
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Behav. Neurosci. , 25 April 2022
Sec. Learning and Memory
Volume 16 - 2022 | https://doi.org/10.3389/fnbeh.2022.806356
This article is part of the Research Topic Horizons in Behavioral Neuroscience View all 9 articles
Long-term potentiation (LTP) and long-term depression (LTD) comprise the principal cellular mechanisms that fulfill established criteria for the physiological correlates of learning and memory. Traditionally LTP, that increases synaptic weights, has been ascribed a prominent role in learning and memory whereas LTD, that decreases them, has often been relegated to the category of “counterpart to LTP” that serves to prevent saturation of synapses. In contradiction of these assumptions, studies over the last several years have provided functional evidence for distinct roles of LTD in specific aspects of hippocampus-dependent associative learning and information encoding. Furthermore, evidence of the experience-dependent “pruning” of excitatory synapses, the majority of which are located on dendritic spines, by means of LTD has been provided. In addition, reports exist of the temporal and physical restriction of LTP in dendritic compartments by means of LTD. Here, we discuss the role of LTD and LTP in experience-dependent information encoding based on empirical evidence derived from conjoint behavioral and electrophysiological studies conducted in behaving rodents. We pinpoint the close interrelation between structural modifications of dendritic spines and the occurrence of LTP and LTD. We report on findings that support that whereas LTP serves to acquire the general scheme of a spatial representation, LTD enables retention of content details. We argue that LTD contributes to learning by engaging in a functional interplay with LTP, rather than serving as its simple counterpart, or negator. We propose that similar spatial experiences that share elements of neuronal representations can be modified by means of LTD to enable pattern separation. Therewith, LTD plays a crucial role in the disambiguation of similar spatial representations and the prevention of generalization.
Hippocampal long-term potentiation (LTP) and long-term depression (LTD) were first described in the 1970s (Bliss and Lomo, 1973; Alger and Teyler, 1976). Comprising a persistent, input-specific increase, or decrease of synaptic strength, respectively, LTP and LTD were initially ascribed roles in information encoding and deletion related to memory acquisition and forgetting (Tsumoto, 1993). Others have argued that LTD silences the memory engram through synaptic weakening (Nabavi et al., 2014; Basu and Siegelbaum, 2015; Luchkina and Bolshakov, 2019; Josselyn and Tonegawa, 2020). Evidence from studies conducted in freely behaving rodents during learning events, indicate, however, that LTP and LTD support different kinds of information storage, and that input-specific information storage can be differentiated according to both the afferent input and the hippocampal subfield involved (Table 1). Furthermore, induction of hippocampal LTP and LTD results in nuclear immediate early gene (IEG) mRNA expression in hippocampal neurons, albeit in distinctly different distributions (Hoang et al., 2021) that correspond to hippocampal gene encoding in response to spatial learning events (Hoang et al., 2018).
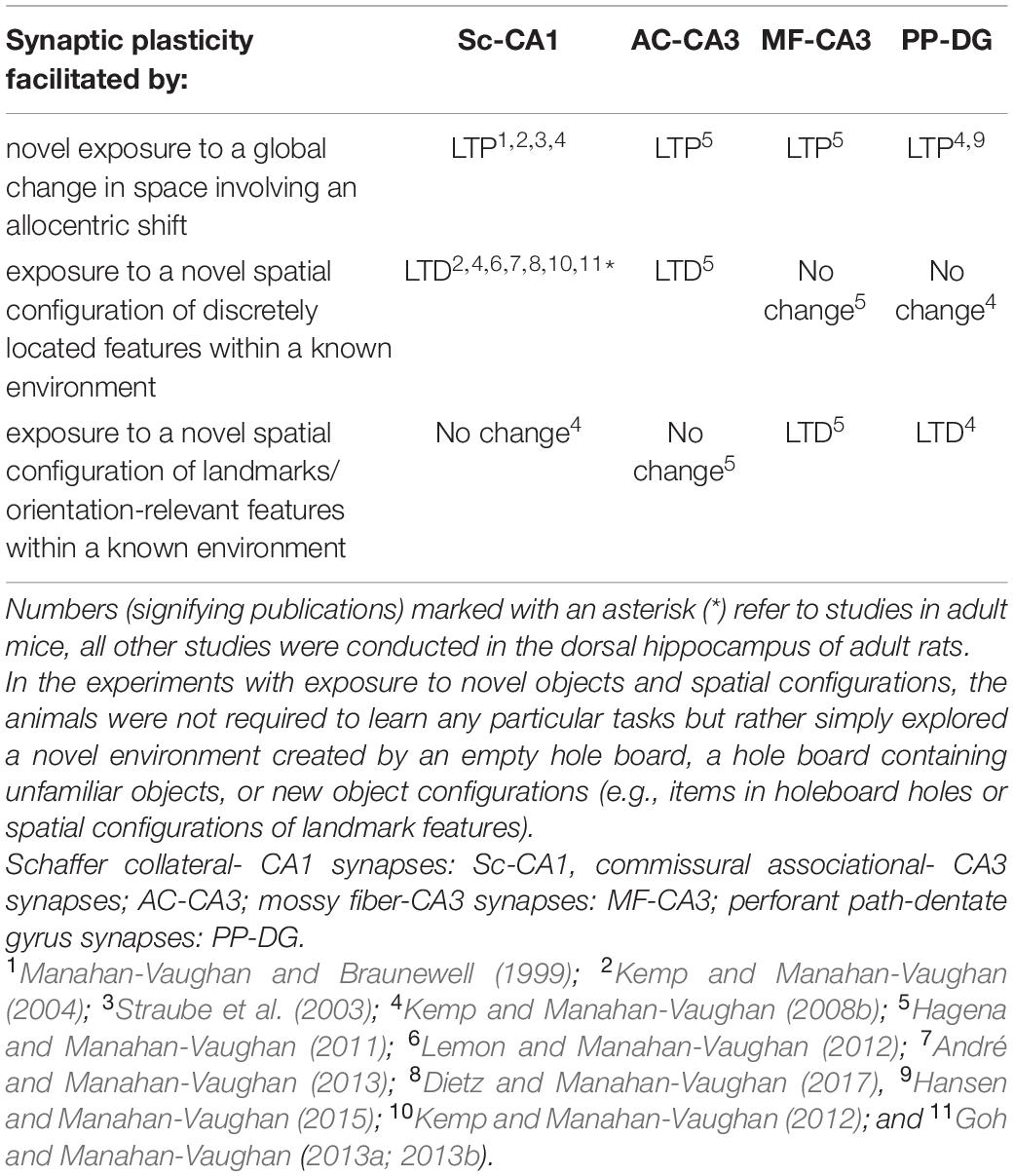
Table 1. Overview of changes in synaptic weights triggered in the hippocampus by specific components of spatial, learning in freely behaving rodents.
A functional role for LTP has been described in the acquisition of conditioned fear memory (Ramirez et al., 2013; Luchkina and Bolshakov, 2019; Josselyn and Tonegawa, 2020), in the acquisition of information about novel space (Kemp and Manahan-Vaughan, 2004), or the gaining of knowledge about the allocentric context of space (Straube et al., 2003; Manahan-Vaughan, 2017). On the other hand, LTD has been implicated in the acquisition of information about novel item configurations (Manahan-Vaughan and Braunewell, 1999; Goh and Manahan-Vaughan, 2013c) spatial information updating (Kemp and Manahan-Vaughan, 2004, 2008a,b) and spatial memory consolidation (Ge et al., 2010; An and Sun, 2018). Furthermore, animals with impaired LTD show deficits in long-term, but not short-term, contextual fear memory (Liu et al., 2014), and either a complete inability to succeed in the Morris Water Maze task (Etkin et al., 2006; Rocchetti et al., 2015), or a deficit in reversal learning when the hidden platform is changed to another quadrant (Nicholls et al., 2008; Kim et al., 2011; Mills et al., 2014). Together with an impaired ability to habituate to novel space and objects (Etkin et al., 2006), this indicates that when LTD is impaired, animals are unable to form a proper detailed representation of space, or modify the representation. Indeed, hippocampal LTD is tightly associated with the de novo acquisition of knowledge of spatial content (Kemp and Manahan-Vaughan, 2008b; Hagena et al., 2016; Manahan-Vaughan, 2018a) or the updating of spatial content information (Kemp and Manahan-Vaughan, 2004; Goh and Manahan-Vaughan, 2013c; Manahan-Vaughan, 2018a). We have proposed in the past that LTP and LTD work together to create a memory “engram” comprised of a neuronal network in which LTP and LTD, of designated synapses, serves to create a unique and discriminable representation of associative experience (Kemp and Manahan-Vaughan, 2007; Manahan-Vaughan, 2017, 2018a,b). Recent evidence suggests that both the structural modifications of synapses, and the temporal and physical restriction of LTP by LTD in dendritic subcompartments may support this process. In this review article, we highlight the role of LTD in spatial content representation and long-term memory and describe how it also leads to information encoding by means of nuclear immediate early gene expression. We then discuss structural modifications of dendritic spines and describe the interrelationship between structural and functional synaptic plasticity. Finally, we describe reports on the physiological interactions of LTD with LTP in the dendritic domain. We propose that LTD creates a robust neuronal representation of spatial content by means of eliminating weakly potentiated synapses and by dictating temporal constraints and the dendritic distribution of LTP. By this means LTD not only enables spatial content encoding and updating, but also supports pattern separation and subverts experience generalization under circumstances where similar experiences are represented by shared neuronal elements.
Causal proof that synaptic plasticity enables learning is not easy to obtain and it is still being discussed that other mechanisms may play a role (Titley et al., 2017; Abraham et al., 2019). However, studies that examined the expression of hippocampal synaptic plasticity during, and as a result of, spatial learning events have shown that LTP emerges when a rat is repeatedly shown a spatial environment in which its allocentric relationship to distal cues is adjusted (Straube et al., 2003; Figure 1A). LTP is also facilitated in an input-specific manner in different synaptic subcompartments of the hippocampus such as the perforant path-dentate gyrus synapse, the mossy fiber-CA3 synapse, commissural associational–CA3 synapse and the Schaffer collateral-CA1 synapse, when a rat is exposed to a global allocentric change in its spatial environment for the first time (e.g., introduction of a novel holeboard into a familiar environment) (Kemp and Manahan-Vaughan, 2004, 2008b; Hagena and Manahan-Vaughan, 2011; Figure 1B). The fact that synapses are potentiated in a distributed manner through the hippocampus by a novel allocentric experience suggests that the initial step in the creation of a spatial representation is the selection of a synaptic network, by means of LTP. Thus, LTP seems to be the “first-responder” event in the hippocampus that occurs in a widespread, albeit input-specific manner, when an animal is confronted with a novel spatial environment, or with salient allocentric changes of a known spatial environment. This property aligns with reports that LTP can be induced with just a single afferent volley (Gustafsson and Wigstrom, 1986; Gustafsson et al., 1987), whereas LTD requires minutes to manifest and stabilize (Dudek and Bear, 1993; Manahan-Vaughan, 1997). Scrutiny of the behavioral learning circumstances, in which LTD emerges, have revealed a more heterogeneous role compared to that observed for LTP. Thus, LTD is associated with very specific forms or components of spatial learning and its expression is localized to discrete subcompartments of the hippocampus (Kemp and Manahan-Vaughan, 2008b; Hagena and Manahan-Vaughan, 2011).
