
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Behav. Neurosci. , 14 July 2023
Sec. Individual and Social Behaviors
Volume 17 - 2023 | https://doi.org/10.3389/fnbeh.2023.1239681
This article is part of the Research Topic Plasticity and Flexibility in the Parental Brain View all 9 articles
Maternal behavior in mammals encompasses a complex repertoire of activities that ensure the survival of the offspring and shape their neural and behavioral development. The laboratory rat has been employed as a classic model for investigating maternal behavior, and recently with the use of advanced techniques, the knowledge of its neural basis has been expanded significantly. However, the standard laboratory testing conditions in which rats take care of a single litter impose constraints on the study of maternal flexibility. Interestingly, the reproductive characteristics of this species, including the existence of a fertile postpartum estrus, allow us to study maternal behavior in more complex and ethologically relevant contexts, even in laboratory settings. Here we review how maternal and sexual motivations interact during the postpartum estrus, shaping the behavioral response of females according to the presence of the pups and males. Next, we describe how impregnation during the postpartum estrus creates a new reproductive context in which mothers simultaneously care for two successive litters, adapting their responses to different behavioral and physiological demands of pups. These findings illustrate the behavioral adaptability of maternal rats to pups’ needs and the presence of other reinforcers, as well as its dependence on the context. In our view, future perspectives in the field, by incorporating the use of cutting-edge techniques, should analyze maternal flexibility and its neural substrates in models that incorporate complex and challenging contexts. This approach would allow a more comprehensive understanding of brain circuits involved in the adaptive and flexible nature of parenting.
Maternal care is a highly conserved behavior in evolution and, although it is present in several vertebrate and invertebrate species, it is most developed and is universal in mammals. In this group, the mother and her offspring forge a long-lasting bond immediately after (or in some species before) parturition. By far, this is the most common enduring bond in mammals, and, importantly for the young, it represents the first social bond in their life (Hrdy, 2009; Numan and Young, 2016; Pereira and Ferreira, 2016). Maternal care received during this first social experience will not only grant nutrition and protection, ensuring their survival, but it will also shape the developmental trajectories of the offspring (Caldji et al., 1998; Champagne et al., 2003; Uriarte et al., 2007, 2014; Zuluaga et al., 2014). Therefore, maternal behavior (MB) must be robust and contingent on the demands of the offspring, but also on the physiological state of the mother and the context in which it occurs.
In the rat, postpartum females display a series of direct caretaking activities of the young that are well characterized and include retrieving the pups to the nest if they are displaced, licking and arranging them inside the nest, and eventually adopting a reflexive posture to nurse them. The expression of these behaviors, although stereotyped, varies according to the needs of the offspring (Reisbick et al., 1975; Hansen et al., 1993; Pereira and Ferreira, 2006; Pereira et al., 2008; Pereira and Morrell, 2011; Grieb et al., 2018). Indeed, as the postpartum advances and the pups become more autonomous, the duration and frequency of maternal responses progressively decline (Reisbick et al., 1975; Cramer et al., 1990; Giovenardi et al., 2000; Pereira and Morrell, 2009). Moreover, although pups have a strong incentive value for postpartum rats, mothers might modify their MB to cope with other incentives such as an intruder in the home cage or food (Mayer et al., 1987; Kinsley et al., 2014). This evidence illustrates the adaptability of MB to pup characteristics and context, however, this maternal flexibility may often be masked in the standard laboratory testing conditions in which rats are exposed to pups from a single litter in their home cages. Interestingly, the reproductive characteristics of this species provide us with the opportunity to study MB in more complex and ethologically relevant contexts, even in laboratory settings.
As in most rodent species, a few hours after parturition female rats show a fertile postpartum estrus (PPE), a brief period of time when females are simultaneously maternally and sexually motivated (Connor and Davis, 1980a; Gilbert et al., 1980; Lonstein and Stern, 1998; Agrati, 2022). In natural conditions the successful mating during this period and the delayed dispersal of the young result in the temporal overlapping of successive litters within the maternal nest (Calhoun, 1963; Gilbert et al., 1983). This situation can be successfully replicated in semi-natural conditions and the laboratory (Gilbert et al., 1983; Uriarte et al., 2008), giving us the possibility to study the coexistence of maternal and sexual motivations during the PPE, as well as the adaptation of MB of mothers simultaneously caring for two overlapping litters with different physiological demands and behavioral capabilities.
Pups are the “apple of the eyes” of maternal rats. Postpartum females prefer to spend time in an environment associated with mother-pups interaction (Fleming et al., 1994; Wansaw et al., 2008), and insatiable lever-press if this action results in the delivery of pups (Wilsoncroft, 1968; Lee et al., 1999). Maternal motivation appears invulnerable, particularly in the first postpartum week, when lactating rats develop conditioned place preference to a compartment associated with pups, but not to a compartment associated with food (Fleming et al., 1994), and even prefer a pup-associated chamber to a cocaine-associated chamber (Mattson et al., 2001; Pereira and Morrell, 2010). Can another stimulus compete with the pups for the attention of the maternal rat? The PPE, when females are simultaneously maternally and sexually motivated (Gilbert et al., 1980; Agrati, 2022), represents an excellent period for exploring how maternal motivation interacts with sexual motivation.
