- Department of Entomology, University of Kentucky, Lexington, KY, United States
Early-life experiences have strong and long-lasting consequences for behavior in a surprising diversity of animals. Determining which environmental inputs cause behavioral change, how this information becomes neurobiologically encoded, and the functional consequences of these changes remain fundamental puzzles relevant to diverse fields from evolutionary biology to the health sciences. Here we explore how insects provide unique opportunities for comparative study of developmental behavioral plasticity. Insects have sophisticated behavior and cognitive abilities, and they are frequently studied in their natural environments, which provides an ecological and adaptive perspective that is often more limited in lab-based vertebrate models. A range of cues, from relatively simple cues like temperature to complex social information, influence insect behavior. This variety provides experimentally tractable opportunities to study diverse neural plasticity mechanisms. Insects also have a wide range of neurodevelopmental trajectories while sharing many developmental plasticity mechanisms with vertebrates. In addition, some insects retain only subsets of their juvenile neuronal population in adulthood, narrowing the targets for detailed study of cellular plasticity mechanisms. Insects and vertebrates share many of the same knowledge gaps pertaining to developmental behavioral plasticity. Combined with the extensive study of insect behavior under natural conditions and their experimental tractability, insect systems may be uniquely qualified to address some of the biggest unanswered questions in this field.
Introduction
Early-life experiences can have profound consequences for adult phenotypes, particularly behaviors (Beach and Jaynes, 1954), a phenomenon called developmental behavioral plasticity (sensu West-Eberhard, 2003, 2005). Although this phenomenon is well-established, its mechanistic basis remains a persistent research puzzle that touches many behavioral neuroscience disciplines and applications (Beldade et al., 2011; Snell-Rood, 2013; Reh et al., 2020). Brain development is fundamentally complex—it is a dynamic interaction between endogenous, gene-guided programs and environmental inputs (Boyce et al., 2020; Reh et al., 2020). Thus, determining how experiences are “embedded” requires knowledge at multiple levels of organization, from molecules to neural structure (Champagne, 2012; Cardoso et al., 2015; Curley and Champagne, 2016; Sinha et al., 2020). Moreover, individual differences can extend to peripheral tissues, which are also shaped by developmental experience and interact with the brain to influence adult behavioral expression (Figure 1). Finally, in addition to triggering behavioral change, environmental conditions dictate the adaptive consequences of behavioral expression. Understanding these consequences may allow researchers to predict the types of experiences that cause lasting or transient behavioral impacts. However, adaptive consequences of behavioral expression are difficult to ascertain in traditional lab-based model systems alone (Yartsev, 2017).
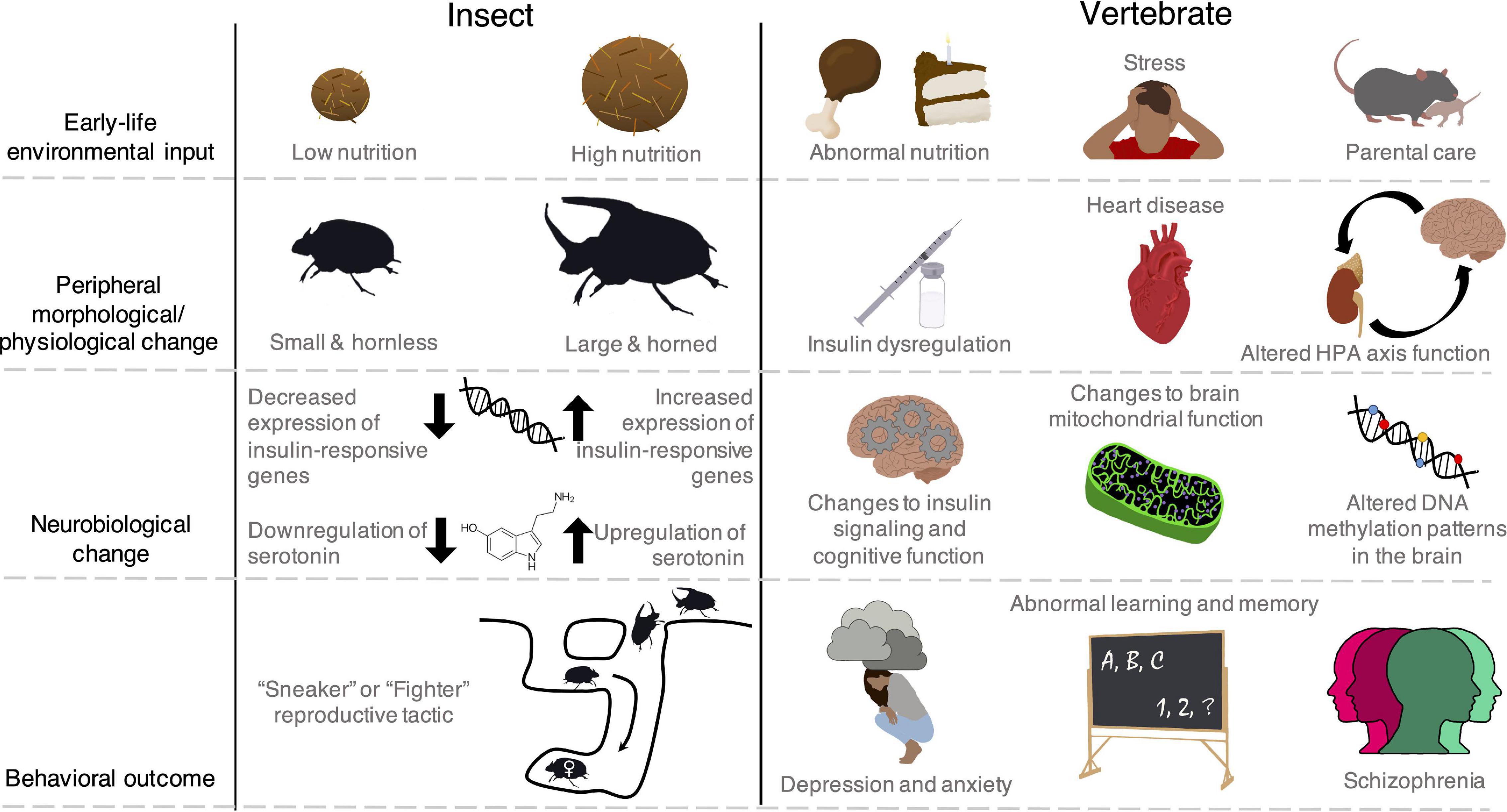
Figure 1. The impacts of early-life experiences extend beyond the brain to peripheral physiological systems and even body morphology in insect and vertebrate species. The brain and peripheral systems interact to shape adult behavioral expression in ways that remain poorly understood. Though these brain-peripheral connections are common across animals including vertebrates, and specifically humans, some insects show particularly conspicuous and discrete changes in morphology, presenting interesting systems to investigate behavioral regulation. Moreover, despite the more noticeable phenotypic differences in some insects, there are examples of common regulatory mechanisms (e.g., insulin signaling) that underpin behavioral dynamics across the insect and vertebrate phylogenetic space. Left: In some beetles (Onthophagus spp.), males that are provided high amounts of nutrition during development emerge as large adults with horns (Emlen, 1997). Horns give males a benefit in competition over female mates, which nest in sub-terranean tunnels under dung piles (Moczek and Emlen, 2000). These morphological changes are associated with changes in brain insulin and serotonin signaling (Snell-Rood and Moczek, 2012; Newsom et al., 2019) and result in two distinct male reproductive tactics. Large, horned males will guard female tunnels and compete with other rivals, while small, hornless males dig side tunnels and sneak around large males to reach the female (Emlen, 1997; Moczek and Emlen, 2000). Right: In vertebrates, early-life nutrition, stress, and social interactions cause coordinated changes in peripheral physiological function (Barker, 1995; Champagne and Curley, 2005; Avitsur et al., 2015) as well as brain hormone signaling, bioenergetics, and gene regulation (Hochberg et al., 2011; Korosi et al., 2012; Hoffmann and Spengler, 2018). These changes can give rise to cognitive and mental health disorders (Avishai-Eliner et al., 2002; van Os et al., 2010; Chen and Baram, 2016; Sripetchwandee et al., 2018).
Fortunately, developmental behavioral plasticity occurs in animals as complex as humans and as simple as nematodes (Jobson et al., 2015; Kundakovic and Champagne, 2015). In this mini review, we explore how the insects are surprisingly well-suited to provide unique contributions to the study of this phenomenon. First, we highlight the strong ecological basis of insect behavior research (Schowalter, 2016), reviewing the exceptionally diverse systems available to explore the neurobiological basis of developmental behavioral plasticity in natural contexts with adaptive significance. Second, we provide an overview of the extensive examples of homology of function between insect and vertebrate nervous systems, despite their phylogenetic distance. We highlight the fact that a variety of mechanisms that embed developmental experience are broadly shared across groups. We conclude that insects offer a fertile and exciting area of future comparative research that explores the complex relationships between early-life experiences and adult behavioral expression.
Insects as Models for Developmental Behavioral Plasticity in Natural Contexts
Extensive previous studies show that the developmental environment has diverse adaptive consequences for insect behavior. Such a perspective is valuable to behavioral neuroscience because environmental context defines the cues, sensory systems, and central processing dynamics that underpin behavioral change. Knowledge of environmental context may also be useful in establishing a general understanding of the types of conditions that give rise to transient versus lasting behavioral effects, a long-term goal in behavioral neuroscience. We highlight some of the established relationships between developmental experience and adult behavioral variation in insects, focusing on three major types of common environmental inputs: season, feeding experience, and interactions with other organisms.
Season
Many insects integrate seasonal cues during development and adaptively tune their adult behavioral expression to match environmental conditions (De Wilde, 1962; Benoit, 2010; Buckley et al., 2012). For example, in the butterfly Bicyclus anynana, males produce a costly nutritional gift they provide to females in order to improve their mating chances. The costs and benefits of this gift change from the wet to the dry season, and accordingly, males adjust their gift production and courtship efforts depending on developmental moisture conditions (Prudic et al., 2011). In ground crickets (Allonemobius fasciatus), developmental temperature constrains male singing ability (Olvido and Mousseau, 1995), and as a result, females adjust their species-specific song preferences in response to their experience of temperature and day length during development (Grace and Shaw, 2004). Subtle differences in developmental temperature (e.g., developing in shaded versus sun-exposed shallow underground nests) can have profound behavioral impacts in female Lasioglossum baleicum bees; they shift from a cooperative reproductive tactic to a solitary one when developing in shadier locations (Hirata and Higashi, 2008). This selection of examples shows that the insects provide opportunities to investigate how simple developmental cues like temperature impact sophisticated phenotypes involving high level sensory integration and complex behaviors.
Feeding Experience
Developmental feeding conditions can convey a variety of information. For example, because many insects are short-lived, developmental diet often predicts the state of nutritional resources available to the adult insect and even its offspring. Females of many insects, particularly moths, prefer to lay eggs on the same species of plant they fed on during development (Petit et al., 2015), a phenomenon often referred to as Hopkins’ Host Selection Principle (Hopkins, 1917). This pattern may minimize search time for suitable host plants for offspring. Though the mechanistic basis of this phenomenon remains controversial, experience-based developmental preferences for or against certain host plants or olfactory cues have been shown in multiple insect clades (Barron, 2001; Rietdorf and Steidle, 2002; Akhtar and Isman, 2003; Blackiston et al., 2008; Akhtar et al., 2009; Videla et al., 2010; Anderson et al., 2013; Anderson and Anton, 2014; König et al., 2015; Lhomme et al., 2017). Developmental feeding conditions can also indirectly signal the degree of intraspecific competition in the immediate environment, triggering mechanisms that alter myriad traits including adult body size, dispersal strategy, activity level, and exploratory behavior (Figure 1; Moczek and Emlen, 2000; Tripet et al., 2002; Tremmel and Müller, 2012).
