- 1Department of Anatomy, Medical School, Ilam University of Medical Sciences, Ilam, Iran
- 2Biotechnology and Medicinal Plants Research Center, Ilam University of Medical Sciences, Ilam, Iran
- 3Department of Pharmacology, Medical School, Ilam University of Medical Sciences, Ilam, Iran
The drug delivery system is valuable in the treatment of the disease. A nanopolymer as a thymol and Thymbra spicata release system was synthesized and its effects on morphine withdrawal syndrome in comparison with clonidine in rats were studied. The nanopolymer was characterized by different methods, namely, IR, HNMR, CNMR, GPC, DLS, and AFM. Thymol in T. spicata extract was assessed. The loading and release rate of thymol and T. spicata extract on the nanopolymer were evaluated by HPLC. The median lethal dose (LD50) of the T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer was studied. The frequency of jumping, rearing, and teeth chattering in naloxone-induced morphine withdrawal syndrome was studied. Synthesized nanopolymer was desirable as a carrier for the drug. The loaded amount of extract and thymol on nanopolymer was estimated 55 ± 3.2% and 48 ± 2.6% and the drug released was 71 and 68%, respectively. LD50 of the T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer was 975, 580, 1,250, and 650 mg/kg, respectively. This study showed that thymol nanopolymer was more effective than clonidine to reduce the frequency of morphine withdrawal symptoms. Our results suggest that T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer are mighty in reducing the narcotic withdrawal signs. The mechanism of action and therapeutic potential is maybe similar to clonidine.
Introduction
Many benefits, namely, improved drug solubility, stability, reduced clearance rate, increased drug exposure, and fewer side effects, have been reported for nanoscale drug delivery (Moghimi et al., 2005; Wagner et al., 2006; Kim et al., 2010). The size of the nanoparticles carrier plays an important role in the production of nanodrugs to improve biodistribution, tissue penetration, and function (Stano et al., 2012; Tang et al., 2014). The amount of drug-loaded on the nanoparticle depends on factors such as the branches of hydrophobic and hydrophilic polymers (Chu et al., 2013). Citric acid is one of the most inexpensive and compatible substances that is also used in the pharmaceutical and food industries (Naeini et al., 2010). In addition to citric acid, glycerol can be used to synthesize a biocompatible polymer (Lee et al., 2001). Fourier transforms infrared (FTIR), nuclear magnetic resonance (NMR), gel permeation chromatography (GPC), dynamic light scattering (DLS), and atomic force microscopy (AFM) are used to investigate the physicochemical properties of the produced polymeric nanoparticles (Adeli et al., 2013).
Today, dependence on opioids is a world health problem that makes many economic, social, and individual problems. In this regard, the use of nanocarriers loaded with naloxone has been considered in the treatment of opioid overdose (Hasan et al., 2021). Nevertheless, the challenge of using nanoparticles loaded with a bioactive component of medicinal plants remains.
Withdrawal symptoms in morphine addicts are relatively severe and include hypertension, diarrhea and vomiting, and dysphoria (Ballantyne and LaForge, 2007). Mu-, kappa-, and delta-opioid receptor agonists, such as methadone, are effective in helping to quit an addiction with analgesic effects, but there are side effects such as constipation, and respiratory depression, physical dependence, and addiction themselves (Li and Zhang, 2012). However, common pharmacological treatments for withdrawal syndrome include MOR agonists (methadone) or multifunctional opioid/nociception-OFQ agonists (buprenorphine) (Kreek et al., 2002).
Medicinal herbs with a variety of active components have been used potentially in the treatment of withdrawal syndrome (Ward et al., 2011; Tabatabai et al., 2014). Numerous studies have shown that various medicinal plants such as Passiflora incarnate, Hypericum perforatu, Avena sativa, and Valeriana officinalis, with their antianxiety, hypnotic, analgesic, and antispasmodic effects, have improved the symptoms of withdrawal syndrome (Ebrahimie et al., 2015). Although there was no evidence of the morphine withdrawal and anti-anxiety effect of Thymbra spicata extract in the previous studies, T. capitata (L.) extract of the same family had a significant antianxiety effect (Motti and de Falco, 2021). The anti-analgesic and antinociceptive effects of the Thymus vulgaris L. extract have also been documented (Rašković et al., 2020). Many medicinal plant oils such as T. spicata, Thymus vulgaris, Monarda fistulosa, Trachyspermum ammi, Thymus ciliates, and Nigella sativa, contain a monoterpene named thymol (Wattanasatcha et al., 2012), which can be used as a food additive under the Food and Drug Administration regulations (Dhaneshwar et al., 2013). Due to the chemical structure of thymol, it can be used to produce nanoparticles to treat various diseases (Jafari et al., 2018; Robledo et al., 2018). Thymol has previously been shown to attenuate the signs and symptoms of an opiate withdrawal syndrome in humans (Khodayar et al., 2014). Thymol not only has been able to reduce the anxiety associated with diazepam withdrawal but also improves motor and memory impairment in rats (Saleem et al., 2021). In this study, in addition to synthesizing a nanopolymer and binding it with thymol, the effects and possible mechanism on morphine withdrawal syndrome in rats were investigated.
Materials and Methods
Preparation of Nanopolymer
By melt polycondensation method, citric acid and glycerol (Carlo Erba) for the synthesis of branched polymer, were combined and dissolved in tetrahydrofuran (THF) (Merck) and filtered. The solution was precipitated in cyclohexane (Merck) and dissolved in THF and then placed in a dialysis bag (Mn cutoff 2000, Sigma-Aldrich) (Adeli et al., 2013). Then, oleic acid was poured into a polymerization capsule and heated at 90, 100, 120, 140, and 160°C, respectively.
Fourier Transforms Infrared Spectroscopy
The nanopolymer IR spectra were performed with an FT–IR (Nicolet 320 spectrophotometer). The spectra were obtained at a resolution of 4 cm–1 in the range 4,000–500 cm–1 (Dong and Gu, 2002).
Nuclear Magnetic Resonance
Identical NMR spectra were obtained by dissolving samples in D2O (Bruker DRX 400 MHz) and the spectra were recorded at 500 MHz. The resulting data were analyzed using ACDLABS/1D NMR software (Neri et al., 1989).
Gel Permeation Chromatography
Using Gel permeation chromatography (Knauer equipped with Smartline Pump 1,000 with an PL Aqua gel-OH mixed-H 8 μm column), molecular weights and distribution of the obtained nanopolymer were determined (Lee, 1978).
Dynamic Light Scattering
Dynamic light scattering data (Malvern Instruments Ltd., United Kingdom were measured in water three times (5 runs to each measurement). The mean size was accounted as the six measurements average (Brown et al., 1992).
Atomic Force Microscopy
A multimode scanning probe microscope (Ara-research Inc., Iran) for atomic force microscopy (AFM) was used and the nanopolymer morphology was determined. The nanopolymer suspension (a droplet) was dried by a freeze dryer (Christ, Germany) onto a clean surface of mica before AFM imaging. The size of the images was 5 × 5 μm. The images were scanned on at least six different areas of the sample (Rezayati Charani et al., 2013).
Thymol Identification by High-Performance Liquid Chromatography
The aerial parts of Thyme spicata were collected from Ilam, Iran, and identity was authenticated by the voucher specimens (NO. 596) deposited in the Department of Horticulture, Faculty of Agriculture, Ilam University. After drying and powdering, the powder was degreased by hexane and 20 g of the plant was used for extraction by a Soxhlet extraction method in a water–ethanol solvent. The plant powder was loaded into the porous cellulose thimble (20 g in a 25- × 80-mm thimble), which was placed inside the Soxhlet extractor. The sidearm was lagged with glass wool. The solvent (250 ml of water–ethanol) was heated and evaporated. By rotary (IKA HB 10, Germany) device, the solvents were removed. The extraction (yield = 5.67%) was lyophilized and kept stored at –20°C and were dissolved in methanol (Lopez et al., 2013). According to the previous procedure, the high-performance liquid chromatography (HPLC) method was done (Aghamohammadi et al., 2016). A reversed-phase UV-HPLC (Smart line; Knauer, Germany) with a C18 column (Nucleosil H.P.; 25 cm × 0.46 cm internal diameter, particle size 3 μm, 100 Å pore size) was developed for the thymol determination. The T. spicata extract peaks were compared with the thymol standard (Sigma Aldrich). A stock solution (0.1 mg/ml) of thymol standard diluted to obtain 15.6, 31.25, 62.5, 125, 250, and 500 μM (Moayeri et al., 2018).
Thymol Encapsulation
Nanopolymer (0.1 g, 1.67 × 10–2 mM) was dissolved in distilled water (5 ml) and stirred for 1 h. Then, thymol and extract dissolved in DMSO (Merck) were added dropwise to a nanopolymer mixture and various concentrations (25, 50, 100, and 150 μM) were obtained (Adams et al., 2003).
Releasing Capacity Assay
Loading capacity was determined by the HPLC method (Lv et al., 2009) and estimate the amount of thymol and extract loaded on the nanopolymer. The mobile phase was 40% methanol and 60% aqueous solution of formic acid (0.1%) (Majumdar et al., 2014). The solution aliquot was injected into the HPLC.
In vitro release of thymol and extract from nanopolymer was done by dissolving of thymol (5 mg) and thymol in extract-loaded nanopolymer in PBS (3 ml, 0.1 M, pH 7.4). At the sampling time, the release medium was replaced by a fresh buffer and injected into the HPLC (Qiao et al., 2005).
Animals
Rats (male albino, 180–260 g, Institute of Medicinal Plants, ACECR, Karaj, Iran) were used. The rats were maintained at standard conditions (22–25°C, 12 h dark-light cycle, standard rodent feed, and water). In total, eight rats were used for each dose of the thymol, extracts, and drugs (n = 3). The method grouping to study morphine withdrawal syndrome was as Table 1.
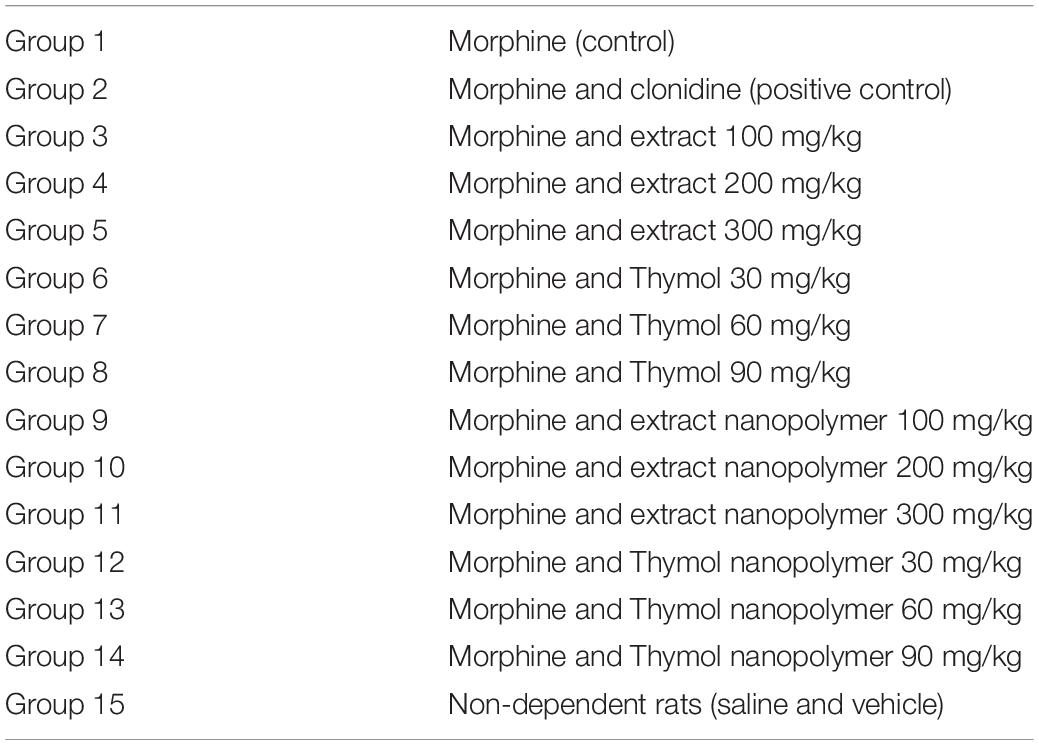
Table 1. Animals grouping and Thymbra spicata extract, thymol, extract nanopolymer, and thymol nanopolymer used to create dependence and morphine withdrawal syndrome.
