
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Behav. Neurosci., 19 January 2023
Sec. Pathological Conditions
Volume 16 - 2022 | https://doi.org/10.3389/fnbeh.2022.1006065
This article is part of the Research TopicTranslational Behavioral Approaches in Animal Models of PsychiatryView all 10 articles
 Karla K. Ausderau1,2,3†‡
Karla K. Ausderau1,2,3†‡ Ricki J. Colman1,4†
Ricki J. Colman1,4† Sabrina Kabakov3
Sabrina Kabakov3 Nancy Schultz-Darken1
Nancy Schultz-Darken1 Marina E. Emborg1,5*‡
Marina E. Emborg1,5*‡Depression and anxiety are some of the most prevalent and debilitating mental health conditions in humans. They can present on their own or as co-morbidities with other disorders. Like humans, non-human primates (NHPs) can develop depression- and anxiety-like signs. Here, we first define human depression and anxiety, examine equivalent species-specific behaviors in NHPs, and consider models and current methods to identify and evaluate these behaviors. We also discuss knowledge gaps, as well as the importance of evaluating the co-occurrence of depression- and anxiety-like behaviors in animal models of human disease. Lastly, we consider ethical challenges in depression and anxiety research on NHPs in order to ultimately advance the understanding and the personalized treatment of these disorders.
Depression is a common mental health disorder that often presents with anxiety. Both mental conditions are frequent comorbidities of a range of diseases, including diabetes, Parkinson’s disease (PD), and asthma. The World Health Organization estimated that more than 280 million people of all ages from around the world suffer from depression and 301 million live with an anxiety disorder (Institute of Health Metrics and Evaluation, 2022). Although the etiologies of both depression and anxiety are not well understood, their association with dysfunction of brain regions within the limbic system or “emotional” brain and the reward system neural network is well documented (Milak et al., 2005; Malhi and Mann, 2018).
Nonhuman primates (NHPs) share genetic, physiological, neuroanatomical, and behavioral attributes with humans that make them valuable for modeling human diseases, including depression and anxiety (Birn et al., 2014; Pessoa and Hof, 2015). NHPs are outbred like humans and thus have a similar degree of inter-individual variability. They undergo developmental stages similar to humans, and their relatively long lifespan compared to rodents aids in the evaluation of changes across the lifespan (Capitanio and Emborg, 2008; Ausderau et al., 2017). Like humans, NHPs (but not other nonhuman species) have 3 unique characteristics of the limbic system: a reduced olfactory lobe and bulb, a cingulate gyrus with greater size in the caudal regions, and an enlarged frontal lobe (Pessoa and Hof, 2015; van Heukelum et al., 2020).
It is difficult to know whether an animal is truly depressed or anxious, as they cannot express with words feelings of hopelessness or distractibility. Yet, NHPs can present depression- and anxiety-like behaviors (Table 1) that can provide insight into the human condition (Bliss-Moreau and Rudebeck, 2021). For example, studies in macaques with the endophenotype [heritable traits derived from laboratory measures (Iacono, 2018)] for “anxious temperament” have detected inheritable patterns of metabolic activity in the hippocampus that were predictors of anxious-like behaviors later in life (Oler et al., 2010). In order to guide clinical and preclinical studies in mental health disorders, the US National Institute of Mental Health developed the Research Domain Criteria (RDoC) that include six psychological domains: cognitive systems, arousal and regulatory systems, social processes, sensorimotor systems, and negative valence systems. Each domain has seven units of analysis: molecules, cells, circuits, physiology, behavior, self-report, and paradigms (RDoC Matrix, 2022). This matrix provides a framework to analyze the data collected in animal models, the presence of a relevant endophenotype, and their overall value for clinical translation. Critical for studies on depression and anxiety, it exposes that, of the six RDoC, the self-report domain cannot be studied in the NHP model (French, 2019; Gururajan et al., 2019).
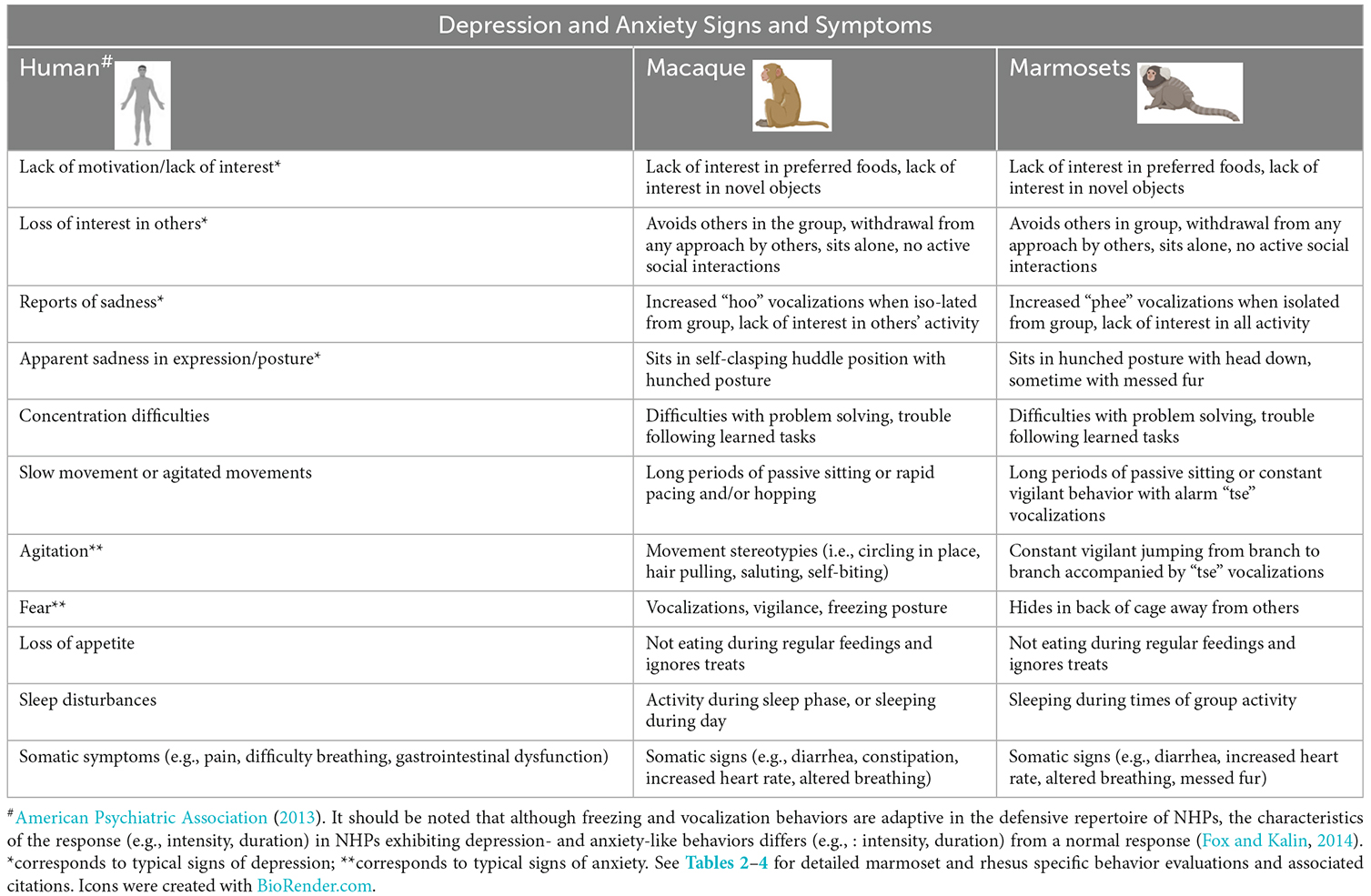
Table 1. Human signs and symptoms of depression and anxiety and their equivalent non-human primate (NHP) depression- and anxiety-like signs.
Advances in identification of alleles associated with human disorders as well as in methods for genomic editing is envisioned as an opportunity to generate NHP disease models based on germline (Aida and Feng, 2020) or somatic cell genomic modification (Saha et al., 2021). These new models are expected to genocopy and phenocopy disorders that may develop depression or anxiety as independent entities or as comorbidities. Here we aim to reflect on human depression and anxiety and equivalent behaviors in NHPs, in order to ethically capitalize on emerging models and methods of evaluation to ultimately advance the understanding and treatment of these disorders.
Depression is defined as extended periods of a sad mood that interferes with everyday functioning (Brody et al., 2018). It is typically diagnosed by a medical professional through a diagnostic evaluation using the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria (American Psychiatric Association, 2013). The prevalence of depression is difficult to estimate as it can occur across the lifespan, its manifestations are heterogeneous and vary by age, sex, income, and health behaviors (Villarroel and Terlizzi, 2020). Although mental, social, and physical symptoms of depression present with individual differences, over 80% have difficulty with their daily activities due to these symptoms (Brody et al., 2018). Different types of depression vary in the range of severity and length of symptomology (Table 1). For example, major depressive disorder is defined by severe symptomology that occurs most of the time compared to milder forms of depression, such as persistent depressive disorder that is a chronic state of milder symptomology but can be equally as debilitating. Depressive symptoms are also a key component of other disorders such as psychotic depression and bipolar disorder and, in addition, they may occur under unique circumstances, like postpartum depression or seasonal affective disorder.
Anxiety disorders are a common mental health condition that typically persists for months if not years (Anxiety Disorders, 2022). Individuals with anxiety disorders often struggle with intense and uncontrollable feelings of anxiety, fear, worry, and/or panic (American Psychiatric Association, 2013). Anxiety disorders include a number of related disorders including generalized anxiety disorder, panic disorder, social anxiety disorder, and various phobia-related disorders (American Psychiatric Association, 2013). While unique in their clinical presentation, the symptoms across disorders are persistent over time and interfere with an individual’s ability to participate in daily activities such as social relationships, work performance, and community engagement (Anxiety Disorders, 2022).
Other mental health conditions often co-occur with depression, including generalized anxiety disorders (Hirschfeld, 2001; Kessler et al., 2008). Shared environmental and genetic risk factors are suggested to contribute to the high rates of co-occurrence (50%) and shared symptomology of depression and anxiety disorders (Wittchen et al., 2000; Pollack, 2005; Lacerda-Pinheiro et al., 2014). Together they can have a cascading impact on an individual’s daily functioning, including self-care, social engagement, and occupational performance (Hirschfeld, 2001; Read et al., 2017).
Depression and anxiety can present independently and as comorbidities or as part of different disease processes. Patients with prevalent chronic disorders such as diabetes, arthritis, asthma, chronic obstructive pulmonary disease, stroke, and PD frequently have depression and/or anxiety that affect the primary illness management and prognosis (Reijnders et al., 2008; Morimoto et al., 2015; Zhang et al., 2017). In fact, people with chronic physical conditions or multiple chronic conditions are 2–3 times more likely to be diagnosed with depression (Read et al., 2017). Due to high rates of co-occurrence and overlap of symptomatology, depression often goes undiagnosed when associated with chronic disease (Morimoto et al., 2015; Mourao et al., 2016; Ismail et al., 2017). Anxiety is hypothesized to be even more prevalent than depression as co-occurring with physical illness and equally undiagnosed and inappropriately treated (Sartorius et al., 2014; Meuret et al., 2020). A strong connection has been established between anxiety disorders and some gastrointestinal diagnoses with up to 95% of patients reporting anxiety in certain conditions, such as irritable bowel syndrome (Whitehead et al., 2002; Härter et al., 2003; Fadgyas-Stanculete et al., 2014). Co-occurrence with chronic pain disorders such as fibromyalgia is also particularly high (Csupak et al., 2018; Montesó-Curto et al., 2018). Accurate diagnosis and treatment of both disorders is essential due to the high economic burden, and the need to guide appropriate clinical decisions and public health policy.
