
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Bioeng. Biotechnol., 05 April 2017
Sec. Bionics and Biomimetics
Volume 5 - 2017 | https://doi.org/10.3389/fbioe.2017.00023
This article is part of the Research TopicBiomimetic fabrication for 3D biologyView all 5 articles
There is a growing demand for alternative fabrication approaches to develop tissues and organs as conventional techniques are not capable of fabricating constructs with required structural, mechanical, and biological complexity. 3D bioprinting offers great potential to fabricate highly complex constructs with precise control of structure, mechanics, and biological matter [i.e., cells and extracellular matrix (ECM) components]. 3D bioprinting is an additive manufacturing approach that utilizes a “bioink” to fabricate devices and scaffolds in a layer-by-layer manner. 3D bioprinting allows printing of a cell suspension into a tissue construct with or without a scaffold support. The most common bioinks are cell-laden hydrogels, decellulerized ECM-based solutions, and cell suspensions. In this mini review, a brief description and comparison of the bioprinting methods, including extrusion-based, droplet-based, and laser-based bioprinting, with particular focus on bioink design requirements are presented. We also present the current state of the art in bioink design including the challenges and future directions.
Tissue engineering is a multidisciplinary field currently focused on two major areas: (i) developing new methods to repair, regenerate, and replace damaged tissues and organs and (ii) creating in vitro tissue models to better understand tissue development, disease development, and progression and to develop and screen drugs (Langer and Vacanti, 1993; Griffith and Naughton, 2002; Benam et al., 2015; Tibbitt et al., 2015; Nguyen et al., 2016; Zhang et al., 2016). Despite recent advances in tissue engineering, there is a continuous lack of tissues and organs for transplantation and a shortage for tissue models for drug discovery and testing (Bajaj et al., 2014). Conventional techniques, such as porogen-leaching, injection molding, and electrospinning, are generally recognized as the bottleneck due to limited control over scaffold architecture, composition, pore shape, size, and distribution (Murphy and Atala, 2014; Groen et al., 2016; Shafiee and Atala, 2016). 3D bioprinting enables fabrication of scaffolds, devices, and tissue models with high complexity (Murphy and Atala, 2014; Mandrycky et al., 2016; Ozbolat et al., 2016, 2017; Shafiee and Atala, 2016). 3D printing allows construction of tissues from commonly used medical images (such as X-ray, magnetic resonance imaging, and computerized tomography scan) using computer-aided design. Custom and patient-specific design, on-demand fabrication, high structural complexity, low-cost, and high-efficiency are some of the major advantages of 3D printing making it very attractive for medicine (Guillemot et al., 2010; Guvendiren et al., 2016).
3D bioprinting is a technology to fabricate constructs from living cells with or without a carrier material in a layer-by-layer manner (Dababneh and Ozbolat, 2014; Murphy and Atala, 2014; Mandrycky et al., 2016; Shafiee and Atala, 2016; Cui et al., 2017). The material that is printed is referred to as a “bioink,” which can be defined as an ink formulation that allows printing of living cells. Here, we would like to note that many of the biomaterial ink formulations are not suitable for cell printing. For instance, polycaprolactone (PCL) and poly(lactic acid) (PLA) are the most widely used biomaterials in 3D printing. However, they could only be printed at elevated temperatures in the form of a polymer melt or when dissolved in organic solvents as a polymer solution. Therefore, they are not considered as bioinks in this review, as both approaches are not suitable for live cell printing (Jose et al., 2016; Munaz et al., 2016). In this paper, we discuss the most commonly used bioinks, including cell-laden hydrogels, extracellular matrix (ECM)-based solutions, and cell suspensions (Levato et al., 2014; Adam et al., 2016; Guvendiren et al., 2016; Panwar and Tan, 2016), and give the current state of the art in bioink design with challenges and future directions. A brief description and comparison of the bioprinting methods with particular focus on bioink design requirements are also given.
3D bioprinting process should be relatively mild and cell friendly as it is required to allow cell printing (Ozbolat et al., 2016, 2017). This requirement limits the number of 3D printing techniques that are suitable for bioprinting (Figure 1). It is important to note that the 3D printing technology determines the requirements for printability of a material, and not all of the 3D printing technologies are suitable for bioprinting. Currently available 3D printing technologies allow a wide range of materials to be printed using diverse ink formulations (Guvendiren et al., 2016). Fused deposition modeling (FDM) is an extrusion-based printing and utilizes synthetic thermoplastics and their composites with ceramics and metals (Turner et al., 2014). For FDM, the form of ink material is a filament, and it is extruded at elevated temperatures (140–250°C) in melt state, which eliminates FDM as an option for bioprinting. Direct ink writing (DIW) is also an extrusion-based printing and allows extrusion of high viscosity solutions, hydrogels, and colloidal suspensions (Ozbolat and Hospodiuk, 2016). DIW allows printing of cell suspensions and/or aggregates with or without a carrier. Inkjet printing is another technology for cell printing. The processing principle is deposition of polymeric solutions, colloidal suspensions, and cell suspensions, with relatively low viscosities [<10 cP (mPa⋅s)] at relatively high shear rates (105–106 s−1) in the form droplets (~50 μm in diameter) (Mironov et al., 2003; Wilson and Boland, 2003a,b; Nakamura et al., 2005; Gudapati et al., 2016). As compared to extrusion-based bioprinters, inkjet bioprinters are not readily available, yet there are commercially available inkjet print heads that are suitable for bioprinting (Nishiyama et al., 2008; Choi et al., 2011). Selective laser sintering utilizes metals, ceramics, polymers, and composites in powder form (10–150 µm in diameter) and is not suitable for bioprinting. In this technique, a directed laser beam locally melts either directly the powder or a polymeric binder onto the bed surface (Shirazi et al., 2015). Layers of fresh powder are continuously supplied after each layer is created. Stereolithography (SLA) requires a viscous photocurable polymer solution or a prepolymer, which is exposed to a directed light (such as UV or laser) to spatially cross-link the solution (Skoog et al., 2014). SLA could potentially be considered for printing live cells as long as a cell-laden prepolymer formulation is used and the photocuring takes place in a mild, cell friendly condition, which are the two major issues for SLA in bioprinting (Elomaa et al., 2015; Wang et al., 2015; Morris et al., 2017). When 3D printing technologies are considered for bioprinting, the most commonly used technologies are DIW and inkjet printing (Ozbolat et al., 2016, 2017). In addition to these technologies laser-induced forward transfer (LIFT) is also shown to be suitable for bioprinting (Barron et al., 2004a,b; Ringeisen et al., 2004; Hopp et al., 2005; Doraiswamy et al., 2006; Koch et al., 2010). In this technique, ink solution is coated onto a glass slide and coated with a laser absorption layer (metal or a metal oxide). Laser is directed to the laser absorption layer with an ablation spot size between 40 and 100 µm in diameter (Barron et al., 2004a,b; Koch et al., 2010) creating a local pressure to eject the ink layer to the substrate.