Diverse neurobiological mechanisms are implicated in the response to developmental feeding experience. For example, plant volatile cues and the olfactory system play a strong role in butterfly and moth larval host plant identification (Petit et al., 2015). In other cases, including in some beetles, bees, aphids, and planthoppers, food intake itself is a cue leading to altered insulin and hormone signaling, which coordinate both peripheral and cognitive processes during development and throughout adulthood (Ament et al., 2008; Snell-Rood and Moczek, 2012; Zhang et al., 2019). More work is needed to understand how physiological processes like insulin signaling affect sensory perception and integration throughout adulthood, a topic that is currently of general interest in vertebrate cognitive neuroscience (Arvanitakis et al., 2020).
Interactions With Other Organisms
Other animals (but see also Schretter et al., 2018; Schwab et al., 2018 for the role of microbiota) commonly shape the insect developmental environment. For example, in a variety of insects, conspecific density and predation pressure induce developmental behavioral plasticity (Walzer and Schausberger, 2011; Müller et al., 2016). One famous case involves the transition from the solitary to gregarious phase in migratory locusts. Increased frequency of physical contact during early life (a result of high conspecific density) gives rise to diverse morphological and behavioral changes, culminating in massive swarming events that disperse individuals to new locations with greater resources (Gillett, 1973; Simpson et al., 2001).
A variety of insect species (e.g., many ants, bees, wasps, and termites) live in complex eusocial societies where certain members forego reproduction to help raise the offspring of their relatives (Oster and Wilson, 1978). Individuals of these species interact socially with conspecifics throughout life, including during development. Female caste differentiation, where females can develop into either a reproductive queen or a non-reproductive worker, is a well-studied example of developmental behavioral plasticity in these eusocial insects (Schwander et al., 2010). Queen/worker caste determination is typically a function of larval nutrition (at least in part) and mediated by adult “nurses” who provide food to larvae (Brian, 1956; Gadagkar et al., 1991; Page and Peng, 2001; Liu et al., 2005; Smith et al., 2008). In some eusocial insects, particularly ants, developmental dietary differences also give rise to behaviorally and morphologically distinct “soldiers” (female workers specialized for defense; Rajakumar et al., 2018).
There are other more subtle effects of the developmental social environment in eusocial insects (Miura, 2004; Traynor et al., 2014; Wang et al., 2014). For example, worker honey bees express different levels of defensiveness during adulthood depending on the defensiveness of the nestmates who rear them; this effect may be mediated by diet, but it is subtle enough that it does not alter body morphology (Rittschof et al., 2015). Adult wasps use vibratory signals directed at larvae, in combination with dietary interventions, to influence adult behavior, again without conspicuous changes in morphology (Jandt et al., 2017). More primitive social insects also show effects of developmental social interactions. For example, in the twig-nesting small carpenter bee (Ceratina calcarata), a mother’s removal from the nest during the larval stage eliminates maternal grooming activity and increases defensive and avoidant behaviors once offspring reach adulthood (Arsenault et al., 2018). Behavioral differentiation in developing insects involves a variety of cue types (e.g., nutrition, pheromone, vibratory, or tactile signals), often acting in combination, suggesting that diverse sensory and physiological systems are integrated to give rise to behavioral effects.
Homology in Insect and Vertebrate Nervous System Function and Plasticity
Insects have a popular reputation of having simplistic, decentralized nervous systems (Schaefer and Ritzmann, 2001). While it is true that some processes are locally guided by “ganglia,” semi-autonomous central nervous system components along the ventral nerve cord (Klowden, 2013), the brain is still required for sensory integration, decision-making, navigation, and learning (Pringle, 1940; Reingold and Camhi, 1977; Zill, 1986; Wessnitzer and Webb, 2006). Indeed, insects are capable of an impressive array of cognitive abilities, such as numeracy and social learning, because of their integrative brains (Chittka and Geiger, 1995; Giurfa et al., 1996, 2001; Dyer, 1998; Crist, 2004; Coolen et al., 2005; Avarguès-Weber, 2012; Pahl et al., 2013; Alem et al., 2016).
Insect brain structure and function is well studied (Ito et al., 2014), giving a strong basis to evaluate mechanisms of developmental plasticity from a comparative perspective. Extensive previous studies illuminate examples of homology of function with vertebrate systems (Simons and Tibbetts, 2019). Below we briefly review these general similarities, and then we focus on the specific neural mechanisms that encode developmental experience, many of which are also shared.
Homology of Function Between Insect and Vertebrate Brains
Insect and vertebrate central nervous systems have similar functions (Kinoshita and Homberg, 2017), and many general features are shared, although notably, the evolutionary origin of these similarities remains controversial (Farris, 2008; Holland et al., 2013). For example, many of the same chemicals act as neurohormones and neurotransmitters, and even in conserved behavioral and cognitive contexts (Bicker et al., 1988; Osborne, 1996; Wu and Brown, 2006; Byrne and Fieber, 2017). In both vertebrates and insects including honey bees, bumble bees, fruit flies, and crickets, dopamine is involved in learning, novelty, reward prediction, and locomotion (Barron et al., 2008; Cohn et al., 2015; Gadagkar et al., 2016; Perry et al., 2016; Hattori et al., 2017; Terao and Mizunami, 2017; Felsenberg et al., 2018; Sovik et al., 2018). Likewise, serotonin modulates appetite, sleep, learning, social behavior, and aggression across a similar range of insect examples (Vleugels et al., 2015; Rillich and Stevenson, 2018; Bubak et al., 2020). Even insect-specific hormones have clear functional analogs in vertebrates. Insect juvenile hormone and vertebrate thyroid hormone both act through type II nuclear receptors, and they show similar growth and developmental functions (Flatt et al., 2006; Charles et al., 2011). Octopamine is an insect-specific neurohormone that is analogous to norepinephrine, and both compounds control stress response, motivation, and aggression (Roeder, 2005; Prieto Peres and Valença, 2010; Alfonso et al., 2019).
Beyond neurochemicals, recent studies suggest extensive homology between insect and vertebrate brain genome dynamics and protein function. Genes responsible for brain developmental patterning are surprisingly conserved (Lichtneckert and Reichert, 2005; Tessmar-Raible et al., 2007; Reichert, 2009; Loesel, 2011; O’Connell, 2013), and there is even evidence for functional conservation of genes associated with complex behaviors like territorial aggression, foraging, and brood care (Toth and Robinson, 2007; Rittschof et al., 2014; Toth et al., 2014; Saul et al., 2019; Shpigler et al., 2019). Cell types in the brain show similarities in structure and function. Like vertebrate brains, insect brains contain neurons and various types of glia (Losada-Perez, 2018), and the metabolic relationships between these cell types are similar across groups (Rittschof and Schirmeier, 2017). Neural activity is well-known for its energetic demands (Peters et al., 2004; Niven and Laughlin, 2008), and insects and vertebrates share some neural adaptations to high energy need (Robertson et al., 2020) and increased cognitive demands; the latter even shows a similar developmental basis (Farris, 2008).
Despite extensive similarities, insects do show some profound differences in nervous system structure and function compared to vertebrates. For example, insect neurons are unmyelinated, they have different classes of olfactory and photoreceptors compared to vertebrates, and neuronal polarity is often different (Chittka and Niven, 2009; Kaupp, 2010; Gutierrez et al., 2011; Rolls and Jegla, 2015; Albert and Kozlov, 2016). Another conspicuous difference between insects and most vertebrates is the structure of early-life development (Figure 2), including the somewhat extreme behavioral and morphological changes that occur during insect metamorphosis. Metamorphosing amphibians and fish are notable exceptions within vertebrates and provide an exciting avenue for comparative work (Gilbert et al., 1996; Heyland and Moroz, 2006; Shi, 2013; Lowe et al., 2021). As with outward appearance, the structure and function of the nervous system can change dramatically during metamorphic developmental transitions in insects (Wolbert and Kubbies, 1983; Weeks and Truman, 1986; Gilbert et al., 1996). For instance, butterflies transition from relatively sessile plant-eating caterpillars to flighted adults with distinct diets, behavioral traits, sensory structures, and motor and cognitive capabilities (André, 1991; Ebenman, 1992). About 80% of all insect species (including ants, bees, wasps, butterflies, beetles, and flies, among others) experience this extreme form of metamorphosis (“complete metamorphosis,” Rolff et al., 2019). Most other insects experience incomplete metamorphosis, where the pupal stage is absent and the body plan in early life is more similar to that of the adult form (except for the absence of wings). Notably, some of these species still show radical differences in life history between juvenile and adult stages (Corbet, 1957; Gabbutt, 1959). The variation in development patterns in insects make them exciting but perhaps challenging subjects for comparative study of developmental behavioral plasticity.
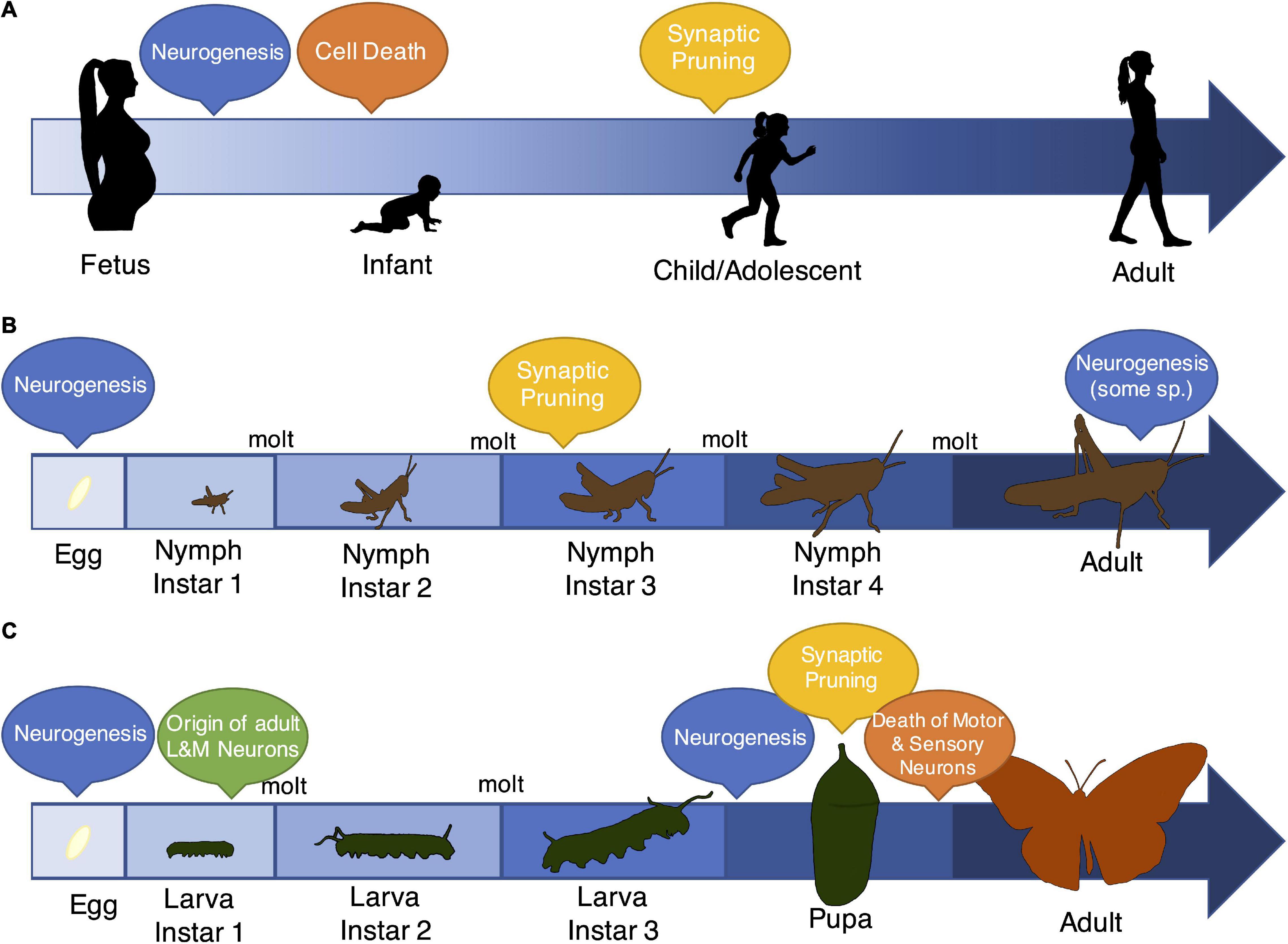
Figure 2. Patterns of development, specifically the timing of neurobiological events, vary across vertebrates and insects. Although insects and vertebrates show remarkable overlap in the types of mechanisms that characterize brain development and entrain early-life experience (Watson, 1992; Pearson, 1993; Reh and Cagan, 1994; Salzberg and Bellen, 1996; Luo and O’Leary, 2005; Bello et al., 2008), the progression of early-life, and specifically the timing of events like neurogenesis, programmed cell death (“Cell Death”), and synaptic pruning, differs markedly across these groups. (A) Most vertebrates show gradual changes in body size and tissue morphology. In the brain, they experience massive neurogenesis early in life followed by cell death and pruning through adolescence and early adulthood (Watson et al., 2006). Notably, more limited neurogenesis also occurs during adulthood (Zhao et al., 2008). (B) Some insects also show a pattern of gradual development (called “incomplete metamorphosis”), where juvenile stages resemble the basic body plan of adults. However, these insects still shed their exoskeletons in order to grow, and as a result, they transition through distinct developmental stages. Relatively little is known about neurobiological events in these species, although there is evidence of extensive neurogenesis both prior to egg hatch and during adulthood (Cayre et al., 1994). There is also evidence for synaptic pruning dynamics that resemble vertebrate mechanisms (Lnenicka and Murphey, 1989). (C) The majority of insects (∼80% of species) show a pattern of complete metamorphosis, where life stages have distinct morphologies, and adult behaviors and body plans vastly differ from juveniles. Data from several representatives of this group again suggest multiple periods of neurogenesis, both early in life and during the pupal stage (Booker and Truman, 1987; Truman and Bate, 1988). Interestingly, the timing of neurogenesis and programmed cell death and the retention of neurons through the life stages is brain region (and thus, functionally) specific (Wegerhoff, 1999; Tissot and Stocker, 2000). For example, a small number of neurons responsible for learning and memory originate early in the larval period and persist through adulthood, but most motor and sensory neurons are completely remodeled during the pupal phase (Cantera et al., 1994).
