- 1Department of Psychology, The University at Albany—The State University of New York (SUNY), Albany, NY, United States
- 2Biological Sciences, The University at Albany—The State University of New York (SUNY), Albany, NY, United States
- 3Centers for Neuroscience, The University at Albany—The State University of New York (SUNY), Albany, NY, United States
- 4Life Sciences Research, The University at Albany—The State University of New York (SUNY), Albany, NY, United States
- 5Department of Biomolecular Sciences, The University of Mississippi, University, MS, United States
The neurosteroid, 5α-pregnan-3α-ol-20-one (known as “allopregnanolone” or 3α,5α-THP), is produced in the midbrain ventral tegmental area (VTA), independent of peripheral sources of progestogens, where it has potential actions at N-methyl-D-aspartate (NMDA) and GABAA receptors to facilitate rodent sexual behavior. Progestogens can also have anti-anxiety effects, but whether these involve actions of centrally-derived 3α,5α-THP or these receptors to support reproductively-relevant behavior is not well understood. We investigated the extent to which 3α,5α-THP’s actions via NMDA and/or GABAA receptors in the midbrain VTA influence reproductive behaviors. Estradiol-primed, ovariectomized/adrenalectomized (OVX/ADX) rats received midbrain VTA infusions of vehicle, an NMDA receptor blocker (MK-801; 200 ng), or a GABAA receptor blocker (bicuculline; 100 ng) followed by a second infusion of vehicle or 3α,5α-THP (100 ng). Reproductively-relevant behaviors were assessed: sexual (paced mating), anxiety-like (elevated plus maze), and social (partner preference, social interaction) behavior. Compared to vehicle, intra-VTA infusions of MK-801 exerted anxiolytic-like effects on elevated plus maze behavior and enhanced lordosis. Unlike prior observations in gonadally-intact rats, intra-VTA bicuculline had no effect on the behavior of OVX/ADX rats (likely due to a floor effect). Subsequent infusions of 3α,5α-THP reversed effects on lordosis and infusions of bicuculline inhibited 3α,5α-THP-facilitated lordosis. Thus, NMDA and GABAA receptors may act as mediators for reproductive behavioral effects of 3α,5α-THP in the midbrain VTA.
Introduction
Progesterone (P4) plays a key role in the regulation of reproductive behavior in female rodents. In the brain, P4 can exerts its effects either via “genomic” or “non-genomic” action. In the hypothalamus, P4 facilitates lordosis, the reflexive posture that allows copulation. In this brain region, P4 actions are mediated by intracellular cognate progestin receptors, which act as nuclear transcription factors to alter RNA transcription and protein synthesis (Meisel and Pfaff, 1985). However, In the midbrain ventral tegmental area (VTA), P4 can exert actions to mediate both “consummatory” reproductive behavior (e.g., lordosis) and “appetitive” reproductive behaviors (e.g., proceptivity indicators such as ear wiggling, hopping, darting, and inhibition of normative anxiety-like behavior) which are etiologically important for successful reproduction. Given that the VTA is largely devoid of progestin receptors, P4 actions in this brain region are mediated by conversion of P4 to its 3α-hydroxy, 5α-reduced metabolite, 5α-pregnan-3α-ol-20-one (known as “allopregnanolone” or 3α,5α-THP). Unlike P4, 3α,5α-THP lacks affinity for progestin receptors and instead acts at neurotransmitter receptors. We have found that engaging in a pseudo-naturalistic mating paradigm (termed “paced mating”) enhances steroidogenesis of 3α,5α-THP in the midbrain and additional brain regions (hippocampus, prefrontal cortex, and diencephalon; Frye et al., 2007). Blocking 3α,5α-THP formation in the VTA increases anxiety-like behavior and attenuates lordosis of female rats or hamsters (Frye and Vongher, 2001; Petralia et al., 2005; Frye et al., 2008a,b, 2009a,b; Frye and Paris, 2011). Thus, 3α,5α-THP actions in the VTA are necessary for the full expression of consummatory and appetitive reproductive behavior. However, the mechanisms of 3α,5α-THP action in this brain region are not well-understood.
One mechanism by which 3α,5α-THP may alter reproductive behavior occurs via actions at inhibitory and excitatory neurotransmitter receptors. A well-characterized, non-genomic signaling pathway by which 3α,5α-THP acts is via modulation of inhibitory γ-aminobutyric acid type A (GABAA) receptor complexes. 3α,5α-THP is among the most potent, positive allosteric modulators of GABAA Cl− channels and a direct agonist in high concentrations (Majewska et al., 1986; Lambert et al., 1987; Morrow et al., 1987; Paul and Purdy, 1992; Gunn et al., 2011). Pharmacologically-enhancing or -attenuating actions at GABAA receptors in the VTA has commensurate effects to enhance or attenuate P4-facilitated lordosis (DeBold and Frye, 1994). Less well understood are 3α,5α-THP’s actions that may involve excitatory N-methyl-D-aspartate (NMDA) receptors. While free 3α,5α-THP has little affinity for NMDA receptors (Maurice et al., 2006), when sulfated it acts as a negative allosteric modulator of NMDA Ca2+ channels, binding the NR2B subunit (Johansson and Le Grevès, 2005). Blocking NMDA receptors in the VTA also enhances progestogen-facilitated lordosis in gonadally-intact, or ovariectomized, rats (Petralia et al., 2007; Frye and Paris, 2011), but it is not known if these effects involve upstream actions of 3α,5α-THP.
