- Department of Psychology, Interdisciplinary Science and Technology Building, Michigan State University, East Lansing, MI, United States
Adolescence is a critical juncture when initiation of drug use intersects with profound developmental changes in the brain. Adolescent drug use increases the risk to develop substance use disorders (SUDs) later in life, but the mechanisms that confer this vulnerability are not understood. SUDs are defined by cycles of use, abstinence, and relapse. Intense craving during drug-free periods is often triggered by cues and environmental contexts associated with previous use. In contrast to our understanding of stimuli that elicit craving and relapse in adults, the behavioral processes that occur during periods of abstinence and relapse in adolescents are poorly understood. The current mini-review will summarize findings from preclinical rodent studies that used cocaine conditioned place preference and operant cocaine self-administration to examine subsequent effects on reward, relapse and incubation of craving.
Introduction
Adolescence is a critical period of development when brain maturation intersects with initiation of drug-taking behavior (Chambers et al., 2003; Stanis and Andersen, 2014; Caballero et al., 2016). Drug use during adolescence increases the risk to develop substance use disorders (SUDs), with the severity of diagnosis at adolescence related to more pronounced negative outcomes later in life (Wagner and Anthony, 2002; Ramo et al., 2012; Winters et al., 2014; McCabe et al., 2022; Volkow and Wargo, 2022). Adults diagnosed with SUDs suffer from chronic relapse often after exposure to drug use environments (contexts), paraphernalia (cues), or stress (Ehrman et al., 1992; Childress et al., 1999; Foltin et al., 2000; Saunders, 2017). Increased craving for drug, or incubation of craving, despite long periods of abstinence poses a particularly difficult challenge for relapse prevention and the successful treatment of SUDs (Gawin and Kleber, 1986; Grant et al., 1996; Garavan et al., 2000; Parvaz et al., 2016). In contrast to our understanding of craving and relapse in adults, the behavioral processes during periods of abstinence and relapse in adolescents are poorly understood.
Human adolescents have distinct patterns of drug use, with longer periods of abstinence between bouts of use compared to adults (Winters et al., 2014; Silvers et al., 2019; Moska et al., 2021). Up to 86% of adolescents relapse after leaving treatment centers, suggesting that the abstinence period is also a critical window of risk for adolescents (Acri et al., 2012; Squeglia et al., 2019; Moska et al., 2021). In regard to cocaine use, existing studies have primarily focused on problematic adult use, cue reactivity, craving and relapse. However, from 2012 to 2018 cocaine-related mortality in adolescent populations increased three-fold. FDA-approved treatments for cocaine use disorders for adults and adolescents are lacking and therefore problematic use during adolescence has the potential to remain untreated and persist into adulthood (LaBossier and Hadland, 2022; McCabe et al., 2023).
Several questions about adolescent drug use, relapse and abstinence remain: what types of drug-associated stimuli trigger craving and relapse in adolescent populations? When is craving highest during abstinence? Are adolescents more sensitive to particular relapse triggers compared to adults? Use of preclinical rodent models of reward and relapse are necessary for a comprehensive understanding of adolescent drug-related behaviors, which is critical to tailor existing behavioral interventions to adolescents and develop new prevention strategies for this vulnerable age group. The current mini-review will summarize findings from preclinical rodent studies that used cocaine conditioned place preference and operant cocaine self-administration to examine subsequent effects on reward, relapse and incubation of craving. We refer the reader to excellent reviews on the behavioral effects of adolescent nicotine, alcohol, cannabis and other stimulant use and the effects of acute or passive adolescent cocaine exposure (Barron et al., 2005; O’Dell, 2009; Marco et al., 2011; Spear, 2016; Walker et al., 2017; Stringfield and Torregrossa, 2021).
Cocaine conditioned place preference (cocaine-CPP) in adolescent and adult rats
Cocaine CPP is a preclinical rodent model used to evaluate the rewarding effects of drug by pairing experimenter-delivered infusions of cocaine in a specific context (Carlezon, 2003; Prus et al., 2009). In general, an initial preference test occurs in a two or three compartment apparatus to assess baseline preferences for a specific compartment. Cocaine is paired in the least preferred compartment in biased designs or randomly assigned to a compartment in unbiased designs. During conditioning trials (15–60 min), rats receive an intraperitoneal (i.p.) injection of saline and are then placed into one compartment (saline-paired). The next day, or 4–5 h later, rats receive an injection of cocaine and are then placed into the second compartment (cocaine-paired). Alternate pairings of cocaine and saline occur 1-2x/day over 2–5 days. On the test day, rodents are placed back in the CPP apparatus (center, for 3 compartment setup) and allowed to freely explore all compartments. Preference for the cocaine-paired side can be measured in several ways (1) ratio of time spent in the cocaine-paired side/ total time in both sides, (2) difference in time spent in the cocaine- vs. saline-paired side or (3) difference in time spent in the cocaine-paired side during pre- vs. post-conditioning. More time spent in the cocaine-paired side suggests that CPP for the cocaine-associated context was formed (Prus et al., 2009; Olmstead, 2011).
Studies that examined age-dependent differences in cocaine reward began behavioral training for adolescent rats at an average post-pubertal age of postnatal day 36 (P36, range from P30–41) and for adults at P72 (range from P55–102) (Figure 1). Studies in the current review delivered cocaine at doses between 3 and 40 mg/kg and used either biased or unbiased designs. Three types of CPP tests were utilized: (1) CPP tests with no cocaine onboard to examine the rewarding property of the cocaine-paired context, (2) CPP re-tests with no cocaine onboard to examine extinction of CPP- rats freely explore all compartments during unpaired extinction training or are confined to the former saline- or cocaine-paired sides during explicit paired extinction, or (3) cocaine-primed CPP in which cocaine (5 or 10 mg/kg) was administered before the CPP test (Table 1).
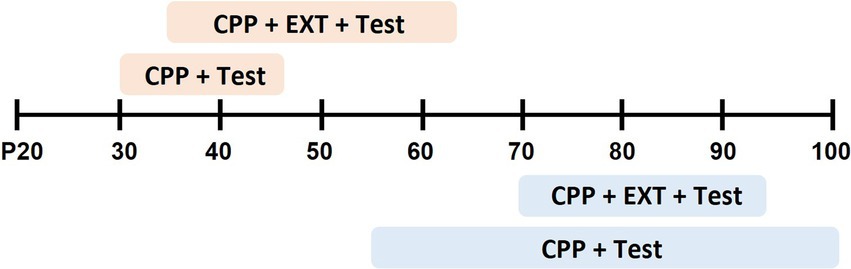
Figure 1. Summary of age range in experiments that employed cocaine conditioned place preference training and test (CPP + Test) or cocaine CPP, extinction training and Test (CPP + EXT + Test). Shaded boxes indicate the length of behavior across postnatal days (P) for adolescent (orange) or adult (blue) rats.