Despite their developmental complexities, one unique benefit to insect study is that in some species, particularly those that undergo complete metamorphosis, only a subset of neurons is retained between the juvenile and adult stages (Figure 2; Cantera et al., 1994; Wegerhoff, 1999; Tissot and Stocker, 2000). This feature narrows the target populations for studies of early-life environmental effects. For example, in the sensory integration and learning and memory centers of the brain (primarily the “mushroom bodies”), adult neurons typically originate during early larval life, suggesting adequate opportunity to retain environmental information into adulthood; this is in contrast to sensory neurons, which are completely distinct between the larval and adult stages (Cayre et al., 1994; Tissot and Stocker, 2000). Moreover, even though the degree of neuronal remodeling may be relatively extreme in insects compared to vertebrates, the components of the remodeling process closely resemble the types of developmental changes that also occur in vertebrates (Luo and O’Leary, 2005; Bello et al., 2008). For example, analogous to developing vertebrates, different neuron populations in circuits associated with learning and memory display a coordinated process of pruning and regrowth during metamorphosis in Drosophila melanogaster (Spear, 2013; Mayseless et al., 2018). These features of insect neurodevelopment provide unique opportunities to study the complex neural mechanisms of developmental behavioral plasticity in careful detail.
Homology of Function in Neural Mechanisms that Encode Developmental Experience
Early-life cues change adult behavior by persistently altering the structure and/or function of the nervous system (Odeon et al., 2013). Though the precise dynamics of these changes remain poorly understood in any system, in general terms, known mechanisms are similar when comparing vertebrates to insects (Watson, 1992; Pearson, 1993; Reh and Cagan, 1994; Salzberg and Bellen, 1996). Major categories of mechanisms include epigenetic modifications, changes in the quantity of neurochemicals and/or their receptors, and brain structural changes (Elekonich and Robinson, 2000; Kretzschmar and Pflugfelder, 2002; Fahrbach, 2006; Schoofs et al., 2017; Glastad et al., 2019). These mechanisms are not mutually exclusive, and one long-term challenge in behavioral neuroscience for insects and vertebrates alike is to understand how these mechanisms are integrated to alter dynamic behaviors (Wolf and Linden, 2011). However, here we highlight some known insect examples of epigenetic, neurochemical, and structural mechanisms that encode developmental experience.
Chemical modifications to brain DNA are proposed to be critical mediators of early-life effects on adult behavior in vertebrates (Aristizabal et al., 2019). DNA methylation and histone post-translational modifications are the most well-studied among these mechanisms (Smallwood and Kelsey, 2012; Paredes et al., 2016). Not all insects possess appreciable levels of DNA methylation (Deobagkar, 2018; Deshmukh et al., 2018), but some, including many social insects, do (Li-Byarlay, 2016; Yagound et al., 2020). Some studies show that developmental experience-induced changes in DNA methylation impact adult behavioral phenotypes (Linksvayer et al., 2012; Patalano et al., 2012; Weiner and Toth, 2012; Yan et al., 2014; Alvarado et al., 2015). For example, the variation in larval diet that gives rise to queen versus worker female honey bees acts at least in part through DNA methylation changes in both the head and peripheral tissues (Kucharski et al., 2008; Shi et al., 2011; Wang et al., 2020). Similarly, studies in termites and locusts demonstrate a relationship between differential DNA methylation and developmentally induced adult behavioral variation (e.g., in the solitary versus gregarious phases of migratory locusts, Lo et al., 2018). Other known epigenetic mechanisms also play a role in developmental behavioral plasticity in insects, including histone modifications and long non-coding RNAs (Simola et al., 2016; Glastad et al., 2019).
The relationship between brain epigenetic modifications and gene expression patterns varies across species and is not well-understood. For example, whereas DNA methylation in gene regulatory regions tends to suppress gene expression in vertebrates, in insects, gene body methylation, which is thought to regulate alternative splicing, is more common (Feng et al., 2010; Zemach et al., 2010; Glastad et al., 2014; Schmitz et al., 2019). Furthermore, some studies have shown surprisingly weak relationships between DNA methylation dynamics and behavioral expression (Herb et al., 2012; Libbrecht et al., 2016). More data is necessary to understand how DNA methylation dynamics correspond to both gene expression dynamics and behavior (Flores et al., 2012; Li-Byarlay, 2016; Jeong et al., 2018), including whether the presence and degree of DNA methylation and other epigenetic modifications predict capacity for behavioral plasticity (Kapheim et al., 2015; Lo et al., 2018). These are general challenges facing vertebrate research as well (Di Sante et al., 2018), which could benefit from a comparative approach.
The developmental environment can cause lasting behavioral effects by altering neurochemical processes, e.g., circulating levels of hormones and neurotransmitters in the central nervous system. For example, changes in brain insulin, juvenile hormone, prothoracicotropic hormone, octopamine, and serotonin signaling are prominent correlates of insect developmental behavioral plasticity (De Wilde and Beetsma, 1982; Rachinsky, 1994; Paulino Simões et al., 1997; Moczek and Emlen, 2000; Snell-Rood and Moczek, 2012; Erion and Sehgal, 2013; Newsom et al., 2019). These chemicals impact behaviors like aggression, gregariousness, feeding, locomotion, and non-aggressive social interactions (Iba et al., 1995; Anstey et al., 2009; Erion and Sehgal, 2013) in a number of species, including the cricket and locust examples above. The degree to which neurochemical systems comparably regulate behaviors across vertebrates and invertebrates is a matter of debate (Bubak et al., 2020), and thus an important area of on-going study, especially in the context of developmental behavioral plasticity.
A final common way the developmental environment affects the nervous system is through brain structural changes (Teicher et al., 2016; Saleh et al., 2017; Hall and Tropepe, 2020). For example, in flies, high conspecific density during development results in larger mushroom bodies and enhanced olfactory processing abilities (Heisenberg et al., 1995). Similar conditions in wasps lead to increased overall adult brain size, and larger-volume mushroom bodies and regions required for visual processing (Groothuis and Smid, 2017). Gregarious locusts have larger integrative mushroom bodies, while solitary individuals show neural adaptations associated with enhanced sensory sensitivity (Ott and Rogers, 2010). Female social insects often show variation in relative brain region size as a function of behavioral specialization (Lucht-Bertram, 1961; Wheeler and Nijhout, 1984; Vitt and Hartfelder, 1998; Page and Peng, 2001; Muscedere and Traniello, 2012). Insect and vertebrate nervous systems not only exhibit many of the same developmental plasticity mechanisms, but they also face many of the same conceptual challenges associated with connecting developmental experience to behavioral expression. These extensive similarities suggest many potential benefits to comparative study.
Discussion
Predicting, and in some cases changing, adult behavioral effects of early-life experience are challenges relevant to diverse fields of behavioral neuroscience (West-Eberhard, 2003; Beldade et al., 2011; Bryck and Fisher, 2012; Snell-Rood, 2013; Stamps and Biro, 2016; Danese, 2020; Reh et al., 2020). Behavioral effects of early-life experience are commonplace among animal species, presenting the opportunity to use comparative approaches to identify the general principles of developmental behavioral plasticity. Many fundamental questions that are common to both insects and vertebrates remain to be resolved, for example, how the brain integrates early-life experience across multiple levels of organization, and whether specific mechanisms like DNA methylation universally predict long term behavioral impacts. Moreover, it remains unclear how developmental experiences are integrated with other sources of information (e.g., genetic variation, parental transgenerational effects) that also influence behavior (Dall et al., 2015; Stamps and Frankenhuis, 2016; Stein et al., 2018; Rösvik et al., 2020), and whether these outcomes can be modified by additional information later in life. Though these sources of complexity apply to both insect and vertebrate species, certain characteristics of insects, like their relatively short lifespans, may alter the ecological selection pressures that shape information integration. With respect to the evolution and expression of behavioral plasticity, diverse comparative approaches may illuminate both broad, general features and taxon-specific patterns.
In insects, studies of behavioral plasticity largely focus on processes during the adult stage, and although many patterns of nervous system development are known (Prillinger, 1981; Rospars, 1988; Hähnlein and Bicker, 1997; Cayre et al., 2000; Awasaki et al., 2008), precisely how these patterns respond to early-life environmental stimuli remains poorly understood. However, the deep research history of insects in natural ecological contexts provides diverse, tractable systems for future work that fills this research gap. The developmental environment, including simple abiotic factors like temperature and moisture, impacts a variety of sophisticated behaviors from dispersal patterns (Zera and Denno, 1997; Alyokhin and Ferro, 1999; Benard and McCauley, 2008) to social and reproductive tactics (Radwan, 1995; Emlen, 1997; Taborsky and Brockmann, 2010; Łukasik, 2010; Kasumovic and Brooks, 2011). Thus, in controlled but environmentally relevant experiments, it is possible to assess how specific types of developmental inputs shape both sensory and integrative processes (Anton and Rossler, 2020; Fernandez et al., 2020; Gonzalez-Tokman et al., 2020) relevant to many different behavioral phenotypes. In addition, the short generation time of insects is ideal for life-long studies of behavior.
