
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci. , 21 April 2022
Sec. Behavioral Endocrinology
Volume 16 - 2022 | https://doi.org/10.3389/fnbeh.2022.846234
Helping behaviors and life satisfaction generally increase after middle-age. Identifying the neural substrates of prosocial behaviors in older adults may offer additional insights into these changes over the lifespan. The present study examines the endogenous release of the neuromodulator oxytocin (OT) in participants aged 18–99 and its relationship to prosocial behaviors. OT has been shown to influence trust, altruism, charity, and generosity, yet the effect of age on OT release has not been well-established. Blood samples before and after a video stimulus were obtained from 103 participants in order to examine the impact of OT on prosocial behaviors. We found that OT release following a social prime increased with age (r = 0.49, p = 0.001) and that OT moderated the relationship between age and donations to charity. We tested for robustness by examining three additional prosocial behaviors, money and goods donated to charity during the past year and social-sector volunteering. OT moderated the impact of age on all three prosocial behaviors (ps < 0.05). The analysis also showed that participants’ change in OT was positively associated with satisfaction with life (p = 0.04), empathic concern (p = 0.015), dispositional gratitude (p = 0.019), and religious commitment (p = 0.001). Our findings indicate that the neural chemistry that helps sustain social relationships and live a fulfilled life appear to strengthen with age.
The physical effects of aging on the body have been well documented (Saxon et al., 2014). Similarly, the effect of aging on cognitive function has been extensively studied (Meaney et al., 1995; Conrad and Bimonte-Nelson, 2010). For example, aging impairs efficiency and processing speed (Salthouse, 1994, 1996; Li et al., 2004), explicit learning (Salthouse, 2006), working memory (Salthouse, 1992; McArdle et al., 2004), and attention and problem-solving (Craik and Salthouse, 2000). These changes may result from the reduction of gray matter volume in brain regions as one ages (Brown and Ridderinkhof, 2009), including the anterior cingulate cortex, the orbital frontal cortex, the superior temporal sulcus, the insula, and the dorsal and ventral striatum (Matochik et al., 2000; Raz et al., 2000; Good et al., 2001; Sowell et al., 2003; Allen et al., 2005; Walhovd et al., 2005).
Social behaviors and emotional responses also change with age (Midlarsky et al., 2015; Matsumoto et al., 2016; Pornpattananangkul et al., 2019). Seniors spend more time volunteering and donate a larger proportion of their income to charity compared to younger adults (Simmons and Emanuele, 2004; Omoto et al., 2009; Choi and Chou, 2010; Bekkers and Wiepking, 2011; Wiles and Jayasinha, 2013). Both of these effects are stronger for religiously active older adults (Lee and Chang, 2007; Bekkers and Wiepking, 2011). In laboratory studies, seniors share more money with strangers than younger adults (Beadle et al., 2015) with and without empathy priming (Matsumoto et al., 2016; Rosen et al., 2016; Rosi et al., 2019).
A number of factors are associated with increased prosociality in seniors compared to younger cohorts. These include increased positive affect (Stanley and Isaacowitz, 2011), the use of intuition rather than calculation during decisions (Rosi et al., 2019), and improved emotional regulation (Larcom and Isaacowitz, 2009; Charles and Carstensen, 2010; Samanez-Larkin and Carstensen, 2011). Older adults may develop skills that improve their moods that younger people are less likely to use such as gazing at happy faces (Isaacowitz et al., 2009) though there is significant variation across individuals (Stanley and Isaacowitz, 2012). Age-related positivity also manifests in older adults as they attend to positive information more than negative information (Reed and Carstensen, 2012). Older adults are also better at reasoning about interpersonal and intergroup conflicts (Artistico et al., 2003; Thornton and Dumke, 2005; Grossmann et al., 2010), and have more accurate evaluations of their own knowledge (Kovalchik et al., 2005). Prosocial behaviors in seniors, relative to younger people, may be more likely to arise because of heightened state-induced affective empathy (Ze et al., 2014; Rosen et al., 2016; Sun et al., 2018; Bailey et al., 2021). Yet, no differences are found in trait empathy between older and younger adults (Konrath et al., 2011; O’Brien et al., 2013; Sun et al., 2018).
Age-varying neurochemical responses might help explain seniors’ prosociality. Correlational and experimental studies of prosocial behaviors suggest oxytocin (OT) as a likely candidate. The release of OT is associated with empathic concern (Barraza and Zak, 2009, 2013) while synthetic OT administration enhances empathy (Hurlemann et al., 2010; Shamay-Tsoory et al., 2013). Both the change in endogenous OT and synthetic OT administration have been associated with donations to charity (Barraza and Zak, 2009; Barraza et al., 2011), generosity toward strangers (Zak et al., 2007; Pornpattananangkul et al., 2017), and trustworthiness (Zak et al., 2004, 2005; Terris et al., 2018) among other prosocial behaviors (Barraza and Zak, 2013) though generosity after OT administration may be biased toward in-groups (Ten Velden et al., 2017) and OT administration may or may not affect trust (Kosfeld et al., 2005; Declerck et al., 2020). In addition, the effect of OT infusion on recognizing emotions in faces shows a positive age gradient (Campbell et al., 2014; Ebner et al., 2015a,b; Horta et al., 2019).
Evidence for the effect of OT on social-emotional responses in older adults comes primarily from intranasal OT administration studies, albeit with mixed findings (Horta et al., 2020). For instance, single-dose OT administration improves emotional recognition in healthy older men (Campbell et al., 2014) and in dementia patients (Jesso et al., 2011). However, replication using a larger sample and additional emotional recognition tasks failed to replicate this finding (Grainger et al., 2018) and two meta analyses show variable effects (Shahrestani et al., 2013; Leppanen et al., 2017). OT administration improves self-reported mood (Ebner et al., 2015a,b) and enhances resting state amygdala-mPFC coupling (Ebner et al., 2013) in older men but not older women. A three-dose OT administration study in a sample of older adults with dementia demonstrated reduced apathy, increased expressions of empathy, and improved caregiver interactions (Finger et al., 2015). A longer (10-day) trial of intranasal OT administration in healthy older adults (mean 80 years) revealed improved dispositional gratitude and reduced fatigue (Barraza et al., 2013).
While synthetic OT administration in older adults may have clinical value, such an intervention may be premature until endogenous OT release in seniors is understood. Some reports find basal OT declines with age with a larger effect for women compared to men (Plasencia et al., 2019), although the opposite effect has also been reported (Kunitake et al., 2020). The current study measures OT reactivity in response to a video stimulus with social content because it is the release of OT that has been associated with prosocial behaviors and the quality of relationships (Zak, 2012). Basal OT is generally unpredictive of behavior in healthy adults (Cochran et al., 2013). The reactivity of peripheral OT to social stimuli occurs within 1 s of stimulus onset and reflects a change in central OT via hypothalamic control (Valstad et al., 2017). The correspondence between the change in central and peripheral OT is the reason why a change in OT measured in blood is related to behaviors (Zak et al., 2004, 2005; Zak, 2012; Barraza and Zak, 2013; Terris et al., 2018). Of interest for the present study, OT-expressing neurons appear to be unaffected by age (Wierda et al., 1991; Ishunina et al., 1999) suggesting that OT is a feasible candidate to influence the age gradient in prosociality.
If OT release in seniors is greater than that in younger adults and is associated with prosociality, it may also be related to the age gradient in satisfaction with life (SWL). Many, though not all, studies report that SWL rises after middle-age and stays high until an advanced age (Diener and Suh, 1997; Diener, 2009; Hansen and Slagsvold, 2012; Jivraj et al., 2014). Part of this effect is due to improved coping skills in the face of stress (Hamarat et al., 2002). More generally, life satisfaction among seniors covaries with health, marital status, religiosity, and cognitive function (Bergan and McConatha, 2001; Bailey and Snyder, 2007; Parra-Rizo and Sanchis-Soler, 2020). Basal OT has been associated with higher SWL in young adults (Garforth et al., 2020) but has not to date been examined in older populations.
The study reported here was designed to test the hypothesis that socially primed OT release will increase with age. We also hypothesized that the increase in OT will positively correlate with changes in age-related prosocial behaviors as well as with participants’ SWL. These three hypotheses seek to shed light on the neural mechanisms that influence social behaviors of older adults compared to younger ones.
Participants were 103 students from the Claremont Colleges, residents from Claremont, CA and neighboring cities in Southern California, and older adults from nearby independent living communities. These participants ranged in age from 18 to 99 (M = 50.39, SD = 25.53; 57.3% female). Participants were moderately diverse, self-identifying as Caucasian (70.9%), Latino/Hispanic (7.8%), African American/Black (3.9%), Asian (2.9%), Pacific Islander (2.9%), and mixed ethnicity/other (11.7%). Participants were divided into young (18–35, n = 41), middle (36–65, n = 28), and older (65–99, n = 34) adult cohorts for comparison. Using the average size effect and standard errors for the change in OT in Barraza and Zak (2009) for 103 observations produces a power of test of 0.97 using G*power (Faul et al., 2007) indicating the sample size is sufficient to detect the hypothesized effects.
This study was approved by the Claremont Graduate University Institutional Review Board (IRB # 1255). Written informed consent was obtained from all participants prior to inclusion in the study. Participants were assigned an alphanumeric code to mask their identities and there was no deception of any kind. Tasks were monetarily incentivized such that participants could earn up to $40 depending on their decisions and the decisions of others. A lab administrator who was not associated with the study paid participants their earnings in private to maintain anonymity.
Figure 1 shows how participants progressed through the study. After consent, participants completed surveys regarding their opinions, attitudes, and demographics. Then, a basal blood sample was obtained after which participants watched a short video. After watching the video, participants rated the emotions they were feeling and a second blood draw was done. Participants then made two decisions involving money to measure prosociality. The first involved sharing money with a stranger in the lab that day and the second was a decision to donate some of their earning to charity.