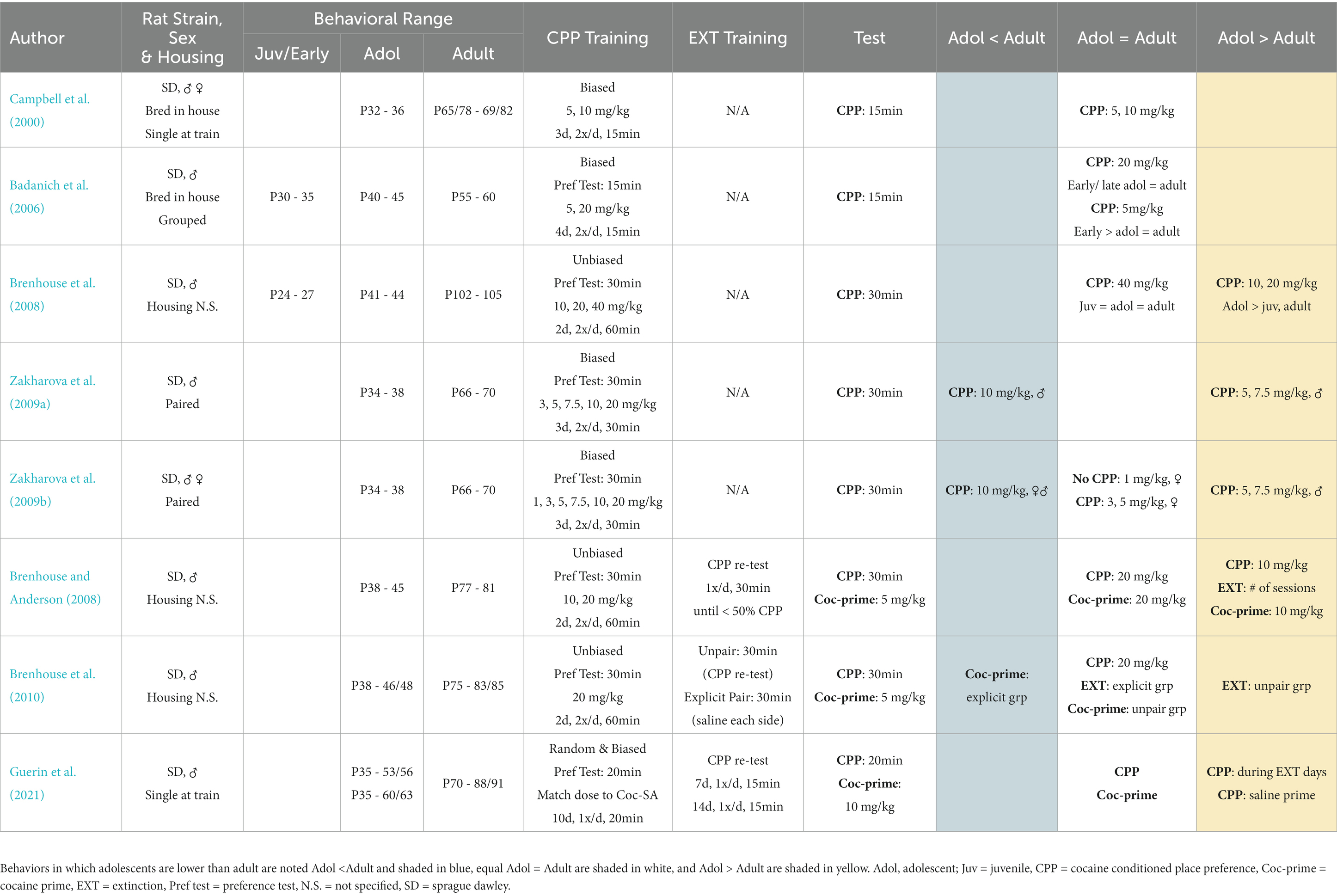
Table 1. Summary of key studies that compared adolescent and adult cocaine conditioned place preference (CPP).
Expression of cocaine-CPP in adolescent and adult rats
The majority of cocaine CPP studies observed similar or higher CPP in adolescent rats compared to adults, with fewer studies showing lower CPP in adolescents. (Campbell et al., 2000; Badanich et al., 2006; Zakharova et al., 2009a,b). Specifically, at a conditioning dose of 5 mg/kg, late adolescent rats (corresponding to post-pubertal age) had similar cocaine CPP as their adult counterparts (Badanich et al., 2006). Another study also found that adolescent and adult rats (male and female combined) had similar cocaine CPP (Campbell et al., 2000). A subsequent study determined that female adolescent and adult rats had similar CPP, but that male adolescents displayed higher CPP than adults (Zakharova et al., 2009a,b).
At conditioning doses of 7.5–10 mg/kg, adolescent and adult rats exhibited similar or higher cocaine CPP (Campbell et al., 2000; Brenhouse et al., 2008; Brenhouse and Andersen, 2008). One study found that adolescent and adult rats (male and female combined) had similar CPP at 10 mg/kg (Campbell et al., 2000). Another set of studies found that adolescent male rats displayed higher CPP at doses of 7.5 and 10 mg/kg- only adolescent rats formed CPP at 7.5 mg/kg (Zakharova et al., 2009b), and adolescent rats displayed higher CPP compared to adult and juvenile groups when conditioned at 10 mg/kg (Brenhouse et al., 2008; Brenhouse and Andersen, 2008). A third set of studies found that both female and male adolescent rats were less sensitive to cocaine CPP than their adult counterparts (Zakharova et al., 2009a,b). Variables that could have contributed to different observations in adolescent sensitivity to CPP at 10 mg/kg include: (1) the age range of adult comparison groups- adolescents showing more CPP than adults at P103–105 age and less CPP when compared to adult at P66–70 age, (2) methodology used calculate cocaine CPP- use of the difference in time spent between saline and cocaine sides vs. the difference in time spent in the cocaine-paired side pre- vs. post-conditioning (Brenhouse et al., 2008; Zakharova et al., 2009a), and (3) results that combined vs. separated male and female behavioral data (Campbell et al., 2000; Zakharova et al., 2009b).
At higher conditioning doses of 20–40 mg/kg, adolescent and adult rats displayed similar or higher cocaine CPP. Adolescent rats had similar or higher cocaine CPP compared to juvenile or adults at 20 mg/kg; however, all three age groups had similar cocaine CPP at 40 mg/kg (Brenhouse et al., 2008). An additional study that matched the dose of cocaine used for CPP training to a separate group trained to self-administer cocaine (7.6–16.5 mg/kg across days) found similar levels of CPP between adult and adolescent rats (Guerin et al., 2021).