Depression is considered to be a chronic but treatable mental disorder with responses ranging from full recovery to ongoing treatment needed. Management plans typically consist of psychotherapy and or medications (e.g., selective serotonin reuptake inhibitors, SSRIs; Cuijpers et al., 2013; Brody and Gu, 2020). Lifestyle changes (e.g., physical activity and mindfulness-based interventions) are now also often recommended as part of the therapeutic approach (Khoury et al., 2013; Rosenbaum et al., 2014; Blanck et al., 2018). Approximately 10%–20% of individuals do not respond to treatment over time (Kessler et al., 2005; Center for Behavioral Health Statistics and Quality, 2018). For treatment-resistant cases, focal neuromodulation (e.g., vagus nerve stimulation, deep brain stimulation, transcranial magnetic stimulation) or, brain stimulation therapies (e.g., electroconvulsive therapy) are considered (Lee et al., 2012; Delaloye and Holtzheimer, 2014; Drobisz and Damborská, 2019; Lv et al., 2019; McAllister-Williams et al., 2020). Although significant gains have been made toward effective depressive disorder interventions, the increased challenge of treating depression with comorbidities (e.g., anxiety and chronic conditions) and the significant number of individuals who do not respond to intervention continue to underscore the complexity of the disorder and further need to better understand its etiology (Evans et al., 2005; Pollack, 2005; Center for Behavioral Health Statistics and Quality, 2018).
Anxiety is treated similarly to depression through psychotherapy (e.g., cognitive behavioral therapy) and medication. Due to the high co-morbidity of depression and anxiety, similar types of pharmacology are often used with the addition of benzodiazepines specifically for anxiety. Additional interventions such as certain types of biofeedback and mindfulness-based interventions have also shown promising results for some individuals with anxiety disorders (Rodrigues et al., 2017; Singh and Gorey, 2018; Tolin et al., 2020). Overall, treatments significantly improve the quality of life for most people with anxiety, but if terminated symptoms often return (Bandelow et al., 2017).
The heterogeneous hypotheses for the development of depression have evolved over time (Shadrina et al., 2018). Today, genetic, biological, environmental, and psychological factors are proposed to contribute to the onset of depression (Sullivan et al., 2000; Krishnan and Nestler, 2008; López-León et al., 2008; Kendler and Gardner, 2016). Anxiety is also considered to have a complex etiology (Bandelow et al., 2017; Thibaut, 2017; Meier and Deckert, 2019). Neuroimaging is emerging as a method to identify depression and related subtypes (Dunlop and Mayberg, 2017; Peng and Yao, 2019). Although initial studies found differences between individuals with depression and controls (Kaiser et al., 2015; Kambeitz et al., 2017; Zhang et al., 2018, 2019) and identified potential predictors of response to intervention (Konarski et al., 2009; Siegle et al., 2012; Dunlop et al., 2017), the neuroimaging findings still need to be sufficiently replicated to have robust clinical implications.
Despite the well-documented physical, social, medical, and economic significance of depression and anxiety, there continues to be limited knowledge of their underlying mechanisms and causes. Further research is needed to better understand depression and anxiety, identify targeted interventions to more effectively manage it, with or without co-occurring conditions.
For this report, we searched PubMed up to February 2022 to find articles describing depression and/or anxiety and evaluation methods in NHPs. Keywords included non-human primate, monkey, marmoset, macaques, prosimian, ape, depression, anxiety, behavior, evaluation, MRI, PET, fMRI, DTI, and SPEC combined with peer-reviewed and English language as filters. Review articles were considered in order to condense multiple previous studies and relevant reference lists were reviewed to gather additional articles. Title and abstracts were screened for topic relevance. Articles were removed based on duplicative findings or not being aligned with the manuscript’s purpose. Fifty articles were selected and analyzed by research team members to extract information on models and tests applied per species and developmental stage. These articles are referenced in Tables 2–4 describing methods of evaluation of depressive and anxious behaviors in species of marmosets and macaques.
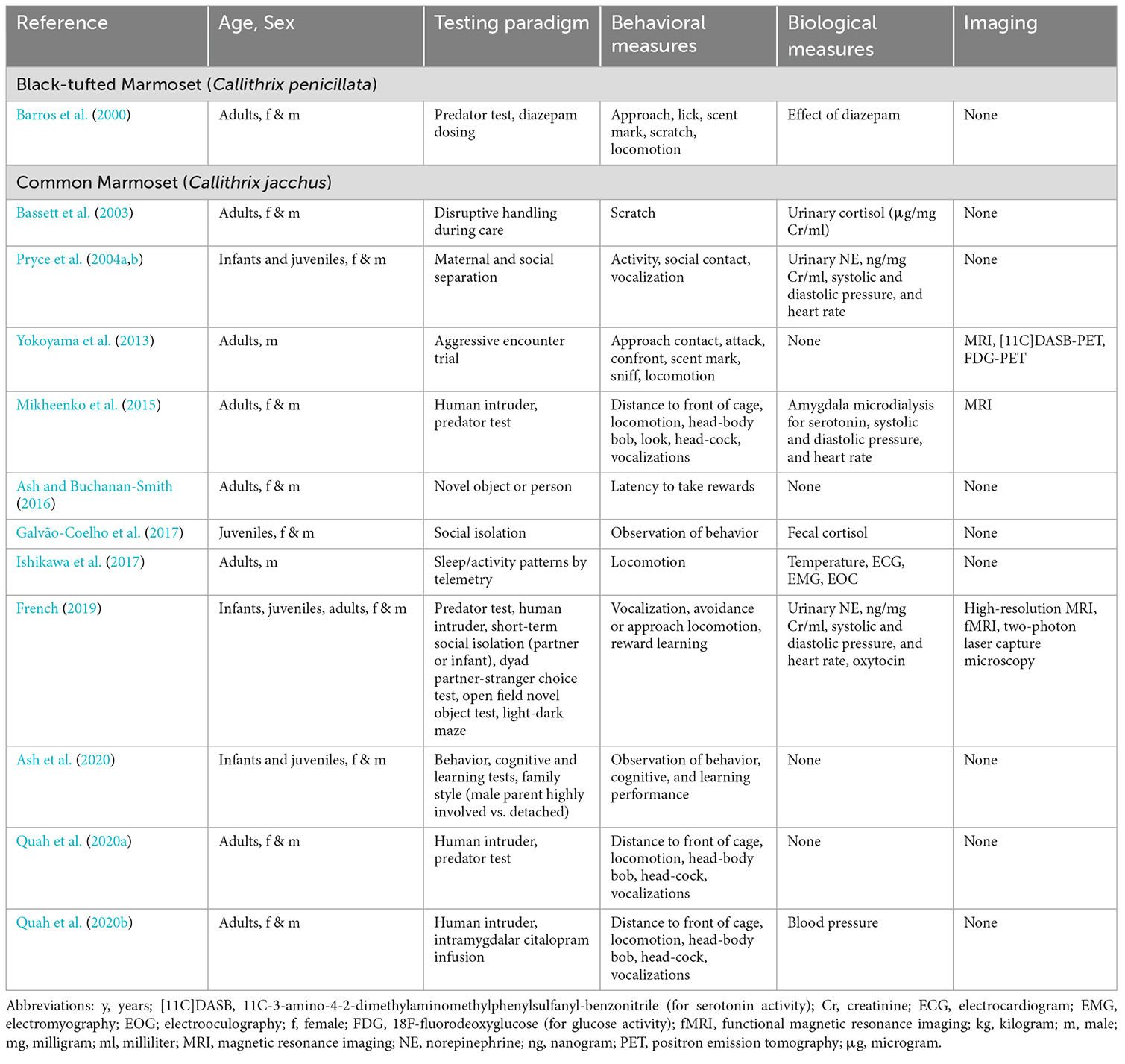
Table 2. Selected peer-reviewed publications describing methods of evaluation of depression- and anxiety-like behaviors in species of marmosets (infant = 0–0.5 y; juvenile = 0.5–2 y; adult = 2–8 y).
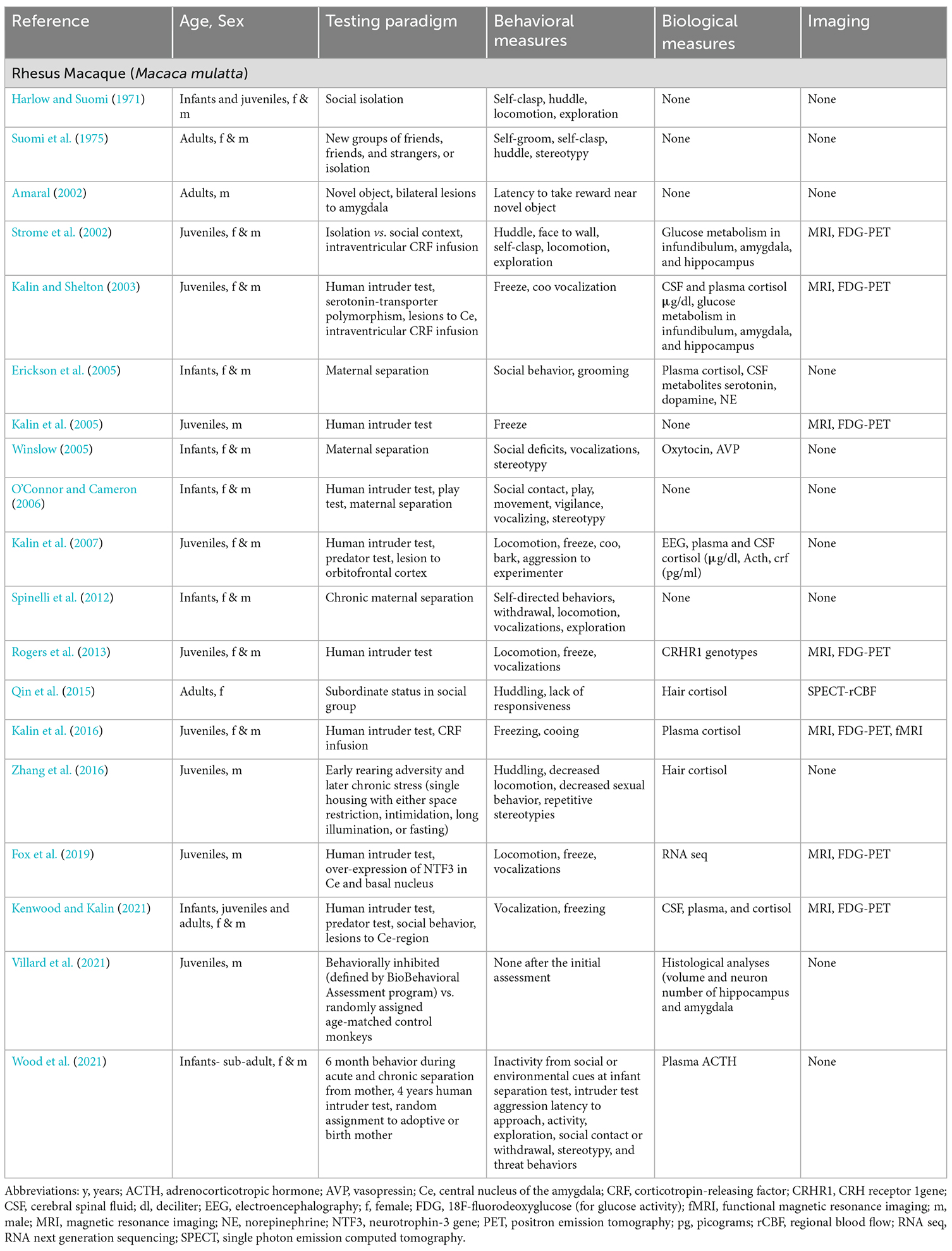
Table 3. Selected peer-reviewed publications describing methods of evaluation of depression- and anxiety-like behaviors in rhesus macaques (infant = 0–1 y; juvenile = 1–4 y; adult = 4–25 y).
