
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci., 24 January 2024
Sec. Learning and Memory
Volume 18 - 2024 | https://doi.org/10.3389/fnbeh.2024.1342589
This article is part of the Research TopicLearning and Memory 2023 - Volume IIView all 30 articles
 Xinran Niu1†
Xinran Niu1† Mia F. Utayde1†
Mia F. Utayde1† Kristin E. G. Sanders1
Kristin E. G. Sanders1 Dan Denis2
Dan Denis2 Elizabeth A. Kensinger3
Elizabeth A. Kensinger3 Jessica D. Payne1*
Jessica D. Payne1*Background: While younger adults are more likely to attend to, process, and remember negative relative to positive information, healthy older adults show the opposite pattern. The current study evaluates when, exactly, this positivity shift begins, and how it influences memory performance for positive, negative, and neutral information.
Methods: A total of 274 healthy early middle-aged (35–47), late middle-aged (48–59), and older adults (>59) viewed scenes consisting of a negative, positive, or a neutral object placed on a plausible neutral background, and rated each scene for its valence and arousal. After 12 h spanning a night of sleep (n = 137) or a day of wakefulness (n = 137), participants completed an unexpected memory test during which they were shown objects and backgrounds separately and indicated whether the scene component was the “same,” “similar,” or “new” to what they viewed during the study session.
Results and conclusions: We found that both late middle-aged and older adults rated positive and neutral scenes more positively compared to early middle-aged adults. However, only older adults showed better memory for positive objects relative to negative objects, and a greater positive memory trade-off magnitude (i.e., remembering positive objects at the cost of their associated neutral backgrounds) than negative memory trade-off magnitude (i.e., remembering negative objects at the cost of their associated neutral backgrounds). Our findings suggest that while the positivity bias may not emerge in memory until older adulthood, a shift toward positivity in terms of processing may begin in middle age.
As we approach older age, we tend to view the world more positively. Younger adults often prioritize negative information over positive information (i.e., a negativity bias; Carstensen and DeLiema, 2018; Barber and Kim, 2021), while older adults are less likely to show this bias and can even show the opposite tendency to preferentially process, attend to, and remember positive over negative information (i.e., a positivity bias). Moreover, even in the face of physical and cognitive declines, healthy aging is associated with more positive life experiences and better psychological well-being (Höglund et al., 2020; Korkmaz and Güloğlu, 2021).
Previous studies find that older adults tend to intrinsically orient their attention toward positive stimuli and away from negative stimuli (Mather and Carstensen, 2003; Rösler et al., 2005; Isaacowitz et al., 2006, 2009; Knight et al., 2007). For example, older adults display gaze preferences for happy faces but tend to look away from fearful faces (Noh and Isaacowitz, 2015; Erbey et al., 2020). Research evaluating an event-related potential (ERP) sensitive to attentional engagement to emotional information and emotion regulation, known as the late positive potential (LPP; Hajcak and Nieuwenhuis, 2006; Ferrari et al., 2008; De Cesarei and Codispoti, 2011), found that the amplitude of the LPP decreases in old age when evoked by negative stimuli, yet the LPP amplitude for positive stimuli remains invariable across the lifespan (Wood and Kisley, 2006; Kisley et al., 2007; Langeslag and van Strien, 2009). While young adults show greater LPP amplitude for negative information than positive information, older adults show comparable LPP amplitude in response to both negative and positive stimuli (Wood and Kisley, 2006; Kisley et al., 2007; Mathieu et al., 2014). These behavioral and ERP studies emphasize that the negativity bias commonly seen in younger adults diminishes as individuals age, through a modulation of attention and affective responding to negative stimuli.
The age-related positivity effect also shows up in memory. A meta-analysis demonstrated that older adults are more likely to remember positive than negative stimuli compared to younger adults (Reed et al., 2014). This positivity effect was consistently observed using incidental encoding procedures, in which participants are not explicitly instructed to remember stimuli and are unaware their memory will be tested later (Charles et al., 2003; Leigland et al., 2004; Kensinger, 2008; Piguet et al., 2008; Spaniol et al., 2008). This is likely because the positivity effect is the most robust when older adults process emotional information naturally and spontaneously during encoding.
There are several theories about why the positivity effect occurs as individuals age. The Socioemotional Selectivity Theory, for instance, states that motivations and priorities shift across the life span, such that older adults become more aware that their future time horizons are limited compared to younger adults (Carstensen et al., 1999; Carstensen and Mikels, 2005; Carstensen, 2006). Thus, while younger adults often grapple with uncertainty about their educational and occupational paths, older adults tend to act in accordance with present-focused goals that promote emotional satisfaction (Waszczuk et al., 2016). These shifts in internal goals result in the selective processing of positive over negative information (Carstensen et al., 1999; Carstensen and Mikels, 2005; Carstensen, 2006). In contrast, other theories propose that the positivity effect results from age-related cognitive or neural deficits. The Dynamic Integration Theory states that due to older adults’ natural cognitive decline, they prioritize the processing of positive more than negative information because negative information tends to be more complex (Labouvie-Vief et al., 2010). Relatedly, the aging brain model proposes that the positivity effect arises as a result of amygdala degradation in old age. Given the importance of the amygdala in evaluating and responding to negative emotional information, amygdala degradation may lead to decreased sensitivity to negative information, therefore resulting in a comparative increase in the processing of positive information (Anderson, 2007; Sergerie et al., 2008).
Contrary to the Dynamic Integration Theory and the aging brain model theory, however, some imaging studies observe structural and functional preservation of the amygdala in normal aging, which explains why the amygdala’s responsiveness to positive stimuli does not vary across age (Allen et al., 2005; Leclerc and Kensinger, 2008; Brabec et al., 2010). Though some fMRI studies do find less amygdala activity in older adults while viewing negative information in comparison to their younger counterparts, these studies concurrently observe higher medial prefrontal cortical activity, which may indicate increased recruitment of cognitive control to regulate negative emotions (Mather et al., 2004; Williams et al., 2006; St Jacques et al., 2009; Leclerc and Kensinger, 2011; Samanez-Larkin and Carstensen, 2011). Therefore, increased cognitive control during emotional processing in old age, suggested by increased PFC activation, may underlie the positivity effect, rather than cognitive deficits in processing complex information or impaired amygdala function. Taken together with the aforementioned ERP findings suggesting that attentional engagement to negative but not positive information decreases with age (Wood and Kisley, 2006; Kisley et al., 2007; Langeslag and van Strien, 2009), older adults may show a more deliberate and controlled form of affective regulatory activity that maintains the emotional processing of positive memories while diminishing the processing of negative memories (Nashiro et al., 2011).
Although increasing age is associated with remembering positive experiences better than negative ones, both positive and negative experiences are disproportionately represented in memory compared to neutral ones (McGaugh, 2000; Christianson, 2014; Williams et al., 2022). This is in part due to the influence of emotional arousal at the time of encoding, which triggers the release of stress-related neuromodulators, such as norepinephrine and cortisol, which “tag” emotional aspects of memories that are worth retaining for future recollection (Holland and Kensinger, 2010; Bennion et al., 2015; Payne and Kensinger, 2018). One of the paradigms that has consistently found enhancements in emotional memory is the emotional memory trade-off task, where recognition memory is assessed separately for objects and backgrounds within scenes containing either an emotional or neutral object placed on a neutral background (Payne et al., 2008, 2012, 2015; Payne and Kensinger, 2011; Chambers et al., 2014; Bennion et al., 2017; Alger et al., 2018; Cummings et al., 2018; Vargas et al., 2019; Denis et al., 2022). Studies using this task consistently demonstrate that participants exhibit better memory for emotional objects than any other type of memory measured by the task, which includes neutral objects and the (also neutral) backgrounds paired with either type of object (Payne et al., 2008, 2012, 2015; Payne and Kensinger, 2011; Chambers et al., 2014; Bennion et al., 2017; Alger et al., 2018; Cummings et al., 2018; Vargas et al., 2019; Denis et al., 2022). Previous studies also find that sleep further enhances this emotional memory trade-off effect, potentially because sleep is a critical stage where emotionally salient information is preferentially consolidated (Payne et al., 2008, 2012; Payne and Kensinger, 2011; Cunningham et al., 2014; Bennion et al., 2015, 2017; Denis et al., 2022; Sanders et al., n.d.).
Importantly, however, most prior studies using the emotional memory trade-off task focus on emotional memory for negatively valenced content, with only a few studies examining positive stimuli (Chambers et al., 2014; Denis et al., 2022). Negative stimuli are often used because negative information tends to elicit higher levels of emotional arousal than positive information, resulting in a stronger effect on memory (Kensinger, 2009; Kauschke et al., 2019; Cook et al., 2023). For example, a vicious looking snake might be perceived as more unpleasant and agitating than a cute puppy is perceived as pleasant and exciting, and thus is more likely to be remembered. However, since positive episodic memories are crucial protective factors against adverse mental health outcomes, it is important to clarify whether there is also a memory trade-off effect for positive information (i.e., whether memory for positive emotional content can also be enhanced at the expense of neutral features of the same event; Holland and Kensinger, 2010; Williams et al., 2022). Moreover, knowing when this positivity shift emerges in adulthood can provide insight into the cognitive-affective mechanisms involved in other key psychological functions, including emotional regulation, decision-making, and psychological well-being as we age (Shohamy and Daw, 2015; Isaacowitz, 2022).
While the age-related positivity effect and emotional memory trade-off effect have been well researched separately, whether and how the positivity effect might interact with the emotional memory trade-off across the age-span remains relatively unexplored. It is unclear whether the age-related positivity effect is so strong that fewer attentional resources would be allocated to neutral features of the same scene, eventually leading to impaired memory performance of the peripheral neutral information in the background (Easterbrook, 1959; Reisberg and Heuer, 2004). Moreover, while it is well established that older adults tend to remember positive over negative information as mentioned above (Reed et al., 2014), it is not yet known if the neutral peripheral background of the attention-capturing object is more poorly remembered when the central event is positive compared to negative. Finally, most prior studies on the age-related positive memory bias have tested memory following brief delays, often because they were focused on how the preferential allocation of attentional resources during encoding leads to superior memory for positive information (Barber and Kim, 2021). As a result, delayed memory tasks that capture consolidation-related processes have not been widely employed.