Participants watched a 100-s video of a father describing his feelings about his 2-year-old son who is dying from brain cancer. The video shows the child playing in the hospital while his father talks. The video was used with permission from St. Jude Children’s Research Hospital. The video is described in detail elsewhere (Barraza and Zak, 2009; Barraza et al., 2015) and has been shown to consistently stimulate OT release and elicit charitable donations (Barraza and Zak, 2013). Since the goal is to induce an increase in OT, no control stimulus is needed following previous studies that expose participants to experiences that may induce an increase in OT (Zak et al., 2004, 2005; Alexander et al., 2015; Curry et al., 2015; Terris et al., 2018).
Participants completed surveys to assess demographics and social behaviors as controls. Another control measures is trait empathy (Interpersonal Reactivity Index, IRI; Davis, 1983) that can affect prosocial behaviors (Rameson et al., 2012). A standard 5 question assessment captured satisfaction with life (SWL; Diener et al., 1985) and in order to examine if religion affected prosocial behaviors and SWL, the 10 question religious commitment inventory was used to assess religious activity (RCI; Worthington et al., 2003). Dispositional gratitude was measured using the GQ-6 (McCullough et al., 2002) because gratitude increased in an OT infusion study (Barraza et al., 2013) and influences prosocial behaviors (Bartlett and DeSteno, 2006).
In order to obtain an observable prosocial behavior, participants were given an opportunity to donate some of their earnings from an unrelated task (Range: $25-$40, M: $25.62, SD: $2.47) to St. Jude Children’s Research Hospital. The video presents the story of a father and his 2-year old son who has cancer. Donation are not solicited in the video. Participants were handed a paper receipt noting their earnings from an interpersonal money sharing task done after video and second blood draw (Figure 1) with a statement offering a choice to make a donation from their earnings to St. Jude. Neutral language was used when describing the donation and the decision was made in private. Participants then carried the receipt to a cashier for payment. At the end of the study, all donations were sent to St. Jude. Previous research using the St. Jude video (Barraza and Zak, 2009) showed that donations are made when participants had increases in OT as well as a marker of attention to, or interest in, the stimulus, cortisol (CORT). Subsequent research has shown that for short videos like the one used here, the faster acting arousal biomarker adrenocorticotropin hormone (ACTH) is a more effective measure (Lin et al., 2013). ACTH is the principle pituitary signal to release CORT from the adrenals and the latter provides a second measure of the arousal response due to the video stimulus. In order to ensure the neurochemical response was captured, OT, CORT, and ACTH were measured before and after the video.
Additional evidence of prosocial behaviors was measured with three questions from the intake questionnaire. Participants were asked to report for the previous year how much money they donated to charity, the value of goods donated to charity, and the number of times they had volunteered at a social sector organization. These measures used ordinal scales from 1 to 5 that were defined for participants.
Participants received two 28 ml blood draws from an antecubital vein by a qualified phlebotomist. Two 8-ml EDTA whole-blood tubes and one 12 ml serum-separator tube were drawn in a sterile field using Vacutainer® blood-collection kits. After phlebotomy, each tube was immediately stored on ice before being placed in a refrigerated centrifuge and spun at 1,500 rpm at 4°C for 12 min following previously published protocols (Zak et al., 2005). Plasma and serum were aliquoted into 2-ml polypropylene Fisher brand microtubes. The microtubes were immediately placed on dry ice and then transferred to an −80°C freezer until analysis.
All hormones were assayed using either radioimmunoassay (RIA) or enzyme-linked immunosorbent assays (ELISA). ACTH (plasma-RIA) samples were assayed using a kit produced by DiaSorin, Inc. (Stillwater, MN, United States); CORT (serum-RIA) samples were assayed using a Diagnostic Systems Laboratories (Webster, TX, United States) kit. OT was assayed using a competitive ELISA assay from Assay Designs, Inc. (Ann Arbor, MI, United States). All tests were performed by the Endocrine Core Laboratory of the Yerkes National Primate Research Center at Emory University (Atlanta, GA, United States). The inter-assay CVs < 15% for all analytes.
Oxytocin assays were not put through an extraction step. While there has been debate on the necessity of extraction (Szeto et al., 2011; McCullough et al., 2013; Leng and Sabatier, 2016), unextracted plasma OT has been shown to be reliable (Plasencia et al., 2019; Chu et al., 2020) and may have a stronger association with psychological factors than extracted OT (MacLean et al., 2019; Saxbe et al., 2019). Using an ELISA also permits comparison to an earlier study using the same video stimulus (Barraza and Zak, 2009) and studies of OT in older adults (Plasencia et al., 2019). Since the need for an extraction step in the OT assay is unclear, we sought to confirm our results by running an assay with extraction for approximately one-third of participants for whom there was sufficient additional plasma. This was done using an RIA kit (Bachem, Inc., Torrance, CA, United States). Our analyses exclusively uses the percentage change in OT in plasma to reflect the release of central OT, reducing likelihood of bias due to the type of assay.
Student’s t-tests were used to examine the hypothesized relationship between the change in OT, age, donation decisions, and SWL. The cited literature identifies ACTH and/or CORT as part of the neural responses to video stimuli that are associated with charitable donations so these are included as covariates. In order to establish inferential validity of the observed prosocial measure, in-experiment donations to charity, data from three self-report prosocial behaviors were also measured, donations of goods, time, and money. This convergent validation, known as triangulation, reduces the likelihood of making a Type 1 error (Fielding, 2012; Loken and Gelman, 2017). The self-report data are ordinal but have enough variation to permit parametric analyses; that is, we cannot reject that with all three are Gaussian using the Jarque-Bera test [donated money: χ(2) = 3.81, p = 0.149; donated goods: χ(2) = 3.031, p = 0.220; donated time: χ(2) = 4.59, p = 0.101]. The moderating impact of age and OT release on prosocial behaviors was examined using least squares and ordered logit regressions. Following the literature relating religiosity and SWL, robustness was examined by examining the relationship between the change in OT and RCI and by including RCI as a covariate in analyses of prosocial behaviors.
Using paired t-tests, we found that watching the video produced significant within-subject changes in both ACTH (M = 9.05%, SD = 45.32%, t(94) = 1.66, p = 0.05) and CORT (M = −9.5%, SD = 34.72%, t(81) = 3.17, p = 0.001). The average ΔOT using the larger ELISA sample was heterogeneous (M1 = 371.56, SD1 = 315.72; M2 = 372.41, SD2 = 273.36) as reported in other studies (Terris et al., 2018) and was not different than zero (M = 1.81%, SD = 25.91, t(86) = 0.08, p = 0.53; Appendix Figure A1). The change in all three biomarkers did not show sex differences (ps > 0.20). Consistent with our hypothesis there was a positive and significant correlation between age and ΔOT (r = 0.49, t(85) = 3.18, p = 0.001; Figure 2).
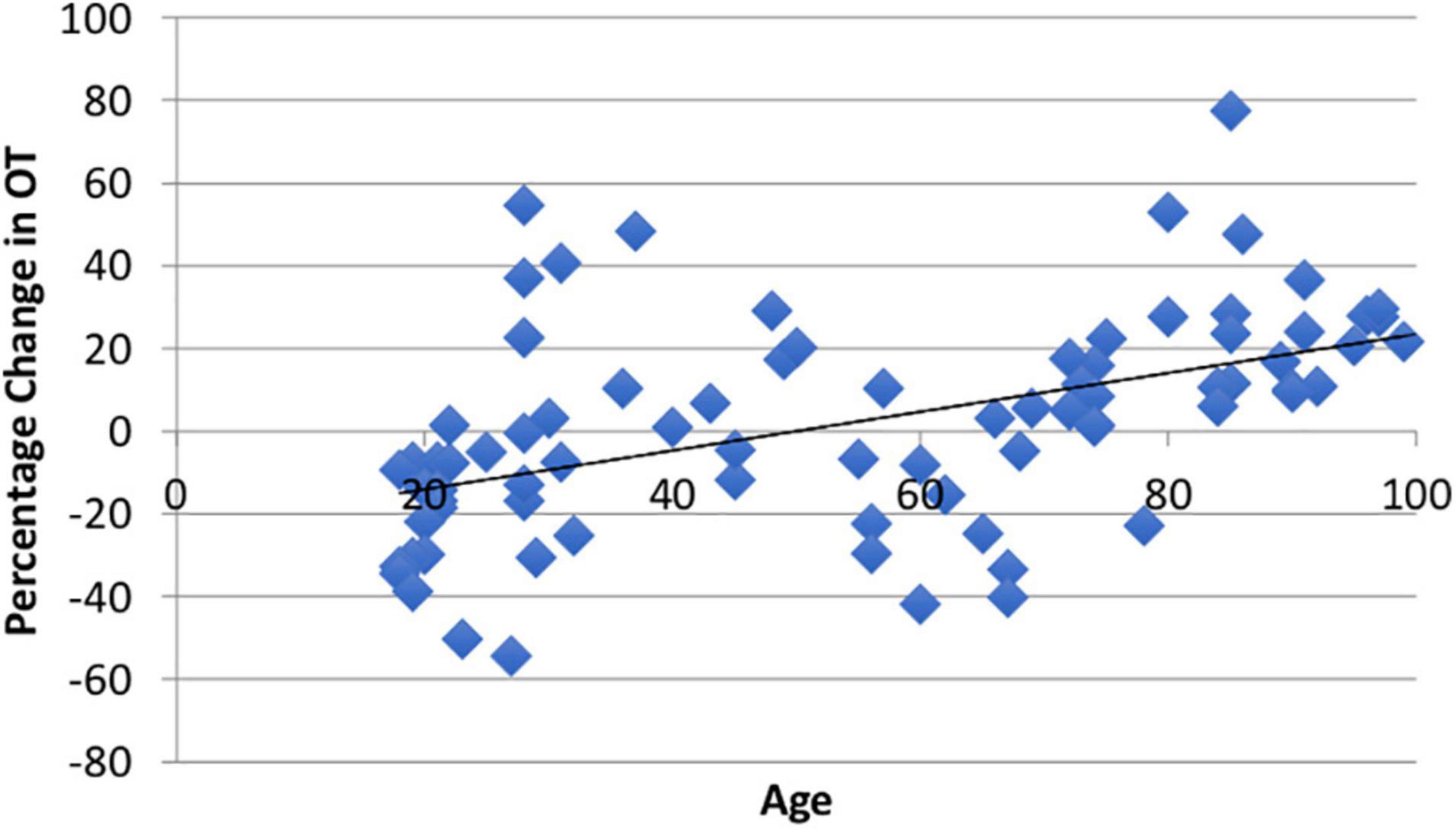
Figure 2. There was a significant positive relationship (r = 0.49, p = 0.001) between age and ΔOT from the video stimulus. The video stimulus increased OT in 55% of the sample.
