- 1Hennepin Healthcare Research Institute, Minneapolis, MN, United States
- 2Department of Medicine, University of Minnesota, Minneapolis, MN, United States
- 3Department of Psychology, University of Minnesota, Minneapolis, MN, United States
- 4Department of Psychology, Arizona State University, Tempe, AZ, United States
Introduction: Sex differences in vulnerability to opioid use disorder (OUD) have been reported in some clinical and preclinical studies, but findings are mixed and further research is needed in this area. The goal of this study was to compare elasticity of demand (reinforcement efficacy) in an i.v. morphine self-administration (SA) model in male and female rats using a translationally relevant behavioral economics approach. Rate of acquisition and predictors of individual differences in demand (e.g., cumulative morphine infusions during acquisition) were also evaluated in both sexes.
Materials, methods, and results: Acquisition of morphine SA (0.4 mg/kg/infusion) under a fixed ratio (FR) 1 schedule of reinforcement was slower and infusions earned were lower in females than in males (n = 30–31/sex), but infusions earned did not differ between sexes during the FR 2 and FR 3 phases of acquisition. Increases in the FR response requirement across sessions during demand testing (FR 1–FR 96) resulted in a progressive reduction in morphine infusions in both sexes. Morphine consumption was well-described by an exponential demand function in both sexes and was associated with considerable individual vulnerability. There were no sex differences in elasticity of demand (rate of decline in morphine consumption with increasing price) or intensity of demand (consumption at zero price). A higher number of infusions earned during the FR 2 and FR 3 phases of acquisition and greater maximum response rates during demand testing were associated with lower demand elasticity (i.e., greater reinforcing efficacy) in both males and females, whereas other relationships were sex-specific (e.g., higher intensity of demand was associated with lower elasticity of demand in males but not in females).
Conclusion: Our findings indicate similar elasticity of demand and predictors of individual differences in demand for morphine in male and female rats, although sex differences were observed in initial rate of acquisition and in some correlations between morphine SA measures. These data are consistent with findings of similar OUD vulnerability in males and females in some human and animal studies.
Introduction
Nearly 100,000 Americans died of opioid overdose in 2022 (Spencer et al., 2024), and the opioid crisis has been declared a nationwide Public Health Emergency. Despite the devastating impact of opioids, only a minority of individuals who experiment with opioids develop opioid use disorder (OUD). Understanding factors contributing to individual differences in vulnerability to OUD may be useful for developing more effective preventions and treatments.
Sex can be an important contributor to OUD vulnerability, but the literature is mixed on whether males or females are more vulnerable. On one hand, opioids produce greater positive subjective effects (e.g., drug liking) and weaker negative subjective effects (e.g., nausea, dizziness) in men compared to women (Fillingim et al., 2005; Lopes et al., 2021; Comer et al., 2010), and prevalence of OUD and overdose has historically been higher in men (McHugh et al., 2021; Marsh et al., 2018; Huang et al., 2006; Back et al., 2010). However, the gap in OUD prevalence between sexes has been narrowing in recent years, with the misuse of several opioids (e.g., oxycodone) increasing at a greater rate in females than in males (McHugh et al., 2021; Jones et al., 2015). In addition, as has been reported for other drugs of abuse (e.g., alcohol) (Piazza et al., 1989; Towers et al., 2023), women can progress from initial opioid misuse to OUD faster than men (i.e., “telescoping”) (Towers et al., 2023; Hernandez-Avila et al., 2004; Back et al., 2011). Some studies have also reported greater opioid craving or relapse in women than men (Back et al., 2011; Maehira et al., 2013), although other studies have reported opposite effects (Nicolas et al., 2022; Gordon et al., 2017; Darke et al., 2007) or no sex differences (Nicolas et al., 2022; Kamal et al., 2007; Kennedy et al., 2013) in these outcomes.
Animal models provide several advantages over human studies (e.g., ability to evaluate the OUD-related effects of opioids in the absence of comorbidities or use of other drugs) and may be useful for better understanding the role of sex in OUD vulnerability. A number of studies have reported greater vulnerability of females than males to the reinforcing effects of opioids using i.v. self-administration (SA) models (e.g., Cicero et al., 2003; Lynch and Carroll, 1999; Smethells et al., 2020; Guha et al., 2022; Towers et al., 2022), which have considerable translational potential because they involve voluntary opioid taking as occurs in humans (Swain et al., 2021). However, some preclinical opioid SA studies have found greater vulnerability in males under at least some conditions (Mavrikaki et al., 2017; Townsend et al., 2019), whereas others have found no sex differences for most or all opioid SA outcomes studied (e.g., Mavrikaki et al., 2017; Stewart et al., 1996; Venniro et al., 2017; Fredriksson et al., 2020). Factors that may contribute to these differences across studies include the opioid used (e.g., morphine versus oxycodone), the phase of OUD modeled (e.g., acquisition versus relapse), or other procedural variables (e.g., schedule of reinforcement). Regardless, these preclinical data parallel the mixed findings regarding sex differences in OUD vulnerability in humans, and emphasize the need for more work in this area.
Behavioral economics, which applies classic economic principles to the experimental analysis of behavior, provides a translationally relevant framework for evaluating the role of sex in OUD vulnerability. Behavioral economics quantifies the change in the consumption of a reinforcer (e.g., opioid) as a function of its unit price, which in drug SA models is operationalized as the cost-benefit ratio of response requirement/unit dose (Hursh, 1991; Bickel et al., 2000; Hursh and Silberberg, 2008). A more rapid decrease in consumption following increases in unit price (greater elasticity of demand) indicates lower abuse liability, while a slower decrease (lower elasticity of demand) indicates greater abuse liability (Hursh, 1991; Bickel et al., 2000; Hursh and Silberberg, 2008). Behavioral economics has served as a sensitive measure of the reinforcing efficacy of opioids and other drugs in both humans and animals (e.g., Aston et al., 2017; Mackillop et al., 2009; Pickover et al., 2016; LeSage et al., 2016; Bentzley et al., 2014), and has been used to study sex differences in the efficacy of several reinforcers including nicotine, cocaine, and food in rodents (Grebenstein et al., 2013; Kohtz et al., 2022; Freeman et al., 2021). However, behavioral economics has been used to only a limited extent to examine sex differences in the preclinical opioid literature, with one study (Townsend et al., 2019) reporting lower elasticity of demand for fentanyl in females and another (Lacy et al., 2020) reporting no overall sex difference in demand for remifentanil.
The goal of this study was to compare elasticity of demand for morphine (0.4 mg/kg/infusion) in an i.v. SA model in male and female rats. We used morphine because it is the prototypical opioid and the primary active metabolite of heroin, and because sex differences have been reported on some measures of morphine SA (e.g., acquisition) (Cicero et al., 2003; Mayberry et al., 2022). Rate of acquisition of morphine SA, predictors of individual differences in demand (e.g., cumulative morphine infusions during acquisition), and relationships between different behavioral economic outcomes (e.g., α and Q0, see below) were also compared across sexes. Such correlational analyses were of interest because sex differences in correlations between measures of opioid SA can be detected even in the absence of sex differences in the SA measures themselves (Guha et al., 2022). We used larger group sizes than are typically used in preclinical studies to facilitate detection of both individual and sex differences in these outcomes, as well as to avoid spurious outcomes that can occur with smaller sample sizes (Szucs and Ioannidis, 2020). Finally, opioid SA can reduce body weight (Chen et al., 2006; Le et al., 2014), a putative marker of physical dependence (Chen et al., 2006). Effects of morphine SA on body weight in both sexes were therefore also examined.
Methods
Animals
Experimentally naïve male and female adult Sprague Dawley rats (Inotiv, West Lafayette, IN) weighing 275–300 g (male) or 200–250 g (female) at arrival were used. All rats were individually housed in a temperature- and humidity-controlled colony room with unlimited access to water under a reversed 12-h light/dark cycle. All behavioral testing occurred during the dark (active) phase. Beginning 1 week following arrival, food was restricted to 16 (females) or 18 (males) g/day to facilitate operant performance, avoid detrimental health effects of long-term ad libitum feeding, and limit catheter migration. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Hennepin Health Research Institute in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Drugs
Morphine sulfate (NIH National Institute on Drug Abuse Drug Supply Program, Bethesda, MD) was dissolved in sterile saline and heparin (30 units/mL) was added to maintain catheter patency. The morphine solution was sterile-filtered and its pH was adjusted to 7.4 prior to use. Morphine doses are expressed as the weight of the salt.
