The Beneficial Effects of Physical Activity on Impaired Adult Neurogenesis and Cognitive Performance
1
Department of Molecular Neurobiochemistry, Faculty for Chemistry and Biochemistry, Ruhr University Bochum, Bochum, Germany
2
Graduate School of Development and Plasticity of the Nervous System, Ruhr University Bochum, Bochum, Germany
3
International Graduate School of Neuroscience, Faculty of Biology and Biotechnology, Ruhr University Bochum, Bochum, Germany
4
Department of Developmental Neurobiology, Faculty of Biology and Biotechnology, Ruhr University Bochum, Bochum, Germany
Running is a potent stimulator of cell proliferation in the adult dentate gyrus and these newly generated hippocampal neurons seem to be implicated in memory functions. Here we have used a mouse model expressing activated Ras under the direction of the neuronal Synapsin I promoter (named synRas mice). These mice develop down-regulated proliferation of adult hippocampal precursor cells and show decreased short-term recognition memory performances. Voluntary physical activity reversed the genetically blocked generation of hippocampal proliferating cells and enhanced the dendritic arborisation of the resulting doublecortin newly generated neurons. Moreover, running improved novelty recognition in both wild type and synRas littermates, compensating their memory deficits. Brain-derived neurotrophic factor (BDNF) has been proposed to be a potential mediator of physical exercise acting in the hippocampus on dentate neurons and their precursors. This was confirmed here by the identification of doublecortin-immunoreactive cells expressing tyrosine receptor kinase B BDNF receptor. While no difference in BDNF levels were detected in basal conditions between the synRas mice and their wild type littermates, running was associated with enhanced BDNF expression levels. Thus increased BDNF signalling is a candidate mechanism to explain the observed effects of running. Our studies demonstrate that voluntary physical activity has a robust beneficial effect even in mice with genetically restricted neurogenesis and cognition.
Wheel running is a general paradigm that has many repercussions on numerous systems, be it cardiovascular, proprioceptive, motor, motivational or general arousal systems. In the last two decades, adult cell proliferation and neurogenesis were found to be differentially regulated by genetic and epigenetic factors (Kempermann, 2002). Indeed, running strongly stimulates adult cell proliferation and neurogenesis in the dentate gyrus but not on the ventricular walls (Brown et al., 2003) and can improve learning and memory abilities in hippocampus-dependent tasks (van Praag et al., 1999; Wojtowicz et al., 2008; Jessberger et al., 2009).
In the hippocampus, Brain-derived neurotrophic factor (BDNF) mRNA and protein have been extensively shown to be increased in response to voluntary physical activity (Berchtold et al., 2001; Johnson et al., 2003; Adlard and Cotman, 2004; Adlard et al., 2004; Russo-Neustadt et al., 2004). BDNF infusion can also increase the number of newly generated neurons of the adult rat hippocampus (Scharfman et al., 2005) even though it is not yet clearly established which type of cells are involved in this response. Recently, nestin-cell specific knock down of tyrosine receptor kinase B (TrkB) in early proliferating cells completely prevented exercise-induced changes in the hippocampus (Li et al., 2008). However, using the neuronal synapsin promoter for selective knock down of TrkB in differentiated hippocampal neurons did not interfere with voluntary exercise stimulation of early proliferating cells underlining the importance of cell type specific signalling mechanisms during lineage development in adult neurogenesis.
The Ras-mediated extracellular signal-regulated cascade (ERK) pathway is considered as a major BDNF/TrkB intracellular signalling pathway in neurons (Vojtek and Der, 1998). In order to gain further insights into the regulation of newly generated neurons we have used here the synapsin I promoter to express permanently activated Ha-Val12 Ras in mice (named synRas). In a number of experimental paradigms this transgenic activation of Ras in brain neurons prevented lesion-induced neuronal degeneration during postnatal development and in the adult brain (Heumann et al., 2000; Felderhoff-Mueser et al., 2004; Chakrabarty et al., 2007; Makwana et al., 2009). Consistently, also in normal newborn hippocampal neurons there is enhanced cell survival in synRas mice. This was associated with a strongly reduced proliferation of non-neuronal early proliferating cells (Manns et al., in revision).
