Salience can broadly be defined as “
a process whereby objects and representations…
[are] attention-grabbing and capture thought and behaviour” (
Jensen and Kapur, 2009
, p.197). Examples of situations where a stimulus might be considered salient include: feature contrast (e.g. a bright light flashing in a dark room); novelty (e.g. a new object in an otherwise familiar environment); and emotional/motivational association (e.g. a previously neutral stimulus that has been paired with reward or punishment). Disrupted salience processing has been proposed as central to explaining positive psychotic symptoms (
Maher, 1974
;
Miller, 1976
;
Shaner, 1999
). The ability to ignore irrelevant stimuli is reliably impaired in schizophrenia (
Hemsley, 1993
), though the exact mechanisms underpinning this deficit remain unclear. However, it is possible that dopaminergic transmission, which is also abnormal in schizophrenia (
Laruelle and Abi-Dargham, 1999
), plays a crucial role in salience processing generally (
Horvitz, 2000
), though the evidence is strongest in the domain of motivational salience (
Berridge and Robinson, 1998
).
Kapur (2003)
, in an elegant attempt to create a framework explaining the symptoms and neurobiology of psychosis-
in-schizophrenia, suggested that stochastic firing of dopamine neurons (
Seeman and Kapur, 2000
) leads to the “aberrant” attribution of salience via context-independent stimulus-reinforcement signalling (
Berridge and Robinson, 1998
). Patients with psychosis have indeed shown impairments on tests of reinforcement learning, particularly on measures that assess adaptive responses to rewards (
Waltz and Gold, 2007
;
Gold et al., 2008
;
Heerey et al., 2008
;
Waltz et al., 2009
). Furthermore, several findings of altered haemodynamic response patterns in patients with psychosis relative to healthy controls during reward processing have provided evidence for the dysfunction of brain circuits innervated by dopamine (
Jensen et al., 2008
;
Murray et al., 2008b
).
To test Kapur’s hypothesis of aberrant salience attribution experimentally,
Roiser et al. (2009)
used a novel paradigm designed to assess implicit and explicit reward learning relating to task-relevant and -irrelevant stimuli (Salience Attribution Test – SAT). They reported decreased “adaptive salience” (task-relevant reward learning) in medicated patients with schizophrenia, consistent with the above studies. Importantly, patients with delusions scored higher on the SAT measure of explicit “aberrant salience” (task-irrelevant reward learning) than those without such symptoms, consistent with the aberrant salience model. Furthermore, healthy control participants with high-schizotypy scores also showed less adaptive salience and more aberrant salience than low scorers, further supporting the model.
In some respects, the aberrant salience framework is not dissimilar from an earlier cognitive model of psychosis proposed by
Gray et al. (1991)
. These authors proposed that a core feature of psychosis is the malfunction of a “comparator” function, which acts continuously to compare current information processing with stored regularities or expectations. This model draws on empirical data from a number of studies utilising animal associative learning paradigms, for example latent inhibition (LI) and Kamin blocking (
Solomon et al., 1981
;
Weiner et al., 1981
,
1984
;
Baruch et al., 1988
;
Jones et al., 1992
;
Gray et al., 1995
). LI refers to the retardation of learning an association between two stimuli after previous inconsequential (non-reinforced) preexposure of these stimuli. Related, learned irrelevance (LIrr) paradigms test a similar preexposure effect, which is thought to reflect the ability to ignore irrelevant stimuli (e.g.
Gal et al., 2005
). Importantly, as has been hypothesised (but not as yet tested) for aberrant salience, LI/LIrr can be modulated pharmacologically by dopamine agonists and antagonists (e.g.
Weiner et al., 1988
;
De la Casa et al., 1993
), though the precise mechanisms by which this process is disrupted are less clear (e.g.
Braunstein-Bercovitz and Lubow, 1998
;
Shrira and Tsakanikos, 2009
).
While both the comparator and aberrant salience accounts of psychosis attempt to explain the generation of the positive symptoms of schizophrenia as a product of a disrupted central cognitive process, driven by dysregulated dopamine transmission, the cognitive concepts on which they are based are, at least superficially, dissimilar. The comparator model suggests a disruption in the “
integration of past regularities of experience with current stimulus recognition, learning, and action” (
Gray, 1998
, p.249). By contrast the aberrant salience model specifies a disruption of (motivational) salience attribution, resulting in the “
aberrant assignment of salience to external objects and internal representations” (
Kapur, 2003
, p.15). A “prediction error” explanation of psychosis has also been proposed, which to some extent bridges the gap between these two accounts, suggesting that “
inappropriate mismatch signals (i.e. prediction errors) are ultimately responsible for the perceptual aberrations, capture of attention and perception of inappropriate causal relationships that are characteristic of psychosis” (
Corlett et al., 2007
, p.241). However, it remains unclear to what extent these models (and the paradigms used to test them) overlap.