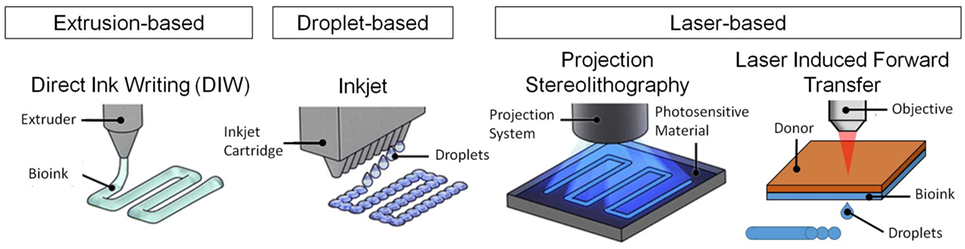
Figure 1. 3D bioprinting techniques for bioprinting of tissues and organs. Figure reproduced with permission from Miller and Burdick (2016). Copyright 2016, American Chemical Society.
The ideal bioink formulation should satisfy certain material and biological requirements. Material properties are printability, mechanics, degradation, and functionalizability. Biological requirements mainly include biocompatibility, cytocompatilibilty, and bioactivity. When material properties are considered, printability is the most important parameter. Printability comprises two parts: (i) the processability of the bioink formulation and (ii) the print fidelity associated with the mechanical strength of the printed construct to self-sustain a 3D structure post-printing. Depending on the printing process, printability could potentially involve solution viscosity, surface tension, and cross-linking properties. Viscosity is a crucial parameter for a bioink formulation as it affects both the print fidelity and cell encapsulation efficiency. High viscosity polymer solutions are less likely to flow easily so that the printed structure could hold its shape at longer times post-printing. However, they require higher pressures to flow, limiting the gage size and smallest achievable print size (mainly for DIW). In this regard, Tirella et al. (2009) investigated the processing window for alginate hydrogels using pressure-assisted microfabrication (DIW technique). They successfully developed a 3D phase diagram showing the interplay between bioink viscosity, print velocity, and applied pressure to obtain high print fidelity (Tirella et al., 2009). The bioink formulation is preferred to have a tunable viscosity to be compatible with different bioprinters. For instance, bioinks for inkjet or droplet-based bioprinters have viscosity values close to 10 mPa⋅s (Gudapati et al., 2016); the viscosity of bioinks for extrusion-based DIW bioprinting ranges from 30 to 6 × 107 mPa⋅s (Hölzl et al., 2016; Ozbolat et al., 2016, 2017); for laser-assisted bioprinting, the bioink viscosity is in the range of 1–300 mPa⋅s (Guillotin et al., 2010; Hölzl et al., 2016). For high viscosity bioinks used in extrusion and droplet-based print, the shear-thinning characteristic is desired to compensate for the high shear stress associated with high viscosity. The overall mechanics, i.e., achievable stiffness, is important not only to create self-supporting constructs but also to control and direct cellular behavior. Degradation is important for the functional integration of the printed construct in vivo by enabling cells to gradually replace the construct with their ECM. Both the bioink and the degradation products should not contain materials that induce inflammatory host response when implanted. Functionalizability is required to incorporate biochemical cues, i.e., bioactivity, to direct cellular behavior, such as adhesion, migration, and differentiation. In addition to biocompatibility and cytocompatibility, high cell viability, both prior- and post-printing, is crucial for the ink formulation. In addition to bioink design, a recent study showed the importance of the print substrate for live cell inkjet printing. In this work, computational and experimental studies confirmed that the stiffness of the print substrate directly influences the impact forces acting on the droplet, which affects the overall cell survival (Tirella et al., 2011). Below we will discuss the commonly used bioinks including current state of the art in ink design.
The most commonly used bioinks for tissue and organ printing are cell-laden hydrogels, decellularized extracellular matrix (dECM)-based solutions, and cell suspensions (Figure 2). Cell-laden hydrogels are particularly attractive due to their tunable properties and their ability to recapitulate the cellular microenvironment (Fedorovich et al., 2007). ECM-based bioink formulations or decellulerized tissue inks are an emerging field due to their inherent bioactivity and ease of formulation into a printable bioink (Pati et al., 2014). Cell suspension inks based on cell aggregates are a viable option to create scaffold-free biological constructs (Forgacs and Foty, 2004; Marga et al., 2007).