Beyond lordosis, progestogens’ actions at GABAA and NMDA receptors in the midbrain VTA may also be important for the expression of additional reproductively-relevant behaviors such as anxiety. Indeed, 3α,5α-THP exerts robust anti-anxiety effects in the VTA (Frye et al., 2006a) and GABAA receptor agonists in the VTA can underlie positive changes in mood and affect (Gifkins et al., 2002; Genud et al., 2009). Less is known about the role that NMDA receptors may play in the VTA; albeit, NMDA receptor antagonism facilitates lordosis and may underlie aspects of anxiolysis, in part, via actions of peripheral glands (adrenals and/or ovaries) and/or neurosteroidogenesis (Frye and Paris, 2011). Notably, the NMDA receptor antagonist, MK-801 (a.k.a. dizocilpine), blocks 3α,5α-THP’s anti-depressant-like effects in the amygdala of rats (Shirayama et al., 2011) supporting a modulatory role for affective behavior. We have previously utilized ovariectomized (OVX) and/or adrenalectomized (ADX) rats to reveal that central neurosteroid enhancement in the VTA is important for the expression of consummatory (i.e., lordosis) and appetitive (i.e., social and anti-anxiety-like) behavior. Antagonizing GABAA and NMDA receptors within the VTA alters the anxiety-like and lordosis response to pharmacologically-promoted increases in steroidogenesis (Frye and Paris, 2011), but the identity of the important steroids that underlie actions at these sites are not known. The present work aimed to assess the importance of GABAA and NMDA receptor targets in the mediation of 3α,5α-THP’s central effects to influence reproductively-relevant behavior. We hypothesized that female OVX/ADX, estradiol (E2)-primed rats administered the NMDA receptor blocker, MK-801, would demonstrate reduced anxiety-like behavior (general anxiety assessed via the elevated plus maze and social anxiety assessed via a social interaction test) and enhanced sexual receptivity (assessed via a propinquity test and a paced mating test), while those administered the GABAA receptor blocker, bicuculline, to the VTA, would demonstrate opposite effects on these behaviors. We further hypothesized that subsequent 3α,5α-THP infusions to the VTA would reverse the effects of blockers.
Materials and Methods
These methods were approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Animals
Adult (50–60 days old), Long-Evans female rats (N = 100) were bred in the Life Sciences Laboratory Animal Care Facility at The University at Albany-SUNY (original stock obtained from Charles River, Raleigh, NC, USA). Rats were housed in polycarbonate cages with woodchip bedding (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1°C) and were maintained on a 12:12 h reversed light cycle (lights off at 08:00 h) with continuous access to Purina Rat Chow and tap water in their home cages.
Surgical Protocol
Rats were stereotaxically-implanted with bilateral guide cannulae aimed over the medial aspect of the VTA (from bregma: AP = −5.3, ML = ± 0.4, DV = −7.0) under xylazine (12 mg/kg) and ketamine (70 mg/kg) anesthesia. Immediately following stereotaxic surgery, rats were OVX/ADX as previously described (Frye and Paris, 2011). Following surgery and prior to testing, animals were monitored for loss of weight, righting response, flank stimulation response, and/or muscle tone (Marshall and Teitelbaum, 1974). One rat failed these assessments and was immediately euthanized. All rats were screened for complete-ADX via post hoc assessment of corticosterone in plasma (methods below). All the rats included in analyses had circulating concentrations of corticosterone that were below baseline levels (<1 μg/dl). Sixteen rats were excluded due to circulating corticosterone >1 μg/dl, which prior work suggests is indicative of incomplete adrenalectomy and can alter behavioral and endocrine measures (Frye and Paris, 2011). Remaining rats all had circulating corticosterone levels <1 μg/dl.
Preparation of Pharmacological Blockers
The NMDA receptor blocker, MK-801 hydrogen maleate (Sigma Chemical Co., St. Louis, MO, USA) was diluted to a concentration of 200 ng/μl in sterile saline as elucidated in prior investigations (Frye and Paris, 2011). MK-801 is a long-lasting non-competitive antagonist that acts in the receptor channel pore, where it blocks opening (Dravid et al., 2007). While, MK-801 and 3α,5α-THP have not previously been co-infused in a mating model, this dose has previously been demonstrated to facilitate reproductive behaviors and antagonize the effects of a general neurosteroidogenesis enhancer (Petralia et al., 2007; Frye and Paris, 2011).
The GABAA blocker, bicuculline (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in sterile saline to a concentration of 100 ng/μl as previously demonstrated (Frye and Paris, 2011). Bicuculline is an antagonist that inhibits the GABAA ion channel by competing for the GABA binding site on GABAA receptors but does not compete with the allosteric steroid-binding site (Ueno et al., 1997). This dose has previously been demonstrated to reduce anxiety-like behavior and lordosis of sexually-receptive rodents (Frye et al., 2006b; Frye and Paris, 2009).
Study Procedure
Seven days after surgery, OVX/ADX rats were primed with E2 (10 μg, SC) and tested 44 h later to evaluate sexual behavior (Figure 1). On the day of testing, E2-primed rats were randomly assigned to receive a bilateral infusion (1 μl) of saline vehicle, bicuculline (100 ng/μl), or MK-801 (200 ng/μl) to the midbrain VTA. Thirty min later, rats received a subsequent infusion of 25% β-cyclodextrin vehicle or 3α,5α-THP (100 ng/μl). Rats were tested 10 min later in all tasks described below (Figure 1). We have previously systematically assessed the effects of exposure to the tasks utilized in this behavioral battery (Frye et al., 2007). We find that performance in one task does not significantly influence subsequent behavioral performance or central/circulating steroid levels, with the exception of an engagement in paced mating which promotes central steroidogenesis of 3α,5α-THP (Frye et al., 2007). As such, the paced mating task is always performed last (Figure 1). There were six experimental conditions based on intra-VTA infusions: vehicle/vehicle (n = 13), vehicle/3α,5α-THP (n = 17), MK-801/vehicle (n = 15), MK-801/3α,5α-THP (n = 12), bicuculline/vehicle (n = 15), and bicuculline/3α,5α-THP (n = 11). The numbers of rats per group were reduced when those with infusions to sites other than the VTA were taken into account.
Behavioral Outcome Measures
Behavioral data were collected using ANY-maze animal tracking software (Stoelting Co., Wood Dale, IL, USA). All rats were placed in an open field apparatus (76 × 57 × 35 cm) consisting of a 48-square grid floor (6 × 8 squares, 9.5 cm/side) for a 5 min habituation to the apparatus and testing room. The frequency of crossings into each of the 48 squares on the floor was recorded as an assessment of general motor behavior. While entries into the central 24 squares of the open field is a common measurement of anti-anxiety behavior (Frye and Rhodes, 2006a), we observed no differences in the number of central squares entered among experimental groups in this study, or prior studies utilizing this OVX/ADX model (Frye and Paris, 2011).
