Introduction
The discovery that signals from the gut reaching the brain can have an impact on affect, emotion and cognition including beliefs and decision-making has been met with considerable public attention. As discussed here, widespread interest in a research field that touches one's personal health also raises expectations and beliefs that are not thoroughly backed by validated scientific evidence. The term “gut feelings,” derived from a lay interpretation of the impact of gut signals on mental activity, is a popular but scientifically ill-defined term that may even lead science astray from its principles of investigation. In these interdependencies, the relation between gut signals and “gut feelings” is a worthwhile theme for analysis in credition research. As outlined in the overview of the Special Topic that this article is part of, credition research is an interdisciplinary approach to understand the nature of beliefs and believing. Based on distinct neuronal processes in the brain, credition refers to the integrative processing of information, its valuation in terms of personal meaning and its impact on a person's behavioral decisions (Seitz et al., 2018; Seitz, 2022).
Bidirectional Gut-Brain Communication
Psychosomatic medicine has gathered ample evidence that gastrointestinal function can be altered by emotions and stress. For instance, 75 years ago Almy and Tulin (1947) published a study in which they performed sigmoidoscopies in volunteer medical students. When during the examination they told the students that they had discovered a carcinoma, they instantly observed strong muscle contractions and an increase in blood flow in the rectum. Once they explained the hoax, the uproar in the rectum subsided rapidly. Although this kind of study would no longer receive ethical approval (Shea-Donohue et al., 2005), it shows that emotional stress can have an immediate impact on the gut. Since then, many studies have confirmed (for reviews see Mayer, 2000; Taché et al., 2001), in a more humane way, that acute physical and emotional stress can affect the digestive tract in a regionally distinct manner, retarding gastric emptying (“being heavy on the stomach”) but hastening colonic propulsion (“soiling one's pants”). The changes in gut function accompanying long-term stress, however, may substantially differ from those in acute settings (McEwen, 2007). The communication from the brain to the gut, often referred to as “brain-gut axis,” is transmitted by several pathways (Figure 1) including the efferent autonomic nervous system with its sympathetic and parasympathetic divisions and neuroendocrine factors of the sympathetic-adrenal medulla and hypothalamus-pituitary-adrenal cortex systems (Holzer et al., 2015).
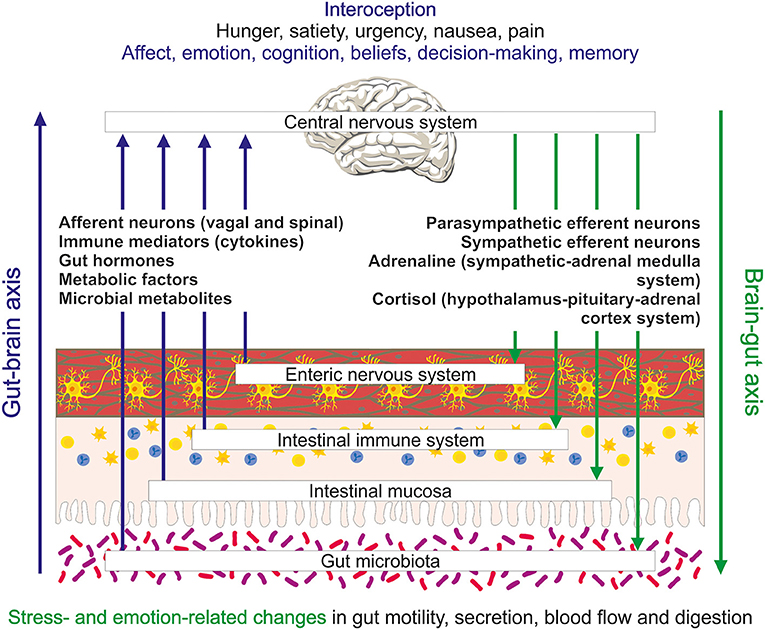
Figure 1. Schematic illustration of the bidirectional exchange of information between the gut and brain and the interoceptive processing of this information in the brain. Several messenger systems including extrinsic afferent neurons, immune mediators, gut hormones, metabolic factors and microbial metabolites carry information from the gut to the brain. They elicit conscious sensations (e.g., hunger, satiety, urgency, nausea, and pain) and influence processes relevant to affect, emotion and cognition. Regulatory outputs from the brain to the gut include emotion- and stress-related changes in motility, secretion, blood flow and digestion.