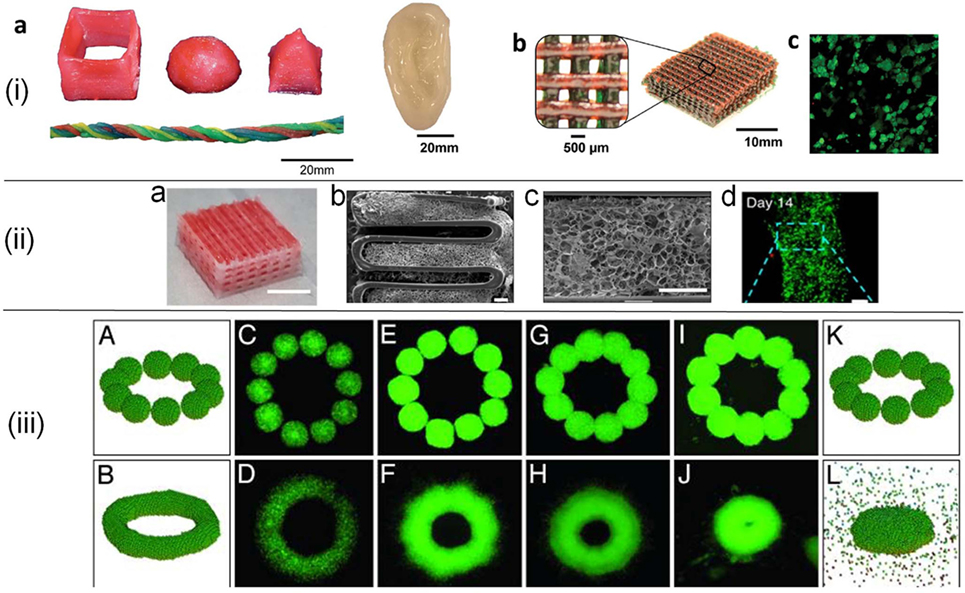
Figure 2. (i) 3D printed constructs in various forms (a,b) using poly(ethylene glycol)–alginate–nanoclay hydrogels. Red food dye was incorporated into some of the bioink formulations for visibility. Live/dead assay of cells (c) in a collagen infused mesh from (b). Reprinted with permission from Hong et al. (2015). Copyright 2015, John Wiley and Sons. (ii) Tissue construct printed from decellularized extracellular matrix (dECM) (a), SEM images of hybrid constructs from dECM supported with polycaprolactone framework (b,c), and fluorescent images of cells (d). Scale bars are 5 mm for (a), 400 µm for (b,c), and 100 µm for (d). Adapted with permission from Pati et al. (2014). Copyright 2014, Nature Publishing Group. (iii) Cell aggregate (500-µm average diameter) configurations in simulations (A,B,K,L) and experiments. C–J correspond to cell aggregates embedded in a neurogel with RGD fragments (C,D) and collagen gels of concentration 1.0 mg/ml (E,F), 1.2 mg/ml (G,H), and 1.7 mg/ml (I,J). Figure adapted with permission from Jakab et al. (2004). Copyright 2004, National Academy of Sciences.
Cell-laden hydrogels are the most commonly used bioinks as they can be easily formulated for extrusion-based (DIW), droplet-based (inkjet), and laser-based (SLA and LIFT) bioprinting technologies. Cell-laden hydrogel bioink formulations utilize natural hydrogels such as agarose, alginate, chitosan, collagen, gelatin, fibrin, and hyaluronic acid (HA), as well as synthetic hydrogels such as pluronic (poloxamer) and poly(ethylene glycol) (PEG), or blends of both. Natural hydrogels offer inherent bioactivity except for agarose and alginate and display a structural resemblance to ECM. For instance, fibrin and collagen hydrogels with inherent filamentous structure display strain-stiffening property, mimicking the non-linear elastic behavior of the soft tissues in our body (Gardel et al., 2004; Storm et al., 2005). Synthetic hydrogels permit but do not promote cellular function, yet there are many ways to tether bioactive cues into synthetic hydrogels (Guvendiren and Burdick, 2013). When compared to natural hydrogels, synthetic hydrogels generally offer tunable mechanical properties. Many natural polymers (such as gelatin and HA) have functionalizable backbone side chains enabling them to be functionalized with chemical moieties to induce cross-linking (chemical- and/or photo-cross-linking) or additional bioactivity (Burdick and Prestwich, 2011). Blends of synthetic and natural polymers have been used to develop mechanically tunable hydrogels with user-defined bioactivity. Finally, the mechanical properties and/or bioactivity can also be tuned by incorporating small amounts of nanoparticles into bioink formulation (Ribeiro et al., 2015).
Usually, all hydrogel bioink formulations require printing of a polymer solution followed by subsequent cross-linking. This requires a highly viscous polymer solution (polymer wt% >3%) and rapid cross-linking to develop self-supporting structures. There are two forms of cross-linking: physical and chemical cross-linking. Physical cross-linking is a non-chemical approach that utilizes hydrophobic interactions, ionic interactions, and hydrogen bonding. Chemical cross-linking relies on the formation of covalent bonds, which could be a radical polymerization (such as photo-cross-linking) or Michael-type addition reaction. The chemically cross-linked hydrogels form a mechanically robust network as compared to the physically cross-linked hydrogels, which is particularly important for the stem cell behavior including differentiation (Huebsch et al., 2010; Khetan et al., 2013).
