- 1Department of Psychiatry and Behavioral Medicine, East Carolina University, Greenville, NC, United States
- 2Internal Medicine and Psychiatry Combined Program, Department of Psychiatry and Behavioral Medicine, East Carolina University, Greenville, NC, United States
- 3East Carolina University Brody School of Medicine, Greenville, NC, United States
Major depressive disorder affects approximately 8.4% of the United States population. The World Health Organization estimates that 280 million adults worldwide are suffering from depression. They have estimated that by 2030 it will be the second most serious condition. Current treatment relies on the monoamine hypothesis, however, one-third of patients with MDD do not respond to monoamine-based antidepressants. For years, it was hypothesized that the primary pathway of MDD involved serotonin as the main neurotransmitter. The monoamine hypothesis, a widely accepted theory, sought to explain the biological basis of MDD as being caused by the depletion of monoamine neurotransmitters, namely norepinephrine and serotonin. This hypothesis regarding monoamines as the pathophysiological basis of MDD led to the design and widespread use of selective serotonin reuptake inhibitors. However, given that only one-third of patients improve with SSRI it is reasonable to infer that the pathway involved is more complex than once hypothesized and there are more neurotransmitters, receptors, and molecules involved. The monoamine hypothesis does not explain why there is a delay in the onset of effect and action of SSRIs. Several studies have demonstrated that chronic stress is a risk factor for the development of MDD. Thus the monoamine hypothesis alone is not enough to fully account for the pathophysiology of MDD highlighting the need for further research involving the pathways of MDD. In this paper, we review the role of inflammation and cytokines on MDD and discuss other pathways involved in the development and persistence of depressive symptoms.
Depression epidemiology and diagnostic criteria
Major depressive disorder (MDD) affects approximately 8.4% of the United States (US) population. Roughly 20 million adults in the US have struggled with MDD at least once in their lives [National Institute of Mental Health (NIMH), n.d.]. The World Health Organization estimates that 280 million adults worldwide are suffering from depression (World Health Organization, 2023). According to a recent study published in the Lancet, depressive disorders had a sharp increase in 2020 since coronavirus disease (COVID) had a global impact on so many people (IHME, 2021). MDD is a disabling condition. It has been estimated by the World Health Organization (WHO) that by 2030 it will be the second most serious condition (Mathers and Loncar, 2006). Current treatment relies on the monoamine hypothesis, however, one-third of patients with MDD do not respond to monoamine-based antidepressants (Berlim and Turecki, 2007). For years, it was hypothesized that the primary pathway of MDD involved serotonin as the main neurotransmitter. The monoamine hypothesis, a widely accepted theory, sought to explain the biological basis of MDD as being caused by the depletion of monoamine neurotransmitters, namely norepinephrine and serotonin (Hirschfeld, 2000). This was initially hypothesized based on studies surrounding the neuroscience of lysergic acid diethylamide (LSD) due to the drug’s peripheral blockade of serotonin receptors and reserpine-induced depletion of serotonin in the brain (Hirschfeld, 2000). Additional evidence was provided upon the discovery of iproniazid, a derivative of isoniazid and antimycobacterial, which was found to improve depressive symptoms in tubercular patients. It was later found that iproniazid was a monoamine oxidase inhibitor and thus prevented the degradation of serotonin and norepinephrine. This hypothesis regarding monoamines as the pathophysiological basis of MDD led to the design and widespread use of selective serotonin reuptake inhibitors (SSRIs) (Hirschfeld, 2000). However, given that only one-third of patients improve with SSRI it is reasonable to infer that the pathway involved is more complex than once hypothesized and there are more neurotransmitters, receptors, and molecules involved. The monoamine hypothesis does not explain why there is a delay in the onset of effect and action of SSRIs. Several studies have demonstrated that chronic stress is a risk factor for the development of MDD (Kendler et al., 1998; Hammen, 2005; Hammen et al., 2009; McLaughlin et al., 2010; Franklin et al., 2018). Thus the monoamine hypothesis alone is not enough to fully account for the pathophysiology of MDD highlighting the need for further research involving the pathways of MDD. Patients with MDD have elevated levels of proinflammatory cytokines, chemokines, and acute-phase proteins (Raison et al., 2006). In this paper, we review the role of inflammation and cytokines on MDD and discuss other pathways involved in the development and persistence of depressive symptoms seen in MDD.
The 12-month prevalence of MDD patients in the US actively treated with pharmacotherapy was 8.9 million and out of them 2.8 million had treatment-resistant MDD. Namely, 30.8% of patients had treatment-resistant MDD (Zhdanava et al., 2021). The purpose of this paper is to review the inflammatory hypothesis and to explain why a subgroup of patients with treatment-resistant depression do not respond to medications based on the monoamine hypothesis. We will review the molecules and biochemical pathways responsible for depressive symptoms in patients with MDD and discuss the role of the inflammatory hypothesis. Inflammation can lead to cytokines directly affecting the metabolism of tryptophan and as a result decreasing the production of serotonin. MDD in the context of inflammation could explain why a subgroup of patients with treatment-resistant depression does not respond to medications that were created based on the monoamine hypothesis and why two-thirds of patients might require anti-inflammatory-immunological-based treatments. Inflammation has been observed to play a striking role in suicidality (Andrew and Miller, 2018). Several studies have examined the role of inflammation and specific cytokine levels in patients who have attempted and in some cases completed suicide. In light of these results, we should reconsider our initial approach and evaluation of patients with MDD. Understanding the inflammatory basis of MDD can aid in the identification of specific biomarkers of inflammation such as IL-6 and CRP to provide a more personalized treatment approach and informed therapeutic strategy in patients with Treatment Resistant Depression (TRD) (Jha and Trivedi, 2018). Previous studies have identified that higher levels of CRP, a nonspecific marker of inflammation, are associated with a greater likelihood of hospitalization due to MDD, increased severity of MDD, and a greater likelihood of completed suicide (Tonelli et al., 2008). A study conducted by Uher et al. (2014) found that patients with CRP < 1mgI-1 showed a greater symptomatic improvement in depression with escitalopram than with Nortriptyline, while those with CRP >1 showed greater improvement with nortriptyline rather than with escitalopram. Drevet’s demonstrated that those who received Anti-IL6 antibody Sirukumab as an adjunct treatment for TRD and had baseline CRP levels of >6mgI-1 showed significant improvements in anhedonia rating scales compared to controls at 12 weeks. Those with baseline CRP values of greater than 8mgI-1 showed a significant decrease in Hamilton Depression Rating Scale scores with Sirukumab as an adjunct treatment (Drevets et al., 2022). These studies among others exemplify a need for more personalized treatment options for those with MDD that is not responsive to conventional methods. In the future, inflammatory levels may become essential in the way we assess and manage patients with MDD (Tonelli et al., 2008; Lindqvist et al., 2009; Steiner et al., 2011; Pandey et al., 2012; Brundin et al., 2017).
Immune pathways
Before studying the correlation between inflammation and psychiatry, we must first review some of the key inflammatory/immune players at the cellular level. Our immune system can be divided into innate and adaptive immune systems. The main cells comprising the innate immune system are granulocytes, mast cells, monocytes, dendritic cells, macrophages, and natural killer cells (Leo et al., 2011). The adaptive immune system is composed of cluster differentiation (CD) 4 T cells, CD8 T cells, B cells, and plasma cells (Leo et al., 2011). The key immune cells in our central nervous system are microglia cells (Nemeroff and Duman, 2015).