Ethical Approval
The study was approved by the Ilam University of Medical Sciences, Ilam, Iran. The ethical approval for this study was obtained from the Animal Care and Ethics Committee (ACEC) of the Ilam University of medical science (IR.MEDILAM.REC.1396.81).
The Median Lethal Dose
To obtain the median lethal dose, different doses of the T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer were administered (orally gavage, 20 rats in each group). The doses were 50, 200, 400, 600, 800, 1,000, 1,600, and 2,400 mg/kg for T. spicata extract and extract nanopolymer and 50, 100, 200, 300, 400, 500, 600, and 700 mg/kg for thymol and thymol nanopolymer, respectively. Then, 72 h after gavage, the median of dead animals was calculated. Mortality graphs were plotted between log-concentration vs. percent mortality and the LD50 was determined (Parmar and Prakash, 2006; El Kabbaoui et al., 2017). Therefore, the T. spicata extract and extract nanopolymer with doses of 100, 200, 300 mg/kg and thymol and thymol nanopolymer with doses of 30, 60, and 90 mg/kg was selected to continue the experiments because none of the used doses caused mortality and were considered in the ED50 range.
Method of Morphine Dependency
Morphine (Darou Pakhsh Pharmaceutical Company, Iran) was injected subcutaneously into the rats two times daily for 7 days. On days 1 and 2, a dose of morphine (2.5 mg/kg) was used two times daily and doubled every day until the day 6, reaching 40 mg/kg. Rats received the last dose (50 mg/kg) on day 7 (Romandini et al., 1984).
Withdrawal Syndrome Method
In all the groups, 4 h after the last dose of morphine, animals received naloxone (3 mg/kg, i.p., Sigma-Aldrich Company, United States). Immediately, the morphine withdrawal symptoms manifestations, namely, jumping, rearing, and teeth chattering, were monitored for half an hour (Feily and Abbasi, 2009).
Animals of all groups received intraperitoneally 3 mg/kg naloxone 4 h after the last injection of morphine on day 7 of the morphine treatment. Immediately after the naloxone injection, each animal was placed in a transparent acrylic cylinder to observe the frequency of withdrawal manifestations (jumping, rearing, and teeth chattering) for 30 min.
Coadministration Use of Thymbra spicata Extract, Thymol, Extract Nanopolymer, Thymol Nanopolymer on Morphine Withdrawal Syndrome
Rats in groups 3, 4, 5, and 15 received T. spicata extract (100, 200, and 300 mg/kg, NG tube), and normal saline, and received thymol at 30, 60, and 90 mg/kg in groups 6, 7, and 8, respectively. Rats in groups 9, 10, and 11 received extract nanopolymer (100, 200, 300 mg/kg, NG tube), and received thymol nanopolymer at 30, 60, and 90 mg/kg in groups 12, 13, and 14, respectively, simultaneous with morphine (two times daily for 6 days). To challenge examine, 4 h before naloxone injection, animals in treatment groups received the last dose on day 70, and group 15 received saline with morphine.
Method of Coadministration Use of Clonidine Hydrochloride and Morphine on Morphine Dependency and Withdrawal Syndrome
Clonidine hydrochloride (0.2 mg/kg i.p.) was used concomitantly with morphine two times daily (6 days) in group 2. The animals received the last dose on day 7, 4 h before receiving naloxone, to examine jumping, rearing, and teeth chattering.
Statistical Analysis
The results were shown as mean ± standard error (SEM). One-way ANOVA followed by Tukey post hoc test was used for data analysis.
Results
Nanopolymer Evaluation
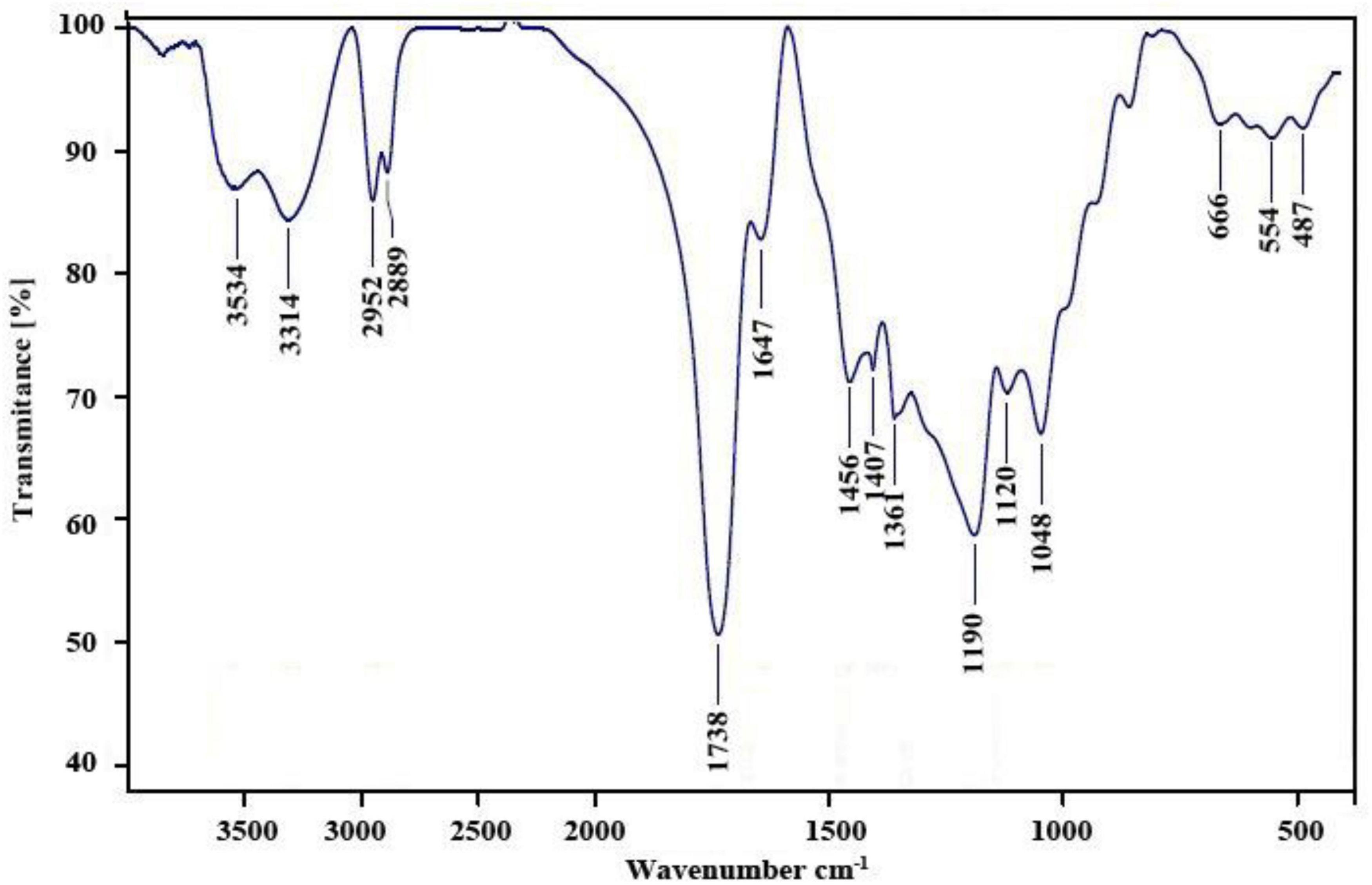
The FT–IR spectrum of the nanopolymer compound was shown (Figure 1). The peak related to the carbonyl ester group, hydroxyl groups, and carbonyl group appears at 1,647, 3,314, and 1,738 cm–1, respectively.
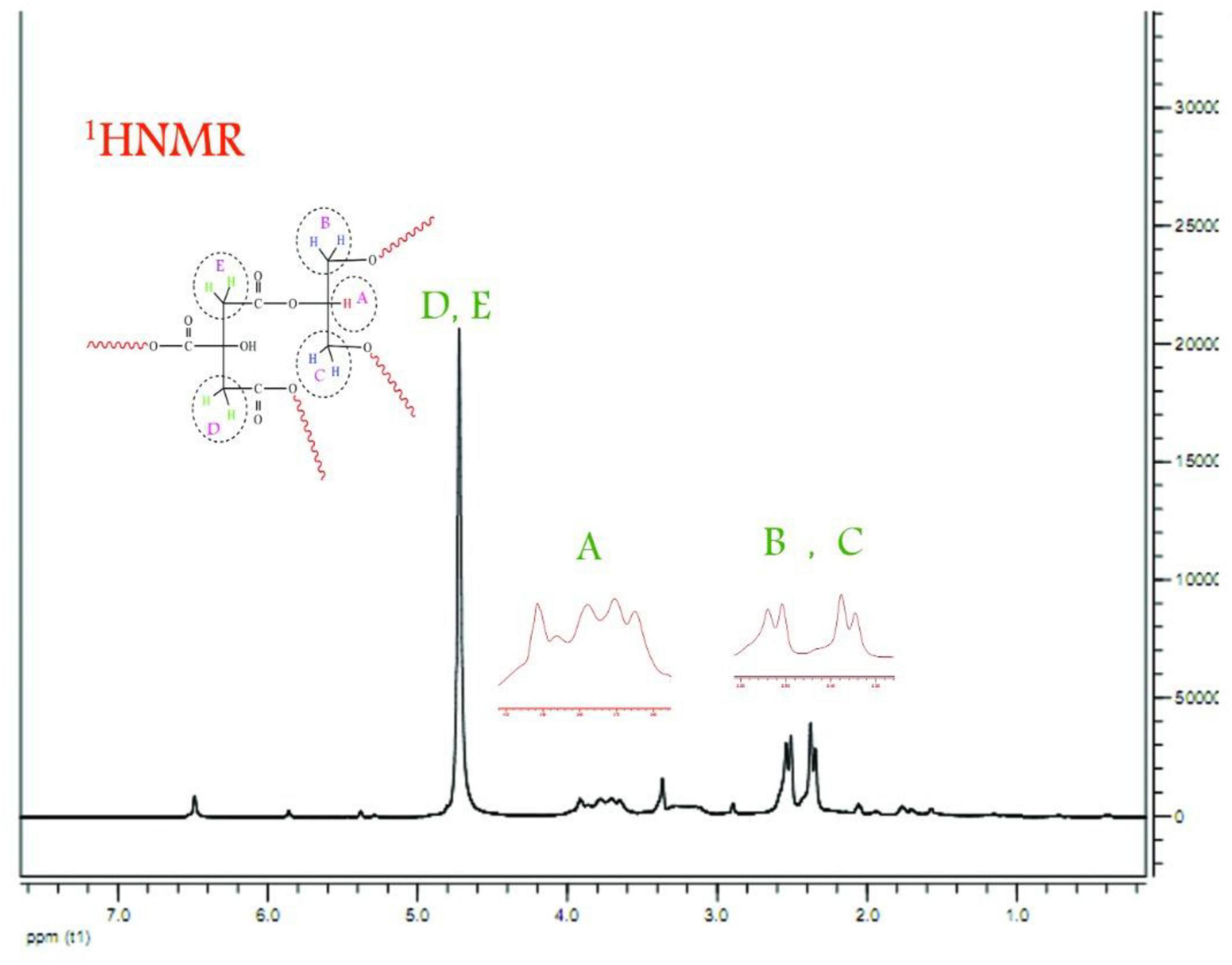
The nanopolymer 1HNMR spectrum was shown (Figure 2). Hydrogen A was carbonyl group methane in the oleic acid monomer (3.82 ppm). Hydrogen B was monomer glycerol (2.68 ppm). Hydrogen C was methylated glycerol. Hydrogens D and E were the citric acid hydrocarbon content (3.36 ppm).