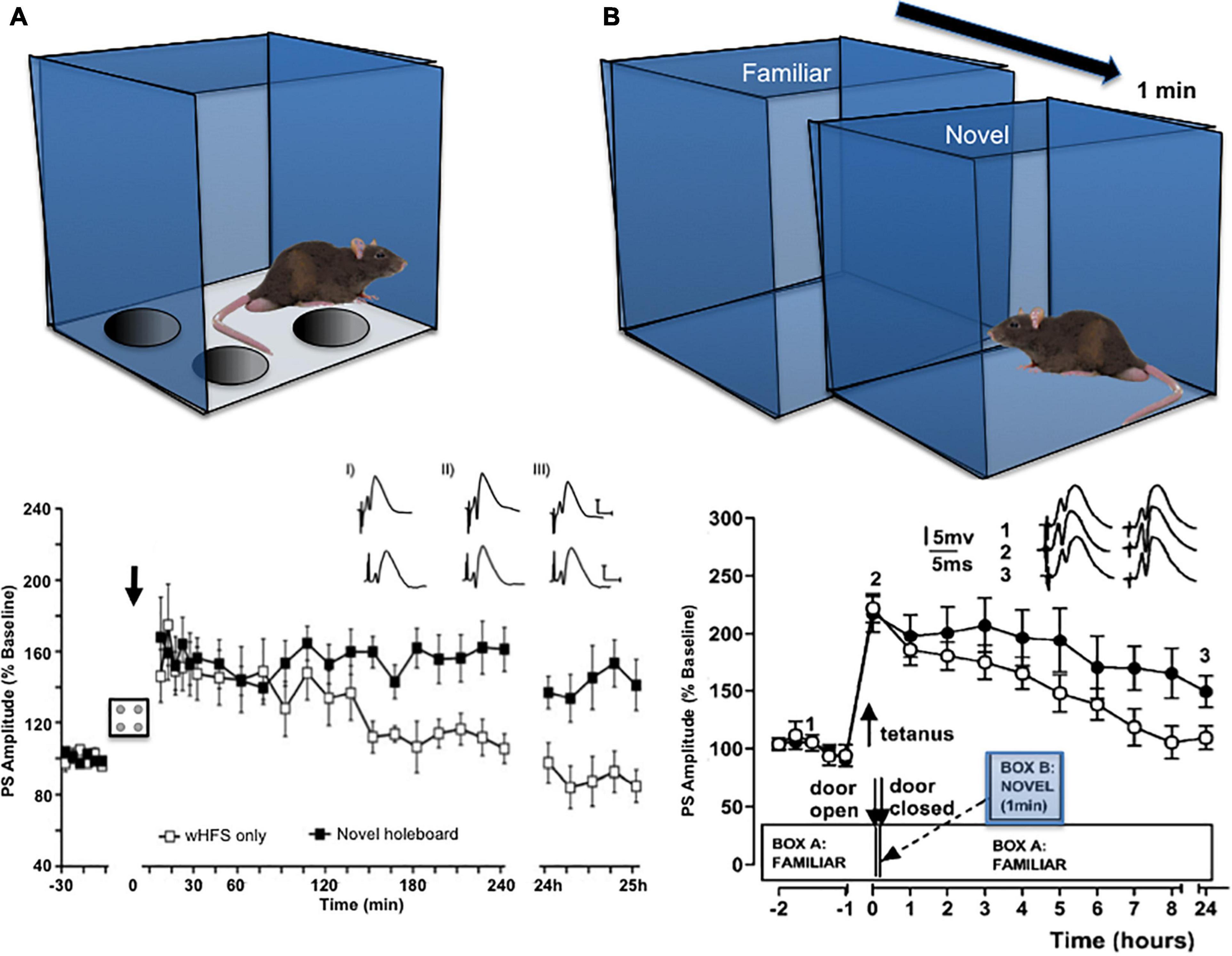
Figure 1. Exposure to novel allocentric space facilitates the induction of hippocampal LTP. (A) Insertion of a novel holeboard into a familiar environment (Ai) promotes the expression of LTP in the hippocampus of rats. In the graph shown, weak high frequency stimulation (wHFS) of perforant path (pp) synapses to the dentate gyrus (DG) triggers short-term potentiation (STP) that lasts for maximally 2 h (unfilled squares). Combination of wHFS (arrow) during exposure to a novel holeboard transforms STP into LTP that lasts for over 24 h (black squares). Inset: analogs show examples of field excitatory post synaptic potentials (fEPSPs) recorded prior to wHFS (iI), 5 min (ii) and 24 h(ii) after wHFS in animals that received wHFS only (top row) and animals that received wHFS during holeboard exploration (bottom row). Scale bar, vertical: 5 mV, horizontal, 5 ms. From Kemp and Manahan-Vaughan, 2008b. (B) Migration from a familiar environment to an adjacent novel environment 2 min after tetanic afferent stimulation (comprising exploration for 1 min, followed by a return to the familiar environment) promotes the expression of LTP at pp-DG synapses in rats. The graph describes how application of the tetanus alone resulted in STP (unfilled circles), compared to when the tetanus was applied in conjunction with novel environmental exposure (filled circles). Inset: analogs show examples of fEPSPs recorded at the time point signified by the digits in animals that received tetanus only (left) and that received tetanus followed by novel environment exploration (right). The graph shown (B) is from Straube et al., 2003, with permission.
The first hint that hippocampal LTD may play a role in information encoding was provided by a study that described the emergence of LTD when afferent stimulation of Schaffer collateral fibers (to induce weak synaptic depression), coupled with exposure of rats to novel objects, transformed short-term depression (STD) into LTD that lasted for days in the hippocampal CA1 region (Manahan-Vaughan and Braunewell, 1999). Subsequent studies in mice revealed a similar relationship: Test pulse stimulation of Schaffer collateral fibers coupled with exposure to novel objects results in LTD in the CA1 region (Goh and Manahan-Vaughan, 2013c). The facilitation of LTD by novel item exploration recruits protein synthesis (Dong et al., 2012; Kemp et al., 2013), a property that has been proposed as a criterion for the qualification of a cellular process as a memory mechanism (Martin et al., 2000). Transgenic, or pharmacological, manipulation of proteins relevant for synaptic plasticity provided additional mechanistic insights into this process. For example, genetic deletion of serum response factor (SRF) (Etkin et al., 2006), or manipulation of neuregulin-signaling (Ledonne et al., 2018), impairs both hippocampal LTD and object recognition memory. Moreover, SPIN90-knockout mice exhibit deficits in hippocampal LTD and object recognition memory (Kim et al., 2017), Bcl-2 associated protein (Bax) knockout mice exhibit deficits in long-term, but not short-term memory, that are accompanied by LTD impairments (Liu et al., 2014), and inhibition of LTD through antagonism of plasticity-related neurotransmitter receptors also prevents object recognition memory (Goh and Manahan-Vaughan, 2013a,b). Here, it is important to point out that a clear delineation has been proposed between the role of the perirhinal cortex in item recognition per se (Aggleton et al., 2010) and the role of the hippocampus in item recognition at the level of item-place recognition and spatial elements of item recognition memory (Wan et al., 1999; Brown and Aggleton, 2001). Although in the former case, LTD in the perirhinal cortex is likely to be involved (Griffiths et al., 2008), closer scrutiny of the relationship between hippocampal LTD and object recognition memory has revealed that it is not the identity of the object itself, but rather the relationship of the object to its location in space that is encoded by hippocampal LTD (Kemp and Manahan-Vaughan, 2004).
The facilitation of hippocampal LTD by visuospatial item-place learning does not only occur when an animal navigates around and explores objects in the physical domain. The viewing of item-place constellations on a computer screen by inert rats also enables LTD (Kemp and Manahan-Vaughan, 2012), suggesting that this phenomenon involves cognitive processing. In line with this, others have shown that inhibition of calcineurin, a key molecular step in the expression of LTD, prevents episodic-like learning in rodents (Zeng et al., 2001). Indeed a comparison of the viewing of visuospatial item constellations at the level of event-related potentials by humans and rats has revealed striking common denominators, including structures such as the posterior parietal cortex (Hauser et al., 2019). The induction of hippocampal LTD by item-place experience is not restricted to the visuospatial domain, however. Spatial configurations of olfactory (André and Manahan-Vaughan, 2013) and auditory items (Dietz and Manahan-Vaughan, 2017) also facilitate the expression of hippocampal LTD (Figures 2A–C).
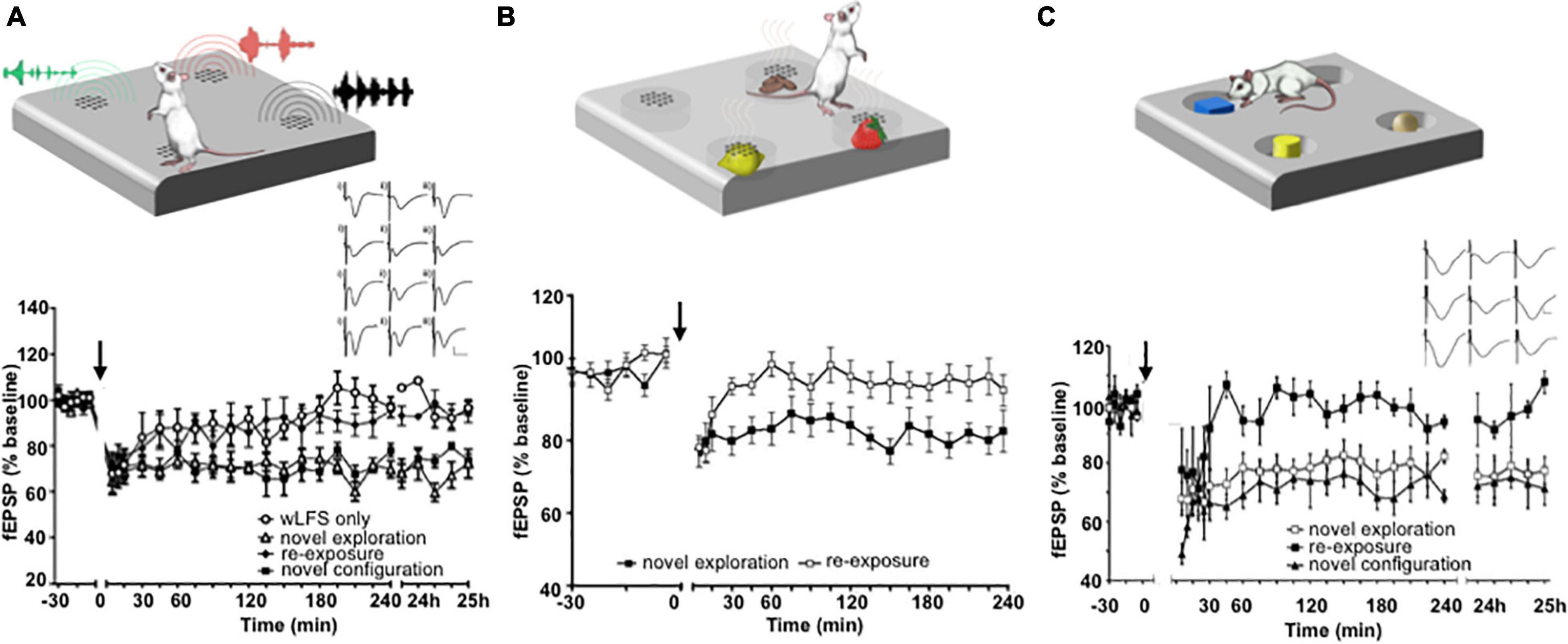
Figure 2. Exposure to item-place experience facilitates the expression of hippocampal LTD. (A) Exploration of novel spatial configurations of auditory items (top) promotes the expression of hippocampal LTD. Graph: weak low frequency stimulation (wLFS, 1 Hz 600 pulses) applied to Schaffer collateral CA1 (SC-CA1) synapses results in short-term depression (STD) in freely behaving rats that lasts for ca. 30 min. Combination of wLFS with novel exploration of audiospatial configurations results in the facilitation of STD into LTD. A subsequent re-exposure to the same items in the same locations at least 7 days after the first exposure during wLFS results in STD. But combining wLFS with the exposure to a novel configuration of the same auditory items results in LTD that lasts for over 24 h. Inset: analogs show examples of field excitatory post synaptic potentials (fEPSPs) recorded prior to wLFS (i), 5 min (ii) and 24 h (iii) after wLFS in animals that received wLFS only (top row), animals that engaged in novel audiospatial cue exploration (2nd row), animals that experienced re-exposure to the cues (3rd row) and animals that were exposed to a novel audiospatial cue configuration (bottom row) Scale bars, vertical: 5 mV, horizontal: 5 ms. From Dietz and Manahan-Vaughan, 2017. (B) Novel Exploration of a spatial configuration of odors (top) also promotes the expression of LTD. The graph shows the expression of LTD when wLFS (applied to SC-CA1 synapses) was combined with de novo exposure to different odors that emanated from holes in the floor of the chamber. Re-exposure to the same odors in the same spatial locations ca. 1 week after the first exposure failed to induced LTD. From André and Manahan-Vaughan (2013). (C) Exploration of spatial configurations of novel visual items promotes LTD (top). Graph: novel exploration of spatially distributed visual items during wLFS of SC-CA1 synapses enables LTD. Re-exposure to the same items in the same spatial configuration during wLFS 1 week later results in STD, whereas exposure to a new spatial configuration of the visual items results in LTD that lasts for over 24 h. Inset: analogs show examples of fEPSPs recorded prior to wLFS (left column), 5 min (middle column) and 24 h (right column) after wLFS in animals that engaged in novel visuospatial cue exploration (top row), animals that experienced re-exposure to the cues (middle row) and animals that were exposed to a novel visuospatial cue configuration (bottom row). Scale bars, vertical: 5 mV, horizontal: 5 ms. From Kemp and Manahan-Vaughan (2004). Cartoons (A–C) were modified from: Manahan-Vaughan (2018b).