Some critical proteins that aid in signaling an immune response belong to the family of cytokines and include interleukins, interferons, and tumor necrosis factors. C-reactive protein (CRP), interleukin 1 (IL-1), interleukin 2 (IL-2) interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-alpha (IFN-α), interferon-gamma (IFN-γ) are cytokines that have been correlated in pathways leading to mental illness and will be briefly discussed in this paper (Levine et al., 1999; Musselman et al., 2001; Lindqvist et al., 2009; Dowlati et al., 2010; Wium-Andersen et al., 2013; Currier, 2015).
Several studies have indicated that inflammation can precipitate depressive symptoms in patients treated with IFN-α or IL-2 (Renault et al., 1987; Buter et al., 1993; Janssen et al., 1994; Capuron et al., 2004; Dieperink et al., 2004). Approximately 30–45% of patients treated with interferon developed depressive symptoms during treatment and even after treatment completion. The purpose of this article is to demonstrate how cytokines including interferon affect the biochemical pathway of serotonin production (Meyers et al., 1991; Miyaoka et al., 1999).
Clinical and preclinical studies have shown that these same proinflammatory cytokines, namely IL-1B, IL-6, and TNF-α contribute to depressive symptoms (Raison et al., 2006; Franklin et al., 2018). Furthermore, other key molecules associated with the development of MDD are damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs), and toll-like receptors (TLRs), DAMPs are endogenous molecular patterns that are released secondary to physical and psychological stress and can stimulate inflammatory pathways even in the absence of pathogens (Franklin et al., 2018). PAMPs are molecules that are derived from pathogen invasion. TLRs are a class of protein recognition receptors (PRRs) that recognize PAMPs from diverse microorganisms and perform a key function in the innate immune system (Franklin et al., 2018). PAMPs are unique to various classes of microorganisms including viruses, bacteria, fungi, and protozoa but some PAMPs are common across various classes of pathogens, these are recognized by various PRRs (Franklin et al., 2018). These PRRs engage a series of downstream signaling pathways that trigger the activation of the innate immune response and the production of inflammatory cytokines (Franklin et al., 2018; Li and Wu, 2021).
Several studies have examined the development of MDD in the context of medical conditions with a high inflammatory burden. Some of these medical conditions will be discussed below. Some studies have investigated the development of MDD in the context of sterile inflammation, namely in otherwise medically healthy patients.
Inflammatory markers and pro-inflammatory cytokines
Most evidence associating depressive symptoms with an inflammatory process includes three main concepts. (1) Systemic diseases with inflammatory processes increase the risk of depression. (2) Elevated pro-inflammatory markers are found in depressed patients. (3) Pro-inflammatory agents often can induce psychiatric side effects (Köhler et al., 2016).
(1) Systemic diseases with inflammatory pathophysiology have been associated with an increased risk of depression. These include rheumatoid arthritis, irritable bowel syndrome (IBS), coronary vascular disease (CVD), diabetes mellitus (DM), hepatitis c, and sepsis. We have reviewed some of these associations in this paper (Köhler et al., 2016).
(2) The associated relationship between proinflammatory markers and depression seems bidirectional since increased markers have been associated with the subsequent development of depression. Similarly, pro-inflammatory markers are found elevated in depressed patients independent of comorbid somatic disease. Specifically, IL-6, CRP, TNF-α, and IL-1 receptor antagonist (IL-1α). Elevated IL-6 levels during childhood have been associated with increased depression in young adults. Setiawan et al. found increased levels of neuroinflammation in individuals suffering from an active depressive episode (Setiawan et al., 2015; Köhler et al., 2016).
(3) The development of depressive disease has been found in patients treated with IFN-α in about 80% of patients. Sarkar et al. found antidepressant treatment before IFN-α treatment lowered mean depression scores and had a lower incidence of MDD (Sarkar and Schaefer, 2014; Köhler et al., 2016).
Given the above, anti-inflammatory interventions may represent opportunities for a more personalized treatment regimen in certain subgroups of depressed patients as discussed below (Köhler et al., 2016).
Medical conditions in association with depression
Depression and inflammation can be seen in the context of several medical conditions with elevated inflammatory markers namely type 2 DM, CVD, hepatitis c, rheumatoid arthritis, and IBS. It can also be seen in the absence of medical conditions as sterile inflammation (Table 1).
One area that has received attention over recent years is the gut microbiome and the gut-brain axis. Immune system dysfunction, which is present in both IBS and depression, can be attributed to alterations in the permeability of the gastrointestinal tract and microbial characteristics. The complex multifactorial systems underlying IBS and depression are mediated by altered cytokines, immunological function, and changes in the HPA axis in response to stress. TNF-α and IL-8 levels that are abnormal are linked to depression. At the same time, depression alone changes IL-1 and IL-10 levels, generating an imbalance between pro- and anti-inflammatory markers, which results in a deviation that can cause IBS symptoms to appear and/or persist (Mudyanadzo et al., 2018). When an immunological response is triggered, psychological stress can make the intestinal lining more permeable, which makes it possible for bacterial liposaccharides (endotoxins) to enter the bloodstream (Peirce and Alviña, 2019). This peripheral inflammation can then move to the central nervous system (CNS) in a variety of ways, impairing neurotransmitters and encouraging neurotoxins, which have an impact on mental health (Peirce and Alviña, 2019). Peirce et al. summarized the mechanisms in the gut microbiome-brain axis that may affect CNS function. The intestinal barrier is strengthened by short-chain fatty acids (SCFAs), tryptophan catabolites (Trycats), and microbial-associated molecular patterns (MAMPs), which stop endotoxins from penetrating the intestinal epithelium, thus decreasing inflammation. Also, the vagus nerve communicates with the gut epithelium, including enteroendocrine cells providing an antidepressant effect. Probiotics, prebiotics, and fecal microbiota transplantation (FMT) have been shown to benefit both the gut microbiome and mental health.
Several medical conditions are associated with higher levels of inflammation. However, some examples of inflammation can be found in the following studies:
Stewart et al. (2009) studied the longitudinal association between depressive symptoms and both IL-6 and CRP among 263 healthy older patients enrolled in the Pittsburgh Healthy Heart Project, a 6-year prospective cohort study. Participants were evaluated for depressive symptoms using the Beck Depression Inventory-II (BDI-II) and had blood work quantifying IL-6 and CRP at baseline and during follow-up visits. Pathology analyses demonstrated that baseline BDI-II was a predictor for a 6-year change in IL-6.
Evidence indicates that MDD patients have elevated levels of circulating blood cytokines (Howren et al., 2009; Dowlati et al., 2010; Valkanova et al., 2013; Haapakoski et al., 2015). Several meta-analyses have found positive correlations between blood levels of both CRP and IL6 with depression (Valkanova et al., 2013; Haapakoski et al., 2015).
Other studies have reported strong associations between depression symptoms and circulating levels of TNF and IL-1β (Howren et al., 2009; Dowlati et al., 2010).
Acute and chronic stress promotes elevations of pro-inflammatory cytokines, including IL-1β, TNF, and IL-6 (You et al., 2011; Wohleb et al., 2012; Hodes et al., 2014; Şahin et al., 2015; Cheng et al., 2016).
