Department of Psychiatry and Behavioral Sciences, Stanford University Stanford, CA, USA
Stressful experiences that are challenging but not overwhelming appear to promote the development of arousal regulation and resilience. Variously described in studies of humans as inoculating, steeling, or toughening, the notion that coping with early life stress enhances arousal regulation and resilience is further supported by longitudinal studies of squirrel monkey development. Exposure to early life stress inoculation diminishes subsequent indications of anxiety, increases exploration of novel situations, and decreases stress-levels of cortisol compared to age-matched monkeys raised in undisturbed social groups. Stress inoculation also enhances prefrontal-dependent cognitive control of behavior and increases ventromedial prefrontal cortical volumes. Larger volumes do not reflect increased cortical thickness but instead represent surface area expansion of ventromedial prefrontal cortex. Expansion of ventromedial prefrontal cortex coincides with increased white matter myelination inferred from diffusion tensor magnetic resonance imaging. These findings suggest that early life stress inoculation triggers developmental cascades across multiple domains of adaptive functioning. Prefrontal myelination and cortical expansion induced by the process of coping with stress support broad and enduring trait-like transformations in cognitive, motivational, and emotional aspects of behavior. Implications for programs designed to promote resilience in humans are discussed.
Adults often cope better with spousal loss, illness, and major accidents if they have previously experienced and coped with stress in childhood (Khoshaba and Maddi, 1999
). Work-related stress likewise has fewer deleterious mental health effects in adults previously exposed to work stress in adolescence (Mortimer and Staff, 2004
). These and related physiological investigations of children suggest that the process of coping with moderate levels of early life stress promotes the development of arousal regulation and resilience (Ellis et al., 2005
; Gunnar et al., 2009
). Variously described in studies of humans as inoculating, steeling, or toughening (Garmezy et al., 1984
; Dienstbier, 1989
; Rutter, 2006
), the notion that coping with stress enhances arousal regulation and resilience is further supported by longitudinal studies of squirrel monkey development.
In natural and semi-natural conditions, squirrel monkey mothers and other group members periodically leave newly weaned offspring beginning at 3–6 months of age to forage for food on their own (Boinski and Fragaszy, 1989
; Lyons et al., 1998
). At this stage of development, offspring are approximately half their adult body size.Initially, brief intermittent separations studied in controlled experimental conditions elicit distress peep-calls and increase plasma levels of cortisol with partial habituation of these measures of arousal observed over repeated separations (Coe et al., 1983
; Hennessy, 1986
). Later in life, monkeys exposed to repeated intermittent separations show fewer behavioral indications of anxiety, diminished stress-levels of cortisol, and increased sensitivity to glucocorticoid feedback regulation of the hypothalamic–pituitary–adrenal (HPA) axis compared to monkeys not exposed to prior separations (Lyons et al., 1999
; Lyons et al., 2000b
, Levine and Mody, 2003
;). These findings are surprising because chronic exposure to severe forms of stress impairs regulation of the HPA axis and increases anxiety in monkeys (Sanchez et al., 2001
; O’Connor and Cameron, 2006
) whereas intermittent separations that simulate a naturally occurring but stressful condition appear to have opposite effects.
Here we review studies that test the hypothesis that brief intermittent separations are a form of stress inoculation that enhances arousal regulation and resilience. Socially housed squirrel monkeys were randomized to either brief intermittent separations or a non-separated control condition at 17 weeks of age (Parker et al., 2004
). For each of 10 total separation sessions, each monkey was removed from the natal group for a 1-h period once a week. In the non-separated control condition, age-matched monkeys remained undisturbed in their natal groups. After completion of these postnatal protocols at 27 weeks of age, all of the monkeys were maintained in identical conditions. Puberty occurs at 2–3 years of age and the average maximum life span is ∼21 years (Brady, 2000
). Behavioral, hormonal, and neuroimaging data were collected at various stages of development as described below. All procedures were conducted in accordance with the NIH Guidelines and were approved by Stanford University’s IACUC.