In particular, critics have emphasised the potential overlap between the constructs of aberrant salience and LI (
Gray, 2004
). Indeed, evidence that acutely psychotic patients and individuals scoring high on measures of schizotypy show disrupted LI (
Baruch et al., 1988
;
Gray et al., 1995
;
Braunstein-Bercovitz and Lubow, 1998
;
Lubow and De la Casa, 2002
;
Vaitl et al., 2002
;
Braunstein-Bercovitz, 2003
;
Schmidt-Hansen et al., 2009
) and LIrr (
Gal et al., 2005
;
Young et al., 2005
;
Orosz et al., 2008
;
Schmidt-Hansen et al., 2009
) should encourage caution in interpreting behavioural findings purporting to demonstrate impaired salience attribution processes in psychosis, since it is conceivable that such a deficit might simply reflect impaired LI. Similarly, the general cognitive impairments characteristic of patients with schizophrenia (
Robbins, 2005
), might also confound the assessment of aberrant salience. For example, in order to perform the SAT, participants must be able to attend continuously for an extended period, use working memory, learn probabilistic associations and guide responses on the basis of such associations, all of which may be impaired in schizophrenia (
Keri et al., 2000
;
Young et al., 2005
;
Waltz and Gold, 2007
).
Therefore, we performed a factor analysis to assess the construct validity of salience attribution measures derived from the SAT (
Roiser et al., 2009
). Our aim was to determine the extent to which the constructs of adaptive and aberrant salience dissociate from other cognitive measures, most specifically LIrr, but also probabilistic learning/sensitivity, sustained attention, and working memory. In order to avoid the potential confound of the general cognitive impairment in schizophrenia, we tested a non-clinical sample, but recorded schizotypy, since individuals scoring higher on this personality trait exhibit similar cognitive impairments to those seen in schizophrenia (
Gray et al., 2002
;
Bedwell et al., 2009
) and are at elevated risk for developing psychosis (
Claridge, 1994
;
Verdoux and van Os, 2002
). We predicted the emergence of implicit aberrant salience, explicit aberrant salience and LIrr on independent factors, and that all three would show associations with schizotypy traits across individuals, as reported previously (
Gray et al., 2002
;
Roiser et al., 2009
).
Participants
Fifty-five volunteers who reported an absence of previous psychiatric or neurological disorders were recruited through online advertising and the University College London Psychology participant pool. Inclusion criteria were: age (18–60 years); native or bilingual English speaker. Exclusion criteria were: previous or current neurological or psychiatric disorders; previous or current substance/alcohol abuse/dependence, excepting a remote (>12 months) history of substance/alcohol abuse. Participants were compensated with a £10 baseline payment for their time and travel and had the opportunity to win up to £20 on study tasks if they cleared further screening measures. This study was approved by the Ealing and West London Mental Health Trust Research Ethics Committee and all participants provided written informed consent.
Screening and Demographic/Personality Assessment
The Mini International Neuropsychiatric Inventory (MINI;
Sheehan et al., 1998
) was administered to participants to confirm the absence of DSM-IV axis-I psychiatric conditions, including alcohol/substance abuse/dependence. Five participants were excluded based on this screen. Intelligence quotient (IQ) was assessed using the Wechsler Test of Adult Reading (WTAR;
Wechsler, 2001
). To assess schizotypy, participants completed the short scales of the Oxford–Liverpool Inventory of Feelings and Experiences (O-LIFE;
Mason et al., 2005
). The O-LIFE consists of four subscales: Unusual Experiences (perceptual aberrations and magical thinking); Cognitive Disorganisation (poor attention, poor decision-making, and social anxiety); Introvertive Anhedonia (avoidance of intimacy and lack of pleasure from social and physical stimuli); and Impulsive Nonconformity (impulsive and eccentric behaviour suggesting a lack of self-control).
Cognitive Assessment
Fifty participants completed the cognitive test battery described below. The order of administration was randomised across participants. Participants had the opportunity to take breaks between measures and were able to win money on two of the tasks (the gambling task and the SAT). Three of the tests were available for use through the authors (Probabilistic reversal learning (PRL):
Swainson et al., 2000
, Choice × Risk gambling task:
Rogers et al., 2003
, SAT:
Roiser et al., 2009
), and two were replicated based on published descriptions (AX-Continuous Performance Test (AX-CPT):
Cohen et al., 1999
; LIrr:
Orosz et al., 2008
) using Cogent 2000 (
http://www.vislab.ucl.ac.uk/cogent.php
; Matlab version 7.1, MathWorks).
The SAT (
Roiser et al., 2009
) aims to tap the attribution of salience to task-relevant and task-irrelevant stimuli. Participants pressed a key in response to a black square (the probe) after seeing one of several conditioned stimuli, which varied along two dimensions (colour and shape). Probability of reinforcement (monetary reward) varied along one of these dimensions (the task-relevant dimension), but not the other (the task-irrelevant dimension). Participants’ response times (implicit) and visual analogue scale ratings (VAS; explicit) provided measures of adaptive (task-relevant) and aberrant (task-irrelevant) salience attribution. A practice session and two experimental sessions, with 64 trials each, were run. VAS ratings of reward probability were made at the end of each session.
Explicit adaptive salience was calculated as the increase in participants’ VAS ratings of the probability of a stimulus to predict monetary reward for high-probability relative to low-probability stimuli. Explicit aberrant salience was calculated as the absolute difference in VAS rating between the two levels of the task-irrelevant dimension. Implicit adaptive salience was calculated as the speeding of responses on high-probability relative to low-probability trials. Implicit aberrant salience was defined as the absolute difference in reaction time between the two levels of the task-irrelevant stimulus dimension (
Roiser et al., 2009
). Implicit and explicit measures of adaptive and aberrant salience measures were included in the factor analysis.