Elevated Plus Maze
The elevated plus maze was conducted per previous methods (File, 1990). Briefly, the maze has four opaque arms (49 cm long, 10 cm wide) elevated off the ground (50 cm high). Two arms (east and west) are enclosed by walls (30 cm high), while the other two arms (north and south) are exposed. The amount of time spent on, and the number of entries into, open or closed arms were recorded during the 5 min task. Open arm time is an index of exploratory and anti-anxiety behavior, while total arm entries are an index of motor behavior.
Partner Preference
Partner preference was conducted as previously described (Frye and Rhodes, 2006a). Briefly, stimulus rats (one diestrous female and one male) are confined to opposite corners of an open field via Plexiglass compartments that are permeated with small holes for olfactory exchange. Sexually-receptive rats will seek an opposite-sex partner for the purposes of copulation (Nofrey et al., 2008). The amount of time that an experimental rat spends in proximity (one body length or less) to either stimulus rat is recorded during a 5 min test. In the lab setting, preference for a male vs. female stimulus rat is considered a measure of sexual receptivity.
Social Interaction
Social interaction was assessed in the open field apparatus per previous methods (File, 1990). Briefly, an experimental female was placed in one corner, while a diestrous stimulus female was placed in the opposite corner of the apparatus. The amount of time that the experimental rat spent interacting (sniffing, crawling over or under, following with contact, tumbling, boxing, or grooming) with the stimulus rats was recorded in a 5 min test. Total time spent in social interaction is a measure of social anxiety-like behavior.
Paced Mating
Paced mating was conducted per previous methods (Erskine, 1985). In brief, the paced mating apparatus (37.5 × 75 × 30 cm) was equally divided by a Plexiglas partition, which contained a small (5 cm in diameter) hole in the bottom center, allowing the female (but not the stimulus male) free access to both sides of the apparatus. Frequency of mounts + intromissions + ejaculations was recorded and a lordosis quotient was calculated [(frequency of female dorsiflexion during a sexual contact/total sexual contacts by a male) * 100] during a 15 min test. As well, the percentage of aggressive behaviors (vocalizing, attack) and the percentage of times the experimental female left the chamber containing the male (% exits) following sexual contacts were recorded.
Cannulae Placement and Neuroendocrine Assessment
Immediately after testing, rats were decapitated and trunk blood and whole brains were collected and stored as described (Frye and Rhodes, 2006a). Infusion site analyses were conducted on fresh tissue, as described (Frye and Paris, 2011). Of the rats that had not been excluded for receiving a partial-ADX, 22 had infusions to sites other than the VTA; however, all experimental groups were not represented, which precluded factorial analyses of behavioral measures based on-site placement. As such, rats with cannulae placement incongruous with a hit to the VTA were excluded from all analyses. Thus, the experimental groups with complete-ADX and verified cannulae placement to the VTA yielded: vehicle/vehicle (n = 9), vehicle/3α,5α-THP (n = 11), MK-801/vehicle (n = 9), MK-801/3α,5α-THP (n = 12), bicuculline/vehicle (n = 11), and bicuculline/3α,5α-THP (n = 9). Plasma was extracted for radioimmunoassay of corticosterone.
Steroid Extraction
Corticosterone was extracted from serum via incubation with ether and 800 cpm of [3H] corticosterone (Frye and Bayon, 1999). Ether-incubated steroids were snap-frozen twice and supernatant was evaporated in a speed drier. Samples were reconstituted with phosphate assay buffer to the original serum volume (Frye et al., 2008a,b).
Radioimmunoassay
Levels of corticosterone were measured by radioimmunoassay, per previously reported methods (Frye et al., 1998). Concentrations of [3H] corticosterone were assessed via the logit-log method of Rodbard and Hutt (1974) with “AssayZap” interpolation software published by Biosoft (1994). Inter- and intra-assay reliability coefficients were 0.04 and 0.07, respectively.
Statistical Analyses
Data were analyzed using StatView (SAS Institute Inc.). Group differences were assessed via two-way analyses of variance (ANOVAs) with central blocker condition (vehicle, MK-801, bicuculline) or central 3α,5α-THP condition (vehicle, 3α,5α-THP) as factors. Significant main effects were followed by Fisher’s protected least significant differences post hoc tests to determine group differences. Significant interactions were delineated via follow-up one-way ANOVA with alpha corrected for all possible comparisons. The alpha-level for statistical significance was p < 0.05.
Results
MK-801 and 3α,5α-THP Infusions to the VTA Altered Anti-anxiety Behavior
Two-way ANOVAs revealed that intra-VTA infusion of blockers and 3α,5α-THP significantly interacted to influence open arm time in the elevated plus-maze (F(2,55) = 3.05, p = 0.05; Figure 2). Contrasts revealed that OVX/ADX rats infused with MK-801/vehicle spent a significantly increased amount of time on the open arms of the elevated plus-maze, compared to rats receiving infusions of bicuculline/vehicle (p = 0.04) or control infusions of vehicle/vehicle (p = 0.046). Rats infused with subsequent 3α,5α-THP did not significantly differ from vehicle-infused controls or any other group. Notably, central manipulations did not significantly alter motor behavior in the open field (indicated by the number of total squares entered) or in the elevated plus-maze (indicated by the number of arms entered; Table 1).
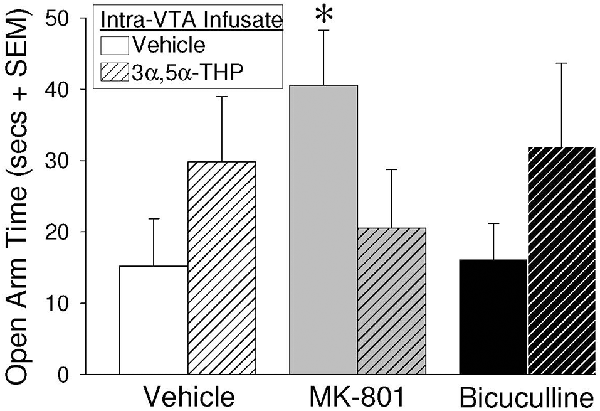
Figure 2. Depicts time spent on the open arms of an elevated plus-maze among estradiol-primed (10 μg, SC), ovariectomized/adrenalectomized (OVX/ADX) female rats infused with the vehicle, MK-801, or bicuculline, followed by subsequent infusions of vehicle or 3α,5α-THP, to the ventral tegmental area (VTA) of the midbrain. *Indicates significantly different from vehicle/vehicle- or bicuculline/vehicle-infused rats, p < 0.05.