Stress-induced sterile inflammation promotes the development of depressive-like behaviors in preclinical stress models of depression. For instance, Iwata et al. demonstrated that the development of stress-induced depressive-like behaviors requires NFkB activation and subsequent cytokine production (Koo and Duman, 2008, 2009; Koo et al., 2010; Iwata et al., 2013).
When reviewing the biochemical pathway of serotonin production we can identify specific steps on the pathway that are affected by inflammation. The information presented below will review the crucial steps in which inflammation can alter the production of serotonin suggesting a direct effect of inflammatory molecules on serotonin production. More clinical research is needed to elucidate altered levels of serotonin production in humans.
Kynurenine pathway
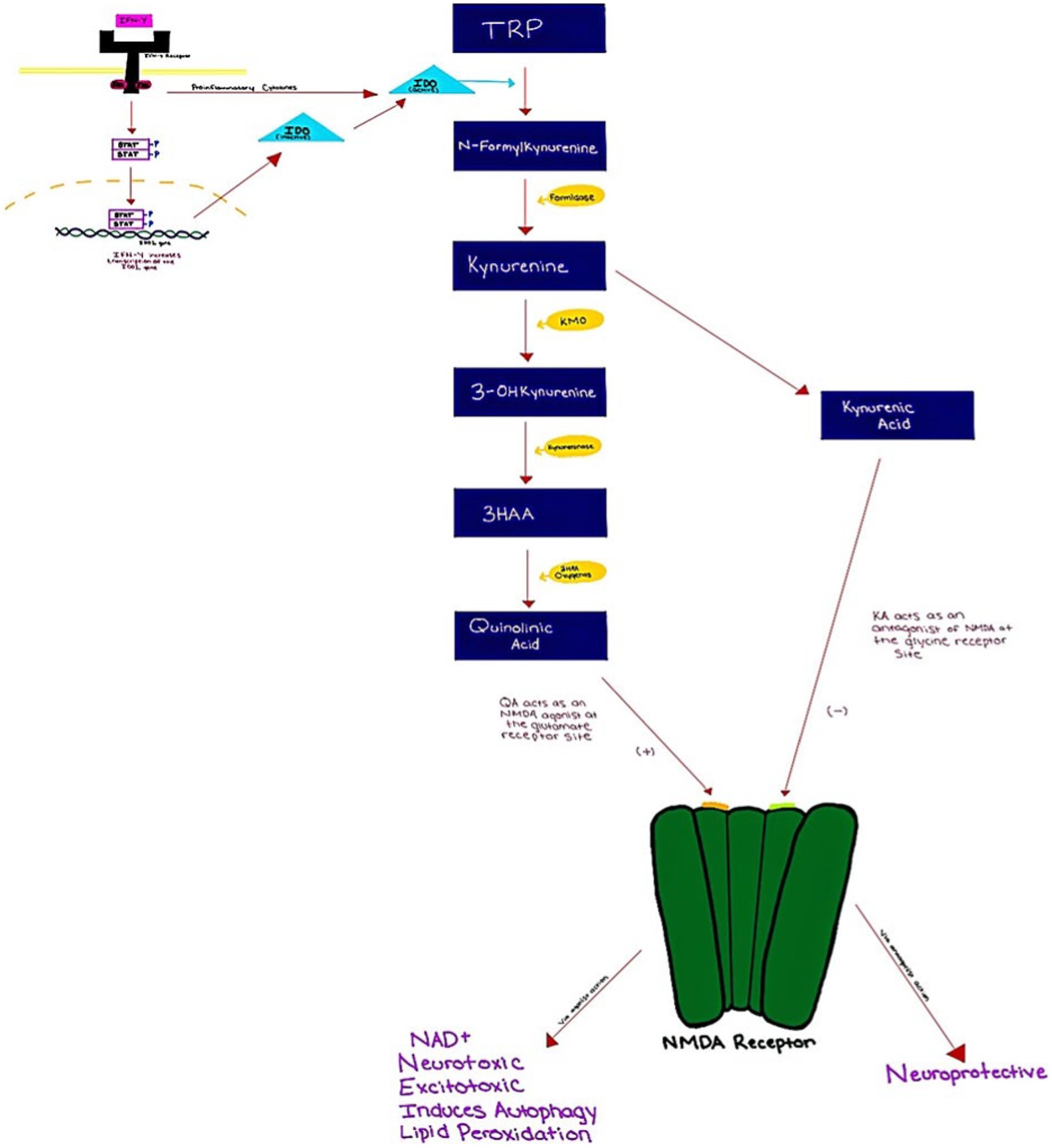
Tryptophan (TRP) is an amino acid that is required for the production of serotonin. It can be metabolized via the serotonin pathway or the kynurenin (KYN) pathway. TRP is metabolized through the KYN pathway rather than the 5-HT pathway more than 90% of the time (Dantzer et al., 2008). The first step in the KYN pathway requires the conversion of TRP to KYN by two key enzymes, tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3 dioxygenase (IDO). Interferon-gamma IFN-γ is a cytokine that regulates cellular processes through transcription and translation (Inserra et al., 2019). IFN-γ binds the IFN-γ receptor, which then induces IDO expression via JAK/STAT signaling (Mellor and Munn, 2004; Okamoto et al., 2007). As a result, by inhibiting IFN-STAT1 activation, TRP may suppress IDO production at the transcriptional level. Additionally, the IFN-γ receptor also secondarily activates transcription factors which then leads to the induction of inflammatory genes (Inserra et al., 2019). During inflammatory states, TDO is suppressed while IDO is activated (Maes et al., 2007). IFN-γ is found to be elevated in MDD patients (Inserra et al., 2019). This could play a major role in the induction/upregulation of IDO, subsequent TRP depletion, decreases in serotonin levels, and the increase in KYN pathway activity (Inserra et al., 2019).
KYN is converted to 3-hydroxykynurenine (3-HK) via kynurenine 3-monooxygenase (KMO) which is then metabolized into 3-hydroxyanthranilic acid (3-HAA) and quinolinic acid (QUIN) in microglia (Agudelo et al., 2014). Quinolinic acid is an agonist of the glutamate N-methyl-d-aspartate (NMDA) receptor and it has been associated with neurotoxicity, excitotoxicity, and preoxidative action (Tavares et al., 2002; Meier et al., 2016) (Figure 1). Past studies have demonstrated abnormal concentrations of kynurenic acid (KYNA) and QUIN, in patients with MDD (Réus et al., 2015; Filho et al., 2016; Więdłocha et al., 2018). These studies indicate increased levels of KYN byproducts during inflammation as opposed to serotonin. Hunt et al. (2020) in their systemic review and meta-analysis sought to examine the relation of KYN pathway metabolites such as TRP, KYN, KYNA, QUIN, and KYN/TRP ratio, in the context of pro-inflammatory activation and immune responses. This was conducted by analyzing 15 studies. Results indicated that there was an association between levels of KYN metabolites and pro-inflammatory activation, IFN-α treatment specifically showed an association between reduction of TRP levels and increase in KYN levels, and subsequently the KYN/TRP ratio. Increases in depression symptoms were also seen similar to increases in KYN metabolites.
Regarding the KYN pathway, possible mechanisms between metabolites and depressive symptoms are based on either the TRP depletion hypothesis or the toxic effects of KYN metabolites produced by KMO in the CNS. In the TPR depletion hypothesis, competition between the KYN pathway and the serotonin pathway for TRP uptake can lower TRP bioavailability. This also aligns with the serotonin hypothesis of depression. On the other hand, toxic KYN metabolites (QUIN in particular) mainly produced in microglia by KMO can be excitotoxic, altering glutaminergic activity, lipid peroxidation, and activating reactive oxygen species (ROS) in the CNS. It is important to note that the findings of this meta-analysis are mainly extended from medically ill patients who were treated with IFN-α and other immune-activating agents; how generalizable the associations drawn from them should be further investigated (Hunt et al., 2020).