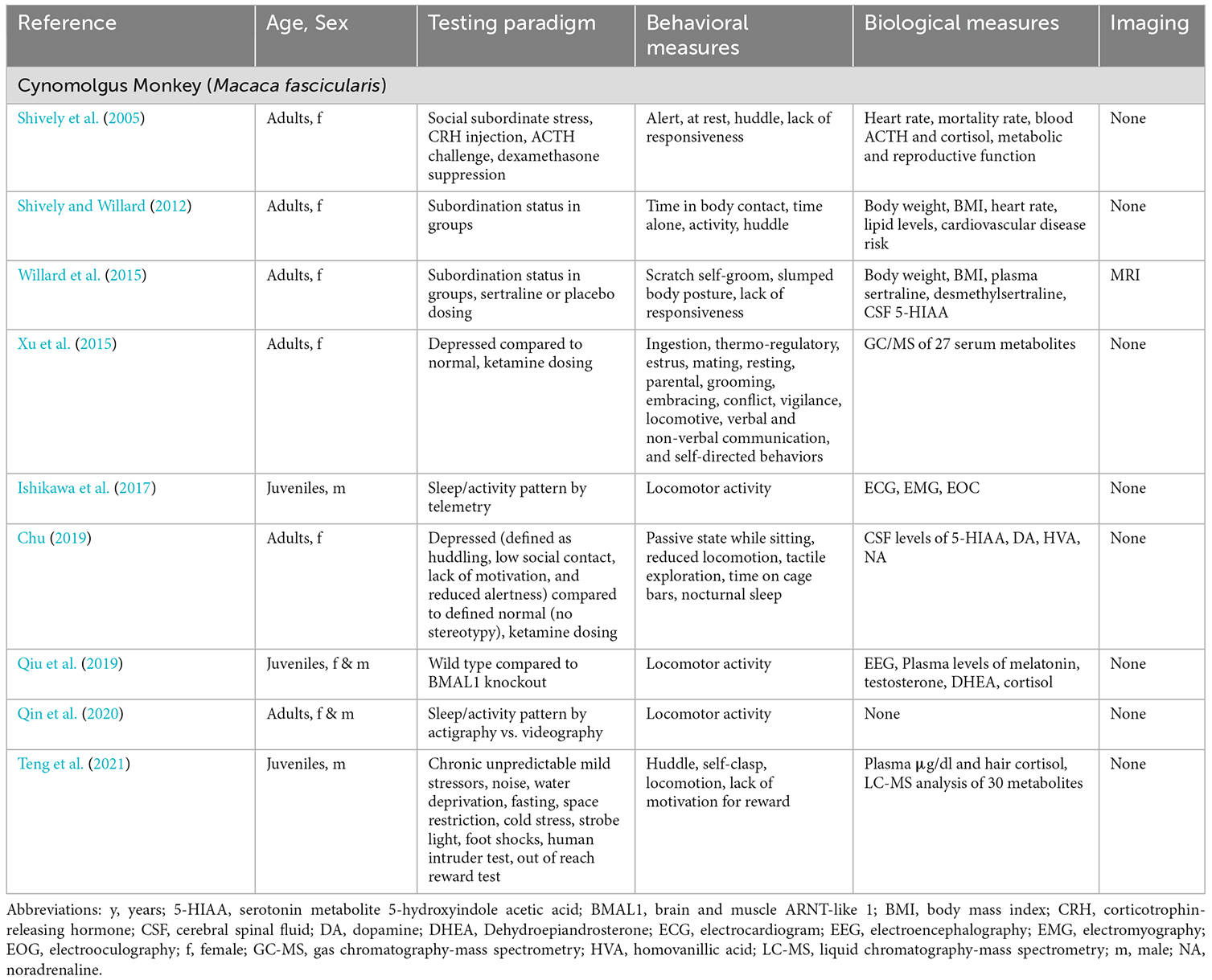
Table 4. Selected peer-reviewed publications describing methods of evaluation of depression- and anxiety-like behaviors in cynomolgus macaques (infant = 0–1 y; juvenile = 1–4 y; adult = 4–25 y).
Our literature search indicates that the most used NHP species in studies of depression and anxiety have been marmosets (genus Callithrix; Table 2) and macaques (genus Macaca; Tables 3 and 4). Isolated publications describe methods to evaluate depressive and anxious behaviors for the gray mouse lemur, owl monkey, capuchin monkey, squirrel monkey, vervet monkey, bonnet monkey, Japanese macaque, olive baboon, and chimpanzee (Table 5). We did not delve deeper into the available literature on chimpanzees given the complicated ethics involved in performing great ape research and the severe restrictions the National Institutes of Health placed on research with this species in 2015.
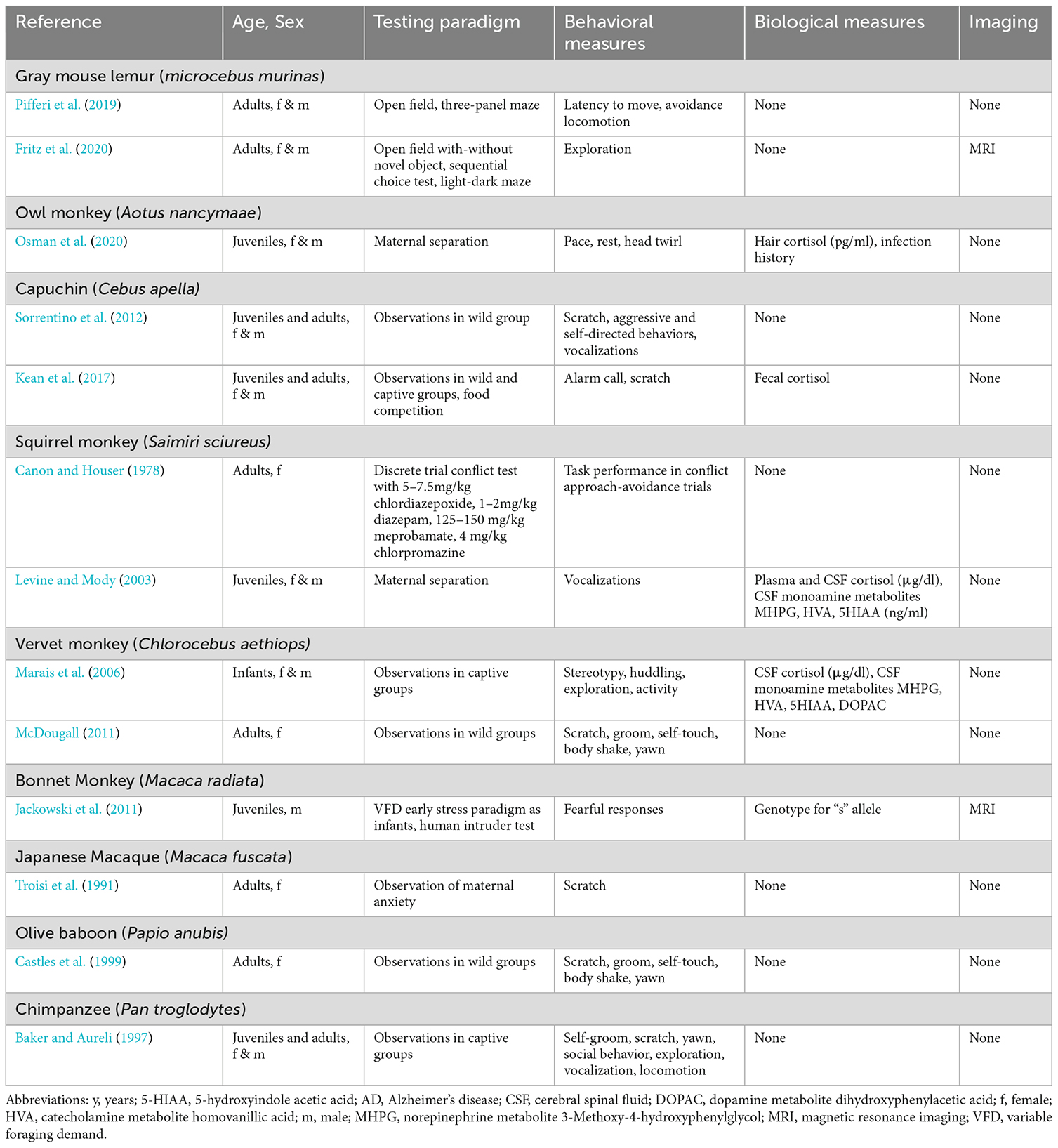
Table 5. Selected peer-reviewed publications describing methods of evaluation of depression- and anxiety-like behaviors in lemur (infant = 0–0.2 y; juvenile = 0.2–1 y; adult = 1–12 y); owl monkey (infant = 0–0.5 y; juvenile = 0.5–2 y; adult = 2–12 y); capuchin (infant = 0–1 y; juvenile = 1–6y; adult = 6–25 y), squirrel monkey (infant = 0–1 y; juvenile = 1–4 y; adult = 4–20 y); vervet (infant = 0–1 y; juvenile = 1–3 y; adult = 3–12 y); baboon (infant = 0–1 y; juvenile = 1–4 y; adult = 4–25 y), chimpanzee (infant = 0–5 y; juvenile = 5–12 y; adult = 12–50 y).
Both, marmoset and macaque monkeys share ~93% sequence identity with the human genome (Marmoset Genome and Analysis, 2014; Warren et al., 2020), yet they have critical species-specific differences to be considered for depression and anxiety studies. Marmosets are small-bodied (300–500 g in captivity), arboreal new world primates native to Brazil. The common marmoset (Callithrix jacchus) has been widely used to model and study depression and anxiety behaviors, compared to its close relative, the black-tufted marmoset (Callithrix penicillata). Among anthropoid primates, marmosets have a smaller, lissencephalic brain and are the shortest-lived (average 5–7 years, max 20) and the most fecund (twins born every 6 months). Marmosets have been used for many years in biomedical research, and within the last decade, their popularity increased dramatically, prompted to a large degree by their use in neuroscience research and their potential for developing germ-line genomic edited subjects (Rodriguez-Callejas et al., 2016; Servick, 2018). In contrast, macaques are old-world NHPs, native to Asia and North Africa. They are relatively large-bodied (2.4–5.5 kg, smaller species; 10–14 kg, larger species) and long-lived (>30 years), with a larger, more complex brain closer to humans. Macaques are the most commonly used NHPs in biomedical research and as such, it is not surprising that they are also the most common NHP used to model depression- and anxiety-like behaviors, specifically the rhesus monkey (Macaca mulatta) and cynomolgus monkey (Macaca fascicularis).