Postpartum estrus is a common physiological phenomenon since all, or almost all, female rats present it [(Blandau and Soderwall, 1941; Connor and Davis, 1980a,b), observations from our lab]. Females are usually sexually active between 6 and 18 h after delivery, although the maximal expression of sexual behavior occurs around 12 h postpartum (Connor and Davis, 1980a; Carrillo-Martínez et al., 2011).1 Gilbert et al. (1980) observed that when given the opportunity to freely interact with both reinforcing stimuli -pups and a male- in a large semi-natural environment, PPE rats exhibit both maternal care and mating, but in separated locations and time windows. Females stay with their pups in the nest area and take care of them until approximately 10 h after parturition, when they leave the pups and copulate with the male away from the nest (Gilbert et al., 1980). The strength of male incentive value for PPE females during these mating bouts is reflected in the little time that females spend with their litters within an ejaculation series, even if the nest area is disturbed (Gilbert et al., 1984).
Given the relevance of the context for females’ sexual responses (Erskine, 1989; Paredes and Alonso, 1997; Guarraci and Frohardt, 2019; Chu and Ågmo, 2023), we wonder how a PPE rat would respond if confronted with the pups and a male in her home cage. As maternal rats vigorously attack intruders in their home cages (Ferreira and Hansen, 1986; Erskine, 1989; De Almeida et al., 2014), while estrous females actively sexually solicit it (Beach, 1976; Pfaus et al., 1999; Blaustein and Erskine, 2002), it was surprising that introducing a male into the home cage of a PPE rat with her pups promoted the merged expression of aggressive responses and sexual solicitation by the female. Therefore, in the context of the home cage, as opposed to the semi-natural environment, the male is perceived as an ambivalent stimulus by the PPE female, who consequently co-expresses maternal aggression and sexual behavior (Agrati et al., 2011). This aggressive response is modulated by the pups. Thus, removing the pups and reintroducing them opposite to the nest, together with the male, increases MB, including pup’s retrieval. This stimulation of MB is associated with an increase of maternal aggression, without affecting sexual behavior (Agrati et al., 2011). In summary, during PPE, when pups and a male are simultaneously available, females are able to flexibly adapt their behavior -maternal behavior and aggression and sexual behavior- depending on the context, even regulating their response according to pup needs.
Unlike what was observed in a semi-natural environment and in the female’s home cage, in a preference test between pups and a male, where no contact with the stimuli was possible, PPE rats ignored the male and preferred the pups (Agrati et al., 2008, 2016). Noteworthy, the time spent near the pups and the attempts made to reach them, were modulated by the female’s maternal motivation. If the maternal motivation of primiparous PPE rats was reduced (by limiting the time of mother-litter interaction), they preferred the male, as opposed to the clear preference for the pups of undisturbed PPE females (Agrati et al., 2016). Moreover, postpartum mothers preferred the pups, while maternal sensitized females2 tested in the proestrus chose the male, and multiparous PPE females exhibited a stronger preference for the pups than primiparous rats (Agrati et al., 2008). Thus, factors known to modulate maternal motivation, such as the endocrine profile and previous reproductive experience of the mothers (Fleming et al., 1994; Scanlan et al., 2006; Afonso et al., 2009; Bridges, 2016), modify the behavior of PPE rats in pups vs. male preference test. Together, these studies indicate that the incentive value of pups and males for PPE females influence one another and change depending upon the context. This dynamic value of pups for PPE rats probably sets the basis that underlies the most appropriate behavioral response according to the circumstances.
The mesocorticolimbic dopaminergic system is considered a general motivational system that promotes the activation of motivated behaviors (Salamone and Correa, 2002; Treadway and Salamone, 2022) including maternal and sexual behaviors [for maternal:(Keer and Stern, 1999; Miller and Lonstein, 2005; Numan et al., 2005a; Pereira et al., 2011) and for sexual: (Pfaus, 2009; Coria-Avila et al., 2014) cf. (Paredes and Ågmo, 2004)]. Interestingly, this system has been implicated in effort-related decision-making between two reinforcers (Salamone and Correa, 2002; Hosking et al., 2015; Correa et al., 2016; Treadway and Salamone, 2022). In our preference task between these two social reinforcers, antagonizing dopaminergic neurotransmission diminished the preference for the pups over the male, as well as the attempts that PPE rats made to gain access to them. Remarkably, the same dopaminergic manipulation did not affect the preference of PPE rats for the pups over a female (social stimulus) or the preference for the male over a female (Ferreño et al., 2018b). This result points to a crucial role for dopaminergic activity in shaping the optimal behavioral output when these two reinforcers for PPE rats, the pups and a male, are inaccessible and compete with each other.
The differential effect of this dopaminergic manipulation on PPE females according to the social stimulus competing with pups (purely social or sexual), exemplifies how challenging situations can help elucidate the contribution of a neurotransmitter system, or even a particular brain area, to the behavioral profile of maternal rats. Similarly, we determined that the pups vs. male preference test was sensitive enough to detect motivational differences between PPE rats differing in their internal state, which did not emerge in the maternal behavior home cage test (Agrati et al., 2016). These studies emphasize the importance of confronting motivations to understand how flexible the behavioral response of mother rats can be, and highlight PPE as an excellent model for this purpose. Beyond its relevance for understanding the unique adaptations of the maternal brain, this model may also be useful for exploring the neural basis of the interaction between motivations.
After the birth of a new litter conceived in the PPE, mother rats will simultaneously raise two overlapping litters (OL) with pups with different physiological demands and behavioral capabilities (i.e., nutritional needs, thermoregulation, waste excretion capacities, mobility, and responses to environmental stimuli), a unique reproductive situation first studied by Gilbert et al. (1983) and Stern and Rogers (1988).