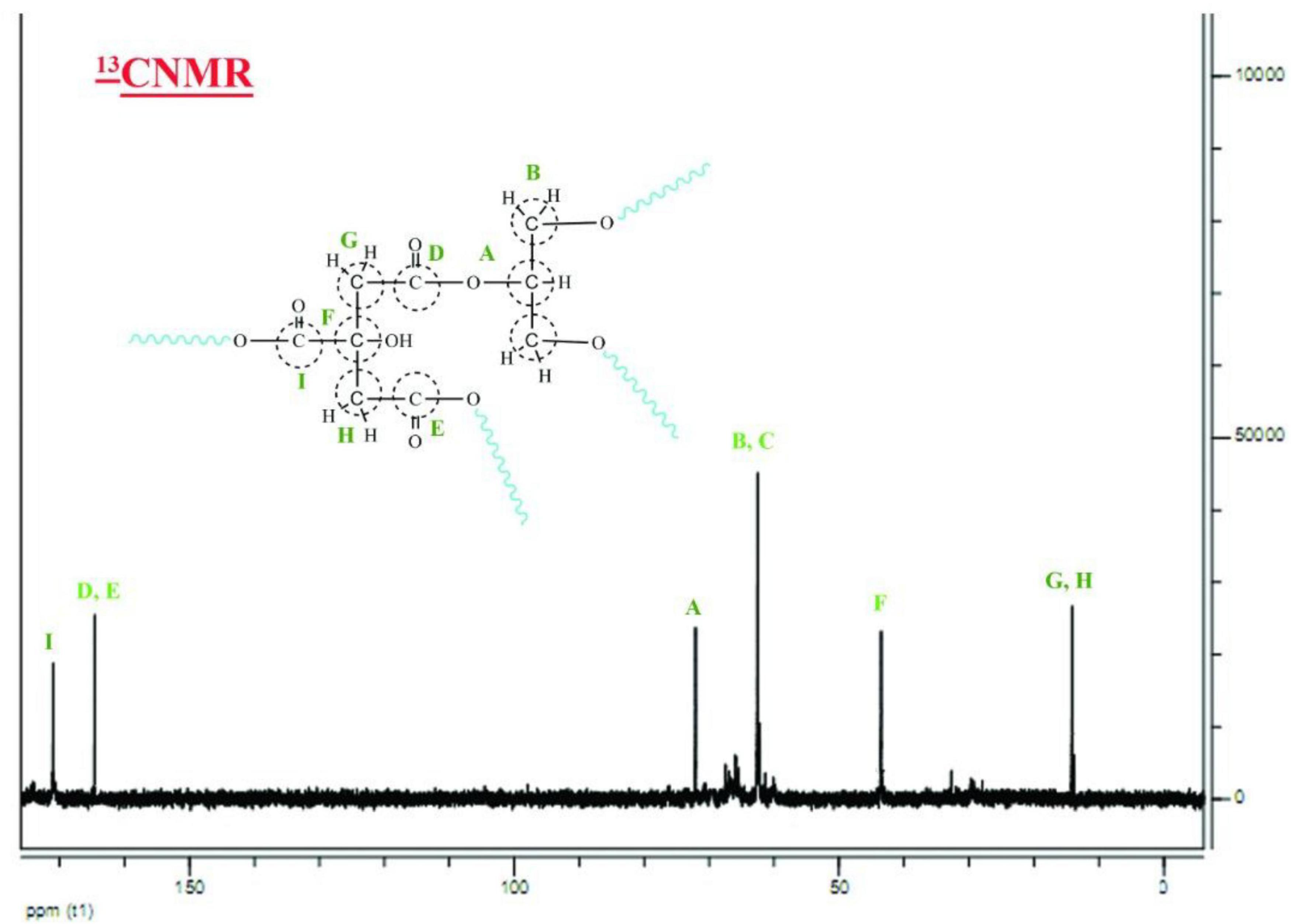
The nanopolymer 13CNMR spectrum was presented (Figure 3). This combination had a 9-peak index in the carbon compounds of this composition (letters A–I). Carbon A the middle carbon of glycerol (74 ppm). Carbons B and C were methyl glycerol carbonates (68 ppm). Carbons D and E were the two Carboniferous carbon (172 ppm). Carbon F was the fourth type of citric acid (44 ppm). Carbons G and H were the methyl group (13 ppm). Carbon G was the carbonyl oleic acid group (183 ppm).
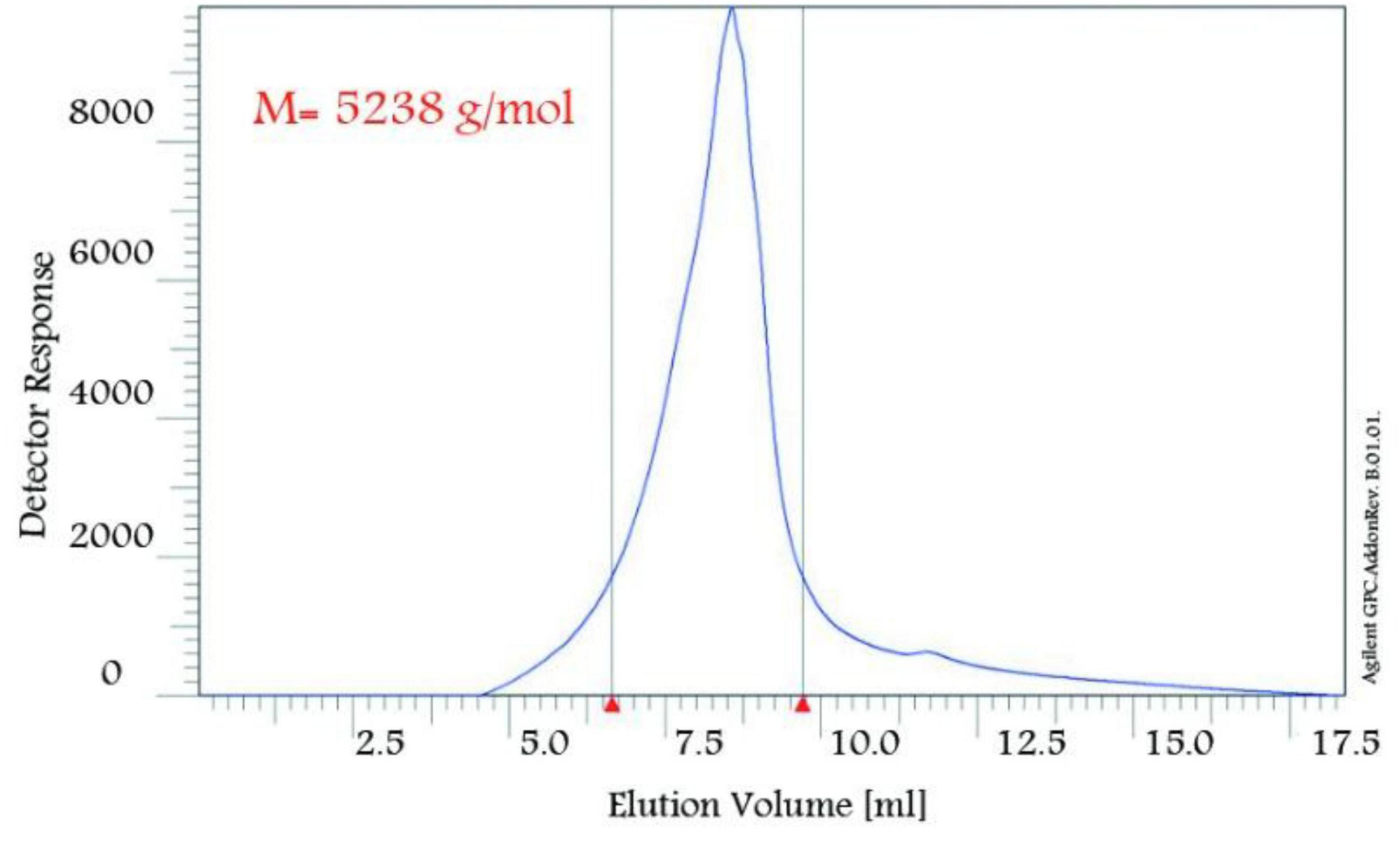
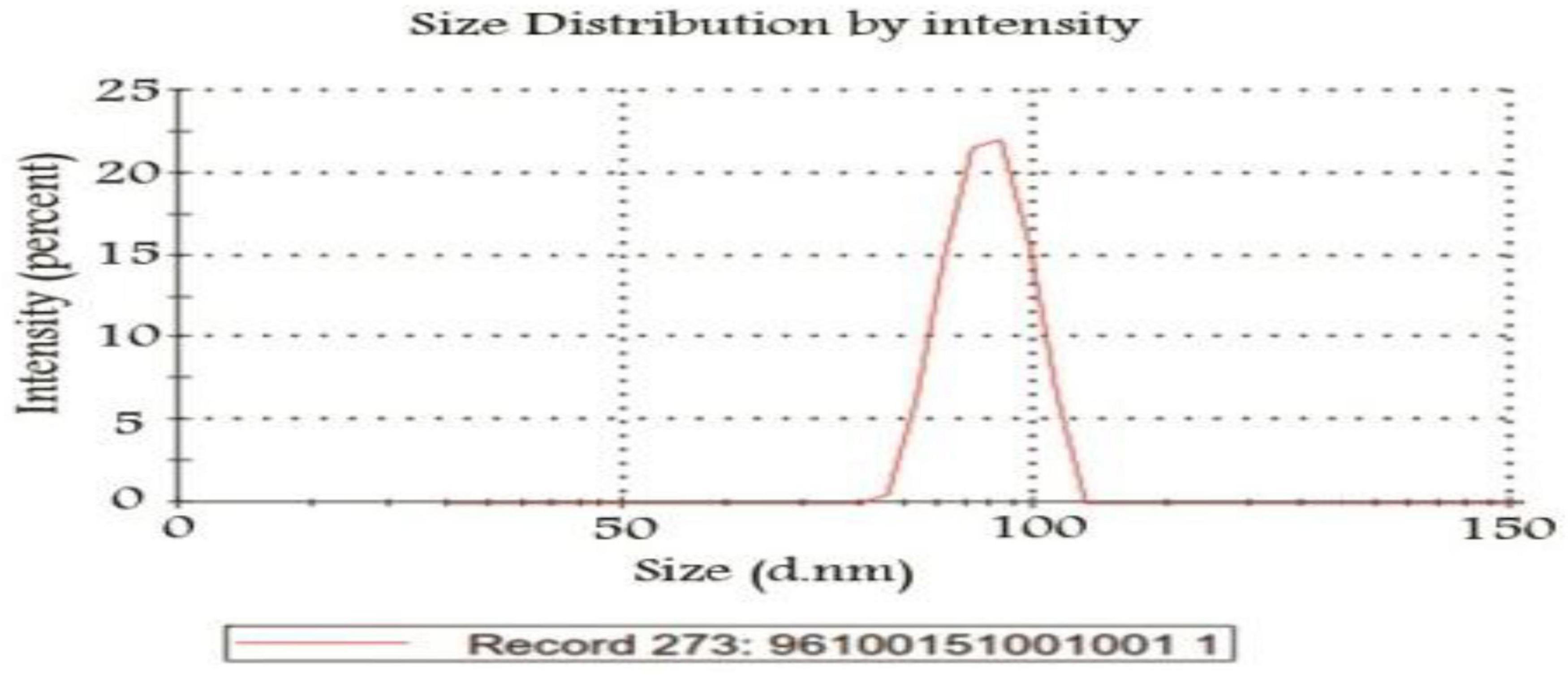
The nanopolymer GPC diagram was shown in Figure 4. This molecular weight was 5,238 g/mol. The size of the nanopolymer was monitored (in water, 25°C) by DLS (Figure 5). The peak width indicated a particle size sample solution larger than 75 nm, but the diameter of most particles was estimated to be about 100 nm.