The current study seeks to investigate the effects of aging on the selective consolidation of emotionally positive and negative central objects at the potential cost of their associated neutral backgrounds. We examined this question using a large sample of middle-aged and older adults (n = 274). Because we were interested in whether consolidation processes would influence these variables, we tested recognition memory after a significant delay (M = 11.86 h). This experiment is part of a larger study examining the impact of sleep (vs. wakefulness) on memory. As such, the memory delay interval spanned either a night of sleep or a day of wakefulness. However, because we were less interested in the specific impact of sleep here and more interested in the impact of a consolidation delay (i.e., the general passage of time), we collapsed these groups for maximum power. We hypothesized that increasing age would be associated with positive and neutral (but not negative) information being rated more positively, and with better memory performance following the delay for neutral and positive (but not negative) objects at the cost of associated neutral backgrounds. Although we did not have a firm prediction about when in the age span these positivity biases would emerge, we suspected that they would arise sometime in late middle age or early older age.
A total of 554 eligible participants were recruited online via Prolific (https://www.prolific.co). Inclusion criteria for eligible participants were (1) be at least 35 years of age, (2) be currently residing in the United States, (3) be fluent in English, (4) have normal or corrected-to-normal vision, and (5) have no history of any diagnosed sleep, psychiatric, or neurological disorders. Of the eligible participants, 277 completed the study. However, three were excluded due to noncompliance with the study instructions (i.e., did not rate the valence or arousal levels of any scenes). Therefore, our final sample consisted of 274 participants (Mage = 55.57, SDage = 12.15). An a priori power analysis indicated that a sample size of 280 participants was sufficient to detect group differences (Cohen’s d = 0.33) between the daytime wake and nighttime sleep conditions with 80% power and α = 0.05 (Denis et al., 2022). A post hoc power analysis showed that a sample size of 274 participants yielded 80% power for detecting age by valence interaction (Cohen’s f = 0.21) with α = 0.05.
The majority of the sample self-identified as white (86.9% White, 6.2% Black/African American, 5.8% Asian, 0.7% American Indian/Native Alaskan, 0.0% Native Hawaiian/Pacific Islander, and 0.4% other; 4.0% Hispanic/Latino/Spanish). Approximately half of the sample reported their biological sex as female (51.8% female, 48.2% male, and 0% intersex). Median annual household income was 65,000 U.S. dollars (range: 0 to 300,000). See Supplementary Table S1 for a detailed breakdown of participant demographic characteristics based on age groups and delay conditions. Procedures were approved by the Institutional Review Board at the University of Notre Dame. Participants gave written informed consent and received compensation through Prolific payments for their participation.
Without being informed about the subsequent memory task, participants viewed a series of 72 scenes in a random order, each shown for 5 s. These scenes stayed the same across all participants, and depicted 24 emotionally negative, 24 positive, and 24 neutral objects, which were always placed on a plausible neutral background (Figure 1A). After each scene was presented, participants had 10 s to rate the scene for its valence (1 = highly negative, 4 = neutral: neither negative nor positive, and 7 = highly positive) and arousal levels (1 = highly calming/subduing, 4 = neither agitating/exciting nor calming/subduing, and 7 = highly agitating/exciting). Previous studies using the same stimuli found that negative scenes were rated as more negative and arousing compared to both positive and neutral scenes, and positive scenes were rated as more positive than negative and neutral scenes, and more arousing than neutral scenes (Sanders et al., n.d.).
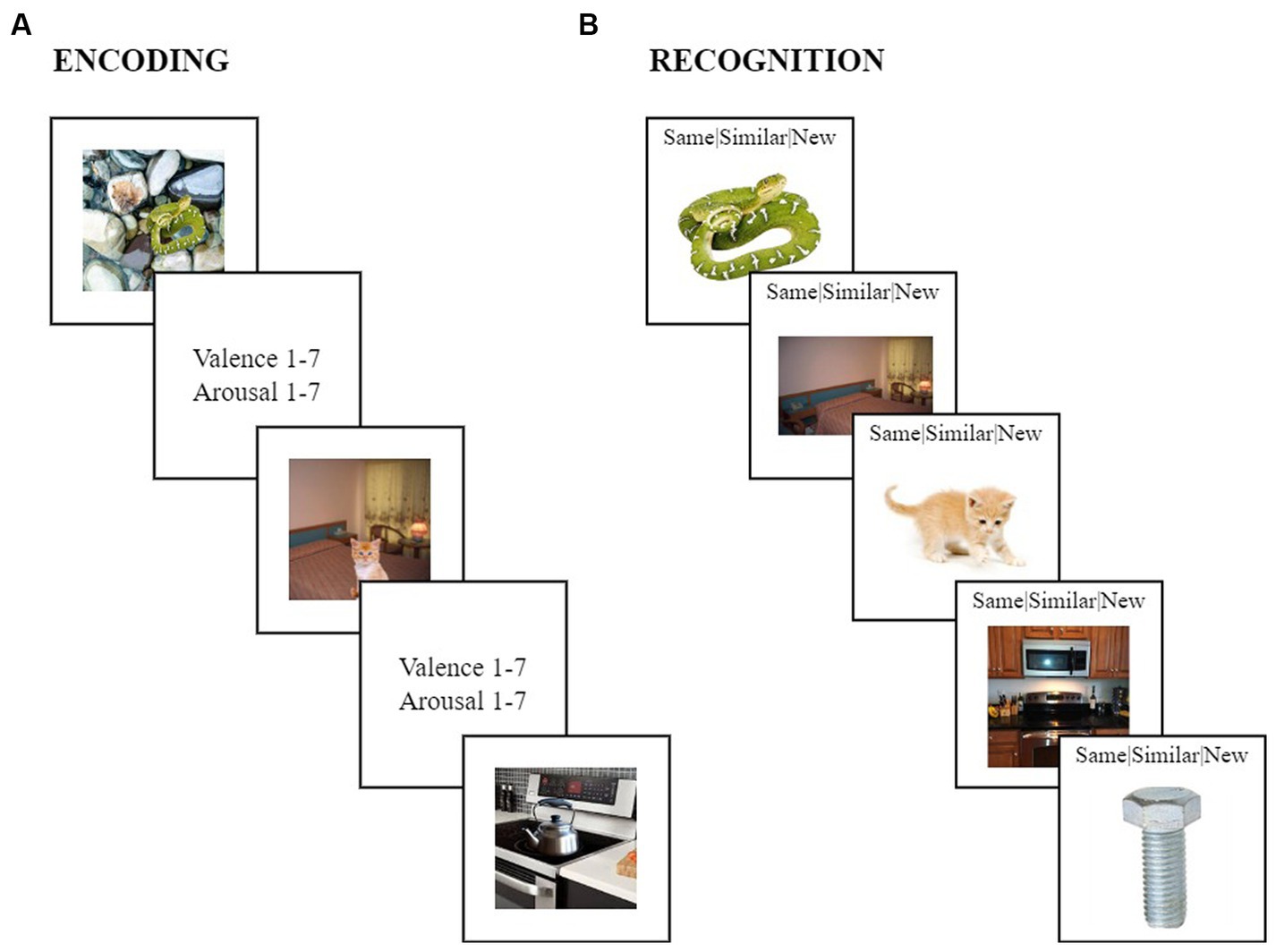
Figure 1. The emotional memory trade-off task. (A) During encoding, participants viewed a series of negative (e.g., a vicious looking snake on wet pebbles), positive (e.g., a cute kitten on a bed) and neutral scenes (e.g., a kettle on a kitchen stove), consisting of objects with negative, positive, or neutral valences always placed on a neutral background. Participants rated the valence and arousal levels of each scene on a scale from one to seven. (B) During recognition, participants indicated whether each scene component was “same” (e.g., the same snake exactly matched what has been viewed during encoding), “similar” (e.g., the similar kitten shared the same verbal label as the kitten during encoding but differed in specific visual details), or “new” (e.g., the bolt was new and was not seen during encoding).
While object and background components were presented together within intact scenes during incidental encoding, objects and backgrounds were shown separately during a surprise memory test (Figure 1B). Specifically, the recognition test consisted of 204 images presented in a random order, each displayed for 10 s. While each image was displaying, participants indicated whether it was “same,” “similar,” or “new” compared to what they had encountered during encoding. Of the 204 images (i.e., objects and backgrounds), 72 were exactly the same as participants had encountered during encoding (same), another 72 shared the same verbal labels as those encountered during encoding but had some variations in visual details (similar), and an additional 60 images were never encountered by participants during encoding (new). All three image sets included 12 negative objects, 12 positive objects, and 12 neutral objects. The same and similar sets were composed of 36 neutral backgrounds, whereas the new set contained 24 neutral backgrounds. Participants were never presented with both the same and similar versions of a particular item during the recognition test, but instead, the presentation of same and similar versions were counterbalanced across participants.
Participants enrolled in a study titled “Emotional Reactivity at Different Times of Day” via Prolific (https://www.prolific.co), which redirected them to an initial screening survey. In the screening survey, participants provided information regarding their demographic characteristics and sleep. Eligible participants were then randomly assigned to either a nighttime sleep or daytime wake condition. For participants in the nighttime sleep condition, session one occurred in the evening of the same day as they completed the screening survey, between 7 and 11 p.m. in their local time, and session two was scheduled for the following morning, between 7 and 11 a.m. in their local time. In contrast, participants in the daytime wake condition performed session one in the morning (7–11 a.m.) of the next day, and session two in the evening of the next day (7–11 p.m.).
During session one, all participants completed questionnaires assessing their subjective sleep and well-being, including a three-night sleep log where participants provided information on the approximate times they went to bed and woke up, how long it took them to fall asleep, and the number of awakenings during the night for the three nights preceding the study. After completing these questionnaires, participants performed a brief 3-min version of the Psychomotor Vigilance Test (Basner et al., 2011). Then, they were presented with the series of 72 scenes, each featuring a negative, positive, or neutral object placed on an (always) neutral background. Participants were instructed to rate the valence and arousal levels of each scene. Participants had the option to take a self-paced break in the middle of the scene viewing task. After the task, they responded to additional questionnaires on their sleep and well-being. Importantly, they were not informed about the later memory task, and instead were told they would take part in another scene viewing task for session two.