Several studies examined differences in cocaine-CPP between juvenile, adolescent, and adult rats; however, we only summarized findings that directly compared adolescent and adult age groups. One study found that juvenile rats showed less CPP than their adult counterparts (age range not specified) at a conditioning dose of 5 mg/kg (Hollis et al., 2012). Another study found that early adolescents (corresponding to pre-pubertal age) had higher CPP at 5 mg/kg when compared to late adolescent or adult rats (Badanich et al., 2006). We refer the reader to seminal papers that compared CPP in juvenile rodents that were not included in the current CPP summary table (Laviola et al., 1992; Pruitt et al., 1995; Bolanos et al., 1996; Brenhouse et al., 2015).
Extinction of cocaine-CPP in adolescent and adult rats
To examine age-dependent differences in the strength of cocaine CPP, several studies incorporated extinction training sessions after the initial CPP test (Brenhouse and Andersen, 2008; Brenhouse et al., 2010; Guerin et al., 2021). During CPP re-tests, the time spent in each compartment was recorded and tests were repeated (daily over 7–14 days) until rats spent significantly less time in the cocaine-paired compartment compared to the initial test day (Brenhouse and Andersen, 2008). One of the first studies to evaluate extinction of cocaine CPP during adolescence found similar levels of CPP between age groups trained at 10 or 20 mg/kg cocaine, but that adolescent rats required more training sessions to reach extinction criteria (Brenhouse and Andersen, 2008). When a passive extinction strategy was used, adolescent rats also required more time than adults to reach extinction criteria; however when an explicit extinction strategy was employed, both age groups reduced CPP to a similar extent (Brenhouse et al., 2010). Consistent with these findings, a subsequent study found that adolescent and adult rats had similar CPP at cocaine doses matched to a separate cohort that received cocaine-SA training (7.6–16.5 mg/kg across days), but that adolescent rats failed to extinguish cocaine CPP when given twice as many extinction sessions as adults (Guerin et al., 2021).
Operant cocaine self-administration (cocaine-SA) in adolescent and adult rats
Adult rodent models of cocaine-SA and relapse have been used to examine volitional drug-taking and -seeking behaviors (Venniro et al., 2016; Khoo et al., 2017; Feltenstein et al., 2021). In general, rats receive all behavioral training in operant boxes. During cocaine-SA, rats learn that active lever presses (or nosepokes) result in intravenous (i.v.) cocaine infusions, whereas inactive presses result in no consequences. Cocaine infusions are delivered on a range of fixed ratio (FR) schedules of reinforcement and length of training sessions can range between 1.5-6 h. Cocaine infusions are paired with discrete cues- tone + light complex, or passive, background stimuli- distinct olfactory, visual, auditory and tactile elements (Fuchs et al., 2008; Perry et al., 2014). After cocaine-SA training, rats undergo 1) 1-60 days of forced abstinence in the home cage, 2) extinction training in the same operant box where responses result in no cocaine or cue presentations, or 3) extinction in a second, distinct context where responses result in no cocaine. During reinstatement testing, rats are re-exposed to cocaine-associated contexts or cues and the number of responses on the previously reinforced lever is measured. An increase in responses after extinction or 1 day of abstinence is a measure of cocaine-seeking behavior, with significant higher responses (i.e., time-dependent increase) after 15–60 days of abstinence considered a measure of incubation of cocaine-seeking (i.e., incubation of craving) (Grimm et al., 2001; Li et al., 2016). During reinstatement tests, the impact of stress (footshock, yohimbine, or corticosterone) or cocaine priming (5–10 mg/kg) on cocaine-seeking can be assessed. A large body of work using adult rodent models of cocaine-SA has demonstrated that cues, context, stress and cocaine priming elicit cocaine-seeking behavior with cocaine-associated cues more likely to elicit incubation of craving (Tran-Nguyen et al., 1998; Grimm et al., 2001; Lu et al., 2004; Venniro et al., 2021). The adult preclinical literature on relapse and abstinence provide a solid foundation on which to compare adolescent studies.
Cocaine-SA in adolescent and adult rats
In general, studies that examined age-dependent differences in cocaine-taking and -seeking behaviors started SA training in post-pubertal adolescents (range: P34–P44) and adults (range: P69–P97) (Figure 2). Studies in the current review used 1.5-3h short access (ShA) procedures (Belluzzi et al., 2005; Frantz et al., 2007; Kantak et al., 2007; Li and Frantz, 2009, 2017; Wong et al., 2013; Wong and Marinelli, 2016; Zbukvic et al., 2016; Li et al., 2018), 6h long-access (LgA) procedures (Wong et al., 2013; Madsen et al., 2017), or a combination of initial LgA training with ShA maintenance periods (Anker and Carroll, 2010; Holtz and Carroll, 2015). A smaller set of studies used intermittent training schedules that alternate between cocaine available vs. non-available (45 vs. 15 min) periods (Anker et al., 2011; Schramm-Sapyta et al., 2011). A last set of studies conduced cocaine-SA (2h ShA) in an an environmental context in the absence of explicit-paired cue (Cho et al., 2020; Guerin et al., 2021; Olekanma et al., 2023) (Table 2).
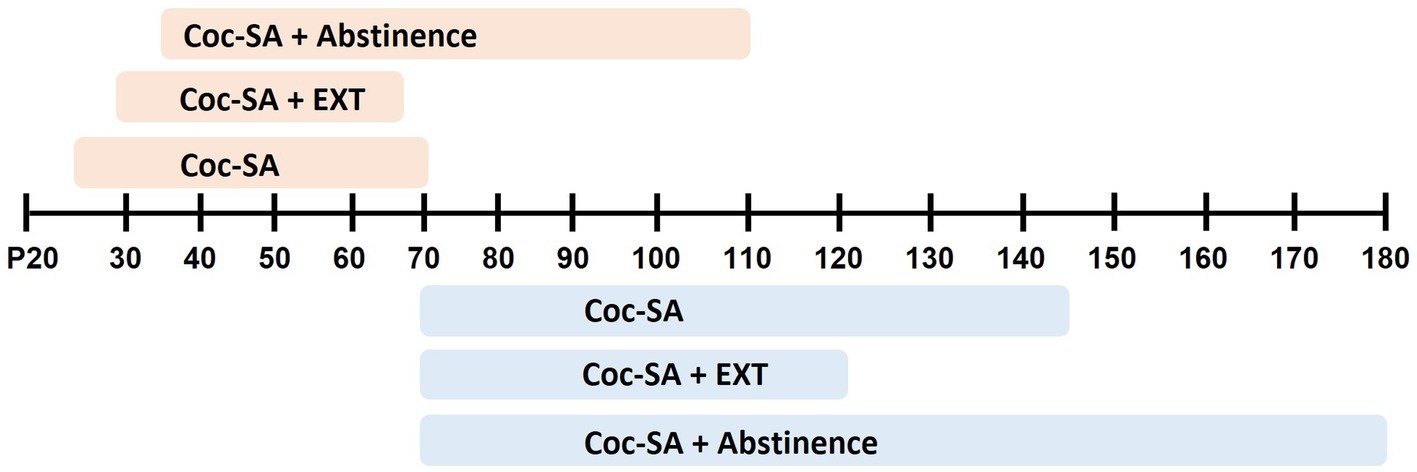
Figure 2. Summary of age range in experiments that employed cocaine self-administration (Coc-SA), Coc-SA and extinction (Coc-SA + EXT), or Coc-SA and drug-free abstinence period (Coc-SA + Abstinence). Shaded boxes indicate the length of behavior across postnatal days (P) for adolescent (orange) or adult (blue) rats.