On the neurobiological level, developmental behavioral plasticity in insects is mediated through familiar neural plasticity mechanisms like epigenetic modifications, neurochemical changes, and changes to neural structure (LeBoeuf et al., 2013). Some of these mechanisms can be, and have been, explored in the context of traditional learning and memory frameworks, which also are well established in insects (Tully et al., 1994; Blackiston et al., 2008; Yang et al., 2012; Alloway, 2015; Tan et al., 2015). Though most learning and memory research has focused on dynamics during the adult stage (Fahrbach et al., 1998; Ravary et al., 2007; Li et al., 2017; Jernigan et al., 2020), many insights from this work are likely applicable to the pre-adult life stages as well. Moreover, in what may be the majority of insect species, only a subset of the brain survives the transition from the juvenile life-stage to adulthood, presenting a narrow range of target areas in which to carefully investigate how neural plasticity mechanisms give rise to complex behaviors. However, some challenges to comparative work remain. For instance, it is unclear which insect life stages are comparable to the early-life timeframe in vertebrates, or whether retention of early-life effects in insects is fundamentally constrained by their extensive morphological and neurobiological remodeling (Vea and Minakuchi, 2020).
Despite these challenges, insects have a history of contributing surprisingly general insights into complex behavioral phenotypes relevant to vertebrate species. For example, eusocial insects present detailed systems to address general neurobiological principles of developmental behavioral plasticity in the context of complex social living. Because insect societies show patterns of organization that can be generalized to other social species (Seeley, 1995; Bonner, 2004; Ireland and Garnier, 2018), they have tremendous promise for investigating both the causes and consequences of developmental plasticity in vertebrates. This comparison may even extend to humans, where many persistent effects of the early-life environment on behavior and mental health are social in nature (Miller et al., 2009; Nothling et al., 2019). It is possible that behavioral plasticity in social contexts has unique neurobiological features (Taborsky and Oliveira, 2012), and social insects will continue to serve as excellent models to examine this idea.
Although this review is specifically focused on insect contributions to behavioral neuroscience in a comparative framework, the uniqueness of this animal group, as well as its ecological and economic importance, cannot be overstated. These aspects provide further motivation for study of developmental behavioral plasticity in this group. Many bee species are important agricultural pollinators (Winfree et al., 2011; Reilly et al., 2020). The ongoing locust outbreak in East Africa is anticipated to cause enormous economic loss and endanger food security (Peng et al., 2020). Many agricultural pests are metamorphosing insects with destructive larval feeding stages (e.g., beetles and moths). Understanding the natural history of these organisms, as well as the range of neural and behavioral responses to developmental experience (Haynes, 1988; Desneux et al., 2007; De França et al., 2017; Müller, 2018; Sehonova et al., 2018) will improve environmental management in addition to deepening our understanding of the general principles of developmental behavioral plasticity.
Author Contributions
RW and CR conceptualized and wrote the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture Hatch Program (accession number 1012993, CR) and the Foundation for Food and Agriculture Research Pollinator Health Fund (549049, CR).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Akhtar, Y., and Isman, M. B. (2003). Larval exposure to oviposition deterrents alters subsequent oviposition behavior in generalist, trichoplusia ni and specialist, Plutella xylostella Moths. J. Chem. Ecol. 29, 1853–1870. doi: 10.1023/A:1024802328458
Akhtar, Y., Shikano, I., and Isman, M. B. (2009). Topical application of a plant extract to different life stages of Trichoplusia ni fails to influence feeding or oviposition behaviour. Entomol. Exp. Appl. 132, 275–282. doi: 10.1111/j.1570-7458.2009.00895.x
Albert, J.T., and Kozlov, A.S. (2016). Comparative aspects of hearing in vertebrates and insects with antennal ears. Curr. Biol. 26, R1050–R1061. doi: 10.1016/j.cub.2016.09.017
Alem, S., Perry, C. J., Zhu, X., Loukola, O. J., Ingraham, T., Søvik, E., et al. (2016). Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol. 14:e1002564. doi: 10.1371/journal.pbio.1002564
Alfonso, S., Sadoul, B., Gesto, M., Joassard, L., Chatain, B., Geffroy, B., et al. (2019). Coping styles in European sea bass: the link between boldness, stress response and neurogenesis. Physiol. Behav. 207, 76–85. doi: 10.1016/j.physbeh.2019.04.020
Alloway, T. M. (2015). Retention of learning through metamorphosis in the grain Beetle Tenebrio molitor. Am. Zool. 12, 471–477. doi: 10.1093/icb/12.3.471
Alvarado, S., Rajakumar, R., Abouheif, E., and Szyf, M. (2015). Epigenetic variation in the Egfr gene generates quantitative variation in a complex trait in ants. Nat. Commun. 6:6513. doi: 10.1038/ncomms7513
Alyokhin, A. V., and Ferro, D. N. (1999). Modifications in dispersal and oviposition of Bt-resistant and Bt-susceptible Colorado potato beetles as a result of exposure to Bacillus thuringiensis subsp. tenebrionis Cry3A toxin. Entomol. Exp. Appl. 90, 93–101. doi: 10.1046/j.1570-7458.1999.00426.x
Ament, S. A., Corona, M., Pollock, H. S., and Robinson, G. E. (2008). Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl. Acad. Sci. U.S.A. 105, 4226–4231. doi: 10.1073/pnas.0800630105
Anderson, P., and Anton, S. (2014). Experience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant Cell Environ. 37, 1826–1835. doi: 10.1111/pce.12342
Anderson, P., Sadek, M. M., Larsson, M., Hansson, B. S., and Thöming, G. (2013). Larval host plant experience modulates both mate finding and oviposition choice in a moth. Anim. Behav. 85, 1169–1175. doi: 10.1016/j.anbehav.2013.03.002
André, H. M. (1991). Stase, metamorphosis and competition in insects and other arthropods. Belg. J. Zool. 121, 3–25.
Anstey, M. L., Rogers, S. M., Ott, S. R., Burrows, M., and Simpson, S. J. (2009). Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323, 627–630. doi: 10.1126/science.1165939
Anton, S., and Rossler, W. (2020). Plasticity and modulation of olfactory circuits in insects. Cell Tissue Res. 383, 149–164. doi: 10.1007/s00441-020-03329-z
Aristizabal, M. J., Anreiter, I., Halldorsdottir, T., Odgers, C. L., McDade, T. W., Goldenberg, A., et al. (2019). Biological embedding of experience: a primer on epigenetics. Proc. Natl. Acad. Sci. U.S.A. 117, 23261–23269. doi: 10.1073/pnas.1820838116
Arsenault, S. V., Hunt, B. G., and Rehan, S. M. (2018). The effect of maternal care on gene expression and DNA methylation in a subsocial bee. Nat. Commun. 9:3468. doi: 10.1038/s41467-018-05903-0
Arvanitakis, Z., Wang, H. Y., Capuano, A. W., Khan, A., Taib, B., Anokye-Danso, F., et al. (2020). Brain insulin signaling, alzheimer disease pathology, and cognitive function. Ann. Neurol. 88, 513–525. doi: 10.1002/ana.25826
Avarguès-Weber, A. (2012). Face recognition: lessons from a Wasp. Curr. Biol. 22, R91–R93. doi: 10.1016/j.cub.2011.12.040
Avishai-Eliner, S., Brunson, K. L., Sandman, C. A., and Baram, T. Z. (2002). Stressed-out, or in (utero)? Trends Neurosci. 25, 518–524. doi: 10.1016/s0166-2236(02)02241-5
Avitsur, R., Levy, S., Goren, N., and Grinshpahet, R. (2015). Early adversity, immunity and infectious disease. Stress 18, 289–296. doi: 10.3109/10253890.2015.1017464
Awasaki, T., Lai, S.-L., Ito, K., and Lee, T. (2008). Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 28, 13742–13753. doi: 10.1523/jneurosci.4844-08.2008
Barker, D. J. P. (1995). Fetal origins of coronary heart disease. BMJ 311, 171–174. doi: 10.1136/bmj.311.6998.171
Barron, A. (2001). The life and death of hopkins’ host-selection principle. J. Insect Behav. 14, 725–737. doi: 10.1023/A:1013033332535
Barron, A. B., Maleszka, R., Helliwell, P. G., and Robinson, G. E. (2008). Effects of cocaine on honey bee dance behaviour. J. Exp. Biol. 212, 163–168. doi: 10.1242/jeb.025361
Beach, F. A., and Jaynes, J. (1954). Effects of early experience upon the behavior of animals. Psychol. Bull. 51, 239–263. doi: 10.1037/h0061176
Beldade, P., Mateus, A. R. A., and Keller, R. A. (2011). Evolution and molecular mechanisms of adaptive developmental plasticity. Mol. Ecol. 20, 1347–1363. doi: 10.1111/j.1365-294X.2011.05016.x
Bello, B. C., Izergina, N., Caussinus, E., and Reichert, H. (2008). Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev. 3:5. doi: 10.1186/1749-8104-3-5
Benard, M. F., and McCauley, S. J. (2008). Integrating across life−history stages: consequences of natal habitat effects on dispersal. Am. Nat. 171, 553–567. doi: 10.1086/587072
Benoit, J. B. (2010). “Water management by dormant insects: comparisons between dehydration resistance during summer aestivation and winter diapause,” in Aestivation: Molecular and Physiological Aspects, eds C. Arturo Navas and J. E. Carvalho (Berlin: Springer), 209–229. doi: 10.1007/978-3-642-02421-4_10
Bicker, G., Schafer, S., Ottersen, O. P., and Storm-Mathisen, J. (1988). Glutamate-like immunoreactivity in identified neuronal populations of insect nervous systems. J. Neurosci. 8, 2108–2122. doi: 10.1523/jneurosci.08-06-02108.1988
Blackiston, D. J., Silva Casey, E., and Weiss, M. R. (2008). Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS One 3:e1736. doi: 10.1371/journal.pone.0001736
Bonner, J. T. (2004). Perspective: the size-complexity rule. Evolution 58, 1883–1890. doi: 10.1111/j.0014-3820.2004.tb00476.x
Booker, R., and Truman, J. W. (1987). Postembryonic neurogenesis in the CNS of the tobacco hornworm, Manduca sexta. I. Neuroblast arrays and the fate of their progeny during metamorphosis. J. Comp. Neurol. 255, 548–559. doi: 10.1002/cne.902550407
Boyce, W. T., Sokolowski, M. B., and Robinson, G. E. (2020). Genes and environments, development and time. Proc. Natl. Acad. Sci. U.S.A. 117, 23235–23241. doi: 10.1073/pnas.2016710117
Brian, M. V. (1956). Studies of caste differentiation inMyrmica rubra L. 4. Controlled larval nutrition. Insectes Soc. 3, 369–394. doi: 10.1007/BF02225758
Bryck, R. L., and Fisher, P. A. (2012). Training the brain: practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. Am. Psychol. 67:87. doi: 10.1037/a0024657
Bubak, A. N., Watt, M. J., Yaeger, J. D. W., Renner, K. J., and Swallow, J. G. (2020). The stalk-eyed fly as a model for aggression - is there a conserved role for 5-HT between vertebrates and invertebrates? J. Exp. Biol. 223, (Pt 1), jeb132159. doi: 10.1242/jeb.132159
Buckley, L. B., Hurlbert, A. H., and Jetz, W. (2012). Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob. Ecol. Biogeogr. 21, 873–885. doi: 10.1111/j.1466-8238.2011.00737.x
Byrne, J. H., and Fieber, L. A. (2017). Neurotransmitters and Neuropeptides of Invertebrates. Oxford: Oxford University Press.
