- 1State Key Laboratory of Cotton Biology, Key Laboratory of Biological and Genetic Breeding of Cotton, The Ministry of Agriculture, Institute of Cotton Research, Chinese Academy of Agricultural Sciences, Anyang, China
- 2School of Medicine, Medical Sciences and Nutrition, The Institute of Medical Sciences, University of Aberdeen, Aberdeen, United Kingdom
- 31-FB, Genetics, Four Brothers Group, Lahore, Pakistan
- 4Institute of Molecular Biology and Biotechnology, The University of Lahore, Lahore, Pakistan
The use of chemicals around the globe in different industries has increased tremendously, affecting the health of people. The modern world intends to replace these noxious chemicals with environmental friendly products for the betterment of life on the planet. Establishing enzymatic processes in spite of chemical processes has been a prime objective of scientists. Various enzymes, specifically microbial proteases, are the most essentially used in different corporate sectors, such as textile, detergent, leather, feed, waste, and others. Proteases with respect to physiological and commercial roles hold a pivotal position. As they are performing synthetic and degradative functions, proteases are found ubiquitously, such as in plants, animals, and microbes. Among different producers of proteases, Bacillus sp. are mostly commercially exploited microbes for proteases. Proteases are successfully considered as an alternative to chemicals and an eco-friendly indicator for nature or the surroundings. The evolutionary relationship among acidic, neutral, and alkaline proteases has been analyzed based on their protein sequences, but there remains a lack of information that regulates the diversity in their specificity. Researchers are looking for microbial proteases as they can tolerate harsh conditions, ways to prevent autoproteolytic activity, stability in optimum pH, and substrate specificity. The current review focuses on the comparison among different proteases and the current problems faced during production and application at the industrial level. Deciphering these issues would enable us to promote microbial proteases economically and commercially around the world.
Introduction
Proteases are a universal entity that is found everywhere, namely, in plants, animals, and microbes. The peptide bond present in the polypeptide chain of amino acids is hydrolyzed by means of proteases (Barrett and McDonald, 1986). Proteases are degradative enzymes and show specificity and selectivity in protein modification (Rao et al., 1998). In the industrial sector, Bacillus sp. are the most active and dynamic extracellular alkaline protease producer. Of the three largest groups of industrial enzymes, proteases are one of them, and their global market is drastically increasing annually. Of the 60% of enzymes marketed worldwide, proteases account for 20% (Kang et al., 1995; Rao et al., 2009; Singhal et al., 2012). Proteases are an integral component of existing life on earth, such as animals, plants, and microbes. By a process of fermentation, proteases can be isolated and purified in a relatively shorter period of time, exhibiting high substrate specificity and catalytic activity (Kumar and Takagi, 1999; Rifaat et al., 2007; Singhal et al., 2012). It is estimated that proteases account for 1–5% of the genome of infectious organisms and 2% of the human genome (Puente et al., 2003). According to researchers, proteases control the activation, synthesis, and turnover of proteins to regulate physiological processes (Rawlings et al., 2004). Different physiological processes, such as formation, birth, aging, and even death are regulated by proteases (Chou et al., 1997, 2000, 2003; Chou and Howe, 2002; Chou, 2004, 2006). Proteases are vital in the imitation and spread of infectious diseases, and because of their significant role in the life cycle, they are imperative for drug discovery. In more than 50 human proteases, a single amino acid mutation may lead to a hereditary disease (Chou et al., 1998). Proteases are involved in normal and pathophysiological processes or conditions. This involvement of proteases may lead them to produce a therapeutic agent against deadly diseases, such as cancer and AIDS (Rawlings et al., 2004). Proteases similar in sequences and structures are grouped into clans and families, which are available in the MEROPS database (Kumar and Takagi, 1999). The proposed review highlights the proteolysis, function, and wide range of sources among different bacteria of microbial proteases. It also discusses the broad range of applications and upcoming advancement for the discovery of new and fresh proteases, especially alkaline proteases from bacteria (Reddy et al., 2008; Haddar et al., 2009a).
Microbial Proteases
Proteases have been successfully produced by researchers from different microbial sources. Microbes account a two-thirds share of commercial protease around the globe (Beg and Gupta, 2003). Since the advent of enzymology, microbial proteolytic proteases have been the most widely studied enzyme. These enzymes have gained interest not only due to their vital role in metabolic activities but also due to their immense utilization in industries (Rao et al., 1998; Sandhya et al., 2005; Younes and Rinaudo, 2015). The proteases available in the market are of microbial origin because of their high yield, less time consumption, less space requirement, lofty genetic manipulation, and cost-effectiveness, which have made them suitable for biotechnological application in the market (Nisha and Divakaran, 2014; Ali et al., 2016). These microbial proteases are preferred to plant and animal proteases because of the presence of all desired characteristics for industrial applications (Palsaniya et al., 2012; Sathishkumar et al., 2015). Proteolytic enzymes found in microbes and mammalian systems are small in size, dense, and structurally spherical (Oberoi et al., 2001). Among different producers of alkaline proteases, Bacillus sp. is of immense importance (Rifaat et al., 2007). The proteases isolated from these microbial sources have a large number of dilutions in various industrial sectors (Pastor et al., 2001; Beg and Gupta, 2003; Das and Prasad, 2010). Usually, extracellular alkaline proteases are secreted out from the producer into the liquid broth from where these proteases are simplified and purified through down streaming to produce an end product. Comparatively, proteases produced by plants and animals are more labor-intensive than microbially produced proteases (Gupta et al., 2002; Kalaiarasi and Sunitha, 2009). Proteases produced by microbial sources are classified into groups based on their acidic or basic properties. They are also classified based on the presence of functional groups and the position of peptide bond (Gessesse, 1997; Panda et al., 2013). Microbial proteases are the most commercially exploited enzyme worldwide. A large number of intracellular proteases are produced by microbes playing a vital role in differentiation, protein turnover, hormone regulation, and cellular protein pool, whereas extracellular proteases are significant in protein hydrolysis (Rao et al., 1998; Johnvesly and Naik, 2001; Adrio and Demain, 2014), such as in processing of photographic film (Kumar and Takagi, 1999; Patil and Chaudhari, 2009), enzymatic synthesis on the basis of solvent and detergent preparation (Simkhada et al., 2010a), substrate specificity (Soroor et al., 2009), thermal tolerance (Amoozegar et al., 2007), and production of zein hydrolysates (Miyaji et al., 2006; Dodia et al., 2008; Jaouadi et al., 2008).
Keratin
Keratins are proteins that are usually present in two forms, namely, hard keratins and soft keratins. Hard keratins mainly include the structural proteins that are prevalently present in fingernails, horns, beaks, upper layer of skin, and mainly hair. Fibers of the keratin proteins are self-assembled into compact follicles that make up the structure of hair. The process of assembling keratin proteins into a complex hair is under the control of multiple genes, cytokines, and growth factors (Charles et al., 2008). In contrast to hard keratins, soft keratins are those that are abundantly present in tissues, such as epithelial tissues. The structure of wool keratin shows great similarity to hair keratin. Three types of hair keratin have been known (Cheng et al., 1995). The first one is the alpha keratins; these range in size from 60 to 80 kDa. Having low sulfur content, these comprise mainly of alpha-helical domains. Overall, alpha keratins make up the structural class of proteins, as they reside in the fiber cortex of hair. The second type is the beta keratins, which are a non-extractable, less-studied class of keratins. These are usually present in the hair cuticle and perform protective functions. The third type is the gamma keratins, which have a high sulfur content; these keratins are ~15 kDa in size. Their size is comparatively smaller than the other classes of keratin. These keratins help to maintain the cortical superstructure by cross-linking the disulfide bonds in the hair (Cheng et al., 1995; Gupta et al., 2002; Prakasham et al., 2006). All these types of keratins can be degraded by the enzyme keratinase, which belongs to a class of protease enzymes. Proteases, which account for 60% of the world's marketed enzymes, is responsible for many applications, such as detergents, food, and leather processing (Suntornsuk and Suntornsuk, 2003; Călin et al., 2017; Adetunji and Adejumo, 2018; Kalaikumari et al., 2019).
The enzyme keratinase (E.C. 3.4.99.11) is one of the serine hydrolase groups that disrupt the disulfide hydrogen bonds in the keratin proteins (Cavello et al., 2015; Bohacz and Korniłłowicz-Kowalska, 2019; Kalaikumari et al., 2019). According to UniProt results, one of the protein keratinases produced by Bacillus subtilis contains two domains. The first one is 59 amino acids long and encodes for inhibitor I9; the other one is 243 amino acids long and encodes for peptidase S8. The first domain occurs from 19 to 77 amino acid sequences and the second domain occurs from 103 to 345 amino acid sequences. The enzyme also has a metal ion binding site for calcium ion. This means that calcium ions act as cofactors for keratinases; the presence of calcium ions in the media can enhance the activity of keratinases. The structure of keratinase makes it very efficient in its function of degrading keratin proteins (Arora and Mishra, 2016; Moraga et al., 2019). Our daily green waste and animal waste includes plenty of keratins, which remain undegraded due to their complexity. Such insoluble keratins may lead to environmental pollution if left untreated. Thus, as a solution, such wastes are treated by keratinase enzymes, which convert the waste into simpler as well as biodegradable substances (Cavello et al., 2015; Hossain et al., 2017). The extracellular keratinases have been successfully isolated from several microbes by using several fermentation techniques and by optimizing conditions, such as pH, temperature, and type of nitrogen and carbon source and the choice of microbe (Govinden and Puchooa, 2012; Lateef et al., 2015). The keratinases from microbes are effective, biodegradable, and economic and provide much better results as compared to chemical treatments (Manirujjaman et al., 2016; Tamreihao et al., 2017).
Alkaline Proteases
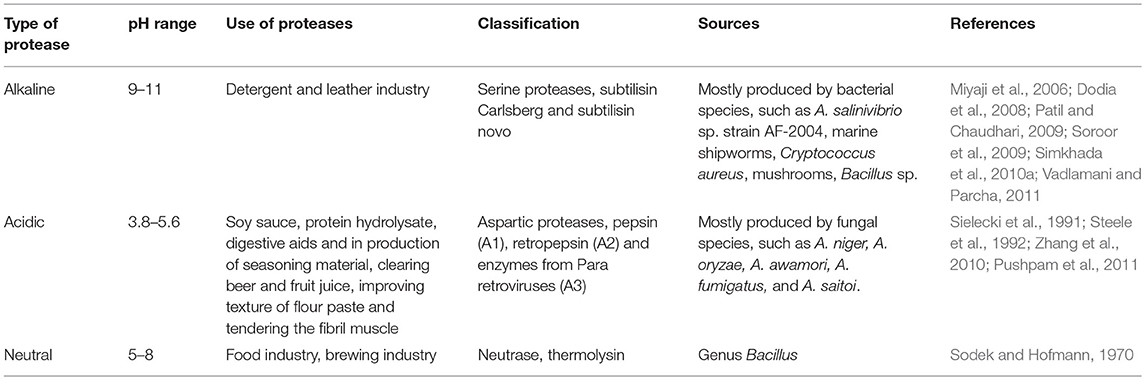
The genus Bacillus is vital for commercially important alkaline protease (EC.3.4.21-24.99), which is active at alkaline pH ranging between 9 and 11 (Varela et al., 1997; Kocher and Mishra, 2009; Singhal et al., 2012). These alkaline protease producers are distributed in water, soil, and highly alkaline conditions. From a variety of sources, such as detergent contamination (Hsiao et al., 1994; Singh et al., 1999), dried fish (Centeno et al., 1996), sand soil, and slaughterhouses, segregation of alkaline proteases has been stated (Adinarayana et al., 2003). The detergent industry consumes alkaline proteases most abundantly, which are serine proteases with an alkaline pH range (Gupta et al., 2002). These alkaline serine proteases, which are easily inactivated by phenyl methane sulfonyl fluoride (PMSF), account for one-third of the share of the enzyme market (Page and Di Cera, 2008). Alkaline proteases are unique in their activity and maintain a constant alkaline pH while being exploited for different formulations in pharmaceutical, food, and other related industries (Banerjee et al., 1999; Joo et al., 2002, 2004; Dias et al., 2008). A broad range of applications of these alkaline proteases are getting more attention from researchers with the hope of discovering new strains with unique properties and substantial activity (Najafi et al., 2005; Saeki et al., 2007). It is reported that for dehairing of animal skin and hides, Bacillus sp. provide the desired hydrolytic, elastolytic, and keratinolytic properties (Bhaskar et al., 2007; Deng et al., 2010; Shankar et al., 2011). These Bacillus strains have been commercially exploited around the globe due to the huge amounts of enzyme secreted with high enzymatic activity (Jacobs, 1995; Ito et al., 1998; Yang J. K. et al., 2000; Beg et al., 2003). Although alkaline proteases are produced by multiple sources (Ellaiah et al., 2002; Prakasham et al., 2005), with the increasing demand of protease in the market, and for cost-effectiveness, only those strains that show greater yield with hyperactivity will be accepted in the current biotechnological advancement (Kumar D. M. et al., 2012). Two essential types of alkaline proteases, such as subtilisin Carlsberg and subtilisin novo are obtained from Bacillus sp., which can be used as an industrial enzyme to produce zein hydrolysates (Miyaji et al., 2006). In halophilic sources, different microbial sp. secreting serine alkaline proteases are also reported (Giménez et al., 2000; Dodia et al., 2008; Vijayaraghavan et al., 2012). The entomopathogenic bacterium Photorhabdus sp. strain EK1 (PhPrtPI) containing Ca2+ alkaline protease is categorized as a metalloprotease. Owing to its broad-spectrum specificity with different proteins and peptides, it is suggested that PhPrtPI provides nutrients to the nematodes by degradation of insect tissues (Soroor et al., 2009). A Salinivibrio sp. strain, AF-2004, produces metallotype protease with a reasonable thermal tolerance and a broad range of pH (5.0–10.0). It is a highly recommended strain due to its thermal and halophilic properties (Amoozegar et al., 2007). Another strain, Bacillus clausii, is also recommended for use at a commercial scale for the production of alkaline protease with the use of peptone, Cu, and fructose as the sole source of energy. The optimum pH and temperature recommended is 8–9 and 37–40°C, respectively (Vadlamani and Parcha, 2011). A strain of Bacillus sp., MPTK 712, isolated from dairy slush producing alkaline protease exhibits a symbiotic relationship with marine shipworms (Greene et al., 1989; Kumar D. M. et al., 2012). Very rare microbes, such as Kurthia spiroforme are also capable of producing alkaline protease (Amoozegar et al., 2007). Some alkaline serine proteases recognized by goat skin metagenomics library shows homology to peptidases (Vadlamani and Parcha, 2011) and Cryptococcus aureus shows good bioactivity with optimum temperature (45–50°C) and pH (9–10) (Kumar D. M. et al., 2012). Different mushrooms producing alkaline protease are also reported (Steele et al., 1992; Li et al., 2009; Pushpam et al., 2011).
Acidic Protease
Acid proteases are stable and active between pH 3.8 and 5.6 and are frequently used in soy sauce, protein hydrolysate, and digestive aids and in the production of seasoning material. The optimum pH of acidic proteases is 3–4 and the isoelectric point range is between 3 and 4.5 with a molecular weight of 30–45 kDa (Zheng et al., 2011; Ravikumar et al., 2012; Machado et al., 2016). Furthermore, acid proteases are also exploited for use in clearing beer and fruit juice, improving texture of flour paste, and tenderizing the fibril muscle (Zhang et al., 2010). In comparison with alkaline proteases, these extracellular acid proteases are mostly produced by fungal species, such as Aspergillus niger (Sielecki et al., 1991), Aspergillus oryzae (Yongquan, 2001), Aspergillus awamori (Ottesen and Rickert, 1970), Aspergillus fumigatus (Shinmyo et al., 1972), and Aspergillus saitoi (Sodek and Hofmann, 1970). Most of the fungal extracellular acid proteases are known as aspergilla opepsins. Aspartic proteases are acid proteases consisting of 380–420 long chains of amino acid residues constituting the active site for catalytic activity. These acidic proteases are endopeptidases and grouped into three families: pepsin (A1), retropepsin (A2), and enzymes from Para retroviruses (A3) (Somkuti and Babel, 1967). These three families are placed in clan AA. It is found that A1 and A2 are closely related to each other while members of the A3 family show some relatedness to families A1 and A2. An active site cleft of the members of the pepsin family is located between lobes of a bilobal structure (Pushpam et al., 2011). A great specificity of acidic proteases is exhibited against aromatic amino acid residues located on both sides of the peptide bond. These aromatic amino acid residues with peptide bonds are similar to pepsin but less stringent in action. Broadly, acidic proteases are divided into two groups: (i) pepsin-like enzymes and (ii) rennin-like enzymes produced by Penicillium, Aspergillus, Rhizopus, Endothia, and Mucor (Tomoda and Shimazono, 1964).
Neutral Proteases
Neutral proteases are defined as, such as they are active at a neutral or weakly acidic or weakly alkaline pH. Mostly neutral proteases belong to the genus Bacillus and with a relatively low thermotolerance ranging from pH 5 to 8 (Table 1). They generate less bitterness in hydrolysis of food proteins due to a medium rate of reaction; therefore, they are considered more valuable in the food industry. Neutrase is incorporated in the brewing industry due to its insensitivity to plant proteinase inhibitors. On the basis of high affinity toward hydrophobic amino acids, neutral proteases are identified and characterized. During production of food hydrolysate, it is slightly advantageous to control the reactivity of neutral proteases due to low thermotolerance. A divalent metal ion is required for the activity of neutral proteases belonging to the metalloprotease type (Barrett, 1995; Woessner et al., 2000; Chavan and Patil, 2007).