Apparatus
Self-administration (SA) sessions were conducted using standard operant conditioning chambers (model ENV-007, Med Associates, Inc.). Each chamber contained two response levers, a green light emitting diode (LED) cue light located 2 cm above each lever, and a house light that provided ambient illumination. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. An infusion pump (model PHM-100-15, Med Associates) placed outside each cubicle delivered infusions in a volume of 0.1 mL/kg over approximately 1 s. MED-PC IV or V software (Med Associates) was used for operating the experimental apparatus and recording data.
Surgery
Each rat was implanted with a chronic indwelling catheter into the right jugular vein under isoflurane (1–3%) anesthesia, using general surgical procedures described in detail elsewhere (Harris et al., 2008; LeSage et al., 2002). The catheter was externalized between the scapulae and attached to either a vascular-access harness (VAH95AB, Instech Laboratories, Plymouth Meeting, PA) or an indwelling vascular-access button (VABR2B, Instech Laboratories). In either case, the catheter was connected mid-scapulae to a tether that ran through a fluid swivel before connecting to the drug pump. Immediately following surgery, rats were administered extended-release meloxicam (4 mg/kg, s.c.) for analgesia. Animals were allowed to recover for 1 week after surgery. During the first 3 days of recovery, they received daily i.v. infusions of heparinized saline and ceftriaxone antibiotic (5.25 mg). Infusions of methohexital (0.1 mL, 10 mg/mL, i.v.) were administered to check catheter patency post-session on Fridays. If a catheter became occluded (indicated by a failure of the animal to exhibit anesthesia within 3–5 s after methohexital infusion), another catheter was implanted into a femoral vein. If that catheter failed, a third catheter was implanted into the contralateral femoral vein. Failure of the third catheter resulted in removal of the animal from the study.
Protocol
Beginning ≈7 days after catheter implantation, rats (n = 61, 30 males, 31 females) were allowed to respond for i.v. infusions of morphine sulfate (0.4 mg/kg/infusion) during daily 2-h sessions conducted 7 days per week using our standard apparatus and procedures (Swain et al., 2020; Swain et al., 2018). This unit dose and access duration support reliable morphine SA without inducing the self-mutilation that can occur with higher unit doses and longer sessions (Swain et al., 2018). Responding on the “active” response lever resulted in an i.v. infusion of morphine accompanied by offset of the cue light above the active response lever. Following a 5-s timeout, the cue light above the active lever was illuminated to signal availability of the next infusion. Responses on the other (“inactive”) response lever were recorded but had no programmed consequences. All rats were weighed immediately prior to each daily SA session throughout the protocol. On the first day of morphine SA, food powder was placed on the active lever to facilitate contact with the drug contingency. Data from this session were not included in the data analysis. Rats were tested under a fixed ratio (FR) 1 schedule for at least 10 sessions and until acquisition criteria were met (≥5 infusions per session and a ≥2:1 response ratio on the active versus inactive lever for at least 5 sessions, with no apparent trend), at which point the FR response requirement was increased to FR 2 for at least 5 sessions and until the same criteria were again met. The FR was then increased to FR 3 for at least 10 sessions and until acquisition criteria were met (same criteria as at FR 1 and FR 2, as well as a coefficient of variation ≤20% across 5 sessions). To measure demand, the FR requirement was increased each day as follows: FR 1, 2, 3, 6, 12, 24, 48, 96. We increased unit price across sessions rather than within each session to account for morphine’s relatively long elimination half-life compared to other opioids (e.g., fentanyl, remifentanil) (e.g., Mullis et al., 1979; Hug and Murphy, 1981). Within-session approaches for increasing unit price are best-suited for drugs with relatively short half-lives, which minimizes effects of prior drug infusions on responding for unit prices later in the session (Lenoir and Ahmed, 2008; Oleson et al., 2011). We found that morphine consumption under this protocol in males at a lower unit dose (0.2 mg/kg/infusion) was well described by the current exponential demand function (Swain et al., 2020; Swain et al., 2018). A negative control group (n = 38, 20 males, 18 females) was allowed to respond for i.v. infusions of saline. Because rats do not reliably self-administer saline, increases in FR during “acquisition” for these animals were not based on SA performance. Rather, they occurred on the same day as for a control-paired rat of the same sex from the morphine SA group that began the protocol at a similar time. Rats in the saline group did not acquire stable SA under the FR 3 schedule and therefore were not tested for demand.
Statistics
Infusions earned during each session during the first 10 acquisition sessions under the FR 1 schedule, the first 5 sessions at FR 2, the first 5 sessions at FR 3, and the final 5 sessions at FR 3 prior to demand testing were compared using separate 3-factor ANOVAs with group (i.e., morphine or saline) and sex as between-subject factors and session as a within-subject factor, followed as appropriate by Bonferroni post hoc tests. Active and inactive lever presses during acquisition were analyzed separately in the same manner as a secondary outcome. The number of sessions needed to acquire morphine SA under the FR 1 schedule as defined above were log-transformed because they were not normally distributed and subsequently compared between sexes using an independent samples t-test with Welch’s correction to account for unequal variances. Average body weights (in g) during the four acquisition phases described above (first 10 sessions at FR 1, etc.) were analyzed using separate 2-factor ANOVAs with group and sex as between-subject factors, followed when appropriate by Bonferonni post hoc tests comparing the morphine and saline group for each sex. Degrees of freedom for all ANOVAs were adjusted using the Greenhouse–Geisser correction to account for possible violations of sphericity. Data for animals lost to attrition during acquisition (see below) were included in analyses for those acquisition phases that they successfully completed.
Infusions at each FR during demand testing in the morphine SA group were compared using a two-factor ANOVA with sex as a between subject factor and FR requirement as a within-subject factor, followed by Dunnett’s post hoc tests comparing infusions at FR 1 to those at subsequent FRs. Total active and inactive lever responses at each FR during demand testing were analyzed in the same manner, except that lever (active versus inactive) was included as an additional within-subject variable. To determine elasticity of demand (reinforcing efficacy) during FR escalation, an exponential demand curve analysis was conducted using the following equation:
In this model, the quantity consumed (Q) of a reinforcer is plotted as a function of its unit price (FR/unit dose). The free parameters, Q0 and α are estimated from the best-fit function and refer to the theoretical maximum level of consumption at zero price (i.e., level or “intensity” of demand) and the rate of change in consumption with increases in unit price (elasticity of demand), respectively. The k parameter is a constant specifying the range of consumption in log units (2.8 in the current dataset) that serves to normalize the free parameters across subjects and allow meaningful statistical comparisons between groups. The k value is held constant across all data sets being compared, because changes in k impact the value of α. The α parameter is considered a measure of reinforcing efficacy, such that rapidly declining (elastic) demand curves have higher α values and indicate lower reinforcing efficacy compared to slower declining (inelastic) demand curves. Because 0 is undefined on a log scale, 0 values in consumption were replaced with 0.04 (1/10th of our lowest non-zero consumption level) to provide better curve fits and more accurate parameter estimates of demand for individual rats (Swain et al., 2020; Koffarnus et al., 2015). Other demand measures of interest included: Q0, the level or intensity of demand as described above; Pmax, or the unit price at which maximal response output occurred; and Omax, or the maximal response output. Pmax and Omax were determined based on their observed rather than their estimated values. These behavioral economic measures were compared between sexes using independent samples t-tests.
Relationships between morphine SA outcomes in each sex were analyzed using linear regression. Outcomes of interest included the behavioral economic measures defined above (i.e., α, Q0, Pmax, and Omax), the number of sessions needed to reach acquisition criteria at FR 1, average infusions during the first 10 days of acquisition at FR 1, average infusions during the first 5 days at FR 2, average infusions during the first and final 5 days at FR 3, cumulative number of infusions earned prior to demand testing, and average number of inactive lever presses during the FR 2 and FR 3 acquisition phases (i.e., those acquisition phases in which inactive lever pressing was higher in the morphine compared to the saline groups, see Results) as a measure of morphine’s locomotor stimulant effects. All outcomes used for regression analyses except α were log-transformed prior to analysis because they were not normally distributed. Slopes for some linear regression analyses were compared between males and females using an F test for equal slopes. All statistical analyses were performed using GraphPad Prism 10, with significance level set at α = 0.05 for all tests.
