
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Big Data , 27 September 2021
Sec. Medicine and Public Health
Volume 4 - 2021 | https://doi.org/10.3389/fdata.2021.654972
This article is part of the Research Topic Internet of Medical Things and Computational Intelligence in Healthcare 4.0 View all 10 articles
Background: Liver is a common metastatic organ for most malignancies, especially the pancreas. However, evidence for prognostic factors of pancreatic cancer metastasis to the liver at different ages is lacking. Thus, we aimed to evaluate the predictors of patients with pancreatic cancer metastasis to liver grouped by age of diagnosis.
Methods: We chose the patients diagnosed between 2004 and 2015 from the SEER database. The primary lesions of metastatic liver cancer between sexes were compared using the Pearson’s chi-square test for categorical variables. The overall survival (OS) and cancer-specific survival (CSS) were the endpoint of the study. The prognostic factors were analyzed with the Kaplan-Meier method and log-rank test, and Cox proportional-hazards regression model.
Results: The main primary sites of metastatic liver cancer for our patients are lung and brunchu, sigmoid colon, pancreas, which in males are lung and bronchu, sigmoid colon and pancreas, while breast, lung and bronchu, sigmoid colon in females. Furthermore, we explored the prognostic factors of pancreatic cancer metastasis to liver grouped by age at diagnosis. Tumor grade, histology and treatment are valid prognostic factors in all age groups. Additionally, gender and AJCC N stage in age<52 years old, while race and AJCC N stage in age >69 years old were predictors. Surgery alone was the optimal treatment in group age>69 years old, whereas surgery combined with chemotherapy was the best option in the other groups.
Conclusion: Our study evaluated the predictors of patients with pancreatic cancer metastasis to liver at various ages of diagnosis.
The liver is the most frequently afflicted metastatic organ second to the lymph nodes for most malignancies (Jaques et al., 1995; Hess et al., 2006; Amankwah et al., 2013; Ryu et al., 2013). The most common tumors with liver metastases arise from the portal venous drainage system, which provides about two-thirds of the liver’s blood supply. Because lesions are usually asymptomatic, liver involvement in metastasis is often neglected and poorly studied, and even extensive infiltration of metastatic tumor may not alter its function or homeostasis until late in the disease (Clark et al., 2016). There are few epidemiological studies on metastatic liver cancer, but 30–70% of patients die of liver metastasis (Pickren J and Lane, 1982) and most patients with liver metastases will die of the primary disease (Gilbert Ha et al., 1982).
As one of the deadliest malignant tumors in the world (Ferlay et al., 2015; Schild and Vokes, 2016), pancreatic cancer is the eighth most common cause of cancer in males and the sixth most common cause of cancer in females. In decades, a large number of studies have shown that the development of pancreatic cancer was closely related to age. The aging trend of the population in the world is challenging the current treatments and caring for patients with pancreatic cancer (Bray et al., 2018; Ferlay et al., 2018). The underlying mechanisms of pancreatic cancer is complicated and uncertain, accompanied with poor prognosis (Maisonneuve, 2019). According to the original site in pancreas, pancreatic cancer is classified as endocrine and exocrine pancreatic cancer, and the latter is more common and has a higher risk of mortality in both females and males (Fesinmeyer et al., 2005). Additionally, the majority of exocrine pancreatic cancer is adenocarcinoma (Li, 2001; Cowgill and Muscarella, 2003). Approximately 50% of pancreatic cancer patients are diagnosed with distant metastases (Mayo et al., 2012), and the most common site of distal metastases found at autopsy was the liver, followed by the peritoneum, lungs and pleura, bones, and adrenal glands (Kamisawa et al., 1995; Mao et al., 1995; Embuscado et al., 2005; Disibio and French, 2008). Previous studies suggested risk factors of pancreatic cancer involving smoking, positive family history and genetics, diabetes, obesity, dietary factors, alcohol consumption, and physical inactivity (Yadav and Lowenfels, 2013; Ilic and Ilic, 2016). Age, race, tumor size, grade, lymph node metastasis (Mayo et al., 2012), AJCC stage (Kamarajah et al., 2017) and treatment (Ansari et al., 2019) are also reported associated with the survival of pancreatic cancer patients. However, evidence for prognostic factors in pancreatic cancer with distant metastasis is rare. However, evidence for prognostic factors in pancreatic cancer with distant metastasis is rare. Moreover, Andrew A et al. and previous studies reported that treatment strategies for pancreatic cancer differentiate in diverse range of ages (Wheeler and Nicholl, 2014). Thus, the objective of this study is to determine the differences in primary sites of metastatic liver cancer between males and females. Furthermore, we evaluated the prognostic risk factors of pancreatic cancer metastasis to liver at different ages of diagnosis through the Cox regression model.
The data was from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program between 2004 and 2015. The program contains the population-based central cancer registries of 18 geographically defined regions. Because all the data used in the study was retrieved from the SEER database with publicly available methods, the study did not require local moral approval or a declaration.
The inclusion criteria included: 1) The disease was diagnosed between 2004 and 2015; 2) metastases of the primary tumor were at the liver; 3) there was only one primary tumor; 4) the diagnosis of the disease was histologically positive; 5) there were more than 0 days of survival.
The exclusion criteria included: 1) age≥85 years old; 2) the demographics of patients were incomplete, including race and marital status; 3) the clinicopathological characteristics of patients were incomplete, including grade, AJCC seventh stage (TNM), tumor size, laterality, causes of death and treatment methods; 4) patients treated with radiotherapy; 5) the type of reporting source was autopsy only or death certificate only. (Figure 1).
We used the histopathology codes from the International Classification of Disease for Oncology third edition (ICD-O-3) to define the primary sites of patients with hepatic metastatic carcinoma. In the ICD-O-3, the codes were defined as follows: code 19-29 (tongue), code 50-69 (gum and other mouth), code 70-89 (salivary gland), code 90-99 (tonsil), code 110-119 (nasopharynx), code 129-139 (hypopharynx), code 150-159 (Esophagus), code 160-169 (stomach), code 170-179 (small intestine), code 180 (cecum), code 181 (appendix), code 182 (ascending colon), code 183 (hepatic flexure), code 184 (transverse colon), code 185 (splenic flexure), code 186 (descending colon), code 187 (sigmoid colon), code 199 (rectosigmoid junction), code 209 (rectum), code 210-218 (Anus, Anal Canal and Anorectum), code 220 (liver), code 221 (intrahepatic bile duct), code 239 (gallbladder), code 240-241 (other biliary), code 250-259 (pancreas), code 300-319 (nose, nasal cavity and middle ear), code 320-329 (larynx), code 340-349 (lung and bronchu), code 380, 472-479, 490-499 (soft tissue including heart), code 381-383 (trachea, mediastinum and other respiratory organs), code 384 (pleura), code 400-419 (bones and joints), code 440-449 (skin excluding basal and squamous), code 480 (retroperitoneum), code 481-482 (peritoneum, omentum and mesentery), code 500-509 (breast), code 510-519 (vulva), code 529 (vagina), code 530-539 (cervix uteri), code 540-549 (corpus uteri), code 569 (ovary), code 570 (other female genital organs), code 601 (penis), code 619 (prostate), code 620-629 (testis), code 649-659 (kidney and renal pelvis), code 669 (ureter), 670-679 (urinary bladder ), code 739 (thyroid) and code 740-755 (other endocrine including thymus).