Besides its neurotrophic effects on survival and differentiation, BDNF activity is acting on branching development of neurons (Borasio et al., 1989; McAllister et al., 1995). Enriched housing conditions (Leggio et al., 2005; Stranahan et al., 2007) and exposure to learning paradigms (Moser et al., 1994; Feria-Velasco et al., 2002) also stimulate dendritic arborisation and spine density. Altogether, these data propose BDNF as a potential mediator of the effects of running on adult neurogenesis and branching development.
There is growing evidence that the level of adult neurogenesis is correlated to behavioural performances (Shors et al., 2001; Drapeau et al., 2003; Dalla et al., 2009; Garthe et al., 2009; Hernández-Rabaza et al., 2009). This has been mainly shown in the most common hippocampal-dependent tasks, namely the trace or contextual fear conditioning. However, the link between novelty recognition and adult neurogenesis has only recently been examined in rats (Jessberger et al., 2009). Indeed, recognition memory relies not only on cortical and paracortical regions (Murray et al., 2000) but also on the integrity of the hippocampus. It is assumed to involve episodic working memory dependent on the interactions between CA3 and the dentate gyrus via mossy fibre inputs (Clark et al., 2001; Manns et al., 2003).
In this study, we first examined whether the reduced level of adult cell proliferation observed in the synRas mouse would be associated with lower dendritic arborisation and lower performance in the object recognition test. Besides, we examined whether running, an epigenetic stimulator of adult neurogenesis, could, on the one hand, reverse the strong phenotypes of this mouse and, on the other hand, enhance the behavioural performance of both wild type and transgenic mice. Furthermore, we hypothesised that running-induced rescue effects were mediated by the neurotrophic factor BDNF and we analysed its expression and the type of cells on which it could act.
Animals and Housing
Two- to three-month-old female synRas and wild type littermate mice, of NMRI background (Heumann et al., 2000) were housed per group of five in standard cages (21 cm × 16 cm × 14 cm) under a 12-h light: 12-h dark cycle (lights on at 7:30 am) with ad libitum access to food and water. Temperature (18°C ± 2°) and humidity (70%) were kept constant. Runner mice were also housed per group of five but in bigger cages (56 cm × 32 cm × 19 cm) with free access to a running wheel (diameter: 15 cm).
Experiment 1: Effects of Running on Adult Proliferation, Adult Neurogenesis and on the Dendritic Tree of Doublecortin-Labeled Immature Neurons in Wild Type and Synras Mice
BrdU injections
For 12 consecutive days control and running animals were daily weighed and received an intraperitoneal injection of 10 mg/ml BrdU (bromodeoxyuridine; Sigma) in 0.9% NaCl solution (daily dose: 50 μg/g body weight).
Histological procedure
One day after the last BrdU injection, animals were deeply anaesthetised with a mix of Ketamin–Rompun (200 μg/g Ketamin Hydrochloride and 5 μg/g Rompun) and were transcardiacally perfused with 50 ml of phosphate buffer saline (0.1 M) followed by 150 ml of ice-cold 4% paraformaldehyde in PBS. Brains were quickly removed and postfixed overnight. 25-μm-sections were cut coronally from an ice-cooled block on a sliding microtome through the entire anteroposterior extension of the hippocampi. Sections were collected and stored at 4°C in phosphate buffer saline containing 0.01% NaN3.
For BrdU-labeling, sections were treated with 0.3% H2O2 in distilled water for 30 min and incubated in SSC-formamid (1:1) for 2 h, washed in SSC buffer and once in phosphate buffer saline. Sections were further incubated in 2 N HCl for 1 h at 37°C and washed in 0.1 M borate buffer (pH 8.5) for 10 min. After extensive washes in PBS, sections were blocked in the PBS containing 0.3% Triton (incubation buffer), 5% horse serum and 2% Mouse-on-Mouse protein (Vector Laboratories) for 1 h, and incubated overnight at 4°C with mouse monoclonal anti-BrdU antibody (1:400; Chemicon) diluted in the incubation buffer. After rinses in PBS, the sections were incubated with the biotinylated horse anti-mouse antibody (1:200; Vector Laboratories) diluted in the incubation medium for 1 h at room temperature. After another set of rinses in PBS, the ABC Elite reagents (Vector Laboratories) were applied for 1 h. The peroxidase reaction was performed using a diaminobenzedine (DAB) black kit (Zymed) according to the manufacturer’s instructions mixing DAB with hydrogen peroxide and nickel-cobalt in an appropriate buffer for 5–10 min.