In contrast to LTP that is expressed, albeit in an input-specific manner, in a widespread distribution across hippocampal subfields in response to a novel allocentric experience (Straube et al., 2003; Kemp and Manahan-Vaughan, 2004, 2008b; Hagena and Manahan-Vaughan, 2011), LTD expression is synaptic subcompartment-specific and this property, in turn, is mediated by specific kinds of item-place experience. Thus, if spatial content pertains to subtle features of the environment that can only be discovered if the animal is physically beside them, LTD is expressed in Schaffer collateral-CA1 synapses (Manahan-Vaughan and Braunewell, 1999; Kemp and Manahan-Vaughan, 2004, 2008b), or commissural-associational-CA3 synapses (Hagena and Manahan-Vaughan, 2011). These features can be visual, olfactory or auditory (André and Manahan-Vaughan, 2013; Dietz and Manahan-Vaughan, 2017; Figure 2). But if the environmental features are large and visible from afar, LTD at perforant path-dentate gyrus, and mossy fiber-CA3 synapses is induced (Kemp and Manahan-Vaughan, 2008b; Hagena and Manahan-Vaughan, 2011). Exposing animals to a novel environment with both distinct novel allocentric and novel content cues triggers hippocampal LTP that segues into LTD (Manahan-Vaughan and Braunewell, 1999). This finding suggests that hippocampal subfields and their synaptic subcompartments are highly specialized with regard to the functional expression of LTD in response to different kinds of item-place experience.
However, it is not only novel item-place constellations that promote the expression of LTD: modifications of spatial configurations conducted by moving familiar items into unfamiliar spatial positions also triggers LTD (Manahan-Vaughan and Braunewell, 1999; Kemp and Manahan-Vaughan, 2004, 2008b,2012; Goh and Manahan-Vaughan, 2013c). This takes place in perforant path-dentate gyrus, and mossy fiber-CA1, synapses when a known spatial arrangement of large landmark features is changed without altering the ostensible content of the spatial environment (Kemp and Manahan-Vaughan, 2008b; Hagena and Manahan-Vaughan, 2011). LTD, at Schaffer collateral-CA1 synapses and commissural associational-CA3 synapses, is also triggered when subtle, less obviously visible visuospatial configurations of familiar items are altered (Manahan-Vaughan and Braunewell, 1999; Kemp and Manahan-Vaughan, 2004; Goh and Manahan-Vaughan, 2013c). Taken together, these findings indicate that LTD supports the fine-tuning of experience-dependent storage of spatial knowledge in a hippocampal neuronal network, that relates, in turn, to the postulated role of the different hippocampal subfields in the acquisition of knowledge about orientational and content features of space (Jacobs and Schenk, 2003).
Further evidence for a role for LTD in information updating comes from studies of reversal and extinction learning. During reversal learning, rodents typically learn the (constant) location of a hidden platform over a series of training trials. Multiple trials result in the animals acquiring an accurate spatial representation of the location of the platform relative to allocentric cues. One can assess reversal learning, and thereby behavioral flexibility, by then changing the location of the platform and examining how rapidly the animal builds a new representation (or continues to look for the platform where it was previously located). Inhibition of LTD prevents reversal learning (Nicholls et al., 2008; Kim et al., 2011, 2017; Dong et al., 2013; Mills et al., 2014) and also prevents extinction learning (Kim et al., 2017), whereby due to changing contingencies a previous behavior should no longer be executed.
These properties of LTD raise the possibility that by serving as a cellular mechanism for representational updating, LTD may also circumvent that very similar experiences become generalized. This not only would serve as an invaluable mechanism to ensure the integrity and reliability of similar memories, but could be expected to support pattern separation, and prevent the generalization of traumatic experience. Evidence for this has been provided by studies that reported on the one hand, that LTD subserves the temporal compartmentalization of acquired memories (Cui et al., 2013) and also that improving LTD prevents the generalization of fear memory (Cao et al., 2021).
We propose that LTD strengthens the robustness of stored experience by pruning away synapses that are weakly integrated into a synaptic network that stores a specific experience. LTD also temporally and physically constrains LTP into specific synaptic and dendritic subcompartments thereby preventing a “seepage” and binding of one discrete memory into another either similar, or recently acquired memory. By this means, erroneous associations are avoided and the integrity of a stored experience is secured. This process can be considered an integral element of pattern separation whereby very similar experiences can be disambiguated from one another. Assuming that LTP creates the memory engram by strengthening selected synapses within a network, and assuming that similar experiences may recruit information storage in overlapping synaptic circuitry, LTD thereby may serve to sharpen the resolution of these representations by minimizing overlap. We propose that without LTD, memory generalization can occur that confounds disambiguation of similar experiences (Figure 3). This possibility is supported by findings that animals with impairments in LTD quickly forget conditioned taste aversion and consume more of the conditioned substance than controls (Toyoda et al., 2020), are unable to learn the platform location in a water maze and show long-term memory deficits in a Barnes maze (Rocchetti et al., 2015). They also display enhanced freezing behavior, in the absence of foot shock, weeks after context-dependent conditioning (Navarrete et al., 2019). This is consistent with behavioral generalization associated with impoverishment of pattern separation. The ability of LTD to refine synaptic networks generated by means of LTP may be of particular importance in contextual learning. Although generalization of memory may also involve depotentiation of LTP at potentiated synapses that creates an instability of the memory representation (Richards and Frankland, 2017; Robertson, 2018), as mentioned above, evidence for a role for LTD in the protection against memory generalization has also been reported (Cui et al., 2013). These truly are fascinating possibilities, and the question arises as to how they could be mechanistically and anatomically realized.
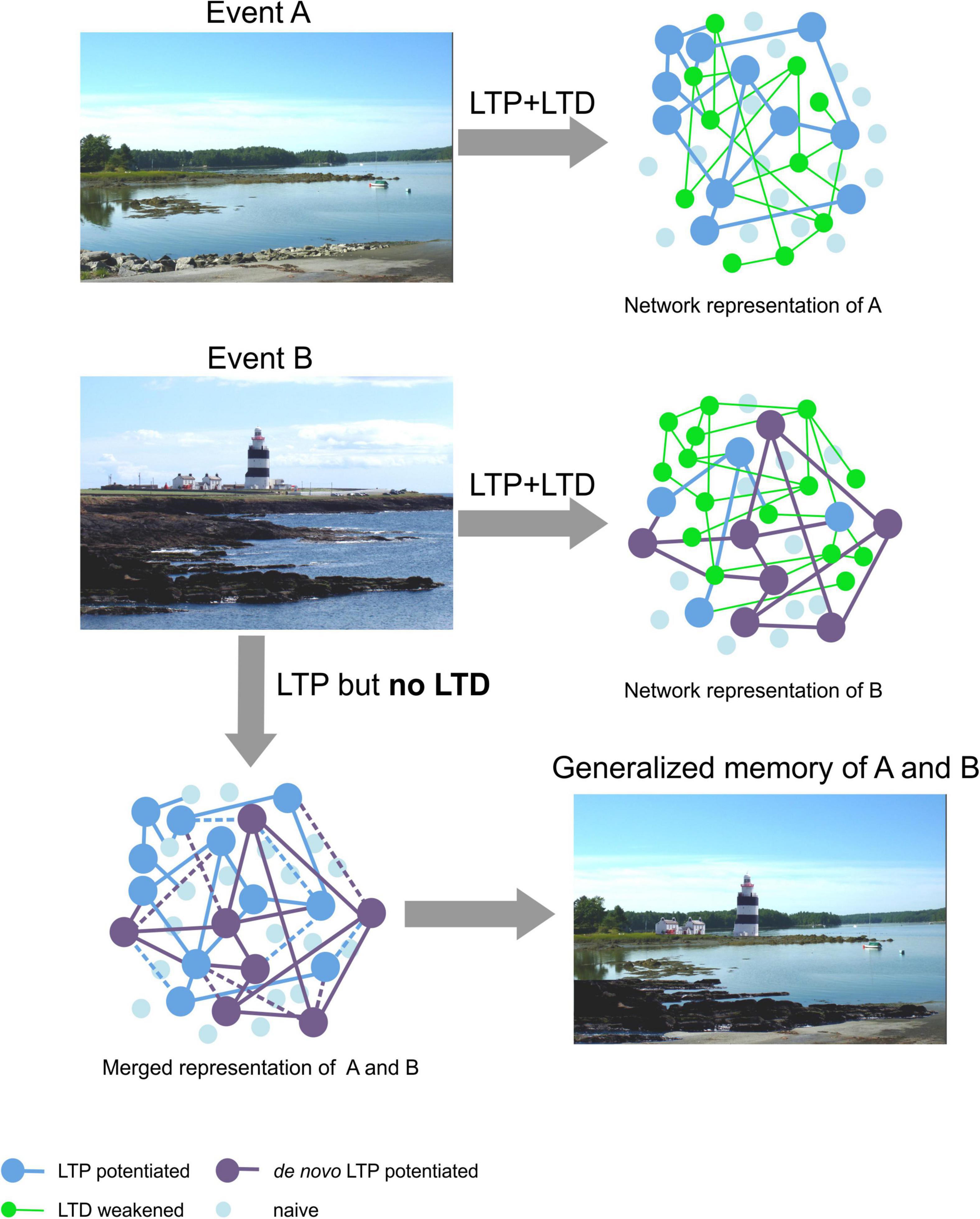
Figure 3. Hypothetical schema of the proposed role for LTD in enabling discriminable spatial representations. Top: The upper photo (Event A, left) is of a landscape near Damariscotta in Maine, United States. By means of LTP the general schema of this landscape is presumably obtained (large dark blue dots, right) (Kemp and Manahan-Vaughan, 2007; Manahan-Vaughan, 2017). Content details are retained by means of LTD (Kemp and Manahan-Vaughan, 2008a; Manahan-Vaughan, 2017) that serves to eliminate weakly potentiated synapses (green dots), or weaken communications between synapses. By this means a robust representation is obtained. Middle: The photo (left) is of Hook Head in Ireland (Event B). When we acquire new memories we are very likely to use blueprints of past memories of similar experiences. Thus, elements of a previously stored neuronal and synaptic network can be re-used as a framework for, in this case, the promontory-like features of the scene, the water inlets and the general global composition of the landscape encoded in Event A. LTD serves to remove superfluous elements, of the new representation compared to the Maine landscape (the asphalt element in the foreground, the trees lining the horizon). De novo LTP is likely to support the retention of new general features of the landscape (large purple dots, right) (Kemp and Manahan-Vaughan, 2004; Manahan-Vaughan, 2018a) and LTD contributes to information encoding through the inclusion of content details such as the houses and the lighthouse (Kemp and Manahan-Vaughan, 2008a; Manahan-Vaughan, 2017, 2018a). Where LTP and LTD work together, LTD serves to modify the new network, thereby enabling pattern separation (Manahan-Vaughan, 2018a; Collitti-Klausnitzer et al., 2021). Bottom left: In the absence of the refinement of signal-to-noise ratios and suppression of redundant synaptic connections, in the new representation by means of LTD, the former potentiated network merges with the new network and the memory of both experiences becomes generalized into one representation (bottom right). Photos: D. Manahan-Vaughan.