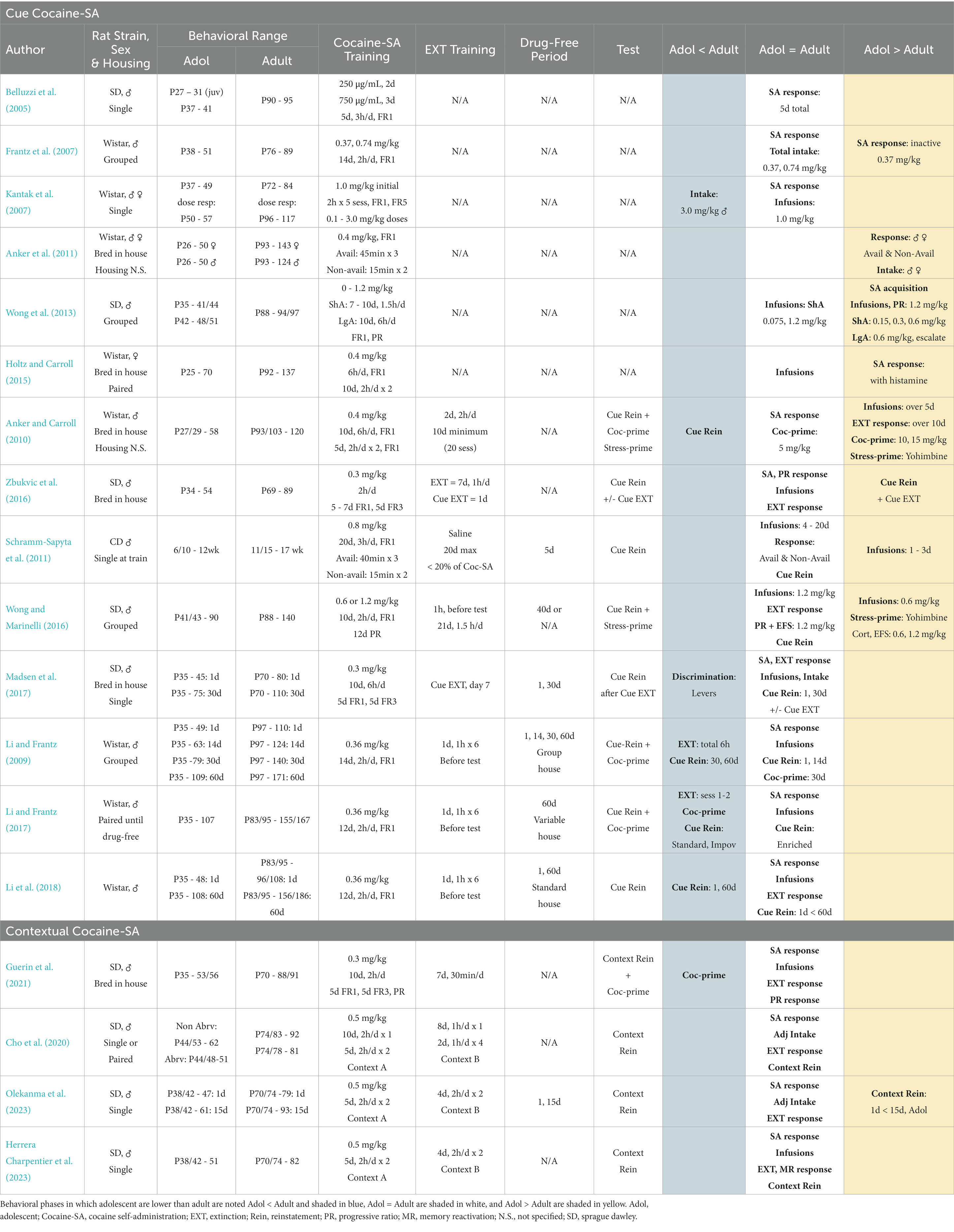
Table 2. Summary of key studies that compared adolescent and adult cocaine self-administration and cue-induced reinstatement (Cue cocaine-SA) or contextual reinstatement (Contextual cocaine-SA).
In general, studies that trained rats under ShA conditions with discrete cues observed that adolescent and adult rats had similar cocaine intake, infusions and active lever responses at doses of 0.3, 0.4, and 1.0 mg/kg (Belluzzi et al., 2005; Frantz et al., 2007; Kantak et al., 2007; Li and Frantz, 2009, 2017; Zbukvic et al., 2016). A study that conducted detailed dose–response curves under ShA conditions, found that adolescent and adult rats had similar intake at low and high doses of cocaine- 0.075 and 1.2 mg/kg. However, adolescents had faster acquisition of cocaine-SA, higher intake at moderate doses of 0.15, 0.3, and 0.6 mg/kg, and higher breakpoints on progressive ratio (PR) schedule of responding compared to adults (Wong et al., 2013). An additional study found that adolescent and adult rats trained under ShA conditions at 1.0 mg/kg had similar intake and PR responding, but that adolescents took less cocaine than their adult counterparts at 3.0 mg/kg (Kantak et al., 2007).
Under LgA conditions, adolescent rats self-administered more cocaine infusions than adults at a dose of 0.6 mg/kg (Wong et al., 2013) but had similar active responses and infusions at a dose of 0.3 mg/kg (Madsen et al., 2017). Adolescent rats that received a combination of LgA and ShA training with 0.4 mg/kg cocaine, had equal or higher active responses and infusions than their adult counterparts (Anker and Carroll, 2010; Holtz and Carroll, 2015). Two studies that used intermittent access schedules found that at 0.4 mg/kg, adolescent male and female rats had higher overall cocaine intake and responded more than adults during available and non-available periods (Anker et al., 2011). At higher doses of 0.8 mg/kg cocaine, adolescents also had higher infusions than adults on training days 1–3, but similar infusions on training days 4–20, with equal responses during available and non-available periods throughout the 20 training days (Schramm-Sapyta et al., 2011). A final set of studies trained rats to self-administer cocaine at 0.3 or 0.6 mg/kg in a distinct environmental context and found that both age groups had similar responses and cocaine intake in the absence of explicit cocaine-paired cues (Cho et al., 2020; Guerin et al., 2021; Olekanma et al., 2023).