Replicating our previous finding (Barraza and Zak, 2009), changes in ACTH were positively correlated with higher scores on empathic concern after the video (r = 0.24, t(93) = 1.77, p = 0.04). Age was not correlated with changes in ACTH (r = −0.10, t(93) = 0.41, p = 0.34) or CORT (r = 0.09, t(80) = 0.35, p = 0.36). As expected, the changes in CORT and ACTH had a positive association (r = 0.47, t(80) = 3.21, p = 0.001). Basal ACTH trended toward a negative age gradient (r = −0.17, t(93) = 1.35, p = 0.09), but basal CORT did not (r = 0.05, t(80) = 0.10, p = 0.54).
In order to confirm our OT results, we assayed OT in a random subsample of 36 participants for whom we had extra plasma using an RIA with extraction, as suggested in McCullough et al. (2013). The data revealed a trend for the positive correlation between ΔOT and age using the RIA (r = 0.24, t(34) = 1.05, p = 0.075, one-tailed). This test is underpowered due to the low sample size but provides additional evidence for the main effect. Matching the finding above, there was no overall change in ΔOT due to the video using the RIA (M = 0.25%, SD = 0.86, t(34) = 0.24, p = 0.81, N = 35).
The average amount donated to charity was $8.19 (SD = $0.89). Charitable donations for the young age group (18–35) were $4.64 (SD = $1.05), increasing to $9.30 (SD = $1.66) for middle-aged participants (36–64), and rising to $12.45 (SD = $1.79) for seniors (>65; Figure 3). There was a significant positive relationship between age and the amount donated (r = 0.32, t(85) = 3.18, p = 0.001). Middle-aged participants donated twice as much to charity compared to young participants ($4.65; t(25) = 1.58, p = 0.06) while seniors donated 168% more than the young cohort ($7.80; t(23) = 3.26, p = 0.001). The age gradient for charitable giving was corroborated by two other measures of prosocial behaviors that increased with age: the amount of time one volunteered to help others (r = 0.19, t(89) = 1.57, p = 0.06) and the percentage of income donated to charity (r = 0.29, t(92) = 2.36, p = 0.01).
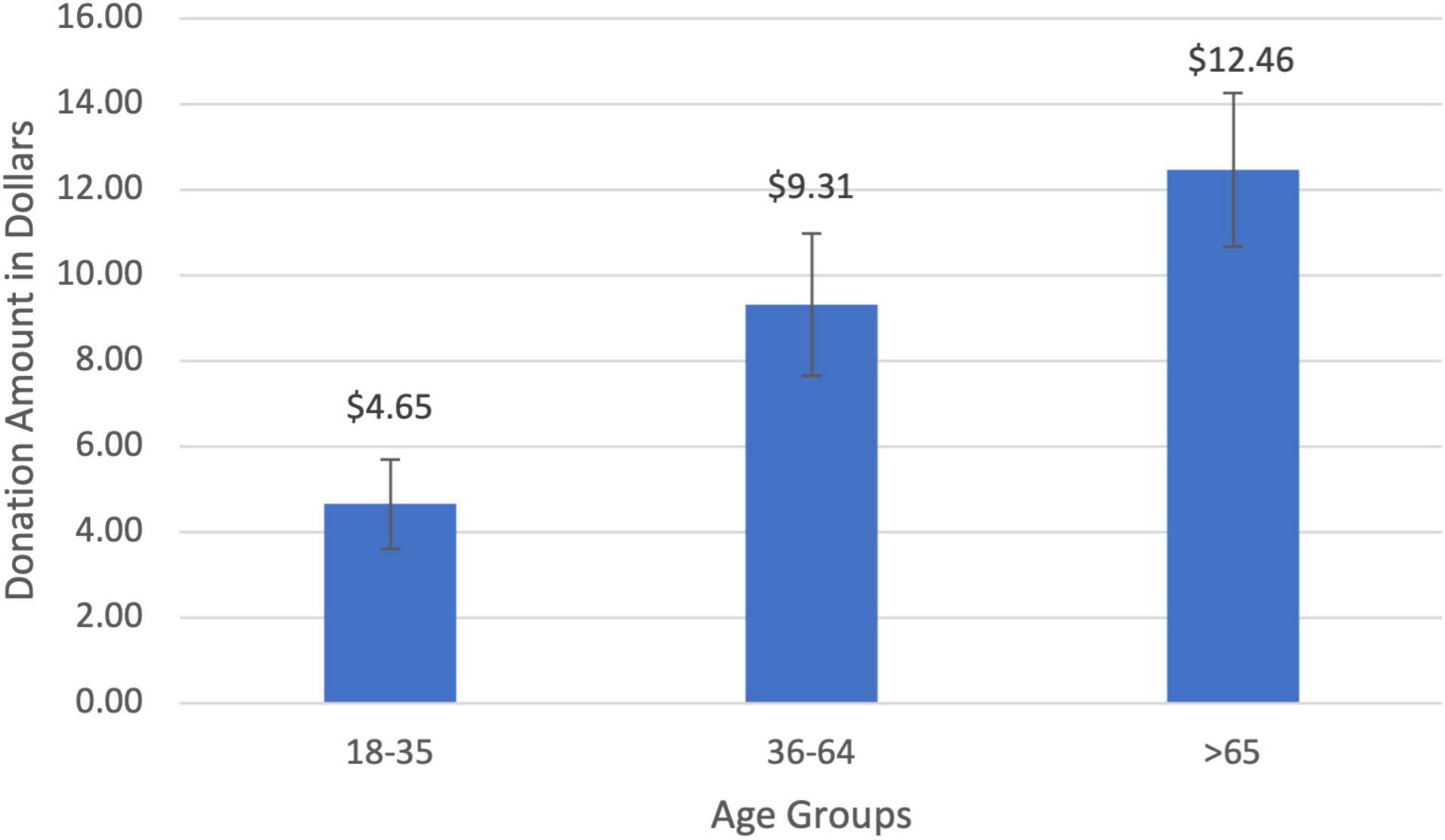
Figure 3. Charitable donation amount by age groups. There was a significant positive correlation between donation amount and age (r = 0.32, p = 0.001).
Consistent with the previous literature (Barraza and Zak, 2009), participants who had increases in both OT and ACTH (N = 40) donated 68% more to charity than participants (N = 63) who did not have these responses (OT+ & ACTH+: M = $12.10; Others: M = $7.19, t = 2.62, p = 0.01). Correlations for the amount donated to charity with the change in ACTH, OT, and CORT alone were not significant (ps > 0.05).
In order to more fully examine the relationship between donations, age, and the changes in ACTH and OT, a set of least squares regression models were estimated. Estimating the linear effects of the changes in neurochemicals and age, only age was significantly related to donations. We hypothesized based on the findings above that age would moderate the effects of ΔOT. The initial estimation identified a mis-specification when both ΔOT and ΔOT*Age were included as explanatory variables due to multicollinearity (ΔOT variable inflation factor VIF > 5). As a result, ΔOT was dropped from the estimation in order to focus on the moderator. Note that this changes the interpretation of the interaction term (Faraway, 2004). In this estimation, the moderator (β = −0.0006, p = 0.029), and age are statistically significant and the model explains 20% of the variance in donations (R2 = 0.20, F(3,60) = 5.03, p = 0.004). The age gradient for donations is steeper when ΔOT is low compared to the slope when ΔOT is high (Figure 4). The variables continue to be significant (R2 = 0.21, F(5,58) = 3.09, p = 0.015) when sex and RCI are included as controls (moderator: β = −0.0005, p = 0.05).