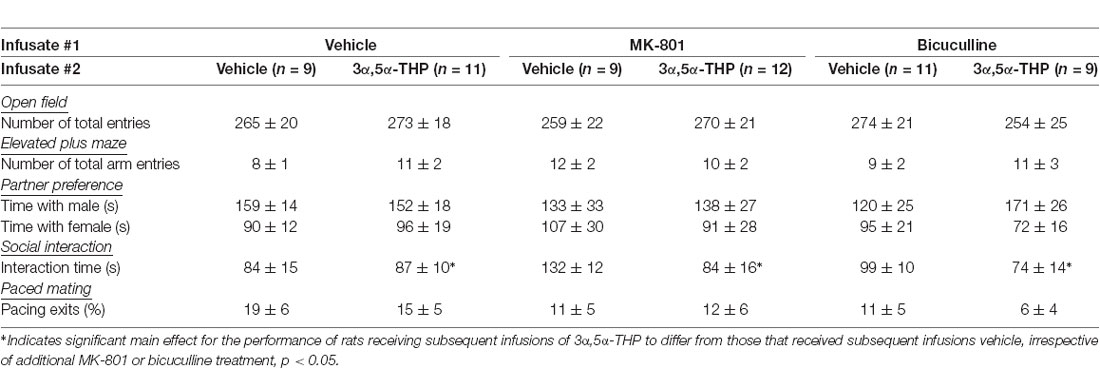
Table 1. Motor and social behavior measures of female ovariectomized/adrenalectomized rats infused with the vehicle, 3α,5α-THP, bicuculline, and/or MK-801 to the ventral tegmental area of the midbrain (mean ± SEM).
3α,5α-THP Influenced Non-sexual Social Behavior
Central manipulations also altered non-sexual social behavior. There was a main effect for subsequent 3α,5α-THP infusions to decrease the duration of time spent in social interaction with a conspecific (F(1,55) = 4.65, p < 0.05); this was observed irrespective of whether vehicle, MK-801, or bicuculline was first infused (Table 1). While, it was apparent that this effect was observed only in MK-801 and bicuculline-infused groups when 3α,5α-THP was co-administered, these factors did not significantly interact.
In the partner preference task, neither infusions of central pharmacological blockers (MK-801, bicuculline) nor infusions of subsequent 3α,5α-THP, significantly influenced the number of time rats spent in proximity to a male (Table 1).
MK-801 and 3α,5α-THP Facilitated Lordosis and Defensive Aggression Behavior in Response to Mounting
Intra-VTA infusions of blockers and 3α,5α-THP significantly interacted to alter lordosis (F(2,55) = 6.54, p < 0.05; Figure 3, top). Infusions of vehicle/3α,5α-THP significantly enhanced lordosis compared to vehicle/vehicle-infused controls (p = 0.03) or bicuculline/vehicle-infused rats (p = 0.002). MK-801/vehicle also significantly enhanced lordosis compared to vehicle/vehicle controls (p = 0.004), or rats infused with MK801/3α,5α-THP (p = 0.01), bicuculline/vehicle (p = 0.0002), or bicuculline/3α,5α-THP (p = 0.02). Thus, infusions 3α,5α-THP or MK-801 with vehicle significantly enhanced lordosis, but 3α,5α-THP attenuated MK-801’s effects.
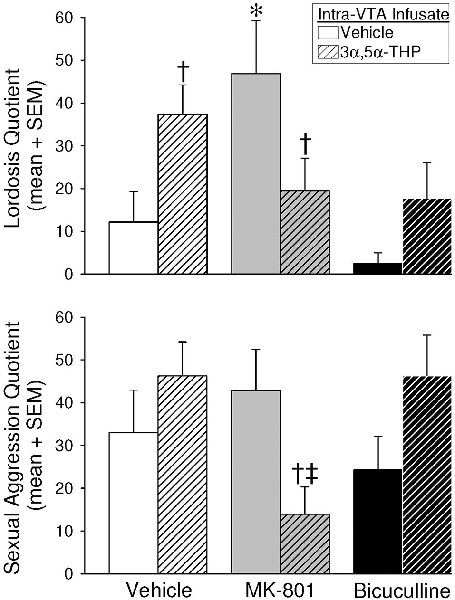
Figure 3. Depicts lordosis (top) and defensive sexual aggression (bottom) quotients in the paced mating paradigm among estradiol-primed (10 μg, SC), OVX/ADX female rats infused with vehicle, MK-801, or bicuculline, followed by subsequent infusions of vehicle or 3α,5α-THP, to the VTA of the midbrain. *Indicates significantly different from vehicle/vehicle-infused controls. †Indicates a significant difference between rats receiving subsequent 3α,5α-THP infusions compared to their respective vehicle/vehicle or MK-801/vehicle infused controls. ‡Indicates significantly different from vehicle/3α,5α-THP or bicuculline/3α,5α-THP-infused rats, p < 0.05.
Defensive aggression in response to mounting was also significantly altered by intra-VTA infusions (Figure 3, bottom). Blockers and 3α,5α-THP interacted (F(2,55) = 5.22, p < 0.05), such that MK-801/3α,5α-THP infusions attenuated defensive aggression compared to MK-801/vehicle infusions (p = 0.02), or infusions of any other compound co-administered with 3α,5α-THP (vehicle/3α,5α-THP, p = 0.006; bicuculline/3α,5α-THP, p = 0.008). The pacing of mating contacts, following mounting by males, was reduced when blockers were infused to the VTA compared to vehicle infusions; however, this was not a statistically significant difference (Table 1). Thus, the co-infusion of MK-801 and 3α,5α-THP to the VTA reduced defensive aggression compared to other manipulations.