Metalloproteases based on specificity in action are grouped into (i) neutral, (ii) alkaline, (iii) Myxobacter I, and (iv) Myxobacter II. A specificity of neutral proteases is shown for hydrophobic acids and inhibited by a chelating agent, such as EDTA (Ethylenediamine tetraacetic acid). Among different types of proteases, metalloproteases are the most diverse. Thermolysin, a well-characterized neutral protease having a single peptide without disulfide bridges, is produced by B. stearothermophilus. It has a molecular weight of 34 kDa. Between the 2-folded lobes of a protein, an essential Zn atom and four Ca atoms are embedded, exhibiting thermotolerance. This thermolysin neutral protease is very stable with a half-life of 1 h at 80°C (Fitzgerald et al., 1990; Dawson and Kent, 2000).
Sources of Proteases
Owing to the high demand of proteases in the global market, the search for proteases has tremendously increased, as they are found everywhere in nature, namely, in plants, animals, and microbes. However, production of plant proteases, such as bromelain, keratinases, and ficin, is time-consuming (Rani et al., 2012). The animal proteases, such as pancreatic, trypsin, pepsin, chymotrypsin, and renin are produced and prepared in pure form in large quantities (Weaver et al., 1977; Boyer and Krebs, 1986). The production of proteases from animal sources is insufficient to fulfill the industrial demand worldwide; therefore, scientists have extended their research of producing protease from bacterial sources (Table 2). Owing to the broad-spectrum biochemical variety and easy genetic manipulation, microbes produce an exceptionally promising number of proteases (Godfrey and West, 1996a; Kuhad et al., 2011). Among different sources, such as plants, animals, and microbes, proteases are generally produced by microbial sources. Among microbes, Bacillus sp. are extensively studied for protease production in a large scale, and they are exploited in various industries like leather, detergent, pharmaceuticals, and textile; some fungal species like Aspergillus sp. have been studied thoroughly for the production of alkaline protease (Singhal et al., 2012; Singh et al., 2016; Rehman et al., 2017). A list of microbes producing proteases is given below. Halophilic enzymes are getting more attention in biotechnological applications due to their thermal stability and ability to retain activity under high stress from organic solvents except for pyridine, which inhibits protease activity. The enzyme activities remained the same up to 80% even at 50, 55, and 60°C for at least 30 min (Madern et al., 2000; Margesin and Schinner, 2001; Xue et al., 2012).
Protease and Substrate Specificity
A number of techniques are being exploited for enzyme production from a dominant microbial source for economic improvement (Eichler, 2001; Haki and Rakshit, 2003), but a quest for good quality grade enzymes for industrial use from bacteria is still under consideration. The use microbial origin proteases in the industrial sector is limited by their quality and cost. The increasing interest in using proteases for the production of various eco-friendly goods in the market is of immense importance, and to make the products cost-effective, scientists are in search of a cheap substrate for enzyme production. Almost two-fourths of production cost is due to microbial growth substrate (Singh et al., 2015; Hamza, 2017a). Both solid substrate and submerged fermentation are exploited for the cost-effective production of microbial proteases. The easily available substrate wheat bran is found to be more promising for protease production in solid substrate fermentation (Priya et al., 2016; Hamza, 2017a,b). Other cheap sources of substrate, such as cow dung, agro-industrial waste, groundnuts, and wheat bran can be remarkable for the production of proteases (Krishnaveni et al., 2012; Verma and Agarwa, 2016; Hamza, 2017a). Additionally, other readily available sources of substrate, such as molasses from sugar industry waste, dairy sludge, and effluents are interestingly promising for value-added product enzyme production and concurrently help to lessen eco-pollution (Prabhavathy et al., 2012; Chatterjee et al., 2015; Rao et al., 2017; Corral et al., 2018). For the commercial production of various enzymes especially proteases, waste from the agriculture industry is expected to be used in the future.
Protease and Yield Improvement
Apart from the use of different substrates for protease production from microbial sources to make them high quality and cost-effective, genetic manipulation provides researchers a new opportunity to make changes in bacterial genome using various biotechnological tools to enhance the yield of proteases with desired characteristics. The diversity in microbes and tools opens a new path for strain improvement for industrial use as well (Rathakrishnan and Nagarajan, 2012; Aruna et al., 2014). Scientists have incorporated different ways to improve protease yield for industrial use, such as cloning and overexpression, screening of strains, fed batch, and chemostat fermentation. Different statistical approaches, such as response surface methodology have also been used for the optimization of different media and growth conditions. Both conventional (UV or chemicals) and modern (rDNA) technology are also used for strain improvement for hyperproduction of proteases (Kumar D. M. et al., 2012; Homaei et al., 2016; Rehman et al., 2017). The rDNA technology is recombinant DNA technology carried out through the combination of our desired gene and the genome of organisms like microbes, plants, and/or animal cells. The new cell (plant, microbes, or animals) produced transgenic organisms called genetically modified organisms. The proteases produced through the transformation of protease genes through microbes like Escherichia coli are called recombinant proteases. Due to thermal instability and the high cost of recovery of enzymes, proteases have been restricted for use in the industry regardless of their advantages. These concerns led to the use of immobilization technology to attempt to increase thermal tolerance, stability to pH, and organic solvents. Immobilization technology has been employed to obtain a high yield of alkaline proteases against a solid support of matrix (Kalisz, 1988; Rao et al., 1998). The proteases are usually immobilized in the alginate–chitosan beads, which exhibit reasonable stability and good activity at 47°C (Mehde et al., 2018; Xu et al., 2018; Özacar et al., 2019; Xing et al., 2019). Genetic engineering with the aim of hyperproduction of enzyme, cost-effectiveness, and quality helps scientists to capture the biotechnology market worldwide. Bioengineered enzymes with greater stability are being generated in the detergent industry, especially using rDNA technology. Under extreme conditions, the expression of gene encoding for proteases through using different vector systems including pHY300PLK, pKL9610, pFX1, and plasmid may be maintained and expressed in Bacillus stearothermophilus, B. stearothermophilus, E. coli, and B. subtilis (Roja Rani et al., 2012; Kostyleva et al., 2016).
Purification of Proteases
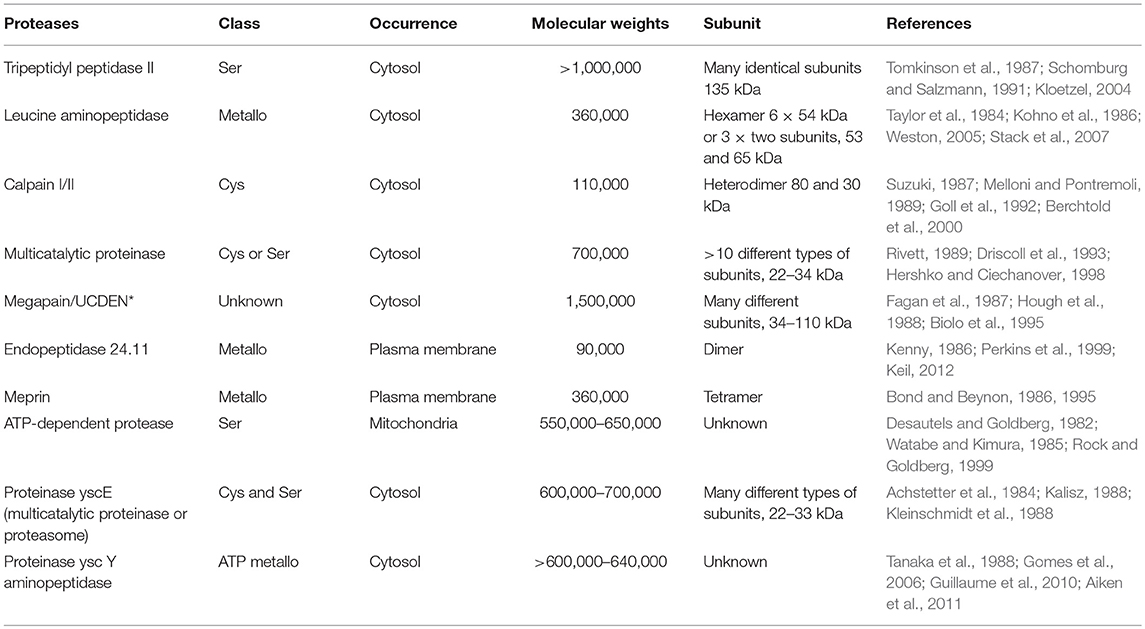
After production of enzymes, purification of these enzymes is a very complex process. A number of methods are in line for their purification. Several techniques are applied for the recovery of value-added product enzymes. The choice of technique depends on the source of enzyme, whether it is extracellular or intracellular (Mienda and Yahya, 2011). During the production and purification of enzymes, the basic consideration is to produce end products that are cost-effective and of high value using economical techniques. Usually, the precipitation method is used for protein recovery from a crude biological mixture. Different reagents, such as salts and organic solvents are used. The most common practice is the use of ammonium sulfate for the precipitation of proteins in an aqueous solution of acidic, neutral, or alkaline pH, which develops ammonium under alkaline conditions. But the use of ammonium sulfate for detergent enzymes has been a choice because under low temperatures, the solubility of salt limits the positive precipitating quality of sodium sulfate salt while the ammonium sulfate enhanced the solubility of salts (Sumantha et al., 2006; Naidu, 2011; Prabhavathy et al., 2013). The use of ion exchange (CM-Sephadex, DEAE-Sephadex) and gel filtration chromatography is expedient for the production of purified proteases, such as alkaline, acidic, and neutral from different bacterial sources, such as Bacillus cereus AT and Bacillus circulans (Kanmani et al., 2011; Annapurna et al., 2012). The preferred technique for the recovery of enzyme formed is the use of dialysis membrane. Ultrafiltration is a pressure-driven separation process that is inexpensive and results in little loss of enzyme activity (Rao et al., 1998; Rani et al., 2012). Such promising techniques like gel filtration are used to determine the molecular mass of proteins using a reference standard of mixture of proteins with known molecular weight (Table 3).
Comparison Among Acidic, Neutral, and Alkaline Proteases
It has been studied extensively that among all enzymes, proteases are being used in various industries abundantly, mainly those of bacterial origin. Acid proteases are obtained from fungal species and neutral proteases are of plant origin. Isolation of both acidic and neutral proteases from fungi and plants is labor-intensive and uneconomical comparatively, while alkaline proteases obtained from bacterial species are demanded by industries because of their cost-effectiveness, ease of production, ready susceptibility to genetic manipulation, less labor intensiveness, and limited space for cultivation.
Microbial Proteases and Industry
Proteases of microbial origin are considered the most significant hydrolytic enzymes, whereas alkaline proteases are ranked the highest in the enzyme market (Mukesh et al., 2012; Mahajan et al., 2016). Interest in studying the proteases has increased not only due to the regulation of different metabolic processes but also due to the significant use in industrial community. The microbes producing substantial numbers of extracellular proteases are of great importance for the industry, and few products of alkaline protease are successfully marketed (Gupta et al., 2002; Gupta and Ramnani, 2006; Vijayaraghavan et al., 2014). Microbial proteases have numerous applications in different industries listed below.
Protein Hydrolysis
In the food industry, proteases are utilized for modification, palatability, and storage life of all available sources of proteins. High nutritional value preparations of protein hydrolysates are achieved by the use of alkaline proteases. In meat tenderization, alkaline proteases of microbial origin are of immense importance (Rao et al., 1998; Sumantha et al., 2006).
Food and Feed Industry
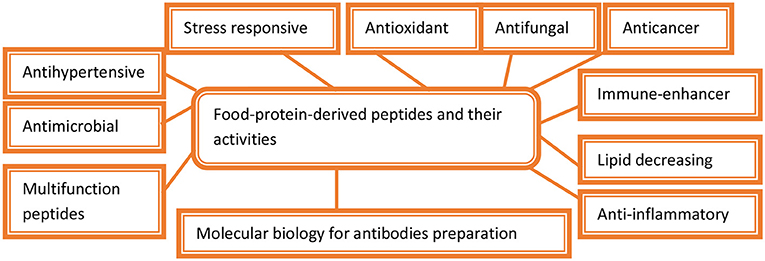
During cheese production from milk, proteases are added to hydrolyze kappa casein to prevent coagulation by stabilizing micelle formation. In the baking industry, for quicker preparation of dough, its gluten is partially hydrolyzed by a heat-labile fungal protease because of its early inactivation in subsequent baking. Protein hydrolysate preparation with high nutritional value has been accomplished by the addition of microbial alkaline proteases. The bioactive peptides play an important role in various pharmaceutical drug formations and as potential molecules under stress environmental conditions (Figure 1). This preparation of hydrolysate is vital in infant food formulation and fortification of soft drinks and juices (Ray, 2012; Singhal et al., 2012; Mótyán et al., 2013; Singh et al., 2016). The mackerel hydrolysates helped in the hydrolysis of protein molecules into free amino acids including carosine, anserine, and other small peptides through the use of proteases. The hydrolysis of proteins into amino acids caused the formation of antioxidants that inhibit autoxidation of linoleic acid and the scavenging effects for α,α-diphenyl-β-picrylhydrazyl free radicals (Wu et al., 2003; Li et al., 2008; Gómez-Guillén et al., 2011). It was found that the long peptides with 1,400 Da molecular weight were stronger antioxidants as compared with smaller peptides with molecular weights of 200 to 900 Da (Clemente, 2000; Foegeding et al., 2002; Tavano, 2013). It has been found that the formation of extensive protein hydrolysates through sequential actions of exoproteases and endopeptidases coupled with the release and development of the post-hydrolysis processes was considered as the most efficient way to produce protein hydrolysates that showed well-defined characteristics during protein hydrolysis (Sarmadi and Ismail, 2010; Chalamaiah et al., 2012; He et al., 2013; Power et al., 2013). The bioactive peptide produced from the hydrolysis of various food proteins plays an important role as antioxidants in cell (Thiansilakul et al., 2007; Nalinanon et al., 2011; Kittiphattanabawon et al., 2012). The protein hydrolysates showed excellent solubility, because of which the antioxidant activities of protein hydrosylates were enhanced (Kumar N. S. et al., 2012; Intarasirisawat et al., 2013; Chi et al., 2015). The bioactive peptides show anticalmodulin, anticancer, and hypocholesterolemic properties, and there are also multifunctional properties of the food-protein-derived peptides (Phoenix et al., 2012; Nicolia et al., 2014; Udenigwe, 2014; Nongonierma and FitzGerald, 2015; Agyei et al., 2016).
Waste Management
The use of chemicals in industries is detrimental to the environment and the surroundings. This hazardous use of chemicals begs for an alternative ecofriendly way for the treatment of waste management. Feathers of poultry containing a very rigid keratin structure accounts for 5% of the body weight and is a rich source of proteins for feed and food. Poultry waste can be degraded into feed and food by the keratinolytic process (Neklyudov et al., 2000; Lasekan et al., 2013). For depilation and cleaning of hairs from drains and clogged pipes, a formulation containing hydrolytic enzymes isolated from B. subtilis, B. amyloliquefaciens, and Streptomyces sp. has been prepared and patented as Genex (Blanch and Moo-Young, 1985; Drew et al., 1985; Ichida et al., 2001; Lasekan et al., 2013).
Leather Industry
Increased application of alkaline protease at emerging leather industries is due to the elastolytic and keratinolytic activity. These influential properties of alkaline protease are very effective in leather processing industries. The particular uses of protease are found to be relevant in the soaking, bating, and dehairing phase of preparing skin and hides. Extermination of unwanted pigments by enzymatic measures helps in clean hide production. Enzymatic proceedings of pancreatic proteases rely on the bating system. Microbial alkaline proteases have become very popular in leather industries (Takami et al., 1992; Brandelli et al., 2010).