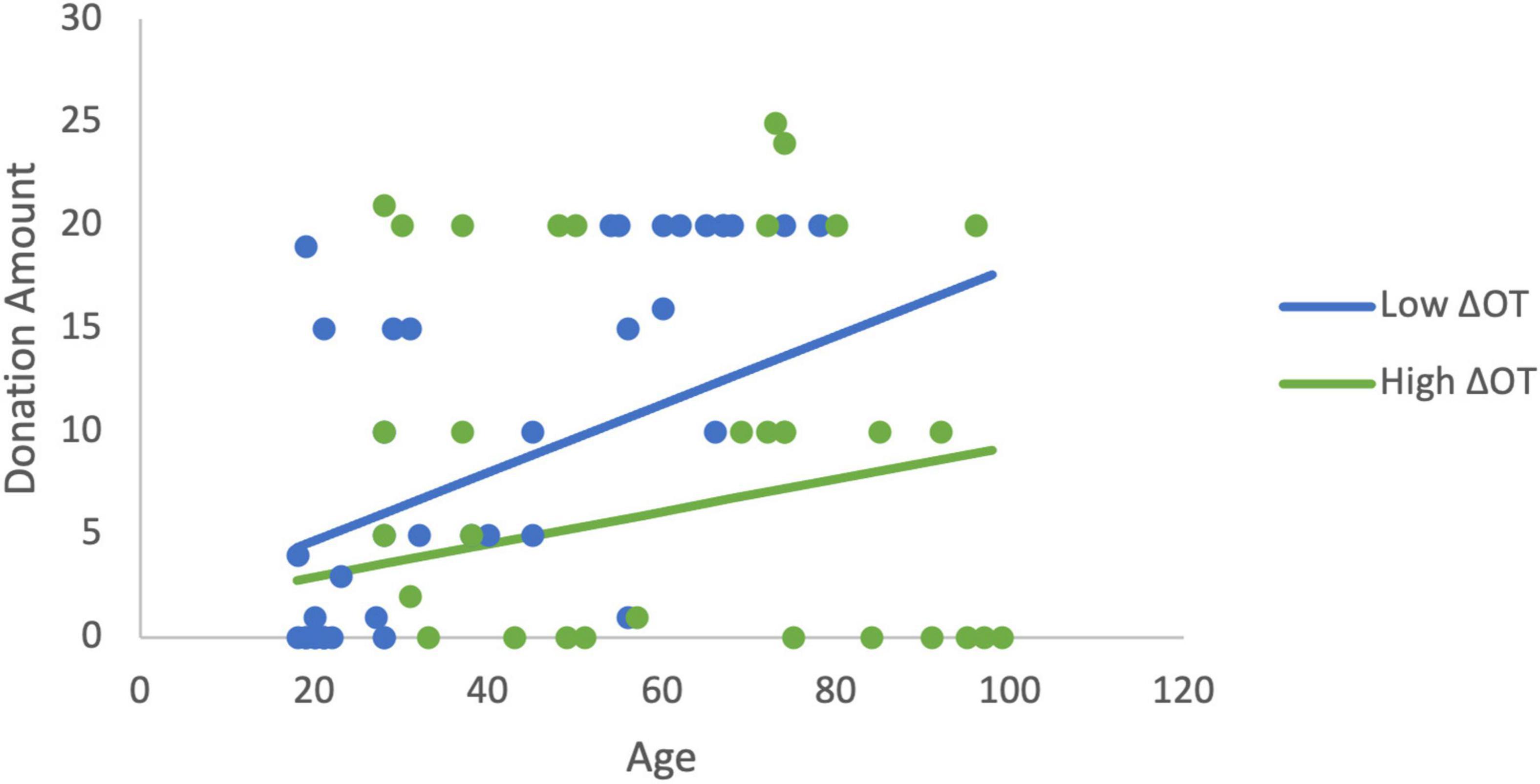
Figure 4. The moderation estimation shows that age has a larger effect on donations to charity when ΔOT is low while the effect of age on donations is positive but has a flatter gradient when ΔOT is high. The low value of ΔOT is the 40th percentile (4.05) and the high value is the 75th percentile (160.96).
Three additional prosocial behaviors were examined to assess the robustness of the charitable donation finding. These are donations to charity of money, goods, and time over the past year. Ordered logits were estimated for the three dependent variables using the same independent variables as in the immediate donation equation. As in the immediate donation equation, ΔOT was excluded due to multicollinearity (VIF > 6) with the moderator ΔOT*Age.
The past year’s donation of money to charity showed a positive age gradient (r = 0.38, p < 0.001). Estimating an ordered logit moderation model with ΔACTH, age, and ΔOT*Age, we found that OT*Age (β = −0.0001, p = 0.024) was a significant predictor in the model (χ2 = 19.71, p = 0.000). The positive age gradient holds for both high and low ΔOT with a steeper gradient when ΔOT is low rather than high (Appendix Figure A2). This relationship continued to be significant when including RCI and sex (OT*Age: χ2 = 24.15, p = 0.000).
The next measure of prosociality tested was goods donated to charity during the last year. As with money donated to charity, there was positive age gradient (r = 0.36, p < 0.001) for donated goods. Estimating the same ordered logit moderation model, OT*Age (β = −0.0001, p = 0.051) was a significant predictor in the model (χ2 = 16.62, p = 0.001). This relationship remained significant when accounting for RCI and sex (OT*Age: χ2 = 20.14, p = 0.001) and increased with age for both high and low ΔOT with a steeper gradient when ΔOT was low (Appendix Figure A3).
The third measure of prosociality was the number of times a participant volunteered at a social sector organization. Older participants trended toward having volunteered more often over the last year compared to younger people in the sample (r = 0.19, p = 0.065). The logit moderation model showed that that OT*Age (β = −0.0002, p = 0.002) was associated with volunteering (χ2 = 15.56, p = 0.001) and the relationship was robust to the inclusion of RCI and sex (OT*Age: χ2 = 21.13, p = 0.001). The positive relationship between age and volunteering is sustained for both low and high ΔOT matching the other two retrospective prosocial measures (Appendix Figure A4).
Both SWL (r = 0.19, t(99) = 1.67. p = 0.05) and RCI (r = 0.54, t(99) = 3.11, p = 0.001) increased with age. Importantly, the change in OT was positively associated with SWL (r = 0.20, t(85) = 1.42, p = 0.04; Figure 5) and religious commitment (r = 0.41, t(85) = 3.17, p = 0.001, Figure 6). The SWL finding stands out because the frequency of contact with friends and loved ones fell with age (r = −0.28, t(92) = 2.36, p = 0.01). ΔOT was also associated with two dispositional traits, gratitude (r = 0.23, t(85) = 2.10, p = 0.019) and empathic concern (IRI, r = 0.28, t(79) = 1.90, p = 0.015).
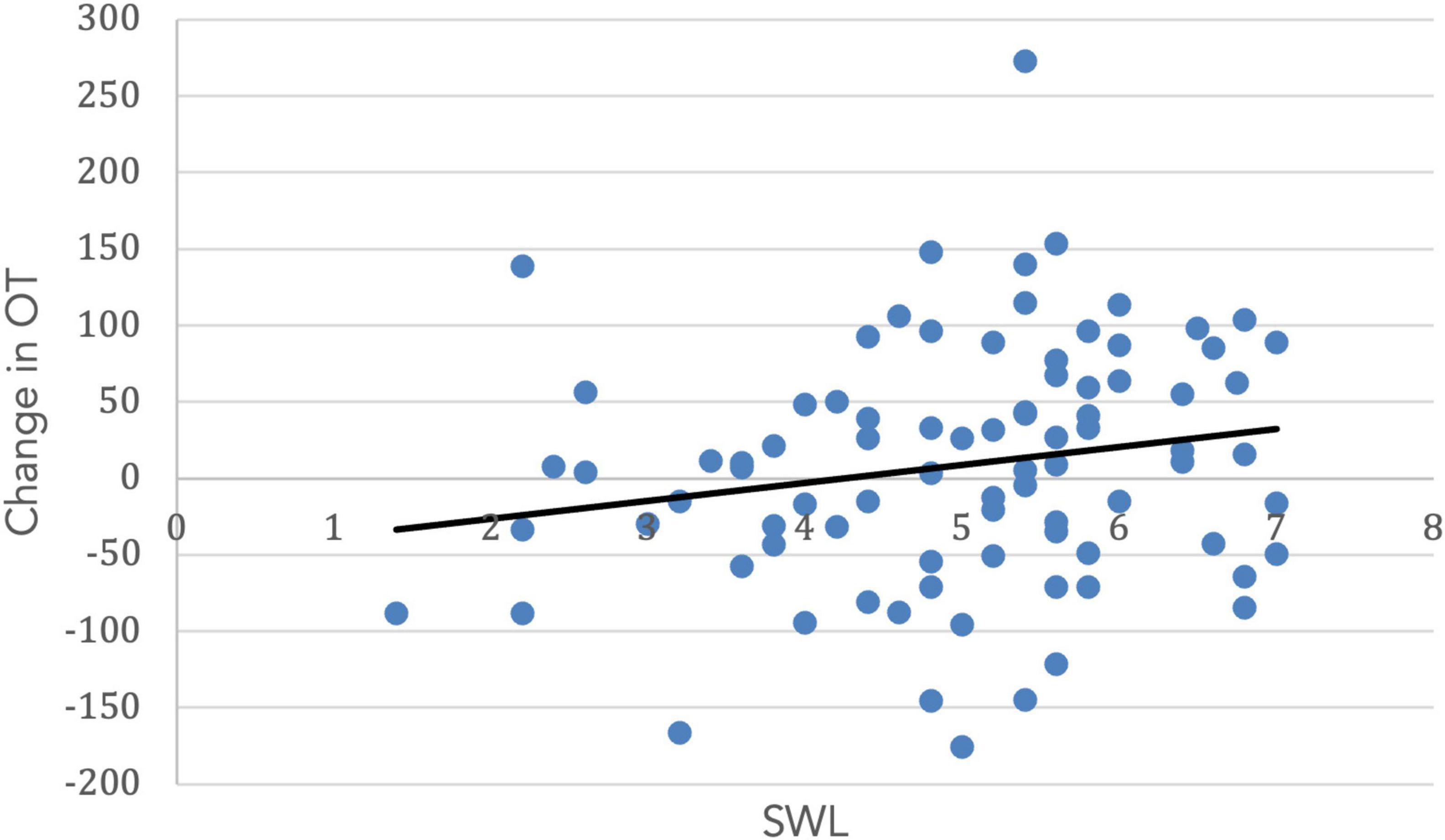
Figure 5. The change in OT had a positive correlation with satisfaction with life (r = 0.20, p = 0.04).
Our analysis has identified a likely neurochemical impetus for prosocial behavior that remains intact with age. The data showed that older participants experienced the largest change in OT in response to an emotional stimulus compared to other age groups. The correlation between neurochemical changes and four measures of prosocial actions suggest that OT impacts prosocial behaviors more strongly in aging adults for small increases in OT. As in previous research, our data show that individuals who dispositionally have high empathic concern have a larger increase in OT after a video prime with social content (Barraza and Zak, 2013; Zak and Barraza, 2013). This dispositional effect partially dampens the age effect on OT from the prime revealing a trait-state interaction that influences the acute donation decision. The “high oxytocin responder” effect has been found for other stimuli and behaviors (Rameson et al., 2012; Procyshyn et al., 2020) and has been previously reported for the video used here (Barraza and Zak, 2009). Nevertheless, the positive age gradient for age on donations was maintained for both low and high ΔOT responders. Note that while there was no average change in OT for the video as in a previous study using the same stimulus (Barraza and Zak, 2009), in most published research using social stimuli to induce OT release, including studies with very large sample sizes, only about 50% of participants will show an increase (Barraza and Zak, 2013; Terris et al., 2018).
