
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Behav. Neurosci. , 20 February 2023
Sec. Motivation and Reward
Volume 17 - 2023 | https://doi.org/10.3389/fnbeh.2023.1080963
This article is part of the Research Topic Alcohol and Energy Drinks: Is This a Really Good Mix? View all 5 articles
Energy drinks (EDs) are beverages similar to soft drinks, characterized by high caffeine concentrations with additional ingredients like taurine and vitamins, marketed for boosting energy, reducing tiredness, increasing concentration, and for their ergogenic effect. The majority of consumers are children, adolescents, and young athletes. Although EDs companies claim about the ergogenic and remineralizing properties of their products, there is a serious lack of evidence at preclinical as well as clinical level to validate their benefits. The regular intake and long-term consequences of these caffeinated drinks are not well documented, especially the possible negative effects in adolescents whose brain is still developing. EDs combined with alcohol are also gaining popularity among adolescents and different publications indicate that this combined consumption might increase the risk to develop an alcohol use disorder, as well as produce serious adverse cardiovascular effects. There is an increasing need to disseminate knowledge on EDs damage on health, so that adolescents can be aware about the potential harmful outcomes of consuming these drinks.
Energy drinks (EDs) are relatively new products that are like soft drinks, with additional additives and higher caffeine concentration that is the cornerstone of these beverages (Howard and Marczinski, 2010). Classic ingredients found in EDs are taurine, glucuronolactone, B vitamins, L-carnitine, sucrose, antioxidants, minerals and other herbal supplements like ginseng, guarana, yerba mate, cocoa, kola nut, and ginkgo biloba (Higgins et al., 2010). These ingredients can differ by brand as well as the list of ingredients that can be proprietary information. Although EDs companies promote EDs for energizing, anti-fatigue, concentration-boosting, and ergogenic properties, there is a serious lack of evidence and clinical trials to validate their benefits (Ruiz and Scherr, 2018).
These products are commonly consumed by adolescents and first consumption usually occurs prior to age 12 (Costa et al., 2016). Compared to girls, adolescent boys have been found to consume EDs more than once a week. Moreover, adolescents who consumed more than one ED per week were those also consuming soft drinks daily, alcohol weekly, and having high screen times or late bedtimes (Lebacq et al., 2020).
The regular intake and long-term consequences of consumption of EDs are not well recognized, most notably the possible dangerous effects on adolescents are not sufficiently studied. Moreover, research on this matter at preclinical level is still limited. Nowadays, the harmful consequences of intake of EDs are the most discussed topic of the scientific literature opening a door to awareness of possible dangers of these drinks to adolescents (Curran and Marczinski, 2017; Hladun et al., 2021; Marinoni et al., 2022).
A bibliographic search in MEDLINE/PubMed was carried out to collect clinical and pre-clinical research or review articles on EDs, and their main ingredients (caffeine, taurine, etc.), from 1987 to September 2022. A special focus was placed on EDs adverse effects (on cardiovascular system and increased risk of drug addiction) especially on children and adolescents. The reference lists of the identified articles were also scanned. Only English-language articles were reviewed. Search terms included: energy drinks/adverse effects OR energy drinks/toxicity; energy drinks/cardiovascular system; energy drinks/alcohol; energy drinks/sport; energy drinks/children OR energy drinks/adolescence; caffeine/adolescence OR caffeine/brain development; taurine/brain development; energy drinks/alcohol use disorders OR energy drinks/drug use disorders.
Since adolescents are the main consumers of EDs (Seifert et al., 2011; Gallimberti et al., 2013) serious concerns have been raised about the detrimental effects of EDs consumption on key brain neurodevelopmental processes. Indeed, childhood and adolescence are critical periods of brain development. While white matter volume, through myelination, is increasing linearly with age, from the third trimester of gestation to the third decade of life (Giedd et al., 1999; Lebel and Deoni, 2018; Geeraert et al., 2019), gray matter volume shows an inverted “U” shaped curve, with a peak increase at adolescence (due to increased synaptogenesis) and then a decrease (due to synaptic pruning) reaching adult volume and organization of neural circuits at about 20–25 years (Lenroot and Giedd, 2006). Therefore, neuronal, and glial cells of the immature brain might be particularly vulnerable to the harmful effects of EDs consumption (Reis et al., 2017; Al-Basher et al., 2018). Caffeine and taurine appear to be the most involved in these effects (Serdar et al., 2019; Brown et al., 2020) considering the role of purinergic signaling in brain development (Rodrigues et al., 2019) and the likely role of taurine in the regulation of network excitability in the immature neocortex and hippocampus (Kilb, 2017).
Caffeine is an antagonist at A1 and A2A adenosine receptors and thus by binding to adenosine receptors can increase the release of various neurotransmitters (glutamate, serotonin, acetylcholine, noradrenaline, dopamine) (McLellan et al., 2016) and, at high concentrations, it is also able to inhibit phosphodiesterase thereby modulating intracellular cyclic adenosine monophosphate and intracellular calcium in brain (Cappelletti et al., 2015). Evidence has accumulated in the last decades pointing to the involvement of purines in fine-tuning different processes during brain development and critical to design brain architecture (Rodrigues et al., 2019). Adenosine, through A1 receptor, also modulates immature synapses in different regions such as hippocampus or developing cortex (Jeong et al., 2003; Kerr et al., 2013; Burnstock and Dale, 2015). Therefore, dysfunction of purinergic signaling by caffeine overconsumption might impair brain development leading to deleterious effects, varying from mild cognitive impairment to severe neurological or psychiatric conditions (Silva et al., 2013; Al-Basher et al., 2018; Serdar et al., 2019; Zhang et al., 2020, 2022; Christensen et al., 2021; Agarwal et al., 2022).