Detergent Industry
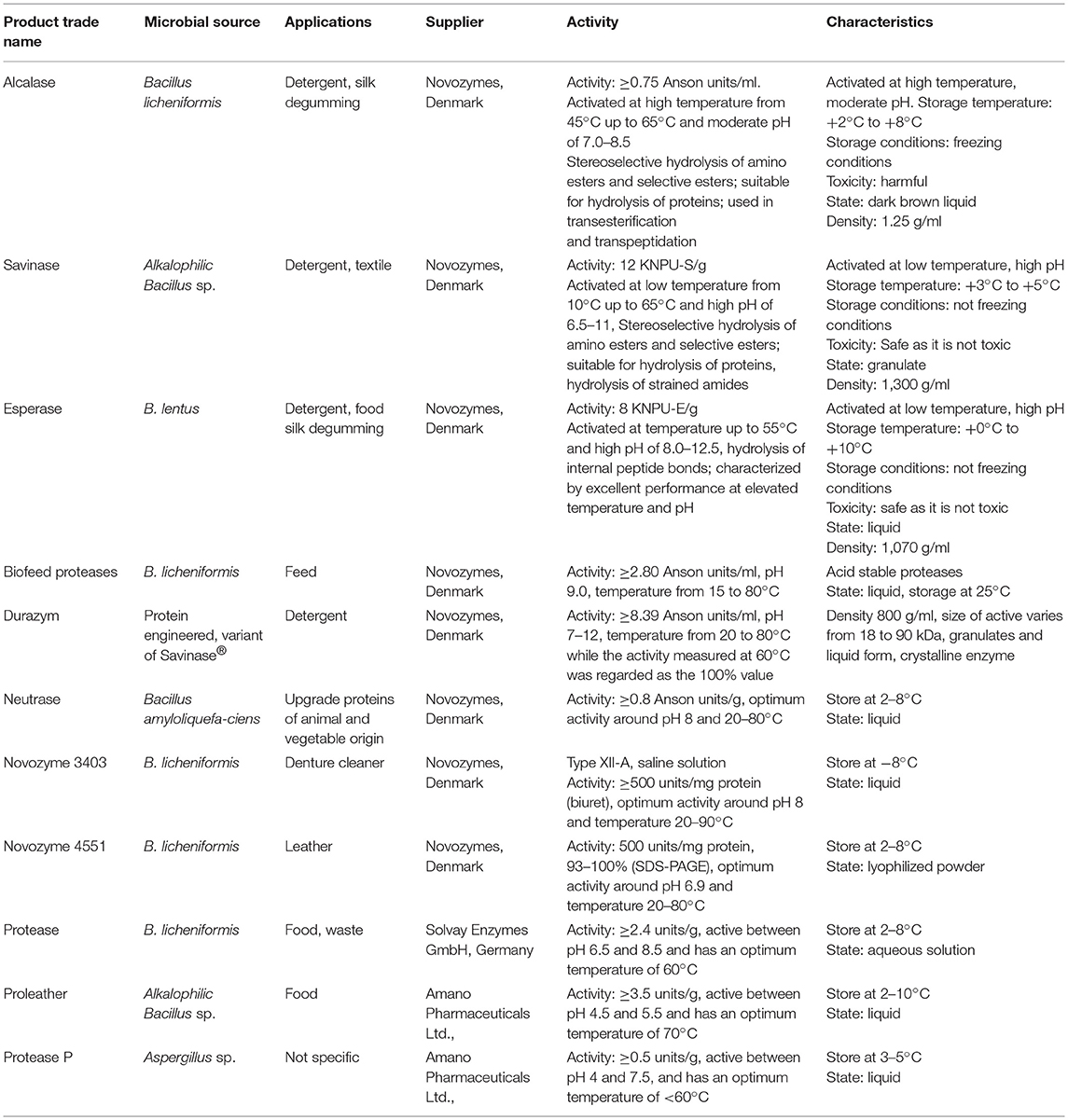
Proteases have been widely used at commercial scale in the detergent industry. The various products in the detergent industry containing proteases as an essential component or ingredient have been used for cleaning of household laundry, dentures, or contact lenses. Of the total sale of enzymes, the utilization of proteases in the detergent industry accounts for ~20%. In 1913, the very first enzymatic preparation, “Brunus,” was prepared consisting of crude pancreatic extract and sodium carbonate. This enzymatic preparation was first marketed in 1956 with a trade name of BIO-40. Alcalase with a trade name of BIOTEX produced by B. licheniformis was introduced into the market by Novo industry A/S in 1960 (Jacobson et al., 1985). Protease produced by B. cereus BM1 was reported as a good detergent ingredient and shows stable activity in a solution of 10% (w/v) commercial detergent (Fabs Perfect), which suggests its commercial consumption (Varela et al., 1997; Illanes, 2008). Isoelectric point is important for the selection of proteases for detergent preparation. Proteases exhibit remarkable results when pH and PI points of these enzymes are approximately concomitant. There are a few other parameters, such as compatibility with surfactants, bleaches and perfumes (De Virgilio et al., 1993; Bayoudh et al., 2000), good activity, optimum pH, and temperature (Aehle et al., 1993; Bech et al., 1993; Kumar et al., 1998; Gupta et al., 1999) ionic strength, stability and removal potential of stain, which have also been considered for the choice of detergent proteases (Beg et al., 2002; Baş and Boyaci, 2007). Traditionally, detergents work at high temperature but the interest has been increased to search and identify alkaline proteases working in a wide range of temperature (Csuk and Glaenzer, 1991; Breuer et al., 2004). Generally, in the presence of bleaching or oxidizing agent, commercially available proteases are not stable. Recently, rDNA technology has been incorporated to produce bioengineered detergent proteases with greater stability and shelf life. By the use of protein engineering, the replacement of few specific amino acid residues has been studied for bleach and oxidation stability of proteases (Oberoi et al., 2001; Sellami-Kamoun et al., 2008; Haddar et al., 2009b). Proteases have been used not only as laundry detergent but also as dishwashing and cleaning detergents both in institutional and industrial sectors (Estell et al., 1985; Shanlin et al., 1997; Bornscheuer et al., 2012).
Photographic Industry
Alkaline proteases produced by B. subtilis, Streptomyces avermectnus, and Conidiobolus coronatus have been successfully reported to recover silver from X-ray films, ensuring that the process is eco-friendlier over the use of chemicals (Godfrey and West, 1996b; Wolff et al., 1996; Yang Y. et al., 2000). Silver recovery by the efficient use of thermally stable mutant alkaline protease produced by Bacillus sp. B21-2 has also been reported for its potential (Bettiol and Showell, 2002; Dhawan and Kaur, 2007; Araujo et al., 2008).
Chemical Industry
Various alkaline proteases producing microorganisms, such as Bacillus pseudofirus SVB1, Aspergillus flavus, and Pseudomonas aeruginosa PseA showed substantial results in peptide synthesis due to stability in organic solvents (Nakiboglu et al., 2001; Ahmed et al., 2008; Shankar et al., 2010). Some alkaline protease producing species of Bacillus and Streptomyces in the water system are active candidates for peptide and organic synthesis (Masui et al., 2004; Jadhav and Hocheng, 2012; Yadav et al., 2015).
Silk Degumming
A proteinaceous substance, “sericin or silk gum,” must be removed by the process of degumming from raw silk in an alkaline solution of soap conventionally. Alkaline protease is the best choice to remove sericin while not attacking the fiber. It has been proven that fiber break is not amenable, and silk threads are found to be much stronger than when previous traditional treatments were used (Yadav et al., 2011; da Silva et al., 2017; Radha et al., 2017).
Medical Field
With the passage of time, scientists have found the broad use of proteases in medical field successfully. In medicine, different formulas, such as gauze, non-woven tissues, and ointment composition containing alkaline proteases produced by B. subtilis show promising therapeutic properties (Sen et al., 2011; Anbu, 2013; Awad et al., 2013). Certain lytic enzyme deficiency syndromes are diagnosed to be aided by an oral administration of alkaline proteases (Gupta and Khare, 2007; Joshi and Satyanarayana, 2013). It has been reported that fibrin degradation has been achieved by alkaline fibrinolytic proteases. The use of this fibrinolytic enzyme suggests its future application as an anticancer drug and in thrombolytic therapy (Jaouadi et al., 2011, 2012). Slow-release dosage form preparation containing collagenases with alkaline proteases is extensively used in therapeutic applications. The hydrolysis of collagen by the enzyme liberates low-molecular-weight peptides without any amino acid release for therapeutic use (Romsomsa et al., 2010; Suwannaphan et al., 2017). For the treatment of various diseases, such as burns, carbuncles, furuncles, and wounds, a preparation of elastoterase immobilized on bandage is used (Davidenko, 1999; Palanivel et al., 2013).
Other Perspectives of Proteases
Apart from vital industrial application of proteases, they are being used for the cleavage of peptide bond to elucidate the association between structure and function of peptides and proteins. Alkaline proteases isolated from Vibrio metschnikovii RH530 can be used as an alternative to proteinase K in DNA isolation (Mukherjee and Rai, 2011; Narasimhan et al., 2015; Vijayaraghavan and Vincent, 2015). Hence, the proteases can be viewed as an alternative to many chemicals involved in various biochemical and physiological processes.
Protease Engineering
Genetic engineering has an enormous contribution on various aspects of life, such as in the field of environmental protection, food production, human health care, animal husbandry, manufacturing of biochemicals, and fuels. In the future, the manipulation of genetic makeup of different organisms will facilitate the production of different therapeutic and industrially important proteins and enzymes to meet the human requirements and combat different serious diseases (Pursel et al., 1989; Cappello et al., 1990; Wang et al., 2003; Mittler and Blumwald, 2010; Hockemeyer et al., 2011).
The production of genetic modified E. coli for the formation of proteases has introduced new and emerging improvement in the development of recombinant proteins (Figure 2). The use of mutations may also be helpful for the formation and isolation of proteases (Simkhada et al., 2010b; Kotb, 2013). Protease engineering in laundry detergents provided improvement in thermal or high-temperature resistance, which allowed proteases to work even under low-temperature conditions. The three protease engineering campaigns presented provide in-depth analysis of protease properties and have identified principles that can be applied to improve or generate enzyme variants for industrial applications beyond laundry detergents (Barthomeuf et al., 1992; Vijayaraghavan and Vincent, 2015; Vojcic et al., 2015; Coker, 2016; Shahid et al., 2016; dos Santos Aguilar and Sato, 2018). The cold-adapted protease subtilisin has been successfully isolated through evolutionary engineering, which is based on the sequential in vitro mutagenesis along with the improved screening method. It was found that the mutation in the subtilisin, termed m-63, exhibited higher efficiency for catalytic activities, which was 100% much higher than that of the wild type at 10°C under N-succinyl-l-Ala-l-Ala-l-Pro-l-Phe-p-nitroanilide as a synthetic substrate for enzyme activities. It was found that the engineering for protease for cold resistance gives cold tolerance in protease, which allowed it to work even under low temperatures (Banerjee and Ray, 2017; Castilla et al., 2017; Onaizi, 2018; Zhou et al., 2019). The mutant proteases from the papain family, such as Glnl9His, Glnl9Glu, and Gin 19Ala, indicated that the Gln19Glu and Glnl9His enzymes participated in the acid-catalyzed hydrolysis in thiomidate, which was converted into amide through the provision of H+ (proton) to form the more reactive protonated thiomidate, which can work at low as well as higher levels of thermal conditions (D'Amico et al., 2002; Siddiqui and Cavicchioli, 2006; Margesin et al., 2007; De Maayer et al., 2014).
Specific inhibition for serin proteases caused crucial switches in a large number of physiological processes for proteases, such as therapeutic applications like ecotin (potential macromolecular inhibitor for serin proteases), which shows as attractive scaffold for engineering the specific proteases inhibitors. The scaffolds showed higher protease inhibition with an apparent dissociation equilibrium constant (Ki*) at 11 pM; however, the Ki* values that were related to proteases [Factor Xa (FXa), thrombin, urokinase-type plasminogen activator (uPA), Factor XIa (FXIa), and membrane-type serine proteases 1] showed four to seven higher orders of magnitudes. The adaptabilities of the scaffolds were also demonstrated though isolation for protease inhibitors up to two additional serine proteases, such as Factor XIIa and membrane-type serine proteases 1/matriptase (Liu et al., 2018; Krasileva, 2019a,b; Zhang et al., 2019).
A large number of serin protease subtilisins require the assistance of N-terminal pro-sequence for precursors for the formation of mature and active protease enzymes. The findings from this study indicates that engineering through the use of pro-sequences, i.e., the site-directed or random mutagenesis for proteases, chimeras, and the gene shufflings between the protease members of the serin protease family, would be a very useful tool for the improvement in functions of the autoprocessing protease enzymes. The conventional or traditional protein engineering techniques now have thus far employed mutagenesis in the protease domains for modification in the enzymatic properties of proteases. The new approach, termed pro-sequencing protein engineering, is not only an important technique for the study of protein folding mechanisms but also a highly promising technology to create unique proteases that have various beneficial catalytic properties (Hosse et al., 2006; Ruigrok et al., 2011; Mascini et al., 2012; Fang et al., 2016, 2017a; Verma et al., 2016; Huang et al., 2017). The Gly216 is an active site for proteases and is specific to the MA190 mutant from α-lytic proteases. It has also been found to be extraordinarily tolerant for an amino acid substitution in proteins. The side chains are usually as long as the Trp, which can be accommodated within the substrate binding pocket without decreasing the catalytic activity of enzymes. The GA216 + MA190 expression for specificities of enzymes was altered due to mutation that produced GL216 + MA190 mutants, which were crystallized both with and without a representative in the series of peptide boronic acid transition state that were analog inhibitors for proteases. Results show that the substrates are the agents that specifically determine the α-lytic protease with distributed properties of the active sites and substrate molecules (Cunningham and Agard, 2003; Ljungdahl, 2009; Liu et al., 2014; Fang et al., 2017b; Yang et al., 2017). The proteases are usually not perfect in acyltransferases. All unwanted proteolytic side reactions of proteases and the protease hydrolysis for the acyl-enzyme during kinetic approaches are the key problems for enzymatic peptide synthesis and activity losses. The planning and optimization for enzymatic peptide synthesis always require the “S” or subsite mapping for proteases along with the knowledge of additional fundamental parameters that determined the reaction courses of proteases (Jakubke, 1994; Jäckel and Koksch, 2005; Baker and Numata, 2012; Asgher et al., 2018; Tavano et al., 2018; Antink et al., 2019; de Souza Vandenberghe et al., 2019; Mota et al., 2019; Siar et al., 2019).
Genetic engineering has been instrumental in understanding the relationship between structure and function of different genetic systems and is an excellent method for manipulating the genes. Genetic engineering is being incorporated for the production of industrially important bacterial enzymes. It has been reported that microbial proteases have been isolated and manipulated with the aim of (i) enzyme overproduction, (ii) studying the primary structure of protein, and (iii) applying protein engineering to suit commercial applications. However, the protease gene from bacteria has been cloned and sequenced (Hogdson, 1994).
The ability of B. subtilis to be nonpathogenic and to produce extracellular proteins in the medium makes it a potential host for the production of recombinant protease enzyme. B. subtilis secretes industrially important proteases subtilisin (apr) or mettaloproteases (npr). This significant study reveals an understanding of the mechanism of overproduction of the proteins. Different strains, such as B. subtilis 168 secretes at least six extracellular proteases into the medium, such as structural genes, neutral protease A and B, minor extracellular protease, bacillopeptidase F, and metalloprotease, which have been cloned. Henner et al. replaced promoters of apr and npr WITH the amylase promoter from B. amyloliquefaciens and B. subtilis, respectively, to increase the expression (Henner et al., 1985; Sloma et al., 1990; Connelly et al., 2004; Bloor and Cranenburgh, 2006; El-Gamal et al., 2012; Idbeaa and Omar, 2016).
A serine protease gene (hspK) of 90 kDa was cloned and sequenced from B. subtilis (Natto) 16 (Yamagata et al., 1995; Satyanarayana et al., 2012; Guleria et al., 2016). A conserved sequence was found between subtilisin BPN and subtilisin Carlsberg from B. amyloliquefaciens and B. licheniformis in the coding region and must have a common precursor (Narhi et al., 1991; Li et al., 2013; Souza et al., 2015). It was also reported that the gene encoding subtilisin amylosacchariticus from B. subtilis subsp. and sequence showed homology to subtilisin E from B. subtilis 168. This gene was then expressed in B. subtilis ISW 1214 using a vector pHY300PLK and showed 20 times more activity than the host (Vasantha et al., 1984; Bordusa, 2002; Gamblin et al., 2008; Heck et al., 2013).
Serratia, a gram-negative bacterium, secretes extracellular protease into the medium. Different strains of Serratia like E-15 produce extracellular metalloprotease, which is used as an anti-inflammatory agent. The corresponding gene was expressed in both S. marcescens and E. coli and an active site and three zinc ligands were revealed. Another study showed that the extracellular serine protease of S. marcescens was excreted through the outer membrane of E. coli. The nucleotide sequence suggested that it produced a preproenzyme of 112 kDa composed of N-terminal sequence and C-terminal sequence (Stabile et al., 1996; Chalker et al., 2009; Dumas et al., 2013).
In the detergent industry, normally, alkaline proteases are preferred over subtilisin with an optimum pH between 8.5 and 10.0. The ale gene was cloned and sequenced, encoding alkaline elastase YaB based on the information available on enzymes (De Vos, 1987; Rao et al., 1998; Sørvig et al., 2005). The resulting amino acid sequence was 55% similar to subtilisin BPN. The positively charged residues are present on the surface of the alkaline elastase YaB molecule, which facilitates its binding to elastin. Another amino acid sequence of alkaline serine protease deduced from B. alcalophilus PB92 shows homology to YaB. Using chromosola integration, the cloned gene was further used to increase the protease production by gene amplification. An ISP-1 encoding gene isolated from alkalophilic Bacillus sp. strain NKS-21 was characterized. It was determined that its nucleotide sequence showed 50% homology to the gene encoding ISP-1 isolated from B. subtilis, B. polymyxa, and Bacillus sp. strain 221 (Kaneko et al., 1989; Gupta et al., 2008; Deng et al., 2010).