Induction of hippocampal synaptic plasticity results in the activation of members of the Fos, Jun, Krox, and Arc families of immediate early genes (IEGs; Manahan-Vaughan, 2017). The temporal pattern and distribution of neuronal expression of IEGs is determined by whether LTP, or LTD, is induced (Yilmaz-Rastoder et al., 2011; Hoang et al., 2021) and also depends on the kind of behavioral learning task implemented (Miyashita et al., 2009; Pevzner et al., 2012), or form of learning-facilitated synaptic plasticity that was instigated (Hoang et al., 2021; Table 2). The persistent (> 24 h) expression of hippocampal LTD requires protein translation in the CA1 region (Manahan-Vaughan, 2000), at commissural associational-CA3 and mossy fiber-CA3 synapses (Hagena and Manahan-Vaughan, 2013), but not in the dentate gyrus (Pöschel and Manahan-Vaughan, 2007). This latter finding might be explained by the fact that electrophysiological recordings were performed from the upper (suprapyramidal) layer of the dentate gyrus in the study by Pöschel and Manahan-Vaughan (2007), whereas more recent studies of gene encoding triggered by LTD have identified the lower (infrapyramidal) layer as being the site of somatic immediate early gene expression triggered by learning-facilitation of LTD (Hoang et al., 2021). IEGs that have either been reported to be essential for learning–mediated LTD, or are triggered by it comprise c-Fos, Homer1a and Arc (Kemp et al., 2013; Hoang et al., 2021). Blocking of c-Fos mRNA prevents learning-mediated LTD facilitation (Kemp et al., 2013), and slices from Arc knockout mice show impaired LTD (Plath et al., 2006), indicating causal contributions of c-Fos and Arc to LTD processes. In a non-behavioral setting, hippocampal LTD (> 24 h) is typically triggered by low frequency stimulation (LFS) of hippocampal afferents. In a behavioral setting LTD is enabled by coupling weak afferent stimulation with a spatial learning event, referred to as learning-facilitated LTD (Manahan-Vaughan, 2018a,b). Homer1a expression is triggered by LFS-induced LTD in all hippocampal regions and by learning-facilitated LTD (landmark exploration) in the CA3 and DG subfields (Hoang et al., 2021; Table 2). Expression is both task and synaptic subcompartment-specific and also relatively sparse. The sparseness depends on the particular IEG, brain region and the specific experience. Experience-dependent expression can be as low as 4–8% in the dentate gyrus, but can also reach levels up to 30% in the cornus ammonis (Hoang et al., 2018, 2021). It should be noted that a sparse population of c-Fos positive neurons in DG (e.g., 6%) may be sufficient to recall the encoded behavior (Liu et al., 2012). Thus sparse and specific IEG expression may enable the selective modulation of discrete hippocampal circuitry. In line with this it was recently shown that Fos expression that was triggered by prolonged exposure of mice to enriched spatial content, results in a differentiated modulation of the inhibitory output of specific populations of interneurons in the hippocampal CA1 region (Yap et al., 2021). By this means, discrete anatomical subcompartments of pyramidal cells can be modulated. This may support the modification and “pruning” of synaptic networks such that stable spatial representations result.
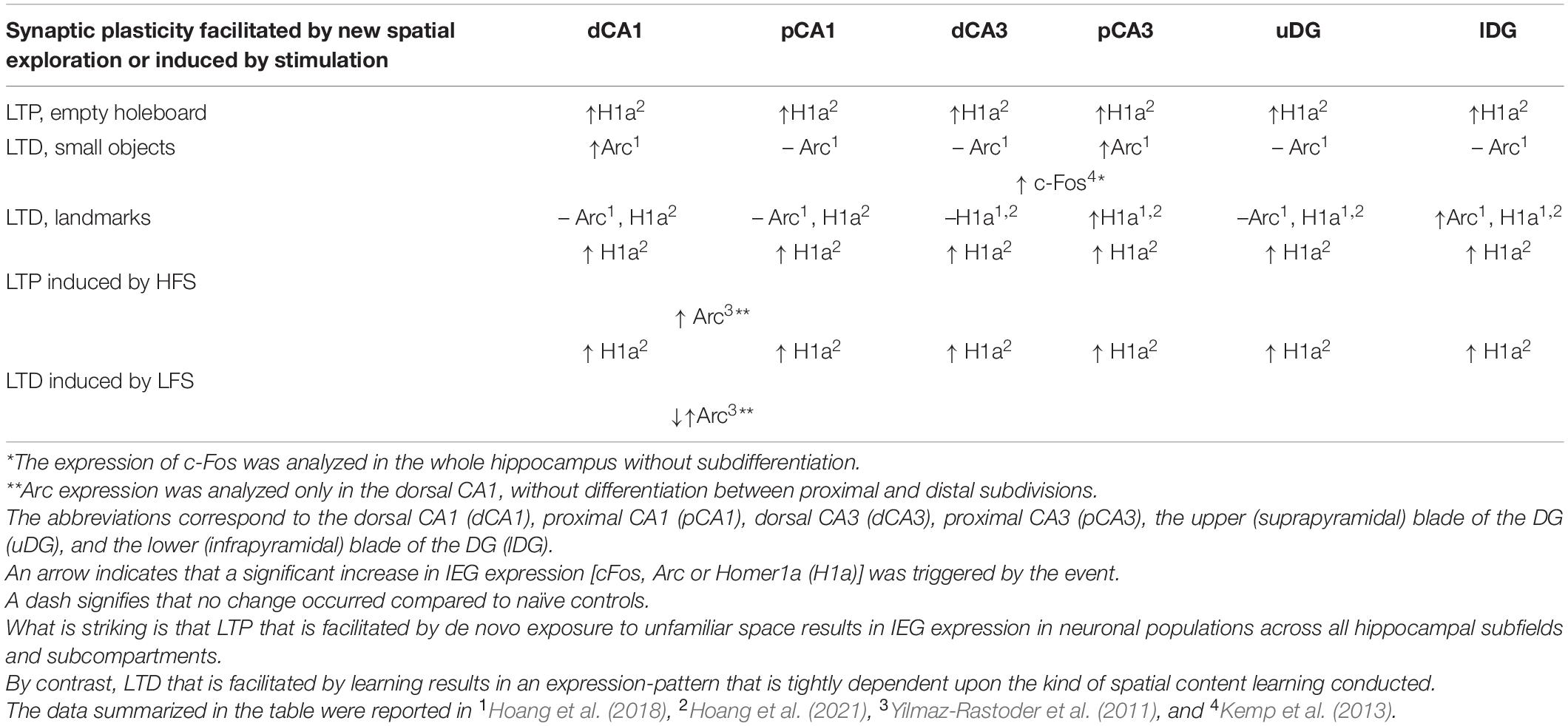
Table 2. Overview of subcompartment-specific cell-nucleus expression of immediate early genes triggered by either different kinds of spatial learning or by task-specific facilitation of hippocampal LTP or LTD.
Studies using fluorescence in situ hybridization to study cell compartment-specific expression of IEGs in neuronal nuclei hint that LTD may indeed modify neuronal networks. Thus, whereas the enablement of LTP by spatial learning results in IEG expression throughout all hippocampal subfields, LTD facilitation by spatial learning results in a differentiated and hippocampal subfield-specific elevation of nuclear IEG expression (Hoang et al., 2018). We believe that this effect is functionally highly meaningful. Consider the abovementioned findings of Yap et al. (2021) who reported that spatial learning results in discrete IEG-dependent modulation of the output of hippocampal interneurons: We recently reported that identical stimulation patterns, when applied to the lateral or medial entorhinal cortex inputs to the dentate gyrus, produce radically different synaptic plasticity outcomes within the same approximate population of granule cells in the dentate gyrus (Collitti-Klausnitzer et al., 2021). Most striking is the preference of the medial perforant path-dentate gyrus synapses to express LTP, and of the lateral perforant path-dentate gyrus to express LTD. Whereas the lateral perforant path provides information from the lateral entorhinal cortex to the hippocampus about the animal’s egocentric relationship to features of space, the medial perforant path provides information about the animal’s allocentric position in space (Lisman, 2007; van Strien et al., 2009; van Cauter et al., 2013; Wang et al., 2018, 2020). Interneurons in the dentate gyrus allow very discrete control of dendritic and axonal compartments (Houser, 2007). IEG expression driven by the experience-dependent induction of LTD in the dentate gyrus may thus enable highly specific modifications of a synaptic and neuronal ensemble, such that spatial information about egocentric and allocentric experience can be disambiguated.
Dendritic spines are highly dynamic and can change their density, morphology and volume in response to neuronal activity and experience (for reviews see Kasai et al., 2010; Fu and Zuo, 2011; Rochefort and Konnerth, 2012; Gipson and Olive, 2017; Chidambaram et al., 2019; Runge et al., 2020). For this reason, they have been proposed to be the site of memory storage in the brain (Nishiyama and Yasuda, 2015; Segal, 2017). Stimulation of single spines induces their enlargement (Maletic-Savatic et al., 1999; Matsuzaki et al., 2004; Nishiyama and Yasuda, 2015) that is, in turn, associated with increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) currents as well as LTP (Matsuzaki et al., 2004). Induction of LTP, conversely, triggers synaptogenesis (Oe et al., 2013; Hasegawa et al., 2015). Particularly, LTP induction (by high frequency stimulation, HFS, or theta burst stimulation, TBS) in CA1 dendrites induces enlargement or de novo growth of spines (Engert and Bonhoeffer, 1999; Matsuzaki et al., 2004; Nägerl et al., 2004, 2007) that can form mature synapses mostly with pre-existing synaptic boutons (Toni et al., 1999; Nägerl et al., 2007). Moreover, LTD, induced by LFS, in the CA1 region has been associated with spine shrinkage or retraction (Nägerl et al., 2004; Zhou et al., 2004), as well as increased synaptic bouton turnover and decreased volume of boutons associated with retracted spines (Becker et al., 2008).