Taurine is a non-essential free amino acid found in high concentrations in the brain, heart, and skeletal muscle (Huxtable, 1992). Taurine inhibits neuronal excitability through interaction with presynaptic glutamatergic receptors (NMDA) and GABAA receptors (positive allosteric modulator of α2 subunit of GABAA receptor) (Tarragon et al., 2021). In addition, taurine has high specificity and affinity for glycine receptors (Albrecht and Schousboe, 2005) and by binding to these receptors mediates glia-neuron signaling in the hypothalamus and excitatory neurotransmission in early development of the neocortex and brain stem (El Idrissi and Trenkner, 2004; Ramírez-Guerrero et al., 2022). High levels of taurine have been found in developing brain and progressively decline until adulthood (Brown et al., 2020), and evidence to date support a role in neuronal development especially in neocortical development (Kilb, 2017; Oja and Saransaari, 2022). Given that during adolescence cortical development is still ongoing and taurine levels are at the highest levels during this period, an excess of taurine intake by EDs consumption might disrupt taurine functions affecting normal development of cortical structures. Indeed, disruption of taurine homeostasis has been reported in numerous studies of neurological disorders included epilepsy and autism (Curran and Marczinski, 2017). A recent preclinical study showed the adverse effects of supplemental taurine consumption during adolescence and early adulthood on learning and memory function, suggesting an altered balance between new synapse formation and synaptic pruning during final cortical maturation as a possible mechanism (Brown et al., 2020). Indeed, taurine activation of GABAA receptors may well interfere with learning and memory processes by affecting glutamate NMDA receptor pathway (Brown et al., 2020).
Another finding (Serdar et al., 2019) showed the detrimental impact of caffeine and taurine on developing oligodendrocytes and neurons. Caffeine and taurine induce degeneration and reduce proliferation of immature oligodendrocytes, accompanied by a decreased myelination capacity. Moreover, caffeine and taurine impair neuronal network formation by reducing dendrite branching and axons fragmentation demonstrating a synergistic effect of the two compounds in oligodendrocyte degeneration (Serdar et al., 2019).
Some studies (Miyake and Marmorstein, 2015; Barrense-Dias et al., 2016) have identified EDs consumption at early adolescence as a predictor of using other legal and illegal substances. EDs frequently are mixed with alcoholic beverages leading to greater alcohol intake and therefore more dangerous blood alcohol levels and consequently a high-risk behavior in adolescents (Thombs et al., 2010; Figure 1). The common habit of mixing EDs with alcohol raises concern since it decreases the perception of drunkenness (Ferreira et al., 2006; Marczinski, 2014; Kaestle et al., 2018) which could lead to further fast and excessive alcohol consumption involving binge drinking and risky behaviors, as driving while intoxicated (Marczinski and Fillmore, 2014).
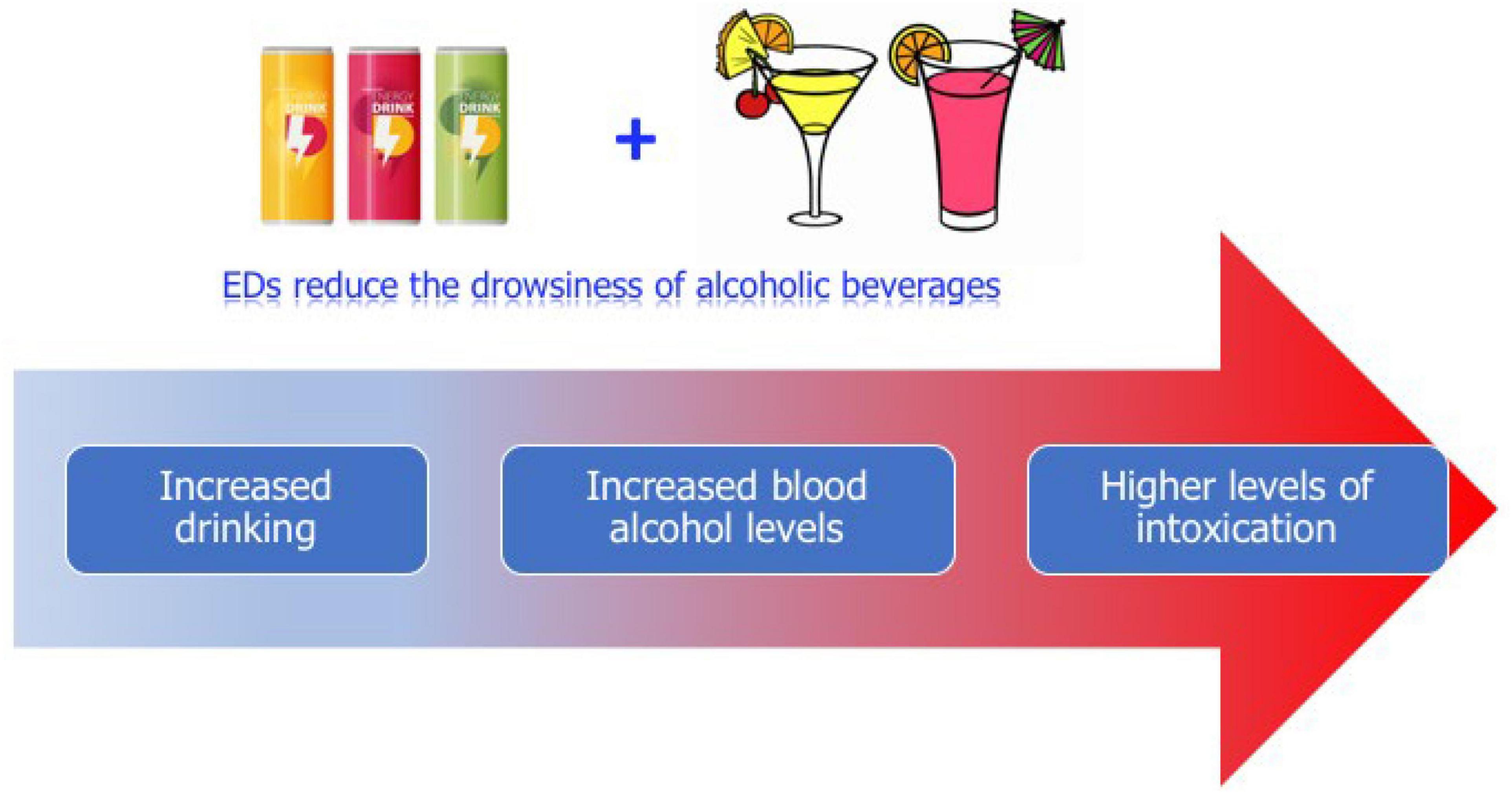
Figure 1. Graphical illustration of likely consequences of mixing Energy Drinks (EDs) with alcoholic drinks. The combined consumption of EDs and alcoholic beverages while reducing drowsiness induced by alcohol (perception of drunkenness) could lead to excessive alcohol intake and thus to higher blood alcohol levels causing more severe intoxications.