A notable finding during the analysis of the literature was that the majority of the NHP publications describe a mix of overlapping signs present in depression or anxiety, complicating the task of distinguishing between studies focused on either disorder, and yet underscoring the difficulty of their differential diagnoses in primates. This mixed reporting of general signs of depression or anxiety was observed in many of the identified marmoset studies, although new reports are emerging specific to depression- (Galvão-Coelho et al., 2017) and anxiety-like (Quah et al., 2020a,b) behaviors in this species (Table 2). In contrast, for macaques (Tables 3 and 4) we were able to identify 10 macaques studies focused on depression (Harlow and Suomi, 1971; Suomi et al., 1975; Strome et al., 2002; Shively et al., 2005; Qin et al., 2015; Willard et al., 2015; Xu et al., 2015; Zhang et al., 2016; Chu, 2019; Teng et al., 2021), eight on anxious temperament (Amaral, 2002; Kalin and Shelton, 2003; Kalin et al., 2005, 2007, 2016; Rogers et al., 2013; Fox et al., 2019; Kenwood and Kalin, 2021), while the rest described general signs of either condition.
As we mentioned in the introduction, it is difficult to know whether an animal is truly depressed or anxious, yet some of its behaviors resemble the human condition (Bliss-Moreau and Rudebeck, 2021). The critical issue is how to generate a NHP model that reliably produces depression- and anxiety-like behaviors. NHPs may spontaneously present depression- and anxiety-like behaviors (Xu et al., 2015; Chu, 2019) but their detection depends on careful observation of a large population of animals. As a modeling alternative, disrupted husbandry or separation, either short or long-term, from either a parent or other social partner has been used to elicit a depressed- or anxious-like condition in both marmosets and macaques (Harlow and Suomi, 1971; Erickson et al., 2005; Winslow, 2005; O’Connor and Cameron, 2006; Spinelli et al., 2012; Zhang et al., 2016; French, 2019; Villard et al., 2021; Wood et al., 2021).
Genotyping has matched specific alleles with spontaneous expression of anxious temperament in macaques, and had helped identified carriers at risk for anxiety (Jackowski et al., 2011; Rogers et al., 2013). Intracerebral delivery of viral vectors encoding for those alleles is proposed as a shortcut to modeling genetic predisposition (Fox et al., 2019). Increased intracerebral corticotropin releasing factor (CRF; Strome et al., 2002; Kalin and Shelton, 2003; Shively et al., 2005) and lesions to the central nucleus of the amygdala or orbitofrontal cortex (Amaral, 2002; Kalin and Shelton, 2003; Kalin et al., 2007; Kenwood and Kalin, 2021) have also been applied to develop a depression- or anxiety-like phenotype.
Although some of these behaviors can present spontaneously, investigators have created testing paradigms to facilitate the detection by evaluating the subjects’ response to what appears to be an aggressive intruder (i.e., human intruder paradigm (Kalin and Shelton, 2003; Kalin et al., 2005, 2007, 2016; O’Connor and Cameron, 2006; Rogers et al., 2013; Mikheenko et al., 2015; French, 2019; Kenwood and Kalin, 2021); a predator (Barros et al., 2000; French, 2019; Kenwood and Kalin, 2021), or a novel object or person (Amaral, 2002; Ash and Buchanan-Smith, 2016). NHPs’ behavioral responses to these paradigms, such as freezing or vocalizations, are typical adaptive defensive behaviors, yet the evaluation does not rely on their presence or absence but rather on the characteristics of the response (e.g., intensity, duration), which in NHPs exhibiting depression- and anxious- like behaviors differ from normal parameters (Fox and Kalin, 2014).
Taken together, these established techniques provide a framework for the use of marmosets and macaques to model and detect depression- and anxiety-like behaviors.
Marmosets and macaques share depression- and anxiety-like behaviors that are similar to human ones but also have species-specific characteristics that change with age. Identifying and scoring these behaviors are the main evaluation tool for studies of these disorders in NHPs (Table 1).
Marmosets’ depressive-like behaviors include less locomotion, increased contact (phee) calling, and/or longer latency to take rewards. Their anxiousness is expressed by avoidance, scent-marking, increased alarm (tsk) or aggressive (chatter, er-er) calls, excessive scratching, licking, and sniffing, or head cocking behavior. Measures of these behaviors need to consider that younger marmosets are more active, sociable, and playful compared to adult animals, who themselves, spend more time grooming and cuddling.
Depressive-like behaviors in macaques also include decreased locomotion, in addition to adopting a self-clasping, huddle posture, increased coo calls, and/or lack of responsiveness for social contact or rewards. Their anxious-like behaviors are freezing, the presence of alarm bark, or excessive scratching, pacing, self-grooming, and yawning. With respect to age, infant rhesus’ behaviors are particularly affected by contact and separation from caregivers, while juveniles are socially active and withdrawal from their group is indicative of possible distress. Adult rhesus are, in general, protectors of the group and affected by strangers, whereas older animals are comfort-seeking with less activity, pursuing contact and grooming.
Like in humans, specific cognitive and learning tests (that can also assess attention) can detect impaired performance in NHPs displaying depression- and anxiety-like behaviors (Sridharan et al., 2012; Ash et al., 2020; Nephew et al., 2020). For rhesus macaques, most of these tests are adaptations of human versions, such as the Wisconsin card sorting task (WCST) and conditioned reward learning (Sridharan et al., 2012). For marmosets, the testing paradigms are often based on rodents ones, like an open field, light-dark maze, classical conditioning, and reward learning (Ash and Buchanan-Smith, 2016; French, 2019; Ash et al., 2020).
Routine clinical observations, including monitoring of food intake and body weight regularly performed in NHP colonies, can indicate loss of appetite and somatic signs associated with depression and anxiety. Although not described in reports studying these disorders, body mass index (BMI; Shively and Willard, 2012; Willard et al., 2015) or dual-energy x-ray absorptiometry (DXA; Yamada et al., 2013; Mattison et al., 2017; Kraynak et al., 2019) can be used in macaques and marmosets to measure body composition and further evaluate animals with loss of appetite manifested by a change in body weight and adiposity. Fitbit-like monitors can be used to assess activity levels and circadian rhythm (Oler et al., 2010; Yamada et al., 2013, 2018; Qiu et al., 2019) and identify sleep disturbance as manifested by increased overnight activity.
Measures of heart rate, blood pressure, and sleep using implanted telemetry and measurements of circulating levels of cortisol, norepinephrine, and serotonin, from plasma, urinary or fecal samples (Bassett et al., 2003; Kalin and Shelton, 2003; Pryce et al., 2004a,b; Erickson et al., 2005; Qin et al., 2015; Kalin et al., 2016; Qiu et al., 2019) along with additional hormone and neurotransmitter metabolites from cerebrospinal fluid (Erickson et al., 2005; Winslow, 2005; Kalin et al., 2007; Mikheenko et al., 2015; Willard et al., 2015; Chu, 2019; French, 2019; Teng et al., 2021) are used to measure physiological responses that correlate with depression and anxiety behaviors. Increased heart rate, blood pressure, cortisol, NE, and serotonin metabolites are often correlated with these behaviors.
Modification of human brain imaging techniques to NHPs is part of the assessment battery in depression and anxiety paradigms (Strome et al., 2002; Kalin and Shelton, 2003; Kalin et al., 2005, 2016; Jackowski et al., 2011; Rogers et al., 2013; Yokoyama et al., 2013; Mikheenko et al., 2015; Qin et al., 2015; Fox et al., 2019; French, 2019; Kenwood and Kalin, 2021). In addition to structural differences with magnetic resonance imaging (MRI) and white matter connections using diffusion tensor imaging, brain activity can be evaluated by functional MRI that utilizes blood oxygen levels to assess neuron activity (Vanduffel et al., 2014), positron emission tomography (PET) with F18 Fluorodeoxiglucose to assess brain consumption of glucose (Strome et al., 2002; Kalin and Shelton, 2003; Rogers et al., 2013; Yokoyama et al., 2013; Kalin et al., 2016; Fox et al., 2019; Kenwood and Kalin, 2021) or single photon emission computed tomography (SPECT) with regional blood flow (rCBF; Qin et al., 2015). PET with C11 3-amino-4-(2-dimethylamino methylphenylsulfanyl)-benzonitrile can be used to assess serotonin pathways in the brain by measuring binding to the serotonin transporter (Yokoyama et al., 2013).
Our analysis was able to identify relevant information for both males and females in multiple species at different developmental timeframes including infant, juvenile, and an adult but not aged NHPs. In humans, the prevalence of depression differs with age. Given that NHP behavior varies across age stages, it is critical to adapt these tests to evaluate age-appropriate activities and responses. Moreover, these behaviors can be identified as comorbid conditions along with various age-related chronic diseases such as diabetes, arthritis, stroke, and PD. The lack of reports regarding depression- and anxiety-like behaviors in older NHPs represents a significant gap in the literature that stymies the use of these highly translational models in addressing important biomedical research questions.
Similar to aging models, research on depression- and anxiety-like behaviors as a comorbidity in NHP models of disease has not been prioritized and is urgently needed in order to help affected patients. These types of studies also provide an opportunity to consider depression and anxiety from a different perspective, unravel pathological mechanisms, and identify novel therapeutic approaches. Two notable examples to consider are NHP models of PD and of interferon alpha (IFN-α)-treatment.
It is estimated that 30%–40% of PD patients present with depression and only 20% are treated. Oral dopamine replacement with L-DOPA (a dopamine precursor), the mainstay therapy for PD, is marginally effective against depression and anxiety (Frisina et al., 2009; Huot et al., 2017). In addition to dopaminergic loss, PD neurodegeneration also affects other neurotransmitters, including norepinephrine and serotonin, which are proposed to contribute to the depression and anxiety of PD (Draoui et al., 2020; Mendonça et al., 2020). Interestingly, monkey models of PD induced by the neurotoxin MPTP also present significant dopaminergic loss as well as loss of norepinephrinergic and serotoninergic innervation (Masilamoni and Smith, 2018). Underdiagnoses of depression and anxiety are common, as frequent indicators of underlying depression can be obscured by some PD symptoms, such as sleep disturbances and slowness of movement. Although depression in rodent models of PD has been widely studied (Schintu et al., 2012; Hussein et al., 2021), reports of depression and anxiety in NHP PD models are missing, which has affected progress in identifying biomarkers and treatments. Anxious pacing has been described in transgenic rhesus macaques overexpressing A53T mutated alpha-synuclein (Niu et al., 2015). Our group has recently identified depression and anxiety behaviors in rhesus macaques with neurotoxin-induced hemiparkinsonism. In addition to typical PD motor signs, the animals presented a lack of interest, anxious pacing, and excessive grooming, indicating a combination of depression- and anxiety-like signs. These behaviors improved after intrastriatal grafting of autologous (from the same animal) induced pluripotent stem cell-derived autologous dopaminergic neurons (Tao et al., 2021). This unexpected finding suggests that, unlike oral dopamine replacement, targeted and localized intracerebral dopamine may be beneficial against depression and anxiety in PD.