The size of the nanopolymer was monitored (in water, 25°C) by DLS (Figure 5). The peak width indicated a particle size sample solution larger than 75 nm, but the diameter of most particles was estimated to be about 100 nm.
The nanopolymer AFM results allowed morphology at the micro-level (Figures 6A–C). As shown in Figure 6C, its length and width were with a cross-sectional profile in which interchange of heterogeneous wrinkles was visible (Mansouri et al., 2013).
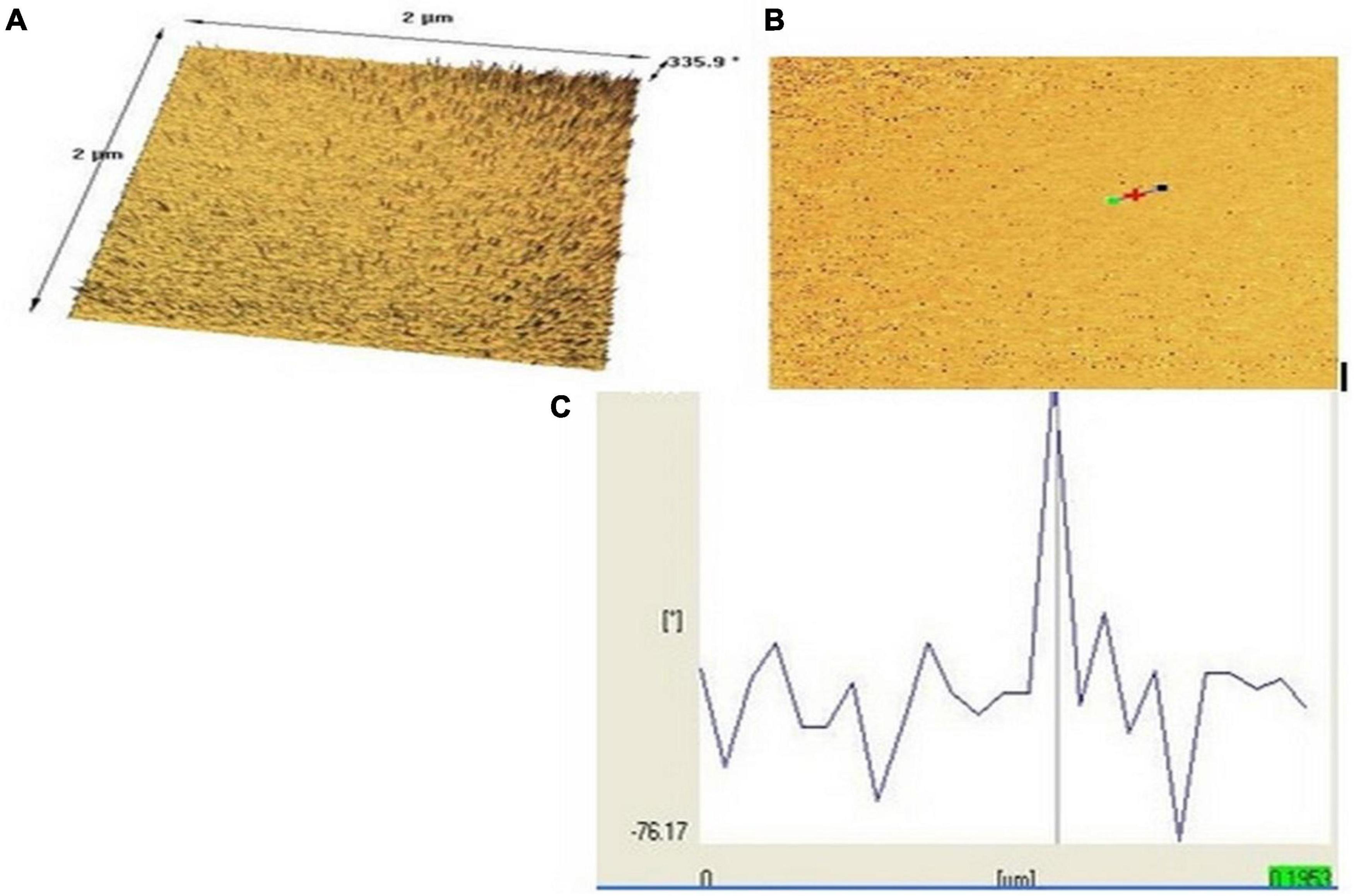
Figure 6. Nanopolymer AFM images of surface morphology [top (A), bottom (B), and (C) topographical view]. (*) has been done by the operator of the atomic force microscopy (AFM) to indicate the particle distance and dimensions.
Extraction and Identification
The HPLC chromatogram of thymol was obtained (retention time = 5.400 min and wavelength = 352 nm). The standard chromatogram of T. spicata extract was similar to that of standard thymol in 5.480 min (in the same conditions, Figures 7A,B). The quantitative analysis revealed that thymol was 3.65 mg/g of T. spicata extract. The method was calibrated with the linear curve (R2 = 0.9989, y = 187,938 × –3,896.03). LOD and LOQ were 52.09 and 173.63, respectively.
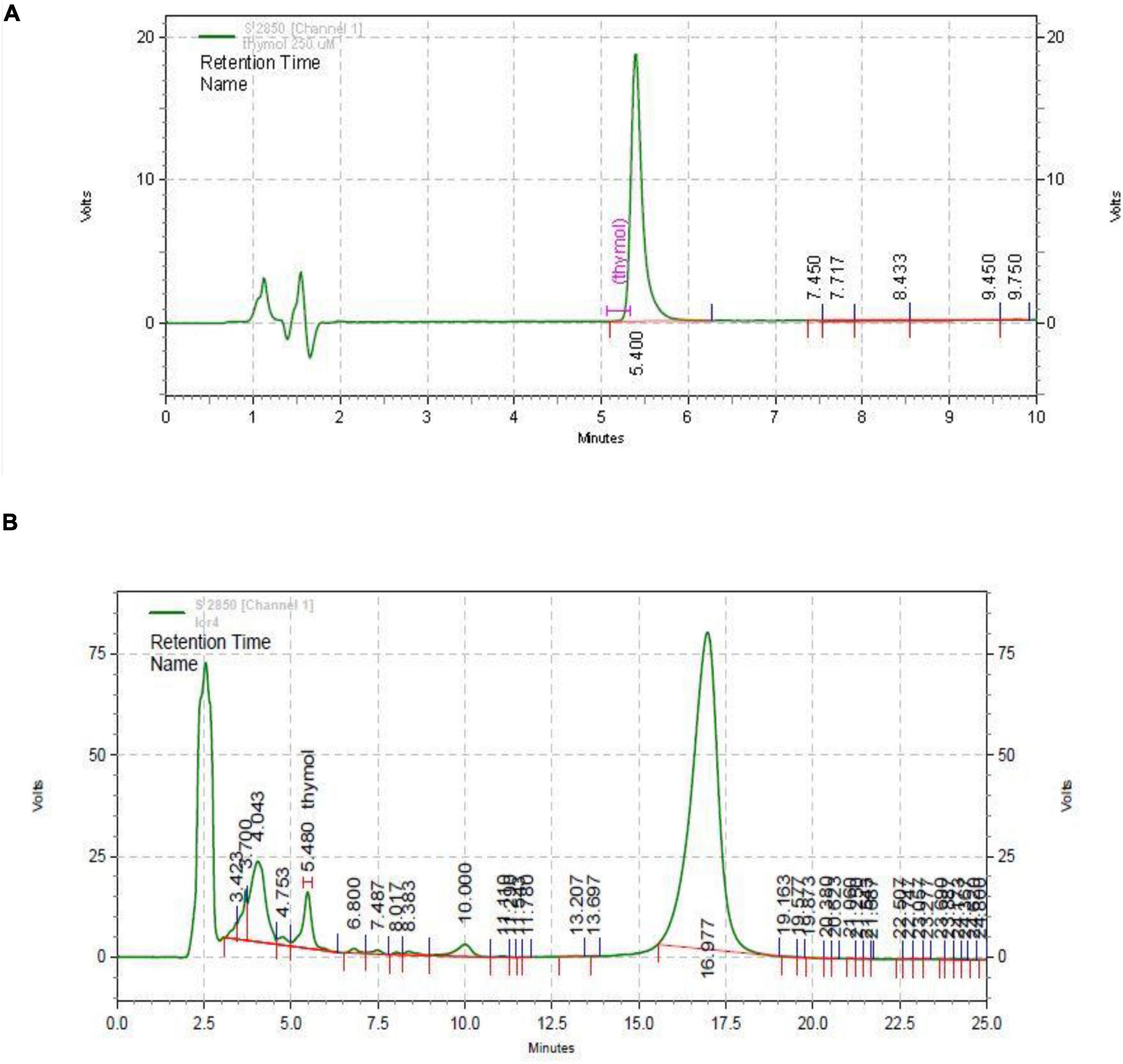
Figure 7. HPLC chromatogram of thymol standard (A) and Thymbra spicata extract (B). HPLC conditions were the same.