Extinction and abstinence in adolescent and adult rats
To examine age-dependent differences in cocaine-seeking or incubation of cocaine-seeking behavior, studies employed various extinction or abstinence procedures: daily extinction training over 7-21 days (Anker and Carroll, 2010; Schramm-Sapyta et al., 2011; Wong and Marinelli, 2016; Zbukvic et al., 2016; Guerin et al., 2021), 7 days of extinction followed by passive presentation of cocaine-paired cues (Cue-EXT), Cue-EXT on day 7 of a 30-day abstinence period (Zbukvic et al., 2016; Madsen et al., 2017) or 1-60 days of abstinence followed by extinction training before reinstatement (Li and Frantz, 2009, 2017; Wong and Marinelli, 2016; Li et al., 2018). Studies that employed cocaine-SA (ShA, 0.3, 0.6 mg/kg) and extinction training found that adolescent and adult rats had similar active responses across 7–21 daily extinction sessions (Wong and Marinelli, 2016; Zbukvic et al., 2016), while studies that used a combination of LgA and ShA training (LgA + ShA) found that adolescent rats had higher extinction responding over a total of 10 days (Anker and Carroll, 2010).
Investigations that incorporated 1–60 days of abstinence, followed by extinction training before reinstatement tests, found that after 30 days of abstinence, both age groups had similar extinction responding across 1 h sessions, but that adolescents had less extinction responses than adults when responses were totaled across all sessions. In a subsequent study, the investigators also found that adolescent rats had lower extinction responses during the 1st two extinction sessions, compared to adults (Li and Frantz, 2009, 2017; Li et al., 2018). A final set of studies utilized passive presentations of a previous cocaine-paired cue (Cue-EXT) on subsequent cocaine-seeking, with or without periods of abstinence. In one of these studies, the investigators found that both age groups responded similarly on daily extinction training that occurred before Cue-EXT (Zbukvic et al., 2016).
Reinstatement of cocaine-seeking in adolescent and adult rats
To understand the impact of adolescent vs. adult cocaine exposure on cocaine-seeking behavior, several studies examined cocaine-seeking elicited by cocaine-paired cues or re-exposure to a cocaine-paired context (Li and Frantz, 2009, 2017; Anker and Carroll, 2010; Schramm-Sapyta et al., 2011; Wong and Marinelli, 2016; Zbukvic et al., 2016; Madsen et al., 2017; Li et al., 2018; Cho et al., 2020; Guerin et al., 2021; Olekanma et al., 2023). In general, rodents that self-administered cocaine during adolescence displayed similar or reduced cue-induced cocaine-seeking when tested immediately after extinction training or after 1 day of abstinence (Li and Frantz, 2009; Schramm-Sapyta et al., 2011; Wong and Marinelli, 2016; Zbukvic et al., 2016). One study exposed rats to passive presentations of cocaine-paired cues (Cue-EXT) after extinction training and found that the adolescent cocaine-exposed group had higher cue-induced reinstatement than adults, suggesting that adolescent rats are less responsive to the Cue-EXT strategy. A set of studies that examined context-induced reinstatement also found that adolescent cocaine-exposed rats had similar levels of cocaine-seeking as adults (Cho et al., 2020; Olekanma et al., 2023).
A subset of the above reinstatement studies incorporated abstinence periods between cocaine-SA and testing to examine age-dependent differences in incubation of craving. Studies that used ShA or LgA conditions found that adolescent cocaine-exposed rats had similar or lower cue-induced cocaine-seeking after 30 or 60 days of abstinence compared to adults (Li and Frantz, 2009, 2017; Madsen et al., 2017; Li et al., 2018). Specifically, one study found that both age groups had higher responses after 60 days of abstinence compared to 1 day, but that overall, adolescents reinstated at lower magnitudes at both timepoints compared to adults. A second study found that adolescent cocaine-exposed rats had lower levels of cue-induced reinstatement after 30 days of abstinence (Li and Frantz, 2009). Another study found that adolescent and adult rats that received Cue-EXT on abstinence day 7, had similar cue-induced reinstatement when tested on abstinence day 30. Taken together with a previous study that found adolescents were less sensitive to Cue-EXT when tested without abstinence, these data suggest that adolescents may be less responsive to cue exposure therapies that are typically used in adult populations (Zbukvic et al., 2016; Madsen et al., 2017). In regard to contextual reinstatement, a study found that adolescent exposed rats had similar responses after 1 day of abstinence, but that only adolescent rats displayed higher responding after 15 days of abstinence (Cho et al., 2020; Olekanma et al., 2023). A timecourse analysis during the contextual reinstatement test showed that adolescent cocaine-exposed rats had higher responding throughout the test, suggesting a potential resistance to extinction within the cocaine-paired context (Olekanma et al., 2023).
Finally, a separate set of studies examined whether cue-induced reinstatement was influenced by exposure to stress or cocaine-priming. In general, adolescent rats displayed similar or reduced cocaine-primed reinstatement at doses of 5 mg/kg when tested immediately after extinction or after 60 days of abstinence (Anker and Carroll, 2010; Li and Frantz, 2017). At a 10 mg/kg priming dose, adolescent rats displayed varying responses- one study found adolescents had lower cocaine-primed reinstatement when tested after extinction or after 60 days of abstinence (Li and Frantz, 2017; Guerin et al., 2021), but that both age groups had similar reinstatement when tested after 30 days of abstinence. A second study found that with priming doses of 10 and 15 mg/kg, adolescents had higher cocaine-seeking than adults when tested after extinction (Anker and Carroll, 2010). Variables that could have contributed to the different cocaine-primed results at 10 mg/kg include ShA vs. LgA + ShA cocaine procedures, extinction training that occurred after an abstinence period (6, 1 h sessions) vs. 7–20 daily extinction sessions (Anker and Carroll, 2010), and use of between-subjects vs. within-subjects testing design during reinstatement tests.
In regard to stress-induced reinstatement, studies have found that adolescent cocaine-exposed rats show heightened stress-induced reinstatement when compared to adults. The heightened sensitivity to stress was observed with ShA vs. LgA + ShA cocaine-SA procedures, extinction training that occurred after 40 days of abstinence vs. 7–21 daily extinction sessions, and with different stressors including yohimbine, corticosterone, or electric footshock (Anker and Carroll, 2010; Wong and Marinelli, 2016).