Results
Acquisition
Infusions
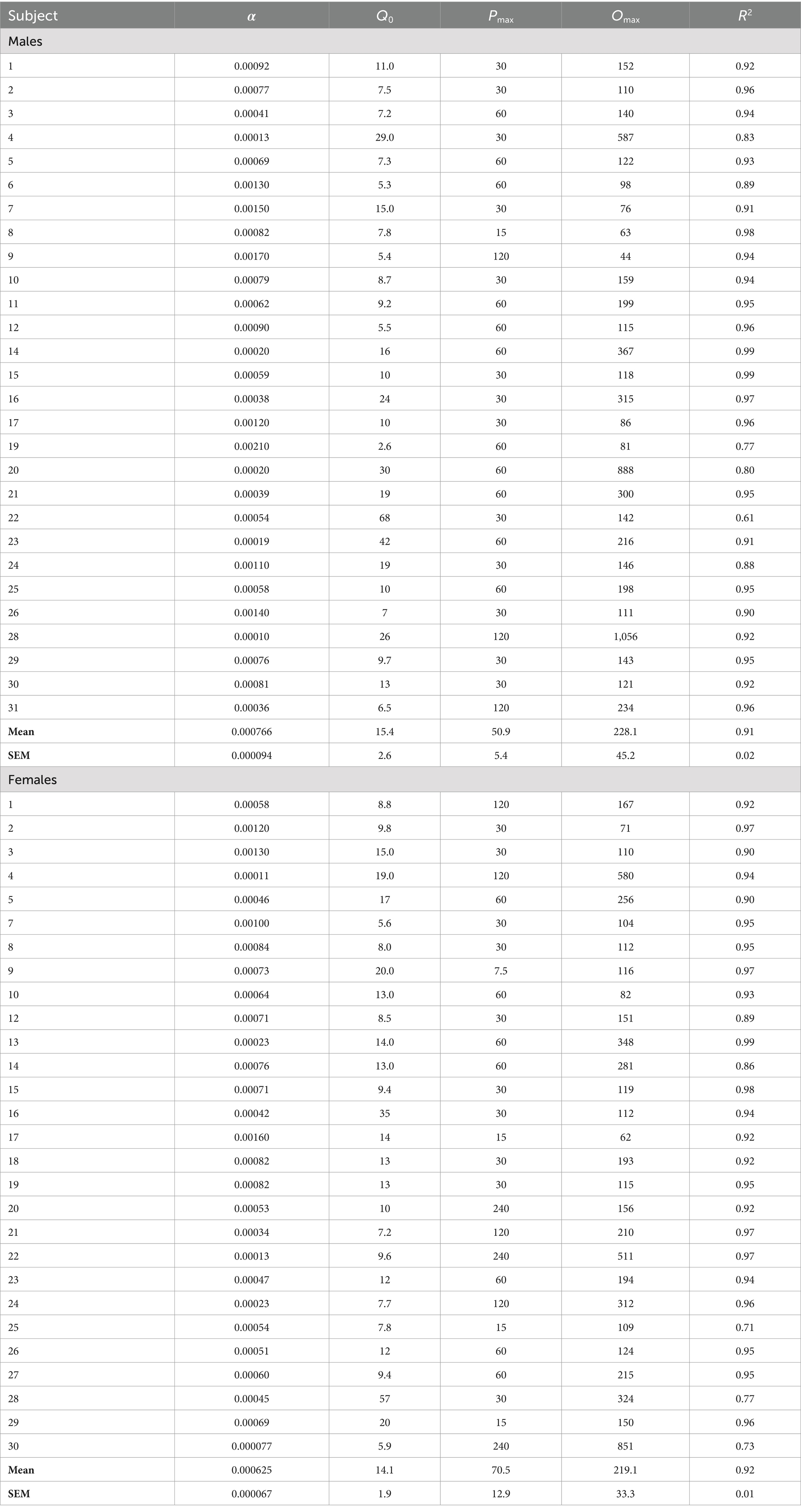
Analysis of infusions earned per session during the first 10 sessions under the FR 1 schedule indicated a significant main effect of group (i.e., morphine versus saline), [F(1, 95) = 26.7, p < 0.0001] and sex, [F(1, 95) = 4.4, p < 0.05], but no significant effects of session or interaction between these variables (Figure 1A). Average infusions across all 10 sessions were significantly higher in the morphine group compared to the saline group for both sexes (t = 3.1 and 4.2 for males and females, respectively, p < 0.01). Bonferroni post-hoc comparisons, which retain their validity despite the absence of a significant interaction between group and sex in the overall ANOVA (see Howell, 2020), indicated that infusions across all 10 sessions at FR 1 were higher in the male morphine group than in the female morphine group (t = 2.3, p < 0.05). In contrast, infusions for the male saline group and female saline group did not differ (Figure 1A).
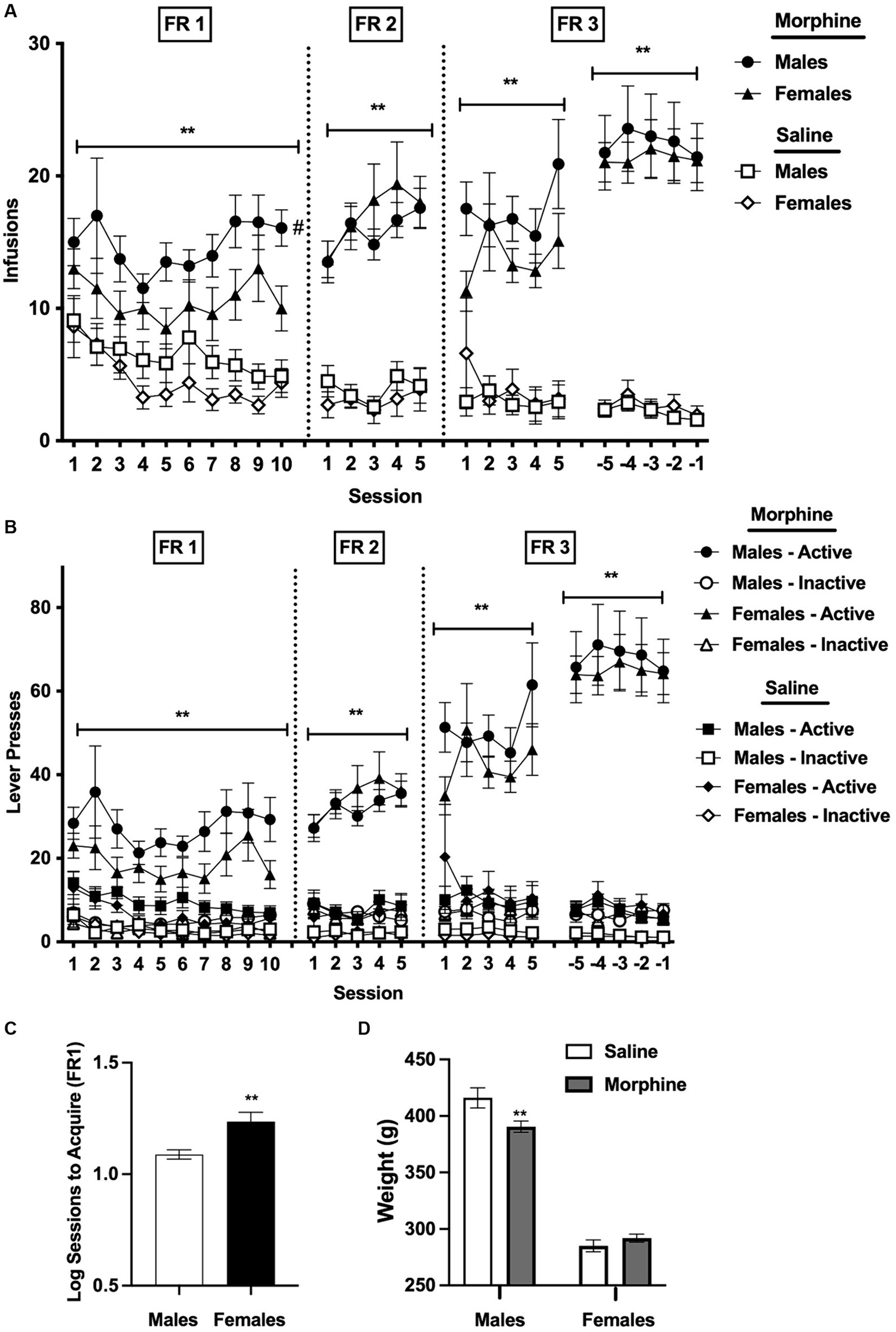
Figure 1. (A) Mean ± SEM infusions earned per session (A) and active/inactive lever responses (B) during acquisition for males and females responding for i.v. morphine (0.4 mg/kg/infusion) or saline. **Significant main effect of group (i.e., morphine versus saline) at that FR, p < 0.01. #Male morphine group different from female morphine group at that FR, p < 0.05. (C) Mean ± SEM sessions required to reach acquisition criteria at FR 1 (log values) in the male and female morphine groups. **Significantly different from males, p = 0.01. (D) Mean ± SEM weight (in g) for males and females in the morphine and saline groups during the last 5 sessions at FR 3. **Significantly different from saline for that sex, p < 0.01.