Pluronic and PEG are the most common synthetic polymers for bioprinting. Pluronic, a poloxamer-based triblock copolymer composed of two hydrophobic groups between a water-soluble group, has been widely used in extrusion-based bioprinting as it gels at room temperature but flows at temperatures below 10°C. However, it is not very stable and erodes within hours. Thus, it is generally used as a supporting material (Kang et al., 2016). Lewis Lab took an advantage of this property and printed pluronic within a photopolymerizable hydrogel to create micro channels (Wu et al., 2011). Müller et al. (2015) developed an acrylated pluronic to create UV cross-linked stable gels post-printing. The most common forms of PEG for bioinks are PEG-diacrylate (PEG-DA) and PEG-methacrylate, which are suitable for extrusion-based, droplet-based, and laser-based printing technologies (Cui et al., 2012; Hribar et al., 2014; Wüst et al., 2015). PEG is hydrophilic and not adhesive to proteins and cells; therefore, it requires blending with other natural polymers or functionalization with biochemical cues. It is possible to form strong robust hydrogels using PEG-based polymers. For instance, Hockaday et al. (2012) printed aortic valve geometries using PEG-DA hydrogels blended with alginate and achieved 10-fold range in elastic modulus from ~5 to ~75 kPa. Hong et al. (2015) reported 3D printing of tough and biocompatible, cell-laden PEG–alginate–nanoclay hydrogels infused with collagen. Rutz et al. (2015) developed partially cross-linked PEG-based multi-material bioink formulations with tunable viscosity to enhance print fidelity and secondary cross-linking ability to stabilize the constructs.
Alginate is one of the most commonly used natural polymers to formulate bioinks for inkjet and DIW printing. For inkjet printing, calcium chloride is jetted onto alginic acid solution (Boland et al., 2007). For extrusion-based printing, alginate is printed as a viscous solution, and the constructs are exposed to CaCl2 solution to induce post-printing cross-linking. Alginate is not cell adhesive, thus it is generally blended with other natural polymers (e.g., gelatin and fibrinogen) to induce cell adhesion and biological activity (Xu et al., 2009; Jia et al., 2014; Yu et al., 2014; Lim et al., 2016; Pan et al., 2016). Note that, the majority of the natural polymers are used as a component of bioink formulation. HA and gelatin that have been utilized extensively in the form of functionalized polymers thus fall into the synthetic polymer category, which is discussed below.
Gelatin is commonly used in the form of gelatin methacryloyl (GelMA)-based hydrogel for DIW (Bertassoni et al., 2014; Loessner et al., 2016). Lim et al. (2016) recently reported a visible light photo-cross-linking system to minimize the oxygen inhibition in photopolymerized GelMA hydrogels. They reported higher print fidelity and cell viability for ruthenium/sodium persulfate visible photo-initiator as compared to UV photo-initiator Igracure 2959. Similar to gelatin, HA has been modified in many ways to create cell-laden bioinks (Highley et al., 2015; Rodell et al., 2015; Ouyang et al., 2016). For instance, Burdick lab reported HA-based supramolecular hydrogels cross-linked by cyclodextrin–adamantane host–guest interactions, which are capable of shear-thinning and self-healing (Highley et al., 2015). The non-covalent bonds allow direct writing of inks into support gels. HA hydrogels were developed to display both shear-thinning behavior due to guest–host bonding and stabilization post-printing via UV-induced covalent cross-linking (Ouyang et al., 2016). Supramolecular hydrogels are particularly attractive for extrusion-based printing as they could flow under shear and self-heal immediately after printing, leading to high print fidelity. In addition to guest–host bonding, self-assembling peptides (Raphael et al., 2017) and polypeptide–DNA hydrogels (Li et al., 2015) are other emerging candidates for bioink design.
Modified inkjet printers have long been used to print cells into cellular assemblies. For instance, endothelial cells were printed from cell suspension (1 × 105 cells/ml) in growth media (Wilson and Boland, 2003a,b). Bioprinting of scaffold-free constructs utilizes cell aggregates in the form of mono- or multicellular spheroids as a bioink (Mironov et al., 2003; Norotte et al., 2009; Jakab et al., 2010; Christensen et al., 2015). The bioink formulation undergoes a fully biological self-assembly without or in the presence of a temporary support layer (Norotte et al., 2009). This technique relies on tissue liquidity and fusion, which allow cells to self-assemble and fuse due to cell–cell interactions (Forgacs et al., 1998; Jakab et al., 2004; Fleming et al., 2010). For instance, Norotte et al. developed spheroids and cylinders of multicellular aggregates with controlled diameter in the range of 300–500 µm and showed that post-printing fusion led to single- and double-layered vascular tubes. Organovo is the first medical research company that uses a similar approach to create functional human tissues toward in vitro disease models. The company has developed liver models using high density bioinks from parenchymal cells or non-parenchymal cells that are printed via extrusion-based printing (Nguyen et al., 2016). Tissues were allowed to mature in a bioreactor for at least 3 days to form scaffold-free tissues. Levato et al. (2014) developed an alternative approach by combining bioprinting with microcarrier technology, which allowed extensive expansion of cells on cell-laden PLA-based microcarriers. Tan et al. (2016) used poly(d,l-lactic-co-glycolic acid) porous microspheres enabling cells to adhere and proliferate before printing.
Decellularized extracellular matrix-based bioinks involve decellularization of a tissue of interest by removing the cells while preserving the ECM. The ECM is then crushed into a powder form and dissolved in a cell friendly buffer solution to formulate the bioink. A carrier polymer could be used to increase the solubility, to tune the viscosity, or to induce/enhance post-cross-linking of the bioink. In this regard, Pati et al. (2014) printed 3D constructs using dECM-based bioinks supported by a PCL framework. For this purpose, dECM was obtained from fat, cartilage, and heart, using a combination of physical, enzymatic, and chemical processes. These ink materials were initially solubilized in an acidic buffer, and pH was adjusted to accommodate cells. This formulation was soluble at 10°C and gelled at 37°C. Following this study, the same group showed that the dECM bioink can be pre-gelled using vitamin B2-induced covalent cross-linking (Jang et al., 2016a,b,c). Using this approach, a 3D printed cardiac patch composed of multiple-cell lines including human cardiac progenitor cells and mesenchymal stem cells was developed (Jang et al., 2016a,b,c). Although dECM bioinks provide novel opportunities to fabricate tissue specific constructs, the decellularization process requires multiple steps including precise quantification of the DNA and the ECM components, making it a costly approach.