Conclusions and future experimental considerations
Overall, preclinical studies that utilized cocaine-CPP and cocaine-SA procedures have increased our knowledge on the impact of adolescent cocaine exposure on cocaine reward, relapse and incubation of craving. Several studies showed that adolescent rats have similar cocaine CPP and SA behavioral profiles when compared to adults, however their responses to subsequent relapse triggers and extinction of cocaine associations are complex. Adolescent rats may initially have similar behavioral profiles as their adult counterparts, however dynamic changes that occur during the drug-free periods may not be revealed until later in life. Future avenues for research include investigating the complex relationship between cocaine exposure during adolescence and subsequent effects on learning. Seminal papers have shown that exposure to cocaine during adolescence can lead to changes in stimulus reward learning, reversal learning, reinforcement-learning trajectories, and habit-like behaviors (Kerstetter and Kantak, 2007; Harvey et al., 2009; Kantak et al., 2014; DePoy et al., 2016; Kantak, 2020; Moin Afshar et al., 2020; Villiamma et al., 2022). Few studies have investigated whether adolescent drug memories may be weakened to prevent subsequent craving and relapse in adulthood. A focus on reducing the reconsolidation of adolescent drug memories would be an interesting avenue to pursue given that adolescent rats are more resistant to extinction of cocaine CPP and operant behavior (Bender and Torregrossa, 2020; Herrera Charpentier et al., 2023). It is known that adolescents are particularly sensitive to the effects of peers and therefore future studies should be designed to examine the impact of adolescent social interactions to exacerbate or mitigate cocaine-SA, reward, relapse, and craving.
An additional avenue for future research should investigate the underlying circuit mechanisms that contribute to craving and relapse in adolescent cocaine-exposed rats. Findings from human literature suggest that prefrontal, limbic and reward regions important for flexible decision making, formation of drug-associations and reward undergo massive reorganization during adolescence (Chambers et al., 2003; Caballero et al., 2016). Key preclinical investigations have found evidence for age-dependent differences in the ventral tegmental area and prelimbic to accumbens circuits during cocaine CPP and operant SA (Brenhouse et al., 2008; Wong et al., 2013). Modulation of these circuits after periods of abstinence would be an important next step in understanding their role in adolescent craving and relapse.
Author contributions
AA: Writing – original draft. CV: Writing – original draft. LV: Writing – review & editing. CR: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the internal grant GE100332.
Acknowledgments
The authors thank Shambhvi Ojha and Victoria Braman for their input on the final manuscript draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acri, M. C., Gogel, L. P., Pollock, M., and Wisdom, J. P. (2012). What adolescents need to prevent relapse after treatment for substance abuse: a comparison of youth, parent, and staff perspectives. J. Child Adolesc. Subst. Abuse 21, 117–129. doi: 10.1080/1067828X.2012.662111
Anker, J. J., and Carroll, M. E. (2010). Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology 208, 211–222. doi: 10.1007/s00213-009-1721-2
Anker, J. J., Zlebnik, N. E., Navin, S. F., and Carroll, M. E. (2011). Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology 215, 785–799. doi: 10.1007/s00213-011-2181-z
Badanich, K. A., Adler, K. J., and Kirstein, C. L. (2006). Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur. J. Pharmacol. 550, 95–106. doi: 10.1016/j.ejphar.2006.08.034
Barron, S., White, A., Swartzwelder, H. S., Bell, R. L., Rodd, Z. A., Slawecki, C. J., et al. (2005). Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol. Clin. Exp. Res. 29, 1720–1725. doi: 10.1097/01.ALC.0000179220.79356.E5
Belluzzi, J. D., Wang, R., and Leslie, F. M. (2005). Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 30, 705–712. doi: 10.1038/SJ.NPP.1300586
Bender, B. N., and Torregrossa, M. M. (2020). Molecular and circuit mechanisms regulating cocaine memory. Cell. Mol. Life Sci. 77, 3745–3768. doi: 10.1007/s00018-020-03498-8
Bolanos, C. A., Garmsen, G. M., Clair, M. A., and McDougall, S. A. (1996). Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur. J. Pharmacol. 317, 1–8. doi: 10.1016/S0014-2999(96)00698-X
Brenhouse, H. C., and Andersen, S. L. (2008). Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav. Neurosci. 122, 460–465. doi: 10.1037/0735-7044.122.2.460
Brenhouse, H. C., Dumais, K., and Andersen, S. L. (2010). Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience 169, 628–636. doi: 10.1016/j.neuroscience.2010.05.063
Brenhouse, H. C., Sonntag, K. C., and Andersen, S. L. (2008). Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 28, 2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008
Brenhouse, H. C., Thompson, B. S., Sonntag, K. C., and Andersen, S. L. (2015). Extinction and reinstatement to cocaine-associated cues in male and female juvenile rats and the role of D1 dopamine receptor. Neuropharmacology 95, 22–28. doi: 10.1016/j.neuropharm.2015.02.017
Caballero, A., Granberg, R., and Tseng, K. Y. (2016). Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. Rev. 70, 4–12. doi: 10.1016/j.neubiorev.2016.05.013
Campbell, J. O., Wood, R. D., and Spear, L. P. (2000). Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol. Behav. 68, 487–493. doi: 10.1016/s0031-9384(99)00225-5
Carlezon, W. A. (2003). Place conditioning to study drug reward and aversion. Methods Mol. Med. 84, 243–249. doi: 10.1385/1-59259-379-8:243
Chambers, R. A., Taylor, J. R., and Potenza, M. N. (2003). Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatr. 160, 1041–1052. doi: 10.1176/appi.ajp.160.6.1041
Childress, A. R., Mozley, P. D., McElgin, W., Fitzgerald, J., Reivich, M., and O’Brien, C. P. (1999). Limbic activation during cue-induced cocaine craving. Am. J. Psychiatr. 156, 11–18. doi: 10.1176/ajp.156.1.11
Cho, B. R., Gerena, J., Olekanma, D. I., Bal, A., Herrera Charpentier, A. N., and Arguello, A. A. (2020). Role of adolescent-formed, context-drug-associations on reinstatement of drug-seeking behavior in rats. Psychopharmacology 237, 2823–2833. doi: 10.1007/s00213-020-05575-z