After an approximately 12-h consolidation delay, participants began session two of the study. All participants completed questionnaires on sleep and well-being. Participants in the nighttime sleep condition additionally answered questions on how they slept between session one and session two. Then, all participants performed another brief version of the Psychomotor Vigilance Test. Next, they viewed a series of 108 objects and 96 backgrounds, and determined if each of the objects and backgrounds was “same,” “similar,” or “new” to what they had encountered during the encoding session. The memory test was divided into three sections to mitigate fatigue, and participants were given the option to take a self-paced break between each section. After the memory task, participants responded to additional questionnaires on sleep and well-being.
The scene rating and memory tasks were programmed in jsPsych (de Leeuw, 2015) and hosted on Cognition.run (https://www.cognition.run). All questionnaires were created and administered on Qualtrics (https://www.qualtrics.com). Refer to Supplementary material for a comprehensive list of measures for sleep, alertness, and well-being.
All data processing and analyses were carried out in R (R Core Team, 2022). For all analyses, we performed two Analyses of Variance (ANOVAs), treating age as both a three-tier category (early middle age: 35–47, late middle age: 48–59, older: 60 and older) and a continuous variable (range: 35–92). The selection of age cutoffs for early middle-aged, late middle-aged, and older adults was made to maintain consistency with other studies conducted by the same research group for cross-experiment comparisons (Denis et al., 2022; Sanders et al., n.d.). Effect sizes were estimated using partial eta-squared (ηp2) for ANOVA models and Cohen’s d for follow-up t-tests. We report both the unadjusted, original p-values and p-values controlled for the false discovery rate (FDR; Benjamini et al., 2001). The threshold for statistical significance was set to FDR-adjusted p < 0.05, two-tailed.
To investigate when the impact of age-related positivity effects on valence and arousal ratings (i.e., positive interpretation bias) emerges in adulthood, we conducted a mixed-model ANOVA with a 3 (Age: Early Middle Age, Late Middle Age, Older Adulthood) × 3 (Valence: Negative, Neutral, Positive) x 2 (Delay: Sleep, Wake) design, for valence and arousing ratings during encoding sessions. We ran pairwise t tests to compare valence and arousal ratings across the following conditions (1) negative, positive, and neutral scenes, (2) negative, positive, and neutral scenes within each age group, and (3) early middle-aged, late middle-aged, and older adults for each scene type. Additionally, we also performed a linear mixed-effects ANOVA, treating age as a continuous variable, and assessed the Pearson’s correlations between age and valence/arousal ratings for each scene type.
For the emotional memory trade-off task, we scored both “specific memory,” which emphasizes the retention of detailed visual properties of each component, and “gist memory” which captures meaning-based general properties of individual scene component (Reyna, 2012). For gist memory, which we focused on in this manuscript, hit rates were calculated by the proportion of trials in which participants responded with “same” or “similar” when they encountered same items. In contrast, specific memory was assessed more stringently, with a hit constituting only “same” response for a same item. False alarm rates for gist and specific memory were computed using the same methods, which was the proportion of trials in which participants incorrectly responded with “same” when encountering new items. To obtain corrected recognition memory, false alarm rates were subtracted from hit rates, yielding the final gist and specific memory scores.
To examine whether and when age-related positively effects extend to memory, we performed a 3 (Age: Early Middle Age, Late Middle Age, Older Adulthood) × 3 (Valence: Negative, Neutral, Positive) × 2 (Component: Object, Background) × 2 (Delay: Sleep, Wake) mixed-model ANOVA, as well as a linear mixed-effects ANOVA treating age as a continuous variable, for both gist and specific memory. T-tests were conducted to examine pairwise comparisons in gist and specific memory across the following conditions, both for the total sample and within each age group: (1) negative, positive and neutral objects, (2) neutral backgrounds associated with negative, positive, and neutral objects, and (3) objects and backgrounds within each scene type. To assess age-related differences in gist and specific memory, we carried out follow-up t tests to compare memory performance between early middle-aged, late middle-aged, and older adults and tested Pearson’s correlations between age and memory for all six scene components. To test how valence and arousal ratings during encoding influence memory performance, we conducted six separate multiple regression analyses. Each regression model included both valence and arousal ratings as predictors on a continuous scale, predicting memory for one of the six scene components.
We calculated a memory trade-off magnitude as corrected recognition memory for (positive, negative, and neutral) objects minus corrected recognition memory for their paired neutral backgrounds during encoding, for both gist and specific memory. A positive memory trade-off magnitude indicates better memory for objects at the cost of their associated backgrounds, a magnitude of zero signifies equal memory performance between objects and backgrounds, while a negative magnitude reflects better memory for backgrounds at the expense of objects.
To analyze whether and when age-related positivity effects on memory trade-offs emerge in adulthood, we conducted a mixed-model ANOVA with a 3 (Age: Early Middle Age, Late Middle Age, Older Adulthood) × 3 (Valence: Negative, Neutral, Positive) × 2 (Delay: Sleep, Wake) design, respectively for the gist and specific memory trade-off measures. We followed-up these ANOVAs with pairwise t-tests across the following conditions: (1) negative, positive, and neutral scenes, (2) negative, positive, and neutral scenes within each age group, and (3) early middle-aged, late middle-aged, and older adults for each scene type. We also tested age as a continuous predictor in linear mixed-effects ANOVA, and assessed age-related correlations with gist and specific memory trade-off magnitudes for negative, positive, and neutral scenes. Finally, to test how valence and arousal ratings during encoding influence memory trade-off effects, we conducted three separate multiple regression analyses, using valence and arousal ratings as predictors in each model for memory trade-off effects on negative, positive, and neutral scenes, respectively.
Treating age as a categorical predictor, we found a significant main effect of scene valence on both valence [F(2, 536) = 1906.28, p < 0.001, adjusted p < 0.001, ηp2 = 0.88] and arousal ratings during encoding [F(2, 536) = 480.86, p < 0.001, adjusted p < 0.001, ηp2 = 0.64]. The main effects on valence [F(2, 540) = 59.77, p < 0.001, adjusted p < 0.001, ηp2 = 0.18] and arousal [F(2, 540) = 9.90, p < 0.001, adjusted p < 0.001, ηp2 = 0.04] were also significant when treating age as a continuous predictor. Follow-up t-tests were all highly significant in the expected directions (Supplementary Table S4).
We also found an interaction between age and scene valence on valence ratings, although this interaction only reached significant when age was considered as a categorical [F(4, 536) = 3.38, p = 0.010, adjusted p = 0.035, ηp2 = 0.03], and was marginal when age was considered as a continuous variable [F(2, 540) = 2.98, p = 0.052, adjusted p = 0.120, ηp2 = 0.01]. Compared to early middle-aged adults, late middle-aged [t(156) = 2.77, p = 0.006, adjusted p = 0.019, d = 0.44] and older adults [t(170) = 2.41, p = 0.017, adjusted p = 0.026, d = 0.35] both rated positive scenes as more positive. Older age was also significantly correlated with rating positive scenes more positively (r = 0.15, p = 0.013, adjusted p = 0.039). Similarly, late middle-aged [t(138) = −2.21, p = 0.029, adjusted p = 0.044, d = −0.35] and older adults [t(181) = −2.35, p = 0.020, adjusted p = 0.044, d = −0.34] also rated neutral scenes more positively compared to early middle-aged adults, although older age was not significantly correlated with more positive ratings for neutral scenes (r = 0.10, p = 0.088, adjusted p = 0.132). In summary, while there was no age-related difference in valence ratings for negative scenes, as age increased, there was an increased positive interpretation bias for both positive and neutral scenes (Figure 2).
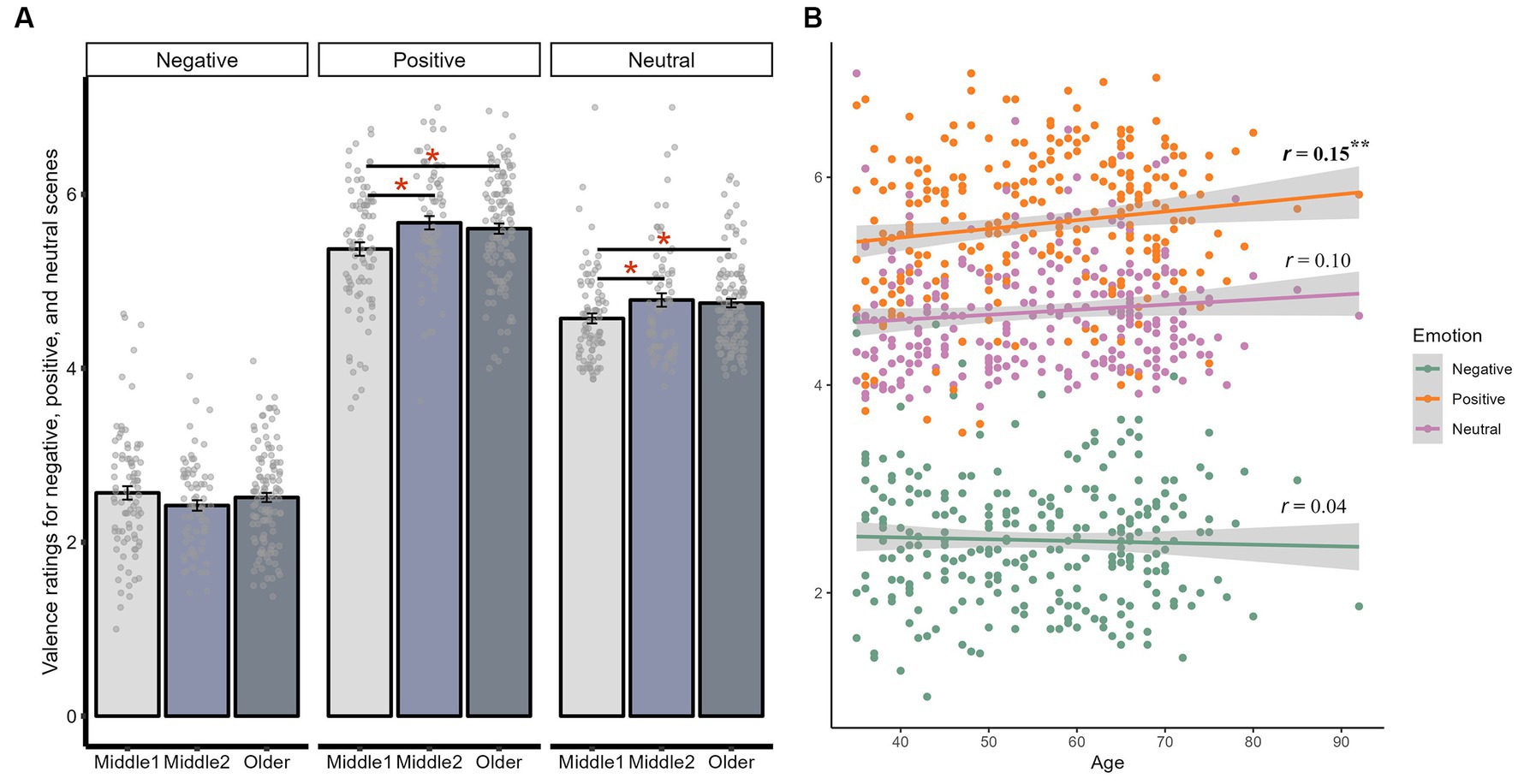
Figure 2. Interaction between age and valence on valence ratings. (A) The average valence ratings for negative, positive, and neutral scenes within early middle-aged, late middle-aged, and older adults. (B) The correlation between age and valence ratings for negative, positive, and neutral scenes. **p < 0.01; *p < 0.05.