The other three retrospective measures of prosocial behaviors also had a positive moderating effect with age and show the same pattern vis-a-vis the magnitude of ΔOT. The retrospective measures of prosocial behaviors were included to increase the likelihood of inferential validity of the statistical test of the observed prosocial donation. That all three measures confirm the relationship with ΔOT is remarkable since retrospective measures are subject to more bias than directly observable behaviors. We believe the relationship between an acute change in OT and retrospective prosocial behaviors is new to the literature and should be replicated to ensure it does not only occur for the sample we collected. This also supports the positive correlation of ΔOT with the self-reports for life satisfaction, trait gratitude, and religious commitment. The larger prosocial response for the ΔOT*Age moderator when ΔOT is low demonstrates the powerful impact of age on helping behaviors.
An additional contribution of the present research is the demonstration that our results hold when analyzing the change in OT using unextracted OT from an ELISA as well as an extracted sample using an RIA. The extracted RIA change in OT trended toward a positive correlation with age (r = 0.27, p = 0.075). The post-stimulus OT values from the RIA and ELISA were positively correlated (r = 0.08, paired t(25), p = 0.0001) as in animal research (Valstad et al., 2017), though the change in OT across assays was not correlated (r = 0.05, paired t(25), p = 0.39).
Age moderated the relationship between ΔOT and immediate donations as well as the history of donations of money and goods. These associations continued to be significant when accounting for biological sex and religious participation. Our findings suggest a possible neurologic explanation for why older adults donate a higher portion of their income to charity (Simmons and Emanuele, 2004; Omoto et al., 2009; Choi and Chou, 2010; Bekkers and Wiepking, 2011). Participants who had positive changes in both ACTH and OT donated 68% more to charity than did those without these responses. The ACTH response is the peripheral signature of attention while the OT response indicates a participant’s emotional resonance with the narrative (Born et al., 1986; Barraza and Zak, 2013). This finding replicates our previous work on immediate donations (Barraza and Zak, 2009; Lin et al., 2013) and extends this to one’s history of donations. Our finding is surprising as older adults tend to be more distracted than younger people (Guerreiro et al., 2010), including while watching videos (Campbell et al., 2015), an area that requires more research. The larger OT response to the video among older adults may be due to heighted subjective emotional responses relative to young adults when viewing films about loss (Kunzmann and Grühn, 2005).
The moderated interaction of OT and age extended to volunteering as well. Seniors have been shown to volunteer more often than younger people (Castle et al., 2012; Sze et al., 2012; Wiles and Jayasinha, 2013; Beadle et al., 2015; Matsumoto et al., 2016). The parametric relationship for volunteering along with the other prosocial behaviors, reveals the strength of the relationship of OT and age. OT administration has been shown to increase dispositional gratitude (Barraza et al., 2013) and the findings here extend this relationship to the release of endogenous OT. Those with higher dispositional gratitude tend to engage in more helping behaviors than those who are less grateful (Bartlett and DeSteno, 2006). OT also functions as an analgesic (González-Hernández et al., 2014). Seniors who suffer from pain, which often reduces physical activity, tend to have fewer social interactions leading to isolation and increased risk of depression (Williamson and Schulz, 1992). Since positive social interactions are an OT stimulus (Barraza and Zak, 2013), there is likely a bidirectional effect of social activities and prosocial behaviors.
The heightened OT response by seniors was associated with an enhanced evaluation of life satisfaction compared to younger participants. While SWL has been shown to increase after middle age (Diener, 2009; Hansen and Slagsvold, 2012; Jivraj et al., 2014), we found that the variation in OT release was linearly associated with SWL. Religiosity is among the factors known to increase SWL (Bergan and McConatha, 2001; Diener and Clifton, 2002) and our analysis found a positive correlation between religious commitment (RCI) and the change in OT. The joint relationship of OT release on SWL, gratitude, and religiosity can help explain why seniors enjoy greater affective well-being than do younger adults (Scheibe and Carstensen, 2010). An innovation in our work is showing the need for a social stimulus to induce OT release so that seniors gain the psychological benefits we identified. Older adults often suffer from loneliness (Cacioppo and Patrick, 2008) that can reduce the social opportunities to release OT.
There are several limitations of the present study. We are unable to establish the causal relationship between OT, prosocial behaviors, and dispositional reports even while our analysis supports the hypotheses that OT influences prosocial behaviors and SWL in older adults. There are likely factors in addition to the release of OT that cause people to share money, donate to charity, participate in religious activities, and have high SWL that we were unable to measure and should be explored in future research. Further, prosocial behaviors may influence the likelihood and amount of OT release (Choi and Kim, 2011; Zak, 2012; Boenigk and Mayr, 2016). Findings in animals suggest a bi-directional causality in which OT release motivates prosocial behaviors that potentiate neural networks that influence future OT excretion (Nelson and Panksepp, 1998). As in nearly all published studies of endogenous OT release, the video stimulus was only effective for some participants that can be due to inattention, stress or interactions with other neurochemicals that additional measures could uncover (Zak et al., 2005; Neumann and Landgraf, 2012; Zak, 2012; Terris et al., 2018). The findings here should be considered preliminary since the sample size is small compared to other published research relating age to prosocial behaviors (Foulkes et al., 2018; Mayr and Freund, 2020). The sample is also geographically homogeneous which might have biased the results even while the main findings continue to hold with the inclusion of covariates such as religious practice. While measuring changes in oxytocin via serial blood draws is the gold standard, this makes large samples logistically difficult. An approach future research could use would be to capture the effect of oxytocin non-invasively for a larger sample through oxytocin’s activation of the vagus nerve using an electrocardiogram (Norman et al., 2011; Barraza et al., 2015). This will allow researchers to extend the sample size to confirm the results reported here.
The confluence of our findings relating OT to prosocial behaviors and the age gradient we report, are a bulwark against expectations that seniors are socially disengaged. Family members, clinical teams, activity directors, and seniors themselves should seek out more opportunities for social activities, even when mobility or pain limits activity choices. Seniors retain the ability and interest in social activity and its positive impact on health and happiness are well-established (Koopmans et al., 2010).
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The data are available at Open IR openicpsr-147581.
The studies involving human participants were reviewed and approved by the Claremont Graduate University Institutional Review Board (IRB # 1255). The patients/participants provided their written informed consent to participate in this study.
PZ and JB conceived and designed the study and collected data. PZ, JB, TO, and BC performed the analysis, interpreted the results, and prepared the manuscript. PZ obtained funding. All authors reviewed the results and approved the final version of the manuscript.
This research was funded by the National Institute on Aging 5R21AG29871 to PZ.
BC was employed by TripActions.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alexander, V., Tripp, S., and Zak, P. J. (2015). Preliminary evidence for the neurophysiologic effects of online coupons: changes in oxytocin, stress, and mood. Psychol. Mark. 32, 977–986. doi: 10.1002/mar.20831
Allen, J. S., Bruss, J., Brown, C. K., and Damasio, H. (2005). Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol. Aging 26, 1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023
Artistico, D., Cervone, D., and Pezzuti, L. (2003). Perceived self-efficacy and everyday problem solving among young and older adults. Psychol. Aging 1868–79. doi: 10.1037/0882-7974.18.1.68
Bailey, P. E., Ebner, N. C., and Stine-Morrow, E. A. (2021). Introduction to the special issue on prosociality in adult development and aging: advancing theory within a multilevel framework. Psychol. Aging 36, 1–9. doi: 10.1037/pag0000598
Bailey, T. C., and Snyder, C. R. (2007). Satisfaction with life and hope: a look at age and marital status. Psychol. Rec. 57, 233–240. doi: 10.1007/BF03395574
Barraza, J., and Zak, P. J. (2013). “Oxytocin instantiates empathy and produces prosocial behaviors Chapter 18,” in Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior, eds E. Choleris, D. W. Pfaff, and M. Kavaliers (Cambridge: Cambridge University Press), 331–342. doi: 10.1017/cbo9781139017855.022
Barraza, J. A., Alexander, V., Beavin, L. E., Terris, E. T., and Zak, P. J. (2015). The heart of the story: peripheral physiology during narrative exposure predicts charitable giving. Biol. Psychol. 105, 138–143. doi: 10.1016/j.biopsycho.2015.01.008
Barraza, J. A., Grewal, N. S., Ropacki, S., Perez, P., Gonzalez, A., and Zak, P. J. (2013). Effects of a 10-day oxytocin trial in older adults on health and well being. Exp. Clin. Psychopharmacol. 21, 85–92. doi: 10.1037/a0031581
Barraza, J. A., McCullough, M. E., Ahmadi, S., and Zak, P. J. (2011). Oxytocin infusion increases charitable donations regardless of monetary resources. Horm. Behav. 60, 148–151. doi: 10.1016/j.yhbeh.2011.04.008
Barraza, J. A., and Zak, P. J. (2009). Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann. N. Y. Acad. Sci. 1167, 182–189. doi: 10.1111/j.1749-6632.2009.04504.x
Bartlett, M. Y., and DeSteno, D. (2006). Gratitude and prosocial behavior: helping when it costs you. Psychol. Sci. 17, 319–325. doi: 10.1111/j.1467-9280.2006.01705.x
Beadle, J. N., Sheehan, A. H., Dahlben, B., and Gutchess, A. H. (2015). Aging, empathy, and prosociality. J. Gerontol. B Psychol. Sci. Soc. Sci. 70, 213–222. doi: 10.1093/geronb/gbt091
Bekkers, R., and Wiepking, P. (2011). Who gives? A literature review of predictors of charitable giving part one: religion, education, age and socialisation. Volunt. Sect. Rev. 2, 337–365. doi: 10.1332/204080511x6087712
Bergan, A., and McConatha, J. T. (2001). Religiosity and life satisfaction. Act. Adapt. Aging 24, 23–34. doi: 10.1300/j016v24n03_02
Boenigk, S., and Mayr, M. L. (2016). The happiness of giving: evidence from the German socioeconomic panel that happier people are more generous. J. Happiness Stud. 17, 1825–1846. doi: 10.1007/s10902-015-9672-2
Born, J., Fehm, H. L., and Voigt, K. H. (1986). ACTH and attention in humans: a review. Neuropsychobiology 15, 165–186. doi: 10.1159/000118261
Brown, S. B. R. E., and Ridderinkhof, K. R. (2009). Aging and the neuroeconomics of decision making: a review. Cogn. Affect. Behav. Neurosci. 9, 365–379. doi: 10.3758/CABN.9.4.365
Cacioppo, J. T., and Patrick, W. (2008). Loneliness: Human Nature and the Need for Social Connection. New York, NY: W. W. Norton & Company.