For the doublecortin staining the sections were not subjected to any pretreatment with the exception of a H2O2 0.3% 30-min incubation. Sections were rinsed in PBS and immediately incubated for 1 h with 5% rabbit serum (Vector laboratories) diluted in PBS-Triton 0.3% followed by an overnight–incubation with the primary antibody anti-doublecortin (Santa Cruz; 1:800). A biotinylated secondary anti-goat antibody (Vector laboratories; 1:200) was used. The subsequent steps were done as indicated above.
Evaluation of the dendritic tree of doublecortin-labelled immature neurons
After labelling the newly born granule cells of the adult dentate gyrus with doublecortin in 60-μm brain sections, the length of their fibres and the number of segments (dendritic fibre section between two arborizations) were determined with a Lucida macroscope and reconstructed with a 100× objective at the granule cell layer of the dorsal hippocampus of five to seven animals per group. The best doublecortin-labelled cells were selected and only doublecortin-positive fibres of the ventral blade of the dentate gyrus were included in the analysis.
Experiment 2. Role of BDNF in Running-Induced Effects on Proliferation and Differentiation
BDNF quantitative real-time PCR
Animals were sacrificed by cervical translocation. Hippocampi were quickly microdissected and frozen in liquid nitrogen before being stored at −80°C up to utilisation. Total RNA was extracted using the Nucleospin RNA II kit (Macherey-Nagel) including DNAseI treatment according to the manufacturer’s instructions. cDNA was synthesised from total RNA in a 20-μl reaction mixture containing 1× reverse transcription buffer (Sensiscript Reverse Transcription kit, Qiagen), 0.5 mM dNTPs (Sensiscript Reverse Transcription kit, Qiagen), 1 μM oligo(dT)s (Invitrogen), 10 units of recombinant RNAsin ribonuclease inhibitor (Promega), and 1 μl of reverse transcriptase (Sensiscript Reverse Transcription kit, Qiagen). After incubation for 1 h at 37°C and 10 min at 93°C in order to inactivate the reverse transcriptase in a thermocycler (Eppendorf Mastercycler), the reaction was terminated by incubation at 4°C. Synthesised cDNA was stored at −20°C up to use. The quantitia was carried out in a 20-μl reaction with 10 μl of 1×QuantiTect SYBR Green PCR Master Mix, 0.5 μM of the forward primer (BDNF: atgccgcaaacatgtctatg; lamin: aggatccaggaattggaggac) and of the reverse primer (BDNF: taatactgtcacacacgctc; lamin: atcactcagctgctgctgctgcat), 1 μl of the synthesised cDNA and RNAse-free water was added up to the final volume.
The reaction was performed in a Lightcycler® with the following programme: a 15-min-initial activation step at 95°C, 50 cycles of 15 s of denaturation at 94°C, 20 s at an annealing temperature of 57°C and 30 s of the extension step at 72°C during which the fluorescent data collection was performed. A melting step of 30 s at 65°C allowed verifying the single amplification of the amplicon of the calculated Tm. Relative ratios (R) were calculated according to the equation introduced by Pfaffl (2001): R = (Etarget)ΔCt(control-sample)/(Ereference)ΔCt(control-sample), where Ct value represents the threshold cycle where the fluorescence signal is above background and E the efficiency of the primers. The BDNF (target) mRNA level was normalised to the level of the reference nuclear gene, lamin B1. Calculation of the ratios and statistical analysis were performed using the Relative Expression Software Tool (REST) (Pfaffl et al., 2002).