Load and Release Evaluation
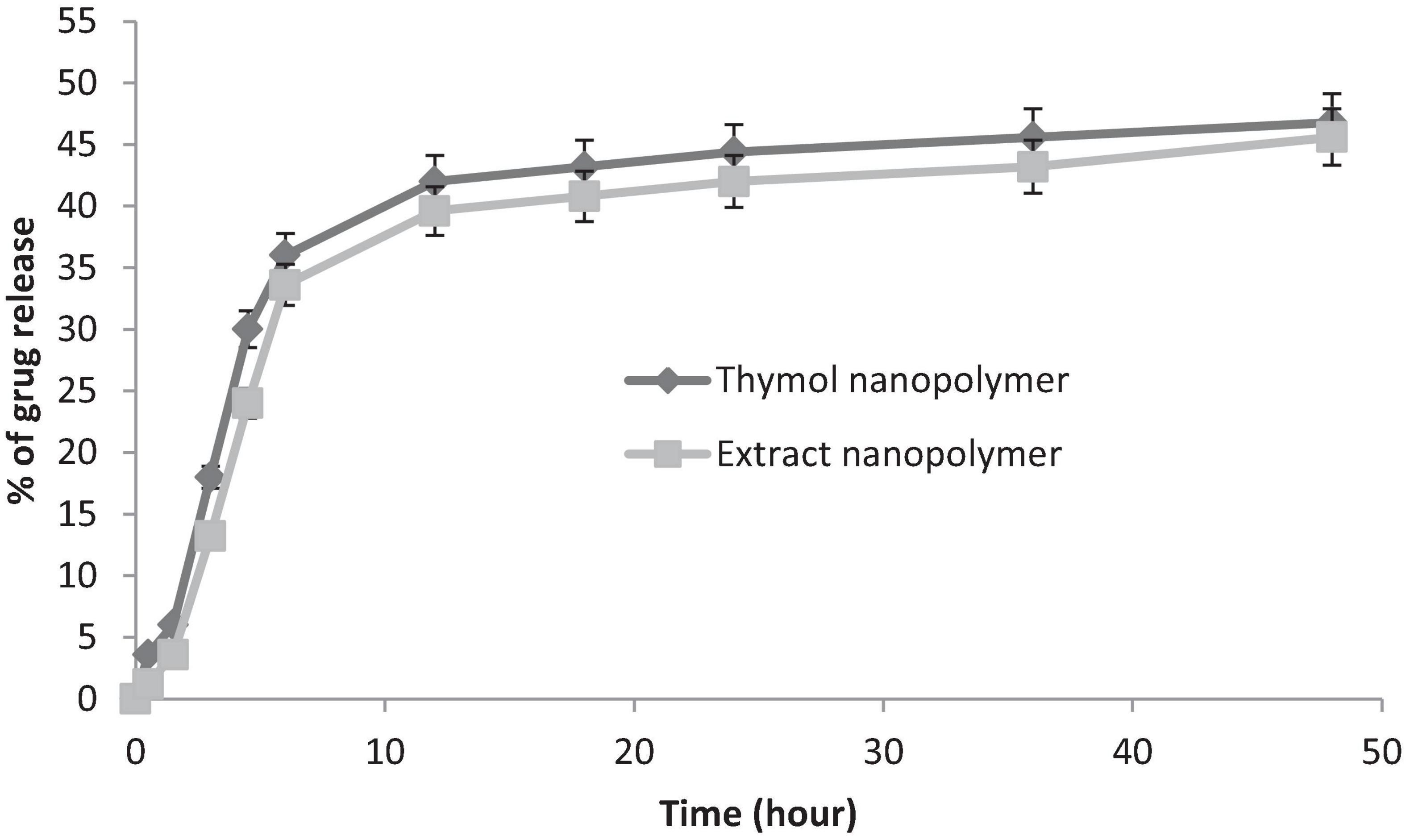
The loading and release capacity of thymol and thymol in the extract was evaluated by HPLC. The loading rate of thymol and extract was estimated at 55 ± 3.2% and 48 ± 2.6%, respectively, (n = 3).
Thymol and extract were released in two phases (fast and slow) of the nanopolymer. Near to 71% of loaded thymol and 68% of loaded extract in the first 12 h and the remaining 90–92% to 48 h were released at a lower rate (Figure 8).
Mortality Determination
LD50 values of the T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer were 975, 580, 1,250, and 650 mg/kg, within 72 h after administration, respectively. No mortality was observed in the treatment range used in this study.
Effect of the Thymbra spicata Extract, Thymol, Extract Nanopolymer, and Thymol Nanopolymer on the Naloxone-Induced Jumping in Morphine-Dependent Rats
Administration of 200 and 300 mg/kg of T. spicata extract and extract nanopolymer (p < 0.05 vs. control and p < 0.001 vs. control, respectively) and all doses of thymol and thymol nanopolymer (30, 60, and 90 mg/kg) decreased the jumps number in morphine-dependent rats significantly (p < 0.001 vs. control) precipitated by naloxone administration (5 mg/kg, i.p.) after the morphine last dose. The effect of T. spicata extract and extract nanopolymer was dose-dependent. Clonidine also reduced the jumps number as expected (Figure 9).
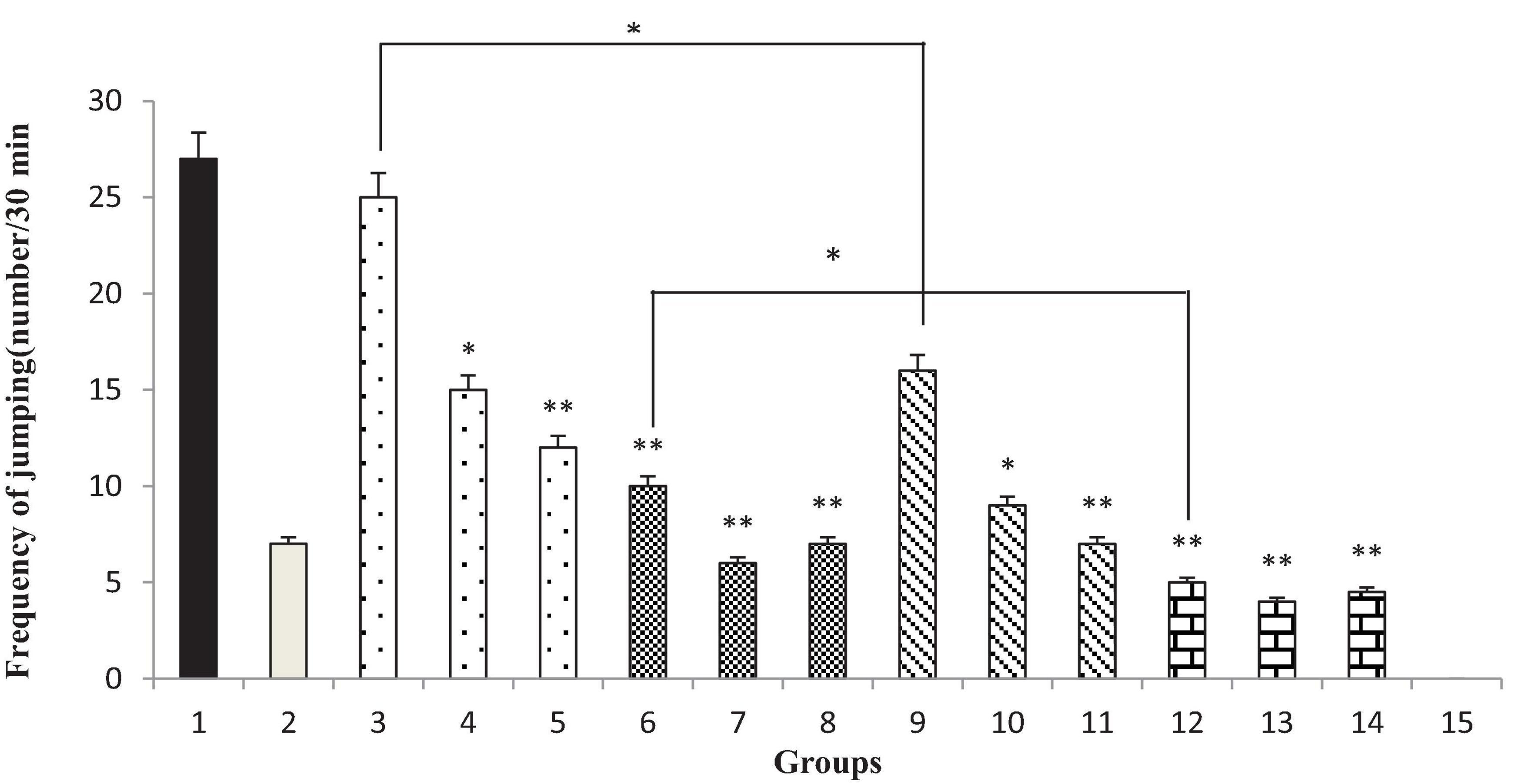
Figure 9. The jumping frequency (number of jumping/30 min) in rats. Group 1; morphine-dependent rats (control). Group 2; morphine-dependent rats and coadministration of clonidine hydrochloride (0.2 mg/kg i.p.) (positive control). Groups 3, 4, and 5; morphine-dependent rats and the coadministration of 100, 200, and 300 mg/kg Thymbra spicata extract, respectively. Groups 6, 7, and 8; morphine-dependent rats and coadministration of 30, 60, and 90 mg/kg thymol, respectively. Groups 9, 10, and 11; morphine-dependent rats and coadministration of 100, 200, and 300 mg/kg extract nanopolymer, respectively. Groups 12, 13, and 14; morphine-dependent rats and coadministration of 30, 60, and 90 mg/kg thymol nanopolymer, respectively. Group 15; non-dependent rats (saline). Data are expressed as mean ± SEM. *P < 0.01 vs. control, **P < 0.001 vs. control.
Effect of the Thymbra spicata Extract, Thymol, Extract Nanopolymer, and Thymol Nanopolymer on the Naloxone-Induced Rearing in Morphine-Dependent Rats
Thymbra spicata extracts and extracts nanopolymer at different doses (200 and 300 mg/kg) and thymol and thymol nanopolymer (30, 60, and 90 mg/kg) decreased the rearing in morphine-dependent rats precipitated by administration of naloxone (5 mg/kg, i.p.) after the last dose of morphine (p < 0.001 vs. control). The effect of T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer was dose-dependent. As expected, clonidine reduced the rearing number in animals (Figure 10).