A species of lactobacillus, such as Lactococcus lactis is used as starter culture in the dairy industry, having a complex system of proteolysis that enables it to grow in milk by the degradation of casein into small peptides and free amino acids. This activity leads to the development of flavor and texture of different dairy products. Lactococcal proteases have been classified into P-I-type protease and P-III-type protease on the basis of differences in caseinolytic specificity. The former degrades predominantly beta casein while the latter degrades alpha S1-, beta-, and K-casein (305), but genetic studies focus more on the P-I-type protease. These protease genes located on plasmids greatly differ in size and genetic organization in different strains (Yamagata et al., 1995; Rao et al., 1998; Helianti et al., 2018; Jeong et al., 2018; Ariyaei et al., 2019).
Extracellular serine proteases A and B are secreted by an organism, Streptomyces griseus, used for commercial production of pronase. The genes encoding protease A (sprA) and protease B (sprB) were isolated from the S. griseus genomic library, and their proteolytic activity was demonstrated in Streptomyces lividans (Henderson et al., 1987; Ramesh et al., 2009; Thirumurugan and Vijayakumar, 2015). Each enzyme is initially secreted as a precursor as suggested by the DNA sequences, which is then incorporated to remove N-terminal propeptide from the mature protease. A strong homology between their coding regions is reported, which suggests that both genes must have originated by gene duplication. Protease B is reported to be one of the major proteases secreted by S. griseus ATCC10137, expressed its gene in S. lividans (Hwang et al., 1993; Tammawong, 2005).
The extracellular enzyme alpha-lytic protease representing the family of trypsin in a soil bacterium Lysobacter enzymogenes 495 is of particular interest. S1 mapping and nucleotide sequence of the structural gene for alpha-lytic protease from L. enzymogenes 495 suggested that it is synthesized as preproprotein with a size of 41 kDa and is processed to its mature extracellular form (20 kDa) (Vasantha et al., 1984; Silen et al., 1988; Palumbo et al., 2003; Qian et al., 2009). Fusing of the promoter and signal sequences of E. coli phoA to the proenzyme portion of the alpha-lytic protease gene was expressed in E. coli for protease enzyme production (Silen et al., 1989; Rattenholl et al., 2001; Mitsuiki et al., 2004). With the following induction, an active enzyme was produced both intra- and extracellularly. Fusion of the mature protein domain alone resulted in the production of an inactive enzyme, indicating that the large N-terminal pro-protein region is necessary for activity. Epstein and Wensink also cloned and sequenced the gene for alpha-lytic protease, a 19.8-kDa serine protease secreted by L. enzymogenes (Qian et al., 2009, 2014). The nucleotide sequence contains an ORF that codes for the 198-residue mature enzyme and a potential prepropeptide, also of 198 residues (Epstein and Wensink, 1988; Reichenbach, 2006; Wang et al., 2013).
Future Prospects
The study of biochemical and molecular aspects of proteolytic systems, such as proteases is gaining interest from researchers due to different reasons. Researchers and engineers are looking for robust and novel bacterial enzymes because of the realization of the commercial value of this enzyme. In the future, protein engineering will play a primary role in producing proteases with new properties. Among proteases, alkaline bacterial proteases play a vital role in different industries due to their potential, and their future use is likely to be increased. Advance strategies like protein/genetic engineering, molecular biology, and computational biology are being adopted by the researchers to generate improved protease-producing strains. Bacterial strains with desirable characteristics will be produced by using in vitro evolutionary changes in the protein primary structure. One major goal of scientists is to achieve bacterial proteases with characteristics, such as yield improvement, changing substrate specificity, enhancement of thermal stability, altering optimum pH, and prevention of auto-proteolytic inactivation.
Conclusions
Since the advent of enzymology, enzymes have been broadly utilized in a wide range of industries like textile, pharmaceuticals, leather, food, and detergent. Globally, its use and production are increasing with the use of cheap raw material and by incorporating genetic manipulation. Now, there is an urgent need for the use of such technology that promises cleaner production as an alternative to the use of hazardous chemicals, such as proteases. The higher-ups and the state should take the responsibility of encouraging investors for a cleaner production to mitigate the risk of eco-pollution.
Author Contributions
AR and SS wrote the initial draft of the manuscript. QA made all necessary corrections and carried out final editing of manuscript. AA, MS, and AM proof read the manuscript. Final approval for publication was given by MA.
Conflict of Interest Statement
AA was employed by the company Four Brothers Private Limited, Pakistan.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Achstetter, T., Emter, O., Ehmann, C., and Wolf, D. H. (1984). Proteolysis in eukaryotic cells. Identification of multiple proteolytic enzymes in yeast. J. Biol. Chem. 259, 13334–13343.
Adetunji, C. O., and Adejumo, I. O. (2018). Efficacy of crude and immobilizedenzymes from Bacillus licheniformis for production of biodegraded feather meal and their assessment on chickens. Environ. Technol. Innov. 11, 116–124. doi: 10.1016/j.eti.2018.05.002
Adinarayana, K., Ellaiah, P., and Prasad, D. S. (2003). Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmSciTech 4, 440–448. doi: 10.1208/pt040456
Adrio, J., and Demain, A. (2014). Microbial enzymes: tools for biotechnological processes. Biomolecules 4, 117–139. doi: 10.3390/biom4010117
Aehle, W., Sobek, H., Amory, A., Vetter, R., Wilke, D., and Schomburg, D. (1993). Rational protein engineering and industrial application: Structure prediction by homology and rational design of protein-variants with improved ‘washing performance’—the alkaline protease from Bacillus alcalophilus. J. Biotechnol. 28, 31–40. doi: 10.1016/0168-1656(93)90123-5
Agyei, D., Ongkudon, C. M., Wei, C. Y., Chan, A. S., and Danquah, M. K. (2016). Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 98, 244–256. doi: 10.1016/j.fbp.2016.02.003
Ahmed, S. A., Al-Domany, R. A., El-Shayeb, N. M., Radwan, H. H., and Saleh, S. A. (2008). Optimization, immobilization of extracellular alkaline protease and characterization of its enzymatic properties. Res. J. Agric. Biol. Sci. 4, 434–446.
Aiken, C. T., Kaake, R. M., Wang, X., and Huang, L. (2011). Oxidative stress-mediated regulation of proteasome complexes. Mol. Cell. Proteomics 10:R110.006924. doi: 10.1074/mcp.R110.006924
Ali, N., Ullah, N., Qasim, M., Rahman, H., Khan, S. N., Sadig, A., et al. (2016). Molecular characterization and growth optimization of halo-tolerant protease producing Bacillus subtilis Strain BLK-1.5 isolated from salt mines of Karak, Pakistan. Extremophiles 20, 395–402. doi: 10.1007/s00792-016-0830-1
Amoozegar, M. A., Fatemi, A. Z., Karbalaei-Heidari, H. R., and Razavi, M. R. (2007). Production of an extracellular alkaline metalloprotease from a newly isolated, moderately halophile, Salinivibrio sp. strain AF-2004. Microbiol. Res. 162, 369–377. doi: 10.1016/j.micres.2006.02.007
Anbu, P. (2013). Characterization of solvent stable extracellular protease from Bacillus koreensis (BK-P21A). Int. J. Biol. Macromol. 56, 162–168. doi: 10.1016/j.ijbiomac.2013.02.014
Annapurna, S. A., Singh, A., Garg, S., Kumar, A., and Kumar, H. (2012). Screening, isolation and characterisation of protease producing moderately halophilic microorganisms. Asian J. Microbiol. Biotech. Environ. Sci. 14, 603–612.
Antink, M. M. H., Sewczyk, T., Kroll, S., Árki, P., Beutel, S., Rezwan, K., et al. (2019). Proteolytic ceramic capillary membranes for the production of peptides under flow. Biochem. Eng. J. 147, 89–99. doi: 10.1016/j.bej.2019.04.005
Araujo, R., Casal, M., and Cavaco-Paulo, A. (2008). Application of enzymes for textile fibres processing. Biocatal. Biotransformation 26, 332–349. doi: 10.1080/10242420802390457
Ariyaei, A., Farhadi, A., Moradian, F., and Mianji, G. R. (2019). Cloning, expression and characterization of a novel alkaline serine protease gene from native Iranian Bacillus sp.; a producer of protease for use in livestock. Gene 69, 310–315. doi: 10.1016/j.gene.2019.01.020
Arora, N. K., and Mishra, J. (2016). Prospecting the roles of metabolites and additives in future bioformulations for sustainable agriculture. Appl. Soil Ecol. 107, 405–407. doi: 10.1016/j.apsoil.2016.05.020
Aruna, K., Shah, J., and Birmole, R. (2014). Production and partial characterization of alkaline protease from Bacillus tequilensis strains CSGAB0139 isolated from spoilt cottage cheese. Int J Appl Biol Pharm. 5, 201–221.
Asgher, M., Bashir, F., and Iqbal, H. M. (2018). Protease-based cross-linked enzyme aggregates with improved catalytic stability, silver removal, dehairing potentials. Int. J. Biol. Macromol. 118, 1247–1256. doi: 10.1016/j.ijbiomac.2018.06.107
Awad, H. M., Mostafa, E.-S. E., Saad, M. M., Selim, M. H., and Hassan, H. M. (2013). Partial purification and characterization of extracellular protease from a halophilic and thermotolerant strain Streptomyces pseudogrisiolus NRC-15. Indian. J. Biochem. Biophys. 50, 305–311.
Baker, P. J., and Numata, K. (2012). Chemoenzymatic synthesis of poly (L-alanine) in aqueous environment. Biomacromolecules 13, 947–951. doi: 10.1021/bm201862z
Banerjee, G., and Ray, A. K. (2017). Impact of microbial proteases on biotechnological industries. Biotechnol. Genet. Eng. Rev. 33, 119–143. doi: 10.1080/02648725.2017.1408256
Banerjee, U. C., Sani, R. K., Azmi, W., and Soni, R. (1999). Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem. 35, 213–219. doi: 10.1016/S0032-9592(99)00053-9
Barrett, A. J. (1995). Methods in Enzymology. Proteolytic Enzymes: Aspartic and Metallo Peptidases, Vol. 248. Cambridge: Academic Press.
Barrett, A. J., and McDonald, J. K. (1986). Nomenclature: protease, proteinase and peptidase. Biochem. J. 237:935. doi: 10.1042/bj2370935
Barthomeuf, C., Pourrat, H., and Pourrat, A. (1992). Collagenolytic activity of a new semi-alkaline protease from Aspergillus niger. Journal of fermentation and bioengineering 73, 233–236. doi: 10.1016/0922-338X(92)90168-T
Baş, D., and Boyaci, I. H. (2007). Modeling and optimization I: usability of response surface methodology. J. Food Eng. 78, 836–845. doi: 10.1016/j.jfoodeng.2005.11.024
Bayoudh, A., Gharsallah, N., Chamkha, M., Dhouib, A., Ammar, S., and Nasri, M. (2000). Purification and characterization of an alkaline protease from Pseudomonas aeruginosa MN1. J. Ind. Microbiol. Biotechnol. 24, 291–295. doi: 10.1038/sj.jim.2900822
Bech, L. M., Branner, S., Breddam, K., and Groen, H. (1993). Oxidation Stable Detergent Enzymes. Google Patents.
Beg, Q. K., and Gupta, R. (2003). Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enzyme Microb. Technol. 32, 294–304. doi: 10.1016/S0141-0229(02)00293-4
Beg, Q. K., Sahai, V., and Gupta, R. (2003). Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem. 39, 203–209. doi: 10.1016/S0032-9592(03)00064-5
Beg, Q. K., Saxena, R., and Gupta, R. (2002). Kinetic constants determination for an alkaline protease from Bacillus mojavensis using response surface methodology. Biotechnol. Bioeng. 78, 289–295. doi: 10.1002/bit.10203
Berchtold, M. W., Brinkmeier, H., and Muntener, M. (2000). Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 80, 1215–1265. doi: 10.1152/physrev.2000.80.3.1215
Bettiol, J.-L. P., and Showell, M. S. (2002). Detergent Compositions Comprising a Mannanase and a Protease. Google Patents.
Bhaskar, N., Sudeepa, E., Rashmi, H., and Selvi, A. T. (2007). Partial purification and characterization of protease of Bacillus proteolyticus CFR3001 isolated from fish processing waste and its antibacterial activities. Bioresour. Technol. 98, 2758–2764. doi: 10.1016/j.biortech.2006.09.033
Biolo, G., Fleming, R. D., and Wolfe, R. R. (1995). Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J. Clin. Invest. 95, 811–819. doi: 10.1172/JCI117731
Blanch, H. W., and Moo-Young, M. (1985). Comprehensive Biotechnology: The Principles, Applications and Regulations of Biotechnology in Industry, Agriculture and Medicine. 3rd Edn. Oxford; Toronto, ON: Pergamon Press.
Bloor, A. E., and Cranenburgh, R. M. (2006). An efficient method of selectable marker gene excision by Xer recombination for gene replacement in bacterial chromosomes. Appl. Environ. Microbiol. 72, 2520–2525. doi: 10.1128/AEM.72.4.2520-2525.2006
Bohacz, J., and Korniłłowicz-Kowalska, T. (2019). Fungal diversity and keratinolytic activity of fungi from lignocellulosic composts with chicken feathers. Process Biochem. 80, 119–128. doi: 10.1016/j.procbio.2019.02.012
Bond, J. S., and Beynon, R. J. (1986). Meprin: a membrane-bound metallo-endopeptidase. Curr. Top. Cell. Regul. 28, 263–290. doi: 10.1016/B978-0-12-152828-7.50009-3
Bond, J. S., and Beynon, R. J. (1995). The astacin family of metalloendopeptidases. Prot. Sci. 4, 1247–1261. doi: 10.1002/pro.5560040701
Bordusa, F. (2002). Proteases in organic synthesis. Chem. Rev. 102, 4817–4868. doi: 10.1021/cr010164d
Bornscheuer, U., Huisman, G., Kazlauskas, R., Lutz, S., Moore, J., and Robins, K. (2012). Engineering the third wave of biocatalysis. Nature 485:185. doi: 10.1038/nature11117
Brandelli, A., Daroit, D. J., and Riffel, A. (2010). Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 85, 1735–1750. doi: 10.1007/s00253-009-2398-5
Breuer, M., Ditrich, K., Habicher, T., Hauer, B., Keßeler, M., Stürmer, R., et al. (2004). Industrial methods for the production of optically active intermediates. Angew. Chem. Int. Ed. 43, 788–824. doi: 10.1002/anie.200300599
Călin, M., Constantinescu-Aruxandei, D., Alexandrescu, E., Răut, I., Doni, M. B., Arsene, M.-L., et al. (2017). Degradation of keratin substrates by keratinolytic fungi. Electron. J. Biotechnol. 28, 101–112. doi: 10.1016/j.ejbt.2017.05.007
Cappello, J., Crissman, J., Dorman, M., Mikolajczak, M., Textor, G., Marquet, M., et al. (1990). Genetic engineering of structural protein polymers. Biotechnol. Prog. 6, 198–202. doi: 10.1021/bp00003a006
Castilla, A., Panizza, P., Rodríguez, D., Bonino, L., Díaz, P., Irazoqui, G., et al. (2017). A novel thermophilic and halophilic esterase from Janibacter sp. R02, the first member of a new lipase family (Family XVII). Enzyme Microb. Technol. 98, 86–95. doi: 10.1016/j.enzmictec.2016.12.010
Cavello, I. A., Crespo, J. M., García, S. S., Zapiola, J. M., Luna, M. F., and Cavalitto, S. F. (2015). Plant growth promotion activity of keratinolytic fungi growing on a recalcitrant waste known as “Hair Waste”. Biotechnol. Res. Int. 2015:952921. doi: 10.1155/2015/952921
Centeno, R., Espino, T., and Mercado, M. (1996). Purification and partial characterization of alkaline protease from Bacillus subtilis NRRL B-3749. Phil. J. Biotechol. 7, 25–34.
Chalamaiah, M., Hemalatha, R., and Jyothirmayi, T. (2012). Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 135, 3020–3038. doi: 10.1016/j.foodchem.2012.06.100
Chalker, J. M., Wood, C. S., and Davis, B. G. (2009). A convenient catalyst for aqueous and protein Suzuki–Miyaura cross-coupling. J. Am. Chem. Soc. 131, 16346–16347. doi: 10.1021/ja907150m
Charles, P., Devanathan, V., Anbu, P., Ponnuswamy, M., Kalaichelvan, P., and Hur, B. K. (2008). Purification, characterization and crystallization of an extracellular alkaline protease from Aspergillus nidulans HA-10. J. Basic Microbiol. 48, 347–352. doi: 10.1002/jobm.200800043
Chatterjee, J., Giri, S., Maity, S., Sinha, A., Ranjan, A., Rajshekhar, et al. (2015). Production and characterization of thermostable alkaline protease of Bacillus subtilis (ATCC 6633) from optimized solid-state fermentation. Biotechnol. Appl. Biochem. 62, 709–718. doi: 10.1002/bab.1309
Chavan, V., and Patil, N. (2007). Study of leukocytic hydrolytic enzymes in patients with acute stage of coronary heart disease. Indian J. Med. Sci. 61, 73–82.