Toll-like receptors
Toll-like receptors (TLRs) also play a key role in the link between inflammatory pathways and the pathogenesis of depressive disorders. The TLR family has ten recognized receptors in humans: TLR1-TLR10 (Onofrio et al., 2020). These TLRs are essential for the detection of PAMPs on bacteria, viruses, and other parasites, cytokine modulation, and the induction of innate immunity (Hedayat et al., 2011; Birra et al., 2020; Debnath et al., 2020; Onofrio et al., 2020). TLRs are located on several immune cells, namely dendritic cells, macrophages, and natural killer cells (Khanmohammadi and Rezaei, 2021). TLRs 7/8 identify RNA viruses such as COVID-19 (Onofrio et al., 2020; Khanmohammadi and Rezaei, 2021). Activation of TLR 7/8 is followed by the release of IL-1, IL-6, TNF-α, and IFN-γ (Duivis et al., 2013). Several studies have investigated the association between COVID and depressive disorders. As research is underway looking at the correlation of pathogens causing depressive symptoms, more information and knowledge will be revealed about the intricate pathways connecting inflammation and mental illnesses. Hung et al. (2014) found TLR mRNA levels to be different in MDD patients compared to healthy controls. Also, TLR4 was discovered to be an independent risk factor for MDD severity. Hou et al. (2018) found that these TLR mRNA levels, which were higher in MDD patients, dropped after four weeks of treatment with SSRIs or SNRIs, implying that antidepressants have a TLR-mediated anti-inflammatory effect. They also showed that IL-6 mRNA expression in MDD patients’ was considerably reduced with an SSRI, but not an SNRI. These findings show that SSRIs and SNRIs influence TLR and cytokine expression differently and that various TLRs may be involved in MDD. Hajebrahimi et al. (2014) found the expression of adaptor proteins that mediate TLR signaling to be higher in 38 depressed medical students in comparison to healthy controls. When compared to healthy controls, depressed patients and postmortem brains had greater amounts of TNF, IL-6, TNF, and IL-1β. The rise in these cytokines could be due to TLR (1, 2, 4, 5, 6, and 10) activation on the cell surface (Kim et al., 2007; Yoshimura et al., 2009; Dowlati et al., 2010; Dahl et al., 2014; Köhler et al., 2018; Figueroa-Hall et al., 2020). TLRs and IL-1 receptors share homology. IL-1 cytokines modulate mu-opioid neurotransmission, this opioid neurotransmitter pathway has been demonstrated to be dysregulated in MDD (Prossin et al., 2016).
PAMPs linked to systemic infection have the potential to cause or worsen psychiatric disorders as evidenced by experimental studies by Irwin et al. (2019) who showed that low-dose lipopolysaccharide (LPS) can cause temporary depressed symptoms. Also, various studies have shown social withdrawal, reduced appetite, decreased motor activity, and altered cognition and sleep in mice administered LPS (a PAMP for TLR4) (Yirmiya, 1996; Yirmiya, 2000; Dantzer, 2001; Konsman et al., 2002; Figueroa-Hall et al., 2020). Lucas and Maes (2013) propose that a vicious loop between the TLR4 complex and the formation of ROS and reactive nitrogen species (RNS), which stimulates the TLR4 complex, might explain why chronic inflammation persists. They show many environmental variables can activate TLR4 pathways, resulting in inflammatory and oxidative, and nitrosative responses; they do so primarily through the actions of ROS/RNS-induced DAMPs on the TLR4 complex.
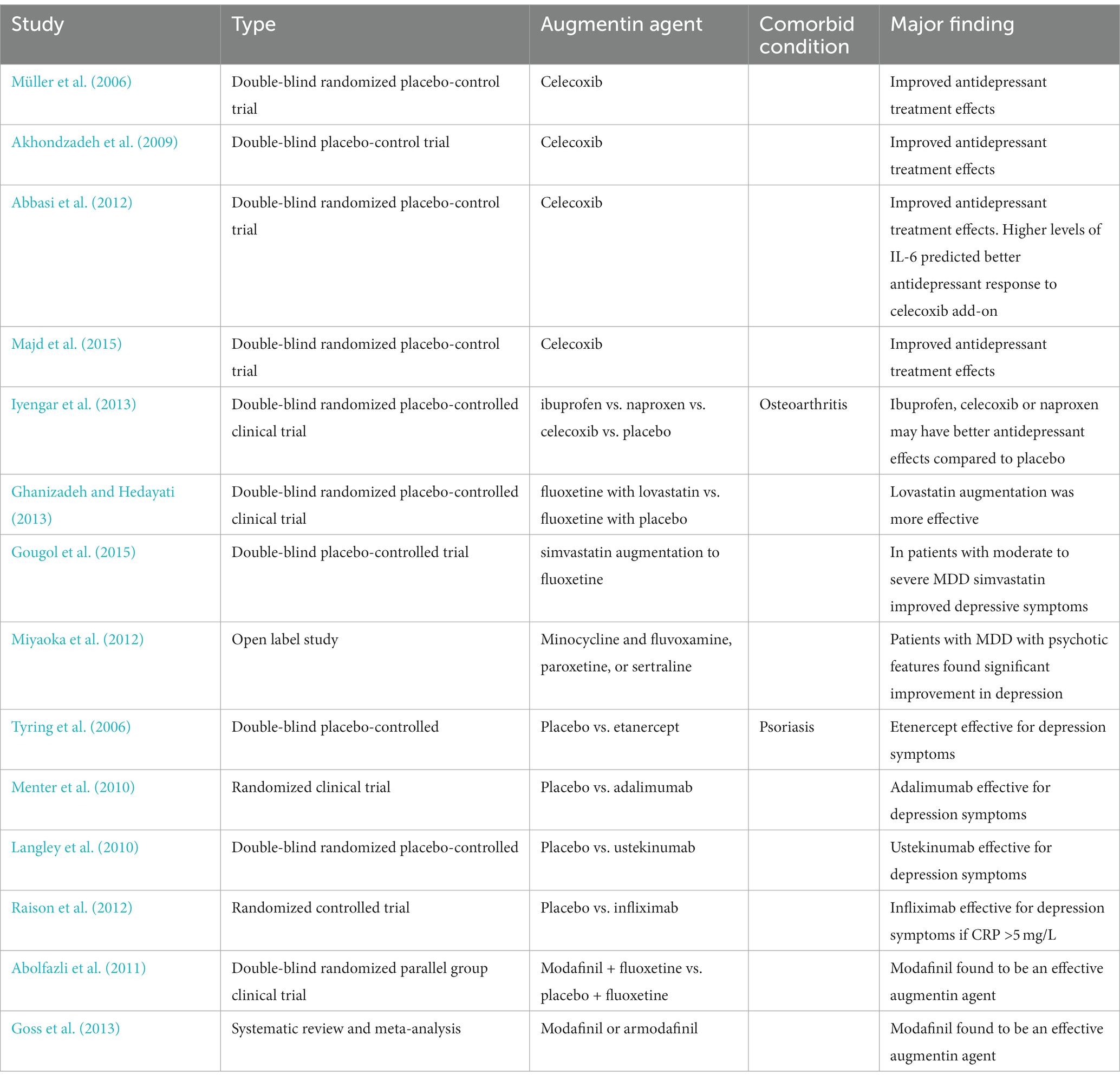
Several medications have been considered as potential anti-inflammatory agents that could augment antidepressant agents. Statins, corticosteroids, minocycline, modafinil, celecoxib, and infliximab have all been considered and investigated. Kohler et al. described several potential augmenting agents that could be used in managing depression (Köhler et al., 2014, 2016). The findings from studies supporting the use of anti-inflammatory agents for MDD treatment augmentation are summarized in the Table 2.