3D printing has a strong potential to become a common fabrication technique in medicine as it enables fabrication of modular and patient-specific scaffolds and devices, and tissue models, with high structural complexity and design flexibility (Murphy and Atala, 2014; Jang et al., 2016a,b,c; Kang et al., 2016; Kuo et al., 2016; Zhang et al., 2016). There is a significant interest in designing novel bioink formulations toward the goal of achieving the “ideal” bioink for each bioprinting technology (Hölzl et al., 2016). Cell-laden hydrogels are the most common bioinks, offering novel strategies including multi-material printing, shear-thinning capability, and sequential cross-linking toward self-supporting constructs. dECM-based bioinks provide an alternative approach utilizing decellulerized tissues, yet the processing of decellulerized tissue increases the cost of the bioinks. Cell aggregate printing enables direct printing of cells into tissue constructs, but the size of these constructs is currently limited as the process requires large quantities of cells. In addition to bioink development, there is also need for bioprinters with high resolution, which is particularly important to develop vascularized constructs. Considering future perspectives, supramolecular hydrogels with reversible cross-linking mechanism (Rodell et al., 2015) and stimuli responsive materials for biomimetic 4D printing (Sydney Gladman et al., 2016) are potentially the most interesting candidates for bioink design. Finally, there are still many regulatory challenges to move the 3D bioprinted constructs into clinic.
SJ and MG wrote the manuscript, and MG edited the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, AA, and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Authors would like to thank Dr. Chya-Yan Liaw for her fruitful comments. Authors are very grateful to National Science Foundation (DMR-1714882) (MG) and New Jersey Institute of Technology (MG and SJ) for the funding.
This work is funded by National Science Foundation (DMR-1714882) and New Jersey Institute of Technology (NJIT) through Faculty Seed Grant and new faculty startup funds.
Adam, E. J., Alexandra, L. R., and Ramille, N. S. (2016). Advancing the field of 3D biomaterial printing. Biomed. Mater. 11, 014102. doi: 10.1088/1748-6041/11/1/014102
Bajaj, P., Schweller, R. M., Khademhosseini, A., West, J. L., and Bashir, R. (2014). 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 16, 247–276. doi:10.1146/annurev-bioeng-071813-105155
Barron, J. A., Spargo, B. J., and Ringeisen, B. R. (2004a). Biological laser printing of three dimensional cellular structures. Appl. Phys. A Mater. Sci. Process. 79, 1027–1030. doi:10.1007/s00339-004-2620-3
Barron, J. A., Wu, P., Ladouceur, H. D., and Ringeisen, B. R. (2004b). Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed. Microdevices 6, 139–147. doi:10.1023/B:BMMD.0000031751.67267.9f
Benam, K. H., Dauth, S., Hassel, B., Herland, A., Jain, A., Jang, K. J., et al. (2015). Engineered in vitro disease models. Annu. Rev. Pathol. 10, 195–265. doi:10.1146/annurev-pathol-012414-040418
Bertassoni, L. E., Cardoso, J. C., Manoharan, V., Cristino, A. L., Bhise, N. S., Araujo, W. A., et al. (2014). Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 6, 024105. doi:10.1088/1758-5082/6/2/024105
Boland, T., Tao, X., Damon, B. J., Manley, B., Kesari, P., Jalota, S., et al. (2007). Drop-on-demand printing of cells and materials for designer tissue constructs. Mater. Sci. Eng. C 27, 372–376. doi:10.1016/j.msec.2006.05.047
Burdick, J. A., and Prestwich, G. D. (2011). Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. Weinheim 23, H41–H56. doi:10.1002/adma.201003963
Choi, W. S., Ha, D., Park, S., and Kim, T. (2011). Synthetic multicellular cell-to-cell communication in inkjet printed bacterial cell systems. Biomaterials 32, 2500–2507. doi:10.1016/j.biomaterials.2010.12.014
Christensen, K., Xu, C., Chai, W., Zhang, Z., Fu, J., and Huang, Y. (2015). Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 112, 1047–1055. doi:10.1002/bit.25501
Cui, H., Nowicki, M., Fisher, J. P., and Zhang, L. G. (2017). 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 6, 1601118. doi:10.1002/adhm.201601118
Cui, X., Breitenkamp, K., Finn, M. G., Lotz, M., and D’Lima, D. D. (2012). Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 18, 1304–1312. doi:10.1089/ten.tea.2011.0543
Dababneh, A. B., and Ozbolat, I. T. (2014). Bioprinting technology: a current state-of-the-art review. J. Manuf. Sci. Eng. 136, 061016. doi:10.1115/1.4028512
Doraiswamy, A., Narayan, R. J., Lippert, T., Urech, L., Wokaun, A., Nagel, M., et al. (2006). Excimer laser forward transfer of mammalian cells using a novel triazene absorbing layer. Appl. Surf. Sci. 252, 4743–4747. doi:10.1016/j.apsusc.2005.07.166
Elomaa, L., Pan, C.-C., Shanjani, Y., Malkovskiy, A., Seppälä, J. V., and Yang, Y. (2015). Three-dimensional fabrication of cell-laden biodegradable poly(ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J. Mater. Chem. B 3, 8348–8358. doi:10.1039/c5tb01468a
Fedorovich, N. E., Alblas, J., De Wijn, J. R., Hennink, W. E., Verbout, A. B. J., and Dhert, W. J. A. (2007). Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 13, 1905–1925. doi:10.1089/ten.2006.0175
Fleming, P. A., Argraves, W. S., Gentile, C., Neagu, A., Forgacs, G., and Drake, C. J. (2010). Fusion of uniluminal vascular spheroids: a model for assembly of blood vessels. Dev. Dyn. 239, 398–406. doi:10.1002/dvdy.22161
Forgacs, G., and Foty, R. A. (2004). “Biological relevance of tissue liquidity and viscoelasticity,” in Function and Regulation of Cellular Systems, eds A. Deutsch, J. Howard, M. Falcke, and W. Zimmermann (Basel: Birkhäuser Basel), 269–277.