Cheng, S.-W., Hu, H.-M., Shen, S.-W., Takagi, H., Asano, M., and Tsai, Y. C. (1995). Production and characterization of keratinase of a feather-degrading Bacillus licheniformis PWD-1. Biosci. Biotechnol. Biochem. 59, 2239–2243. doi: 10.1271/bbb.59.2239
Chi, C.-F., Wang, B., Wang, Y.-M., Zhang, B., and Deng, S.-G. (2015). Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 12, 1–10. doi: 10.1016/j.jff.2014.10.027
Chou, J. J., Matsuo, H., Duan, H., and Wagner, G. (1998). Solution structure of the RAIDD CARD and model for CARD/CARD interaction in caspase-2 and caspase-9 recruitment. Cell 94, 171–180. doi: 10.1016/S0092-8674(00)81417-8
Chou, K.-C. (2004). Structural bioinformatics and its impact to biomedical science. Curr. Med. Chem. 11, 2105–2134. doi: 10.2174/0929867043364667
Chou, K.-C. (2006). Structural bioinformatics and its impact to biomedical science and drug discovery. Front. Med. Chem. 3, 455–502. doi: 10.2174/978160805206610603010455
Chou, K.-C., and Howe, W. J. (2002). Prediction of the tertiary structure of the β-secretase zymogen. Biochem. Biophys. Res. Commun. 292, 702–708. doi: 10.1006/bbrc.2002.6686
Chou, K.-C., Jones, D., and Heinrikson, R. L. (1997). Prediction of the tertiary structure and substrate binding site of caspase-8. FEBS Lett. 419, 49–54. doi: 10.1016/S0014-5793(97)01246-5
Chou, K.-C., Tomasselli, A. G., and Heinrikson, R. L. (2000). Prediction of the tertiary structure of a caspase-9/inhibitor complex. FEBS Lett. 470, 249–256. doi: 10.1016/S0014-5793(00)01333-8
Chou, K.-C., Wei, D.-Q., and Zhong, W.-Z. (2003). Binding mechanism of coronavirus main proteinase with ligands and its implication to drug design against SARS. Biochem. Biophys. Res. Commun. 308, 148–151. doi: 10.1016/S0006-291X(03)01342-1
Clemente, A. (2000). Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 11, 254–262. doi: 10.1016/S0924-2244(01)00007-3
Coker, J. A. (2016). Extremophiles and biotechnology: current uses and prospects. F1000Res. 5:F1000 Faculty Rev-396. doi: 10.12688/f1000research.7432.1
Connelly, M. B., Young, G. M., and Sloma, A. (2004). Extracellular proteolytic activity plays a central role in swarming motility in Bacillus subtilis. J. Bacteriol. 186, 4159–4167. doi: 10.1128/JB.186.13.4159-4167.2004
Corral, J. C. C., de los Santos Villalobos, S., Barrgàn, L. A. P., Figueroa, J. J. B., Vásquez-Murrieta, M. S., Estrada Alvarado, M. I., et al. (2018). Isolation of moderately halophilic bacteria in saline environments of Sonora State searching for proteolytic hydrolases. Open Agric. 3, 207–213. doi: 10.1515/opag-2018-0021
Csuk, R., and Glaenzer, B. I. (1991). Baker's yeast mediated transformations in organic chemistry. Chem. Rev. 91, 49–97. doi: 10.1021/cr00001a004
Cunningham, E. L., and Agard, D. A. (2003). Interdependent folding of the N- and C-terminal domains defines the cooperative folding of α-lytic protease. Biochemistry 42, 13212–13219. doi: 10.1021/bi035409q
da Silva, O. S., Gomes, M. H. G., de Oliveira, R. L., Porto, A. L. F., Converti, A., and Porto, T. S. (2017). Partitioning and extraction protease from Aspergillus tamarii URM4634 using PEG-citrate aqueous two-phase systems. Biocatal. Agric. Biotechnol. 91, 68–73. doi: 10.1016/j.bcab.2016.12.012
D'Amico, S., Claverie, P., Collins, T., Georlette, D., Gratia, E., Hoyoux, A., et al. (2002). Molecular basis of cold adaptation. Philos. Trans. R. Soc. Lond. Series B Biol. Sci. 357, 917–925. doi: 10.1098/rstb.2002.1105
Das, G., and Prasad, M. (2010). Isolation, purification & mass production of protease enzyme from Bacillus subtilis. Int. Res. J. Microbiol. 1, 26–31.
Davidenko, T. (1999). Immobilization of alkaline protease on polysaccharides of microbial origin. Pharma. Chem. J. 33, 487–489. doi: 10.1007/BF02510074
Dawson, P. E., and Kent, S. B. (2000). Synthesis of native proteins by chemical ligation. Annu. Rev. Biochem. 69, 923–960. doi: 10.1146/annurev.biochem.69.1.923
De Maayer, P., Anderson, D., Cary, C., and Cowan, D. A. (2014). Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 15, 508–517. doi: 10.1002/embr.201338170
de Souza Vandenberghe, L. P., Karp, S. G., Pagnoncelli, M. G. B., Rodrigues, C., Medeiros, A. B. P., and Soccol, C. R. (2019). “Digestive enzymes: industrial applications in food products,” in Green Bio-processes, eds B. Parameswaran, S. Varjani, and S. Raveendran (Singapore: Springer), 267–291. doi: 10.1007/978-981-13-3263-0_14
De Virgilio, C., Bürckert, N., Bell, W., Jenö, P., Boller, T., and Wiemken, A. (1993). Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur. J. Biochem. 212, 315–323. doi: 10.1111/j.1432-1033.1993.tb17664.x
De Vos, W. M. (1987). Gene cloning and expression in lactic streptococci. FEMS Microbiol. Rev. 3, 281–295. doi: 10.1016/0378-1097(87)90113-3
Deng, A., Wu, J., Zhang, Y., Zhang, G., and Wen, T. (2010). Purification and characterization of a surfactant-stable high-alkaline protease from Bacillus sp. B001. Bioresour. Technol. 101, 7100–7106. doi: 10.1016/j.biortech.2010.03.130
Desautels, M., and Goldberg, A. (1982). Demonstration of an ATP-dependent, vanadate-sensitive endoprotease in the matrix of rat liver mitochondria. J. Biol. Chem. 257, 11673–11679.
Dhawan, S., and Kaur, J. (2007). Microbial mannanases: an overview of production and applications. Crit. Rev. Biotechnol. 27, 197–216. doi: 10.1080/07388550701775919
Dias, D. R., Vilela, D. M., Silvestre, M. P. C., and Schwan, R. F. (2008). Alkaline protease from Bacillus sp. isolated from coffee bean grown on cheese whey. World J. Microbiol. Biotechnol. 24, 2027–2034. doi: 10.1007/s11274-008-9706-6
Dodia, M., Rawal, C., Bhimani, H., Joshi, R., Khare, S., and Singh, S. P. (2008). Purification and stability characteristics of an alkaline serine protease from a newly isolated Haloalkaliphilic bacterium sp. AH-6. J. Ind. Microbiol. Biotechnol. 35, 121–131. doi: 10.1007/s10295-007-0273-x
dos Santos Aguilar, J. G., and Sato, H. H. (2018). Microbial proteases: production and application in obtaining protein hydrolysates. Food Res. Int. 103, 253–262. doi: 10.1016/j.foodres.2017.10.044
Drew, S., Daniel, I. W., Moo-Young, M., and Blanch, H. W. (1985). Comprehensive Biotechnology: The Principles, Applications and Regulations of Biotechnology in Industry, Agriculture and Medicine. Oxford: Pergamon Press.
Driscoll, J., Brown, M. G., Finley, D., and Monaco, J. J. (1993). MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature 365:262. doi: 10.1038/365262a0
Dumas, A., Spicer, C. D., Gao, Z., Takehana, T., Lin, Y. A., Yasukohchi, T., et al. (2013). Self-liganded Suzuki–Miyaura coupling for site-selective protein PEGylation. Angew. Chem. Int. Ed. 52, 3916–3921. doi: 10.1002/anie.201208626
Eichler, J. (2001). Biotechnological uses of archaeal extremozymes. Biotechnol. Adv. 19, 261–278. doi: 10.1016/S0734-9750(01)00061-1
El-Gamal, M. S., Abdel-Shakour, E. H., Abdel-Rahman, M. A., and Attia, A. A. (2012). Protease productivity by some thermoalkalotolerant gram positive bacteria isolated from manure. Egypt J. Biotechnol. 41, 1–25.
Ellaiah, P., Srinivasulu, B., and Adinarayana, K. (2002). A review on microbial alkaline proteases. J. Sci. Ind. Res. 61, 690–704.
Epstein, D., and Wensink, P. (1988). The alpha-lytic protease gene of Lysobacter enzymogenes. The nucleotide sequence predicts a large prepro-peptide with homology to pro-peptides of other chymotrypsin-like enzymes. J. Biol. Chem. 263, 16586–16590.
Estell, D. A., Graycar, T. P., and Wells, J. A. (1985). Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. J. Biol. Chem. 260, 6518–6521.
Fagan, J. M., Waxman, L., and Goldberg, A. L. (1987). Skeletal muscle and liver contain a soluble ATP+ ubiquitin-dependent proteolytic system. Biochem. J. 243, 335–343. doi: 10.1042/bj2430335
Fang, Z., Yong, Y.-C., Zhang, J., Du, G., and Chen, J. (2017a). Keratinolytic protease: a green biocatalyst for leather industry. Appl. Microbiol. Biotechnol. 101, 7771–7779. doi: 10.1007/s00253-017-8484-1
Fang, Z., Zhang, J., Du, G., and Chen, J. (2017b). Rational protein engineering approaches to further improve the keratinolytic activity and thermostability of engineered keratinase KerSMD. Biochem. Eng. J. 127, 147–153. doi: 10.1016/j.bej.2017.08.010
Fang, Z., Zhang, J., Liu, B., Du, G., and Chen, J. (2016). Enhancement of the catalytic efficiency and thermostability of Stenotrophomonas sp. keratinase KerSMD by domain exchange with KerSMF. Microb. Biotechnol. 9, 35–46. doi: 10.1111/1751-7915.12300
Fitzgerald, P., McKeever, B., VanMiddlesworth, J. F., Springer, J. P., Heimbach, J. C., Leu, C. T., et al. (1990). Crystallographic analysis of a complex between human immunodeficiency virus type 1 protease and acetyl-pepstatin at 2.0-A resolution. J. Biol. Chem. 265, 14209–14219. doi: 10.2210/pdb5hvp/pdb
Foegeding, E. A., Davis, J. P., Doucet, D., and McGuffey, M. K. (2002). Advances in modifying and understanding whey protein functionality. Trends Food Sci. Technol. 13, 151–159. doi: 10.1016/S0924-2244(02)00111-5
Gamblin, D. P., Scanlan, E. M., and Davis, B. G. (2008). Glycoprotein synthesis: an update. Chem. Rev. 109, 131–163. doi: 10.1021/cr078291i
Gessesse, A. (1997). The use of nug meal as a low-cost substrate for the production of alkaline protease by the alkaliphilic Bacillus sp. AR-009 and some properties of the enzyme. Bioresour. Technol. 62, 59–61. doi: 10.1016/S0960-8524(97)00059-X
Giménez, M. I., Studdert, C. A., Sánchez, J. J., and De Castro, R. E. (2000). Extracellular protease of Natrialba magadii: purification and biochemical characterization. Extremophiles 4, 181–188. doi: 10.1007/s007920070033
Godfrey, T., and West, S. (1996b). “Introduction to industrial enzymology,” in Industrial Enzymology, eds T. Godfrey and S. West (London: Mac. Millan Press).
Goll, D. E., Thompson, V. F., Taylor, R. G., and Zalewska, T. (1992). Is calpain activity regulated by membranes and autolysis or by calcium and calpastatin? Bioessays 14, 549–556. doi: 10.1002/bies.950140810
Gomes, A. V., Zong, C., Edmondson, R. D., Li, X., Stefani, E., Jones, R. C., et al. (2006). Mapping the murine cardiac 26S proteasome complexes. Circ. Res. 99, 362–371. doi: 10.1161/01.RES.0000237386.98506.f7
Gómez-Guillén, M., Giménez, B., López-Caballero, M., and Montero, M. P. (2011). Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 25, 1813–1827. doi: 10.1016/j.foodhyd.2011.02.007
Govinden, G., and Puchooa, D. (2012). Isolation and characterization of feather degrading bacteria from Mauritian soil. Afr. J. Biotechnol. 11, 13591–13600. doi: 10.5897/AJB12.1683
Greene, R. V., Cotta, M. A., and Griffin, H. L. (1989). A novel, symbiotic bacterium isolated from marine shipworm secretes proteolytic activity. Curr. Microbiol. 19, 353–356. doi: 10.1007/BF01570881
Guillaume, B., Chapiro, J., Stroobant, V., Colau, D., Van Holle, B., Parvizi, G., et al. (2010). Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc. Natl. Acad. Sci. U.S.A. 107, 18599–18604. doi: 10.1073/pnas.1009778107
Guleria, S., Walia, A., Chauhan, A., and Shirkot, C. (2016). Molecular characterization of alkaline protease of Bacillus amyloliquefaciens SP1 involved in biocontrol of Fusarium oxysporum. Int. J. Food Microbiol. 232, 134–143. doi: 10.1016/j.ijfoodmicro.2016.05.030
Gupta, A., Joseph, B., Mani, A., and Thomas, G. (2008). Biosynthesis and properties of an extracellular thermostable serine alkaline protease from Virgibacillus pantothenticus. World J. Microbiol. Biotechnol. 24, 237–243. doi: 10.1007/s11274-007-9462-z
Gupta, A., and Khare, S. (2007). Enhanced production and characterization of a solvent stable protease from solvent tolerant Pseudomonas aeruginosa PseA. Enzyme Microb. Technol. 42, 11–16. doi: 10.1016/j.enzmictec.2007.07.019
Gupta, R., Beg, Q., and Lorenz, P. (2002). Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 59, 15–32. doi: 10.1007/s00253-002-0975-y
Gupta, R., Gupta, K., Saxena, R., and Khan, S. (1999). Bleach-stable, alkaline protease from Bacillus sp. Biotechnol. Lett. 21, 135–138. doi: 10.1023/A:1005478117918
Gupta, R., and Ramnani, P. (2006). Microbial keratinases and their prospective applications: an overview. Appl. Microbiol. Biotechnol. 70:21. doi: 10.1007/s00253-005-0239-8
Haddar, A., Agrebi, R., Bougatef, A., Hmidet, N., Sellami-Kamoun, A., and Nasri, M. (2009a). Two detergent stable alkaline serine-proteases from Bacillus mojavensis A21: purification, characterization and potential application as a laundry detergent additive. Bioresour. Technol. 100, 3366–3373. doi: 10.1016/j.biortech.2009.01.061
Haddar, A., Bougatef, A., Agrebi, R., Sellami-Kamoun, A., and Nasri, M. (2009b). A novel surfactant-stable alkaline serine-protease from a newly isolated Bacillus mojavensis A21. Purification and characterization. Process Biochem. 44, 29–35. doi: 10.1016/j.procbio.2008.09.003
Haki, G., and Rakshit, S. (2003). Developments in industrially important thermostable enzymes: a review. Bioresour. Technol. 89, 17–34. doi: 10.1016/S0960-8524(03)00033-6
Hamza, T. A. (2017a). Bacterial protease enzyme: Safe and good alternative for industrial and commercial use. Int. J. Chem. Biomol. Sci. 3, 1–10.
Hamza, T. A. (2017b). Isolation and screening of protease producing bacteria from local environment for detergent additive. Am. J. Life Sci. 5:116. doi: 10.11648/j.ajls.20170505.11
He, R., Girgih, A. T., Malomo, S. A., Ju, X., and Aluko, R. E. (2013). Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 5, 219–227. doi: 10.1016/j.jff.2012.10.008
Heck, T., Faccio, G., Richter, M., and Thöny-Meyer, L. (2013). Enzyme-catalyzed protein crosslinking. Appl. Microbiol. Biotechnol. 97, 461–475. doi: 10.1007/s00253-012-4569-z
Helianti, I., Furgeva, N., Mulyawati, L., Ferniah, R. S., and Kusumaningrum, H. P. (2018). Cloning of a gene encoding protease from Bacillus halodurans CM1 into Escherichia coli DH5α and expression analyses of the gene product. Makara J. Sci. 22, 113–120. doi: 10.7454/mss.v22i3.9900
Henderson, G., Krygsman, P., Liu, C., Davey, C. C., and Malek, L. T. (1987). Characterization and structure of genes for proteases A and B from Streptomyces griseus. J. Bacteriol. 169, 3778–3784. doi: 10.1128/jb.169.8.3778-3784.1987
Henner, D. J., Yang, M., Band, L., and Wells, J. (1985). “Expression of cloned protease genes in Bacillus subtilis,” in Proceedings of the 9th International Spore Conference, 95–103.