Since SSRI’s have been used for years to treat depression one can naturally wonder whether there is also a potential effect of SSRI’s on inflammation. Recent studies have shown that certain SSRI’s can have an effect on murine microglia response to inflammation (Hwang et al., 2008; Hashioka et al., 2009; Horikawa et al., 2010; Tynan et al., 2012). Further research is required and eagerly anticipated when exploring this topic.
Bottom line
A considerable fraction of the global population suffers from a depressive disorder. It is a very complicated illness with a wide range of manifestations and many co-occurring medical and mental conditions. There has been a rigorous effort to better manage symptoms of MDD. We now know more about the underlying mechanisms of depression other than the monoaminergic hypothesis. Chronic low-grade inflammation has been associated with depression via many mechanisms as we discussed above. The pathways are more intricate than previously thought as our brain is undoubtedly incredibly complex. There are a multitude of studies describing the role that inflammation plays in MDD and this could explain why two-thirds of patients continue to struggle with depressive symptoms despite having tried multiple SSRI’s and SNRI’s. Given this body of evidence, it would be remiss to disregard this association. Current studies are examining immunotherapy treatments for MDD ultimately aiming to better manage patients with MDD (Evrensel et al., 2021; Drevets et al., 2022). As future studies continue to explore MDD in the context of inflammation new paths can be forged in the treatment of this disorder giving hope to patients with treatment-resistant MDD (Evrensel et al., 2021; Drevets et al., 2022).
Author contributions
IP: Conceptualization, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing. MS: Conceptualization, Investigation, Resources, Writing – original draft, Writing – review & editing. AP: Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi, S. H., Hosseini, F., Modabbernia, A., Ashrafi, M., and Akhondzadeh, S. (2012). Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J. Affect. Disord. 141, 308–314. doi: 10.1016/j.jad.2012.03.033
Abolfazli, R., Hosseini, M., Ghanizadeh, A., Ghaleiha, A., Tabrizi, M., Raznahan, M., et al. (2011). Double-blind randomized parallel-group clinical trial of efficacy of the combination fluoxetine plus modafinil versus fluoxetine plus placebo in the treatment of major depression. Depress. Anxiety 28, 297–302. doi: 10.1002/da.20801
Agudelo, L. Z., Femenía, T., Orhan, F., Porsmyr-Palmertz, M., Goiny, M., Martinez-Redondo, V., et al. (2014). Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced Depression. Cells 159, 33–45. doi: 10.1016/j.cell.2014.07.051
Akhondzadeh, S., Jafari, S., Raisi, F., Nasehi, A. A., Ghoreishi, A., Salehi, B., et al. (2009). Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress. Anxiety 26, 607–611. doi: 10.1002/da.20589
Andrew, H., and Miller, M. D. (2018). Five Things to Know About Inflammation and Depression. Available at: https://www.psychiatrictimes.com/view/five-things-know-about-inflammation-and-depression (Accessed November 20, 2023)
Berlim, M. T., and Turecki, G. (2007). What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 17, 696–707. doi: 10.1016/j.euroneuro.2007.03.009
Birra, D., Benucci, M., Landolfi, L., Merchionda, A., Loi, G., Amato, P., et al. (2020). COVID 19: a clue from innate immunity. Immunol. Res. 68, 161–168. doi: 10.1007/s12026-020-09137-5
Brundin, L., Bryleva, E. Y., and Thirtamara, R. K. (2017). Role of inflammation in suicide: from mechanisms to treatment. Neuropsychopharmacology 42, 271–283. doi: 10.1038/npp.2016.116
Buter, J., de Vries, E. G., Sleijfer, D. T., Willemse, P. H., and Mulder, N. H. (1993). Neuropsychiatric symptoms during treatment with interleukin-2. Lancet Lond Engl. 341:628. doi: 10.1016/0140-6736(93)90384-s
Capuron, L., Ravaud, A., Miller, A. H., and Dantzer, R. (2004). Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav. Immun. 18, 205–213. doi: 10.1016/j.bbi.2003.11.004
Cheng, S. W., Li, J. X., Chen, D. T. L., Chien, Y. C., Chang, J. P. C., Huang, S. Y., et al. (2021). Predictive genetic variations in the kynurenine pathway for interferon-α-induced depression in patients with hepatitis C viral infection. J. Pers. Med. 11:192. doi: 10.3390/jpm11030192
Cheng, Y., Pardo, M., Armini, R. S., Martinez, A., Mouhsine, H., Zagury, J. F., et al. (2016). Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav. Immun. 53, 207–222. doi: 10.1016/j.bbi.2015.12.012
Currier, M. B. (2015). The role of inflammation in mediating risk for medical disorders in depressed patients. Psychiatr. Ann. 45, 249–254. doi: 10.3928/00485713-20150501-07
Dahl, J., Ormstad, H., Aass, H. C. D., Malt, U. F., Bendz, L. T., Sandvik, L., et al. (2014). The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45, 77–86. doi: 10.1016/j.psyneuen.2014.03.019
Dantzer, R. (2001). Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 15, 7–24. doi: 10.1006/brbi.2000.0613
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W., and Kelley, K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. doi: 10.1038/nrn2297
Debnath, M., Banerjee, M., and Berk, M. (2020). Genetic gateways to COVID-19 infection: implications for risk, severity, and outcomes. FASEB J. 34, 8787–8795. doi: 10.1096/fj.202001115R
Dieperink, E., Ho, S. B., Tetrick, L., Thuras, P., Dua, K., and Willenbring, M. L. (2004). Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen. Hosp. Psychiatry 26, 237–240. doi: 10.1016/j.genhosppsych.2004.01.003
Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. doi: 10.1016/j.biopsych.2009.09.033
Drevets, W. C., Wittenberg, G. M., Bullmore, E. T., and Manji, H. K. (2022). Immune targets for therapeutic development in depression: towards precision medicine. Nat. Rev. Drug Discov. 21, 224–244. doi: 10.1038/s41573-021-00368-1
Duivis, H. E., Kupper, N., Penninx, B. W., Na, B., de Jonge, P., and Whooley, M. A. (2013). Depressive symptoms and white blood cell count in coronary heart disease patients: prospective findings from the heart and soul study. Psychoneuroendocrinology 38, 479–487. doi: 10.1016/j.psyneuen.2012.07.006
Evrensel, A., Ünsalver, B. Ö., Ceylan, M. E., and Tarhan, N. (2021). Vaccination and immunotherapy for major Depression. Adv. Exp. Med. Biol. 1305, 503–513. doi: 10.1007/978-981-33-6044-0_25
Figueroa-Hall, L. K., Paulus, M. P., and Savitz, J. (2020). Toll-like receptor signaling in Depression. Psychoneuroendocrinology 121:104843. doi: 10.1016/j.psyneuen.2020.104843
Filho, C. B., Jesse, C. R., Donato, F., del Fabbro, L., Gomes de Gomes, M., Rossito Goes, A. T., et al. (2016). Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 260, 154–162. doi: 10.1016/j.cbi.2016.11.005
Franklin, T. C., Xu, C., and Duman, R. S. (2018). Depression and sterile inflammation: essential role of danger associated molecular patterns. Brain Behav. Immun. 72, 2–13. doi: 10.1016/j.bbi.2017.10.025
Ghanizadeh, A., and Hedayati, A. (2013). Augmentation of fluoxetine with lovastatin for treating major depressive disorder, a randomized double-blind placebo controlled-clinical trial. Depress. Anxiety 30, 1084–1088. doi: 10.1002/da.22195
Goldstein, B. I., Carnethon, M. R., Matthews, K. A., McIntyre, R., Miller, G. E., Raghuveer, G., et al. (2015). Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease. Circulation 132, 965–986. doi: 10.1161/CIR.0000000000000229