At 9 months of age, each monkey was placed along with its mother in a novel test environment that contained an assortment of foods and various toy-like objects (Parker et al., 2004
). Similar test conditions have been used to demonstrate that severe forms of early life stress increase subsequent indications of anxiety in marmoset monkeys and macaques (Hinde and Spencer-Booth, 1971
; Andrews and Rosenblum, 1993
; Dettling et al., 2002
). Here the novel environment test was used to determine whether intermittent separations enhance arousal regulation during forced exposure to an unfamiliar situation.
In the novel environment test, the previously separated and non-separated monkeys were initially similar but differences emerged over repeated test sessions. Gradually, the previously separated monkeys showed fewer signs of anxiety inferred from decreased maternal clinging, increased object exploration, and lower post-test cortisol levels compared to monkeys not exposed to early intermittent separations. As described elsewhere in greater detail (Parker et al., 2004
), these results support the suggestion that intermittent separations represent a form of stress inoculation that enhances arousal regulation during forced exposure to a novel situation.
At 1.5 years of age, all monkeys were administered a behavioral test of cognitive control that specifically focused on response inhibition and flexible goal-directed action (Parker et al., 2005
). Monkeys were required to inhibit a previously rewarded reaching response in order to efficiently retrieve food treats from a plastic box. In marmoset monkeys and macaques, performance on this test is impaired by lesions of the prefrontal cortex (Diamond, 1990
; Dias et al., 1996
; Wallis et al., 2001
) but not lesions of the hippocampus (Diamond et al., 1989
). Test performance is also impaired in a marmoset model of parental neglect (Pryce et al., 2004
) and in squirrel monkeys chronically treated with high doses of cortisol (Lyons et al., 2000a
). Here we used this test to assess whether intermittent separations improve cognitive control of behavior in the context of changing task-related demands.
The ability to inhibit a previously rewarded behavioral response and flexibly adapt to changing demands gradually improved over repeated test trials. At peak levels of performance, however, all of the previously separated monkeys successfully completed all test trials whereas fewer than half of the monkeys not exposed to intermittent separations achieved similar levels of success (Parker et al., 2005
). These and related findings suggest that intermittent separations are a form of stress inoculation that enhances cognitive control of behavior in situations that require flexibility and response inhibition.
At 2.5 years of age, all monkeys were administered a test to examine curiosity in a stress-free condition (Parker et al., 2007
). Each monkey was provided free access to explore a familiar or novel toy-like object secured to the wall of a compartment affixed to the monkey’s home cage. Similar test conditions have been used to demonstrate that severe forms of early life stress diminish subsequent exploratory tendencies in macaques (Hinde and Spencer-Booth, 1971
; Sackett, 1972
; Roder et al., 1989
). Here exploration of novelty was used to assess rearing-related differences in curiosity.
All previously separated and all but one of the non-separated monkeys approached the compartment and subsequently peered inside. More of the previously separated monkeys left their home cage to enter the compartment, and they entered faster, with greater frequency, and spent more time inside the compartment compared to non-separated monkeys. Inside the compartment, more previously separated than non-separated monkeys explored one or both objects, and the previously separated monkeys showed a significant preference for the novel versus familiar object (Parker et al., 2007
).
In the initial novel environment test conducted at 9 months of age, significantly more object exploration was observed in the previously separated compared to non-separated monkeys. Object exploration at 9 months of age was assessed in stressful conditions that involved involuntary removal from the home cage and forced exposure to an unfamiliar situation. In keeping with results from similar studies of forced exposure to novelty in rodents (Hughes, 2007
), both previously separated and non-separated monkeys had significantly higher cortisol levels after forced exposure to the novel test environment compared to baseline levels measured in undisturbed home cage conditions (Parker et al., 2004
). Moreover, post-test cortisol levels were significantly higher in the non-separated compared to the previously separated monkeys so that differences in object exploration were confounded with physiological indications of anxiety.