Hershko, A., and Ciechanover, A. (1998). The ubiquitin system. Ann. Rev. 67, 425–479. doi: 10.1007/978-1-4899-1922-9_1
Hockemeyer, D., Wang, H., Kiani, S., Lai, C. S., Gao, Q., Cassady, J. P., et al. (2011). Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 29:731. doi: 10.1038/nbt.1927
Hogdson, J. (1994). The changing bulk biocatalyst market: recombinant DNA techniques have changed bulk enzyme production dramatically. Biotechnology 12, 789–790. doi: 10.1038/nbt0894-789
Homaei, A., Lavajoo, F., and Sariri, R. (2016). Development of marine biotechnology as a resource for novel proteases and their role in modern biotechnology. Int. J. Biol. Macromol. 88, 542–552. doi: 10.1016/j.ijbiomac.2016.04.023
Hossain, M. M., Sultana, F., and Islam, S. (2017). “Plant growth-promoting fungi (PGPF): phytostimulation and induced systemic resistance,” in Plant-Microbe Interactions in Agro-Ecological Perspectives, eds D. P. Singh, H. B. Singh, and R. Prabha (Singapore: Springer), 135–191. doi: 10.1007/978-981-10-6593-4_6
Hosse, R. J., Rothe, A., and Power, B. E. (2006). A new generation of protein display scaffolds for molecular recognition. Prot. Sci. 15, 14–27. doi: 10.1110/ps.051817606
Hough, R. F., Pratt, G. W., and Rechsteiner, M. (1988). “Ubiquitin/ATP-dependent protease,” in Ubiquitin, ed M. Rechsteiner (Boston, MA: Springer), 101–134. doi: 10.1007/978-1-4899-2049-2_5
Hsiao, H.-Y., Fodge, D. W., and LaLonde, J. J. (1994). Alkaline Proteases Stable in Heavy-Duty Detergent Liquids. Google Patents.
Huang, M., Chen, R., and Ren, G. (2017). Secretory expression and purification of Bacillus licheniformis keratinase in insect cells. PLoS ONE 12:e0183764. doi: 10.1371/journal.pone.0183764
Hwang, D. H., Kim, J. C., Kim, J., Byun, S. M., and Chun, M. (1993). Molecular cloning and nucleotide sequence of the protease B gene from Streptomyces griseus ATCC 10137. Korean Biochem. J. 181, 707–713.
Ichida, J. M., Krizova, L., LeFevre, C. A., Keener, H. M., Elwell, D. L., Burtt, Jr., et al. (2001). Bacterial inoculum enhances keratin degradation and biofilm formation in poultry compost. J. Microbiol. Methods 47, 199–208. doi: 10.1016/S0167-7012(01)00302-5
Idbeaa, A., and Omar, I. (2016). Alkaline Protease Production Using Submerged Fermentation From Different Species of Bacillus. Biotechnology and Bioengineering Sam Higginbottom Institute of Agriculture, Technology and Sciences, Allahabad.
Illanes, A. (2008). Enzyme Biocatalysis. Principles and Applications. New York, NY: Springer-Verlag New York Inc. doi: 10.1007/978-1-4020-8361-7
Intarasirisawat, R., Benjakul, S., Wu, J., and Visessanguan, W. (2013). Isolation of antioxidative and ACE inhibitory peptides from protein hydrolysate of skipjack (Katsuwana pelamis) roe. J. Funct. Foods 5, 1854–1862. doi: 10.1016/j.jff.2013.09.006
Ito, S., Kobayashi, T., Ara, K., Ozaki, K., Kawai, S., and Hatada, Y. (1998). Alkaline detergent enzymes from alkaliphiles: enzymatic properties, genetics, and structures. Extremophiles 2, 185–190. doi: 10.1007/s007920050059
Jäckel, C., and Koksch, B. (2005). Fluorine in peptide design and protein engineering. Eur. J. Org. Chem. 2005, 4483–4503. doi: 10.1002/ejoc.200500205
Jacobs, M. F. (1995). Expression of the subtilisin Carlsberg-encoding gene in Bacillus licheniformis and Bacillus subtilis. Gene 152, 69–74. doi: 10.1016/0378-1119(94)00655-C
Jacobson, J. W., Glick, J. L., and Madello, K. L. (1985). Composition for Cleaning Drains Clogged With Deposits Containing Hair. Google Patents.
Jadhav, U., and Hocheng, H. (2012). A review of recovery of metals from industrial waste. J. Achiev. Mater. Manufac. Eng. 54, 159–167.
Jakubke, H. D. (1994). Protease-catalyzed peptide synthesis: basic principles, new synthesis strategies and medium engineering. J. Chinese Chem. Soc. 41, 355–370. doi: 10.1002/jccs.199400049
Jaouadi, B., Abdelmalek, B., and Jaouadi Zaraî, B. N. (2011). “The bioengineering and industrial applications of bacterial alkaline proteases: the case of SAPB and KERAB,” in Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications, ed A. Carpi (InTechOpen) 445–466. doi: 10.5772/23850
Jaouadi, B., Ellouz-Chaabouni, S., Rhimi, M., and Bejar, S. (2008). Biochemical and molecular characterization of a detergent-stable serine alkaline protease from Bacillus pumilus CBS with high catalytic efficiency. Biochimie 90, 1291–1305. doi: 10.1016/j.biochi.2008.03.004
Jaouadi, N. Z., Jaouadi, B., Aghajari, N., and Bejar, S. (2012). The overexpression of the SAPB of Bacillus pumilus CBS and mutated sapB-L31I/T33S/N99Y alkaline proteases in Bacillus subtilis DB430: new attractive properties for the mutant enzyme. Bioresour. Technol. 105, 142–151. doi: 10.1016/j.biortech.2011.11.115
Jeong, Y. J., Baek, S. C., and Kim, H. (2018). Cloning and characterization of a novel intracellular serine protease (IspK) from Bacillus megaterium with a potential additive for detergents. Int. J. Biol. Macromol. 108, 808–816. doi: 10.1016/j.ijbiomac.2017.10.173
Johnvesly, B., and Naik, G. (2001). Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem. 37, 139–144. doi: 10.1016/S0032-9592(01)00191-1
Joo, H.-S., Kumar, C. G., Park, G.-C., Kim, K. T., Paik, S. R., and Chang, C.-S. (2002). Optimization of the production of an extracellular alkaline protease from Bacillus horikoshii. Process Biochem. 38, 155–159. doi: 10.1016/S0032-9592(02)00061-4
Joo, H.-S., Kumar, C. G., Park, G.-C., Paik, S. R., and Chang, C.-S. (2004). Bleach-resistant alkaline protease produced by a Bacillus sp. isolated from the Korean polychaete, Periserrula leucophryna. Process Biochem. 39, 1441–1447. doi: 10.1016/S0032-9592(03)00260-7
Joshi, S., and Satyanarayana, T. (2013). Characteristics and applications of a recombinant alkaline serine protease from a novel bacterium Bacillus lehensis. Bioresour. Technol. 131, 76–85. doi: 10.1016/j.biortech.2012.12.124
Kalaiarasi, K., and Sunitha, P. (2009). Optimization of alkaline protease production from Pseudomonas fluorescens isolated from meat waste contaminated soil. Afr. J. Biotechnol. 8, 7035–7041. doi: 10.5897/AJB2009.000-9547
Kalaikumari, S. S., Vennila, T., Monika, V., Chandraraj, K., Gunasekaran, P., and Rajendhran, J. (2019). Bioutilization of poultry feather for keratinase production and its application in leather industry. J. Clean. Prod. 208, 44–53. doi: 10.1016/j.jclepro.2018.10.076
Kalisz, H. M. (1988). “Microbial proteinasesm” in Enzyme Studies. Advances in Biochemical Engineering/Biotechnology, Vol. 36, ed A. Fiechter (Berlin; Heidelberg: Springer), 1–65. doi: 10.1007/BFb0047944
Kaneko, R., Koyama, N., Tsai, Y.-C., Juang, R.-Y., Yoda, K., and Yamasaki, M. (1989). Molecular cloning of the structural gene for alkaline elastase YaB, a new subtilisin produced by an alkalophilic Bacillus strain. J. Bacteriol. 171, 5232–5236. doi: 10.1128/jb.171.9.5232-5236.1989
Kang, S. G., Kim, I. S., Rho, Y. T., and Lee, K. J. (1995). Production dynamics of extracellular proteases accompanying morphological differentiation of Streptomyces albidoflavus SMF301. Microbiology 141, 3095–3103. doi: 10.1099/13500872-141-12-3095
Kanmani, R., Dhivya, S., Jayalakshmi, S., and Vijayabaskar, P. (2011). Studies on detergent additives of protease enzyme from an estuarine bacterium Bacillus cereus. Int. Res. J. Biotechnol. 2, 157–163.
Keil, B. (2012). Specificity of Proteolysis. Berlin; Heidelberg: Springer Science and Business Media; Springer-Verlag.
Kenny, J. (1986). Cell surface peptidases are neither peptide-nor organ-specific. Trends Biochem. Sci. 11, 40–42. doi: 10.1016/0968-0004(86)90231-8
Kittiphattanabawon, P., Benjakul, S., Visessanguan, W., and Shahidi, F. (2012). Gelatin hydrolysate from blacktip shark skin prepared using papaya latex enzyme: antioxidant activity and its potential in model systems. Food Chem. 135, 1118–1126. doi: 10.1016/j.foodchem.2012.05.080
Kleinschmidt, J. A., Escher, C., and Wolf, D. H. (1988). Proteinase yscE of yeast shows homology with the 20 S cylinder particles of Xenopus laevis. FEBS Lett. 239, 35–40. doi: 10.1016/0014-5793(88)80540-4
Kloetzel, P. M. (2004). Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nat. Immunol. 5:661. doi: 10.1038/ni1090
Kocher, G., and Mishra, S. (2009). Immobilization of Bacillus circulans MTCC 7906 for enhanced production of alkaline protease under batch and packed bed fermentation conditions. Internet J. Microbiol. 7, 359–378. doi: 10.5580/2599
Kohno, H., Kanda, S., and Kanno, T. (1986). Immunoaffinity purification and characterization of leucine aminopeptidase from human liver. J. Biol. Chem. 261, 10744–10748.
Kostyleva, E., Sereda, A., Velikoretskaya, I., Nefedova, L., Sharikov, A. Y., Tsurikova, N., et al. (2016). A new Bacillus licheniformis mutant strain producing serine protease efficient for hydrolysis of soy meal proteins. Microbiology 85, 462–470. doi: 10.1134/S0026261716040123
Kotb, E. (2013). Activity assessment of microbial fibrinolytic enzymes. Appl. Microbiol. Biotechnol. 97, 6647–6665. doi: 10.1007/s00253-013-5052-1
Krasileva, K. V. (2019a). The role of transposable elements and DNA damage repair mechanisms in gene amplification and protein domain shuffling in plant genomes. PeerJ Preprints 7:e27486v1. doi: 10.7287/peerj.preprints.27486v1
Krasileva, K. V. (2019b). The role of transposable elements and DNA damage repair mechanisms in gene duplications and gene fusions in plant genomes. Curr. Opin. Plant Biol. 48, 18–25. doi: 10.1016/j.pbi.2019.01.004
Krishnaveni, K., Kumar, D. M., Balakumaran, M., Ramesh, S., and Kalaichelvan, P. (2012). Production and optimization of extracellular Alkaline Protease from Bacillus subtilis isolated from dairy effluent. Der Pharm. Lett. 4, 98–109.
Kuhad, R. C., Gupta, R., and Singh, A. (2011). Microbial cellulases and their industrial applications. Enzyme Res. (2011) 2011:280696. doi: 10.4061/2011/280696
Kumar, C. G., Malik, R., and Tiwari, M. (1998). Novel enzyme-based detergents: an Indian perspective. Curr. Sci. 75, 1312–1318.
Kumar, C. G., and Takagi, H. (1999). Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol. Adv. 17, 561–594. doi: 10.1016/S0734-9750(99)00027-0
Kumar, D. M., Premavathi, V., Govindarajan, N., Balakumaran, M., and Kalaichelvan, P. (2012). Production and purification of alkaline protease from Bacillus sp. MPTK 712 isolated from dairy sludge. Global Vet. 8, 433–439.
Kumar, N. S., Nazeer, R., and Jaiganesh, R. (2012). Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 42, 1641–1649. doi: 10.1007/s00726-011-0858-6
Lasekan, A., Bakar, F. A., and Hashim, D. (2013). Potential of chicken by-products as sources of useful biological resources. Waste Manage. 33, 552–565. doi: 10.1016/j.wasman.2012.08.001
Lateef, A., Adelere, I. A., and Gueguim-Kana, E. B. (2015). Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications. Biotech. Biotechnol. Equip. 29, 54–63. doi: 10.1080/13102818.2014.986360
Li, J., Chi, Z., Wang, X., Peng, Y., and Chi, Z. (2009). The selection of alkaline protease-producing yeasts from marine environments and evaluation of their bioactive peptide production. Chinese J. Oceanol. Limnol. 27:753. doi: 10.1007/s00343-009-9198-8
Li, Q., Yi, L., Marek, P., and Iverson, B. L. (2013). Commercial proteases: present and future. FEBS Lett. 587, 1155–1163. doi: 10.1016/j.febslet.2012.12.019
Li, Y., Jiang, B., Zhang, T., Mu, W., and Liu, J. (2008). Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 106, 444–450. doi: 10.1016/j.foodchem.2007.04.067
Liu, B., Zhang, J., Fang, Z., Du, G., Chen, J., and Liao, X. (2014). Functional analysis of the C-terminal propeptide of keratinase from Bacillus licheniformis BBE11-1 and its effect on the production of keratinase in Bacillus subtilis. Process Biochem. 49, 1538–1542. doi: 10.1016/j.procbio.2014.04.021
Liu, J., Sharma, A., Niewiara, M. J., Singh, R., Ming, R., and Yu, Q. (2018). Papain-like cysteine proteases in Carica papaya: lineage-specific gene duplication and expansion. BMC Genomics 19:26. doi: 10.1186/s12864-017-4394-y
Ljungdahl, P. O. (2009). Amino-Acid-Induced Signalling via the SPS-Sensing Pathway in Yeast. Stockholm: Portland Press Limited, 242–247. doi: 10.1042/BST0370242
Machado, A. R. G. M., Teixeira, M. F. S., de Souza Kirsch, L., Campelo, M. d C. L., and de Aguiar Oliveira, I. M. (2016). Nutritional value and proteases of Lentinus citrinus produced by solid state fermentation of lignocellulosic waste from tropical region. Saudi J. Biol. Sci. 23, 621–627. doi: 10.1016/j.sjbs.2015.07.002
Madern, D., Ebel, C., and Zaccai, G. (2000). Halophilic adaptation of enzymes. Extremophiles 4, 91–98. doi: 10.1007/s007920050142
Mahajan, R., Chaudhari, G., and Chopadaa, M. (2016). Report on Biotechnological applications of proteolytic enzymes from lattices of euphorbian plants. J. Appl. Biotechnol. Rep. 2, 333–337. doi: 10.21276/ijlssr.2016.2.4.7
Manirujjaman, M., Amin, R., Nahid, A., and Alam, M. (2016). Isolation and characterization of feather degrading bacteria from poultry waste. Afr. J. Bacteriol. Res. 8, 14–21.
Margesin, R., Neuner, G., and Storey, K. B. (2007). Cold-loving microbes, plants, and animals—fundamental and applied aspects. Naturwissenschaften 94, 77–99. doi: 10.1007/s00114-006-0162-6
Margesin, R., and Schinner, F. (2001). Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5, 73–83. doi: 10.1007/s007920100184
Mascini, M., Palchetti, I., and Tombelli, S. (2012). Nucleic acid and peptide aptamers: fundamentals and bioanalytical aspects. Angew. Chem. Int. Ed. 51, 1316–1332. doi: 10.1002/anie.201006630
Masui, A., Yasuda, M., Fujiwara, N., and Ishikawa, H. (2004). Enzymatic hydrolysis of gelatin layers on used lith film using thermostable alkaline protease for recovery of silver and PET film. Biotechnol. Prog. 20, 1267–1269. doi: 10.1021/bp030058s
Mehde, A. A., Mehdi, W. A., Özacar, M., and Özacar, Z. Z. (2018). Evaluation of different saccharides and chitin as eco-friendly additive to improve the magnetic cross-linked enzyme aggregates (CLEAs) activities. Int. J. Biol. Macromol. 118, 2040–2050. doi: 10.1016/j.ijbiomac.2018.07.075
Melloni, E., and Pontremoli, S. (1989). The calpains. Trends Neurosci. 12, 438–444. doi: 10.1016/0166-2236(89)90093-3
Mienda, B. S., and Yahya, A. (2011). Engineering of microbial proteases: improving stability and catalytic performances. IIOAB J. 2, 10–15.
Mitsuiki, S., Ichikawa, M., Oka, T., Sakai, M., Moriyama, Y., Sameshima, Y., et al. (2004). Molecular characterization of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Enzyme Microb. Technol. 34, 482–489. doi: 10.1016/j.enzmictec.2003.12.011
Mittler, R., and Blumwald, E. (2010). Genetic engineering for modern agriculture: challenges and perspectives. Annu. Rev. Plant Biol. 61, 443–462. doi: 10.1146/annurev-arplant-042809-112116
Miyaji, T., Otta, Y., Nakagawa, T., Watanabe, T., Niimura, Y., and Tomizuka, N. (2006). Purification and molecular characterization of subtilisin-like alkaline protease BPP-A from Bacillus pumilus strain MS-1. Lett. Appl. Microbiol. 42, 242–247. doi: 10.1111/j.1472-765X.2005.01851.x
Moraga, J., Gomes, W., Pinedo, C., Cantoral, J. M., Hanson, J. R., Carbú, M., et al. (2019). The current status on secondary metabolites produced by plant pathogenic Colletotrichum species. Phytochem. Rev. 18, 1–25. doi: 10.1007/s11101-018-9590-0
Mota, T. R., Linhares, H. V., Araújo-Filho, J. H., Veras, D. M., Costa, H. P., Souza, C. M., et al. (2019). Protein extract from Cereus jamacaru (DC.) inhibits Colletotrichum gloeosporioides growth by stimulating ROS generation and promoting severe cell membrane damage. Microb. Pathog. 130, 71–80. doi: 10.1016/j.micpath.2019.02.033
Mótyán, J., Tóth, F., and Tozsér, J. (2013). Research applications of proteolytic enzymes in molecular biology. Biomolecules 3, 923–942. doi: 10.3390/biom3040923
Mukesh, D., Rajan, R., Lawrence, L., Priyadarshini, S., Chittybabu, S., and Kalaichelvan, P. (2012). Distaining and de-hairing capability of partially purified Bacillus subtilis protease from optimized fermentation medium. Asian J. Exp. Biol. Sci. 3, 613–620.