Interferon alpha (IFN-α), an inflammatory cytokine, has been successfully used as immunotherapy for patients with chronic hepatitis B and C, and certain cancers, like malignant melanoma and Karposi sarcoma (Pestka et al., 2004). Yet its administration can induce numerous side effects, including depression and anxiety (Sleijfer et al., 2005; Pinto and Andrade, 2016). IFN-α dosing in rhesus macaques also induced depression- and anxiety-like behaviors, identified as increased huddling, self-scratching, body shakes, and yawning. These behaviors were associated with increased ACTH levels in plasma and lower homovanillic acid (a dopamine metabolite) levels in CSF (Felger et al., 2007). Follow up NHP studies demonstrated that IFN-α reduces the striatal availability of dopamine precursors that can be reversed by L-DOPA administration. This research has led to a number of clinical studies testing DA replacement in depression associated with high levels of inflammatory cytokines (Escalona and Fawcett, 2017; Rutherford et al., 2019).
The information gathered from seemingly two different lines of research, PD, and IFN-α, provides new insight in the role of dopaminergic neurotransmission and inflammation in depression and anxiety. It also emphasizes the need to develop precision medicine approaches to optimize treatments depending on the underlying condition associated with depression and anxiety that can be further facilitated by studies in a new generation of NHP models of disease based on germline (Aida and Feng, 2020) or somatic cell genomic modification (Saha et al., 2021).
The welfare of animals used in biomedical research is extremely important both ethically and because well cared for animals make the best research subjects. Worldwide, consideration of the use of animals in research is based on the principle of the three R’s; replacement, reduction, and refinement (Russel and Burch, 1959). These principles guide researchers to use non-animal alternatives when possible and if not possible to use lower phylogenetic species of animals, use the fewest animals necessary to appropriately address the research question, refine techniques to minimize animal suffering, and use assessments tools that are most efficient at collecting the necessary data.
A number of established and validated techniques can be applied to assess NHPs’ depression- and anxiety-like behaviors, which were not specifically designed to assess these disorders, but can be easily adapted for such use. As we mentioned above, routine clinical observations performed in NHP colonies are great resources for assessing loss of appetite and somatic signs often associated with depression. The use of fitbit-like monitors is another non-invasive, easy approach to record activity levels and evaluate circadian rhythm and sleep patterns (Yamada et al., 2013, 2018; Kraynak et al., 2019; Qin et al., 2020). Traditional cognitive testing in macaques has relied on the Wisconsin general testing apparatus (WGTA) and conditioned reward learning (Sridharan et al., 2012). These tests require devoted, highly trained personnel and extensive training for the animals that can last many months, yet some animals even without depression and anxiety, are still nonreliable or nonperformers. Alternative cognitive testing such as the use of a multilevel puzzle feeder task that the animals can perform in their home cage, can begin to address these challenges (Watson et al., 1999).
Behavioral data can be collected either in person or by scoring videos, using focal animal sampling, all occurrence sampling, or instantaneous scan sampling. In-person scoring can be expeditious and provide greater insight into the animal’s overall demeanor, but it can be heavily influenced by the scorer. To minimize data variability, it is critical that beyond blind evaluation, the investigators are well-known by the animals, trained for reliability, and have time to dedicate to the testing. Scoring of videorecorded sessions can be done anytime, off-site, and inter-rater-reliability tests can be easily performed. Yet, good quality recordings require animals’ habituation to the set-up and personnel, plus the application of systematic methods by blind investigators. The behavioral data can be used to assess animals for motivation and interest levels, locomotor patterns, social interactions, anxious-like behavior, apparent sadness, and any stereotypical behaviors (Harlow and Suomi, 1971; Suomi et al., 1975; Troisi et al., 1991; Barros et al., 2000; Strome et al., 2002; Erickson et al., 2005; Winslow, 2005; O’Connor and Cameron, 2006; Shively et al., 2009; Shively and Willard, 2012; Spinelli et al., 2012; Willette et al., 2012; Rogers et al., 2013; Qin et al., 2015; Kalin et al., 2016; Ash et al., 2020; Kenwood and Kalin, 2021).
The scientific value and utility of NHP models for the study of depression and anxiety are mentioned above, but the ethical decision to use NHP models requires a somewhat different calculus. These decisions must be based on consideration of a harm-benefit analysis rooted in scientifically valid information. Specifically, regarding whether the benefit gained from using the model outweighs any potential harm to the animals. For example, for some forms of depression and anxiety research one should ask, does the information garnered using a stress-induced model outweigh the level of harm experienced by the animals in the study? Can spontaneous behaviors provide enough insight in the condition? Could a terminal analysis of the brain be replaced by an in vivo imaging study? The inverse must be considered as well. Does the potential harm in lack of knowledge outweigh the benefit to the animal in not being used in research? For example, without the use of animal research will we fail to identify effective new therapies for depression and anxiety, or will the lack of animal studies lead to an ineffective or dangerous treatment being employed? It is our obligation as researchers to use animal models respectfully and appropriately. This includes using the most appropriate model to answer our questions, inducing the minimal amount of stress possible to achieve the aims of the study, using the fewest animals possible while maintaining an appropriately powered study, and to reduce the harm to each individual through the use of refinements whenever possible.
Depression and anxiety affect millions of people worldwide across the lifespan. NHP research is providing clues on pathophysiology, diagnoses, and treatments for these disorders, but much work remains to be done. The gaps in knowledge on depression and anxiety with aging and comorbidities are opportunities to advance the field, ethically leveraging the resources already generated. These emerging datasets in NHPs can be the canvas for the application of novel targeted testing approaches (e.g.,: genetic manipulation, imaging in awake behaving animals) to help identify mechanisms, create precise interventions and improve treatment outcomes. They will require a team-oriented approach combining disease-specific experts and NHP researchers in order to create encompassing experimental designs, maximize results, and ultimately help patients affected by these disorders.
KA provided insight on the human condition. RC and NS-D contributed expertise in nonhuman primate evaluations and interpretation. SK and NS-D critically contributed to the creation of Tables 2–5. ME conceived the idea for the manuscript and provided neurobiological basis. All authors contributed to the article and approved the submitted version.
This publication was made possible by NIH grants R61NS115102, U34AG068466, and P51OD011106 and the Kellett Mid-Career Award, University of Wisconsin-Madison.
We thank Dr. Ned Kalin and the reviewers for their insightful suggestions to the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aida, T., and Feng, G. (2020). The dawn of non-human primate models for neurodevelopmental disorders. Curr. Opin. Genet. Dev. 65, 160–168. doi: 10.1016/j.gde.2020.05.040
Amaral, D. G. (2002). The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol. Psychiatry 51, 11–17. doi: 10.1016/s0006-3223(01)01307-5
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders 5th ed. Arlington, VA: American Psychiatric Association.
Anxiety Disorders (2022). National Institute of Mental Health (NIMH). Available online at: https://www.nimh.nih.gov/health/topics/anxiety-disorders. Accessed July 26, 2022.
Ash, H., and Buchanan-Smith, H. M. (2016). The long-term impact of infant rearing background on the affective state of adult common marmosets (Callithrix jacchus). Appl. Anim. Behav. Sci. 174, 128–136. doi: 10.1016/j.applanim.2015.10.009
Ash, H., Ziegler, T. E., and Colman, R. J. (2020). Early learning in the common marmoset (Callithrix jacchus): behavior in the family group is related to preadolescent cognitive performance. Am. J. Primatol. 82:e23159. doi: 10.1002/ajp.23159
Ausderau, K. K., Dammann, C., McManus, K., Schneider, M., Emborg, M. E., and Schultz-Darken, N. (2017). Cross-species comparison of behavioral neurodevelopmental milestones in the common marmoset monkey and human child. Dev. Psychobiol. 59, 807–821. doi: 10.1002/dev.21545
Baker, K. C., and Aureli, F. (1997). Behavioural indicators of anxiety: an empirical test in chimpanzees. Behaviour 134, 1031–1050.
Bandelow, B., Michaelis, S., and Wedekind, D. (2017). Treatment of anxiety disorders. Dialogues Clin. Neurosci. 19, 93–107. doi: 10.31887/DCNS.2017.19.2/bbandelow
Barros, M., Boere, V., Huston, J. P., and Tomaz, C. (2000). Measuring fear and anxiety in the marmoset (Callithrix penicillata) with a novel predator confrontation model: effects of diazepam. Behav. Brain Res. 108, 205–211. doi: 10.1016/s0166-4328(99)00153-9
Bassett, L., Buchanan-Smith, H. M., McKinley, J., and Smith, T. E. (2003). Effects of training on stress-related behavior of the common marmoset (Callithrix jacchus) in relation to coping with routine husbandry procedures. J. Appl. Anim. Welf. Sci. 6, 221–233. doi: 10.1207/S15327604JAWS0603_07
Birn, R. M., Shackman, A. J., Oler, J. A., Williams, L. E., McFarlin, D. R., Rogers, G. M., et al. (2014). Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol. Psychiatry 19, 915–922. doi: 10.1038/mp.2014.46
Blanck, P., Perleth, S., Heidenreich, T., Kröger, P., Ditzen, B., Bents, H., et al. (2018). Effects of mindfulness exercises as stand-alone intervention on symptoms of anxiety and depression: systematic review and meta-analysis. Behav. Res. Therapy 102, 25–35. doi: 10.1016/j.brat.2017.12.002
Bliss-Moreau, E., and Rudebeck, P. H. (2021). Animal models of human mood. Neurosci. Biobehav. Rev. 120, 574–582. doi: 10.1016/j.neubiorev.2020.06.024
Brody, D. J., and Gu, Q. (2020). Antidepressant use among adults: united states, 2015–2018. NCHS Data Brief 12:8. Available online at: https://www.cdc.gov/nchs/data/databriefs/db377-H.tif.
Brody, D. J., Pratt, L. A., and Hughes, J. P. (2018). Prevalence of depression among adults aged 20 and over: United States, 2013–2016. NCHS Data Brief 303, 1–8. Available online at: https://www.cdc.gov/nchs/products/databriefs/db377.htm.
Canon, J. G., and Houser, V. P. (1978). Squirrel monkey active conflict test. Psychobiology 6, 215–222.
Capitanio, J. P., and Emborg, M. E. (2008). Contributions of non-human primates to neuroscience research. Lancet 371, 1126–1135. doi: 10.1016/S0140-6736(08)60489-4
Castles, D. L., Whiten, A., and Aureli, F. (1999). Social anxiety, relationships and self-directed behaviour among wild female olive baboons. Anim. Behav. 58, 1207–1215. doi: 10.1006/anbe.1999.1250
Center for Behavioral Health Statistics and Quality (2018). 2017 national survey on drug use and health: detailed tables. Substance Abuse Mental Health Services Administration 62, 1–2871. Available online at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.tif.