Cantera, R., Nässel, D. R., and Veenstra, J. A. (1994). Postembryonic development of corazonin-containing neurons and neurosecretory cells in the blowfly, Phormia terraenovae. J. Comp. Neurol. 350, 559–572. doi: 10.1002/cne.903500405
Cardoso, S. D., Teles, M. C., and Oliveira, R. F. (2015). Neurogenomic mechanisms of social plasticity. J. Exp. Biol. 218(Pt 1), 140–149. doi: 10.1242/jeb.106997
Cayre, M., Malaterre, J., Charpin, P., Strambi, C., and Strambi, A. (2000). Fate of neuroblast progeny during postembryonic development of mushroom bodies in the house cricket, Acheta domesticus. J. Insect Physiol. 46, 313–319. doi: 10.1016/S0022-1910(99)00184-5
Cayre, M., Strambi, C., and Strambi, A. (1994). Neurogenesis in an adult insect brain and its hormonal control. Nature 368, 57–59. doi: 10.1038/368057a0
Champagne, F. A. (2012). Interplay between social experiences and the genome: epigenetic consequences for behavior. Adv. Genet. 77, 33–57. doi: 10.1016/B978-0-12-387687-4.00002-7
Champagne, F. A., and Curley, J. P. (2005). How social experiences influence the brain. Curr. Opin. Neurobiol. 15, 704–709. doi: 10.1016/j.conb.2005.10.001
Charles, J.-P., Iwema, T., Epa, V. C., Takaki, K., Rynes, J., and Jindra, M. (2011). Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. U.S.A. 108, 21128–21133. doi: 10.1073/pnas.1116123109
Chen, Y., and Baram, T. Z. (2016). Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 41, 197–206. doi: 10.1038/npp.2015.181
Chittka, L., and Geiger, K. (1995). Can honey bees count landmarks? Anim. Behav. 49, 159–164. doi: 10.1016/0003-3472(95)80163-4
Chittka, L., and Niven, J. (2009). Are bigger brains better? Curr. Biol. 19, R995–R1008. doi: 10.1016/j.cub.2009.08.023
Cohn, R., Morantte, I., and Ruta, V. (2015). Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell 163, 1742–1755. doi: 10.1016/j.cell.2015.11.019
Coolen, I., Dangles, O., and Casas, J. (2005). Social learning in noncolonial insects? Curr. Biol. 15, 1931–1935. doi: 10.1016/j.cub.2005.09.015
Corbet, P. S. (1957). The life-history of the emperor dragonfly Anax imperator Leach (Odonata: Aeshnidae). J. Anim. Ecol. 26, 1–69. doi: 10.2307/1781
Crist, E. (2004). Can an insect speak? The case of the honeybee dance language. Soc. Stud. Sci. 34, 7–43. doi: 10.1177/0306312704040611
Curley, J. P., and Champagne, F. A. (2016). Influence of maternal care on the developing brain: mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 40:52–66. doi: 10.1016/j.yfrne.2015.11.001
Dall, S. R., McNamara, J. M., and Leimar, O. (2015). Genes as cues: phenotypic integration of genetic and epigenetic information from a Darwinian perspective. Trends Ecol. Evol. 30, 327–333. doi: 10.1016/j.tree.2015.04.002
Danese, A. (2020). Annual research review: rethinking childhood trauma-new research directions for measurement, study design and analytical strategies. J. Child Psychol. Psychiatry 61, 236–250. doi: 10.1111/jcpp.13160
De França, S. M., Breda, M. O., Barbosa, D. R., Araujo, A. M., and Guedes, C. A. (2017). “The sublethal effects of insecticides in insects,” in Biological Control of Pest and Vector Insects, ed. V. D. C. Shields (London: IntechOpen), 23–39.
De Wilde, J. (1962). Photoperiodism in insects and mites. Annu. Rev. Entomol. 7, 1–26. doi: 10.1146/annurev.en.07.010162.000245
De Wilde, J., and Beetsma, J. (1982). “The physiology of caste development in social insects,” in Advances in Insect Physiology, eds M. J. Berridge, J. E. Treherne, and V. B. Wigglesworth (Cambridge, MA: Academic Press), 167–246. doi: 10.1016/s0065-2806(08)60154-x
Deobagkar, D. (2018). Epigenetics with special reference to the human X chromosome inactivation and the enigma of Drosophila DNA methylation. J. Genet. 97, 371–378. doi: 10.1007/s12041-018-0937-5
Deshmukh, S., Ponnaluri, V. C., Dai, N., Pradhan, S., and Deobagkar, D. (2018). Levels of DNA cytosine methylation in the Drosophila genome. PeerJ 6:e5119. doi: 10.7717/peerj.5119
Desneux, N., Decourtye, A., and Delpuech, J.-M. (2007). The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106. doi: 10.1146/annurev.ento.52.110405.091440
Di Sante, J., Ismaylova, E., Nemoda, Z., Gouin, J. P., Yu, W. J., Caldwell, W., et al. (2018). Peripheral DNA methylation of HPA axis-related genes in humans: Cross-tissue convergence, two-year stability and behavioural and neural correlates. Psychoneuroendocrinology 97, 196–205. doi: 10.1016/j.psyneuen.2018.07.019
Dyer, F. C. (1998). “5 - Spatial cognition: lessons from central-place foraging insects,” in Animal Cognition in Nature, eds R. P. Balda, I M. Pepperberg, and A. C. Kamil (London: Academic Press), 119–154.
Ebenman, B. (1992). Evolution in organisms that change their niches during the life cycle. Am. Nat. 139, 990–1021. doi: 10.1086/285370
Elekonich, M. M., and Robinson, G. E. (2000). Organizational and activational effects of hormones on insect behavior. J. Insect Physiol. 46, 1509–1515. doi: 10.1016/S0022-1910(00)00101-3
Emlen, D. J. (1997). Alternative reproductive tactics and male-dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 41, 335–341. doi: 10.1007/s002650050393
Erion, R., and Sehgal, A. (2013). Regulation of insect behavior via the insulin-signaling pathway. Front. Physiol. 4:353. doi: 10.3389/fphys.2013.00353
Fahrbach, S. E. (2006). Structure of the mushroom bodies of the insect brain. Annu. Rev. Entomol. 51, 209–232. doi: 10.1146/annurev.ento.51.110104.150954
Fahrbach, S. E., Moore, D., Capaldi, E. A., Farris, S. M., and Robinson, G. E. (1998). Experience-expectant plasticity in the mushroom bodies of the honeybee. Learn. Mem. 5, 115–123.
Farris, S. M. (2008). Structural, functional and developmental convergence of the insect mushroom bodies with higher brain centers of vertebrates. Brain Behav. Evol. 72, 1–15. doi: 10.1159/000139457
Felsenberg, J., Jacob, P. F., Walker, T., Barnstedt, O., Edmondson-Stait, A. J., Pleijzier, M. W., et al. (2018). Integration of parallel opposing memories underlies memory extinction. Cell 175, 709.e15–722.e15. doi: 10.1016/j.cell.2018.08.021
Feng, S., Cokus, S. J., Zhang, X., Chen, P.-Y., Bostick, M., Goll, M. G., et al. (2010). Conservation and divergence of methylation patterning in plants and animals. Proc. Natl. Acad. Sci. U.S.A. 107, 8689–8694. doi: 10.1073/pnas.1002720107
Fernandez, M. P., Pettibone, H. L., Bogart, J. T., Roell, C. J., Davey, C. E., Pranevicius, A., et al. (2020). Sites of circadian clock neuron plasticity mediate sensory integration and entrainment. Curr. Biol. 30, 2225.e5–2237.e5. doi: 10.1016/j.cub.2020.04.025
Flatt, T., Moroz, L. L., Tatar, M., and Heyland, A. (2006). Comparing thyroid and insect hormone signaling. Integr. Comp. Biol. 46, 777–794. doi: 10.1093/icb/icl034
Flores, K., Wolschin, F., Corneveaux, J. J., Allen, A. N., Huentelman, M. J., and Amdam, G. V. (2012). Genome-wide association between DNA methylation and alternative splicing in an invertebrate. BMC Genomics 13:480. doi: 10.1186/1471-2164-13-480
Gabbutt, P. D. (1959). The bionomics of the wood cricket, Nemobius sylvestris (Orthoptera: Gryllidae). J. Anim. Ecol. 28, 15–42. doi: 10.2307/2011
Gadagkar, R., Bhagavan, S., Chandrashekara, K., and Vinutha, C. (1991). The role of larval nutrition in pre-imaginal biasing of caste in the primitively eusocial wasp Ropalidia marginata (Hymenoptera: Vespidae). Ecol. Entomol. 16, 435–440. doi: 10.1111/j.1365-2311.1991.tb00236.x
Gadagkar, V., Puzerey, P. A., Chen, R., Baird-Daniel, E., Farhang, A. R., and Goldberg, J. H. (2016). Dopamine neurons encode performance error in singing birds. Science 354, 1278–1282. doi: 10.1126/science.aah6837
Gilbert, L. I., Tata, J. R., and Atkinson, B. G. (1996). Metamorphosis: Postembryonic Reprogramming of Gene Expression in Amphibian and Insect Cells. London: Academic Press.
Gillett, S. D. (1973). Social determinants of aggregation behaviour in adults of the desert locust. Anim. Behav. 21, 599–606. doi: 10.1016/S0003-3472(73)80022-3
Giurfa, M., Eichmann, B., and Menzel, R. (1996). Symmetry perception in an insect. Nature 382, 458–461. doi: 10.1038/382458a0
Giurfa, M., Zhang, S., Jenett, A., Menzel, R., and Srinivasan, M. V. (2001). The concepts of ‘sameness’ and ‘difference’in an insect. Nature 410, 930–933. doi: 10.1038/35073582
Glastad, K. M., Hunt, B. G., and Goodisman, M. A. D. (2014). Evolutionary insights into DNA methylation in insects. Curr. Opin. Insect Sci. 1, 25–30. doi: 10.1016/j.cois.2014.04.001
Glastad, K. M., Hunt, B. G., and Goodisman, M. A. D. (2019). Epigenetics in insects: genome regulation and the generation of phenotypic diversity. Annu. Rev. Entomol. 64, 185–203. doi: 10.1146/annurev-ento-011118-111914
Gonzalez-Tokman, D., Cordoba-Aguilar, A., Dattilo, W., Lira-Noriega, A., Sanchez-Guillen, R. A., and Villalobos, F. (2020). Insect responses to heat: physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. Camb. Philos. Soc. 95, 802–821. doi: 10.1111/brv.12588
Grace, J. L., and Shaw, K. L. (2004). Effects of developmental environment on signal-preference coupling in a Hawaiian cricket. Evolution 58, 1627–1633. doi: 10.1111/j.0014-3820.2004.tb01744.x
Groothuis, J., and Smid, H. M. (2017). Nasonia parasitic wasps escape from Haller’s rule by diphasic, partially isometric brain-body size scaling and selective neuropil adaptations. Brain Behav. Evol. 90, 243–254. doi: 10.1159/000480421
Gutierrez, D. V., Oh, E., and Herlitze, S. (2011). “Vertebrate and invertebrate rhodopsins: light control of G-Protein signaling,” in Photosensitive Molecules for Controlling Biological Function, eds J. J. Chambers, and R. H. Kramer (Berlin: Springer), 133–146. doi: 10.1007/978-1-61779-031-7_7
Hähnlein, I., and Bicker, G. (1997). Glial patterning during postembryonic development of central neuropiles in the brain of the honeybee. Dev. Genes Evol. 207, 29–41. doi: 10.1007/s004270050089
Hall, Z. J., and Tropepe, V. (2020). Using teleost fish to discern developmental signatures of evolutionary adaptation from phenotypic plasticity in brain structure. Front. Neuroanat. 14:10. doi: 10.3389/fnana.2020.00010
Hattori, D., Aso, Y., Swartz, K. J., Rubin, G. M., Abbott, L. F., and Axel, R. (2017). Representations of novelty and familiarity in a mushroom body compartment. Cell 169, 956.e17–969.e17. doi: 10.1016/j.cell.2017.04.028
Haynes, K. F. (1988). Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 33, 149–168. doi: 10.1146/annurev.en.33.010188.001053
Heisenberg, M., Heusipp, M., and Wanke, C. (1995). Structural plasticity in the Drosophila brain. J. Neurosci. 15, 1951–1960. doi: 10.1523/jneurosci.15-03-01951.1995
Herb, B. R., Wolschin, F., Hansen, K. D., Aryee, M. J., Langmead, B., Irizarry, R., et al. (2012). Reversible switching between epigenetic states in honeybee behavioral subcastes. Nat. Neurosci. 15, 1371–1373. doi: 10.1038/nn.3218
Heyland, A., and Moroz, L. L. (2006). Signaling mechanisms underlying metamorphic transitions in animals. Integr. Comp. Biol. 46, 743–759. doi: 10.1093/icb/icl023
Hirata, M., and Higashi, S. (2008). Degree-day accumulation controlling allopatric and sympatric variations in the sociality of sweat bees, Lasioglossum (Evylaeus) baleicum (Hymenoptera: Halictidae). Behav. Ecol. Sociobiol. 62:1239. doi: 10.1007/s00265-008-0552-1
Hochberg, Z., Feil, R., Constancia, M., Fraga, M., Junien, C., Carel, J. C., et al. (2011). Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 32, 159–224. doi: 10.1210/er.2009-0039
Hoffmann, A., and Spengler, D. (2018). The mitochondrion as potential interface in early-life stress brain programming. Front. Behav. Neurosci. 12:306. doi: 10.3389/fnbeh.2018.00306
Holland, L. Z., Carvalho, J. E., Escriva, H., Laudet, V., Schubert, M., Shimeld, S. M., et al. (2013). Evolution of bilaterian central nervous systems: a single origin. EvoDevo 4:27. doi: 10.1186/2041-9139-4-27
Hopkins, A. (1917). A discussion of CG Hewitt’s paper on “Insect Behaviour”. J. Econ. Entomol. 10, 92–93.