According to the age at diagnosis of patients, we divided them into three groups, including age at diagnosis <52 years old, age at diagnosis 52–69 years old and age at diagnosis 69–84 years old.
Information on demographic factors (age, race, sex and marital status), tumor-related factors (tumor size, grade, histology and AJCC TNM staging system), therapeutic factors (surgery and chemotherapy) and follow-up were collected from the SEER database. And follow-up period ended in 2015. Based on the Surgery Codes of the SEER program and information about other treatments, we divided the treatment options into categories: no treatment (N), surgery alone (S), chemotherapy alone (C), surgery combined with chemotherapy (SC).
OS and CSS were the interesting endpoint, and the cancer-specific death was based on the code of “SEER cause-specific death classification” in the SEER database. OS was measured from the date on which the first-time definite diagnosis was made until the date of death caused by any cause or the most recent follow-up.
Age and tumor size are categorized according to the best cut-off value produced by the x-tile software version 3.6.1 (Yale University School of Medicine, US). (S2) The incidence rates were calculated by using R software. And baseline patients’ demographics and clinicopathological characteristics were compared using the Pearson’s chi-square test for categorical variables. The independent risk factors were identified by univariate and multivariate Cox proportional-hazards regression analyses for OS. R software version 4.0.2 (R Project, Vienna, Austria) was used for all analysis. Statistically significant cutoff value was set up as p < 0.05, two-sided. p < 0.2 was selected as filter value for univariate to multivariate analysis.
Regardless of gender, the most common primary site of hepatic metastatic carcinoma was lung and brunchu that accounted for 15.18% of all primary lesions, followed by sigmoid colon (11.11%), pancreas (9.15%), breast (8.92%), cecum (8.18%) and rectum (7.81%). The result of Pearson’s chi-square test showed that the primary sites of hepatic metastatic carcinoma were significantly different between males and females including anus, anal canal and anorectum (p < 0.001), ascending colon (p < 0.05), breast (p < 0.001), cervix uteri (p < 0.001), corpus colon (p < 0.001), descending colon (p < 0.05), esophagus (p < 0.001), gallbladder (p < 0.001), hepatic flexure (p < 0.05), kidney and renal pelvis (p < 0.001), larynx (p < 0.05), liver (p < 0.001), lung and bronchus (p < 0.001), nasopharynx (p < 0.01), other female genital organs (p < 0.001), ovary (p < 0.001), pancreas (p < 0.001), peritoneum, omentum and mesentery (p < 0.001), prostate (p < 0.001), rectosigmoid junction (p < 0.001), rectum (p < 0.001), sigmoid colon (p < 0.001), splenic flexure (p < 0.001), stomach (p < 0.001), testis (p < 0.01), urinary bladder (p < 0.001) and vulva (p < 0.05). (Table 1). In females, the top five most common primary lesions of hepatic metastases were breast (18.39%), lung and bronchu (13.51%), sigmoid colon (9.55%), cecum (8.51%) and pancreas (8.17%), while in males were lung and bronchu (16.75%), sigmoid colon (12.56%), pancreas (10.06%), rectum (10.02%) and cecum (7.87%). (Figures 2,3).
The Kaplan Meier survival curve showed significant difference in overall survival for patients diagnosed at different age groups (p < 0.001). The overall survival time was negatively correlated with the age at diagnosis. Among the three groups, the prognosis of patients diagnosed at age less than 52 years old was the best, and of which the median survival time was 1 year. (Figure 4).
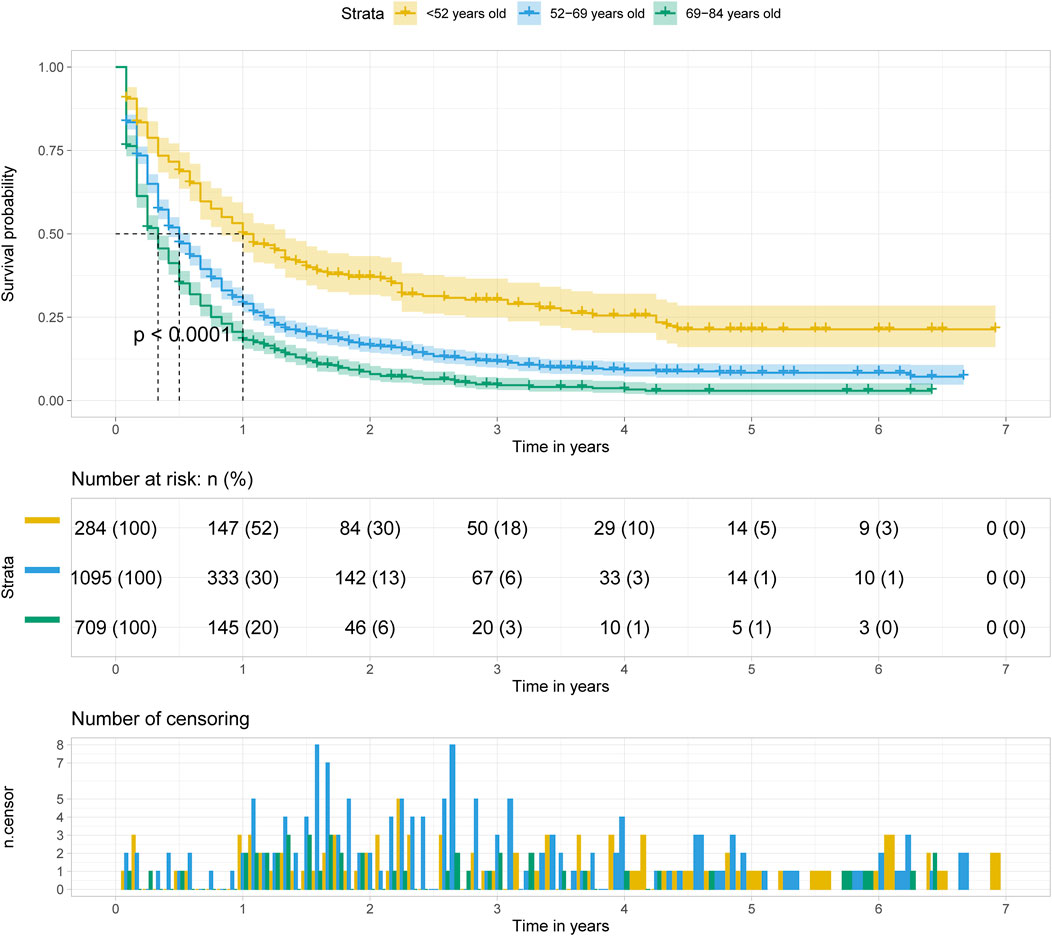
FIGURE 4. Kalpan Meier survival curve showing the effect of age at diagnosis with pancreatic cancer metastasis to liver.
Regardless of age at diagnosis, surgery alone (S) was the optimal treatment option for patients with pancreatic cancer metastasis to liver, followed by surgery combined with chemotherapy (SC), chemotherapy alone (C) and no treatment (N) (p < 0.001). And the median survival time of patients with surgery alone was approximately 3.5–4 years. (Figure 5).