A within-subject LIrr task (
Orosz et al., 2008
) lasting approximately 7 min was administered to test for the relative retardation of associative learning following preexposure, as indexed by the speeding of responses to associations of non-preexposed (NPE) relative to preexposed (PE) stimuli with a target stimulus. Participants were instructed to respond as quickly as possible to a target letter during continuous sequential presentation of letters, which varied between presentation blocks containing PE, NPE, and random (R) letter cues preceding the target. LIrr is defined as the speeding of responses during the NPE relative to the PE conditions. A LIrr value greater than zero indicates successful LIrr, while a value around or below 0 is evidence for a disruption of LIrr (
Orosz et al., 2008
). The LIrr measure was included in the factor analysis.
This game taps into aspects of decision making based on the manipulation of reinforcement properties. Participants were presented with a forced-choice trial of two gambles, a control gamble (consisting of a 50% probability of winning, with the amounts to win or lose both at 10 points), and an experimental gamble (with varying probabilities of winning, and amounts to win or lose). The experimental gamble varied according to potential wins (80 or 20 points), losses (80 or 20 points), and the probability of winning (25% or 75%;
Rogers et al., 2003
). Participants were instructed to try to win as many points as possible (in this case corresponding to monetary reward) over four games consisting of 20 trials each. Measures of sensitivity to probability, wins and losses were calculated by comparing the proportion of experimental gambles chosen when the probability of winning, potential wins and losses were high relative to low, respectively. Sensitivity to probability was included in the factor analysis.
In the PRL task, participants responded by clicking on one of two abstract patterns (coloured either red of green) presented simultaneously in a forced-choice paradigm. Subjects learned to respond to the “correct” stimulus, which was reinforced 80% of the time, and avoid the “incorrect” stimulus, which was reinforced 20% of the time. Reinforcement consisted of either “correct” or “incorrect” being displayed on the computer screen, accompanied by a high- or low-frequency tone, respectively. The association between the stimuli and feedback was reversed after 40 trials (i.e. the previously high-reinforced stimulus became the low-reinforced stimulus). The number of errors during initial acquisition (Stage 1) and reversal (Stage 2) were calculated. An “error” was defined as the choice of the low-reinforced stimulus, regardless of the feedback given on that trial. The number of errors made during acquisition (Stage 1) was included in the factor analysis.
The AX-CPT tests participants’ ability to actively maintain context information in order to mediate behaviour accordingly (
Cohen et al., 1999
). Participants were required to respond to a target combination of letters – “A” followed by “X” – while ignoring all other letter sequences. Individual letters were displayed sequentially and participants responded by pressing the space bar. Rates of inappropriate responses and the proportion of correct responses to target pairs were calculated (“A–X” letter sequences); the latter was included in the factor analysis.
Working memory was assessed using forward and backward digit span (
Wechsler, 1981
). Participants were required to listen to sequences of digits, read out loud, and then repeat the sequence back in either forward or reverse order. Sequences were presented in ascending order of difficulty, from 3 to 8 digits (forwards) and 2 to 7 digits (backwards). The total number of correctly repeated sequences in the forwards condition was included in the factor analysis.
Analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences, version 16.0 (SPSS Inc., Chicago, IL., USA). Regardless of the transformations carried out for statistical analysis, raw values are presented in the text and tables for ease of interpretation. The implicit and explicit aberrant salience variables from the SAT were square-root transformed. Stage 1 errors from the probabilistic reversal task were double-log transformed. The proportion of correct responses measure on the AX-CPT was arcsin transformed. To ensure that participants performed as expected on the various tasks, t-tests were performed on the outcome measures where appropriate (one-sample or related-samples).
Data were assessed for the adequacy of factor analytic methods. The R matrix showed satisfactory correlations between variables, with all included variables yielding at least one correlation with another above 0.3. While some variables showed fairly weak correlations with others, re-running the analysis without these yielded similar results to those reported below, justifying their retention. Bartlett’s test was highly significant [χ2(45) = 87.65, p < 0.001], rejecting the possibility that variable correlations formed an identity matrix, and measures of sampling adequacy were sufficient (KMO = 0.54).
The measures listed below, thought to be most reflective of the constructs investigated, were standardised using z-scores (after transformation if appropriate) and entered into a principal components analysis (PCA) with direct oblimin (delta = 0) oblique rotation. The variables entered included: implicit (reaction time) and explicit (VAS rating) measures of adaptive and aberrant salience; LIrr; sensitivity to probability (from the gambling task); percentage of hits to A–X (from the AX-CPT); Stage 1 errors from the PRL; working memory (forward digit span); and estimated IQ (WTAR). The number of variables was limited in order to perform a valid analysis with the sample size available. Oblique rotation was chosen on the premise that some the possible correlations between constructs needed to be taken into account in the factor interpretation.
Solutions extracting two to eight components were examined. After inspection of the factor structures emerging with different extraction and rotation methods, a five-factor solution was chosen. Although Kaiser’s criterion for eigenvalues (>1.0) initially yielded a four-factor solution, it was found from the scree plot and close comparisons of the structure and pattern matrices that a slightly lower eigenvalue cut-off (>0.9) provided a clearer structure of the data (see Figure
1
), with less noisy loadings on some of the variables, and strong loadings on the additional factor. The rotation for the final five-factor solution converged in eight iterations. For the final solution, factor loadings below 0.2 were considered as noise, although weak loadings above 0.2 were taken into account. Factor interpretation was based on the strength and combination of variable loadings. The five extracted components showed no problematic intercorrelations (all correlation coefficients ≤0.1).