Based on undisturbed recording conducted multiple times a day for several days, we reported that mothers direct most of their maternal responses to the newborn pups from the second litter. However, they also continue to provide care for the juvenile pups from the first litter (Uriarte et al., 2008). The mothers do not wean the first litter upon the birth of the new litter, and they invest more in the care of the juveniles compared to same-aged pups raised in single litters (Uriarte et al., 2008). Notably, the presence of the juveniles also influences the OL dam’s MB toward the newborns. They exhibit reduced licking of the newborns and spend more time outside the nest, as compared to mothers rearing single litters (Uriarte et al., 2008). Thus, within the context of OL, mothers display a distinctive behavioral profile, adapting their caregiving activities to the specific characteristics of pups from both litters.
The reduced maternal response exhibited by mothers with OL toward juvenile pups may be attributed to the decreased incentive value of pups at weaning age. This decline in incentive value has been linked to the evolving behavioral capacities and physiological needs of the growing pups. Thus, younger pups tend to be more rewarding for mother rats in specific behavioral tests assessing motivation, such as the conditioned place preference test, compared to older pups (Wansaw et al., 2008). In accordance, we have shown that multiparous mothers during the early postpartum period strongly prefer newborns over juveniles in a preference test without physical access to them, while late postpartum mothers do not express a preference toward either of them (Ferreño et al., 2018a).
Interestingly, OL mothers performed more attempts to gain access to newborns, similar to those observed in early postpartum mothers, suggesting that newborns have a higher incentive value than juveniles for them (Ferreño et al., 2018a). However, OL mothers, similar to late postpartum rats, do not differ in the time spent in the newborns’ and juveniles’ chambers of the maze (Ferreño et al., 2018a). This evidence suggests that juvenile pups have an incentive value for OL mothers, who chose to spend time near them. Therefore, the differential maternal response of these mothers according to the pups’ age observed may be an adaptation to the reduced demands of these more autonomous pups.
The adaptation of the maternal response to the changing needs of growing pups of a single litter throughout lactation has been proposed to rely on changes in the functional role of areas involved in MB regulation, such as the medial preoptic area (mPOA) and the medial prefrontal cortex (mPFC) (Pereira and Morrell, 2009, 2020; Pereira et al., 2011). Transient inactivation of mPOA in early postpartum abolishes the active components of MB such as retrieval, licking and nest building, while this procedure on postpartum days 13–14 restores these behaviors to early postpartum levels (Pereira and Morrell, 2009), indicating a different function of this area throughout the postpartum period. Regarding the distinct subregions of the mPFC, Pereira and Morrell (2011) proposed that the infralimbic subregion (IL-mPFC) has a central role during early postpartum, which wanes as it progresses, whereas the prelimbic subregion (PrL-mPFC) appears to be recruited later in the postpartum.
Similarly, we described specific and distinctive neural activation patterns in OL mothers exposed to newborns or juveniles. Although during the behavioral test the mothers displayed high levels of MB toward the newborns and had minimal interaction with the juveniles, c-Fos expression in the mPOA and the nucleus accumbens was similar after interacting with each type of pup. In contrast, a differential activation according to pups age was quantified in the ventral region of the bed nucleus of the stria terminalis, and in neural areas involved in the flexibility and cognitive processes of the behavior, including subregions of the amygdala and the mPFC (Pose et al., 2019). In line with Pereira and Morrell’s results, the IL-mPFC of OL mothers exhibited the highest c-Fos expression after interacting with newborns. The PrL-mPFC showed high c-Fos expression after interacting with newborns, but even more after interacting with juveniles, pointing toward a particular profile of activation of this area in OL dams with elements that resemble both early and late postpartum periods. This might be part of the unique neural adaptations that allow them to respond adequately to newborn and juvenile pups simultaneously (Pose et al., 2019). Therefore, we posit that the specific profile of activation of the neural circuitry controlling MB enables dams to respond adequately to the requirements of pups with different characteristics and physiological demands. An interesting perspective to deepen this hypothesis includes the analysis of maternal motivation and behavior following the transient inactivation of these specific areas of the maternal neural circuit in mothers taking care of successive litters. Furthermore, delving into the functioning of maternal circuits during OL could help identify differences between areas crucial for being a mother and those that process and evaluate the offspring.
Which factors contribute to this maternal adaptation? Recent studies indicate that the levels of MB toward newborns are lower during late- compared to early postpartum period (Grieb et al., 2018), suggesting a role of the gestation hormonal profile in high maternal response toward neonates. In addition, extensive research conducted throughout the peripartum period demonstrates that gestational and parturition hormones, along with reproductive experience, induce structural and functional remodeling of brain areas associated with motherhood and its adaptations (Kinsley and Bridges, 1988; Leuner and Gould, 2010; Bridges, 2016; Uriarte et al., 2020; Pawluski et al., 2022). To elucidate whether the behavioral adaptation of OL mothers that allows them to care for both types of pups providing differential maternal responses, depends on the endocrine profile of their second gestation and/or on their recent maternal experience, it would be interesting to compare the relative incentive value of newborn and juvenile pups for OL mothers differing in their hormonal exposure and maternal experience.
Studies presented in this section show that the model of rats raising OL provides useful insights into how maternal behavior implicates flexible and contingent processes and can help us better understand the neuroendocrine mechanisms underlying parenting. Additionally, the OL model provides a socially enriching environment for juveniles, promoting increased interaction with their mother and fostering parental behavior toward newborns. As these alterations in early experience can lead to long-term changes in affective behavior (Uriarte et al., 2009, 2014), this model also offers a valuable approach to study the impact of early social environments on individual development and highlights the notion that diverse early social backgrounds can give rise to multiple developmental trajectories.