Campbell, A., Ruffman, T., Murray, J. E., and Glue, P. (2014). Oxytocin improves emotion recognition for older males. Neurobiol. Aging 35, 2246–2248. doi: 10.1016/j.neurobiolaging.2014.04.021
Campbell, K. L., Shafto, M. A., Wright, P., Tsvetanov, K. A., Geerligs, L., Cusack, R., et al. (2015). Idiosyncratic responding during movie-watching predicted by age differences in attentional control. Neurobiol. Aging 36, 3045–3055. doi: 10.1016/j.neurobiolaging.2015.07.028
Castle, E., Eisenberger, N. I., Seeman, T. E., Moons, W. G., Boggero, I. A., Grinblatt, M. S., et al. (2012). Neural and behavioral bases of age differences in perceptions of trust. Proc. Natl. Acad. Sci. U.S.A. 109, 20848–20852. doi: 10.1073/pnas.1218518109
Charles, S. T., and Carstensen, L. L. (2010). Social and emotional aging. Ann. Rev. Psychol. 61, 383–409. doi: 10.1146/annurev.psych.093008.100448
Choi, N. G., and Chou, R. J. A. (2010). Time and money volunteering among older adults: the relationship between past and current volunteering and correlates of change and stability. Ageing Soc. 30, 559–581. doi: 10.1017/s0144686x0999064x
Choi, N. G., and Kim, J. (2011). The effect of time volunteering and charitable donations in later life on psychological wellbeing. Ageing Soc. 31, 590–610. doi: 10.1017/s0144686x10001224
Chu, C., Hammock, E. A., and Joiner, T. E. (2020). Unextracted plasma oxytocin levels decrease following in-laboratory social exclusion in young adults with a suicide attempt history. J. Psychiatr. Res. 121, 173–181. doi: 10.1016/j.jpsychires.2019.11.015
Cochran, D., Fallon, D., Hill, M., and Frazier, J. A. (2013). The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv. Rev. Psychiatry 21, 219–247. doi: 10.1097/HRP.0b013e3182a75b7d
Conrad, C. D., and Bimonte-Nelson, H. A. (2010). Impact of the hypothalamic–pituitary–adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog. Brain Res. 18, 31–76. doi: 10.1016/S0079-6123(10)82002-3
Craik, F. I., and Salthouse, T. A. (eds) (2000). The Handbook of Aging and Cognition, 2nd Edn. Mahwah, NJ: Lawrence Erlbaum.
Curry, B. A., Donaldson, B., Vercoe, M., Filippo, M., and Zak, P. J. (2015). Oxytocin responses after dog and cat interactions depend on pet ownership and may affect interpersonal trust. Hum. Anim. Interact. Bull. 3, 56–71.
Davis, M. H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126. doi: 10.3389/fpsyg.2021.588934
Declerck, C. H., Boone, C., Pauwels, L., Vogt, B., and Fehr, E. (2020). A registered replication study on oxytocin and trust. Nat. Hum. Behav. 4, 646–655. doi: 10.1038/s41562-020-0878-x
Diener, E., and Clifton, D. (2002). Life satisfaction and religiosity in broad probability samples. Psychol. Inq. 13, 206–209.
Diener, E. (ed.). (2009). “Subjective well-being,” in The Science of Well-Being, (Dordrecht: Springer), 11–58.
Diener, E., Emmons, R. A., Larsen, R. J., and Griffin, S. (1985). The satisfaction with life scale. J. Pers. Assess. 49, 71–75.
Diener, E. D., and Suh, M. E. (1997). Subjective well-being and age: an international analysis. Annu. Rev. Gerontol. Geriatr. 17, 304–324.
Ebner, N. C., Horta, M., Lin, T., Feifel, D., Fischer, H., and Cohen, R. A. (2015a). Oxytocin modulates meta-mood as a function of age and sex. Front. Aging Neurosci. 7:175. doi: 10.3389/fnagi.2015.00175
Ebner, N. C., Kamin, H., Diaz, V., Cohen, R. A., and MacDonald, K. (2015b). Hormones as “difference makers” in cognitive and socioemotional aging processes. Front. Psychol. 5:1595. doi: 10.3389/fpsyg.2014.01595
Ebner, N. C., Maura, G. M., MacDonald, K., Westberg, L., and Fischer, H. (2013). Oxytocin and socioemotional aging: current knowledge and future trends. Front. Hum. Neurosci. 7:487. doi: 10.3389/fnhum.2013.00487
Faul, F., Erdfelder, E., Lang, A.-G., and Buchner, A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/bf03193146
Fielding, N. G. (2012). Triangulation and mixed methods designs: data integration with new research technologies. J. Mix. Methods Res. 6, 124–136. doi: 10.1177/1558689812437101
Finger, E. C., MacKinley, J., Blair, M., Oliver, L. D., Jesso, S., Tartaglia, M. C., et al. (2015). Oxytocin for frontotemporal dementia: a randomized dose-finding study of safety and tolerability. Neurology 84, 174–181. doi: 10.1212/WNL.0000000000001133
Foulkes, L., Leung, J. T., Fuhrmann, D., Knoll, L. J., and Blakemore, S. J. (2018). Age differences in the prosocial influence effect. Dev. Sci. 21:e12666. doi: 10.1111/desc.12666
Garforth, B., Degnbol, H., Terris, E. T., Zak, P. J., and Winterdahl, M. (2020). Elevated plasma oxytocin levels and higher satisfaction with life in young oral contraceptive users. Sci. Rep. 10:8208. doi: 10.1038/s41598-020-64528-w
González-Hernández, A., Rojas-Piloni, G., and Condés-Lara, M. (2014). Oxytocin and analgesia: future trends. Trends Pharmacol. Sci. 35, 549–551. doi: 10.1016/j.tips.2014.09.004
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N. A., Friston, K. J., and Frackowiak, R. S. J. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36. doi: 10.1006/nimg.2001.0786
Grainger, S. A., Henry, J. D., Steinvek, H. R., Vanman, E. J., Rendell, P. G., and Labuschagne, I. (2018). Intranasal oxytocin does not reduce age-related difficulties in social cognition. Horm. Behav. 99, 25–34. doi: 10.1016/j.yhbeh.2018.01.009
Grossmann, I., Na, J., Varnum, M. E., Park, D. C., Kitayama, S., and Nisbett, R. E. (2010). Reasoning about social conflicts improves into old age. Proc. Nat. Acad. Sci. 107, 7246–7250. doi: 10.1073/pnas.1001715107
Guerreiro, M. J., Murphy, D. R., and Van Gerven, P. W. (2010). The role of sensory modality in age-related distraction: a critical review and a renewed view. Psychol. Bull. 136, 975–1022. doi: 10.1037/a0020731
Hamarat, E., Thompson, D., Steele, D., Matheny, K., and Simons, C. (2002). Age differences in coping resources and satisfaction with life among middle-aged, young-old, and oldest-old adults. J. Genet. Psychol. 163, 360–367. doi: 10.1080/00221320209598689
Hansen, T., and Slagsvold, B. (2012). The age and subjective well-being paradox revisited: a multidimensional perspective. Nor. Epidemiol. 22, 187–195.
Horta, M., Pehlivanoglu, D., and Ebner, N. C. (2020). The role of intranasal oxytocin on social cognition: an integrative human lifespan approach. Curr. Behav. Neurosci. Rep. 7, 175–192. doi: 10.1007/s40473-020-00214-5
Horta, M., Ziaei, M., Lin, T., Porges, E. C., Fischer, H., Feifel, D., et al. (2019). Oxytocin alters patterns of brain activity and amygdalar connectivity by age during dynamic facial emotion identification. Neurobiol. Aging 78, 42–51. doi: 10.1016/j.neurobiolaging.2019.01.016
Hurlemann, R., Patin, A., Onur, O. A., Cohen, M. X., Baumgartner, T., Metzler, S., et al. (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 30, 4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010
Isaacowitz, D. M., Toner, K., and Neupert, S. D. (2009). Use of gaze for real-time mood regulation: effects of age and attentional functioning. Psychol. Aging 24, 989–994. doi: 10.1037/a0017706
Ishunina, T. A., Salehi, A., Hofman, M. A., and Swaab, D. F. (1999). Activity of vasopressinergic neurones of the human supraoptic nucleus is age-and sex-dependent. J. Neuroendocrinol. 11, 251–258. doi: 10.1046/j.1365-2826.1999.00318.x
Jesso, S., Morlog, D., Ross, S., Pell, M. D., Pasternak, S. H., Mitchell, D. G., et al. (2011). The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain 134, 2493–2501. doi: 10.1093/brain/awr171
Jivraj, S., Nazroo, J., Vanhoutte, B., and Chandola, T. (2014). Aging and subjective well-being in later life. J. Gerontol. B Psychol. Sci. Soc. Sci. 69, 930–941.