Figure 1. Scree plot showing the eigenvalues of the five extracted components and residual components.
Factor scores were calculated for each individual based on a regression model and correlated with the schizotypy subscales using Pearson’s r. Those factors significantly correlated with schizotypy were entered into a single-step regression model as predictors of the related schizotypy subscale to assess the amount of variance in schizotypy traits that could be explained with the extracted factors. To compare our results with previous findings, the unstandardised salience attribution measures derived from the SAT, and LIrr, were also correlated with the O-LIFE subscales.
Demographics
One participant was excluded from analysis due to outlying values on several tests. The remaining 49 participants (23 male, 26 female) had a mean age of 24.80 years (SD = 7.03), and an average estimated IQ of 109.55 (SD = 4.32).
Schizotypy
Mean scores on the O-LIFE schizotypy scales were 1.41 (SD = 1.81) for Unusual Experiences, 2.86 (SD = 2.60) for Cognitive Disorganisation, 1.12 (SD = 1.32) for Introvertive Anhedonia, and 2.14 (SD = 1.95) for Impulsive Nonconformity.
Behavioural Tests
Table
1
shows the descriptive statistics and tests of difference for the behavioural variables of the cognitive test battery. Performance on all tests was similar to previous reports.
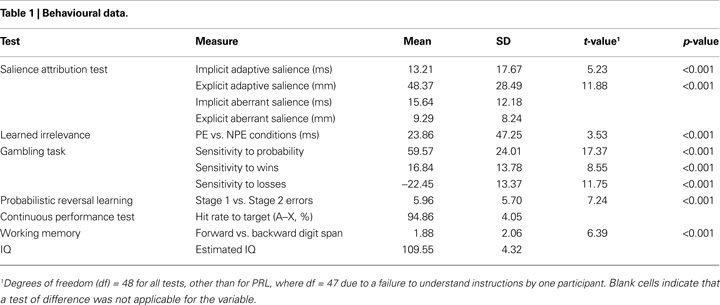
On the SAT, high-probability trials were rated as significantly more likely to yield reward (mean = 64.82, SD = 19.97) relative to low-probability trials (mean = 16.45, SD = 13.03; Cohen’s d = 1.70), and were responded to faster (mean = 227.26 ms, SD = 27.45) than low-probability trials (mean = 240.47, SD = 30.07, Cohen’s d = 0.75). A significant learned irrelevance effect was present on the LIrr (mean reaction times: R = 393.00 ms (SD = 47.87); PE = 377.35 ms (SD = 51.27); and NPE = 353.49 ms (SD = 61.83); Cohen’s d = 0.50). The median false alarm, premature, and miss rates were 3 (range: 0–22), 0 (range: 0–12), and 1 (range: 0–7), respectively. On the gambling task, participants chose the experimental gamble significantly more often when the probability of winning was high (mean = 77.87, SD = 13.65) relative to low (mean = 18.30, SD = 17.67; Cohen’s d = 2.48). The experimental gamble was chosen significantly more often when potential wins were high (mean = 56.50, SD = 14.30) relative to low (mean = 39.67, SD = 1.43; Cohen’s d = 1.22), and significantly less often when potential losses were high (mean = 36.86, SD = 1.90) relative to low (mean = 59.31, SD = 1.58; Cohen’s d = 1.68). On the PRL, participants made significantly fewer errors on Stage 1 (mean = 1.73 errors, SD = 3.03) than on Stage 2 (mean = 7.69 errors, SD = 6.40; Cohen’s d = 1.05). On the AX-CPT, mean correct response latency was 279.71 ms (SD = 40.61), mean response rate to “AY” (“A” not followed by “X”) trials was 15.45% (SD = 13.39), mean response rate to “BX” (“not A” followed by “X”) was 6.94% (SD = 6.91) and median response rate to “BY” (“not A” followed by “not X”) trials was 0 (range: 0–5%). On the Digit Span, participants recalled significantly more items on the forward stage (mean = 9.33, SD = 1.95) than the backwards stage (mean = 7.45, SD = 2.63; Cohen’s d = 0.91).
Factor Analysis
Five factors were retained from the PCA, which are depicted with their variable loadings in Table
2
. Overall, the factors accounted for 74.48% of the variance and were interpreted as reflecting the following constructs: (1)
Operant/explicit learning; (2)
General cognitive ability;
(3)
Contingency-based speeding; (4)
Implicit aberrant salience; and (5)
Attentional vigilance.
We labelled the first factor “Operant/explicit learning”, based on the strong negative loadings of explicit aberrant salience and Stage 1 errors, and the strong positive loading of explicit adaptive salience. Digit span also loaded weakly onto this factor.
The second factor was labelled “General cognitive ability”, since high loadings consisted of the IQ, sensitivity to probability (from the gambling task), and the highest loading of the forward digit span. However, the explicit aberrant and adaptive salience measures also weakly loaded on this factor.
Contrary to our expectations, the third factor included high loadings of LIrr and the implicit measure of adaptive salience, as well as a weak residual loading of explicit adaptive salience. Based on the LIrr and implicit adaptive salience loadings, this factor was termed “Contingency-based speeding”.
Interestingly, the implicit aberrant salience measure loaded highly onto its own factor, and was therefore labelled “Implicit aberrant salience”. Some residual loadings were evident from the implicit adaptive salience measure, IQ, and sensitivity to probability.