DePoy, L. M., Allen, A. G., and Gourley, S. L. (2016). Adolescent cocaine self-administration induces habit behavior in adulthood: sex differences and structural consequences. Transl. Psychiatry 6:e875. doi: 10.1038/tp.2016.150
Ehrman, R. N., Robbins, S. J., Childress, A. R., and O’Brien, C. P. (1992). Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107, 523–529. doi: 10.1007/BF02245266
Feltenstein, M. W., See, R. E., and Fuchs, R. A. (2021). Neural substrates and circuits of drug addiction. Cold Spring Harb. Perspect. Med. 11:a039628. doi: 10.1101/CSHPERSPECT.A039628
Foltin, R. W., and Haney, M. (2000). Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology 149, 24–33. doi: 10.1007/s002139900340
Frantz, K. J., O’Dell, L. E., and Parsons, L. H. (2007). Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology 32, 625–637. doi: 10.1038/sj.npp.1301130
Fuchs, R. A., Lasseter, H. C., Ramirez, D. R., and Xie, X. (2008). Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov. Today Dis. Model. 5, 251–258. doi: 10.1016/j.ddmod.2009.03.001
Garavan, H., Pankiewicz, J., Bloom, A., Cho, J. K., Sperry, L., Ross, T. J., et al. (2000). Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatr. 157, 1789–1798. doi: 10.1176/appi.ajp.157.11.1789
Gawin, F. H., and Kleber, H. D. (1986). Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Arch. Gen. Psychiatry 43, 107–113. doi: 10.1001/archpsyc.1986.01800020013003
Grant, S., London, E. D., Newlin, D. B., Villemagne, V. L., Liu, X., Contoreggi, C., et al. (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U. S. A. 93, 12040–12045. doi: 10.1073/pnas.93.21.12040
Grimm, J. W., Hope, B. T., Wise, R. A., and Shaham, Y. (2001). Incubation of cocaine craving after withdrawal. Nature 412, 141–142. doi: 10.1038/35084134
Guerin, A. A., Zbukvic, I. C., Luikinga, S. J., Drummond, K. D., Lawrence, A. J., Madsen, H. B., et al. (2021). Extinction and drug-induced reinstatement of cocaine seeking following self-administration or conditioned place preference in adolescent and adult rats. Dev. Psychobiol. 63, 125–137. doi: 10.1002/dev.22017
Harvey, R. C., Dembro, K. A., Rajagopalan, K., Mutebi, M. M., and Kantak, K. M. (2009). Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology 206, 61–71. doi: 10.1007/s00213-009-1579-3
Herrera Charpentier, A. N., Olekanma, D. I., Valade, C. T., Reeves, C. A., Cho, B. R., and Arguello, A. A. (2023). Influence of reconsolidation in maintenance of cocaine-associated contextual memories formed during adolescence or adulthood. Sci. Rep. 13:13936. doi: 10.1038/s41598-023-39949-y
Hollis, F., Gaval-Cruz, M., Carrier, N., Dietz, D. M., and Kabbaj, M. (2012). Juvenile and adult rats differ in cocaine reward and expression of zif268 in the forebrain. Neuroscience 200, 91–98. doi: 10.1016/j.neuroscience.2011.10.012
Holtz, N. A., and Carroll, M. E. (2015). Cocaine self-administration punished by intravenous histamine in adolescent and adult rats. Behav. Pharmacol. 26, 393–397. doi: 10.1097/FBP.0000000000000136
Kantak, K. M. (2020). Adolescent-onset vs. adult-onset cocaine use: impact on cognitive functioning in animal models and opportunities for translation. Pharmacol. Biochem. Behav. 196:172994. doi: 10.1016/j.pbb.2020.172994
Kantak, K. M., Barlow, N., Tassin, D. H., Brisotti, M. F., and Jordan, C. J. (2014). Performance on a strategy set shifting task in rats following adult or adolescent cocaine exposure. Psychopharmacology 231, 4489–4501. doi: 10.1007/s00213-014-3598-y
Kantak, K. M., Goodrich, C. M., and Uribe, V. (2007). Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus). Exp. Clin. Psychopharmacol. 15, 37–47. doi: 10.1037/1064-1297.15.1.37
Kerstetter, K. A., and Kantak, K. M. (2007). Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology 194, 403–411. doi: 10.1007/s00213-007-0852-6
Khoo, S. Y. S., Gibson, G. D., Prasad, A. A., and McNally, G. P. (2017). How contexts promote and prevent relapse to drug seeking. Genes Brain Behav. 16, 185–204. doi: 10.1111/gbb.12328
LaBossier, N. J., and Hadland, S. E. (2022). Stimulant misuse among youth. Curr. Probl. Pediatr. Adolesc. Health Care 52:101265. doi: 10.1016/J.CPPEDS.2022.101265
Laviola, G., Dell’Omo, G., Alleva, E., and Bignami, G. (1992). Ontogeny of cocaine hyperactivity and conditioned place preference in mice. Psychopharmacology 107, 221–228. doi: 10.1007/BF02245141
Li, C., and Frantz, K. J. (2017). Abstinence environment contributes to age differences in reinstatement of cocaine seeking between adolescent and adult male rats. Pharmacol. Biochem. Behav. 158, 49–56. doi: 10.1016/j.pbb.2017.06.003
Li, C., and Frantz, K. J. (2009). Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology 204, 725–733. doi: 10.1007/s00213-009-1502-y
Li, C., White, A. C., Schochet, T., McGinty, J. F., and Frantz, K. J. (2018). ARC and BDNF expression after cocaine self-administration or cue-induced reinstatement of cocaine seeking in adolescent and adult male rats. Addict. Biol. 23, 1233–1241. doi: 10.1111/adb.12689
Li, X., Venniro, M., and Shaham, Y. (2016). Translational research on incubation of cocaine craving. JAMA Psychiatry 73, 1115–1116. doi: 10.1001/jamapsychiatry.2016.2110
Lu, L., Grimm, J. W., Dempsey, J., and Shaham, Y. (2004). Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology 176, 101–108. doi: 10.1007/S00213-004-1860-4
Madsen, H. B., Zbukvic, I. C., Luikinga, S. J., Lawrence, A. J., and Kim, J. H. (2017). Extinction of conditioned cues attenuates incubation of cocaine craving in adolescent and adult rats. Neurobiol. Learn. Mem. 143, 88–93. doi: 10.1016/j.nlm.2016.09.002
Marco, E. M., Adriani, W., Ruocco, L. A., Canese, R., Sadile, A. G., and Laviola, G. (2011). Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci. Biobehav. Rev. 35, 1722–1739. doi: 10.1016/J.NEUBIOREV.2011.02.011
McCabe, S. E., Schulenberg, J. E., Schepis, T. S., McCabe, V. V., and Veliz, P. T. (2022). Longitudinal analysis of substance use disorder symptom severity at age 18 years and substance use disorder in adulthood. JAMA Netw. Open 5:e225324. doi: 10.1001/JAMANETWORKOPEN.2022.5324
McCabe, S. E., Figueroa, O., McCabe, V. V., Schepis, T. S., Schulenberg, J. E., Veliz, P. T., et al. (2023). Is age of onset and duration of stimulant therapy for ADHD associated with cocaine, methamphetamine, and prescription stimulant misuse? J. Child Psychol. Psychiatry. doi: 10.1111/JCPP.13807