In the study conducted at 2.5 years of age, exploratory behavior was assessed by providing free access to a novel test compartment that was connected to the home cage. To further reduce the potentially stressful nature of the test situation, all monkeys were acclimated to the test procedures prior to the start of the study. Post-test cortisol levels were not significantly different from baseline levels measured in undisturbed home cage conditions at 2.5 years of age (Parker et al., 2007
). Moreover, neither baseline nor post-test cortisol levels correlated with exploratory behavior in the stress-free test condition at 2.5 years of age. Individual differences previously observed in object exploration at 9 months of age were, however, correlated with differences in test compartment entries and total time spent inside the compartment at 2.5 years of age. Individual differences previously observed in cognitive control at 1.5 years of age also correlated with test compartment entries and total time spent inside the compartment at 2.5 years of age (Lyons and Parker, 2007
).
Taken together these findings suggest that brief intermittent separations that simulate a naturally occurring but stressful condition promote enduring trait-like transformations in multiple domains of adaptive functioning. Stress inoculation-induced arousal regulation enhances curiosity inferred from increased exploration of novel situations (Parker et al., 2004
). Curiosity reflects more than diminished anxiety because the previously separated monkeys are more curious than non-separated monkeys even in the absence of physiological indications of anxiety (Parker et al., 2007
). Curiosity is not an indication of impulsivity because response inhibition is greater in monkeys exposed to intermittent separations compared to non-separated controls (Parker et al., 2005
). Curiosity motivates individuals to seek new opportunities for action (Silvia, 2008
). Engagement in new situations that entail challenging but not overwhelmingly stressful experiences have cascading effects that promote further adaptations in cognitive, motivational, and socioemotional aspects of behavior (Mason, 1971
; Gibson, 1988
).
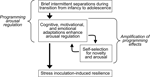
Based on these considerations we believe that the process of coping with early life stress enhances arousal regulation which promotes curiosity and thereby amplifies the effects induced by coping with early life stress (Figure 1
). The initial step of “programming” arousal regulation in response to early life stress may be adaptive in adolescence and adulthood when animals reside in environments that are similar to the one in which they are born (Cameron et al., 2005
). Programming effects are not necessarily fixed, however, as subsequent cascades linking multiple domains of behavior across life span development may amplify (or diminish) the initial effects induced by the process of coping with early life stress. Cascade effects in life span models of development are increasingly viewed as important in humans (Schaffer, 2000
; Burt et al., 2008
) but are seldom considered in animal behavior research (O’Connor and Cameron, 2006
).
Figure 1. Developmental cascade model of stress inoculation-induced resilience. Coping with brief intermittent separations that simulate a naturally occurring but stressful condition enhances arousal regulation which promotes curiosity and thereby amplifies the initial effects induced by coping with stress.
Arousal regulation, cognitive control, and curiosity are mediated, in part, by prefrontal cortical brain regions in human and nonhuman primates (Price, 2005
; Barbas and Zikopoulos, 2007
). Therefore, we used non-invasive neuroimaging to examine prefrontal development at 3.3 years of age (Katz et al., 2009
). In keeping with previous observations (Lyons et al., 2002
) we found that monkeys exposed to intermittent separations had larger ventromedial but not larger dorsolateral prefrontal cortical volumes compared to monkeys not exposed to prior intermittent separations. Larger volumes do not reflect increased cortical thickness but instead represent surface area expansion of ventromedial prefrontal cortex (Katz et al., 2009
).
These findings are of interest because ventromedial prefrontal cortical size in humans predicts diminished impulsivity (Matsuo et al., 2009
), lower harm avoidance (Yamasue et al., 2008
), and greater retention of learned extinction of fear (Milad et al., 2005
). Recent neuroimaging studies of humans support results from animal research confirming that learned extinction of fear is mediated by prefrontal downregulation of arousal via inhibitory connections that diminish neural output from the amygdala (Delgado et al., 2008
; Quirk and Mueller, 2008
). Additional evidence likewise suggests that differences in the balance between top-down prefrontal regulation and arousal-inducing amygdala activation may account for trait-like differences in successfully coping with stress (Bishop, 2007
; Drabant et al., 2009
).