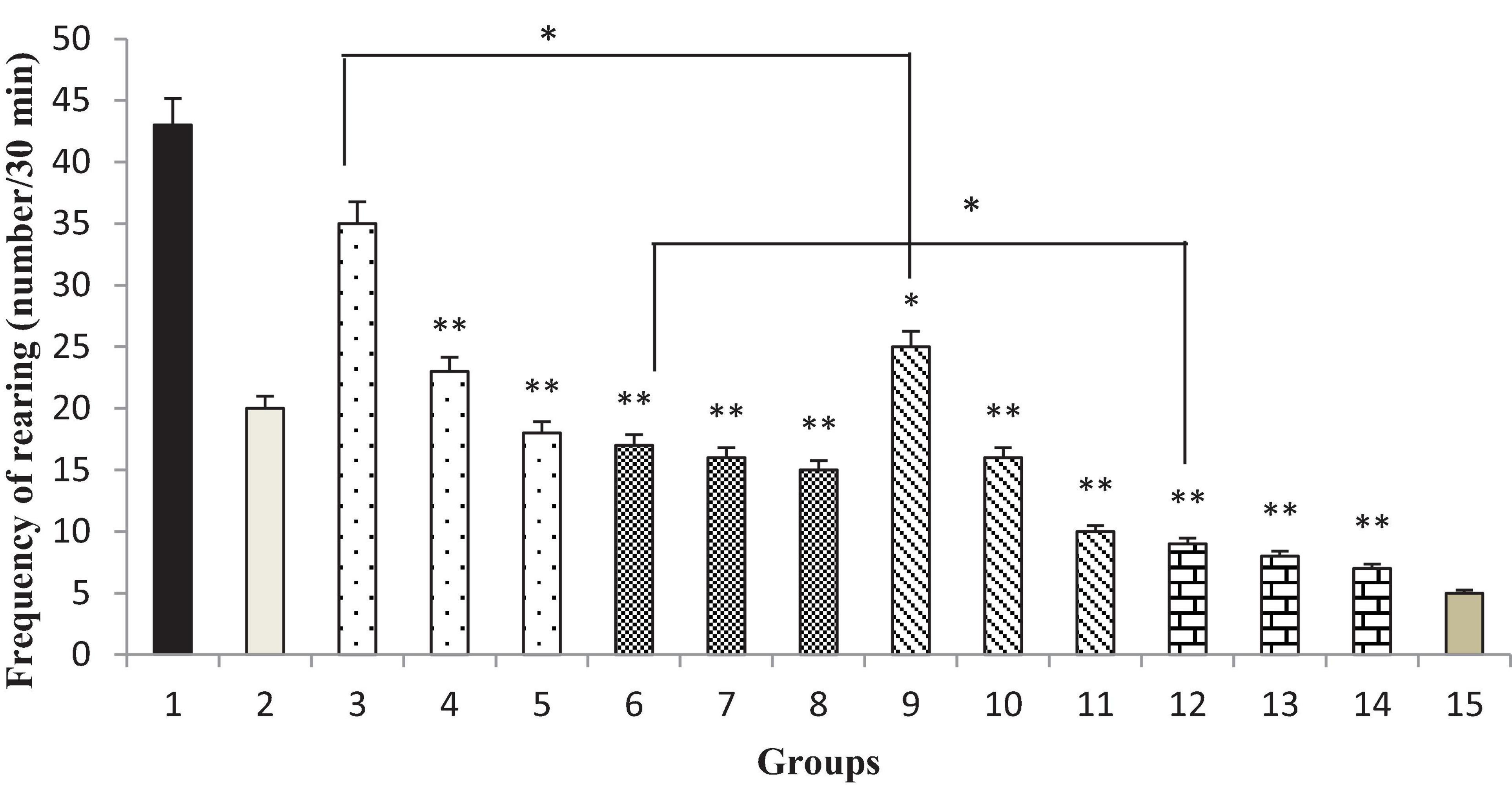
Figure 10. The frequency (number of rearing/30 min) of rearing in rats. Group 1; morphine-dependent rats (control). Group 2; morphine-dependent rats and coadministration of clonidine hydrochloride (0.2 mg/kg i.p.) (positive control). Groups 3, 4, and 5; morphine-dependent rats and the coadministration of 100, 200, and 300 mg/kg Thymbra spicata extract, respectively. Groups 6, 7, and 8; morphine-dependent rats and coadministration of 30, 60, and 90 mg/kg thymol, respectively. Groups 9, 10, and 11; morphine-dependent rats and coadministration of 100, 200, and 300 mg/kg extract nanopolymer, respectively. Groups 12, 13, and 14; morphine-dependent rats and coadministration of 30, 60, and 90 mg/kg thymol nanopolymer, respectively. Group 15; non-dependent rats (saline). Data are expressed as mean ± SEM. *P < 0.01 vs. control, **P < 0.001 vs. control.
Effect of the Thymbra spicata Extract, Thymol, Extract Nanopolymer, and Thymol Nanopolymer on the Naloxone-Induced Teeth Chattering in Morphine-Dependent Rats
Different doses of the T. spicata extract and extract nanopolymer (200 and 300 mg/kg) and thymol and thymol nanopolymer (30, 60, and 90 mg/kg) morphine-dependent rats decreased the teeth chattering in precipitated by administration of naloxone (5 mg/kg, i.p.) after the morphine last dose (p < 0.001 vs. control). The effect of T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer was dose-dependent. Also, clonidine reduced the teeth chattering number (Figure 11).
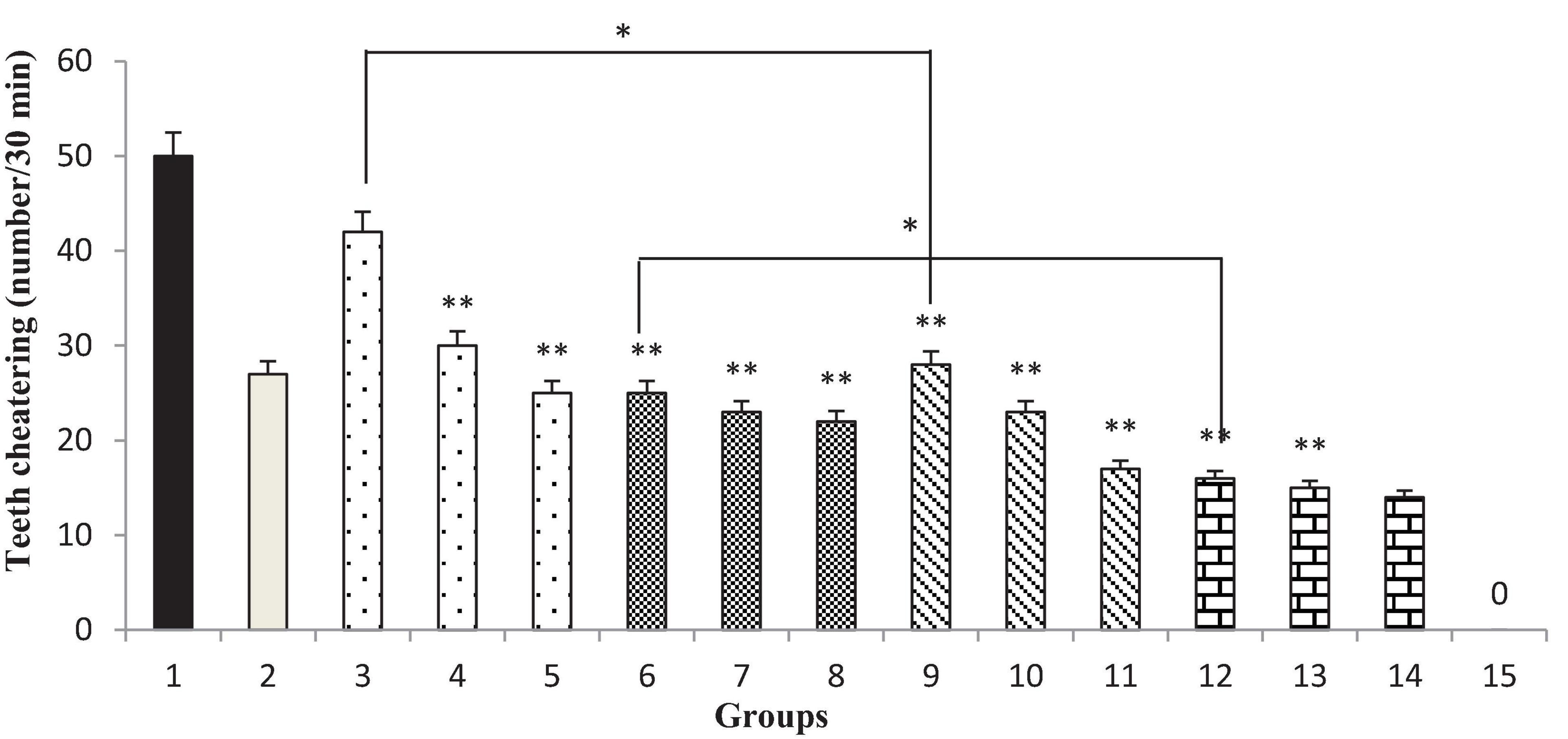
Figure 11. The frequency (number of teeth chattering/30 min) of teeth chattering in rats. Group 1; morphine-dependent rats (control). Group 2; morphine-dependent rats and coadministration of clonidine hydrochloride (0.2 mg/kg i.p.) (positive control). Groups 3, 4, and 5; morphine-dependent rats and the coadministration of 100, 200, and 300 mg/kg T. spicata extract, respectively. Groups 6, 7, and 8; morphine-dependent rats and coadministration of 30, 60, and 90 mg/kg thymol, respectively. Groups 9, 10, and 11; morphine-dependent rats and coadministration of 100, 200, and 300 mg/kg extract nanopolymer, respectively. Groups 12, 13, and 14; morphine-dependent rats and coadministration of 30, 60, and 90 mg/kg thymol nanopolymer, respectively. Group 15; non-dependent rats (saline). Data are expressed as mean ± SEM. * P < 0.01 vs. control, **P < 0.001 vs. control.
Discussion
This study showed that the design of drug delivery systems and the production of nanodrugs of T. spicata and its bioactive component thymol can be used more effectively in reducing the symptoms of a morphine withdrawal syndrome in rats. In this regard, to reduce the adverse effects, the researchers are now focusing on drug delivery systems. Thymol, a terpenoids structure, which is a bioactive component of some medicinal plants is used for a variety of disorders and diseases (Alvarez Echazu et al., 2017).
Various nanoparticles have been used for many research (Byrne et al., 2008). Recent research has focused on using hyperbranched polymers to increase efficiency due to particular characteristics such as lots of terminal groups, low viscosity, and high solubility (Kolhe et al., 2003; Zhang et al., 2010). They have a similar design to dendrimers, but they are more easily synthesized (Rokicki et al., 2005; Zhu et al., 2006; Wang et al., 2011). In addition, to arrange their compatibility, solubility, chemical recognizability, and reactivity, hyperbranched polymers end-groups can be modified easily (Seiler, 2006). Due to characteristics, namely, excellent biocompatibility and small size, nanoparticles are easily fluid in the bloodstream to increase the likelihood of cell receptors binding (Dulak et al., 2008). In the present study, nanopolymer was synthesized with high biocompatibility and solubility that use for biomedical applications. It has some branches used for loading drugs thymol and extracts as a drug delivery system and all interactions between thymol and nanopolymer were non-covalent. It has been documented that nanopolymer through various mechanisms, such as direct diffusion can penetrate the cell (Meissner, 1986; Verma et al., 2008).