The fifth factor emerged independently with only one high loading of the continuous performance measure and a weak residual loading of explicit adaptive salience. This factor was labelled “Attentional vigilance”.
Correlational Analysis
Factor scores
As displayed in Table
3
, the only significant associations that emerged between the factor scores and the O−LIFE schizotypy subscales were negative correlations between Introvertive Anhedonia and the
General cognitive ability factor, and the Introvertive Anhedonia subscale and the
Implicit aberrant salience factor.
A single-step regression model with the General cognitive ability and Implicit aberrant salience factor scores as predictors explained approximately one-quarter of the variation in Introvertive Anhedonia scores (r2 = 0.252, p < 0.01; standardised betas = −0.415 (p < 0.01), and −0.306 (p < 0.05), respectively).
Raw scores
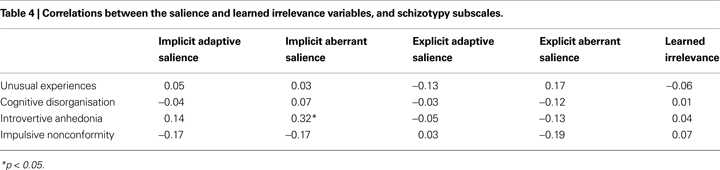
Pearson’s
r correlations between schizotypy and the unstandardised measures derived from the SAT and LIrr are presented in Table
4
. The only significant association that emerged was a positive correlation between Introvertive Anhedonia schizotypy score and implicit aberrant salience, replicating our previous finding (
Roiser et al., 2009
).
We used factor analysis to assess the construct validity of salience attribution measures derived from the SAT. In particular, the implicit aberrant salience measure was found to show excellent construct validity, and was independent from all other measures, including LIrr. However, LIrr and implicit adaptive salience loaded onto the same factor, which we interpret as reflecting the ability to use informative stimuli to speed responses. The explicit aberrant and adaptive salience measures loaded primarily onto the same factor, which also included the Stage 1 errors measure from the PRL. The schizotypy trait of Introvertive Anhedonia showed moderate associations with the
General cognitive ability and
Implicit aberrant salience factors, as well as the raw implicit aberrant salience measure, the latter of which is consistent with our previous finding (
Roiser et al., 2009
). However, we were unable to replicate previously reported associations between explicit aberrant salience and schizotypy, or between LIrr and schizotypy. This may be in part due to the relatively low variance of schizotypy scores in our sample.
The SAT implicit aberrant salience measure loaded very strongly onto its own factor, which did not include LIrr. We therefore interpret this factor as representing automatic salience misattribution, as indexed by the inappropriate speeding of responses to one level of the task-irrelevant stimulus dimension, relative to the other. This result suggests that the implicit aberrant salience measure derived from the SAT is indeed a valid construct, and is not to any great extent confounded by other cognitive processes, at least those included in the present study. The weak loadings of IQ and sensitivity to probability on this factor point to a possible minor association between aberrant salience and general cognitive ability. The minor contribution of implicit adaptive salience to this factor most likely simply reflects the use of a similar response format (reaction times) in the calculation of both measures. Importantly, the implicit aberrant salience factor, and the raw implicit aberrant salience measure, correlated significantly with schizotypy, replicating our previous result and providing further evidence for the face validity of this measure.
The emergence of LIrr and implicit aberrant salience as independent constructs is important in terms of understanding cognitive deficits in psychosis and schizophrenia. In contrast to previous suggestions (e.g.
Gray, 2004
), we speculate that different learning and attentional processes contribute to these constructs. While the mechanisms of both disrupted LI/LIrr and elevated aberrant salience are hypothesised to reflect dopaminergic dysfunction, implicit aberrant salience may be more closely related to an impairment in the motivational aspects of reward learning. To put it another way, we suggest that the implicit aberrant salience measure represents the tendency to inappropriately and automatically tag irrelevant cues with importance or relevance, as
Kapur (2003)
hypothesised occurs in psychosis.
Since LIrr and aberrant salience were orthogonal in our dataset, it would appear that low scores on the measure of LIrr we employed do not necessarily reflect impaired irrelevance attribution, or at least not in the same way that high scores on the SAT implicit aberrant salience measure do. Supporting this contention, other studies have suggested that LI/LIrr may rely not only on the attribution of irrelevance but also on switching (
Braunstein-Bercovitz, 2003
). Our data further clarify the interpretation of LIrr measures, since the implicit adaptive salience and LIrr measures jointly defined the factor we termed
Contingency-based speeding. Both loaded highly in the same direction, indicating that the same process may underlie higher LIrr (more retardation of learning an association with a PE stimulus relative to a NPE stimulus) and higher adaptive salience (speeding of responses on high relative to low-probability reward trials). Both these measures reflect changes in the speed at which participants respond in response to associative manipulations. Thus the
Contingency-based speeding factor appears to reflect either the successful learning of associations between stimuli, or possibly the extent to which responses are guided by such learning; it is difficult to disambiguate between these two possibilities on the basis of the current dataset.
Either way, our data challenge the interpretation of LIrr as reflecting solely the ability to attribute stimuli as irrelevant (
Gal et al., 2005
;
Young et al., 2005
). Moreover, schizotypy, which was associated with implicit aberrant salience, did not show a significant relationship with LIrr. While this pattern of results might be interpreted as suggesting that aberrant salience is possibly be more relevant to psychosis than LI, therefore supporting the aberrant salience model over the comparator model, we feel that such a conclusion would be premature. Ours was a non-clinical sample with low variance in schizotypy, and further work is needed to determine whether aberrant salience and LI are similarly dissociable in patients suffering from psychotic symptoms.