Mukherjee, A. K., and Rai, S. K. (2011). A statistical approach for the enhanced production of alkaline protease showing fibrinolytic activity from a newly isolated Gram-negative Bacillus sp. strain AS-S20-I. New Biotechnol. 28, 182–189. doi: 10.1016/j.nbt.2010.11.003
Naidu, K. S. (2011). Characterization and purification of protease enzyme. J. Appl. Pharma. Sci. 1:17.
Najafi, M. F., Deobagkar, D., and Deobagkar, D. (2005). Potential application of protease isolated from Pseudomonas aeruginosa PD100. Electron. J. Biotechnol. 8, 79–85. doi: 10.2225/vol8-issue2-fulltext-5
Nakiboglu, N., Toscali, D., and Yaşa, I. (2001). Silver recovery from waste photographic films by using enzymatic method. Turk. J. Chem. 25, 349–353.
Nalinanon, S., Benjakul, S., Kishimura, H., and Shahidi, F. (2011). Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 124, 1354–1362. doi: 10.1016/j.foodchem.2010.07.089
Narasimhan, M. K., Chandrasekaran, M., and Rajesh, M. (2015). Fibrinolytic enzyme production by newly isolated Bacillus cereus SRM-001 with enhanced in-vitro blood clot lysis potential. J. Gen. Appl. Microbiol. 61, 157–164. doi: 10.2323/jgam.61.157
Narhi, L., Stabinsky, Y., Levitt, M., Miller, L., Sachdev, R., Finley, S., et al. (1991). Enhanced stability of subtilisin by three point mutations. Biotechnol. Appl. Biochem. 13, 12–24.
Neklyudov, A., Ivankin, A., and Berdutina, A. (2000). Properties and uses of protein hydrolysates. Appl. Biochem. Microbiol. 36, 452–459. doi: 10.1007/BF02731888
Nicolia, A., Manzo, A., Veronesi, F., and Rosellini, D. (2014). An overview of the last 10 years of genetically engineered crop safety research. Crit. Rev. Biotechnol. 34, 77–88. doi: 10.3109/07388551.2013.823595
Nisha, N., and Divakaran, J. (2014). Optimization of alkaline protease production from Bacillus subtilis NS isolated from sea water. Afr. J. Biotechnol. 13, 1707–1713. doi: 10.5897/AJB2014.13652
Nongonierma, A. B., and FitzGerald, R. J. (2015). The scientific evidence for the role of milk protein-derived bioactive peptides in humans: a review. J. Funct. Foods 17, 640–656. doi: 10.1016/j.jff.2015.06.021
Oberoi, R., Beg, Q. K., Puri, S., Saxena, R., and Gupta, R. (2001). Characterization and wash performance analysis of an SDS-stable alkaline protease from a Bacillus sp. World J. Microbiol. Biotechnol. 17, 493–497. doi: 10.1023/A:1011918806201
Onaizi, S. A. (2018). Enzymatic removal of protein fouling from self-assembled cellulosic nanofilms: experimental and modeling studies. Eur. Biophys. J. 47, 951–960. doi: 10.1007/s00249-018-1320-4
Ottesen, M., and Rickert, W. (1970). The isolation and partial characterization of an acid protease produced by Mucor miehei. C. R. Trav. Lab. Carlsberg 37, 301–325.
Özacar, M., Mehde, A. A., Mehdi, W. A., Özacar, Z. Z., and Severgün, O. (2019). The novel multi cross-linked enzyme aggregates of protease, lipase, and catalase production from the sunflower seeds, characterization and application. Colloids Surf. B Biointerfaces 173, 58–68. doi: 10.1016/j.colsurfb.2018.09.042
Page, M. J., and Di Cera, E. (2008). Evolution of peptidase diversity. J. Biol. Chem. 283, 30010–30014. doi: 10.1074/jbc.M804650200
Palanivel, P., Ashokkumar, L., and Balagurunathan, R. (2013). Production, purification and fibrinolytic characterization of alkaline protease from extremophilic soil fungi. Int. J. Pharm. Bio. Sci. 4, 101–110.
Palsaniya, P., Mishra, R., Beejawat, N., Sethi, S., and Gupta, B. L. (2012). Optimization of alkaline protease production from bacteria isolated from soil. J. Microbiol. Biotechnol. Res. 2, 695–701.
Palumbo, J. D., Sullivan, R. F., and Kobayashi, D. Y. (2003). Molecular characterization and expression in Escherichia coli of three β-1, 3-glucanase genes from Lysobacter enzymogenes strain N4-7. J. Bacteriol. 185, 4362–4370. doi: 10.1128/JB.185.15.4362-4370.2003
Panda, M. K., Sahu, M. K., and Tayung, K. (2013). Isolation and characterization of a thermophilic Bacillus sp. with protease activity isolated from hot spring of Tarabalo, Odisha, India. Iran. J. Microbiol. 5:159.
Pastor, M. D., Lorda, G. S., and Balatti, A. (2001). Protease obtention using Bacillus subtilis 3411 and amaranth seed meal medium at different aeration rates. Braz. J. Microbiol. 32, 6–9. doi: 10.1590/S1517-83822001000100002
Patil, U., and Chaudhari, A. (2009). Purification and characterization of solvent-tolerant, thermostable, alkaline metalloprotease from alkalophilic Pseudomonas aeruginosa MTCC 7926. J. Chem. Technol. Biotechnol. 84, 1255–1262. doi: 10.1002/jctb.2169
Perkins, D.N., Pappin, D. J., Creasy, D. M., and Cottrell, J. S. (1999). Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2
Phoenix, D. A., Dennison, S. R., and Harris, F. (2012). Antimicrobial Peptides. Wiley-VCH Verlag GmbH & Co. KGaA.
Power, O., Jakeman, P., and FitzGerald, R. (2013). Antioxidative peptides: enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino acids 44, 797–820. doi: 10.1007/s00726-012-1393-9
Prabhavathy, G., Rajasekara Pandian, M., and Senthilkumar, B. (2012). Optimization and production of extracellular alkaline protease by solid state fermentation using Bacillus subtilis. J. Acad. Ind. Res. 17, 427–430. doi: 10.1016/j.jtusci.2014.04.010
Prabhavathy, G., Rajasekara, M., and Senthilkumar, B. (2013). Identification of industrially important alkaline protease producing Bacillus subtilis by 16s rRNA sequence analysis and its applications. Int. J. Res. Pharma. Biomed. Sci. 4, 332–338.
Prakasham, R., Rao, C. S., Rao, R. S., Rajesham, S., and Sarma, P. (2005). Optimization of alkaline protease production by Bacillus sp. using Taguchi methodology. Appl. Biochem. Biotechnol. 120, 133–144. doi: 10.1385/ABAB:120:2:133
Prakasham, R., Rao, C. S., and Sarma, P. (2006). Green gram husk—an inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresour. Technol. 97, 1449–1454. doi: 10.1016/j.biortech.2005.07.015
Priya, V., Preethi, S., Karthikeyan, S., and Babu, R. (2016). Isolation and identification of protease producing bacteria from soil. Int. J. Res. Eng. Technol. 3, 1362–1365.
Puente, X. S., Sánchez, L. M., Overall, C. M., and López-Otín, C. (2003). Human and mouse proteases: a comparative genomic approach. Nat. Rev. Genet. 4:544. doi: 10.1038/nrg1111
Pursel, V. G., Pinkert, C. A., Miller, K. F., Bolt, D. J., Campbell, R. G., Palmiter, R. D., et al. (1989). Genetic engineering of livestock. Science 244, 1281–1288. doi: 10.1126/science.2499927
Pushpam, P. L., Rajesh, T., and Gunasekaran, P. (2011). Identification and characterization of alkaline serine protease from goat skin surface metagenome. AMB Express 1:3. doi: 10.1186/2191-0855-1-3
Qian, G., Xu, F., Venturi, V., Du, L., and Liu, F. (2014). Roles of a solo LuxR in the biological control agent Lysobacter enzymogenes strain OH11. Phytopathology 104, 224–231. doi: 10.1094/PHYTO-07-13-0188-R
Qian, G.-L., Hu, B.-S., Jiang, Y.-H., and Liu, F.-Q. (2009). Identification and characterization of Lysobacter enzymogenes as a biological control agent against some fungal pathogens. Agric. Sci. China 8, 68–75. doi: 10.1016/S1671-2927(09)60010-9
Radha, S., Sridevi, A., HimakiranBabu, R., Nithya, V., Prasad, N., and Narasimha, G. (2017). Medium optimization for acid protease production from Aspergillus sps under solid state fermentation and mathematical modelling of protease activity. J. Microbiol. Biotechnol. Res. 2, 6–16.
Ramesh, S., Rajesh, M., and Mathivanan, N. (2009). Characterization of a thermostable alkaline protease produced by marine Streptomyces fungicidicus MML1614. Bioprocess Biosyst. Eng. 32, 791–800. doi: 10.1007/s00449-009-0305-1
Rani, K., Rana, R., and Datt, S. (2012). Review on latest overview of proteases. Int. J. Curr. Life Sci. 2, 12–18.
Rao, C. S., Sathish, T., Ravichandra, P., and Prakasham, R. (2009). Characterization of thermo-and detergent stable serine protease from isolated Bacillus circulans and evaluation of eco-friendly applications. Process Biochem. 44, 262–268. doi: 10.1016/j.procbio.2008.10.022
Rao, M. B., Tanksale, A. M., Ghatge, M. S., and Deshpande, V. V. (1998). Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 62, 597–635.
Rao, R. R., Vimudha, M., Kamini, N., Gowthaman, M., Chandrasekran, B., and Saravanan, P. (2017). Alkaline protease production from Brevibacterium luteolum (MTCC 5982) under solid-state fermentation and its application for sulfide-free unhairing of cowhides. Appl. Biochem. Biotechnol. 182, 511–528. doi: 10.1007/s12010-016-2341-z
Rathakrishnan, P., and Nagarajan, P. (2012). Optimizing factors affecting protease production by a Bacillus cereus using groundnut shell under solid substrate fermentation. Int. J. Sci. Invent. Todays 1, 114–129.
Rattenholl, A., Lilie, H., Grossmann, A., Stern, A., Schwarz, E., and Rudolph, R. (2001). The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur. J. Biochem. 268, 3296–3303. doi: 10.1046/j.1432-1327.2001.02232.x
Ravikumar, G., Gomathi, D., Kalaiselvi, M., and Uma, C. (2012). A protease from the medicinal mushroom Pleurotus sajor-caju; production, purification and partial characterization. Asian Pac. J. Trop. Biomed. 2, S411–S417. doi: 10.1016/S2221-1691(12)60198-1
Rawlings, N. D., Tolle, D. P., and Barrett, A. J. (2004). MEROPS: the peptidase database. Nucleic Acids Res. 32, D160–D164. doi: 10.1093/nar/gkh071
Reddy, L., Wee, Y.-J., Yun, J.-S., and Ryu, H.-W. (2008). Optimization of alkaline protease production by batch culture of Bacillus sp. RKY3 through Plackett–Burman and response surface methodological approaches. Bioresour. Technol. 99, 2242–2249. doi: 10.1016/j.biortech.2007.05.006
Rehman, R., Ahmed, M., Siddique, A., Hasan, F., Hameed, A., and Jamal, A. (2017). Catalytic role of thermostable metalloproteases from Bacillus subtilis KT004404 as dehairing and destaining agent. Appl. Biochem. Biotechnol. 181, 434–450. doi: 10.1007/s12010-016-2222-5
Reichenbach, H. (2006). The Genus Lysobacter. The Prokaryotes: Volume 6: Proteobacteria: Gamma Subclass. Singapore: Springer Science+Business Media LLC, 939–957.
Rifaat, H. M., El-Said, O. H., Hassanein, S. M., and Selim, M. S. (2007). Protease activity of some mesophilic streptomycetes isolated from Egyptian habitats. J. Cult. Collect. 5, 16–24.
Rivett, A. J. (1989). The multicatalytic proteinase of mammalian cells. Arch. Biochem. Biophys. 268, 1–8. doi: 10.1016/0003-9861(89)90558-4
Rock, K. L., and Goldberg, A. L. (1999). Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu. Rev. Immunol. 17, 739–779. doi: 10.1146/annurev.immunol.17.1.739
Roja Rani, M., Lalitha Kumara, B., and Siva Prasad, D. (2012). Isolation and screening of alkaline protease producing bacteria and induction of overproducing Bacillus licheniformis mutants through UV irradiation. IOSR J. Pharmacy 1, 1–14. doi: 10.9790/3013-0110114
Romsomsa, N., Chim-anagae, P., and Jangchud, A. (2010). Optimization of silk degumming protease production from Bacillus subtilis C4 using Plackett-Burman design and response surface methodology. Sci. Asia 36, 118–124. doi: 10.2306/scienceasia1513-1874.2010.36.118
Ruigrok, V. J., Levisson, M., Eppink, M. H., Smidt, H., and Van Der Oost, J. (2011). Alternative affinity tools: more attractive than antibodies? Biochem. J. 436, 1–13. doi: 10.1042/BJ20101860
Saeki, K., Ozaki, K., Kobayashi, T., and Ito, S. (2007). Detergent alkaline proteases: enzymatic properties, genes, and crystal structures. J. Biosci. Bioeng. 103, 501–508. doi: 10.1263/jbb.103.501
Sandhya, C., Sumantha, A., Szakacs, G., and Pandey, A. (2005). Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process Biochem. 40, 2689–2694. doi: 10.1016/j.procbio.2004.12.001
Sarmadi, B.H., and Ismail, A. (2010). Antioxidative peptides from food proteins: a review. Peptides 31, 1949–1956. doi: 10.1016/j.peptides.2010.06.020
Sathishkumar, R., Ananthan, G., and Arun, J. (2015). Production, purification and characterization of alkaline protease by ascidian associated Bacillus subtilis GA CAS8 using agricultural wastes. Biocatal. Agric. Biotechnol. 4, 214–220. doi: 10.1016/j.bcab.2014.12.003
Satyanarayana, T., Sharma, A., Mehta, D., Puri, A. K., Kumar, V., Nisha, M., et al. (2012). “Biotechnological applications of biocatalysts from the Firmicutes bacillus and Geobacillus species,” in Microorganisms in Sustainable Agriculture and Biotechnology, eds T. Satyanarayana, B. Johri, and Anil Prakash (Dordrecht: Springer), 343–379. doi: 10.1007/978-94-007-2214-9_17
Schomburg, D., and Salzmann, M. (1991). Enzyme Handbook. Berlin; Heidelberg: Springer-Verlag, 1–1175. doi: 10.1007/978-3-642-76729-6_1
Sellami-Kamoun, A., Haddar, A., Ali, N. E.-H., Ghorbel-Frikha, B., Kanoun, S., and Nasri, M. (2008). Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbiol. Res. 163, 299–306. doi: 10.1016/j.micres.2006.06.001
Sen, S., Dasu, V., Dutta, K., and Mandal, B. (2011). Characterization of a novel surfactant and organic solvent stable high-alkaline protease from new Bacillus pseudofirmus SVB1. Res. J. Microbiol. 6, 769–783. doi: 10.3923/jm.2011.769.783
Shahid, M., Mohammad, F., Chen, G., Tang, R.-C., and Xing, T. (2016). Enzymatic processing of natural fibres: white biotechnology for sustainable development. Green Chem. 18, 2256–2281. doi: 10.1039/C6GC00201C
Shankar, S., More, S., and Laxman, R. S. (2010). Recovery of silver from waste X-ray film by alkaline protease from Conidiobolus coronatus. Kathmandu Univ. J. Sci. Eng. Technol. 6, 60–69. doi: 10.3126/kuset.v6i1.3311
Shankar, S., Rao, M., and Laxman, R. S. (2011). Purification and characterization of an alkaline protease by a new strain of Beauveria sp. Process Biochem. 46, 579–585. doi: 10.1016/j.procbio.2010.10.013
Shanlin, F., Stocker, R., and Davies, M. J. (1997). Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 324, 1–18. doi: 10.1042/bj3240001
Shinmyo, A., Mitsushima, K., and Terui, G. (1972). Kinetic studies on enzyme production by microbes. IX. Some properties of auto-induction of acid protease in Aspergillus niger. J. Ferment. Technol. 50, 647–654.