In a recent review analyzing the likely increased risk to develop substance use disorders by EDs consumers it has been reported that the most consistent finding is the association between EDs consumption and alcohol use disorders (AUD), while association with the use of other substances (nicotine, cannabis, stimulants, and analgesics) is controversial (Yasuma et al., 2021). Although different studies support the notion that EDs consumers are at increased risk for AUD, the possible mechanisms underlying this association is unknown (Miller, 2008; Arria et al., 2011). Some researchers postulate that EDs could function as a gateway (a priming) to drug dependence (Reissig et al., 2009). Some potential mechanisms could explain the link between these caffeinated drinks and AUD. Caffeine may prolong drinking episodes by delaying the normal sleepiness with a consequent increase in overall alcohol ingestion. On the other hand, antagonism by caffeine of the neuromodulator adenosine (Fredholm et al., 1999) might have an important role in facilitating many neuropharmacological and behavioral properties of alcohol (Sharma et al., 2010). In a recent preclinical study (Vargiu et al., 2021) it has been shown that chronic consumption of the ED Red Bull during adolescence increases dopamine in the nucleus accumbens (NAcb) shell dopamine via a non-adaptive mechanism, as observed with drugs of abuse. The repeated increase of shell dopamine following repeated exposure to EDs might be at the basis of a gateway effect toward abuse of other drugs.
Preclinical findings in rats, have revealed that caffeine may induce alcohol self-administration (Kunin et al., 2000; Arolfo et al., 2004). With regard to the possible mechanism of this effect, the antagonism of adenosine action by caffeine on A2A receptors might be at the basis of this effect. Indeed, adenosine by activating A2A receptors inhibits striatal dopamine transmission and elevation of dopamine levels is pivotal in the reinforcing properties of alcohol (as well as of all abused substances) (Nam et al., 2013; Ulenius et al., 2019).
Taurine does not affect levels of dopamine, serotonin, noradrenaline, and glutamate (Wu et al., 2017) but it can modulate dopamine neurotransmission in brain areas relevant for drug consumption (Adermark et al., 2011; Chen et al., 2018). Moreover, systemic injection of ethanol can increase taurine and dopamine in the NAcb and taurine, administered locally in the NAcb, elevates dopamine in a similar pattern as ethanol (Ericson et al., 2011). A recent study by Pulcinelli et al. (2020) shows that chronic taurine administration increases voluntary alcohol intake and preference in rats. The authors speculate that this effect might be due to an anxiolytic effect of taurine through an interaction with the GABA and dopamine systems. Relevant is the finding that acute taurine does not affect locomotor stimulation induced by alcohol administration, at variance with caffeine, but co-administration with caffeine is able to increase ethanol induced locomotor stimulation to a greater extent than each drug alone or in combination with ethanol (Ulenius et al., 2019). Since locomotor stimulation is considered an index of increased dopamine levels in the NAcb (Wise and Bozarth, 1987) and given that the increase of mesolimbic dopamine transmission is a key feature of all addictive drugs (Di Chiara et al., 2004; Di Chiara and Bassareo, 2007), it can be speculated that taurine in combination with caffeine might have a role in the increased consumption of alcohol observed in humans drinking EDs mixed with alcohol.
Despite claims of being safe and beneficial, consumption of EDs have been linked to fatal outcomes associated with their effects on cardiovascular system including atrial and ventricular arrhythmias, myocardial infarctions, cardiomyopathies, and sudden cardiac death (Mangi et al., 2017; Ehlers et al., 2019; Cao et al., 2021; Piccioni et al., 2021; Figure 2).

Figure 2. A graphical summary of positive and negative effects of EDs consumption in vulnerable individuals. In the circle are depicted the vulnerable population most sensitive to the harmful outcomes of excessive consumption of EDs. The red arrows mean negative outcomes while green arrows represent likely beneficial effects of moderate EDs consumption.
Often these negative outcomes are associated with the high caffeine concentrations in EDs, but it should be considered that other ingredients (taurine, sugars, and B-vitamins) may well contribute, through different mechanisms, to these consequences by increasing heart rate, blood pressure, and heart contractility in addition to prolonging the QTc interval (Kaur et al., 2022). When considering the potential negative outcomes of caffeine, it should be considered also the contribution of the caffeine from herbal extracts, present in EDs, such as guarana and yerba mate, whose amount is often not declared in the product label.
The mechanisms through which EDs might produce negative outcomes on cardiovascular system are different depending on the ingredient considered.
Caffeine, antagonizing adenosine action at A2A receptors in vascular tissues, blocks its vasodilatory effects, increases catecholamine levels (sympathetic tone) thus increasing vascular resistance, reduces myocardial perfusion, and renin secretion. Moreover, by its competitive inhibition of phosphodiesterase, leading to an elevation of myocardial cyclic adenosine monophosphate, caffeine exerts a positive inotropic action on myocardium (Kaur et al., 2022).
As regards the possible contribution of taurine to cardiovascular negative outcomes the results of preclinical and clinical studies are inconclusive (Schaffer et al., 2014; Ehlers et al., 2019). This might be due to different effects of taurine alone or in combination with caffeine and depending on dose and acute or long-term exposure. Although several beneficial effects have been attributed to taurine in different studies as anti-inflammatory, antioxidant, and neuroprotective in neurodegenerative disorders, antiarrhythmic, and hypotensive activity (Schaffer et al., 2014; Caine and Geracioti, 2016), when taurine is ingested with caffeine led to an increase in blood pressure (Grasser et al., 2016). A recent study has investigated a likely role of taurine combined with caffeine in inducing arrhythmias in an animal whole-organ model. The authors found that low doses of taurine decreased the number of ventricular arrhythmias whereas higher concentrations provoked significantly more arrhythmias in a dose-dependent fashion (Ellermann et al., 2022). This finding is not surprising based on the inotropic effect of taurine on myocardium (Schaffer et al., 2014) consistent with the ability of taurine to release intracellular calcium thus having a role in the regulation of calcium homeostasis and contractile function (Ehlers et al., 2019).
Most of EDs consumed by adolescents contain high amounts of sugars with a variable combination of glucose, sucrose, fructose, or high corn-fructose syrup. Among these, fructose has the greatest autonomic effect, increasing blood pressure after consumption (Somers and Svatikova, 2020). Glucose, fructose, and sucrose also increase heart rate after ingestion and caffeine may have a synergistic effect by increasing blood glucose and insulin after consumption (Nowak et al., 2018). These effects might exacerbate not only cardiovascular effects of caffeine but also, with excessive and long-term use, lead to obesity, insulin resistance, and diabetes, with the latter being a risk factor for cardiovascular diseases.