The findings presented in this article, derived from studies conducted in highly demanding reproductive contexts, show that female rats are able to adapt their behavior specifically to the needs of the pups, the presence of other reinforcers, and the context. This behavioral flexibility allows them to care for and protect their offspring while also engaging in mating or differentially attending to pups of different ages. Consequently, each reproductive context has its own characteristics that not only shed light on, but also facilitate, the examination of complementary aspects of motherhood: coping simultaneously with competing motivations or with different and specific demands of pups (Figure 1).
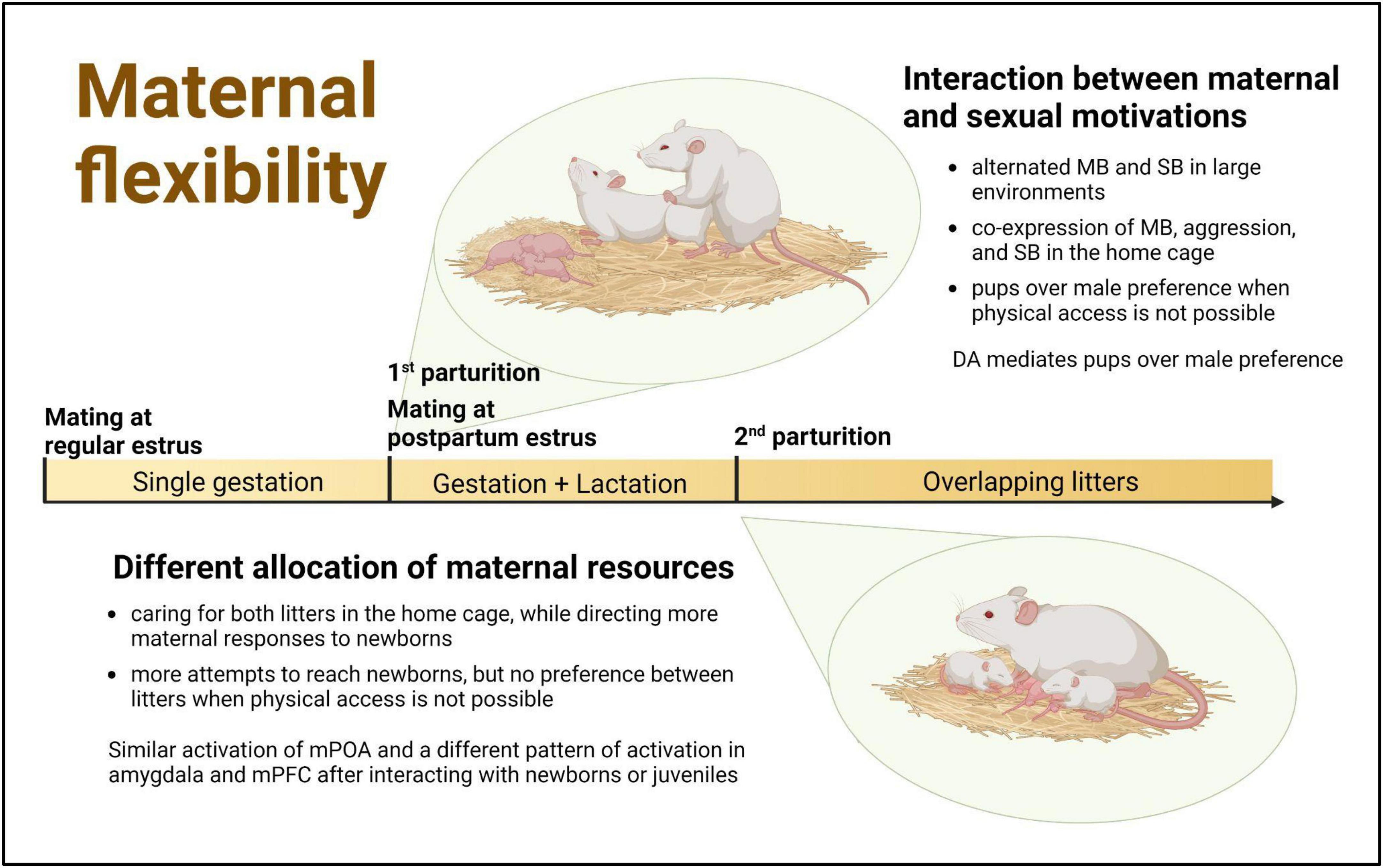
Figure 1. Challenging reproductive contexts in the female rat as a window of opportunity to study maternal flexibility. Within the first 24 h after parturition female rats usually exhibit a fertile postpartum estrus, when they are simultaneously maternally and sexually motivated The successful mating during this period and the delayed dispersal of pups from the first litter can result in the temporal overlapping within the maternal nest of successive litters with different behavioral and physiological demands. MB, maternal behavior; SB, sexual behavior; DA, dopamine; mPOA, medial preoptic area; mPFC, medial prefrontal cortex. Created with BioRender.com.
Traditionally, rodents have been used as models in the study of the neural mechanisms underlying evolutionarily conserved behaviors, including MB. Using these experimental models has proven advantageous as they enable researchers to reduce the number of variables involved, facilitating the study of the neurobiology of this behavior (Ferreira et al., 1987; Fleming and Korsmit, 1996; Numan et al., 2005b; Numan, 2007; Fleming et al., 2008; Pereira and Morrell, 2009, 2011; Olazábal et al., 2013). Based upon these foundations, over the past few decades, technological advances have allowed the identification of neuronal phenotypes and the specificity of their connections, as well as the detection of epigenetic marks in the maternal neural circuitry (Champagne et al., 2006; Fang et al., 2018; Kohl et al., 2018; Mayer et al., 2019). However, as our understanding of this neural circuit continues to evolve, it becomes crucial to test it in situations that closely mimic the complex and natural environmental conditions in which MB actually unfolds, as exemplified by the scenarios mentioned earlier. For example, in comparison to mothers caring for pups in standard conditions, the behavioral flexibility of PPE females when confronted with pups and a male and that of mothers raising OL, likely demands a greater involvement of cognitive functions (Pose et al., 2019), processes depending on the integrity of mPFC circuits and their subcortical connections (Dalley et al., 2004).
