
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Behav. Neurosci., 31 March 2022
Sec. Pathological Conditions
Volume 16 - 2022 | https://doi.org/10.3389/fnbeh.2022.893662
This article is part of the Research TopicGene and Environment Interactions in Neurodevelopmental DisordersView all 11 articles
Editorial on the Research Topic
Gene and Environment Interactions in Neurodevelopmental Disorders
The knowledge that both the genetic patrimony and lifetime environmental exposures define disease risk is well-accepted. However, the so-called Gene-Environment effects—when the consequences of an environmental exposure vary according to the genetic background—are less understood (Kloke et al., 2013). This is especially true in early life when individuals undergo a series of dynamic and tightly linked developmental processes (Miguel et al., 2019a). The brain and the periphery reorganize during specific developmental time periods to adapt to changes in the environment the subjects are being raised. These are known as “critical periods” (Hensch, 2004).
There has been an increased interest in unraveling how certain types of exposure occurring during these critical periods affect developmental trajectories. Characterizing relevant genes and proteins involved and their precise timing of action in relationship with the type and severity of the environmental exposure is critically needed and was the main aim of this Research Topic. The articles enclosed in this Research Topic represent important advances in the understanding of these relationships.
Focusing on main genetic effects, Zhongling et al. reported a case of Joubert syndrome associated with a new mutation in IFT74, a gene responsible for regulating cilia composition. Wang Y. et al. identified increased allele frequencies of single nucleotide polymorphisms (SNPs) from the Interleukin-23 (IL-23) gene in children with cerebral palsy compared to healthy controls. The perspective from Malatesta et al. focuses on the environmental stimulus, proposing the existence of a critical period during which caregiver's postural and motor lateral biases influence offspring hemispheric lateralization.
During gestation, fetal environment is defined by the maternal metabolic milieu, which influences fetal development. Wang H. et al. investigated the effects of high maternal estradiol on proliferation and differentiation of fetal hypothalamic neural stem/progenitor cells (NSC/NPCs) in mice and identified critical effects on neurogenesis related genes. Sandoval et al. explored another maternal internal state during pregnancy: immune activation, which usually results from infections. Though maternal immune activation induced behavioral alterations compatible with autism spectrum disorders and schizophrenia in the offspring, this was not exacerbated by the loss of vesicular zinc, another known risk factor for neurodevelopmental disorders.
Szekely et al. compiled five candidate polymorphisms (one per gene: DAT1, DRD4, DRD2, COMT, BDNF) in a multilocus score, to explore their interaction with prenatal adversity and postnatal parenting behavior on the development of attentional competence skills in 18- and 24-months children. The same candidate polymorphisms representing COMT and BDNF were used in the study from Cao-Lei et al., investigating the effects of prenatal maternal stress (defined by exposure to a natural disaster during pregnancy) on hippocampal volumes at 11–12 years of age. The SNP located on COMT gene moderated the effect of maternal distress on hippocampal volumes, suggesting that gene-environment interactions have long-term effects on brain neuroanatomical features.
de Mendonça Filho et al. used a novel approach to genomic profiling (Silveira et al., 2017; Hari Dass et al., 2019; Miguel et al., 2019b), representing variations in a prefrontal cortex-specific BDNF gene co-expression network in children, and show that this biological mechanism moderates the effect of prenatal adversity on cognitive developmental trajectories between 6 and 36 months. Intriguingly, epigenetics-related components of the BDNF gene network moderate the effects of prenatal adversity on gray matter content in cortical regions later in childhood. The same approach (Silveira et al., 2017; Hari Dass et al., 2019; Miguel et al., 2019b) was employed by Potter-Dickey et al. to investigate if prefrontal, striatal and hippocampal Cannabinoid Receptor 1 (CNR1) gene co-expression networks moderate the effect of parental caregiving quality on infant attachment styles. Finally, Batra et al. demonstrated that the genetic background associated with higher fasting insulin regulates the effects of early adversity on the development of inhibitory control in young children, corroborating the programming effects of insulin on executive functions in response to early adversity (Batra et al., 2021).
The Research Topic compiles evidence that gene-environment interactions influence neurodevelopment, proposing mechanisms by which this moderation occurs (Figure 1). The studies illustrate novel techniques that can uncover biological targets and pathways with important implications for early detection, prevention, and treatment of neurodevelopmental disturbances.