BDNF ELISA quantification
Mice were sacrificed by cervical translocation. Hippocampi were quickly dissected and frozen in liquid nitrogen before being stored at −80°C up to utilisation. Samples were thawed and processed with a commercially available BDNF ELISA immunoassay (Promega). The tissue was first homogenised in lysis buffer (18-μl/mg tissue), containing 137 mM NaCl, 20 mM Tris-HCl, 1% NP40, 10% glycerol, 1 mM Phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml aprotinin, 1 μg/ml leupeptin and 0.5 mM sodium vanadate. Samples were further diluted 4:1 with DPBS buffer. The BDNF ELISA immunoassay was then performed according to the manufacturer’s instructions. Samples were subjected to acid pretreatment for 15 min with 1 μl of HCl 1 N per 50 μl of undiluted sample. A standard curve ranging from 0 to 500 pg/ml was obtained by serial dilution of BDNF protein standard in Block & Sample 1× buffer. All samples and standards were prepared in duplicates. The optical density of the wells was analysed in a TECAN microplate reader at 450 nm and protein concentrations in the samples were calculated from the standard curve.
Histological procedure for double immunofluorescence labelling
For double immunofluorescence, sections from wild type mice were incubated for 1 h in the incubation buffer with 10% donkey serum, and then incubated overnight with rabbit anti-TrkB (TK-) (1:50; Santa Cruz) in combination with goat anti-doublecortin (1:100; Santa Cruz). The following day, the sections were rinsed in PBS and incubated in the dark with the respective dye-conjugated secondary antibodies (FITC-anti-rabbit, 1:200; Cy3-anti-goat, 1:100; biotinylated anti-mouse, 1:200, later incubated with Cy3-streptavidin, 1:1000) diluted in the incubation buffer. The sections were then rinsed thrice in PBS, the cell nuclei stained for 10 min with the Hoechst 33342 dye (1:1000) and rinsed again with distilled water before mounting with the ProLong Antifade kit (Molecular Probes).
Experiment 3: Effects of Running on Novelty Recognition Memory
Novel object recognition task
The behavioural procedure was similar to that previously described (Sargolini et al., 2003). It consisted of three different phases: a familiarisation phase, a sample phase and a test phase. On the first day mice individually underwent a single 10-min-familiarisation session, during which they were introduced for the first time in a PVC square arena (50cm × 50cm) with 40-cm-high walls and a white floor divided into 16 sectors (12.5 cm × 12.5 cm) by black lines. During this phase, the baseline level of locomotor activity, internal and external crossings, was recorded. On the second day animals were submitted to a single 10-min-sample session (Figure 5A), during which two identical objects (O1 and O2) were placed in a symmetric position from the centre of the arena, each 10 cm from the sidewalls. After a 10-min delay during which mice returned to their home-cage, they were reintroduced into the arena (test session) and exposed for 10 min to 2 objects, a familiar object: triplicate of the objects presented in the sample session (O3) and a novel object (NO), which substituted one of the previous sample object. Object exploration was evaluated by the time spent in contact by the animals with the objects during the sample and the test sessions. A contact was defined as the subject’s snout touching the object from the floor.
Statistical analysis
All graphs and statistical analysis were performed using the Prism programme (GraphPad) except for the real-time PCR analysis where the REST software (Pfaffl et al., 2002) was used. For the number of labelled cells, BDNF protein levels, the number of segments and the dendritic length, non parametric Mann–Whitney tests were appliedon the mean data of each individual whereas for the behavioural data, parametric analysis of variance were applied. A p value was considered statistically relevant when inferior to 0.05.
Experiment 1: Effects of Running on Adult Proliferation, Adult Neurogenesis and on the Dendritic Tree of Doublecortin-Labeled Immature Neurons
To obtain a better understanding of the mechanisms underlying adult neurogenesis, we examined whether free access to a running wheel could stimulate the reduced adult hippocampal proliferation observed in the synRas mice. All mice were housed per group of five; however, the control mice were in normal standard cages, the running mice were in a bigger cage with free access to a running wheel for 13 days. Control and running mice of both genotypes were daily injected with BrdU (12 injections). The proliferation rate was estimated by counting the number of cells labelled for BrdU per mm2 of the area of the granule cell layer of the hippocampus on one section out of six through the entire hippocampus (from −1.40 mm to −3.40 mm distance from bregma, according to Paxinos and Franklin, 2001).