B-vitamins (thiamine, riboflavin, niacin, pantothenic acid, pyridoxine hydrochloride, biotin, inositol, and cyanocobalamin) are involved in many metabolic processes essential for a proper cell function, especially mitochondrial function and energy production (Depeint et al., 2006; Kennedy, 2016). Since EDs contain large amounts of sugars, addition of these vitamins has likely the purpose to provide cofactors for energy utilization and metabolism. However, despite the beneficial effects of these vitamins, it cannot be ruled out any adverse effect of an overload of these nutrients (Seifert et al., 2011; Wolk et al., 2012; De Sanctis et al., 2017). Indeed, while it was reported a beneficial effect of vitamin B12 (cyanocobalamin) in reducing homocysteine levels and thus cardiovascular risk, recently it has been suggested that an excess of vitamin B12 might have harmful cerebrovascular consequences (Duschek et al., 2016). Among the effects of EDs consumption on cardiovascular system it should be included the increased platelet aggregation and endothelial dysfunction leading to risk of thrombosis (Worthley et al., 2010). This effect could not be related to caffeine (Natella et al., 2008) while many different ingredients of EDs may well interact to produce this negative outcome on platelet and endothelial function (Olas and Bryś, 2019).
The ergogenic effect of EDs is mainly attributed to caffeine content, although all the other ingredients might potentiate this effect. Until 2004, caffeine was in the doping list of substances banned by the World Anti-Doping Agency. Since its removal from the banned substances list caffeine has been used by athletes in any quantity and form without the burden of being sanctioned. Evidence supports the ergogenic effects of these caffeinated drinks, involving (1) prolonged endurance exercise; (2) muscle strength and muscle endurance; and (3) high-intensity exercise and intermittent sprints (McLellan et al., 2016). The ergogenic effect seems similar in male and female athletes. However, their efficacy is linked to the need of a minimum intake of caffeine of 3 mg/kg (Jiménez et al., 2021), considered the optimal dose for the ergogenic effects (Guest et al., 2021). Nonetheless, often this dose is overcome thus raising concerns about the risk-benefit ratio given the side effects on cardiovascular function (de Souza et al., 2022).
The efficacy of caffeine and EDs on sports performance mainly depends on various factors such as timing and dose consumed, the type of sport, and the individual response to caffeine (Burke, 2008). Caffeine seems to be effective in long-lasting sport activities, with the greatest effects in sports involving fatigue during or toward the end of the event, while it does not seem to be effective in single events short lasting, involving strength and power, requiring very high intensity (Burke, 2008).
Other components of EDs may act synergistically with caffeine in increasing physical performance. Taurine modulates skeletal muscle contractile function and may attenuate exercise-induced DNA damage, with some evidence showing the ability to improve exercise capacity and performance (Higgins et al., 2010). Moreover, since taurine plays a role as an antioxidant it could improve ATP turnover in the muscle cell, leading to an increase in high intensity exercise performance (Karayigit et al., 2021). L-carnitine, another component of EDs, plays an important role in reducing inflammatory responses, oxidative stress, and apoptosis. Thus, preventing cellular damage L-carnitine favorable affects recovery from exercise stress. It has been shown that dietary supplementation with L-carnitine increases maximal oxygen consumption and lowers the respiratory quotient, indicating stimulation of lipid metabolism (Karlic and Lohninger, 2004).
In addition to the dangerous consequences on cardiovascular system, other systems have been reported to be negatively affected by EDs consumption. Among acute side effects observed following EDs consumption there are headache, irritability, excitability, malaise, dehydration, nervousness, insomnia, nausea/vomiting, abdominal pain (Wolk et al., 2012; Nadeem et al., 2021; Khouja et al., 2022). More serious and even fatal consequences have been reported following large amount of EDs including seizures, intracerebral hemorrhage, acute hepatitis, acute renal failure, psychosis, suicidal ideation (Hernandez-Huerta et al., 2017; Kim et al., 2018; Kelsey et al., 2019), Figure 2. The side effects associated with EDs consumption are mainly related to their caffeine content (Breda et al., 2014). In adolescents, who are not usual caffeine consumers, susceptibility to caffeine intoxication could be significantly augmented due to a lack of pharmacological tolerance (Thombs et al., 2010; Thomson et al., 2014). In addition, genetic factors could similarly contribute to an individual susceptibility to caffeine illnesses as well as caffeine intoxication, dependence, and withdrawal (Turagam et al., 2015). Compounds such as guarana, yerba mate, cocoa, and kola nut may increase biological effects of caffeine in EDs (Babu et al., 2008). In particular, guarana and caffeine, alone or in association with taurine, could induce neurotoxicological effects as well as an interference on redox homeostasis (Zeidán-Chuliá et al., 2013). In a case report, the consumption of EDs in adolescents, produced a dental enamel erosion resulting from the acidity of EDs (Duchan et al., 2010).
The use of EDs is an issue hotly debated due to concerns regarding the consumption by adolescents of high caffeine amounts with consequent deleterious effects on the developing brain and cardiovascular systems. Avoidance of caffeine use in young people poses a great societal challenge because of the widespread availability of caffeine-containing beverages and a lack of awareness of the potential risks.
The present review focused mostly on insidious nature of EDs consumption given the likely dangerous effects on adolescents and deleterious outcomes of their ingredients on developing brain. Although clinical research points to the negative effects of EDs in adolescents, there is a need for further studies. Among current research gaps there is the fact that most of clinical studies are indeed cross-sectional and therefore not suitable to draw definitive conclusions since the cause or effect cannot be determined where an association is found. In this regard longitudinal studies could provide stronger evidence. Moreover, although pre-clinical research is helping to elucidate the negative outcomes of EDs in adolescents, it is still scarce and not exhaustive. Notably, different studies are conducted on EDs in toto and not on the singular components at well-defined concentrations and this is a significant critical issue precluding identification of any synergism between components.
Future development in the field should take into account the above weaknesses providing more information on acute and long-term effects of the use of EDs. There is a need of spreading the knowledge on EDs damage on health to let adolescents be aware that these drinks are potentially devious.