Iba, M., Nagao, T., and Urano, A. (1995). Effects of population density on growth, behavior and levels of biogenic amines in the cricket, Gryllus bimaculatus. Zool. Sci. 12, 695–702. doi: 10.2108/zsj.12.695
Ireland, T., and Garnier, S. (2018). Architecture, space and information in constructions built by humans and social insects: a conceptual review. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20170244. doi: 10.1098/rstb.2017.0244
Ito, K., Shinomiya, K., Ito, M., Armstrong, J. D., Boyan, G., Hartenstein, V., et al. (2014). A systematic nomenclature for the insect brain. Neuron 81, 755–765. doi: 10.1016/j.neuron.2013.12.017
Jandt, J. M., Suryanarayanan, S., Hermanson, J. C., Jeanne, R. L., and Toth, A. L. (2017). Maternal and nourishment factors interact to influence offspring developmental trajectories in social wasps. Proc. Biol. Sci. 284:20170651. doi: 10.1098/rspb.2017.0651
Jeong, H., Wu, X., Smith, B., and Yi, S. V. (2018). Genomic landscape of methylation islands in hymenopteran insects. Genome Biol. Evol. 10, 2766–2776. doi: 10.1093/gbe/evy203
Jernigan, C. M., Halby, R., Gerkin, R. C., Sinakevitch, I., Locatelli, F., and Smith, B. H. (2020). Experience-dependent tuning of early olfactory processing in the adult honey bee, Apis mellifera. J. Exp. Biol. 223(Pt 1):jeb206748. doi: 10.1242/jeb.206748
Jobson, M. A., Jordan, J. M., Sandrof, M. A., Hibshman, J. D., Lennox, A. L., and Baugh, L. R. (2015). Transgenerational effects of early life starvation on growth, reproduction, and stress resistance in Caenorhabditis elegans. Genetics 201, 201–212. doi: 10.1534/genetics.115.178699
Kapheim, K. M., Pan, H., Li, C., Salzberg, S. L., Puiu, D., Magoc, T., et al. (2015). Genomic signatures of evolutionary transitions from solitary to group living. Science 348, 1139–1143.
Kasumovic, M. M., and Brooks, R. C. (2011). It’s all who you know: the evolution of socially cued anticipatory plasticity as a mating strategy. Q. Rev. Biol. 86, 181–197. doi: 10.1086/661119
Kaupp, U. B. (2010). Olfactory signalling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 11, 188–200. doi: 10.1038/nrn2789
Kinoshita, M., and Homberg, U. (2017). “Insect brains: minute structures controlling complex behaviors,” in Brain Evolution by Design: From Neural Origin to Cognitive Architecture, eds S. Shigeno, Y. Murakami, and T. Nomura (Tokyo: Springer), 123–151. doi: 10.1007/978-4-431-56469-0_6
König, K., Krimmer, E., Brose, S., Gantert, C., Buschlüter, I., König, C., et al. (2015). Does early learning drive ecological divergence during speciation processes in parasitoid wasps? Proc. R. Soc. B Biol. Sci. 282:20141850. doi: 10.1098/rspb.2014.1850
Korosi, A., Naninck, E. F., Oomen, C. A., Schouten, M., Krugers, H., Fitzsimons, C., et al. (2012). Early-life stress mediated modulation of adult neurogenesis and behavior. Behav. Brain Res. 227, 400–409. doi: 10.1016/j.bbr.2011.07.037
Kretzschmar, D., and Pflugfelder, G. O. (2002). Glia in development, function, and neurodegeneration of the adult insect brain. Brain Res. Bull. 57, 121–131. doi: 10.1016/S0361-9230(01)00643-8
Kucharski, R., Maleszka, J., Foret, S., and Maleszka, R. (2008). Nutritional control of reproductive status in honeybees via DNA methylation. Science 319, 1827–1830. doi: 10.1126/science.1153069
Kundakovic, M., and Champagne, F. A. (2015). Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology 40, 141–153. doi: 10.1038/npp.2014.140
LeBoeuf, A. C., Benton, R., and Keller, L. (2013). The molecular basis of social behavior: models, methods and advances. Curr. Opin. Neurobiol. 23, 3–10. doi: 10.1016/j.conb.2012.08.008
Lhomme, P., Carrasco, D., Larsson, M., Hansson, B., and Anderson, P. (2017). A context-dependent induction of natal habitat preference in a generalist herbivorous insect. Behav. Ecol. 29, 360–367. doi: 10.1093/beheco/arx173
Li, L., MaBouDi, H., Egertova, M., Elphick, M. R., Chittka, L., and Perry, C. J. (2017). A possible structural correlate of learning performance on a colour discrimination task in the brain of the bumblebee. Proc. Biol. Sci. 284:20171323. doi: 10.1098/rspb.2017.1323
Libbrecht, R., Oxley, P. R., Keller, L., and Kronauer, D. J. (2016). Robust DNA methylation in the clonal raider ant brain. Curr. Biol. 26, 391–395. doi: 10.1016/j.cub.2015.12.040
Li-Byarlay, H. (2016). The function of DNA methylation marks in social insects. Front. Ecol. Evol. 4:57. doi: 10.3389/fevo.2016.00057
Lichtneckert, R., and Reichert, H. (2005). Insights into the urbilaterian brain: conserved genetic patterning mechanisms in insect and vertebrate brain development. Heredity 94, 465–477. doi: 10.1038/sj.hdy.6800664
Linksvayer, T. A., Fewell, J. H., Gadau, J., and Laubichler, M. D. (2012). Developmental evolution in social insects: regulatory networks from genes to societies. J. Exp. Zool. Part B Mol. Dev. Evol. 318, 159–169. doi: 10.1002/jez.b.22001
Liu, Y., Henderson, G., Mao, L., and Laine, R. A. (2005). Effects of temperature and nutrition on juvenile hormone titers of Coptotermes formosanus (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 98, 732–737. doi: 10.1603/0013-87462005098[0732:Eotano]2.0.Co;2
Lnenicka, G. A., and Murphey, R. K. (1989). The refinement of invertebrate synapses during development. J. Neurobiol. 20, 339–355. doi: 10.1002/neu.480200507
Lo, N., Simpson, S. J., and Sword, G. A. (2018). Epigenetics and developmental plasticity in orthopteroid insects. Curr. Opin. Insect Sci. 25, 25–34. doi: 10.1016/j.cois.2017.11.003
Loesel, R. (2011). “Neurophylogeny: retracing early metazoan brain evolution,” in Evolutionary Biology – Concepts, Biodiversity, Macroevolution and Genome Evolution, ed. P. Pontarotti (Berlin: Springer), 169–191. doi: 10.1007/978-3-642-20763-1_11
Losada-Perez, M. (2018). Glia: from ‘just glue’ to essential players in complex nervous systems: a comparative view from flies to mammals. J. Neurogenet. 32, 78–91. doi: 10.1080/01677063.2018.1464568
Lowe, W. H., Martin, T. E., Skelly, D. K., and Woods, H. A. (2021). Metamorphosis in an era of increasing climate variability. Trends Ecol. Evol. 36, 360–375. doi: 10.1016/j.tree.2020.11.012
Lucht-Bertram, E. (1961). Das postembryonale wachstum von hirnteilen bei Apis mellifica L. und Myrmeleon europaeus L. Zeitschrift für Morphol. Ökol. Tiere 50, 543–575. doi: 10.1007/bf00389832
Łukasik, P. (2010). Trophic dimorphism in alternative male reproductive morphs of the acarid mite Sancassania berlesei. Behav. Ecol. 21, 270–274. doi: 10.1093/beheco/arp186
Luo, L., and O’Leary, D. D. (2005). Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156. doi: 10.1146/annurev.neuro.28.061604.135632
Mayseless, O., Berns, D. S., Yu, X. M., Riemensperger, T., Fiala, A., and Schuldiner, O. (2018). Developmental coordination during olfactory circuit remodeling in Drosophila. Neuron 99, 1204.e5–1215.e5. doi: 10.1016/j.neuron.2018.07.050
Miller, G. E., Chen, E., Fok, A. K., Walker, H., Lim, A., Nicholls, E. F., et al. (2009). Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 14716–14721. doi: 10.1073/pnas.0902971106
Miura, T. (2004). Proximate mechanisms and evolution of caste polyphenism in social insects: from sociality to genes. Ecol. Res. 19, 141–148. doi: 10.1111/j.1440-1703.2003.00618.x
Moczek, A. P., and Emlen, D. J. (2000). Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim. Behav. 59, 459–466. doi: 10.1006/anbe.1999.1342
Müller, C. (2018). Impacts of sublethal insecticide exposure on insects — Facts and knowledge gaps. Basic Appl. Ecol. 30, 1–10. doi: 10.1016/j.baae.2018.05.001
Müller, T., Küll, C. L., and Müller, C. (2016). Effects of larval versus adult density conditions on reproduction and behavior of a leaf beetle. Behav. Ecol. Sociobiol. 70, 2081–2091. doi: 10.1007/s00265-016-2212-1
Muscedere, M. L., and Traniello, J. F. (2012). Division of labor in the hyperdiverse ant genus Pheidole is associated with distinct subcaste-and age-related patterns of worker brain organization. PLoS One 7:e31618. doi: 10.1371/journal.pone.0031618
Newsom, K. D., Moczek, A. P., and Schwab, D. B. (2019). Serotonin differentially affects morph-specific behavior in divergent populations of a horned beetle. Behav. Ecol. 31, 352–360. doi: 10.1093/beheco/arz192
Niven, J. E., and Laughlin, S. B. (2008). Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. doi: 10.1242/jeb.017574
Nothling, J., Malan-Mueller, S., Abrahams, N., Hemmings, S. M. J., and Seedat, S. (2019). Epigenetic alterations associated with childhood trauma and adult mental health outcomes: a systematic review. World J. Biol. Psychiatry 21, 493–512. doi: 10.1080/15622975.2019.1583369
O’Connell, L. A. (2013). Evolutionary development of neural systems in vertebrates and beyond. J. Neurogenet. 27, 69–85. doi: 10.3109/01677063.2013.789511
Odeon, M. M., Salatino, A. E., and Acosta, G. B. (2013). Consequences of postnatal stress: maternal separation in rats induces long-lasting changes on glutamate transporters. Clin. Exp. Pharmacol. 3:121.
Olvido, A. E., and Mousseau, T. A. (1995). Effect of rearing environment on calling-song plasticity in the striped ground cricket. Evolution 49, 1271–1277. doi: 10.2307/2410452
Osborne, R. H. (1996). Insect neurotransmission: neurotransmitters and their receptors. Pharmacol. Therapeut. 69, 117–142. doi: 10.1016/0163-7258(95)02054-3
Oster, G. F., and Wilson, E. O. (1978). Caste and Ecology in the Social Insects. Princeton, NJ: Princeton University Press.