Could synaptic plasticity, and most specifically LTD, serve as a cellular mechanism whereby synaptic remodeling, in conjunction with long-term memory formation, is enabled? A causal link between LTD and spine structural plasticity is supported by several experimental findings (Matsuzaki et al., 2001, 2004; Becker et al., 2008; Kwon and Sabatini, 2011; Wiegert and Oertner, 2013; Bosch et al., 2014; for reviews see Bosch and Hayashi, 2012; Nishiyama and Yasuda, 2015; Suratkal et al., 2021). A further link between synaptic plasticity and spine remodeling is provided by the postsynaptic actin cytoskeleton that plays a major role in structural and functional aspects of dendritic spines (Gipson and Olive, 2017; Runge et al., 2020; Suratkal et al., 2021). It undergoes a constant turnover of polymerized filamentous (F)-actin, or depolymerized globular (G)-actin. It was shown that HFS of the hippocampus switches the equilibrium toward F-actin, whereas LFS increases G-actin (Okamoto et al., 2004). Importantly, these frequency-dependent modulations of actin polymerization/de-polymerization occur concomitantly with spine enlargement (in case of HFS) and spine shrinkage (in case of LFS). Thus, induction of synaptic plasticity is accompanied by actin modifications underlying dendritic spine remodeling, further supporting the link between these two processes. These findings also suggest that spine remodeling and synaptic plasticity may share molecular mechanisms.
Indeed, structural and functional synaptic plasticity share a common denominator in terms of the time sequence of the synaptic changes upon activation (Nishiyama and Yasuda, 2015) and also share molecular mechanisms (Table 3). The actin cytoskeleton grows within minutes after LTP induction, with actin and actin-binding proteins (such as cofilin) accumulating in the spine. The actin cytoskeleton is first rapidly remodeled and subsequently stabilized (Bosch et al., 2014). The actin modification by actin-binding proteins contributes to enhanced stabilization (decreased de-polymerization) of F-actin which by a continuous actin polymerization possibly leads to spine expansion (Bosch and Hayashi, 2012; Nishiyama, 2019). After a few hours, the postsynaptic density (PSD) size increases, followed also by growth of presynaptic terminals (Bosch et al., 2014; Meyer et al., 2014; Nishiyama and Yasuda, 2015). Interestingly, the increase in PSD size, in PSD components (such as PSD-95) and in the presynaptic bouton re-establishes the correlation of these components to spine volume (that increased shortly after stimulation) and allows stabilization of the enlarged synapse (Meyer et al., 2014). If one or more of these subsynaptic components do not increase in size, the spine volume and synapse size return to their initial state.
Hippocampal LTD includes pre-and postsynaptic components (Pöschel and Stanton, 2007). LTD that occurs under circumstances of spatial learning mediates AMPAR endocytosis, suggesting that synapse-specific modifications take place (Ge et al., 2010; Ashby et al., 2021). In line with this, it has been shown that following induction of hippocampal LTD, pre-and postsynaptic structures become segregated (Bastrikova et al., 2008). Furthermore, after induction of hippocampal LTD, depressed synapses are eliminated from hippocampal circuitry (Hasegawa et al., 2015) and effects are N-methyl-D-aspartate receptor (NMDAR)-dependent (Wiegert and Oertner, 2013). Strikingly, this process is accompanied by a stabilization of the persistency of the retained fraction of spines on affected dendrites (Wiegert and Oertner, 2013). Moreover, whereas LTP increases synaptic stability at the level of dendritic spines, LTD weakens it, but LTD is not able to destabilize potently induced LTP (Wiegert et al., 2018). This latter finding aligns with our own findings that the potent induction of hippocampal LTP using strong HFS protocols induces higher levels and more widespread distribution of nuclear IEG expression than LTP induced by behavioral learning (Hoang et al., 2021). Others have reported that LTP is induced by fear conditioning (Whitlock et al., 2006; Subramaniyan et al., 2021) and that fear conditioning is associated with generalization of fear memory to a non-threatening environment (Liu et al., 2012). This raises the possibility that potently induced LTP may be associated with the generalization of memories, and that this may occur because it is invulnerable to modification by LTD.
The stabilization of new spines has also been linked to synaptic plasticity mechanisms involving calcium/calmodulin-dependent protein kinase II (CaMKII; Wilbrecht et al., 2010. Furthermore, changes in the number and persistency of synaptic spines is also driven by sensory experience (Holtmaat et al., 2006). Thus, both neuronal activity and sensory experience lead to spine remodeling (Rochefort and Konnerth, 2012); but how are these processes connected to learning and behavior? Bidirectional spine changes have been demonstrated in in vivo studies (Fu and Zuo, 2011; Rochefort and Konnerth, 2012). For example, although motor skill learning initially triggers spine formation, and the fraction of new spines that emerges correlates with task acquisition performance, memory retention after training over a period of several days correlates with the degree of spine elimination (Xu et al., 2009; Yang et al., 2009). Auditory fear conditioning and extinction learning are also associated with spine elimination and new spine formation in the cortex (Lai et al., 2012, 2018; Yang et al., 2016). Others have reported similar results in the hippocampus following contextual fear conditioning, whereby spine elimination (measured 24 h after fear conditioning) occurred particularly on hippocampal neurons that were activated during learning (Sanders et al., 2012). Thus, spine remodeling appears to comprise an important building block of memory circuits. Furthermore, structural changes in dendritic spines appear highly specifically in conjunction with cued learning, and correlate with memory performance.
Taken together, it is possible that experience-dependent LTD mediates synapse elimination and pruning of “LTP circuitry” at the level of spine insertion and enlargement, thereby refining the resolution, stability and integrity of synaptic networks. We propose that the benefit to synaptic circuitry, that is occupied with long- term experience-dependent information storage, is an increase in signal-to-noise ratios of those networks involved in retaining memories, such that similar experiences can be more easily disambiguated from one another.
From a temporal point of view, LTP is induced very rapidly (Gustafsson and Wigström, 1990), whereas LTD requires minutes to emerge (Dudek and Bear, 1993; Manahan-Vaughan and Braunewell, 1999; Klausnitzer and Manahan-Vaughan, 2008). This property may enable LTD to refine a recently acquired memory and help optimize accurate memory retention. In line with this possibility, LTD has been implicated in spatial memory consolidation (Ge et al., 2010), and pharmacological prevention of LTD prevents both the acquisition of an accurate memory of spatial experience and learning-facilitation of LTD (Kemp and Manahan-Vaughan, 2008a; Popkirov and Manahan-Vaughan, 2011; Hagena and Manahan-Vaughan, 2012; Lemon and Manahan-Vaughan, 2012; Hagena et al., 2016; Dietz and Manahan-Vaughan, 2017). Under circumstances where a spatial paradigm, used to trigger LTP, was combined with spatial content elements that enable LTD, it became apparent that an initial potentiation of synapses was followed minutes later by LTD (Manahan-Vaughan and Braunewell, 1999) suggesting that LTP and LTD are processes that can occur concomitantly in the same synaptic population. This property has, in fact, been reported within the entorhinal cortex, where it was shown that LTP and LTD can be expressed in the basal and apical dendritic compartments of the same pyramidal cell population (Solger et al., 2004). The same property was later reported in the hippocampus (Pavlowsky and Alarcon, 2012). These studies show that LTP and LTD can be triggered within the same dendritic compartment, but their dual manifestation is spatially regulated and activity-dependent.
Coincident expression of LTP and LTD has also been reported at hippocampal synapses. Here, for example induction of homosynaptic LTP results in heterosynaptic LTD (Stanton and Sejnowski, 1989). But coincident activity in the (subsequently) depressed synapse must occur at the time-point of the LTP event in order for heterosynaptic LTD to occur (Abraham et al., 2007), suggesting that heterosynaptic LTD is not a passive side effect of homosynaptic LTP induction, but is actually an active part of network modification. Moreover, the degree of heterosynaptic interactions between LTP and LTD is determined by the degree of overlap of the terminal fields of afferent inputs, and thereby of dendritic fields (White et al., 1990), These observations fit well with the possibility that one of the tasks of LTD is to improve signal-to-noise ratios during information encoding by means of LTP, and with the likelihood that LTD can constrain the physical distribution of LTP in a synaptic network.
Earlier in this article, we described how different components of spatial learning can result in the synaptic subcompartment-specific expression of LTD in the hippocampus. Specific afferent inputs corresponding to the dorsal and ventral streams (Mishkin et al., 1983) can determine to some extent which kind of information content is delivered to specific hippocampal subfields (Amaral and Witter, 1989; Burke et al., 2011; Sauvage et al., 2013; Hoang et al., 2018). But a further disambiguation of this information needs to take place at the level of the dendritic field, so that experience-dependent encoding at the level of LTP and LTD can take place, assuming these are the primary determinants of disambiguated spatial information storage. In this context, it has been reported that, in the CA1 region, both LTP and LTD induced by patterned afferent stimulation (e.g., HFS at 10 Hz, LFS at 1 Hz) is greater in magnitude in the dendritic compartment that is distal to the pyramidal cell layer, compared to plasticity that is expressed proximally to the pyramidal cell layer (Aihara et al., 2005). By contrast, if a stochastic-like stimulation pattern was used the LTP expression pattern remained the same (distal > proximal) with its magnitude being determined by the stimulation frequency used. By contrast, LTD expression occurred in a uniformal distributed manner across all dendritic subcompartments (distal = proximal) (Aihara et al., 2005). Furthermore, it has been reported that LTD that is expressed in the distal dendrites persists for longer than LTD expression in proximal dendrites (Ramachandran et al., 2015). This suggests that depending on the afferent input and the dendritic subcompartment in which LTD is expressed, the influence of LTD on expression patterns of LTP will vary. This influence also extends into the domain of metaplasticity, through which the prior history of activity-dependent experience of a synapse influences subsequent plasticity events (Abraham, 2008): prior induction of LTD reduces the magnitude of a subsequent induction of LTP (and vice versa), and simultaneous induction of LTP and LTD reduces the magnitude of LTP expressed (Pavlowsky and Alarcon, 2012). These processes suggest that LTD can temporally and physically constrain LTP into discrete synaptic subcompartments. One consequence of this process would be the limitation of the building of associations with other recently acquired representations, which at a plasticity level could be expected to prevent processes such as synaptic tagging (Frey and Morris, 1998). At a behavioral level this process would prevent generalization of memories and serve to optimize pattern separation. Behavioral evidence for this derives from studies that show that enhancement of LTD reduces fear memory generalization (Cao et al., 2021) and that inhibition of LTD prevents both item-place recognition memory (Dong et al., 2012; Goh and Manahan-Vaughan, 2013b; Kim et al., 2017; Ledonne et al., 2018) and spatial information updating (Kemp and Manahan-Vaughan, 2008b; Popkirov and Manahan-Vaughan, 2011; Lemon and Manahan-Vaughan, 2012; Dong et al., 2013).
Findings derived from the anatomical and cellular scrutiny of neuronal changes in learning and plasticity events, long with studies that interlink hippocampal LTP and LTD with spatial learning, indicate the interdependence of these processes and provide a plausible explanation as to how learning can be related to both structural and functional plasticity in the brain. Furthermore, the close interrelationship of these processes provides fascinating insights as to how persistent increases and decreases of synaptic efficacy are implemented in the brain to support memory formation, on both structural and functional levels.