Moreover, the refinement in the analysis of classically recorded variables, as well as the incorporation of new ones, is proving a useful insight into the characterization of MB (Carcea et al., 2021; Rivas et al., 2021; Tuncali et al., 2023; Winters et al., 2023), and may be relevant for a more accurate description of the maternal repertoire displayed in challenging contexts. For example, Tuncali et al. (2023), through the analysis of ultrasonic vocalizations of the mother-litter dyad, propose a role for the expression of maternal positive affect in the mother-pup interaction. Will females in PPE similarly vocalize toward a male and their pups? Will this affective communication differ in mother rats during an interaction with younger or older litters?
We believe that future perspectives in this field should integrate advanced techniques such as neuroimaging, optogenetics, and molecular biology and refinement in behavioral analysis with models that incorporate complex and challenging contexts. This integrated approach would provide a more comprehensive understanding of the neural circuits implicated in the adaptive and flexible nature of parenting.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
DA and NU worked equally on the conception, development of the ideas, and writing of the manuscript. Both authors contributed to the article and approved the submitted version.
This work was supported by CSIC I + D grant 2020 to DA and grant 2022 to NU and by PEDECIBA, SNI-ANII, and DT CSIC-UdelaR funding to DA and NU.
We would like to thank Annabel Ferreira for the careful review of the manuscript, but above all, we are deeply grateful to her for always inspiring us and encouraging us to question the established paradigms.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Afonso, V., King, S., Chatterjee, D., and Fleming, A. (2009). Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm. Behav. 56, 11–23. doi: 10.1016/j.yhbeh.2009.02.003
Agrati, D. (2022). Adolescence and postpartum: two life periods to deepen our understanding of the complexity of female rat sexual behavior. Sexes 3, 282–297. doi: 10.3390/sexes3020022
Agrati, D., Fernández-Guasti, A., and Ferreira, A. (2008). The reproductive stage and experience of sexually receptive mothers alter their preference for pups or males. Behav. Neurosci. 122, 998–1004. doi: 10.1037/a0012585
Agrati, D., Fernández-Guasti, A., Ferreno, M., and Ferreira, A. (2011). Coexpression of sexual behavior and maternal aggression: the ambivalence of sexually active mother rats toward male intruders. Behav. Neurosci. 125, 446–451. doi: 10.1037/a0023085
Agrati, D., Ferreño, M., Marin, G., Uriarte, N., Zuluaga, M., Fernández-Guasti, A., et al. (2016). Previous and recent maternal experiences modulate pups’ incentive value relative to a male without affecting maternal behavior in postpartum estrous rats. J. Physiol. Paris 110, 140–148. doi: 10.1016/j.jphysparis.2016.11.002
Beach, F. (1976). Sexual attractivity, proceptivity, and receptivity in female mammals. Horm. Behav. 7, 105–138. doi: 10.1016/0018-506X(76)90008-8
Blandau, R., and Soderwall, A. (1941). Post-parturitional heat and the time of ovulation in the albino rat. Data on parturition. Anat. Rec. 81, 419–431. doi: 10.1002/ar.1090810402
Blaustein, J., and Erskine, M. (2002). “Feminine sexual behavior,” in Hormones, brain and behavior, ed. Academic Press (Amsterdam: Elsevier), 139–214.
Bridges, R. (2016). Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Horm. Behav. 77, 193–203. doi: 10.1016/j.yhbeh.2015.09.001
Caldji, C., Tannenbaum, B., Sharma, S., Francis, D., Plotsky, P., and Meaney, M. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U.S.A. 95, 5335–5340. doi: 10.1073/pnas.95.9.5335
Calhoun, J. (1963). The ecology and sociology of the Norway rat. Bethesda, MD: U.S. Dept. of Health, Education, and Welfare.
Carcea, I., Caraballo, N., Marlin, B., Ooyama, R., Riceberg, J., Mendoza Navarro, J., et al. (2021). Oxytocin neurons enable social transmission of maternal behaviour. Nature 596, 553–557. doi: 10.1038/s41586-021-03814-7
Carrillo-Martínez, G., Gómora-Arrati, P., González-Arenas, A., Morimoto, S., Camacho-Arroyo, I., and González-Flores, O. (2011). Role of progesterone receptors during postpartum estrus in rats. Horm. Behav. 59, 37–43. doi: 10.1016/j.yhbeh.2010.10.008
Champagne, F., Francis, D., Mar, A., and Meaney, M. (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 79, 359–371. doi: 10.1016/S0031-9384(03)00149-5
Champagne, F., Weaver, I., Diorio, J., Dymov, S., Szyf, M., and Meaney, M. (2006). Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial Preoptic area of female offspring. Endocrinology 147, 2909–2915. doi: 10.1210/en.2005-1119
Chu, X., and Ågmo, A. (2023). “Evaluation of sexual behavior in laboratory vs seminatural conditions,” in Animal models of reproductive behavior, Vol. 200, eds R. Paredes, W. Portillo, and M. Bedos (New York, NY: Springer US), 171–195.