There was a significant main effect of group on infusions during the first 5 sessions at FR 2, [F(1, 95) = 92.4, p < 0.0001], the first 5 sessions at FR 3, [F(1, 92) = 84.0, p < 0.0001], and the final 5 sessions at [FR 3, F(1, 90) = 91.0, p < 0.0001], reflecting higher infusions in the male and female morphine groups compared to the male and female saline groups (Figure 1A). There was also a significant effect of session during the final 5 sessions at [FR 3, F(3.3, 300.3) = 2.7, p < 0.05]. There were no other significant main effects or interactions at either FR 2 or FR 3.
Lever presses
Analysis of active lever presses under the FR 1 schedule indicated a significant main effect of group at FR 1, [F(1, 95) = 21.8, p < 0.0001], reflecting higher levels of active lever pressing in the morphine compared to the saline group for both sexes (Figure 1B), and a trend toward a main effect of sex, [F(1, 95) = 3.3, p = 0.07]. There were also significant effects of group during the first 5 sessions at FR 2 [F(1, 95) = 92.6, p < 0.0001], the first 5 sessions at FR 3 [F(1, 94) = 53.7, p < 0.0001], and the final 5 sessions at FR 3, [F(1, 90) = 86.8, p < 0.0001], reflecting continued higher active lever pressing in the morphine compared to the saline groups during these sessions, as well as a significant main effect of session during the final 5 sessions at FR 3, [F(3.3, 297.5) = 2.8, p < 0.05]. There were no other significant main effects or interactions for active lever presses during acquisition.
Analysis of inactive lever presses indicated a significant main effect of session at FR 1, [F(2.7, 252.6) = 3.2, p < 0.05], but no effects of group, sex, or interaction between these variables (Figure 1B). There were significant main effects of group at FR 2, [F(1, 95) = 19.7, p < 0.001], the first 5 sessions at FR 3, [F(1, 94.0) = 11.1, p < 0.01], and the final 5 sessions at FR 3, [F(1, 90) = 16.2, p < 0.01], reflecting slightly higher inactive lever pressing in the male and female morphine groups compared to the male and female saline groups (Figure 1B), but no effects of session, sex, or interactions.
Sessions to acquire under the FR 1 schedule
Females required significantly more sessions than males to achieve acquisition criteria under the FR 1 schedule (mean ± SEM sessions to acquire in males and females = 12.7 ± 0.7 and 20.0 ± 2.3 sessions, respectively; t = 3.2, p < 0.01; Figure 1C).
Body weights
Body weights did not differ between the morphine and saline group for either males or females under the FR 1 schedule, FR 2 schedule, or the first 5 sessions under the FR 3 schedule (data not shown). Analysis of body weights averaged across the final 5 sessions at FR 3 indicated no significant effect of group, but there was a significant effect of sex, [F(1, 90) = 400.0, p < 0.0001], and a significant interaction between sex and group, [F(1, 90) = 7.9, p < 0.01]. Body weights were lower in the morphine group compared to the saline group in males (t = 3.2, p < 0.01; Figure 1D), but did not differ between the morphine and saline group in females (Figure 1D).
Demand
A total of 6 rats (3/sex) in the morphine group were lost to attrition during acquisition due to catheter issues, illness, or other problems. Analysis of infusions during FR escalation in the remaining 56 rats (28/sex) indicated a significant effect of FR value, [F (2.4, 128.6) = 97.9, p < 0.0001], but no effect of sex or FR × sex interaction. Comparison of data collapsed across sex indicated that infusions were decreased compared to FR 1 at all FR values ≥ FR 3 (Dunnett q = 4.5–12.3, p < 0.01; Figure 2A). Analysis of total active and inactive responses indicated a significant effect of FR value [F (7, 378) = 11.8, p < 0.0001] and lever (active versus inactive) [F (0.4, 23.0) = 86.0, p < 0.0001], as well as a significant interaction between FR value and lever [F (3.1, 163.7) = 11.3, p < 0.0001]. There was no significant effect of sex and no significant interactions related to sex. Comparison of data collapsed across sex indicated that total active lever responses were increased compared to FR 1 at all FR values ≥FR 6 (q = 3.0–8.8, p < 0.05 or 0.01; Figure 2A). The similar number of infusions at FR 1 and FR 2 in both sexes (Figure 2A) suggests that total active lever responses should double between these FRs, yet this was not the case for either sex (Figure 2B). This reflects the higher levels of non-reinforced active lever responses during the 5 s timeout at FR 1 compared to FR 2 in both sexes (Figure 2B, grey symbols).
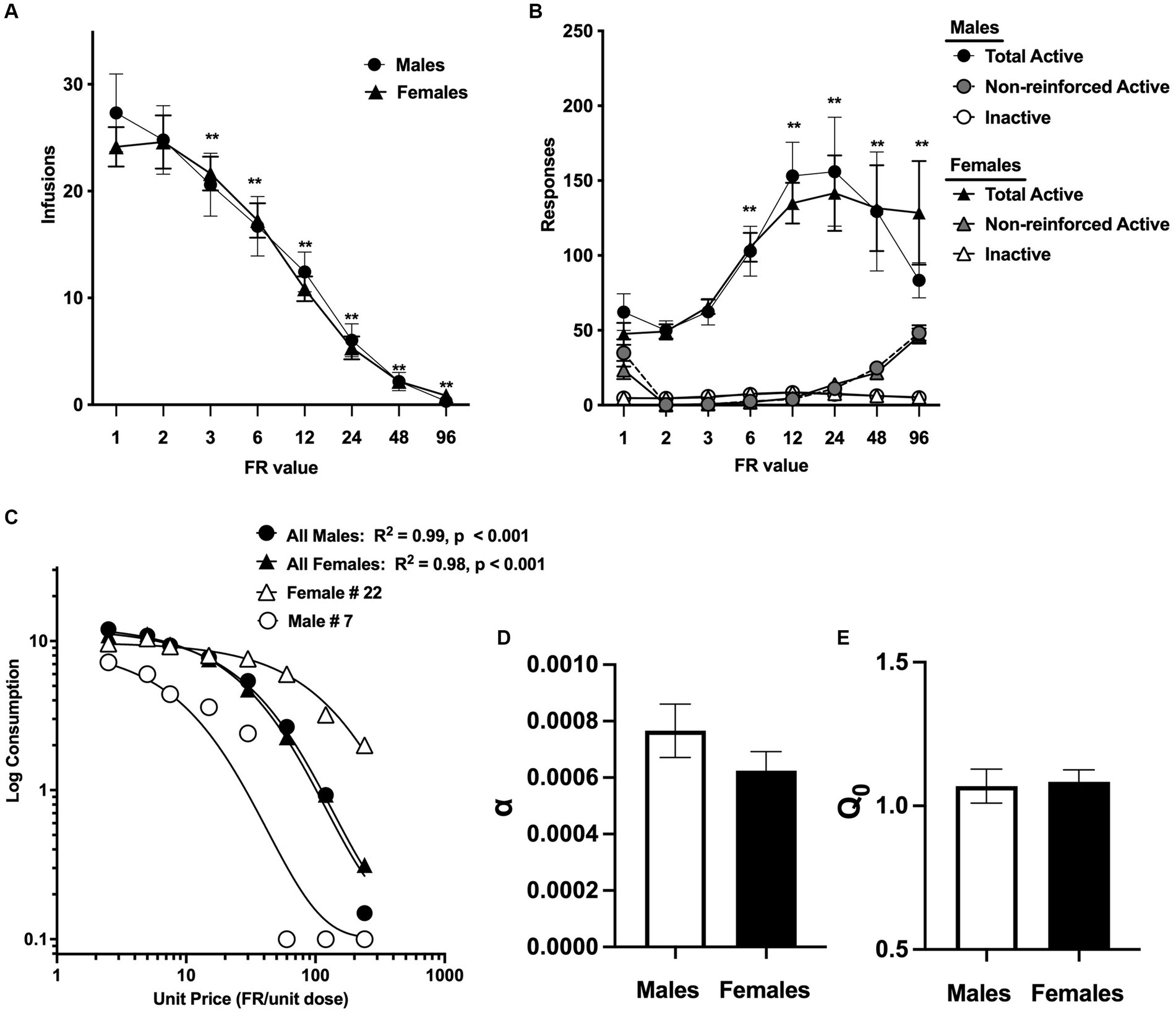
Figure 2. Mean ± SEM infusions earned per session (A) and total active lever responses, non-reinforced active lever responses (i.e., active lever responses during the 5 s timeout period), and inactive lever responses (B) at each FR during demand testing in the male and female morphine groups. **Significantly different compared to infusions or active lever responses at FR1 (collapsed across sexes), p < 0.01. (C) Exponential demand curve describing morphine consumption as a function of unit price for rats as a group, and for individual rats with relatively low (female #22) and high (male #7) elasticity of demand (α). Mean ± SEM α values (D) and log-transformed Q0 values (E) in the male and female morphine groups.