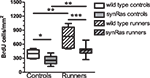
One day after the last of the 12 daily injections, we could observe that under standard control conditions, there were less newborn cells in the synRas mice compared to the wild type NMRI mice (214.83 ± 23.27 cells/mm2 versus 394.17 ± 37.53 cells/mm2 respectively, Mann–Whitney test, p < 0.01) (Figure 1). This was in accordance with previously reported data (Manns et al., in revision). Running stimulated the proliferation rate as revealed by the 160%-increase in the number of BrdU-labeled cells in the wild type running mice compared to the wild type control mice (673.08 ± 60.27 cells/mm2 versus 394.17 ± 37.53 cells/mm2 respectively, Mann–Whitney test, p < 0.01). A comparable increase (128%) of the proliferation rate was also observed in the synRas mice (214.83 ± 23.27 cells/mm2 in standard conditions compared to 442.20 ± 25.44 cells/mm2 in the running conditions, Mann–Whitney test, p < 0.01). Thus, the basal proliferation rate of the synRas mice was stimulated by running to reach similar levels as in wild type control mice.
Figure 1. Quantification of the number of bromodeoxyuridine-labeled cells per mm2 in the four different groups: wild type controls (n = 6), synRas controls (n = 8), wild type runners (n = 11), synRas runners (n = 13).
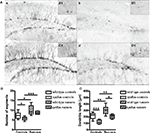
In order to show that this paradigm also affected the rate of adult neurogenesis, an immunohistochemical staining for doublecortin, a marker for immature neurons (Figure 2) was performed in wild type and synRas mice housed under standard conditions and with free access to a running wheel after 13 days. The pictures (Figure 2A) gave a qualitative impression of the density and general aspect of the DCX-labeled immature neurons in the dentate gyrus of wild type control mice, wild type running mice, synRas control mice and synRas running mice. The overall density of doublecortin cells in the synRas control mice was lower when compared to the wild type control mice. Yet, this reduction was compensated when the synRas mice were exposed to a bigger space with free access to the running wheel, leading to a similar level of doublecortin expression as in wild type control animals. The density of the immature neurons in the running wild type mice was also increased compared to the wild type control mice. The influence of physical activity on the dendritic arborisation of the immature neurons was then estimated by the number of segments and the length of the dendrites of the best labeled doublecortin cells. Physical activity induced an increased number of segments in running wild type and synRas mice compared to their respective non-running controls (p < 0.05 and p < 0.01, respectively) (Figure 2B). No difference in the number of segments was observed between the two groups in any housing conditions. Similarly, physical activity lead to longer dendrites in wild type and synRas mice (Figure 2C, p < 0.05) suggesting that running was able to overcome the Ras supressed dendrite growth in doublecortin neuronal precursor cells.
Figure 2. Effects of physical activity on the dendritic arborisation of immature doublecortin-labeled neurons. (A) Representative photographs and islets of doublecortin-labeled cells at high magnifier (×100) in wild type controls (n = 6) (a), synRas controls (n = 6) (b), wild type runners (n = 7) (c) and synRas runners (n = 5) (d). (B) Effects of physical activity on the number of the segments of immature doublecortin-labeled neurons. (C) Effects of physical activity on the length of the dendrites of immature doublecortin-labeled neurons. (* = p < 0.05; ** = p < 0.01; *** = p < 0.001 according to Mann–Whitney.