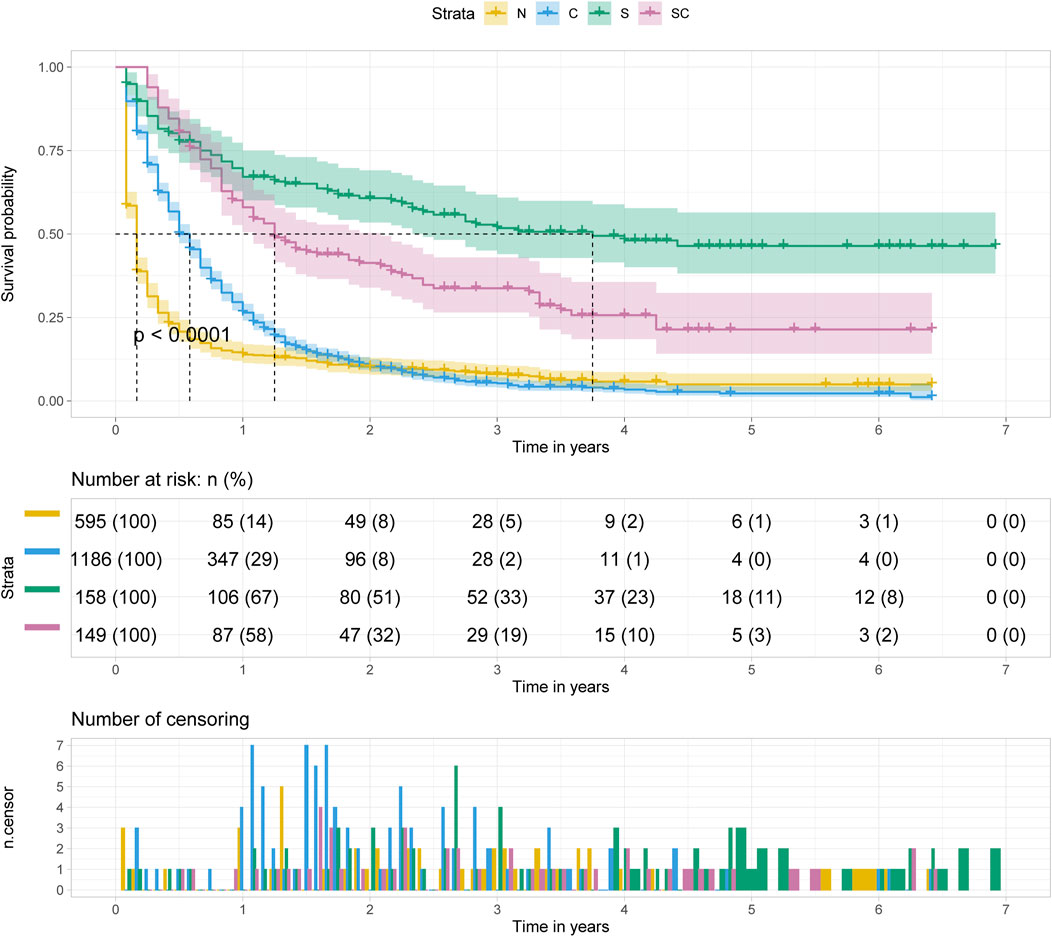
FIGURE 5. Kalpan Meier survival curve showing the effect of treatment with pancreatic cancer metastasis to liver.
As the multivariable hazard ratio of in prognosis displayed in Figure 6, with the increase of the age of diagnosis, treatment showed significantly protective effect, while grade had a significant effect on prognosis only in younger age. And other prognostic factors had almost no significant change. (Figure 6A). Thus, we further analyzed the relative hazard ratio of diverse treatment options and age of diagnosis in patients, we found that when patients were diagnosed at a younger age, chemotherapy alone was the most adverse risk factor, while when diagnosed at an older age, age at diagnosis was the most adverse risk factor for the outcome. What’s more, for patients diagnosed at all ages, chemotherapy alone was the treatment with the worst effect on prognosis, while for patients diagnosed at age more than 69 years old, surgery was better than combined with chemotherapy. (Figure 6B).
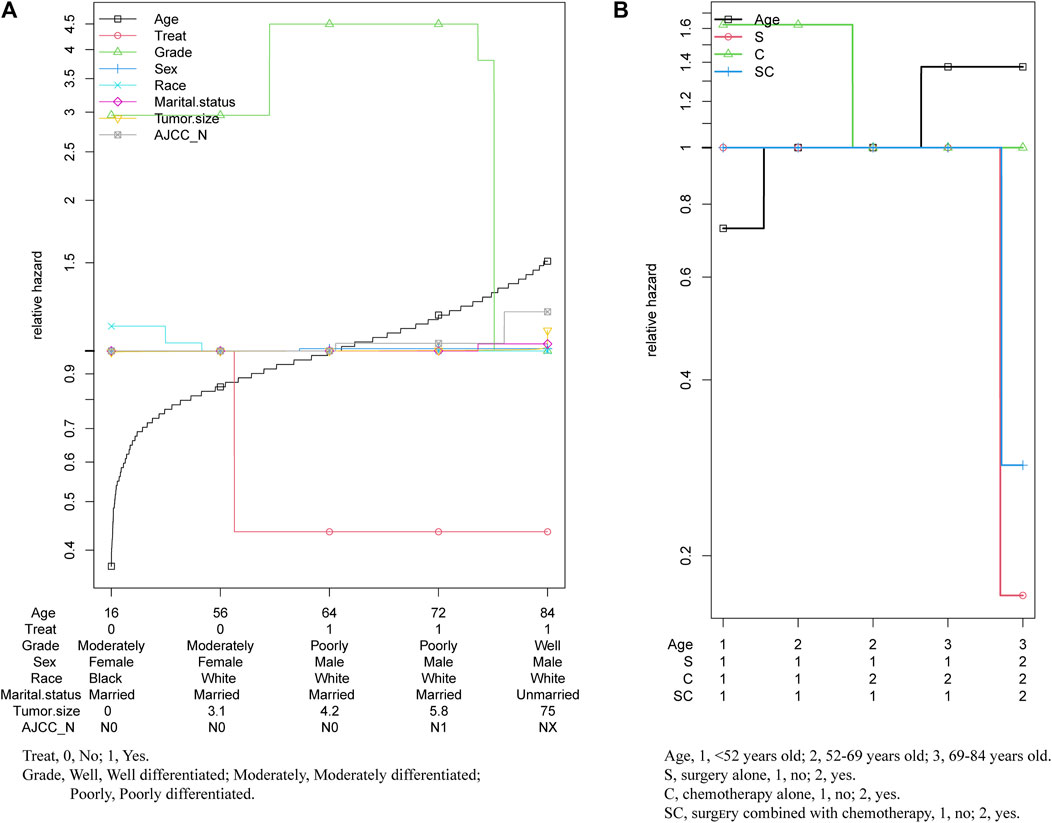
FIGURE 6. Relative hazard ratio of multivariables in patients with pancreatic cancer metastasis to liver.
Demographic characteristics of 2088 patients with pancreatic cancer metastasis to liver grouped by age at diagnosed during the 12-years study period (between 2004 and 2015) in the SEER database are shown in Table 2. In this study, sex (p = 0.002), race (p = 0.031), marital status (p < 0.001), tumor grade (p < 0.001), AJCC N stage (p = 0.005), treatment (p < 0.001), median survival time (p < 0.001) and vital status (p < 0.001) were the parameters with significant difference among different groups. On the whole, most patients were married white males whose tumors were poorly differentiated and less than 4.9 cm in size, treated with chemotherapy alone (C). The most common histological type of tumors was adenomas and adenocarcinomas. Compared with the other groups, well differentiated tumors (25%), surgery alone (S, 14.44%) or surgery combined with chemotherapy (SC, 11.27%) for treatment strategies and longer survival time (12 months) would more likely to occur in age <52 years old group.