Morphine consumption during demand testing was well described by an exponential demand function for both males and females, with R2 values ≥0.85 for the majority of rats of each sex (Table 1) and R2 = 0.98 or 0.99 for females and males as a group, respectively (Figure 2C). There was considerable individual variability in α values (i.e., elasticity of demand) in both sexes, with some rats showing a rapid decline in morphine consumption following increases in FR (e.g., male #7 in Figure 2C) and others maintaining significant consumption despite the increases in unit price (e.g., female #22 in Figure 2C). There was a 21.0-fold and 18.1-fold range in α values across individual males and females, respectively (Table 1; see also scatterplots of α values shown in Figures 3A–C). There were no sex differences in α (Figure 2D), Q0 (Figure 2E), Pmax, or Omax (Table 1).
Figure 3. Scatterplots with regression line depicting the relationship between Omax and α (A), infusions during the final 5 sessions at FR 3 and α (B), Q0 and α (C), and Omax and cumulative morphine infusions earned prior to demand testing (D) in males and females. Regression lines are solid for males and dashed for females. Lower α values = greater reinforcement efficacy.
Correlates
Higher Omax values (maximal response output) and a higher number of infusions earned during the FR 2 and FR 3 phases of acquisition were associated with lower α values (greater reinforcing efficacy) in both sexes (Figures 3A,B and Table 2). In contrast, higher Q0 values were associated with lower α values in males but not females (Figure 3C), while higher Pmax values were associated with lower α values in females but not males (Table 2). Several other relationships between morphine SA measures were similar in both sexes, whereas others were sex-specific (Table 2). For example, Omax values were significantly correlated with infusions earned during the FR 2 and FR 3 phases and cumulative morphine infusions earned prior to demand testing in both sexes (Table 2 and Figure 3D), while Omax was associated with Q0 in males but not females (Table 2).
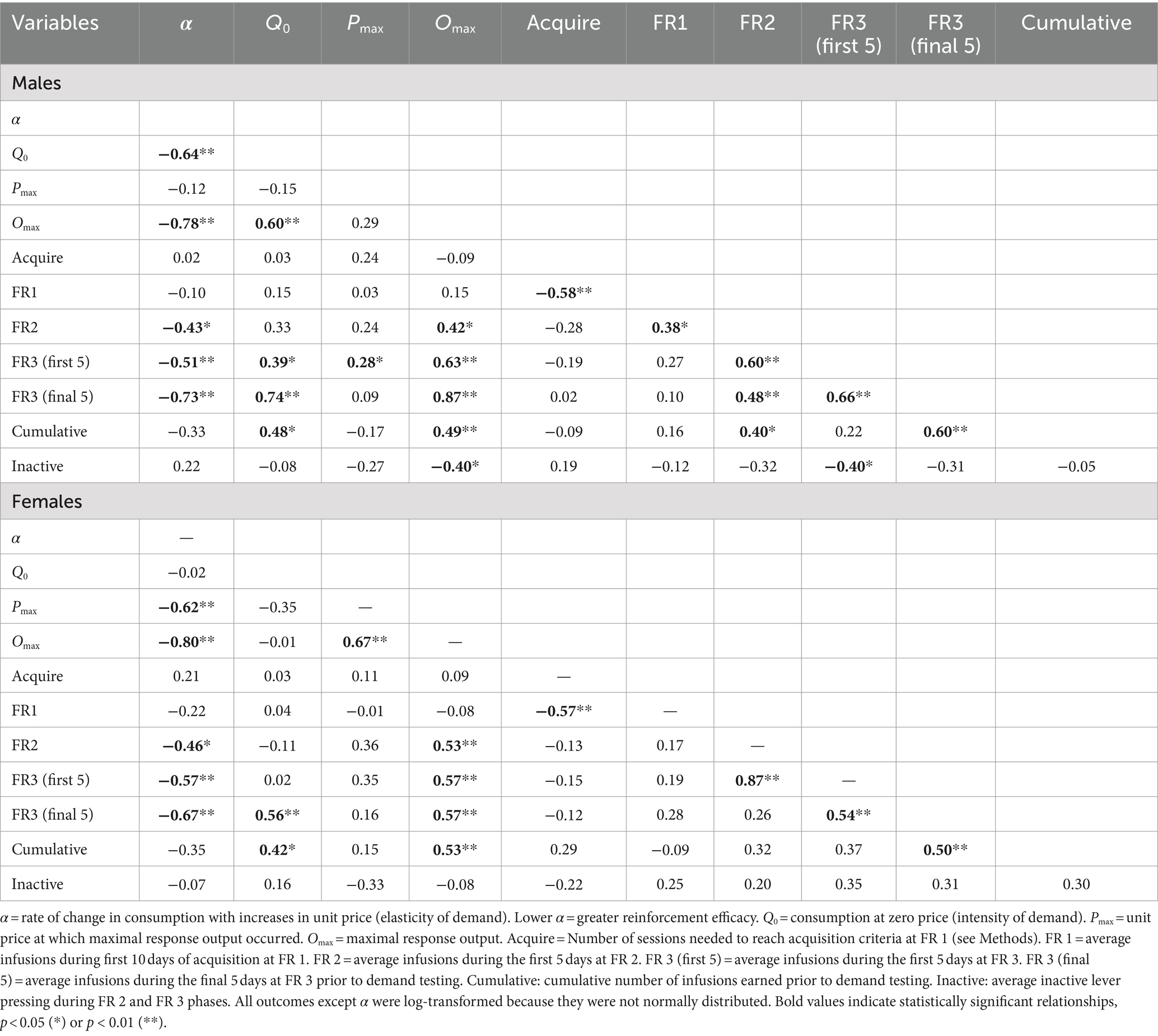
Table 2. Correlation (Pearson’s R) between various measures of morphine SA in males (top panel) and females (bottom panel).
Analysis of data portrayed in Figures 3A–C indicated a significant difference in the slope of the linear regression lines describing the relationship between Q0 and α values in males versus females [F (1, 52) = 7.9, p ≤ 0.001; Figure 3C]. In contrast, there were no sex differences in slopes of the regression lines describing the relationships between the other variables (Figures 3A,B,D).
Discussion
The main finding of this study was that elasticity of demand in an i.v. morphine SA model did not differ between male and female rats. Most predictors of individual differences in demand also generalized across sexes. Acquisition of morphine SA under a FR 1 schedule of reinforcement was slower and infusions earned were lower in females than in males, but morphine SA did not differ between sexes during the subsequent FR 2 and FR 3 phases of acquisition.
Our data are consistent with findings of similar OUD vulnerability in males and females in some human and animal studies (e.g., Nicolas et al., 2022; Kennedy et al., 2013; Stewart et al., 1996) Nonetheless, we acknowledge that a number of studies have demonstrated clear sex differences in OUD vulnerability in both species (e.g., Hernandez-Avila et al., 2004; Lynch and Carroll, 1999; Townsend et al., 2019). Taken together, these studies suggest that factors mediating the relationship between sex and OUD vulnerability may be complex and only apparent under certain conditions.
The factor(s) contributing to differences in our findings versus other preclinical studies that found sex differences in i.v. opioid SA are not clear. It is unlikely that this discrepancy is due to our study being underpowered to detect sex differences, as our group sizes were actually similar to or larger than those often used in this literature. It also is not attributable to our use of morphine, as two prior studies have reported differences in i.v SA of this opioid (Cicero et al., 2003; Mayberry et al., 2022). Specifically, Mayberry et al. (2022) reported that females earned a higher number of morphine infusions than males during a 10 day acquisition period under a FR 1 schedule, which is opposite of the current findings, although there were no sex differences of incubation of morphine-seeking in that study (Mayberry et al., 2022). Furthermore, Cicero et al. (2003) reported greater reinforcing efficacy (i.e., higher breakpoints) in females than males responding for i.v. morphine under a progressive ratio (PR) schedule of reinforcement. Methodological factors that could account for the difference between our findings and those of Mayberry et al. (2022) include rat strain (Sprague Dawley in this study versus Long Evans in the Mayberry study), training dose (0.4 mg/kg/infusion versus 0.75 mg/kg/infusion), and duration of morphine SA access (2 h/day versus 12 h/day), among others. Although the Cicero et al. (2003) study used the same rat strain and vendor/source as our study, it differed in other respects including its use of a longer (4 h/day) access schedule and a considerably different acquisition procedure in which rats were first trained to respond for food, then switched to i.v. heroin SA, and then switched to morphine SA. Perhaps more importantly, the morphine unit dose in Cicero et al. (2003) was 150 μg/infusion regardless of body weight, resulting in females receiving a higher morphine unit dose due to their lower body weight compared to males (e.g., ≈ 0.7 versus 0.4 mg/kg/infusion for ≈ 90-day old females and males, respectively). This may have contributed to the higher breakpoints in females because higher unit doses in drug SA models generally support higher breakpoints (Stafford et al., 1998; Grasing et al., 2003).