CC and AP equally contributed to the literature review and in writing the manuscript. Both authors were responsible for manuscript revision and approved the submitted version.
This research was supported by funds from Fondazione di Sardegna (2017), DR rep. 406, prot. 12822, 17/02/2020 awarded to AP.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adermark, L., Clarke, R. B., Olsson, T., Hansson, E., Söderpalm, B., and Ericson, M. (2011). Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict. Biol. 16, 43–54. doi: 10.1111/j.1369-1600.2010.00206.x
Agarwal, K., Manza, P., Tejeda, H. A., Courville, A. B., Volkow, N. D., and Joseph, P. V. (2022). Prenatal caffeine exposure is linked to elevated sugar intake and BMI, altered reward sensitivity, and aberrant insular thickness in adolescents: an ABCD investigation. Nutrients 14:4643. doi: 10.3390/nu14214643
Al-Basher, G. I., Aljabal, H., Almeer, R. S., Allam, A. A., and Mahmoud, A. M. (2018). Perinatal exposure to energy drink induces oxidative damage in the liver, kidney and brain, and behavioral alterations in mice offspring. Biomed. Pharmacother. 102, 798–811. doi: 10.1016/j.biopha.2018.03.139
Albrecht, J., and Schousboe, A. (2005). Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem. Res. 30, 1615–1621. doi: 10.1007/s11064-005-8986-6
Arolfo, M., Yao, L., Gordon, A. S., Diamond, I., and Janak, P. H. (2004). Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol. Clin. Exp. Res. 28, 1308–1316.
Arria, A. M., Caldeira, K. M., Kasperski, S. J., Vincent, K. B., Griffiths, R. R., and O’Grady, K. E. (2011). Energy drink consumption and increased risk for alcohol dependence. Alcohol. Clin. Exp. Res. 35, 365–375. doi: 10.1111/j.1530-0277.2010.01352.x
Babu, K. M., James, C. R., and Lewander, W. (2008). Energy drinks: the new eye-opener for adolescents. Clin. Ped. Emerg. Med. 9, 35–42.
Barrense-Dias, Y., Berchtold, A., Akre, C., and Surís, J. C. (2016). Consuming energy drinks at the age of 14 predicted legal and illegal substance use at 16. Acta Paediatr. 105, 1361–1368. doi: 10.1111/apa.13543
Breda, J. J., Whiting, S. H., Encarnação, R., Norberg, S., Jones, R., Reinap, M., et al. (2014). Energy drink consumption in Europe: a review of the risks, adverse health effects, and policy options to respond. Front. Public Health 2:134. doi: 10.3389/fpubh.2014.00134
Brown, J., Villalona, Y., Weimer, J., Ludwig, C. P., Hays, B. T., Massie, L., et al. (2020). Supplemental taurine during adolescence and early adulthood has sex-specific effects on cognition, behavior and neurotransmitter levels in C57BL/6J mice dependent on exposure window. Neurotoxicol. Teratol. 79:106883. doi: 10.1016/j.ntt.2020.106883
Burke, L. M. (2008). Caffeine and sports performance. Appl. Physiol. Nutr. Metab. 33, 1319–1334. doi: 10.1139/H08-130
Burnstock, G., and Dale, N. (2015). Purinergic signalling during development and ageing. Purinergic Signal. 11, 277–305. doi: 10.1007/s11302-015-9452-9
Caine, J. J., and Geracioti, T. D. (2016). Taurine, energy drinks, and neuroendocrine effects. Cleve. Clin. J. Med. 83, 895–904. doi: 10.3949/ccjm.83a.15050
Cao, D. X., Maiton, K., Nasir, J. M., Estes, N. A. M., and Shah, S. A. (2021). Energy drink-associated electrophysiological and ischemic abnormalities: a narrative review. Front. Cardiovasc. Med. 8:679105. doi: 10.3389/fcvm.2021.679105
Cappelletti, S., Piacentino, D., Sani, G., and Aromatario, M. (2015). Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 13, 71–88. doi: 10.2174/1570159X13666141210215655
Chen, V. C., Chiu, C. C., Chen, L. J., Hsu, T. C., and Tzang, B. S. (2018). Effects of taurine on striatal dopamine transporter expression and dopamine uptake in SHR rats. Behav. Brain. Res. 348, 219–226. doi: 10.1016/j.bbr.2018.04.031
Christensen, Z. P., Freedman, E. G., and Foxe, J. J. (2021). Caffeine exposure in utero is associated with structural brain alterations and deleterious neurocognitive outcomes in 9-10 year old children. Neuropharmacology 186:108479. doi: 10.1016/j.neuropharm.2021.108479
Costa, B. M., Hayley, A., and Miller, P. (2016). Adolescent energy drink consumption: an Australian perspective. Appetite 105, 638–642. doi: 10.1016/j.appet.2016.07.001
Curran, C. P., and Marczinski, C. A. (2017). Taurine, caffeine, and energy drinks: reviewing the risks to the adolescent brain. Birth Defects Res. 109, 1640–1648. doi: 10.1002/bdr2.1177
De Sanctis, V., Soliman, N., Soliman, A. T., Elsedfy, H., Di Maio, S., El Kholy, M., et al. (2017). Caffeinated energy drink consumption among adolescents and potential health consequences associated with their use: a significant public health hazard. Acta Biomed. 88, 222–231. doi: 10.23750/abm.v88i2.6664
de Souza, J. G., Del Coso, J., Fonseca, F. S., Silva, B. V. C., de Souza, D. B., da Silva Gianoni, R. L., et al. (2022). Risk or benefit? Side effects of caffeine supplementation in sport: a systematic review. Eur. J. Nutr. 61, 3823–3834. doi: 10.1007/s00394-022-02874-3
Depeint, F., Bruce, W. R., Shangari, N., Mehta, R., and O’Brien, P. J. (2006). Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 163, 94–112. doi: 10.1016/j.cbi.2006.04.014
Di Chiara, G., and Bassareo, V. (2007). Reward system and addiction: what dopamine does and doesn’t do. Curr. Opin. Pharmacol. 7, 69–76. doi: 10.1016/j.coph.2006.11.003
Di Chiara, G., Bassareo, V., Fenu, S., De Luca, M. A., Spina, L., Cadoni, C., et al. (2004). Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47(Suppl. 1), 227–241. doi: 10.1016/j.neuropharm.2004.06.032
Duchan, E., Patel, N. D., and Feucht, C. (2010). Energy drinks: a review of use and safety for athletes. Phys. Sportsmed. 38, 171–179. doi: 10.3810/psm.2010.06.1796
Duschek, N., Basic, J., Falkensammer, J., Taher, F., and Assadian, A. (2016). B-vitamin serum concentrations predicting long-term overall and stroke-free survival after carotid endarterectomy. J. Stroke Cerebrovasc. Dis. 25, 1235–1243. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.044
Ehlers, A., Marakis, G., Lampen, A., and Hirsch-Ernst, K. I. (2019). Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe. Food Chem. Toxicol. 130, 109–121. doi: 10.1016/j.fct.2019.05.028
El Idrissi, A., and Trenkner, E. (2004). Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem. Res. 29, 189–197. doi: 10.1023/b:nere.0000010448.17740.6e
Ellermann, C., Hakenes, T., Wolfes, J., Wegner, F. K., Willy, K., Leitz, P., et al. (2022). Cardiovascular risk of energy drinks: caffeine and taurine facilitate ventricular arrhythmias in a sensitive whole-heart model. J. Cardiovasc. Electrophysiol. 33, 1290–1297. doi: 10.1111/jce.15458
Ericson, M., Chau, P., Clarke, R. B., Adermark, L., and Söderpalm, B. (2011). Rising taurine and ethanol concentrations in nucleus accumbens interact to produce dopamine release after ethanol administration. Addict. Biol. 16, 377–385. doi: 10.1111/j.1369-1600.2010.00245.x
Ferreira, S. E., de Mello, M. T., Pompéia, S., and de Souza-Formigoni, M. L. (2006). Effects of energy drink ingestion on alcohol intoxication. Alcohol. Clin. Exp. Res. 30, 598–605.