Forgacs, G., Foty, R. A., Shafrir, Y., and Steinberg, M. S. (1998). Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys. J. 74, 2227–2234. doi:10.1016/S0006-3495(98)77932-9
Gardel, M. L., Shin, J. H., MacKintosh, F. C., Mahadevan, L., Matsudaira, P., and Weitz, D. A. (2004). Elastic behavior of cross-linked and bundled actin networks. Science 304, 1301. doi:10.1126/science.1095087
Griffith, L. G., and Naughton, G. (2002). Tissue engineering – current challenges and expanding opportunities. Science 295, 1009–1014. doi:10.1126/science.1069210
Groen, N., Guvendiren, M., Rabitz, H., Welsh, W. J., Kohn, J., and de Boer, J. (2016). Stepping into the omics era: opportunities and challenges for biomaterials science and engineering. Acta Biomater. 34, 133–142. doi:10.1016/j.actbio.2016.02.015
Gudapati, H., Dey, M., and Ozbolat, I. (2016). A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102, 20–42. doi:10.1016/j.biomaterials.2016.06.012
Guillemot, F., Mironov, V., and Nakamura, M. (2010). Bioprinting is coming of age: report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B’09). Biofabrication 2, 010201. doi:10.1088/1758-5082/2/1/010201
Guillotin, B., Souquet, A., Catros, S., Duocastella, M., Pippenger, B., Bellance, S., et al. (2010). Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 31, 7250–7256. doi:10.1016/j.biomaterials.2010.05.055
Guvendiren, M., and Burdick, J. A. (2013). Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 24, 841–846. doi:10.1016/j.copbio.2013.03.009
Guvendiren, M., Molde, J., Soares, R. M. D., and Kohn, J. (2016). Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2, 1679–1693. doi:10.1021/acsbiomaterials.6b00121
Highley, C. B., Rodell, C. B., and Burdick, J. A. (2015). Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 27, 5075–5079. doi:10.1002/adma.201501234
Hockaday, L. A., Kang, K. H., Colangelo, N. W., Cheung, P. Y. C., Duan, B., Malone, E., et al. (2012). Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 4, 035005. doi:10.1088/1758-5082/4/3/035005
Hölzl, K., Lin, S., Tytgat, L., Van Vlierberghe, S., Gu, L., and Ovsianikov, A. (2016). Bioink properties before, during and after 3D bioprinting. Biofabrication 8, 032002. doi:10.1088/1758-5090/8/3/032002
Hong, S., Sycks, D., Chan, H. F., Lin, S., Lopez, G. P., Guilak, F., et al. (2015). 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater. Weinheim 27, 4035–4040. doi:10.1002/adma.201501099
Hopp, B., Smausz, T., Kresz, N., Barna, N., Bor, Z., Kolozsvári, L., et al. (2005). Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 11, 1817–1823. doi:10.1089/ten.2005.11.1817
Hribar, K. C., Soman, P., Warner, J., Chung, P., and Chen, S. (2014). Light-assisted direct-write of 3D functional biomaterials. Lab. Chip 14, 268–275. doi:10.1039/C3LC50634G
Huebsch, N., Arany, P. R., Mao, A. S., Shvartsman, D., Ali, O. A., Bencherif, S. A., et al. (2010). Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526. doi:10.1038/nmat2732
Jakab, K., Marga, F., Norotte, C., Murphy, K., Vunjak-Novakovic, G., and Forgacs, G. (2010). Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2, 022001–022001. doi:10.1088/1758-5082/2/2/022001
Jakab, K., Neagu, A., Mironov, V., Markwald, R. R., and Forgacs, G. (2004). Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc. Natl. Acad. Sci. U.S.A 101, 2864–2869. doi:10.1073/pnas.0400164101
Jang, J., Kim, T. G., Kim, B. S., Kim, S. W., Kwon, S. M., and Cho, D. W. (2016a). Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 33, 88–95. doi:10.1016/j.actbio.2016.01.013
Jang, J., Park, H. J., Kim, S. W., Kim, H., Park, J. Y., Na, S. J., et al. (2016b). 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112, 264–274. doi:10.1016/j.biomaterials.2016.10.026
Jang, J., Yi, H. G., and Cho, D. W. (2016c). 3D printed tissue models: present and future. ACS Biomater. Sci. Eng. 2, 1722–1731. doi:10.1021/acsbiomaterials.6b00129
Jia, J., Richards, D. J., Pollard, S., Tan, Y., Rodriguez, J., Visconti, R. P., et al. (2014). Engineering alginate as bioink for bioprinting. Acta Biomater. 10, 4323–4331. doi:10.1016/j.actbio.2014.06.034
Jose, R. R., Rodriguez, M. J., Dixon, T. A., Omenetto, F., and Kaplan, D. L. (2016). Evolution of bioinks and additive manufacturing technologies for 3D bioprinting. ACS Biomater. Sci. Eng. 2, 1662–1678. doi:10.1021/acsbiomaterials.6b00088
Kang, H. W., Lee, S. J., Ko, K. I., Kengla, C., Yoo, J. J., and Atala, A. (2016). A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319. doi:10.1038/nbt.3413
Khetan, S., Guvendiren, M., Legant, W. R., Cohen, D. M., Chen, C. S., and Burdick, J. A. (2013). Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465. doi:10.1038/nmat3586
Koch, L., Kuhn, S., Sorg, H., Gruene, M., Schlie, S., Gaebel, R., et al. (2010). Laser printing of skin cells and human stem cells. Tissue Eng. Part C Methods 16, 5. doi:10.1089/ten.tec.2009.0397