In this study, to get nanopolymer structure, different analytical techniques have been used. The nanopolymer FT–IR spectrum demonstrated carbon derived from the carbonyl group at 1,738 cm–1 and a hydroxyl group at 3,314 cm–1. The NMR spectrums showed 5 types of hydrogens and 9 types of carbon. The molecular weight of the nanopolymer was obtained by GPC technique around 5,238 g/mol and by DLS tests, the hydrodynamic diameter of the nanopolymer was approximately 75 nm. AFM results showed that nanopolymer was a natural microporous and mesoporous material with polymodal pore size distribution.
Nanopolymer loading capacity was evaluated by HPLC. Based on this analysis, the loading rate of thymol and extract was estimated at 55 ± 3.2% and 48 ± 2.6%, respectively. It has been shown that thymol chitosan nanopolymer had a loading capacity of about 2.5% with antibacterial effects (Medina et al., 2019). But, similar to our study, thymol nanospheres loading was reported to be about 43% (Wattanasatcha et al., 2012).
In this study, it was documented that coadministration of T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer with morphine reduced the severity of the signs of morphine withdrawal syndrome induced by naloxone. These findings demonstrated that the extract and thymol nanopolymer were able to reduce the symptoms of morphine withdrawal syndrome. The effects observed with extract nanopolymer, thymol nanopolymer, and clonidine in reducing the symptoms of morphine withdrawal syndrome were in one direction. Therefore, one of the mechanisms of action of the nanopolymers may be similar to clonidine. Also, the results showed that increasing the dose of T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer decreased the frequency of the signs of withdrawal syndrome, namely, jumping, teeth chattering, and rearing. It has been reported that some supplements and drugs such as magnesium, beta-carbolines, and midazolam reduce the symptoms of withdrawal in the rat (Aricioglu-Kartal and Uzbay, 1997). Moreover, for the management of heroin or methadone withdrawal, clonidine and lofexidine are more effective than placebo (Gowing et al., 2014). Recently, the use of medicinal plants to improve the symptoms of morphine withdrawal has been considered.
It has been reported that some medicinal plants, such as Zingiber officinale, Glycyrrhiza glabra, and Ziziphus jujube relieve morphine withdrawal symptoms (Doosti et al., 2013). Thymus species are the medicinal plants whose leaves and flowering parts are widely used as a tonic, antiseptic, antitussive, and carminative (Ghasemi Pirbalouti, 2009; Jahani et al., 2013; Bakhshi et al., 2014) and also used in pharmaceutical, cosmetic, and preservation of several food products (Surburg and Panten, 2006). The medicinal plant Zataria multiflora and Thymus vulgaris, thymol-containing, reversed the effects of naloxone due to their analgesic effects (Hosseinzadeh et al., 2000; Taherian et al., 2009). Also, Thymus daenensis extract and essential oil attenuate morphine withdrawal behaviors in mice (Khodayar et al., 2014). However, no study has been investigated the effects of the T. spicata extract, extract nanopolymer, and thymol nanopolymer on morphine withdrawal syndrome in rats.
Although the Thymus vulgaris and its main constituents (thymol and carvacrol) have reduced the symptoms of experimental autoimmune encephalomyelitis in an animal model (Mahmoodi et al., 2019), and thymol has anti-Alzheimer’s (Azizi et al., 2012), antiseizure (Sancheti et al., 2014), antidepressant (Deng et al., 2015), and nAch receptor activity (Sammi et al., 2017), but the effectiveness of T. spicata on the nervous system was not observed.
It was also found that thymol treatment increased the Aβ protein levels (Azizi et al., 2012) and the prolonged onset of myoclonic jerk and glutathione levels (Sancheti et al., 2014), and decreased oxidative stress (Lee et al., 2015), norepinephrine in the hippocampus (Deng et al., 2015), and synaptic Ach levels (Sammi et al., 2017).
Our results demonstrate that the ameliorative effects of T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer against withdrawal syndrome by naloxone challenge may be modulated by α2 receptor or PKA pathway in rats. However, extract nanopolymer, and thymol nanopolymer alleviate the morphine withdrawal syndrome more than the total T. spicata extract and thymol.
LD50 of the T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer were 975, 580, 1,250, and 650 mg/kg, respectively. It has been documented that agents with LD50 values in the range of 50–500 and 500–5,000 mg/kg showed moderately and slightly toxic, respectively (Ruiz et al., 2012). By our studies, previous studies have shown that LD50 of thymol and thymol nanopolymer were 435 and 583 mg/kg, respectively (Karimi et al., 2019), and other studies have shown that LD50 of thymol was 980 mg/kg (Jamil et al., 2010).
Conclusion
Our results suggest that T. spicata extract, thymol, extract nanopolymer, and thymol nanopolymer can reduce the narcotic drugs withdrawal symptoms, and its reducing effect is equivalent to clonidine and may have the potential of narcotic addiction treatment. The efficacy of thymol nanopolymer was greater than the others, and since it affected equivalent to clonidine, its pharmacological mechanism of action is likely to be similar.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by the Ilam University of Medical Sciences.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported by the Ilam University of Medical Sciences (Grant No. 87-04-33-8073).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, M. L., Lavasanifar, A., and Kwon, G. S. (2003). Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 92, 1343–1355.
Adeli, M., Rasoulian, B., Saadatmehr, F., and Zabihi, F. (2013). Hyperbranched poly(citric acid) and its application as anticancer drug delivery system. J. Appl. Polymer Sci. 129, 3665–3671. doi: 10.2147/IJN.S17336
Aghamohammadi, A., Azadbakht, M., and Hosseinimehr, S. J. (2016). Quantification of thymol content in different extracts of Zataria multiflora by HPLC method. Pharm. Biomed. Res. 2, 8–13.
Alvarez Echazu, M. I., Olivetti, C. E., Anesini, C., Perez, C. J., Alvarez, G. S., and Desimone, M. F. (2017). Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. C. Mater. Biol. Appl. 81, 588–596. doi: 10.1016/j.msec.2017.08.059
Aricioglu-Kartal, F., and Uzbay, I. T. (1997). Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats. Life Sci. 61, 1775–1781. doi: 10.1016/s0024-3205(97)00801-1
Azizi, Z., Ebrahimi, S., Saadatfar, E., Kamalinejad, M., and Majlessi, N. (2012). Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav. Pharmacol. 23, 241–249. doi: 10.1097/FBP.0b013e3283534301
Bakhshi, H., Amin, G., Zolfi, R., Pirmohammadi, M., Bakhshi, A., Taghinezhad, F., et al. (2014). Larvicidal properties of botanical extracts of lawsonia inermis against Anopheles stephensi. Adv. Infect. Dis. 4, 178–185.
Ballantyne, J. C., and LaForge, S. K. (2007). Opioid dependence and addiction during opioid treatment of chronic pain. Pain 129, 235–255.
Brown, W., Schillen, K., and Hvidt, S. (1992). Triblock copolymers in aqueous solution studied by static and dynamic light scattering and oscillatory shear measurements: influence of relative block sizes. J. Phys. Chem. 96, 6038–6044.
Byrne, J. D., Betancourt, T., and Brannon-Peppas, L. (2008). Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 60, 1615–1626. doi: 10.1016/j.addr.2008.08.005
Chu, K. S., Schorzman, A. N., Finniss, M. C., Bowerman, C. J., Peng, L., Luft, J. C., et al. (2013). Nanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and efficacy. Biomaterials 34, 8424–8429. doi: 10.1016/j.biomaterials.2013.07.038
Deng, X. Y., Li, H. Y., Chen, J. J., Li, R. P., Qu, R., Fu, Q., et al. (2015). Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav. Brain Res. 291, 12–19. doi: 10.1016/j.bbr.2015.04.052
Dhaneshwar, S., Patel, V., Patil, D., and Meena, G. (2013). Studies on synthesis, stability, release and pharmacodynamic profile of a novel diacerein-thymol prodrug. Bioorg. Med. Chem. Lett. 23, 55–61. doi: 10.1016/j.bmcl.2012.11.016
Dong, Q., and Gu, L. (2002). Synthesis of AN-g-casein copolymer in concentrated aqueous solution of sodium thiocyanate and AN-g-casein fiber’s structure and property. Eur. Polymer J. 38, 511–519.
Doosti, F., Dashti, S., Tabatabai, S. M., and Hosseinzadeh, H. (2013). Traditional Chinese and Indian medicine in the treatment of opioid-dependence: a review. Avicenna J. Phytomed. 3, 205–215.
Dulak, J., Deshane, J., Jozkowicz, A., and Agarwal, A. (2008). Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117, 231–241. doi: 10.1161/CIRCULATIONAHA.107.698316
Ebrahimie, M., Bahmani, M., Shirzad, H., Rafieian-Kopaei, M., and Saki, K. (2015). A review study on the effect of iranian herbal medicines on opioid withdrawal syndrome. J. Evid. Based Complementary Altern. Med. 20, 302–309. doi: 10.1177/2156587215577896
El Kabbaoui, M., Chda, A., El-Akhal, J., Azdad, O., Mejrhit, N., Aarab, L., et al. (2017). Acute and sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. J. Ethnopharmacol. 209, 147–156. doi: 10.1016/j.jep.2017.07.029
Feily, A., and Abbasi, N. (2009). The inhibitory effect of Hypericum perforatum extract on morphine withdrawal syndrome in rat and comparison with clonidine. Phytother. Res. 23, 1549–1552. doi: 10.1002/ptr.2807
Ghasemi Pirbalouti, A. (2009). Medicinal plants used in Chaharmahal and Bakhtyari districts, Iran. Herba Polonica 55, 69–77.
Gowing, L., Farrell, M. F., Ali, R., and White, J. M. (2014). Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst. Rev. 3:CD002024.
Hasan, N., Imran, M., Kesharwani, P., Khanna, K., Karwasra, R., Sharma, N., et al. (2021). Intranasal delivery of Naloxone-loaded solid lipid nanoparticles as a promising simple and non-invasive approach for the management of opioid overdose. Int. J. Pharm. 599:120428. doi: 10.1016/j.ijpharm.2021.120428
Hosseinzadeh, H., Ramezani, M., and Salmani, G. (2000). Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J. Ethnopharmacol. 73, 379–385. doi: 10.1016/s0378-8741(00)00238-5
Jafari, A., Rasmi, Y., Hajaghazadeh, M., and Karimipour, M. (2018). Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: biochemical and histological evidences. Environ. Toxicol. Pharmacol. 58, 29–36. doi: 10.1016/j.etap.2017.12.010
Jahani, M., Rezaei, O., Khodadadi, M., and Alemzadeh, E. (2013). A survey of fungal contamination associated with barberry (Berberis spp.) in Iran. Am. J. Plant Sci. 04, 1594–1596.
Jamil, D. M., Brown, J. E., Driscoll, D., and Howell, N. K. (2010). Characterization and antioxidant activity of the volatile oils of Thymus Syriacus Boiss. var syriacus and Thymbra spicata L. grown wild in Kurdistan-Iraq. Proc. Nutr. Soc. 69:E104.
Karimi, E., Abbasi, S., Aidy, A. L. I., Ghaneialvar, H., Mohammadpour, S., Akhavan, M. M., et al. (2019). Anti-diabetic effect of a novel nano polymer of thymol in streptozotocin-induced diabetic wistar rats. Int. J. Appl. Pharm. 11, 81–89.