Connor, J., and Davis, H. (1980a). Postpartum estrus in Norway rats. I. Behavior. Biol. Reprod. 23, 994–999. doi: 10.1095/biolreprod23.5.994
Connor, J., and Davis, H. (1980b). Postpartum estrus in Norway rats. II. Physiology. Biol. Reprod. 23, 1000–1006. doi: 10.1095/biolreprod23.5.1000
Coria-Avila, G., Manzo, J., Garcia, L., Carrillo, P., Miquel, M., and Pfaus, J. (2014). Neurobiology of social attachments. Neurosci. Biobehav. Rev. 43, 173–182. doi: 10.1016/j.neubiorev.2014.04.004
Correa, M., Pardo, M., Bayarri, P., López-Cruz, L., San Miguel, N., Valverde, O., et al. (2016). Choosing voluntary exercise over sucrose consumption depends upon dopamine transmission: effects of haloperidol in wild type and adenosine A2AKO mice. Psychopharmacology 233, 393–404. doi: 10.1007/s00213-015-4127-3
Cramer, C., Thiels, E., and Alberts, J. (1990). Weaning in rats: I. Maternal behavior. Dev. Psychobiol. 23, 479–493. doi: 10.1002/dev.420230604
Dalley, J., Cardinal, R., and Robbins, T. (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 28, 771–784. doi: 10.1016/j.neubiorev.2004.09.006
De Almeida, R., Ferreira, A., and Agrati, D. (2014). “Sensory, hormonal, and neural basis of maternal aggression in rodents,” in Neuroscience of aggression, Vol. 17, eds K. Miczek and A. Meyer-Lindenberg (Berlin: Springer Berlin Heidelberg), 111–130. doi: 10.1007/7854_2014_312
Erskine, M. (1989). Solicitation behavior in the estrous female rat: a review. Horm. Behav. 23, 473–502. doi: 10.1016/0018-506x(89)90037-8
Fang, Y., Yamaguchi, T., Song, S., Tritsch, N., and Lin, D. A. (2018). Hypothalamic midbrain pathway essential for driving maternal behaviors. Neuron 98, 192.e10–207.e10. doi: 10.1016/j.neuron.2018.02.019
Ferreira, A., Dahlöf, L., and Hansen, S. (1987). Olfactory mechanisms in the control of maternal aggression, appetite, and fearfulness: effects of lesions to olfactory receptors, mediodorsal thalamic nucleus, and insular prefrontal cortex. Behav. Neurosci. 101, 709–717. doi: 10.1037//0735-7044.101.5.709
Ferreira, A., and Hansen, S. (1986). Sensory control of maternal aggression in Rattus norvegicus. J. Comp. Psychol. 100, 173–177. doi: 10.1037/0735-7036.100.2.173
Ferreño, M., Uriarte, N., Zuluaga, M., Ferreira, A., and Agrati, D. (2018b). Dopaminergic activity mediates pups’ over male preference of postpartum estrous rats. Physiol. Behav. 188, 134–139. doi: 10.1016/j.physbeh.2018.02.002
Ferreño, M., Pose, S., Agrati, D., Zuluaga, M., Ferreira, A., and Uriarte, N. (2018a). Incentive value of newborn pups relative to juveniles for mother rats raising overlapping litters. Behav. Processes. 157, 333–336. doi: 10.1016/j.beproc.2018.07.016
Fleming, A., Gonzalez, A., Afonso, V., and Lovic, V. (2008). “Plasticity in the maternal neural circuit,” in Neurobiology of the parental brain, ed. R. S. Bridges (Amsterdam: Elsevier), 516–535.
Fleming, A., and Korsmit, M. (1996). Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav. Neurosci. 110, 567–582. doi: 10.1037//0735-7044.110.3.567
Fleming, A., Korsmit, M., and Deller, M. (1994). Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology 22, 44–53. doi: 10.3758/BF03327079
Fleming, A., and Rosenblatt, J. (1974). Maternal behavior in the virgin and lactating rat. J. Comp. Physiol. Psychol. 86, 957–972. doi: 10.1037/h0036414
Gilbert, A., Burgoon, D., Sullivan, K., and Adler, N. (1983). Mother-weanling interactions in norway rats in the presence of a successive litter produced by postpartum mating ✩. Physiol. Behav. 30, 267–271. doi: 10.1016/0031-9384(83)90017-3
Gilbert, A., Pelchat, R., and Adler, N. (1980). Postpartum copulatory and maternal behaviour in Norway rats under seminatural conditions. Anim. Behav. 28, 989–995. doi: 10.1016/s0003-3472(80)80087-x
Gilbert, A., Pelchat, R., and Adler, N. (1984). Sexual and maternal behaviour at the postpartum oestrus: the role of experience in time-sharing. Anim. Behav. 32, 1045–1053. doi: 10.1016/S0003-3472(84)80220-1
Giovenardi, M., Consiglio, A., Barros, H., and Lucion, A. (2000). Pup age and aggressive behavior in lactating rats. Braz. J. Med. Biol. Res. 33, 1083–1088. doi: 10.1590/S0100-879X2000000900015
Grieb, Z., Holschbach, M., and Lonstein, J. (2018). Interaction between postpartum stage and litter age on maternal caregiving and medial preoptic area orexin. Physiol. Behav. 194, 430–436. doi: 10.1016/j.physbeh.2018.06.025
Guarraci, F., and Frohardt, R. (2019). “What a Girl Wants”: what can we learn from animal models of female sexual motivation? Front. Behav. Neurosci. 13:216. doi: 10.3389/fnbeh.2019.00216
Hansen, S., Bergvall, Å, and Nyiredi, S. (1993). Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol. Biochem. Behav. 45, 673–676. doi: 10.1016/0091-3057(93)90523-v
Hosking, J., Floresco, S., and Winstanley, C. (2015). Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology 40, 1005–1015. doi: 10.1038/npp.2014.285
Keer, S., and Stern, J. (1999). Dopamine receptor blockade in the nucleus Accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol. Behav. 67, 659–669. doi: 10.1016/s0031-9384(99)00116-x