Fredholm, B. B., Battig, K., Holmen, J., Nehlig, A., and Zvartau, E. E. (1999). Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51, 83–133.
Gallimberti, L., Buja, A., Chindamo, S., Vinelli, A., Lazzarin, G., Terraneo, A., et al. (2013). Energy drink consumption in children and early adolescents. Eur. J. Pediatr. 172, 1335–1340. doi: 10.1007/s00431-013-2036-1
Geeraert, B. L., Lebel, R. M., and Lebel, C. (2019). A multiparametric analysis of white matter maturation during late childhood and adolescence. Hum. Brain Mapp. 40, 4345–4356. doi: 10.1002/hbm.24706
Giedd, J. N., Blumenthal, J., Jeffries, N. O., Castellanos, F. X., Liu, H., Zijdenbos, A., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863. doi: 10.1038/13158
Grasser, E. K., Miles-Chan, J. L., Charrière, N., Loonam, C. R., Dulloo, A. G., and Montani, J. P. (2016). Energy drinks and their impact on the cardiovascular system: potential mechanisms. Adv. Nutr. 7, 950–960. doi: 10.3945/an.116.012526
Guest, N. S., VanDusseldorp, T. A., Nelson, M. T., Grgic, J., Schoenfeld, B. J., Jenkins, N. D. M., et al. (2021). International society of sports nutrition position stand: caffeine and exercise performance. J. Int. Soc. Sports Nutr. 18:1. doi: 10.1186/s12970-020-00383-4
Hernandez-Huerta, D., Martin-Larregola, M., Gomez-Arnau, J., Correas-Lauffer, J., and Dolengevich-Segal, H. (2017). Psychopathology related to energy drinks: a psychosis case report. Case Rep. Psychiatry 2017:5094608. doi: 10.1155/2017/5094608
Higgins, J. P., Tuttle, T. D., and Higgins, C. L. (2010). Energy beverages: content and safety. Mayo Clin. Proc. 85, 1033–1041. doi: 10.4065/mcp.2010.0381
Hladun, O., Papaseit, E., Martín, S., Barriocanal, A. M., Poyatos, L., Farré, M., et al. (2021). Interaction of energy drinks with prescription medication and drugs of abuse. Pharmaceutics 13:1532. doi: 10.3390/pharmaceutics13101532
Howard, M. A., and Marczinski, C. A. (2010). Acute effects of a glucose energy drink on behavioral control. Exp. Clin. Psychopharmacol. 18, 553–561. doi: 10.1037/a0021740
Huxtable, R. J. (1992). Physiological actions of taurine. Physiol. Rev. 72, 101–163. doi: 10.1152/physrev.1992.72.1.101
Jeong, H. J., Jang, I. S., Nabekura, J., and Akaike, N. (2003). Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J. Neurophysiol. 89, 1214–1222. doi: 10.1152/jn.00516.2002
Jiménez, S. L., Díaz-Lara, J., Pareja-Galeano, H., and Del Coso, J. (2021). Caffeinated drinks and physical performance in sport: a systematic review. Nutrients 13:2944. doi: 10.3390/nu13092944
Kaestle, C. E., Droste, N., Peacock, A., Bruno, R., and Miller, P. (2018). Perception of intoxication in a field study of the night-time economy: blood alcohol concentration, patron characteristics, and event-level predictors. Addict. Behav. 76, 195–200. doi: 10.1016/j.addbeh.2017.08.018
Karayigit, R., Naderi, A., Saunders, B., Forbes, S. C., Coso, J. D., Berjisian, E., et al. (2021). Combined but not isolated ingestion of caffeine and taurine improves Wingate sprint performance in female team-sport athletes habituated to caffeine. Sports 9:162. doi: 10.3390/sports9120162
Karlic, H., and Lohninger, A. (2004). Supplementation of L-carnitine in athletes: does it make sense? Nutrition 20, 709–715. doi: 10.1016/j.nut.2004.04.003
Kaur, A., Yousuf, H., Ramgobin-Marshall, D., Jain, R., and Jain, R. (2022). Energy drink consumption: a rising public health issue. Rev. Cardiovasc. Med. 23:83. doi: 10.31083/j.rcm2303083
Kelsey, D., Berry, A. J., Swain, R. A., and Lorenz, S. (2019). A case of psychosis and renal failure associated with excessive energy drink consumption. Case Rep. Psychiatry 2019:3954161. doi: 10.1155/2019/3954161
Kennedy, D. O. (2016). B vitamins and the brain: mechanisms, dose and efficacy-A review. Nutrients 8:68. doi: 10.3390/nu8020068
Kerr, M. I., Wall, M. J., and Richardson, M. J. (2013). Adenosine A1 receptor activation mediates the developmental shift at layer 5 pyramidal cell synapses and is a determinant of mature synaptic strength. J. Physiol. 591, 3371–3380. doi: 10.1113/jphysiol.2012.244392
Khouja, C., Kneale, D., Brunton, G., Raine, G., Stansfield, C., Sowden, A., et al. (2022). Consumption and effects of caffeinated energy drinks in young people: an overview of systematic reviews and secondary analysis of UK data to inform policy. BMJ Open 12, e047746. doi: 10.1136/bmjopen-2020-047746
Kilb, W. (2017). Putative role of Taurine as neurotransmitter during perinatal cortical development. Adv. Exp. Med. Biol. 975(Pt 1), 281–292. doi: 10.1007/978-94-024-1079-2_25
Kim, J.-S., Kim, K., and Seo, Y. (2018). Associations between Korean adolescents’ energy drink consumption and suicidal ideation and attempts. Arch. Psychiatr. Nurs. 32, 331–336.