Goss, A. J., Kaser, M., Costafreda, S. G., Sahakian, B. J., and Fu, C. H. Y. (2013). Modafinil augmentation therapy in unipolar and bipolar depression: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Psychiatry 74, 1101–1107. doi: 10.4088/JCP.13r08560
Gougol, A., Zareh-Mohammadi, N., Raheb, S., Farokhnia, M., Salimi, S., Iranpour, N., et al. (2015). Simvastatin as an adjuvant therapy to fluoxetine in patients with moderate to severe major depression: a double-blind placebo-controlled trial. J. Psychopharmacol. 29, 575–581. doi: 10.1177/0269881115578160
Haapakoski, R., Mathieu, J., Ebmeier, K. P., Alenius, H., and Kivimäki, M. (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 49, 206–215. doi: 10.1016/j.bbi.2015.06.001
Hajebrahimi, B., Bagheri, M., Hassanshahi, G., Nazari, M., Bidaki, R., Khodadadi, H., et al. (2014). The adapter proteins of TLRs, TRIF and MYD88, are upregulated in depressed individuals. Int. J. Psychiatry Clin. Pract. 18, 41–44. doi: 10.3109/13651501.2013.859708
Hammen, C. (2005). Stress and Depression. Annu. Rev. Clin. Psychol. 1, 293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938
Hammen, C., Kim, E. Y., Eberhart, N. K., and Brennan, P. A. (2009). Chronic and acute stress and the prediction of major depression in women. Depress. Anxiety 26, 718–723. doi: 10.1002/da.20571
Hashioka, S., McGeer, P. L., Monji, A., and Kanba, S. (2009). Anti-inflammatory effects of antidepressants: possibilities for preventives against Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 9, 12–19. doi: 10.2174/187152409787601897
Hayashino, Y., Mashitani, T., Tsujii, S., and Ishii, H. (2014). Diabetes distress and care registry at Tenri study group. Elevated levels of hs-CRP are associated with high prevalence of depression in japanese patients with type 2 diabetes: the diabetes distress and care registry at Tenri (DDCRT 6). Diabetes Care 37, 2459–2465. doi: 10.2337/dc13-2312
Hedayat, M., Netea, M. G., and Rezaei, N. (2011). Targeting of toll-like receptors: a decade of progress in combating infectious diseases. Lancet Infect. Dis. 11, 702–712. doi: 10.1016/S1473-3099(11)70099-8
Hirschfeld, R. M. (2000). History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 61, 4–6.
Hodes, G. E., Pfau, M. L., Leboeuf, M., Golden, S. A., Christoffel, D. J., Bregman, D., et al. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. U. S. A. 111, 16136–16141. doi: 10.1073/pnas.1415191111
Horikawa, H., Kato, T. A., Mizoguchi, Y., Monji, A., Seki, Y., Ohkuri, T., et al. (2010). Inhibitory effects of SSRIs on IFN-γ induced microglial activation through the regulation of intracellular calcium. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 34, 1306–1316. doi: 10.1016/j.pnpbp.2010.07.015
Hou, T. Y., Huang, T. L., Lin, C. C., Wu, M. K., and Hung, Y. Y. (2018). Effects of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors on toll-like-receptors expression profiles. Neuropsychiatry 8, 243–248. doi: 10.4172/Neuropsychiatry.1000345
Howren, M. B., Lamkin, D. M., and Suls, J. (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186. doi: 10.1097/PSY.0b013e3181907c1b
Hung, Y. Y., Kang, H. Y., Huang, K. W., and Huang, T. L. (2014). Association between toll-like receptors expression and major depressive disorder. Psychiatry Res. 220, 283–286. doi: 10.1016/j.psychres.2014.07.074
Hunt, C., Cordeiro, T. M. E., Suchting, R., de Dios, C., Cuellar Leal, V. A., Soares, J. C., et al. (2020). Effect of immune activation on the kynurenine pathway and depression symptoms – a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 118, 514–523. doi: 10.1016/j.neubiorev.2020.08.010
Hwang, J., Zheng, L. T., Ock, J., Lee, M. G., Kim, S. H., Lee, H. W., et al. (2008). Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology 55, 826–834. doi: 10.1016/j.neuropharm.2008.06.045
IHME . (2021). The Lancet: COVID-19 pandemic led to stark rise in depressive and anxiety disorders globally in 2020, with women and younger people most affected. Institute for Health Metrics and Evaluation. Available at: https://www.healthdata.org/news-release/lancet-covid-19-pandemic-led-stark-rise-depressive-and-anxiety-disorders-globally-2020 (Accessed March 20, 2023)
Inserra, A., Mastronardi, C. A., Rogers, G., Licinio, J., and Wong, M. L. (2019). Neuroimmunomodulation in major depressive disorder: focus on caspase 1, inducible nitric oxide synthase, and interferon-gamma. Mol. Neurobiol. 56, 4288–4305. doi: 10.1007/s12035-018-1359-3
Irwin, M. R., Cole, S., Olmstead, R., Breen, E. C., Cho, J. J., Moieni, M., et al. (2019). Moderators for depressed mood and systemic and transcriptional inflammatory responses: a randomized controlled trial of endotoxin. Neuropsychopharmacology 44, 635–641. doi: 10.1038/s41386-018-0259-6
Iwata, M., Ota, K. T., and Duman, R. S. (2013). The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 31, 105–114. doi: 10.1016/j.bbi.2012.12.008
Iyengar, R. L., Gandhi, S., Aneja, A., Thorpe, K., Razzouk, L., Greenberg, J., et al. (2013). NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am. J. Med. 126, 1017.e11–1017.e18. doi: 10.1016/j.amjmed.2013.02.037
Janssen, H. L., Brouwer, J. T., van der Mast, R. C., and Schalm, S. W. (1994). Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J. Hepatol. 21, 241–243. doi: 10.1016/s0168-8278(05)80402-7
Jha, M. K., and Trivedi, M. H. (2018). Personalized antidepressant selection and pathway to novel treatments: clinical utility of targeting inflammation. Int. J. Mol. Sci. 19:233. doi: 10.3390/ijms19010233
Kendler, K. S., Karkowski, L. M., and Prescott, C. A. (1998). Stressful life events and major Depression: risk period, long-term contextual threat, and diagnostic specificity. J. Nerv. Ment. Dis. 186, 661–669. doi: 10.1097/00005053-199811000-00001
Khanmohammadi, S., and Rezaei, N. (2021). Role of toll-like receptors in the pathogenesis of COVID-19. J. Med. Virol. 93, 2735–2739. doi: 10.1002/jmv.26826
Kim, Y. K., Na, K. S., Shin, K. H., Jung, H. Y., Choi, S. H., and Kim, J. B. (2007). Cytokine imbalance in the pathophysiology of major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 31, 1044–1053. doi: 10.1016/j.pnpbp.2007.03.004
Köhler, O., Benros, M. E., Nordentoft, M., Farkouh, M. E., Iyengar, R. L., Mors, O., et al. (2014). Effect of anti-inflammatory treatment on Depression, depressive symptoms, and adverse effects: a systematic review and Meta-analysis of randomized clinical trials. JAMA Psychiatry 71, 1381–1391. doi: 10.1001/jamapsychiatry.2014.1611
Köhler, C. A., Freitas, T. H., Stubbs, B., Maes, M., Solmi, M., Veronese, N., et al. (2018). Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol. Neurobiol. 55, 4195–4206. doi: 10.1007/s12035-017-0632-1
Köhler, O., Krogh, J., Mors, O., and Benros, M. E. (2016). Inflammation in Depression and the potential for anti-inflammatory treatment. Curr. Neuropharmacol. 14, 732–742. doi: 10.2174/1570159X14666151208113700
Konsman, J. P., Parnet, P., and Dantzer, R. (2002). Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 25, 154–159. doi: 10.1016/s0166-2236(00)02088-9
Koo, J. W., and Duman, R. S. (2008). IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. U. S. A. 105, 751–756. doi: 10.1073/pnas.0708092105
Koo, J. W., and Duman, R. S. (2009). Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr. Opin. Investig. Drugs 10, 664–671.