Kinsley, C., Blair, J., Karp, N., Hester, N., McNamara, I., Orthmeyer, A., et al. (2014). The mother as hunter: significant reduction in foraging costs through enhancements of predation in maternal rats. Horm. Behav. 66, 649–654. doi: 10.1016/j.yhbeh.2014.09.004
Kinsley, C., and Bridges, R. (1988). Parity-associated reductions in behavioral sensitivity to Opiates1. Biol. Reprod. 39, 270–278. doi: 10.1095/biolreprod39.2.270
Kohl, J., Babayan, B., Rubinstein, N., Autry, A., Marin-Rodriguez, B., Kapoor, V., et al. (2018). Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. doi: 10.1038/s41586-018-0027-0
Lee, A., Clancy, S., and Fleming, A. (1999). Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res. 100, 15–31. doi: 10.1016/S0166-4328(98)00109-0
Leuner, B., and Gould, E. (2010). Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. J. Neurosci. 30, 13499–13503. doi: 10.1523/JNEUROSCI.3388-10.2010
Lonstein, J., and Stern, J. (1998). Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Res. 804, 21–35. doi: 10.1016/s0006-8993(98)00642-8
Mattson, B., Williams, S., Rosenblatt, J., and Morrell, J. (2001). Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav. Neurosci. 115, 683–694. doi: 10.1037/0735-7044.115.3.683
Mayer, A., Reisbick, S., Siegel, H., and Rosenblatt, J. (1987). Maternal aggression in rats: changes over pregnancy and lactation in a sprague-dawley strain. Aggress. Behav. 13, 29–43. doi: 10.1002/1098-2337(1987)13:1<29::AID-AB2480130106>3.0.CO;2-1
Mayer, H., Crepeau, M., Duque-Wilckens, N., Torres, L., Trainor, B., and Stolzenberg, D. (2019). Histone deacetylase inhibitor treatment promotes spontaneous caregiving behaviour in non-aggressive virgin male mice. J. Neuroendocrinol. 31:e12734. doi: 10.1111/jne.12734
Miller, S., and Lonstein, J. (2005). Dopamine D1 and D2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav. Neurosci. 119, 1072–1083. doi: 10.1037/0735-7044.119.4.1072
Numan, M. (2007). Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 49, 12–21. doi: 10.1002/dev.20198
Numan, M., Numan, M., Pliakou, N., Stolzenberg, D., Mullins, O., Murphy, J., et al. (2005a). The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav. Neurosci. 119, 1588–1604. doi: 10.1037/0735-7044.119.6.1588
Numan, M., Numan, M., Schwarz, J., Neuner, C., Flood, T., and Smith, C. (2005b). Medial preoptic area interactions with the nucleus accumbens–ventral pallidum circuit and maternal behavior in rats. Behav. Brain Res. 158, 53–68. doi: 10.1016/j.bbr.2004.08.008
Numan, M., and Young, L. (2016). Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav. 77, 98–112. doi: 10.1016/j.yhbeh.2015.05.015
Olazábal, D., Pereira, M., Agrati, D., Ferreira, A., Fleming, A., González-Mariscal, G., et al. (2013). Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci. Biobehav. Rev. 37, 1875–1892. doi: 10.1016/j.neubiorev.2013.04.004
Paredes, R., and Ågmo, A. (2004). Has dopamine a physiological role in the control of sexual behavior? Prog. Neurobiol. 73, 179–225. doi: 10.1016/j.pneurobio.2004.05.001
Paredes, R., and Alonso, A. (1997). Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav. Neurosci. 111, 123–128. doi: 10.1037/0735-7044.111.1.123
Pawluski, J., Hoekzema, E., Leuner, B., and Lonstein, J. (2022). Less can be more: fine tuning the maternal brain. Neurosci. Biobehav. Rev. 133:104475. doi: 10.1016/j.neubiorev.2021.11.045
Pereira, M., Farrar, A., Hockemeyer, J., Müller, C., Salamone, J., and Morrell, J. (2011). Effect of the adenosine A2A receptor antagonist MSX-3 on motivational disruptions of maternal behavior induced by dopamine antagonism in the early postpartum rat. Psychopharmacology 213, 69–79. doi: 10.1007/s00213-010-2015-4
Pereira, M., and Ferreira, A. (2006). Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav. Brain Res. 175, 139–148. doi: 10.1016/j.bbr.2006.08.013
Pereira, M., and Ferreira, A. (2016). Neuroanatomical and neurochemical basis of parenting: dynamic coordination of motivational, affective and cognitive processes. Horm. Behav. 77, 72–85. doi: 10.1016/j.yhbeh.2015.08.005
Pereira, M., and Morrell, J. (2009). The changing role of the medial preoptic area in the regulation of maternal behavior across the postpartum period: facilitation followed by inhibition. Behav. Brain Res. 205, 238–248. doi: 10.1016/j.bbr.2009.06.026
Pereira, M., and Morrell, J. (2010). The medial preoptic area is necessary for motivated choice of pup- over cocaine-associated environments by early postpartum rats. Neuroscience 167, 216–231. doi: 10.1016/j.neuroscience.2010.02.015
Pereira, M., and Morrell, J. (2011). Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J. Neuroendocr. 23, 1020–1035. doi: 10.1111/j.1365-2826.2011.02200.x
Pereira, M., and Morrell, J. (2020). Infralimbic cortex biases preference decision making for offspring over competing cocaine-associated stimuli in new mother rats. eNeuro 7:ENEURO.0460-19.2020. doi: 10.1523/ENEURO.0460-19.2020
Pereira, M., Seip, K., and Morrell, J. (2008). “Maternal motivation and its neural substrate across the postpartum period,” in Neurobiol parent brain, ed. R. S. Bridges (Cambridge, MA: Academic Press), 39–59.