Konrath, S. H., O’Brien, E. H., and Hsing, C. (2011). Changes in dispositional empathy in American college students over time: a meta-analysis. Pers. Soc. Psychol. Rev. 15, 180–198. doi: 10.1177/1088868310377395
Koopmans, T. A., Geleijnse, J. M., Zitman, F. G., and Giltay, E. J. (2010). Effects of happiness on all-cause mortality during 15 years of follow-up: the Arnhem Elderly Study. J. Happiness Stud. 11, 113–124. doi: 10.1007/s10902-008-9127-0
Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U., and Fehr, E. (2005). Oxytocin increases trust in humans. Nature 435, 673–676. doi: 10.1038/nature03701
Kovalchik, S., Camerer, C. F., Grether, D. M., Plott, C. R., and Allman, J. M. (2005). Aging and decision making: a comparison between neurologically healthy elderly and young individuals. J. Econ. Behav. Organ. 58, 79–94. doi: 10.1016/j.jebo.2003.12.001
Kunitake, Y., Imamura, Y., Mizoguchi, Y., Matsushima, J., Tateishi, H., Murakawa-Hirachi, T., et al. (2020). Serum oxytocin levels and logical memory in older people in rural Japan. J. Geriatr. Psychiatry Neurol. 34, 156–161. doi: 10.1177/0891988720915526
Kunzmann, U., and Grühn, D. (2005). Age differences in emotional reactivity: the sample case of sadness. Psychol. Aging 20, 47–59. doi: 10.1037/0882-7974.20.1.47
Larcom, M. J., and Isaacowitz, D. M. (2009). Rapid emotion regulation after mood induction: age and individual differences. J. Gerontol. B Psychol. Sci. Soc. Sci. 64B, 733–741. doi: 10.1093/geronb/gbp077
Lee, Y. K., and Chang, C. T. (2007). Who gives what to charity? Characteristics affecting donation behavior. Soc. Behav. Pers. 35, 1173–1180. doi: 10.1080/17470919.2019.1599421
Leng, G., and Sabatier, N. (2016). Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J. Neuroendocrinol. 28:10.1111/jne.12413. doi: 10.1111/jne.12413
Leppanen, J., Ng, K. W., Tchanturia, K., and Treasure, J. (2017). Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci. Biobehav. Rev. 78, 125–144. doi: 10.1016/j.neubiorev.2017.04.010
Li, S. C., Lindenberger, U., Hommel, B., Aschersleben, G., Prinz, W., and Baltes, P. B. (2004). Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol. Sci. 15, 155–163. doi: 10.1111/j.0956-7976.2004.01503003.x
Lin, P.-Y., Grewal, N. S., Morin, C., Johnson, W. D., and Zak, P. J. (2013). Oxytocin increases the influence of public service advertisements. PLoS One 8:e56934. doi: 10.1371/journal.pone.0056934
Loken, E., and Gelman, A. (2017). Measurement error and the replication crisis. Science 355, 584–585. doi: 10.1126/science.aal3618
MacLean, E. L., Wilson, S. R., Martin, W. L., Davis, J. M., Nazarloo, H. P., and Carter, C. S. (2019). Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology 107, 225–231. doi: 10.1016/j.psyneuen.2019.05.018
Matochik, J. A., Chefer, S. I., Lane, M. A., Woolf, R. I., Morris, E. D., Ingram, D. K., et al. (2000). Age-related decline in striatal volume in monkeys as measured by magnetic resonance imaging. Neurobiol. Aging 21, 591–598. doi: 10.1016/s0197-4580(00)00134-2
Matsumoto, Y., Yamagishi, T., Li, Y., and Kiyonari, T. (2016). Prosocial behavior increases with age across five economic games. PLoS One 11:e0158671. doi: 10.1371/journal.pone.0158671
Mayr, U., and Freund, A. M. (2020). Do we become more prosocial as we age, and if so, why? Curr. Dir. Psychol. Sci. 29, 248–254. doi: 10.1177/0963721420910811
McArdle, J. J., Hamgami, F., Jones, K., Jolesz, F., Kikinis, R. Spiro III, A., et al. (2004). Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 59, P294–P304. doi: 10.1093/geronb/59.6.p294
McCullough, M. E., Churchland, P. S., and Mendez, A. J. (2013). Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 37, 1485–1492. doi: 10.1016/j.neubiorev.2013.04.018
McCullough, M. E., Emmons, R. A., and Tsang, J.-A. (2002). The grateful disposition: a conceptual and empirical topography. J. Pers. Soc. Psychol. 82, 112–127. doi: 10.1037/0022-3514.82.1.112
Meaney, M. J., O’Donnell, D., Rowe, W., Tannenbaum, B., Steverman, A., Walker, M., et al. (1995). Individual differences in hypothalamic-pituitary-adrenal activity in later life and hippocampal aging. Exp. Gerontol. 30, 229–251. doi: 10.1016/0531-5565(94)00065-b
Midlarsky, E., Kahana, E., and Belser, A. (2015). “Prosocial behavior in late life,” in Oxford Library of Psychology. The Oxford Handbook of Prosocial Behavior, eds D. A. Schroeder and W. G. Graziano (Oxford: Oxford University Press), 415–432. doi: 10.1093/oxfordhb/9780195399813.013.030
Nelson, E. E., and Panksepp, J. (1998). Brain substrates of infant–mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev. 22, 437–452. doi: 10.1016/s0149-7634(97)00052-3
Neumann, I. D., and Landgraf, R. (2012). Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649–659. doi: 10.1016/j.tins.2012.08.004
Norman, G. J., Cacioppo, J. T., Morris, J. S., Malarkey, W. B., Berntson, G. G., and DeVries, A. C. (2011). Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol. Psychol. 86, 174–180. doi: 10.1016/j.biopsycho.2010.11.006
O’Brien, E., Konrath, S. H., Grühn, D., and Hagen, A. L. (2013). Empathic concern and perspective taking: linear and quadratic effects of age across the adult life span. J. Gerontol. B Psychol. Sci. Soc. Sci. 68, 168–175. doi: 10.1093/geronb/gbs055
Omoto, A. M., Malsch, A. M., and Barraza, J. A. (2009). “Compassionate acts: motivations for and correlates of volunteerism among older adults,” in The Science of Compassionate Love: Theory, Research, and Applications, eds B. Fehr, S. Sprecher, and L. G. Underwood (Hoboken, NJ: Wiley Blackwell), 257–282. doi: 10.1002/9781444303070.ch9
Parra-Rizo, M., and Sanchis-Soler, G. (2020). Satisfaction with life, subjective well-being and functional skills in active older adults based on their level of physical activity practice. Int. J. Environ. Res. Public Health 17:1299. doi: 10.3390/ijerph17041299
Plasencia, G., Luedicke, J. M., Nazarloo, H. P., Carter, C. S., and Ebner, N. C. (2019). Plasma oxytocin and vasopressin levels in young and older men and women: functional relationships with attachment and cognition. Psychoneuroendocrinology 110:104419. doi: 10.1016/j.psyneuen.2019.104419
Pornpattananangkul, N., Chowdhury, A., Feng, L., and Yu, R. (2019). Social discounting in the elderly: senior citizens are good samaritans to strangers. J. Gerontol. B Psychol. Sci. Soc. Sci. 74, 52–58. doi: 10.1093/geronb/gbx040
Pornpattananangkul, N., Zhang, J., Chen, Q., Kok, B. C., and Yu, R. (2017). Generous to whom? The influence of oxytocin on social discounting. Psychonueroendocrinology 79, 93–97. doi: 10.1016/j.psyneuen.2017.02.016
Procyshyn, T. L., Watson, N. V., and Crespi, B. J. (2020). Experimental empathy induction promotes oxytocin increases and testosterone decreases. Horm. Behav. 117:104607. doi: 10.1016/j.yhbeh.2019.104607
Rameson, L. T., Morelli, S. A., and Lieberman, M. D. (2012). The neural correlates of empathy: experience, automaticity, and prosocial behavior. J. Cogn. Neurosci. 24, 235–245. doi: 10.1162/jocn_a_00130
Raz, N., Williamson, A., Gunning-Dixon, F., Head, D., and Acker, J. D. (2000). Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc. Res. Tech. 51, 85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0
Reed, A. E., and Carstensen, L. L. (2012). The theory behind the age-related positivity effect. Front. Psychol. 3:339. doi: 10.3389/fpsyg.2012.00339
Rosen, J. B., Brand, M., and Kalbe, E. (2016). Empathy mediates the effects of age and sex on altruistic moral decision making. Front. Behav. Neurosci. 10:67. doi: 10.3389/fnbeh.2016.00067
Rosi, A., Nola, M., Lecce, S., and Cavallini, E. (2019). Prosocial behavior in aging: which factors can explain age-related differences in social-economic decision making? Int. Psychogeriatr. 31, 1747–1757. doi: 10.1017/S1041610219000061
Salthouse, T. A. (1992). Influence of processing speed on adult age differences in working memory. Acta Psychol. 79, 155–170. doi: 10.1016/0001-6918(92)90030-H
Salthouse, T. A. (1994). The aging of working memory. Neuropsychology 8, 535–543. doi: 10.1037/0894-4105.8.4.535
Salthouse, T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychol. Rev. 103, 403–429. doi: 10.1037/0033-295x.103.3.403
Salthouse, T. A. (2006). Mental exercise and mental aging: evaluating the validity of the “use it or lose it” hypothesis. Perspect. Psychol. Sci. 1, 68–87. doi: 10.1111/j.1745-6916.2006.00005.x
Samanez-Larkin, G. R., and Carstensen, L. L. (2011). “Socioemotional functioning and the aging brain”, in The Oxford Handbook of Social Neuroscience, eds J. Decety and Cacioppo, J. T. (New York, NY: Oxford: Oxford University Press), 507–521. doi: 10.1093/oxfordhb/9780195342161.013.0034
Saxbe, D., Khaled, M., Horton, K. T., and Mendez, A. J. (2019). Maternal prenatal plasma oxytocin is positively associated with prenatal psychological symptoms, but method of immunoassay extraction may affect results. Biol. Psychol. 147:107718. doi: 10.1016/j.biopsycho.2019.107718
Saxon, S. V., Etten, M. J., and Perkins, E. A. (2014). Physical Change and Aging: A Guide for the Helping Professions, 6th Edn. New York, NY: Springer.