This study provided the opportunity to extend a behavioral economic framework to morphine SA in females. Consistent with our previous studies in male rats trained using a lower morphine SA unit dose (Swain et al., 2018; Liu et al., 2021; Swain et al., 2021), an exponential demand function provided an excellent fit for morphine consumption in both sexes, with considerable individual differences in α. As such, these data further support the generalizability and utility of behavioral economics for evaluating determinants of OUD vulnerability.
We evaluated predictors of individual differences in elasticity of demand (α) that were measured prior to demand testing (e.g., rate of acquisition), as well as correlates derived from demand testing itself (e.g., Q0). Among the former, higher infusions during the FR 2 and FR 3 acquisition phases predicted lower elasticity of demand (greater reinforcing efficacy) in both sexes. This is consistent with preclinical findings indicating that higher baseline levels of nicotine SA predict lower demand elasticity (Grebenstein et al., 2015). Higher typical (e.g., past-year) levels of consumption of opioids and other drugs also predicts lower demand elasticity in humans (Pickover et al., 2016; Bertholet et al., 2015). Together these findings implicate baseline drug intake as a sensitive prospective indicator of demand. Among the correlates derived from demand testing, Omax (maximal response output) was closely correlated with elasticity of demand and several other morphine SA outcomes (e.g., infusions during the FR 2 and FR 3 acquisition phases) in both sexes. This close correspondence between Omax and demand and other measures of drug use has also previously been reported in humans (Murphy et al., 2011; Murphy et al., 2009). Together, these findings support the sensitivity and generalizability of Omax as a measure of addiction vulnerability in both species.
As is common in this literature (Guha et al., 2022; Townsend et al., 2019; Mayberry et al., 2022) and in line with the suggestion that estrous cycle monitoring is not essential when first evaluating sex differences in a preclinical model (Becker and Koob, 2016), we did not track the estrous cycle. Nonetheless, given that sex hormones can influence SA of opioids and other drugs (e.g., Towers et al., 2022; Becker et al., 2017; Lopresti et al., 2020), we cannot rule out the possibility that sex differences could have been detected had we analyzed data according to estrous phase. This seems unlikely, however, as any increases in morphine SA in females compared to males during certain phases of the cycle would need to be fully offset by decreases in morphine SA during other phases to yield the almost superimposable mean levels of SA across sexes. This would also result in greater day-to-day variability in morphine SA for females compared to males, which was not observed (e.g., mean coefficient of variation during final 5 sessions at FR 3 for males and females was 12.1 and 12.2%, respectively).
A further potential limitation is that only one morphine SA unit dose was studied. Future studies could evaluate the generality of our findings to other morphine unit doses by increasing unit price during demand testing using unit dose reduction rather than FR escalation. To the extent that these two approaches for increasing unit price produce functionally equivalent effects on drug consumption (Bickel et al., 1990; Smith et al., 2016; DeGrandpre et al., 1993), a unit dose reduction protocol would likely yield similar findings as our FR escalation protocol in terms of morphine’s reinforcing efficacy. Nonetheless, a unit dose reduction protocol would allow analysis of sex differences in morphine’s reinforcing potency (lowest dose that maintains SA).
The reduced weight gain in males during morphine SA is consistent with prior studies (Le et al., 2014; Lee et al., 2017) and may reflect the development of physical dependence, although future studies should measure other signs of dependence (e.g., somatic withdrawal signs such as wet-dog shakes, etc.) to confirm this interpretation. The effect of morphine SA on weight in males was only observed at the end of the FR 3 acquisition period, suggesting that it was a consequence of the higher levels of morphine SA (see Figure 1A) and/or higher cumulative morphine exposure during this phase compared to previous phases. This effect of morphine SA on weight was not due to a reduction in food intake, as all rats finished their daily allotment of food throughout the protocol, although it is unclear what other factors (e.g., increased metabolism, hyperthermia, increased locomotor activity, diarrhea during overnight withdrawal periods) may have contributed. In contrast, morphine SA did not affect weight in females, consistent with findings that repeated noncontingent injections of morphine reduced body weight to a greater extent in males compared to females (Boghossian et al., 2001).
In conclusion, our findings indicate generally similar acquisition, demand, and predictors/correlates of demand in an i.v. morphine SA model in male and female rats. Given that distinct neurobiological mechanisms can mediate addiction-related behavior in males and females even in the absence of sex differences in the behavior itself (Mayberry et al., 2022; Becker and Koob, 2016; Becker et al., 2017), comparison of the mechanisms mediating morphine SA in males and females in this model is warranted. Evaluating genetic, transcriptomic, and epigenetic mechanisms underlying demand in both sexes is of particular interest given the involvement of these mechanisms in individual differences in SA of morphine and other opioids (Ambrosio et al., 1995; Browne et al., 2023; Browne et al., 2020). Indeed, the minimal sex differences in morphine SA in this model could be an advantage for this purpose, as the mechanisms underlying morphine SA in males and females could be compared in the absence of sex differences in opioid intake that could complicate data interpretation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Hennepin Health Research Institute. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PM: Data curation, Methodology, Writing – original draft, Writing – review & editing. SL: Data curation, Methodology, Writing – original draft, Writing – review & editing. JS: Writing – original draft, Writing – review & editing. ML: Writing – original draft, Writing – review & editing. JG: Conceptualization, Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by NIH/NIDA Grant U01 DA051993 (Gewirtz and Harris, MPI), a University of Minnesota Doctoral Dissertation Fellowship (Liu), and the Hennepin Healthcare Research Institute Career Development Award (Harris, LeSage, and Smethells, PIs).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ambrosio, E., Goldberg, S. R., and Elmer, G. I. (1995). Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav. Pharmacol. 6, 229–237. doi: 10.1097/00008877-199504000-00003
Aston, E. R., Farris, S. G., MacKillop, J., and Metrik, J. (2017). Latent factor structure of a behavioral economic marijuana demand curve. Psychopharmacology 234, 2421–2429. doi: 10.1007/s00213-017-4633-6
Back, S. E., Lawson, K. M., Singleton, L. M., and Brady, K. T. (2011). Characteristics and correlates of men and women with prescription opioid dependence. Addict. Behav. 36, 829–834. doi: 10.1016/j.addbeh.2011.03.013
Back, S. E., Payne, R. L., Simpson, A. N., and Brady, K. T. (2010). Gender and prescription opioids: findings from the National Survey on Drug Use and Health. Addict. Behav. 35, 1001–1007. doi: 10.1016/j.addbeh.2010.06.018
Back, S. E., Payne, R. L., Wahlquist, A. H., Carter, R. E., Stroud, Z., Haynes, L., et al. (2011). Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am. J. Drug Alcohol Abuse 37, 313–323. doi: 10.3109/00952990.2011.596982
Becker, J. B., and Koob, G. F. (2016). Sex differences in animal models: focus on addiction. Pharmacol. Rev. 68, 242–263. doi: 10.1124/pr.115.011163