Kunin, D., Gaskin, S., Rogan, F., Smith, B., and Amit, Z. (2000). Caffeine promotes ethanol drinking in rats: examination using a limited-access free choice paradigm. Alcohol 21, 271–277. doi: 10.1016/s0741-8329(00)00101-4
Lebacq, T., Desnouck, V., Dujeu, M., Holmberg, E., Pedroni, C., and Castetbon, K. (2020). Determinants of energy drink consumption in adolescents: identification of sex-specific patterns. Public Health 185, 182–188. doi: 10.1016/j.puhe.2020.05.040
Lebel, C., and Deoni, S. (2018). The development of brain white matter microstructure. Neuroimage 182, 207–218. doi: 10.1016/j.neuroimage.2017.12.097
Lenroot, R. K., and Giedd, J. N. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729. doi: 10.1016/j.neubiorev.2006.06.001
Mangi, M. A., Rehman, H., Rafique, M., and Illovsky, M. (2017). Energy drinks and the risk of cardiovascular disease: a review of current literature. Cureus 9, e1322. doi: 10.7759/cureus.1322
Marczinski, C. A. (2014). Combined alcohol and energy drink use: hedonistic motives, adenosine, and alcohol dependence. Alcohol. Clin. Exp. Res. 38, 1822–1825. doi: 10.1111/acer.12493
Marczinski, C. A., and Fillmore, M. T. (2014). Energy drinks mixed with alcohol: what are the risks? Nutr. Rev. 72(Suppl. 1), 98–107. doi: 10.1111/nure.12127
Marinoni, M., Parpinel, M., Gasparini, A., Ferraroni, M., and Edefonti, V. (2022). Risky behaviors, substance use, and other lifestyle correlates of energy drink consumption in children and adolescents: a systematic review. Eur. J. Pediatr. 181, 1307–1319. doi: 10.1007/s00431-021-04322-6
McLellan, T. M., Caldwell, J. A., and Lieberman, H. R. (2016). A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 71, 294–312. doi: 10.1016/j.neubiorev.2016.09.001
Miller, K. E. (2008). Energy drinks, race, and problem behaviors among college students. J. Adolesc. Health 43, 490–497.
Miyake, E. R., and Marmorstein, N. R. (2015). Energy drink consumption and later alcohol use among early adolescents. Addict. Behav. 43, 60–65.
Nadeem, I. M., Shanmugaraj, A., Sakha, S., Horner, N. S., Ayeni, O. R., and Khan, M. (2021). Energy drinks and their adverse health effects: a systematic review and meta-analysis. Sports Health 13, 265–277. doi: 10.1177/1941738120949181
Nam, H. W., Bruner, R. C., and Choi, D. S. (2013). Adenosine signaling in striatal circuits and alcohol use disorders. Mol. Cells 36, 195–202. doi: 10.1007/s10059-013-0192-9
Natella, F., Nardini, M., Belelli, F., Pignatelli, P., Di Santo, S., Ghiselli, A., et al. (2008). Effect of coffee drinking on platelets: inhibition of aggregation and phenols incorporation. Br. J. Nutr. 100, 1276–1282. doi: 10.1017/S0007114508981459
Nowak, D., Gośliński, M., and Nowatkowska, K. (2018). The effect of acute consumption of energy drinks on blood pressure, heart rate and blood glucose in the group of young adults. Int. J. Environ. Res. Public. Health 15:544. doi: 10.3390/ijerph15030544
Oja, S. S., and Saransaari, P. (2022). Taurine and the brain. Adv. Exp. Med. Biol. 1370, 325–331. doi: 10.1007/978-3-030-93337-1_31
Olas, B., and Bryś, M. (2019). Effects of coffee, energy drinks and their components on hemostasis: the hypothetical mechanisms of their action. Food Chem. Toxicol. 127, 31–41. doi: 10.1016/j.fct.2019.02.039
Piccioni, A., Covino, M., Zanza, C., Longhitano, Y., Tullo, G., Bonadia, N., et al. (2021). Energy drinks: a narrative review of their physiological and pathological effects. Intern. Med. J. 51, 636–646. doi: 10.1111/imj.14881
Pulcinelli, R. R., de Paula, L. F., Nietiedt, N. A., Bandiera, S., Hansen, A. W., Izolan, L. D. R., et al. (2020). Taurine enhances voluntary alcohol intake and promotes anxiolytic-like behaviors in rats. Alcohol 88, 55–63. doi: 10.1016/j.alcohol.2020.07.004
Ramírez-Guerrero, S., Guardo-Maya, S., Medina-Rincón, G. J., Orrego-González, E. E., Cabezas-Pérez, R., and González-Reyes, R. E. (2022). Taurine and astrocytes: a homeostatic and neuroprotective relationship. Front. Mol. Neurosci. 15:937789. doi: 10.3389/fnmol.2022.937789
Reis, R., Charehsaz, M., Sipahi, H., Ekici, A. I. D., Macit, C., Akkaya, H., et al. (2017). Energy drink induced lipid peroxidation and oxidative damage in rat liver and brain when used alone or combined with alcohol. J. Food Sci. 82, 1037–1043. doi: 10.1111/1750-3841.13662
Reissig, C. J., Strain, E. C., and Griffiths, R. R. (2009). Caffeinated energy drinks-a growing problem. Drug Alcohol Depend. 99, 1–10. doi: 10.1016/j.drugalcdep.2008.08.001
Rodrigues, R. J., Marques, J. M., and Cunha, R. A. (2019). Purinergic signalling and brain development. Semin. Cell. Dev. Biol. 95, 34–41. doi: 10.1016/j.semcdb.2018.12.001
Ruiz, L. D., and Scherr, R. E. (2018). Risk of energy drink consumption to adolescent health. Am. J. Lifestyle Med. 13, 22–25. doi: 10.1177/1559827618803069