Experiment 2: Involvement of BDNF in Running-Induced Effects on Proliferation and Differentiation
In order to analyse whether BDNF was associated with the effects of running on cell proliferation, its relative hippocampal mRNA level was determined. No significant difference in the BDNF expression between the wild type and the synRas groups was detected in runners and non-runners (Figure 3). However, after 13 days of voluntary physical activity, the mRNA level of the BDNF gene was significantly increased compared to the wild type control group in the wild type runner group (2.2-fold increase, p < 0.05) and in the synRas runner group (2.6-fold increase, p < 0.05; 2.0-fold increase compared to the synRas control group, p < 0.05). An ELISA assay was performed in order to test whether the BDNF mRNA increase translated into an increase of BDNF protein. No significant difference was found between the wild type and the synRas mice in runners and non-runners (Mann–Whitney, p > 0.05). After 13 days of exercise, the level of BDNF protein was significantly increased in the wild type mice (p < 0.05) whereas in the synRas mice the increase was apparent but did not reach significance (p > 0.05).
Figure 3. Effects of physical activity on BDNF mRNA expression in wild type controls (n = 6), synRas controls (n = 5), wild type runners (n = 5), synRas runners (n = 5) (A) and protein expression (B) in wild type controls (n = 4), synRas controls (n = 4), wild type runners (n = 5), synRas runners (n = 4).
Determination of TrkB expressing cells
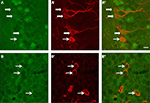
During adult neurogenesis, cells undergo successive developmental stages from a precursor cell to a mature granule cell (Kempermann et al., 2004). In order to determine whether immature neurons could react to the running-induced increase of BDNF, a double immunohistochemical staining for the BDNF receptor TrkB and doublecortin was performed on wild type sections. As shown in Figure 4, some doublecortin cells expressed the TrkB receptors. However, the expression of the TrkB receptor can occur at different stages of differentiation because it was observed on DCX-positive cells with no or short dendrites as well as on cells with complex dendritic arbors. This suggested that BDNF could act not only on very immature proliferative DCX cells but also on more differentiated neurons.
Figure 4. Expression of TrkB receptors (green) by doublecortin (red)-labeled cells of the dentate gyrus of wild type mice. (A,B) TrkB expression; (A’,B’) Doublecortin expression; (A”,B”) merge. White arrows point to doublecortin-immunoreactive cells (with or without processes) that express the TrkB receptors. The bar represents 8 μm.
Experiment 3: Effects of Running on Novelty Recognition Memory
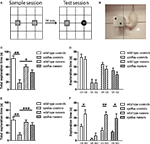
As it has been suggested a strong correlation between the level of adult neurogenesis and the behavioural performances of hippocampal-dependent tasks (Shors et al., 2001; Drapeau et al., 2003; Jessberger et al., 2009), the possible correlation between adult neurogenesis and the reaction to object substitution was examined. Indeed, in the test session (Figure 5), 10 min after the presentation of the sample objects, the wild type female animals were able to selectively explore the NO, compared to the familiar one (t-test, p < 0.05), indicating that they had recognised the object substitution. In contrast, the synRas mice with a highly reduced adult hippocampal cell proliferation and neurogenesis, did not spend more time exploring the NO compared to the familiar object (t-test, p > 0.05), suggesting that wild type mice interacted more with the NO whereas the synRas mice did not (genotype effect p < 0.01, object category effect p < 0.01). Moreover, the two-way ANOVA revealed a high genotype effect (p < 0.01) and a significant object effect (p < 0.01) but no interaction genotype × object category (p > 0.05). Simple effect analysis showed high significant difference between the wild type and the synRas mice in the exploration of the familiar object (t-test, p < 0.01) and the NO (t-test, p < 0.05). As running stimulated adult proliferation and neurogenesis, voluntary physical activity enhanced object recognition performance in wild type mice as they selectively explored the NO, compared to the familiar one (t-test, p < 0.05). Similarly, runner synRas mice were able to react to the object substitution (t-test, p < 0.05).
Figure 5. Effects of physical activity on object recognition memory. (A) Schematic representation of the experimental protocol. During the sample session, mice were exposed to two identical objects O1 and O2. During the test session, mice were exposed to a triplicate of the familiar object O3 and a novel object NO. (B) Example of a snout contact with an object. (C) Total exploration duration during the sample session. (D) Exploration duration of the specific objects during the sample session. (E) Total exploration duration for each group during the test session. (F) Duration of object exploration for each object (triplicate O3 and novel object NO) during the test session. Wild type controls (n = 10), synRas controls (n = 8), wild type runners (n = 12), synRas runners (n = 11). * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
We demonstrate here that the decrease in adult proliferation and neurogenesis and the morphological deficits in DCX-positive immature neurons observed in the synRas mouse model are reversed by voluntary physical activity. Our data support the view of an involvement of BDNF. Most interestingly, the running-induced rescue of proliferation and differentiation correlates with a rescue of recognition memory as indicated by an improvement of object exploration and discrimination.