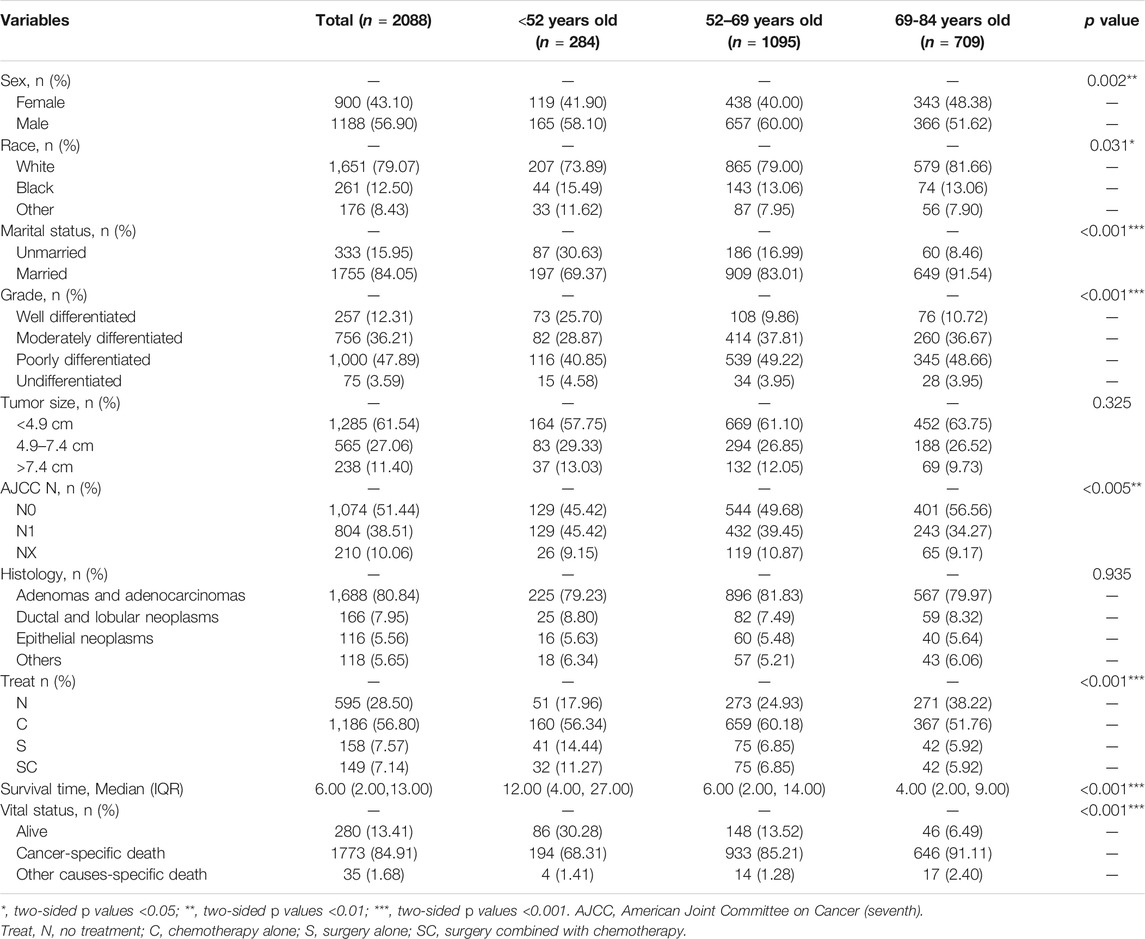
TABLE 2. Clinical characteristics of the patients with pancreatic cancer metastasis to liver grouped by age at diagnosis.
As illustrated in Table 3, on the basis of the overall survival (OS), univariate analysis showed that the significant indicators were sex, grade, tumor size, AJCC N stage, histology and treatment in group age <52 years old; marital status, grade, tumor size, histology and treatment in group age 52–69 years old; and race, grade, AJCC N stage, histology and treatment in group age 69–84 years old.
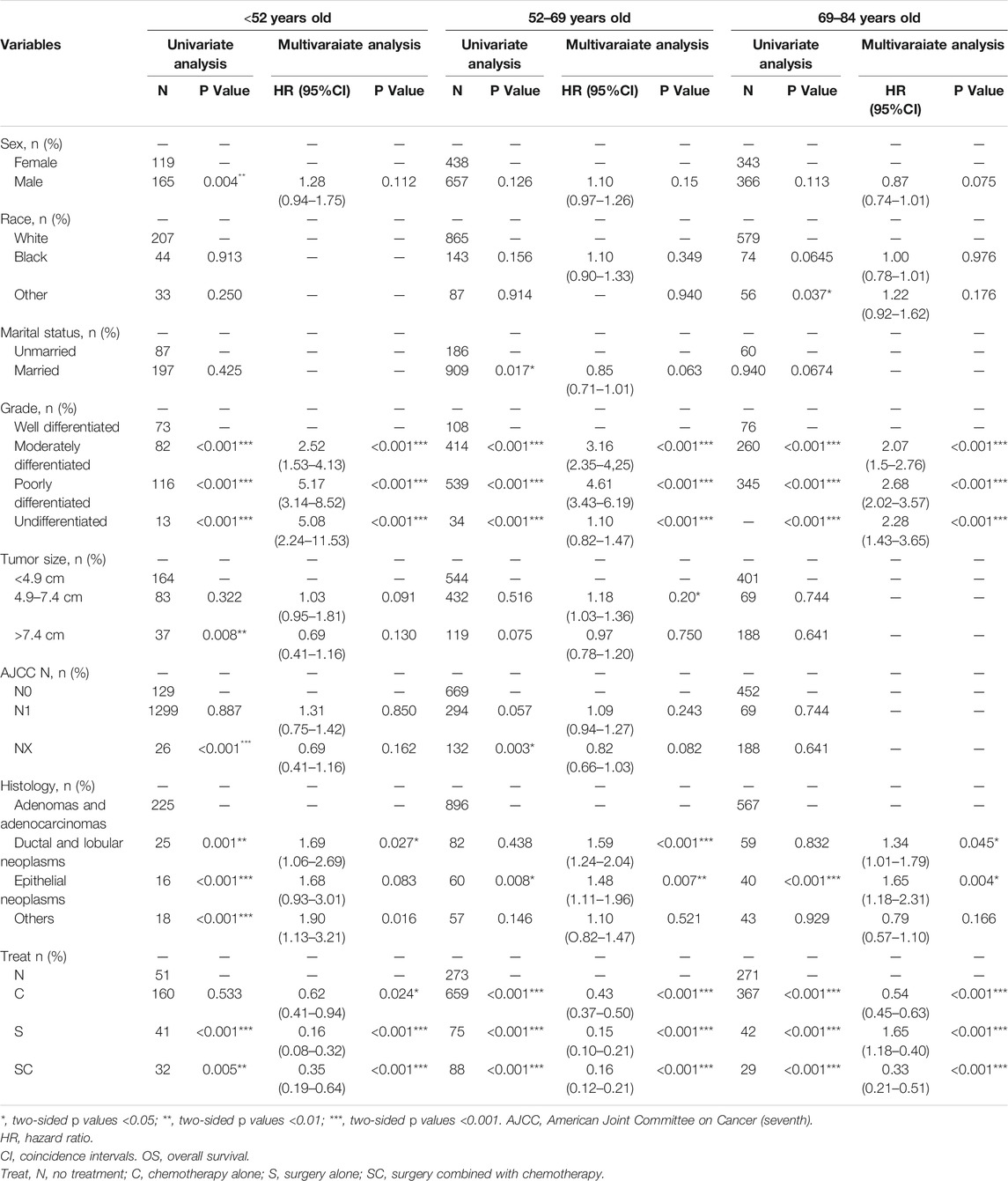
TABLE 3. Univariate and multivariate of OS in the patients with pancreatic cancer metastasis to liver grouped by age at diagnosis.