Chu, X. (2019). Preliminary validation of natural depression in macaques with acute treatments of the fast-acting antidepressant ketamine. Behav. Brain Res. 360, 60–68. doi: 10.1016/j.bbr.2018.11.044
Csupak, B., Sommer, J. L., Jacobsohn, E., and El-Gabalawy, R. (2018). A population-based examination of the co-occurrence and functional correlates of chronic pain and generalized anxiety disorder. J. Anxiety Disord. 56, 74–80. doi: 10.1016/j.janxdis.2018.04.005
Cuijpers, P., Sijbrandij, M., Koole, S. L., Andersson, G., Beekman, A. T., and Reynolds, C. F., III (2013). The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta-analysis of direct comparisons. World Psychiatry 12, 137–148. doi: 10.1002/wps.20038
Delaloye, S., and Holtzheimer, P. E. (2014). Deep brain stimulation in the treatment of depression. Dialogues Clin. Neurosci. 16, 83–91. doi: 10.31887/DCNS.2014.16.1/sdelaloye
Draoui, A., El Hiba, O., Aimrane, A., El Khiat, A., and Gamrani, H. (2020). Parkinson’s disease: from bench to bedside. Rev. Neurol. (Paris) 176, 543–559. doi: 10.1016/j.neurol.2019.11.002
Drobisz, D., and Damborská, A. (2019). Deep brain stimulation targets for treating depression. Behav. Brain Res. 359, 266–273. doi: 10.1016/j.bbr.2018.11.004
Dunlop, B. W., and Mayberg, H. S. (2017). Neuroimaging advances for depression. Cerebrum 2017, 1–15. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6132047/.
Dunlop, B. W., Rajendra, J. K., Craighead, W. E., Sweeney, J. A., Jia, Z. Y., Gong, Q. Y., et al. (2017). Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am. J. Psychiatry 174, 533–545. doi: 10.1176/appi.ajp.2016.16050518
Erickson, K., Gabry, K. E., Schulkin, J., Champoux, M., Schulkin, J., Gold, P., et al. (2005). Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Dev. Psychobiol. 46, 331–339. doi: 10.1002/dev.20061
Escalona, R., and Fawcett, J. (2017). Pramipexole in treatment resistant-depression, possible role of inflammatory cytokines. Neuropsychopharmacology 42:363. doi: 10.1038/npp.2016.217
Evans, D. L., Charney, D. S., Lewis, L., Golden, R. N., Gorman, J. M., Krishnan, K. R., et al. (2005). Mood disorders in the medically ill: scientific review and recommendations. Biol. Psychiatry 58, 175–189. doi: 10.1016/j.biopsych.2005.05.001
Fadgyas-Stanculete, M., Buga, A. M., Popa-Wagner, A., and Dumitrascu, D. L. (2014). The relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestations. J. Mol. Psychiatry 2:4. doi: 10.1186/2049-9256-2-4
Felger, J. C., Alagbe, O., Hu, F., Mook, D., Freeman, A. A., Sanchez, M. M., et al. (2007). Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol. Psychiatry 62, 1324–1333. doi: 10.1016/j.biopsych.2007.05.026
Fox, A. S., and Kalin, N. H. (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am. J. Psychiatry 171, 1162–1173. doi: 10.1176/appi.ajp.2014.14040449
Fox, A. S., Souaiaia, T., Oler, J. A., Kovner, R., Kim, J. M. H., Nguyen, J., et al. (2019). Dorsal amygdala neurotrophin-3 decreases anxious temperament in primates. Biol. Psychiatry 86, 881–889. doi: 10.1016/j.biopsych.2019.06.022
French, J. A. (2019). “The marmoset as a model in behavioral neuroscience and psychiatric research,” in The Common Marmoset in Captivity and Biomedical Research (Cambridge, MA: Elsevier), 477–491.
Frisina, P. G., Haroutunian, V., and Libow, L. S. (2009). The neuropathological basis for depression in Parkinson’s disease. Parkinsonism Relat. Disord. 15, 144–148. doi: 10.1016/j.parkreldis.2008.04.038
Fritz, R. G., Zimmermann, E., Meier, M., Mestre-Francés, N., Radespiel, U., and Schmidtke, D. (2020). Neurobiological substrates of animal personality and cognition in a nonhuman primate (Microcebus murinus). Brain Behav. 10:e01752. doi: 10.1002/brb3.1752
Galvão-Coelho, N. L., Galvão, A. C. de. M., Silva, F. S. da., and Sousa, M. B. C. de. (2017). Common marmosets: a potential translational animal model of juvenile depression. Front. Psychiatry 8:175. doi: 10.3389/fpsyt.2017.00175
Gururajan, A., Reif, A., Cryan, J. F., and Slattery, D. A. (2019). The future of rodent models in depression research. Nat. Rev. Neurosci. 20, 686–701. doi: 10.1038/s41583-019-0221-6
Härter, M. C., Conway, K. P., and Merikangas, K. R. (2003). Associations between anxiety disorders and physical illness. Eur. Arch. Psychiatry Clin. Neurosci. 253, 313–320. doi: 10.1007/s00406-003-0449-y
Harlow, H. F., and Suomi, S. J. (1971). Production of depressive behaviors in young monkeys. J. Autism. Dev. Disord. 1, 246–255. doi: 10.1007/BF01557346
Hirschfeld, R. M. A. (2001). The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim. Care Companion J. Clin. Psychiatry 3, 244–254. doi: 10.4088/pcc.v03n0609
Huot, P., Sgambato-Faure, V., Fox, S. H., and McCreary, A. C. (2017). Serotonergic approaches in Parkinson’s disease: translational perspectives, an update. ACS Chem. Neurosci. 8, 973–986. doi: 10.1021/acschemneuro.6b00440
Hussein, A., Guevara, C. A., Del Valle, P., Gupta, S., Benson, D. L., and Huntley, G. W. (2021). Non-motor symptoms of Parkinson’s disease: the neurobiology of early psychiatric and cognitive dysfunction. Neuroscientist . [Epub ahead of print]. doi: 10.1177/10738584211011979
Iacono, W. G. (2018). Endophenotypes in psychiatric disease: prospects and challenges. Genome Med. 10:11. doi: 10.1186/s13073-018-0526-5
Institute of Health Metrics and Evaluation (2022). Global Health Data Exchange (GHDx). Available online at: https://vizhub.healthdata.org/gbd-results/. Accessed July 15, 2022
Ishikawa, A., Sakai, K., Maki, T., Mizuno, Y., Niimi, K., Oda, Y., et al. (2017). Investigation of sleep-wake rhythm in non-human primates without restraint during data collection. Exp. Anim. 66, 51–60. doi: 10.1538/expanim.16-0073
Ismail, Z., Elbayoumi, H., Fischer, C. E., Hogan, D. B., Millikin, C. P., Schweizer, T., et al. (2017). Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry 74, 58–67. doi: 10.1001/jamapsychiatry.2016.3162
Jackowski, A., Perera, T. D., Abdallah, C. G., Garrido, G., Tang, C. Y., Martinez, J., et al. (2011). Early-life stress, corpus callosum development, hippocampal volumetrics and anxious behavior in male nonhuman primates. Psychiatry Res. 192, 37–44. doi: 10.1016/j.pscychresns.2010.11.006
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., and Pizzagalli, D. A. (2015). Large-scale network dysfunction in majord depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611. doi: 10.1001/jamapsychiatry.2015.0071
Kalin, N. H., Fox, A. S., Kovner, R., Riedel, M. K., Fekete, E. M., Roseboom, P. H., et al. (2016). Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit. Biol. Psychiatry 80, 345–355. doi: 10.1016/j.biopsych.2016.01.010
Kalin, N. H., and Shelton, S. E. (2003). Nonhuman primate models to study anxiety, emotion regulation and psychopathology. Ann. N Y Acad. Sci. 1008, 189–200. doi: 10.1196/annals.1301.021
Kalin, N. H., Shelton, S. E., and Davidson, R. J. (2007). Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol. Psychiatry 62, 1134–1139. doi: 10.1016/j.biopsych.2007.04.004
Kalin, N. H., Shelton, S. E., Fox, A. S., Oakes, T. R., and Davidson, R. J. (2005). Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol. Psychiatry 58, 796–804. doi: 10.1016/j.biopsych.2005.05.021
Kambeitz, J., Cabral, C., Sacchet, M. D., Gotlib, I. H., Zahn, R., Serpa, M. H., et al. (2017). Detecting neuroimaging biomarkers for depression: a meta-analysis of multivariate pattern recognition studies. Biol. Psychiatry 82, 330–338. doi: 10.1016/j.biopsych.2016.10.028
Kean, D., Tiddi, B., Fahy, M., Heistermann, M., Schino, G., and Wheeler, B. C. (2017). Feeling anxious? The mechanisms of vocal deception in tufted capuchin monkeys. Anim. Behav. 130, 37–46. doi: 10.1016/j.anbehav.2017.06.008
Kendler, K. S., and Gardner, C. O. (2016). Depressive vulnerability, stressful life events and episode onset of major depression: a longitudinal model. Psychol. Med. 46, 1865–1874. doi: 10.1017/S0033291716000349
Kenwood, M. M., and Kalin, N. H. (2021). Nonhuman primate models to explore mechanisms underlying early-life temperamental anxiety. Biol. Psychiatry 89, 659–671. doi: 10.1016/j.biopsych.2020.08.028
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., and Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–602. doi: 10.1001/archpsyc.62.6.593
Kessler, R. C., Gruber, M., Hettema, J. M., Hwang, I., Sampson, N., and Yonkers, K. A. (2008). Comorbid major depression and generalized anxiety disorders in the National comorbidity survey follow-up. Psychol. Med. 38, 365–374. doi: 10.1017/S0033291707002012
Khoury, B., Lecomte, T., Fortin, G., Masse, M., Therien, P., Bouchard, V., et al. (2013). Mindfulness-based therapy: a comprehensive meta-analysis. Clin. Psychol. Rev. 33, 763–771. doi: 10.1016/j.cpr.2013.05.005
Konarski, J. Z., Kennedy, S. H., Segal, Z. V., Lau, M. A., Bieling, P. J., McIntyre, R. S., et al. (2009). Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J. Psychiatry Neurosci. 34, 175–180. Available online at: https://www.jpn.ca/content/34/3/175.long.