In the synRas model, endogenously regulated Ras is coexpressed with permanently activated Ras in neurons under the direction of the synapsin-1 promoter leading to enhanced morphological differentiation (Heumann et al., 2000) and survival of mature neurons (Manns et al., in revision). This is associated with a decreased proliferation of early proliferating cells due to a non cell autonomous mechanism because neural precursor cells do not express the Ras transgene (Chakrabarty and Heumann, 2008). However, the underlying mechanisms are yet tob e elucidated.
We report here that voluntary physical activity, an ethologically relevant paradigm, reverses this strong synRas phenotype even after a relatively short exposure to a running wheel, demonstrating the plasticity if this system. The effects of voluntary physical activity on adult neurogenesis were observed in the NMRI mice. This corroborates with previous results obtained in the C57BL/6 and Hsd:ICR strains (van Praag et al., 1999) and argues for strain-independent effects of running.
The degree to which proliferation was stimulated was not different in the wild type and the synRas mice. However, the basal level of proliferation was significantly lower in the synRas mice. Yet the hippocampal level of BDNF was not significantly different which suggested that BDNF is not the cause for the generally low proliferation in synRas mice. Consequently, these data suggest that the ways in which basal proliferation is negatively regulated by differentiated neurons differ from the mechanisms by which physical activity promotes proliferation. This is in accordance with a report that suggested regulatory context-dependent mechanisms of proliferation (Kitamura et al., 2003). Indeed the basal level of adult proliferation in mice lacking the NMDA receptor ε1 subunit were as high as in the wild type mice (Kitamura et al., 2003) but physical activity failed to stimulate adult hippocampal proliferation of these mice. Besides, voluntary physical activity is not always sufficient and failed to rescue hippocampal proliferation in a mouse model of Huntington disease (Kohl et al., 2007).
Previous work has implicated BDNF in adult neurogenesis and differentiation. BDNF infusion can increase the number of newly generated neurons in the adult rat hippocampus (Scharfman et al., 2005). Indeed, the TrkB receptor is present on progenitor cells and its deficiency impairs adult neurogenesis (Bergami et al., 2008; Li et al., 2008). Donovan et al. (2008) demonstrated the receptor on cells in various stages of differentiation. Our double-labeling confirms the presence of the TrkB receptor on immature and more differentiated DCX-positive cells suggesting that BDNF can act on different stages to stimulate adult proliferation and neurogenesis.
As expected, running for 13 days increased the hippocampal BDNF mRNA in wild type and a comparable increase was seen in synRas mice. Moreover, the BDNF protein levels increased in both genotypes. The beneficial effects of physical activity could thus be mediated by BDNF which is secreted in an activity-dependend manner (Canossa et al., 2001; Lessmann et al., 2003) and could stimulate the cell division of early proliferating cells either directly as suggested by the recent TrkB deletion models (Li et al., 2008) or indirectly e.g. by promoting synaptic activity and neurotransmitter release (Chan et al., 2008). Further, a wealth of studies has implicated BDNF in dendritogenesis (Davies et al., 1986; McAllister et al., 1995, 1997) suggesting that the enhanced production of BDNF in mice of both genotypes directly explains the running-induced increase in dendritic complexity. Moreover, BDNF is important for cognition as shown at the level of synaptic transmission and plasticity (Schuman, 1999; Lu, 2003) and higher functions in animals (Mizuno et al., 2000; Monteggia et al., 2004), and humans carrying mutations causing an impairment of activity-dependent BDNF secretion (Egan et al., 2003). The enhanced production of BDNF thus correlates well with the clear improvement of wild type and synRas mice in the behavioral task employed in the present study.