Synaptic connections between neurons are considered to be the major site of information storage in the brain. Consequently, sensory experience and learning elicit physiological and structural modifications of synapses that are the neuronal substrate correlated with this experience. Meticulous research has provided us with substantial knowledge about synaptic modifications related to learning and memory. The size or number of dendritic spines can both increase and decrease in response to learning. Increase in spine formation and shrinkage of spines have been associated with LTP and LTD, respectively. Furthermore, LTD has been shown to modulate the magnitude of LTP and to constrain its expression both on physical (dendritic subcompartment) and temporal (metaplastic) levels. Behavioral studies that integrate the scrutiny of memory acquisition and retention have demonstrated a role for LTD in item-place memory, spatial content learning and representation updating. Furthermore, studies using pharmacology or transgenic manipulations infer a role for LTD in preventing memory generalization, pattern separation and optimization of the integrity of memories of spatial experience. Taken together, current evidence suggests that hippocampal LTD uniquely contributes to spatial learning and memory, most particularly in the support of the acquisition, updating and unadulterated long-term memory of spatial content.
Both authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
German Research Foundation (DFG); Grant Number: SFB 874/B10, Project No.: 122679504; SFB 1280/A04, Project No.: 316803389.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The research work cited, which originated from the authors’ own work, could not have been made possible without the extraordinary efforts of the co-authors of those publications, along with the technical staff who supported this work. We use this opportunity to extend our most heartfelt gratitude to these individuals.
Abraham, W. C. (2008). Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9:387. doi: 10.1038/nrn2356
Abraham, W. C., Jones, O. D., and Glanzman, D. L. (2019). Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 4:9. doi: 10.1038/s41539-019-0048-y
Abraham, W. C., Logan, B., Wolff, A., and Benuskova, L. (2007). Heterosynaptic” LTD in the dentate gyrus of anesthetized rat requires homosynaptic activity. J. Neurophysiol. 98, 1048–1051. doi: 10.1152/jn.00250.2007
Aggleton, J. P., Albasser, M. M., Aggleton, D. J., Poirier, G. L., and Pearce, J. M. (2010). Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav. Neurosci. 124, 55–68. doi: 10.1037/a0018320
Aihara, T., Kobayashi, Y., and Tsukada, M. (2005). Spatiotemporal visualization of long-term potentiation and depression in the hippocampal CA1 area. Hippocampus 15, 68–78. doi: 10.1002/hipo.20031
Alger, B. E., and Teyler, T. J. (1976). Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 110, 463–480. doi: 10.1016/0006-8993(76)90858-1
Amaral, D. G., and Witter, M. P. (1989). The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591. doi: 10.1016/0306-4522(89)90424-7
An, L., and Sun, W. (2018). Acute melamine affects spatial memory consolidation via inhibiting hippocampal NMDAR-dependent LTD in rats. Toxicol. Sci. 163, 385–396. doi: 10.1093/toxsci/kfx039
André, M. A. E., and Manahan-Vaughan, D. (2013). Spatial olfactory learning facilitates long-term depression in the hippocampus. Hippocampus 23, 963–968. doi: 10.1002/hipo.22158
Ashby, D. M., Floresco, S. B., Phillips, A. G., McGirr, A., Seamans, J. K., and Wang, Y. T. (2021). LTD is involved in the formation and maintenance of rat hippocampal CA1 place-cell fields. Nat. Commun. 12:100. doi: 10.1038/s41467-020-20317-7
Bastrikova, N., Gardner, G. A., Reece, J. M., Jeromin, A., and Dudek, S. M. (2008). Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 105, 3123–3127. doi: 10.1073/pnas.0800027105
Basu, J., and Siegelbaum, S. A. (2015). The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb. Perspect. Biol. 7:a021733. doi: 10.1101/cshperspect.a021733
Baumgärtel, K., and Mansuy, I. M. (2012). Neural functions of calcineurin in synaptic plasticity and memory. Learn. Mem. 19, 375–384. doi: 10.1101/lm.027201.112
Becker, N., Wierenga, C. J., Fonseca, R., Bonhoeffer, T., and Nägerl, U. V. (2008). LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron 60, 590–597. doi: 10.1016/j.neuron.2008.09.018
Bliss, T. V., and Lomo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. doi: 10.1113/jphysiol.1973.sp010273
Borovac, J., Bosch, M., and Okamoto, K. (2018). Regulation of actin dynamics during structural plasticity of dendritic spines: signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 91, 122–130. doi: 10.1016/j.mcn.2018.07.001
Bosch, M., Castro, J., Saneyoshi, T., Matsuno, H., Sur, M., and Hayashi, Y. (2014). Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82, 444–459. doi: 10.1016/j.neuron.2014.03.021
Bosch, M., and Hayashi, Y. (2012). Structural plasticity of dendritic spines. Curr. Opin. Neurobiol. 22, 383–388. doi: 10.1016/j.conb.2011.09.002
Brown, M. W., and Aggleton, J. P. (2001). Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2, 51–61. doi: 10.1038/35049064
Burke, S. N., Maurer, A. P., Nematollahi, S., Uprety, A. R., Wallace, J. L., and Barnes, C. A. (2011). The influence of objects on place field expression and size in distal hippocampal CA1. Hippocampus 21, 783–801. doi: 10.1002/hipo.20929
Cao, Q., Wei, Y., Deng, J., Li, J., Huang, Y., Li, Y., et al. (2021). NRG1 accelerates the forgetting of fear memories and facilitates the induction of long-term depression in adult mice. Psychopharmacology 238, 2535–2542. doi: 10.1007/s00213-021-05877-w
Chidambaram, S. B., Rathipriya, A. G., Bolla, S. R., Bhat, A., Ray, B., Mahalakshmi, A. M., et al. (2019). Dendritic spines: revisiting the physiological role. Prog. Neuropsychopharmacol. Biol. Psychiatry 92, 161–193. doi: 10.1016/j.pnpbp.2019.01.005
Collitti-Klausnitzer, J., Hagena, H., Dubovyk, V., and Manahan-Vaughan, D. (2021). Preferential frequency-dependent induction of synaptic depression by the lateral perforant path and of synaptic potentiation by the medial perforant path inputs to the dentate gyrus. Hippocampus 31, 957–981. doi: 10.1002/hipo.23338
Costa, J. F., Dines, M., and Lamprecht, R. (2020). The Role of Rac GTPase in dendritic spine morphogenesis and memory. Front. Synaptic Neurosci. 12:12. doi: 10.3389/fnsyn.2020.00012
Coultrap, S. J., and Bayer, K. U. (2012). CaMKII regulation in information processing and storage. Trends Neurosci. 35, 607–618. doi: 10.1016/j.tins.2012.05.003
Cui, Z., Feng, R., Jacobs, S., Duan, Y., Wang, H., Cao, X., et al. (2013). Increased NR2A:NR2B ratio compresses long-term depression range and constrains long-term memory. Sci. Rep. 3:1036. doi: 10.1038/srep01036
Dietz, B., and Manahan-Vaughan, D. (2017). Hippocampal long-term depression is facilitated by the acquisition and updating of memory of spatial auditory content and requires mGlu5 activation. Neuropharmacology 115, 30–41. doi: 10.1016/j.neuropharm.2016.02.026
Dong, Z., Bai, Y., Wu, X., Li, H., Gong, B., Howland, J. G., et al. (2013). Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology 64, 65–73. doi: 10.1016/j.neuropharm.2012.06.027
Dong, Z., Gong, B., Li, H., Bai, Y., Wu, X., Huang, Y., et al. (2012). Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J. Neurosci. 32, 11980–11990. doi: 10.1523/JNEUROSCI.0984-12.2012
Dudek, S. M., and Bear, M. F. (1993). Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J. Neurosci. 13, 2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993
Engert, F., and Bonhoeffer, T. (1999). Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70. doi: 10.1038/19978
Etkin, A., Alarcón, J. M., Weisberg, S. P., Touzani, K., Huang, Y. Y., Nordheim, A., et al. (2006). A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron 50, 127–143. doi: 10.1016/j.neuron.2006.03.013
Frey, U., and Morris, R. (1998). Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology 37, 545–552. doi: 10.1016/S0028-3908(98)00040-9
Fu, M., and Zuo, Y. (2011). Experience-dependent structural plasticity in the cortex. Trends Neurosci. 34, 177–187. doi: 10.1016/j.tins.2011.02.001
Ge, Y., Dong, Z., Bagot, R. C., Howland, J. G., Phillips, A. G., Wong, T. P., et al. (2010). Hippocampal long-term depression is required for the consolidation of spatial memory. Proc. Natl. Acad. Sci. U.S.A. 107, 16697–16702. doi: 10.1073/pnas.1008200107
Gipson, C. D., and Olive, M. F. (2017). Structural and functional plasticity of dendritic spines - root or result of behavior? Genes Brain Behav. 16, 101–117. doi: 10.1111/gbb.12324
Goh, J. J., and Manahan-Vaughan, D. (2013c). Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb. Cortex 23, 1118–1125. doi: 10.1093/cercor/bhs089
Goh, J. J., and Manahan-Vaughan, D. (2013a). Endogenous hippocampal LTD that is enabled by spatial object recognition requires activation of NMDA receptors and the metabotropic glutamate receptor, mGlu5. Hippocampus 23, 129–138. doi: 10.1002/hipo.22072
Goh, J. J., and Manahan-Vaughan, D. (2013b). Hippocampal long-term depression in freely behaving mice requires the activation of beta-adrenergic receptors. Hippocampus 23, 1299–1308. doi: 10.1002/hipo.22168
Griffiths, S., Scott, H., Glover, C., Bienemann, A., Ghorbel, M. T., Uney, J., et al. (2008). Expression of long-term depression underlies visual recognition memory. Neuron 58, 186–194. doi: 10.1016/j.neuron.2008.02.022
Gustafsson, B., and Wigstrom, H. (1986). Hippocampal long-lasting potentiation produced by pairing single volleys and brief conditioning tetani evoked in separate afferents. J. Neurosci. 6, 1575–1582. doi: 10.1523/JNEUROSCI.06-06-01575.1986
Gustafsson, B., and Wigström, H. (1990). “Chapter 16 long-term potentiation in the hippocampal CA1 region: its induction and early temporal development,” in Understanding the Brain through the Hippocampus: The Hippocampal Region as a Model for Studying Brain Structure and Function, eds J. Storm-Mathisen, J. Zimmer, O. P. Ottersen, and T. W. Blackstad (Amsterdam: Elsevier), 223–232. doi: 10.1016/s0079-6123(08)61252-2
Gustafsson, B., Wigstrom, H., Abraham, W. C., and Huang, Y. Y. (1987). Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J. Neurosci. 7, 774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987
Hagena, H., Hansen, N., and Manahan-Vaughan, D. (2016). β-adrenergic control of hippocampal function: subserving the choreography of synaptic information storage and memory. Cereb. Cortex 26, 1349–1364. doi: 10.1093/cercor/bhv330
Hagena, H., and Manahan-Vaughan, D. (2011). Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb. Cortex 21, 2442–2449. doi: 10.1093/cercor/bhq271