Communication between brain and gut is in fact a bidirectional process that, besides efferent connections, also involves afferent pathways that carry information from the gastrointestinal tract to the central nervous system (CNS) (Figure 1). Extrinsic sensory neurons of the vagal and spinal nerves transmitting mechanical and chemical stimuli are one component of this “gut-brain axis.” Endocrine chemical messages carried by gut hormones released from enteroendocrine cells in the gut mucosa, mediators (cytokines) of the gastrointestinal immune system, metabolic factors related to nutrient absorption and digestion, and messengers generated by the gut microbiota (Figure 1) constitute other important components of the gut-brain communication network. The enteric nervous system intrinsic to the gut (Perez-Burgos et al., 2014), enteroendocrine cells (Kaelberer et al., 2020) as well as immune and microbial messengers also use, in part, extrinsic sensory neurons to signal to the brain (Holzer et al., 2015).
This complex afferent communication system provides the brain with integrated information on gut function. In this task, the gut-brain axis contributes to interoception, a process that enables the brain to “know” the internal state of the body (Craig, 2002) and align its mental activity and homeostatic body control (Chen et al., 2021). Functional imaging studies have shown that information coming from the gut reaches brain regions relevant to emotion, affect and cognition. A good deal of what we now know about the bidirectional information exchange between gut and brain has been disclosed by research efforts to understand irritable bowel syndrome (IBS). Characterized by recurrent abdominal pain associated with alterations in bowel habits, IBS is frequently comorbid with anxiety disorders and depression and now widely considered a disorder of gut-brain-gut communication (Black et al., 2020a; Mayer et al., 2022).
Interoceptive Gut-Brain Communication: Impact on Mental Processes
The complex gut-brain-gut communication network contributes to interoception (Mayer, 2011; Holzer, 2017), a process that integrates information from all internal organs to impact on various domains of brain activity and behavior. In view of the neuronal and endocrine messaging modes of the signaling pathways it is obvious that interoceptive processes take place both at the conscious and subconscious level (Chen et al., 2021). Neuroanatomical and functional imaging studies have provided a detailed mapping of the brain regions in which interoceptive input is received, integrated and distributed to output relays. Whether delivered through neuronal or endocrine pathways, interoceptive information is first processed in subcortical structures of the CNS such as the spinal cord, brainstem and thalamus before it is passed on to higher brain regions including the hypothalamus, insula, anterior cingulate cortex, and somatosensory cortex (Critchley and Harrison, 2013; Wang et al., 2019; Chen et al., 2021). In this way interoceptive signals inform not only regulatory functions of the CNS to maintain internal homeostasis, but also influence feelings (mood, affect, emotion) and their valence as well as motivational and cognitive processes related to preferences, beliefs and decision-making (Figure 1) (Critchley and Harrison, 2013; Wang et al., 2019; Chen et al., 2021). In an experimental setting it has been shown that interoceptive signals from the gut carried by the vagus nerve support memory function in the hippocampus (Suarez et al., 2018).
Particular implications of gut-derived interoceptive signals in mental activity can be deduced from its disturbance in IBS which commonly is associated with visceral hyperalgesia as well as hypersensitivity to emotional challenge. A shown by functional imaging studies, painful rectal distension in healthy controls activates brain regions associated with visceral sensation and interoceptive processing (thalamus, anterior insula, anterior midcingulate cortex), emotional arousal (perigenual anterior cingulate gyrus) and attention and modulation of arousal (inferior parietal, lateral and medial prefrontal cortex) (Tillisch et al., 2011). In IBS patients, the activation of brain regions associated with visceral sensation, interoceptive processing and emotional arousal is significantly increased (Tillisch et al., 2011). In addition, psychological stress in IBS patients enhances the neuronal activation, which rectal distension induces in brain regions such as the insula, midcingulate cortex and ventrolateral prefrontal cortex, to a larger degree than in healthy controls (Elsenbruch et al., 2010). Likewise, IBS patients respond to contextual threat with increased neuronal activity within the salience, attention, default mode and emotional arousal networks within the CNS as compared with healthy controls, which appears to reflect the propensity of IBS subjects to overestimate the likelihood and severity of future abdominal pain (Hong et al., 2016). Furthermore, the hypersensitivity to rectal distension in IBS is related to changes in functional connectivity within resting-state networks associated with interoception, salience and sensory processing, changes that appear to be relevant to the hypervigilance and intestinal hyperalgesia seen in IBS patients (Icenhour et al., 2017). Meta-analyses have shown that patients with IBS present with significantly higher levels of anxiety and depression than healthy controls (Fond et al., 2014). Accordingly, cognitive behavioral therapy and gut-directed hypnotherapy have proved beneficial in a part of IBS patients (Peter et al., 2018; Black et al., 2020b), attesting to gut-brain-gut communication as a viable treatment target.