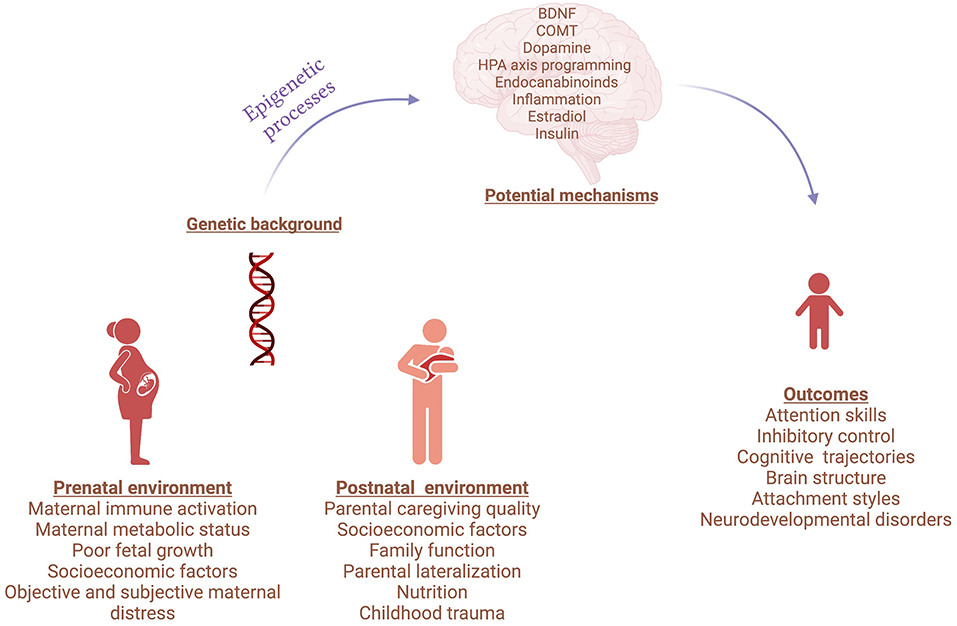
Figure 1. Gene and environment interactions in neurodevelopmental disorders. Created with BioRender.com.
All authors wrote and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the authors of the Research Topic for their contributions.
Batra, A., Latsko, M., Portella, A. K., and Silveira, P. P. (2021). Early adversity and insulin: neuroendocrine programming beyond glucocorticoids. Trends Endocrinol. Metab. 32, 1031–1043. doi: 10.1016/j.tem.2021.09.003
Hari Dass, S. A., McCracken, K., Pokhvisneva, I., Chen, L. M., Garg, E., Nguyen, T. T. T., et al. (2019). A biologically-informed polygenic score identifies endophenotypes and clinical conditions associated with the insulin receptor function on specific brain regions. EBioMedicine 42, 188–202. doi: 10.1016/j.ebiom.2019.03.051
Hensch, T. K. (2004). Critical period regulation. Ann. Rev. Neurosci. 27, 549–579. doi: 10.1146/annurev.neuro.27.070203.144327
Kloke, V., Heiming, R. S., Bolting, S., Kaiser, S., Lewejohann, L., Lesch, K. P., et al. (2013). Unexpected effects of early-life adversity and social enrichment on the anxiety profile of mice varying in serotonin transporter genotype. Behav. Brain Res. 247, 248–258. doi: 10.1016/j.bbr.2013.03.039
Miguel, P. M., Pereira, L. O., Barth, B., de Mendonca Filho, E. J., Pokhvisneva, I., Nguyen, T. T. T., et al. (2019b). Transporter gene network moderates the effect of perinatal hypoxic-ischemic conditions on cognitive flexibility and brain gray matter density in children. Biol. Psychiatry 86, 621–630. doi: 10.1016/j.biopsych.2019.03.983
Miguel, P. M., Pereira, L. O., Silveira, P. P., and Meaney, M. J. (2019a). Early environmental influences on the development of children's brain structure and function. Dev. Med. Child Neurol. 61, 1127–1133. doi: 10.1111/dmcn.14182
Silveira, P. P., Pokhvisneva, I., Parent, C., Cai, S., Rema, A. S. S., Broekman, B. F. P., et al. (2017). Cumulative prenatal exposure to adversity reveals associations with a broad range of neurodevelopmental outcomes that are moderated by a novel, biologically informed polygenetic score based on the serotonin transporter solute carrier family C6, member 4 (SLC6A4) gene expression. Dev. Psychopathol. 29, 1601–1617. doi: 10.1017/S0954579417001262
Keywords: gene environment interaction, development, early adversity, prenatal stress, genetics
Citation: Silveira PP, More L and Gottfried C (2022) Editorial: Gene and Environment Interactions in Neurodevelopmental Disorders. Front. Behav. Neurosci. 16:893662. doi: 10.3389/fnbeh.2022.893662
Received: 10 March 2022; Accepted: 15 March 2022;
Published: 31 March 2022.
Edited and reviewed by: Rainer Spanagel, University of Heidelberg, Germany
Copyright © 2022 Silveira, More and Gottfried. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrícia Pelufo Silveira, cGF0cmljaWEuc2lsdmVpcmFAbWNnaWxsLmNh
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.