Moin Afshar, N., Keip, A. J., Taylor, J. R., Lee, D., and Groman, S. M. (2020). Reinforcement learning during adolescence in rats. J. Neurosci. 40, 5857–5870. doi: 10.1523/JNEUROSCI.0910-20.2020
Moska, C., Goudriaan, A. E., Blanken, P., van de Mheen, D., Spijkerman, R., Schellekens, A., et al. (2021). Youth in transition: study protocol of a prospective cohort study into the long-term course of addiction, mental health problems and social functioning in youth entering addiction treatment. BMC Psychiatry 21:605. doi: 10.1186/S12888-021-03520-8
O’Dell, L. E. (2009). A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology 56, 263–278. doi: 10.1016/J.NEUROPHARM.2008.07.039
Olekanma, D. I., Reeves, C. A., Cho, B. R., Herrera Charpentier, A. N., Gerena, J., Bal, A., et al. (2023). Context-drug-associations and reinstatement of drug-seeking behavior in male rats: adolescent and adult time-dependent effects. Neurobiol. Learn. Mem. 199:107722. doi: 10.1016/j.nlm.2023.107722
Parvaz, M. A., Moeller, S. J., and Goldstein, R. Z. (2016). Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73, 1127–1134. doi: 10.1001/jamapsychiatry.2016.2181
Perry, C. J., Zbukvic, I., Kim, J. H., and Lawrence, A. J. (2014). Role of cues and contexts on drug-seeking behaviour. Br. J. Pharmacol. 171, 4636–4672. doi: 10.1111/bph.12735
Pruitt, D. L., Bolanos, C. A., and McDougall, S. A. (1995). Effects of dopamine D1 and D2 receptor antagonists on cocaine-induced place preference conditioning in preweanling rats. Eur. J. Pharmacol. 283, 125–131. doi: 10.1016/0014-2999(95)00309-9
Prus, A. J., James, J. R., and Rosecrans, J. A. (2009). “Conditioned place preference” in Methods of behavior analysis in neuroscience. ed. J. J. Buccafusco. 2nd ed (Boca Raton, FL: CRC Press/Taylor & Francis)
Ramo, D. E., Prince, M. A., Roesch, S. C., and Brown, S. A. (2012). Variation in substance use relapse episodes among adolescents: a longitudinal investigation. J. Subst. Abus. Treat. 43, 44–52. doi: 10.1016/j.jsat.2011.10.003
Saunders, J. B. (2017). Substance use and addictive disorders in DSM-5 and ICD 10 and the draft ICD 11. Curr. Opin. Psychiatry 30, 227–237. doi: 10.1097/YCO.0000000000000332
Schramm-Sapyta, N. L., Cauley, M. C., Stangl, D. K., Glowacz, S., Stepp, K. A., Levin, E. D., et al. (2011). Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology 215, 493–504. doi: 10.1007/S00213-011-2216-5
Silvers, J. A., Squeglia, L. M., Rømer Thomsen, K., Hudson, K. A., and Feldstein Ewing, S. W. (2019). Hunting for what works: adolescents in addiction treatment. Alcohol. Clin. Exp. Res. 43, 578–592. doi: 10.1111/acer.13984
Spear, L. P. (2016). Consequences of adolescent use of alcohol and other drugs: studies using rodent models. Neurosci. Biobehav. Rev. 70, 228–243. doi: 10.1016/J.NEUBIOREV.2016.07.026
Squeglia, L. M., Fadus, M. C., McClure, E. A., Tomko, R. L., and Gray, K. M. (2019). Pharmacological treatment of youth substance use disorders. J. Child. Adolesc Psychopharmacol. 29, 559–572. doi: 10.1089/cap.2019.0009
Stanis, J. J., and Andersen, S. L. (2014). Reducing substance use during adolescence: a translational framework for prevention. Psychopharmacology 231, 1437–1453. doi: 10.1007/s00213-013-3393-1
Stringfield, S. J., and Torregrossa, M. M. (2021). Disentangling the lasting effects of adolescent cannabinoid exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 104:110067. doi: 10.1016/J.PNPBP.2020.110067
Tran-Nguyen, L. T. L., Fuchs, R. A., Coffey, G. P., Baker, D. A., O'Dell, L. E., and Neisewander, J. L. (1998). Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 19, 48–59. doi: 10.1016/S0893-133X(97)00205-4
Venniro, M., Caprioli, D., and Shaham, Y. (2016). Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog. Brain Res. 224, 25–52. doi: 10.1016/bs.pbr.2015.08.004
Venniro, M., Reverte, I., Ramsey, L. A., Papastrat, K. M., D’Ottavio, G., Milella, M. S., et al. (2021). Factors modulating the incubation of drug and non-drug craving and their clinical implications. Neurosci. Biobehav. Rev. 131, 847–864. doi: 10.1016/j.neubiorev.2021.09.050
Villiamma, P., Casby, J., and Groman, S. M. (2022). Adolescent reinforcement-learning trajectories predict cocaine-taking behaviors in adult male and female rats. Psychopharmacology 239, 2885–2901. doi: 10.1007/S00213-022-06174-W
Volkow, N. D., and Wargo, E. M. (2022). Association of Severity of adolescent substance use disorders and long-term outcomes. JAMA Netw. Open 5:e225656. doi: 10.1001/jamanetworkopen.2022.5656
Wagner, F. A., and Anthony, J. C. (2002). From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 26, 479–488. doi: 10.1016/S0893-133X(01)00367-0
Walker, D. M., Bell, M. R., Flores, C., Gulley, J. M., Willing, J., and Paul, M. J. (2017). Adolescence and reward: making sense of neural and behavioral changes amid the Chaos. J. Neurosci. 37, 10855–10866. doi: 10.1523/JNEUROSCI.1834-17.2017
Winters, K. C., Tanner-Smith, E. E., Bresani, E., and Meyers, K. (2014). Current advances in the treatment of adolescent drug use. Adolesc. Health Med. Ther. 5, 199–210. doi: 10.2147/AHMT.S48053
Wong, W. C., Ford, K. A., Pagels, N. E., McCutcheon, J. E., and Marinelli, M. (2013). Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J. Neurosci. 33, 4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013
Wong, W. C., and Marinelli, M. (2016). Adolescent-onset of cocaine use is associated with heightened stress-induced reinstatement of cocaine seeking. Addict. Biol. 21, 634–645. doi: 10.1111/adb.12284
Zakharova, E., Leoni, G., Kichko, I., and Izenwasser, S. (2009a). Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav. Brain Res. 198, 45–50. doi: 10.1016/j.bbr.2008.10.019
Zakharova, E., Wade, D., and Izenwasser, S. (2009b). Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Behav. 92, 131–134. doi: 10.1016/j.pbb.2008.11.002
Keywords: adolescence, context, self-administration, cocaine seeking, reinstatement, cocaine conditioned place preference, incubation, cue
Citation: Arguello AA, Valade CT, Voutour LS and Reeves CA (2024) Cocaine reward and reinstatement in adolescent versus adult rodents. Front. Behav. Neurosci. 17:1278263. doi: 10.3389/fnbeh.2023.1278263
Edited by:
Brandon Warren, University of Florida, United StatesReviewed by:
Marco Venniro, University of Maryland, United StatesCopyright © 2024 Arguello, Valade, Voutour and Reeves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy A. Arguello, YXJndWVsbDVAbXN1LmVkdQ==
 Amy A. Arguello
Amy A. Arguello Christian T. Valade
Christian T. Valade