Ott, S. R., and Rogers, S. M. (2010). Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proc. R. Soc. B Biol. Sci. 277, 3087–3096. doi: 10.1098/rspb.2010.0694
Page, R. E., and Peng, C. Y. S. (2001). Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 36, 695–711. doi: 10.1016/S0531-5565(00)00236-9
Pahl, M., Si, A., and Zhang, S. (2013). Numerical cognition in bees and other insects. Front. Psychol. 4:162. doi: 10.3389/fpsyg.2013.00162
Paredes, U., Radersma, R., Cannell, N., While, G. M., and Uller, T. (2016). Low incubation temperature induces DNA hypomethylation in lizard brains. J. Exp. Zool. A Ecol. Genet. Physiol. 325, 390–395. doi: 10.1002/jez.2024
Patalano, S., Hore, T. A., Reik, W., and Sumner, S. (2012). Shifting behaviour: epigenetic reprogramming in eusocial insects. Curr. Opin. Cell Biol. 24, 367–373. doi: 10.1016/j.ceb.2012.02.005
Paulino Simões, Z. L., Boleli, I. C., and Hartfelder, K. (1997). Occurrence of a prothoracicotropic hormone-like peptide in the developing nervous system of the honey bee (Apis mellifera L). Apidologie 28, 399–409. doi: 10.1051/apido:19970607
Pearson, K. (1993). Common principles of motor control in vertebrates and invertebrates. Annu. Rev. Neurosci. 16, 265–297. doi: 10.1146/annurev.ne.16.030193.001405
Peng, W., Ma, N. L., Zhang, D., Zhou, Q., Yue, X., Khoo, S. C., et al. (2020). A review of historical and recent locust outbreaks: links to global warming, food security and mitigation strategies. Environ. Res. 191:110046. doi: 10.1016/j.envres.2020.110046
Perry, C. J., Baciadonna, L., and Chittka, L. (2016). Unexpected rewards induce dopamine-dependent positive emotion-like state changes in bumble bees. Science 353, 1529–1531. doi: 10.1126/science.aaf4454
Peters, A., Schweiger, U., Pellerin, L., Hubold, C., Oltmanns, K. M., Conrad, M., et al. (2004). The selfish brain: competition for energy resources. Neurosci. Biobehav. Rev. 28, 143–180. doi: 10.1016/j.neubiorev.2004.03.002
Petit, C., Le, Ru, B., Dupas, S., Frérot, B., Ahuya, P., et al. (2015). Influence of dietary experience on the induction of preference of adult moths and larvae for a new olfactory cue. PLoS One 10:e0136169. doi: 10.1371/journal.pone.0136169
Prieto Peres, M. F., and Valença, M. M. (2010). “Chapter 60 - Headache: endocrinological aspects,” in Handbook of Clinical Neurology, eds M. J. Aminoff, F. Boller, and D. F. Swaab (Amsterdam: Elsevier), 717–737.
Prillinger, L. (1981). Postembryonic development of the antennal lobes in Periplaneta americana L. Cell Tissue Res. 215, 563–575. doi: 10.1007/BF00233532
Prudic, K. L., Jeon, C., Cao, H., and Monteiro, A. (2011). Developmental plasticity in sexual roles of butterfly species drives mutual sexual ornamentation. Science 331, 73–75. doi: 10.1126/science.1197114
Rachinsky, A. (1994). Octopamine and serotonin influence on corpora allata activity in honey bee (Apis mellifera) larvae. J. Insect Physiol. 40, 549–554. doi: 10.1016/0022-1910(94)90141-4
Radwan, J. (1995). Male morph determination in two species of acarid mites. Heredity 74, 669–673. doi: 10.1038/hdy.1995.91
Rajakumar, R., Koch, S., Couture, M., Fave, M. J., Lillico-Ouachour, A., Chen, T., et al. (2018). Social regulation of a rudimentary organ generates complex worker-caste systems in ants. Nature 562, 574–577. doi: 10.1038/s41586-018-0613-1
Ravary, F., Lecoutey, E., Kaminski, G., Chaline, N., and Jaisson, P. (2007). Individual experience alone can generate lasting division of labor in ants. Curr. Biol. 17, 1308–1312. doi: 10.1016/j.cub.2007.06.047
Reh, R. K., Dias, B. G., Nelson, C. A. III, Kaufer, D., Werker, J. F., Kolb, B., et al. (2020). Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. U.S.A. 117, 23242–23251. doi: 10.1073/pnas.1820836117
Reh, T., and Cagan, R. (1994). Intrinsic and extrinsic signals in the developing vertebrate and fly eyes: viewing vertebrate and invertebrate eyes in the same light. Perspect. Dev. Neurobiol. 2, 183–190.
Reichert, H. (2009). Evolutionary conservation of mechanisms for neural regionalization, proliferation and interconnection in brain development. Biol. Lett. 5, 112–116. doi: 10.1098/rsbl.2008.0337
Reilly, J., Artz, D., Biddinger, D., Bobiwash, K., Boyle, N., Brittain, C., et al. (2020). Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B 287:20200922. doi: 10.1098/rspb.2020.0922
Reingold, S. C., and Camhi, J. M. (1977). A quantitative analysis of rhythmic leg movements during three different behaviors in the cockroach, Periplaneta americana. J. Insect Physiol. 23, 1407–1420. doi: 10.1016/0022-1910(77)90166-4
Rietdorf, K., and Steidle, J. L. (2002). Was Hopkins right? Influence of larval and early adult experience on the olfactory response in the granary weevil Sitophilus granarius (Coleoptera, Curculionidae). Physiol. Entomol. 27, 223–227. doi: 10.1046/j.1365-3032.2002.00289.x
Rillich, J., and Stevenson, P. A. (2018). Serotonin mediates depression of aggression after acute and chronic social defeat stress in a model insect. Front. Behav. Neurosci. 12:233. doi: 10.3389/fnbeh.2018.00233
Rittschof, C. C., and Schirmeier, S. (2017). Insect models of central nervous system energy metabolism and its links to behavior. Glia 66, 1160–1175. doi: 10.1002/glia.23235
Rittschof, C. C., Bukhari, S. A., Sloofman, L. G., Troy, J. M., Caetano-Anolles, D., Cash-Ahmed, A., et al. (2014). Neuromolecular responses to social challenge: common mechanisms across mouse, stickleback fish, and honey bee. Proc. Natl. Acad. Sci. U.S.A. 111, 17929–17934. doi: 10.1073/pnas.1420369111
Rittschof, C. C., Coombs, C. B., Frazier, M., Grozinger, C. M., and Robinson, G. E. (2015). Early-life experience affects honey bee aggression and resilience to immune challenge. Sci. Rep. 5:15572. doi: 10.1038/srep15572
Robertson, R. M., Dawson-Scully, K. D., and Andrew, R. D. (2020). Neural shutdown under stress: an evolutionary perspective on spreading depolarization. J. Neurophysiol. 123, 885–895. doi: 10.1152/jn.00724.2019
Roeder, T. (2005). Tyramine and Octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50, 447–477. doi: 10.1146/annurev.ento.50.071803.130404
Rolff, J., Johnston, P. R., and Reynolds, S. (2019). Complete metamorphosis of insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20190063. doi: 10.1098/rstb.2019.0063
Rolls, M. M., and Jegla, T. J. (2015). Neuronal polarity: an evolutionary perspective. J. Exp. Biol. 218, 572–580. doi: 10.1242/jeb.112359
Rospars, J. P. (1988). Structure and development of the insect antennodeutocerebral system. Int. J. Insect Morphol. Embryol. 17, 243–294. doi: 10.1016/0020-7322(88)90041-4
Rösvik, A., Lhomme, P., Khallaf, M. A., and Anderson, P. (2020). Plant-induced transgenerational plasticity affecting performance but not preference in a polyphagous moth. Front. Ecol. Evol. 8:254. doi: 10.3389/fevo.2020.00254
Saleh, A., Potter, G. G., McQuoid, D. R., Boyd, B., Turner, R., MacFall, J. R., et al. (2017). Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 47, 171–181. doi: 10.1017/S0033291716002403
Salzberg, A., and Bellen, H. J. (1996). Invertebrate versus vertebrate neurogenesis: variations on the same theme? Dev. Genet. 18, 1–10. doi: 10.1002/(sici)1520-6408(1996)18:1<1::aid-dvg1>3.0.co;2-d
Saul, M. C., Blatti, C., Yang, W., Bukhari, S. A., Shpigler, H. Y., Troy, J. M., et al. (2019). Cross-species systems analysis of evolutionary toolkits of neurogenomic response to social challenge. Genes Brain Behav. 18:e12502. doi: 10.1111/gbb.12502
Schaefer, P. L., and Ritzmann, R. E. (2001). Descending influences on escape behavior and motor pattern in the cockroach. J. Neurobiol. 49, 9–28. doi: 10.1002/neu.1062
Schmitz, R. J., Lewis, Z. A., and Goll, M. G. (2019). DNA methylation: shared and divergent features across eukaryotes. Trends Genet. 35, 818–827. doi: 10.1016/j.tig.2019.07.007
Schoofs, L., De Loof, A., and Van Hiel, M. B. (2017). Neuropeptides as regulators of behavior in insects. Annu. Rev. Entomol. 62, 35–52. doi: 10.1146/annurev-ento-031616-035500
Schretter, C. E., Vielmetter, J., Bartos, I., Marka, Z., Marka, S., Argade, S., et al. (2018). A gut microbial factor modulates locomotor behaviour in Drosophila. Nature 563, 402–406. doi: 10.1038/s41586-018-0634-9
Schwab, D. B., Casasa, S., and Moczek, A. P. (2018). On the reciprocally causal and constructive nature of developmental plasticity and robustness. Front. Genet. 9:735. doi: 10.3389/fgene.2018.00735
Schwander, T., Lo, N., Beekman, M., Oldroyd, B. P., and Keller, L. (2010). Nature versus nurture in social insect caste differentiation. Trends Ecol. Evol. 25, 275–282. doi: 10.1016/j.tree.2009.12.001
Seeley, T. D. (1995). The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies. Cambridge, MA: Harvard University Press.
Sehonova, P., Svobodova, Z., Dolezelova, P., Vosmerova, P., and Faggio, C. (2018). Effects of waterborne antidepressants on non-target animals living in the aquatic environment: a review. Sci. Total Environ. 63, 789–794. doi: 10.1016/j.scitotenv.2018.03.076
Shi, Y. Y., Huang, Z. Y., Zeng, Z. J., Wang, Z. L., Wu, X. B., and Yan, W. Y. (2011). Diet and cell size both affect queen-worker differentiation through DNA methylation in honey bees (Apis mellifera, Apidae). PLoS One 6:e18808. doi: 10.1371/journal.pone.0018808
Shpigler, H. Y., Saul, M. C., Murdoch, E. E., Corona, F., Cash-Ahmed, A. C., Seward, C. H., et al. (2019). Honey bee neurogenomic responses to affiliative and agonistic social interactions. Genes Brain Behav. 18:e12509. doi: 10.1111/gbb.12509
Simola, D. F., Graham, R. J., Brady, C. M., Enzmann, B. L., Desplan, C., Ray, A., et al. (2016). Epigenetic (re)programming of caste-specific behavior in the ant Camponotus floridanus. Science 351:aac6633. doi: 10.1126/science.aac6633
Simons, M., and Tibbetts, E. A. (2019). Insects as models for studying the evolution of animal cognition. Curr. Opin. Insect Sci. 34, 117–122. doi: 10.1016/j.cois.2019.05.009
Simpson, S. J., Despland, E., Hägele, B. F., and Dodgson, T. (2001). Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl. Acad. Sci. U.S.A. 98, 3895–3897. doi: 10.1073/pnas.071527998
Sinha, S., Jones, B. M., Traniello, I. M., Bukhari, S. A., Halfon, M. S., Hofmann, H. A., et al. (2020). Behavior-related gene regulatory networks: a new level of organization in the brain. Proc. Natl. Acad. Sci. U.S.A. 117, 23270–23279. doi: 10.1073/pnas.1921625117
Smallwood, S. A., and Kelsey, G. (2012). De novo DNA methylation: a germ cell perspective. Trends Genet. 28, 33–42. doi: 10.1016/j.tig.2011.09.004
Smith, C. R., Anderson, K. E., Tillberg, C. V., Gadau, J., and Suarez, A. V. (2008). Caste determination in a polymorphic social insect: nutritional, social, and genetic factors. Am. Nat. 172, 497–507. doi: 10.1086/590961
Snell-Rood, E. C. (2013). An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004–1011. doi: 10.1016/j.anbehav.2012.12.031
Snell-Rood, E. C., and Moczek, A. P. (2012). Insulin signaling as a mechanism underlying developmental plasticity: the role of FOXO in a nutritional polyphenism. PLoS One 7:e34857. doi: 10.1371/journal.pone.0034857
Sovik, E., Berthier, P., Klare, W. P., Helliwell, P., Buckle, E. L. S., Plath, J. A., et al. (2018). Cocaine directly impairs memory extinction and alters brain DNA methylation dynamics in honey bees. Front. Physiol. 9:79. doi: 10.3389/fphys.2018.00079
Sripetchwandee, J., Chattipakorn, N., and Chattipakorn, S. C. (2018). Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia. Front. Endocrinol. 9:496. doi: 10.3389/fendo.2018.00496
Stamps, J. A., and Biro, P. A. (2016). Personality and individual differences in plasticity. Curr. Opin. Behav. Sci. 12, 18–23.