Background anxiety can strongly influence attitudes, beliefs and decisions, which is most evident in psychiatric disorders associated with generalized emotional disturbances. Decision-making depends on the computation of the value of available options, which in turn are a function of the environment and the internal state of the individual (Paulus and Yu, 2012). Engelmann et al. (2015) have shown that incidental anxiety disrupts the neural valuation of risky decision-making and shifts the valuation focus from possible positive consequences to anticipated negative consequences, a process in which the activity of the anterior insula plays a particular role. Transient anxiety states normally carry adaptive value since they may increase vigilance and attention to possible negative outcomes (Engelmann et al., 2015). This functional anxiety, however, can turn into a maladaptive state if anxious behavior is permanently adopted and becomes detached from the environment (Grupe and Nitschke, 2013). Affect can likewise have an adverse influence on decision-making (Paulus and Yu, 2012; Lerner et al., 2015). For instance, aversive affect appears to be a key source for irrational decision-making, especially with respect to trust in the context of social behavior (Engelmann et al., 2019).
Interoceptive Gut Signals and “Gut Feelings”: Science Versus Belief
The term “gut feelings” is a popular expression used in everyday language and refers to instinctive feelings, intuition, beliefs and decisions without rational underpinnings (Holzer, 2017). In this context, “gut feelings” are related to positive outcomes as exemplified by notions such as “gut feelings are guardian angels.” The view that feelings originate in the gut may have also been fostered by the labeling of the enteric nervous system as “little brain” or “second brain” (Gershon, 1998). However, feelings and other mental capacities cannot be attributed to this nervous system in the gut, which is indispensable for the neural regulation of digestion (Holzer et al., 2001). Feelings or emotions do not originate in the gut but are generated in the brain, and the term “gut feelings” is a scientifically ill-defined and misleading expression. The impact of gut-derived interoceptive signals and sensations on mental health can be either positive or negative, the latter being aptly exemplified by the neuropsychiatric disturbances in IBS. There is no scientific evidence based on validated data that “gut feelings” have the power to direct judgements and decisions such that they have primarily a beneficial or happy payoff. To the contrary, instantaneous gut sensations known in neurogastroenterology, such as abdominal pain, flatulence, diarrhea-related urgency and nausea, are rather distressful. Notwithstanding these opposing views, the bidirectional communication network between gut and brain and the process of gastrointestinal interoception provide a neurobiological frame to explain emotions, beliefs, judgements and decisions under the influence of signals from the gut (Mayer et al., 2022).
The term “gut feeling” has also entered the scientific literature, which was fuelled not only by the elucidation of the gut-brain communication network but also driven by the entry of the gut microbiota as a factor of the gut-brain axis. Research in experimental models has provided a wealth of information on how the vast microbial community in the gut can participate in gut-brain signaling and interact with the neuronal, immune, endocrine and metabolic messengers of the gut-brain axis (Cryan et al., 2019; Farzi et al., 2019; Gershon and Margolis, 2021; Hassan et al., 2022). However, evidence for a direct impact of the gut microbiota on emotional-affective and cognitive behavior in humans lags behind, and microbiota-directed interventions with proven efficacy in the management of neuropsychiatric disease are not yet available (Dinan and Cryan, 2019; Federici et al., 2020; Simpson et al., 2020; Le Morvan de Sequeira et al., 2022). While changes in the composition and diversity of the gut microbiome are associated with many neurological and psychiatric disorders (Simpson et al., 2021), causal relationships between particular aberrations of the gut microbiome and particular disorders of the human brain remain to be delineated. Despite the insufficient evidence, the hype in microbiome research is also mirrored in the popular press, the vast majority of articles (>90%) reporting health benefits associated with the gut microbiome without mentioning the limitations of such claims (Marcon et al., 2021). “Hope or hype” has become a common phrase in biomedical research areas in which a research boost raises expectations and beliefs in health benefits that await to be fulfilled.