Discussion
The hypothesis that intra-VTA infusions of MK-801 would enhance sexual and reproductively-relevant (exploratory, affective, social) behaviors, and bicuculline would reduce these behaviors, was partly upheld. E2-primed, OVX/ADX rats that were infused with MK-801 demonstrated significantly enhanced lordosis in the paced mating task, and significant anxiolysis as assessed via the elevated plus-maze, compared to bicuculline- or vehicle -infused controls. Similarly, investigations in mouse models have revealed that systemic administration of MK-801 has commensurate anxiolytic effects to those of 3α,5α-THP (Reddy and Kulkarni, 1997) and genetically perturbing global NMDA receptor expression yields an aberrant sexual, social, and anxiety-like phenotype (Mohn et al., 1999). However, MK-801 infusions in the present study did not significantly alter non-sexual social behavior (partner preference or free social interaction). Alternatively, infusions of bicuculline significantly blocked the effects of 3α,5α-THP to enhance lordosis, but did not significantly reduce sexual, social or affective behaviors on their own. These findings are commensurate with those of past observations wherein sexually-receptive rodents that were gonadally-intact or OVX (and/or ADX and hormone primed with E2 and P4) demonstrated enhanced lordosis when NMDA receptors were blocked in the VTA (Petralia et al., 2007; Frye et al., 2008a,b; Frye and Paris, 2011) and reduced lordosis when bicuculline was infused to the midbrain VTA or central gray (McCarthy et al., 1991; Frye and Paris, 2009, 2011). These effects are dampened when peripheral steroid glands (ovaries and adrenals) are completely extirpated such that sexual and anxiety-like behavior of rats is greater in gonadally-intact > OVX > OVX/ADX rats (Fernández-Guasti et al., 1991; Gorzalka and Moe, 1994; Frye and Paris, 2009, 2011). E2-priming alone may not sufficiently reinstate anti-anxiety and lordosis in the OVX/ADX model to a level that intra-VTA bicuculline can efficaciously be observed to attenuate these behaviors (Frye and Paris, 2011). Indeed, circulatory P4 is a critical factor for several aspects of paced mating including its reinforcing properties (Paredes and Alonso, 1997; Paredes and Vazquez, 1999; González-Flores et al., 2004). For these reasons, we may have observed a “floor effect” on anxiety-like responding with exogenous 3α,5α-THP non-significantly increasing anti-anxiety-like behavior when administered alone. Thus, NMDA and/or GABAA receptors in the midbrain VTA regulate lordosis and anti-anxiety-like behavior of rats; but, appetitive sexual behaviors (e.g., ear wiggling, pacing) and social interactions may require circulatory progestogens for assessment of full expression.
The second hypothesis, that subsequent 3α,5α-THP infusions to the VTA would reverse effects of pharmacological blockers, was partly supported. Enhancements of anxiolysis and lordosis that were promoted by MK-801 infusions were not observed when 3α,5α-THP was co-administered. Co-infusion of MK-801 and 3α,5α-THP also reduced defensive aggression, but this was not observed when either compound was infused with the vehicle. These data support the notion that 3α,5α-THP may play an important reproductive regulatory role via intra-VTA NMDA receptors. Similarly, others have seen intracerebroventricular infusions of an NMDA receptor antagonist to block P4-enhancement of lordosis among OVX, E2-primed rats (Gargiulo et al., 1992; Gargiulo and Donoso, 1995). We also observed 3α,5α-THP-mediated lordosis to be significantly attenuated when bicuculline was co-infused, supporting a regulatory role for 3α,5α-THP at intra-VTA GABAA receptors. Indeed, 3α,5α-THP has been observed to regulate GABAA subunit expression in vitro and ex vivo (Shen et al., 2005; Zhou and Smith, 2007) and orally-active micronized P4, which can metabolize to 3α,5α-THP, is seen to enhance the positive and negative effects of benzodiazepines in premenopausal women (Babalonis et al., 2011a,b). In the present animal model, it is known that neither gonadal nor adrenal, P4 are necessary for the expression of lordosis (Foreman and Moss, 1977; Auger et al., 1997); rather, central neurosteroidogenesis in the VTA is critical for expression and maintenance of this behavior (Frye and Paris, 2011). The present investigation extends these findings to reveal 3α,5α-THP as the important neurosteroid product acting in the VTA to mediate reproductively-relevant anxiety and sexual behavior of female rats.
We have previously observed 3α,5α-THP infusions to the VTA to be associated with enhanced 3α,5α-THP production in other brain regions (hippocampus, prefrontal cortex, diencephalon; Frye and Rhodes, 2006a). These effects are not thought to be due to infusate diffusion given that we have previously observed central infusate to spread ~1 mm and for intra-VTA infusions not to diffuse beyond the midbrain (Frye and Rhodes, 2008). Moreover, 3α,5α-THP infusions targeted to sites in proximity to the VTA (substantia nigra or central gray) are not observed to promote 3α,5α-THP formation in midbrain, hippocampus, or striatum (Frye and Rhodes, 2006b, 2008; Frye et al., 2008a,b). Rather, steroids can be synthesized in neural cells, independent of peripheral sources (King, 2008). Notably, women administered P4 exhibited shifts in metabolite:prohormone ratio that was indicative of depression status, supporting the importance of steroid metabolites (Girdler et al., 2012). Others find that exogenous progestins and oral contraceptives that do not metabolize 3α,5α-THP increase anxiety-like behavior of rodents (Porcu et al., 2012). Formation of 3α,5α-THP may play an important role in the benefits of pregnane steroids.