The emergence of the separate
Operant/explicit learning factor suggests that implicit aberrant salience and LIrr are both distinct from the ability to form a conscious appraisal of stimulus-reinforcement associations. The explicit adaptive and the explicit aberrant salience measures loaded strongly onto this factor, in opposite directions. This factor also explained variance in the number of Stage 1 errors on the PRL. The three variables loading most highly onto this factor all tapped aspects of participants’ explicit knowledge of probabilistic stimulus-reinforcement associations. However, the strong loadings of the explicit adaptive and aberrant salience measures on the same factor complicates the interpretation of our previous findings (
Roiser et al., 2009
), where delusions were associated with explicit aberrant salience, but not with explicit adaptive salience, in patients with schizophrenia. One possible reason for this strong joint loading is that participants who did not learn any stimulus-reinforcement associations might simply respond randomly on the VAS. Such individuals would be expected to exhibit low scores (perhaps even negative) on the explicit adaptive salience measure, but higher (non-zero) on the explicit aberrant salience measure. This is because the latter measure is calculated using absolute scores, and will therefore detect any variability in ratings on the task-irrelevant dimension, random or otherwise. One strategy would be to exclude participants who did not show at least some explicit adaptive salience, and especially those exhibiting high explicit aberrant salience ratings, to attempt to exclude variability simply related to random responding. Unfortunately, we did not have a sufficiently large enough sample in the present study to make this strategy viable.
The General cognitive ability factor explained the sensitivity to probability measure and estimated IQ very clearly. It also best accounted for variance in forwards digit span, although this measure also showed medium loadings on other constructs, notably the Operant/explicit learning factor. Thus it appears that sensitivity to probability, as assessed by the decision-making task, is independent from probabilistic learning, as assessed by the SAT and PRL. Interestingly, the General cognitive ability factor was moderately associated with Introvertive Anhedonia schizotypy, in fact more strongly than was the Implicit aberrant salience factor. There was also a trend towards an association with Cognitive Disorganisation schizotypy. These associations may reflect the general cognitive impairment commonly reported in schizophrenia and high-schizotypy individuals.
The factor of Attentional vigilance appeared to be a direct reflection of the ability to maintain contextual information and apply it to guide responses, with nearly no residual variance of the proportion of hits to “AX” remaining unexplained. However, it is important to note that with the initial four-factor solution, this attentional measure was distributed across the other four factors with weak to medium loadings, suggesting that rather than being strongly related to any particular variable included, attentional vigilance appears to be implicated to some extent in most of the constructs described when it is not treated as a construct in its own right.
In fact, most measures included in the factor analysis showed weak residual loadings, indicating that many cognitive variables are impossible to fully dissociate from each other. For example, while the measures derived from the SAT show good validity based on the current analysis, some maintenance of working memory capacity is logically necessary to accurately report probabilistic associations using VAS, supported by a weak residual loading of explicit SAT measures on the
Operant/explicit learning factor. Crucially, the
General cognitive ability and the
Attentional vigilance factors were clearly dissociable from those explaining variance on SAT measures, suggesting that the SAT provides valid measures of salience attribution largely unconfounded by attention, working memory or IQ. Thus while other cognitive processes are clearly necessary to complete the SAT, it appears that these processes can be dissociated sufficiently to detect aberrant salience attribution independent of any generalised cognitive deficit (
Murray et al., 2008a
also raise this point with regards to incentive motivation processes).
We were unable to replicate an association between schizotypy and explicit aberrant salience; neither was schizotypy associated with either of the adaptive salience measures (
Roiser et al., 2009
). The reason for this failure to replicate is not clear, but may be related to the sample characteristics. The variability in schizotypy in our sample was relatively low, likely reflecting the recruitment methods employed and stringent screening criteria (compare, for example, with
Shrira and Tsakanikos, 2009
). However, it is notable that, while not statistically significant, there was a trend towards an association between the
Operant/explicit learning factor and Unusual Experiences schizotypy, though whether this reflects an association with explicit adaptive or aberrant salience is unclear. Future studies in healthy volunteers may consider pre-selecting participants on the basis of schizotypy in order to avoid such interpretative difficulties.
A weakness of our analysis was that two of the factors were interpreted based on a strong loading of only a single variable each (Implicit aberrant salience and Attentional vigilance). This is not ideal in a PCA, which sets out to reduce the constructs based on shared variance, thus requiring loadings of several items to accept latent variables as valid constructs. However, these factors could be interpreted clearly, since the variables entered into the PCA were secondary (construct) variables based on previously validated paradigms, which yielded similar effect sizes to those reported elsewhere in the literature.
In summary, we found that the SAT can dissociate implicit aberrant salience processing from other aspects of reward learning and attention, in particular LIrr. Furthermore, mechanisms of LIrr and adaptive salience attribution appear to be associated, but both were largely independent from other cognitive processes, including attentional vigilance and working memory. We replicated an association between implicit aberrant salience and Introvertive Anhedonia schizotypy in healthy volunteers, but we could not replicate previously reported associations between schizotypy and explicit aberrant salience or LIrr. These data support the use of aberrant salience, particularly the implicit measure, as a valid construct in future studies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Robert D. Rogers providing the Choice × Risk gambling task and Luke Clark for providing the Probabilistic Reversal Learning task.