Despite evidence that coping with stress depends on myelinated prefrontal cortical and subcortical (e.g., amygdala)interconnections, myelination is not often included in discussions of neural plasticity as a mechanism for experience-dependent arousal regulation and resilience. In the study described above we used diffusion tensor imaging to examine prefrontal myelination in terms of fractional anisotropy at 3.3 years of age (Katz et al., 2009
). Increased fractional anisotropy occurs when tissue microstructure constrains water proton diffusion directionality as exemplified by myelination of axons in white matter. Myelination increases nerve conduction velocities and facilitates synchronous firing of neurons by reducing travel distance effects in distributed networks (Fields, 2008
). Coordination of firing inputs to maximize temporal summation at postsynaptic neurons is the foundation for a key concept in neuroplasticity and development – neurons that fire together, wire together. In children and adolescents, myelination of prefrontal interconnections determined by fractional anisotropy increases with age (Barnea-Goraly et al., 2005
) and maturation of prefrontal-dependent functions (Casey et al., 2007
).
In monkeys exposure to intermittent separations increases ventromedial but not dorsolateral prefrontal white matter measures of fractional anisotropy (Katz et al., 2009
). This difference coincides with the difference in ventromedial and not dorsolateral prefrontal cortical volumes measured in the same sample of monkeys. White matter measures of fractional anisotropy in ventromedial but not dorsolateral regions correlate with their respective volumes of prefrontal cortex (Katz et al., 2009
). Taken together these findings suggest that the process of coping with early life stress increases prefrontal myelination and expands a region of cortex that broadly controls arousal regulation and resilience.
The effect size for these neuroadaptations is small but similar in magnitude to that observed for environmental enrichment in marmoset monkeys (Kozorovitskiy et al., 2005
) and rats (Rosenzweig, 2003
). Enrichment entails repeated exposure to novel inanimate and/or social stimulation (Fox et al., 2006
) and appears to elicit neuroendocrine indications of mild stress in rats (Moncek et al., 2004
). After exposure to enrichment, however, rats show diminished indications of anxiety, increased exploration, and enhanced prefrontal-dependent learning compared to non-enriched controls (Schrijver et al., 2004
; Fox et al., 2006
). This evidence from rodents combined with our primate findings suggest that enrichment effects may be mediated, in part, by the process of coping with stress.
Brief intermittent exposure to foot shock during infancy enhances arousal regulation and resilience in rats (Levine, 1962
). Evidently, this outcome reflects the effects of increased maternal stimulation received after foot shock when rat pups are returned to the nest (Smotherman and Bell, 1980
). That increased maternal stimulation enhances arousal regulation is supported by evidence that licking, grooming, and arched-back nursing correlate inversely with HPA axis activation in pups studied as adults (Cameron et al., 2005
).
To determine whether separation effects in monkeys are maternally mediated, maternal behavior and cortisol levels were examined in monkeys randomized to three postnatal conditions (Parker et al., 2006
). In one condition, each monkey was separated from its mother and the natal group for 10 weekly sessions that each lasted 1 h in duration. In the second condition, each monkey and its mother were removed together as a pair and separated from the natal group for 10 weekly 1 h sessions. Both of these social separation conditions elicit locomotor agitation, distress vocalizations, and HPA axis activation with baseline measures of arousal restored soon after reunion with members of the natal group (Coe et al., 1983
; Jordan et al., 1985
). In the third condition, non-separated monkeys were raised in undisturbed groups.
Rearing-related differences in maternal behavior did not correspond with differences in the development of arousal regulation. Monkeys exposed along with their mother to intermittent separations received less maternal care in the home cage, yet both intermittent separation conditions enhanced arousal regulation inferred from diminished stress-levels of cortisol compared to the levels measured in monkeys raised in undisturbed groups (Parker et al., 2006
). These and related findings suggest that changes in arousal regulation more closely correspond to stress exposure than to separation-induced changes in maternal care. Similar conclusions have been reported in recent investigations of early life stress and developmental plasticity in rats (Macri and Wurbel, 2006
; Tang et al., 2006
).
