367. doi: 10.1177/0963689718780304
Ylostalo, J. H., Bartosh, T. J., Coble, K., and Prockop, D. J. (2012). Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30, 2283–2296. doi: 10.1002/stem.1191
Yoshida, K., Nakashima, A., Doi, S., Ueno, T., Okubo, T., Kawano, K. I., et al. (2018). Serum-free medium enhances the immunosuppressive and antifibrotic abilities of mesenchymal stem cells utilized in experimental renal fibrosis. Stem Cells Transl. Med. 7, 893–905. doi: 10.1002/sctm.17-0284
Keywords: mesenchymal stromal cells, colitis, therapeutic effect, mechanism, serum free, macrophage polarization
Citation: Wu X, Wu D, Mu Y, Zhao Y and Ma Z (2020) Serum-Free Medium Enhances the Therapeutic Effects of Umbilical Cord Mesenchymal Stromal Cells on a Murine Model for Acute Colitis. Front. Bioeng. Biotechnol. 8:586. doi: 10.3389/fbioe.2020.00586
Received: 06 March 2020; Accepted: 14 May 2020;
Published: 26 June 2020.
Edited by:
Pedro M. Baptista, University of Zaragoza, SpainReviewed by:
Elisabeth Ferreira, University of Arkansas for Medical Sciences, United StatesCristina Carvalho Barrias, INEB-Instituto de Engenharia Biomédica/i3S-Instituto de Investigação e Inovação em Saúde, Portugal
Copyright © 2020 Wu, Wu, Mu, Zhao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijie Ma, MTM4MTE2NDcwOTFAMTYzLmNvbQ==
 Xiaoyun Wu
Xiaoyun Wu Daocheng Wu2
Daocheng Wu2 Zhijie Ma
Zhijie Ma