The behavioral effects of inhibitors observed herein likely involve modulation of VTA efferents to limbic and extralimbic brain regions. In the present report, we observed either 3α,5α-THP or MK-801 actions in the VTA to facilitate lordosis. The midbrain VTA consists of a mixture of dopaminergic (~65%) and non-dopaminergic neurons, the latter of which are largely GABAergic (~30%) or glutaminergic (~5%; Zessen et al., 2012). Activation of dopaminergic efferents from the VTA to forebrain structures (particularly, within the striatum) are generally observed to inhibit lordosis and lesioning or quiescing these neurons facilitates lordosis (Caggiula et al., 1979; Sirinathsinghji et al., 1986; Pednekar and Mascarenhas, 1993; Frye et al., 2010). By virtue of 3α,5α-THP’s potent affinity for GABAA receptors, it rapidly promotes Cl− influx into neurons, reducing excitability (Majewska et al., 1986; Lambert et al., 1987). When acting in the VTA, 3α,5α-THP may dampen the activity of dopaminergic efferents, thereby promoting lordosis. As well, intra-VTA NMDA receptors are important for dopamine neurotransmission (Gu and Lu, 2018) and infusion of MK-801 decreases Ca2+ influx into neurons, similarly attenuating excitation and promoting lordosis. Despite achieving their endpoints by different mechanisms, actions of 3α,5α-THP and MK-801 to inhibit dopaminergic projection neurons to other brain regions, particularly the hippocampus, mPFC, and striatum (caudate/putamen and nucleus accumbens), may underlie their effects to facilitate reproductive behavior. It is of interest that the subsequent addition of 3α,5α-THP reversed MK-801’s effects on lordosis. The capacity for 3α,5α-THP to restore behavioral homeostasis may be conferred by its capacity to potently activate GABAA Cl− channels, restoring ion homeostasis. Following inhibition of Ca2+ channels via MK-801, cells may become hyperpolarized; under these circumstances, subsequent activation of GABAA channels via 3α,5α-THP may efflux, rather than influx, Cl−, thus, restoring the excitatory/inhibitory ion balance within the VTA.
The present study reveals the capacity for modulation of intra-VTA NMDA receptors to influence appetitive and consummatory reproductive behaviors in female rats. Subsequent administration of 3α,5α-THP restored behavioral homeostasis, presumably via actions at GABAA receptors to re-establish ion homeostasis within the VTA. These data further reveal the importance of excitatory/inhibitory substrates within the VTA for reproductively-relevant behaviors. Formation of 3α,5α-THP may act to balance ion homeostasis within the VTA, thereby influencing efferents to regions involved in the processing of natural reward.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Author Contributions
CF participated in experimental design. DL and JP acquired data. CF, DL, and JP performed data analyses. CF, AQ, DL, and JP wrote and contributed to the writing of the manuscript. All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This research was supported by a grant from the National Institute of Mental Health (MH06769801). The funding for the publication costs was provided by the Denali Center, Albany, NY, USA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Technical assistance, provided by Eric Zhou and Sehee Kim, is appreciated. The authors also appreciate the assistance of Dr. Alicia Walf in the review of this manuscript.
References
Auger, A. P., Moffatt, C. A., and Blaustein, J. D. (1997). Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology 138, 511–514. doi: 10.1210/endo.138.1.4986
Babalonis, S., Lile, J. A., Martin, C. A., and Kelly, T. H. (2011a). Progesterone effects on the discriminative stimulus, subjective and performance effects of triazolam in healthy, premenopausal women. Behav. Pharmacol. 22, 441–449. doi: 10.1097/fbp.0b013e328349fc02
Babalonis, S., Lile, J. A., Martin, C. A., and Kelly, T. H. (2011b). Physiological doses of progesterone potentiate the effects of triazolam in healthy, premenopausal women. Psychopharmacology 215, 429–439. doi: 10.1007/s00213-011-2206-7
Caggiula, A. R., Herndon, J. G. Jr., Scanlon, R., Greenstone, D., Bradshaw, W., and Sharp, D. (1979). Dissociation of active form immobility components of sexual behavior on female rats by central 6-hydroxydopamine: implications for ca involvement in sexual behavior and sensorimotor responsiveness. Brain Res. 3, 505–520. doi: 10.1016/0006-8993(79)90582-1
DeBold, J. F., and Frye, C. A. (1994). Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm. Behav. 28, 445–453. doi: 10.1006/hbeh.1994.1042
Dravid, S. M., Erreger, K., Yuan, H., Nicholson, K., Le, P., Lyuboslavsky, P., et al. (2007). Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J. Physiol. 581, 107–128. doi: 10.1113/jphysiol.2006.124958
Erskine, M. S. (1985). Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav. Neurosci. 99, 151–161. doi: 10.1037/0735-7044.99.1.151
Fernández-Guasti, A., Vega-Matuszczyk, J., and Larsson, K. (1991). Synergistic action of estradiol, progesterone and testosterone on rat proceptive behavior. Physiol. Behav. 50, 1007–1011. doi: 10.1016/0031-9384(91)90429-r
Foreman, M. M., and Moss, R. L. (1977). Effects of subcutaneous injection and intrahypothalamic infusion of releasing hormones upon lordotic response to repetitive coital stimulation. Horm. Behav. 8, 219–234. doi: 10.1016/0018-506x(77)90039-3
Frye, C. A., and Bayon, L. E. (1999). Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. J. Neuroendocrinol. 11, 839–847. doi: 10.1046/j.1365-2826.1999.00379.x