Schaffer, S. W., Shimada, K., Jong, C. J., Ito, T., Azuma, J., and Takahashi, K. (2014). Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids 46, 1147–1157. doi: 10.1007/s00726-014-1708-0
Seifert, S. M., Schaechter, J. L., Hershorin, E. R., and Lipshultz, S. E. (2011). Health effects of energy drinks on children, adolescents, and young adults. Pediatrics 127:511–528. doi: 10.1542/peds.2009-3592
Serdar, M., Mordelt, A., Müser, K., Kempe, K., Felderhoff-Müser, U., Herz, J., et al. (2019). Detrimental impact of energy drink compounds on developing oligodendrocytes and neurons. Cells 8:1381. doi: 10.3390/cells8111381
Sharma, R., Engemann, S. C., Sahota, P., and Thakkar, M. M. (2010). Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol Clin. Exp. Res. 34, 813–818. doi: 10.1111/j.1530-0277.2010.01153.x
Silva, C. G., Métin, C., Fazeli, W., Machado, N. J., Darmopil, S., Launay, P. S., et al. (2013). Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci. Transl. Med. 5:197ra104. doi: 10.1126/scitranslmed.3006258
Somers, K. R., and Svatikova, A. (2020). Cardiovascular and autonomic responses to energy drinks-clinical implications. J. Clin. Med. 9:431. doi: 10.3390/jcm9020431
Tarragon, E., Calleja-Conde, J., Giné, E., Segovia-Rodríguez, L., Durán-González, P., and Echeverry-Alzate, V. (2021). Alcohol mixed with energy drinks: what about taurine? Psychopharmacology 238, 1–8. doi: 10.1007/s00213-020-05705-7
Thombs, D. L., O’Mara, R. J., Tsukamoto, M., Rossheim, M. E., Weiler, R. M., Merves, M. L., et al. (2010). Event-level analyses of energy drink consumption and alcohol intoxication in bar patrons. Addict. Behav. 35, 325–330. doi: 10.1016/j.addbeh.2009.11.004
Thomson, B. M., Campbell, D. M., Cressey, P., Egan, U., and Horn, B. (2014). Energy drink consumption and impact on caffeine risk. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 31, 1476–1488. doi: 10.1080/19440049.2014.940608
Turagam, M. K., Velagapudi, P., Kocheril, A. G., and Alpert, M. A. (2015). Commonly consumed beverages in daily life: do they cause atrial fibrillation? Clin. Cardiol. 38, 317–322. doi: 10.1002/clc.22385
Ulenius, L., Adermark, L., Söderpalm, B., and Ericson, M. (2019). Energy drink constituents (caffeine and taurine) selectively potentiate ethanol-induced locomotion in mice. Pharmacol. Biochem. Behav. 187:172795. doi: 10.1016/j.pbb.2019.172795
Vargiu, R., Broccia, F., Lobina, C., Lecca, D., Capra, A., Bassareo, P. P., et al. (2021). Chronic red bull consumption during adolescence: effect on mesocortical and mesolimbic dopamine transmission and cardiovascular system in adult rats. Pharmaceuticals 14:609. doi: 10.3390/ph14070609
Wise, R. A., and Bozarth, M. A. (1987). A psychomotor stimulant theory of addiction. Psychol. Rev. 94, 469–492.
Wolk, B. J., Ganetsky, M., and Babu, K. M. (2012). Toxicity of energy drinks. Curr. Opin. Pediatr. 24, 243–251. doi: 10.1097/MOP.0b013e3283506827
Worthley, M. I., Prabhu, A., Sciscio, P. D., Schultz, C., Sanders, P., and Willoughby, S. R. (2010). Detrimental effects of energy drink consumption on platelet and endothelial function. Am. J. Med. 123, 184–187. doi: 10.1016/j.amjmed.2009.09.013
Wu, G. F., Ren, S., Tang, R. Y., Xu, C., Zhou, J. Q., Lin, S. M., et al. (2017). Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci. Rep. 7:4989. doi: 10.1038/s41598-017-05051-3
Yasuma, N., Imamura, K., Watanabe, K., Nishi, D., Kawakami, N., and Takano, A. (2021). Association between energy drink consumption and substance use in adolescence: a systematic review of prospective cohort studies. Drug Alcohol. Depend. 219:108470. doi: 10.1016/j.drugalcdep.2020.108470
Zeidán-Chuliá, F., Gelain, D. P., Kolling, E. A., Rybarczyk-Filho, J. L., Ambrosi, P., Terra, S. R., et al. (2013). Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxid. Med. Cell. Longev. 2013:791795. doi: 10.1155/2013/791795
Zhang, H., Lee, Z. X., and Qiu, A. (2020). Caffeine intake and cognitive functions in children. Psychopharmacology 237, 3109–3116. doi: 10.1007/s00213-020-05596-8
Keywords: energy drinks, adolescence, alcohol, alcohol use disorders, sport, caffeine, taurine
Citation: Cadoni C and Peana AT (2023) Energy drinks at adolescence: Awareness or unawareness? Front. Behav. Neurosci. 17:1080963. doi: 10.3389/fnbeh.2023.1080963
Received: 26 October 2022; Accepted: 06 February 2023;
Published: 20 February 2023.
Edited by:
Mercè Correa, Jaume I University, SpainReviewed by:
Maria Lucia O. Souza-Formigoni, Federal University of São Paulo, BrazilCopyright © 2023 Cadoni and Peana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Cadoni,  Y2NhZG9uaUB1bmljYS5pdA==,
Y2NhZG9uaUB1bmljYS5pdA==,  Y3Jpc3RpbmEuY2Fkb25pQGluLmNuci5pdA==; Alessandra Tiziana Peana,
Y3Jpc3RpbmEuY2Fkb25pQGluLmNuci5pdA==; Alessandra Tiziana Peana,  YXBlYW5hQHVuaXNzLml0
YXBlYW5hQHVuaXNzLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.