Khodayar, M. J., Taherzadeh, E., Siahpoosh, A., Mansourzadeh, Z., and Tabatabaei, S. A. (2014). Thymus daenensis extract and essential oils effects on morphine withdrawal signs in mice. Jundishapur J. Nat. Pharm. Prod. 9:e9959. doi: 10.17795/jjnpp-9959
Kolhe, P., Misra, E., Kannan, R. M., Kannan, S., and Lieh-Lai, M. (2003). Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int. J. Pharm. 259, 143–160. doi: 10.1016/s0378-5173(03)00225-4
Kreek, M. J., LaForge, K. S., and Butelman, E. (2002). Pharmacotherapy of addictions. Nat. Rev. Drug Discov. 1, 710–726.
Lee, B. H., Nam, T. G., Park, W. J., Kang, H., Heo, H. J., Chung, D. K., et al. (2015). Antioxidative and neuroprotective effects of volatile components in essential oils from Chrysanthemum indicum Linné flowers. Food Sci. Biotechnol. 24, 717–723.
Lee, D. P., Deonarine, A. S., Kienetz, M., Zhu, Q., Skrzypczak, M., Chan, M., et al. (2001). A novel pathway for lipid biosynthesis: the direct acylation of glycerol. J. Lipid Res. 42, 1979–1986.
Lee, W. Y. (1978). Calibration of the gel permeation chromatography polyester resins. J. Appl. Polymer Sci. 22, 3343–3344. doi: 10.1016/j.ijbiomac.2018.12.181
Li, J.-X., and Zhang, Y. (2012). Emerging drug targets for pain treatment. Eur. J. Pharmacol. 681, 1–5.
Lopez, A., de Tangil, M. S., Vega-Orellana, O., Ramirez, A. S., and Rico, M. (2013). Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 18, 4942–4954. doi: 10.3390/molecules18054942
Lv, Q., Yu, A., Xi, Y., Li, H., Song, Z., Cui, J., et al. (2009). Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int. J. Pharm. 372, 191–198. doi: 10.1016/j.ijpharm.2009.01.014
Mahmoodi, M., Ayoobi, F., Aghaei, A., Rahmani, M., Taghipour, Z., Hosseini, A., et al. (2019). Beneficial effects of Thymus vulgaris extract in experimental autoimmune encephalomyelitis: Clinical, histological and cytokine alterations. Biomed. Pharmacother. 109, 2100–2108. doi: 10.1016/j.biopha.2018.08.078
Majumdar, D., Jung, K.-H., Zhang, H., Nannapaneni, S., Wang, X., Amin, A. R. M. R., et al. (2014). Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity. Cancer Prev. Res. 7, 65–73. doi: 10.1158/1940-6207.CAPR-13-0230
Mansouri, N., Rikhtegar, N., Panahi, H., Atabi, F., and Karimi Shahraki, B. (2013). Porosity, characterization and structural properties of natural zeolite - Clinoptilolite - As a sorbent. Environ. Prot. Eng. 39:139–152.
Medina, E., Caro, N., Abugoch, L., Gamboa, A., íaz-Dosque, M. D., and Tapia, C. (2019). Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 240, 191–198.
Meissner, G. (1986). Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 261, 6300–6306.
Moayeri, A., Azimi, M., Karimi, E., Aidy, A., and Abbasi, N. (2018). Attenuation of morphine withdrawal syndrome by prosopis farcta extract and its bioactive component luteolin in comparison with clonidine in rats. Med. Sci. Monit. Basic Res. 24, 151–158. doi: 10.12659/MSMBR.909930
Moghimi, S. M., Hunter, A. C., and Murray, J. C. (2005). Nanomedicine: current status and future prospects. FASEB J. 19, 311–330.
Motti, R., and de Falco, B. (2021). Traditional herbal remedies used for managing anxiety and insomnia in italy: an ethnopharmacological overview. Horticulturae 7:523.
Naeini, A. T., Adeli, M., and Vossoughi, M. (2010). Poly(citric acid)-block-poly(ethylene glycol) copolymers–new biocompatible hybrid materials for nanomedicine. Nanomedicine 6, 556–562. doi: 10.1016/j.nano.2009.11.008
Neri, D., Szyperski, T., Otting, G., Senn, H., and Wuethrich, K. (1989). Stereospecific nuclear magnetic resonance assignments of the methyl groups of valine and leucine in the DNA-binding domain of the 434 repressor by biosynthetically directed fractional carbon-13 labeling. Biochemistry 28, 7510–7516. doi: 10.1021/bi00445a003
Parmar, N. S., and Prakash, S. (2006). Screening Methods in Pharmacology. Oxford: Alpha Science International.
Qiao, M., Chen, D., Ma, X., and Liu, Y. (2005). Injectable biodegradable temperature-responsive PLGA–PEG–PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int. J. Pharm. 294, 103–112. doi: 10.1016/j.ijpharm.2005.01.017
Rašković, A. L., Kvrgić, M. P., Tomas, A. D., Stilinović, N. P., Čabarkapa, V. S., Stojšić-Milosavljević, A. Đ., et al. (2020). Antinociceptive activity of thyme (Thymus vulgaris L.) and interactions with neurotropics and analgesics. Braz. J. Pharm. Sci. 56:e18819. doi: 10.1590/s2175-97902020000318819
Rezayati Charani, P., Dehghani-Firouzabadi, M., Afra, E., and Shakeri, A. (2013). Rheological characterization of high concentrated MFC gel from kenaf unbleached pulp. Cellulose 20, 727–740.
Robledo, N., Vera, P., Lopez, L., Yazdani-Pedram, M., Tapia, C., and Abugoch, L. (2018). Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 246, 211–219. doi: 10.1016/j.foodchem.2017.11.032
Rokicki, G., Rakoczy, P., Parzuchowski, P., and Sobiecki, M. (2005). Hyperbranched aliphatic polyethers obtained from environmentally benign monomer: glycerol carbonate. Green Chem. 7, 529–539.
Romandini, S., Cervo, L., and Samanin, R. (1984). Evidence that drugs increasing 5-hydroxytryptamine transmission block jumping but not wet dog shakes in morphine-abstinent rats: a comparison with clonidine. J. Pharm. Pharmacol. 36, 68–70. doi: 10.1111/j.2042-7158.1984.tb02995.x
Ruiz, P., Begluitti, G., Tincher, T., Wheeler, J., and Mumtaz, M. (2012). Prediction of acute mammalian toxicity using QSAR methods: a case study of sulfur mustard and its breakdown products. Molecules 17, 8982–9001. doi: 10.3390/molecules17088982
Saleem, S., Naqvi, F., Batool, A., Naqvi, S. H., Naqvi, F., Batool, Z., et al. (2021). Neuroprotective role of a monoterpene (thymol) on diazepam induced withdrawal symptoms in rats. Pak. J. Pharm. Sci. 34(4 Suppl.), 1615–1620.
Sammi, S. R., Trivedi, S., Rath, S. K., Nagar, A., Tandon, S., Kalra, A., et al. (2017). 1-Methyl-4-propan-2-ylbenzene from Thymus vulgaris attenuates cholinergic dysfunction. Mol. Neurobiol. 54, 5468–5481. doi: 10.1007/s12035-016-0083-0
Sancheti, J., Shaikh, M. F., Chaudhari, R., Somani, G., Patil, S., Jain, P., et al. (2014). Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Naunyn Schmiedebergs Arch. Pharmacol. 387, 59–66. doi: 10.1007/s00210-013-0917-5
Seiler, M. (2006). Hyperbranched polymers: phase behavior and new applications in the field of chemical engineering. Fluid Phase Equilib. 241, 155–174.
Stano, A., Nembrini, C., Swartz, M. A., Hubbell, J. A., and Simeoni, E. (2012). Nanoparticle size influences the magnitude and quality of mucosal immune responses after intranasal immunization. Vaccine 30, 7541–7546. doi: 10.1016/j.vaccine.2012.10.050
Surburg, H., and Panten, J. (2006). Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th Edn. Hoboken, NJ: Wiley, 1–318.
Tabatabai, S. M., Dashti, S., Doosti, F., and Hosseinzadeh, H. (2014). Phytotherapy of opioid dependence and withdrawal syndrome: a review. Phytother. Res. 28, 811–830. doi: 10.1002/ptr.5073
Taherian, A. A., Babaei, M., Vafaei, A. A., Jarrahi, M., Jadidi, M., and Sadeghi, H. (2009). Antinociceptive effects of hydroalcoholic extract of Thymus vulgaris. Pak. J. Pharm. Sci. 22, 83–89.
Tang, L., Yang, X., Yin, Q., Cai, K., Wang, H., Chaudhury, I., et al. (2014). Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. U.S.A. 111, 15344–15349. doi: 10.1073/pnas.1411499111
Verma, A., Uzun, O., Hu, Y., Hu, Y., Han, H. S., Watson, N., et al. (2008). Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 7, 588–595. doi: 10.1038/nmat2202
Wagner, V., Dullaart, A., Bock, A. K., and Zweck, A. (2006). The emerging nanomedicine landscape. Nat. Biotechnol. 24, 1211–1217. doi: 10.1038/nbt1006-1211
Wang, T., Li, M., Gao, H., and Wu, Y. (2011). Nanoparticle carriers based on copolymers of poly(epsilon-caprolactone) and hyperbranched polymers for drug delivery. J. Colloid Interface Sci. 353, 107–115. doi: 10.1016/j.jcis.2010.09.053
Ward, J., Rosenbaum, C., Hernon, C., McCurdy, C. R., and Boyer, E. W. (2011). Herbal medicines for the management of opioid addiction: safe and effective alternatives to conventional pharmacotherapy? CNS Drugs 25, 999–1007. doi: 10.2165/11596830-000000000-00000
Wattanasatcha, A., Rengpipat, S., and Wanichwecharungruang, S. (2012). Thymol nanospheres as an effective anti-bacterial agent. Int. J. Pharm. 434, 360–365. doi: 10.1016/j.ijpharm.2012.06.017
Zhang, H., Zhao, C., Cao, H., Wang, G., Song, L., Niu, G., et al. (2010). Hyperbranched poly(amine-ester) based hydrogels for controlled multi-drug release in combination chemotherapy. Biomaterials 31, 5445–5454. doi: 10.1016/j.biomaterials.2010.03.034
Keywords: nano polymer, thymol, Thymbra spicata, morphine, withdrawal syndrome
Citation: Moayeri A, Mehdizadeh R, Karimi E, Aidy A, Ghaneialvar H and Abbasi N (2022) Thymol Nanopolymer Synthesis and Its Effects on Morphine Withdrawal Syndrome in Comparison With Clonidine in Rats. Front. Behav. Neurosci. 16:843951. doi: 10.3389/fnbeh.2022.843951
Received: 27 December 2021; Accepted: 24 May 2022;
Published: 29 June 2022.
Edited by:
Phillip R. Zoladz, Ohio Northern University, United StatesReviewed by:
Jolanta Orzelska-Gorka, Medical University of Lublin, PolandMustafa Sevindik, Osmaniye Korkut Ata University, Turkey
Copyright © 2022 Moayeri, Mehdizadeh, Karimi, Aidy, Ghaneialvar and Abbasi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naser Abbasi, YWJiYXNpLW5AbWVkaWxhbS5hYy5pcg==
 Ardeshir Moayeri1
Ardeshir Moayeri1 Naser Abbasi
Naser Abbasi