Frye, C. A., Bayon, L. E., Pursnani, N. K., and Purdy, R. H. (1998). The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity and proceptivity in female rats. Brain Res. 808, 72–83. doi: 10.1016/s0006-8993(98)00764-1
Frye, C. A., Marrone, J., and Walf, A. A. (2008a). Effects of manipulating progesterone and NMDA receptors in the ventral tegmental area for lordosis of hamsters and rats. Psychopharmacology 200, 71–80. doi: 10.1007/s00213-008-1143-6
Frye, C. A., Paris, J. J., and Rhodes, M. E. (2008b). Estrogen is necessary for 5α-pregnan-3α-ol-20-one (3α,5α-THP) infusion to the ventral tegmental area to facilitate social and sexual, but neither exploratory nor affective behavior of ovariectomized rats. Pharmacol. Biochem. Behav. 91, 261–270. doi: 10.1016/j.pbb.2008.08.018
Frye, C. A., and Paris, J. J. (2009). Infusions of bicuculline to the ventral tegmental area attenuates sexual, exploratory, and anti-anxiety behavior of proestrous rats. Pharmacol. Biochem. Behav. 93, 474–481. doi: 10.1016/j.pbb.2009.06.012
Frye, C. A., and Paris, J. J. (2011). Effects of neurosteroid actions at N-methyl-D-aspartate and GABAA receptors in the midbrain ventral tegmental area for anxiety-like and mating behavior of female rats. Psychopharmacology 213, 93–103. doi: 10.1007/s00213-010-2016-3
Frye, C. A., Paris, J. J., and Rhodes, M. E. (2007). Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction 133, 663–674. doi: 10.1530/rep.1.01208
Frye, C. A., Paris, J. J., and Rhodes, M. E. (2009a). Exploratory, anti-anxiety, social, and sexual behaviors of rats in behavioral estrus is attenuated with inhibition of 3α,5α-THP formation in the midbrain ventral tegmental area. Behav. Brain Res. 2, 269–276. doi: 10.1016/j.bbr.2008.06.005
Frye, C. A., Paris, J. J., and Rhodes, M. E. (2009b). Increasing 3α,5α-THP following inhibition of neurosteroid biosynthesis in the ventral tegmental area reinstates anti-anxiety, social, and sexual behavior of naturally receptive rats. Reproduction 1, 119–128. doi: 10.1530/REP-08-0250
Frye, C. A., Petralia, S. M., Rhodes, M. E., and DeBold, J. F. (2010). 6-hydroxydopamine lesions enhance progesterone-facilicated lordosis of rats and hamsters, independent of effects in motor behavior. Physiol. Behav. 2, 218–224. doi: 10.1016/j.physbeh.2009.09.006
Frye, C. A., and Rhodes, M. E. (2006a). Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology 83, 336–347. doi: 10.1159/000096051
Frye, C. A., and Rhodes, M. E. (2006b). Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J. Neuroendocrinol. 18, 960–975. doi: 10.1111/j.1365-2826.2006.01494.x
Frye, C. A., and Rhodes, M. E. (2008). Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon and cortex of female rats. Behav. Brain Res. 187, 88–99. doi: 10.1016/j.bbr.2007.08.031
Frye, C. A., Rhodes, M. E., Petralia, S. M., Walf, A. A., Sumida, K., and Edinger, K. L. (2006a). 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience 138, 1007–1014. doi: 10.1016/j.neuroscience.2005.06.015
Frye, C. A., Walf, A. A., and Petralia, S. M. (2006b). In the ventral tegmental area, progestins have actions at D1 receptors for lordosis of hamsters and rats that involve GABAA receptors. Horm. Behav. 50, 332–337. doi: 10.1016/j.yhbeh.2006.04.001
Frye, C. A., and Vongher, M. J. (2001). Ventral tegmental area infusions of inhibitors of the biosynthesis and metabolism of 3α,5α-THP attenuate lordosis of hormone-primed and behavioral oestrous rats and hamster. J. Neuroendocrinol. 12, 1076–1086. doi: 10.1046/j.1365-2826.2001.00731.x
Gargiulo, P. A., and Donoso, A. O. (1995). Interaction between glutamate and luteinizing hormone-releasing hormone (LHRH) in lordosis behavior and luteinizing hormone release (LH): further studies on NMDA receptor mediation. Physiol. Behav. 58, 169–173. doi: 10.1016/0031-9384(95)00040-p
Gargiulo, P. A., Muñoz, V., and Donoso, A. O. (1992). Inhibition by N-methyl-D-aspartic acid (NMDA) receptor antagonist of lordosis behavior induced by estrogen followed by progesterone or luteinizing hormone-releasing hormone (LHRH) in the rat. Anat. Cell Biol. 52, 737–739. doi: 10.1016/0031-9384(92)90407-s
Genud, R., Merenlender, A., Gispan-Herman, I., Maayan, R., Weizman, A., and Yadid, G. (2009). DHEA lessens depressive-like behavior via GABA-ergic modulation of the mesolimbic system. Neuropsychopharmacology 34, 577–584. doi: 10.1038/npp.2008.46
Gifkins, A., Greba, Q., and Kokkinidis, L. (2002). Ventral tegmental area dopamine neurons mediate the shock sensitization of acoustic startle: a potential site of action for benzodiazepine anxiolytics. Behav. Neurosci. 116, 785–794. doi: 10.1037/0735-7044.116.5.785
Girdler, S. S., Lindgren, M., Porcu, P., Rubinow, D. R., Johnson, J. L., and Morrow, A. L. (2012). A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology 37, 543–553. doi: 10.1016/j.psyneuen.2011.08.004
González-Flores, O., Camacho, F. J., Domínguez-Salazar, E., Ramírez-Orduna, J. M., Beyer, C., and Paredes, R. G. (2004). Progestins and place preference conditioning after paced mating. Horm. Behav. 46, 151–157. doi: 10.1016/j.yhbeh.2004.02.006
Gorzalka, B. B., and Moe, I. V. (1994). Adrenal role in proceptivity and receptivity induced by two modes of estradiol treatment. Physiol. Behav. 55, 29–34. doi: 10.1016/0031-9384(94)90005-1
Gu, X., and Lu, W. (2018). Genetic deletion of NMDA receptors suppresses GABAergic synaptic transmission in two distinct types of central neurons. Neurosci. Lett. 6, 147–153. doi: 10.1016/j.neulet.2018.01.024
Gunn, B. G., Brown, A. R., Lambert, J. J., and Belelli, D. (2011). Neurosteroids and GABAA receptor interactions: a focus on stress. Front. Neurosci. 5:131. doi: 10.3389/fnins.2011.00131