Controlled exposure to stress-related cues is a key feature of resiliency training for people that work in conditions where performance in the face of adversity is required, e.g., medical and military personnel, aviators, police, firefighters, and rescue workers (Meichenbaum, 2007
; Stetz et al., 2007
). A similar process also occurs during cognitive behavior exposure therapy for stress-induced psychopathology. Patients are taught to imagine a graded series of stressful situations relevant to their particular condition, and then to interact in vivo with these relevant stressful situations. Repeated exposure to relevant stressors is thought to activate cognitive and emotional processing within and between exposure sessions, and thereby modify erroneous conditions that underlie the disorder (Foa and Kozak, 1986
). A critical feature of this approach is the idea that simultaneous cognitive and emotional processing is required for intervention-based changes in behavior to occur (De Raedt, 2006
).
Although exposure therapy for patients and resiliency training for healthy humans are administered by professional psychologists and psychiatrists, results from our studies of monkeys support Epstein’s (1983
) suggestion that these interventions build on a process that commonly occurs spontaneously without formal guidance. A better understanding of this process may help to enhance interventions designed to improve human mental health.
Consider, for example, the role of habituation to stressors in exposure therapy. Within-session habituation is thought to reflect the cognitive and emotional processing required to achieve adaptive long-term changes in behavior (Foa and Kozak, 1986
). As a consequence, the duration of each session has generally ranged from 45 to 120 min. Recently, however, a trial of shorter 30-min exposure sessions has raised the possibility that between- and not within-session habituation effectively reduces anxiety (van Minnen and Foa, 2006
). Animal studies designed to compare within- and between-session habituation as predictors of stress inoculation effects may provide relevant and clinically useful information. A better understanding of this issue is needed because of concerns that prolonged exposure sessions may cause patient dropout from therapy and impede recovery (Hembree et al., 2003
).
Further research is likewise needed to determine whether medications aid or interfere with recovery from stress-induced psychopathology. Benzodiazapines have been prescribed to immediately reduce anxiety in humans after exposure to severe forms of stress, but placebo controlled studies have failed to demonstrate short- or long-term efficacy (Braun et al., 1990
; Gelpin et al., 1996
). In some studies, early administration of benzodiazapines have actually been associated with less favorable outcomes (Braun et al., 1990
; Mellman et al., 2002
). If simultaneous cognitive and emotional processing of stressors is required to achieve long-term adaptations, then drugs that interfere with these processes may impede or prevent recovery. In animal models, benzodiazepines impair learned extinction of conditioned fear (Bouton et al., 1990
) and similar studies are needed to determine whether benzodiazapines interfer with the development of stress inoculation-induced resilience.
In this review, we have discussed aspects of stress neurobiology that warrant greater attention in behavioral neuroscience research. Although stress is commonly viewed as having deleterious effects, the process of coping with stress is also considered a potential mediating mechanism for the positive effects induced in laboratory rodents by environmental enrichment (Fox et al., 2006
). Controlled exposure to stress-related cues is a key feature of resiliency training for healthy humans (Meichenbaum, 2007
; Stetz et al., 2007
) and is exploited in therapeutic interventions for patients with mood and anxiety disorders (Foa and Kozak, 1986
; De Raedt, 2006
). In monkeys, myelination and prefrontal cortical expansion induced by coping with brief intermittent separations that simulate a naturally occurring but stressful experience supports transformations in multiple domains of behavior and physiology (Parker et al., 2004
, 2005
, 2007
; Lyons et al., 1999
; Levine and Mody, 2003
; Katz et al., 2009
). Stress is an inevitable aspect of life that cannot be eliminated or avoided for very long. To consider stress solely as inherently destructive overlooks its broader role in shaping adaptive outcomes in the course of life span development.
The research reviewed in this report was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supported by Public Health Service grants MH47573, MH66537, MH77884, and DA16902.
Casey, B. J., Epstein, J. N., Buhle, J., Liston, C., Davidson, M. C., Tonev, S. T., Spicer, J., Niogi, S., Millner, A. J., Reiss, A., Garrett, A., Hinshaw, S. P., Greenhill, L. L., Shafritz, K. M., Vitolo, A., Kotler, L. A., Jarrett, M. A., and Glover, G. (2007). Frontostriatal connectivity and its role in cognitive control in parent–child dyads with ADHD. Am. J. Psychiatry 164, 1729–1736.