In multivariate analysis, we further observed the variables selected from univariate analysis (p < 0.2). Cox regression analysis was performed to compete hazard ratios and 95% confidence intervals. In the three groups, tumor grade was all associated with poor overall survival, and surgery alone (S) was the best treatment option for the overall survival of patients.
Using AJCC N0 stage as reference, AJCC N1 stage (p = 0.020, HR = 1.18, 95%CI, 1.03–1.36) in group age 52–69 years and AJCC NX stage (p = 0.039, HR = 1.33, 95%CI, 1.01–1.74) in group age 69–84 years old were indicated to be associated with poor overall survival, while in group age <52 years old, AJCC N stage was not correlated with the prognosis. Choosing adenomas and adenocarcinomas as reference in histological types, in addition to ductal and lobular neoplasms (age <52 years, p = 0.027, HR = 1.69, 95%CI, 1.06-2.69; age 52–69 years old, p < 0.001, HR = 1.59, 95%CI, 1.24-2.04; age 69–84 years old, p = 0.045, HR = 1.34, 95%CI, 1.01-1.79) in the three groups, other histological types (p = 0.016, HR = 1.90, 95%CI, 1.13-3.21) in group age <52 years old, and epithelial neoplasms (age 52–69 years old, p = 0.007, HR = 1.48, 95%CI, 1.11-1.96; age 69–84 years old, p = 0.004, HR = 1.65, 95%CI, 1.18-2.31) in the other two groups (age >52 years old) were associated with a poor overall survival.
As illustrated in Table 4, on the basis of the cancer-specific survival (CSS), univariate analysis showed that the significant indicators were sex, grade, histology and treatment methods in group age <52 years old; grade, histology and treatment methods in group age 52–69 years old; and race, grade, AJCC T stage and treatment methods in group age 69–84 years old.
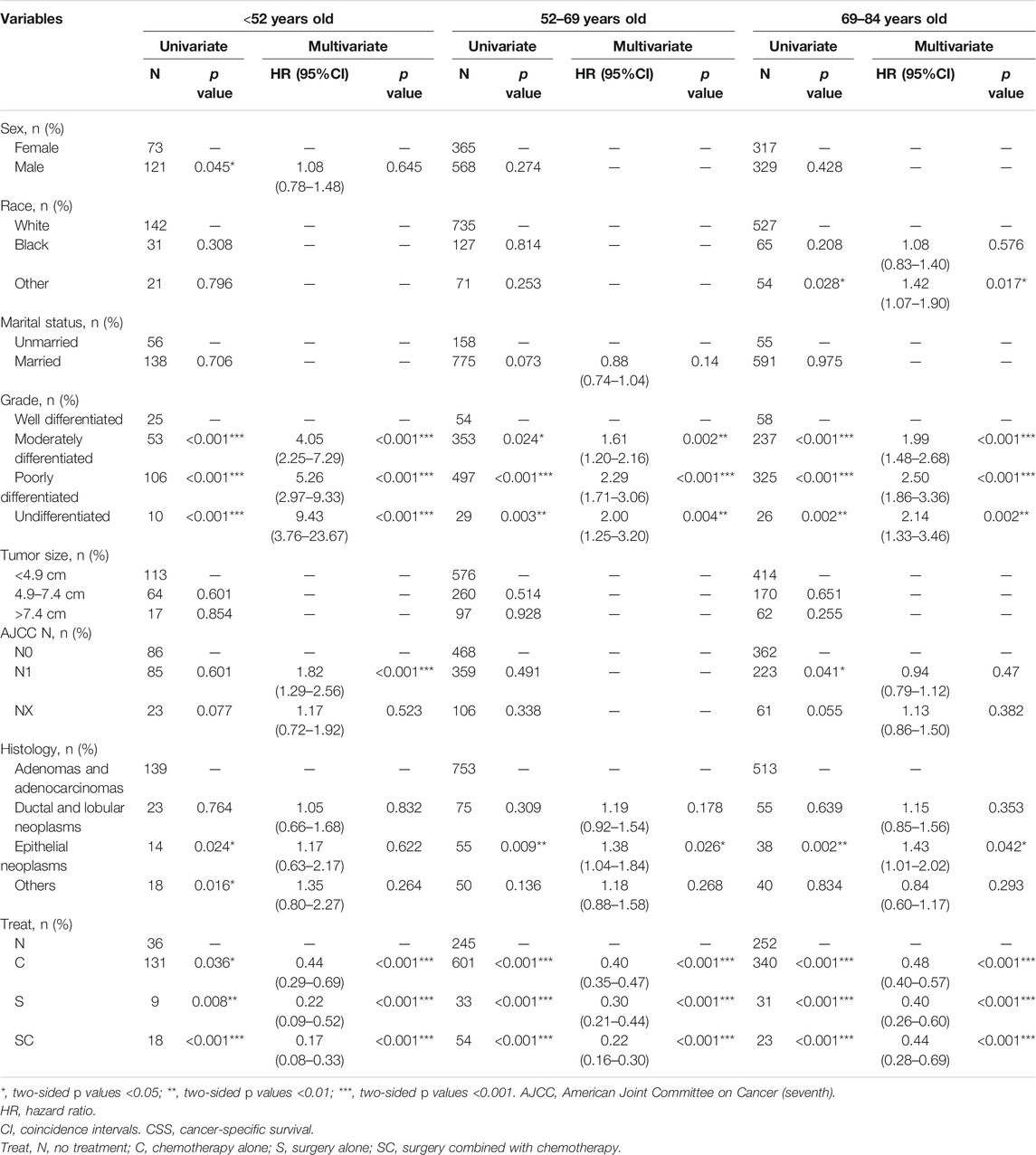
TABLE 4. Univariate and multivariate of CSS in the patients with pancreatic cancer metastasis to liver grouped by age at diagnosis.
Using well differentiated grade as reference, multivariate analysis in Table 4 indicated tumor grade was associated with poor overall survival at different ages. In addition, treatment (S, C, SC) was associated with better cancer-specific survival in all three groups compared with no treatment. Notably, in group age 69–84 years old, surgery alone (S, p < 0.001, HR = 0.40, 95%CI, 0.26-0.60) was the optimal treatment, whereas surgery combined with chemotherapy (SC, group age <52 years old, p < 0.01, HR = 0.17, 95%CI, 0.08-0.33; group age 52–69 years old, p < 0.001, HR = 0.22, 95%CI, 0.16-0.30) was the best option in the other groups.