Kraynak, M., Colman, R. J., Flowers, M. T., Abbott, D. H., and Levine, J. E. (2019). Ovarian estradiol supports sexual behavior but not energy homeostasis in female marmoset monkeys. Int. J. Obes. (Lond) 43, 1034–1045. doi: 10.1038/s41366-018-0156-4
Krishnan, V., and Nestler, E. J. (2008). The molecular neurobiology of depression. Nature 455, 894–902. doi: 10.1038/nature07455
López-León, S., Janssens, A. C. J. W., Ladd, A. M. G.-Z., Del-Favero, J., Claes, S. J., Oostra, B. A., et al. (2008). Meta-analyses of genetic studies on major depressive disorder. Mol. Psychiatry 13, 772–785. doi: 10.1038/sj.mp.4002088
Lacerda-Pinheiro, S. F., Pinheiro Junior, R. F. F., de Lima, M. A. P., da Silva, G. G. L., dos Santos, M. d. S. V., Júnior, A. G. T., et al. (2014). Are there depression and anxiety genetic markers and mutations? A systematic review. J. Affect. Disord. 168, 387–398. doi: 10.1016/j.jad.2014.07.016
Lee, J. C., Blumberger, D. M., Fitzgerald, P. B., Daskalakis, Z. J., and Levinson, A. J. (2012). The role of transcranial magnetic stimulation in treatment-resistant depression: a review. Curr. Pharm. Des. 18, 5846–5852. doi: 10.2174/138161212803523644
Levine, S., and Mody, T. (2003). The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci. Biobehav. Rev. 27, 83–89. doi: 10.1016/s0149-7634(03)00011-3
Lv, H., Zhao, Y.-H., Chen, J.-G., Wang, D.-Y., and Chen, H. (2019). Vagus nerve stimulation for depression: a systematic review. Front. Psychol. 10:64. doi: 10.3389/fpsyg.2019.00064
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet 392, 2299–2312. doi: 10.1016/S0140-6736(18)31948-2
Marais, L., Daniels, W., Brand, L., Viljoen, F., Hugo, C., and Stein, D. J. (2006). Psychopharmacology of maternal separation anxiety in vervet monkeys. Metab. Brain Dis. 21, 201–210. doi: 10.1007/s11011-006-9011-8
Marmoset Genome, S., and Analysis, C. (2014). The common marmoset genome provides insight into primate biology and evolution. Nat. Genet. 46, 850–857. doi: 10.1038/ng.3042
Masilamoni, G. J., and Smith, Y. (2018). Chronic MPTP administration regimen in monkeys: a model of dopaminergic and non-dopaminergic cell loss in Parkinson’s disease. J. Neural Transm. (Vienna) 125, 337–363. doi: 10.1007/s00702-017-1774-z
Mattison, J. A., Colman, R. J., Beasley, T. M., Allison, D. B., Kemnitz, J. W., Roth, G. S., et al. (2017). Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8:14063. doi: 10.1038/ncomms14063
McAllister-Williams, R. H., Arango, C., Blier, P., Demyttenaere, K., Falkai, P., Gorwood, P., et al. (2020). The identification, assessment and management of difficult-to-treat depression: an international consensus statement. J. Affect. Disord. 267, 264–282. doi: 10.1016/j.jad.2020.02.023
McDougall, P. (2011). Scratching our heads Rethinking social anxiety in vervets (Chlorocebus aethiops). Int. J. Primatol. 32, 335–345. doi: 10.1007/s10764-010-9471-x
Meier, S. M., and Deckert, J. (2019). Genetics of anxiety disorders. Curr. Psychiatry Rep. 21:16. doi: 10.1007/s11920-019-1002-7
Mendonça, I. P., Duarte-Silva, E., Chaves-Filho, A. J. M., da Costa, A. B. L. d. S., and Peixoto, C. A. (2020). Neurobiological findings underlying depressive behavior in Parkinson’s disease: a review. Int. Immunopharmacol. 83:106434. doi: 10.1016/j.intimp.2020.106434
Meuret, A. E., Tunnell, N., and Roque, A. (2020). “Anxiety disorders and medical comorbidity: treatment implications,” in Anxiety Disorders: Rethinking and Understanding Recent Discoveries. Advances in Experimental Medicine and Biology, Vol. 1191, ed Y. K. Kim (Singapore: Springer), 237–261. doi: 10.1007/978-981-32-9705-0_15
Mikheenko, Y., Shiba, Y., Sawiak, S., Braesicke, K., Cockcroft, G., Clarke, H., et al. (2015). Serotonergic, brain volume and attentional correlates of trait anxiety in primates. Neuropsychopharmacology 40, 1395–1404. doi: 10.1038/npp.2014.324
Milak, M. S., Parsey, R. V., Keilp, J., Oquendo, M. A., Malone, K. M., and Mann, J. J. (2005). Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch. Gen. Psychiatry 62, 397–408. doi: 10.1001/archpsyc.62.4.397
Montesó-Curto, P., García-Martinez, M., Romaguera, S., Popa-Wagner, A., Dumitrascu, D. L., Malone, K. M., et al. (2018). Problems and solutions for patients with fibromyalgia: building new helping relationships. J. Adv. Nurs. 74, 339–349. doi: 10.1111/jan.13412
Morimoto, S. S., Kanellopoulos, D., Manning, K. J., and Alexopoulos, G. S. (2015). Diagnosis and treatment of depression and cognitive impairment in late-life. Ann. N Y Acad. Sci. 1345, 36–46. doi: 10.1111/nyas.12669
Mourao, R. J., Mansur, G., Malloy-Diniz, L. F., Costa, E. C., and Diniz, B. S. (2016). Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 31, 905–911. doi: 10.1002/gps.4406
Nephew, B. C., Febo, M., Cali, R., Workman, K. P., Payne, L., Moore, C. M., et al. (2020). Robustness of sex-differences in functional connectivity over time in middle-aged marmosets. Sci. Rep. 10:16647. doi: 10.1038/s41598-020-73811-9
Niu, Y., Guo, X., Chen, Y., Wang, C.-E., Gao, J., Yang, W., et al. (2015). Early Parkinson’s disease symptoms in α-synuclein transgenic monkeys. Hum. Mol. Genet. 24, 2308–2317. doi: 10.1093/hmg/ddu748
O’Connor, T. G., and Cameron, J. L. (2006). Translating research findings on early experience to prevention: animal and human evidence on early attachment relationships. Am. J. Prev. Med. 31, S175–S181. doi: 10.1016/j.amepre.2006.07.005
Oler, J. A., Fox, A. S., Shelton, S. E., Rogers, J., Dyer, T. D., Davidson, R. J., et al. (2010). Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 466, 864–868. doi: 10.1038/nature09282
Osman, M., Olkun, A., Maldonado, A. M., Lopez-Tremoleda, J., Sanchez-Perea, N., and Paredes, U. M. (2020). Rejection in early life is associated with high and long-lasting stress and higher incidence of infections in owl monkeys. bioRxiv [Preprint]. doi: 10.1101/2020.10.17.343921
Peng, D., and Yao, Z. (2019). “Neuroimaging advance in depressive disorder,” in Depressive Disorders: Mechanisms, Measurement and Management. Advances in Experimental Medicine and Biology, Vol. 1180, ed Y. Fang (Singapore: Springer), 59–83. doi: 10.1007/978-981-32-9271-0_3
Pessoa, L., and Hof, P. R. (2015). From Paul Broca’s great limbic lobe to the limbic system. J. Comp. Neurol. 523, 2495–2500. doi: 10.1002/cne.23840
Pestka, S., Krause, C. D., and Walter, M. R. (2004). Interferons, interferon-like cytokines and their receptors. Immunol. Rev. 202, 8–32. doi: 10.1111/j.0105-2896.2004.00204.x
Pifferi, F., Epelbaum, J., and Aujard, F. (2019). Strengths and weaknesses of the gray mouse lemur (Microcebus murinus) as a model for the behavioral and psychological symptoms and neuropsychiatric symptoms of dementia. Front. Pharmacol. 10:1291. doi: 10.3389/fphar.2019.01291
Pinto, E. F., and Andrade, C. (2016). Interferon-related depression: a primer on mechanisms, treatment and prevention of a common clinical problem. Curr. Neuropharmacol. 14, 743–748. doi: 10.2174/1570159x14666160106155129
Pollack, M. H. (2005). Comorbid anxiety and depression. J. Clin. Psychiatry 66, 22–29. Available online at: https://www.psychiatrist.com/jcp/medical/comorbidity/comorbid-anxiety-depression/.
Pryce, C. R., Dettling, A., Spengler, M., Spaete, C., and Feldon, J. (2004a). Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Ann. N Y Acad. Sci. 1032, 245–249. doi: 10.1196/annals.1314.030
Pryce, C. R., Dettling, A. C., Spengler, M., Schnell, C. R., and Feldon, J. (2004b). Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol. Psychiatry 56, 72–79. doi: 10.1016/j.biopsych.2004.05.002
Qin, D. D., Feng, S.-F., Zhang, F.-Y., Wang, N., Sun, W.-J., Zhou, Y., et al. (2020). Potential use of actigraphy to measure sleep in monkeys: comparison with behavioral analysis from videography. Zool. Res. 41, 437–443. doi: 10.24272/j.issn.2095-8137.2020.056
Qin, D., Rizak, J., Chu, X., Li, Z., Yang, S., Lü, L., et al. (2015). A spontaneous depressive pattern in adult female rhesus macaques. Sci. Rep. 5:11267. doi: 10.1038/srep11267
Qiu, P., Jiang, J., Liu, Z., Cai, Y., Huang, T., Wang, Y., et al. (2019). BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. Natl. Sci. Rev. 6, 87–100. doi: 10.1093/nsr/nwz002
Quah, S. K. L., Cockcroft, G. J., McIver, L., Santangelo, A. M., and Roberts, A. C. (2020a). Avoidant coping style to high imminence threat is linked to higher anxiety-like behavior. Front. Behav. Neurosci. 14:34. doi: 10.3389/fnbeh.2020.00034
Quah, S. K. L., McIver, L., Roberts, A. C., and Santangelo, A. M. (2020b). Trait anxiety mediated by amygdala serotonin transporter in the common marmoset. J. Neurosci. 40, 4739–4749. doi: 10.1523/JNEUROSCI.2930-19.2020
RDoC Matrix (2022). National Institute of Mental Health (NIMH). Available online at: https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/constructs/rdoc-matrix. Accessed December 7, 2022.
Read, J. R., Sharpe, L., Modini, M., and Dear, B. F. (2017). Multimorbidity and depression: a systematic review and meta-analysis. J. Affect. Disord. 221, 36–46. doi: 10.1016/j.jad.2017.06.009
Reijnders, J. S. A. M., Ehrt, U., Weber, W. E. J., Aarsland, D., and Leentjens, A. F. G. (2008). A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 23, 183–189. doi: 10.1002/mds.21803
Rodrigues, M. F., Nardi, A. E., and Levitan, M. (2017). Mindfulness in mood and anxiety disorders: a review of the literature. Trends Psychiatry Psychother. 39, 207–215. doi: 10.1590/2237-6089-2016-0051
Rodriguez-Callejas, J. D., Fuchs, E., and Perez-Cruz, C. (2016). Evidence of Tau hyperphosphorylation and dystrophic microglia in the common marmoset. Front. Aging Neurosci. 8:315. doi: 10.3389/fnagi.2016.00315
Rogers, J., Raveendran, M., Fawcett, G. L., Fox, A. S., Shelton, S. E., Oler, J. A., et al. (2013). CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol. Psychiatry 18, 700–707. doi: 10.1038/mp.2012.152
Rosenbaum, S., Tiedemann, A., Sherrington, C., Curtis, J., and Ward, P. B. (2014). Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J. Sci. Med. Sport 18:e150. doi: 10.1016/j.jsams.2014.11.161
Russel, W., and Burch, R. (1959). The Principles of Humane Experimental Technique. London: Methuen & Co. Limited.
Rutherford, B. R., Slifstein, M., Chen, C., Abi-Dargham, A., Brown, P. J., Wall, M. W., et al. (2019). Effects of L-DOPA monotherapy on psychomotor speed and [11C]raclopride binding in high-risk older adults With depression. Biol. Psychiatry 86, 221–229. doi: 10.1016/j.biopsych.2019.04.007
Saha, K., Sontheimer, E. J., Brooks, P. J., Dwinell, M. R., Gersbach, C. A., Liu, D. R., et al. (2021). The NIH somatic cell genome editing program. Nature 592, 195–204. doi: 10.1038/s41586-021-03191-1
Sartorius, N., Holt, R. I. G., and Maj, M. (2014). Comorbidity of Mental and Physical Disorders. Basel, Switzerland: Karger Medical and Scientific Publishers.