The interaction between age and valence on arousal ratings, whether looking at age as a categorical [F(4, 536) = 2.79, p = 0.026, adjusted p = 0.091, ηp2 = 0.02] or continuous predictor [F(2, 540) = 3.91, p = 0.021, adjusted p = 0.072, ηp2 = 0.01], was only significant before adjusting for multiple comparisons. None of the pairwise age comparisons or age-related correlations were significant either (ps > 0.8, Supplementary Tables S4, S14). Notably, although both early [t(85) = 3.27, p = 0.002, adjusted p = 0.002, d = 0.28] and late middle-aged adults [t(72) = 2.78, p = 0.007, adjusted p = 0.007, d = 0.29] perceived positive scenes as more arousing than neutral scenes, this effect was not significant for older adults [t(114) = 0.81, p = 0.421, adjusted p = 0.421, d = 0.06]. However, the correlation between age and perceived arousal of positive information was not significant (r = 0.10, p = 0.088, adjusted p = 0.132).
We focused on gist memory given its resilience against age-related cognitive deficits and memory delay, especially over extended delay periods (Davis et al., 2003; Titz and Verhaeghen, 2010; Matorina and Poppenk, 2021). Results for specific memory mostly followed the same patterns as for gist memory (see Supplementary material).
We found a significant interaction between valence and component on gist memory, both with age as a three-tier category [F(2, 536) = 136.41, p < 0.001, adjusted p < 0.001, ηp2 = 0.34] and on a continuous scale [F(2, 1,350) = 4.37, p = 0.013, adjusted p = 0.048, ηp2 = 0.01]. Comparing object and background memory within negative, positive, and neutral scene type, memory for objects was better than that for backgrounds for both negative [t(273) = 17.06, p < 0.001, adjusted p < 0.001, d = 1.11] and positive scenes [t(273) = 19.41, p < 0.001, adjusted p < 0.001, d = 1.22], although this object-background comparison was not significant for neutral scenes [t(273) = 1.59, p = 0.113, adjusted p = 0.113, d = 0.09].
In addition, both negative [t(273) = 9.33, p < 0.001, adjusted p < 0.001, d = 0.53] and positive objects [t(273) = 11.91, p < 0.001, adjusted p < 0.001, d = 0.62] were remembered better than neutral objects. In contrast, backgrounds associated with negative [t(273) = −8.24, p < 0.001, adjusted p < 0.001, d = −0.44] and positive objects [t(273) = −10.08, p < 0.001, adjusted p < 0.001, d = −0.47] were remembered worse than those associated with neutral objects, although memory did not differ for backgrounds presented on negative and positive scenes [t(273) = 0.94, p = 0.349, adjusted p = 0.349, d = 0.05]. Although memory for positive objects was numerically better than that for negative objects, this difference was not significant [t(273) = 1.66, p = 0.099, adjusted p = 0.099, d = 0.09].
In line with the valence by component interaction on memory, we also found a significant main effect of valence on memory trade-off effects treating age as both a three-tier category [F(2, 536) = 136.41, p < 0.001, adjusted p < 0.001, ηp2 = 0.34] and a continuous variable [F(2, 540) = 59.77, p < 0.001, adjusted p < 0.001, ηp2 = 0.18]. Follow-up t-tests indicated that negative [t(273) = 13.07, p < 0.001, adjusted p < 0.001, d = 0.96] and positive memory trade-off magnitudes [t(273) = 15.68, p < 0.001, adjusted p < 0.001, d = 1.09] were both greater than neutral one. Interestingly, the positive memory trade-off magnitude was numerically greater than the negative one [t(273) = 1.90, p = 0.059, adjusted p = 0.059, d = 0.13], although this effect was not significant.
We found a significant interaction between age and valence on memory, both with age as a three-tier category [F(4, 536) = 4.84, p = 0.001, adjusted p = 0.004, ηp2 = 0.04] and on a continuous scale [F(2, 1,350) = 6.10, p = 0.002, adjusted p = 0.011, ηp2 = 0.01]. Although the three-way interaction between age, valence, and component was not significant with age being either a categorical [F(4, 536) = 0.53, p = 0.713, adjusted p = 0.823, ηp2 = 0.00] or continuous predictor [F(2, 1,350) = 0.33, p = 0.720, adjusted p = 0.806, ηp2 = 0.00], we conducted exploratory analyses to compare memory for objects and backgrounds separately as our research focus was not on memory collapsed across objects and backgrounds within each scene.
Comparing memory between negative and positive objects (Figure 3), older adults had significantly better memory for positive objects compared to negative ones [t(114) = 2.65, p = 0.009, adjusted p = 0.009, d = 0.26], while early and late middle-aged adults did not show significant differences (adjusted ps > 0.8, Supplementary Table S10). However, pairwise comparisons of memory between early middle-aged, late middle-aged, and older adults (adjusted ps > 0.9, Supplementary Table S9) and age-related correlations (adjusted ps > 0.6, Supplementary Table S14) were mostly not significant. Instead, all age groups showed similar patterns of better memory for both negative and positive objects compared to neutral objects, and worse memory for backgrounds presented on negative and positive scenes than neutral scenes (adjusted ps < 0.020, Supplementary Table S10).
The interaction between age and valence was not significant, either with age being a categorical [F(4, 536) = 0.53, p = 0.713, adjusted p = 0.897, ηp2 = 0.00] or continuous predictor [F(2, 540) = 0.44, p = 0.647, adjusted p = 0.755, ηp2 = 0.00]. Exploratory analyses indicated that, when comparing positive and negative memory trade-off effects (Figure 4), only older adults had a numerically greater positive than negative memory trade-off magnitude [t(114) = 1.91, p = 0.059, adjusted p = 0.059, d = 0.21]. In contrast, neither early [t(85) = 1.14, p = 0.258, adjusted p = 0.258, d = 0.13] nor late middle-aged groups [t(72) = −0.17, p = 0.862, adjusted p = 0.862, d = −0.02] showed significant differences.
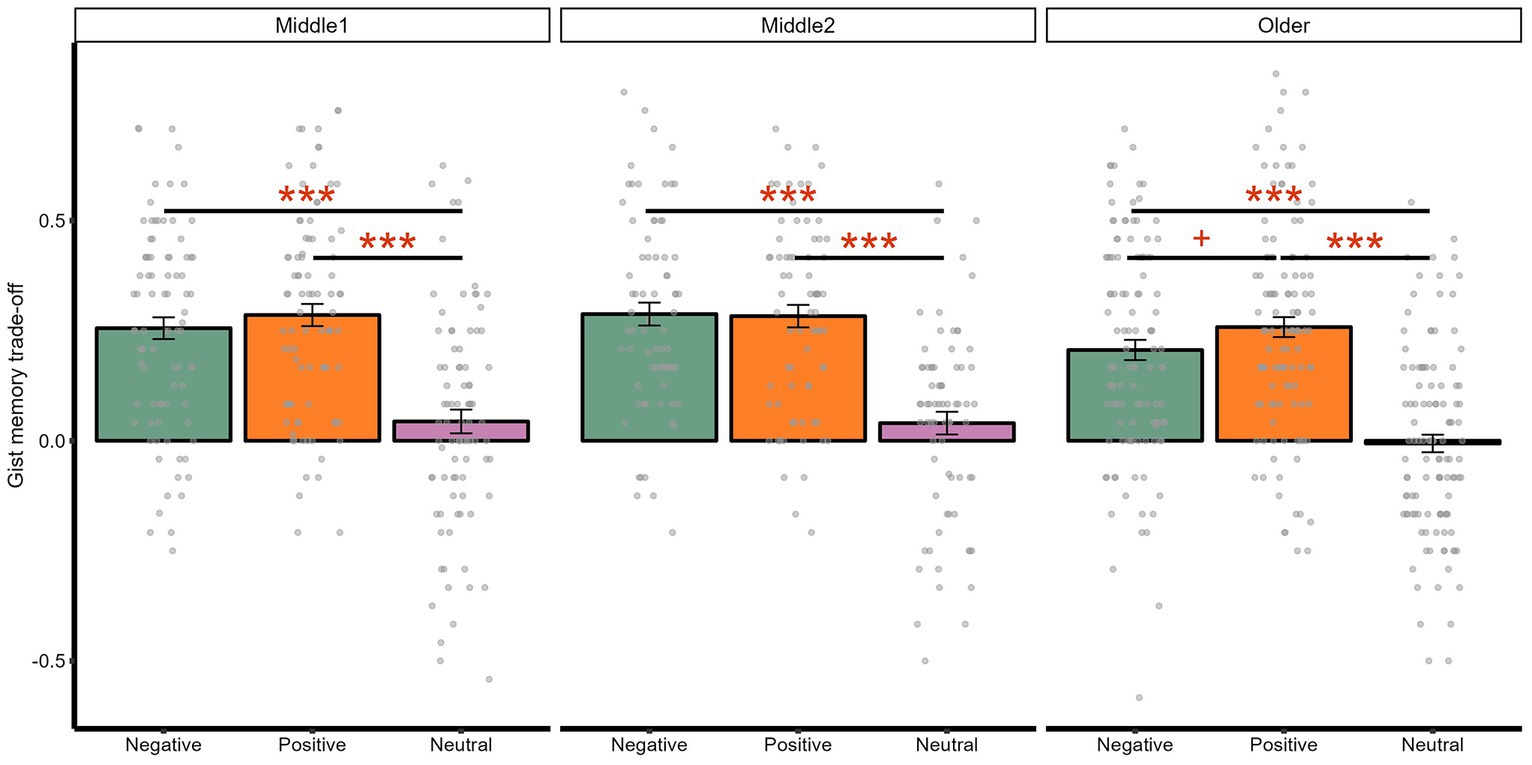
Figure 4. Interaction between age and valence on gist memory trade-off effects. ***p < 0.001; +p < 0.10.