When using AJCC N0 as reference, patients with AJCC N1 stage (p < 0.001, HR = 1.82, 95%CI, 1.29-2.56) had a poor prognosis only in group age <52 years old. And epithelial neoplasms (age 52-69, p = 0.026, HR = 1.38, 95%CI, 1.04-1.84; age 69–84 years old, p = 0.042 HR = 1.43, 95%CI, 1.01-2.02) were associated with a poor cancer-specific survival only in group age >52 years old when using adenomas and adenocarcinomas as reference. Additionally, in group age 69–84 years old, other racial patients (p = 0.017, HR = 1.42, 95%CI, 1.07-1.90) had a worse prognosis.
It was reported that 90% cancer-related deaths resulted from metastasis of the primary tumor. The formation of local infiltrates and metastases are clinically most relevant to the progression of cancer (Christofori, 2006). Organ damage due to growth-related lesions, paraneoplastic syndromes, or treatment complications was significantly associated with morbidity and mortality of metastatic disease (Steeg, 2006). In general, cancer metastasis can be divided into different stages from local invasion, intravasation, survival in circulation, extravasation, finally to colonization and metastasis (Hanahan and Weinberg, 2011). The unique biological characteristics of the liver make it a vulnerable site for tumor metastasis: 1) structural and hemodynamic features - characteristic microcirculation in the liver makes it easier for diffuse tumor cells carried in the blood to enter. In addition, molecules on the surface of hepatic nonparenchymal cells (NPCs) lining the hepatic capillaries contribute to the adhesion and retention of circulating tumor cells. The pore on the hepatic sinusoidal endothelial cell (LSECs) facilitates the tumor cells to enter the basement membrane directly; 2) regenerative capabilities—the cellular tissue remodeling mechanism involved in self-renewal and reconstruction that promotes intratumoral stroma and blood vessel formation through signals generated by tumor cells, creating an enabling environment for survival and growth; 3) regional immunosuppression—the general foreign body reaction is reduced to limit potential damage to the liver, resulting in a relatively tolerant microenvironment that allows for the survival and growth of foreign tumor cells (Vidal-Vanaclocha, 2011; Clark et al., 2016).
Pancreatic cancer is the fourth leading cause of cancer-related death worldwide, and its main metastatic site is liver (Stott et al., 2010). Studies have shown that, in addition to smoking, a family history of pancreatic cancer, black race, diabetes, and increased body mass index were also predictors of pancreatic cancer mortality (Coughlin et al., 2000). A lack of early signs and symptoms, as well as high aggressiveness, leads to a low survival rate. The prognosis of patients with pancreatic cancer is closely related to tumor stage and tumor grade/aggressiveness (Bolm et al., 2015) that can only be evaluated by biopsy or surgery. Our present data showed tumor grade was also a significant predictor of overall survival and cancer-specific survival in patients with liver metastasis, independent of age at diagnosis (Table 3, Table 4). In addition, 85% of the histology types of pancreatic cancer are ductal adenocarcinoma of the pancreas (PDAC) (Ryan et al., 2014; Hogendorf et al., 2018). For patients with PDAC, younger age, male sex, larger tumor size, low ALT level and high CA 19-9 level could predict unexpected distant metastasis (Liu et al., 2018). Histologically, pancreatic adenocarcinoma accounts for the largest proportion in pancreatic cancer (Simard et al., 2012), accompanied with the worst prognosis, and the most common site of metastasis is liver (Lemke et al., 2013; Deeb et al., 2015; Kumar et al., 2015), which is consistent with our results (Table 2). Our data suggested that most histologic types of pancreatic metastases to liver were adenocarcinomas. The prognosis of patients with pancreatic cancer with liver metastasis was poorer than that of patients with distant lymph node metastasis or lung metastasis. The factors predicting the better prognosis included age<65 years, white race, being married, female sex and surgery treatment (Oweira et al., 2017). Furthermore, our study showed that the younger the age, the higher the overall survival rate of patients with pancreatic cancer with liver metastasis (Figure 4). In addition, we found differences in prognostic factors among the groups after grouping by age at diagnosis. Histologically, compared with pancreatic adenocarcinoma, ductal and lobular neoplasms and epithelial neoplasms were associated with poor overall survival in the group age >52 years old, while the latter were not correlated with the prognosis in group age <52 years old. In the multivariate regression analysis, histological type was a significant predictor for cancer-specific survival only for patients diagnosed at age >52 years old. AJCC N1 stage with significance in predicting poor overall survival only in group age 52–69 years old, and predicting poor cancer-specific survival only in group age <52 years old. (Table 3, Table 4).
At present, the only treatment for pancreatic cancer is surgery, and adjuvant therapy based on chemotherapy can improve the survival rate (McGuigan et al., 2018). For elderly patients (age>80 years old), postoperative adjuvant chemotherapy is critical to the prognosis (Sho et al., 2016). Surgery is limited to patients with localized disease, and metastatic spread is often considered a contraindication to resection, regardless of whether it is observed synchronously or ectopic (Seufferlein et al., 2012). However, metastatic excision or local treatment is occasionally performed in centers around the world based on individual clinical experience, and there is no objective evidence to guide treatment methods taking into account patient choice or metastatic spread (Gleisner et al., 2007; Shrikhande et al., 2007; De Jong et al., 2010; Nentwich et al., 2012; Edwards et al., 2013). In general, palliative chemotherapy with FOLFIRONOX (mFOLFIRINOX with 5-fluorouracil) is the preferred chemotherapy regimen for metastatic pancreatic cancer (McGuigan et al., 2018). Despite this, T. Hackert (Hackert et al., 2017) proved that resection of liver or interaortocaval lymph nodes (ILN) metastases could be superior to palliative treatment for pancreatic cancer patients with metastasis. Mitsuka et al. (2020) found that the median survival time was significantly improved for patients diagnosed between 44 and 83 years old who underwent liver resection or pancreatectomy. Other study (Warschkow et al., 2020) showed that lymphadenectomy had only 18% direct effect on improved overall survival, while 82% of its effect were mediated by other factors like treatment at high-volume hospitals and adjuvant chemotherapy for patients whose median age were 66 years. However, the analysis on differences among different ages of patients is scarce. As we know, there is insufficient evidence that the efficacy of different therapies in patients with metastatic pancreatic cancer is age-related. Our data showed chemotherapy alone was the most important prognostic factor for patients who diagnosed at younger age, and age of diagnosis was the most prognostic factor for patients diagnosed at an older age. For the diagnosis of pancreatic cancer at all ages, surgery was the best treatment method to improve the overall survival rate of patients with pancreatic cancer with liver metastasis (Table 3). Considering the tumor-specific survival rate, surgery combined with chemotherapy is the best choice for patients under 69 years of age at the time of diagnosis, while surgery alone is the best choice for patients aged 69–84 years at the time of diagnosis. In addition, surgery alone and combined chemotherapy were significantly superior to chemotherapy alone in terms of overall survival and tumor-specific survival (Table 3, Table 4). Interestingly, in a case report (Katsura et al., 2019), after the combination therapy of pancreatoduodenectomy and chemotherapy, a 66-year-old patient with pancreatic ductal carcinoma metastasis to liver showed the disappearance of liver metastasis and without other new metastasis. This case report partially confirms the conclusion from our analysis that surgery alone was the optimal treatment in group age>69 years old, while surgery combined with chemotherapy was the best option in the other groups. Both surgical treatment and chemotherapy cause damage to human bodies. Especially, the elderly can hardly bear the double blow, as surgical treatment and chemotherapy both exerting in therapy. In addition, chemotherapy is often accompanied with many side effects. The analyzed data in this manuscript demonstrates that surgical treatment alone is superior to surgery plus chemotherapy in patients older than 69 years of age. It suggests that surgery should be a priority for the older population (age>69) with pancreatic cancer metastasis to liver. Certainly, clinical treatment selection depends on the multiple assessment of patient, and this manuscript provides an epidemiological reference for the selection of clinical treatment.