Here we could observe a rescue not only of the reduced adult hippocampal neurogenesis but also of impaired cognitive functions. The relationship between neurogenesis and object recognition has recently been shown in rats (Jessberger et al., 2009). This test relies on the integrity of the hippocampus and specifically of the connectivity between the dentate gyrus and the CA3. Indeed, novelty recognition memory is assimilated to an episodic-like memory (Kesner, 2007). For unknown reasons, the synRas mice show a general lack of interest for the objects and a reduced exploration in the sample and test sessions. It has recently been shown that the TrkB signalling cascade is involved in the modulation of anxiety levels: genetic deletion of this receptor in the dentate gyrus leads to a decrease in exploratory behaviour in a novel environment and in the elevated plus maze (Bergami et al., 2008). Therefore, a possible explanation could have been that the synRas mice show higher levels of anxiety in control conditions. This was however not the case in our system as no difference in any of the anxiety tests (open-field, elevated plus maze and dark–light box) were observed (data not shown). This low exploration level did not allow mice to properly encode information about the objects and to later discriminate them. However, when the synRas mice were exposed to a running wheel for 13 days, there was a dramatical increase of this ability. The present data not only show that the wild type running mice performed better but also that the running synRas mice could detect and react to the object substitution after exercising.
Chronic forced exercise could restore interferon-alpha-induced learning deficits in NO recognition (Fahey et al., 2008). Here we demonstrate that voluntary physical activity enhances object recognition memory in unlesioned synRas mice, suggesting that in some cases, exercise can be a way to recover from genetically-induced impaired behavioural abilities. It has already been proposed that hippocampal-dependent task performance predicts hippocampal neurogenesis (Shors et al., 2001; Drapeau et al., 2003) or that there is a reciprocal action of learning and adult neurogenesis (van Praag et al., 1999; Cao et al., 2004; Snyder et al., 2005; Olson et al., 2006; Dupret et al., 2008). Running has been shown to improve performance in other behavioural tasks (van Praag et al., 1999; Binder et al., 2004) and exercise-induced neurogenesis was associated with spatial memory recovery in the Morris water maze in a stroke model (Luo et al., 2007). The present data complement the idea that there is an important correlation between adult neurogenesis and episodic-like memory. A possible explanation would be that exercise would induce long-term potentiation (LTP) in hippocampal neurons leading to better performance in recognition memory tasks and stimulating the proliferation and differentiation of newly generated neurons. Indeed, exercise has been shown to induce LTP, a cellular correlate of learning and memory (Vasuta et al., 2007). Moreover, recognition memory and LTP are intimately linked (Wang et al., 2004; Hennigan et al., 2008).
A number of environmental stimuli such as social stress or long-term light deprivation or normal ageing may lead to a downregulation of neurogenesis and cognitive functions. Using a genetically modified mouse model exhibiting a BDNF-independent downregulation of hippocampal neurogenesis with genetically induced deficiencies in cognition we demonstrate voluntary physical activity has a robust beneficial effect that could also be beneficial in therapies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the DFG Graduate Programme 736 and the GK “Development and Plasticity of the Nervous System” for their financial support and Dr. Martina Manns for her technical expertise and advice.
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., Zaitsev, E., Gold, B., Goldman, D., Dean, M., Lu, B., and Weinberger, D. R. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269.
Hernández-Rabaza, V., Llorens-Martín, M., Velázquez-Sánchez, C., Ferragud, A., Arcusa, A., Gumus, H. G., Gómez-Pinedo, U., Pérez-Villalba, A., Roselló, J., Trejo, J. L., Barcia, J. A., and Canales, J. J. (2009). Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience 159, 59–68.
Heumann, R., Goemans, C., Bartsch, D., Lingenhohl, K., Waldmeier, P. C., Hengerer, B., Allegrini, P. R., Schellander, K., Wagner, E. F., Arendt, T., Kamdem, R. H., Obst-Pernberg, K., Narz, F., Wahle, P. and Berns, H. (2000). Transgenic activation of Ras in neurons promotes hypertrophy and protects from lesion-induced degeneration. J. Cell Biol. 151, 1537–1548.