Negative objects were remembered significantly better when scenes containing the negative objects were rated as more arousing during encoding (B = 0.06, p = 0.003, adjusted p = 0.016, R2 = 0.03). Descriptively, memory trade-off effects for negative scenes were greater when negative scenes were rated as more negative (B = −0.06, p = 0.012, adjusted p = 0.059, R2 = 0.02) and more arousing (B = 0.05, p = 0.030, adjusted p = 0.089, R2 = 0.02). There were no other significant effects of valence and arousal ratings on recognition memory or memory trade-off effects (ps > 0.9, Supplementary Tables S15, S16).
Delay conditions did not yield significant main effects or interaction effects with age and/or scene valences on valence/arousal ratings, corrected recognition memory, or memory trade-off magnitude, either with age being a categorical or continuous predictor (ps > 0.9, Supplementary Tables S3, S6, S12).
Our findings show that individuals interpret both positive and neutral information more positively as they approach older age, demonstrating the expected age-related positivity effect on valence ratings (i.e., positive interpretation bias). The age-related positivity effect also extends to memory. While none of the age groups exhibited a preference for remembering negative over positive information, older adults were more likely to remember positive than negative information. This was revealed as better memory for positive objects compared to negative objects on separate scenes, and descriptively speaking, greater positive than negative memory trade-off magnitude (i.e., the degree to which emotional objects were remembered better at the cost of their neutral peripheral backgrounds on the same scenes).
Consistent with prior research (Mather and Knight, 2005; Grühn and Scheibe, 2008; Gallo et al., 2009; Gilet et al., 2012; Schryer and Ross, 2012), we found that older age is correlated with perceiving positive events more positively. It is intriguing that older adults in our study also rated neutral scenes more positively, even though these stimuli have been previously normed as neutral in younger adults (Payne et al., 2008, 2012; Payne and Kensinger, 2011; Cunningham et al., 2014; Bennion et al., 2015, 2017; Denis et al., 2022; Sanders et al., n.d.). These results align with a previous study that this age-related positive interpretation bias also extends to neutral information (Mather and Knight, 2005). One possible mechanism underlying this interpretation bias is related to emotion regulation strategies that evolve with age. Specifically, compared to younger adults, older individuals tend to employ cognitive reappraisal to reframe their life experiences with positive interpretations (Liang et al., 2017; Nakagawa et al., 2017). This use of cognitive reappraisal and increased positive interpretation bias may contribute to a more positive overall outlook in older age, potentially promoting psychological resilience and satisfaction throughout the aging process (Höglund et al., 2020; Korkmaz and Güloğlu, 2021).
However, we failed to find a parallel association of increasing age and perceiving negative information less negatively. This is possibly because our negative stimuli consisted of highly negative content (e.g., snake, weapon, blood, vomit) that may have reached a functional ceiling with older participants, limiting the extent to which middle-aged adults could perceive them even more negatively. This potential ceiling effect might explain why universally negative information is not consistently perceived less negatively as individuals approach older age (Grühn and Scheibe, 2008; Streubel and Kunzmann, 2011; Gilet et al., 2012; Lee et al., 2023). In addition, our findings show that while middle-aged adults rated positive scenes as more arousing compared to neutral scenes, this pattern did not persist in older adults. Although age-related differences in arousal ratings did not reach significance, this may still point to a subtle shift in perceived arousal with age, consistent with previous findings on age-related decreases in perceived arousal to positive information (Keil and Freund, 2009; Gilet et al., 2012; Lee et al., 2023).
The finding that older adults remembered positive objects better than negative ones aligns with prior research that advancing age increases the positivity effect on memory (Reed et al., 2014). Notably, although this shift to a positivity memory bias is consistently found in studies where participants are instructed to passively view image or word stimuli (Charles et al., 2003; Leigland et al., 2004; Kensinger, 2008; Piguet et al., 2008; Spaniol et al., 2008), it is not observed in paradigms that impose informational processing constraints while viewing the stimuli (Kensinger et al., 2002; Denburg et al., 2003; Comblain et al., 2004; Grühn et al., 2005; Emery and Hess, 2008; Majerus and D’Argembeau, 2011; Leshikar et al., 2015; Eich and Castel, 2016). For example, when participants are explicitly instructed to remember stimuli (i.e., intentional encoding; Kensinger et al., 2002; Grühn et al., 2005; Emery and Hess, 2008; Majerus and D’Argembeau, 2011; Eich and Castel, 2016), or to make judgments about the stimuli during encoding, such as evaluating the complexity of visual images and determining whether verbal stimuli are self-referential (Comblain et al., 2004; Majerus and D’Argembeau, 2011), their ability to freely and naturally process emotional information may be hindered (Grühn et al., 2005; Gallo et al., 2009; Majerus and D’Argembeau, 2011). Therefore, the positivity effect may be attenuated when participants’ goals or focus are influenced by the experimental instruction (Carstensen and DeLiema, 2018; Sakaki et al., 2019).
Although we failed to find a linear association between older age and increasing positive memory bias, this is in accordance with prior research that age-related differences are only found when directly comparing older and younger, but not middle-aged adults (Fung et al., 2010; Reed et al., 2014). Critically, because most previous studies showing an age-related positivity effect focus on the comparisons between young and older adults (Leigland et al., 2004; Mather and Carstensen, 2005; Mikels et al., 2005; Kensinger, 2008; Piguet et al., 2008; Spaniol et al., 2008), it is not yet known whether this positivity effect already arises in middle age. The current study revealed that both early and late middle-aged adults do not show better memory for negative than positive information. This may suggest that the negativity bias and its influence on memory diminish in middle age, although a full transition to a positivity effect has not yet taken place. Another possibility for the null association between older age and increasing positive memory bias is that age-associated memory impairments counteract this positivity effect. Specifically, older adults tend to experience memory-related deficits due to declines in the structure and functionality of the hippocampus (Daselaar et al., 2006; de Flores et al., 2015). Although we focused on gist memory due to its resilience against forgetfulness, age-related memory deficits might still increase exponentially during the 12-h delay period (Davis et al., 2003; Titz and Verhaeghen, 2010; Matorina and Poppenk, 2021). Future research should incorporate retention intervals of varying lengths to explore the optimal delay condition during which positive aspects of memory are selectively consolidated more than accelerated forgetfulness impairs overall memory performance.
We replicated previous research findings on emotional memory trade-off effects, such that negative and positive objects were remembered better than both neutral objects on separate scenes and paired neutral backgrounds on the same scenes (Payne et al., 2008, 2012; Payne and Kensinger, 2011; Cunningham et al., 2014; Bennion et al., 2015, 2017; Denis et al., 2022; Sanders et al., n.d.). Interestingly, the typical pattern in younger adults, where neutral information in the background is less well-remembered when paired with a negative central object compared to a positive one (Denis et al., 2022; Sanders et al., n.d.), is not observed with middle-aged and older adults in the current study. Instead, in older age, the trade-off in emotional memory is more pronounced when the emotional object is positive as opposed to negative. This is potentially because emotionally positive events captures more attention than negative ones in older age (Noh and Isaacowitz, 2015; Erbey et al., 2020), thereby overshadowing the neutral and less salient aspects of the same event to a greater extent (Easterbrook, 1959; Reisberg and Heuer, 2004).
It is important to acknowledge that we did not explore how highly arousing, emotional information might have differential effects on memory bias from non-arousing, emotional information, especially because arousal and valence may influence memory processes through distinct mechanisms (Kensinger, 2004; Kensinger and Corkin, 2004). Prior research has shown that emotional memory induced by highly arousing information involves automatic processes and is well preserved with age (Kensinger, 2008), especially for negative stimuli (Mather and Knight, 2006; Leclerc and Kensinger, 2008). In contrast, emotional memory induced by non-arousing, negatively-valenced information tends to decline with age (Nashiro and Mather, 2011; Lee et al., 2023), as older adults may be utilizing cognitive control mechanisms via prefrontal cortex pathways to down-regulate their negative affect processing (Williams et al., 2006; St Jacques et al., 2009; Peña-Gómez et al., 2011). In line with prior research, our findings show that negative information is better remembered among middle-aged and older adults when it is perceived as arousing, but not when it is perceived as unpleasant. These results suggest that the age-related shift from negative to positive memory bias is most robust when comparing non-arousing or low-arousing, negative vs. positive information.
In our study, where most negative stimuli were rated as highly arousing relative to positive stimuli, it is not surprising that older age was not associated with worse memory for negative scenes, as they are likely still protected by the arousal pathway. Nevertheless, older adults still demonstrated superior memory for positive compared to negative information, which may suggest that age-related positivity effect observed in our study is likely driven primarily by valence-related emotional processing. Still, more studies should incorporate a broader spectrum of valence and arousal levels in their negative and positive stimuli, which is essential for exploring the nuanced impact of valence and arousal, both independently and interactively, on memory-related processes.