Kuo, C.-Y., Eranki, A., Placone, J. K., Rhodes, K. R., Aranda-Espinoza, H., Fernandes, R., et al. (2016). Development of a 3D printed, bioengineered placenta model to evaluate the role of trophoblast migration in preeclampsia. ACS Biomater. Sci. Eng. 2, 1817–1826. doi:10.1021/acsbiomaterials.6b00031
Langer, R., and Vacanti, J. P. (1993). Tissue engineering. Am. Assoc. Adv. Sci. 260, 920–926. doi:10.1126/science.8493529
Levato, R., Visser, J., Planell, J. A., Engel, E., Malda, J., and Mateos-Timoneda, M. A. (2014). Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 6, 035020. doi:10.1088/1758-5082/6/3/035020
Li, C., Faulkner-Jones, A., Dun, A. R., Jin, J., Chen, P., Xing, Y., et al. (2015). Rapid formation of a supramolecular polypeptide–DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem. Int. Ed. 54, 3957–3961. doi:10.1002/anie.201411383
Lim, K. S., Schon, B. S., Mekhileri, N. V., Brown, G. C. J., Chia, C. M., Prabakar, S., et al. (2016). New visible-light photoinitiating system for improved print fidelity in gelatin-based bioinks. ACS Biomater. Sci. Eng. 2, 1752–1762. doi:10.1021/acsbiomaterials.6b00149
Loessner, D., Meinert, C., Kaemmerer, E., Martine, L. C., Yue, K., Levett, P. A., et al. (2016). Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 11, 727–746. doi:10.1038/nprot.2016.037
Mandrycky, C., Wang, Z., Kim, K., and Kim, D. H. (2016). 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 34, 422–434. doi:10.1016/j.biotechadv.2015.12.011
Marga, F., Neagu, A., Kosztin, I., and Forgacs, G. (2007). Developmental biology and tissue engineering. Birth Defects Res. C Embryo Today 81, 320–328. doi:10.1002/bdrc.20109
Miller, J. S., and Burdick, J. A. (2016). Editorial: special issue on 3D printing of biomaterials. ACS Biomater. Sci. Eng. 2, 1658–1661. doi:10.1021/acsbiomaterials.6b00566
Mironov, V., Boland, T., Trusk, T., Forgacs, G., and Markwald, R. R. (2003). Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 21, 157–161. doi:10.1016/S0167-7799(03)00033-7
Morris, V. B., Nimbalkar, S., Younesi, M., McClellan, P., and Akkus, O. (2017). Mechanical properties, cytocompatibility and manufacturability of chitosan:PEGDA hybrid-gel scaffolds by stereolithography. Ann. Biomed. Eng. 45, 286–296. doi:10.1007/s10439-016-1643-1
Müller, M., Becher, J., Schnabelrauch, M., and Zenobi-Wong, M. (2015). Nanostructured pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 7, 035006. doi:10.1088/1758-5090/7/3/035006
Munaz, A., Vadivelu, R. K., St John, J., Barton, M., Kamble, H., and Nguyen, N.-T. (2016). Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 1, 1–17. doi:10.1016/j.jsamd.2016.04.001
Murphy, S. V., and Atala, A. (2014). 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785. doi:10.1038/nbt.2958
Nakamura, M., Kobayashi, A., Takagi, F., Watanabe, A., Hiruma, Y., Ohuchi, K., et al. (2005). Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 11, 1658–1666. doi:10.1089/ten.2005.11.1658
Nguyen, D. G., Funk, J., Robbins, J. B., Crogan-Grundy, C., Presnell, S. C., Singer, T., et al. (2016). Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS ONE 11:e0158674. doi:10.1371/journal.pone.0158674
Nishiyama, Y., Nakamura, M., Henmi, C., Yamaguchi, K., Mochizuki, S., Nakagawa, H., et al. (2008). Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J. Biomech. Eng. 131, 035001. doi:10.1115/1.3002759
Norotte, C., Marga, F. S., Niklason, L. E., and Forgacs, G. (2009). Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30, 5910–5917. doi:10.1016/j.biomaterials.2009.06.034
Ouyang, L., Highley, C. B., Rodell, C. B., Sun, W., and Burdick, J. A. (2016). 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater. Sci. Eng. 2, 1743–1751. doi:10.1021/acsbiomaterials.6b00158
Ozbolat, I. T., and Hospodiuk, M. (2016). Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76, 321–343. doi:10.1016/j.biomaterials.2015.10.076
Ozbolat, I. T., Moncal, K. K., and Gudapati, H. (2017). Evaluation of bioprinter technologies. Addit. Manuf. 13, 179–200. doi:10.1016/j.addma.2016.10.003
Ozbolat, I. T., Peng, W., and Ozbolat, V. (2016). Application areas of 3D bioprinting. Drug Discov. Today 21, 1257–1271. doi:10.1016/j.drudis.2016.04.006
Pan, T., Song, W., Cao, X., and Wang, Y. (2016). 3D bioplotting of gelatin/alginate scaffolds for tissue engineering: influence of crosslinking degree and pore architecture on physicochemical properties. J. Mater. Sci. Technol. 32, 889–900. doi:10.1016/j.jmst.2016.01.007
Panwar, A., and Tan, L. P. (2016). Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 21, 6. doi:10.3390/molecules21060685
Pati, F., Jang, J., Ha, D. H., Won Kim, S., Rhie, J. W., Shim, J. H., et al. (2014). Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5, 3935. doi:10.1038/ncomms4935