Becker, J. B., McClellan, M. L., and Reed, B. G. (2017). Sex differences, gender and addiction. J. Neurosci. Res. 95, 136–147. doi: 10.1002/jnr.23963
Bentzley, B. S., Jhou, T. C., and Aston-Jones, G. (2014). Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc. Natl. Acad. Sci. U.S.A. 111, 11822–11827. doi: 10.1073/pnas.1406324111
Bertholet, N., Murphy, J. G., Daeppen, J. B., Gmel, G., and Gaume, J. (2015). The alcohol purchase task in young men from the general population. Drug Alcohol Depend. 146, 39–44. doi: 10.1016/j.drugalcdep.2014.10.024
Bickel, W. K., DeGrandpre, R. J., Higgins, S. T., and Hughes, J. R. (1990). Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci. 47, 1501–1510. doi: 10.1016/0024-3205(90)90178-T
Bickel, W. K., Marsch, L. A., and Carroll, M. E. (2000). Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with Behavioral economics: a theoretical proposal. Psychopharmacology 153, 44–56. doi: 10.1007/s002130000589
Boghossian, S., Jourdan, D., Dacher, M., and Alliot, J. (2001). Effect of morphine on caloric intake and macronutrient selection in male and female Lou/c/jall rats during ageing. Mech. Ageing Dev. 122, 1825–1839. doi: 10.1016/s0047-6374(01)00321-9
Browne, C. J., Futamura, R., Minier-Toribio, A., Hicks, E. M., Ramakrishnan, A., Martinez-Rivera, F. J., et al. (2023). Transcriptional signatures of heroin intake and relapse throughout the brain reward circuitry in male mice. Sci. Adv. 9:eadg8558. doi: 10.1126/sciadv.adg8558
Browne, C. J., Godino, A., Salery, M., and Nestler, E. J. (2020). Epigenetic mechanisms of opioid addiction. Biol. Psychiatry 87, 22–33. doi: 10.1016/j.biopsych.2019.06.027
Chen, S. A., O'Dell, L. E., Hoefer, M. E., Greenwell, T. N., Zorrilla, E. P., and Koob, G. F. (2006). Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31, 2692–2707. doi: 10.1038/sj.npp.1301008
Cicero, T. J., Aylward, S. C., and Meyer, E. R. (2003). Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol. Biochem. Behav. 74, 541–549. doi: 10.1016/S0091-3057(02)01039-0
Comer, S. D., Cooper, Z. D., Kowalczyk, W. J., Sullivan, M. A., Evans, S. M., Bisaga, A. M., et al. (2010). Evaluation of potential sex differences in the subjective and analgesic effects of morphine in normal, healthy volunteers. Psychopharmacology 208, 45–55. doi: 10.1007/s00213-009-1703-4
Darke, S., Ross, J., Mills, K. L., Williamson, A., Havard, A., and Teesson, M. (2007). Patterns of sustained heroin abstinence amongst long-term, dependent heroin users: 36 months findings from the Australian Treatment Outcome Study (ATOS). Addict. Behav. 32, 1897–1906. doi: 10.1016/j.addbeh.2007.01.014
DeGrandpre, R. J., Bickel, W. K., Hughes, J. R., Layng, M. P., and Badger, G. (1993). Unit price as a useful metric in analyzing effects of reinforcer magnitude. J. Exp. Anal. Behav. 60, 641–666. doi: 10.1901/jeab.1993.60-641
Fillingim, R. B., Ness, T. J., Glover, T. L., Campbell, C. M., Hastie, B. A., Price, D. D., et al. (2005). Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J. Pain 6, 116–124. doi: 10.1016/j.jpain.2004.11.005
Fredriksson, I., Applebey, S. V., Minier-Toribio, A., Shekara, A., Bossert, J. M., and Shaham, Y. (2020). Effect of the dopamine stabilizer (-)-OSU6162 on potentiated incubation of opioid craving after electric barrier-induced voluntary abstinence. Neuropsychopharmacology 45, 770–779. doi: 10.1038/s41386-020-0602-6
Freeman, L. R., Bentzley, B. S., James, M. H., and Aston-Jones, G. (2021). Sex differences in demand for highly palatable foods: role of the orexin system. Int. J. Neuropsychopharmacol. 24, 54–63. doi: 10.1093/ijnp/pyaa040
Gordon, M. S., Kinlock, T. W., Schwartz, R. P., O'Grady, K. E., Fitzgerald, T. T., and Vocci, F. J. (2017). A randomized clinical trial of buprenorphine for prisoners: findings at 12-months post-release. Drug Alcohol Depend. 172, 34–42. doi: 10.1016/j.drugalcdep.2016.11.037
Grasing, K., Li, N., He, S., Parrish, C., Delich, J., and Glowa, J. (2003). A new progressive ratio schedule for support of morphine self-administration in opiate dependent rats. Psychopharmacology 168, 387–396. doi: 10.1007/s00213-003-1442-x
Grebenstein, P. E., Burroughs, D., Roiko, S. A., Pentel, P. R., and LeSage, M. G. (2015). Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alcohol Depend. 151, 181–193. doi: 10.1016/j.drugalcdep.2015.03.030
Grebenstein, P., Burroughs, D., Zhang, Y., and LeSage, M. G. (2013). Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol. Biochem. Behav. 114-115, 70–81. doi: 10.1016/j.pbb.2013.10.020
Guha, S. K., Alonso-Caraballo, Y., Driscoll, G. S., Babb, J. A., Neal, M., Constantino, N. J., et al. (2022). Ranking the contribution of behavioral measures comprising oxycodone self-administration to reinstatement of drug-seeking in male and female rats. Front. Behav. Neurosci. 16:1035350. doi: 10.3389/fnbeh.2022.1035350
Harris, A. C., Burroughs, D., Pentel, P. R., and LeSage, M. G. (2008). Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp. Clin. Psychopharmacol. 16, 86–97. doi: 10.1037/1064-1297.16.1.86
Hernandez-Avila, C. A., Rounsaville, B. J., and Kranzler, H. R. (2004). Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 74, 265–272. doi: 10.1016/j.drugalcdep.2004.02.001
Huang, B., Dawson, D. A., Stinson, F. S., Hasin, D. S., Ruan, W. J., Saha, T. D., et al. (2006). Prevalence, correlates, and comorbidity of nonmedical prescription drug use and drug use disorders in the United States: results of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 67, 1062–1073. doi: 10.4088/jcp.v67n0708
Hug, C. C. Jr., and Murphy, M. R. (1981). Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology 55, 369–375. doi: 10.1097/00000542-198110000-00006
Hursh, S. R. (1991). Behavioral economics of drug self-administration and drug abuse policy. J. Exp. Anal. Behav. 56, 377–393. doi: 10.1901/jeab.1991.56-377
Hursh, S. R., and Silberberg, A. (2008). Economic demand and essential value. Psychol. Rev. 115, 186–198. doi: 10.1037/0033-295X.115.1.186
Jones, C. M., Logan, J., Gladden, R. M., and Bohm, M. K. (2015). Vital signs: demographic and substance use trends among heroin users—United States, 2002–2013. MMWR Morb. Mortal Wkly. Rep. 64, 719–725
Kamal, F., Flavin, S., Campbell, F., Behan, C., Fagan, J., and Smyth, R. (2007). Factors affecting the outcome of methadone maintenance treatment in opiate dependence. Ir. Med. J. 100, 393–397
Kennedy, A. P., Epstein, D. H., Phillips, K. A., and Preston, K. L. (2013). Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 132, 29–37. doi: 10.1016/j.drugalcdep.2012.12.025
Koffarnus, M. N., Franck, C. T., Stein, J. S., and Bickel, W. K. (2015). A modified exponential behavioral economic demand model to better describe consumption data. Exp. Clin. Psychopharmacol. 23, 504–512. doi: 10.1037/pha0000045
Kohtz, A. S., Lin, B., Davies, H., Presker, M., and Aston-Jones, G. (2022). Hormonal milieu drives economic demand for cocaine in female rats. Neuropsychopharmacology 47, 1484–1492. doi: 10.1038/s41386-022-01304-6
Lacy, R. T., Austin, B. P., and Strickland, J. C. (2020). The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addict. Biol. 25:e12716. doi: 10.1111/adb.12716
Le, T., Xia, M., Jia, M., Sarkar, N., Chen, J., Li, H., et al. (2014). Association between initial morphine intake and body weight change, acoustic startle reflex and drug seeking in rats. Psychopharmacology 231, 4569–4577. doi: 10.1007/s00213-014-3606-2
Lee, B. H., Park, T. Y., Lin, E., Li, H., Yang, C. H., and Choi, K. H. (2017). Altered acoustic startle reflex, prepulse inhibition, and peripheral brain-derived neurotrophic factor in morphine self-administered rats. Int. J. Neuropsychopharmacol. 20, pyw107–pyw191. doi: 10.1093/ijnp/pyw107
Lenoir, M., and Ahmed, S. H. (2008). Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology 33, 2272–2282. doi: 10.1038/sj.npp.1301602
LeSage, M. G., Keyler, D. E., Shoeman, D., Raphael, D., Collins, G., and Pentel, P. R. (2002). Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol. Biochem. Behav. 72, 279–289. doi: 10.1016/S0091-3057(01)00775-4