We failed to find sleep-dependent benefit for gist memory, which aligns with previous research indicating that meaning-based general properties in gist memory, relative to episodic-like details in specific memory, are less susceptible to forgetting over time and are therefore not as protected by sleep-related consolidation processes (Stickgold and Walker, 2013; Lutz et al., 2017; Matorina and Poppenk, 2021). Another plausible explanation could involve the fact that most participants in the sleep condition did not complete the encoding session immediately before their bedtime, whereas prior research demonstrates that sleep may only enhance memory consolidation when it occurs closely after learning (Solano et al., 2023). In addition, contrary to what we initially hypothesized based upon prior research, sleep did not magnify the emotional memory trade-off effect to result in better memory for emotional objects at the expense of associated neutral backgrounds, as has been seen before (Payne et al., 2008, 2012; Payne and Kensinger, 2011; Cunningham et al., 2014; Bennion et al., 2015, 2017). However, it is worth noting that most of these previous studies examined college-aged adults (Payne et al., 2008, 2012; Payne and Kensinger, 2011; Cunningham et al., 2014; Bennion et al., 2015, 2017), and the functional link between sleep and memory likely weakens with advancing age (Tucker et al., 2011; Scullin, 2013). Overall, this is in line with the existing literature that the prioritization of emotional memory during sleep is more of an exception than the norm (Prehn-Kristensen et al., 2009, 2013; Baran et al., 2012; Morgenthaler et al., 2014; Göder et al., 2015; Bolinger et al., 2018; Kurz et al., 2019; Davidson et al., 2021). Additionally, previous studies indicate that the proportion of rapid eye movement sleep and slow-wave sleep (Cairney et al., 2015) and the local coupling between sleep spindles and slow oscillations (Solano et al., 2021) both play crucial roles in memory consolidation. Future research with polysomnography-monitored sleep data is needed to investigate how different aspects of sleep architecture may facilitate age-related shifts in positive memory bias.
With a large online sample drawn from various U.S. communities, our findings provide better generalizability compared to laboratory studies with participants limited to college students or a single community. However, our sample may not fully represent the broader U.S. population as it consisted of healthy adults who were primarily White and had the technological literacy to participate using Prolific. It is also important to note that the attrition rate (50%) in our study was relatively high compared to in-person laboratory studies, as half of the eligible participants did not complete the follow-up experimental sessions. Although our attrition rate is still considered typical for longitudinal online studies, it may still impact the generalizability of our findings (Khadjesari et al., 2011; Rübsamen et al., 2017).
This is one of the first studies to examine emotional processing and delayed emotional memory performance in a sample of healthy middle age and older adults. Our findings support the age-related positivity effect in memory across a relatively long consolidation delay (approximately 12-h). Moreover, we provide well-powered evidence that while a shift toward positive processing emerges in middle age, the positivity bias in memory may not emerge until older adulthood. Efforts to further understand these age-related positivity effects, their mechanisms, boundary conditions, and impact on cognitive and emotional processing will be important if we are to better understand and promote mental well-being and cognitive functioning in aging adults.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.17605/OSF.IO/8Q2VW.
The studies involving humans were approved by The Institutional Review Board at the University of Notre Dame. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XN: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MU: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. KS: Data curation, Investigation, Methodology, Resources, Software, Supervision, Visualization, Writing – review & editing. EK: Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing. JP: Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing. DD: Data curation, Investigation, Methodology, Resources, Software, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Science Foundation under Grant BCS-2001025, awarded to JP as the Principal Investigator and EK as the co-Principal Investigator.
We thank Brooke Borton and Skye Harris for contributions to participant recruitment and data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2024.1342589/full#supplementary-material
Alger, S. E., Kensinger, E. A., and Payne, J. D. (2018). Preferential consolidation of emotionally salient information during a nap is preserved in middle age. Neurobiol. Aging 68, 34–47. doi: 10.1016/j.neurobiolaging.2018.03.030
Allen, J. S., Bruss, J., Brown, C. K., and Damasio, H. (2005). Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol. Aging 26, 1245–1260; discussion 1279-1282. doi: 10.1016/j.neurobiolaging.2005.05.023
Anderson, A. K. (2007). Feeling emotional: the amygdala links emotional perception and experience. Soc. Cogn. Affect. Neurosci. 2, 71–72. doi: 10.1093/scan/nsm022
Baran, B., Pace-Schott, E. F., Ericson, C., and Spencer, R. M. C. (2012). Processing of emotional reactivity and emotional memory over sleep. J. Neurosci. 32, 1035–1042. doi: 10.1523/JNEUROSCI.2532-11.2012
Barber, S. J., and Kim, H. (2021). “The positivity effect: a review of theories and recent findings” in Multiple pathways of cognitive aging: Motivational and contextual influences. eds. G. Sedek, T. Hess, and D. Touron (Oxford, UK: Oxford University Press).
Basner, M., Mollicone, D., and Dinges, D. F. (2011). Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to Total and partial sleep deprivation. Acta Astronaut. 69, 949–959. doi: 10.1016/j.actaastro.2011.07.015
Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N., and Golani, I. (2001). Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284. doi: 10.1016/S0166-4328(01)00297-2
Bennion, K. A., Mickley Steinmetz, K. R., Kensinger, E. A., and Payne, J. D. (2015). Sleep and cortisol interact to support memory consolidation. Cereb. Cortex 25, 646–657. doi: 10.1093/cercor/bht255
Bennion, K. A., Payne, J. D., and Kensinger, E. A. (2017). Residual effects of emotion are reflected in enhanced visual activity after sleep. Cogn. Affect. Behav. Neurosci. 17, 290–304. doi: 10.3758/s13415-016-0479-3
Bolinger, E., Born, J., and Zinke, K. (2018). Sleep divergently affects cognitive and automatic emotional response in children. Neuropsychologia 117, 84–91. doi: 10.1016/j.neuropsychologia.2018.05.015
Brabec, J., Rulseh, A., Hoyt, B., Vizek, M., Horinek, D., Hort, J., et al. (2010). Volumetry of the human amygdala—an anatomical study. Psychiatry Res. Neuroimaging 182, 67–72. doi: 10.1016/j.pscychresns.2009.11.005
Cairney, S. A., Durrant, S. J., Power, R., and Lewis, P. A. (2015). Complementary roles of slow-wave sleep and rapid eye movement sleep in emotional memory consolidation. Cereb. Cortex 25, 1565–1575. doi: 10.1093/cercor/bht349
Carstensen, L. L. (2006). The influence of a sense of time on human development. Science 312, 1913–1915. doi: 10.1126/science.1127488
Carstensen, L. L., and DeLiema, M. (2018). The positivity effect: a negativity bias in youth fades with age. Curr. Opin. Behav. Sci. 19, 7–12. doi: 10.1016/j.cobeha.2017.07.009
Carstensen, L. L., Isaacowitz, D. M., and Charles, S. T. (1999). Taking time seriously: a theory of socioemotional selectivity. Am. Psychol. 54, 165–181. doi: 10.1037/0003-066X.54.3.165
Carstensen, L. L., and Mikels, J. A. (2005). At the intersection of emotion and cognition: aging and the positivity effect. Curr. Dir. Psychol. Sci. 14, 117–121. doi: 10.1111/j.0963-7214.2005.00348.x
Chambers, A. M., Payne, J. D., Chambers, A. M., and Payne, J. D. (2014). The influence of sleep on the consolidation of positive emotional memories: preliminary evidence. AIMSN 1, 39–51. doi: 10.3934/Neuroscience.2014.1.39
Charles, S. T., Mather, M., and Carstensen, L. L. (2003). Aging and emotional memory: the forgettable nature of negative images for older adults. J. Exp. Psychol. Gen. 132, 310–324. doi: 10.1037/0096-3445.132.2.310
Christianson, S.-A. (Ed.). (2014). The handbook of emotion and memory: Research and theory. New York: Psychology Press.