Baruch, I., Hemsley, D. R., and Gray, J. A. (1988). Differential performance of acute and chronic schizophrenics in a latent inhibition task.
J. Nerv. Ment. Dis. 176, 598–606.
Bedwell, J. S., Kamath, V., and Compton, M. T. (2009). The relationship between interview-based schizotypal personality dimension scores and the continuous performance test.
Schizophr. Res. 108, 158–162.
Berridge, K. C., and Robinson, T. E. (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience?
Brain Res. Brain Res. Rev. 28, 309–369.
Braunstein-Bercovitz, H. (2003). The modulation of latent inhibition by field-dependency: is it related to attentional dysfunction in schizotypy?
Pers. Individ. Dif. 35, 1719–1729.
Braunstein-Bercovitz, H., and Lubow, R. E. (1998). Are high-schizotypal normal participants distractible or limited in attentional resources? A study of latent inhibition as a function of masking task load and schizotypy level.
J. Abnorm. Psychol. 107, 659–670.
Claridge, G. (1994). Single indicator of risk for schizophrenia: probable fact or likely myth?
Schizophr. Bull. 20, 151–168.
Cohen, J. D., Barch, D. M., Carter, C., and Servan-Schreiber, D. (1999). Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks.
J. Abnorm. Psychol. 108, 120–133.
Corlett, P. R., Honey, G. D., and Fletcher, P. C. (2007). From prediction error to psychosis: ketamine as a pharmacological model of delusions.
J. Psychopharmacol. (Oxford) 21, 238–252.
De la Casa, L. G., Ruiz, G., and Lubow, R. E. (1993). Amphetamine-produced attenuation of latent inhibition is modulated by stimulus preexposure duration: implications for schizophrenia.
Biol. Psychiatry 33, 707–711.
Gal, G., Mendlovic, S., Bloch, Y., Beitler, G., Levkovitz, Y., Young, A. M., Feldon, J., and Ratzoni, G. (2005). Learned irrelevance is disrupted in first-episode but not chronic schizophrenia patients.
Behav. Brain Res. 159, 267–275.
Gold, J. M., Waltz, J. A., Prentice, K. J., Morris, S. E., and Heerey, E. A. (2008). Reward processing in schizophrenia: a deficit in the representation of value.
Schizophr. Bull. 34, 835–847.
Gray, J. A. (1998). Integrating Schizophrenia.
Schizophr. Bull. 24, 249–266.
Gray, J. A. (2004). [Letter to the Editor.]
. Am. J. Psychiatry 161, 377.
Gray, J. A., Feldon, J., Rawlins, J. N. R., Hemsley, D. R., and Smith, A. D. (1991). The neuropsychology of schizophrenia.
Behav. Brain Res. 14, 1–20.
Gray, N. S., Fernandez, M., Williams, J., Ruddle, R. A., and Snowden, R. J. (2002). Which schizotypal dimensions abolish latent inhibition?
Br. J. Clin. Psychol. 41, 271–284.
Gray, N. S., Pilowsky, L. S., Gray, J. A., and Kerwin, R. W. (1995). Latent inhibition in drug naive schizophrenics: relationship to duration of illness and dopamine D2 binding using SPET.
Schizophr. Res. 17, 95–107.
Heerey, E. A., Bell-Warren, K. R., and Gold, J. M. (2008). Decision-making impairments in the context of intact reward sensitivity in schizophrenia.
Biol. Psychiatry 64, 62–69.
Hemsley, D. R. (1993). A simple (or simplistic?) cognitive model for schizophrenia.
Behav. Res. Ther. 31, 633–645.
Horvitz, J. C. (2000). Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events.
Neuroscience 96, 651–656.
Jensen, J., and Kapur, S. (2009). Salience and psychosis: moving from theory to practise.
Psychol. Med. 39, 197–198.
Jensen, J., Willeit, M., Zipursky, R. B., Savina, I., Smith, A. J., Menon, M., Crawley, A. P., and Kapur, S. (2008). The formation of abnormal associations in schizophrenia: neural and behavioral evidence.
Neuropsychopharmacology 33, 473–479.
Jones, S. H., Gray, J. A., and Hemsley, D. R. (1992). Loss of the Kamin blocking effect in acute but not chronic schizophrenics.
Biol. Psychol. 32, 739–755.
Kapur, S. (2003). Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia.
Am. J. Psychiatry 160, 13–23.
Keri, S., Kelemen, O., Szekeres, G., Bagoczky, N., Erdelyi, R., Antal, A., Benedek, G., and Janka, Z. (2000). Schizophrenics know more than they can tell: probabilistic classification learning in schizophrenia.
Psychol. Med. 30, 149–155.
Laruelle, M., and Abi-Dargham, A. (1999). Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies.
J. Psychopharmacol. (Oxford) 13, 358–371.
Lubow, R. E., and De la Casa, G. (2002). Latent inhibition as a function of schizotypality and gender: implications for schizophrenia.
Biol. Psychol. 59, 69–86.
Maher, B. A. (1974). Delusional thinking and perceptual disorder.
J. Individ. Psychol. 30, 98–113.
Mason, O., Linney, Y., and Claridge, G. (2005). Short scales for measuring schizotypy.
Schizophr. Res. 78, 293–296.