LeSage, M. G., Staley, M., Muelken, P., Smethells, J. R., Stepanov, I., Vogel, R. I., et al. (2016). Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats. Drug Alcohol Depend. 168, 76–88. doi: 10.1016/j.drugalcdep.2016.08.628
Liu, S. X., Gades, M. S., Swain, Y., Ramakrishnan, A., Harris, A. C., Tran, P. V., et al. (2021). Repeated morphine exposure activates synaptogenesis and other neuroplasticity-related gene networks in the dorsomedial prefrontal cortex of male and female rats. Drug Alcohol Depend. 221:108598. doi: 10.1016/j.drugalcdep.2021.108598
Lopes, G. S., Bielinski, S., Moyer, A. M., Jacobson, D. J., Wang, L., Jiang, R., et al. (2021). Sex differences in type and occurrence of adverse reactions to opioid analgesics: a retrospective cohort study. BMJ Open 11:e044157. doi: 10.1136/bmjopen-2020-044157
Lopresti, N. M., Esguerra, M., and Mermelstein, P. G. (2020). Sex differences in animal models of opioid reward. Curr. Sex. Health Rep. 12, 186–194. doi: 10.1007/s11930-020-00266-4
Lynch, W. J., and Carroll, M. E. (1999). Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144, 77–82. doi: 10.1007/s002130050979
Mackillop, J., Murphy, J. G., Tidey, J. W., Kahler, C. W., Ray, L. A., and Bickel, W. K. (2009). Latent structure of facets of alcohol reinforcement from a behavioral economic demand curve. Psychopharmacology 203, 33–40. doi: 10.1007/s00213-008-1367-5
Maehira, Y., Chowdhury, E. I., Reza, M., Drahozal, R., Gayen, T. K., Masud, I., et al. (2013). Factors associated with relapse into drug use among male and female attendees of a three-month drug detoxification-rehabilitation programme in Dhaka, Bangladesh: a prospective cohort study. Harm Reduct. J. 10:14. doi: 10.1186/1477-7517-10-14
Marsh, J. C., Park, K., Lin, Y. A., and Bersamira, C. (2018). Gender differences in trends for heroin use and nonmedical prescription opioid use, 2007–2014. J. Subst. Abus. Treat. 87, 79–85. doi: 10.1016/j.jsat.2018.01.001
Mavrikaki, M., Pravetoni, M., Page, S., Potter, D., and Chartoff, E. (2017). Oxycodone self-administration in male and female rats. Psychopharmacology 234, 977–987. doi: 10.1007/s00213-017-4536-6
Mayberry, H. L., Bavley, C. C., Karbalaei, R., Peterson, D. R., Bongiovanni, A. R., Ellis, A. S., et al. (2022). Transcriptomics in the nucleus accumbens shell reveal sex- and reinforcer-specific signatures associated with morphine and sucrose craving. Neuropsychopharmacology 47, 1764–1775. doi: 10.1038/s41386-022-01289-2
McHugh, R. K., Nguyen, M. D., Chartoff, E. H., Sugarman, D. E., and Greenfield, S. F. (2021). Gender differences in the prevalence of heroin and opioid analgesic misuse in the United States, 2015–2019. Drug Alcohol Depend. 227:108978. doi: 10.1016/j.drugalcdep.2021.108978
Mullis, K. B., Perry, D. C., Finn, A. M., Stafford, B., and Sadee, W. (1979). Morphine persistence in rat brain and serum after single doses. J. Pharmacol. Exp. Ther. 208, 228–231
Murphy, J. G., MacKillop, J., Skidmore, J. R., and Pederson, A. A. (2009). Reliability and validity of a demand curve measure of alcohol reinforcement. Exp. Clin. Psychopharmacol. 17, 396–404. doi: 10.1037/a0017684
Murphy, J. G., MacKillop, J., Tidey, J. W., Brazil, L. A., and Colby, S. M. (2011). Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 113, 207–214. doi: 10.1016/j.drugalcdep.2010.08.004
Nicolas, C., Zlebnik, N. E., Farokhnia, M., Leggio, L., Ikemoto, S., and Shaham, Y. (2022). Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharmacol. Rev. 74, 119–140. doi: 10.1124/pharmrev.121.000367
Oleson, E. B., Richardson, J. M., and Roberts, D. C. (2011). A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology 214, 567–577. doi: 10.1007/s00213-010-2058-6
Piazza, N. J., Vrbka, J. L., and Yeager, R. D. (1989). Telescoping of alcoholism in women alcoholics. Int. J. Addict. 24, 19–28. doi: 10.3109/10826088909047272
Pickover, A. M., Messina, B. G., Correia, C. J., Garza, K. B., and Murphy, J. G. (2016). A Behavioral economic analysis of the nonmedical use of prescription drugs among young adults. Exp. Clin. Psychopharmacol. 24, 38–47. doi: 10.1037/pha0000052
Smethells, J. R., Greer, A., Dougen, B., and Carroll, M. E. (2020). Effects of voluntary exercise and sex on multiply-triggered heroin reinstatement in male and female rats. Psychopharmacology 237, 453–463. doi: 10.1007/s00213-019-05381-2
Smith, T. T., Rupprecht, L. E., Sved, A. F., and Donny, E. C. (2016). Characterizing the relationship between increases in the cost of nicotine and decreases in nicotine content in adult male rats: implications for tobacco regulation. Psychopharmacology 233, 3953–3964. doi: 10.1007/s00213-016-4426-3
Spencer, M., Garnett, M. F., and Minino, A. M. (2024). “Drug overdose deaths in the United States, 2002–2022” in NCHS Data Brief No. 491. Hyattsville, MD: National Center for Health Statistics.
Stafford, D., LeSage, M. G., and Glowa, J. R. (1998). Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology 139, 169–184. doi: 10.1007/s002130050702
Stewart, J., Woodside, B., and Shaham, Y. (1996). Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology 24, 154–159. doi: 10.3758/BF03331967
Swain, Y., Gewirtz, J. C., and Harris, A. C. (2021). Behavioral predictors of individual differences in opioid addiction vulnerability as measured using i.v. self-administration in rats. Drug Alcohol Depend. 221:108561. doi: 10.1016/j.drugalcdep.2021.108561
Swain, Y., Muelken, P., LeSage, M. G., Gewirtz, J. C., and Harris, A. C. (2018). Locomotor activity does not predict individual differences in morphine self-administration in rats. Pharmacol. Biochem. Behav. 166, 48–56. doi: 10.1016/j.pbb.2018.01.008
Swain, Y., Muelken, P., Skansberg, A., Lanzdorf, D., Haave, Z., LeSage, M. G., et al. (2020). Higher anhedonia during withdrawal from initial opioid exposure is protective against subsequent opioid self-administration in rats. Psychopharmacology 237, 2279–2291. doi: 10.1007/s00213-020-05532-w
Swain, Y., Waller, N. G., Gewirtz, J. C., and Harris, A. C. (2021). Individual differences in different measures of opioid self-administration in rats are accounted for by a single latent variable. Front Psychiatry 12:712163. doi: 10.3389/fpsyt.2021.712163
Szucs, D., and Ioannidis, J. P. (2020). Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990-2012) and of latest practices (2017–2018) in high-impact journals. NeuroImage 221:117164. doi: 10.1016/j.neuroimage.2020.117164
Towers, E. B., Setaro, B., and Lynch, W. J. (2022). Sex- and dose-dependent differences in the development of an addiction-like phenotype following extended-access fentanyl self-administration. Front. Pharmacol. 13:841873. doi: 10.3389/fphar.2022.841873
Towers, E. B., Williams, I. L., Qillawala, E. I., Rissman, E. F., and Lynch, W. J. (2023). Sex/gender differences in the time-course for the development of substance use disorder: a focus on the telescoping effect. Pharmacol. Rev. 75, 217–249. doi: 10.1124/pharmrev.121.000361
Townsend, E. A., Negus, S. S., Caine, S. B., Thomsen, M., and Banks, M. L. (2019). Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 44, 2022–2029. doi: 10.1038/s41386-019-0356-1
Keywords: opioid use disorder, individual differences, sex differences, behavioral economics, morphine
Citation: Harris AC, Muelken P, Liu SX, Smethells JR, LeSage MG and Gewirtz JC (2024) Magnitude and predictors of elasticity of demand for morphine are similar in male and female rats. Front. Behav. Neurosci. 18:1443364. doi: 10.3389/fnbeh.2024.1443364
Edited by:
Polymnia Georgiou, University of Wisconsin–Milwaukee, United StatesReviewed by:
Christopher Olsen, Medical College of Wisconsin, United StatesHelen Kamens, The Pennsylvania State University (PSU), United States
Copyright © 2024 Harris, Muelken, Liu, Smethells, LeSage and Gewirtz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew C. Harris, aGFycjA1N0B1bW4uZWR1
 Andrew C. Harris
Andrew C. Harris Peter Muelken1
Peter Muelken1 John R. Smethells
John R. Smethells Mark G. LeSage
Mark G. LeSage