Although the SEER database provides a large amount of clinical data, there are still many limitations in our research. First, we need to further conduct a follow-up clinical trial to verify this result. Second, we did not include patients undergoing radiotherapy because of the small number of cases, and we need to compare the effects of radiotherapy, chemotherapy and surgery on prognosis. Finally, the sequence of chemotherapy and surgery, the diverse methods of surgery and chemotherapy can be further studied.
In this population-based analysis, we found the main primary sites of metastatic liver cancer are lung and brunchu, sigmoid colon and pancreas. Furthermore, we explored the prognostic factors of pancreatic cancer metastasis to liver grouped by age at diagnosis. Tumor grade, histology and treatment are valid prognostic factors in all age groups. Additionally, gender and AJCC N stage in age<52 years old, while race and AJCC N stage in age>69 years old were predictors. Surgery alone was the optimal treatment in group age>69 years old, whereas surgery combined with chemotherapy was the best option in the other groups. In conclusion, these findings would help to choose better treatment for patients with metastatic liver cancer.
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.The data was from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program between 2004 and 2015.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was founded by National Natural Science Foundation of China (81974032, 81570337 to SL), Natural Science Foundation of Hubei Province (2015CFB455 to SL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We wish to thank the help from the Division of Infectious Disease as well as Division of Cardiology, Department of Internal Medicine in Tongji Hospital, and the Department and Institute of Infectious Disease, Xi'an Children's Hospital.
AJCC, American Joint Committee on Cancer (seventh); CI, coincidence intervals; CSS, cancer-specific survival; HR, hazard ratio; OS, overall survival; SEER, Surveillance Epidemiology and End Results.
Ansari, D., Althini, C., Ohlsson, H., and Andersson, R. (2019). Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbecks Arch. Surg. 404 (5), 565–571. doi:10.1007/s00423-019-01810-0
Bolm, L., Janssen, S., Käsmann, L., Wellner, U., Bartscht, T., Schild, S. E., et al. (2015). Predicting Survival after Irradiation of Metastases from Pancreatic Cancer. Anticancer Res. 35 (7), 4105–4108.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clinicians 68 (6), 394–424. doi:10.3322/caac.21492
Christofori, G. (2006). New signals from the invasive front. Nature 441 (7092), 444–450. doi:10.1038/nature04872
Clark, A. M., Ma, B., Taylor, D. L., Griffith, L., and Wells, A. (2016). Liver metastases: Microenvironments and ex-vivo models. Exp. Biol. Med. (Maywood) 241 (15), 1639–1652. doi:10.1177/1535370216658144
Coughlin, S. S., Calle, E. E., Patel, A. V., and Thun, M. J. (2000). Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control 11 (10), 915–923. doi:10.1023/a:1026580131793
Cowgill, S. M., and Muscarella, P. (2003). The genetics of pancreatic cancer. Am. J. Surg. 186 (3), 279–286. doi:10.1016/s0002-9610(03)00226-5
De Jong, M. C., Farnell, M. B., Sclabas, G., Cunningham, S. C., Cameron, J. L., Geschwind, J.-F., et al. (2010). Liver-Directed Therapy for Hepatic Metastases in Patients Undergoing Pancreaticoduodenectomy. Ann. Surg. 252 (1), 142–148. doi:10.1097/SLA.0b013e3181dbb7a7
Deeb, A., Haque, S. U., and Olowokure, O. (2015). Pulmonary metastases in pancreatic cancer, is there a survival influence? J. Gastrointest. Oncol. 6 (3), E48–E51. doi:10.3978/j.issn.2078-6891.2014.114
Disibio, G., and French, S. W. (2008). Metastatic patterns of cancers: results from a large autopsy study. Arch. Pathol. Lab. Med. 132 (6), 931–939. doi:10.5858/2008-132-931-mpocrf
Edwards, J., Scoggins, C., McMasters, K., and Martin, R. (2013). Combined pancreas and liver therapies: resection and ablation in hepato-pancreatico-biliary malignancies. J. Surg. Oncol. 107 (7), 709–712. doi:10.1002/jso.23318
Embuscado, E. E., Laheru, D., Ricci, F., Yun, K. J., de Boom Witzel, S., Seigel, A., et al. (2005). Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol. Ther. 4 (5), 548–554. doi:10.4161/cbt.4.5.1663
Ferlay, J., Colombet, M., Soerjomataram, I., Dyba, T., Randi, G., Bettio, M., et al. (2018). Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 103, 356–387. doi:10.1016/j.ejca.2018.07.005
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136 (5), E359–E386. doi:10.1002/ijc.29210
Fesinmeyer, M. D., Austin, M. A., Li, C. I., De Roos, A. J., and Bowen, D. J. (2005). Differences in Survival by Histologic Type of Pancreatic Cancer. Cancer Epidemiol. Biomarkers Prev. 14 (7), 1766–1773. doi:10.1158/1055-9965.epi-05-0120
Gilbert HA, K. A., Hintz, B. L., Rao, A. R., and Nussbaum, H. (1982). “Patterns of metastases,” in Liver Metastases. Editor W. LGH (Boston: GK Hall Medical Publishers), 19–39.
Gleisner, A. L., Assumpcao, L., Cameron, J. L., Wolfgang, C. L., Choti, M. A., Herman, J. M., et al. (2007). Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 110 (11), 2484–2492. doi:10.1002/cncr.23074
Hackert, T., Niesen, W., Hinz, U., Tjaden, C., Strobel, O., Ulrich, A., et al. (2017). Radical surgery of oligometastatic pancreatic cancer. Eur. J. Surg. Oncol. (Ejso) 43 (2), 358–363. doi:10.1016/j.ejso.2016.10.023
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144 (5), 646–674. doi:10.1016/j.cell.2011.02.013
Hess, K. R., Varadhachary, G. R., Taylor, S. H., Wei, W., Raber, M. N., Lenzi, R., et al. (2006). Metastatic patterns in adenocarcinoma. Cancer 106 (7), 1624–1633. doi:10.1002/cncr.21778
Hogendorf, P., Durczyński, A., and Strzelczyk, J. (2018). Metastatic Pancreatic Cancer. J. Invest. Surg. 31 (2), 151–152. doi:10.1080/08941939.2017.1291774
Ilic, M., and Ilic, I. (2016). Epidemiology of pancreatic cancer. Wjg 22 (44), 9694–9705. doi:10.3748/wjg.v22.i44.9694
Jaques, D. P., Coit, D. G., Casper, E. S., and Brennan, M. F. (1995). Hepatic metastases from soft-tissue sarcoma. Ann. Surg. 221 (4), 392–397. doi:10.1097/00000658-199504000-00010
Kamarajah, S. K., Burns, W. R., Frankel, T. L., Cho, C. S., and Nathan, H. (2017). Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann. Surg. Oncol. 24 (7), 2023–2030. doi:10.1245/s10434-017-5810-x
Kamisawa, T., Isawa, T., Koike, M., Tsuruta, K., and Okamoto, A. (1995). Hematogenous metastases of pancreatic ductal carcinoma. Pancreas 11 (4), 345–349. doi:10.1097/00006676-199511000-00005
Katsura, Y., Takeda, Y., Ohmura, Y., Sakamoto, T., Kawai, K., Inatome, J., et al. (2019). A Case Report of Conversion Surgery for Pancreatic Ductal Adenocarcinoma with Liver Metastasis after Chemotherapy. Gan To Kagaku Ryoho 46 (4), 802–804.