Pfaus, J., Smith, W., and Coopersmith, C. (1999). Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. Horm. Behav. 35, 224–240. doi: 10.1006/hbeh.1999.1516
Pfaus, J. G. (2009). Pathways of sexual desire. J. Sex Med. 6, 1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x
Pose, S., Zuluaga, M., Ferreño, M., Agrati, D., Bedó, G., and Uriarte, N. (2019). Raising overlapping litters: differential activation of rat maternal neural circuitry after interacting with newborn or juvenile pups. J. Neuroendocrinol. 31:e12701. doi: 10.1111/jne.12701
Reisbick, S., Rosenblatt, J., and Mayer, A. (1975). Decline of maternal behavior in the virgin and lactating rat. J. Comp. Physiol. Psychol. 89, 722–732. doi: 10.1037/h0077059
Rivas, M., Serantes, D., Peña, F., González, J., Ferreira, A., Torterolo, P., et al. (2021). Role of hypocretin in the medial preoptic area in the regulation of sleep, maternal behavior and body temperature of lactating rats. Neuroscience 475, 148–162. doi: 10.1016/j.neuroscience.2021.08.034
Salamone, J., and Correa, M. (2002). Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav. Brain Res. 137, 3–25. doi: 10.1016/S0166-4328(02)00282-6
Scanlan, V., Byrnes, E., and Bridges, R. (2006). Reproductive experience and activation of maternal memory. Behav. Neurosci. 120, 676–686. doi: 10.1037/0735-7044.120.3.676
Stern, J., and Rogers, L. (1988). Experience with younger siblings facilitates maternal responsiveness in pubertal norway rats. Dev. Psychobiol. 21, 575–589. doi: 10.1002/dev.420210608
Treadway, M., and Salamone, J. (2022). “Vigor, effort-related aspects of motivation and Anhedonia,” in Anhedonia: preclinical, translational, and clinical integration, Vol. 58, ed. D. Pizzagalli (Cham: Springer International Publishing), 325–353.
Tuncali, I., Sorial, N., Torr, K., and Pereira, M. (2023). Positive maternal affect during mother–litter interaction is reduced in new mother rats exhibiting a depression-like phenotype. Sci. Rep. 13:6552. doi: 10.1038/s41598-023-33035-z
Uriarte, N., Breigeiron, M., Benetti, F., Rosa, X., and Lucion, A. (2007). Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev. Psychobiol. 49, 451–462. doi: 10.1002/dev.20241
Uriarte, N., Fernandez, M., Agrati, D., Zuluaga, M., Ferreno, M., and Ferreira, A. (2014). Maternal and affective behaviors of lactating rats reared in overlapping litters. J. Physiol. Paris 108, 221–230. doi: 10.1016/j.jphysparis.2014.04.001
Uriarte, N., Ferreira, A., Rosa, X., and Lucion, A. (2009). Effects of litter-overlapping on emotionality, stress response, and reproductive functions in male and female rats. Dev. Psychobiol. 51, 259–267. doi: 10.1002/dev.20360
Uriarte, N., Ferreira, A., Rosa, X., Sebben, V., and Lucion, A. (2008). Overlapping litters in rats: effects on maternal behavior and offspring emotionality. Physiol. Behav. 93, 1061–1070. doi: 10.1016/j.physbeh.2008.02.004
Uriarte, N., Ferreño, M., Méndez, D., and Nogueira, J. (2020). Reorganization of perineuronal nets in the medial Preoptic area during the reproductive cycle in female rats. Sci. Rep. 10:5479. doi: 10.1038/s41598-020-62163-z
Wansaw, M., Pereira, M., and Morrell, J. (2008). Characterization of maternal motivation in the lactating rat: contrasts between early and late postpartum responses. Horm. Behav. 54, 294–301. doi: 10.1016/j.yhbeh.2008.03.005
Wilsoncroft, W. (1968). Babies by bar-press: maternal behavior in the rat. Behav. Res. Methods Instrum. 1, 229–230. doi: 10.3758/BF03208105
Winters, C., Gorssen, W., Wöhr, M., and D’Hooge, R. (2023). BAMBI: a new method for automated assessment of bidirectional early-life interaction between maternal behavior and pup vocalization in mouse dam-pup dyads. Front. Behav. Neurosci. 17:1139254. doi: 10.3389/fnbeh.2023.1139254
Keywords: postpartum estrus, overlapping litters, maternal motivation and behavior, sexual motivation, maternal flexibility
Citation: Agrati D and Uriarte N (2023) What can challenging reproductive contexts tell us about the rat’s maternal behavior? Front. Behav. Neurosci. 17:1239681. doi: 10.3389/fnbeh.2023.1239681
Received: 13 June 2023; Accepted: 03 July 2023;
Published: 14 July 2023.
Edited by:
Daniela Schulz, Boğaziçi University, TürkiyeReviewed by:
Fay A. Guarraci, Southwestern University, United StatesCopyright © 2023 Agrati and Uriarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniella Agrati, ZGFncmF0aUBmY2llbi5lZHUudXk=; Natalia Uriarte, bmF0aXVyaWFAZmNpZW4uZWR1LnV5
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.