Miller, R. (1976). Schizophrenic psychology, associative learning and the role of forebrain dopamine.
Med. Hypotheses 2, 203–211.
Murray, G. K., Clark, L., Corlett, P. R., Blackwell, A. D., Cools, R., Jones, P. B., Robbins, T. W., and Poustka, L. (2008a). Incentive motivation in first-episode psychosis: a behavioural study.
BMC Psychiatry 8, 34.
Murray, G. K., Corlett, P. R., Clark, L., Pessiglione, M., Blackwell, A. D., Honey, G., Jones, P. B., Bullmore, E. T., Robbins, T. W., and Fletcher, P. C. (2008b). Substantia nigra/ventral tegmental reward prediction error disruption in psychosis.
Mol. Psychiatry 13, 239, 267–276.
Orosz, A. T., Feldon, J., Gal, G., Simon, A. E., and Cattapan-Ludewig, K. (2008). Deficient associative learning in drug-naive first-episode schizophrenia: results obtained using a new visual within-subjects learned irrelevance paradigm.
Behav. Brain Res. 193, 101–107.
Robbins, T. W. (2005). Synthesizing schizophrenia: a bottom-up, symptomatic approach.
Schizophr. Bull. 31, 854–864.
Rogers, R. D., Blackshaw, A. J., Middleton, H. C., Matthews, K., Hawtin, K., Crowley, C., Hopwood, A., Wallace, C., Deakin, J. F., Sahakian, B. J., and Robbins, T. W. (1999). Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour.
Psychopharmacology (Berl.) 146, 482–491.
Rogers, R. D., Tunbridge, E. M., Bhagwagar, Z., Drevets, W. C., Sahakian, B. J., and Carter, C. S. (2003). Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues.
Neuropsychopharmacology 28, 153–162.
Roiser, J. P., Stephan, K. E., den Ouden, H. E., Barnes, T. R., Friston, K. J., and Joyce, E. M. (2009). Do patients with schizophrenia exhibit aberrant salience?
Psychol. Med. 39, 199–209.
Schmidt-Hansen, M., Killcross, A. S., and Honey, R. C. (2009). Latent inhibition, learned irrelevance, and schizotypy: assessing their relationship.
Cogn. Neuropsychiatry 14, 11–29.
Seeman, P., and Kapur, S. (2000). Schizophrenia: more dopamine, more D2 receptors.
Proc. Natl. Acad. Sci. U.S.A. 97, 7673–7675.
Shaner, A. (1999). Delusions, superstitious conditioning and chaotic dopamine neurodynamics.
Med. Hypotheses 53, 119–123.
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., and Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I
.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD
-10.
J. Clin. Psychiatry 59(Suppl 20),
22–33;quiz 34–57.
Shrira, A., and Tsakanikos, E. (2009). Latent inhibition as a function of positive and negative schizotypal symptoms: evidence for a bi-
directional model.
Pers. Individ. Dif. 47, 434–438.
Solomon, P. R., Crider, A., Winkelman, J. W., Turi, A., Kamer, R. M., and Kaplan, L. J. (1981). Disrupted latent inhibition in the rat with chronic amphetamine or haloperidol-induced supersensitivity: Relationship to schizophrenic attention disorder.
Biol. Psychol. 16, 519–537.
Swainson, R., Rogers, R. D., Sahakian, B. J., Summers, B. A., Polkey, C. E., and Robbins, T. W. (2000). Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication.
Neuropsychologia 38, 596–612.
Vaitl, D., Lipp, O., Bauer, U., Schuler, G., Stark, R., Zimmermann, M., and Kirsch, P. (2002). Latent inhibition and schizophrenia: Pavlovian conditioning of autonomic responses.
Schizophr. Res. 55, 147–158.
Verdoux, H., and van Os, J. (2002). Psychotic symptoms in non-clinical populations and the continuum of psychosis.
Schizophr. Res. 54, 59–65.
Waltz, J. A., and Gold, J. M. (2007). Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction.
Schizophr. Res. 93, 296–303.
Waltz, J. A., Schweitzer, J. B., Gold, J. M., Kurup, P. K., Ross, T. J., Salmeron, B. J., Rose, E. J., McClure, S. M., and Stein, E. A. (2009). Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers.
Neuropsychopharmacology 34, 1567–1577.
Wechsler D. (1981). Manual for the Wechsler Adult Intelligence Scale-Revised. New York, NY, The Psychological Corporation.
Wechsler, D. (2001). Wechsler Test of Adult Reading manual. San Antonio, TX, The Psychological Corporation.
Weiner, I., Lubow, R. E., and Feldon, J. (1981). Chronic amphetamine and latent inhibition.
Behav. Brain Res. 2, 285–286.
Weiner, I., Lubow, R. E., and Feldon, J. (1984). Abolition of the expression but not the acquisition of latent inhibition by chronic amphetamine in rats.
Psychopharmocology 83, 194–199.
Weiner, I., Lubow, R. E., and Feldon, J. (1988). Disruption of latent inhibition by acute administration of low doses of amphetamine.
Pharmacol. Biochem. Behav. 30, 871–878.
Young, A. M., Kumari, V., Mehrotra, R., Hemsley, D. R., Andrew, C., Sharma, T., Williams, S. C., and Gray, J. A. (2005). Disruption of learned irrelevance in acute schizophrenia in a novel continuous within-subject paradigm suitable for fMRI.
Behav. Brain Res. 156, 277–288.