Stamps, J. A., and Frankenhuis, W. E. (2016). Bayesian models of development. Trends Ecol. Evol. 31, 260–268. doi: 10.1016/j.tree.2016.01.012
Stein, L. R., Bukhari, S. A., and Bell, A. M. (2018). Personal and transgenerational cues are nonadditive at the phenotypic and molecular level. Nat. Ecol. Evol. 2, 1306–1311. doi: 10.1038/s41559-018-0605-4
Taborsky, B., and Oliveira, R. F. (2012). Social competence: an evolutionary approach. Trends Ecol. Evol. 27, 679–688. doi: 10.1016/j.tree.2012.09.003
Taborsky, M., and Brockmann, H. J. (2010). “Alternative reproductive tactics and life history phenotypes,” in Animal Behaviour: Evolution and Mechanisms, ed. P. Kappeler (Berlin: Springer), 537–586. doi: 10.1007/978-3-642-02624-9_18
Tan, K., Chen, W., Dong, S., Liu, X., Wang, Y., and Nieh, J. C. (2015). A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 5:10989. doi: 10.1038/srep10989
Teicher, M. H., Samson, J. A., Anderson, C. M., and Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 17, 652–666. doi: 10.1038/nrn.2016.111
Terao, K., and Mizunami, M. (2017). Roles of dopamine neurons in mediating the prediction error in aversive learning in insects. Sci. Rep. 7:14694. doi: 10.1038/s41598-017-14473-y
Tessmar-Raible, K., Raible, F., Christodoulou, F., Guy, K., Rembold, M., Hausen, H., et al. (2007). Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell 129, 1389–1400. doi: 10.1016/j.cell.2007.04.041
Tissot, M., and Stocker, R. F. (2000). Metamorphosis in Drosophila and other insects: the fate of neurons throughout the stages. Prog. Neurobiol. 62, 89–111. doi: 10.1016/s0301-0082(99)00069-6
Toth, A. L., and Robinson, G. E. (2007). Evo-devo and the evolution of social behavior. Trends Genet. 23, 334–341. doi: 10.1016/j.tig.2007.05.001
Toth, A. L., Tooker, J. F., Radhakrishnan, S., Minard, R., Henshaw, M. T., and Grozinger, C. M. (2014). Shared genes related to aggression, rather than chemical communication, are associated with reproductive dominance in paper wasps (Polistes metricus). BMC Genomics 15:75. doi: 10.1186/1471-2164-15-75
Traynor, K. S., Le Conte, Y., and Page, R. E. (2014). Queen and young larval pheromones impact nursing and reproductive physiology of honey bee (Apis mellifera) workers. Behav. Ecol. Sociobiol. 68, 2059–2073. doi: 10.1007/s00265-014-1811-y
Tremmel, M., and Müller, C. (2012). Insect personality depends on environmental conditions. Behav. Ecol. 24, 386–392. doi: 10.1093/beheco/ars175
Tripet, F., Jacot, A., and Richner, H. (2002). Larval competition affects the life histories and dispersal behavior of an avian ectoparasite. Ecology 83, 935–945. doi: 10.1890/0012-9658(2002)083[0935:lcatlh]2.0.co;2
Truman, J. W., and Bate, M. (1988). Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev. Biol. 125, 145–157. doi: 10.1016/0012-1606(88)90067-x
Tully, T., Cambiazo, V., and Kruse, L. (1994). Memory through metamorphosis in normal and mutant Drosophila. J. Neurosci. 14, 68–74. doi: 10.1523/jneurosci.14-01-00068.1994
van Os, J., Kenis, G., and Rutten, B. P. (2010). The environment and schizophrenia. Nature 468, 203–212. doi: 10.1038/nature09563
Vea, I. M., and Minakuchi, C. (2020). Atypical insects: molecular mechanisms of unusual life history strategies. Curr. Opin. Insect Sci. 43, 46–53. doi: 10.1016/j.cois.2020.09.016
Videla, M., Valladares, G., and Salvo, A. (2010). Differential effects of experience on feeding and ovipositing preferences of a polyphagous leafminer. Entomol. Exp. Appl. 137, 184–192. doi: 10.1111/j.1570-7458.2010.01053.x
Vitt, H. H., and Hartfelder, K. (1998). Scientific Note neurogenesis detected by BrdU incorporation in brains of larval honey bees, Apis mellifera L. (HYMENOPTERA : APIDAE). Int. J. Insect Morphol. Embryol. 27, 351–354. doi: 10.1016/S0020-7322(98)00028-2
Vleugels, R., Verlinden, H., and Vanden Broeck, J. (2015). Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2, 1–14.
Walzer, A., and Schausberger, P. (2011). Threat-sensitive anti-intraguild predation behaviour: maternal strategies to reduce offspring predation risk in mites. Anim. Behav. 81, 177–184. doi: 10.1016/j.anbehav.2010.09.031
Wang, H., Liu, Z., Wang, Y., Ma, L., Zhang, W., and Xu, B. (2020). Genome-wide differential DNA methylation in reproductive, morphological, and visual system differences between queen bee and worker bee (Apis mellifera). Front. Genet. 11:770. doi: 10.3389/fgene.2020.00770
Wang, Y., Kaftanoglu, O., Fondrk, M. K., and Page, R. E. (2014). Nurse bee behaviour manipulates worker honeybee (Apis mellifera L.) reproductive development. Anim. Behav. 92, 253–261. doi: 10.1016/j.anbehav.2014.02.012
Watson, A. H. D. (1992). Presynaptic modulation of sensory afferents in the invertebrate and vertebrate nervous system. Comp. Biochem. Physiol. Part A Physiol. 103, 227–239. doi: 10.1016/0300-9629(92)90573-9
Watson, R. E., Desesso, J. M., Hurtt, M. E., and Cappon, G. D. (2006). Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res. B Dev. Reprod. Toxicol. 77, 471–484. doi: 10.1002/bdrb.20090
Weeks, J., and Truman, J. (1986). Hormonally mediated reprogramming of muscles and motoneurones during the larval-pupal transformation of the tobacco hornworm, Manduca sexta. J. Exp. Biol. 125, 1–13.
Wegerhoff, R. (1999). GABA and serotonin immunoreactivity during postembryonic brain development in the beetle Tenebrio molitor. Microsc. Res. Techn. 45, 154–164. doi: 10.1002/(sici)1097-0029(19990501)45:3<154::Aid-jemt3<3.0.Co;2-5
Weiner, S. A., and Toth, A. L. (2012). Epigenetics in social insects: a new direction for understanding the evolution of castes. Genet. Res. Int. 2012:609810.
Wessnitzer, J., and Webb, B. (2006). Multimodal sensory integration in insects—towards insect brain control architectures. Bioinspir. Biomimet. 1:63. doi: 10.1088/1748-3182/1/3/001
West-Eberhard, M. J. (2003). Developmental Plasticity and Evolution. Oxford: Oxford University Press.
West-Eberhard, M. J. (2005). Developmental plasticity and the origin of species differences. Proc. Natl. Acad. Sci. U.S.A. 102, (Suppl. 1), 6543–6549. doi: 10.1073/pnas.0501844102
Wheeler, D. E., and Nijhout, H. F. (1984). Soldier determination in Pheidole bicarinata: inhibition by adult soldiers. J. Insect Physiol. 30, 127–135. doi: 10.1016/0022-1910(84)90116-1
Winfree, R., Gross, B. J., and Kremen, C. (2011). Valuing pollination services to agriculture. Ecol. Econ. 71, 80–88. doi: 10.1016/j.ecolecon.2011.08.001
Wolbert, P., and Kubbies, M. (1983). The cell cycle of epidermal cells during reprogramming in the pupa of Galleria. J. Insect Physiol. 29, 787–794. doi: 10.1016/0022-1910(83)90008-2
Wolf, C., and Linden, D. E. J. (2011). Biological pathways to adaptability – interactions between genome, epigenome, nervous system and environment for adaptive behavior. Genes Brain Behav. 11, 3–28. doi: 10.1111/j.1601-183X.2011.00752.x
Wu, Q., and Brown, M. R. (2006). Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 51, 1–24. doi: 10.1146/annurev.ento.51.110104.151011
Yagound, B., Remnant, E. J., Buchmann, G., and Oldroyd, B. P. (2020). Intergenerational transfer of DNA methylation marks in the honey bee. Proc. Natl. Acad. Sci. U.S.A. 117, 32519–32527. doi: 10.1073/pnas.2017094117
Yan, H., Simola, D. F., Bonasio, R., Liebig, J., Berger, S. L., and Reinberg, D. (2014). Eusocial insects as emerging models for behavioural epigenetics. Nat. Rev. Genet. 15, 677–688. doi: 10.1038/nrg3787
Yang, E.-C., Chang, H.-C., Wu, W.-Y., and Chen, Y.-W. (2012). Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS One 7:e49472. doi: 10.1371/journal.pone.0049472
Yartsev, M. M. (2017). Rebalancing diversity of animal models in neuroscience research. Science 358, 466–469. doi: 10.1126/science.aan8865
Zemach, A., McDaniel, I. E., Silva, P., and Zilberman, D. (2010). Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328, 916–919. doi: 10.1126/science.1186366
Zera, A. J., and Denno, R. F. (1997). Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 42, 207–230. doi: 10.1146/annurev.ento.42.1.207
Zhang, C.-X., Brisson, J. A., and Xu, H.-J. (2019). Molecular mechanisms of wing polymorphism in insects. Annu. Rev. Entomol. 64, 297–314. doi: 10.1146/annurev-ento-011118-112448
Zhao, C., Deng, W., and Gage, F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660. doi: 10.1016/j.cell.2008.01.033
Keywords: critical period, phenotypic plasticity, genetic toolkit, trauma, DNA methylation
Citation: Westwick RR and Rittschof CC (2021) Insects Provide Unique Systems to Investigate How Early-Life Experience Alters the Brain and Behavior. Front. Behav. Neurosci. 15:660464. doi: 10.3389/fnbeh.2021.660464
Received: 29 January 2021; Accepted: 16 March 2021;
Published: 21 April 2021.
Edited by:
Tom V. Smulders, Newcastle University, United KingdomReviewed by:
Bryan Kolb, University of Lethbridge, CanadaLars Chittka, Queen Mary University of London, United Kingdom
Copyright © 2021 Westwick and Rittschof. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clare C. Rittschof, Y2xhcmUucml0dHNjaG9mQHVreS5lZHU=
 Rebecca R. Westwick
Rebecca R. Westwick Clare C. Rittschof
Clare C. Rittschof