Kumar, A., Dagar, M., Herman, J., Iacobuzio-Donahue, C., and Laheru, D. (2015). CNS involvement in pancreatic adenocarcinoma: a report of eight cases from the Johns Hopkins Hospital and review of literature. J. Gastrointest. Canc 46 (1), 5–8. doi:10.1007/s12029-014-9667-y
Lemke, J., Scheele, J., Kapapa, T., Wirtz, C., Henne-Bruns, D., and Kornmann, M. (2013). Brain metastasis in pancreatic cancer. Ijms 14 (2), 4163–4173. doi:10.3390/ijms14024163
Liu, X., Fu, Y., Chen, Q., Wu, J., Gao, W., Jiang, K., et al. (2018). Predictors of distant metastasis on exploration in patients with potentially resectable pancreatic cancer. BMC Gastroenterol. 18 (1), 168. doi:10.1186/s12876-018-0891-y
Maisonneuve, P. (2019). Epidemiology and burden of pancreatic cancer. La Presse Médicale 48 (3 Pt 2), e113–e123. doi:10.1016/j.lpm.2019.02.030
Mao, C., Domenico, D. R., Kim, K., Hanson, D. J., and Howard, J. M. (1995). Observations on the Developmental Patterns and the Consequences of Pancreatic Exocrine Adenocarcinoma. Arch. Surg. 130 (2), 125–134. doi:10.1001/archsurg.1995.01430020015001
Mayo, S. C., Nathan, H., Cameron, J. L., Olino, K., Edil, B. H., Herman, J. M., et al. (2012). Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 118 (10), 2674–2681. doi:10.1002/cncr.26553
McGuigan, A., Kelly, P., Turkington, R. C., Jones, C., Coleman, H. G., and McCain, R. S. (2018). Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. Wjg 24 (43), 4846–4861. doi:10.3748/wjg.v24.i43.4846
Mitsuka, Y., Yamazaki, S., Yoshida, N., Yan, M., Higaki, T., and Takayama, T. (2020). Time interval-based indication for liver resection of metastasis from pancreatic cancer. World J. Surg. Onc 18 (1), 294. doi:10.1186/s12957-020-02058-5
Nentwich, M. F., Bockhorn, M., König, A., Izbicki, J. R., and Cataldegirmen, G. (2012). Surgery for advanced and metastatic pancreatic cancer--current state and trends. Anticancer Res. 32 (5), 1999–2002.
Oweira, H., Petrausch, U., Helbling, D., Schmidt, J., Mannhart, M., Mehrabi, A., et al. (2017). Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. Wjg 23 (10), 1872–1880. doi:10.3748/wjg.v23.i10.1872
Pickren J, T. Y., and Lane, W. (1982). “Liver metastases: Analysis of autopsy data,” in Liver Metastases. Editor G. H. L. Weiss (Boston: GK Hall Medical Publishers), 2–18.
Reed, D., Amankwah, A. P., and Conley, D. R. (2013). Epidemiology and therapies for metastatic sarcoma. Clep 5, 147–162. doi:10.2147/clep.s28390
Ryan, D. P., Hong, T. S., and Bardeesy, N. (2014). Pancreatic adenocarcinoma. N. Engl. J. Med. 371 (11), 1039–1049. doi:10.1056/NEJMra1404198
Ryu, S. W., Saw, R., Scolyer, R. A., Crawford, M., Thompson, J. F., and Sandroussi, C. (2013). Liver resection for metastatic melanoma: equivalent survival for cutaneous and ocular primaries. J. Surg. Oncol. 108 (2), 129–135. doi:10.1002/jso.23361
Schild, S. E., and Vokes, E. E. (2016). Pathways to improving combined modality therapy for stage III nonsmall-cell lung cancer. Ann. Oncol. 27 (4), 590–599. doi:10.1093/annonc/mdv621
Seufferlein, T., Bachet, J. B., Van Cutsem, E., and Rougier, P. (2012). Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 23 (Suppl. 7), vii33–vii40. doi:10.1093/annonc/mds224
Sho, M., Murakami, Y., Kawai, M., Motoi, F., Satoi, S., Matsumoto, I., et al. (2016). Prognosis after surgical treatment for pancreatic cancer in patients aged 80 years or older: a multicenter study. J. Hepatobiliary Pancreat. Sci. 23 (3), 188–197. doi:10.1002/jhbp.320
Shrikhande, S. V., Kleeff, J., Reiser, C., Weitz, J., Hinz, U., Esposito, I., et al. (2006). Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 14 (1), 118–127. doi:10.1245/s10434-006-9131-8
Simard, E. P., Ward, E. M., Siegel, R., and Jemal, A. (2012). Cancers with increasing incidence trends in the United States: 1999 through 2008. CA: A Cancer J. Clinicians 62 (2), 118–128. doi:10.3322/caac.20141
Steeg, P. S. (2006). Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 12 (8), 895–904. doi:10.1038/nm1469
Stott, S. L., Hsu, C.-H., Tsukrov, D. I., Yu, M., Miyamoto, D. T., Waltman, B. A., et al. (2010). Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. 107 (43), 18392–18397. doi:10.1073/pnas.1012539107
Vidal-Vanaclocha, F. (2011). “Architectural and Functional Aspects of the Liver with Implications for Cancer Metastasis,” in Liver Metastasis: Biology and Clinical Management. Editor P. Brodt (Dordrecht: Springer Netherlands), 9–42. doi:10.1007/978-94-007-0292-9_2
Warschkow, R., Tsai, C., Köhn, N., Erdem, S., Schmied, B., Nussbaum, D. P., et al. (2020). Role of lymphadenectomy, adjuvant chemotherapy, and treatment at high-volume centers in patients with resected pancreatic cancer-a distinct view on lymph node yield. Langenbecks Arch. Surg. 405 (1), 43–54. doi:10.1007/s00423-020-01859-2
Wheeler, A. A., and Nicholl, M. B. (2014). Age Influences Likelihood of Pancreatic Cancer Treatment, but not Outcome. World J. Oncol. 5 (1), 7–13. doi:10.14740/wjon789w
Keywords: pancreatic cancer, liver, prognosis, metastasis, treatment
Citation: Liu M, Wang M and Li S (2021) Prognostic Factors of Survival in Pancreatic Cancer Metastasis to Liver at Different Ages of Diagnosis: A SEER Population-Based Cohort Study. Front. Big Data 4:654972. doi: 10.3389/fdata.2021.654972
Received: 18 January 2021; Accepted: 16 August 2021;
Published: 27 September 2021.
Edited by:
Shi-Cong Tao, Shanghai Jiao Tong University, ChinaReviewed by:
Raghvendra Mall, St. Jude Children’s Research Hospital, United StatesCopyright © 2021 Liu, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Li, c2hlbmdsaTQxMEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.