Hagena, H., and Manahan-Vaughan, D. (2012). Learning-facilitated long-term depression and long-term potentiation at mossy fiber-CA3 synapses requires activation of β-adrenergic receptors. Front. Integr. Neurosci. 6:23. doi: 10.3389/fnint.2012.00023
Hagena, H., and Manahan-Vaughan, D. (2013). Differentiation in the protein synthesis-dependency of persistent synaptic plasticity in mossy fiber and associational/commissural CA3 synapses in vivo. Front. Integr. Neurosci. 7:10. doi: 10.3389/fnint.2013.00010
Hansen, N., and Manahan-Vaughan, D. (2015). Hippocampal long-term potentiation that is elicited by perforant path stimulation or that occurs in conjunction with spatial learning is tightly controlled by beta-adrenoreceptors and the locus coeruleus. Hippocampus 25, 1285–1298. doi: 10.1002/hipo.22436
Hasegawa, S., Sakuragi, S., Tominaga-Yoshino, K., and Ogura, A. (2015). Dendritic spine dynamics leading to spine elimination after repeated inductions of LTD. Sci. Rep. 5:7707. doi: 10.1038/srep07707
Hauser, M. F. A., Wiescholleck, V., Colitti-Klausnitzer, J., Bellebaum, C., and Manahan-Vaughan, D. (2019). Event-related potentials evoked by passive visuospatial perception in rats and humans reveal common denominators in information processing. Brain Struct. Funct. 224, 1583–1597. doi: 10.1007/s00429-019-01854-4
Hayama, T., Noguchi, J., Watanabe, S., Takahashi, N., Hayashi-Takagi, A., Ellis-Davies, G. C. R., et al. (2013). GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat. Neurosci. 16, 1409–1416. doi: 10.1038/nn.3496
Hoang, T.-H., Aliane, V., and Manahan-Vaughan, D. (2018). Novel encoding and updating of positional, or directional, spatial cues are processed by distinct hippocampal subfields: evidence for parallel information processing and the “what” stream. Hippocampus 28, 315–326. doi: 10.1002/hipo.22833
Hoang, T.-H., Böge, J., and Manahan-Vaughan, D. (2021). Hippocampal subfield-specific Homer1a expression is triggered by learning-facilitated long-term potentiation and long-term depression at medial perforant path synapses. Hippocampus 31, 897–915. doi: 10.1002/hipo.23333
Holbro, N., Grunditz, A., and Oertner, T. G. (2009). Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl. Acad. Sci. U.S.A. 106, 15055–15060. doi: 10.1073/pnas.0905110106
Holtmaat, A., Wilbrecht, L., Knott, G. W., Welker, E., and Svoboda, K. (2006). Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441, 979–983. doi: 10.1038/nature04783
Houser, C. R. (2007). “Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity,” in The Dentate Gyrus: A Comprehensive Guide to Structure Function and Clinical Implications, ed. H. E. Scharfman (Amsterdam: Elsevier), 217–811. doi: 10.1016/S0079-6123(07)63013-1
Jacobs, L. F., and Schenk, F. (2003). Unpacking the cognitive map: the parallel map theory of hippocampal function. Psychol. Rev. 110, 285–315. doi: 10.1037/0033-295x.110.2.285
Josselyn, S. A., and Tonegawa, S. (2020). Memory engrams: recalling the past and imagining the future. Science 367:eaaw4325. doi: 10.1126/science.aaw4325
Kasai, H., Fukuda, M., Watanabe, S., Hayashi-Takagi, A., and Noguchi, J. (2010). Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33, 121–129. doi: 10.1016/j.tins.2010.01.001
Kemp, A., and Manahan-Vaughan, D. (2004). Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Natl. Acad. Sci. U.S.A. 101, 8192–8197. doi: 10.1073/pnas.0402650101
Kemp, A., and Manahan-Vaughan, D. (2007). Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 30, 111–118. doi: 10.1016/j.tins.2007.01.002
Kemp, A., and Manahan-Vaughan, D. (2008a). Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb. Cortex 18, 1326–1334. doi: 10.1093/cercor/bhm164
Kemp, A., and Manahan-Vaughan, D. (2008b). The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb. Cortex 18, 968–977. doi: 10.1093/cercor/bhm136
Kemp, A., and Manahan-Vaughan, D. (2012). Passive spatial perception facilitates the expression of persistent hippocampal long-term depression. Cereb. Cortex 22, 1614–1621. doi: 10.1093/cercor/bhr233
Kemp, A., Tischmeyer, W., and Manahan-Vaughan, D. (2013). Learning-facilitated long-term depression requires activation of the immediate early gene, c-fos, and is transcription dependent. Behav. Brain Res. 254, 83–91. doi: 10.1016/j.bbr.2013.04.036
Kim, D. H., Kang, M., Kim, C.-H., Huh, Y. H., Cho, I. H., Ryu, H.-H., et al. (2017). SPIN90 modulates long-term depression and behavioral flexibility in the hippocampus. Front. Mol. Neurosci. 10:295. doi: 10.3389/fnmol.2017.00295
Kim, I. H., Wang, H., Soderling, S. H., and Yasuda, R. (2014). Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall. eLife 3:e02839. doi: 10.7554/eLife.02839
Kim, J.-I., Lee, H.-R., Sim, S., Baek, J., Yu, N.-K., Choi, J.-H., et al. (2011). PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat. Neurosci. 14, 1447–1454. doi: 10.1038/nn.2937
Klausnitzer, J., and Manahan-Vaughan, D. (2008). Frequency facilitation at mossy fiber-CA3 synapses of freely behaving rats is regulated by adenosine A1 receptors. J. Neurosci. 28, 4836–4840. doi: 10.1523/JNEUROSCI.3729-07.2008
Kwon, H.-B., and Sabatini, B. L. (2011). Glutamate induces de novo growth of functional spines in developing cortex. Nature 474, 100–104. doi: 10.1038/nature09986
Lai, C. S. W., Adler, A., and Gan, W.-B. (2018). Fear extinction reverses dendritic spine formation induced by fear conditioning in the mouse auditory cortex. Proc. Natl. Acad. Sci. U.S.A. 115, 9306–9311. doi: 10.1073/pnas.1801504115
Lai, C. S. W., Franke, T. F., and Gan, W.-B. (2012). Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483, 87–91. doi: 10.1038/nature10792
Ledonne, A., Mango, D., Latagliata, E. C., Chiacchierini, G., Nobili, A., Nisticò, R., et al. (2018). Neuregulin 1/ErbB signalling modulates hippocampal mGluRI-dependent LTD and object recognition memory. Pharmacol. Res. 130, 12–24. doi: 10.1016/j.phrs.2018.02.003
Lemon, N., and Manahan-Vaughan, D. (2012). Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb. Cortex 22, 2131–2138. doi: 10.1093/cercor/bhr297
Lisman, J. E. (2007). “Role of the dual entorhinal inputs to hippocampus: a hypothesis based on cue/action (non-self/self) couplets,” in The Dentate Gyrus: A Comprehensive Guide to Structure Function and Clinical Implications, ed. H. E. Scharfman (Amsterdam: Elsevier), 615–818. doi: 10.1016/S0079-6123(07)63033-7
Liu, X., Gu, Q.-H., Duan, K., and Li, Z. (2014). NMDA receptor-dependent LTD is required for consolidation but not acquisition of fear memory. J. Neurosci. 34, 8741–8748. doi: 10.1523/JNEUROSCI.2752-13.2014
Liu, X., Ramirez, S., Pang, P. T., Puryear, C. B., Govindarajan, A., Deisseroth, K., et al. (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385. doi: 10.1038/nature11028
Luchkina, N. V., and Bolshakov, V. Y. (2019). Mechanisms of fear learning and extinction: synaptic plasticity-fear memory connection. Psychopharmacology 236, 163–182. doi: 10.1007/s00213-018-5104-4
Maletic-Savatic, M., Malinow, R., and Svoboda, K. (1999). Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927. doi: 10.1126/science.283.5409.1923
Manahan-Vaughan, D. (1997). Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J. Neurosci. 17, 3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997
Manahan-Vaughan, D. (2000). Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb. Cortex 10, 482–487. doi: 10.1093/cercor/10.5.482
Manahan-Vaughan, D. (2017). “Learning-related hippocampal long-term potentiation and long-term depression,” in Learning and Memory: A Comprehensive Reference, ed. J. H. Byrne (San Diego, CA: Elsevier Science), 585–609. doi: 10.1016/b978-0-12-809324-5.21104-8
Manahan-Vaughan, D. (2018a). “Item-place encoding through hippocampal long-term depression,” in Handbook of Object Novelty Recognition, eds A. Ennaceur and M. A. de Souza Silva (Amsterdam: Academic Press), 273–289. doi: 10.1016/b978-0-12-812012-5.00019-7
Manahan-Vaughan, D. (2018b). “Recording field potentials and synaptic plasticity from freely behaving rodents,” in Handbook of in vivo Neural Plasticity Techniques: A Systems Neuroscience Approach to the Neural Basis of Memory and Cognition, ed. D. Manahan-Vaughan (San Diego CA: Elsevier), 1–42. doi: 10.1016/b978-0-12-812028-6.00001-x
Manahan-Vaughan, D., and Braunewell, K. H. (1999). Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Natl. Acad. Sci. U.S.A. 96, 8739–8744. doi: 10.1073/pnas.96.15.8739
Martin, S. J., Grimwood, P. D., and Morris, R. G. (2000). Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711. doi: 10.1146/annurev.neuro.23.1.649
Matsuzaki, M., Ellis-Davies, G. C., Nemoto, T., Miyashita, Y., Iino, M., and Kasai, H. (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092. doi: 10.1038/nn736
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. R., and Kasai, H. (2004). Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766. doi: 10.1038/nature02617
Meyer, D., Bonhoeffer, T., and Scheuss, V. (2014). Balance and stability of synaptic structures during synaptic plasticity. Neuron 82, 430–443. doi: 10.1016/j.neuron.2014.02.031
Mills, F., Bartlett, T. E., Dissing-Olesen, L., Wisniewska, M. B., Kuznicki, J., Macvicar, B. A., et al. (2014). Cognitive flexibility and long-term depression (LTD) are impaired following β-catenin stabilization in vivo. Proc. Natl. Acad. Sci. U.S.A. 111, 8631–8636. doi: 10.1073/pnas.1404670111
Mishkin, M., Ungerleider, L. G., and Macko, K. A. (1983). Object vision and spatial vision: two cortical pathways. Trends Neurosci. 6, 414–417. doi: 10.1016/0166-2236(83)90190-X
Miyashita, T., Kubik, S., Haghighi, N., Steward, O., and Guzowski, J. F. (2009). Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J. Neurosci. 29, 898–906. doi: 10.1523/JNEUROSCI.4588-08.2009
Nabavi, S., Fox, R., Proulx, C. D., Lin, J. Y., Tsien, R. Y., and Malinow, R. (2014). Engineering a memory with LTD and LTP. Nature 511, 348–352. doi: 10.1038/nature13294
Nägerl, U. V., Eberhorn, N., Cambridge, S. B., and Bonhoeffer, T. (2004). Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44, 759–767. doi: 10.1016/j.neuron.2004.11.016
Nägerl, U. V., Köstinger, G., Anderson, J. C., Martin, K. A. C., and Bonhoeffer, T. (2007). Protracted synaptogenesis after activity-dependent spinogenesis in hippocampal neurons. J. Neurosci. 27, 8149–8156. doi: 10.1523/JNEUROSCI.0511-07.2007
Navarrete, M., Cuartero, M. I., Palenzuela, R., Draffin, J. E., Konomi, A., Serra, I., et al. (2019). Astrocytic p38α MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nat. Commun. 10:2968. doi: 10.1038/s41467-019-10830-9
Nicholls, R. E., Alarcon, J. M., Malleret, G., Carroll, R. C., Grody, M., Vronskaya, S., et al. (2008). Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 58, 104–117. doi: 10.1016/j.neuron.2008.01.039