Comblain, C., D’Argembeau, A., Van der Linden, M., and Aldenhoff, L. (2004). The effect of ageing on the recollection of emotional and neutral pictures. Memory 12, 673–684. doi: 10.1080/09658210344000477
Cook, A. M., Klin, C. M., and Westerman, D. L. (2023). Surviving with story characters: what do we remember? Mem. Cogn. 51, 1303–1316. doi: 10.3758/s13421-022-01391-2
Cummings, L. R., Graur, S., McMakin, D. L., and Fournier, J. C. (2018). 0973 sleep disturbance and emotion regulation dysfunction in depression: self-report and neural evidence. Sleep 41:A361. doi: 10.1093/sleep/zsy061.972
Cunningham, T. J., Chambers, A. M., and Payne, J. D. (2014). Prospection and emotional memory: how expectation affects emotional memory formation following sleep and wake. Front. Psychol. 5:862. doi: 10.3389/fpsyg.2014.00862
Daselaar, S. M., Fleck, M. S., Dobbins, I. G., Madden, D. J., and Cabeza, R. (2006). Effects of healthy aging on hippocampal and Rhinal memory functions: an event-related fMRI study. Cereb. Cortex 16, 1771–1782. doi: 10.1093/cercor/bhj112
Davidson, P., Jönsson, P., Carlsson, I., and Pace-Schott, E. (2021). Does sleep selectively strengthen certain memories over others based on emotion and perceived future relevance? Nat. Sci. Sleep 13, 1257–1306. doi: 10.2147/NSS.S286701
Davis, H. P., Small, S. A., Stern, Y., Mayeux, R., Feldstein, S. N., and Keller, F. R. (2003). Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex 39, 1063–1091. doi: 10.1016/S0010-9452(08)70878-5
De Cesarei, A., and Codispoti, M. (2011). Affective modulation of the LPP and α-ERD during picture viewing. Psychophysiology 48, 1397–1404. doi: 10.1111/j.1469-8986.2011.01204.x
de Flores, R., La Joie, R., and Chételat, G. (2015). Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience 309, 29–50. doi: 10.1016/j.neuroscience.2015.08.033
de Leeuw, J. R. (2015). jsPsych: a JavaScript library for creating behavioral experiments in a web browser. Behav. Res. Methods 47, 1–12. doi: 10.3758/s13428-014-0458-y
Denburg, N. L., Buchanan, T. W., Tranel, D., and Adolphs, R. (2003). Evidence for preserved emotional memory in normal older persons. Emotion 3, 239–253. doi: 10.1037/1528-3542.3.3.239
Denis, D., Sanders, K. E. G., Kensinger, E. A., and Payne, J. D. (2022). Sleep preferentially consolidates negative aspects of human memory: well-powered evidence from two large online experiments. Proc. Natl. Acad. Sci. U. S. A. 119:e2202657119. doi: 10.1073/pnas.2202657119
Easterbrook, J. A. (1959). The effect of emotion on cue utilization and the organization of behavior. Psychol. Rev. 66, 183–201. doi: 10.1037/h0047707
Eich, T. S., and Castel, A. D. (2016). The cognitive control of emotional versus value-based information in younger and older adults. Psychol. Aging 31, 503–512. doi: 10.1037/pag0000106
Emery, L., and Hess, T. M. (2008). Viewing instructions impact emotional memory differently in older and young adults. Psychol. Aging 23, 2–12. doi: 10.1037/0882-7974.23.1.2
Erbey, M., Roebbig, J., Babayan, A., Kumral, D., Reinelt, J., Reiter, A. M. F., et al. (2020). Positivity in younger and in older age: associations with future time perspective and socioemotional functioning. Front. Psychol. 11:567133. doi: 10.3389/fpsyg.2020.567133
Ferrari, V., Codispoti, M., Cardinale, R., and Bradley, M. M. (2008). Directed and motivated attention during processing of natural scenes. J. Cogn. Neurosci. 20, 1753–1761. doi: 10.1162/jocn.2008.20121
Fung, H. H., Isaacowitz, D. M., Lu, A. Y., and Li, T. (2010). Interdependent self-construal moderates the age-related negativity reduction effect in memory and visual attention. Psychol. Aging 25, 321–329. doi: 10.1037/a0019079
Gallo, D. A., Foster, K. T., and Johnson, E. L. (2009). Elevated false recollection of emotional pictures in young and older adults. Psychol. Aging 24, 981–988. doi: 10.1037/a0017545
Gilet, A.-L., Grühn, D., Studer, J., and Labouvie-Vief, G. (2012). Valence, arousal, and imagery ratings for 835 French attributes by young, middle-aged, and older adults: the French emotional evaluation list (FEEL). Eur. Rev. Appl. Psychol. 62, 173–181. doi: 10.1016/j.erap.2012.03.003
Göder, R., Graf, A., Ballhausen, F., Weinhold, S., Baier, P. C., Junghanns, K., et al. (2015). Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 16, 564–569. doi: 10.1016/j.sleep.2014.12.022
Grühn, D., and Scheibe, S. (2008). Age-related differences in valence and arousal ratings of pictures from the international affective picture system (IAPS): do ratings become more extreme with age? Behav. Res. Methods 40, 512–521. doi: 10.3758/brm.40.2.512
Grühn, D., Smith, J., and Baltes, P. B. (2005). No aging bias favoring memory for positive material: evidence from a heterogeneity-homogeneity list paradigm using emotionally toned words. Psychol. Aging 20, 579–588. doi: 10.1037/0882-7974.20.4.579
Hajcak, G., and Nieuwenhuis, S. (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn. Affect. Behav. Neurosci. 6, 291–297. doi: 10.3758/cabn.6.4.291
Höglund, P., Hakelind, C., and Nordin, S. (2020). Severity and prevalence of various types of mental ill-health in a general adult population: age and sex differences. BMC Psychiatry 20:209. doi: 10.1186/s12888-020-02557-5
Holland, A. C., and Kensinger, E. A. (2010). Emotion and autobiographical memory. Phys Life Rev 7, 88–131. doi: 10.1016/j.plrev.2010.01.006
Isaacowitz, D. M. (2022). What do we know about aging and emotion regulation? Perspect. Psychol. Sci. 17, 1541–1555. doi: 10.1177/17456916211059819
Isaacowitz, D. M., Toner, K., and Neupert, S. D. (2009). Use of gaze for real-time mood regulation: effects of age and attentional functioning. Psychol. Aging 24, 989–994. doi: 10.1037/a0017706
Isaacowitz, D. M., Wadlinger, H. A., Goren, D., and Wilson, H. R. (2006). Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychol. Aging 21, 40–48. doi: 10.1037/0882-7974.21.1.40
Kauschke, C., Bahn, D., Vesker, M., and Schwarzer, G. (2019). The role of emotional valence for the processing of facial and verbal stimuli—positivity or negativity Bias? Front. Psychol. 10, 10:1654. doi: 10.3389/fpsyg.2019.01654
Keil, A., and Freund, A. M. (2009). Changes in the sensitivity to appetitive and aversive arousal across adulthood. Psychol. Aging 24, 668–680. doi: 10.1037/a0016969
Kensinger, E. A. (2004). Remembering emotional experiences: the contribution of valence and arousal. Rev. Neurosci. 15, 241–251. doi: 10.1515/revneuro.2004.15.4.241
Kensinger, E. A. (2008). Age differences in memory for arousing and nonarousing emotional words. J. Gerontol. B Psychol. Sci. Soc. Sci. 63, P13–P18. doi: 10.1093/geronb/63.1.p13
Kensinger, E. A. (2009). Remembering the details: effects of emotion. Emot. Rev. 1, 99–113. doi: 10.1177/1754073908100432
Kensinger, E. A., Brierley, B., Medford, N., Growdon, J. H., and Corkin, S. (2002). Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion 2, 118–134. doi: 10.1037/1528-3542.2.2.118
Kensinger, E. A., and Corkin, S. (2004). Two routes to emotional memory: distinct neural processes for valence and arousal. Proc. Natl. Acad. Sci. U. S. A. 101, 3310–3315. doi: 10.1073/pnas.0306408101
Khadjesari, Z., Murray, E., Kalaitzaki, E., White, I. R., McCambridge, J., Thompson, S. G., et al. (2011). Impact and costs of incentives to reduce attrition in online trials: two randomized controlled trials. J. Med. Internet Res. 13:e1523:e26. doi: 10.2196/jmir.1523
Kisley, M. A., Wood, S., and Burrows, C. L. (2007). Looking at the sunny side of life: age-related change in an event-related potential measure of the negativity bias. Psychol. Sci. 18, 838–843. doi: 10.1111/j.1467-9280.2007.01988.x
Knight, M., Seymour, T. L., Gaunt, J. T., Baker, C., Nesmith, K., and Mather, M. (2007). Aging and goal-directed emotional attention: distraction reverses emotional biases. Emotion 7, 705–714. doi: 10.1037/1528-3542.7.4.705
Korkmaz, H., and Güloğlu, B. (2021). The role of uncertainty tolerance and meaning in life on depression and anxiety throughout Covid-19 pandemic. Personal. Individ. Differ. 179:110952. doi: 10.1016/j.paid.2021.110952
Kurz, E., Conzelmann, A., Barth, G. M., Hepp, L., Schenk, D., Renner, T. J., et al. (2019). Signs of enhanced formation of gist memory in children with autism spectrum disorder – a study of memory functions of sleep. J. Child Psychol. Psychiatry 60, 907–916. doi: 10.1111/jcpp.13048
Labouvie-Vief, G., Grühn, D., and Studer, J. (2010). “Dynamic integration of emotion and cognition: equilibrium regulation in development and aging” in The handbook of life-span development, Vol 2: Social and emotional development (Hoboken, NJ: John Wiley & Sons, Inc.), 79–115.
Langeslag, S. J. E., and van Strien, J. W. (2009). Aging and emotional memory: the co-occurrence of neurophysiological and behavioral positivity effects. Emotion 9, 369–377. doi: 10.1037/a0015356
Leclerc, C. M., and Kensinger, E. A. (2008). Effects of age on detection of emotional information. Psychol. Aging 23, 209–215. doi: 10.1037/0882-7974.23.1.209
Leclerc, C. M., and Kensinger, E. A. (2011). Neural processing of emotional pictures and words: a comparison of young and older adults. Dev. Neuropsychol. 36, 519–538. doi: 10.1080/87565641.2010.549864
Lee, K., Sayre, B., James, T. A., and Duarte, A. (2023). Age-related reductions in arousal-enhanced memory are moderated by trait emotion regulation. Sci. Rep. 13:15469. doi: 10.1038/s41598-023-41741-x
Leigland, L. A., Schulz, L. E., and Janowsky, J. S. (2004). Age related changes in emotional memory. Neurobiol. Aging 25, 1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015
Leshikar, E. D., Dulas, M. R., and Duarte, A. (2015). Self-referencing enhances recollection in both young and older adults. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 22, 388–412. doi: 10.1080/13825585.2014.957150
Liang, Y., Huo, M., Kennison, R., and Zhou, R. (2017). The role of cognitive control in older adult cognitive reappraisal: detached and positive reappraisal. Front. Behav. Neurosci. 11:27. doi: 10.3389/fnbeh.2017.00027
Lutz, N. D., Diekelmann, S., Hinse-Stern, P., Born, J., and Rauss, K. (2017). Sleep supports the slow abstraction of gist from visual perceptual memories. Sci. Rep. 7:42950. doi: 10.1038/srep42950
Majerus, S., and D’Argembeau, A. (2011). Verbal short-term memory reflects the organization of long-term memory: further evidence from short-term memory for emotional words. J. Mem. Lang. 64, 181–197. doi: 10.1016/j.jml.2010.10.003