Research hypes also carry the risk of deviating to questionable conceptions. One example relates to the purported mediator of the microbiome-gut-brain axis, 5-hydroxytryptamine (5-HT, serotonin), which both in the scientific and lay press is sometimes said to be an interface between gut microbiota and brain and to act as a “feel-good hormone”. 5-HT synthesized in distinct brain neurons can in fact sustain good mood, and drugs (selective serotonin reuptake inhibitors) targeting the cerebral 5-HT system are efficacious in depression and certain anxiety disorders. However, more than 90% of the body's 5-HT is produced in the gut, primarily in enterochromaffin cells, but also in enteric neurons. Although the gut microbiota can indirectly modify the synthesis of 5-HT in gut and brain through regulating the availability of its precursor L-tryptophan (Gheorghe et al., 2019; Legan et al., 2022), intestinal 5-HT is unlikely to contribute to the “feel-good” action of cerebral 5-HT because it does not pass the blood-brain barrier. To the contrary, an excess of 5-HT in the gut can elicit nausea and emesis associated with chemo- and radiotherapy, facilitate intestinal inflammation, mediate diarrhea associated with bacterial infection, and contribute to IBS-related pain (Gershon, 2013; Legan et al., 2022).
Conclusion
The gut-brain-gut communication network is part of the interoceptive circuits which enable the brain to sense and interpret the physiological condition in the body and regulate its autonomic and mental activity accordingly. While this relationship has become an important research area in neuroscience, it also provides an example where solid science is at risk going uncritical and fostering unproven conceptions and expectations. It is here that credition research can find fruitful ground to analyze the working of science at the interface of “hype or hope” and to understand how interoceptive signals from the gut impact on mental activity to influence affect, emotion, beliefs, predictions and decisions. In its interdisciplinary approach, credition research is relevant to many areas in which belief processes shape religious, social, societal, economic, legal as well as scientific and medical conceptions and expectations. In analyzing these relationships, credition research bears considerable responsibility to unveil the misinterpretation of scientific data and the neglect of their validity status, which champion unproven notions and predictions. The placebo and nocebo effects represent a particular outcome of belief processes in which a complex set of input information convinces the patient that a certain choice of treatment is better or worse than the other although scientific evidence indicates that they are equivalent in their action. Importantly, placebo and nocebo effects are real, and they work either way, influencing brain activity as indicated by functional imaging studies and altering organ function in the periphery (Meissner, 2014; Bingel et al., 2022).
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This paper was funded by Dr. Rüdiger Seitz, via the Volkswagen Foundation, Siemens Healthineers, and the Betz Foundation. Siemens Healthineers was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author thanks Judith C.J. Holzer-Geissler and Ulrike Holzer-Petsche for drawing Figure 1.
References
Almy, T. P., and Tulin, M. (1947). Alterations in colonic function in man under stress: experimental production of changes simulating the irritable colon. Gastroenterology 8, 616–626.
Bingel, U., Wiech, K., Ritter, C., Wanigasekera, V., Mhuircheartaigh, R., Lee, M. C., et al. (2022). Hippocampus mediates nocebo impairment of opioid analgesia through changes in functional connectivity. Eur J Neurosci in press. doi: 10.1111/ejn.15687
Black, C. J., Drossman, D. A., Talley, N. J., Ruddy, J., and Ford, A. C. (2020a). Functional gastrointestinal disorders: advances in understanding and management. Lancet 396, 1664–1674. doi: 10.1016/S0140-6736(20)32115-2
Black, C. J., Thakur, E. R., Houghton, L. A., Quigley, E. M. M., Moayyedi, P., and Ford, A. C. (2020b). Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Gut 69, 1441–1451. doi: 10.1136/gutjnl-2020-321191