Schintu, N., Zhang, X., and Svenningsson, P. (2012). Studies of depression-related states in animal models of Parkinsonism. J. Parkinsons Dis. 2, 87–106. doi: 10.3233/JPD-2012-12076
Servick, K. (2018). U.S. labs clamor for marmosets. Science 362, 383–384. doi: 10.1126/science.362.6413.383
Shadrina, M., Bondarenko, E. A., and Slominsky, P. A. (2018). Genetics factors in major depression disease. Front. Psychiatry 9:334. doi: 10.3389/fpsyt.2018.00334
Shively, C. A., Musselman, D. L., and Willard, S. L. (2009). Stress, depression and coronary artery disease: modeling comorbidity in female primates. Neurosci. Biobehav. Rev. 33, 133–144. doi: 10.1016/j.neubiorev.2008.06.006
Shively, C. A., Register, T. C., Friedman, D. P., Morgan, T. M., Thompson, J., and Lanier, T. (2005). Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biol. Psychol. 69, 67–84. doi: 10.1016/j.biopsycho.2004.11.006
Shively, C. A., and Willard, S. L. (2012). Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp. Neurol. 233, 87–94. doi: 10.1016/j.expneurol.2011.09.026
Siegle, G. J., Thompson, W. K., Collier, A., Berman, S. R., Feldmiller, J., Thase, M. E., et al. (2012). Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners and patient characteristics. Arch. Gen. Psychiatry 69, 913–924. doi: 10.1001/archgenpsychiatry.2012.65
Singh, S. K., and Gorey, K. M. (2018). Relative effectiveness of mindfulness and cognitive behavioral interventions for anxiety disorders: meta-analytic review. Soc. Work Mental Health 16, 238–251. doi: 10.1080/15332985.2017.1373266
Sleijfer, S., Bannink, M., Van Gool, A. R., Kruit, W. H. J., and Stoter, G. (2005). Side effects of interferon-alpha therapy. Pharm. World Sci. 27, 423–431. doi: 10.1007/s11096-005-1319-7
Sorrentino, E. P. di., Schino, G., Tiddi, B., and Aureli, F. (2012). Scratching as a window into the emotional responses of wild tufted capuchin monkeys. Ethology 118, 1072–1084. doi: 10.1111/eth.12008
Spinelli, S., Schwandt, M. L., Lindell, S. G., Heilig, M., Suomi, S. J., Higley, J. D., et al. (2012). The serotonin transporter gene linked polymorphic region is associated with the behavioral response to repeated stress exposure in infant rhesus macaques. Dev. Psychopathol. 24, 157–165. doi: 10.1017/S0954579411000745
Sridharan, A., Willette, A. A., Bendlin, B. B., Alexander, A. L., Coe, C. L., Voytko, M. L., et al. (2012). Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front. Aging Neurosci. 4:31. doi: 10.3389/fnagi.2012.00031
Strome, E. M., Wheler, G. H. T., Higley, J. D., Loriaux, D. L., Suomi, S. J., and Doudet, D. J. (2002). Intracerebroventricular corticotropin-releasing factor increases limbic glucose metabolism and has social context-dependent behavioral effects in nonhuman primates. Proc. Natl. Acad. Sci. U S A 99, 15749–15754. doi: 10.1073/pnas.232480899
Sullivan, P. F., Neale, M. C., and Kendler, K. S. (2000). Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry 157, 1552–1562. doi: 10.1176/appi.ajp.157.10.1552
Suomi, S. J., Eisele, C. D., Grady, S. A., and Harlow, H. F. (1975). Depressive behavior in adult monkeys following separation from family environment. J. Abnorm. Psychol. 84, 576–578. doi: 10.1037/h0077066
Tao, Y., Vermilyea, S. C., Zammit, M., Lu, J., Olsen, M., Metzger, J. M., et al. (2021). Autologous transplant therapy alleviates motor and depressive behaviors in parkinsonian monkeys. Nat. Med. 27, 632–639. doi: 10.1038/s41591-021-01257-1
Teng, T., Shively, C. A., Li, X., Jiang, X., Neigh, G. N., Yin, B., et al. (2021). Chronic unpredictable mild stress produces depressive-like behavior, hypercortisolemia and metabolic dysfunction in adolescent cynomolgus monkeys. Transl. Psychiatry 11:9. doi: 10.1038/s41398-020-01132-6
Thibaut, F. (2017). Anxiety disorders: a review of current literature. Dialogues Clin. Neurosci. 19, 87–88. doi: 10.31887/DCNS.2017.19.2/fthibaut
Tolin, D. F., Davies, C. D., Moskow, D. M., and Hofmann, S. G. (2020). “Biofeedback and neurofeedback for anxiety disorders: a quantitative and qualitative systematic review,” in Anxiety Disorders: Rethinking and Understanding Recent Discoveries. Advances in Experimental Medicine and Biology, Vol. 1191, ed Y. K. Kim (Singapore: Springer), 265–289.
Troisi, A., Schino, G., D’Antoni, M., Pandolfi, N., Aureli, F., and D’Amato, F. R. (1991). Scratching as a behavioral index of anxiety in macaque mothers. Behav. Neural Biol. 56, 307–313. doi: 10.1016/0163-1047(91)90469-7
van Heukelum, S., Mars, R. B., Guthrie, M., Buitelaar, J. K., Beckmann, C. F., Tiesinga, P. H. E., et al. (2020). Where is cingulate cortex? a cross-species view. Trends Neurosci. 43, 285–299. doi: 10.1016/j.tins.2020.03.007
Vanduffel, W., Zhu, Q., and Orban, G. A. (2014). Monkey cortex through fMRI glasses. Neuron 83, 533–550. doi: 10.1016/j.neuron.2014.07.015
Villard, J., Bennett, J. L., Bliss-Moreau, E., Capitanio, J. P., Fox, N. A., Amaral, D. G., et al. (2021). Structural differences in the hippocampus and amygdala of behaviorally inhibited macaque monkeys. Hippocampus 31, 858–868. doi: 10.1002/hipo.23329
Villarroel, M. A., and Terlizzi, E. P. (2020). Symptoms of depression among adults: United States, 2019. NCHS Data Brief 379, 1–8. Available online at: https://www.cdc.gov/nchs/products/databriefs/db379.htm.
Warren, W. C., Harris, R. A., Haukness, M., Fiddes, I. T., Murali, S. C., Fernandes, J., et al. (2020). Sequence diversity analyses of an improved rhesus macaque genome enhance its biomedical utility. Science 370:eabc6617. doi: 10.1126/science.abc6617
Watson, S. L., Shively, C. A., and Voytko, M. L. (1999). Can puzzle feeders be used as cognitive screening instruments? Differential performance of young and aged female monkeys on a puzzle feeder task. Am. J. Primatol. 49, 195–202. doi: 10.1002/(SICI)1098-2345(199910)49:2<195::AID-AJP9>3.0.CO;2-J
Whitehead, W. E., Palsson, O., and Jones, K. R. (2002). Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 122, 1140–1156. doi: 10.1053/gast.2002.32392
Willard, S. L., Uberseder, B., Clark, A., Daunais, J. B., Johnston, W. D., Neely, D., et al. (2015). Long term sertraline effects on neural structures in depressed and nondepressed adult female nonhuman primates. Neuropharmacology 99, 369–378. doi: 10.1016/j.neuropharm.2015.06.011
Willette, A. A., Coe, C. L., Colman, R. J., Bendlin, B. B., Kastman, E. K., Field, A. S., et al. (2012). Calorie restriction reduces psychological stress reactivity and its association with brain volume and microstructure in aged rhesus monkeys. Psychoneuroendocrinology 37, 903–916. doi: 10.1016/j.psyneuen.2011.10.006
Winslow, J. T. (2005). Neuropeptides and non-human primate social deficits associated with pathogenic rearing experience. Int. J. Dev. Neurosci. 23, 245–251. doi: 10.1016/j.ijdevneu.2004.03.003
Wittchen, H. U., Kessler, R. C., Pfister, H., Höfler, M., and Lieb, R. (2000). Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatr. Scand. Suppl. 102, 14–23. doi: 10.1111/j.0065-1591.2000.acp29-03.x
Wood, E. K., Espinel, W. F., Hunter, J., Emmett, A., Skowbo, A. N., Schwandt, M. L., et al. (2021). The effects of at-birth adoption on atypical behavior and anxiety: a nonhuman primate model. J. Am. Acad. Child Adolesc. Psychiatry 60, 1382–1393. doi: 10.1016/j.jaac.2021.04.021
Xu, F., Wu, Q., Xie, L., Gong, W., Zhang, J., Zheng, P., et al. (2015). Macaques exhibit a naturally-occurring depression similar to humans. Sci. Rep. 5:9220. doi: 10.1038/srep09220
Yamada, Y., Colman, R. J., Kemnitz, J. W., Baum, S. T., Anderson, R. M., Weindruch, R., et al. (2013). Long-term calorie restriction decreases metabolic cost of movement and prevents decrease of physical activity during aging in rhesus monkeys. Exp. Gerontol. 48, 1226–1235. doi: 10.1016/j.exger.2013.08.002
Yamada, Y., Kemnitz, J. W., Weindruch, R., Anderson, R. M., Schoeller, D. A., and Colman, R. J. (2018). Caloric restriction and healthy life span: frail phenotype of nonhuman primates in the Wisconsin National Primate Research Center Caloric Restriction Study. J. Gerontol. A Biol. Sci. Med. Sci. 73, 273–278. doi: 10.1093/gerona/glx059
Yokoyama, C., Kawasaki, A., Hayashi, T., and Onoe, H. (2013). Linkage between the midline cortical serotonergic system and social behavior traits: positron emission tomography studies of common marmosets. Cereb. Cortex 23, 2136–2145. doi: 10.1093/cercor/bhs196
Zhang, L., Fu, T., Yin, R., Zhang, Q., and Shen, B. (2017). Prevalence of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. BMC Psychiatry 17:70. doi: 10.1186/s12888-017-1234-1
Zhang, Z.-Y., Mao, Y., Feng, X.-L., Zheng, N., Lü, L. B., Ma, Y. Y., et al. (2016). Early adversity contributes to chronic stress induced depression-like behavior in adolescent male rhesus monkeys. Behav. Brain Res. 306, 154–159. doi: 10.1016/j.bbr.2016.03.040
Zhang, F. F., Peng, W., Sweeney, J. A., Jia, Z. Y., and Gong, Q. Y. (2018). Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci. Ther. 24, 994–1003. doi: 10.1111/cns.12835
Keywords: depression, anxiety, non-human primates, macaque, marmoset
Citation: Ausderau KK, Colman RJ, Kabakov S, Schultz-Darken N and Emborg ME (2023) Evaluating depression- and anxiety-like behaviors in non-human primates. Front. Behav. Neurosci. 16:1006065. doi: 10.3389/fnbeh.2022.1006065
Received: 28 July 2022; Accepted: 29 December 2022;
Published: 19 January 2023
Edited by:
Patricio Huerta, Feinstein Institute for Medical Research, United StatesReviewed by:
Mar Sanchez, Emory University, United StatesCopyright © 2023 Ausderau, Colman, Kabakov, Schultz-Darken and Emborg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina E. Emborg, ZW1ib3JnQHByaW1hdGUud2lzYy5lZHU=
† These authors have contributed equally to this work and share first authorship
‡ORCID: Karla K. Ausderau, https://orcid.org/0000-0003-0799-1022; Marina E. Emborg, https://orcid.org/0000-0002-9351-6641
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.