Koo, J. W., Russo, S. J., Ferguson, D., Nestler, E. J., and Duman, R. S. (2010). Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. U. S. A. 107, 2669–2674. doi: 10.1073/pnas.0910658107
Kraus, M. R., Schäfer, A., Csef, H., Scheurlen, M., and Faller, H. (2000). Emotional state, coping styles, and somatic variables in patients with chronic hepatitis C. Psychosomatics 41, 377–384. doi: 10.1176/appi.psy.41.5.377
Kudlow, P. A., Cha, D. S., Lam, R. W., and McIntyre, R. S. (2013). Sleep architecture variation: a mediator of metabolic disturbance in individuals with major depressive disorder. Sleep Med. 14, 943–949. doi: 10.1016/j.sleep.2013.04.017
Laake, J. P. S., Stahl, D., Amiel, S. A., Petrak, F., Sherwood, R. A., Pickup, J. C., et al. (2014). The association between depressive symptoms and systemic inflammation in people with type 2 diabetes: findings from the South London diabetes study. Diabetes Care 37, 2186–2192. doi: 10.2337/dc13-2522
Langley, R. G., Feldman, S. R., Han, C., Schenkel, B., Szapary, P., Hsu, M. C., et al. (2010). Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J. Am. Acad. Dermatol. 63, 457–465. doi: 10.1016/j.jaad.2009.09.014
Leo, O., Cunningham, A., and Stern, P. (2011). Vaccine immunology. Perspect Vaccinol. 1, 25–59. doi: 10.1016/j.pervac.2011.05.002
Levine, J., Barak, Y., Chengappa, K. N., Rapoport, A., Rebey, M., and Barak, V. (1999). Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 40, 171–176. doi: 10.1159/000026615
Li, D., and Wu, M. (2021). Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 6, 291–224. doi: 10.1038/s41392-021-00687-0
Lichtman, J. H., Froelicher, E. S., Blumenthal, J. A., Carney, R. M., Doering, L. V., Frasure-Smith, N., et al. (2014). Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations. Circulation 129, 1350–1369. doi: 10.1161/CIR.0000000000000019
Lindqvist, D., Janelidze, S., Hagell, P., Erhardt, S., Samuelsson, M., Minthon, L., et al. (2009). Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry 66, 287–292. doi: 10.1016/j.biopsych.2009.01.030
Lucas, K., and Maes, M. (2013). Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 48, 190–204. doi: 10.1007/s12035-013-8425-7
Maes, M., Mihaylova, I., Ruyter, M. D., Kubera, M., and Bosmans, E. (2007). The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression – and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol. Lett. 28, 826–831.
Majd, M., Hashemian, F., Hosseini, S. M., Vahdat Shariatpanahi, M., and Sharifi, A. (2015). A randomized, double-blind, placebo-controlled trial of celecoxib augmentation of sertraline in treatment of drug-naive depressed women: A pilot study. Iran. J. Pharm. Res. 14, 891–899.
Mathers, C. D., and Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3:e442. doi: 10.1371/journal.pmed.0030442
McLaughlin, K. A., Conron, K. J., Koenen, K. C., and Gilman, S. E. (2010). Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med. 40, 1647–1658. doi: 10.1017/S0033291709992121
Meier, T. B., Drevets, W. C., Wurfel, B. E., Ford, B. N., Morris, H. M., Victor, T. A., et al. (2016). Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav. Immun. 53, 39–48. doi: 10.1016/j.bbi.2015.11.003
Mellor, A. L., and Munn, D. H. (2004). Ido expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4, 762–774. doi: 10.1038/nri1457
Menter, A., Augustin, M., Signorovitch, J., Yu, A. P., Wu, E. Q., Gupta, S. R., et al. (2010). The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J. Am. Acad. Dermatol. 62, 812–818. doi: 10.1016/j.jaad.2009.07.022
Meyers, C. A., Scheibel, R. S., and Forman, A. D. (1991). Persistent neurotoxicity of systemically administered interferon-alpha. Neurology 41, 672–676. doi: 10.1212/wnl.41.5.672
Miyaoka, H., Otsubo, T., Kamijima, K., Ishii, M., Onuki, M., and Mitamura, K. (1999). Depression from interferon therapy in patients with hepatitis C. Am. J. Psychiatry 156:1120. doi: 10.1176/ajp.156.7.1120
Miyaoka, T., Wake, R., Furuya, M., Liaury, K., Ieda, M., Kawakami, K., et al. (2012). Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 37, 222–226. doi: 10.1016/j.pnpbp.2012.02.002
Mudyanadzo, T. A., Hauzaree, C., Yerokhina, O., Architha, N. N., and Ashqar, H. M. (2018). Irritable bowel syndrome and depression: a shared pathogenesis. Cureus 10:e3178. doi: 10.7759/cureus.3178
Müller, N., Schwarz, M. J., Dehning, S., Douhe, A., Cerovecki, A., Goldstein-Müller, B., et al. (2006). The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11, 680–684. doi: 10.1038/sj.mp.4001805
Musselman, D. L., Miller, A. H., Porter, M. R., Manatunga, A., Gao, F., Penna, S., et al. (2001). Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am. J. Psychiatry 158, 1252–1257. doi: 10.1176/appi.ajp.158.8.1252
National Institute of Mental Health (NIMH) . (n.d.) Major Depression. Available at: https://www.nimh.nih.gov/health/statistics/major-depression (Accessed February 1, 2023)
Nemeroff, C. B., and Duman, R. S. (2015). This issue: inflammation and psychiatric disorders. Psychiatr. Ann. 45, 222–224. doi: 10.3928/00485713-20150501-03
Nerurkar, L., Siebert, S., McInnes, I. B., and Cavanagh, J. (2019). Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry 6, 164–173. doi: 10.1016/S2215-0366(18)30255-4
Okamoto, T., Toné, S., Kanouchi, H., Miyawaki, C., Ono, S., and Minatogawa, Y. (2007). Transcriptional regulation of indoleamine 2,3-dioxygenase (IDO) by tryptophan and its analogue: Down-regulation of the indoleamine 2,3-dioxygenase (IDO) transcription by tryptophan and its analogue. Cytotechnology 54, 107–113. doi: 10.1007/s10616-007-9081-4
Onofrio, L., Caraglia, M., Facchini, G., Margherita, V., Placido, S. D., and Buonerba, C. (2020). Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci. OA 6:FSO605. doi: 10.2144/fsoa-2020-0091
Pandey, G. N., Rizavi, H. S., Ren, X., Fareed, J., Hoppensteadt, D. A., Roberts, R. C., et al. (2012). Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J. Psychiatr. Res. 46, 57–63. doi: 10.1016/j.jpsychires.2011.08.006