Raphael, B., Khalil, T., Workman, V. L., Smith, A., Brown, C. P., Streuli, C., et al. (2017). 3D cell bioprinting of self-assembling peptide-based hydrogels. Mater. Lett. 190, 103–106. doi:10.1016/j.matlet.2016.12.127
Ribeiro, M., de Moraes, M. A., Beppu, M. M., Garcia, M. P., Fernandes, M. H., Monteiro, F. J., et al. (2015). Development of silk fibroin/nanohydroxyapatite composite hydrogels for bone tissue engineering. Eur. Polymer J. 67, 66–77. doi:10.1016/j.eurpolymj.2015.03.056
Ringeisen, B. R., Kim, H., Barron, J. A., Krizman, D. B., Chrisey, D. B., Jackman, S., et al. (2004). Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 10, 483–491. doi:10.1089/107632704323061843
Rodell, C. B., MacArthur, J. W., Dorsey, S. M., Wade, R. J., Wang, L. L., Woo, Y. J., et al. (2015). Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 25, 636–644. doi:10.1002/adfm.201403550
Rutz, A. L., Hyland, K. E., Jakus, A. E., Burghardt, W. R., and Shah, R. N. (2015). A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 27, 1607–1614. doi:10.1002/adma.201405076
Shafiee, A., and Atala, A. (2016). Printing technologies for medical applications. Trends Mol. Med. 22, 254–265. doi:10.1016/j.molmed.2016.01.003
Shirazi, S. F. S., Gharehkhani, S., Mehrali, M., Yarmand, H., Metselaar, H. S. C., Kadri, N. A., et al. (2015). A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci. Tech. Adv. Mat. 16, 033502. doi:10.1088/1468-6996/16/3/033502
Skoog, S. A., Goering, P. L., and Narayan, R. J. (2014). Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 25, 845–856. doi:10.1007/s10856-013-5107-y
Storm, C., Pastore, J. J., MacKintosh, F. C., Lubensky, T. C., and Janmey, P. A. (2005). Nonlinear elasticity in biological gels. Nature 435, 191–194. doi:10.1038/nature03521
Sydney Gladman, A., Matsumoto, E. A., Nuzzo, R. G., Mahadevan, L., and Lewis, J. A. (2016). Biomimetic 4D printing. Nat. Mater. 15, 413–418. doi:10.1038/nmat4544
Tan, Y. J., Tan, X., Yeong, W. Y., and Tor, S. B. (2016). Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: a new biofabrication strategy. Sci. Rep. 6, 39140. doi:10.1038/srep39140
Tibbitt, M. W., Rodell, C. B., Burdick, J. A., and Anseth, K. S. (2015). Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. U.S.A. 112, 14444–14451. doi:10.1073/pnas.1516247112
Tirella, A., Orsini, A., Vozzi, G., and Ahluwalia, A. (2009). A phase diagram for microfabrication of geometrically controlled hydrogel scaffolds. Biofabrication 1, 4. doi:10.1088/1758-5082/1/4/045002
Tirella, A., Vozzi, F., De Maria, C., Vozzi, G., Sandri, T., Sassano, D., et al. (2011). Substrate stiffness influences high resolution printing of living cells with an ink-jet system. J. Biosci. Bioeng. 112, 79–85. doi:10.1016/j.jbiosc.2011.03.019
Turner, B. N., Strong, R., and Gold, S. A. (2014). A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 20, 192–204. doi:10.1108/rpj-01-2013-0012
Wang, Z., Abdulla, R., Parker, B., Samanipour, R., Ghosh, S., and Kim, K. (2015). A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 7, 045009. doi:10.1088/1758-5090/7/4/045009
Wilson, W. C. Jr., and Boland, T. (2003a). Cell and organ printing 1: protein and cell printers. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 272, 491–496. doi:10.1002/ar.a.10057
Wilson, W. C. Jr., and Boland, T. (2003b). Cell and organ printing 1: protein and cell printers. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 272, 491–496. doi:10.1002/ar.a.10057
Wu, W., DeConinck, A., and Lewis, J. A. (2011). Omnidirectional printing of 3D microvascular networks. Adv. Mater. Weinheim 23, H178–H183. doi:10.1002/adma.201004625
Wüst, S., Müller, R., and Hofmann, S. (2015). 3D Bioprinting of complex channels – effects of material, orientation, geometry, and cell embedding. J. Biomed. Mater. Res. A. 103, 2558–2570. doi:10.1002/jbm.a.35393
Xu, T., Baicu, C., Aho, M., Zile, M., and Boland, T. (2009). Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication 1, 035001–035001. doi:10.1088/1758-5082/1/3/035001
Yu, Z., Rui, Y., Liliang, O., Hongxu, D., Ting, Z., Kaitai, Z., et al. (2014). Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6, 035001. doi:10.1088/1758-5082/6/3/035001
Keywords: additive manufacturing, biofabrication, tissue engineering, regenerative medicine, hydrogel, cell printing, extracellular matrix
Citation: Ji S and Guvendiren M (2017) Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 5:23. doi: 10.3389/fbioe.2017.00023
Received: 25 January 2017; Accepted: 21 March 2017;
Published: 05 April 2017
Edited by:
Giovanni Vozzi, University of Pisa, ItalyReviewed by:
Piergiorgio Gentile, University of Sheffield, UKCopyright: © 2017 Ji and Guvendiren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Murat Guvendiren, bXVyYXRnQG5qaXQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.