Nishiyama, J. (2019). Plasticity of dendritic spines: molecular function and dysfunction in neurodevelopmental disorders. Psychiatry Clin. Neurosci. 73, 541–550. doi: 10.1111/pcn.12899
Nishiyama, J., and Yasuda, R. (2015). Biochemical computation for spine structural plasticity. Neuron 87, 63–75. doi: 10.1016/j.neuron.2015.05.043
Oe, Y., Tominaga-Yoshino, K., Hasegawa, S., and Ogura, A. (2013). Dendritic spine dynamics in synaptogenesis after repeated LTP inductions: dependence on pre-existing spine density. Sci. Rep. 3:1957. doi: 10.1038/srep01957
Oh, W. C., Hill, T. C., and Zito, K. (2013). Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc. Natl. Acad. Sci. U.S.A. 110, E305–E312. doi: 10.1073/pnas.1214705110
Okamoto, K.-I., Nagai, T., Miyawaki, A., and Hayashi, Y. (2004). Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci. 7, 1104–1112. doi: 10.1038/nn1311
Pavlowsky, A., and Alarcon, J. M. (2012). Interaction between long-term potentiation and depression in CA1 synapses: temporal constrains, functional compartmentalization and protein synthesis. PLoS One 7:e29865. doi: 10.1371/journal.pone.0029865
Pevzner, A., Miyashita, T., Schiffman, A. J., and Guzowski, J. F. (2012). Temporal dynamics of Arc gene induction in hippocampus: relationship to context memory formation. Neurobiol. Learn. Mem. 97, 313–320. doi: 10.1016/j.nlm.2012.02.004
Plath, N., Ohana, O., Dammermann, B., Errington, M. L., Schmitz, D., Gross, C., et al. (2006). Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52, 437–444. doi: 10.1016/j.neuron.2006.08.024
Popkirov, S. G., and Manahan-Vaughan, D. (2011). Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb. Cortex 21, 501–509. doi: 10.1093/cercor/bhq093
Pöschel, B., and Manahan-Vaughan, D. (2007). Persistent (24h) long-term depression in the dentate gyrus of freely moving rats is not dependent on activation of NMDA receptors, L-type voltage-gated calcium channels or protein synthesis. Neuropharmacology 52, 46–54. doi: 10.1016/j.neuropharm.2006.07.019
Pöschel, B., and Stanton, P. K. (2007). “Comparison of cellular mechanisms of long-term depression of synaptic strength at perforant path–granule cell and Schaffer collateral–CA1 synapses,” in The Dentate Gyrus: A Comprehensive Guide to Structure Function and Clinical Implications, ed. H. E. Scharfman (Amsterdam: Elsevier), 473–500. doi: 10.1016/S0079-6123(07)63026-X
Ramachandran, B., Ahmed, S., and Dean, C. (2015). Long-term depression is differentially expressed in distinct lamina of hippocampal CA1 dendrites. Front. Cell. Neurosci. 9:23. doi: 10.3389/fncel.2015.00023
Ramirez, S., Liu, X., Lin, P.-A., Suh, J., Pignatelli, M., Redondo, R. L., et al. (2013). Creating a false memory in the hippocampus. Science 341, 387–391. doi: 10.1126/science.1239073
Richards, B. A., and Frankland, P. W. (2017). The persistence and transience of memory. Neuron 94, 1071–1084. doi: 10.1016/j.neuron.2017.04.037
Robertson, E. M. (2018). Memory instability as a gateway to generalization. PLoS Biol. 16:e2004633. doi: 10.1371/journal.pbio.2004633
Rocchetti, J., Isingrini, E., Dal Bo, G., Sagheby, S., Menegaux, A., Tronche, F., et al. (2015). Presynaptic D2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol. Psychiatry 77, 513–525. doi: 10.1016/j.biopsych.2014.03.013
Rochefort, N. L., and Konnerth, A. (2012). Dendritic spines: from structure to in vivo function. EMBO Rep. 13, 699–708. doi: 10.1038/embor.2012.102
Runge, K., Cardoso, C., and de Chevigny, A. (2020). Dendritic spine plasticity: function and mechanisms. Front. Synaptic Neurosci. 12:36. doi: 10.3389/fnsyn.2020.00036
Sanders, J., Cowansage, K., Baumgärtel, K., and Mayford, M. (2012). Elimination of dendritic spines with long-term memory is specific to active circuits. J. Neurosci. 32, 12570–12578. doi: 10.1523/JNEUROSCI.1131-12.2012
Sauvage, M. M., Nakamura, N. H., and Beer, Z. (2013). Mapping memory function in the medial temporal lobe with the immediate-early gene Arc. Behav. Brain Res. 254, 22–33. doi: 10.1016/j.bbr.2013.04.048
Segal, M. (2017). Dendritic spines: morphological building blocks of memory. Neurobiol. Learn. Mem. 138, 3–9. doi: 10.1016/j.nlm.2016.06.007
Solger, J., Wozny, C., Manahan-Vaughan, D., and Behr, J. (2004). Distinct mechanisms of bidirectional activity-dependent synaptic plasticity in superficial and deep layers of rat entorhinal cortex. Eur. J. Neurosci. 19, 2003–2007. doi: 10.1111/j.1460-9568.2004.03292.x
Stanton, P. K., and Sejnowski, T. J. (1989). Associative long-term depression in the hippocampus induced by hebbian covariance. Nature 339, 215–218. doi: 10.1038/339215a0
Stein, I. S., Park, D. K., Flores, J. C., Jahncke, J. N., and Zito, K. (2020). Molecular mechanisms of non-ionotropic NMDA receptor signaling in dendritic spine shrinkage. J. Neurosci. 40, 3741–3750. doi: 10.1523/JNEUROSCI.0046-20.2020
Stein, I. S., and Zito, K. (2019). Dendritic spine elimination: molecular mechanisms and implications. Neuroscientist 25, 27–47. doi: 10.1177/1073858418769644
Straube, T., Korz, V., and Frey, J. U. (2003). Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci. Lett. 344, 5–8. doi: 10.1016/S0304-3940(03)00349-5
Subramaniyan, M., Manivannan, S., Chelur, V., Tsetsenis, T., Jiang, E., and Dani, J. A. (2021). Fear conditioning potentiates the hippocampal CA1 commissural pathway in vivo and increases awake phase sleep. Hippocampus 31, 1154–1175. doi: 10.1002/hipo.23381
Suratkal, S. S., Yen, Y.-H., and Nishiyama, J. (2021). Imaging dendritic spines: molecular organization and signaling for plasticity. Curr. Opin. Neurobiol. 67, 66–74. doi: 10.1016/j.conb.2020.08.006
Titley, H. K., Brunel, N., and Hansel, C. (2017). Toward a neurocentric view of learning. Neuron 95, 19–32. doi: 10.1016/j.neuron.2017.05.021
Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R., and Muller, D. (1999). LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425. doi: 10.1038/46574
Toyoda, H., Katagiri, A., Kato, T., and Sato, H. (2020). Intranasal administration of rotenone reduces GABAergic inhibition in the mouse insular cortex leading to impairment of LTD and conditioned taste aversion memory. Int. J. Mol. Sci. 22:259. doi: 10.3390/ijms22010259
Tsumoto, T. (1993). Long-term depression in cerebral cortex: a possible substrate of “forgetting” that should not be forgotten. Neurosci. Res. 16, 263–270. doi: 10.1016/0168-0102(93)90036-P
van Cauter, T., Camon, J., Alvernhe, A., Elduayen, C., Sargolini, F., and Save, E. (2013). Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb. Cortex 23, 451–459. doi: 10.1093/cercor/bhs033
van Strien, N. M., Cappaert, N. L. M., and Witter, M. P. (2009). The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci. 10, 272–282. doi: 10.1038/nrn2614
Wan, H., Aggleton, J. P., and Brown, M. W. (1999). Different contributions of the hippocampus and perirhinal cortex to recognition memory. J. Neurosci. 19, 1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999
Wang, C., Chen, X., and Knierim, J. J. (2020). Egocentric and allocentric representations of space in the rodent brain. Curr. Opin. Neurobiol. 60, 12–20. doi: 10.1016/j.conb.2019.11.005
Wang, C., Chen, X., Lee, H., Deshmukh, S. S., Yoganarasimha, D., Savelli, F., et al. (2018). Egocentric coding of external items in the lateral entorhinal cortex. Science 362, 945–949. doi: 10.1126/science.aau4940
White, G., Levy, W. B., and Steward, O. (1990). Spatial overlap between populations of synapses determines the extent of their associative interaction during the induction of long-term potentiation and depression. J. Neurophysiol. 64, 1186–1198. doi: 10.1152/jn.1990.64.4.1186
Whitlock, J. R., Heynen, A. J., Shuler, M. G., and Bear, M. F. (2006). Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097. doi: 10.1126/science.1128134
Wiegert, J. S., and Oertner, T. G. (2013). Long-term depression triggers the selective elimination of weakly integrated synapses. Proc. Natl. Acad. Sci. U.S.A. 110, E4510–E4519. doi: 10.1073/pnas.1315926110
Wiegert, J. S., Pulin, M., Gee, C. E., and Oertner, T. G. (2018). The fate of hippocampal synapses depends on the sequence of plasticity-inducing events. eLife 7:e39151. doi: 10.7554/eLife.39151
Wilbrecht, L., Holtmaat, A., Wright, N., Fox, K., and Svoboda, K. (2010). Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J. Neurosci. 30, 4927–4932. doi: 10.1523/JNEUROSCI.6403-09.2010
Woolfrey, K. M., O’Leary, H., Goodell, D. J., Robertson, H. R., Horne, E. A., Coultrap, S. J., et al. (2018). CaMKII regulates the depalmitoylation and synaptic removal of the scaffold protein AKAP79/150 to mediate structural long-term depression. J. Biol. Chem. 293, 1551–1567. doi: 10.1074/jbc.M117.813808
Xu, T., Yu, X., Perlik, A. J., Tobin, W. F., Zweig, J. A., Tennant, K., et al. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919. doi: 10.1038/nature08389
Yang, G., Pan, F., and Gan, W.-B. (2009). Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924. doi: 10.1038/nature08577
Yang, Y., Liu, D.-Q., Huang, W., Deng, J., Sun, Y., Zuo, Y., et al. (2016). Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nat. Neurosci. 19, 1348–1355. doi: 10.1038/nn.4370
Yap, E.-L., Pettit, N. L., Davis, C. P., Nagy, M. A., Harmin, D. A., Golden, E., et al. (2021). Bidirectional perisomatic inhibitory plasticity of a Fos neuronal network. Nature 590, 115–121. doi: 10.1038/s41586-020-3031-0
Yilmaz-Rastoder, E., Miyamae, T., Braun, A. E., and Thiels, E. (2011). LTP- and LTD-inducing stimulations cause opposite changes in arc/arg3.1 mRNA level in hippocampal area CA1 in vivo. Hippocampus 21, 1290–1301. doi: 10.1002/hipo.20838
Zeng, H., Chattarji, S., Barbarosie, M., Rondi-Reig, L., Philpot, B. D., Miyakawa, T., et al. (2001). Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 107, 617–629. doi: 10.1016/S0092-8674(01)00585-2
Zhang, L., Zhang, P., Wang, G., Zhang, H., Zhang, Y., Yu, Y., et al. (2018). Ras and Rap signal bidirectional synaptic plasticity via distinct subcellular microdomains. Neuron 98, 783–800.e4. doi: 10.1016/j.neuron.2018.03.049
Keywords: LTD, LTP, hippocampus, spatial learning and memory, rodent
Citation: Stacho M and Manahan-Vaughan D (2022) The Intriguing Contribution of Hippocampal Long-Term Depression to Spatial Learning and Long-Term Memory. Front. Behav. Neurosci. 16:806356. doi: 10.3389/fnbeh.2022.806356
Received: 31 October 2021; Accepted: 10 March 2022;
Published: 25 April 2022.
Edited by:
André Fiala, University of Göttingen, GermanyReviewed by:
Yu-zhang Liu, University of Pittsburgh, United StatesCopyright © 2022 Stacho and Manahan-Vaughan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denise Manahan-Vaughan, ZGVuaXNlLm1hbmFoYW4tdmF1Z2hhbkBydWIuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.