Peirce, J. M., and Alviña, K. (2019). The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 97, 1223–1241. doi: 10.1002/jnr.24476
Prossin, A. R., Koch, A. E., Campbell, P. L., Barichello, T., Zalcman, S. S., and Zubieta, J. K. (2016). Acute experimental changes in mood state regulate immune function in relation to central opioid neurotransmission: a model of human CNS-peripheral inflammatory interaction. Mol. Psychiatry 21, 243–251. doi: 10.1038/mp.2015.110
Raison, C. L., Capuron, L., and Miller, A. H. (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27, 24–31. doi: 10.1016/j.it.2005.11.006
Raison, C., Rutherford, R. E., Woolwine, B., Shuo, C., Schettler, P., Drake, D., et al. (2012). 176. The tumor necrosis factor-alpha antagonist infliximab reduces depressive symptoms in patients with treatment resistant depression and high inflammation. Brain Behav. Immun. 26:S49. doi: 10.1016/j.bbi.2012.07.200
Renault, P. F., Hoofnagle, J. H., Park, Y., Mullen, K. D., Peters, M., Jones, D. B., et al. (1987). Psychiatric complications of long-term interferon alfa therapy. Arch. Intern. Med. 147, 1577–1580. doi: 10.1001/archinte.1987.00370090055011
Réus, G. Z., Jansen, K., Titus, S., Carvalho, A. F., Gabbay, V., and Quevedo, J. (2015). Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J. Psychiatr. Res. 68, 316–328. doi: 10.1016/j.jpsychires.2015.05.007
Şahin, T. D., Karson, A., Balcı, F., Yazır, Y., Bayramgürler, D., and Utkan, T. (2015). TNF-alpha inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behav. Brain Res. 292, 233–240. doi: 10.1016/j.bbr.2015.05.062
Sarkar, S., and Schaefer, M. (2014). Antidepressant pretreatment for the prevention of interferon alfa-associated depression: a systematic review and meta-analysis. Psychosomatics 55, 221–234. doi: 10.1016/j.psym.2013.06.015
Setiawan, E., Wilson, A. A., Mizrahi, R., Rusjan, P. M., Miler, L., Rajkowska, G., et al. (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275. doi: 10.1001/jamapsychiatry.2014.2427
Steiner, J., Walter, M., Gos, T., Guillemin, G. J., Bernstein, H. G., Sarnyai, Z., et al. (2011). Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflammation 8:94. doi: 10.1186/1742-2094-8-94
Stewart, J. C., Rand, K. L., Muldoon, M. F., and Kamarck, T. W. (2009). A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav. Immun. 23, 936–944. doi: 10.1016/j.bbi.2009.04.011
Tavares, R. G., Tasca, C. I., Santos, C. E. S., Alves, Ĺ. B., Porciúncula, L. O., Emanuelli, T., et al. (2002). Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 40, 621–627. doi: 10.1016/S0197-0186(01)00133-4
Tonelli, L. H., Stiller, J., Rujescu, D., Giegling, I., Schneider, B., Maurer, K., et al. (2008). Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand. 117, 198–206. doi: 10.1111/j.1600-0447.2007.01128.x
Tynan, R. J., Weidenhofer, J., Hinwood, M., Cairns, M. J., Day, T. A., and Walker, F. R. (2012). A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav. Immun. 26, 469–479. doi: 10.1016/j.bbi.2011.12.011
Tyring, S., Gottlieb, A., Papp, K., Gordon, K., Leonardi, C., Wang, A., et al. (2006). Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet Lond Engl. 367, 29–35. doi: 10.1016/S0140-6736(05)67763-X
Uher, R., Tansey, K. E., Dew, T., Maier, W., Mors, O., Hauser, J., et al. (2014). An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry 171, 1278–1286. doi: 10.1176/appi.ajp.2014.14010094
Valkanova, V., Ebmeier, K. P., and Allan, C. L. (2013). CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 150, 736–744. doi: 10.1016/j.jad.2013.06.004
Więdłocha, M., Marcinowicz, P., Krupa, R., Janoska-Jaździk, M., Janus, M., Dębowska, W., et al. (2018). Effect of antidepressant treatment on peripheral inflammation markers – a meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 80, 217–226. doi: 10.1016/j.pnpbp.2017.04.026
Wium-Andersen, M. K., Ørsted, D. D., Nielsen, S. F., and Nordestgaard, B. G. (2013). Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 70, 176–184. doi: 10.1001/2013.jamapsychiatry.102
Wohleb, E. S., Fenn, A. M., Pacenta, A. M., Powell, N. D., Sheridan, J. F., and Godbout, J. P. (2012). Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37, 1491–1505. doi: 10.1016/j.psyneuen.2012.02.003
World Health Organization . (2023). Depression. Available at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed March 20, 2023)
Yirmiya, R. (1996). Endotoxin produces a depressive-like episode in rats. Brain Res. 711, 163–174. doi: 10.1016/0006-8993(95)01415-2
Yirmiya, R. (2000). Depression in medical illness: the role of the immune system. West. J. Med. 173, 333–336. doi: 10.1136/ewjm.173.5.333
Yoshimura, R., Hori, H., Ikenouchi-Sugita, A., Umene-Nakano, W., Ueda, N., and Nakamura, J. (2009). Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 33, 722–726. doi: 10.1016/j.pnpbp.2009.03.020
You, Z., Luo, C., Zhang, W., Chen, Y., He, J., Zhao, Q., et al. (2011). Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depression. Behav. Brain Res. 225, 135–141. doi: 10.1016/j.bbr.2011.07.006
Keywords: major depressive disorder, inflammation, monoamine hypothesis, immune pathways, cytokines, kynurenine, toll-like receptor
Citation: Pastis I, Santos MG and Paruchuri A (2024) Exploring the role of inflammation in major depressive disorder: beyond the monoamine hypothesis. Front. Behav. Neurosci. 17:1282242. doi: 10.3389/fnbeh.2023.1282242
Edited by:
Serge Campeau, University of Colorado Boulder, United StatesReviewed by:
Oliver Ambree, Osnabrück University, GermanyCopyright © 2024 Pastis, Santos and Paruchuri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melody G. Santos, bWVsb2R5Z3JhY2VtZEBnbWFpbC5jb20=
 Irene Pastis
Irene Pastis Melody G. Santos
Melody G. Santos Akshita Paruchuri3
Akshita Paruchuri3