Mather, M., Canli, T., English, T., Whitfield, S., Wais, P., Ochsner, K., et al. (2004). Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol. Sci. 15, 259–263. doi: 10.1111/j.0956-7976.2004.00662.x
Mather, M., and Carstensen, L. L. (2003). Aging and attentional biases for emotional faces. Psychol. Sci. 14, 409–415. doi: 10.1111/1467-9280.01455
Mather, M., and Carstensen, L. L. (2005). Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn. Sci. 9, 496–502. doi: 10.1016/j.tics.2005.08.005
Mather, M., and Knight, M. (2005). Goal-directed memory: the role of cognitive control in older adults’ emotional memory. Psychol. Aging 20, 554–570. doi: 10.1037/0882-7974.20.4.554
Mather, M., and Knight, M. R. (2006). Angry faces get noticed quickly: threat detection is not impaired among older adults. J. Gerontol. Ser. B 61, P54–P57. doi: 10.1093/geronb/61.1.P54
Mathieu, N. G., Gentaz, E., Harquel, S., Vercueil, L., Chauvin, A., Bonnet, S., et al. (2014). Brain processing of emotional scenes in aging: effect of arousal and affective context. PLoS One 9:e99523. doi: 10.1371/journal.pone.0099523
Matorina, N., and Poppenk, J. (2021). Memory decay distinguishes subtypes of gist. Neurobiol. Learn. Mem. 185:107519. doi: 10.1016/j.nlm.2021.107519
McGaugh, J. L. (2000). Memory--a century of consolidation. Science 287, 248–251. doi: 10.1126/science.287.5451.248
Mikels, J. A., Larkin, G. R., Reuter-Lorenz, P. A., and Carstensen, L. L. (2005). Divergent trajectories in the aging mind: changes in working memory for affective versus visual information with age. Psychol. Aging 20, 542–553. doi: 10.1037/0882-7974.20.4.542
Morgenthaler, J., Wiesner, C. D., Hinze, K., Abels, L. C., Prehn-Kristensen, A., and Göder, R. (2014). Selective REM-sleep deprivation does not diminish emotional memory consolidation in young healthy subjects. PLoS One 9:e89849. doi: 10.1371/journal.pone.0089849
Nakagawa, T., Gondo, Y., Ishioka, Y., and Masui, Y. (2017). Age, emotion regulation, and affect in adulthood: the mediating role of cognitive reappraisal. Jpn. Psychol. Res. 59, 301–308. doi: 10.1111/jpr.12159
Nashiro, K., and Mather, M. (2011). How arousal affects younger and older adults’ memory binding. Exp. Aging Res. 37, 108–128. doi: 10.1080/0361073X.2011.536746
Nashiro, K., Sakaki, M., and Mather, M. (2011). Age differences in brain activity during emotion processing: reflections of age-related decline or increased emotion regulation. Gerontology 58, 156–163. doi: 10.1159/000328465
Noh, S. R., and Isaacowitz, D. M. (2015). The effects of varying contextual demands on age-related positive gaze preferences. Psychol. Aging 30, 356–368. doi: 10.1037/a0039233
Payne, J., Chambers, A., and Kensinger, E. (2012). Sleep promotes lasting changes in selective memory for emotional scenes. Front. Integr. Neurosci. 6:108. doi: 10.3389/fnint.2012.00108
Payne, J. D., and Kensinger, E. A. (2011). Sleep leads to changes in the emotional memory trace: evidence from fMRI. J. Cogn. Neurosci. 23, 1285–1297. doi: 10.1162/jocn.2010.21526
Payne, J. D., and Kensinger, E. A. (2018). Stress, sleep, and the selective consolidation of emotional memories. Curr. Opin. Behav. Sci. 19, 36–43. doi: 10.1016/j.cobeha.2017.09.006
Payne, J. D., Kensinger, E. A., Wamsley, E. J., Spreng, R. N., Alger, S. E., Gibler, K., et al. (2015). Napping and the selective consolidation of negative aspects of scenes. Emotion 15, 176–186. doi: 10.1037/a0038683
Payne, J. D., Stickgold, R., Swanberg, K., and Kensinger, E. A. (2008). Sleep preferentially enhances memory for emotional components of scenes. Psychol. Sci. 19, 781–788. doi: 10.1111/j.1467-9280.2008.02157.x
Peña-Gómez, C., Vidal-Piñeiro, D., Clemente, I. C., Pascual-Leone, Á., and Bartrés-Faz, D. (2011). Down-regulation of negative emotional processing by transcranial direct current stimulation: effects of personality characteristics. PLoS One 6:e22812. doi: 10.1371/journal.pone.0022812
Piguet, O., Connally, E., Krendl, A. C., Huot, J. R., and Corkin, S. (2008). False memory in aging: effects of emotional valence on word recognition accuracy. Psychol. Aging 23, 307–314. doi: 10.1037/0882-7974.23.2.307
Prehn-Kristensen, A., Göder, R., Chirobeja, S., Bressmann, I., Ferstl, R., and Baving, L. (2009). Sleep in children enhances preferentially emotional declarative but not procedural memories. J. Exp. Child Psychol. 104, 132–139. doi: 10.1016/j.jecp.2009.01.005
Prehn-Kristensen, A., Munz, M., Molzow, I., Wilhelm, I., Wiesner, C. D., and Baving, L. (2013). Sleep promotes consolidation of emotional memory in healthy children but not in children with attention-deficit hyperactivity disorder. PLoS One 8:e65098. doi: 10.1371/journal.pone.0065098
R Core Team (2022). R: a language and environment for statistical computing. Available at: https://www.R-project.org/.
Reed, A. E., Chan, L., and Mikels, J. A. (2014). Meta-analysis of the age-related positivity effect: age differences in preferences for positive over negative information. Psychol. Aging 29, 1–15. doi: 10.1037/a0035194
Reisberg, D., and Heuer, F. (2004). “Memory for emotional events” in Memory and emotion. eds. D. Reisberg and P. Hertel (Oxford, UK: Oxford University Press).
Reyna, V. F. (2012). A new intuitionism: meaning, memory, and development in fuzzy-trace theory. Judgm. Decis. Mak. 7, 332–359. doi: 10.1017/S1930297500002291
Rösler, A., Ulrich, C., Billino, J., Sterzer, P., Weidauer, S., Bernhardt, T., et al. (2005). Effects of arousing emotional scenes on the distribution of visuospatial attention: changes with aging and early subcortical vascular dementia. J. Neurol. Sci. 229-230, 109–116. doi: 10.1016/j.jns.2004.11.007
Rübsamen, N., Akmatov, M. K., Castell, S., Karch, A., and Mikolajczyk, R. T. (2017). Factors associated with attrition in a longitudinal online study: results from the HaBIDS panel. BMC Med. Res. Methodol. 17:132. doi: 10.1186/s12874-017-0408-3
Sakaki, M., Raw, J. A. L., Findlay, J., and Thottam, M. (2019). Advanced aging enhances the positivity effect in memory: due to cognitive control or age-related decline in emotional processing? Collabra. Psychology 5:49. doi: 10.1525/collabra.222
Samanez-Larkin, G. R., and Carstensen, L. L. (2011). “Socioemotional functioning and the aging brain” in The Oxford handbook of social neuroscience Oxford library of psychology (New York, NY, US: Oxford University Press), 507–521. doi: 10.1093/oxfordhb/9780195342161.001.0001
Sanders, K. E. G., Denis, D., Niu, X., Kensinger, E. A., and Payne, J. D. (n.d.). Sleep’s relative effect on emotional memory from young adulthood to middle age.
Schryer, E., and Ross, M. (2012). Evaluating the valence of remembered events: the importance of age and self-relevance. Psychol. Aging 27, 237–242. doi: 10.1037/a0023283
Scullin, M. K. (2013). Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol. Aging 28, 105–114. doi: 10.1037/a0028830
Sergerie, K., Chochol, C., and Armony, J. L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 32, 811–830. doi: 10.1016/j.neubiorev.2007.12.002
Shohamy, D., and Daw, N. D. (2015). Integrating memories to guide decisions. Curr. Opin. Behav. Sci. 5, 85–90. doi: 10.1016/j.cobeha.2015.08.010
Solano, A., Lerner, G., Griffa, G., Deleglise, A., Caffaro, P., Riquelme, L., et al. (2023). Sleep consolidation potentiates skill maintenance. bioRxiv: 2023.10.26.564031. doi: 10.1101/2023.10.26.564031
Solano, A., Riquelme, L. A., Perez-Chada, D., and Della-Maggiore, V. (2021). Local coupling between sleep spindles and slow oscillations supports the stabilization of motor memories. bioRxiv: 2020.08.24.264697. doi: 10.1101/2020.08.24.264697
Spaniol, J., Voss, A., and Grady, C. L. (2008). Aging and emotional memory: cognitive mechanisms underlying the positivity effect. Psychol. Aging 23, 859–872. doi: 10.1037/a0014218
St Jacques, P. L., Dolcos, F., and Cabeza, R. (2009). Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol. Sci. 20, 74–84. doi: 10.1111/j.1467-9280.2008.02258.x
Stickgold, R., and Walker, M. P. (2013). Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 16, 139–145. doi: 10.1038/nn.3303
Streubel, B., and Kunzmann, U. (2011). Age differences in emotional reactions: arousal and age-relevance count. Psychol. Aging 26, 966–978. doi: 10.1037/a0023424
Titz, C., and Verhaeghen, P. (2010). Aging and directed forgetting in episodic memory: a meta-analysis. Psychol. Aging 25, 405–411. doi: 10.1037/a0017225
Tucker, M., McKinley, S., and Stickgold, R. (2011). Sleep optimizes motor skill in the elderly. J. Am. Geriatr. Soc. 59, 603–609. doi: 10.1111/j.1532-5415.2011.03324.x
Vargas, I., Payne, J. D., Muench, A., Kuhlman, K. R., and Lopez-Duran, N. L. (2019). Acute sleep deprivation and the selective consolidation of emotional memories. Learn. Mem. 26, 176–181. doi: 10.1101/lm.049312.119
Waszczuk, M. A., Zavos, H. M. S., Gregory, A. M., and Eley, T. C. (2016). The stability and change of etiological influences on depression, anxiety symptoms and their co-occurrence across adolescence and young adulthood. Psychol. Med. 46, 161–175. doi: 10.1017/S0033291715001634
Williams, L. M., Brown, K. J., Palmer, D., Liddell, B. J., Kemp, A. H., Olivieri, G., et al. (2006). The mellow years?: neural basis of improving emotional stability over age. J. Neurosci. 26, 6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006
Williams, S. E., Ford, J. H., and Kensinger, E. A. (2022). The power of negative and positive episodic memories. Cogn. Affect. Behav. Neurosci. 22, 869–903. doi: 10.3758/s13415-022-01013-z
Keywords: emotional memory, aging, positivity effect, middle age, older adulthood, memory consolidation, memory trade-off, valence
Citation: Niu X, Utayde MF, Sanders KEG, Denis D, Kensinger EA and Payne JD (2024) Age-related positivity effect in emotional memory consolidation from middle age to late adulthood. Front. Behav. Neurosci. 18:1342589. doi: 10.3389/fnbeh.2024.1342589
Received: 22 November 2023; Accepted: 08 January 2024;
Published: 24 January 2024.
Edited by:
David Clewett, University of California, Los Angeles, United StatesReviewed by:
Andrea C. Medina, National Autonomous University of Mexico, MexicoCopyright © 2024 Niu, Utayde, Sanders, Denis, Kensinger and Payne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica D. Payne, anBheW5lN0BuZC5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.