Chen, W. G., Schloesser, D., Arensdorf, A. M., Simmons, J. M., Cui, C., Valentino, R., et al. (2021). https://pubmed.ncbi.nlm.nih.gov/33378655/The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 44, 3–16. doi: 10.1016/j.tins.2020.10.007
Craig, A. D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. doi: 10.1038/nrn894
Critchley, H. D., and Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron 77, 624–638. doi: 10.1016/j.neuron.2013.02.008
Cryan, J. F., O'Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Dinan, T. G., and Cryan, J. F. (2019). Gut microbes and depression: still waiting for godot. Brain Behav. Immun. 79, 1–2. doi: 10.1016/j.bbi.2019.02.007
Elsenbruch, S., Rosenberger, C., Bingel, U., Forsting, M., Schedlowski, M., and Gizewski, E. R. (2010). Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology 139, 1310–1319. doi: 10.1053/j.gastro.2010.06.054
Engelmann, J. B., Meyer, F., Fehr, E., and Ruff, C. C. (2015). Anticipatory anxiety disrupts neural valuation during risky choice. J. Neurosci. 35, 3085–3099. doi: 10.1523/JNEUROSCI.2880-14.2015
Engelmann, J. B., Meyer, F., Ruff, C. C., and Fehr, E. (2019). The neural circuitry of affect-induced distortions of trust. Sci. Adv. 5, eaau3413. doi: 10.1126/sciadv.aau3413
Farzi, A., Hassan, A. M., Zenz, G., and Holzer, P. (2019). Diabesity and mood disorders: Multiple links through the microbiota-gut-brain axis. Mol. Aspects Med. 66, 80–93. doi: 10.1016/j.mam.2018.11.003
Federici, S., Suez, J., and Elinav, E. (2020). Our microbiome: On the challenges, promises, and hype. Results Probl. Cell. Differ. 69, 539–557. doi: 10.1007/978-3-030-51849-3_20
Fond, G., Loundou, A., Hamdani, N., Boukouaci, W., Dargel, A., Oliveira, J., et al. (2014). Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 264, 651–660. doi: 10.1007/s00406-014-0502-z
Gershon, M. D. (2013). 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 20, 14–21. doi: 10.1097/MED.0b013e32835bc703
Gershon, M. D., and Margolis, K. G. (2021). The gut, its microbiome, and the brain: Connections and communications. J. Clin. Invest. 131, e143768. doi: 10.1172/JCI143768
Gheorghe, C. E., Martin, J. A., Manriquez, F. V., Dinan, T. G., Cryan, J. F., and Clarke, G. (2019). Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 48:137–145. doi: 10.1016/j.coph.2019.08.004
Grupe, D. W., and Nitschke, J. B. (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 14, 488–501. doi: 10.1038/nrn3524
Hassan, A. M., Mancano, G., Kashofer, K., Liebisch, G., Farzi, A., Zenz, G., et al. (2022). Anhedonia induced by high-fat diet in mice depends on gut microbiota and leptin. Nutr. Neurosci. 25, 299–312. doi: 10.1080/1028415X.2020.175150
Holzer, P. (2017). “Interoception and gut feelings: unconscious body signals' impact on brain function, behavior and belief processes,” in Processes of Believing: The Acquisition, Maintenance, and Change in Creditions, eds H. F. Angel, L. Oviedo, R. F. Paloutzian, A. L. C. Runehov, R. J. Seitz (Berlin: Springer), 435–442. doi: 10.1007/978-3-319-50924-2
Holzer, P., Hassan, A. M., Jain, P., Reichmann, F., and Farzi, A. (2015). Neuroimmune pharmacological approaches. Curr. Opin. Pharmacol. 25, 13–22. doi: 10.1016/j.coph.2015.09.003
Holzer, P., Schicho, R., Holzer-Petsche, U., and Lippe, I. T. (2001). The gut as a neurological organ. Wien. Klin. Wochenschr. 113, 647–660
Hong, J. Y., Naliboff, B., Labus, J. S., Gupta, A., Kilpatrick, L. A., Ashe-McNalley, C., et al. (2016). Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterol. Motil. 28, 127–138. doi: 10.1111/nmo.12710
Icenhour, A., Witt, S. T., Elsenbruch, S., Lowén, M., Engström, M., Tillisch, K., et al. (2017). Brain functional connectivity is associated with visceral sensitivity in women with irritable bowel syndrome. Neuroimage Clin. 15, 449–457. doi: 10.1016/j.nicl.2017.06.001
Kaelberer, M. M., Rupprecht, L. E., Liu, W. W., Weng, P., and Bohórquez, D. V. (2020). Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu. Rev. Neurosci. 43, 337–353. doi: 10.1146/annurev-neuro-091619-022657