Siar, E.-H., Morellon-Sterling, R., Zidoune, M. N., and Fernandez-Lafuente, R. (2019). Amination of ficin extract to improve its immobilization on glyoxyl-agarose: improved stability and activity versus casein. Int. J. Biol. Macromol. 133, 412–419. doi: 10.1016/j.ijbiomac.2019.04.123
Siddiqui, K.S., and Cavicchioli, R. (2006). Cold-adapted enzymes. Annu. Rev. Biochem. 75, 403–433. doi: 10.1146/annurev.biochem.75.103004.142723
Sielecki, A., Fujinaga, M., Read, R., and James, M. (1991). Refined structure of porcine pepsinogen at 1 · 8 Å resolution. J. Mol. Biol. 219, 671–692. doi: 10.1016/0022-2836(91)90664-R
Silen, J., Frank, D., Fujishige, A., Bone, R., and Agard, D. A. (1989). Analysis of prepro-alpha-lytic protease expression in Escherichia coli reveals that the pro region is required for activity. J. Bacteriol. 171, 1320–1325. doi: 10.1128/jb.171.3.1320-1325.1989
Silen, J., McGrath, C., Smith, K., and Agard, D. (1988). Molecular analysis of the gene encoding α-lytic protease: evidence for a preproenzyme. Gene 69, 237–244. doi: 10.1016/0378-1119(88)90434-9
Simkhada, J.R., Mander, P., Cho, S. S., and Yoo, J. C. (2010b). A novel fibrinolytic protease from Streptomyces sp. CS684. Process Biochem. 45, 88–93. doi: 10.1016/j.procbio.2009.08.010
Simkhada, J. R., Cho, S. S., Park, S. J., Mander, P., Choi, Y. H., Lee, H. J., et al. (2010a). An oxidant-and organic solvent-resistant alkaline metalloprotease from Streptomyces olivochromogenes. Appl. Biochem. Biotechnol. 162, 1457–1470. doi: 10.1007/s12010-010-8925-0
Singh, J., Vohra, R., and Sahoo, D. K. (1999). Alkaline protease from a new obligate alkalophilic isolate of Bacillus sphaericus. Biotechnol. Lett. 21, 921–924. doi: 10.1023/A:1005502824637
Singh, P., Rani, A., and Chaudhary, N. (2015). Isolation and characterization of protease producing Bacillus sp from soil. Int. J. Pharma Sci. Res. 6, 633–639.
Singh, R., Mittal, A., Kumar, M., and Mehta, P. K. (2016). Microbial protease in commercial applications. J. Pharm. Chem. Biol. Sci. 4, 365–374.
Singhal, P., Nigam, V., and Vidyarthi, A. (2012). Studies on production, characterization and applications of microbial alkaline proteases. Int. J. Adv. Biotechnol. Res. 3, 653–669.
Sloma, A., Rudolph, C., Rufo, G., Sullivan, B., Theriault, K., Ally, D., et al. (1990). Gene encoding a novel extracellular metalloprotease in Bacillus subtilis. J. Bacteriol. 172, 1024–1029. doi: 10.1128/jb.172.2.1024-1029.1990
Sodek, J., and Hofmann, T. (1970). Large-scale preparation and some properties of penicillopepsin, the acid proteinase of Penicillium janthinellum. Can. J. Biochem. 48, 425–431. doi: 10.1139/o70-069
Somkuti, G., and Babel, F. (1967). Conditions influencing the synthesis of acid protease by Mucor pusillus Lindt. Appl. Environ. Microbiol. 15, 1309–1312.
Soroor, M. A., Hendawy, H., Ghazy, A., Semary, N., Khalil, K., and Aziz, A. (2009). Characterization of an alkaline metalloprotease secreted by the entomopathogenic bacterium Photorhabdus sp. strain EK1. Res. J. Agric. Biol. Sci. 5, 349–360.
Sørvig, E., Mathiesen, G., Naterstad, K., Eijsink, V. G., and Axelsson, L. (2005). High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology 151, 2439–2449. doi: 10.1099/mic.0.28084-0
Souza, P. M., Bittencourt, M. L. d. A., Caprara, C. C., Freitas, M., de, Almeida, R. P. C., de Silveira, D., et al. (2015). A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 46, 337–346. doi: 10.1590/S1517-838246220140359
Stabile, M. R., Lai, W. G., DeSantis, G., Gold, M., Jones, J. B., Mitchinson, C., et al. (1996). Probing the specificity of the S1 binding site of M222 mutants of subtilisin B. lentus with boronic acid inhibitors. Bioorg. Med. Chem. Lett. 6, 2501–2506. doi: 10.1016/0960-894X(96)00466-0
Stack, C. M., Lowther, J., Cunningham, E., Donnelly, S., Gardiner, D. L., Trenholme, K. R., et al. (2007). Characterization of the Plasmodium falciparum M17 leucyl aminopeptidase A protease involved in amino acid regulation with potential for antimalarial drug development. J. Biol. Chem. 282, 2069–2080. doi: 10.1074/jbc.M609251200
Steele, D. B., Fiske, M. J., Steele, B. P., and Kelley, V. C. (1992). Production of a low-molecular-weight, alkaline-active, thermostable protease by a novel, spiral-shaped bacterium, Kurthia spiroforme sp. nov. Enzyme Microb. Technol. 14, 358–360. doi: 10.1016/0141-0229(92)90003-7
Sumantha, A., Larroche, C., and Pandey, A. (2006). Microbiology and industrial biotechnology of food-grade proteases: a perspective. Food Technol. Biotechnol. 44:211.
Suntornsuk, W., and Suntornsuk, L. (2003). Feather degradation by Bacillus sp. FK 46 in submerged cultivation. Bioresour. Technol. 86, 239–243. doi: 10.1016/S0960-8524(02)00177-3
Suwannaphan, S., Fufeungsombut, E., Promboon, A., and Chim-Anage, P. (2017). A serine protease from newly isolated Bacillus sp. for efficient silk degumming, sericin degrading and colour bleaching activities. Int. Biodeter. Biodegrad. 117, 141–149. doi: 10.1016/j.ibiod.2016.12.009
Suzuki, K. (1987). Calcium activated neutral protease: domain structure and activity regulation. Trends Biochem. Sci. 12, 103–105. doi: 10.1016/0968-0004(87)90048-X
Takami, H., Nakamura, S., Aono, R., and Horikoshi, K. (1992). Degradation of human hair by a thermostable alkaline protease from alkaliphilic Bacillus sp. no. AH-101. Biosci. Biotechnol. Biochem. 56, 1667–1669. doi: 10.1271/bbb.56.1667
Tammawong, S. (2005). Cloning and Expression of Neutral Protease (npr) Gene from Bacillus cereus MFKU33 in Escherichia coli. Bangkok: Kasetsart University.
Tamreihao, K., Devi, L. J., Khunjamayum, R., Mukherjee, S., Ashem, R. S., and Ningthoujam, D. S. (2017). Biofertilizing potential of feather hydrolysate produced by indigenous keratinolytic Amycolatopsis sp. MBRL 40 for rice cultivation under field conditions. Biocatal. Agric. Biotechnol. 10, 317–320. doi: 10.1016/j.bcab.2017.04.010
Tanaka, K., Yoshimura, T., Kumatori, A., Ichihara, A., Ikai, A., Nishigai, M., et al. (1988). Proteasomes (multi-protease complexes) as 20 S ring-shaped particles in a variety of eukaryotic cells. J. Biol. Chem. 263, 16209–16217.
Tavano, O. L. (2013). Protein hydrolysis using proteases: an important tool for food biotechnology. J. Mol. Catal. B Enzym. 90, 1–11. doi: 10.1016/j.molcatb.2013.01.011
Tavano, O. L., A. Berenguer-Murcia Secundo, F., and Fernandez-Lafuente, R. (2018). Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 17, 412–436. doi: 10.1111/1541-4337.12326
Taylor, A., Volz, K. W., Lipscomb, W. N., and Takemoto, L. (1984). Leucine aminopeptidase from bovine lens and hog kidney. Comparison using immunological techniques, electron microscopy, and X-ray diffraction. J. Biol. Chem. 259, 14757–14761.
Thiansilakul, Y., Benjakul, S., and Shahidi, F. (2007). Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food Chem. 103, 1385–1394. doi: 10.1016/j.foodchem.2006.10.055
Thirumurugan, D., and Vijayakumar, R. (2015). Characterization and structure elucidation of antibacterial compound of Streptomyces sp. ECR77 isolated from East Coast of India. Curr. Microbiol. 70, 745–755. doi: 10.1007/s00284-015-0780-3
Tomkinson, B., Wernstedt, C., Hellman, U., and Zetterqvist, O. (1987). Active site of tripeptidyl peptidase II from human erythrocytes is of the subtilisin type. Proc. Natl. Acad. Sci. U.S.A. 84, 7508–7512. doi: 10.1073/pnas.84.21.7508
Tomoda, K., and Shimazono, H. (1964). Acid protease produced by trametes sanguinea, a wood-destroying fungus: part purification I, and crystallization of the enzyme part I physical I, and enzymological properties of the enzyme. Agric. Biol. Chem. 28, 770–778. doi: 10.1080/00021369.1964.10858303
Udenigwe, C. C. (2014). Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 36, 137–143. doi: 10.1016/j.tifs.2014.02.004
Vadlamani, S., and Parcha, S. R. (2011). Studies on industrially important alkaline protease production from locally isolated superior microbial strain from soil microorganisms. Int. J. Biotechnol. Appl. 3, 102–105. doi: 10.9735/0975-2943.3.3.102-105
Varela, H., Ferrari, M. D., Belobrajdic, L., Vazquez, A., and Loperena, M. L. (1997). Skin unhairing proteases of Bacillus subtilis: production andpartial characterization. Biotechnol. Lett. 19, 755–758. doi: 10.1023/A:1018384025181
Vasantha, N., Thompson, L., Rhodes, C., Banner, C., Nagle, J., and Filpula, D. (1984). Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J. Bacteriol. 159, 811–819.
Verma, A., Singh, H., Anwar, M. S., Kumar, S., Ansari, M. W., and Agrawal, S. (2016). Production of thermostable organic solvent tolerant keratinolytic protease from Thermoactinomyces sp. RM4: IAA production and plant growth promotion. Front. Microbiol. 7:1189. doi: 10.3389/fmicb.2016.01189
Verma, T., and Agarwa, S. (2016). Isolation and screening of haloalkaline protease producing bacteria from tannery solid waste. Int. J. Res. Eng. Technol. 5, 237–244. doi: 10.15623/ijret.2016.0501048
Vijayaraghavan, P., Jebamalar, T. R. J., and Vincent, S. G. P. (2012). Biosynthesis optimization and purification of a solvent stable alkaline serine protease from Halobacterium sp. Ann. Microbiol. 62, 403–410. doi: 10.1007/s13213-011-0276-8
Vijayaraghavan, P., Lazarus, S., and Vincent, S. G. P. (2014). De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: biosynthesis and properties. Saudi J. Biol. Sci. 21, 27–34. doi: 10.1016/j.sjbs.2013.04.010
Vijayaraghavan, P., and Vincent, S. P. (2015). A low cost fermentation medium for potential fibrinolytic enzyme production by a newly isolated marine bacterium, Shewanella sp. IND20. Biotechnol. Rep. 7, 135–142. doi: 10.1016/j.btre.2015.06.005
Vojcic, L., Pitzler, C., Koerfer, G., Jakob, F., Martinez, R., Maurer, K.-H., et al. (2015). Advances in protease engineering for laundry detergents. New Biotechnol. 32, 629–634. doi: 10.1016/j.nbt.2014.12.010
Wang, W., Vinocur, B., and Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14. doi: 10.1007/s00425-003-1105-5
Wang, Y., Qian, G., Li, Y., Wang, Y., Wang, Y., Wright, S., et al. (2013). Biosynthetic mechanism for sunscreens of the biocontrol agent Lysobacter enzymogenes. PLoS ONE 8:e66633. doi: 10.1371/journal.pone.0066633
Watabe, S., and Kimura, T. (1985). ATP-dependent protease in bovine adrenal cortex. Tissue specificity, subcellular localization, and partial characterization. J. Biol. Chem. 260, 5511–5517.
Weaver, L., Kester, W., and Matthews, B. (1977). A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of extended substrates. J. Mol. Biol. 114, 119–132. doi: 10.1016/0022-2836(77)90286-8
Weston, J. (2005). Mode of action of bi-and trinuclear zinc hydrolases and their synthetic analogues. Chem. Rev. 105, 2151–2174. doi: 10.1021/cr020057z
Woessner, J. F., Woessner, J. F., and Nagase, H. (2000). Matrix Metalloproteinases and TIMPs. Oxford: Oxford University Press.
Wolff, A., Showell, M., Venegas, M., Barnett, B., and Wertz, W. (1996). “Laundry performance of subtilisin proteases,” in Subtilisin Enzymes. Advances in Experimental Medicine and Biology, Vol. 379, eds R. Bott, and C. Betzel (Boston, MA: Springer), 113–120. doi: 10.1007/978-1-4613-0319-0_12
Wu, H.-C., Chen, H.-M., and Shiau, C.-Y. (2003). Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 36, 949–957. doi: 10.1016/S0963-9969(03)00104-2
Xing, X., Jia, J.-Q., Zhang, J.-F., Zhou, Z.-W., Li, J., Wang, N., et al. (2019). CALB immobilized onto magnetic nanoparticles for efficient kinetic resolution of racemic secondary alcohols: long-term stability and reusability. Molecules 24:490. doi: 10.3390/molecules24030490
Xu, M.-Q., Wang, S.-S., Li, L.-N., Gao, J., and Zhang, Y.-W. (2018). Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 8:460. doi: 10.3390/catal8100460
Xue, B., Dunker, A. K., and Uversky, V. N. (2012). Orderly order in protein intrinsic disorder distribution: disorder in 3500 proteomes from viruses and the three domains of life. J. Biomol. Struct. Dyn. 30, 137–149. doi: 10.1080/07391102.2012.675145
Yadav, S. K., Bisht, D., Shikha, S., and Darmwal, N. S. (2011). Oxidant and solvent stable alkaline protease from Aspergillus flavus and its characterization. Afr. J. Biotechnol. 10, 8630–8640. doi: 10.5897/AJB10.1611
Yadav, S. K., Bisht, D., Tiwari, S., and Darmwal, N. S. (2015). Purification, biochemical characterization and performance evaluation of an alkaline serine protease from Aspergillus flavus MTCC 9952 mutant. Biocatal. Agric. Biotechnol. 4, 667–677. doi: 10.1016/j.bcab.2015.08.007
Yamagata, Y., Abe, R., Fujita, Y., and Ichishima, E. (1995). Molecular cloning and nucleotide sequence of the 90k serine protease gene, hspK, from Bacillus subtilis (natto) No. 16. Curr. Microbiol. 31, 340–344. doi: 10.1007/BF00294696
Yang, J.-K., Shih, L., Tzeng, Y.-M., and Wang, S.-L. (2000). Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microb. Technol. 26, 406–413. doi: 10.1016/S0141-0229(99)00164-7
Yang, S., Du, G., Chen, J., and Kang, Z. (2017). Characterization and application of endogenous phase-dependent promoters in Bacillus subtilis. Appl. Microbiol. Biotechnol. 101, 4151–4161. doi: 10.1007/s00253-017-8142-7
Yang, Y., Jiang, L., Zhu, L., Wu, Y., and Yang, S. (2000). Thermal stable and oxidation-resistant variant of subtilisin E. J. Biotechnol. 81, 113–118. doi: 10.1016/S0168-1656(00)00272-8
Younes, I., and Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs 13, 1133–1174. doi: 10.3390/md13031133
Zhang, G., Wang, H., Zhang, X., and Ng, T. (2010). Helvellisin, a novel alkaline protease from the wild ascomycete mushroom Helvella lacunosa. J. Biosci. Bioeng. 109, 20–24. doi: 10.1016/j.jbiosc.2009.06.022
Zhang, S., Xu, Z., Sun, H., Sun, L., Shaban, M., Yang, X., et al. (2019). Genome-wide identification of papain-like cysteine proteases in Gossypium hirsutum and functional characterization in response to Verticillium dahliae. Front. Plant Sci. 10:134. doi: 10.3389/fpls.2019.00134
Zheng, S., Wang, H., and Zhang, G. (2011). A novel alkaline protease from wild edible mushroom Termitomyces albuminosus. Acta Biochim. Pol. 58, 269–273. doi: 10.18388/abp.2011_2277
Keywords: microbial proteases, proteolytic enzymes, bacterial enzymes, industrial enzyme, substrate-specific proteases
Citation: Razzaq A, Shamsi S, Ali A, Ali Q, Sajjad M, Malik A and Ashraf M (2019) Microbial Proteases Applications. Front. Bioeng. Biotechnol. 7:110. doi: 10.3389/fbioe.2019.00110
Received: 07 February 2019; Accepted: 01 May 2019;
Published: 12 June 2019.
Edited by:
Xiao-Jun Ji, Nanjing Tech University, ChinaReviewed by:
Jiufu Qin, Technical University of Denmark, DenmarkJin-Song Gong, Jiangnan University, China
Copyright © 2019 Razzaq, Shamsi, Ali, Ali, Sajjad, Malik and Ashraf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qurban Ali, YmlvbGZvcm1hbml0ZUBnbWFpbC5jb20=; c2FpbTE2MjlAZ21haWwuY29t
 Abdul Razzaq1
Abdul Razzaq1 Arfan Ali
Arfan Ali Qurban Ali
Qurban Ali