Scheibe, S., and Carstensen, L. L. (2010). Emotional aging: recent findings and future trends. J. Gerontol. Series B Psychol. Sci. Soc. Sci. 65, 135–144. doi: 10.1093/geronb/gbp132
Shahrestani, S., Kemp, A. H., and Guastella, A. J. (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology 38, 1929–1936. doi: 10.1038/npp.2013.86
Shamay-Tsoory, S. G., Abu-Akel, A., Palgi, S., Sulieman, R., Fischer-Shofty, M., Levkovitz, Y., et al. (2013). Giving peace a chance: oxytocin increases empathy to pain in the context of the Israeli–Palestinian conflict. Psychoneuroendocrinology 38, 3139–3144. doi: 10.1016/j.psyneuen.2013.09.015
Simmons, W. O., and Emanuele, R. (2004). Does government spending crowd out donations of time and money? Public Financ. Rev. 32, 498–511. doi: 10.1177/1091142104264364
Sowell, E. R., Peterson, B. S., Thompson, P. M., Welcome, S. E., Henkenius, A. L., and Toga, A. W. (2003). Mapping cortical change across the human life span. Nat. Neurosci. 6, 309–315. doi: 10.1038/nn1008
Stanley, J. T., and Isaacowitz, D. M. (2011). Age-related differences in profiles of mood-change trajectories. Dev. Psychol. 47, 318–330. doi: 10.1037/a0021023
Stanley, J. T., and Isaacowitz, D. M. (2012). “Socioemotional perspectives on adult development,” in The Wiley-Blackwell Handbook of Adulthood and Aging, eds S. K. Whitbourne and Sliwinski, M. J. (Hoboken, NJ: Wiley Blackwell), 236–253). doi: 10.1002/9781118392966.ch12
Sun, B., Luo, Z., Zhang, W., Li, W., and Li, X. (2018). Age-related differences in affective and cognitive empathy: self-report and performance-based evidence. Aging Neuropsychol. Cogn. 25, 655–672. doi: 10.1080/13825585.2017.1360835
Sze, J. A., Gyurak, A., Goodkind, M. S., and Levenson, R. W. (2012). Greater emotional empathy and prosocial behavior in late life. Emotion 12, 1129–1140. doi: 10.1037/a0025011
Szeto, A., McCabe, P. M., Nation, D. A., Tabak, B. A., Rossetti, M. A., McCullough, M. E., et al. (2011). Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 73, 393–400. doi: 10.1097/PSY.0b013e31821df0c2
Ten Velden, F. S., Daughters, K., and De Dreu, C. K. (2017). Oxytocin promotes intuitive rather than deliberated cooperation with the in-group. Horm. Behav. 92, 164–171. doi: 10.1016/j.yhbeh.2016.06.005
Terris, E. T., Beavin, L. E., Barraza, J. A., Schloss, J., and Zak, P. J. (2018). Endogenous oxytocin release eliminates in-group bias in monetary transfers with perspective-taking. Front. Behav. Neurosci. 12:35. doi: 10.3389/fnbeh.2018.00035
Thornton, W. J., and Dumke, H. A. (2005). Age differences in everyday problem-solving and decision-making effectiveness: a meta-analytic review. Psychol. Aging, 20(1), 85.
Valstad, M., Alvares, G. A., Egknud, M., Matziorinis, A. M., Andreassen, O. A., Westlye, L. T., et al. (2017). The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 78, 117–124. doi: 10.1016/j.neubiorev.2017.04.017
Walhovd, K. B., Fjell, A. M., Reinvang, I., Lundervold, A., Dale, A. M., Eilertsen, D. E., et al. (2005). Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol. Aging 26, 1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020
Wierda, M., Goudsmit, E., Van der Woude, P. F., Purba, J. S., Hofman, M. A., Bogte, H., et al. (1991). Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol. Aging 12, 511–516. doi: 10.1016/0197-4580(91)90081-t
Wiles, J. L., and Jayasinha, R. (2013). Care for place: the contributions older people make to their communities. J. Aging Stud. 27, 93–101. doi: 10.1016/j.jaging.2012.12.001
Williamson, G. M., and Schulz, R. (1992). Pain, activity restriction, and symptoms of depression among community-residing elderly adults. J. Gerontol. 47, 367–372. doi: 10.1093/geronj/47.6.p367
Worthington, E. L. Jr., Wade, N. G., Hight, T. L., and Ripley, J. S. (2003). The religious commitment inventory—10: development, refinement, and validation of a brief scale for research and counseling. J. Couns. Psychol. 50, 84–96. doi: 10.1037/0022-0167.50.1.84
Zak, P. J., and Barraza, J. A. (2013). The neurobiology of collective action. Front. Neurosci. 7:211. doi: 10.3389/fnins.2013.00211
Zak, P. J., Kurzban, R., and Matzner, W. T. (2004). The neurobiology of trust. Ann. N. Y. Acad. Sci. 1032, 224–227.
Zak, P. J., Kurzban, R., and Matzner, W. T. (2005). Oxytocin is associated with human trustworthiness. Horm. Behav. 48, 522–527. doi: 10.1016/j.yhbeh.2005.07.009
Zak, P. J., Stanton, A. A., and Ahmadi, S. (2007). Oxytocin increases generosity in humans. PLoS One 2:e1128. doi: 10.1371/journal.pone.0001128
Ze, O., Thoma, P., and Suchan, B. (2014). Cognitive and affective empathy in younger and older individuals. Aging Ment. Health 18, 929–935. doi: 10.1080/13607863.2014.899973
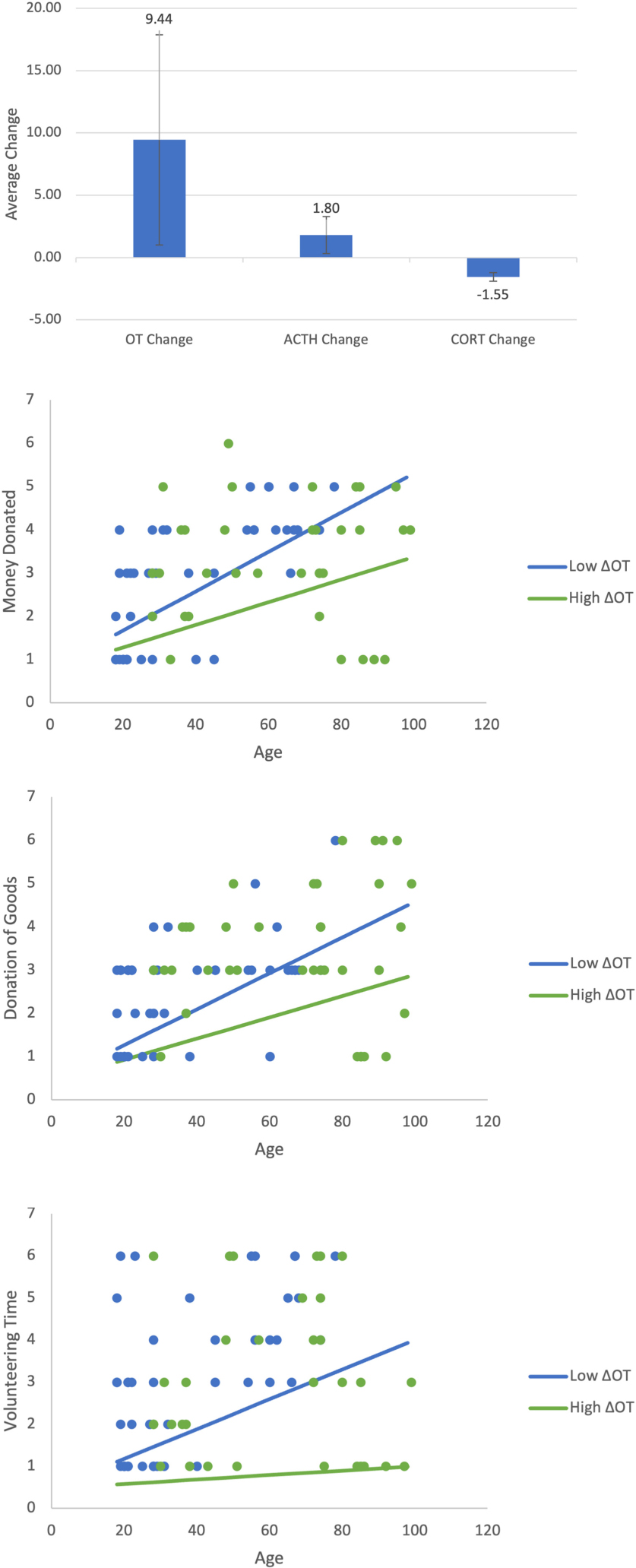
Appendix A1-A4. Shows the change in all three neurochemicals in response to the video stimulus. Show the moderating effect of age and ΔOT on retrospective prosocial behaviors. All three figures show that age increases prosocial behaviors irrespective of whether the positive change in ΔOT is high or low. This pattern is similar to that found for immediate donations. As in Figure 4, the low value of ΔOT is the value at the 40th percentile (4.05) and the high value is the 75th percentile (160.96).
Keywords: positive psychology, lifespan, neuroendocrinology, behavior, gratitude
Citation: Zak PJ, Curry B, Owen T and Barraza JA (2022) Oxytocin Release Increases With Age and Is Associated With Life Satisfaction and Prosocial Behaviors. Front. Behav. Neurosci. 16:846234. doi: 10.3389/fnbeh.2022.846234
Received: 30 December 2021; Accepted: 14 March 2022;
Published: 21 April 2022.
Edited by:
James Cherry, Boston University, United StatesCopyright © 2022 Zak, Curry, Owen and Barraza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul J. Zak, cGF1bC56YWtAY2d1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.