Le Morvan de Sequeira, C., Hengstberger, C., Enck, P., and Mack, I. (2022). Effect of probiotics on psychiatric symptoms and central nervous system functions in human health and disease: a systematic review and meta-analysis. Nutrients 14, 621. doi: 10.3390/nu14030621
Legan, T. B., Lavoie, B., and Mawe, G. M. (2022). Direct and indirect mechanisms by which the gut microbiota influence host serotonin systems. Neurogastroenterol. Motil. e14346. doi: 10.1111/nmo.14346
Lerner, J. S., Li, Y., Valdesolo, P., and Kassam, K. S. (2015). Emotion and decision making. Annu. Rev. Psychol. 66, 799–823. doi: 10.1146/annurev-psych-010213-115043
Marcon, A. R., Turvey, S., and Caulfield, T. (2021). 'Gut health' and the microbiome in the popular press: a content analysis. BMJ Open 11, e052446. doi: 10.1136/bmjopen-2021-052446
Mayer, E. A. (2000). The neurobiology of stress and gastrointestinal disease. Gut 47, 861–869. doi: 10.1136/gut.47.6.861
Mayer, E. A. (2011). Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 12, 453–466. doi: 10.1038/nrn3071
Mayer, E. A., Nance, K., and Chen, S. (2022). The gut-brain axis. Annu. Rev. Med. 73, 439–453. doi: 10.1146/annurev-med-042320-014032
McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904. doi: 10.1152/physrev.00041.2006
Meissner, K. (2014). Placebo responses on cardiovascular, gastrointestinal, and respiratory organ functions. Handb. Exp. Pharmacol. 225, 183–203. doi: 10.1007/978-3-662-44519-8_11
Paulus, M. P., and Yu, A. J. (2012). https://pubmed.ncbi.nlm.nih.gov/22898207/Emotion and decision-making: affect-driven belief systems in anxiety and depression. Trends Cogn. Sci. 16, 476–483. doi: 10.1016/j.tics.2012.07.009
Perez-Burgos, A., Mao, Y. K., Bienenstock, J., and Kunze, W. A. (2014). The gut-brain axis rewired: adding a functional vagal “sensory synapse”. FASEB J. 28, 3064–3074. doi: 10.1096/fj.13-245282
Peter, J., Tran, U. S., Michalski, M., and Moser, G. (2018). The structure of resilience in irritable bowel syndrome and its improvement through hypnotherapy: cross-sectional and prospective longitudinal data. PLoS One 13, e0202538. doi: 10.1371/journal.pone.0202538
Seitz, R. J. (2022). Believing and beliefs – neurophysiological underpinnings. Front. Behav. Neurosci. 16, 880504. doi: 10.3389/fnbeh.2022.880504
Seitz, R. J., Angel, H. F., and Paloutzian, R. F. (2018). From believing to belief: a general theoretical model. J. Cogn. Neurosci. 30, 1254–1264. doi: 10.1162/jocn_a_01292
Shea-Donohue, T., Cook, I. J., de Giorgio, R., Tonini, M., Dent, J., Costa, M., et al. (2005). A teaching module on irritable bowel syndrome. Neurogastroenterol. Motil. 17(Suppl 3), 20–40. doi: 10.1111/j.1365-2982.2005.00713.x
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression - a systematic review. Clin. Psychol. Rev. 83, 101943. doi: 10.1016/j.cpr.2020.101943
Simpson, C. A., Schwartz, O. S., and Simmons, J. G. (2020). The human gut microbiota and depression: widely reviewed, yet poorly understood. J. Affect. Disord. 274, 73–75. doi: 10.1016/j.jad.2020.05.115
Suarez, A. N., Hsu, T. M., Liu, C. M., Noble, E. E., Cortella, A. M., Nakamoto, E. M., et al. (2018). Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 9, 2181. doi: 10.1038/s41467-018-04639-1
Taché, Y., Martinez, V., Million, M., and Wang, L. (2001). Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am. J. Physiol. 280, G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173
Tillisch, K., Mayer, E. A., and Labus, J. S. (2011). Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140, 91–100. doi: 10.1053/j.gastro.2010.07.053
Keywords: brain-gut axis, gut-brain axis, interoception, emotion, affect, cognition, beliefs
Citation: Holzer P (2022) Gut Signals and Gut Feelings: Science at the Interface of Data and Beliefs. Front. Behav. Neurosci. 16:929332. doi: 10.3389/fnbeh.2022.929332
Received: 26 April 2022; Accepted: 01 June 2022;
Published: 05 July 2022.
Edited by:
Rüdiger J. Seitz, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Hirokata Fukushima, Kansai University, JapanTian Yuan, Wake Forest School of Medicine, United States
Patrice Duquette, Private Practitioner, Birmingham, MI, United States
Copyright © 2022 Holzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Holzer, cGV0ZXIuaG9semVyQG1lZHVuaWdyYXouYXQ=
 Peter Holzer
Peter Holzer