Johansson, T., and Le Grevès, P. (2005). The effect of dehydroepiandrosterone sulfate and allopregnanolone sulfate on the binding of [3H]ifenprodil to the N-methyl-D-aspartate receptor in rat frontal cortex membrane. J. Steroid Biochem. Mol. Biol. 94, 263–266. doi: 10.1016/j.jsbmb.2005.01.020
King, S. R. (2008). Emerging roles for neurosteroids in sexual behavior and function. J. Androl. 29, 524–533. doi: 10.2164/jandrol.108.005660
Lambert, J. J., Peters, J. A., and Cottrell, G. A. (1987). Actions of synthetic and endogenous steroids on the GABAA receptor. Trends Pharmacol. Sci. 8, 224–227. doi: 10.1016/0165-6147(87)90067-8
Majewska, M. D., Harrison, N. L., Schwartz, R. D., Barker, J. L., and Paul, S. M. (1986). Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007. doi: 10.1126/science.2422758
Marshall, J. F., and Teitelbaum, P. (1974). Further analysis of sensory inattention following lateral hypothalamic damage in rats. J. Comp. Physiol. Psychol. 86, 375–395. doi: 10.1037/h0035941
Maurice, T., Grégoire, C., and Espallergues, J. (2006). Neuro(active)steroids actions at the neuromodulatory sigma1 (sigma1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol. Biochem. Behav. 84, 581–597. doi: 10.1016/j.pbb.2006.07.009
McCarthy, M. M., Pfaff, D. W., and Schwartz-Giblin, S. (1991). Midbrain central gray GABAA receptor activation enhances, and blockade reduces, sexual behavior in the female rat. Exp. Brain Res. 86, 108–116. doi: 10.1007/bf00231045
Meisel, R. L., and Pfaff, D. W. (1985). Brain region specificity in estradiol effects on neuronal ultrastructure in rats. Mol. Cell. Endocrinol. 40, 159–166. doi: 10.1016/0303-7207(85)90171-6
Mohn, A. R., Gainetdinov, R. R., Caron, M. G., and Koller, B. H. (1999). Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98, 427–436. doi: 10.1016/s0092-8674(00)81972-8
Morrow, A. L., Suzdak, P. D., and Paul, S. M. (1987). Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur. J. Pharmacol. 142, 483–485. doi: 10.1016/0014-2999(87)90094-x
Nofrey, B., Rocha, B., Lopez, H. H., and Ettenberg, A. (2008). The effects of sexual experience and estrus on male-seeking motivated behavior in the female rat. Physiol. Behav. 3, 533–538. doi: 10.1016/j.physbeh.2008.08.002
Paredes, R. G., and Alonso, A. (1997). Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav. Neurosci. 111, 123–128. doi: 10.1037/0735-7044.111.1.123
Paredes, R. G., and Vazquez, B. (1999). What do female rats like about sex? Paced mating. Behav. Brain Res. 105, 117–127. doi: 10.1016/s0166-4328(99)00087-x
Pednekar, J. R., and Mascarenhas, J. F. (1993). Role of striatal dopamine in the lordosis behavior. Indian J. Physiol. Pharmacol. 4, 333–336.
Petralia, S. M., DeBold, J. F., and Frye, C. A. (2007). MK-801 infusions to the ventral tegmental area and ventromedial hypothalamus produce opposite effects on lordosis of hormone-primed rats. Pharmacol. Biochem. Behav. 86, 377–385. doi: 10.1016/j.pbb.2007.01.005
Petralia, S. M., Jahagirdar, V., and Frye, C. A. (2005). inhibition biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioural oestrus. J. Endocrinol. 17, 545–552. doi: 10.1111/j.1365-2826.2005.01342.x
Porcu, P., Mostallino, M. C., Sogliano, C., Santoru, F., Berretti, R., and Concas, A. (2012). Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABAA receptor subunit expression and anxiety-like behavior. Pharmacol. Biochem. Behav. 102, 366–372. doi: 10.1016/j.pbb.2012.05.011
Reddy, D. S., and Kulkarni, S. K. (1997). Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 752, 61–71. doi: 10.1016/s0006-8993(96)01447-3
Rodbard, D., and Hutt, D. M. (1974). “Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting,” in Symposium on Radioimmunoassay and Related Procedures in Medicine, International Atomic Energy Agency, (New York, NY: Uniset), 209–233.
Shen, H., Gong, Q. H., Yuan, M., and Smith, S. S. (2005). Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology 49, 573–586. doi: 10.1016/j.neuropharm.2005.04.026
Shirayama, Y., Muneoka, K., Fukumoto, M., Tadokoro, S., Fukami, G., Hashimoto, K., et al. (2011). Infusions of allopregnanolone into the hippocampus and amygdala, but not into the nucleus accumbens and medial prefrontal cortex, produce antidepressant effects on the learned helplessness rats. Hippocampus 21, 1105–1113. doi: 10.1002/hipo.20824
Sirinathsinghji, D. J., Whittington, P. E., and Audsley, A. R. (1986). Regulation of mating behavior in the female rats by gonadotropin-releasing hormone in the ventral tegmental area: effects of selective destruction of the A10 dopamine neurons. Brain Res. 4, 167–173. doi: 10.1016/0006-8993(86)90406-3
Ueno, S., Bracamontes, J., Zorumski, C., Weiss, D. S., and Steinbach, J. H. (1997). Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J. Neurosci. 17, 625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997
Zessen, R. V., Phillips, J. L., Budygin, E., and Stuber, G. D. (2012). Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194. doi: 10.1016/j.neuron.2012.02.016
Keywords: allopregnanolone, anxiety, bicuculline, dizocilpine, lordosis
Citation: Frye CA, Qrareya A, Llaneza DC and Paris JJ (2020) Central Actions of 3α,5α-THP Involving NMDA and GABAA Receptors Regulate Affective and Sexual Behavior of Female Rats. Front. Behav. Neurosci. 14:11. doi: 10.3389/fnbeh.2020.00011
Received: 08 September 2019; Accepted: 20 January 2020;
Published: 11 February 2020.
Edited by:
Patrizia Porcu, Institute of Neuroscience (CNR), ItalyReviewed by:
Alonso Fernandez-Guasti, Center for Research and Advanced Studies (CINVESTAV), MexicoJonathan Paul Fry, University College London, United Kingdom
Copyright © 2020 Frye, Qrareya, Llaneza and Paris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheryl A. Frye, cafrye@albany.edu
 Cheryl A. Frye
Cheryl A. Frye